Abstract
Alzheimer's disease (AD) is an increasingly common neurodegenerative disease. Since the intestinal microbiome is closely related to nervous system diseases, alterations in the composition of intestinal microbiota could potentially contribute to the pathophysiology of AD. However, how the initial interactions with intestinal microbes alter events later in life, such as during neurodegenerative diseases, is still unclear. This review summarizes what is known about the relationship between the intestinal microbiome and AD.
Keywords: Alzheimer's disease, blood‐brain barrier, intestinal barrier, intestinal microbiota, neuroinflammation
1. INTRODUCTION
Alzheimer's disease (AD) is the most common chronic neurodegenerative disease. Patients suffer from short‐term memory loss, verbal memory decline, mood swings, and loss of motivation, planning and intellectual coordination skills.1, 2 AD is characterized by cortex atrophy, loss of neurons and synapses, amyloid plaques caused by the aggregation of Aβ1–42 peptide and neurofibrillary tangles formed by tau, a microtubule‐associated protein. In addition to the deposition of Aβ and neurofibrillary tangles, neuroinflammation is also a pathological hallmark of AD.3, 4 Alarmingly, the number of individuals suffering from Alzheimer's disease has increased from 21.7 million to 46 million in a quarter century.5 However, over 95% of cases are sporadic AD patients and the pathogenesis is still unclear. There are no effective curative treatments. The results of recent genome‐wide association studies (GWAS) have identified and validated 20 novel AD genetic risk loci, such as the ABOEe4 allele, ABCA7, clusterin (CLU), fermitin family member 2 (FERMT2), phosphatidylinositol‐binding clathrin assembly protein (PICALM),6 but none of the markers are located within coding regions, suggesting a comparatively strong contribution of epigenetic or environmental factors to AD risk.7 In humans, it is estimated that the microbiome encodes >100‐fold more genes than the human genome8 and is impacted by host and environmental factors. Recent studies indicate that there is a close correlation between the intestinal microbiome and AD, which raises a new hypothesis about the pathogenesis of AD. In this review, we will discuss what is known about the possible relationship between the intestinal microbiome and AD.
2. COMPOSITION OF THE INTESTINAL MICROBIOME
Microbes are found throughout the human body9 and the majority of metabolites in human plasma are microbe‐derived.10 The microbes residing in the body are classified into three groups: symbionts, commensal organisms and pathobionts.11 Numerous small molecules synthesized by microbiota influence human health. The microflora take part in the regulation of many physiological functions, including synthesizing vitamins and amino acids, influencing the biotransformation of bile acids, increasing the bioavailability of minerals, forming a barrier against colonization by pathogenic bacteria and stimulating the production of substances that inhibit the adhesion of pathogens to enterocytes.12, 13
The alimentary tract is the most substantial contributor to the total bacterial population within the human body,9 and it is one of the largest interfaces (250‐400 m2) between the host and what can be regarded as a continuation of the external environment in the human body.14 Simultaneously, intestinal mucosal lymphoid tissue is the largest and most important human immune organ, containing 70%‐80% of the immune system in the whole body.15 The human gastrointestinal tract is inhabited by nearly 1014 microorganisms from at least 1000 distinct microbial species which are collectively known as intestinal microbiota.16 The cell density of human intestinal microbiota varies at different positions along the gastrointestinal tract, with 102/mL in the stomach, duodenum and jejunum, 103‐108/mL in the ileum and 1012/mL in the colon.9, 17 Almost the entire gut intestinal microbiome is composed of species from the phyla Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria and Verrucomicrobia.17 The composition of the microbiota differs in various regions of the digestive tract (Table 1). Lactobacillaceae, Clostridum, Streptococcus Bacteroides, Actinomycinae, Corynebacteria dominate in the small intestine, while the colon is colonized by Bacteroides, Clostridum, Bifidobacterium and Enterobacteriaceae.
Table 1.
The composition of the microbiota in various regions of the digestive tract
| Position | Density number/mL | Bacteria species |
|---|---|---|
| Stomach | 0‐102 | Lactobacillus, Candida, Streptococcus, Helicobacter pylori, Peptostreptococcus |
| Duodenum | 102 | Streptococcus, Lactobacillus |
| Jejunum | 102 | Streptococcus, Lactobacillus |
| Proximal ileum | 103 | Streptococcus, Lactobacillus |
| Distal ileum | 107‐108 | Clostridum, Streptococcus, Bacteroides, Actinomycinae, Corynebacteria |
| Colon | 1011‐1012 | Bacteroides, Clostridum groups IV and XIV, Bifidobacterium, Enterobacteriaceae |
The microbial composition of the intestinal tract is impacted by host and environmental factors, the mode of delivery at birth, the postnatal environment, diet, use of drugs, as well as age, sex, microflora transplantation and physiological influences, and holds distinctive compositional and functional features across different life periods.18 The microbial community is relatively stable in adults, and starts to shift about 65 years of age, the age of predilection for AD.14 It has been shown that the microbiome in the elderly population is less diverse and resilient, which makes it more susceptible to environmental factors and interventions than that of younger adults. Similarly, the intestinal microbiome of centenarians was found to differ significantly from that of other adults. Biagi and his team found that the genera Coprococcus, Roseburia, and Faecalibacterium were negatively correlated with age, while Oscillospira and Akkermansia had a positive age correlation.19 Alterations in the intestinal microbiome are associated with the physical state of the elderly. The frail tend to have a decreasing abundance of Lactobacilli, Bacteroides Prevotella and Faecalibacterium prausnitzii, and increasing proportions of Ruminococcus, Atopobium and Enterobacteriaceae.20
3. INTESTINAL MICROBIOME, NEURODEVELOPMENT AND NEUROINFLAMMATION
The Human Microbiome Project (HMP) is revealing the beneficial effects of intestinal microorganisms on human health.20 Neurodevelopment is a complex process that is partly dependent on environmental signals from the intestines, which largely originate from the microbiome. The intestinal microbiome and its metabolites participate in neuroinflammation and basic neurogenerative processes such as the formation of the blood‐brain barrier (BBB), myelination, neurogenesis, and microglia maturation, from the prenatal period to old age. The microbiota can influence neuroinflammation by modulating microglia and astrocytes. During maturation, microglia remain in an immature status in the absence of microbiota and can be rescued by the administration of short‐chain fatty acids (SCFAs).21 Lactobacillus reuteri can suppress neuroinflammation in astrocytes in the brain by promoting the production of indole‐3‐aldehyde and indole‐3‐propionic acid, which are then transported across the BBB.22
