ABSTRACT
Little is known about the expression and function of Retinoic acid-related orphan receptors (RORA, B, and C) in pancreatic β cells. Here in, we utilized cDNA microarray and RNA sequencing approaches to investigate the expression pattern of ROR receptors in normal and diabetic human pancreatic islets. Possible correlations between RORs expression and HbA1c levels as well as insulin secretory capacity in isolated human islets were evaluated. The impact of RORB and RORC expression on insulin secretion in INS-1 (832/13) cells was validated as well. While RORA was the highest expressed gene among the three RORs in human islet cells, RORC was the highest expressed in INS-1 cells (832/13) and while RORB was the lowest expressed gene in human islet cells, RORA was the highest expressed in INS-1 cells (832/13). The expression of RORB and RORC was significantly lower in diabetic/hyperglycemic donors as compared with non-diabetic counterparts. Furthermore, while the expression of RORB correlated positively with insulin secretion and negatively with HbA1c, that of RORC correlated negatively with HbA1c. The expression pattern of RORA did not correlate with either of the two parameters. siRNA silencing of RORB or RORC in INS-1 (832/13) cells resulted in a significant downregulation of insulin mRNA expression and insulin secretion. These findings suggest that RORB and RORC are part of the molecular cascade that regulates insulin secretion in pancreatic β cells; and insight that provides for further work on the potential therapeutic utility of RORB and RORC genes in β cell dysfunction in type 2 diabetes.
KEYWORDS: Diabetes, insulin secretion, INS-1 (832/13), gene expression microarray, human islets, Retinoic acid-related orphan receptor
1. Introduction
Diabetes mellitus (DM is a global health crisis that leads to significant morbidity and mortality and associates with increased economic burden. Based on epidemiological data compiled by the International Diabetes Federation (IDF), the expected number of diabetics will reach 460 million by 2040; most of whom (90%) with type 2 diabetes (T2D). The disease is characterized by increased blood glucose (hyperglycemia) resulting from impaired insulin secretion, insulin action or both.1 Hence, elucidating the molecular cascade that regulates insulin expression and secretion and factors affect this process should provide for a better understanding of the pathophysiology of DM. Vitamin A and its metabolites (Retinoic acids) are crucial for embryonic cell differentiation and early pancreatic β cell determination and maturation.2,3 Retinoic-acid-receptor-related orphan receptors belong to the nuclear receptor superfamily and are comprised of three members ROR alpha (RORA), ROR beta (RORB) and ROR gamma (RORC). RORs modulate gene expression by binding as a monomer to ROR response elements (ROREs) in the promoters of target genes.4 All three ROR genes exhibit significant sequence similarities and each is known to have multiple isoforms.5–7 While RORA is expressed by multiple tissues including skeletal muscles, liver, purkinje cells, thymus, kidney and adipose tissue among others8,9 the expression of RORB is limited to the central nervous system, retina, and pineal gland.10 The expression pattern of RORC is similar to that of RORA except that it is highly expressed in the thymus.11
Previous reports have shown that both RORA and RORC regulate the expression of genes important to lipid/glucose homeostasis, hence their potential involvement in metabolic syndrome, insulin resistance and inflammation.12–14 RORA gene deficiency was reported to protect against diet-induced obesity and insulin resistance in mice.14 Moreover, RORA was identified as a T2D susceptibility locus in Mexican Americans and Han Chinese.15,16 RORB was shown to play a critical role in retinal progenitor cell proliferation and differentiation.17 Recently, it has been suggested that RORB is an important regulator of osteogenesis.18 The expression pattern of RORC was shown to be predictive of adipocyte size in obese subjects and hence a likely player in the modulation of obesity-associated insulin resistance.19 Collectively, RORs appear to have tissue-specific distribution and play an important role in the pathophysiology of diabetes. However, little is known about the expression patterns of RORs in human pancreatic tissue and their possible involvement in regulating insulin gene expression and/or insulin protein secretion. In this study, we used cDNA microarray and RNA-seq gene expression data to investigate the expression patterns of RORs in human pancreatic islets and to assess as to whether their expression correlates with such metabolic phenotypes such as glucose-stimulated insulin secretion and HbA1c levels. Furthermore, the impact of ROR receptor expression on insulin mRNA expression and insulin secretion was functionally validated by a series of gene silencing experiments in INS-1 (832/13) cells.
2. Materials and methods
2.1. Human pancreatic islets
Preparation of human pancreatic islets and insulin secretion was done as previously described.20 Briefly, were obtained from 67 non-diabetic donors (30 females, 37 males, age 59 ± 10, BMI 25.9 ± 3.5 and HbA1c 5.5 ± 1.1) and 10 T2D donors (4 females, 6 males, age 60.7 ± 12, BMI 28.1 ± 4.5 and HbA1c 7.1 ± 1.2). Islets were cultured in CMRL 1066 (ICN Biomedicals, Costa Mesa, CA, USA). For insulin secretion, islets were hand-picked under a stereomicroscope and incubated for 30 min in Krebs Ringer Bicarbonate (KRB) buffer (pH 7.4) containing (in mM) 120 NaCl, 25 NaHCo3, 4.7 KCl, 1.2 MgSO4, 2.5 CaCl2, 1.2 KH2PO, 10 HEPES supplemented with 0.1% bovine serum albumin, N-2 hydroxyethylpiperazine-N’-2-ethanesulfonic acid (10 mmol/1) and 1 mmol/l glucose at 37°C (12 islets/vial containg 1 ml KRB buffer). To obtain constant pH and oxygenation, each vial containing islets was gased with 95% O2-5% CO2. The buffer was changed to a KRB buffer containing either 1 mM (basal secretion) or 16.7 68 mM glucose (stimulated secretion). Islets were then incubated for 1h at 37°C in a metabolic shaker. An aliquot of the medium was removed for analysis of insulin using a radioimmunoassay kit (EuroDiagnostica, Malmö, Sweden).
