Abstract
Basic research on obesity is becoming more important due to an increasing number of obese people. Experiments using obesity-model animals often require surgical interventions, such as gastric operation, and proper selection of anesthesia is important. Avertin, an agent mainly composed of 2,2,2-Tribromoethanol, has been used as general anesthesia for a long time, without the use of narcotic drugs. In the current study, we found that a single injection of avertin can decrease body weight (BW) in male and female C57BL/6J and ICR mice with high fat-diet (HFD)-induced obesity, but not in standard diet-fed nonobese males and females. Because the BW-reducing effect was more prominent in the female mice, we compared the effects of avertin and a mixture of three types of anesthetic agents (3MIX), which was developed in 2011, on BW reduction in HFD-induced obese female mice. Although both avertin and 3MIX decreased food intake and BW, the effects of avertin were significantly more potent than those of 3MIX. C-Fos expression, a neural activation marker, was dramatically increased in the brain regions related to the regulation of both food intake and the autonomic nervous system after avertin injection, but not after 3MIX injection. This suggests that avertin strongly stimulates the center of feeding regulation and the autonomic nervous system and therefore decreases BW. The current study suggests the advantages of using 3MIX for surgical interventions in mice in obesity research, as it is ideal to prevent anesthesia-induced BW decline.
Keywords: avertin, high fat diet, mixture of three types of anesthetic agents, obesity
Introduction
Obesity has become a global epidemic [17], and elucidation of the mechanisms involved in obesity development is important. This has brought rise to an increase in basic experimentation using obese animal models.
There are numerous genetic obesity-model animals, such as Zucker fatty rats [24], ob/ob mice [23] and db/db mice [5], which are deficient in leptin or leptin receptors, and OLETF rats, which are deficient in cholecystokinin receptors [16]. However, high-fat diet (HFD)-induced obese mouse models are frequently used, since they can reach obesity easily without genetic mutation and their obese state is closer to that of humans, when compared with other models [11].
When performing experiments in HFD-induced obesity mouse models, surgical interventions, such as gastric operation and osmotic pump implantation, are often required [13, 14]. In such cases, it is important to select anesthetics that have less effect on body weight (BW) and food intake.
Avertin, which is mainly composed of tribromoethanol, induces depression of the respiratory and cardiovascular center in the central nervous system [15] and is commonly used as a general anesthetic, since it is easily made and is known to rapidly induce short-term anesthesia with rapid recovery [6]. Papaioannou and Fox [22] reported that tribromoethanol is an agent with a low mortality and morbidity rate (<1%), and no significant abdominal adhesions or inflammatory responses are experienced as a result. However, side effects, such as peritonitis, intestinal ileus, or serositis of the abdominal organs, and deaths are reported in mice [12]. In addition, repeated tribromoethanol injections are reported to lead to high mortality [15].
In 2011, a mixture of three types of anesthetic agents (3MIX) was developed in Japan [7]. 3MIX is composed of an α2 adrenaline receptor agonist (medetomidine), GABAA receptor agonist (midazolam), and opioid κ receptor agonist (butorphanol) [7]. When combined, these three agents suppress autonomic reflexes, consciousness, and pain, as well as induce muscle relaxant effects [7,8,9]. 3MIX is easily made, shows a rapid induction of short-term anesthesia, and also enables induction of prompt recovery through application of the antagonist of the α2 adrenaline receptor (atipamezole) [8, 9]. In the present study, we aimed to compare the effects of avertin and 3MIX on BW and food intake in an obese mouse model. We also aimed to identify the advantages of the use of 3MIX for experiments in obesity models with surgical intervention.
Materials and Methods
Animals
Male and female C57BL/6J and ICR mice aged six weeks were purchased from Japan SLC (Hamamatsu, Japan).
All animals in this study were housed in individual cages and were maintained on a 12-hour light/dark cycle with lights on at 7 AM. The mice were fed a HFD (HFD32, CLEA Japan, Osaka, Japan) or standard diet (CE7, CLEA Japan), which served as the control, for eight (males) or 12 weeks (females). Thus, 14-week-old male mice and 18-week-old female mice were used for the experiment, and the effects of avertin were compared.
For comparison of the effects of avertin and 3MIX, female C57BL/6J mice, aged six weeks, were purchased from Japan SLC and fed HFD for 14 weeks. Thus, 20-week-old female mice were used for this experiment. All animals in this study were housed in individual cages. All experimental procedures and animal care were carried out according to the relevant guidelines and regulations and were approved by the Fukushima Medical University Institute of Animal Care and Use Committee.
Preparing anesthetic agents
Avertin: 2,2,2-Tribromoethanol (2%, Sigma-Aldrich, St. Louis, MO, USA), 100% ethanol (8%, Wako Pure Chemical Industries Osaka, Japan), and 2-Methyl-2-butanol (1.2%, Wako Pure Chemical Industries) were dissolved in 0.9% sterile NaCl.
3MIX: Medetomidine (0.003%, Domitor, Nippon Zenyaku Kogyo Co., Ltd., Koriyama, Japan), midazolam (0.04%, Dormicum, Astellas Pharma Inc., Tokyo, Japan), and butorphanol tartrate (0.05%, Vetorphale, Meiji Seika Pharma Co., Ltd., Tokyo, Japan) were dissolved in 0.9% sterile NaCl.
Comparison of the effect of avertin on blood glucose, rectal temperature, food intake, and BW in male and female mice
HFD-fed and standard diet-fed male and female mice (C57BL/6J and ICR) were anesthetized by intraperitoneal (IP) injection of sterile avertin (tribromoethanol: 200 mg/10 ml/kg) at 13:00. This was the lowest cutoff dose of tribromoethanol, as reported in previous studies (200–250 mg/kg) [2, 10, 20, 21]. Blood glucose level and rectal temperature were measured at 0, 15, 30, 60, 120, and 180 min after avertin injection (room temperature was 21–21.5°C). The animals were fasted during the measurement period, and both BW and food intake were measured daily at 17:00 for seven days. The following formula was used to evaluate food efficiency: (BW change for 7 days) / (total amount of food intake for 7 days).
