Abstract
Neurological diseases, including acute attacks (e.g., ischemic stroke) and chronic neurodegenerative diseases (e.g., Alzheimer’s disease), have always been one of the leading cause of morbidity and mortality worldwide. These debilitating diseases represent an enormous disease burden, not only in terms of health suffering but also in economic costs. Although the clinical presentations differ for these diseases, a growing body of evidence suggests that oxidative stress and inflammatory responses in brain tissue significantly contribute to their pathology. However, therapies attempting to prevent oxidative damage or inhibiting inflammation have shown little success. Identification and targeting endogenous “upstream” mediators that normalize such processes will lead to improve therapeutic strategy of these diseases. Thioredoxin-interacting protein (TXNIP) is an endogenous inhibitor of the thioredoxin (TRX) system, a major cellular thiol-reducing and antioxidant system. TXNIP regulating redox/glucose-induced stress and inflammation, now is known to get upregulated in stroke and other brain diseases, and represents a promising therapeutic target. In particular, there is growing evidence that glucose strongly induces TXNIP in multiple cell types, suggesting possible physiological roles of TXNIP in glucose metabolism. Recently, a significant body of literature has supported an essential role of TXNIP in the activation of the NOD-like receptor protein (NLRP3)-inflammasome, a well-established multi-molecular protein complex and a pivotal mediator of sterile inflammation. Accordingly, TXNIP has been postulated to reside centrally in detecting cellular damage and mediating inflammatory responses to tissue injury. The majority of recent studies have shown that pharmacological inhibition orgenetic deletion of TXNIP is neuroprotective and able to reduce detrimental aspects of pathology following cerebrovascular and neurodegenerative diseases. Conspicuously, the mainstream of the emerging evidences is highlighting TXNIP link to damaging signals in endothelial cells. Thereby, here, we keep the trend to present the accumulative data on CNS diseases dealing with vascular integrity. This review aims to summarize evidence supporting the significant contribution of regulatory mechanisms of TXNIP with the development of brain diseases, explore pharmacological strategies of targeting TXNIP, and outline obstacles to be considered for efficient clinical translation.
Keywords: Alzheimer’s disease, Inflammation, Neurological diseases, NLRP3-inflammasome, Oxidative stress, Stroke, Subarachnoid hemorrhage, Thioredoxin-interacting protein, Thioredoxin
Introduction
Cerebrovascular and neurological diseases including cerebral stroke, subarachnoid hemorrhage (SAH), and Alzheimer’s disease (AD) are among the most serious health problems faced by the modern society [1–5]. The burden of these diseases is growing inexorably with enormous economic and human costs. Although the clinical presentations differ for these diseases, a growing body of evidence suggests that oxidative stress [6–8], mitochondrial dysfunction [9, 10], and altered calcium homeostasis [11, 12] as well as inflammatory responses [8, 13] in brain tissue significantly contribute in their pathology [14–17]. Nevertheless, many have demonstrated that the reactive oxygen species (ROS) overproduction is a common pivotal mediator in different deteriorating cascades. Above that, high relative oxygen consumption (20% of total body consumption), abundance of easily oxidizable substrates [18, 19], and relatively low availability of ROS scavengers compared to other organs [20, 21] render the brain particularly sensitive to oxidative damage. As such, there is a strong postulation that oxidative damage is laid upstream to many of the abovementioned perilous signals. Despite overwhelming evidence of the damaging consequences of redox imbalance, large-scale clinical trials with classic antioxidants (e.g., NXY-059) failed to demonstrate benefit for stroke [22, 23] or other age-related CNS diseases [24, 25]. However, there are not enough mature evidences to reach a definitive conclusion on this issue. There is still a gap in understanding how early redox changes affect the neurovascular unit to sustain neuronal injury. We believe an alternative strategy—activation of endogenous defense mechanisms—can provide better protection in these diseases.
Several antioxidant systems including the two major thiol-reductase systems, glutathione and thioredoxin (TRX), can protect cells from oxidative damage. The TRX system detoxifies ROS by thiol-reducing mechanism [26]. In addition to its antioxidant properties, thiol-disulfide exchange reactions serve as control mechanisms for signal transduction. This system consists of TRX, TRX reductase (TRX-R), TXNIP, and nicotinamide adenine dinucleotide phosphate reduced form (NADPH). TRX activity and function are regulated by an intracellular endogenous inhibitor, thioredoxin-interacting protein (TXNIP) [27], or vitamin D3 upregulated protein 1 (VDUP1) which was first discovered to overexpress in HL-60 cells treated with 1,25-dihydroxyvitamin D [28]. Forced overexpression of TXNIP decreases TRX activity, increases oxidative stress, and inhibits cell growth [29, 30]. In addition, glucose strongly induces TXNIP in multiple cell types including retinal neurovascular tissue [31], endothelial cells [29], and pancreatic beta cells [32], highlighting the physiological roles of TXNIP in glucose metabolism [33]. TXNIP also acts as a redox-sensitive signaling protein by interacting with apoptosis signal [34], which in turn is involved in a variety of biological [35] and pathological events [36]. TXNIP is a central signaling hub that links oxidative/glucose stress and inflammation to cellular injury, making it a “multiple pathway” target and thus a promising new approach for brain therapy [37, 38]. In this connection, there is growing body of evidences suggesting TRX system modulation may provide promising therapeutic approaches in acute and chronic brain injuries. The primary aim of the current review is to provide the existing evidences supporting a role of TXNIP in pathology of neurological diseases like Alzheimer’s disease and stroke. As there are much more available findings, TXNIP involvement in ischemic stroke and associated endothelial dysfunction would be of specific interest to explore the potential efficacy of TXNIP modulation as a promising therapeutic target in pathology of CNS diseases. We also outline obstacles that need to be overcome for successful translation of these therapies to effective clinical practice.
Thioredoxin System
As a highly conserved protein from prokaryotes to mammals, TRX was initially identified as a hydrogen donor to ribonucleotide reductase, the essential enzyme for DNA synthesis in Escherichia coli [39]. Then, recognized as a part of cellular disulfide oxidoreductase complex, TRX was known to be regenerated by TRX-R at the expense of reducing equivalents (NADPH), playing a critical role in maintaining the cellular redox homeostasis.
That is, the oxidized thioredoxin (Trx-S2) is reduced by NADPH through a reaction catalyzed by TRX-R activity. The reduced Trx-(SH)2 may then directly reduce the disulfide in the substrate proteins [40]. Encoded by two different genes, cytosolic (TRX1) and mitochondrial (TRX2) isoforms have few distinctions [41]. While TRX1 and TRX2 are widely expressed in rat brain, TXR1 specifically shows a high expression level in regions of high metabolic activity and oxidative burden (e.g., substantia nigra and subthalamic nucleus) [42, 43].
Often known as the main TRX isoform, TRX1 is localized in the cytosol, plasma membrane (PM), and nucleus as well as the extra-cellular space, in contrast to TRX2 which is only specific to mitochondria. For its redox regulation function, TRX system and, in particular, TXR1 have been empirically shown to provide enormous protective effects [44]. Mice with TRX overexpression are more resistant to oxidative stress with longer life span, suggesting its role in cellular survival. Conspicuously, either overexpression [45] or intravenous administration of TRX [46] has been reported to provide neuro-protection against ischemic stroke insult and improve life span [47]. TRX2 as the mitochondrial isoform may also directly block cytochrome-c release and protects cells from ROS-induced apoptosis [48–50]. Besides its primary role in ROS scavenging system, TRX reduces cystine moieties in the DNA-binding sites of several transcription factors [51, 52] and has established transcriptional and post-transcriptional roles to adjust biological functions, e.g., through HIF-α and VEGF modulation [53, 54].
Thioredoxin-Interacting Protein
TRX-interacting protein (TXNIP) is the endogenous negative regulator in TRX system belonging to α-arrestin protein family. TXNIP is widely expressed almost in all normal tissue cells [55]. In subcellular fractions, TXNIP has reserved a conserved expression patterns in Drosophila and rat nervous systems demonstrating cytoplasmic enrichment in neurons and nuclear expression in glial cells [56]. The redox-related protein complex TRX/TXNIP, named as “Redoxisome,” is a critical regulator for ROS signaling and is involved in the pathogenesis of various diseases autoimmune disease and degenerative diseases [44]. Genetic silencing of TXNIP in human aortic endothelial cells under high glucose condition produced less reactive oxygen species than wild-type control cells, supporting the inhibitory effect of TXNIP on redox activity of TRX [29]. Beside its governing role in ROS signaling, TXNIP is central to glucose hemostasis [33] and regulates the transcription of several genes [51, 52, 57] each of which is opening new venues to explain TXNIP implication in several disorders.
TXNIP Structure and Intracellular Localization
Human TXNIP is a 46-kDa ubiquitously expressed protein that contains 391 amino acid residues [28] and is encoded on chromosome 1q21.1 [58, 59]. Initially thought to be a cytoplasmic protein [60, 61], TXNIP was later found by Saxena et al., to primarily reside in the nucleus under normal condition. However, TXNIP translocates to the mitochondria in response to oxidative stress, where it oxidizes the TRX2 and induces the activation of ASK1 resulting in induction of apoptotic signal cascade, cytochrome-c release, and caspase-3 cleavage [62]. Following rigorous investigations by Wu et al. on rat primary hepatocytes, they conclude shuttling of TXNIP to plasma membrane is of significance in acute suppression of glucose transport. This was supposed to take place via a model in which TXNIP augments GLUT-1 endocytosis through recruiting it into clathrin-coated pits in the plasma membrane. Furthermore, TXNIP shRNA was also found to reduce GLUT-1 protein level and re-localization of TXNIP to nucleus was suggested to mediate a lasting suppression of glucose uptake [63]. Through inhibiting the activity of signal transducer and activator of transcription 3 (STAT3), TXNIP has been also described to regulate expression of microRNA-204 at nuclear level and thereby controlling insulin expression [57]. Additionally, TXNIP shuttling to the plasma membrane contributes to TRX1 shuttling to VEGFR2 receptor where it mediates VEGF signaling [64]. TXNIP is expressed in many organs; however, the expression in the healthy brain is prominently rather lower in others [56]. In vitro TXNIP is induced by a variety of stress stimuli, including heat shock and H2O2 in human embryonic kidney cells [60], UV light and ROS in cultured Bosc cells [65], and high glucose in human aortic smooth muscle cells [66]. Interestingly, some of these instigating stimuli may regulate TRX independent of TXNIP. Instantly, hydrogen peroxide in cardiomyocytes [67] or SAH in rat brains [67] has been shown to directly reduce TRX reductase activity, suggesting its role in redox regulation.
TXNIP Downstream Effectors
In addition to cellular redox regulation, TXNIP has been documented to modulate several effectors either laid downstream to TRX or the TXNIP molecule itself. Given that almost all damaging stimuli may improve ROS generation and instigate on TXNIP, this molecule provides an exceptional link between the cells’ metabolic and redox status to the pathological inflammation and programmed cell death or dysfunction. Being extensively interconnected particularly through ROS signaling, several detrimental signals have been shown to get induced by TXNIP for which the available data is briefed as follows.
a) TXNIP and apoptotic pathways
TXNIP, well characterized for mediating glucocorticoid-induced apoptosis in stress conditions [34], may provoke apoptotic signals mainly via antagonizing TRX anti-apoptotic effect. TRX has been long discovered to provide anti-apoptotic effects by binding and inhibiting the pro-apoptotic protein and apoptosis signal-regulating kinase 1 (ASK-1). In response to various stress types, TXNIP translocates to mitochondria where it prevents the inhibitory association of TRX2 on ASK-1, leading to activation of ASK-1 and apoptotic kinase pathway. ASK1 is usually bound to TRX2 under basal conditions. Unbound ASK1 is phosphorylated and instigates cytochrome C release and caspase-3 cleavage [68]. Progressive beta cell apoptosis is a hallmark in pathophysiology of types 1 and 2 diabetes. TXNIP overexpressing beta cells are more susceptible to apoptosis suggesting that TXNIP as a therapeutic target to protect against apoptosis [32]. Genetic deletion of TXNIP protects beta cell from glucotoxicity-induced apoptosis by modulating mitochondrial death pathway [69]. It has been reported that TXNIP has a pro-apoptotic role in some acute brain injuries. In our recent study, we detected a remarkable attenuation in apoptotic effectors caspase-1, caspase-3, and PARP following embolic stroke in TXNIP −/− mice [70]. The more recent findings by Zhau et al. demonstrated that enhanced TXNIP expression is associated with escalated brain cell apoptosis in early brain injury (EBI) following subarachnoid hemorrhage (SAH) in rats and resveratrol (60 mg/kg) or TXNIP small interfering RNA (siRNA) improve apoptotic cell death leading to better prognosis for SAH [71]. In fact, TXNIP contribution to apoptosis has been also shown in various in vitro models including thrombin exposed to microglia [72], endothelial cells stressed with palmitate [73], and retinal pericytes exposed to high glucose [74]. While counting for detrimental effects in diabetes and thromboembolic disease, the TXNIP-associated apoptosis is still regarded as a promising target in oncology medicine. In this line, several in vitro examinations have demonstrated TXNIP pro-apoptotic role in prostate cancer [75], hepatocellular carcinoma [76], and in vitro acute myeloid leukemia [77].
b) TXNIP and oxidative phosphorylation
Mitochondrial biogenesis and respiration may be disrupted in a variety of CNS disorders. Supported by concrete evidences, TXNIP reduces uptake of glucose working as the main energy source in many organs. Instantly, TXNIP over-expression in cultured adipocytes has been shown to reduce glucose uptake and TXNIP silencing with siRNA enhances glucose uptake in adipocytes and in skeletal muscle [33]. Importantly, thioredoxin-protein that is induced in response to glucose rise, e.g., in diabetes, provides a negative feedback loop to regulate glucose uptake. In the meticulous study by Wu and colleagues on primary rat hepatocytes, the reduced glucose uptake was found to rely on two distinct mechanisms by TXNIP. Firstly, in a direct approach, TXNIP may bind to the glucose transporter GLUT1 and induce GLUT1 internalization. Secondly, through an indirect influence on transcription, TXNIP abolishes the level of GLUT1 messenger RNA. Furthermore, they showed that energy stress in hepatocytes results the phosphorylation of TXNIP by AMP-dependent protein kinase (AMPK) and subsequent rapid degradation of TXNIP [63].