2.2. Microarray gene expression
The microarrays (GeneChip Human Gene 1.0 ST) and (GeneChip Rat 2.0 ST) were performed using the Affymetrix standard protocol as previously described.20 The array data were summarized and normalized with robust multiarray analysis (RMA) method. All human islets data are MIAME compliant, and the raw data have been deposited in a MIAME database (GEO, accession number: GSE 50398 and GSE 50397).
2.3. Expression of ROR receptors in human metabolic tissues as analyzed by RNA-sequencing
RNA-sequencing sample preparation was performed using Illumina’s TruSeq RNA Sample Preparation Kit. Resulting libraries were quality-checked on a 2200 Tape-station (Agilent Technologies) before combining 6 samples into one pool for sequencing on one lane on Flow cell sequencing on a HiSeq 2000 (Illumina). The output reads were aligned to the human reference genome (hg19) with STAR.21 Raw data was normalized using trimmed mean of M-values and presented as Fragments/Kilobase of Exon Per Million Fragments Mapped (FPKM) or transformed into log2 counts per million using the voom-function (edgeR/limma R-packages).
2.4. Culturing of INS-1 cell line
INS-1 (832/13) cells were cultured in RPMI 1640 medium (Gibco) supplemented with 11.1 mM D-glucose, 10% fetal bovine serum (Sigma), 5 ml penicillin/streptomycin (1000 µ/10 mg/ml; Sigma), 5.6 ml Hepes (1 M, Sigma), 2 mML-glutamine (200 mM, Sigma), 1 mM sodium pyruvate (Sigma) and 50 µM ß-mercaptoethanol (14.3 M, Sigma).
2.5. RNA interference and insulin secretion assay
A day before transfection, 250,000 INS-1 (832/13) cells/well were seeded in a 24-well plate with 1 mL/well of complete RPMI 1640 medium without antibiotics. After reaching ≈ 60% confluence, cells were transfected with siRNA for RORB (siRNA ID: S159636) and RORC (siRNA ID: S173516) using a mixture of lipofectamine 3000 transfection reagent (Thermo Fisher Scientific). A previously described negative control sequence from Ambion was employed at a final concentration of 40 nM siRNA and 1 µL of lipofectamine 3000 transfection reagent in 1 ml Opti-MEM media.22 Insulin secretion assay was performed 72 hr post-transfection. Cells were washed with 1 ml pre-warmed Secretion Assay Buffer (SAB), pH 7.2 (114 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 1.16 mMMgSO4, 20 mM HEPES, 2.5 mM CaCl2, 25.5 mM NaHCO3 and 0.2% bovine serum albumin) containing 2.8 mM glucose. Cells were then pre-incubated for 2 hours in new 2 ml SAB with 2.8 mM glucose. Subsequently, cells were stimulated for 1 hour by incubation in 1 ml SAB containing either 2.8 mM or 16.7 mM glucose. Secreted insulin was determined using rat insulin ELISA kit (Elabscience, China) according to manufacturer’s instructions.
2.6. Quantitative RT-qPCR
Total RNA extraction from transfected INS-1 cells was performed 48 hr post-transfection using RNeasy Plus Mini Kit 50 (Qiagen, Hilden, Germany). Quality and quantity of extracted RNA was assessed using NanoDrop ND-1000 Spectrophotometer. cDNA synthesis was performed using revertaid H minus first strand cDNA synthesis kit (Thermo Fisher Scientific). Knockdown efficiency was assessed by qPCR using TaqMan gene expression assay; RORB (Rn01451215_m1), RORC (Rn1533719_m1), INS1 (Rn02121433_g1) and INS2 (Rn01774648_g1). All reactions were performed in triplicate in 48-well plate step-one real-time PCR system (Applied Biosystems, Foster City, CA, USA). Rat HPRT1 (Rn01527840_m1) primer assay was used as endogenous control for normalizing expression of target mRNA. Relative gene expression was performed using 2− ΔΔCt method.
2.7. Apoptosis and proliferation analysis
For apoptosis analysis, 24 hr post-transfection cells were cultured with or without a cockatiel of pro-apoptotic cytokines ((IL-1β, 100 ng/ml; TNFα, 125 ng/ml; and INFγ, 125 ng/ml) for 24 hr in RMPI 1640 complete medium. Cells were then re-suspended in 500 µl of Annexin-V (1X) Binding Buffer (BD, USA). A 5 µl of Annexin V-FITC and 5 µl Propidium Iodide were incubated at room temperature for 10 min in the dark and then analyzed by Flow cytometry (FACSAria III, BD). Cell proliferation was measured using the CFSE labeling assay as previously described.23 Briefly, cells were washed with PBS and incubated with 1 μM CFSE dye (Ebioscience) for 10 min. Cells were then subjected to siRNA transfection as explained above and analyzed by flow cytometry (FACSCalibur; BD Biosciences) after 24 or 48 hr.
2.8. MTT assay
MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide colorimetric assay (Sigma-Aldrich) was used as a colorimetric assay to assess cell proliferation/viability. Forty-eight hours after siRNA silencing, 20,000 transfected cells were seeded in 0.2 ml culture medium in 96 well plate and cultured for 24 hr. A 10 uL of MTT solution was added to each well and incubated at 37°C for 120 minutes. MTT formzan product was dissolved in DMSO and absorbance was read at 570 nm on a microplate reader. Percentage cell viability was calculated based on average 570 nm absorbance values using the formula: % cell viability = (OD 570 nm of sample/OD 570 nm of control) x 100.
2.9. Statistical analysis
Differences in expression levels were analyzed by the student test or the nonparametric Mann-Whitney test. Correlation was done between variables was assessed using the nonparametric Spearman’s test. All statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) version 23.0 software (SPSS, Chicago, IL, USA).
