Abstract
Enhancer is a positive regulator for spatiotemporal development in eukaryotes. As a cluster, super‐enhancer is closely related to cell identity‐ and fate‐determined processes. Both of them function tightly depending on their targeted transcription factors, cofactors, and genes through distal genomic interactions. They have been recognized as critical components and played positive roles in transcriptional regulatory network or factory. Recent advances of next‐generation sequencing have dramatically expanded our ability and knowledge to interrogate the molecular mechanism of enhancer and super‐enhancer for transcription. Here, we review the history, importance, advances and challenges on enhancer and super‐enhancer field. This will benefit our understanding of their function mechanism for transcription underlying precise gene expression.
Keywords: enhancer, next‐generation sequencing, super‐enhancer, transcription regulation
1. TRANSCRIPTION REGULATION IN EUKARYOTES
DNA is the genetic information storage in cell/organisms. Transcription is an intermediate process that synthesizes RNA and then RNA translates the message into protein to perform a specific biological function. As the first step, transcription switches on and regulates gene expression. Therefore, scientists put lots of effort and attention to the field in the long run. In 1860s, scientists proposed genetic factor to explain “one gene‐one trait” which was based on Mendel's pea experiments.1 In 1941, Beadle and Eatum proposed “one gene‐one enzyme” to explain inborn errors of metabolism.2 In 1957, “one gene‐one polypeptide” was introduced due to the progress of biochemical genetics.3 In 1958, Crick proposed central dogma which is often stated as “DNA makes RNA and RNA makes protein” (Figure 1).4 Central dogma defines the genetic information flow of DNA, RNA, and protein. It has clarified the role of these three macromolecules in transcription. Since then, transcription has become the central field of biologists. In 1970s, “one gene‐multiple RNAs” hypothesis was proposed due to splicing and other progresses on molecular biology.5 Meanwhile, transcription has been recognized as a dynamic process. Scientists divide it into multiple sub‐processes, mainly including initiation, elongation, and termination (Figure 2).6 RNA polymerase II (RNAPII) is identified as the core factor to initiate and regulate gene expression by coordinating with lots of other factors, including general transcription factors, enhancers, mediators, cohesions, insulators, and silencers accompanying with other epigenetic mechanisms.7 In the past decades, next generation sequencing (NGS) has been innovated into transcription research.8, 9, 10 Genome architecture, methylation, acetylation, and other histone modifications have also been brought into the field, which dramatically extended the view of transcription regulation.10 Among them, as the vigorous positive factor, enhancer attracts special interests of scientists.11, 12, 13
Figure 1.
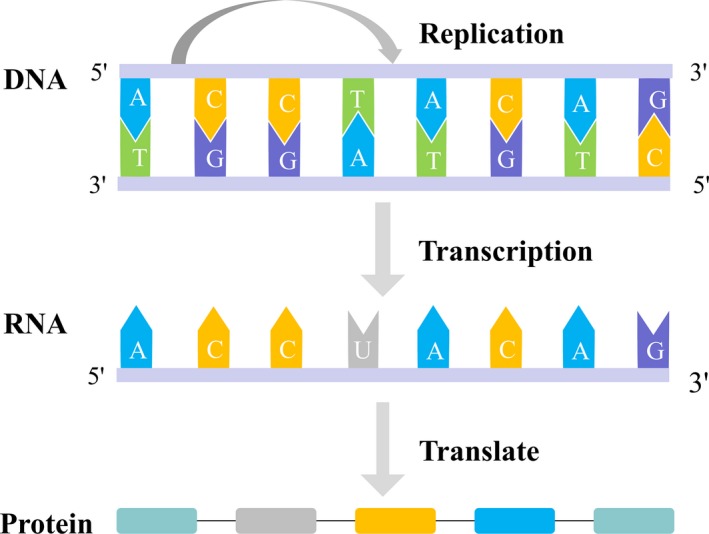
Diagram of central dogma
Figure 2.
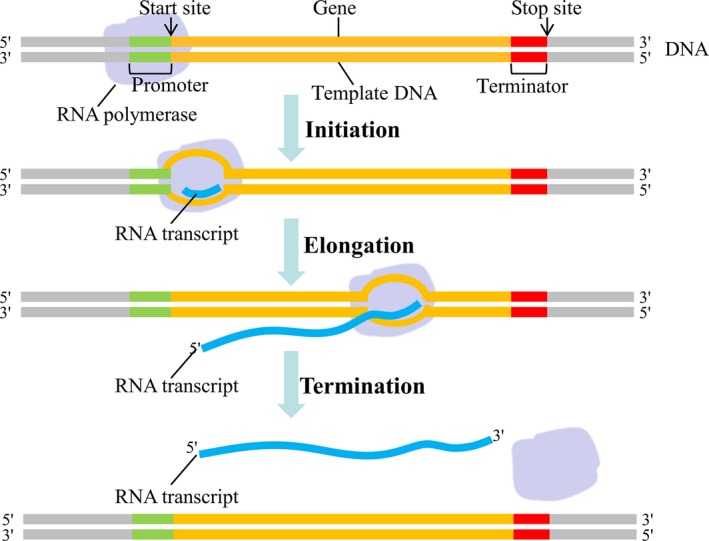
Diagram of transcription sub‐processes, including initiation, elongation and termination
2. ENHANCER IS A POSITIVE REGULATOR IN TRANSCRIPTION
2.1. Enhancer is a positive regulator
Enhancer is a short region of DNA that can be bound by proteins (activators) to activate transcription of a gene.14 It can positively regulate spatiotemporal gene expression during development through either cis‐ or trans‐ interaction manner (Figure 3).13, 15, 16, 17 In 1981, enhancer was first described as a 72‐bp repeated sequence in simian virus 40 (SV40) genome, which could increase the ectopic expression of a reporter gene by ~200‐fold.18, 19 In 1983, enhancer was discovered within a mouse immunoglobulin heavy chain gene in mammals.20 Subsequently, different enhancers in various cells and tissues have been reported.14, 15, 16, 17
Figure 3.
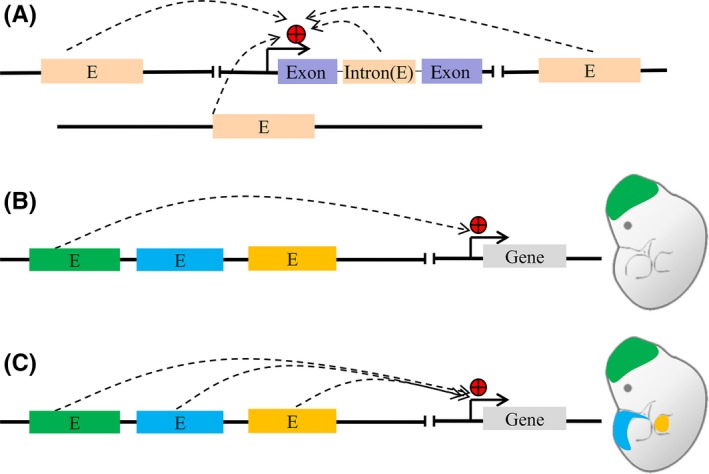
A, Enhancers are cis‐regulatory elements that can increase expression of target genes in cis and trans‐acting manner; (B and C) Enhancer regulate spatiotemporal gene expression
2.2. Properties of enhancer chromatin
Enhancers activity are usually linked with certain properties of chromatin (Figure 4). Active enhancers are typically bound with transcription factors (TFs).21 The flanking of enhancers are commonly marked by histone modifications such as histone H3 lysine 4 monomethylation (H3K4me1) and H3K27 acetylation (H3K27ac).22, 23, 24 Active enhancers are marked by both H3K4me1 and H3K27ac, with depletion of histone H3 lysine 4 trimethylation (H3K4me3);22 inactive, poised enhancers are marked only with H3K4me1.24 In addition, enhancers are typically depleted of nucleosomes and sensitive to DNase I digestion.25 Distal enhancers are brought into close proximity with their target promoters through chromatin looping,14 which is facilitated by mediators and cofactors.11, 21 Moreover, active enhancer can recruit RNAPII and produce RNAs that contributes to its function and gene regulation.26, 27
Figure 4.
