Abstract
Exosomes are membrane-bound nanovesicles that transport molecular signals between cells. This study determined changes in maternal plasma exosome proteomics contents in term and preterm births. Maternal plasma (MP) samples were collected from group 1: term not in labor (TNIL, n = 13); group 2: term in labor (TL, n = 11); group 3: preterm premature rupture of membranes (pPROM, n = 8); and group 4: preterm birth (PTB, n = 13). Exosomes isolated from plasma by differential density centrifugation followed by size exclusion chromatography were characterized by morphology (electron microscopy), quantity and size (nanoparticle tracking analysis), and markers (western blot). A quantitative, information-independent acquisition [sequential windowed acquisition of all theoretical mass spectra (SWATH-MS)] approach was used to determine the protein profile in exosomes. Ingenuity Pathway Analysis determined pathways associated with the protein profile identified in exosomes. MP exosomes were spherical, had a mean diameter of 120 nm, and were positive for exosomal proteins CD63 and TSG101 irrespective of pregnancy status. No distinct changes in exosome quantities were seen in maternal circulation across the groups. SWATH-MS identified 72 statistically significant proteins across the groups studied. Bioinformatics analysis showed the proteins within the exosomes in TNIL, TL, pPROM, and PTB target pathways mainly associated with inflammatory and metabolic signals. Exosomal data suggest that homeostatic imbalances, specifically inflammatory and endocrine signaling, might disrupt pregnancy maintenance resulting in labor-related changes both at term and preterm. Reflection of physiologic changes in exosomes is suggestive of its usefulness as biomarkers and cellular function indicators.
Preterm birth (PTB; birth before the 37th completed week of pregnancy) is a major pregnancy complication and socioeconomic problem that affects 15% to 20% of all pregnancies worldwide (1). The PTB rate has increased in the United States by as much as 30% during the past 25 years despite advances in perinatal care. The most common (60%) phenotype of PTB occurs spontaneously (2, 3). To address the problem of PTB, a clear understanding of the signals that induce normal labor is needed. Normal term human parturition is initiated when fetal organs are matured around the 37th to 40th weeks of gestation. Conventional theories of initiation signaling of parturition are primarily linked to fetal and maternal endocrine changes including cortisol production and functional progesterone withdrawal, as well as immune changes such as leukocyte infiltration into the fetomaternal interface tissues, which correlate with fetal growth and development (4–10). Imbalances in endocrine and immune functions lead to an inflammatory overload that disrupts the maintenance of pregnancy resulting in labor-related changes (11–14). Nonetheless, the nature of these signals and their mechanism in initiating parturition needs further investigation. Understanding these signals and their mechanisms in normal term pregnancies can provide insights into pathologic activation of such signals that can cause spontaneous preterm parturitions.
Fetal endocrine signals are generally considered to contribute to the timing of birth (15). However, endocrine signals have not fully explained how preterm labor is initiated and how specific pregnancy risk factors may override endocrine signals to effect PTB (9). These ambiguities in our existing theories of signaling suggest that timing of birth may also be controlled by paracrine factors (5, 16). We report a paracrine signaling mechanism mediated by extracellular vesicles, specifically exosomes (17). Exosomes are small vesicles (∼30 to 150 nm) originated from endosomal compartments secreted to the extracellular milieu after fusion of multivesicular bodies with the plasma membrane (18). Exosomes can function as vectors to transport signals between the fetal and maternal tissues, where they deposit their cargo into target cells to produce a functional effect (19). Several cells/tissues have been reported to release the exosomes including neuronal cells (20), fibroblast cells (21), adipocytes (22), intestinal (23), and lung epithelial cells (24), and in both fetal (placenta, umbilical cord, and fetal membranes) and maternal (decidua, myometrium, and cervix) reproductive tissues (25–27). Exosome cargo includes proteins, nucleic acids, and fatty acids. Using the same cohort of subjects, our group recently reported exosomes cargo characteristics in amniotic fluid during pregnancy (28).
Descriptive data support an association between exosomes and their potential functional contributions during pregnancy and parturition (29). Exosome-mediated signaling is an unexplored mechanism for fetal-maternal communication (26, 27, 30, 31). Exosome contents represent the physiologic state of the cell of origin making them a good vector of paracrine signaling. Several lines of evidence support a role for exosomes as a potential communication channel. They include, but not limited to: (i) Exosomes quantity increase in human maternal plasma (MP) as gestation progress (26); (ii) increase in MP exosome concentration is contributed by fetal/placental specific exosomes (26, 32, 33); (iii) placental cells and amnion epithelial cells release exosomes and have unique cargo that changes in response to exposure to oxidative stress inducers, infection, and inflammatory agents and environmental toxins (27, 34, 35); (iv) fetal cell–derived exosomes can traverse through fetomaternal tissues and can produce inflammatory changes in maternal tissues (29); (v) in animal models of pregnancy, total exosome concentration (fetal and maternal) and inflammatory cargo progressively increase as gestation progress; (vi) exosomes from placental trophoblasts in MP show antiviral effects (36, 37); and (vii) amniotic fluid exosomes show unique proteomic profile at term and preterm pregnancies (28).
Based on these data, we hypothesized that differential expression of exosome contents will reflect the underlying physiology during term and preterm pregnancies. Therefore, the objective of this study was to isolate, characterize, and establish the proteomic content of circulating exosomes present in MP samples obtained from a well-characterized cohort of spontaneous PTB with or without preterm premature rupture of the membranes (pPROMs) and normal term birth. Exosomes were isolated, and quantitative proteomics-based cargo characterization followed by bioinformatics analysis of differentially expressed proteins were performed. We report the differences between the MP exosome protein profile in late spontaneous PTB, pPROM (between 34 and 36 weeks of gestation), and at term between deliveries with and without labor. Changes in exosomal cargo reflect distinct functional pathways and biological mechanisms that underlines normal and pathologic pregnancies. This information is expected to help define exosomal signaling as well as its value as a biomarker to be tested in MP from longitudinal cohorts.
Materials and Methods
Subject recruitment and sample collection protocols were approved by Western institutional review board for TriStar Nashville, TN. All samples were collected after receiving informed written consent from subjects. Use of samples for this study was approved by the institutional review board at The University of Texas Medical Branch, Galveston, TX. Subjects were recruited at the Centennial Medical Center, Nashville, TN. Institutional review boards at TriStar, Nashville, TN, approved this study. All included pregnancies were singleton live births. Subjects were recruited between September 2003 and December 2006 (38–40).
For this study, our cohort of late preterm (PTB, pPROM) and term [term in labor (TL) and term not in labor (TNIL)] consisted of 45 subjects. Mothers between the ages of 18 and 40 years were recruited on admission for term or preterm deliveries. Gestational age was determined by last menstrual period and corroborated by ultrasound dating. Plasma samples were collected from women who underwent: (i) PTB (n = 13), defined as having 2 contractions/10 minutes followed by delivery between 340/7 and <370/7 weeks’ gestation; (ii) pPROM (n = 13), defined as rupture of membranes (confirmed by tests such as amniotic fluid pooling, “ferning,” nitrazine, and Amnisure positivity) followed by PTB before onset of labor at <370/7 weeks’ gestation; (iii) TL (n = 10), defined as having two contractions/10 minutes followed by delivery at ≥370/7 weeks with no medical or obstetrical complications during pregnancy; and (iv) normal TNIL (n = 12), undergoing elective cesarean deliveries between 37 and 42 weeks’ gestation. Subjects with multiple gestations, preeclampsia, placental previa, fetal anomalies, gestational diabetes, poly- and oligohydramnios, intrauterine growth restrictions, and other complications such as surgeries during pregnancies were excluded. Samples from late PTBs were used for this study as these cases are more homogeneous, generally devoid of infectious etiology and are similar in clinical presentations compared with early PTBs (<32 weeks). Late preterm groups also resemble normal term births in many perspectives from the fetal side. Amniotic fluid sample exosome analysis was conducted using the same cohort of subjects as previously; hence, clinical and demographics of subjects and experimental approaches are the same in this study (28).