The BBB is formed by capillary endothelial cells, astrocytes, and pericytes and develops during the early period of intrauterine life.23 It is the gateway for the passage and exchange of molecules and nutrients between the circulatory system and the brain parenchyma. Intestinal microbiota impact the permeability of the BBB during gestation, and the effects are propagated throughout life. Germ‐free mice displayed increased BBB permeability compared to pathogen‐free mice with normal intestinal flora,24 and increasing BBB permeability may facilitate an increased rate of pathogen entry into the brain, allowing for neuroinflammation.25 Microbial signals have been found to reduce the rates of neurogenesis very early in life during cortical development,26 but rescue decreasing neurogenesis in the hippocampi of adult mice undergoing long‐term antibiotic treatment.27 Bacterial cell wall components also induce the proliferation of neurons in the frontal cortex.28 The microbiome is also necessary for the regulation of myelin‐related genes, with clear implications for cortical myelination, which is a critical process in the development of a healthy brain.29 In addition to their effects on the central nervous system, intestinal microbiota regulate the enteric nervous system (ENS) and their effects depend on vagus nerve and myenteric neuron activities.30 The ENS degenerates with age. Cholinergic nerves, as well as enteric glial cells, are lost in both the myenteric and the submucosal plexus.31
Metabolites produced by intestinal microbes are important molecules for neurological function. γ‐Aminobutyric acid (GABA), the chief inhibitory neurotransmitter in the mammalian central nervous system, can be produced by Lactobacillus and Bifidobacterium species. It plays an important role in developing brain. As an excitatory transmitter, GABA improves the intracellular Ca2+ concentration of postsynaptic neurons in the early stages of CNS development. GABA also takes part in the proliferation and development of neural progenitor cells via brain‐derived neurotrophic factor (BDNF) and the formation of synapses,32 and after development, it acts as an inhibitory transmitter by decreasing the intracellular Cl− concentration.33 In addition, GABA has been shown to suppress inflammatory immune responses in type 1 diabetes animal models,34 and lower numbers of enteric Lactobacillus and Bifidobacterium decrease the amount of GABA in the intestines and CNS.12
Intestinal microbiota play an important role in the synthesis of serotonin (5‐hydroxytryptamine, 5‐HT),35 which in turn plays a crucial role in the regulation of cognitive function. 5‐HT is produced in the enteric nervous system of gastrointestinal tract and central nervous system. It regulates intestinal movements, mood, appetite, and sleep. It is also associated with memory and learning. Gut microbiota indirectly control the production of neurotransmitters by stimulating host enterochromaffin (EC) cells to produce 5‐HT.36 Escherichia, Bacillus, and Saccharomyces spp. can produce norepinephrine and dopamine.30 Short‐chain fatty‐ acids (SCFAs) are produced by probiotics in the colon, chief among them being acetic, propionic, butyric and lactic acids.36
Among the SCFAs, butyrate has received particular attention due to its beneficial effects on maintaining health. In fact, butyrate which is produced by Butyricicoccus and Clostridium, is the main energy source of colonocytes.37 It increases the mitochondrial respiration rate and ATP production. Butyrate is a histone deacetylase inhibitor that induces the sprouting of dendrites, increases the number of synapses, and reinstates learning behavior and access to long‐term memories.38, 39 Butyrate can facilitate increased gut intestine motility by stimulating cholinergic neurons of the ENS, whereas propionate decreases motility and increases secretion.
In addition to their role in neuroinflammation, commensal microbiota are closely connected to the systemic inflammatory responses of the host. It has been shown that germ‐free animals show lack of growth of CD4 + T‐cell populations. The intestinal microbiome stimulates epithelial cells and gut mucosal lymphoid tissue to release serum amyloid A1, and also stimulates the T‐cell compartment and upregulates innate intestinal defense mediators. Particular bacterial strains, such as Akkermansia muciniphila and Faecalibacterium prausnitzii also possess anti‐inflammatory properties,14 and butyrate acts as an anti‐inflammatory agent by suppressing the nuclear factor kappa‐light‐chain‐enhancer of the signaling pathways of activated B cells (NF‐κB).40 It is also a potent agonist of the G protein‐coupled receptors (GPRs), including free fatty acid receptor 2 (FFAR2), FFAR3 and GPR109. FFAR3 is highly expressed in the sympathetic nervous system and is associated with inhibiting ganglia activity,41 and can promote the development of dendritic and T‐cell precursors from bone marrow. GPR109A promotes the generation of Treg cells.22 Microbial dysbiosis and increased intestinal permeability trigger systemic inflammation by elevating the levels of serum interleukin 6 (IL‐6),42 and IL‐6 also increases in the serum and brain tissue of AD patients.43
4. NEURONFLAMMATION AND AD
It has been demonstrated that both acute and chronic systemic inflammation are associated with an increase in cognitive decline in AD patients.44 The classical pro‐inflammatory cytokine tumor necrosis factor α (TNF‐α) is produced by microglia and is an early cellular marker of AD pathology, and has also been shown to induce neuronal cell cycle events.45
Microglia and astrocytes are important players in the development and progression of neuroinflammation and are critical for the maintenance of normal brain homeostasis. Microglia located throughout the brain and spine account for about 15% of the neuroglia in the brain.46 Microglia can clean up Aβ fibrils via phagocytosis. They surround amyloid plaques, and the presence of Aβ deposits in activated microglia in the brains of AD patients has been shown.43 However, chronic neuroinflammation caused by the accumulation of Aβ and neuronal debris can damage the BBB, which induces the activated microglia to release pro‐inflammatory cytokines.47, 48 In AD, the induction of an inflammatory response by microglia may influence neuronal integrity and function and contribute to neurodegeneration. Under inflammatory conditions, cytokines upregulate β‐secretase mRNA protein and increase NF‐κB signaling by activating TNF‐α, resulting in increased Aβ production, which has a neurotoxic effect.43
Astrocytes also play a role in the progression of AD. Reactive astrocytes, as well as reactive microglia, contribute to neuroinflammation and BBB dysfunction. They also induce a pro‐inflammatory profile, interacting with Aβ.49 The accumulation of activated astrocytes correlates strongly with Braak staging in AD.50, 51 Moreover, metabolic alterations in neuronal and glial cells and the disruption of calcium homeostasis in neurons and astrocytes induce large‐scale neuroinflammation and the loss of neurons, which is associated with hypervascularity and hyperpermeability.52
Recognition of the inflammatory hypothesis of AD has been gradually accepted over recent years. Some pathogens are associated with most of the changes seen in AD, such as inflammation, brain cell atrophy, immunological aberrations, amyloidogenesis, altered gene expression and cognitive deficits.7 The Aβ peptide is a cleavage product generated by β‐ and γ‐secretases, derived from Aβ protein precursor distributed in the neuronal membrane. Aβ42 peptides form the central core of senile plaques.53 Consideration has been given to possible physiological roles of Aβ as an antimicrobial peptide (AMP), utilizing fibrillation to protect the host from a wide range of infectious agents.54