On the other side, TXNIP may hypothetically elevate mitochondrial oxygen consumption. In fact, there are empirical evidences indicating TXNIP may enhance mitochondrial respiration through blocking hypoxia-inducible transcription factors (HIFs) or inducing peroxisome proliferators-activated receptor (PPAR)α. HIF-1α is long known to repress mitochondrial oxygen consumption by inhibiting pyruvate dehydrogenase (PDH) complex and interfering with tricarboxylic acid (TCA) cycle [78, 79] which ends with reduced ROS in mitochondria [80]. In both in vivo and in vitro models of gastric cancer, Shin et al. showed TXNIP may assemble with the beta-domain of byprolyl-hydroxylases and von Hippel–Lindau protein (pVHL) and improve its interaction with HIF-1α to augment the ubiquitin-induced degradation of HIF-1α concluding TXNIP mediates nuclear export of HIF-1α, resulting in HIF-1α degradation [81]. PPARα is a transcription factor required for activation of the mitochondrial biogenic response [82] and might be inhibited by TRX interaction. Nuclear translocation of TRX has been shown to directly attenuate PPARα transcriptional activity in Hela cancer cell lines [83]. Through an alternative indirect and less documented pathway, cytosolic TRX may bind to ASK1 and inhibits its downstream p38MAP kinase [84] which has been shown may activate PPARα in cardiomyocytes [85]. Therefore, cytosolic and nuclear TXNIP both work to improve PPARα transcriptional activity, thus enhancing mitochondrial biogenesis, oxygen consumption, and ROS generation.
c) TXNIP and NLRP3 inflammasomes
Formation of large multi-protein complexes “inflammasomes” is an early event in the primary immune responses. TXNIP regulates inflammatory responses by modulating different switch points along inflammatory cascades in different cell populations. Retinal endothelial cells exposed to high glucose (HG) or agonist of advanced glycation end-products (RAGE) found remarkable overexpression of TXNIP in parallel with some inflammatory genes like VEGF-A and ICAM1. Interestingly, they found that TXNIP silencing by siRNA blocks RAGE and HG inflammatory effects. According to their findings, TXNIP is supposed to produce its anti-inflammatory effects through p38 MAPK-NF-kappaB signaling pathway and modifications in histone H3 lysine (K) nine regions [86]. The intriguing study by Wang et al. further revealed that TXNIP inhibits the expression of Kruppel-like factor 2 (KLF2), a key anti-inflammatory transcription factor in endothelial cells, a finding which well describes TXNIP-induced inflammation in endothelial cells exposed to ex vivo disturbed blood flow modeling [87]. Above all, however, there is accumulating evidence suggesting that the all-inclusive mechanism describing TXNIP pro-inflammatory role is the direct activation of NOD-like receptor protein 3 (NLRP3) inflammasomes in several cell types (Fig. 1).
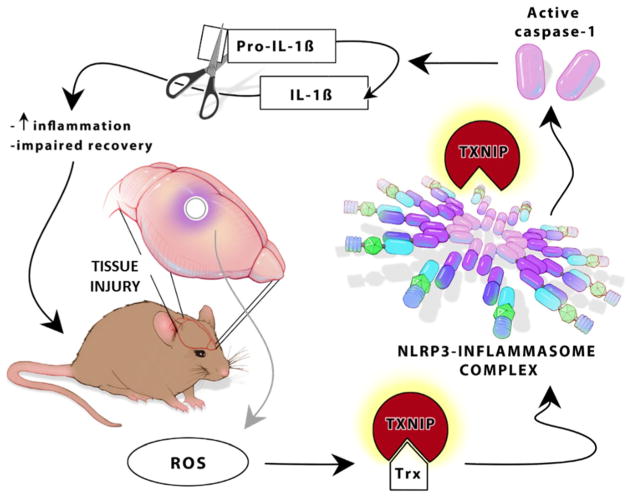
Fig. 1.
Schematic representation of TXNIP as a direct NLRP3 activator. ROS generation is a common feature in several types of acute and chronic brain tissue injuries. ROS is well characterized to dissociate TXNIP from its endogenous ligand TRX. This leads to direct TXNIP attachment to NLRP3-inflammasome resulting in activation of caspase-1 that in turn causes maturation and cleavage of pro-IL-1β to IL-1β. This further ends with increased inflammation and impaired recovery after brain injury in mice. ROS, reactive oxygen species; TXNIP, thioredoxin-interacting protein; NLRP3, Nod-like receptor protein 3; IL-1β, interleukin-1-beta
NLRP3 inflammasome is a key component in sterile inflammation and is involved in several diseases. Typically classified as cytosolic receptors sensing damage associated molecules (i.e., cell debris, urate crystals, ATP), NLRPs are of critical damage sensing molecules which form NLRP inflammasome upon priming, activation, and oligomerization. NLRP3 biology is described elsewhere in full details [88]. In brief, NLRP3 inflammasome is the assembly of NLRP3 oligomers and apoptosis-associated speck-like (ASC) adapter protein. In the priming step, the transcripts of all NLRP3 inflammasome constituents including ASC, NLRP3, and pro-caspase-1 are produced to sufficient levels to contribute to inflammasome assembly. Upon activation, NLRP3 triggers caspase-1 activation and subsequent maturation of IL-1β and IL-18, a critical step in inflammatory responses. NLRP3 is activated by a variety “danger” signals including whole RNA, RNA/DNA hybrids [89], and proteins from gram-positive [90] and gram-negative bacteria [91], viruses [92], and protozoa [93] as well as monosodium urate crystals [94] and calcium pyrophosphate dihydrate crystals [95] which might be classified as disease-associated molecular patterns (DAMPs). This posits NLRP3 inflammasome in an almost all-existing place in any pathological process dealing with inflammation independent of the initiating source.
Noteworthy, much of NLRP3-activating stimuli converge on TXNIP followed by TXNIP dissociation from TRX and its direct association with NLRP3 inflammasome. The phenomena were first identified by Zhou et al. in cultured macrophages [96]. According to their observations, a variety of NLRP3-inflammasome activators including ATP, monosodium urate crystals, and silica was shown to result in TXNIP dissociation from TRX and binding to the NLRP3 inflammasome. In fact, they showed TXNIP binding is an essential step for NLRP3 inflammasome activation. Pursued by other scientists, it was further understood that TXNIP/NLRP3 activation is centrally involved in several disease models. Instantly, inhibition of TXNIP produces profound amelioration as it has been documented in high fat-exposed human retinal endothelial cells (subjected to TXNIP silencing) [97], non-alcoholic fatty liver disease (treated with quercetin) (NAFLD) [98], and diabetic cardiomyopathy (treated with rosuvastatin) [99] as well as in high glucose-exposed human retinal microvasculature (subjected to TXNIP silencing) [100] and thrombin-exposed BV2 cells (treated with N-acetyl-l-cysteine) [72].
There are extensive investigations suggesting TXNIP may work as a main mediator to link a vast variety of deteriorating stimuli, e.g., oxidative stress [101], inflammation [102], and even senescence [103] to reach to NLRP3 inflammasome. Direct TXNIP/NLRP3 interaction, yet not fully characterized though, has been established based on the empirical evidences produced ever. As is detailed later in this review, ROS-induced activation of NLRP3-inflammasome in macrophages is reported to be associated with increased localization of TXNIP into the mitochondria [104] resulting in oxidation of TRX, liberation of TXNIP, and increased interaction with NLRP3 [105]. As predicted based on molecular modeling, the enhanced TXNIP/NLRP3 association appears to bring a conformational change in the NLRP3 protein pyrin domain [106]. On the contrary side, in macrophages of TXNIP knockout mice, it has been shown that NLRP3 carry more S-nitrosylated sites compared to wild-type controls. This modification might be responsible for less IL-1β maturation in response to LPS stimulation in TXNIP knockout cells [107].
d) TXNIP and oxidative stress
Conspicuously, activation of NLRP3 inflammasome through distinct upstream signals is associated with increased ROS levels [96, 108]. Noteworthy, ROS-generating stimuli may potentiate TXNIP/NLRP3 pathway through enhancing TXNIP transcription and expression. Instantly, the typical hepatic inflammation following high fructose diet has been shown to mediate both ROS and TXNIP overexpression either in vivo or in vitro which is followed by NLRP3 inflammasome activation [109]. Paclitaxel and lentinan as anticancer medications have been also shown to increase TXNIP expression as well as TXNIP-NLRP3 interaction, in parallel with ROS generation [110]. Such transcriptional augmentation may efficiently explain how ROS enhances TXNIP protein levels which in turn may bind to NLRP3 inflammasome to instigate inflammatory responses.
TXNIP shuttling to the mitochondria is the other well-known event following ROS creating stimuli. Soon after their first investigations [96], using T helper cells and NLRP3 −/− mice, Zhou et al. described more details on NLRP3 and its adaptor ASC re-localization in different states of activity. Accordingly, resting NLRP3 localizes to endoplasmic reticulum structures and upon sensing activating stimuli, both NLRP3 and ASC redistribute to the perinuclear space and co-localize with endoplasmic reticulum and mitochondria. Then, they showed nigericin as an established NLRP3 stimulator causes TXNIP to redistribute to mitochondria in a ROS-dependent manner [104]. This was in consistent with similar findings reported by others on pancreatic beta cells [62]. By blocking mitochondrial voltage-dependent anion channel, Zhou and colleagues showed the inhibition of all the pertinent changes in NLRP3 and TXNIP, indicating the prominent role of NLRP3 inflammasome in sensing mitochondrial dysfunction and explaining association of mitochondrial damage with inflammatory diseases [104]. Mitochondrial shuttling of TXNIP initiates downstream apoptotic signaling through ASK1 release from mitochondrial TRX2. The subsequent cleavage of caspase-3 leads to apoptosis which besides the cytosolic activation of caspase-1-induced pyroptosis ends with substantial DAMP release serving as a secondary signal for NLRP3 activation. A schematic diagram of TXNIP role in different subcellular compartments is represented in Fig. 2.
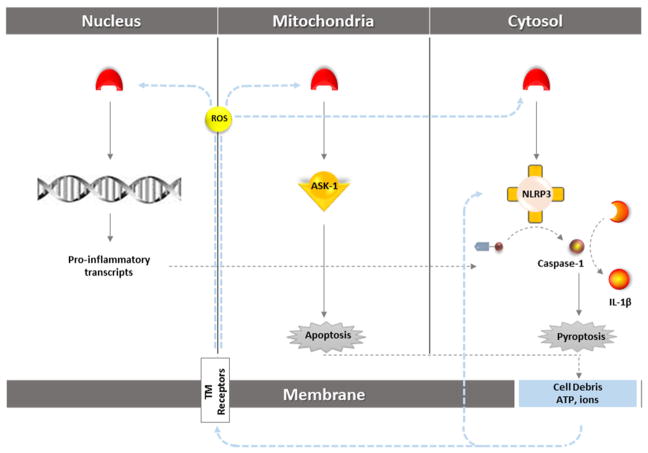
Fig. 2.
Schematic representation of ROS/TXNIP interplay in NLRP3 activation in different cell compartments. TXNIP might affect both priming and activation steps in NLRP3 metabolism. Typically in cytosolic milieu, TXNIP directly stimulates NLRP3 inflammasome’s cleavage activity on caspase-1 and subsequent IL-1β maturation. Nuclear TXNIP works as a transcription factor for several genes, contributing to the NLRP3 priming step through amplifying the required transcripts of NLRP3 inflammasome components namely pro-caspase-3 and pro-IL-1β. Mitochondrial TXNIP levels which increase subsequent to TXNIP shuttling in stress conditions release the pro-apoptotic ASK-1 from TRX inhibition. The consequent apoptotic cell death besides the pyroptosis induced by caspase-1 activation ends with release of cell debris and intracellular ions. The induced sterile inflammation produces a positive feedback to amplify NLRP3 inflammasome activation either by ROS-induced TXNIP attachment to NLRP3 inflammasome or through TXNIP-independent pathways. TXNIP, thioredoxin-interacting protein; NLRP3, Nod-like receptor protein 3; ASK-1, apoptosis signal-regulating kinase 1
TXNIP Regulation
Various endogenous and exogenous stimuli control TXNIP expression. Metabolic status of the cells is closely reflected at TXNIP transcription instantly depending on glucose and oxygen availability. TXNIP also relies downstream to many deteriorating signals triggering inflammation, oxidative stress, and excitotoxicity which are common grounds in a variety of CNS diseases. However, such stimuli intricately interact with each other; each may independently instigate TXNIP expression as follows.
A) Glucose
The expression of TXNIP is inducible by high glucose concentration through the carbohydrate response element-binding protein (ChREBP) located in the upstream region of the TXNIP prompter [111]. Glucose stress is a well-known feature following acute brain injury contributing to TXNIP activation [61]. Human pancreatic islet gene expression microarray study demonstrated that TXNIP is the most strongly upregulated gene in response to glucose [112]. Cardiac TXNIP and cleaved caspase-3 are overexpressed in streptozotocin- and obesity-induced diabetic mice [113]. Furthermore, poor prognosis in diabetic patients is associated with hypomethylation of TXNIP gene addressing still less investigated aspects of TXNIP-diabetes connection [114]. Elevated expression of TXNIP has been observed in the muscle of pre-diabetic and diabetic patients [33]. Consistently, TXNIP is strongly induced in human and mice pancreatic beta cells by glucocorticoids in a p38 mitogen-activated protein kinase (MAPK) manner [115]. While high glucose inhibits TXNIP, palmitate, a 16 carbon fatty acid, was shown to reserve TXNIP expression in rat islet cells [116].
B) AMP-activated protein kinase
AMP-activated protein kinase (AMPK) is of cellular energy sensing molecules to control metabolic homeostasis which is involved in glucose-mediated TXNIP overexpression. The accurate inspection of TXNIP biochemistry by Wu et al. described AMPK-dependent phosphorylation of TXNIP following energy stress, e.g., low glucose levels in various cancerous cell lines, i.e., HepG2, MCF7, and T47D. This TXNIP phosphorylation leads to its rapid degradation and explains how high glucose may reserve TXNIP lasting activity [63]. In addition to TXNIP post-translational modifications, AMPK may also affect TXNIP transcription. In rats’ beta cell line and islets, high glucose-induced ChREBP nuclear entry and recruitment to the TXNIP promoter were inhibited by pharmacologic activation of AMPK but reversed in AMPK −/− cells. Accordingly, AMPK is an important regulator of TXNIP through modulation of ChREBP activity [116]. Either of these ways, TXNIP suppression increase in GLUT1 function and expression may compensate for long-term adaptation.
C) Oxidative stress
As described earlier in this review, ROS is a common pathway for many deteriorating signals to stimulate TXNIP. However, there is not enough information to conclude an established mechanism by which ROS may govern TXNIP. In 2011, Fang et al. reported that high glucose exposure in rat mesangial cells modulates TXNIP expression at mRNA and protein levels. Using a p38 MAPK, inhibitor abolished TXNIP expression, suggesting MAPK signaling involvement in ROS-TXNIP pathway [117]. Soon after, Kim et al. showed oxidative stress without glucose can induce TXNIP expression, as they detected remarkable TXNIP in both in vivo and in vitro models of ischemic reperfusion injury. Inhibition Ca2+ channels by verapamil partially prevented expression of TXNIP in vivo [61]. In this connection, the transcription factor Forkhead box, class O (FOXO) may link ROS to TXNIP expression. FOXO1 and FOXO3 are the predominant forms of the four existing FOXOs in neural cells. Active FOXO is a direct enhancer of TXNIP transcription in neural cells in which the high synaptic activity has been shown to suppress FOXO1 expression [118]. Insulin signaling through Akt activation works as a significant regulator of FOXO so that Akt-induced FOXO phosphorylation ends with its dissociation from the TXNIP promoter and redistribution to the cytosol [119]. Nevertheless, for the existing controversies, it seems FOXO-TXNIP pathway is rather complex and context sensitive to define a single FOXO-TXNIP connection. Interestingly, in liver cells, FOXO1 works as a repressor and downregulates TXNIP [120]. In pancreatic beta cells, FOXO1 still works as a TXNIP suppressor with more known mechanisms. Kibbe et al. showed FOXO1 and ChREBP bind to the same region in the TXNIP promoter and FOXO1 inhibit ChREBP-induced TXNIP expression [121].