Comparison between the effects of avertin and 3MIX on rectal temperature, blood glucose, BW, and food intake
The BW of the HFD-fed female C57BL/6J mice was measured, and either saline, avertin (tribromoethanol: 200 mg/10 ml/kg), or 3MIX (10 ml/kg) was IP injected. Blood glucose and rectal temperature were measured at 0, 15, 30, 60, 120, 180, 240, and 300 min after avertin or 3MIX injection (room temperature was 22.5°C). The animals were fasted during the measurement period, and both BW and food intake were measured daily after IP injection at 17:00 for seven days. In order to determine the effect of 3MIX, we did not use the α2 adrenaline receptor antagonist for recovery.
Comparison between neural activity after avertin and 3MIX injection
Standard diet-fed male C57BL/6J mice (16 weeks old) were used for this experiment. On the day of the experiment, food was removed 4 h before avertin or 3MIX injection. At 13:00, avertin (tribromoethanol: 200 mg/10 ml/kg) or 3MIX (10 ml/kg) was IP injected. Two hours later, the mice were perfused with 4% paraformaldehyde and 0.2% picric acid under anesthesia. Then, the brain was removed, and brain sections 40-µm thick were cut using a freezing microtome. The sections at 160 µm intervals between −1.3 and −2.0 mm from the bregma were used for immunostaining. The brain sections were washed in PBS (0.01 M, pH7.4) and incubated for 20 min with 0.3% H2O2. After incubation for 1 h in blocking solution comprising 0.3% Triton X-100, 2% bovine serum albumin, and 2% normal goat serum, the sections were incubated with rabbit anti-c-Fos antibody (sc-52, 1:1,000, Santa Cruz Biotechnology, Dallas, TX, USA) in a blocking solution overnight at 4°C. Next, the sections were incubated with biotinylated goat anti-rabbit IgG (diluted to 1:500, Vector Laboratories, Burlingame, CA, USA) for 30 min, followed by incubation with an avidin-biotin-peroxidase complex (Vectastain Elite ABC Kit, Vector Laboratories, Burlingame, CA, USA) for 60 min. Immunoreactions were visualized by incubation in 0.02% diaminobenzidine solution containing 0.3% nickel ammonium and 0.015% H2O2 for 5 min. After color development, the sections were mounted on glass slides and covered.
The number of c-Fos-immunoreactive (IR) cells in each section was counted manually under a microscope. An average cell count per section was then obtained in each investigated nucleus of each animal.
Statistical analysis
All data are presented as the mean ± SEM. The interactions between time and treatment factors in rectal temperature, blood glucose level, change of blood glucose, BW, and food intake measurements over seven days between the control and HFD groups were analyzed by two-way ANOVA followed by Tukey’s multiple range test. The differences in percentage of BW change and food efficiency after injection of saline, avertin, or 3MIX among the three groups were analyzed by one-way ANOVA followed by Tukey’s multiple range test. Student’s t-test was used for two-group comparisons. P<0.05 was considered statistically significant.
Results
Effect of avertin on blood glucose and rectal temperature in male and female C57BL/6J mice
In the male C57BL/6J mice (14 weeks old), the initial BW was 26.32 ± 0.55 g for the standard diet group (n=5) and 34.88 ± 2.1 g for the HFD group (n=5). The mean rectal temperature was decreased after injection of avertin in both groups. There were no significant differences in the mean rectal temperature at any time point between the two groups (F5, 40=0.62, P>0.05; Fig. 1A). The mean blood glucose level was significantly lower at 15 and 30 min and significantly higher at 120 and 180 min in the HFD group (F5, 40=8.37; P<0.01; Fig. 1B). The mean change in blood glucose level was also significantly lower at 15 and 30 min and significantly higher at 120 and 180 min in the HFD group (F5, 40=20.07, P<0.01; Fig. 1C).
Fig. 1.
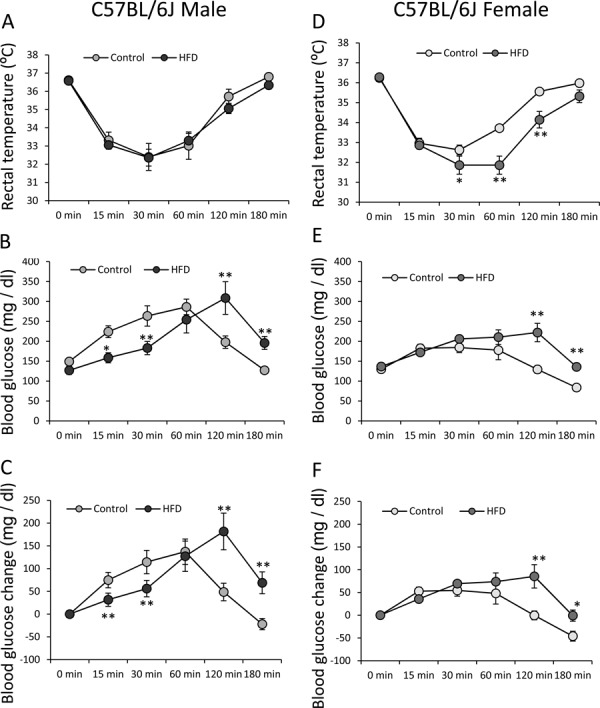
Effects of avertin on blood glucose and rectal temperature in male and female C57BL/6J mice. Changes in rectal temperature (A), blood glucose (B), and blood glucose change (C) after avertin injection in standard diet- and high fat diet (HFD)-fed male C57BL/6J mice (n=5 in both groups). Changes in rectal temperature (D), blood glucose (E), and blood glucose change (F) after avertin injection in standard diet- and HFD-fed female C57BL/6J mice (n=5 in both groups). *P<0.05, two-way ANOVA followed by Tukey’s multiple range test. **P<0.01, two-way ANOVA followed byTukey’s multiple range test.