It has been reported that AD brains contain higher bacterial levels than the brains of non‐demented controls.55 An estimated 90% of Aβ plaques in AD patients’ brains contained HSV‐1 (herpes simplex virus) DNA.56 Moreover, the risk of dementia increased 2.56‐fold in patients with HSV.57 Human herpes viruses 6A and 7 (HHV‐6A, HHV‐7) were also found to be abundant in the lobes of AD patients. These viruses interact with many of the known AD risk genes, including γ‐secretase subunit presenilin‐1 (PSEN1), BACE1, amyloid β precursor protein binding family B member 2 (APBB2), CLU, bridging integrator 1 (BIN1), and PICALM.58 Moreover, they showed a correlation with neuron loss.58 Similarly, postmortem investigations showed the presence of Chlamydia pneumoniae in the brains of AD patients.59 Aβ deposition has also been found in olfactory bulbs infiltrated by Chlamydia pneumonia.60 Some observations suggest AD may also be associated with Helicobacter pylori and Toxoplasma gondii infection.61, 62
Clinical anti‐Aβ trials also found an increased incidence of infections, including meningoencephalitis,63 orolabial herpes, skin infections64 and upper respiratory infections,65 among the study participants. The main pathways of pathogen infiltration of the CNS include the lymphatic pathways,66 the BBB and the blood‐labyrinth barrier (BLB).67 Lymphatic vessels are spread along the transverse sinuses and superior sagittal sinus (SSS) and reach the olfactory bulb.68 The entorhinal cortex‐hippocampus axis and the olfactory system have been suggested as the earliest anatomical regions targeted by AD.69 Accordingly, amyloid deposits can be found in the brains of non‐transgenic BALB/c mice following intranasal infection with Chlamydia pneumonia.60
The BBB is also responsible for the strict control of the molecules transported into the brain. The increase in BBB permeability seen during physiological aging may facilitate an increased rate of pathogen entry into the brain.25 This implies that age is a potential risk factor of Alzheimer's disease, and the intestinal microbiome may be responsible for pathogen infection in the brain and AD by changing the permeability of BBB.
Taken together, these studies suggest that microglial priming can affect brain development and, later, the onset and progression of neurodegenerative diseases. It has been found that the incidence of AD is reduced in patients who use nonsteroidal anti‐inflammatory drugs (NSAIDs).70
5. INTESTINAL MICROBIOME ALTERATIONS AND AD
Dysbiosis of the intestinal microbiome has been implicated in multiple diseases, including intestinal disorders and extra‐intestinal disorders such as inflammatory bowel disease, diabetes mellitus, asthma, obesity, autism and rheumatoid arthritis.13 Recent studies have also linked microbial dysbiosis to neurodegenerative disease.71, 72, 73 A small number of studies have demonstrated different intestinal microbial populations both in human patients and in animal models of AD (Table 2). Observations of the microbiota of AD patients at the phylum level indicate that these participants harbor decreased numbers of Firmicutes and Actinobacteria, and an increase in Bacteroidetes compared to controls. Within the Firmicutes, the families Ruminococcaceae, Turicibacteraceae, Clostridiaceae, and Clostridium sensu stricto were all less abundant in AD patients.74 Recent reports indicate similar alterations in animal models of AD. For example, APPswe/PS1▵E9 (PAP) transgenic mice had less Firmicutes but more Bacteroidetes.72 These intestinal microbial alterations in AD animal models are associated with chronic neuroinflammation.
Table 2.
Altered composition of intestinal microbiota of AD patients and animal models of AD
| Increased numbers of microbiota | Decreased numbers of microbiota | |
|---|---|---|
| AD patients | Bacteroidetes, Proteobacteria, Bilopila, Bacteroidaceae, Gemellaceae, Rikenellaceae, Bacteroides, Phascolarctobacterium, Gemella, Alistipes, Blautia | Firmicutes, Actinobacteria, Ruminococcaceae, Turicibacteraceae, Peptostreptococcaceae, Clostridiaceae, Mogibacteriaceae, Bifidobacteriaceae, Erysipdotrichaceae CC115, Clostridiaceae SMB53, Dialister, Clostridium, Bifidobacterium, Turicibacter, Bifidobacterium, Adlereutzia |
| PAP transgenic mice | Tenericutes, Proteobacteria, Veriucomicrobia, Erysipelotrichales, Erysipeiotrichaceae, Rikenellacaea unclassified genera | Verrucomicrobia, Proteoacteria, Ruminococcus, Butyricicoccus, Allobaculum, Akkermansia |
| 5xFAD mice | Firmicutes, Clostridium leptum | Bacteroidetes |
Neuroinflammation triggered by pro‐inflammatory molecules causes increased permeability of the BBB,75, 76 resulting in immune cell infiltration, exacerbation of the inflammatory response, leading to reactive gliosis, and eventually causing neurodegeneration.77 Bacteroidetes is a family of Gram‐negative bacteria, and thus have lipopolysaccharide (LPS) as the major outer membrane component, which can trigger systemic inflammation and promote the release of pro‐inflammatory cytokines.78 Injection of LPS during brain development enhances microglial activity and results in increased levels of the pro‐inflammatory cytokines IL‐1β, IL‐6 and TNF‐α.79, 80 Additionally, LPS is associated with AD pathology, since it can potentiate amyloid fibrillogenesis when co‐incubated with Aβ peptide,81 and systemic injection of LPS in animals results in amyloid deposition and tau‐related pathology.81, 82 In humans, LPS and Gram‐negative Escherichia coli fragments have been found to co‐localize with amyloid plaques in the brains of AD patients, and the levels of LPS and Gram‐negative E. coli fragments were greater in AD patients compared to control brains.83 Antibiotic exposure in pregnancy can reduce postnatal production of granulocyte colony stimulating factor (G‐CSF), which has neuroprotective effects, promoting neural tissue repair and improvement in functional recovery.84 Additionally, G‐CSF has played a protective role in spatial learning and memory formation in animal models.85, 86
Animal studies showed that Akkermansia and seven other bacterial genera were correlated with the levels of cerebral soluble Aβ42.72 Akkermansia and Butyricicoccus, which can increase gut barrier integrity,87 were negatively correlated with the amount of pathogenic Aβ42 in the brain. Akkermansia is involved in intestinal remodeling and controls the intestinal absorptive capacity.88 Butyricicoccus produces butyrate, which is one of the SCFAs that drives the maturation of microglia and is needed for the maintenance of mature microglia.21 It regulates the size and function of the colonic Treg cell pool and protects against colitis by inhibiting NF‐κB activation in colon cells.89, 90 Accordingly, a decrease in protection of the intestinal barrier may result in increased expression of inflammatory components and the influx of LPS into the AD brain. Consistent with this hypothesis, Leblhuber et al. found elevated fecal calprotectin levels in patients with AD, indicating a leaky intestinal barrier.91 Thus, increased abundance of Bacteroides may result in increased translocation of LPS from the intestines into systemic circulation and the brain, and thus contribute to or exacerbate AD pathology. In human studies, postmortem examinations of the brains of AD patients showed increased histone deacetylase levels, which are associated with butyrate.92 This may indicate a relationship between the decreased abundance of butyrate‐producing bacteria and AD. Decreased amounts of enteric Bifidobacterium in PAP transgenic mice were found to be correlated with decreased levels of GABA, resulting in CNS dysfunction.72 Moreover, a postmortem study showed that GABA levels were decreased in the cortices of AD patients.93 Dysbiosis of intestinal microbes was also found to decrease the levels of brain‐derived neurotrophic factor (BDNF) in the hippocampus and cortex, inducing cognitive disorders via the intestine‐brain axis.94