D) Calcium and ER Stress
Elevated calcium influx is a common feature in a variety of brain diseases ranging from acute ischemic stroke to Alzheimer’s associated degeneration. Shalev team started to discover calcium role in TXNIP biology when in their in vivo and in vitro experiments, they indicated calcium channel blockers dose dependently reduce TXNIP transcription and protein levels in cardiomyocytes even in face to severe diabetes [113]. They also found calcium channel blockers may efficiently inhibit TXNIP expression in INS-1 cells and oral verapamil reduces TXNIP expression and β-cell apoptosis and eventually enhances endogenous insulin levels [122]. In continuation of their investigations in cardiomyocytes and in vivo in diabetic mice, they proposed a mechanism suggesting verapamil inhibits the calcineurin signaling which in turn increases the binding and repression activity of the transcription nuclear factor Y (NFY) on its target genes including TXNIP [123]. In a parallel study by Kim et al., it was suggested that the elevated intracellular calcium ions may also contribute to TXNIP expression during ischemic injury as calcium channel blockers, diltiazem, and verapamil could inhibit induction of TXNIP after OGD and re-oxygenation [61]. Intracellular calcium overload may take place downstream to excitotoxicity, mitochondrial dysfunction, and endoplasmic reticulum (ER) stress. The accumulation of unfolded proteins during ER stress leading to the induction of progressive cascade termed as unfolded protein response (UPR) may induce TXNIP expression in beta cells in a Ca2+-independent manner. According to the existing data, UPR leads to TXNIP expression via two of its apoptotic effectors PERK and IRE1α in beta cells [124, 125]. IRE1α has been shown to increase TXNIP mRNA stability through its kinase/RNase function that reduces the levels of miR-17, a TXNIP-destabilizing microRNA [124]. Alternatively, PERK signaling may boost TXNIP transcription through enhanced expression and nuclear translocation of ChREBP. In the transcription factor ATF5, a ATF/cAMP response element may also mediate this ER stress effect [125].
TXNIP in Central Nervous System Diseases
Decades of extensive research have established the role of TRX/TXNIP signaling in pathogenesis of several disorders associated with oxidative stress, including diabetes [126] and endothelial dysfunction [127]. Numerous emerging reports are also pointing to increased TXNIP expression in neurodegenerative and cerebrovascular diseases including AD [128] and stroke [70]. Reportedly, TXNIP molecule is overexpressed in the hippocampus of 5xFAD mice at the first appearance of cognitive decline [128]. This is associated with augmented hippocampus expression of glial fibrillary acidic protein (GFAP). The contributory function of beta amyloid to upregulate TXNIP was demonstrated in RBE4 cells, rat cerebral capillary endothelial cells lines exposed to beta amyloid (Aβ) which was suggested to enhance TXNIP expression through “receptor for glycation end-products” (RAGE) [86]. Augmented expression of TXNIP and downregulation of TRX have been also reported in D-galactose-induced rat model of Alzheimer’s disease. In this study, treatment with salidroside, a herbal phenylpropanoid glycoside, enhanced the cognitive performances by regulating the expressions of TRX, TXNIP, and NF-kB proteins [129].
According to several reports, increased mRNA expression of TXNIP has been also detected in brains affected with sub-arachnoid hemorrhage (SAH) [130, 131]. In a rabbit model of SAH, the brain stem levels of TRX1 and TRX reductase (TRXR) decreased in parallel with a rise in the expression of TXNIP [130]. Telmisartan treatment was then shown to ameliorate morphological changes of cerebral vasospasm in parallel with restoring the reduced levels of Trx1 and TRXR and decreasing TXNIP expression [131]. Recently, Zhao et al. showed that TXNIP is highly expressed in neurons of the SAH rat brain and inhibition of TXNIP by pharmacological or siRNA attenuated apoptosis and early brain injury after SAH. Given pharmacological inhibition of PERK was associated with decreased expression of TXNIP, they concluded the increased SAH-induced TXNIP expression mediated RNA-like endoplasmic reticulum (ER) kinase (PERK) a trans-membrane protein in the ER [71]. TXNIP also acts as mediator of inflammation and apoptosis in intravitreal NMDA injection. TXNIP knockout mice demonstrated less glial activation and NLRP3 inflammasome-induced inflammation I parallel with ameliorated microvascular degeneration compared to wild-type animals [132]. This was in consistent with earlier reports indicating genetic deletion or pharmacological inhibition of TXNIP prevented neuronal cell death and inflammation and preserved retinal NMDA-induced retinal neurotoxicity [133]. Similarly, increased expression of TXNIP associated with high-fructose diet has been shown to induce hypothalamic inflammation and insulin signaling dysfunction in rats. In this study, pharmacological treatment with quercetin, a natural flavonoid prevented fructose reduced hypothalamic inflammatory lesion by modulating AMPK/TXNIP signaling [134]. Thrombin exposure is an established notorious feature in SAH. In a study on thrombin-exposed microglia, ROS mediated TXNIP expression leading to NLRP3 inflammasome activation and microglial apoptosis [72]. Looking for solid evidences in TXNIP implication in CNS disease, recent investigations are increasingly addressing TXNIP modulation approaches to provide protection against CNS diseases. A concise view on the published reports in this field is provided in Table 1.
Table 1.
Summary of studies on TXNIP modulation in cerebrovascular and neurodegenerative diseases
| Disease model | TXNIP modulation | Main findings | Reference | ||
|---|---|---|---|---|---|
|
| |||||
| Pharmacological | Genetic | ||||
| Stroke | tMCAO Hyperglycemic rats |
TXNIP siRNA 72 h prior to I/R (500 pmol, i.c.v.) |
Reverse the effect of hyperbaric oxygen preconditioning on hyperglycemic rats | [135] | |
| tMCAO Mice |
Ruscogenin 1 h prior to I/R (10 mg/kg, p.o.) |
Decreased brain infarction, edema, improved neurological outcome by suppressing a TXNIP/NLRP3 inflammasome activation and MAPK pathway | [70] | ||
| tMCAO Rats |
Umbelliferone 7 days prior to I/R (15, 30 mg/kg, i.p.) |
Protected against cerebral ischemia reperfusion injury by suppressing TXNIP/NLRP3 inflammasome activation | [136] | ||
| eMCAO Mice |
Resveratrol 3 h after I/R (5 mg/Kg, i.v.) |
TXNIP Knockout |
Protected from ischemic injury characterized by decreased infarct size, improved neurological score suppressing a TXNIP/NLRP3 inflammasome activation and apoptosis | [137] | |
| OGD Ex vivo hippocampus |
Curcumin 1 h prior to I/R (50 mg/kg, i.p.) |
Attenuated ischemic brain injury. Modulation of TXNIP/NLRP3 inflammasome activation by suppression of ER stress |
[138] | ||
| tMCAO Rats |
Compound 10b I/R onset (3 mg/kg) |
Attenuated cerebral ischemia by upregulating endogenous antioxidant system and downregulation of markers of oxidative stress | [139] | ||
| OGD Primary neuron |
TXNIP siRNA 2 h prior to OGD (25 nM) |
Reduced lactate dehydrogenase release | [61] | ||
| Subarachnoid hemorrhage | Rat | Resveratrol (60 mg/kg) | TXNIP siRNA | Reduction in inflammatory and apoptotic factors, as well as attenuation of the prognostic indices | [140] |
| Rat | Resveratrol (60 mg/kg) GSK2656157 PERK inhibitor |
TXNIP siRNA | Attenuation of apoptosis and prognostic indicators, including SAH grade, neurological deficits, brain water content, and BBB permeability | [69] | |
| Rabbit | Telmisartan 72 h prior to I/R (5 mg/kg, i.p.) |
Reduced morphological changes of cerebral vasospasm and attenuated endothelial apoptosis significantly | [129] | ||
| Dementia and Alzheimer’s disease | Okadaic acid (OA) induced SH-SY5Y cells | Quercetin | Attenuated tau phosphorylation, suppressed ER stress with decreased phosphorylation of IRE1α and PERK, thereby inhibited TXNIP and NLRP3 inflammasome activation | [141] | |
| D-galactose-induced rats | Salidroside (20, 40 mg/kg) for 28 days | Significantly attenuated cognitive impairment and neuroinflammation in hippocampus | [127] | ||
| Aβ-induced excitotoxicity | Nobiletin | Reversed the inhibition of glutamate-induced increases in CREB. Phosphorylation, TXNIP, and NMDA receptor subunits | [142] | ||
| ZDF rats | Ac-Cys-Pro-Cys-amide (CB3) (Thioredoxin agonist) |
Diminished apoptotic markers, most likely acting via the MAPK-AMPK-mTOR pathway | [143] | ||
TXNIP in Endothelial Dysfunction
Microvascular complications have a critical causative role in several organ system diseases including diabetes [37] and migraine attacks [144]. Figure 3 represents the putative molecular pathways may explain the involvement of TXNIP in signals affecting vascular dysfunction. In fact, cerebrovascular dysfunction may play a causative role or work as a predictor for poor prognosis in some CNS disorders like dementia or Alzheimer’s disease which are not typically classified in cerebrovascular diseases [145, 146]. According to the existing strong evidence, many of TXNIP abrogating roles in the CNS diseases might hypothetically root from the associated vascular pathology. This might be supported by evidences indicating TXNIP modulation may confer protection in experimental models of endothelial dysfunction (Table 2). In fact, TXVIP may play a central role in endothelial oxidative stress and inflammation [158], alignment [159], and proliferation [160] all of which contribute to atherosclerotic and angiopathies. Interestingly, as confirmed by modulating TXNIP by siRNA in vascular endothelia cells [161] or HUVECs [103], there are empirical evidences indicating that endothelial senescence as a major contributing factor to vascular complications depends on sufficient TXNIP/NLRP3 activation and pro-atherogenic ECs’ inflammation. TXNIP involvement in endothelial dysfunction is not confined but grossly might be classified to three categories as follows.
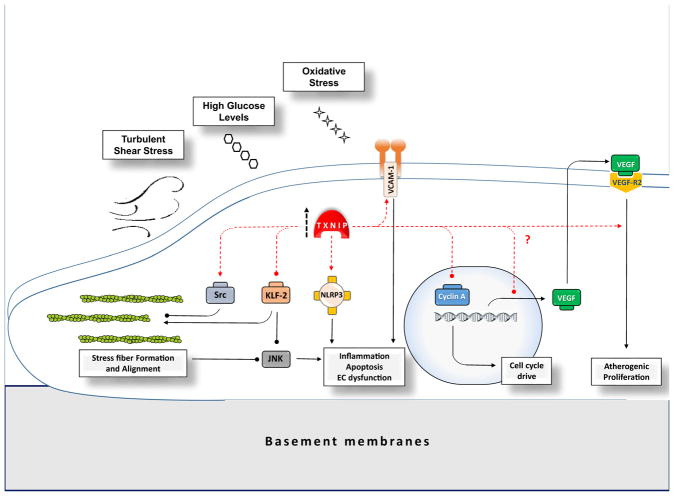
Fig. 3.
Simplified illustration of TXNIP role in presumptive pathways involved in endothelial dysfunction. Endothelial dysfunction and pro-atherogenic phenotypes may rise from pro-inflammatory or aberrant proliferating signals. EC dysfunctions are more likely in vascular curvatures with disturbed laminar blood flow with high risk for atherosclerotic plaques development. TXNIP expression elevates in response to high glucose, oxidative stress, and also in sites of DSS in vascular tree and might directly contribute to pro-inflammatory signals and atherogenic changes through different pathways. TXNIP basically is well known to instigate NLRP3/IL-1β signaling as a pivotal inflammatory signal. Such locally inflammatory triggers might be amplified by DSS-induced VCAM-1 overexpression and subsequent monocyte adhesion which are dependent on TXNIP activity. Actin stress fibers and the linked intracellular signals may also be affected by TXNIP. ECs’ stress fibers are essential components to determine EC phenotype which in turn would affect major apoptotic and inflammatory pathways. Laminar shear stress in straight blood vessels makes ECs and their stress fibers align in the longitudinal direction of the vessels in a quiescence state. Appropriate formation and alignment of stress fibers are associated with the least JNK signaling and apoptosis in ECs. KLF-2 a well characterized anti-inflammatory checkpoint in ECs may also contribute to stress fiber formation and alignment. DSS-associated TXNIP overexpression may release pro-inflammatory effectors from KLF-2 and aggravate the appropriately designed actin fibers which in turn may escalate the inflammation through JNK activation. This might be augmented by TXNIP-induced Src phosphorylation and inhibition which correlates with less actin stress fiber formation. TXNIP may play different roles in EC abnormal proliferation and angiogenesis which besides its key pro-inflammatory roles may provide other prominent pathway to form atherosclerotic plaques along the vessels. Basically, TXNIP activity may result in suppression of VEGF transcripts through association with TRX which contributes to elevated endothelial VEGF levels. On the other side, it has been well documented that TXNIP is required for VEGF-R2 activation by VEGF and the consequent angiogenesis and EC proliferation. Nevertheless, concluding proliferative effects for TXNIP is hard to conclude, given TXNIP is extensively addressed as a component of combating against cancerous proliferation by inducing G0/G1 cell cycle arrest through different effectors like cycline-A. DSS, disturbed shear stress; TXNIP, thioredoxin-interacting protein; NLRP3, Nod-like receptor protein 3; IL-1β, interleukin-1-beta; JNK, c-Jun N-terminal kinases; VCAM-1, vascular cell adhesion protein 1; KLF-2, Kruppel-like factor 2
Table 2.