Isolation of exosomes from MP
Exosomes were isolated from plasma as previously described (26, 32, 41). In brief, plasma samples were diluted with an equal volume of PBS (pH 7.4) and centrifuged at 2000g for 30 minutes at 4°C (Sorvall®, high-speed microcentrifuge, fixed rotor; Thermo Fisher Scientific Inc., Asheville, NC). The 2000g supernatant fluid was then centrifuged at 12,000g for 45 minutes at 4°C (Sorvall, high-speed microcentrifuge, fixed rotor). The resultant supernatant fluid (2 mL) was transferred to an ultracentrifuge tube (10 mL; Beckman Coulter, Brea, CA) and centrifuged at 100,000g for 2 hours (Sorvall, T-8100, fixed ultracentrifuge rotor). The 100,000g pellet was resuspended in PBS (10 mL) and filtered through a 0.22-μm filter (Steritop™; Merck Millipore, Billerica, MA) and then centrifuged at 100,000g for 2 hours. The pellet containing the enriched small vesicles was resuspended in 500 μL PBS and placed onto a size-exclusion chromatography column (Sepharose® CL-2B resin); the exosomes were obtained by elution with PBS. Vesicles were concentrated using a 100,000 Nominal Molecular Weight Limit Amicon Ultra-15 Centrifugal Filter Unit (Merck Millipore) by centrifugation at 4000g for 10 minutes (4°C). Exosomes were characterized by size distribution, abundance of proteins associated with exosomes, and morphology in accordance with the recommendation of the international society of extracellular vesicles, using nanoparticle tracking analysis (NTA), western blot analysis, and electron microscopy, respectively (32). The exosome isolation, characterization, and proteomic analysis were conducted within an ISO17025-accredited (National Association of Testing Authorities, Australia) research facility (Exosome Biology Laboratory, Centre for Clinical Diagnostics, Brisbane, Australia). All data were recorded within a 21 Code of Federal Regulation part 11–compliant electronic laboratory notebook (Laboratory Archives, Carlsbad, CA).
Exosomes were characterized by size distribution, enrichment of CD63, and morphology by NTA, western blot, and electron microscopy, respectively.
Light scatter and fluorescence NTA
NTA measurements were performed using a NanoSight NS500 instrument (NanoSight NTA 3.0 Nanoparticle Tracking and Analysis Release Version Build 0064; Malvern Instruments, Malvern, United Kingdom), as previously described (28). Exosomes were diluted to 100 µg/mL protein, and five videos were processed and analyzed. A minimum of 200 completed tracks per video were collected for each analyzed sample. NTA postacquisition settings were optimized and kept constant between samples, and each video was then analyzed to give the mean, mode, and median particle size together with an estimated number of particles per milliliter of amniotic fluid. A spreadsheet (Excel; Microsoft Corp., Redmond, WA) was automatically generated, recording the concentration of each particle size. One hundred-nanometer polystyrene latex microspheres (Malvern NTA 4088) were routinely analyzed to confirm instrument performance. For fluorescence NTA, Qdots (Qdot® nanocrystals) were conjugated to anti-CD63, placental alkaline phosphatase (PLAP, clone H17E2; Thermo Fisher) or IgG1 isotype control antibody (IgG1 sc-34665, Santa Cruz Biotechnology, Dallas, TX) with a SiteClick Qdot 605 Antibody Conjugation Kit (Life Technologies, Carlsbad, CA), executed according to the manufacturer’s instructions, as previously described (30, 32, 42). Exosomes were diluted in PBS and incubated with FcR blocking reagent (10 µL, 10 minutes at 4°C) (MACS Miltenyi Biotec, Bergisch Gladbach, Germany), followed by incubation with anti-CD63-Qdot605, PLAP-Qdot605, or IgG1-Qdot605 (10 µL, 1:100) for 30 minutes in the dark at room temperature. Samples were then diluted to 500 µL with PBS and analyzed using the NanoSight NS500 instrument and NTA software: (i) exosomes alone; (ii) exosomes + IgG1-Qdot605; (iii) exosomes + anti-CD63-Qdot605 and exosomes + anti-PLAP-Qdot605; (iv) background controls; (v) FcR blocking reagent + IgG1-Qdot605; (vi) FcR blocking reagent + anti-CD63-Qdot605; and (vii) FcR blocking reagent + anti-PLAP-Qdot605). In fluorescence mode (i.e., camera level 9, shutter speed 11.25 ms, and slider gain 250), five 60-second videos were captured for each sample and analyzed.
Western blot analysis and transmission electron microscopy
Exosome proteins separated by polyacrylamide gel electrophoresis were transferred onto polyvinylidene difluoride membrane using the Trans-Blot® Turbo™ Transfer System (BioRad Laboratories, Hercules, CA). After transfer, the blot was blocked with Odyssey® Blocking Buffer (LI-COR®, Lincoln, NE) at room temperature for 1 hour. The blocking buffer was removed and the antibodies [CD63 (sc15363; Santa Cruz Biotechnology); CD9 (sc13118; Santa Cruz Biotechnology); and TSG101 and EPR7130 (Abcam, Cambridge, United Kingdom)], and a negative control for Grp94, were added and incubated overnight at 4°C. The blots were then washed for 5 minutes in Tris-buffered saline supplemented with 0.1% v/v Tween 20, for three times in total. Antirabbit conjugated with DyLight 800 secondary antibody diluted 1:15,000 in Odyssey Blocking Buffer was added. Washes of 5 minutes each of Tween 20 and an additional wash of Tris-buffered saline for 5 minutes to remove any residual Tween 20. The blots were scanned using a LI-COR Biosciences Odyssey IR Imaging System and data quantified using Image Studio software version 4.0. For electron microscopy analysis, exosome pellets were fixed in 3% (w/v) glutaraldehyde and analyzed under an FEI Tecnai 12 transmission electron microscope (Hillsboro, OR), as we previously described (27).
Sequential window acquisition of all theoretical mass spectrometry
Ion library generation
To generate the ion library used in the sequential window acquisition of all theoretical (SWATH) mass spectrometry (MS) analysis, total exosomes derived from MP were reduced, alkylated, and trypsinized using an in-gel digestion method, as previously described (28). The resulting peptide samples were processed in an information-dependent acquisition (IDA) on an AB Sciex 5600 TripleTOF mass spectrometer with the top 20 precursor ions automatically selected for fragmentation.
SWATH
For SWATH acquisition, the 5600 TripleTOF was operated in a looped product ion mode. Using an isolation width of 26 m/z, a set of 32 overlapping windows (1 m/z overlap) was constructed covering the mass range 400 to 1200 m/z. For individual patient samples, 10 µg of exosomes were digested using the Filter Aided Sample Preparation, as previously described (43).
Data processing
ProteinPilot, version 4.5b, software and the Paragon™ Algorithm were used to search against a human SwissProt database. A global false discovery rate (FDR) of 1% was used as the threshold for the number of proteins for import. For SWATH processing, the SWATH Acquisition Microapp (version 2.0) within PeakView (version 2.2) was used. Within Microapp, a setting of three peptides per protein, four transitions per peptide, peptide confidence threshold corresponding to 1% global FDR, and FDR threshold of 1% was used. The retention time was then manually realigned with a minimum of five peptides with constantly high signal intensities and distributed along the time axis. The resulting peak area for each protein after SWATH processing was exported to MakerView (version 1.3.1); the resulting data were normalized using the most likely ratio method.