3. Results
1. Expression analysis of RORs in human pancreatic islets and INS-1 cells
The expression of ROR genes in the pancreatic islets of 67 non-diabetic and 10 diabetic cadaveric human donors were analyzed using Human Gene 1.0 ST expression arrays. At first, we calculated mean values of the negative control probe-sets to estimate the background control signal on the array. As showed in Figure 1(a), expression values of all ROR genes were above the background control value (P < 0.001). Both of RORA and RORC genes showed higher levels of expression as compared with that of RORB. To further confirm the expression of RORs, we analyzed RNA sequencing (RNA-seq) data from human pancreatic islets (n = 162). RNA-seq showed expression patters similar to those observed in the microarray data. RORA was the highest expressed gene (top 43th percentile of genes expressed) (Figure 1(d)) followed by RORC (45th percentile) (Figure 1(e)). Both RORA and RORC showed expression levels comparable to those of the ion channel gene KCNJ11, a marker of high expression of functional gene in human islets.24 RORB receptor mRNA showed low expression (86th percentile) (Figure 1(f)) as compared with that of RORA and RORC. Additionally, expression of RORs in metabolic tissues (adipose tissue, islets, liver and muscle) obtained from cadaveric human organ donors showed higher levels of expression of RORB in pancreatic islets as compared with that in other tissues (Figure 1(h)). However, expression levels of RORA and RORC were higher in liver and muscles tissues as compared with that of pancreatic islets (Figure 1(g–i)). Contrasting expression patterns were noted between human islets and the clonal rat (INS-1, 832/13) β cells. While RORA was the highest expressed gene among the three RORs in human islet cells, RORC was the highest expressed in INS-1 cells (832/13) and while RORB was the lowest expressed gene in human islet cells, RORA was the highest expressed in INS-1 cells (832/13) (Figure 1(a–b)). As RORC transcript was not available in the rat 1.0 ST array, we analyzed its expression by qPCR. As shown in Figure 1(c), the expression of RORC was very high in INS-1 (832/13) cells as compared with that of RORB (p = 0.0001).
2. Differential expression analysis of RORs in diabetic islets
Figure 1.
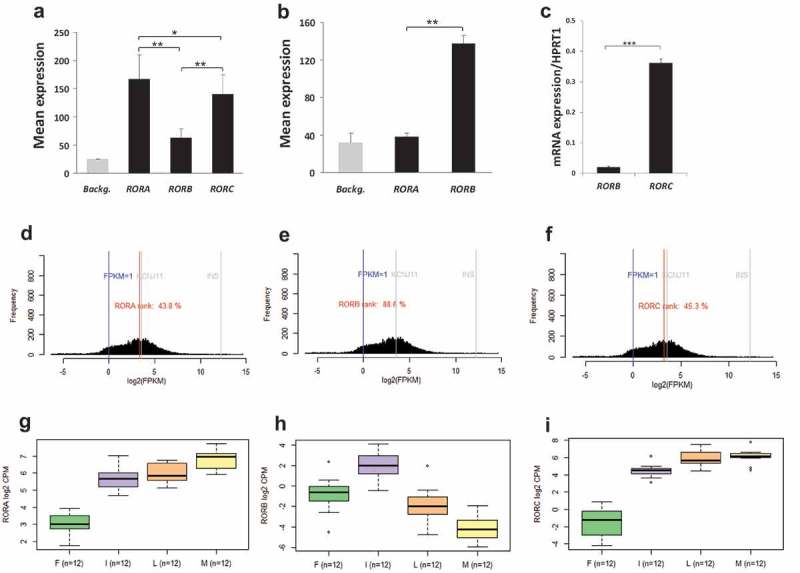
Expression profile of ROR genes in human pancreatic islets and rat INS-1 (832/13) cells: Microarray gene expression profiles of RORs in human pancreatic islets (n = 67) (a) and rat INS-1 (832/13) cells (n = 3) (b). Background signals were estimated as the mean of all negative control probe-sets on Human Gene 1.0 ST (∼2900) or Rat 2.0 ST array (∼2900). (c) RT-PCR analysis of RORB and RORC in INS-1 (832/13) cells. RNA-seq expression data from normal human islets (n = 162) showing a histogram of RORA-transcript (d), RORB-transcript (e) and RORC-transcript (f) expression frequency (FPKMs) compared to KCNJ11 as expression marker (g, h and i). Expression profile of ROR transcripts in human adipose tissue (F) (n = 14), pancreatic islets (I) (n = 15), liver (L) (n = 13) and skeletal muscle tissues (M) (n = 13) as revealed by RNA-seq. Bars represent mean ± SEM; * denotes P<0.05 and ** denotes P<0.01.
Here we addressed the question of whether ROR genes are differentially expressed in human islets. Pancreatic donors were stratified into diabetics vs. non-diabetics (based on independent clinical diagnosis) and normoglycemics (HbA1c < 6%) vs. hyperglycemics (HbA1C ≥ 6%). The expression of RORB (p = 0.03) and RORC (p = 0.01) was significantly lower in diabetic donors as compared with non-diabetic counterparts (Figure 2(a)). No significant differences were observed in the level of RORA between the two groups (p = 0.09). Furthermore, expression of both RORB (p = 0.04) and RORC (p = 0.003) was significantly lower in hyperglycemic donors as compared with that in normoglycemics (Figure 2(b)). Although the expression RORA was lower in hyperglycemic donors as compared with that in normoglycemic counterparts, the difference was statistically insignificant (p = 0.06). Furthermore, RORB expression correlated positively (r2 = 0.3; p = 0.02) with insulin secretion and negatively (r2 = – 0.34; p = 0.006) with HbA1c (Figure 2(d)). Expression of RORC correlated only negatively (r2 = – 0.37; p = 0.003) with HbA1c. No correlation of any type was discernable between RORC expression and insulin secretion (r2 = 0.2; p = 0.08) (Figure 2(c)) or between RORA expression and insulin secretion or HbA1c (Figure 2(e)).