Evidences addressing alleviating impact of TXNIP modulation in endothelial dysfunction
| Endothelial complication | Model | TXNIP inhibition | Main findings | Reference | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Subject | Stimuli | Pharmacological | Genetic | |||
| Diabetic endothelial dysfunction | Human aortic endothelial cells | High glucose | Metformin | Reduced nuclear entry of ChREBP and FOXO1 as well as their recruitment on the TXNIP promoter, partially through AMPK | [147] | |
| In vitro HUVECs |
High glucose | Mangiferin | Attenuate IRE1α phosphorylation Reduce ROS/TXNIP/NLRP3 inflammasome activation |
[148] | ||
| In vitro HUVECs In vivo |
High glucose HGD rats |
Rutin | Reduced Nox4 and ROS Inhibited TXNIP/NLRP3/Caspase-1 Restored phenylephrine-mediated contractions and acetylcholine-induced relaxations in aortic tissue |
[149] | ||
| In vitro HUVECs |
High glucose | Teneligliptin | Reduced TXNIP And ER stress, apoptosis, and cell cycle inhibitors As well as EC proliferation |
[150] | ||
| Diabetic retinopathy | In vitro Rat retina ECs Ex vivo Rat retia |
High glucose STZ rats |
Azaserine (inhibitor of HBP pathway) | siRNAs | Reduced sclerotic fibronectin and COX-II (in vitro) Abolishes diabetes-induced retinal gliosis and ganglion injury (ex vivo) |
[126] |
| In vivo | STZ rats | Curcumin | Reduced leukocyte-endothelium interaction, and inhibiting ICAM-1 and NOX2 expression. | [151] | ||
| In vitro human retinal microvascular ECs |
High glucose | Minocycline | siRNA | Attenuated ROS/TXNIP/NLRP3 inflammasome activation Decrease cell permeability and apoptosis |
[100] | |
| In vitro human retinal microvascular ECs |
Moderately high glucose | siRNA | Reduced ROS and uncontrolled angiogenesis By blocking VEGFR2 and the downstream Akt/mTOR |
[152] | ||
| Endothelial ER stress | In vitro Rat aortic ECs |
Palmitate | Salicylate AICAR |
siRNA | Activate AMPK Suppressed ROS-associated ER stress Reduced TXNIP/NLRP3 inflammasome activation |
[153] |
| In vitro HUVECs |
Palmitate | Astragaloside IV Cycloastragenol |
Ameliorated endothelial dysfunction by inhibiting inflammation and reducing cell apoptosis, through AMPK regulation | [154] | ||
| In vitro Rat aortic EC In vivo |
palmitate HFD rats |
Ilexgenin A | Enhance AMPK activity Suppressed ROS-associated TXNIP/NLRP3 induction, through AMPK activation Reduced EC inflammation and apoptosis |
[155] | ||
| HUVECs | Palmitate | Quercetin Luteolin EGCG |
Suppressed ROS-associated TXNIP/NLRP3 induction, partly through AMPK activation Reduced EC inflammation and apoptosis |
[73] | ||
| In vitro HUVECs |
Thapsigargin | Mangiferin | Prevents TG-induced loss of NO production inhibited TG-induced TXNIP/IL-1β secretion |
[148] | ||
| In vivo | HFD rats | Mangiferin | Prevented ER stress-associated TXNIP/NLRP3 inflammasome through AMPK activity Improved endothelium-dependent vasodilation in response to insulin/insulin resistance |
[156] | ||
| Endothelial aging | In vivo | Natural/diet-induced aged mice | Resveratrol | KO mice | Prevent rise in NADPH oxidase by diet-induced aging Decrease collagen accumulation Decrease nuclear TXNIP |
[157] |
TXNIP and Endothelial Inflammation and Oxidative Stress
TXNIP-associated vasculopathy can be best exemplified by diabetes-induced EC dysfunction leading to the very irreversible nephropathy and retinopathy. As supporting evidence for this role, TXNIP shows significantly higher levels in kidneys from patients with diabetic nephropathy as well as HG-exposed mesangial cells [162]. Rat retinal EC treated with S100B (RAGE ligand) or HG represent high expression of TXNIP and inflammatory markers Cox2, VEGF-A, and ICAM1 [86]. TXNIP ablation in vitro has been also shown to prevent ROS generation and inflammation in HG-exposed rat Muller cell lines [158]. Interestingly, there are emerging evidences indicating diabetes induced pro-atherosclerotic events might be ascribed to TXNIP. Recent comprehensive investigation by Byon et al. showed in vitro ablation of TXNIP provides protection against oxidative stress and dramatically reduces macrophage adhesion in vascular smooth muscle cells (VSMC) exposed oxidized phospholipids hydrogen peroxide. Intriguingly, by in vivo examinations, they reported TXNIP ablation led to 49 to 71% reduction in atherosclerotic lesions in TXNIP-ApoE double knockout mice, compared to control ApoE knockout mice [163]. In clinics, plasma levels of TXNIP have been also shown to work as a reliable predictor of subclinical atherosclerosis in type 2 diabetic patients [164].
Given the high prevalence of dyslipidemia in diabetic subjects, it might be of note that either in vivo or in vitro models of high-fat diet (HFD) fed spontaneously hypertensive rats (SHR) and Palmitate-BSA-exposed human retinal show higher endothelial expression of TXNIP and NLRP3 interaction and IL-1β production. Importantly, HFD fed animals acquire more acellular capillaries, a hallmark of retinal ischemic lesions [97].
As an early event in atherosclerosis, ECs’ inflammation, growth, and monocyte adhesion might arise from turbulent shear stress commonly known as disturbed stress (DS). As of the earliest investigators, Yamawaki et al. described decreased TXNIP expression in response to chronic EC exposure to normal flow. They concluded the associated mitigation of cytokine and the JNK-p38 pathway inhibits proinflammatory effectors such as vascular cell adhesion molecule 1 (VCAM1) expression [127]. On the other side, the intriguing data obtained by Obikane et al. implied that DS may induce TXNIP and VCAM-1 overexpression which was reversed with p21-overexpression [165]. Later in vivo examinations by Wang and colleagues indicated the increased TXNIP was found to be required for DS-induced endothelial inflammation as EC-specific TXNIP knockout mice showed significant decreases in aortal VCAM-1 and ICAM-1 mRNA expression and less EC-monocyte rolling in animal retina. Their obtained in vitro data provided first evidence for TXNIP link with a key anti-inflammatory transcription factor Kruppel-like factor 2 (KLF-2) in ECs. KLF-2 was shown to get repressed by TXNIP in HUVECs in flow chamber model, explaining much of detrimental TXNIP effects [87].
TXNIP and Stress Fiber Malformation
Integrating biophysics to endothelial cell morphology and intracellular signaling implies that laminar shear stress of blood flow contributes to stress fiber (short basal actin filaments) formation and alignment in the longitudinal direction of the vessel [166]. Interestingly, as demonstrated in response to uniaxial stretch on bovine aortic endothelial cells, such alignment has been shown to minimize JNK signaling and EC dysfunction [167]. Recent findings are indicating TXNIP is involved in the mechanotransduction effect of shear stress through indirect regulation of Src tyrosine 527 dephosphorylation and inactivation [159]. Some Src family members have established key role in ECs’ stress fiber formation [168]. As such, the pro-atherogenic effect of TXNIP might be presumably attributed to interfering with stress fiber formation and alignment. This hypothesis might be more confirmed based on evidences supporting TXNIP-KLF2 link to stress fiber formation.
Besides its anti-inflammatory function, on in vitro examinations by Boon et al, KLF2 has been shown to work as an essential component to direct the formation of stress fibers and thereby the ECs’ alignment and morphology. Accordingly, KLF2-induced JNK inhibition is partly mediated through stress fiber formation and alignment [169]. Not surprisingly, the described established TXNIP-NLRP3 inflammasomes link is an alternative well-characterized pathway which may mediate TXNIP-induced dysfunction in HUVECs [170].
TXNIP and Aberrant Endothelial Proliferation
The pathological development of atherosclerotic lesions roots from diverse events including anomalous angiogenesis subsequent to aberrant endothelial cell proliferation, migration, or tube formation [171, 172]. Increase EC division rate may contribute to increased endothelial permeability [173] and enhanced EC proliferation presumably contributes to atherosclerosis development [165]. Interestingly, ECs’ proliferation together with monocytes’ adhesion has been shown to increase at branch orifices which are prone to atherosclerotic plaques and exposed to disturbed shear stress [172]. Involvement of TRX system in angiogenesis has been extensively studied and referred in previous reviews [174]. However, according to the contradicting data, there is not enough compelling evidence to conclude about TXNIP role.
As of earliest works in this field, forced expression of TXNIP was reported to increase ROS production and proliferation and tube formation in ECs [175]. HUVEC examinations later showed TXNIP knockdown abolishes VEGFR2 internalization and phosphorylation and abrogates VEGF-induced EC proliferation and tube formation [160], consistent with other reports indicating VEGF is not efficient to stimulate vascular sprouting from aortic rings in hypoxia [176]. According to mechanistic studies on HUVECs, physiological levels of ROS cause TXNIP to translocate to the plasma membrane as an essential carrier for TRX1 to activate vascular VEGFR2 signaling [64]. As augmented by PARP1 siRNA treatment, TXNIP translocation and VEGFR2 activation in cell membrane are probably under PPAR1 control [177].
On the contrary side, there are several evidences indicating TXNIP suppress angiogenic events in ECs exposed to diabetic-like environment. In this line, high glucose exposure to endothelial progenitor cells is reported to impair EC proliferation, migration, and tube formation which is efficiently ameliorated by TXNIP silencing by siRNA [178, 179]. According to further in vitro experimentations, TXNIP has been suggested to mediate HG-induced impairment by abolishing VEGF production and angiogenic effects in diabetic conditions, which requires adequate TRX1 activity [180]. Such findings support the recent report on the antioxidant teneligliptin to antagonize TXNIP overexpression and reverse the reduced EC proliferation in HUVECs exposed to HG. Teneligliptin effects have been suggested to involve cell cycle inhibitors like P21 [150].
The unraveled information suggesting an anti-proliferative effect of TXNIP on endothelial cells and angiogenesis is well in agreement with the established effect of TXNIP (VDUP1) on cell cycle arrest in G0/G1 [181] and may partly explain vitamin D benefits to combat cancerous proliferation [182, 183]. However, co-localization of TXNIP overexpression with enhanced EC proliferation in vascular branch orifices with disturbed sheer stress supports the hypothesis that TXNIP contribute to EC proliferation and angiogenesis [172, 184]. Undoubtedly, taking all the existing controversies together, it seems TXNIP role in EC proliferation highly depends on experimental settings and needs to be precisely addressed in translational context. Interestingly, as a vivid example, according to the most recent report using moderately high glucose (MHG) levels instead of HG, human retinal microvascular endothelial cells’ proliferation in vitro is not affected by MHG level. Alternatively, MHG remarkably stimulated EC migration and tube formation well resembling proliferative diabetic retinopathy leading to visual disturbance. HMG-induced ROS and uncontrolled angiogenesis were shown to disappear in TXNIP knockout cells supporting the hypotheses that TXNIP contribute to pathologic angiogenesis [152].
TXNIP as an Experimental Target for Ischemic Stroke
Ischemic/reperfusion injury is exceptionally associated with oxidative stress given the profound oxygen rebound flow after spontaneous thrombolysis or therapeutic revascularization [185]. Regarding the governing role of TXNIP in TRX system and thereby in oxidative stress injury, it is rather reasonable that TXNIP stands among promising targets in stroke therapy. Several studies have demonstrated a correlation between increased TXNIP expression and stress-related diseases including ischemic stroke in mice [70], in vivo NMDA neurotoxicity [132, 136], and metabolic stress in high-fat animals [134]. TXNIP expression has been empirically shown to rise in experimental ischemic–reperfusion injury in eMCAO mice [70], intraluminal MCAO in rats [186] and mice, and in vitro OGD model [61]. TXNIP overexpression might be expected based on the described stimuli like oxidative stress and excitotoxicity following stroke, although recent findings in ischemic human pancreatic cancer tissues imply TXNIP transcription is dependent to HIF-1α and highly affected by putative hypoxia-regulated element-binding sequence in its promoter region [187]. Interestingly, mice with overexpression of TRX [45] or primary neurons treated with TXNIP siRNA silencing appear more resistant against ischemic reperfusion damage [61]. Resveratrol as a TXNIP antagonist has also been previously reported to provide protection in hepatic ischemic injury in rats [137]. Recently, we reported that genetic ablation of TXNIP (TXNIP−/−) as well as pharmacological inhibition of TXNIP by resveratrol protects against brain infarction and neurological outcome in mouse model of embolic stroke [70]. According to our obtained data, TXNIP−/− mice showed higher expression of TRX with reciprocal decrease in the makers of oxidative stress including nitrotyrosine (NT) in parallel with inflammatory quiescence. We concluded that protective effect of TXNIP inhibition is partly due to inhibition of NLRP3 inflammasome components including cleaved caspase and IL-1β. The existing knowledge supports the multiple deteriorating TXNIP effectors that can contribute to acute ischemic stroke injury through redox imbalance and inflammasome activation. In Fig. 4, the potential implication of TXNIP in neural injury is illustrated including those involved in the typical cerebrovascular disease stroke or degenerative disorders. However, little has been unraveled in this context; much more triggering pathways may be posited to explain stroke-induced TXNIP pathological role.
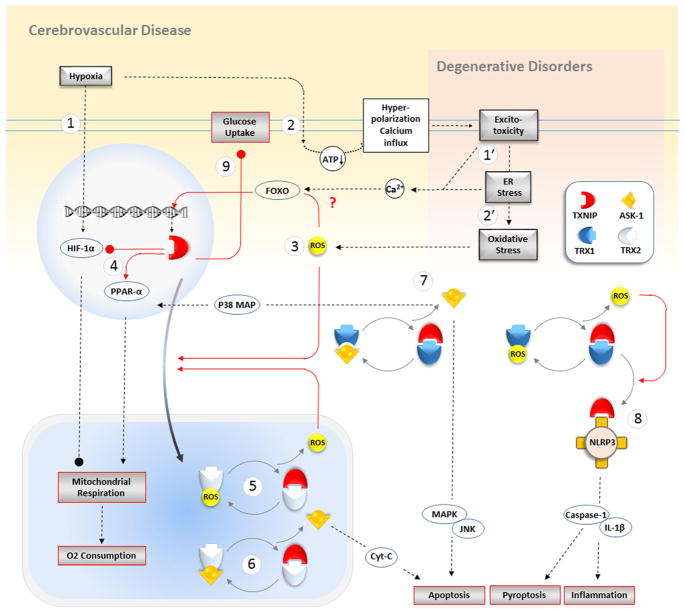
Fig. 4.