Modes of enhancer action
2.3. Enhancer identification
Traditionally, enhancers have been identified based on their ability to increase transcription by using reporter gene assays.14, 18 Transgenic reporters are widely used for enhancer identification in animal models such as nematode, fruit fly, and mouse.14 Traditional transgenic reporter assays, for example, those based on luciferase, are usually low throughput as they could only validate individual enhancer in a relative simple mode.14, 18 In the recent years, with the advent of NGS, high‐throughput computational and experimental methods have been adapted to predict enhancers.14, 28 These are mainly included in several categories: (a) Computational analysis of conserved noncoding sequences and TF binding motif29, 30, 31; (b) Chromatin immunoprecipitation and sequencing (ChIP‐seq)28 for transcription factors,32, 33 mediators and cofactors such as P300,34, 35 and histone modifications such as H3K4me1 and H3K27ac23, 24; (c) Chromatin accessibility assays, including DNase I digestion coupled to sequencing (DNase‐seq),25, 36 formaldehyde‐assisted isolation and sequencing (FAIRE‐seq),37 and transposase‐accessible chromatin followed by sequencing (ATAC‐seq)38; (d) Multiple methods depending on the detection of enhancer RNAs,28 including global run‐on sequencing (GRO‐seq),39 precision nuclear run on and sequencing (PRO‐seq),40 native elongating transcript sequencing (NET‐seq),41 cap‐analysis gene expression (CAGE)42; (e) Methods based on enhancer‐promoter interactions, including chromosome conformation capture (3C),43 4C,44 5C,45 Hi‐C,46 and chromatin interaction analysis by paired‐end tag sequencing (ChIA‐PET)47; (f) Methods of testing enhancer activity,28 such as massively parallel reporter assays (MPRAs),48 self‐transcribing active regulatory region sequencing (STARR‐seq),49 and functional identification of regulatory elements within accessible chromatin (FIREWACh).50 Currently, enhancers can be defined by using one or combinations of these methods.
Accordingly, thousands of enhancers in different model animals such as fruit fly, nematode and mouse, as well as human have been annotated by different international genome annotation consortia, such as ENCODE,51 NIH Epigenome Roadmap,36 FANTOM5,42, 52 and Blueprint/IHEC.53 At the same time, enhancer related databases such as VISTA Enhancer Browser,54 Enhancer Atlas,55 and HEDD56 have been developed for visualizing and sharing information of enhancers annotations across mammalians. These useful resources provide new insight into their roles and mechanism of enhancers‐mediated gene regulation.
3. ROLE AND ADVANCE ON TRANSCRIPTION RESERCHES
3.1. H3K4me1 and H3K27ac
H3K4me1 and H3K27ac are commonly used hallmarks to identify putative genome‐wide enhancers.11, 57 H3K4me1 and H3K27ac are conferred by the mixed lineage leukemia (MLL) family of methyltransferease (MLL2/3/4) and the CREB‐binding protein (CBP)/P300 acetyltransfereases, respectively.11, 57 Knocking out H3K4 methytransferases MLL3 and MLL4 have resulted in a global loss of H3K4me1 binding, and subduction of H3K27ac, mediators and RNAPII bindings as well.58, 59 It has been found that H3K4me1 can facilitate recruitment of the cohesion complex to chromatin, which provides a potential mechanism for MLL3/4 to promote chromatin interactions between enhancers and promoters.60 In addition, a recent study has suggested that H3K4me1 might play a fine‐tune role in enhancer activity by facilitating binding of the BAF complex and possibly other chromatin regulators.61 Meanwhile, active enhancers in both flies62 and mice63 are not necessarily marked by H3K27ac, but H3K27ac has been supposed to affect enhancer activity through destabilizing nucleosomes or recruiting H3K27ac‐binding proteins.64 All these evidences imply that H3K4me1 and H3K27ac themselves are not required for enhancer activity.
3.2. Diverse modes of enhancer action
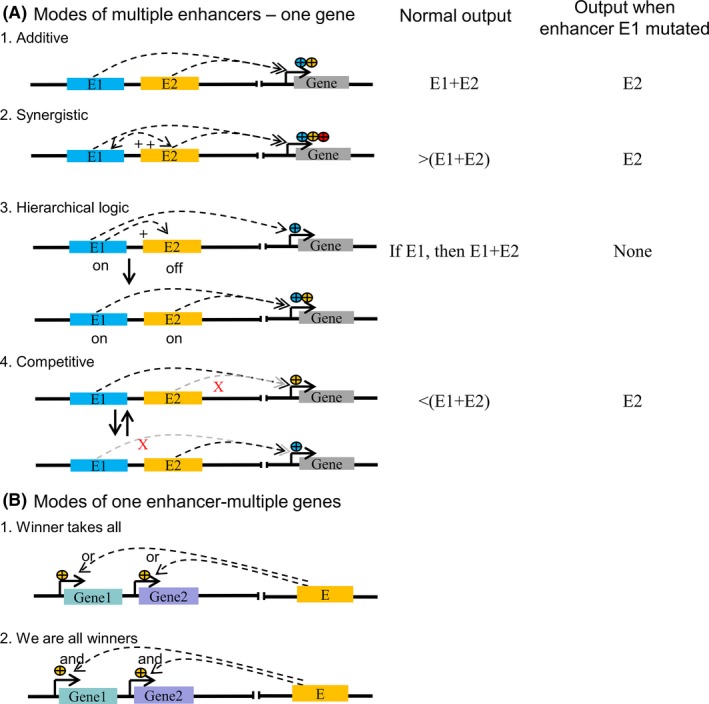
As time goes by, enhancer has been recognized that it could regulate gene expression in quite diverse manners, which are summarized as “multiple enhancers—one target gene” (Figure 4A) and “one enhancer—multiple target genes” patterns (Figure 4B).65, 66 The former pattern includes addictive, synergistic, hierarchical, and redundant mode. (a) An additive mode represents that gene transcription is determined by the superimposed effect of multiple enhancers (Figure 4A‐1). For example, the even skipped (eve) gene is expressed in seven pair‐rule stripes along the length of Drosophila embryo due to five separate enhancers,16 so as enhancers of α‐ and β‐globin genes in mouse erythroid cells,67, 68 within the developing limb69; (b) A synergistic mode proposes that multiple enhancers produce an effect greater than the sum of their individuals (Figure 4A‐2),70 for example, enhancers near hunchback and knirps in Drosophila, 71 and murine Fgf8 locus72; (c) A hierarchical logic mode supposes that one or some enhancers can first activate one gene transcription to a basal level, while these enhancers could initiate the activity of their nearby enhancers to amplify its expression (Figure 4A‐3). As an example, a conditional relationship between two enhancers near the PU.1 locus in mouse myeloid cells70; (d) A redundancy mode describes that lossing one of gene‐associated enhancers would not greatly affect its expression pattern due to their functional redundancy.73 A potential mechanism of this might be a competition model that two enhancers compete for one target gene, which could ensure a relative constant gene expression in the case of one enhancer loss (Figure 4A‐4).73 Enhancer redundancy is a remarkably widespread feature in mammalian genome.66, 74, 75
On the other hand, the solo enhancer is able to regulate multiple genes (Figure 4B). Two types of competition modes, “winner takes all” and “we are all winners,” have been proposed to explain this.65 For the first one, only one target gene is activated and expressed in each cell (Figure 4B‐1). As an example, to ensure unique identity of neurons, only one olfactory receptor gene or protocadherin gene is expressed in each cell of its sensory system and brain.76, 77 For the second one, multiple genes are activated and expressed in all cells, but they are not necessarily expressed at maximum levels (Figure 4B‐2). This mode can be detectable when the deletion of one such gene would increase other gene expression,78 or the introduction of an extra gene copy would decrease other gene expression.79, 80 The interaction of multiple genes expression are switched by a shared enhancer in one cell is thought to belong this mode.81
3.3. Enhancer‐promoter interactions
3.3.1. DNA‐Looping
Enhancer‐promoter interactions can be commonly found to determine spatiotemporal gene expression pattern in eukaryotes.82, 83 This has been well presented by studies of the globin locus control region (LCR) and its target gene.84, 85 During erythroid development, LCR activates distinct globin genes in a stage specific manner through the formation of DNA looping.86 LCR‐β‐globin interactions are established dependent on gene‐specific transcription factors, including the hematopoietic‐specific factors GATA1 and FOG1,87 KLF,88 and the widely expressed factor LDB1.89 The depletion of LDB1 has been previously reported to disrupt long‐range LCR loop formation, and thus affect gene transcription.89 There are other examples of specific gene regulation involving in enhancer‐promoter looping. The Satb1 gene is silent when its promoter does not contact with enhancers in the brain, whereas it is highly expressed when enhancer‐promoter looping has been de novo formed in the thymus.90 In the latest study, a distal enhancer of Sox9 can reverse sex in mouse,91 which suggests DNA‐Looping could also determine specific traits.