Figure 2.
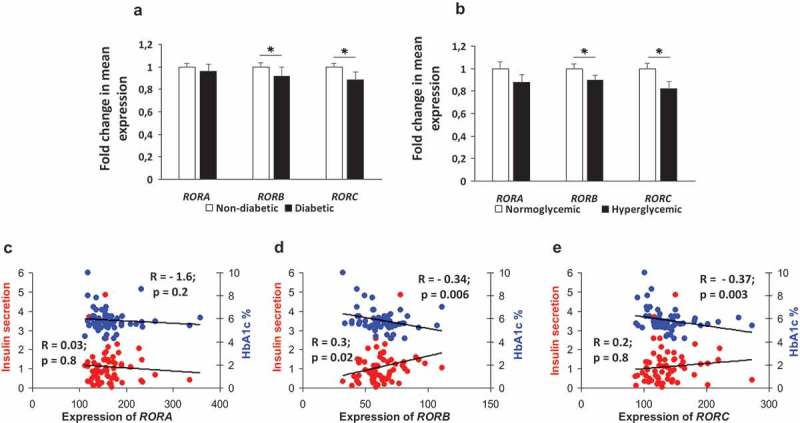
Expression profile of ROR genes in diabetics versus non-diabetics and hyperglycemics versus normo-glycemics. Fold change in mean expression of ROR genes in diabetic (n = 10) versus nondiabetic (n = 67) donors (a). RORB and RORC showed a significant expression reduction. Fold changes in mean expression genes in 20 donors with hyperglycemia versus 30 donors with normoglycemia (b). RORB and RORC showed a significant expression reduction. Correlation of expression of RORA (c), RORB (d) and RORC (e) with stimulated insulin secretion (ng/islet/hr) measured at 16.7 mM glucose (n= 65) and HbA1c levels (n = 64). Bars represent mean ± SEM, * denotes P<0.05.
3.3. Functional validation of RORB and RORC association with insulin secretion in INS-1 (832/13) cells
To explore the influence of ROR receptors on glucose-stimulated insulin secretion, we used RNA interference (siRNA) to silence the expression RORB and RORC in INS-1 (832/13) cells. RORA was excluded as it has been implicated previously in insulin secretion.25 Knockdown efficiency as measured by qRT-PCR after 48 hr showed a 64% and 66% drop in RORB and RORC expression respectively (Figure 3(a)). Expression silencing of RORB or RORC resulted in a significant decrease in glucose-stimulated insulin secretion after 1 h of incubation with 16.7 mM glucose (p = 0.01) (Figure 3(b–c)). However, no significant change was observed at basal (2.8 mM) glucose-stimulated insulin secretion (Figure 3(b–c)). As shown in Figure 3(d), percentage apoptosis in transfected cells cultured in the absence of cytokines was 3% while that in the presence of cytokines was 75%. No significant difference in apoptotic potential was observed in RORB or RORC knockdown cells as compared with siRNA negative controls. This was further confirmed by MTT cell viability and cell proliferation data. RORB and RORC knockdown cells showed no reduction in cell vialibity as compared with negative siRNA controls (Figure 3(e)). Furthermore, no differences in the proliferative response were observed between RORB or RORC knockdown cells as compared with siRNA negative controls at 24 or 48 hr post culture (Figure 3(f)).
Figure 3.
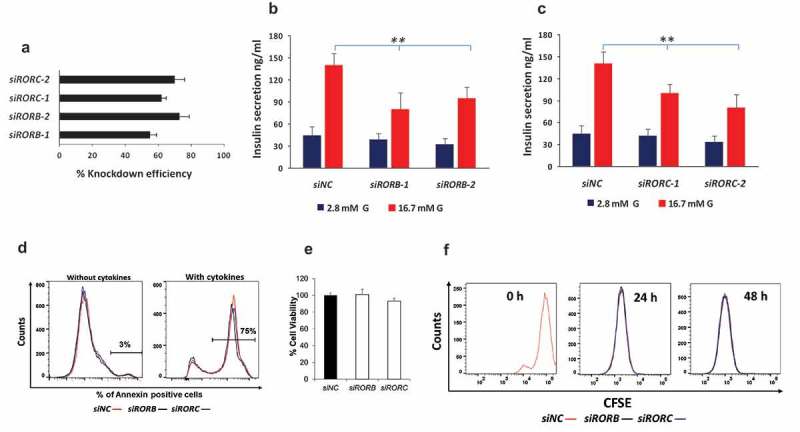
Functional competence of INS-1 (832/13) cells following siRNA silencing of RORB and RORC genes. Knockdown efficiency of siRNA of RORB and RORC in INS-1 (832/13) cells (a). Insulin secretion in response to 2.8 mM glucose and 16.7 mM glucose 72 hr following siRNA silencing of RORB (b) and RORC (c) at 1 hr static incubation; data shown is representative of three independent experiments for each gene. Apoptotic potential of INS-1 cells transfected with RORB or RORC siRNA and as assayed in the presence or absence of cytokine stimulation (d). Percentage cell viability in siRNA RORB- and RORC-silenced INS-1 cells as determined by MTT assay (e). Proliferative potential of siRNA RORB- and RORC-silenced INS-1 cells as determined by CFSE staining (f). Bars represent mean ± SD, * denotes P<0.01; siNC: siRNA negative control.