At present, AD is thought to be associated with innate immunity. Several risk factors for AD, including aging, systemic infection, inflammation, obesity in middle life and brain trauma, all involve an activation of the innate immune system.95, 96 Furthermore, PAP transgenic mice raised in the absence of microbiota have less Aβ.72, 97 Recent studies have found Aβ deposition not only in the brain, but also in the intestinal villous stroma in PAP transgenic mice older than 5 months age and in 5xFAD mice, indicating considerable expression of human amyloid‐β protein precursor (AβPP). This may be the reason for the shorter, sparser and irregularly arranged intestinal villi of PAP transgenic mice with altered intestinal microbiota.73, 98
6. THE INTESTINAL MUCOSAL BARRIER AND AD
The intestinal mucosal barrier is composed of the mucosal layer, intestinal epithelial layer and microbiota.99 The mucosal layer consists of mucin molecules secreted by goblet cells and the secreted mucus prevents the direct contact of microorganisms with the epithelial layer.100 Epithelial cells are held together by tight junction proteins including claudins, occludin, junctional adhesion molecules, and tricellulin. Microbiota indirectly support the integrity of the intestinal barrier by stimulating epithelial cell proliferation and producing SCFAs indirectly.99 The pro‐inflammatory cytokines TNF‐α, IL‐4, IL‐6, and IL‐13 are known to increase the permeability of intestinal epithelial cell monolayers, and this effect has been related to increased expression of claudin.99
An increase in gut dysbiosis and a disrupted intestinal mucosal barrier can allow the passage of microbes, microbial products, and foreign antigens into the body, resulting in activation of the immune system.101 Notably, host‐microbe interactions contribute to a broad range of extra‐intestinal autoimmune and inflammatory diseases.102 Experiments have shown that the increased colonic permeability that comes with age is due to age‐associated remodeling of intestinal epithelial tight junction proteins. Accordingly, the dysbiosis of the intestinal barrier leads to geriatric vulnerability to gastrointestinal dysfunction.103 Intestinal microbial alterations and intestinal barrier injury in animal models of AD are associated with several risk factors for AD, such as intestinal inflammation, stroke, diabetes and hypertension. The abundance of microbes belonging to the family Erysipelotrichaceae correlated with inflammatory bowel disease (IBD) and showed a tendency to increase with age in PAP transgenic mice.98, 104 Moreover, it has been showed that diabetes is associated with intestinal barrier dysfunction. Hyperglycemia increases intestinal barrier permeability via alteration of tight and adherence junction integrity, resulting in systemic influx of microbial products and enhanced dissemination of enteric infection.105
Stroke and hypertension are also risk factors for AD. Dysbiosis of the intestinal microbiome has also been implicated in stroke, and is correlated with increasing vascular and epithelial permeability.106 It has been showed that kidney cells and blood vessels express receptors for SCFAs and they are associated with hypertension. Moreover, the bacteria that show a decrease in numbers in the intestines of AD patients regulate blood pressure via their fermentation products.107
Exercise, which is an important factor that reduces the prevalence of AD, is also associated with the intestinal barrier. Intestinal mucosal damage partly arises from imbalances in pro‐inflammatory and anti‐inflammatory cytokines. Repeated exercise results in reduced pro‐inflammatory cytokine expression and increased anti‐inflammatory IL‐10 expression.108 One study has demonstrated that regular exercise can decrease colonic oxidative insult in a rat model of colitis.109 Regular exercise also has an anti‐inflammatory effect that improves the immunological profile in type 2 diabetes mellitus, coronary artery disease, peripheral arterial disease and obesity.20
There is a lack of evidence that the cognitive reserve is correlated with the intestinal barrier and intestinal microbes. However, it can the improve symptoms of AD in the elderly whose intestinal barrier and BBB have been injured already.3
7. PROBIOTICS AND THERAPY
Probiotics and fecal microbiota transplantation have been used to treat intestinal disease, diabetes, neuropsychological diseases and multiple diseases in animal models and human patients.110 The effects of probiotics include correcting gastrointestinal barrier defects and the composition of intestinal microbiota, reducing inflammation and releasing biogenic factors.30 They can improve clinical symptoms, histological alterations and mucus production by reducing inflammation and are well tolerated, effective, and safe in patients with IBD.111 Probiotics are also helpful in controlling blood glucose levels by affecting SIRT1 and fetuin‐A levels to a certain extent.112 Interestingly, recent studies found that probiotics had a beneficial impact on mental disorders. Dietary supplementation with Bifidobacterium cultures can correct behavioral deficits of rats in the forced swim test and elevate plasma tryptophan, a precursor of a highly potent neuroprotective antioxidant. Similarly, Bacteroides fragilis was found to be able to correct communication defects, anxiety‐like behaviors and sensorimotor behaviors by decreasing the level of 4‐ethylphenylsulfate. Oral administration of Lactobacillus and Bifidobacterium can improve mood and alleviate anxiety and depressive symptoms, as well as decrease urinary cortisol.113 Lactobacillus has also been found to alleviate the gastrointestinal symptoms of people under stress, while also reducing the level of salivary cortisol.114 It has been reported that the transplantation of fecal microbiota cured a patient with seizures and Crohn's disease, who remained without relapse for more than 20 months.115
8. FUTURE DIRECTIONS
The pathogenesis of AD is still unclear. Current treatments that pursue the classical hypotheses of AD causation, such as the amyloid hypothesis, the tau hypothesis, and the cholinergic hypothesis, are ineffective. Intestinal microbiota influence human health and disease. An increasing number of scientific reports suggest an important role of the intestinal bacterial flora in multiple disorders. Notably, intestinal microbiota can promote the occurrence of neuropsychiatric disorders. Intestinal bacteria can also impact the function and structure of the brain via the intestine‐brain axis. The intestinal microbiome and intestinal barrier are closely related to nervous system diseases, although the specific mechanisms remain unclear. Studies have shown that dysbiosis of intestinal microbiota can induce brain pathology. The intestinal barrier and BBB, as the main protectors against pathogen infection, may play a crucial role in the pathogenesis of AD. The increasing permeability of the intestinal mucosa and BBB in the elderly may intensify inflammatory reactions, and induce amyloid aggregation. Moreover, dysbiosis of the intestinal microbiome facilitates the entry of LPS and pathogens into the circulatory system and CNS, which in turn may lead to a vicious cycle of neuronal destruction (Figure 1). Thus, in the future, we might find biomarkers associated with intestinal microbes and inflammation that can be used for early diagnosis and prevention of AD by treating neuroinflammation, improving the intestinal barrier, and regulating the microbiome. However, such progress is predicated on a better understanding of the bidirectional communication between the brain and microbiota, which should be a focus of future research.