The protein yin and yang (YY1) has been recognized as a structural mediator of DNA looping in recent study.92 YY1 could globally mediate enhancer‐promoter interactions by binding to DNA and facilitate the formation of chromatin loops, probably through its dimerization.92 In addition, YY1 has been further indicated to positively regulate transcription by targeting promoters and enhancers to through the BAF complexes in embryonic stem cells.93
3.3.2. TADs
Along with the 3D genome architecture, topologically associating domain (TAD) has been realized as a popular pattern for enhancer function. TAD is a proposed selfing‐interaction genomic territory, meaning that DNA sequences physically interact with each other more frequently within than outside.90 Recent studies have indicated that TADs might ensure proper physical interactions between promoters and distal enhancers.94 For example, Shh expression is not affected by changing the distance between Shh gene and its associated enhancer (ZRS) within TAD.94 Conversely, it has been altered by inversions disrupting the TAD between them.94
The mechanism leading to the TAD boundary formation have attracted the study interest of many biologists. TADs are suspected to be bordered by dimerization of the zinc finger protein CTCF bound to chromatin.95 Disruption of a conserved CTCF‐cohesion boundary extends the sub‐TAD of the mouse α‐globin gene cluster to adjacent CTCF‐cohesin‐binding sites.96 This in turn allows α‐globin enhancers to interact with more additional promoters located within extended sub‐TAD. In addition, a study of the Sox9 locus has showed that duplication of boundary‐containing regions results in the formation of a new TAD that is insulated from its neighbors by the duplicated boundary.97 However, the research field of TAD remains controversial, more efforts and data will be eager for further interpreting its mechanism.
3.4. Enhancer RNAs
Enhancer RNAs (eRNAs) are a new class of long noncoding RNAs synthesized at enhancers,98 which are correlated with enhancer activity and contribute to gene regulation.98, 99 The transcription of enhancer was first reported in the locus control region (LCR) of the β‐globin gene.100 Subsequently, enhancers have been found to be broadly transcribed.26, 101, 102, 103 Unlike messenger RNAs (mRNAs), eRNAs are generally short, non‐coding, bidirectionally transcribed, and their 3′‐end are not polyadenylated.42, 102, 104 Meanwhile, they are susceptible to exosome‐mediated degradation and express at very low levels.104, 105 Recent studies have revealed that eRNAs can be generated through unidirectional transcription, that are longer and contain a poly A tail.106 eRNAs could promote transcription by facilitating nucleosomes depletion and establishing DNA accessibility.107, 108 Moreover, nascent eRNAs have been found to contribute to the stabilization of TF binding,109 the recruitment and activation of cofactors,110, 111, 112, 113 the release of negative elongation factor (NELF) from promoters.114 In addition, eRNAs have been indicated to play a role in gene regulatory networks by controlling promoter and enhancer interactions and topology of higher order chromatin structure.115
4. SUPER‐ENHANCER DETERMINES CELL INDENTITY AND FATE
4.1. Super‐enhancer is a cluster of enhancers
Super‐enhancer is emerging as cluster of enhancers that is densely occupied by the master regulators and mediators, which is speculated to act as switches to determine cell identity and fate.116, 117 This notion was first described as genomic regions with high levels of five master transcription factors (Oct4, Sox2, Nanog, Klf4, and Esrrb) and the Mediators in mESCs.117 Subsequent studies have extended the concept of super‐enhancers as genomic regions densely occupied by high levels of H3K4me1, H3K27ac, p300 or master transcription factors in multiple cell types and tissues.116, 118 The main identification procedure has been summarized as five steps (taking H3K27ac as example, Figure 5): (A) performing H3K27ac ChIP‐seq experiment in the interested cell types or tissues; (B) mapping H3K27ac ChIP‐seq data to reference genome; (C) calling peaks using peak calling algorithm, for example, MACS2119; (D) stitching enhancers within 12.5 kb of each other (performing in ROSE); (E) plotting the ranked stitched enhancers and the remaining individual enhancers by the total background‐normalized levels of H3K27ac within the genomic region; a line with a slope of one tangent to the curve is used as a cutoff to distinguish super‐enhancers above the point and typical enhancers below the point of tangency (performing in ROSE).
Figure 5.
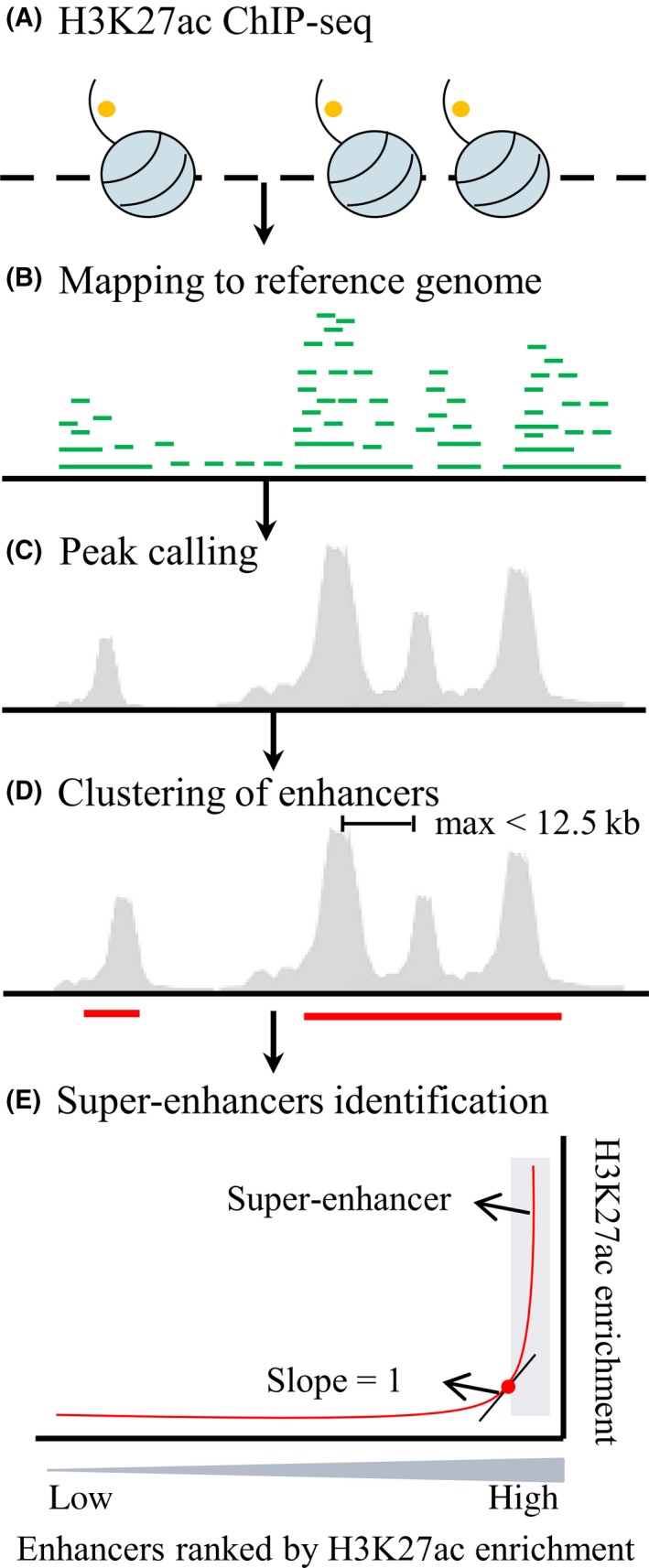
Identification procedure of super‐enhancers
4.2. Properties of super‐enhancers
Super‐enhancers differ from typical enhancers in size, transcription factor density and content, and ability to activate transcription (Figure 6). In addition, super‐enhancers produce higher level of eRNAs than typical enhancers,116, 117 for example, about 93% of super‐enhancers and about 30% of intergenic typical enhancers are associated with eRNAs during toll‐like receptor 4 (TLR4) signaling in macrophages, respectively.120 Super‐enhancer and its associated genes are frequently located within a loop connected by two CTCF sites co‐occupied by cohesion within TADs, as an example, 84% of super‐enhancers and 48% of typical enhancers are located within such structures in mESCs, respectively.121 Remarkably, super‐enhancers are capable of maintaining cell identity, determining cell fate, driving oncogene transcription in cancer cells.118, 122
Figure 6.