To explore whether RORs are involved in insulin exocytosis machinery, insulin secretion was measured in ROR gene knockdown cells stimulated with 35 mM KCl (depolarizing agent. Insulin secretion in RORC knockdown cells showed a significant reduction prior to KCL stimulation as compared with siRNA negative controls (P < 0.05) (Figure 4(b)). RORB knockdown cells showed no significant difference in insulin secretion between transfected cells and controls (Figure 4(a)). However, qRT-PCR analysis showed a significant decrease of Ins1 and Ins2 (P < 0.05) mRNA expression in transfected cells as compared with negative siRNA controls (Figure 4(c–d)). No significant effect of RORB or RORC silencing on PDX1 was observed (Figure 4€) suggesting that RORB or RORC regulate Ins1 and Ins2 genes in an indirect manner.
Figure 4.
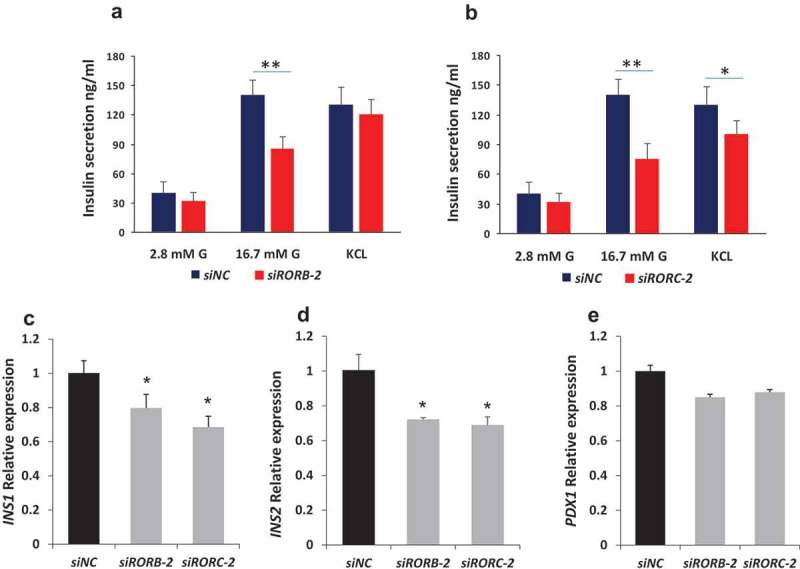
Effect of RORB and RORC silencing on exocytosis machinery and insulin expression. Cells transfected with siRNA against RORB-2 and RORC-2 were stimulated by a depolarizing agent (35 mM KCL) and insulin was measured by ELISA (a, b). Only RORB showed a significant difference between transfected and control cells. qPCR expression analysis showing a significant downregulation of insulin mRNA gene ins1 (c) and ins2 (d) in RORB and RORC knockdown cells. qPCR expression analysis of PDX1 in RORB and RORC knockdown cells (e). Bars represent mean ± SD; * denotes P<0.05* and ** denotes P<0.01; siNC: siRNA negative control.
3.4. Expression correlation between RORs and GPRC5C
G-protein coupled receptor C5C (GPRC5C) has recently been shown to trigger all-trans retinoic acid (ATRA)-mediated signals that are criti-cal for pancreatic β cells function and proliferation.26 In this context, our data show that RORC, but not RORA or RORB, positively correlate (r2 = 0.38; p = 0.001) with GPRC5C (Figure 5(a)). Additionally, GPRC5C expression was significantly down-regulated (19%) in RORC, but not in RORB, knockdown cells (Figure 5(b)).
Figure 5.
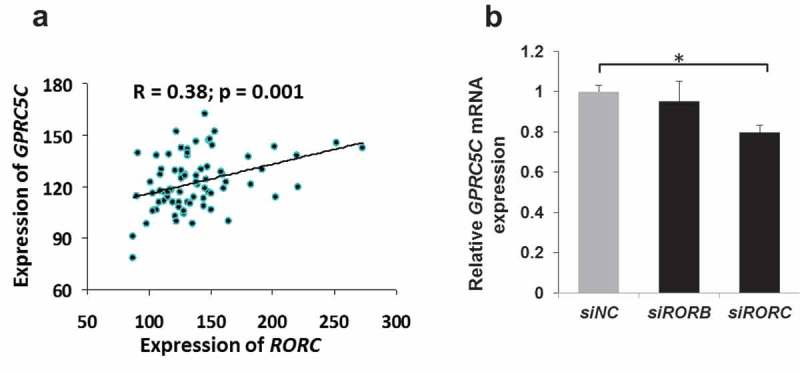
Co-expression of GPRC5C with RORC in human pancreatic islets and rat INS-1 cells. Co-expression analysis of GPRC5C with RORC in human pancreatic islets (n = 77) (a). RT-PCR expression analysis of GPRC5C following siRNA silencing of RORB or ROBC genes in INS-1 cells (b). Bars represent mean ± SEM, ∗ denotes P<0.05; siNC: siRNA negative control.
4. Discussion
In this study, we profiled the expression of ROR receptors in human pancreatic islets and INS-1 (832/13) rat β cells. The data demonstrates that the expression of RORB and RORC is reduced in diabetic/hyperglycemic human islets and that it inversely correlates with HbA1c levels. Silencing of RORB or RORC genes in rat INS-1 cells resulted in reduced expression of Ins1 and Ins2 genes at the mRNA level and hence reduced insulin secretion.