In the female C57BL/6J mice (18 weeks old), the initial BW was 22.18 ± 0.38 g for the standard diet group (n=5) and 32.04 ± 1.07 g for the HFD groups (n=5). As with the male mice, the mean rectal temperature was decreased in both groups after injection of avertin (Fig. 1D). However, the mean rectal temperature in the HFD group was significantly lower (F5, 40=5.27; P<0.01) at 30, 60 and 120 min (Fig. 1D). The mean blood glucose level was slightly increased in both groups; however, the mean blood glucose level in the HFD group was significantly higher (F5, 40=4.68; P<0.01) at 120 and 180 min (Fig. 1E). In the analyses of the mean change in blood glucose level, the HFD group showed a significantly higher increase at 120 and 180 min (F5, 40=4.68; P<0.01) (Fig. 1F).
Effect of avertin on blood glucose and rectal temperature in male and female ICR mice
We measured the same parameters in a different mouse strain, ICR mice. In the male ICR mice (14 weeks old), the initial BW was 44.04 ± 1.01 g for the standard diet group (n=5) and 67.84 ± 3.18 g for the HFD group (n=5). As with the male C57BL/6J mice, the mean rectal temperature was decreased in both groups after avertin injection (Fig. 2A). The mean rectal temperature in the HFD group was significantly higher at 15 min and significantly lower at 180 min (F5, 40=3.32; P<0.05) (Fig. 2A). The mean blood glucose level was increased in both groups. However, the mean blood glucose level in the HFD group was significantly higher (F5, 40=2.88; P<0.05) at all time points including 0 min (Fig. 2B). On the other hand, in the analysis of mean change in blood glucose level, the HFD group showed a significantly higher increase at 120 min (F5, 40=2.88; P<0.05) (Fig. 2C).
Fig. 2.
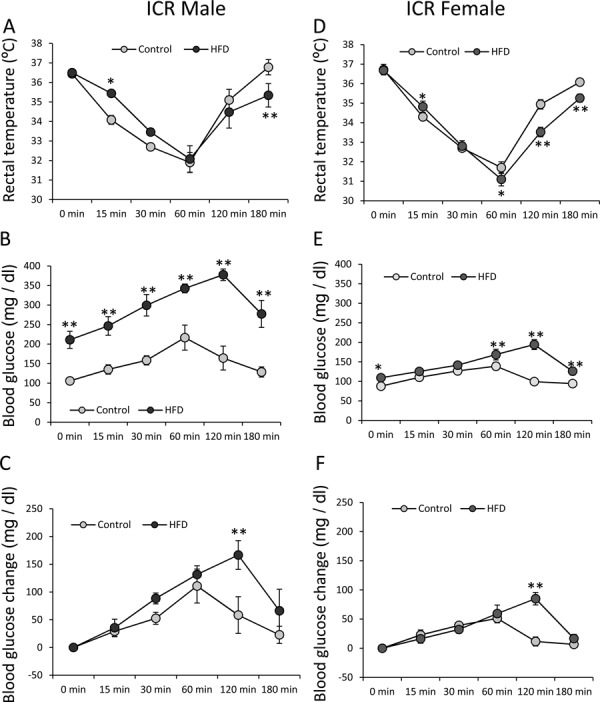
Effects of avertin on blood glucose and rectal temperature in male and female ICR mice. Changes in rectal temperature (A), blood glucose (B), and blood glucose change (C) after avertin injection in standard diet- and HFD-fed male ICR mice (n=5 in both groups). Changes in rectal temperature (D), blood glucose (E), and blood glucose change (F) after avertin injection in standard diet- and HFD-fed female ICR mice (n=5 in both groups). *P<0.05, two-way ANOVA followed by Tukey’s multiple range test. **P<0.01, two-way ANOVA followed by Tukey’s multiple range test.
In the female ICR mice (18 weeks old), the initial BW was 38.10 ± 1.34 g for the standard diet group (n=5) and 60.64 ± 1.50 g for the HFD group (n=5). The mean rectal temperature was decreased in both groups after avertin injection. In comparison with the control group, the mean rectal temperature in the HFD group was significantly lower (F5, 40=8.23; P<0.01) at 60, 120, and 180 min (Fig. 2D). The mean blood glucose level was slightly increased in both groups (Fig. 2E), although that in the HFD group was significantly higher (F5, 40=10.38; P<0.01) at 0, 60, 120, and 180 min (Fig. 2E). In the analysis of mean change in blood glucose level, the HFD group showed a significantly higher increase at 120 min (F5, 40=10.38; P<0.01) (Fig. 2F).
Effect of avertin on BW and food intake in male and female C57BL/6J mice
In the HFD-fed male mice (14 weeks old), avertin injection slightly decreased BW gain, but it was not significantly different (F7, 56=1.50; P>0.05) when compared with the control mice (Fig. 3A). At Day 7, the percentage of BW change in the control group revealed a 4% increase in BW from the initial BW (Fig. 3C), while that in the HFD group revealed a 1% increase in BW. No significant differences were found between the two groups. Food intake was significantly decreased at Day 2 (F7, 56=4.56; P<0.01) in the HFD group (Fig. 3B). There were no significant differences in food efficiency between the groups (Fig. 3D).
Fig. 3.