Putative pathways of TXNIP activation in neural damage associated with cerebrovascular and neurodegenerative diseases. A few molecules are assumed to augment TXNIP expression following acute or chronic brain diseases; however, little is established in details. Boxes with black outline are immediate disease consequences to stimulate TXNIP in steps which might be simplified as follows. As illustrated in the top orange background, as the specific feature in stroke and cerebrovascular complications, (1) hypoxia is thought to engage TXNIP promoter region to enhance TXNIP transcription and HIF-1α likewise. (2) The likely ATP shortage following severe O2 deficiency may lead to deregulation of ion homeostasis and partial membrane hyperpolarization. This would be a progressive cascade of excitotoxicity, Ca2+ overload, and ER stress leading to intracellular accumulation of ROS. On the other side, as described in the top pink background as a common feature in neurodegenerative disorders, (1′) the long-lasting excitotoxicity leads to ER stress together which may lead to intracellular Ca2+ overload. (2′) The persisting oxidative stress fueled by continuous excitotoxicity complicated with other multiple aggravating signals strongly contributes to keep ROS in high levels. (3) The accumulated ROS either in vascular injuries or degenerative disorders is known to induce TXNIP expression probably through its suppressor FOXO modulation. ROS with different sources within the cell may also induce intracellular shuttling of TXNIP to mitochondria and partly to cytosol and plasma membrane. (4) TXNIP still residing in nucleus may substantially affect mitochondrial biogenesis by modulating transcription of HIF-1α or PPAR-α inhibiting and enhancing mitochondrial oxygen consumption respectively. This way, TXNIP plays a pivotal role in cell death and ROS generation in neural injury. (5) Within mitochondria, TXNIP assembles with TRX-2 to prevent its function as a ROS scavenger leaving the ROS distributes to exacerbate TXNIP expression and shuttering. (6) Binding to TXR-2 simultaneously leads to ASK-1 release and activation to induce Cyt-c-dependent apoptosis. (7) The parallel similar cytosolic event leads to p38 MAP production which amplifies nuclear PPAR-α transcripts. (8) Intracytosolic TXNIP when exposed to ROS overload undergoes some structural changes improving its affinity for NLRP3 inflammasome leading to NLRP3 oligomerization and activation to produce mature cytokines. (9) Concurrent with the vast damaging signals, TXNIP may instigate in several cellular compartments, and while translocated to plasma membrane, TXNIP abolishes GLUT-1 transporter function and less glucose uptake abrogating cellular metabolism danger. ROS, reactive oxygen species; TXNIP, thioredoxin-interacting protein; TXR, thioredoxin; HIF-1α, hypoxia-inducible factor 1-alpha; PPAR-1α, peroxisome proliferator-activated receptor 1-alpha; GLUT, glucose transporter; ASK-1, apoptosis signal-regulating kinase 1; ER, endoplasmic reticulum; NLRP3, Nod-like receptor protein 3; IL-1, interleukin-1; MAPK, mitogen-activated protein kinases; JNK, c-Jun N-terminal kinases; FOXO, Forkhead box transcription factor, class O; Cyt C, cytochrome C
Briefing some of existing evidences might help to understand the significance of TXNIP role in stroke pathology. In investigations performed by Wang et al., increased expression of TXNIP has been reported in rat model of cerebral ischemia [186]. In their experimentations, 7 days pretreatment with umbelliferon, a natural antioxidant ameliorated neurological deficit as well as brain infarction and edema in MCAO rats. They ascribed the beneficial effects of umbelliferone to inhibition of TXNIP/NLRP3 inflammasome activation and upregulation of PPAR-γ with maintenance of redox status. These findings were supported with later reports showing ischemic stroke-induced BBB dysfunction is associated with increased expression of TXNIP and activation of NLRP3 inflammasome in MCAO mice and OGD injured mouse brain microvascular ECs. Such alterations were reversed by ruscogenin, a bioactive steroid sapogenin either in vivo or in vitro. In vivo, ruscogenin also improved cerebral brain flow and upregulated the expression of tight junctions while in cerebrovascular ECs, reduced production of ROS, p38 MAPK, and c-Jun N-terminal kinase (JNK) phosphorylation [138].
Ischemic neuronal injury is associated with increased ER stress and activation of inflammatory response which might arise from ER-induced TXNIP/NLRP3 activation. The data obtained by Li et al. (2015) in vitro excitotoxicity modeling showed remarkable ER stress in hippocampus or SH-SY5Y cells associates a remarkable ER stress and TXNIP/NLRP3 activation. Curcumin could reduce NLRP3 activation in hippocampal CA1 region in MCAO rats while it attenuated ER stress through AMPK activation in vitro in parallel with TXNIP repression suggesting the role of ER stress in the induction of TXNIP expression [139]. Hua and colleagues also demonstrated increased TXNIP expression, elevated oxidative stress markers and reciprocal decrease in the expression TRX in rat ischemic stroke model [135]. In their study on compound 10b, a novel free radical scavenger, they found a significant decrease in the expression of TXNIP concurrent with Bax, caspase 3, and caspase 9 attenuation implicative of TXNIP activation by stroke-induced oxidative stress. In a recent work examining hyperglycemic ischemic stroke in mice, Guo et al. provided first evidences implying TXNIP is also involved in preconditioning against ischemic stroke, showing animals receiving intracerebral TXNIP siRNA did not gain protection against ischemic stroke after hyperbaric oxygen preconditioning [188]. The most recent additions on TXNIP suggest that nuclear factor erythroid 2-related factor 2 (Nrf2) has a pivotal role in regulating TRX/TXNIP in cerebral ischemia. Accordingly, Nrf2 upregulation by systemic tert-butylhydroquinone (tBHQ) has been shown to enhance cytosolic TXNIP expression leading to NLRP3 inflammasome activation in MCAO animals, the effects which vanished with either Nrf2 or TRX siRNA treatment in vivo [140]. All together, these evidences are highlighting activation of TXNIP as a key event associated with inflammation and cell death in response to ischemic brain injury. Evidences showing transfection with TXNIP siRNA prevent cellular damage in model of cerebral ischemia [61, 189], supporting the idea of TXNIP inhibition as a therapeutic approach to prevent the detrimental consequences of hemorrhagic or ischemic stroke.
Summary and Future Directions
In recent decades, prevention of neurological diseases has received intensive attention due to the increasing global economic burden for growing elderly population. However, owing to the complexity of the underlying molecular mechanisms, the treatment options for ischemic stroke still have several limitations. Accumulating evidences demonstrate that activation of TXNIP plays a prominent role in the pathogenesis and progression of several CNS diseases inducing degenerative and acute brain injury. Hence, efficient inhibition of TXNIP function or expression at the molecular level may provide an insight into development of new therapeutics. Although a number of neuroprotective agents including resveratrol, curcumin, and umbellifereone could modulate the expression of TXNIP and ameliorate injury in animal models of CNS diseases particularly in ischemic stroke, none have passed clinical trials with effects comparable to animal findings. Noteworthy, increasing evidences are suggesting TXNIP deteriorating role is intimately linked to the brain microvasculature. This comes to high significance in ischemic stroke as a typical cerebrovascular disease which vastly engages all TXNIP regulators in different stages of ischemia and reperfusion. The current lack of specific inhibitors however limits TXNIP use as a direct therapeutic target for stroke and other cerebrovascular diseases. As such, further investigation to develop specific TXNIP inhibitor and their validation appears to shed light on better management of neurological diseases. Nonetheless, restraining TXNIP to induce cell cycle arrest and drive apoptotic events and the potential consequent neoplastic risk is a major concern in therapeutic application of TXNIP inhibitors [141, 190]. Therefore, applicable TXNIP partial agonists may provide appropriate therapeutic tools to avoid potential side effect due to intensive TXNIP ablation. This may also suggest TXNIP inhibitors might be better candidates in acute brain injury like that occurs in ischemic stroke and brain trauma requiring less chronic medications.
Acknowledgments
Authors would like to thank Ms. Colby Polonsky, Augusta University, Augusta, Georgia, for her assistance with Fig. 1.
Funding This work was supported by the National Institute of Health [R01-NS097800 (TI)].
Abbreviations
- AD
Alzheimer’s disease
- AIM2
Absence in melanoma
- AMPK
AMP-activated protein kinase
- ASC
Apoptosis-associated speck-like
- ASK-1
Apoptosis signal-regulating kinase
- BBB
Blood-brain barrier
- Cyt-c
Cytochrome C
- ChREBP
Carbohydrate response element-binding protein
- DAMP
Damage-associated molecular patterns
- DSS
Disturbed shear stress
- ECs
Endothelial cells
- ER
Endoplasmic reticulum
- eMCAO
Embolic MCAO
- FOXO
Forkhead box transcription factor, class O
- GFAP
Glial fibrillary acidic protein
- GLUT
Glucose transporter
- HFD
High-fat diet
- HGD
High-glucose diet
- HUVECs
Human umbilical vein endothelial cells
- HRE
Human retinal endothelial cells
- ICH
Intracerebral hemorrhage
- IL-1β
Interleukin-1-beta
- IRE1α
Serine/threonine-protein kinase/endoribonuclease
- JNK
c-Jun-N-terminal kinase
- KO
Knockout
- KLF-2
Kruppel-like factor 2
- LPS
Lipopolysaccharide
- LRR
Leucine-rich repeat domain
- MAP
Mitogen-activated protein
- MCAO
Middle cerebral artery occlusion
- MI
Myocardial infarction
- MAPK
Mitogen-activated protein kinase
- miRNA
Microribonucleic acid
- MMP-9
Matrix metalloproteinase 9
- MyD-88
Myeloid differentiation primary response 88
- NADPH
Nicotinamide adenine dinucleotide phosphateoxidase
- NAD
Nicotinamide adenine dinucleotide phosphate
- NADH
Nicotinamide adenine dinucleotide phosphate
- NBD
Nucleotide-binding domain
- NF-κB
Nuclear factor kappa-B
- NLR
Nucleotide-binding oligomerization domain-like receptor
- NLRP
NOD-like receptor proteins
- NMDA
N-Methyl-D-aspartate
- NOD
Nucleotide-binding oligomerization domain
- NOX
Nicotinamide adenine dinucleotide phosphate oxidase
- OGD
Oxygen glucose deprivation
- PAMP
Pathogen-associated molecular patterns
- PERK
Protein kinase R-like ER kinase
- PRR
Pattern recognition receptor
- PTP
Phospho-tyrosine phosphatases
- pVHL
von Hippel–Lindau protein
- RAGE
Advanced glycation end-products
- stz
Streptozotocin
- SAH
Subarachnoid hemorrhage
- SUR1
Sulfonylurea receptor 1
- TLR
Toll-like receptor
- tMCAO
Transient MCAO
- TNF-α
Tumor necrotizing factor-α
- TRX
Thioredoxin
- TXNIP
Thioredoxin-interacting protein
- UPR
Unfolded protein response
- VDUP1
Vitamin D3 upregulated protein 1
- VCAM
Vascular cell adhesion protein
- VEGF
Vascular endothelial growth factor
- WT
Wild type
- ZDF
Zucker diabetic fatty
Footnotes
Compliance with Ethical Standards
Conflict of Interest The authors declare that they have no conflict of interest.
References
- 1.Global Burden of Disease Study C. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kowal SL, Dall TM, Chakrabarti R, et al. The current and projected economic burden of Parkinson’s disease in the United States. Mov Disord. 2013;28:311–318. doi: 10.1002/mds.25292. [DOI] [PubMed] [Google Scholar]
- 3.Murray CJ, Atkinson C, Bhalla K, et al. The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310:591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett DA, Krishnamurthi RV, Barker-Collo S, et al. The global burden of ischemic stroke: findings of the GBD 2010 study. Glob Heart. 2014;9:107–112. doi: 10.1016/j.gheart.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Rubiano AM, Carney N, Chesnut R, et al. Global neurotrauma research challenges and opportunities. Nature. 2015;527:S193–S197. doi: 10.1038/nature16035. [DOI] [PubMed] [Google Scholar]
- 6.Fischer R, Maier O. Interrelation of oxidative stress and inflammation in neurodegenerative disease: role of TNF. Oxidative Med Cell Longev. 2015;2015 doi: 10.1155/2015/610813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim GH, Kim JE, Rhie SJ, et al. The role of oxidative stress in neurodegenerative diseases. Exp Neurobiol. 2015;24:325–340. doi: 10.5607/en.2015.24.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chamorro Á, Dirnagl U, Urra X, et al. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016;15:869–881. doi: 10.1016/S1474-4422(16)00114-9. [DOI] [PubMed] [Google Scholar]
- 9.Witte ME, Geurts JJ, de Vries HE, et al. Mitochondrial dysfunction: a potential link between neuroinflammation and neurodegeneration? Mitochondrion. 2010;10:411–418. doi: 10.1016/j.mito.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Chan PH. Mitochondrial dysfunction and oxidative stress as determinants of cell death/survival in stroke. Ann N YAcad Sci. 2005;1042:203–209. doi: 10.1196/annals.1338.022. [DOI] [PubMed] [Google Scholar]
- 11.Rothwell PM, Howard SC, Dolan E, et al. Effects of β blockers and calcium-channel blockers on within-individual variability in blood pressure and risk of stroke. Lancet Neurol. 2010;9:469–480. doi: 10.1016/S1474-4422(10)70066-1. [DOI] [PubMed] [Google Scholar]
- 12.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol. 2006;100:328–335. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- 13.Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015;16:358. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- 14.Grotta JC, Albers GW, Broderick JP, et al. Stroke: pathophysiology, diagnosis, and management. Elsevier Health Sciences; 2015. [Google Scholar]
- 15.Kristián T, Siesjö BK. Calcium in ischemic cell death. Stroke. 1998;29:705–718. doi: 10.1161/01.str.29.3.705. [DOI] [PubMed] [Google Scholar]
- 16.Chan PH. Role of oxidants in ischemic brain damage. Stroke. 1996;27:1124–1129. doi: 10.1161/01.str.27.6.1124. [DOI] [PubMed] [Google Scholar]