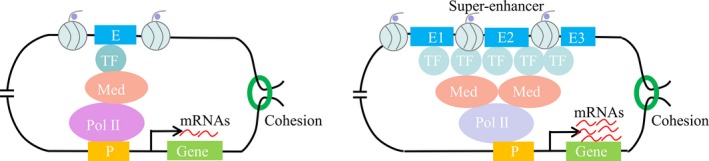
Differences between organization and function of typical enhancers and super‐enhancers
4.2.1. Maintaining cell identity
A series of studies have indicated that super‐enhancers are capable of maintaining cell identity. In mESCs, both super‐enhancers and typical enhancers are co‐occupied by master TFs Oct4, Sox2, and Nanog, which are important for pluripotency; but only super‐enhancers are densely occupied by TFs KLF and ESrrb, which play important role in cell identity.117 In the same study, the crucial role of super‐enhancers in cell identity has been further revealed by that reduced levels of Oct4 or Mediators cause preferential loss of expression of super‐enhancer‐associated genes relative to other genes in mESCs.117 Likewise, key TFs that control cell identity have been found to bind at super‐enhancer in other differentiated cell types, such as myotubes (MyoD), T helper (Th) cells (T‐Bet) and macrophages (C/EBPα).117 Subsequently, super‐enhancers co‐occupied by lineage‐specific factors have been identified in diverse cell types such as adipocytes, hair follicle stem cells, and mammary epithelial cells.12, 123, 124, 125, 126 For example, in the mammary epithelium, mammary‐specific super‐enhancers have been identified with mammary‐enriched transcription factors, such as signal transducer and activator of transcription 5 (STAT5), glucocorticoid receptor (GR), E74 like ETS transcription factors 5 (ELF5), and nuclear factor I B (NFIB).124
In addition, super‐enhancers are correlated with lineage‐specific transcriptional factors and oncogenes in a broad spectrum of cancers, such as neuroblastoma,127 small‐cell lung cancer (SCLC),128 medulloblastoma,129 breast,57 esophageal,130 gastric cancers,131 and melanoma.132 Moreover, in medulloblastoma, super‐enhancers are able to distinguish its four main subtypes based on underlying biochemical and genetic signatures, suggesting that super‐enhancers are correlated with tumor heterogeneity and define cell identity.129 In addition, studies have revealed that different super‐enhancers have same target oncogenes in various tumor types, for example, tumor‐specific super‐enhancer profiles have been found at the MYC and MYCN loci,127, 128 which further indicate the importance of super‐enhancers on maintaining cell identity.
4.2.2. Determining cell fate
Analyses of the super‐enhancer dynamics during lineage commitment of specific cell types have shown that super‐enhancers are remodeled during differentiation, having crucial roles in cell fate determination.123, 133, 134 Deletion of super‐enhancer constituents at the Nanog 135 or Sox2 136 locus in mouse embryonic stem cells reduces the corresponding target gene expression and impaired some other key pluripotency genes, resulting in cellular differentiation. In another example, distinct super‐enhancer landscape and super‐enhancer‐associated TF network have been identified for mesenchymal and adrenergic cells, and the state of adrenergic cells towards mesenchymal is associated with the changes of super‐enhancers landscape.137
4.2.3. Driving oncogene transcription in cancer cells
Super‐enhancers have been found to drive the expression of a few critical oncogenes in several types of tumor cells.122 In Nasopharyngeal carcinoma, super‐enhancers are linked to genes important for oncogenesis including ETV6.138 In Oesophageal squamous cell carcinoma (OSCC), super‐enhancers are associated with oncogenes including PAK4, RUNX1, DNAJB1, SREBF2, and YAP1.130 Deletion of a super‐enhancer reduces the expression of cancer‐related genes and impairs some oncogenic properties.139 In contrast, duplication of super‐enhancers leads to overexpression of a key oncogenic transcription factor, which then activates other cancer‐related genes in squamous cell carcinomas.140 Super‐enhancers can be targeted through inhibition of chromatin and transcriptional regulators that disproportionately bound to these regulatory elements super‐enhancers.122 Recent studies have demonstrated that JQ1 (a competitive inhibitor of BRD4, and a covalent inhibitor of CDKs), selectively kill cancer cells by inhibiting SE‐driven oncogenic transcription, with both agents lacking systemic toxic effects in vivo.127, 128, 141, 142
5. CHALLENGES AND ONGOING STUDIES
As positive transcription regulators, scientists have put lots of efforts and made significant progress on enhancer and super‐enhancer related studies. So far, there are still a few challenges remained for understanding their role and mechanism in gene transcription: (a) precisely identifying enhancer motif across the genome; (b) validating vast enhancer candidates identified by ChIP‐seq and other methods; (c) precisely annotating enhancers to their target genes in genome; (d) the ambiguous definition and unclear composition of super‐enhancer.
5.1. Precision identification of motif
Precisely, identification of motifs is essential for understanding the enhancer function mechanism and genome constitution. Motif is a degenerate short (6‐10 bp) DNA sequence pattern that summarizes the DNA sequence binding preference of a transcription factor.14 Enhancer motifs recruit transcription factors, which in turn enroll cofactors, and thus activate transcription.14 They are highly linked to enhancer activities and gene expression.66 The space between motifs is one of factors contributing to enhancer activities. For example, the neural plate‐specific Otx‐a enhancer in Ciona controls Otx‐a expression in a moderate and proper manner.143 This enhancer contains GATA and ETS DNA sequence motifs.143 A 3 bp insertion between one set of them has been found to result in a threefold increase of Otx expression.143 Thus, precisely motifs are important for understanding enhancers function. However, up to now, the identified potential enhancer candidates, by various methods such as ChIP‐seq, bestrow hundreds of base pairs along the genome (Figure 7).28, 34 The conflict size differences between motif and potential enhancer candidates would result in the difficulty for dissecting enhancer, its function and genome annotation.
Figure 7.
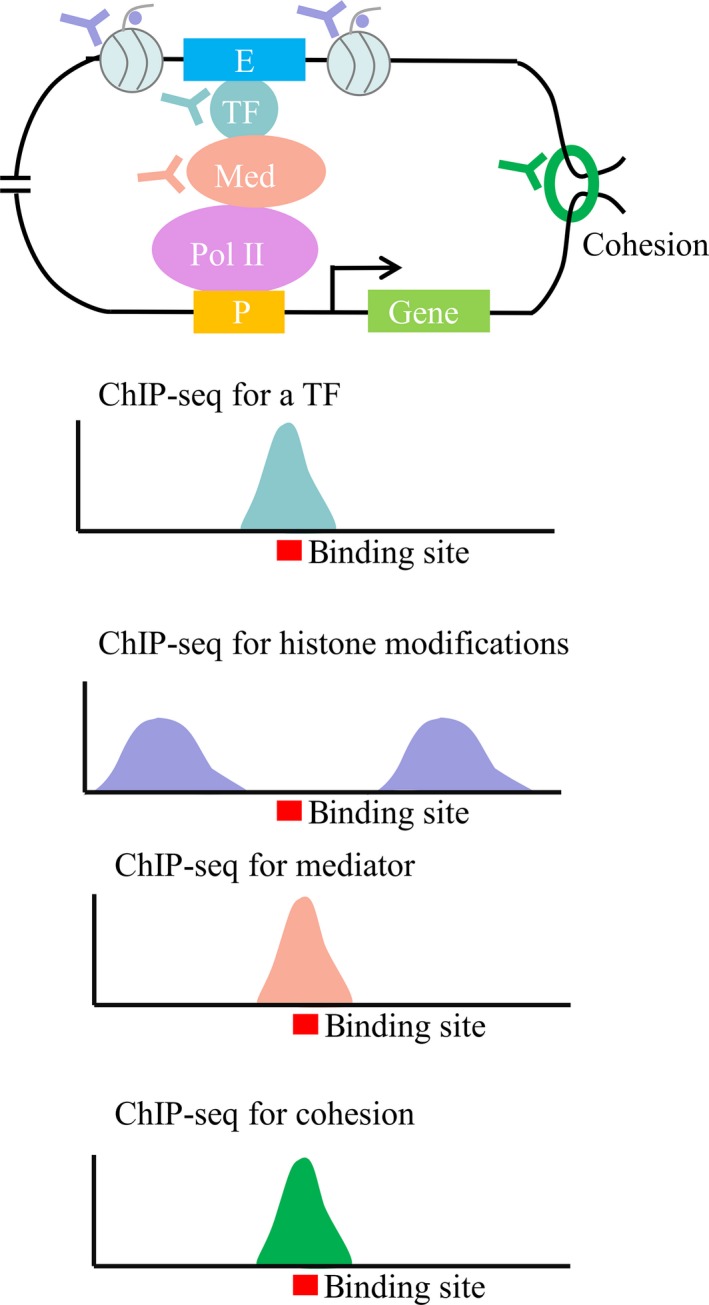
Scheme of binding site for one TF
Scientists have started to put their effort to position motif precisely. There are several methods developed to identify enhancers at high resolution and low background. For example, ChIP‐exo, a derivation of ChIP‐seq, has been adapted.144 Compared to ChIP‐seq, ChIP‐exo includes an additional step of exonuclease digestion that trims DNA fragments.144 This step allows identifying putative enhancer candidates at high resolution and low background noise, and in turn positioning motifs more precisely.144 However, the current ChIP‐exo technique has been applied to limited cell types. Thus, more efforts are required for developing new experimental methods and algorithms of enhancer identification and motif position in the future.