ROR receptors play a critical role in the regulation of various metabolic pathways, circadian rhythm, immunity and embryonic development among other physiological processes.27 RORA was shown to modulate the expression and secretion of the FGF21 hormone, which regulates peripheral glucose tolerance and hepatic lipid metabolism.28 RORA was also shown to modulate the expression of several genes important in glucose and lipid metabolism including glucose 6-phosphatase and apolipoprotein A1, A5 and C3 genes.29–31 Overexpression of RORA in rat INS-1 cells was reported to associate with increased expression of Ins1/Ins2 mRNA and insulin secretion25 perhaps by directly binding to insulin gene promoter and/or influencing the activity of β-cell enriched transcription factors like BETA2.25 These observations notwithstanding, no discernable differences in expression of RORA were observed between diabetics or hyperglycemics on one hand and control donors on the other. Furthermore, the expression of RORA did not correlate with insulin secretion or HbA1c levels. Although RORA was found to be highly expressed in human islets and more so in muscle tissue its expression in rat INS-1 cells was significantly reduced. This is consistent with Munhlbauer et al who documented its lack of expression in INS-1 cells and preferential expression in pancreatic α-cells.32
Previous reports have suggested that the expression of RORB is mostly restricted to the central nervous system, retina, and pineal gland.5 Recently however, it has been shown that RORB is differentially expressed in bone, pancreatic and endometrial cancer tissues.33,34 Our findings show that although the expression of RORB is relatively lower than that of RORA or RORC, human islets expressed the receptor at much higher levels as compared with that in liver, muscle or fat tissue. RORB expression was low in diabetic/hyperglycemic islets and correlated positively with insulin secretion and negatively with HbA1c levels. siRNA silencing of RORB in INS-1 further demonstrate a possible involvement of RORB in pancreatic β cell function. This is consistent with previous reports which have shown that RORB suppress the Wnt pathway by enhancing the transcription of HMG family transcription factor HBP1.35 Wnt signaling is known to be involved in β cell proliferation and insulin secretion.36–39 The possibility that RORB might affect β cell function by modulating Wnt activity is speculative and requires further investigation.
The 2 isoforms of RORC (1 and 2) are expressed in a tissue-specific manner. RORC (RORC1 in particular) has been shown capable of regulating obesity-associated insulin resistance and modulating the transcription of genes involved in lipid and carbohydrate metabolism.40–42 Recently, RORC has been shown to be expressed in insulin-producing pancreatic β cells in rodents.32 The current study demonstrates high expression of RORC in human pancreatic islets and rat INS-1 cells alike. More importantly though was the finding that RORC expression is reduced in diabetic islets and that it correlates positively with insulin secretion and inversely with HbA1c levels. This suggested that RORC may functionally impact β cells; a possibility that was confirmed by the observation that disrupted RORC expression in INS-1 cells associates with reduced insulin mRNA expression and attenuated glucose-stimulated insulin secretion. This is in contrast with previous reports which have shown that mice lacking RORC exhibit improved insulin sensitivity and glucose tolerance on a high-fat diet.19,43 However, such effects of RORC in mice were observed in adipose and liver tissue but not pancreatic β cells. Our findings are further corroborated by the recent observation that RORC is required for glucose-induced proliferation in INS-1E and β cells in primary rat islets and hence its positive effect on β cell adaptive responsiveness to metabolic stress.44
Lastly, it is worth noting that pancreatic islets express a wide range of receptors, many of which are still designated as orphan receptors.45,46 Consistent with previous reports, data presented here further implicates Retinoic acid in the induction of a wide range of receptors in pancreatic β cells with the action site distal to RORB and RORC.26,47 A possible limitation of our study relates to the utility of correlating ROR gene expression with insulin secretion or HbA1c levels in diabetic donors who are on DM medications that could normalize fasting blood glucose and glucose tolerance. Further work is needed to dissect the role of the RORB and RORC genes in pancreatic β cell function.
Funding Statement
JT is funded by a seed grant from AL-Jalila foundation (AJF201723), University of Sharjah (1701090119-P) and Boehringer Ingelheim.
Acknowledgments
Human pancreatic islets were obtained from The Nordic Network for Clinical Islet Transplantation. We wish to thank Manju Nidagodu Jayakumar for FCAS flow analysis.
Disclosure of potential conflicts of interest
The authors declare that there is no duality of interest associated with this article.
References
- 1.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martín M, Gallego-Llamas J, Ribes V, Kedinger M, Niederreither K, Chambon P, Dollé P, Gradwohl G.. Dorsal pancreas agenesis in retinoic acid-deficient Raldh2 mutant mice. Dev Biol. 2005;284(2):399–411. doi: 10.1016/j.ydbio.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 3.Molotkov A, Molotkova N, Duester G.. Retinoic acid generated by Raldh2 in mesoderm is required for mouse dorsal endodermal pancreas development. Dev Dyn. 2005;232(4):950–957. doi: 10.1002/dvdy.20256. [DOI] [PubMed] [Google Scholar]