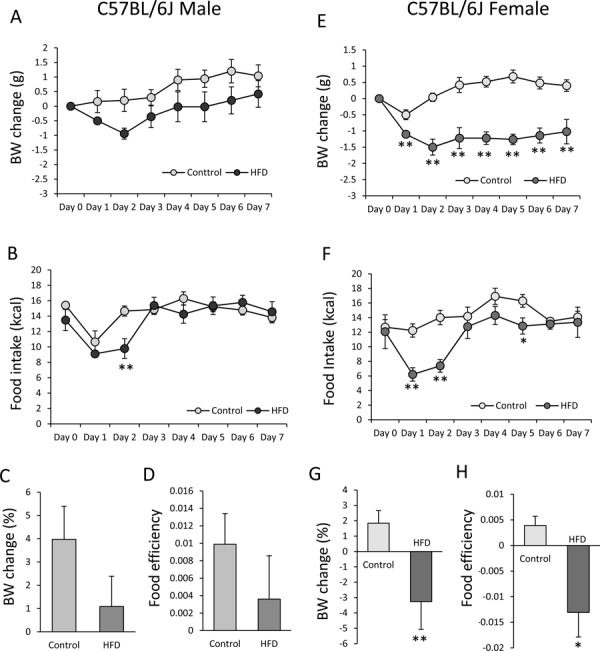
Effects of avertin on body weight (BW) and food intake in male and female C57BL/6J mice. Changes in BW (A), food intake (kcal) (B), and percentage of BW change from initial BW (Day 0) (C) and food efficiency (BW change (g)/total calorie intake for seven days) (D) after avertin injection in standard diet- and HFD-fed male C57BL/6J mice (n=5 in both groups). Changes in BW (E), food intake (kcal) (F), percentage of BW change from the initial BW (Day 0) (G), and food efficiency (H) after avertin injection in standard diet- and HFD-fed female C57BL/6J mice (n=5 in both groups). A, B, E, F: *P<0.05, two-way ANOVA followed by Tukey’s multiple range test; **P<0.01, two-way ANOVA followed by Tukey’s multiple range test. C, D, G, H: *P<0.05, unpaired t-test; **P<0.01, unpaired t-test.
In the HFD-fed female mice (18 weeks old), BW dramatically decreased (F7, 56=10.31; P<0.01) from the Day 1 level after avertin injection compared with the control mice, and it did not recover throughout the experimental period (Fig. 3E). The percentage of BW change in the control group revealed a 2% increase in BW (Fig. 3G). However, the percentage of BW change revealed a 3% decrease in BW in the HFD group, which was a significant decrease compared with the control group (Fig. 3G). Food intake was also significantly decreased (F7, 56=2.84; P<0.05) at Days 1, 2, and 5 (Fig. 3F), and food efficiency was significantly decreased in the HFD group compared with the control group (Fig. 3H).
Effect of avertin on BW and food intake in male and female ICR mice
In the HFD-fed male mice (14 weeks old), one of the five mice used in this study died at Day 3 after avertin injection. Thus, details of the deceased mouse were removed from the data for food intake and BW. The BW change in the HFD group was significantly decreased (F7, 49=2.92; P<0.05) compared with the control group (Fig. 4A). At Day 7, the percentage of BW change in the control group revealed a 1.5% increase in BW relative to the initial BW (Fig. 4C), while that in the HFD group revealed a 3% decrease (Fig. 4C). However, there was no significant difference in the percentage of BW change between the two groups (Fig. 4C). Food intake was significantly lower (F7, 49=7.49; P<0.05) between Days 0 and 4 in the HFD-fed mice (Fig. 4B). There was no significant difference between the two groups in terms of food efficiency (Fig. 4D).
Fig. 4.
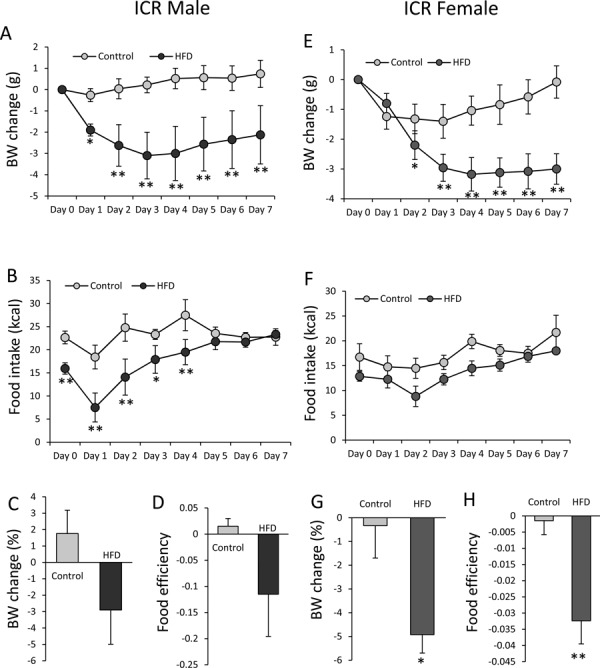
Effects of avertin on body weight (BW) and food intake in male and female ICR mice. Changes in BW (A), food intake (kcal) (B), and percentage of BW change from the initial BW (Day 0) (C), and food efficiency (BW change (g)/total calorie intake for seven days) (D) after avertin injection in standard diet- (n=5) and HFD-fed ICR male mice (n=4). Changes in BW (E), food intake (kcal) (F), percentage of BW change from the initial BW (Day 0) (G), and food efficiency (H) after avertin injection in standard diet- and HFD-fed female ICR mice (n=5 in both groups). A, B, E, F: *P<0.05, two-way ANOVA followed by Tukey’s multiple range test; **P<0.01, two-way ANOVA followed by Tukey’s multiple range test. C, D, G, H: *P<0.05, unpaired t-test; **P<0.01, unpaired t-test.
In the HFD-fed female mice (18 weeks old), the BW change after avertin injection showed a significant decrease (F7, 56=9.95; P<0.01) from Day 2 compared with the control mice and did not recover thereafter (Fig. 4E). The percentage of BW change in the control group was −0.3% (Fig. 4G). However, the percentage of BW change in the HFD group was −5%, which was significantly larger compared with the control group (Fig. 4G). There were no significant differences in food intake between the two groups (F7, 56=0.74; P>0.05) (Fig. 4F). Food efficiency dramatically decreased in the HFD group compared with the control group (Fig. 4H).