5.2. The validation of enhancer activity
Identifying functional enhancers is an important step for understanding their mechanism in gene transcription. Up to now, hundred thousands of putative enhancer candidates have been identified across human and multiple model animals,23, 24 but not all of them are representative of functional ones. Indeed, with the data generated by the ENCODE Project, only a fraction (26%) of enhancer candidates display enhancer activity with reporter assays.51, 145 In addition, the data in VISTA Enhancer Browser reveals that only 50% of putative candidate elements exhibit enhancer activity in transgenic mouse (up to date 23 June 2018). With the development of NGS, several high‐throughput screening methods, such as MPRA, STARR‐seq and FIREWACh, have been adapted to validate enhancers activity.48, 49, 50 These related methods have greatly improved our ability to validate enhancers activity, but there are still a lot of putative enhancer candidates have not been functionally tested.23, 24 Therefore, enhancers activity validating remains as a challenge for biologists.
5.3. The assignment of enhancers to their target promoters
Enhancer‐promoter interaction is important for gene transcription and has commonly occurred in eukaryotes. However, their related information or data in multiple cell types/tissues is still lacking. A few years ago, enhancers have been typically assigned to their neighboring promoters based on linear proximity or shared chromatin states.116 However, enhancers do not always regulate their neighboring genes. A well‐characterized example is that the ZRS enhancer, which resides in an intron of Lmbr1 (encoding limb region 1 protein), contributes to the limb bud activation of the Shh gene, which locates in nearly 1 Mb away.146, 147 Sanyal et al148 have found that only 7% of distal regulatory elements control their closed promoters in human cell lines. Moreover, Zhang et al149 have found 76% of the putative enhancers do not interact with their neighboring promoters in mESCs. Thus, direct approaches for detecting enhancer‐promoter interactions are required. Several three‐dimensional technologies, such as 3C,43 4C,44 and 5C,45 Hi‐C46 and ChIA‐PET47 have been adapted to directly identify physical contacts. However, the available data of these associations is still far more insufficient. Data accumulation might be an option to solve this, which might need global efforts to achieve.
5.4. Definition and composition of super‐enhancers
Despite of biological effects of super‐enhancers, its definition is ambiguous and molecular composition is unclear.118 Super‐enhancer can be termed as enhancer cluster. However, according to its identification procedure (Figure 5), a few defined super‐enhancers are single enhancers, for example, 15% (35 of 231) are proposed as single in mESCs.117 Most defined super‐enhancers contain several ones, which are difficult to distinguish their boundaries. Accordingly, these would be an obstacle for understanding their functional mechanism in gene transcription. The ambiguous definition and unclear composition of super‐enhancers would be caused by the current low resolution methods. The concept indeed fits researcher's need to shrink the list of regulatory candidates. Therefore, the related studies have been explosively increased during the last a few years. However, its ambiguous definition and molecular composition remind us that it is a long way to uncover their mechanism.
6. CONCLUDING REMARKS
Enhancer and super‐enhancer are positive regulators for gene transcription. Scientists have made great processes on their effect and mechanism research. Their function is tightly dependent of the recruitment of transcriptional factors, cofactors, and mediators, as well as the formation of enhancer‐promoter interactions. Recent advent of NGS has greatly expanded our knowledge and skill to explore genome‐wide composition. We review their history, definition, importance advance and challenge with different aspects. Currently, precision motif, activity validation, targeted gene, and molecular mechanism are the central of the field. To achieve these goals, more efforts on developing new methods and accumulating data across different cell types/tissues are required. We hope this essay would be beneficial for further understanding the role and mechanism of enhancers and super‐enhancers in transcription, as well as for providing future clues in the field.
CONFLICT OF INTEREST
None.
ACKNOWLEDGEMENTS
The writing of this review article was supported by the China Postdoctoral Science Foundation (2017M620977, 2018T110169); Technology Research and Development Project of Dapeng New Zone (KY20160308); the Agricultural Science and Technology Innovation Program Cooperation and Innovation Mission (CAAS‐XTCX2016001‐3).
Peng YL, Zhang YB. Enhancer and super‐enhancer: Positive regulators in gene transcription. Animal Model Exp Med. 2018;1:169–179. 10.1002/ame2.12032
REFERENCES
- 1. Corcos AF, Monaghan FV. Gregor Mendel's Experiments on Plant Hybrids: a Guided Study. New Brunswick, NJ: Rutgers University Press; 1993. [Google Scholar]
- 2. Beadle GW, Tatum EL. Genetic control of biochemical reactions in Neurospora . PNAS. 1941;27(11):499‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berg P, Singer M. George Beadle, an Uncommon Farmer: the emergence of genetics in the 20th century. Cold Spring Harbor, NY: Cold Spring Harbor Labratory Press; 2003:335. [Google Scholar]
- 4. Crick FHC. On Protein Synthesis In: Sanders FK, ed. Symposia of the Society for Experimental Biology, Number XII: The Biological Replication of Macromolecules. Cambridge, UK: Cambridge University Press; 1985:138‐163. [Google Scholar]
- 5. Chow LT, Gelinas RE, Broker TR, Roberts RJ. An amazing sequence arrangement at the 5′ ends of adenovirus 2 messenger RNA. Cell. 1977;12(1):1‐8. [DOI] [PubMed] [Google Scholar]
- 6. Watson JD, Baker TA, Bell SP, Gann AA, Levine M, Losick RM. Molecular Biology of the Gene, (7th ed.). Pearson; 2013. [Google Scholar]
- 7. Juven‐Gershon T, Kadonaga JT. Regulation of gene expression via the core promoter and the basal transcriptional machinery. Dev Biol. 2010;339(2):225‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Albert I, Mavrich TN, Tomsho LP, et al. Translational and rotational settings of H2A.Z nucleosomes across the Saccharomyces cerevisiae genome. Nature. 2007;446(7135):572‐576. [DOI] [PubMed] [Google Scholar]
- 9. Dalal CK, Johnson AD. How transcription circuits explore alternative architectures while maintaining overall circuit output. Gene Dev. 2017;31(14):1397‐1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Levine M, Cattoglio C, Tjian R. Looping back to leap forward: transcription enters a new era. Cell. 2014;157(1):13‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buecker C, Wysocka J. Enhancers as information integration hubs in development: lessons from genomics. Trends Genet. 2012;28(6):276‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ko JY, Oh S, Yoo KH. Functional enhancers as master regulators of tissue‐specific gene regulation and cancer development. Mol Cells. 2017;40(3):169‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ong CT, Corces VG. Enhancer function: new insights into the regulation of tissue‐specific gene expression. Nat Rev Genet. 2011;12(4):283‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shlyueva D, Stampfel G, Stark A. Transcriptional enhancers: from properties to genome‐wide predictions. Nat Rev Genet. 2014;15(4):272‐286. [DOI] [PubMed] [Google Scholar]
- 15. Bulger M, Groudine M. Functional and mechanistic diversity of distal transcription enhancers. Cell. 2011;144(3):327‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Levine M. Transcriptional enhancers in animal development and evolution. Curr Biol. 2010;20(17):R754‐R763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Blackwood EM, Kadonaga JT. Going the distance: a current view of enhancer action. Science. 1998;281(5373):60‐63. [DOI] [PubMed] [Google Scholar]
- 18. Banerji J, Rusconi S, Schaffner W. Expression of a beta‐globin gene is enhanced by remote SV40 DNA sequences. Cell. 1981;27(2 Pt 1):299‐308. [DOI] [PubMed] [Google Scholar]
- 19. Moreau P, Hen R, Wasylyk B, Everett R, Gaub MP, Chambon P. The SV40 72 base repair repeat has a striking effect on gene expression both in SV40 and other chimeric recombinants. Nucleic Acids Res. 1981;9(22):6047‐6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Banerji J, Olson L, Schaffner W. A lymphocyte‐specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell. 1983;33(3):729‐740. [DOI] [PubMed] [Google Scholar]