- 4.Medvedev A, Yan Z-H, Hirose T, Giguère V, Jetten AM. Cloning of a cDNA encoding the murine orphan receptor RZR/RORγ and characterization of its response element. Gene. 1996;181(1):199–206. [DOI] [PubMed] [Google Scholar]
- 5.André E, Gawlas K, Steinmayr M, Becker-André M. A novel isoform of the orphan nuclear receptor RORβ is specifically expressed in pineal gland and retina. Gene. 1998;216(2):277–283. [DOI] [PubMed] [Google Scholar]
- 6.Giguere V, Tini M, Flock G, Ong E, Evans RM, Otulakowski G. Isoform-specific amino-terminal domains dictate DNA-binding properties of ROR alpha, a novel family of orphan hormone nuclear receptors. Genes Dev. 1994;8(5):538–553. [DOI] [PubMed] [Google Scholar]
- 7.He Y-W, Deftos ML, Ojala EW, Bevan MJ. RORγt, a novel isoform of an orphan receptor, negatively regulates Fas ligand expression and IL-2 production in T cells. Immunity. 1998;9(6):797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamilton BA, Frankel WN, Kerrebrock AW, Hawkins TL, FitzHugh W, Kusumi K, Russell LB, Mueller KL, Van BV, Birren BW, et al. Disruption of the nuclear hormone receptor RORα in staggerer mice. Nature. 1996;379(6567):736–739. [DOI] [PubMed] [Google Scholar]
- 9.Steinmayr M, André E, Conquet F, Rondi-Reig L, Delhaye-Bouchaud N, Auclair N, Daniel H, Crépel F, Mariani J, Sotelo C, et al. Staggerer phenotype in retinoid-related orphan receptor α-deficient mice. Proc National Acad Sci. 1998;95(7):3960–3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andre E, Conquet F, Steinmayr M, Stratton SC, Porciatti V, Becker-André M. Disruption of retinoid‐related orphan receptor β changes circadian behavior, causes retinal degeneration and leads to vacillans phenotype in mice. Embo J. 1998;17(14):3867–3877. doi: 10.1093/emboj/17.14.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126(6):1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 12.Lau P, Fitzsimmons RL, Pearen MA, Watt MJ, Muscat GEO. Homozygous staggerer (sg/sg) mice display improved insulin sensitivity and enhanced glucose uptake in skeletal muscle. Diabetologia. 2011;54(5):1169–1180. doi: 10.1007/s00125-011-2046-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fitzsimmons RL, Lau P, Muscat GE. Retinoid-related orphan receptor alpha and the regulation of lipid homeostasis. J Steroid Biochem Mol Biol. 2012;130(3):159–168. doi: 10.1016/j.jsbmb.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Jetten AM, Kang HS, Takeda Y. Retinoic acid-related orphan receptors α and γ: key regulators of lipid/glucose metabolism, inflammation, and insulin sensitivity. Front Endocrinol (Lausanne). 2013;4(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayes MG, Pluzhnikov A, Miyake K, Sun Y, Ng MCY, Roe CA, Below JE, Nicolae RI, Konkashbaev A, Bell GI, et al. Identification of type 2 diabetes genes in Mexican Americans through genome-wide association studies. Diabetes. 2007;56(12):3033–3044. doi: 10.2337/db07-0482. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Liu Y, Liu Y, Zhang Y, Su Z. Genetic variants of retinoic acid receptor-related orphan receptor alpha determine susceptibility to type 2 diabetes mellitus in han chinese. Genes. 2016;7(8):54. doi: 10.3390/genes7080054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chow L, Levine EM, Reh TA. The nuclear receptor transcription factor, retinoid-related orphan receptor β, regulates retinal progenitor proliferation. Mech Dev. 1998;77:149–164. [DOI] [PubMed] [Google Scholar]
- 18.Roforth MM, Liu G, Khosla S, Monroe DG. Examination of nuclear receptor expression in osteoblasts reveals Rorβ as an important regulator of osteogenesis. J Bone Min Res. 2012;27(4):891–901. doi: 10.1002/jbmr.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meissburger B, Ukropec J, Roeder E, Beaton N, Geiger M, Teupser D, Civan B, Langhans W, Nawroth PP, Gasperikova D, et al. Adipogenesis and insulin sensitivity in obesity are regulated by retinoid‐related orphan receptor gamma. EMBO Mol Med. 2011;3(11):637–651. doi: 10.1002/emmm.201100172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taneera J, Lang S, Sharma A, Fadista J, Zhou Y, Ahlqvist E, Jonsson A, Lyssenko V, Vikman P, Hansson O, et al. A systems genetics approach identifies genes and pathways for type 2 diabetes in human islets. Cell Metab. 2012;16(1):122–134. doi: 10.1016/j.cmet.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25(9):1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Y, Oskolkov N, Shcherbina L, Ratti J, Kock K-H, Su J, Martin B, Oskolkova MZ, Göransson O, Bacon J, et al. HMGB1 binds to the rs7903146 locus in TCF7L2 in human pancreatic islets. Mol Cell Endocrinol. 2016;430:138–145. doi: 10.1016/j.mce.2016.01.027. [DOI] [PubMed] [Google Scholar]
- 23.Kang W, Nielsen O, Fenger C, Leslie G, Holmskov U, Reid KBM. Induction of DMBT1 expression by reduced ERK activity during a gastric mucosa differentiation-like process and its association with human gastric cancer. Carcinogenesis. 2005;26(6):1129–1137. doi: 10.1093/carcin/bgi045. [DOI] [PubMed] [Google Scholar]
- 24.Gloyn AL, Pearson ER, Antcliff JF, Proks P, Bruining GJ, Slingerland AS, Howard N, Srinivasan S, Silva JMCL, Molnes J, et al. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6. 2 and permanent neonatal diabetes. N Engl J Med. 2004;350(18):1838–1849. doi: 10.1056/NEJMoa032922. [DOI] [PubMed] [Google Scholar]
- 25.Kuang J, Hou X, Zhang J, Chen Y, Su Z. Identification of insulin as a novel retinoic acid receptor‐related orphan receptor α target gene. FEBS Lett. 2014;588(6):1071–1079. doi: 10.1016/j.febslet.2014.02.029. [DOI] [PubMed] [Google Scholar]
- 26.Amisten S, Mohammad Al-Amily I, Soni A, Hawkes R, Atanes P, Persaud SJ, Rorsman P, Salehi A. Anti-diabetic action of all-trans retinoic acid and the orphan G protein coupled receptor GPRC5C in pancreatic β-cells. Endocr J. 2017;64(3):325–338. doi: 10.1507/endocrj.EJ16-0338. [DOI] [PubMed] [Google Scholar]