Figure 1.
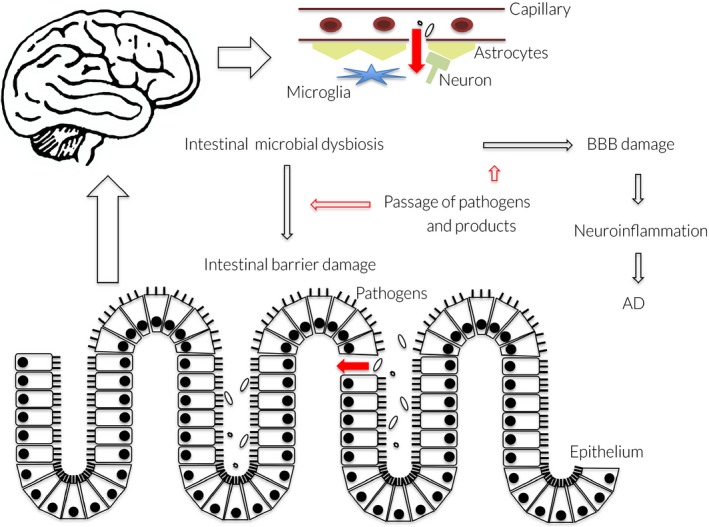
The potential relationship between intestinal microbiome alterations, mucosal barrier dysfunction, neuroinflammation and the pathogenesis of AD. Dysbiosis of the intestinal microbiome facilitates intestinal barrier and BBB damage and the entry of pathogens and their products into the circulatory system. Pathogens and their products pass into the brain via damaged BBB, and may intensify inflammatory reactions, and induce amyloid aggregation and the occurrence of AD
CONFLICT OF INTEREST
None.
ACKNOWLEDGEMENTS
Supported by grants from CAMS Innovation Fund for Medical Science (CIFMS) (2017‐12M‐2‐005), International Science and Technology Cooperation Program of China (2015DFG32230), National Science Fund for Young Scholars (81500938 and CIFMS, 2016‐12M‐1‐010).
Li Z, Zhu H, Zhang L, Qin C. The intestinal microbiome and Alzheimer's disease: A review. Animal Models Exp Med. 2018;1:180–188. 10.1002/ame2.12033
REFERENCES
- 1. Burns A, Iliffe S. Alzheimer's disease. BMJ. 2009;338:b158. [DOI] [PubMed] [Google Scholar]
- 2. Mistridis P, Krumm S, Monsch AU, Berres M, Taylor KI. The 12 years preceding mild cognitive impairment due to Alzheimer's disease: the temporal emergence of cognitive decline. J Alzheimers Dis. 2015;48(4):1095‐1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer's disease. The Lancet. 2011;377(9770):1019‐1031. [DOI] [PubMed] [Google Scholar]
- 4. Trovato Salinaro A, Pennisi M, Di Paola R, et al. Neuroinflammation and neurohormesis in the pathogenesis of Alzheimer's disease and Alzheimer‐linked pathologies: modulation by nutritional mushrooms. Immun Ageing. 2018;15:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990‐2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545‐1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Apostolova LG, Risacher SL, Duran T, et al. Associations of the top 20 Alzheimer disease risk variants with brain amyloidosis. JAMA Neurol. 2018;75(3):328‐341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hill JM, Clement C, Pogue AI, Bhattacharjee S, Zhao Y, Lukiw WJ. Pathogenic microbes, the microbiome, and Alzheimer's disease (AD). Front Aging Neurosci. 2014;6:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016;14(8):e1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nicholson JK, Holmes E, Kinross J, et al. Host‐gut microbiota metabolic interactions. Science. 2012;336(6086):1262‐1267. [DOI] [PubMed] [Google Scholar]
- 11. Koboziev I, Reinoso Webb C, Furr KL, Grisham MB. Role of the enteric microbiota in intestinal homeostasis and inflammation. Free Radic Biol Med. 2014;68:122‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Szablewski L. Human gut microbiota in health and Alzheimer's disease. J Alzheimers Dis. 2018;62(2):549‐560. [DOI] [PubMed] [Google Scholar]
- 13. Hall AB, Tolonen AC, Xavier RJ. Human genetic variation and the gut microbiome in disease. Nat Rev Genet. 2017;18(11):690‐699. [DOI] [PubMed] [Google Scholar]
- 14. Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474(11):1823‐1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sochocka M, Donskow‐Lysoniewska K, Diniz BS, Kurpas D, Brzozowska E, Leszek J. The gut microbiome alterations and inflammation‐driven pathogenesis of Alzheimer's disease—a critical review. Mol Neurobiol. 2018; 10.1007/s12035-018-1188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gill SR, Pop M, Deboy RT, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312(5778):1355‐1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134(2):577‐594. [DOI] [PubMed] [Google Scholar]
- 18. Kundu P, Blacher E, Elinav E, Pettersson S. Our Gut Microbiome: the Evolving Inner Self. Cell. 2017;171(7):1481‐1493. [DOI] [PubMed] [Google Scholar]
- 19. Biagi E, Franceschi C, Rampelli S, et al. Gut microbiota and extreme longevity. Curr Biol. 2016;26(11):1480‐1485. [DOI] [PubMed] [Google Scholar]
- 20. Cresci GA, Bawden E. Gut microbiome: what we do and don't know. Nutr Clin Pract. 2015;30(6):734‐746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Erny D, Hrabe de Angelis AL, Jaitin D, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18(7):965‐977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cox LM, Weiner HL. Microbiota signaling pathways that influence neurologic disease. Neurotherapeutics. 2018;15(1):135‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood‐brain barrier integrity during embryogenesis. Nature. 2010;468(7323):562‐566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Braniste V, Al‐Asmakh M, Kowal C, et al. The gut microbiota influences blood‐brain barrier permeability in mice. Sci Transl Med. 2014;6(263):263ra158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Erdo F, Denes L, de Lange E. Age‐associated physiological and pathological changes at the blood‐brain barrier: a review. J Cereb Blood Flow Metab. 2017;37(1):4‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The central nervous system and the gut microbiome. Cell. 2016;167(4):915‐932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mohle L, Mattei D, Heimesaat MM, et al. Ly6C(hi) monocytes provide a link between antibiotic‐induced changes in gut microbiota and adult hippocampal neurogenesis. Cell Rep. 2016;15(9):1945‐1956. [DOI] [PubMed] [Google Scholar]
- 28. Humann J, Mann B, Gao G, et al. Bacterial peptidoglycan traverses the placenta to induce fetal neuroproliferation and aberrant postnatal behavior. Cell Host Microbe. 2016;19(3):388‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hoban AE, Stilling RM, Ryan FJ, et al. Regulation of prefrontal cortex myelination by the microbiota. Transl Psychiatry. 2016;6:e774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim N, Yun M, Oh YJ, Choi HJ. Mind‐altering with the gut: modulation of the gut‐brain axis with probiotics. J Microbiol. 2018;56(3):172‐182. [DOI] [PubMed] [Google Scholar]