- 21. Spitz F, Furlong MM. Transcription factors: from enhancer binding to developmental control. Nat Rev Genet. 2012;13(9):613‐626. [DOI] [PubMed] [Google Scholar]
- 22. Calo E, Wysocka J. Modification of enhancer chromatin: what, how, and why? Mol Cell. 2013;49(5):825‐837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Creyghton MP, Cheng AW, Welstead GG, et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. PNAS. 2010;107(50):21931‐21936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rada‐Iglesias A, Bajpai R, Swigut T, Brugmann SA, Flynn RA, Wysocka J. A unique chromatin signature uncovers early developmental enhancers in humans. Nature. 2011;470(7333):279‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thurman RE, Rynes E, Humbert R, et al. The accessible chromatin landscape of the human genome. Nature. 2012;489(7414):75‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lam MT, Li W, Rosenfeld MG, Glass CK. Enhancer RNAs and regulated transcriptional programs. Trends Biochem Sci. 2014;39(4):170‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li W, Notani D, Rosenfeld MG. Enhancers as non‐coding RNA transcription units: recent insights and future perspectives. Nat Rev Genet. 2016;17(4):207‐223. [DOI] [PubMed] [Google Scholar]
- 28. Murakawa Y, Yoshihara M, Kawaji H, et al. Enhanced identification of transcriptional enhancers provides mechanistic insights into diseases. Trends Genet. 2016;32(2):76‐88. [DOI] [PubMed] [Google Scholar]
- 29. Woolfe A, Goodson M, Goode DK, et al. Highly conserved non‐coding sequences are associated with vertebrate development. PLoS Biol. 2005;3(1):e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pennacchio LA, Ahituv N, Moses AM, et al. In vivo enhancer analysis of human conserved non‐coding sequences. Nature. 2006;444(7118):499‐502. [DOI] [PubMed] [Google Scholar]
- 31. Visel A, Prabhakar S, Akiyama JA, et al. Ultraconservation identifies a small subset of extremely constrained developmental enhancers. Nat Genet. 2008;40(2):158‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen X, Xu H, Yuan P, et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell. 2008;133(6):1106‐1117. [DOI] [PubMed] [Google Scholar]
- 33. Zinzen RP, Girardot C, Gagneur J, Braun M, Furlong EE. Combinatorial binding predicts spatio‐temporal cis‐regulatory activity. Nature. 2009;462(7269):65‐70. [DOI] [PubMed] [Google Scholar]
- 34. Visel A, Blow MJ, Li Z, et al. ChIP‐seq accurately predicts tissue‐specific activity of enhancers. Nature. 2009;457(7231):854‐858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. May D, Blow MJ, Kaplan T, et al. Large‐scale discovery of enhancers from human heart tissue. Nat Genet. 2011;44(1):89‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bernstein BE, Stamatoyannopoulos JA, Costello JF, et al. The NIH roadmap epigenomics mapping consortium. Nat Biotechnol. 2010;28(10):1045‐1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Giresi PG, Kim J, McDaniell RM, Iyer VR, Lieb JD. FAIRE ((F)under‐barormaldehyde‐(A)under‐barssisted (I)under‐barsolation of (R)under‐baregulatory (E)under‐barlements) isolates active regulatory elements from human chromatin. Genome Res. 2007;17(6):877‐885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Buenrostro JD, Giresi PG, Zaba LC, Chang HY, Greenleaf WJ. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA‐binding proteins and nucleosome position. Nat Methods. 2013;10(12):1213‐1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Melgar MF, Collins FS, Sethupathy P. Discovery of active enhancers through bidirectional expression of short transcripts. Genome Biol. 2011;12(11):R113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kwak H, Fuda NJ, Core LJ, Lis JT. Precise maps of RNA polymerase reveal how promoters direct initiation and pausing. Science. 2013;339(6122):950‐953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mayer A, di Iulio J, Maleri S, et al. Native elongating transcript sequencing reveals human transcriptional activity at nucleotide resolution. Cell. 2015;161(3):541‐554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Andersson R, Gebhard C, Miguel‐Escalada I, et al. An atlas of active enhancers across human cell types and tissues. Nature. 2014;507(7493):455‐461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dekker J, Rippe K, Dekker M, Kleckner N. Capturing chromosome conformation. Science. 2002;295(5558):1306‐1311. [DOI] [PubMed] [Google Scholar]
- 44. Simonis M, Klous P, Splinter E, et al. Nuclear organization of active and inactive chromatin domains uncovered by chromosome conformation capture‐on‐chip (4C). Nat Genet. 2006;38(11):1348‐1354. [DOI] [PubMed] [Google Scholar]
- 45. Dostie J, Richmond TA, Arnaout RA, et al. Chromosome conformation capture carbon copy (5C): a massively parallel solution for mapping interactions between genomic elements. Genome Res. 2006;16(10):1299‐1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lieberman‐Aiden E, van Berkum NL, Williams L, et al. Comprehensive mapping of long‐range interactions reveals folding principles of the human genome. Science. 2009;326(5950):289‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fullwood MJ, Liu MH, Pan YF, et al. An oestrogen‐receptor‐alpha‐bound human chromatin interactome. Nature. 2009;462(7269):58‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Melnikov A, Murugan A, Zhang X, et al. Systematic dissection and optimization of inducible enhancers in human cells using a massively parallel reporter assay. Nat Biotechnol. 2012;30(3):271‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Arnold CD, Gerlach D, Stelzer C, Boryń ŁM, Rath M, Stark A. Genome‐wide quantitative enhancer activity maps identified by STARR‐seq. Science. 2013;339(6123):1074‐1077. [DOI] [PubMed] [Google Scholar]
- 50. Murtha M, Tokcaer‐Keskin Z, Tang Z, et al. FIREWACh: high‐throughput functional detection of transcriptional regulatory modules in mammalian cells. Nat Methods. 2014;11(5):559‐565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. ENCODE Project Consortium . An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489(7414):57‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. FANTOM Consortium and the RIKEN PMI and CLST (DGT) . A promoter‐level mammalian expression Atlas. Nature. 2014;507(7493):462‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fernández JM, de la Torre V, Richardson D, et al. The BLUEPRINT data analysis portal. Cell Syst. 2016;3(5):491‐495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Visel A, Minovitsky S, Dubchak I, Pennacchio LA. VISTA Enhancer Browser—a database of tissue‐specific human enhancers. Nucleic Acids Res. 2007;35:D88‐D92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gao T, He B, Liu S, Zhu H, Tan K, Qian J. EnhancerAtlas: a resource for enhancer annotation and analysis in 105 human cell/tissue types. Bioinformatics. 2016;32(23):3543‐3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang Z, Zhang Q, Zhang W, et al. HEDD: Human Enhancer Disease Database. Nucleic Acids Res. 2018;46(D1):D113‐D120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang A, Yue F, Li Y, et al. Epigenetic priming of enhancers predicts developmental competence of hESC‐derived endodermal lineage intermediates. Cell Stem Cell. 2015;16(4):386‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hu D, Gao X, Morgan MA, Herz HM, Smith ER, Shilatifard A. The MLL3/MLL4 branches of the COMPASS family function as major histone H3K4 monomethylases at enhancers. Mol Cell Biol. 2013;33(23):4745‐4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lee JE, Wang C, Xu S, et al. H3K4 mono‐ and di‐methyltransferase MLL4 is required for enhancer activation during cell differentiation. Elife. 2013;2:e01503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yan J, Chen SA, Local A, et al. Histone H3 lysine 4 monomethylation modulates long‐range chromatin interactions at enhancers. Cell Res. 2018;28(2):204‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Local A, Huang H, Albuquerque CP, et al. Identification of H3K4me1‐associated proteins at mammalian enhancers. Nat Genet. 2018;50(1):73‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bonn S, Zinzen RP, Girardot C, et al. Tissue‐specific analysis of chromatin state identifies temporal signatures of enhancer activity during embryonic development. Nat Genet. 2012;44(2):148‐156. [DOI] [PubMed] [Google Scholar]