Comparison between the effects of avertin and 3MIX on rectal temperature, blood glucose, BW, and food intake in obese female C57BL/6J mice
Because BW and food intake in obese female C57BL/6J mice were largely affected by avertin injection, we used them (20 weeks old) to compare the effects of avertin and 3MIX on rectal temperature, blood glucose, BW, and food intake. The initial BW was 33.68 ± 1.1 g for the saline-injected control group (n=5), 35.74 ± 1.67 g for the avertin group (n=5), and 36.8 ± 1.57 g for the 3MIX-injected group (n=4).
The mean rectal temperature in both the avertin and 3MIX groups was significantly decreased (F14, 77=268.66; P<0.01) (Fig. 5A). The mean rectal temperature in the 3MIX group continued to decrease during the measurement period and was significantly lower between 30 and 300 min than in the avertin group. The mean blood glucose level in both the avertin and 3MIX groups was increased compared with the control group (F14, 77=44.77; P<0.01) (Fig. 5B). However, the increase in blood glucose level was greater in the 3MIX group, and it did not recover during the measurement period (Fig. 5B).
Fig. 5.
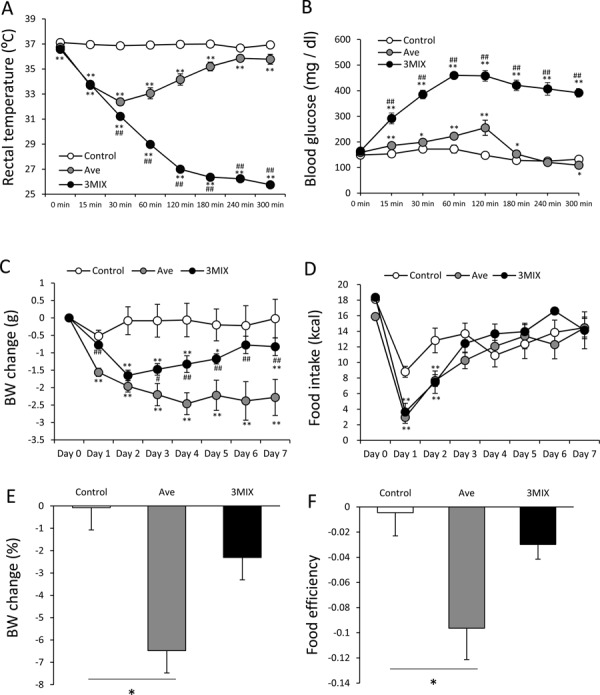
Comparison between the effects of avertin and 3MIX on rectal temperature, blood glucose, body weight (BW), and food intake in HFD-fed female C57BL/6J mice. Changes in rectal temperature (A), blood glucose (B), BW change (C), and food intake (kcal) (D), percentage of BW changes from initial BW (Day 0) (E), and food efficiency (BW change (g)/total calorie intake for seven days) (F) after injection of saline (n=5), avertin (n=5), or 3MIX (n=4). A–D: Two-way ANOVA followed by Tukey’s multiple range test. *P<0.05, compared with the control group; **P<0.01, compared with the control group; #P<0.05, compared with the avertin-injected group; ##P<0.01, compared with the avertin-injected group. E, F: *P<0.05, one-way ANOVA followed by Tukey’s multiple range test.
The BW changes in both the avertin and 3MIX groups were significantly decreased compared with that in the control group (F14, 77=3.46; P<0.01) (Fig. 5C). The BW decline was larger in the avertin group than in the 3MIX group (Fig. 5C). The BW in the 3MIX group was almost recovered at Day 7. The percentages of BW change from the initial BW were −0.08%, −6.5%, and −2.3% for the control, avertin, and 3MIX groups, respectively, with a significant difference observed between the control and avertin groups (Fig. 5E). Food intake in the avertin and 3MIX groups was similarly significantly decreased (F14, 77=1.85; P<0.05) compared with control group at Days 1 and 2. Food efficiency was significantly lower in the avertin group than in the control group (Fig. 5F).
The distribution of c-Fos in the brain after avertin and 3MIX administration
In order to clarify the effects of avertin and 3MIX on brain regions related to the regulation of feeding, body temperature, and energy expenditure, c-Fos expression after either avertin or 3MIX injection was examined in standard diet-fed nonobese male C57BL/6J mice (16 weeks old).
IP injection of avertin significantly increased c-Fos expression in various regions related to the regulation of feeding and energy expenditure, including the preoptic area (POA), supraoptic nucleus (SON), paraventricular nucleus (PVN), ventromedial hypothalamic nucleus (VMH), arcuate nucleus (ARC), and dorsomedial hypothalamic nucleus (DMH) (Figs. 6A–P). However, in the 3MIX group, almost no c-Fos expression was found in these brain regions (Figs. 6A–P).
Fig. 6.