- 27.Cook DN, Kang HS, Jetten AM. Retinoic acid-related orphan receptors (RORs): regulatory functions in immunity, development, circadian rhythm, and metabolism. Nucl Receptor Res. 2015;2. doi: 10.11131/2015/101185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Solt LA, Burris TP. Regulation of FGF21 expression and secretion by retinoic acid receptor-related orphan receptor α. J Biol Chem. 2010;285(21):15668–15673. doi: 10.1074/jbc.M110.102160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chauvet C, Vanhoutteghem A, Duhem C, Saint-Auret G, Bois-Joyeux B, Djian P, Staels B, Danan J-L, Laudet V. Control of gene expression by the retinoic acid-related orphan receptor alpha in HepG2 human hepatoma cells. PLoS One. 2011;6(7):e22545. doi: 10.1371/journal.pone.0022545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Genoux A, Dehondt H, Helleboid-Chapman A, Duhem C, Hum DW, Martin G, Pennacchio LA, Staels B, Fruchart-Najib J, Fruchart J-C. Transcriptional regulation of apolipoprotein A5 gene expression by the nuclear receptor RORα. Arterioscler Thromb Vasc Biol. 2005;25(6):1186–1192. doi: 10.1161/01.ATV.0000163841.85333.83. [DOI] [PubMed] [Google Scholar]
- 31.Vu-Dac N, Gervois P, Grötzinger T, De Vos P, Schoonjans K, Fruchart JC, Auwerx J, Mariani J, Tedgui A, Staels B. Transcriptional regulation of apolipoprotein AI gene expression by the nuclear receptor RORα. J Biol Chem. 1997;272:22401–22404. [DOI] [PubMed] [Google Scholar]
- 32.Mühlbauer E, Bazwinsky-Wutschke I, Wolgast S, Labucay K, Peschke E. Differential and day-time dependent expression of nuclear receptors RORα, RORβ, RORγ and RXRα in the rodent pancreas and islet. Mol Cell Endocrinol. 2013;365(2):129–138. doi: 10.1016/j.mce.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Risinger J, Allard J, Chandran U, Day R, Chandramouli GVR, Miller C, Zahn C, Oliver J, Litzi T, Marcus C, et al. Gene expression analysis of early stage endometrial cancers reveals unique transcripts associated with grade and histology but not depth of invasion. Front Oncol. 2013;3:139. doi: 10.3389/fonc.2013.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roforth MM, Khosla S, Monroe DG. Identification of Rorβ targets in cultured osteoblasts and in human bone. Biochem Biophys Res Commun. 2013;440(4):768–773. doi: 10.1016/j.bbrc.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wen Z, Pan T, Yang S, Liu J, Tao H, Zhao Y, Xu D, Shao W, Wu J, Liu X, et al. Up-regulated NRIP2 in colorectal cancer initiating cells modulates the Wnt pathway by targeting RORβ. Mol Cancer. 2017;16(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Papadopoulou S, Edlund H. Attenuated Wnt signaling perturbs pancreatic growth but not pancreatic function. Diabetes. 2005;54:2844–2851. [DOI] [PubMed] [Google Scholar]
- 37.Heiser PW, Lau J, Taketo MM, Herrera PL, Hebrok M. Stabilization of β-catenin impacts pancreas growth. Development. 2006;133(10):2023–2032. [DOI] [PubMed] [Google Scholar]
- 38.Fujino T, Asaba H, Kang MJ, Ikeda Y, Sone H, Takada S, Kim DH, Ioka RX, Ono M, Tomoyori H, et al. Low-density lipoprotein receptor-related protein 5 (LRP5) is essential for normal cholesterol metabolism and glucose-induced insulin secretion. Proc National Acad Sci. 2003;100(1):229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin T. Current understanding on role of the Wnt signaling pathway effector TCF7L2 in glucose homeostasis. Endocr Rev. 2016;37:254–277. [DOI] [PubMed] [Google Scholar]
- 40.Tinahones FJ, Moreno‐Santos I, Vendrell J, Chacon MR, Garrido‐Sanchez L, García‐Fuentes E, Macias‐González M. The retinoic acid receptor‐related orphan nuclear receptor γ1 (RORγ1): a novel player determinant of insulin sensitivity in morbid Obesity. Obesity. 2012;20(3):488–497. [DOI] [PubMed] [Google Scholar]
- 41.Takeda Y, Kang HS, Lih FB, Jiang H, Blaner WS, Jetten AM. Retinoid acid-related orphan receptor γ, RORγ, participates in diurnal transcriptional regulation of lipid metabolic genes. Nucleic Acids Res. 2014;42(16):10448–10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raichur S, Lau P, Staels B, Muscat GE. Retinoid-related orphan receptor γ regulates several genes that control metabolism in skeletal muscle cells: links to modulation of reactive oxygen species production. J Mol Endocrinol. 2007;39(1):29–44. [DOI] [PubMed] [Google Scholar]
- 43.Takeda Y, Kang HS, Freudenberg J, DeGraff LM, Jothi R, Jetten AM. Retinoic acid-related orphan receptor γ (RORγ): a novel participant in the diurnal regulation of hepatic gluconeogenesis and insulin sensitivity. PLoS Genet. 2014;10(5):e1004331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt SF, Madsen JG, Frafjord KØ, Poulsen Ll, Salö S, Boergesen M, Loft A, Larsen BD, Madsen MS, Holst JJ. Integrative genomics outlines a biphasic glucose response and a ChREBP-RORγ axis regulating proliferation in β cells. Cell Rep. 2016;16(9):2359–2372. [DOI] [PubMed] [Google Scholar]
- 45.Amisten S, Atanes P, Hawkes R, Ruz-Maldonado I, Liu B, Parandeh F, Zhao M, Huang GC, Salehi A, Persaud SJ. A comparative analysis of human and mouse islet G-protein coupled receptor expression. Sci Rep. 2017;7:46600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Atanes P, Ruz-Maldonado I, Hawkes R, Liu B, Zhao M, Huang GC, Al-Amily IM, Salehi A, Amisten S, Persaud SJ. Defining G protein-coupled receptor peptide ligand expressomes and signalomes in human and mouse islets. Cell Mol Life Sci. 2018:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soni A, Amisten S, Rorsman P, Salehi A. GPRC5B a putative glutamate-receptor candidate is negative modulator of insulin secretion. Biochem Biophys Res Commun. 2013;441(3):643–648. [PubMed] [Google Scholar]