- 17.Xia W, Han J, Huang G, et al. Inflammation in ischaemic brain injury: current advances and future perspectives. Clin Exp Pharmacol Physiol. 2010;37:253–258. doi: 10.1111/j.1440-1681.2009.05279.x. [DOI] [PubMed] [Google Scholar]
- 18.Clarke D. Circulation and energy metabolism of the brain. Basic Neurochem: Mol Cell Med Asp. 1999:637–669. [Google Scholar]
- 19.Gerlach M, Ben-Shachar D, Riederer P, et al. Altered brain metabolism of iron as a cause of neurodegenerative diseases? J Neurochem. 1994;63:793–807. doi: 10.1046/j.1471-4159.1994.63030793.x. [DOI] [PubMed] [Google Scholar]
- 20.Cooper AJ, Meister A. Glutathione in the brain: disorders of glutathione metabolism. Mol Genet Basis Neurol Dis. 1997:35. [Google Scholar]
- 21.Drechsel DA, Patel M. Respiration-dependent H2O2 removal in brain mitochondria via the thioredoxin/peroxiredoxin system. J Biol Chem. 2010;285:27850–27858. doi: 10.1074/jbc.M110.101196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bereczki D, Fekete I. Vinpocetine for acute ischaemic stroke. The Cochrane Library. 2008 doi: 10.1002/14651858.CD000480.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geeganage C, Beavan J, Ellender S, et al. Interventions for dysphagia and nutritional support in acute and subacute stroke. The Cochrane Library. 2012 doi: 10.1002/14651858.CD000323.pub2. [DOI] [PubMed] [Google Scholar]
- 24.Farina N, Isaac M, Clark AR, et al. Vitamin E for Alzheimer’s dementia and mild cognitive impairment. Cochrane Database Syst Rev. 2012:11. doi: 10.1002/14651858.CD002854.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans JR, Henshaw K. Antioxidant vitamin and mineral supplements for preventing age-related macular degeneration. Cochrane Database Syst Rev. 2008:CD000253. doi: 10.1002/14651858.CD000253.pub2. [DOI] [PubMed] [Google Scholar]
- 26.Holmgren A, Johansson C, Berndt C, et al. Thiol redox control via thioredoxin and glutaredoxin systems. Portland Press Limited; 2005. [DOI] [PubMed] [Google Scholar]
- 27.Schulze PC, De Keulenaer GW, Yoshioka J, et al. Vitamin D3-upregulated protein-1 (VDUP-1) regulates redox-dependent vascular smooth muscle cell proliferation through interaction with thioredoxin. Circ Res. 2002;91:689–695. doi: 10.1161/01.res.0000037982.55074.f6. [DOI] [PubMed] [Google Scholar]
- 28.Chen K-S, DeLuca HF. Isolation and characterization of a novel cDNA from HL-60 cells treated with 1, 25-dihydroxyvitamin D-3. Biochim Biophys Acta (BBA) Gene Struct Expr. 1994;1219:26–32. doi: 10.1016/0167-4781(94)90242-9. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Rong Y, Zhang M, et al. Up-regulation of thioredoxin interacting protein (Txnip) by p38 MAPK and FOXO1 contributes to the impaired thioredoxin activity and increased ROS in glucose-treated endothelial cells. Biochem Biophys Res Commun. 2009;381:660–665. doi: 10.1016/j.bbrc.2009.02.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morrison JA, Pike LA, Sams SB, et al. Thioredoxin interacting protein (TXNIP) is a novel tumor suppressor in thyroid cancer. Mol Cancer. 2014;13:62. doi: 10.1186/1476-4598-13-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh LP. Thioredoxin interacting protein (TXNIP) and pathogenesis of diabetic retinopathy. J Clin Exp Ophthalmol. 2013:4. doi: 10.4172/2155-9570.1000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shalev A. Lack of TXNIP protects β-cells against glucotoxicity. Portland Press Limited; 2008. [DOI] [PubMed] [Google Scholar]
- 33.Parikh H, Carlsson E, Chutkow WA, et al. TXNIP regulates peripheral glucose metabolism in humans. PLoS Med. 2007;4:e158. doi: 10.1371/journal.pmed.0040158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Z, Rong Y, Malone M, et al. Thioredoxin-interacting protein (txnip) is a glucocorticoid-regulated primary response gene involved in mediating glucocorticoid-induced apoptosis. Oncogene. 2006;25:1903–1913. doi: 10.1038/sj.onc.1209218. [DOI] [PubMed] [Google Scholar]
- 35.Bellamy CO, Malcolmson RD, Harrison DJ, et al. Seminars in cancer biology. Elsevier; 1995. Cell death in health and disease: the biology and regulation of apoptosis. [DOI] [PubMed] [Google Scholar]
- 36.Zhou J, Chng W-J. Roles of thioredoxin binding protein (TXNIP) in oxidative stress, apoptosis and cancer. Mitochondrion. 2013;13:163–169. doi: 10.1016/j.mito.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Abais JM, Xia M, Zhang Y, et al. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid Redox Signal. 2015;22:1111–1129. doi: 10.1089/ars.2014.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshihara E, Masaki S, Matsuo Y, et al. Thioredoxin/Txnip: redoxisome, as a redox switch for the pathogenesis of diseases. Front Immunol. 2014;4:514. doi: 10.3389/fimmu.2013.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nordlund P, Reichard P. Ribonucleotide reductases. Annu Rev Biochem. 2006;75:681–706. doi: 10.1146/annurev.biochem.75.103004.142443. [DOI] [PubMed] [Google Scholar]
- 40.Luthman M, Holmgren A. Rat liver thioredoxin and thioredoxin reductase: purification and characterization. Biochemistry. 1982;21:6628–6633. doi: 10.1021/bi00269a003. [DOI] [PubMed] [Google Scholar]
- 41.Sabens EA, Mieyal JJ. Glutaredoxin and thioredoxin enzyme systems: catalytic mechanisms and physiological functions. In: Masella R, Massa G, editors. Glutathione and sulfur amino acids in human health and disease. Hoboken, NJ: John Wiley & Sons, Inc; 2009. pp. 121–156. [Google Scholar]
- 42.Lippoldt A, Padilla C, Gerst H, et al. Localization of thioredoxin in the rat brain and functional implications. J Neurosci. 1995;15:6747–6756. doi: 10.1523/JNEUROSCI.15-10-06747.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rybnikova E, Damdimopoulos AE, Gustafsson JÅ, et al. Expression of novel antioxidant thioredoxin-2 in the rat brain. Eur J Neurosci. 2000;12:1669–1678. doi: 10.1046/j.1460-9568.2000.00059.x. [DOI] [PubMed] [Google Scholar]
- 44.Yoshihara E, Masaki S, Matsuo Y, et al. Thioredoxin/Txnip: redoxisome, as a redox switch for the pathogenesis of diseases. Front Immunol. 2013:4. doi: 10.3389/fimmu.2013.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takagi Y, Mitsui A, Nishiyama A, et al. Overexpression of thioredoxin in transgenic mice attenuates focal ischemic brain damage. Proc Natl Acad Sci. 1999;96:4131–4136. doi: 10.1073/pnas.96.7.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hattori I, Takagi Y, Nakamura H, et al. Intravenous administration of thioredoxin decreases brain damage following transient focal cerebral ischemia in mice. Antioxid Redox Signal. 2004;6:81–87. doi: 10.1089/152308604771978372. [DOI] [PubMed] [Google Scholar]
- 47.Mitsui A, Hamuro J, Nakamura H, et al. Overexpression of human thioredoxin in transgenic mice controls oxidative stress and life span. Antioxid Redox Signal. 2002;4:693–696. doi: 10.1089/15230860260220201. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka T, Hosoi F, Yamaguchi-Iwai Y, et al. Thioredoxin-2 (TRX-2) is an essential gene regulating mitochondria-dependent apoptosis. EMBO J. 2002;21:1695–1703. doi: 10.1093/emboj/21.7.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang D, Masutani H, S-i O, et al. Control of mitochondrial outer membrane permeabilization and Bcl-xL levels by thioredoxin 2 in DT40 cells. J Biol Chem. 2006;281:7384–7391. doi: 10.1074/jbc.M509876200. [DOI] [PubMed] [Google Scholar]
- 50.Chen Y, Cai J, Murphy T, et al. Overexpressed human mitochondrial thioredoxin confers resistance to oxidant-induced apoptosis in human osteosarcoma cells. J Biol Chem. 2002;277:33242–33248. doi: 10.1074/jbc.M202026200. [DOI] [PubMed] [Google Scholar]
- 51.Matthews JR, Wakasugi N, Virelizier J-L, et al. Thiordoxin regulates the DNA binding activity of NF-κB by reduction of a disulphid bond involving cysteine 62. Nucleic Acids Res. 1992;20:3821–3830. doi: 10.1093/nar/20.15.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Okamoto T, Ogiwara H, Hayashi T, et al. Human thioredoxin/adult T cell leukemia-derived factor activates the enhancer binding protein of human immunodeficiency virus type 1 by thiol redox control mechanism. Int Immunol. 1992;4:811–819. doi: 10.1093/intimm/4.7.811. [DOI] [PubMed] [Google Scholar]
- 53.Welsh SJ, Bellamy WT, Briehl MM, et al. The redox protein thioredoxin-1 (Trx-1) increases hypoxia-inducible factor 1α protein expression. Cancer Res. 2002;62:5089–5095. [PubMed] [Google Scholar]
- 54.Baker AF, Dragovich T, Tate WR, et al. The antitumor thioredoxin-1 inhibitor PX-12 (1-methylpropyl 2-imidazolyl di-sulfide) decreases thioredoxin-1 and VEGF levels in cancer patient plasma. J Lab Clin Med. 2006;147:83–90. doi: 10.1016/j.lab.2005.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bodnar JS, Chatterjee A, Castellani LW, et al. Positional cloning of the combined hyperlipidemia gene Hyplip1. Nat Genet. 2002;30:110. doi: 10.1038/ng811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Levendusky MC, Basle J, Chang S, et al. Expression and regulation of vitamin D3 upregulated protein 1 (VDUP1) is conserved in mammalian and insect brain. J Comp Neurol. 2009;517:581–600. doi: 10.1002/cne.22195. [DOI] [PubMed] [Google Scholar]
- 57.Xu G, Chen J, Jing G, et al. Thioredoxin-interacting protein regulates insulin transcription through microRNA-204. Nat Med. 2013;19:1141–1146. doi: 10.1038/nm.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elbein SC, Hoffman MD, Teng K, et al. A genome-wide search for type 2 diabetes susceptibility genes in Utah Caucasians. Diabetes. 1999;48:1175–1182. doi: 10.2337/diabetes.48.5.1175. [DOI] [PubMed] [Google Scholar]
- 59.Vionnet N, Dupont S, Gallina S, et al. Genomewide search for type 2 diabetes–susceptibility genes in French Whites: evidence for a novel susceptibility locus for early-onset diabetes on chromosome 3q27-qter and independent replication of a type 2–diabetes locus on chromosome 1q21–q24. Am J Hum Genet. 2000;67:1470–1480. doi: 10.1086/316887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Junn E, Han SH, Im JY, et al. Vitamin D3 up-regulated protein 1 mediates oxidative stress via suppressing the thioredoxin function. J Immunol. 2000;164:6287–6295. doi: 10.4049/jimmunol.164.12.6287. [DOI] [PubMed] [Google Scholar]
- 61.Kim GS, Jung JE, Narasimhan P, et al. Induction of thioredoxin-interacting protein is mediated by oxidative stress, calcium, and glucose after brain injury in mice. Neurobiol Dis. 2012;46:440–449. doi: 10.1016/j.nbd.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saxena G, Chen J, Shalev A. Intracellular shuttling and mitochondrial function of thioredoxin-interacting protein. J Biol Chem. 2010;285:3997–4005. doi: 10.1074/jbc.M109.034421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu N, Zheng B, Shaywitz A, et al. AMPK-dependent degradation of TXNIP upon energy stress leads to enhanced glucose uptake via GLUT1. Mol Cell. 2013;49:1167–1175. doi: 10.1016/j.molcel.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spindel ON, Berk BC. Thioredoxin-interacting protein mediates TRX1 translocation to the plasma membrane in response to tumor necrosis factor-α. Arterioscler Thromb Vasc Biol. 2011;31:1890–1897. doi: 10.1161/ATVBAHA.111.226340. [DOI] [PubMed] [Google Scholar]
- 65.K-y K, Shin SM, Kim JK, et al. Heat shock factor regulates VDUP1 gene expression. Biochem Biophys Res Commun. 2004;315:369–375. doi: 10.1016/j.bbrc.2004.01.047. [DOI] [PubMed] [Google Scholar]
- 66.Schulze PC, Yoshioka J, Takahashi T, et al. Hyperglycemia promotes oxidative stress through inhibition of thioredoxin function by thioredoxin-interacting protein. J Biol Chem. 2004;279:30369–30374. doi: 10.1074/jbc.M400549200. [DOI] [PubMed] [Google Scholar]
- 67.Wang Y, De Keulenaer GW, Lee RT. Vitamin D3-up-regulated protein-1 is a stress-responsive gene that regulates cardiomyocyte viability through interaction with thioredoxin. J Biol Chem. 2002;277:26496–26500. doi: 10.1074/jbc.M202133200. [DOI] [PubMed] [Google Scholar]
- 68.Saitoh M, Nishitoh H, Fujii M, et al. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen J, Fontes G, Saxena G, et al. Lack of TXNIP protects against mitochondria-mediated apoptosis but not against fatty acid–induced ER stress–mediated β-cell death. Diabetes. 2010;59:440–447. doi: 10.2337/db09-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ishrat T, Mohamed IN, Pillai B, et al. Thioredoxin-interacting protein: a novel target for neuroprotection in experimental thromboembolic stroke in mice. Mol Neurobiol. 2015;51:766–778. doi: 10.1007/s12035-014-8766-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao Q, Che X, Zhang H, et al. Thioredoxin-interacting protein mediates apoptosis in early brain injury after subarachnoid haemorrhage. Int J Mol Sci. 2017;18:854. doi: 10.3390/ijms18040854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ye X, Zuo D, Yu L, et al. ROS/TXNIP pathway contributes to thrombin induced NLRP3 inflammasome activation and cell apoptosis in microglia. Biochem Biophys Res Commun. 2017;485:499–505. doi: 10.1016/j.bbrc.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 73.Wu J, Xu X, Li Y, et al. Quercetin, luteolin and epigallocatechin gallate alleviate TXNIP and NLRP3-mediated inflammation and apoptosis with regulation of AMPK in endothelial cells. Eur J Pharmacol. 2014;745:59–68. doi: 10.1016/j.ejphar.2014.09.046. [DOI] [PubMed] [Google Scholar]
- 74.Devi TS, Hosoya K-I, Terasaki T, et al. Critical role of TXNIP in oxidative stress, DNA damage and retinal pericyte apoptosis under high glucose: implications for diabetic retinopathy. Exp Cell Res. 2013;319:1001–1012. doi: 10.1016/j.yexcr.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wei M, Jiao D, Han D, et al. Knockdown of RNF2 induces cell cycle arrest and apoptosis in prostate cancer cells through the upregulation of TXNIP. Oncotarget. 2017;8:5323. doi: 10.18632/oncotarget.14142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li J, Yue Z, Xiong W, et al. TXNIP overexpression suppresses proliferation and induces apoptosis in SMMC7221 cells through ROS generation and MAPK pathway activation. Oncol Rep. 2017;37:3369–3376. doi: 10.3892/or.2017.5577. [DOI] [PubMed] [Google Scholar]
- 77.Zhou J, Bi C, Cheong L-L, et al. The histone methyltransferase inhibitor, DZNep, up-regulates TXNIP, increases ROS production, and targets leukemia cells in AML. Blood. 2011;118:2830–2839. doi: 10.1182/blood-2010-07-294827. [DOI] [PubMed] [Google Scholar]