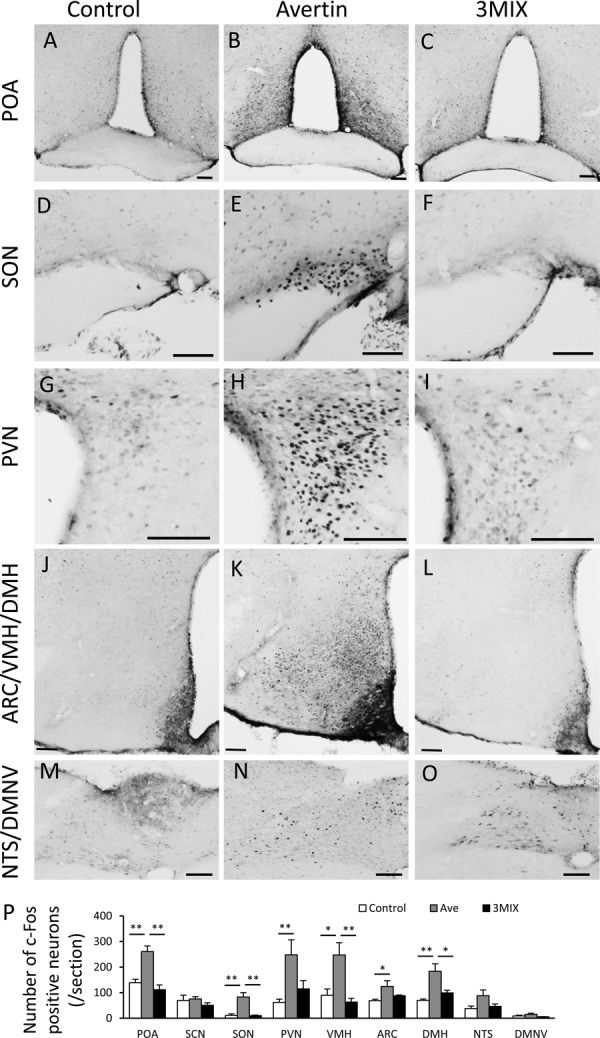
Distribution of c-Fos in the central nervous systems after saline, avertin, or 3MIX injection. Photomicrographs of c-Fos expression in the preoptic area (POA) (A–C), supraoptic nucleus (SON) (D–E), paraventricular nucleus (PVN) (G–H), arcuate (ARC)/ ventromedial hypothalamic nucleus (VMH)/ dorsomedial hypothalamic nucleus (DMH) (J–L), and nucleus tractus solitaries (NTS)/ dorsal motor nucleus of the vagus (DMNV) (M–O). The right, middle, and left columns indicate saline, avertin, and 3MIX injection, respectively. All scale bars in these photomicrographs indicate 100 µm. The number of c-Fos positive neurons in the brain regions related food intake/energy expenditure (P). *P<0.05, one-way ANOVA followed by Tukey’s multiple range test. **P<0.01, one-way ANOVA followed by Tukey’s multiple range test.
Discussion
The present study showed that avertin injection decreased food intake and BW in all mice (both male and female C57BL/6J and ICR) fed a HFD compared with control mice fed a standard diet. The effects of avertin on BW and feeding were larger in the ICR mice than in the C57BL/6J mice. This difference may be due to the difference in obesity levels.
The BWs of the HFD-fed ICR mice of both sexes were approximately twice those of the HFD-fed C57BL/6J mice in the present study. This result indicates that body fat accumulates more easily in ICR mice fed a HFD than in C57BL/6J mice. It is known that alcohols, as components (tribromoethanol, ethanol, and amyl alcohol) of avertin, are distributed to the white adipose tissue and affect the physiological properties of the adipocyte [25]. Adipose tissue secretes more than 50 adipokines, including leptin, which are related to glucose, lipid, energy metabolism, and appetite regulation [1, 25, 26]. Although there is no evidence indicating that a component of avertin affects the secretion or production of these adipokines, leading to alteration of energy metabolism, it could be considered that the avertin is prone to causing an adverse effect in individuals with a larger fat mass. The differences in BW and food intake between C57BL/6J and ICR mice observed in the present study might be related to the degree of fat accumulation.
As shown by the c-Fos expression, avertin injection strongly activated the hypothalamic brain regions related to feeding and energy expenditure. A HFD is known to induce dynamic metabolic changes such as chronic inflammation in the central nervous system, as well as in peripheral organs [29]. We examined the distribution of Iba1, which is an inflammation marker [29], in the hypothalamus of the 8-week standard diet- and HFD-fed C57BL/6J mice. We found that Iba1 was remarkably increased in the hypothalamus, including the VMH and DMH, of the HFD-fed mice, indicating central inflammation induction (data not shown). HFD consumption is also known to induce cytokine production, neuronal stress, and leptin/insulin resistance, which promote food intake and obesity development [3]. In addition, dysfunction of the autonomic neural circuits which regulate body temperature and blood glucose can occur in cases of obesity [19].
Therefore, the cause of changes in BW and food intake observed after avertin injections in HFD-induced obese mice, but not in standard diet-fed nonobese mice, may be related to neural vulnerability induced by inflammation of the feeding center and dysfunction of the autonomic neural circuit induced by HFD consumption. However, we found no evidence to suggest that HFD consumption induces neural vulnerability; thus, further studies are required.
The BW decline was larger in the females than in the males in both C57BL/6J and ICR mice fed a HFD after avertin injection. Although BW decline was observed in the male C57BL/6J and ICR mice fed a HFD, there were no significant differences in the percentage of BW change at seven days after avertin injection. One of the causes of BW decline in the female HFD-fed mice was considered to be a significant decrease in their food efficiency compared with the control female mice. Thus it was considered that the BW decline after avertin injection in the HFD-fed female mice was induced by enhancement of energy expenditure, as well as decline in food intake.
Because food efficiency is more prominent in obese female mice than in obese male mice, contributions of sex differences and pharmacokinetics under a HFD should be taken into account when considering the mechanisms for BW decline when using anesthesia. The factors that affect the pharmacokinetics of tribromoethanol remain unknown. Generally, hepatic drug-metabolizing enzymes are known to play an important role in drug metabolism [30]; however, thousands of genes with sexual dimorphism exist in the mouse liver [31]; therefore substantial sex differences exist in its metabolic function [30]. In addition, HFD feeding itself also alters the expression levels of hepatic drug-metabolizing enzymes in mice [18]. Although there are no reports on the effects of avertin on the hepatic drug-metabolizing enzymes, the major effect of avertin on the HFD-fed female mice in the present study may have been due to the sex difference of the drug metabolizing enzymes as well the changes of expression levels of said enzymes induced by HFD feeding. However, further studies are required to confirm this.
When considering the effects of avertin regarding the different strains of mice, only rectal temperature was different between the C57BL/6J and ICR mice after injection. As shown in Figs. 1A, D and 2A, D, the rectal temperature of the C57BL/6J mice showed U-shaped changes, whereas that of ICR mice showed V-shaped changes. This difference may be due to the difference in their processes of decline and recovery of rectal temperature. The rectal temperature of the C57BL/6J mice was acutely decreased after avertin injection, and it recovered slowly. On the other hand, the rectal temperature of the ICR mice was slowly decreased after avertin injection, and it rapidly recovered. Furthermore, at 30 min after avertin injection in the present study, 20% (4/20) of the C57BL/6J mice woke from the avertin anesthesia compared with 55% (11/20) of the ICR mice. The difference in drug clearance speed may be due to the different strains.