Ingenuity Pathway Analysis of identified proteins
Pathway enrichment analyses were performed with Ingenuity Pathway Analysis (IPA; Qiagen, Hilden, Germany). IPA was performed to identify canonical pathways, diseases and functions, and protein networks. Heat map analysis was used to demonstrate the expression patterns of biological functions based on z scores. Significantly enriched pathways for the proteins and pathways were identified with the criterion P < 0.05.
Statistical analysis of nonproteomic data
Maternal demographic and clinical data were compared using Student t, χ2, or Mann-Whitney U tests, where appropriate. All MS data are provided in an online repository (44).
Results
Patient cohort data
Maternal age was between 24 and 29 years and not different between case groups (PTB and pPROM) and control groups (TNIL and TL) (P = 0.3) and had a similar number of nulliparous women and smokers in each group (P = 0.1 and P = 0.7, respectively). Race, marital status, level of high school, and college education (determined socioeconomic status) were similar across all groups. As per definition of cases, women in the PTB and pPROM groups delivered babies at an earlier gestational age and with a lower birth weight than women in the TNIL and TL groups (P < 0.001 and P < 0.001, respectively; Table 1) (28). This study has restricted late PTB and pPROM based on sample availability.
Table 1.
Clinical and Demographic Information
| TNIL (n = 13) | TL (n = 11) | PTB (n = 13) | pPROM (n = 8) | P Value | |
|---|---|---|---|---|---|
| Age, y | 28.6 ± 4 | 27 ± 5.6 | 26.5 ± 5.5 | 24 ± 6.8 | 0.3 |
| Gestational age, wk | 38.6 [38.2–39.3] | 39 [37.3–39.5] | 36 [35.1–36.3] | 34 [32.2–34.5] | <0.001 |
| Birth weight, g | 3950 [3225–3724] | 3322 [2863–3402] | 2948 [2291–3058] | 2346 [2182–2723] | <0.001 |
| Gravidity | 2 [2–3] | 2 [1–4] | 2 [1–4.5] | 2 [1.25–2] | 0.31 |
| Nulliparity | 0 (0) | 4 (36) | 5 (38) | 3 (38) | 0.1 |
| History of PTD | 1 (8) | 0 (0) | 5 (38) | 0 (0) | 0.02 |
| Smoking | 1 (8) | 0 (0) | 1 (8) | 1 (12.5) | 0.7 |
| GBS | 0.04 | ||||
| Negative | 8 (62) | 11 (100) | 7 (54) | 4 (57) | |
| Positive | 4 (31) | 0 (0) | 1 (8) | 1 (14) | |
| Unknown | 1 (8) | 0 (0) | 5 (38) | 2 (29) | |
| Race | 0.32 | ||||
| White | 12 (92) | 8 (73) | 11 (85) | 8 (100) | |
| Black | 1 (8) | 3 (27) | 2 (15) | 0 (0) | |
| Marital status | 0.28 | ||||
| Single | 2 (15) | 5 (45) | 6 (46) | 4 (50) | |
| Married | 11 (85) | 5 (45) | 7 (54) | 4 (50) | |
| Divorced | 0 (0) | 1 (9) | 0 (0) | 0 (0) | |
| High school education, y | 12 [12–12] | 12 [12–12] | 12 [12–12] | 12 [12–12] | 0.48 |
| College education, y | 2 [0–4] | 0 [0–2.5] | 0 [0–2] | 0 [0–2.25] | 0.48 |
Data are reported as mean ± SD, median [interquartile range] or n (%).
Abbreviation: GBS, group B Streptococcus.
Exosome isolation and characterization
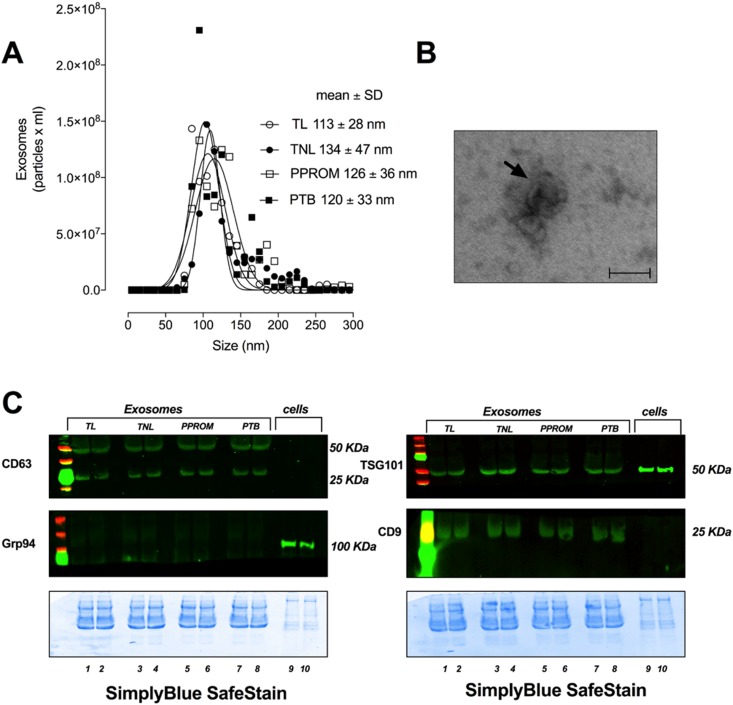
The characteristics of exosomes isolated and purified using a well-established and validated method (28) are presented in Fig. 1. NTA identified particles of ∼120 nm (Fig. 1A). Transmission electron microscopy identified vesicles with a cup shape morphology, which is characteristic of an exosome (Fig. 1B). Furthermore, exosomes were positive for exosomal protein markers CD63, TSG101, and CD9, and negative for Grp94, which is an endoplasmic reticulum marker demonstrating the purity of the exosome isolation (Fig. 1C). No substantial differences on the exosomes’ characteristics among PTB, pPROM, TL, and TNIL were identified.
Figure 1.
Characterization of exosome isolated from MP. (A) Representative size distribution of exosomes isolated from maternal plasma at TNIL, TL, pPROMs, and PTB. (B) Electron micrograph indicating the morphology and size of exosomes. (C) Representative western blot and corresponding SDS-gel stained with SimplyBlue SafeStain (bottom) for exosome-enriched marker CD63, TSG101, and CD9 and negative marker Grp94. Scale bar, 100 nm.