- 31. Phillips RJ, Powley TL. Innervation of the gastrointestinal tract: patterns of aging. Auton Neurosci. 2007;136(1–2):1‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Obrietan K, Gao XB, van den Pol AN. Excitatory actions of GABA increase BDNF expression via a MAPK‐CREB‐dependent mechanism—a positive feedback circuit in developing neurons. J Neurophysiol. 2002;88(2):1005‐1015. [DOI] [PubMed] [Google Scholar]
- 33. Li K, Xu E. The role and the mechanism of γ‐aminobutyric acid during central nervous system development. Neuroscience Bulletin. 2008;24(3):195‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tian J, Lu Y, Zhang H, Chau CH, Dang HN, Kaufman DL. Aminobutyric acid inhibits T cell autoimmunity and the development of inflammatory responses in a mouse type 1 diabetes model. J Immunol. 2004;173(8):5298‐5304. [DOI] [PubMed] [Google Scholar]
- 35. Yano JM, Yu K, Donaldson GP, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161(2):264‐276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wong JMW, de Souza R, Kendall CWC, Emam A, Jenkins DJA. Colonic Health: fermentation and Short Chain Fatty Acids. J Clin Gastroenterol. 2006;40(3):235‐243. [DOI] [PubMed] [Google Scholar]
- 37. Topping DL, Clifton PM. Short‐chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81(3):1031‐1064. [DOI] [PubMed] [Google Scholar]
- 38. Liu H, Wang J, He T, et al. Butyrate: a double‐edged sword for health? Adv Nutr. 2018;9(1):21‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007;447(7141):178‐182. [DOI] [PubMed] [Google Scholar]
- 40. Schwab M, Reynders V, Loitsch S, Steinhilber D, Stein J, Schroder O. Involvement of different nuclear hormone receptors in butyrate‐mediated inhibition of inducible NF kappa B signalling. Mol Immunol. 2007;44(15):3625‐3632. [DOI] [PubMed] [Google Scholar]
- 41. Bourassa MW, Alim I, Bultman SJ, Ratan RR. Butyrate, neuroepigenetics and the gut microbiome: can a high fiber diet improve brain health? Neurosci Lett. 2016;625:56‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thevaranjan N, Puchta A, Schulz C, et al. Age‐associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe. 2017;21(4):455‐466. e454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Calsolaro V, Edison P. Neuroinflammation in Alzheimer's disease: current evidence and future directions. Alzheimers Dement. 2016;12(6):719‐732. [DOI] [PubMed] [Google Scholar]
- 44. Holmes C, Cunningham C, Zotova E, et al. Systemic inflammation and disease progression in Alzheimer disease. Neurology. 2009;73(10):768‐774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bhaskar K, Maphis N, Xu G, et al. Microglial derived tumor necrosis factor‐alpha drives Alzheimer's disease‐related neuronal cell cycle events. Neurobiol Dis. 2014;62:273‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Varley J, Brooks DJ, Edison P. Imaging neuroinflammation in Alzheimer's disease and other dementias: recent advances and future directions. Alzheimers Dement. 2015;11(9):1110‐1120. [DOI] [PubMed] [Google Scholar]
- 47. Heneka MT, Carson MJ, Khoury JE, et al. Neuroinflammation in Alzheimer's disease. Lancet Neurol. 2015;14(4):388‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Swanson A, Wolf T, Sitzmann A, Willette AA. Neuroinflammation in Alzheimer's disease: pleiotropic roles for cytokines and neuronal pentraxins. Behav Brain Res. 2018;347:49‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Batarseh YS, Duong QV, Mousa YM, Al Rihani SB, Elfakhri K, Kaddoumi A. Amyloid‐beta and astrocytes interplay in amyloid‐beta related disorders. Int J Mol Sci. 2016;17(3):338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Simpson JE, Ince PG, Lace G, et al. Astrocyte phenotype in relation to Alzheimer‐type pathology in the ageing brain. Neurobiol Aging. 2010;31(4):578‐590. [DOI] [PubMed] [Google Scholar]
- 51. Simon E, Obst J, Gomez‐Nicola D. The evolving dialogue of microglia and neurons in Alzheimer's disease: microglia as necessary transducers of pathology. Neuroscience. 2018; 10.1016/j.neuroscience.2018.01.059 [DOI] [PubMed] [Google Scholar]
- 52. Biron KE, Dickstein DL, Gopaul R, Jefferies WA. Amyloid triggers extensive cerebral angiogenesis causing blood brain barrier permeability and hypervascularity in Alzheimer's disease. PLoS ONE. 2011;6(8):e23789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang H, Ma Q, Zhang YW, Xu H. Proteolytic processing of Alzheimer's beta‐amyloid precursor protein. J Neurochem. 2012;120(Suppl 1):9‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gosztyla ML, Brothers HM, Robinson SR. Alzheimer's Amyloid‐beta is an antimicrobial peptide: a review of the evidence. J Alzheimers Dis. 2018;62(4):1495‐1506. [DOI] [PubMed] [Google Scholar]
- 55. Emery DC, Shoemark DK, Batstone TE, et al. 16S rRNA next generation sequencing analysis shows bacteria in Alzheimer's post‐mortem brain. Front Aging Neurosci. 2017;9:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wozniak MA, Mee AP, Itzhaki RF. Herpes simplex virus type 1 DNA is located within Alzheimer's disease amyloid plaques. J Pathol. 2009;217(1):131‐138. [DOI] [PubMed] [Google Scholar]
- 57. Tzeng NS, Chung CH, Lin FH, et al. Anti‐herpetic medications and reduced risk of Dementia in patients with herpes simplex virus infections‐a Nationwide, population‐based cohort study in Taiwan. Neurotherapeutics. 2018;15(2):417‐429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Readhead B, Haure‐Mirande JV, Funk CC, et al. Multiscale analysis of independent Alzheimer's cohorts finds disruption of molecular, genetic, and clinical networks by human herpesvirus. Neuron. 2018;99(1):64‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gerard HC, Dreses‐Werringloer U, Wildt KS, et al. Chlamydophila (Chlamydia) pneumoniae in the Alzheimer's brain. FEMS Immunol Med Microbiol. 2006;48(3):355‐366. [DOI] [PubMed] [Google Scholar]