When comparing the effects of avertin and 3MIX in the HFD-induced obese female mice, both anesthetic agents decreased food intake and BW compared with the saline control group. However, the decline in BW and food intake was significantly larger in the avertin group than in the 3MIX group. In addition, BW in the 3MIX group was almost recovered at seven days after injection. Surprisingly, even after complete recovery of food intake, BW remained low at seven days after injection in the avertin group compared with the control and 3MIX groups. Taking the results for rectal temperature and blood glucose after injection of avertin and 3MIX into consideration, it is estimated that the cause of decline in food intake and BW after administration is independent from the cause of changes in blood glucose and rectal temperature. Although BW and food intake were almost recovered at Day 7, larger changes in rectal temperature and blood glucose were observed in the 3MIX group compared with those in the avertin group.
As shown in Fig. 5E, avertin injection decreased BW by 6.5% in the female C57BL/6J mice fed a HFD; however, Fig. 3G shows that avertin decreased BW by only 3% in the same mice. The details of the mechanism underlying this difference are unclear. It is possible that there is a large variation in the effect of avertin on the BW in HFD-fed female mice.
We have shown that avertin injection dramatically induced c-Fos expression in the POA, PVN, VMH, ARC, and DMH, which are regulation centers for feeding, the autonomic nervous system, and energy expenditure [4, 27, 28]. Although the avertin mechanism that induces depression of the respiratory and cardiovascular centers in the central nervous system remains unknown [15], our present data revealed for the first time that avertin remarkably activates the brain regions related to the regulation of feeding and energy expenditure. On the other hand, the mechanisms of all three components in 3MIX have been known to involve an α2 adrenaline receptor agonist (medetomidine), GABAA receptor agonist (midazolam), and opioid κ receptor agonist (butorphanol) [7]. Regarding c-Fos expression in the brain after 3MIX injection, no changes between the control and 3MIX groups were observed. As for the decline in BW change, food intake, food efficiency, and strong c-Fos expression in the hypothalamus after avertin injection, avertin may stimulate the feeding center, autonomic nervous system, and energy expenditure and decrease food intake as well as decrease food efficiency for long durations in obese mice.
In conclusion, this study indicates that 3MIX is a useful anesthetic for HFD-induced obese mice and clearly shows the advantage of using 3MIX in obesity research. However, when we injected 3MIX into even more obese male C57BL/6J mice (fed a HFD for 15 weeks, aged 21 weeks old; 47.7 ± 0.5 g, n=12), one of 12 failed to recover from anesthesia and died at Day 2 after injection. It is therefore necessary to use 3MIX with caution for obese mice fed a HFD.
Acknowledgments
The authors thank Mr. Takato Suzuki of the Laboratory Animal Center of Fukushima Medical University for his technical advice. We thank Dr. Shingen Misaka of the Department of Bioregulation and Pharmacological Medicine, Fukushima Medical University, for his critical advice regarding pharmacokinetics.
References
- 1.Blüher M., Mantzoros C.S.2015. From leptin to other adipokines in health and disease: facts and expectations at the beginning of the 21st century. Metabolism 64: 131–145. doi: 10.1016/j.metabol.2014.10.016 [DOI] [PubMed] [Google Scholar]
- 2.Brown E.T., Umino Y., Loi T., Solessio E., Barlow R.2005. Anesthesia can cause sustained hyperglycemia in C57/BL6J mice. Vis. Neurosci. 22: 615–618. doi: 10.1017/S0952523805225105 [DOI] [PubMed] [Google Scholar]
- 3.Dorfman M.D., Thaler J.P.2015. Hypothalamic inflammation and gliosis in obesity. Curr. Opin. Endocrinol. Diabetes Obes. 22: 325–330. doi: 10.1097/MED.0000000000000182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elmquist J.K., Maratos-Flier E., Saper C.B., Flier J.S.1998. Unraveling the central nervous system pathways underlying responses to leptin. Nat. Neurosci. 1: 445–450. doi: 10.1038/2164 [DOI] [PubMed] [Google Scholar]
- 5.Halaas J.L., Gajiwala K.S., Maffei M., Cohen S.L., Chait B.T., Rabinowitz D., Lallone R.L., Burley S.K., Friedman J.M.1995. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269: 543–546. doi: 10.1126/science.7624777 [DOI] [PubMed] [Google Scholar]
- 6.Hill W.A., Tubbs J.T., Carter C.L., Czarra J.A., Newkirk K.M., Sparer T.E., Rohrbach B., Egger C.M.2013. Repeated administration of tribromoethanol in C57BL/6NHsd mice. J. Am. Assoc. Lab. Anim. Sci. 52: 176–179. [PMC free article] [PubMed] [Google Scholar]
- 7.Kawai S., Takagi Y., Kaneko S., Kurosawa T.2011. Effect of three types of mixed anesthetic agents alternate to ketamine in mice. Exp. Anim. 60: 481–487. doi: 10.1538/expanim.60.481 [DOI] [PubMed] [Google Scholar]
- 8.Kirihara Y., Takechi M., Kurosaki K., Kobayashi Y., Saito Y., Takeuchi T.2015. Anesthetic effects of a three-drugs mixture--comparison of administrative routes and antagonistic effects of atipamezole in mice. Exp. Anim. 64: 39–47. doi: 10.1538/expanim.14-0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirihara Y., Takechi M., Kurosaki K., Kobayashi Y., Saito Y., Takeuchi T.2016. Effects of an anesthetic mixture of medetomidine, midazolam, and butorphanol in rats-strain difference and antagonism by atipamezole. Exp. Anim. 65: 27–36. doi: 10.1538/expanim.15-0036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kubo Y., Tahara Y., Hirao A., Shibata S.2012. 2,2,2-Tribromoethanol phase-shifts the circadian rhythm of the liver clock in Per2:Luciferase knockin mice: lack of dependence on anesthetic activity. J. Pharmacol. Exp. Ther. 340: 698–705. doi: 10.1124/jpet.111.188615 [DOI] [PubMed] [Google Scholar]