Quantification of exosomes
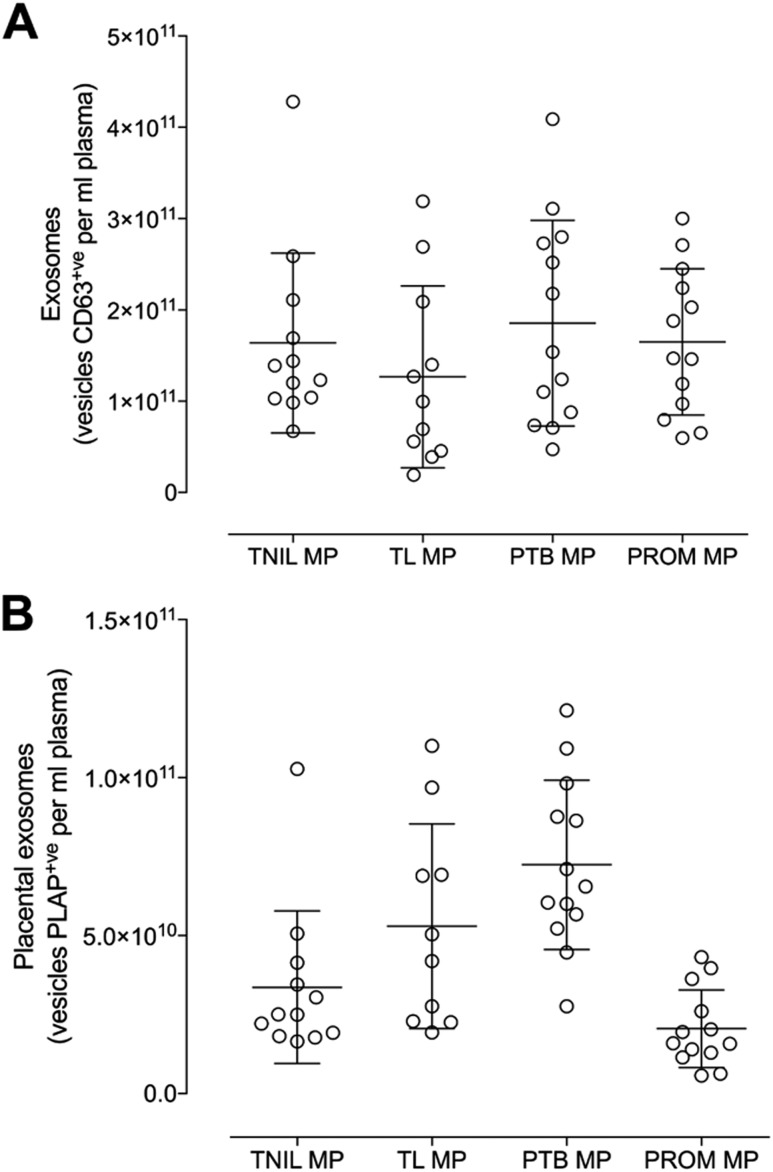
The total number of exosomes (Qdot-CD63+ve) and placenta-derived exosomes (Qdot-PLAP+ve) are presented in Fig. 2A and 2B. We used NTA in fluorescence mode to quantify individual vesicles present in maternal circulation. There were no substantial differences in the MP concentration of total circulating exosomes across all the groups studies (i.e., TL, TNIL, pPROM, and PTB; ANOVA P = 0.426) (Fig. 2A). However, significant changes (ANOVA P < 0.0001) on the levels of circulating placenta-derived exosomes present in maternal circulation across the groups were identified (Fig. 2B). A Dunn multiple comparisons test was used to identify statistically significant (P < 0.05) differences between pairwise comparisons (Fig. 2B). The levels of circulating placenta-derived exosomes were significantly increased in PTB compared with TNIL (P = 0.001), and significantly decreased in pPROM compared with TL (P = 0.001) and pPROM compared with PTB (P < 0.0001).
Figure 2.
Profile of exosomes in maternal plasma. Quantification of total and placenta-derived exosomes obtained from women at TNIL, TL, pPROMs, and PTB was performed using NTA in fluorescence mode by Qdot coupled with CD63 (total exosomes) and PLAP (placental exosomes). (A) Total exosomes (CD63+ve). (B) Placenta-derived exosomes (CD63+ve and PLAP+ve).
Differentially expressed proteins quantified by SWATH-MS in circulating exosomes in MP
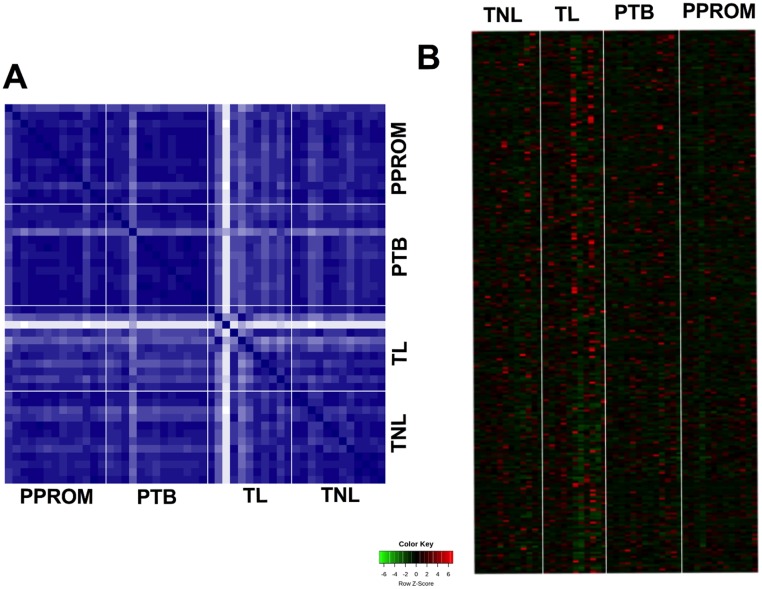
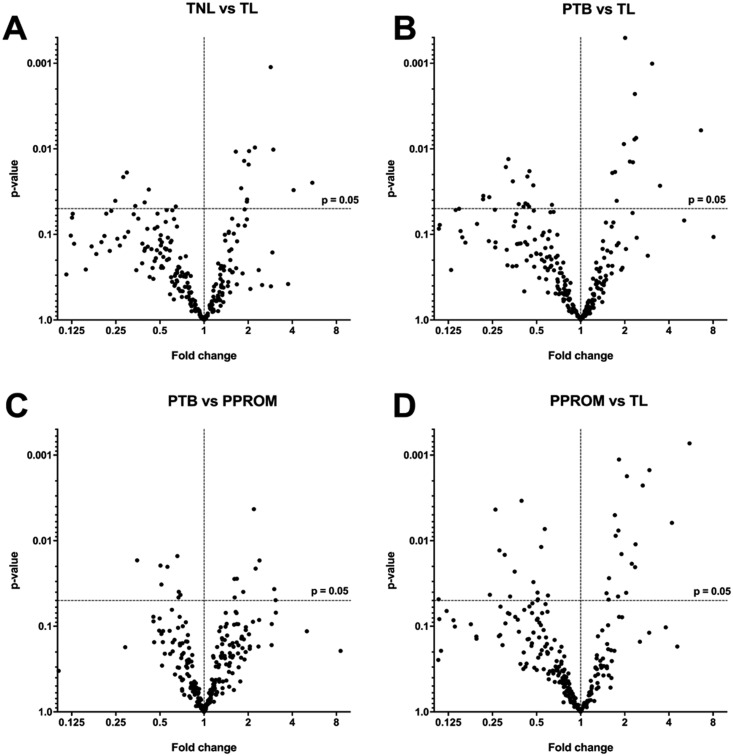
IDA and SWATH profiles were generated using exosomes isolated from women with TL, TNIL, pPROM, and PTB using independent samples per group. For this analysis, we have used total exosomes and not PLAP+ exosomes. The IDA library was used to identify peptide ions that were present in SWATH ion profiles. Proteins were identified and quantified by comparing SWATH-generated peptide ion profiles for each individual’s samples against the IDA library (PeakView). IDA of MS from all individual exosome samples was initially performed, identifying 250 total proteins (44), and analyzed using IDA and SWATH. The proteins contributing to the differences between samples were subjected to hierarchal clustering analysis and displayed as heat maps (Fig. 3A and 3B). The variation in the relative abundance of exosomal proteins between selected pair groups (i.e., TNIL vs TL; PTB vs TL; PTB vs pPROM; and pPROM vs TL) was established by comparison with the SWATH profile against the IDA library and presented as a volcano plot (Fig. 4A and 4D). A total of 17, 30, 37, and 16 statistically significant protein identifications (P < 0.05) in the relative expression of exosomal proteins in PTB vs pPROM, PTB vs TL, pPROM vs TL, and TNIL vs TL were identified, respectively (44). A correlation analysis was performed to establish the association between the clinical characteristics of the patients (i.e., gestational age and birth weight) and the exosomal proteomic profile. Interestingly, 18 proteins that correlate (P < 0.05) with the gestational age (9 proteins) and birth weight (9 proteins) were identified (44). Finally, we performed a bioinformatic analysis in the proteins that correlates with gestational age and birth weight and identified that these proteins were associated with immune and inflammation response, and lipid metabolism, respectively (44).