- 60. Little CS, Hammond CJ, MacIntyre A, Balin BJ, Appelt DM. Chlamydia pneumoniae induces Alzheimer‐like amyloid plaques in brains of BALB/c mice. Neurobiol Aging. 2004;25(4):419‐429. [DOI] [PubMed] [Google Scholar]
- 61. Kountouras J, Boziki M, Zavos C, et al. A potential impact of chronic Helicobacter pylori infection on Alzheimer's disease pathobiology and course. Neurobiol Aging. 2012;33(7):e3‐e4. [DOI] [PubMed] [Google Scholar]
- 62. Prandota J. Possible link between Toxoplasma gondii and the anosmia associated with neurodegenerative diseases. Am J Alzheimers Dis Other Demen. 2014;29(3):205‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gilman S, Koller M, Black RS, et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64(9):1553‐1562. [DOI] [PubMed] [Google Scholar]
- 64. Doody RS, Raman R, Farlow M, et al. A phase 3 trial of semagacestat for treatment of Alzheimer's disease. N Engl J Med. 2013;369(4):341‐350. [DOI] [PubMed] [Google Scholar]
- 65. Green RC, Schneider LS, Amato DA, et al. Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: a randomized controlled trial. JAMA. 2009;302(23):2557‐2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Aspelund A, Antila S, Proulx ST, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212(7):991‐999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Le TN, Blakley BW. Mannitol and the blood‐labyrinth barrier. J Otolaryngol Head Neck Surg. 2017;46(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Izen RM, Yamazaki T, Nishinaka‐Arai Y, Hong YK, Mukouyama YS. Postnatal development of lymphatic vasculature in the brain meninges. Dev Dyn. 2018;247(5):741‐753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hyman BT, Van Hoesen GW, Kromer LJ, Damasio AR. Perforant pathway changes and the memory impairment of Alzheimer's disease. Ann Neurol. 1986;20(4):472‐481. [DOI] [PubMed] [Google Scholar]
- 70. Imbimbo BP, Solfrizzi V, Panza F. Are NSAIDs useful to treat Alzheimer's disease or mild cognitive impairment? Front Aging Neurosci. 2010; 10.3389/fnagi.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sampson TR, Debelius JW, Thron T, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson's disease. Cell. 2016;167(6):1469‐1480.e1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Harach T, Marungruang N, Duthilleul N, et al. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci Rep. 2017;7:41802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Brandscheid C, Schuck F, Reinhardt S, et al. Altered Gut Microbiome Composition and Tryptic Activity of the 5xFAD Alzheimer's Mouse Model. J Alzheimers Dis. 2017;56(2):775‐788. [DOI] [PubMed] [Google Scholar]
- 74. Vogt NM, Kerby RL, Dill‐McFarland KA, et al. Gut microbiome alterations in Alzheimer's disease. Sci Rep. 2017;7(1):13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. McCusker RH, Kelley KW. Immune‐neural connections: how the immune system's response to infectious agents influences behavior. J Exp Biol. 2013;216:84‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tohidpour A, Morgun AV, Boitsova EB, et al. Neuroinflammation and infection: molecular mechanisms associated with dysfunction of neurovascular unit. Front Cell Infect Microbiol. 2017;7:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood‐brain barrier. Nat Med. 2013;19(12):1584‐1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761‐1772. [DOI] [PubMed] [Google Scholar]
- 79. Cai Z, Pan ZL, Pang Y, Evans OB, Rhodes PG. Cytokine induction in fetal rat brains and brain injury in neonatal rats after maternal lipopolysaccharide administration. Pediatr Res. 2000;47(1):64‐72. [DOI] [PubMed] [Google Scholar]
- 80. Liverman CS, Kaftan HA, Cui L, et al. Altered expression of pro‐inflammatory and developmental genes in the fetal brain in a mouse model of maternal infection. Neurosci Lett. 2006;399(3):220‐225. [DOI] [PubMed] [Google Scholar]
- 81. Asti A, Gioglio L. Can a bacterial endotoxin be a key factor in the kinetics of amyloid fibril formation? J Alzheimers Dis. 2014;39(1):169‐179. [DOI] [PubMed] [Google Scholar]
- 82. Sheng JG, Bora SH, Xu G, Borchelt DR, Price DL, Koliatsos VE. Lipopolysaccharide‐induced‐neuroinflammation increases intracellular accumulation of amyloid precursor protein and amyloid beta peptide in APPswe transgenic mice. Neurobiol Dis. 2003;14(1):133‐145. [DOI] [PubMed] [Google Scholar]
- 83. Zhan X, Stamova B, Jin LW, DeCarli C, Phinney B, Sharp FR. Gram‐negative bacterial molecules associate with Alzheimer disease pathology. Neurology. 2016;87(22):2324‐2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Deshmukh HS, Liu Y, Menkiti OR, et al. The microbiota regulates neutrophil homeostasis and host resistance to Escherichia coli K1 sepsis in neonatal mice. Nat Med. 2014;20(5):524‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wallner S, Peters S, Pitzer C, Resch H, Bogdahn U, Schneider A. The Granulocyte‐colony stimulating factor has a dual role in neuronal and vascular plasticity. Front Cell Dev Biol. 2015;3:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Diederich K, Schabitz WR, Minnerup J. Seeing old friends from a different angle: novel properties of hematopoietic growth factors in the healthy and diseased brain. Hippocampus. 2012;22(5):1051‐1057. [DOI] [PubMed] [Google Scholar]
- 87. Everard A, Belzer C, Geurts L, et al. Cross‐talk between Akkermansia muciniphila and intestinal epithelium controls diet‐induced obesity. Proc Natl Acad Sci U S A. 2013;110(22):9066‐9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Chevalier C, Stojanovic O, Colin DJ, et al. Gut microbiota orchestrates energy homeostasis during cold. Cell. 2015;163(6):1360‐1374. [DOI] [PubMed] [Google Scholar]