- 63. Pradeepa MM, Grimes GR, Kumar Y, et al. Histone H3 globular domain acetylation identifies a new class of enhancers. Nat Genet. 2016;48(6):681‐686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Catarino RR, Stark A. Assessing sufficiency and necessity of enhancer activities for gene expression and the mechanisms of transcription activation. Genes Dev. 2018;32(3–4):202‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Krijger PH, de Laat W. Regulation of disease‐associated gene expression in the 3D genome. Nat Rev Mol Cell Bio. 2016;17(12):771‐782. [DOI] [PubMed] [Google Scholar]
- 66. Long HK, Prescott SL, Wysocka J. Ever‐changing landscapes: transcriptional enhancers in development and evolution. Cell. 2016;167(5):1170‐1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hay D, Hughes JR, Babbs C, et al. Genetic dissection of the alpha‐globin super‐enhancer in vivo. Nat Genet. 2016;48(8):895‐903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bender MA, Roach JN, Halow J, et al. Targeted deletion of 5′ HS1 and 5′ HS4 of the beta‐globin locus control region reveals additive activity of the DNaseI hypersensitive sites. Blood. 2001;98(7):2022‐2027. [DOI] [PubMed] [Google Scholar]
- 69. Visel A, Akiyama JA, Shoukry M, Afzal V, Rubin EM, Pennacchio LA. Functional autonomy of distant‐acting human enhancers. Genomics. 2009;93(6):509‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Maeda RK, Karch F. Gene expression in time and space: additive vs hierarchical organization of cis‐regulatory regions. Curr Opin Genet Dev. 2011;21(2):187‐193. [DOI] [PubMed] [Google Scholar]
- 71. Perry MW, Boettiger AN, Levine M. Multiple enhancers ensure precision of gap gene‐expression patterns in the Drosophila embryo. PNAS. 2011;108(33):13570‐13575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Marinić M, Aktas T, Ruf S, Spitz F. An integrated holo‐enhancer unit defines tissue and gene specificity of the Fgf8 regulatory landscape. Dev Cell. 2013;24(5):530‐542. [DOI] [PubMed] [Google Scholar]
- 73. Hong JW, Hendrix DA, Levine MS. Shadow enhancers as a source of evolutionary novelty. Science. 2008;321(5894):1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bothma JP, Garcia HG, Ng S, Perry MW, Gregor T, Levine M. Enhancer additivity and non‐additivity are determined by enhancer strength in the Drosophila embryo. Elife. 2015;4:e07956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Osterwalder M, Barozzi I, Tissières V, et al. Enhancer redundancy provides phenotypic robustness in mammalian development. Nature. 2018;554(7691):239‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Khan M, Vaes E, Mombaerts P. Regulation of the probability of mouse odorant receptor gene choice. Cell. 2011;147(4):907‐921. [DOI] [PubMed] [Google Scholar]
- 77. Tasic B, Nabholz CE, Baldwin KK, et al. Promoter choice determines splice site selection in protocadherin alpha and ‐gamma pre‐mRNA splicing. Mol Cell. 2002;10(1):21‐33. [DOI] [PubMed] [Google Scholar]
- 78. Lower KM, Hughes JR, De Gobbi M, et al. Adventitious changes in long‐range gene expression caused by polymorphic structural variation and promoter competition. PNAS. 2009;106(51):21771‐21776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Dillon N, Trimborn T, Strouboulis J, Fraser P, Grosveld F. The effect of distance on long‐range chromatin interactions. Mol Cell. 1997;1(1):131‐139. [DOI] [PubMed] [Google Scholar]
- 80. De Gobbi M, Viprakisit V, Hughes JR, et al. A regulatory SNP causes a human genetic disease by creating a new transcriptional promoter. Science. 2006;312(5777):1215‐1217. [DOI] [PubMed] [Google Scholar]
- 81. Wijgerde M, Grosveld F, Fraser P. Transcription complex stability and chromatin dynamics in‐vivo. Nature. 1995;377(6546):209‐213. [DOI] [PubMed] [Google Scholar]
- 82. Novo CL, Javierre BM, Cairns J, et al. Long‐range enhancer interactions are prevalent in mouse embryonic stem cells and are reorganized upon pluripotent state transition. Cell Rep. 2018;22(10):2615‐2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kagey MH, Newman JJ, Bilodeau S, et al. Mediator and cohesin connect gene expression and chromatin architecture. Nature. 2010;467(7314):430‐435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Tolhuis B, Palstra RJ, Splinter E, Grosveld F, de Laat W. Looping and interaction between hypersensitive sites in the active beta‐globin locus. Mol Cell. 2002;10(6):1453‐1465. [DOI] [PubMed] [Google Scholar]
- 85. Snetkova V, Skok JA. Enhancer talk. Epigenomics. 2018;10(4):483‐498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Will AJ, Cova G, Osterwalder M, et al. Composition and dosage of a multipartite enhancer cluster control developmental expression of Ihh (Indian hedgehog). Nat Genet. 2017;49(10):1539‐1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Vakoc CR, Letting DL, Gheldof N, et al. Proximity among distant regulatory elements at the beta‐globin locus requires GATA‐1 and FOG‐1. Mol Cell. 2005;17(3):453‐462. [DOI] [PubMed] [Google Scholar]
- 88. Drissen R, Palstra RJ, Gillemans N, et al. The active spatial organization of the beta‐globin locus requires the transcription factor EKLF. Genes Dev. 2004;18(20):2485‐2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Song SH, Hou C, Dean A. A positive role for NLI/Ldb1 in long‐range beta‐globin locus control region function. Mol Cell. 2007;28(5):810‐822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. van de Werken HJ, Landan G, Holwerda SJ, et al. Robust 4C‐seq data analysis to screen for regulatory DNA interactions. Nat Methods. 2012;9(10):969‐972. [DOI] [PubMed] [Google Scholar]
- 91. Gonen N, Futtner CR, Wood S, et al. Sex reversal following deletion of a single distal enhancer of Sox9 . Science. 2018;360(6396):1469‐1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Weintraub AS, Li CH, Zambudi AV, et al. YY1 Is a structural regulator of enhancer‐promoter loops. Cell. 2017;171(7):1573‐1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wang J, Wu XG, Wei C, et al. YY1 positively regulates transcription by targeting promoters and super‐Enhancers through the BAF complex in embryonic stem cells. Stem Cell Reports. 2018;10(4):1324‐1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Symmons O, Pan L, Remeseiro S, et al. The Shh Topological domain facilitates the action of remote enhancers by reducing the effects of genomic distances. Dev Cell. 2016;39(5):529‐543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Soutourina J. Transcription regulation by the Mediator complex. Nat Rev Mol Cell Bio. 2018;19(4):262‐274. [DOI] [PubMed] [Google Scholar]
- 96. Hanssen LLP, Kassouf MT, Oudelaar AM, et al. Tissue‐specific CTCF‐cohesin‐mediated chromatin architecture delimits enhancer interactions and function in vivo. Nat Cell Biol. 2017;19(8):952‐961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Franke M, Ibrahim DM, Andrey G, et al. Formation of new chromatin domains determines pathogenicity of genomic duplications. Nature. 2016;538(7624):265‐269. [DOI] [PubMed] [Google Scholar]
- 98. Kim TK, Hemberg M, Gray JM. Enhancer RNAs: a class of long noncoding RNAs synthesized at enhancers. CSH Perspect Biol. 2015;7(1):a018622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Cheng JH, Pan DZ, Tsai ZT, Tsai HK. Genome‐wide analysis of enhancer RNA in gene regulation across 12 mouse tissues. Sci Rep. 2015;5:12648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Tuan D, Kong S, Hu K. Transcription of the hypersensitive site HS2 enhancer in erythroid cells. PNAS. 1992;89(23):11219‐11223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. De Santa F, Barozzi I, Mietton F, et al. A large fraction of extragenic RNA Pol II transcription sites overlap enhancers. PLoS Biol. 2010;8(5):e1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kim TK, Hemberg M, Gray JM, et al. Widespread transcription at neuronal activity‐regulated enhancers. Nature. 2010;465(7295):182‐187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lam MT, Cho H, Lesch HP, et al. Rev‐Erbs repress macrophage gene expression by inhibiting enhancer‐directed transcription. Nature. 2013;498(7455):511‐515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Djebali S, Davis CA, Merkel A, et al. Landscape of transcription in human cells. Nature. 2012;489(7414):101‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Pefanis E, Wang J, Rothschild G, et al. RNA exosome‐regulated long non‐coding RNA transcription controls super‐enhancer activity. Cell. 2015;161(4):774‐789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Koch F, Fenouil R, Gut M, et al. Transcription initiation platforms and GTF recruitment at tissue‐specific enhancers and promoters. Nat Struct Mol Biol. 2011;18(8):956‐963. [DOI] [PubMed] [Google Scholar]