Figure 3.
Protein profile in exosomes isolated from maternal plasma. The protein profile in exosomes obtained from women at TNIL, TL, pPROMs, and PTB was identified and quantified using SWATH MS analysis (see “Materials and Methods”). (A) Hierarchical clustering of SWATH data performed across proteomics samples. (B) Heat map represents the relative abundance of proteins in exosomes across the groups.
Figure 4.
Comparison of protein abundance in exosomes isolated from maternal plasma. Volcano plots were using to establish the differences in the protein abundance within exosomes obtained from women at TNIL, TL, pPROMs, and PTB in a selected pair of groups. Volcano plot showing differentially expressed protein between (A) TNL vs TL, (B) PTB vs TL, (C) PTB vs PPROMs, and (D) PPROMs vs TL. The horizontal axis represents the log2 of fold change; the vertical axis represents P value. The horizontal dotted line shows P = 0.05. Each black dot represents a protein, with black dots on the right above the dashed line being proteins upregulated and on the left being downregulated.
IPA
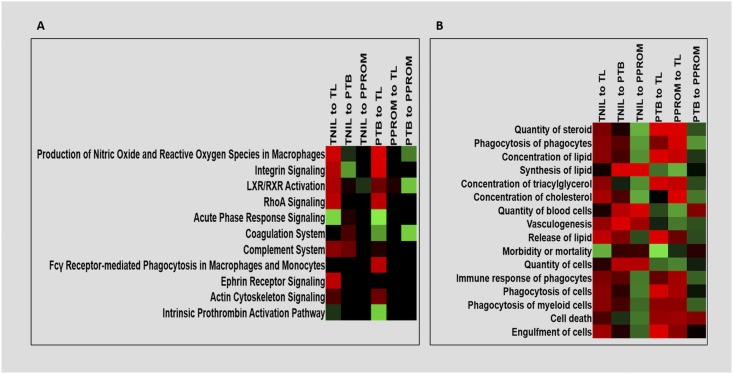
To investigate the potential functions of these differentially expressed proteins, IPA of the exosomal proteomic profile per pair of analysis (i.e., TNIL vs TL, PTB vs TL, PTB vs pPROM, and pPROM vs TL) was performed (44). As shown in Fig. 5, the z scores indicate differences observed in various functional pathways between various group analyses. Nonspecific inflammation is one of the key mediators of labor pathways at term and preterm; however, heterogeneity is evident within the type of inflammation associated with each condition. For example, upregulated hematological dysfunctions such as decidual hemorrhage and other coagulopathies (e.g., thrombin activation) may contribute to pathological activation of inflammation that affects preterm labor. Similarly, inflammation, coagulopathies, and oxidative stress differentiates PTB and pPROM pathways and biological functions (Fig. 5A and 5B). Similarities in TL and pPROM pathways have been reported based on molecular, histologic, and cellular biological studies in fetal membranes (45, 46), and we report such similarities reflected in exosomes between these groups. Canonical pathway analysis shows similarities in the pathway activation between TNIL and PTB when compared with TL (Fig. 5A). These similarities are also apparent in the biological function analysis, showing similar upregulation of phagocytosis and cell death (Fig. 5B). MP exosome proteomic signature appears confirmatory of this finding because we did not see any substantial upregulation of any pathways between these groups (Fig. 5A and 5B).
Figure 5.
Ingenuity pathway analysis heat maps comparing canonical pathways and biological functions for all conditions studied. Differentially expressed proteins were used to identify the (A) top canonical pathways and (B) biological functions associated with each comparison group. Upregulation (green, positive z score), downregulation (red, negative z score).
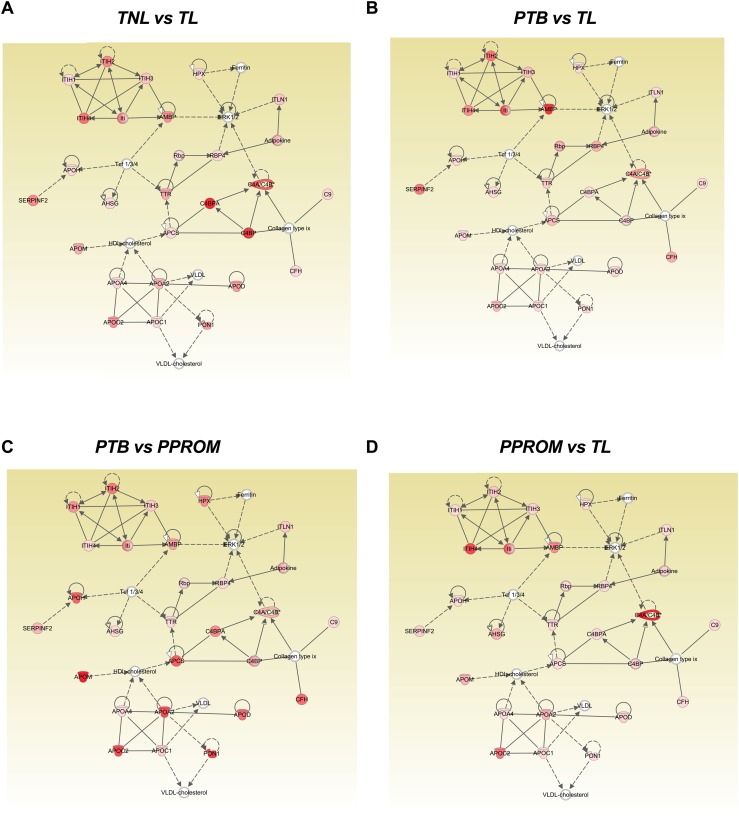
When network analysis of differentially regulated molecules was performed, no significant difference (no difference in the P value per each analysis) on the top canonical pathways, top disease and biological functions, and top molecular and cellular functions regulated by the proteins for each pair of analysis was identified. For all the conditions analyzed, the protein profile across groups was associated with acute phase response signaling, liver X receptor/retinoid X receptor activation, organismal injury and abnormalities, inflammatory response, cell-to-cell signaling, and interaction and cellular movement. Finally, we evaluated the networks associated with the difference in the protein profile per each pairs of analysis. We compared the protein profile using network analysis: (i) TNIL vs TL (Fig. 6A), (ii) PTB vs TL (Fig. 6B), (iii) PTB vs pPROM (Fig. 6C), and (iv) pPROM vs TL (Fig. 6D). The higher score was for the network on lipid metabolism, molecular transport, and small molecule biochemistry in each pair of analysis; however, differential activation of molecules involved in each pair of analysis was identified (Fig. 6A–6D) as indicated by intensity of color and type of arrow. Molecules associated with high-density lipoprotein cholesterol signaling pathways such as apolipoprotein M, apolipoprotein A2, apolipoprotein C2, and paraoxonase 1 were upregulated in PTB compared with pPROM (Fig. 6C). On the other hand, the alpha-1-microglobulin/bikunin precursor, which leads to ERK1/2 seem to be activated when we compared the protein profile between PTB and TL (Fig. 6B). Interestingly, complement 4 binding (C4B) protein, which is associated with the classical immune activation pathway, was upregulated in pPROM compared with TL (Fig. 6D). Similarly, complement C4B protein alpha and C4B upregulated exosomal protein profile between TNIL with TL were compared.