- 89. Inan MS, Rasoulpour RJ, Yin L, Hubbard AK, Rosenberg DW, Giardina C. The luminal short‐chain fatty acid butyrate modulates NF‐kappaB activity in a human colonic epithelial cell line. Gastroenterology. 2000;118(4):724‐734. [DOI] [PubMed] [Google Scholar]
- 90. Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short‐chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569‐573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Leblhuber F, Geisler S, Steiner K, Fuchs D, Schutz B. Elevated fecal calprotectin in patients with Alzheimer's dementia indicates leaky gut. J Neural Transm (Vienna). 2015;122(9):1319‐1322. [DOI] [PubMed] [Google Scholar]
- 92. Graff J, Rei D, Guan JS, et al. An epigenetic blockade of cognitive functions in the neurodegenerating brain. Nature. 2012;483(7388):222‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Solas M, Puerta E, Ramirez MJ. Treatment options in Alzheimer´s disease: the GABA story. Curr Pharm Des. 2015;21(34):4960‐4971. [DOI] [PubMed] [Google Scholar]
- 94. Bercik P, Denou E, Collins J, et al. The intestinal microbiota affect central levels of brain‐derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141(2):599‐609, 609 e591‐593. [DOI] [PubMed] [Google Scholar]
- 95. Heneka MT, Golenbock DT, Latz E. Innate immunity in Alzheimer's disease. Nat Immunol. 2015;16(3):229‐236. [DOI] [PubMed] [Google Scholar]
- 96. Balducci C, Forloni G. Novel targets in Alzheimer's disease: a special focus on microglia. Pharmacol Res. 2018;130:402‐413. [DOI] [PubMed] [Google Scholar]
- 97. Minter MR, Zhang C, Leone V, et al. Antibiotic‐induced perturbations in gut microbial diversity influences neuro‐inflammation and amyloidosis in a murine model of Alzheimer's disease. Sci Rep. 2016;6:30028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zhang L, Wang Y, Xiayu X, et al. Altered gut microbiota in a mouse model of Alzheimer's disease. J Alzheimers Dis. 2017;60(4):1241‐1257. [DOI] [PubMed] [Google Scholar]
- 99. Sanchez de Medina F, Romero‐Calvo I, Mascaraque C, Martinez‐Augustin O. Intestinal inflammation and mucosal barrier function. Inflamm Bowel Dis. 2014;20(12):2394‐2404. [DOI] [PubMed] [Google Scholar]
- 100. Kurashima Y, Kiyono H. Mucosal ecological network of epithelium and immune cells for gut homeostasis and tissue healing. Annu Rev Immunol. 2017;35:119‐147. [DOI] [PubMed] [Google Scholar]
- 101. Marquez M, Fernandez Gutierrez del Alamo C, Giron‐Gonzalez JA. Gut epithelial barrier dysfunction in human immunodeficiency virus‐hepatitis C virus coinfected patients: influence on innate and acquired immunity. World J Gastroenterol. 2016;22(4):1433‐1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14(3):141‐153. [DOI] [PubMed] [Google Scholar]
- 103. Tran L, Greenwood‐Van Meerveld B. Age‐associated remodeling of the intestinal epithelial barrier. J Gerontol A Biol Sci Med Sci. 2013;68(9):1045‐1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Schaubeck M, Clavel T, Calasan J, et al. Dysbiotic gut microbiota causes transmissible Crohn's disease‐like ileitis independent of failure in antimicrobial defence. Gut. 2016;65(2):225‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Thaiss CA, Levy M, Grosheva I, et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science. 2018;359(6382):1376‐1383. [DOI] [PubMed] [Google Scholar]
- 106. Wen SW, Wong CHY. An unexplored brain‐gut microbiota axis in stroke. Gut Microbes. 2017;8(6):601‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Miyamoto J, Kasubuchi M, Nakajima A, Irie J, Itoh H, Kimura I. The role of short‐chain fatty acid on blood pressure regulation. Curr Opin Nephrol Hypertens. 2016;25(5):379‐383. [DOI] [PubMed] [Google Scholar]
- 108. Hoffman‐Goetz L, Spagnuolo PA, Guan J. Repeated exercise in mice alters expression of IL‐10 and TNF‐alpha in intestinal lymphocytes. Brain Behav Immun. 2008;22(2):195‐199. [DOI] [PubMed] [Google Scholar]
- 109. Kasimay O, Guzel E, Gemici A, et al. Colitis‐induced oxidative damage of the colon and skeletal muscle is ameliorated by regular exercise in rats: the anxiolytic role of exercise. Exp Physiol. 2006;91(5):897‐906. [DOI] [PubMed] [Google Scholar]
- 110. Moayyedi P, Surette MG, Kim PT, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology. 2015;149(1):102‐109. e106. [DOI] [PubMed] [Google Scholar]
- 111. Plaza‐Diaz J, Ruiz‐Ojeda FJ, Vilchez‐Padial LM, Gil A. Evidence of the anti‐inflammatory effects of probiotics and synbiotics in intestinal chronic diseases. Nutrients. 2017;9(6): 10.3390/nu9060555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Khalili L, Alipour B, Asghari Jafar‐Abadi M, et al. The effects of lactobacillus casei on glycemic response, Serum Sirtuin1 and Fetuin‐A levels in patients with type 2 diabetes mellitus: a randomized controlled trial. Iran Biomed J. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Liu X, Cao S, Zhang X. Modulation of gut microbiota‐brain axis by probiotics, prebiotics, and diet. J Agric Food Chem. 2015;63(36):7885‐7895. [DOI] [PubMed] [Google Scholar]
- 114. Kato‐Kataoka A, Nishida K, Takada M, et al. Fermented milk containing lactobacillus casei strain shirota preserves the diversity of the gut microbiota and relieves abdominal dysfunction in healthy medical students exposed to academic stress. Appl Environ Microbiol. 2016;82(12):3649‐3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. He Z, Cui BT, Zhang T, et al. Fecal microbiota transplantation cured epilepsy in a case with Crohn's disease: the first report. World J Gastroenterol. 2017;23(19):3565‐3568. [DOI] [PMC free article] [PubMed] [Google Scholar]