- 107. Gilchrist DA, Dos Santos G, Fargo DC, et al. Pausing of RNA polymerase II disrupts DNA‐specified nucleosome organization to enable precise gene regulation. Cell. 2010;143(4):540‐551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Mousavi K, Zare H, Dell'Orso SK, et al. eRNAs promote transcription by establishing chromatin accessibility at defined genomic coci. Mol Cell. 2013;51(5):606‐617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Sigova AA, Abraham BJ, Ji X, et al. Transcription factor trapping by RNA in gene regulatory elements. Science. 2015;350(6263):978‐981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kaikkonen MU, Spann NJ, Heinz S, et al. Remodeling of the enhancer landscape during macrophage activation is coupled to enhancer transcription. Mol Cell. 2013;51(3):310‐325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Gardini A, Baillat D, Cesaroni M, et al. Integrator regulates transcriptional initiation and pause release following activation. Mol Cell. 2014;56(1):128‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lai F, Gardini A, Zhang A, Shiekhattar R. Integrator mediates the biogenesis of enhancer RNAs. Nature. 2015;525(7569):399‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Bose DA, Donahue G, Reinberg D, Shiekhattar R, Bonasio R, Berger SL. RNA binding to CBP stimulates histone acetylation and transcription. Cell. 2017;168(1‐2):135‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Schaukowitch K, Joo JY, Liu X, Watts JK, Martinez C, Kim TK. Enhancer RNA facilitates NELF release from immediate early genes. Mol Cell. 2014;56(1):29‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Rothschild G, Basu G. Lingering questions about enhancer RNA and enhancer transcription‐coupled genomic instability. Trends Genet. 2017;33(2):143‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Hnisz D, Abraham BJ, Lee TI, et al. Super‐enhancers in the control of cell identity and disease. Cell. 2013;155(4):934‐947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Whyte WA, Orlando DA, Hnisz D, et al. Master transcription factors and mediator establish super‐enhancers at key cell identity genes. Cell. 2013;153(2):307‐319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Pott S, Lieb JD. What are super‐enhancers? Nat Genet. 2015;47(1):8‐12. [DOI] [PubMed] [Google Scholar]
- 119. Zhang Y, Liu T, Meyer CA, et al. Model‐based Analysis of ChIP‐Seq (MACS). Genome Biol. 2008;9(9):R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Hah N, Benner C, Chong LW, Yu RT, Downes M, Evans RM. Inflammation‐sensitive super enhancers form domains of coordinately regulated enhancer RNAs. PNAS. 2015;112(3):E297‐E302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Dowen JM, Fan ZP, Hnisz D, et al. Control of cell identity genes occurs in insulated neighborhoods in mammalian chromosomes. Cell. 2014;159(2):374‐387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Sengupta S, George RE. Super‐enhancer‐driven transcriptional dependencies in cancer. Trends Cancer. 2017;3(4):269‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Adam RC, Yang H, Rockowitz S, et al. Pioneer factors govern super‐enhancer dynamics in stem cell plasticity and lineage choice. Nature. 2015;521(7552):366‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Shin HY, Willi M, HyunYoo K, et al. Hierarchy within the mammary STAT5‐driven Wap super‐enhancer. Nat Genet. 2016;48(8):904‐911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Siersbaek R, Rabiee A, Nielsen R, et al. Transcription factor cooperativity in early adipogenic hotspots and super‐Enhancers. Cell Rep. 2014;7(5):1443‐1455. [DOI] [PubMed] [Google Scholar]
- 126. Gosselin D, Link VM, Romanoski CE, et al. Environment drives selection and function of enhancers controlling tissue‐specific macrophage identities. Cell. 2014;159(6):1327‐1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Chipumuro E, Marco E, Christensen CL, et al. CDK7 inhibition suppresses super‐enhancer‐Linked oncogenic transcription in MYCN‐driven cancer. Cell. 2014;159(5):1126‐1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Christensen CL, Kwiatkowski N, Abraham BJ, et al. Targeting transcriptional addictions in small cell lung cancer with a covalent CDK7 inhibitor. Cancer Cell. 2014;26(6):909‐922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Lin CY, Erkek S, Tong YA, et al. Active medulloblastoma enhancers reveal subgroup‐specific cellular origins. Nature. 2016;530(7588):57‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Jiang YY, Lin DC, Mayakonda A, et al. Targeting super‐enhancer‐associated oncogenes in oesophageal squamous cell carcinoma. Gut. 2017;66(8):1358‐1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Ooi WF, Xing M, Xu C, et al. Epigenomic profiling of primary gastric adenocarcinoma reveals super‐enhancer heterogeneity. Nat Commun. 2016;7:12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Zhou B, Wang L, Zhang S, et al. INO80 governs superenhancer‐mediated oncogenic transcription and tumor growth in melanoma. Genes Dev. 2016;30(12):1440‐1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Thakurela S, Sahu SK, Garding A, Tiwari VK. Dynamics and function of distal regulatory elements during neurogenesis and neuroplasticity. Genome Res. 2015;25(9):1309‐1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Vahedi G, Kanno Y, Furumoto Y, et al. Super‐enhancers delineate disease‐associated regulatory nodes in T cells. Nature. 2015;520(7548):558‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Blinka S, Reimer MH Jr, Pulakanti K, Rao S. Super‐Enhancers at the nanog locus differentially regulate neighboring pluripotency‐associated genes. Cell Rep. 2016;17(1):19‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Li Y, Rivera CM, Ishii H, et al. CRISPR reveals a distal super‐enhancer required for Sox2 expression in mouse embryonic stem cells. PLoS ONE. 2014;9(12):e114485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. van Groningen T, Koster J, Valentijn LJ, et al. Neuroblastoma is composed of two super‐enhancer‐associated differentiation states. Nat Genet. 2017;49(8):1261‐1266. [DOI] [PubMed] [Google Scholar]
- 138. Ke L, Zhou H, Wang C, et al. Nasopharyngeal carcinoma super‐enhancer‐driven ETV6 correlates with prognosis. PNAS. 2017;114(36):9683‐9688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Zhang X, Choi PS, Francis JM, et al. Identification of focally amplified lineage‐specific super‐enhancers in human epithelial cancers. Nat Genet. 2016;48(2):176‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Zhang X, Choi PS, Francis JM, et al. Somatic superenhancer duplications and hotspot mutations lead to oncogenic activation of the KLF5 transcription tactor. Cancer Discov. 2018;8(1):108‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Chapuy B, Michael M, Lin C, et al. Disruption of super enhancer‐driven cancer dependencies In diffuse large B‐cell lymphoma. Blood. 2013;122(21):3021. [Google Scholar]
- 142. Kwiatkowski N, Zhang T, Rahl PB, et al. Targeting transcription regulation in cancer with a covalent CDK7 inhibitor. Nature. 2014;511(7511):616‐620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Landt SG, Marinov GK, Kundaje A, et al. ChIP‐seq guidelines and practices of the ENCODE and modENCODE consortia. Genome Res. 2012;22(9):1813‐1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Rhee HS, Pugh BF. ChIP‐exo: a method to identify genomic location of DNA‐binding proteins at near single nucleotide accuracy. Curr Protoc Mol Biol. 2012;Chapter 21:Unit 21.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Kwasnieski JC, Fiore C, Chaudhari HG, Cohen BA. High‐throughput functional testing of ENCODE segmentation predictions. Genome Res. 2014;24(10):1595‐1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Lettice LA, Heaney SJH, Purdie LA, et al. A long‐range Shh enhancer regulates expression in the developing limb and fin and is associated with preaxial polydactyly. Hum Mol Genet. 2003;12(14):1725‐1735. [DOI] [PubMed] [Google Scholar]
- 147. Sagai T, Hosoya M, Mizushina Y, Tamura M, Shiroishi T. Elimination of a long‐range cis‐regulatory module causes complete loss of limb‐specific Shh expression and truncation of the mouse limb. Development. 2005;132(4):797‐803. [DOI] [PubMed] [Google Scholar]
- 148. Sanyal A, Lajoie BR, Jain G, Dekker J. The long‐range interaction landscape of gene promoters. Nature. 2012;489(7414):109‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Zhang Y, Wong CH, Birnbaum RY, et al. Chromatin connectivity maps reveal dynamic promoter‐enhancer long‐range associations. Nature. 2013;504(7479):306‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]