Figure 6.
IPA to identify the signaling pathway associated with the differentially expressed proteins across all the conditions studied. Differently expressed proteins obtained from the pair of analysis between (A) TNL vs TL, (B) PTB vs TL, (C) PTB vs PPROM, and (D) PPROM vs TL were submitted to IPA analysis. The networks representing the higher score were lipid metabolism, molecular transport, and small molecule biochemistry in each pair of analysis. Each network displays the genes as nodes and the relationships between the nodes as lines. The color intensity of each node indicates the degree of regulation (high = red; or low = pink) of the respective gene transcript.
Discussion
Circulating exosomes in maternal blood during pregnancy and parturition might function as communicators between fetomaternal systems by carrying fetal physiologic cargo to maternal uterine tissues to cause immune activation (29) and determine timing of parturition as well as prime them for labor-associated inflammatory changes. All these functions raise the potential of exosomes as biomarkers of pregnancy-associated functions (28, 29, 47). Before initiating functional and biomarker studies, we determined the circulating levels and protein profile of MP exosomes in a well-characterized cohort of samples obtained from women with different signals of parturition (i.e., PTB with pPROM) and without rupture and normal term birth.
In this study, we did not find any differences in the total number of circulating exosomes in either at spontaneous PTB either with rupture or intact membranes matched by gestational age or at term irrespective of labor status. Ambiguity persists in placental-specific exosome isolation and characterization as antibodies available from commercial sources are not totally specific to determine placental-specific alkaline phosphatases. Therefore, a discussion based on differences in the quantity of PLAP exosomes is not attempted here because their functional contributions will be purely speculative without performing additional mechanistic studies. However, based on prior reports, it is clear that changes in their quantity are indicative of changes in placental function contributing to pregnancy outcome (48–50).
Exosomes are involved in cell-to-cell communication because they can protect and deliver specific cargo (e.g., proteins) that are readily degraded to regulate the biological function of a recipient cell either locally or at a distant site (18, 51, 52). We next investigated the protein content of the total circulating exosomes using a quantitative approach (SWATH MS/MS). Interestingly, differential expression of proteins in exosomes per condition were identified, and the main signaling networks associated with these changes were lipid metabolism and molecular transport, primarily associated with inflammation during parturition. This study shows distinct signals of parturition in MP exosomes associated with different pregnancy outcomes. These data denote that changes in exosome cargo reflect functional changes contributing to pregnancy outcome as well as the potential of exosome as a biomarker. The latter point is somewhat weak in this study because samples were obtained after labor onset.
As shown in Fig. 6A–6D, we used bioinformatics to dissect the information carried in differentially regulated exosomal peptides in various conditions. Although the networking of various molecules represented by exosomal cargo peptides are somewhat similar, their involvement and interactions in various conditions are distinct. This is represented by differences in color intensity and the type of arrows (e.g., broken, solid, double pointed). For example, high-density lipoprotein cholesterol signaling pathways such as apolipoprotein M, apolipoprotein A2, apolipoprotein C2, and paraoxonase 1 were upregulated in PTB compared with pPROM (Fig. 6A). On the other hand, alpha-1-microglobulin/bikunin precursor, which leads to ERK1/2, seems to be activated when we compared the protein profile between PTB and TL (Fig. 6B). Interestingly, complement C4B, which is associated with the classical immune activation pathway, was upregulated when pPROM was compared with TL (Fig. 6D). These data are suggestive of common physiologic state of pregnant subjects reflected in exosomes and alterations in physiology suggestive of deviations in functional pathways that may determine various outcomes.
Comparing canonical and biological pathways as shown in the heat map (Fig. 5) depicted by differentially regulated exosomal cargo peptides also suggest oxidative stress (TL, pPROM) (46), nonspecific inflammation (TL, PTB, and pPROM) and other reported parturition associated functional changes (acute phase signaling, coagulation, complement activation, liver X receptor/retinoid X receptor activation) (53, 54). Lack of functional data thwart further discussion of bioinformatics data. However, we conclude that our exosomal cargo analysis summarizes the following: (i) TL is associated with nonspecific inflammation compared with TNIL (55); (ii) PTB and term birth shares inflammation; however, inflammation is overrepresented in PTB along with hematological dysfunctions in which the latter could be the pathological initiator of PTB and inflammation is likely an effecter of labor process (56); (iii) confirming our prior data, pPROM and TL are similar in mechanistic pathways (45); and (iv) PTB and pPROM differ phenotypically and in pathways (46). None of these are novel findings and have been reported by many laboratories using tissue/cell culture models, and by analyzing samples (amniotic fluid, maternal and cord blood), placenta, and membranes; however, we report representation of these pathways and functions. Our findings have two implications: (i) potential role of exosomes in both physiologic and pathologic parturition as a communicator or as a disposal mechanism of physiologic waste that (ii) enhances the usefulness of exosomes in MP as a potential biomarker of underlying physiology.
Strengths and limitation of this study are highlighted in this section. Profile of extracellular vesicles, quantification approach using MS/MS, and generating supportive evidence for reported functional pathways associated with normal and abnormal pregnancies through exosome analysis are some of the strengths. Although we quantitated placental exosomes, their isolation from these samples were not attempted to better understand the precise contributions of placenta in these conditions. Sample size is adequate to generate differential proteome profile in a discovery approach and not appropriate for any further stratified analysis based on specific risk profiles of subjects in PTB and pPROM groups. In this study, we analyzed a total of 48 women with different signals of parturition; thus, a larger trial is required to further validate the results of this study. In vitro and/or animal studies are needed to establish the function of the exosomal proteins associated with different signals of parturition. Moreover, because of availability of samples, our study is restricted to late PTBs and pPROMs (between 34 and 37 weeks). Commercially available PLAP antibodies were used to test placental specific exosome concentration changes; however, we used total exosomes for further characterization and discussion of our work. This is partially because of questionable specificity of commercially available antibodies in detecting placental specific exosomes. Additional studies are needed to understand any differences in MP exosomal proteomic cargo between this group and early PTBs (<32 weeks’ gestation). Validation of these data in additional cohorts is also required. This study is a descriptive study; functional validation of these findings is beyond the scope of this report.
Acknowledgments
The authors thank the contributions of Poorna Ram Menon, a Clear Falls High School student who worked as a summer intern at the Menon laboratory and helped with sample preparation.