- 78.Zhang H, Gao P, Fukuda R, et al. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell. 2007;11:407–420. doi: 10.1016/j.ccr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 79.Goda N, Kanai M. Hypoxia-inducible factors and their roles in energy metabolism. Int J Hematol. 2012;95:457–463. doi: 10.1007/s12185-012-1069-y. [DOI] [PubMed] [Google Scholar]
- 80.Kelly DP. Hypoxic reprogramming. Nat Genet. 2008;40:132–134. doi: 10.1038/ng0208-132. [DOI] [PubMed] [Google Scholar]
- 81.Shin D, Jeon J-H, Jeong M, et al. VDUP1 mediates nuclear export of HIF1α via CRM1-dependent pathway. Biochimica et Biophysica Acta (BBA)-Molecular Cell Res. 2008;1783:838–848. doi: 10.1016/j.bbamcr.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 82.Duncan JG, Fong JL, Medeiros DM, et al. Insulin-resistant heart exhibits a mitochondrial biogenic response driven by the peroxisome proliferator-activated receptor-α/PGC-1α gene regulatory pathway. Circulation. 2007;115:909–917. doi: 10.1161/CIRCULATIONAHA.106.662296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu G-H, Qu J, Shen X. Thioredoxin-mediated negative autoregulation of peroxisome proliferator-activated receptor α transcriptional activity. Mol Biol Cell. 2006;17:1822–1833. doi: 10.1091/mbc.E05-10-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zarubin T, Jiahuai H. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005;15:11–18. doi: 10.1038/sj.cr.7290257. [DOI] [PubMed] [Google Scholar]
- 85.Barger PM, Browning AC, Garner AN, et al. p38 mitogen-activated protein kinase activates peroxisome proliferator-activated receptor α a potential role in the cardiac metabolic stress response. J Biol Chem. 2001;276:44495–44501. doi: 10.1074/jbc.M105945200. [DOI] [PubMed] [Google Scholar]
- 86.Perrone L, Devi TS, Ki H, et al. Thioredoxin interacting protein (TXNIP) induces inflammation through chromatin modification in retinal capillary endothelial cells under diabetic conditions. J Cell Physiol. 2009;221:262–272. doi: 10.1002/jcp.21852. [DOI] [PubMed] [Google Scholar]
- 87.Wang X-Q, Nigro P, Fujiwara K, et al. Thioredoxin interacting protein promotes endothelial cell inflammation in response to disturbed flow by increasing leukocyte adhesion and repressing Kruppel-like factor 2Novelty and significance. Circ Res. 2012;110:560–568. doi: 10.1161/CIRCRESAHA.111.256362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ishrat T, Nasoohi S. The NLRP3 inflammasome: a possible therapeutic target for treatment of stroke, in cellular and molecular approaches to regeneration and repair. Springer; 2018. pp. 427–480. [Google Scholar]
- 89.Gupta R, Ghosh S, Monks B, et al. RNA and β-hemolysin of group B Streptococcus induce interleukin-1β (IL-1β) by activating NLRP3 inflammasomes in mouse macrophages. J Biol Chem. 2014;289:13701–13705. doi: 10.1074/jbc.C114.548982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Costa A, Gupta R, Signorino G, et al. Activation of the NLRP3 inflammasome by group B streptococci. J Immunol. 2012;188:1953–1960. doi: 10.4049/jimmunol.1102543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rathinam VA, Vanaja SK, Waggoner L, et al. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell. 2012;150:606–619. doi: 10.1016/j.cell.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Allen IC, Scull MA, Moore CB, et al. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30:556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gonçalves VM, Matteucci KC, Buzzo CL, et al. NLRP3 controls Trypanosoma cruzi infection through a caspase-1-dependent IL-1R-independent NO production. PLoS Negl Trop Dis. 2013;7:e2469. doi: 10.1371/journal.pntd.0002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Duewell P, Kono H, Rayner KJ, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pazár B, Ea H-K, Narayan S, et al. Basic calcium phosphate crystals induce monocyte/macrophage IL-1β secretion through the NLRP3 inflammasome in vitro. J Immunol. 2011;186:2495–2502. doi: 10.4049/jimmunol.1001284. [DOI] [PubMed] [Google Scholar]
- 96.Zhou R, Tardivel A, Thorens B, et al. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 97.Mohamed IN, Hafez SS, Fairaq A, et al. Thioredoxin-interacting protein is required for endothelial NLRP3 inflammasome activation and cell death in a rat model of high-fat diet. Diabetologia. 2014;57:413–423. doi: 10.1007/s00125-013-3101-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang W, Wang C, Ding XQ, et al. Quercetin and allopurinol reduce liver thioredoxin-interacting protein to alleviate inflammation and lipid accumulation in diabetic rats. Br J Pharmacol. 2013;169:1352–1371. doi: 10.1111/bph.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Luo B, Li B, Wang W, et al. Rosuvastatin alleviates diabetic cardiomyopathy by inhibiting NLRP3 inflammasome and MAPK pathways in a type 2 diabetes rat model. Cardiovasc Drugs Ther. 2014;28:33–43. doi: 10.1007/s10557-013-6498-1. [DOI] [PubMed] [Google Scholar]
- 100.Chen W, Zhao M, Zhao S, et al. Activation of the TXNIP/NLRP3 inflammasome pathway contributes to inflammation in diabetic retinopathy: a novel inhibitory effect of minocycline. Inflamm Res. 2017;66:157–166. doi: 10.1007/s00011-016-1002-6. [DOI] [PubMed] [Google Scholar]
- 101.Gao P, He F-F, Tang H, et al. NADPH oxidase-induced NALP3 inflammasome activation is driven by thioredoxin-interacting protein which contributes to podocyte injury in hyperglycemia. Journal of Diabetes Research. 2015;2015 doi: 10.1155/2015/504761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lv H, Liu Q, Wen Z, et al. Xanthohumol ameliorates lipopolysaccharide (LPS)-induced acute lung injury via induction of AMPK/GSK3β-Nrf2 signal axis. Redox Biol. 2017;12:311–324. doi: 10.1016/j.redox.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yin Y, Zhou Z, Liu W, et al. Vascular endothelial cells senescence is associated with NOD-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome activation via reactive oxygen species (ROS)/thioredoxin-interacting protein (TXNIP) pathway. Int J Biochem Cell Biol. 2017;84:22–34. doi: 10.1016/j.biocel.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 104.Zhou R, Yazdi AS, Menu P, et al. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 105.van Hooijdonk D. Theoredoxin oxidation mediated by mitochondrial ROS is a key event in NLRP3 inflammasome activation 2013 [Google Scholar]
- 106.Lunov O, Syrovets T, Loos C, et al. Amino-functionalized polystyrene nanoparticles activate the NLRP3 inflammasome in human macrophages. ACS Nano. 2011;5:9648–9657. doi: 10.1021/nn203596e. [DOI] [PubMed] [Google Scholar]
- 107.Park YJ, Yoon SJ, Suh HW, et al. TXNIP deficiency exacerbates endotoxic shock via the induction of excessive nitric oxide synthesis. PLoS Pathog. 2013;9:e1003646. doi: 10.1371/journal.ppat.1003646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Joshi S, Wang W, Peck AB, et al. Activation of the NLRP3 inflammasome in association with calcium oxalate crystal induced reactive oxygen species in kidneys. J Urol. 2015;193:1684–1691. doi: 10.1016/j.juro.2014.11.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang X, Zhang J-H, Chen X-Y, et al. Reactive oxygen species-induced TXNIP drives fructose-mediated hepatic inflammation and lipid accumulation through NLRP3 inflammasome activation. Antioxid Redox Signal. 2015;22:848–870. doi: 10.1089/ars.2014.5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu W, Gu J, Qi J, et al. Lentinan exerts synergistic apoptotic effects with paclitaxel in A549 cells via activating ROS-TXNIP-NLRP3 inflammasome. J Cell Mol Med. 2015;19:1949–1955. doi: 10.1111/jcmm.12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Minn AH, Hafele C, Shalev A. Thioredoxin-interacting protein is stimulated by glucose through a carbohydrate response element and induces β-cell apoptosis. Endocrinology. 2005;146:2397–2405. doi: 10.1210/en.2004-1378. [DOI] [PubMed] [Google Scholar]
- 112.Shalev A, Pise-Masison CA, Radonovich M, et al. Oligonucleotide microarray analysis of intact human pancreatic islets: identification of glucose-responsive genes and a highly regulated TGFβ signaling pathway. Endocrinology. 2002;143:3695–3698. doi: 10.1210/en.2002-220564. [DOI] [PubMed] [Google Scholar]
- 113.Chen J, Cha-Molstad H, Szabo A, et al. Diabetes induces and calcium channel blockers prevent cardiac expression of proapoptotic thioredoxin-interacting protein. Am J Physiol Endocrinol Metab. 2009;296:E1133–E1139. doi: 10.1152/ajpendo.90944.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Soriano-Tárraga C, Jiménez-Conde J, Giralt-Steinhauer E, et al. Epigenome-wide association study identifies TXNIP gene associated with type 2 diabetes mellitus and sustained hyperglycemia. Hum Mol Genet. 2015;25:609–619. doi: 10.1093/hmg/ddv493. [DOI] [PubMed] [Google Scholar]
- 115.Reich E, Tamary A, Sionov RV, et al. Involvement of thioredoxin-interacting protein (TXNIP) in glucocorticoid-mediated beta cell death. Diabetologia. 2012;55:1048–1057. doi: 10.1007/s00125-011-2422-z. [DOI] [PubMed] [Google Scholar]
- 116.Shaked M, Ketzinel-Gilad M, Cerasi E, et al. AMP-activated protein kinase (AMPK) mediates nutrient regulation of thioredoxin-interacting protein (TXNIP) in pancreatic beta-cells. PLoS One. 2011;6:e28804. doi: 10.1371/journal.pone.0028804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fang S, Jin Y, Zheng H, et al. High glucose condition up-regulated Txnip expression level in rat mesangial cells through ROS/MEK/MAPK pathway. Mol Cell Biochem. 2011;347:175–182. doi: 10.1007/s11010-010-0626-z. [DOI] [PubMed] [Google Scholar]
- 118.Mubarak B, Soriano FX, Hardingham GE. Synaptic NMDAR activity suppresses FOXO1 expression via a cis-acting FOXO binding site: FOXO1 is a FOXO target gene. Channels. 2009;3:233–239. doi: 10.4161/chan.3.4.9381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Brunet A, Bonni A, Zigmond MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 120.de Candia P, Blekhman R, Chabot AE, et al. A combination of genomic approaches reveals the role of FOXO1a in regulating an oxidative stress response pathway. PLoS One. 2008;3:e1670. doi: 10.1371/journal.pone.0001670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kibbe C, Chen J, Xu G, et al. FOXO1 competes with carbohydrate response element-binding protein (ChREBP) and inhibits thioredoxin-interacting protein (TXNIP) transcription in pancreatic beta cells. J Biol Chem. 2013;288:23194–23202. doi: 10.1074/jbc.M113.473082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xu G, Chen J, Jing G, et al. Preventing β-cell loss and diabetes with calcium channel blockers. Diabetes. 2012;61:848–856. doi: 10.2337/db11-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cha-Molstad H, Xu G, Chen J, et al. Calcium channel blockers act through nuclear factor Y to control transcription of key cardiac genes. Mol Pharmacol. 2012;82:541–549. doi: 10.1124/mol.112.078253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lerner AG, Upton J-P, Praveen P, et al. IRE1α induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metab. 2012;16:250–264. doi: 10.1016/j.cmet.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Oslowski CM, Hara T, O’Sullivan-Murphy B, et al. Thioredoxin-interacting protein mediates ER stress-induced β cell death through initiation of the inflammasome. Cell Metab. 2012;16:265–273. doi: 10.1016/j.cmet.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Perrone L, Devi T, Hosoya K, et al. Inhibition of TXNIP expression in vivo blocks early pathologies of diabetic retinopathy. Cell Death Dis. 2010;1:e65. doi: 10.1038/cddis.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yamawaki H, Pan S, Lee RT, et al. Fluid shear stress inhibits vascular inflammation by decreasing thioredoxin-interacting protein in endothelial cells. J Clin Investig. 2005;115:733. doi: 10.1172/JCI200523001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gouget T, Djelloul M, Boucraut J, et al. TXNIP, the major player in insulin resistance, is early over-expressed in the brain of the 5XFAD Alzheimer’s mice model and is induced by Aβ in vitro: emerging role of TXNIP and inflammation in Alzheimer’s disease progression. Alzheimers Dement: J Alzheimers Assoc. 2011;7:S684. [Google Scholar]
- 129.Gao J, He H, Jiang W, et al. Salidroside ameliorates cognitive impairment in a d-galactose-induced rat model of Alzheimer’s disease. Behav Brain Res. 2015;293:27–33. doi: 10.1016/j.bbr.2015.06.045. [DOI] [PubMed] [Google Scholar]
- 130.Kaya B, Erdi F, Kilinc I, et al. Alterations of the thioredoxin system during subarachnoid hemorrhage-induced cerebral vasospasm. Acta Neurochir. 2015;157:793. doi: 10.1007/s00701-015-2390-z. [DOI] [PubMed] [Google Scholar]
- 131.Erdi F, Keskin F, Esen H, et al. Telmisartan ameliorates oxidative stress and subarachnoid haemorrhage-induced cerebral vasospasm. Neurol Res. 2016;38:224–231. doi: 10.1080/01616412.2015.1105626. [DOI] [PubMed] [Google Scholar]
- 132.El-Azab M, Baldowski B, Mysona B, et al. Deletion of thioredoxin-interacting protein preserves retinal neuronal function by preventing inflammation and vascular injury. Br J Pharmacol. 2014;171:1299–1313. doi: 10.1111/bph.12535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Al-Gayyar MM, Abdelsaid MA, Matragoon S, et al. Thioredoxin interacting protein is a novel mediator of retinal inflammation and neurotoxicity. Br J Pharmacol. 2011;164:170–180. doi: 10.1111/j.1476-5381.2011.01336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang Q-Y, Pan Y, Wang R, et al. Quercetin inhibits AMPK/TXNIP activation and reduces inflammatory lesions to improve insulin signaling defect in the hypothalamus of high fructose-fed rats. J Nutr Biochem. 2014;25:420–428. doi: 10.1016/j.jnutbio.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 135.Hua K, Sheng X, T-t L, et al. The edaravone and 3-n-butylphthalide ring-opening derivative 10b effectively attenuates cerebral ischemia injury in rats. Acta Pharmacol Sin. 2015;36:917–927. doi: 10.1038/aps.2015.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Matragoon S, Al-Gayyar MM, Abdelsaid MA, et al. Thioredoxin interacting protein is a novel mediator of retinal inflammation and neurotoxicity. Invest Ophthalmol Vis Sci. 2011;52:1006–1006. doi: 10.1111/j.1476-5381.2011.01336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nivet-Antoine V, Cottart C-H, Lemaréchal H, et al. Trans-resveratrol downregulates Txnip overexpression occurring during liver ischemia-reperfusion. Biochimie. 2010;92:1766–1771. doi: 10.1016/j.biochi.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 138.Cao G, Jiang N, Hu Y, et al. Ruscogenin attenuates cerebral ischemia-induced blood-brain barrier dysfunction by suppressing TXNIP/NLRP3 inflammasome activation and the MAPK pathway. Int J Mol Sci. 2016;17:1418. doi: 10.3390/ijms17091418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li Y, Li J, Li S, et al. Curcumin attenuates glutamate neurotoxicity in the hippocampus by suppression of ER stress-associated TXNIP/NLRP3 inflammasome activation in a manner dependent on AMPK. Toxicol Appl Pharmacol. 2015;286:53–63. doi: 10.1016/j.taap.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 140.Hou Y, Wang Y, He Q, et al. Nrf2 inhibits NLRP3 inflammasome activation through regulating Trx1/TXNIP complex in cerebral ischemia reperfusion injury. Behav Brain Res. 2018;336:32–39. doi: 10.1016/j.bbr.2017.06.027. [DOI] [PubMed] [Google Scholar]