- 11.Lemonnier D.1972. Effect of age, sex, and sites on the cellularity of the adipose tissue in mice and rats rendered obese by a high-fat diet. J. Clin. Invest. 51: 2907–2915. doi: 10.1172/JCI107115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lieggi C.C., Artwohl J.E., Leszczynski J.K., Rodriguez N.A., Fickbohm B.L., Fortman J.D.2005. Efficacy and safety of stored and newly prepared tribromoethanol in ICR mice. Contemp. Top. Lab. Anim. Sci. 44: 17–22. [PubMed] [Google Scholar]
- 13.Maejima Y., Aoyama M., Sakamoto K., Jojima T., Aso Y., Takasu K., Takenosihita S., Shimomura K.2017. Impact of sex, fat distribution and initial body weight on oxytocin’s body weight regulation. Sci. Rep. 7: 8599. doi: 10.1038/s41598-017-09318-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maejima Y., Iwasaki Y., Yamahara Y., Kodaira M., Sedbazar U., Yada T.2011. Peripheral oxytocin treatment ameliorates obesity by reducing food intake and visceral fat mass. Aging (Albany NY) 3: 1169–1177. doi: 10.18632/aging.100408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer R.E., Fish R.E.2005. A review of tribromoethanol anesthesia for production of genetically engineered mice and rats. Lab Anim. (NY) 34: 47–52. doi: 10.1038/laban1105-47 [DOI] [PubMed] [Google Scholar]
- 16.Miyasaka K., Kanai S., Ohta M., Kawanami T., Kono A., Funakoshi A.1994. Lack of satiety effect of cholecystokinin (CCK) in a new rat model not expressing the CCK-A receptor gene. Neurosci. Lett. 180: 143–146. doi: 10.1016/0304-3940(94)90507-X [DOI] [PubMed] [Google Scholar]
- 17.Ng M., Fleming T., Robinson M., Thomson B., Graetz N., Margono C., Mullany E.C., Biryukov S., Abbafati C., Abera S.F., et al. 2014. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384: 766–781. doi: 10.1016/S0140-6736(14)60460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ning M., Jeong H.2017. High-fat diet feeding alters expression of hepatic drug-metabolizing enzymes in mice. Drug Metab. Dispos. 45: 707–711. doi: 10.1124/dmd.117.075655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nonogaki K.1999. Obesity: autonomic circuits versus feeding. Nat. Med. 5: 742–743. doi: 10.1038/10464 [DOI] [PubMed] [Google Scholar]
- 20.Norton W.B., Scavizzi F., Smith C.N., Dong W., Raspa M., Parker-Thornburg J.V.2016. Refinements for embryo implantation surgery in the mouse: comparison of injectable and inhalant anesthesias - tribromoethanol, ketamine and isoflurane - on pregnancy and pup survival. Lab. Anim. 50: 335–343. doi: 10.1177/0023677215616530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oh S.S., Hayes J.M., Sims-Robinson C., Sullivan K.A., Feldman E.L.2010. The effects of anesthesia on measures of nerve conduction velocity in male C57Bl6/J mice. Neurosci. Lett. 483: 127–131. doi: 10.1016/j.neulet.2010.07.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papaioannou V.E., Fox J.G.1993. Efficacy of tribromoethanol anesthesia in mice. Lab. Anim. Sci. 43: 189–192. [PubMed] [Google Scholar]
- 23.Pelleymounter M.A., Cullen M.J., Baker M.B., Hecht R., Winters D., Boone T., Collins F.1995. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269: 540–543. doi: 10.1126/science.7624776 [DOI] [PubMed] [Google Scholar]
- 24.Phillips M.S., Liu Q., Hammond H.A., Dugan V., Hey P.J., Caskey C.J., Hess J.F.1996. Leptin receptor missense mutation in the fatty Zucker rat. Nat. Genet. 13: 18–19. doi: 10.1038/ng0596-18 [DOI] [PubMed] [Google Scholar]
- 25.Pravdova E., Fickova M.2006. Alcohol intake modulates hormonal activity of adipose tissue. Endocr. Regul. 40: 91–104. [PubMed] [Google Scholar]
- 26.Schwartz G.J., Azzara A.V., Heaner M.K.2013. Roles for central leptin receptors in the control of meal size. Appetite 71: 466–469. doi: 10.1016/j.appet.2013.04.017 [DOI] [PubMed] [Google Scholar]
- 27.Schwartz M.W., Woods S.C., Porte D., Jr, Seeley R.J., Baskin D.G.2000. Central nervous system control of food intake. Nature 404: 661–671. doi: 10.1038/35007534 [DOI] [PubMed] [Google Scholar]
- 28.Tan C.L., Knight Z.A.2018. Regulation of Body Temperature by the Nervous System. Neuron 98: 31–48. doi: 10.1016/j.neuron.2018.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thaler J.P., Yi C.X., Schur E.A., Guyenet S.J., Hwang B.H., Dietrich M.O., Zhao X., Sarruf D.A., Izgur V., Maravilla K.R., Nguyen H.T., Fischer J.D., Matsen M.E., Wisse B.E., Morton G.J., Horvath T.L., Baskin D.G., Tschöp M.H., Schwartz M.W.2012. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Invest. 122: 153–162. doi: 10.1172/JCI59660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waxman D.J., Holloway M.G.2009. Sex differences in the expression of hepatic drug metabolizing enzymes. Mol. Pharmacol. 76: 215–228. doi: 10.1124/mol.109.056705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang X., Schadt E.E., Wang S., Wang H., Arnold A.P., Ingram-Drake L., Drake T.A., Lusis A.J.2006. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res. 16: 995–1004. doi: 10.1101/gr.5217506 [DOI] [PMC free article] [PubMed] [Google Scholar]