Financial Support: This study is supported by the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant 1R01HD084532-01A1 (to R.M.). C.S. is supported by the Lions Medical Research Foundation and Fondo Nacional de Desarrollo Científico y Tecnológico Grant 1170809.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- C4B
complement 4 binding
- FDR
false discovery rate
- IDA
information-dependent acquisition
- IPA
Ingenuity Pathway Analysis
- MP
maternal plasma
- MS
mass spectrometry
- NTA
nanoparticle tracking analysis
- PLAP
placental alkaline phosphatase
- pPROM
preterm premature rupture of the membranes
- PTB
preterm birth
- SWATH
sequential window acquisition of all theoretical
- TL
term in labor
- TNIL
term not in labor
References
- 1. Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, Rubens C, Menon R, Van Look PF. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ. 2010;88(1):31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345(6198):760–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Menon R. Spontaneous preterm birth, a clinical dilemma: etiologic, pathophysiologic and genetic heterogeneities and racial disparity. Acta Obstet Gynecol Scand. 2008;87(6):590–600. [DOI] [PubMed] [Google Scholar]
- 4. Fuchs AR, Fields MJ, Freidman S, Shemesh M, Ivell R. Oxytocin and the timing of parturition. Influence of oxytocin receptor gene expression, oxytocin secretion, and oxytocin-induced prostaglandin F2 alpha and E2 release. Adv Exp Med Biol. 1995;395:405–420. [PubMed] [Google Scholar]
- 5. Menon R, Bonney EA, Condon J, Mesiano S, Taylor RN. Novel concepts on pregnancy clocks and alarms: redundancy and synergy in human parturition. Hum Reprod Update. 2016;22(5):535–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Plunkett J, Doniger S, Orabona G, Morgan T, Haataja R, Hallman M, Puttonen H, Menon R, Kuczynski E, Norwitz E, Snegovskikh V, Palotie A, Peltonen L, Fellman V, DeFranco EA, Chaudhari BP, McGregor TL, McElroy JJ, Oetjens MT, Teramo K, Borecki I, Fay J, Muglia L. An evolutionary genomic approach to identify genes involved in human birth timing. PLoS Genet. 2011;7(4):e1001365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Keelan JA. Intrauterine inflammatory activation, functional progesterone withdrawal, and the timing of term and preterm birth. J Reprod Immunol. 2018;125:89–99. [DOI] [PubMed] [Google Scholar]
- 8. Gao L, Rabbitt EH, Condon JC, Renthal NE, Johnston JM, Mitsche MA, Chambon P, Xu J, O’Malley BW, Mendelson CR. Steroid receptor coactivators 1 and 2 mediate fetal-to-maternal signaling that initiates parturition. J Clin Invest. 2015;125(7):2808–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Challis JR, Smith SK. Fetal endocrine signals and preterm labor. Biol Neonate. 2001;79(3-4):163–167. [DOI] [PubMed] [Google Scholar]
- 10. Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev. 2007;65(12 Pt 2):S194–S202. [DOI] [PubMed] [Google Scholar]
- 11. Gomez-Lopez N, Vega-Sanchez R, Castillo-Castrejon M, Romero R, Cubeiro-Arreola K, Vadillo-Ortega F. Evidence for a role for the adaptive immune response in human term parturition. Am J Reprod Immunol. 2013;69(3):212–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bukowski R, Sadovsky Y, Goodarzi H, Zhang H, Biggio JR, Varner M, Parry S, Xiao F, Esplin SM, Andrews W, Saade GR, Ilekis JV, Reddy UM, Baldwin DA. Onset of human preterm and term birth is related to unique inflammatory transcriptome profiles at the maternal fetal interface. PeerJ. 2017;5:e3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fragiadakis GK, Baca QJ, Gherardini PF, Ganio EA, Gaudilliere DK, Tingle M, Lancero HL, McNeil LS, Spitzer MH, Wong RJ, Shaw GM, Darmstadt GL, Sylvester KG, Winn VD, Carvalho B, Lewis DB, Stevenson DK, Nolan GP, Aghaeepour N, Angst MS, Gaudilliere BL. Mapping the fetomaternal peripheral immune system at term pregnancy. J Immunol. 2016;197(11):4482–4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blank V, Hirsch E, Challis JR, Romero R, Lye SJ. Cytokine signaling, inflammation, innate immunity and preterm labour - a workshop report. Placenta.2008;29(Suppl A):S102-S104. [DOI] [PubMed] [Google Scholar]
- 15. Smith R, Mesiano S, McGrath S. Hormone trajectories leading to human birth. Regul Pept. 2002;108(2-3):159–164. [DOI] [PubMed] [Google Scholar]
- 16. Menon R, Mesiano S, Taylor RN. Programmed fetal membrane senescence and exosome-mediated signaling: a mechanism associated with timing of human parturition. Front Endocrinol (Lausanne). 2017;8:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Salomon C, Nuzhat Z, Dixon CL, Menon R. Placental exosomes during gestation: liquid biopsies carrying signals for the regulation of human parturition. Curr Pharm Des. 2018;24(9):974–982. [DOI] [PubMed] [Google Scholar]
- 18. Desrochers LM, Antonyak MA, Cerione RA. Extracellular vesicles: satellites of information transfer in cancer and stem cell biology. Dev Cell. 2016;37(4):301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sheller-Miller S, Lei J, Saade G, Salomon C, Burd I, Menon R. Feto-maternal trafficking of exosomes in murine pregnancy models. Front Pharmacol. 2016;7:432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chivet M, Hemming F, Pernet-Gallay K, Fraboulet S, Sadoul R. Emerging role of neuronal exosomes in the central nervous system. Front Physiol. 2012;3:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haj-Salem I, Plante S, Gounni AS, Rouabhia M, Chakir J. Fibroblast-derived exosomes promote epithelial cell proliferation through TGF-β2 signalling pathway in severe asthma. Allergy. 2018;73(1):178–186. [DOI] [PubMed] [Google Scholar]
- 22. Wang L, Hu L, Zhou X, Xiong Z, Zhang C, Shehada HMA, Hu B, Song J, Chen L. Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling [published correction appears in Sci Rep. 2018;8:7066]. Sci Rep. 2017;7(1):13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barreto A, Rodríguez LS, Rojas OL, Wolf M, Greenberg HB, Franco MA, Angel J. Membrane vesicles released by intestinal epithelial cells infected with rotavirus inhibit T-cell function. Viral Immunol. 2010;23(6):595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yuan Z, Bedi B, Sadikot RT Bronchoalveolar lavage exosomes in lipopolysaccharide-induced septic lung injury. J Vis Exp 2018(135). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mitchell MD, Peiris HN, Kobayashi M, Koh YQ, Duncombe G, Illanes SE, Rice GE, Salomon C. Placental exosomes in normal and complicated pregnancy. Am J Obstet Gynecol. 2015;213(4Suppl):S173–S181. [DOI] [PubMed] [Google Scholar]