- 141.Yamaguchi F, Takata M, Kamitori K, et al. Rare sugar D-allose induces specific up-regulation of TXNIP and subsequent G1 cell cycle arrest in hepatocellular carcinoma cells by stabilization of p27kip1. Int J Oncol. 2008;32:377–385. [PubMed] [Google Scholar]
- 142.Chen J, Deng X, Liu N, et al. Quercetin attenuates tau hyperphosphorylation and improves cognitive disorder via suppression of ER stress in a manner dependent on AMPK pathway. J Funct Foods. 2016;22:463–476. [Google Scholar]
- 143.Ohizumi Y. A new strategy for preventive and functional therapeutic methods for dementia—approach using natural products. Yakugaku Zasshi: J Pharm Soc Jpn. 2015;135:449–464. doi: 10.1248/yakushi.14-00245. [DOI] [PubMed] [Google Scholar]
- 144.Hoon JN, Willigers JM, Troost J, et al. Vascular effects of 5-HT1B/1D-receptor agonists in patients with migraine headaches. Clin Pharmacol Ther. 2000;68:418–426. doi: 10.1067/mcp.2000.110502. [DOI] [PubMed] [Google Scholar]
- 145.Gao Y, Li W, Liu Y, et al. Effect of telmisartan on preventing learning and memory deficits via peroxisome proliferator-activated receptor-γ in vascular dementia spontaneously hypertensive rats. Journal of Stroke and Cerebrovascular Diseases. 2017 doi: 10.1016/j.jstrokecerebrovasdis.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 146.Varma A, Adams W, Lloyd J, et al. Diagnostic patterns of regional atrophy on MRI and regional cerebral blood flow change on SPECT in young onset patients with Alzheimer’s disease, frontotemporal dementia and vascular dementia. Acta Neurol Scand. 2002;105:261–269. doi: 10.1034/j.1600-0404.2002.1o148.x. [DOI] [PubMed] [Google Scholar]
- 147.Li X, Kover KL, Heruth DP, et al. New insight into metformin action: regulation of ChREBP and FoXO1 activities in endothelial cells. Mol Endocrinol. 2015;29:1184–1194. doi: 10.1210/ME.2015-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Song J, Li J, Hou F, et al. Mangiferin inhibits endoplasmic reticulum stress-associated thioredoxin-interacting protein/NLRP3 inflammasome activation with regulation of AMPK in endothelial cells. Metabolism. 2015;64:428–437. doi: 10.1016/j.metabol.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 149.Wang W, Wu Q-h, Sui Y, et al. Rutin protects endothelial dysfunction by disturbing Nox4 and ROS-sensitive NLRP3 inflammasome. Biomed Pharmacother. 2017;86:32–40. doi: 10.1016/j.biopha.2016.11.134. [DOI] [PubMed] [Google Scholar]
- 150.Pujadas G, De Nigris V, Prattichizzo F, et al. The dipeptidyl peptidase-4 (DPP-4) inhibitor teneligliptin functions as antioxidant on human endothelial cells exposed to chronic hyperglycemia and metabolic high-glucose memory. Endocrine. 2017;56:509–520. doi: 10.1007/s12020-016-1052-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wongeakin N, Bhattarakosol P, Patumraj S. Molecular mechanisms of curcumin on diabetes-induced endothelial dysfunctions: Txnip, ICAM-1, and NOX2 expressions. Biomed Res Int. 2014;2014 doi: 10.1155/2014/161346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Duan J, Du C, Shi Y, et al. Thioredoxin-interacting protein deficiency ameliorates diabetic retinal angiogenesis. Int J Biochem Cell Biol. 2017 doi: 10.1016/j.biocel.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 153.Li J, Wang Y, Wang Y, et al. Pharmacological activation of AMPK prevents Drp1-mediated mitochondrial fission and alleviates endoplasmic reticulum stress-associated endothelial dysfunction. J Mol Cell Cardiol. 2015;86:62–74. doi: 10.1016/j.yjmcc.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 154.Zhao Y, Li Q, Zhao W, et al. Astragaloside IV and cycloastragenol are equally effective in inhibition of endoplasmic reticulum stress-associated TXNIP/NLRP3 inflammasome activation in the endothelium. J Ethnopharmacol. 2015;169:210–218. doi: 10.1016/j.jep.2015.04.030. [DOI] [PubMed] [Google Scholar]
- 155.Li Y, Yang J, Chen M-H, et al. Ilexgenin A inhibits endoplasmic reticulum stress and ameliorates endothelial dysfunction via suppression of TXNIP/NLRP3 inflammasome activation in an AMPK dependent manner. Pharmacol Res. 2015;99:101–115. doi: 10.1016/j.phrs.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 156.Xu X, Chen Y, Song J, et al. Mangiferin suppresses endoplasmic reticulum stress in perivascular adipose tissue and prevents insulin resistance in the endothelium. Eur J Nutr. 2017:1–13. doi: 10.1007/s00394-017-1441-z. [DOI] [PubMed] [Google Scholar]
- 157.Bedarida T, Baron S, Vibert F, et al. Resveratrol decreases TXNIP mRNA and protein nuclear expressions with an arterial function improvement in old mice. J Gerontol A: Biomed Sci Med Sci. 2015;71:720–729. doi: 10.1093/gerona/glv071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Devi TS, Lee I, Hüttemann M, et al. TXNIP links innate host defense mechanisms to oxidative stress and inflammation in retinal Muller glia under chronic hyperglycemia: implications for diabetic retinopathy. Exp Diabetes Res. 2012;2012 doi: 10.1155/2012/438238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Spindel ON, Burke RM, Yan C, et al. Thioredoxin-interacting protein is a biomechanical regulator of Src activity novelty and significance. Circ Res. 2014;114:1125–1132. doi: 10.1161/CIRCRESAHA.114.301315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Park S-Y, Shi X, Pang J, et al. Thioredoxin-interacting protein mediates sustained VEGFR2 signaling in endothelial cells required for angiogenesis significance. Arterioscler Thromb Vasc Biol. 2013;33:737–743. doi: 10.1161/ATVBAHA.112.300386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Riahi Y, Kaiser N, Cohen G, et al. Foam cell-derived 4-hydroxynonenal induces endothelial cell senescence in a TXNIP-dependent manner. J Cell Mol Med. 2015;19:1887–1899. doi: 10.1111/jcmm.12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Advani A, Gilbert RE, Thai K, et al. Expression, localization, and function of the thioredoxin system in diabetic nephropathy. J Am Soc Nephrol. 2009;20:730–741. doi: 10.1681/ASN.2008020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Byon CH, Han T, Wu J, et al. Txnip ablation reduces vascular smooth muscle cell inflammation and ameliorates atherosclerosis in apolipoprotein E knockout mice. Atherosclerosis. 2015;241:313–321. doi: 10.1016/j.atherosclerosis.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhao Y, Li X, Tang S. Retrospective analysis of the relationship between elevated plasma levels of TXNIP and carotid intima-media thickness in subjects with impaired glucose tolerance and early type 2 diabetes mellitus. Diabetes Res Clin Pract. 2015;109:372–377. doi: 10.1016/j.diabres.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 165.Obikane H, Abiko Y, Ueno H, et al. Effect of endothelial cell proliferation on atherogenesis: a role of p21 Sdi/Cip/Waf1 in monocyte adhesion to endothelial cells. Atherosclerosis. 2010;212:116–122. doi: 10.1016/j.atherosclerosis.2010.05.029. [DOI] [PubMed] [Google Scholar]
- 166.Malek AM, Izumo S. Mechanism of endothelial cell shape change and cytoskeletal remodeling in response to fluid shear stress. J Cell Sci. 1996;109:713–726. doi: 10.1242/jcs.109.4.713. [DOI] [PubMed] [Google Scholar]
- 167.Kaunas R, Usami S, Chien S. Regulation of stretch-induced JNK activation by stress fiber orientation. Cell Signal. 2006;18:1924–1931. doi: 10.1016/j.cellsig.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 168.Xu D, Kishi H, Kawamichi H, et al. Involvement of Fyn tyrosine kinase in actin stress fiber formation in fibroblasts. FEBS Lett. 2007;581:5227–5233. doi: 10.1016/j.febslet.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 169.Boon RA, Leyen TA, Fontijn RD, et al. KLF2-induced actin shear fibers control both alignment to flow and JNK signaling in vascular endothelium. Blood. 2010;115:2533–2542. doi: 10.1182/blood-2009-06-228726. [DOI] [PubMed] [Google Scholar]
- 170.Sun X, Jiao X, Ma Y, et al. Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome. Biochem Biophys Res Commun. 2016;481:63–70. doi: 10.1016/j.bbrc.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 171.Park H-Y, Kwon HM, Lim HJ, et al. Potential role of leptin in angiogenesis: leptin induces endothelial cell proliferation and expression of matrix metalloproteinases in vivo and in vitro. Exp Mol Med. 2001;33:95–102. doi: 10.1038/emm.2001.17. [DOI] [PubMed] [Google Scholar]
- 172.Azuma K, Watada H, Niihashi M, et al. A new En face method is useful to quantitate endothelial damage in vivo. Biochem Biophys Res Commun. 2003;309:384–390. doi: 10.1016/j.bbrc.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 173.Caplan BA, Schwartz CJ. Increased endothelial cell turnover in areas of in vivo Evans Blue uptake in the pig aorta. Atherosclerosis. 1973;17:401–417. doi: 10.1016/0021-9150(73)90031-2. [DOI] [PubMed] [Google Scholar]
- 174.Dunn LL, Buckle AM, Cooke JP, et al. The emerging role of the thioredoxin system in angiogenesis. Arterioscler Thromb Vasc Biol. 2010;30:2089–2098. doi: 10.1161/ATVBAHA.110.209643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Piao Z, Yoon S, Kim M, et al. VDUP1 potentiates Ras-mediated angiogenesis via ROS production in endothelial cells. Cellular and molecular biology (Noisy-le-Grand, France) 2009;55:OL1096–103. [PubMed] [Google Scholar]
- 176.Abdelsaid MA, Matragoon S, El-Remessy AB. Thioredoxin-interacting protein expression is required for VEGF-mediated angiogenic signal in endothelial cells. Antioxid Redox Signal. 2013;19:2199–2212. doi: 10.1089/ars.2012.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Spindel ON, Yan C, Berk BC. Thioredoxin-interacting protein mediates nuclear–to–plasma membrane communication. Arterioscler Thromb Vasc Biol. 2012;32:1264–1270. doi: 10.1161/ATVBAHA.111.244681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Dunn L, Simpson P, Lecce L, et al. TXNIP knockdown rescues diabetes-related impairment in endothelial progenitor cell-mediated angiogenesis. Am Heart Assoc 2012 [Google Scholar]
- 179.Buckle A, Dunn L, Ng MK. Hyperglycaemia inhibits thioredoxin-mediated angiogenesis: implications for impairment of neovascularisation in diabetes mellitus. Heart Lung Circ. 2007;16:S214–S215. [Google Scholar]
- 180.Dunn LL, Simpson PJ, Prosser HC, et al. A critical role for thioredoxin-interacting protein in diabetes-related impairment of angiogenesis. Diabetes. 2014;63:675–687. doi: 10.2337/db13-0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Han SH, Jeon JH, Ju HR, et al. VDUP1 upregulated by TGF-[beta] 1 and 1, 25-dihydorxyvitamin D3 inhibits tumor cell growth by blocking cell-cycle progression. Oncogene. 2003;22:4035. doi: 10.1038/sj.onc.1206610. [DOI] [PubMed] [Google Scholar]
- 182.Kallay E, Pietschmann P, Toyokuni S, et al. Characterization of a vitamin D receptor knockout mouse as a model of colorectal hyperproliferation and DNA damage. Carcinogenesis. 2001;22:1429–1435. doi: 10.1093/carcin/22.9.1429. [DOI] [PubMed] [Google Scholar]
- 183.Zinser GM, Suckow M, Welsh J. Vitamin D receptor (VDR) ablation alters carcinogen-induced tumorigenesis in mammary gland, epidermis and lymphoid tissues. J Steroid Biochem Mol Biol. 2005;97:153–164. doi: 10.1016/j.jsbmb.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 184.Maleszewska M, Vanchin B, Harmsen MC, et al. The decrease in histone methyltransferase EZH2 in response to fluid shear stress alters endothelial gene expression and promotes quiescence. Angiogenesis. 2016;19:9–24. doi: 10.1007/s10456-015-9485-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Candelario-Jalil E, Mhadu NH, Al-Dalain SM, et al. Time course of oxidative damage in different brain regions following transient cerebral ischemia in gerbils. Neurosci Res. 2001;41:233–241. doi: 10.1016/s0168-0102(01)00282-6. [DOI] [PubMed] [Google Scholar]
- 186.Wang X, Li R, Wang X, et al. Umbelliferone ameliorates cerebral ischemia–reperfusion injury via upregulating the PPAR gamma expression and suppressing TXNIP/NLRP3 inflammasome. Neurosci Lett. 2015;600:182–187. doi: 10.1016/j.neulet.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 187.Baker AF, Koh MY, Williams RR, et al. Identification of thioredoxin-interacting protein 1 as a hypoxia-inducible factor 1α-induced gene in pancreatic cancer. Pancreas. 2008;36:178–186. doi: 10.1097/MPA.0b013e31815929fe. [DOI] [PubMed] [Google Scholar]
- 188.Guo Z-N, Xu L, Hu Q, et al. Hyperbaric oxygen preconditioning attenuates hemorrhagic transformation through reactive oxygen species/thioredoxin-interacting protein/Nod-like receptor protein 3 pathway in hyperglycemic middle cerebral artery occlusion rats. Crit Care Med. 2016;44:e403–e411. doi: 10.1097/CCM.0000000000001468. [DOI] [PubMed] [Google Scholar]
- 189.Zhao Q, Che X, Zhang H, et al. Thioredoxin-interacting protein links endoplasmic reticulum stress to inflammatory brain injury and apoptosis after subarachnoid haemorrhage. J Neuroinflammation. 2017;14:104. doi: 10.1186/s12974-017-0878-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sheth SS, Bodnar JS, Ghazalpour A, et al. Hepatocellular carcinoma in Txnip-deficient mice. Oncogene. 2006;25:3528–3536. doi: 10.1038/sj.onc.1209394. [DOI] [PubMed] [Google Scholar]