- 26. Sarker S, Scholz-Romero K, Perez A, Illanes SE, Mitchell MD, Rice GE, Salomon C. Placenta-derived exosomes continuously increase in maternal circulation over the first trimester of pregnancy. J Transl Med. 2014;12(1):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sheller S, Papaconstantinou J, Urrabaz-Garza R, Richardson L, Saade G, Salomon C, Menon R. Amnion-epithelial-cell-derived exosomes demonstrate physiologic state of cell under oxidative stress. PLoS One. 2016;11(6):e0157614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dixon CL, Sheller-Miller S, Saade GR, Fortunato SJ, Lai A, Palma C, Guanzon D, Salomon C, Menon R. Amniotic fluid exosome proteomic profile exhibits unique pathways of term and preterm labor. Endocrinology. 2018;159(5):2229–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hadley EE, Sheller-Miller S, Saade G, Salomon C, Mesiano S, Taylor RN, Taylor BD, Menon R. Amnion epithelial cell-derived exosomes induce inflammatory changes in uterine cells. Am J Obstet Gynecol. 2018;219(5):478.e1–478.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Truong G, Guanzon D, Kinhal V, Elfeky O, Lai A, Longo S, Nuzhat Z, Palma C, Scholz-Romero K, Menon R, Mol BW, Rice GE, Salomon C. Oxygen tension regulates the miRNA profile and bioactivity of exosomes released from extravillous trophoblast cells - Liquid biopsies for monitoring complications of pregnancy. PLoS One. 2017;12(3):e0174514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chang G, Mouillet JF, Mishima T, Chu T, Sadovsky E, Coyne CB, Parks WT, Surti U, Sadovsky Y. Expression and trafficking of placental microRNAs at the feto-maternal interface. FASEB J. 2017;31(7):2760–2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Salomon C, Torres MJ, Kobayashi M, Scholz-Romero K, Sobrevia L, Dobierzewska A, Illanes SE, Mitchell MD, Rice GE. A gestational profile of placental exosomes in maternal plasma and their effects on endothelial cell migration. PLoS One. 2014;9(6):e98667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Samantha Sheller-Miller YA. S-134: Environmental toxicant induced cellular injury is reflected in placental exosomes. Reprod Sci.2018;25:296A. [Google Scholar]
- 34. Sheller-Miller S, Urrabaz-Garza R, Saade G, Menon R. Damage-associated molecular pattern markers HMGB1 and cell-free fetal telomere fragments in oxidative-stressed amnion epithelial cell-derived exosomes. J Reprod Immunol. 2017;123:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Salomon C, Yee S, Scholz-Romero K, Kobayashi M, Vaswani K, Kvaskoff D, Illanes SE, Mitchell MD, Rice GE. Extravillous trophoblast cells-derived exosomes promote vascular smooth muscle cell migration. Front Pharmacol. 2014;5:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Delorme-Axford E, Bayer A, Sadovsky Y, Coyne CB. Autophagy as a mechanism of antiviral defense at the maternal-fetal interface. Autophagy. 2013;9(12):2173–2174. [DOI] [PubMed] [Google Scholar]
- 37. Mouillet JF, Ouyang Y, Bayer A, Coyne CB, Sadovsky Y. The role of trophoblastic microRNAs in placental viral infection. Int J Dev Biol. 2014;58(2-4):281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Menon R, Velez DR, Simhan H, Ryckman K, Jiang L, Thorsen P, Vogel I, Jacobsson B, Merialdi M, Williams SM, Fortunato SJ. Multilocus interactions at maternal tumor necrosis factor-alpha, tumor necrosis factor receptors, interleukin-6 and interleukin-6 receptor genes predict spontaneous preterm labor in European-American women. Am J Obstet Gynecol. 2006;194(6):1616–1624. [DOI] [PubMed] [Google Scholar]
- 39. Menon R, Fortunato SJ, Edwards DR, Williams SM. Association of genetic variants, ethnicity and preterm birth with amniotic fluid cytokine concentrations. Ann Hum Genet. 2010;74(2):165–183. [DOI] [PubMed] [Google Scholar]
- 40. Fortunato SJ, Menon R, Velez DR, Thorsen P, Williams SM. Racial disparity in maternal-fetal genetic epistasis in spontaneous preterm birth. Am J Obstet Gynecol. 2008;198(6):666.e1–666.e9, discussion 666.e9–666.e10. [DOI] [PubMed] [Google Scholar]
- 41. Salomon C, Guanzon D, Scholz-Romero K, Longo S, Correa P, Illanes SE, Rice GE. Placental exosomes as early biomarker of preeclampsia: potential role of exosomal microRNAs across gestation. J Clin Endocrinol Metab. 2017;102(9):3182–3194. [DOI] [PubMed] [Google Scholar]
- 42. Rice GE, Scholz-Romero K, Sweeney E, Peiris H, Kobayashi M, Duncombe G, Mitchell MD, Salomon C. The effect of glucose on the release and bioactivity of exosomes from first trimester trophoblast cells. J Clin Endocrinol Metab. 2015;100(10):E1280–E1288. [DOI] [PubMed] [Google Scholar]
- 43. Vaswani K, Ashman K, Reed S, Salomon C, Sarker S, Arraztoa JA, Pérez-Sepúlveda A, Illanes SE, Kvaskoff D, Mitchell MD, Rice GE. Applying SWATH mass spectrometry to investigate human cervicovaginal fluid during the menstrual cycle. Biol Reprod. 2015;93(2):39. [DOI] [PubMed] [Google Scholar]
- 44.Menon R, Dixon CL, Sheller-Miller S, Fortunato SJ, Saade GR, Palma C, Lai A, Guanzon D, Salomon C. Data from: Quantitative proteomics by SWATH-MS of maternal plasma exosome determine pathways associated with term and preterm birth. MassIVE 2018. Deposited 18 December 2018. ftp://massive.ucsd.edu/MSV000083292. [DOI] [PMC free article] [PubMed]
- 45. Menon R, Boldogh I, Hawkins HK, Woodson M, Polettini J, Syed TA, Fortunato SJ, Saade GR, Papaconstantinou J, Taylor RN. Histological evidence of oxidative stress and premature senescence in preterm premature rupture of the human fetal membranes recapitulated in vitro. Am J Pathol. 2014;184(6):1740–1751. [DOI] [PubMed] [Google Scholar]
- 46. Dutta EH, Behnia F, Boldogh I, Saade GR, Taylor BD, Kacerovský M, Menon R. Oxidative stress damage-associated molecular signaling pathways differentiate spontaneous preterm birth and preterm premature rupture of the membranes. Mol Hum Reprod. 2016;22(2):143–157. [DOI] [PubMed] [Google Scholar]
- 47. Jin J, Menon R. Placental exosomes: a proxy to understand pregnancy complications. Am J Reprod Immunol. 2018;79(5):e12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kshirsagar SK, Alam SM, Jasti S, Hodes H, Nauser T, Gilliam M, Billstrand C, Hunt JS, Petroff MG. Immunomodulatory molecules are released from the first trimester and term placenta via exosomes. Placenta. 2012;33(12):982–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Miranda J, Paules C, Nair S, Lai A, Palma C, Scholz-Romero K, Rice GE, Gratacos E, Crispi F, Salomon C. Placental exosomes profile in maternal and fetal circulation in intrauterine growth restriction - Liquid biopsies to monitoring fetal growth. Placenta. 2018;64:34–43. [DOI] [PubMed] [Google Scholar]
- 50. Nair S, Salomon C. Extracellular vesicles and their immunomodulatory functions in pregnancy. Semin Immunopathol. 2018;40(5):425–437. [DOI] [PubMed] [Google Scholar]
- 51. Bullerdiek J, Flor I. Exosome-delivered microRNAs of “chromosome 19 microRNA cluster” as immunomodulators in pregnancy and tumorigenesis. Mol Cytogenet. 2012;5(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kourembanas S. Exosomes: vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu Rev Physiol. 2015;77(1):13–27. [DOI] [PubMed] [Google Scholar]
- 53. Capece A, Vasieva O, Meher S, Alfirevic Z, Alfirevic A. Pathway analysis of genetic factors associated with spontaneous preterm birth and pre-labor preterm rupture of membranes. PLoS One. 2014;9(9):e108578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lee SY, Park KH, Jeong EH, Oh KJ, Ryu A, Park KU. Relationship between maternal serum C-reactive protein, funisitis and early-onset neonatal sepsis. J Korean Med Sci. 2012;27(6):674–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Martin LF, Moço NP, Ramos BR, Camargo RP, Silva MG. Pentraxin-3 concentration in the amniotic fluid of women at term, in spontaneous preterm labor and when not in labor. Eur J Obstet Gynecol Reprod Biol. 2014;176:86–89. [DOI] [PubMed] [Google Scholar]
- 56. Velez DR, Fortunato SJ, Thorsen P, Lombardi SJ, Williams SM, Menon R. Preterm birth in Caucasians is associated with coagulation and inflammation pathway gene variants. PLoS One. 2008;3(9):e3283. [DOI] [PMC free article] [PubMed] [Google Scholar]