Abstract
Dyslexia is a common neurobiological disorder in which a child fails to acquire typical word reading skills despite adequate opportunity and intelligence. The visual word form area (VWFA) is a region within the left fusiform gyrus that specializes for print over the course of reading acquisition and is often hypoactivated in individuals with dyslexia. It is currently unknown whether atypicalities in this brain region are already present in kindergarten children who will subsequently develop dyslexia. Here, we measured fMRI activation in response to letters and false fonts in bilateral fusiform gyrus in children with and without risk for dyslexia (defined by family history or low scores on assessments of pre-reading skills, such as phonological awareness). We then followed these children longitudinally through the end of second grade to evaluate whether brain activation patterns in kindergarten were related to second-grade reading outcomes. Compared to typical readers who exhibited no risk factors for reading impairment in kindergarten, there was significant hypoactivation to both letters and false-fonts in the left fusiform gyrus in at-risk children who subsequently developed reading impairment, but not in at-risk children who developed typical reading skills. There were no significant differences in letter- or false-font responses in the right fusiform gyrus among the groups. The finding that hypoactivation to print in the VWFA is present in children who subsequently develop reading impairment even prior to the onset of formal reading instruction suggests that atypical responses to print play an early role in the development of reading impairments such as dyslexia.
Keywords: Reading outcomes, Dyslexia, Reading impairment, VWFA, Diagnosis
Highlights
-
•
Measured kindergarten VWFA activation in children with and without dyslexia risk.
-
•
At-risk children who became impaired readers had reduced left VWFA activation.
-
•
Reduced activation was present in response to both letters and false fonts.
-
•
At-risk but typical readers had reduced specificity for letters in left VWFA.
-
•
Evidence for early brain difference in left VWFA in future struggling readers.
1. Introduction
Developmental dyslexia is a common neurodevelopmental disorder that is diagnosed in individuals with average nonverbal IQ and adequate schooling who persistently fail to develop typical word reading ability (Katzir et al., 2004; Peterson and Pennington, 2015). Dyslexia is now understood to have a genetic (reviewed in Eicher and Gruen, 2013; Galaburda et al., 2006; Mascheretti et al., 2017) and neural basis (Maisog et al., 2008; Norton et al., 2015; Richlan et al., 2009, Richlan et al., 2011; Vandermosten et al., 2012). Individuals with dyslexia exhibit brain differences in several areas of the reading network. Multiple studies have identified hypoactivation in regions related to phonological processing, including left superior temporal gyrus (STG), temporo-parietal region, and inferior frontal gyrus (IFG) (Hoeft et al., 2007; Richards and Aylward, 2006; Richlan, 2012). Further, hypoactivation in regions specialized for orthographic processing are well-established, especially in the left fusiform gyrus in a functional region specialized for print termed the visual word form area (VWFA) (Cohen et al., 2002; Van der Mark et al., 2009; Olulade et al., 2015; Richlan et al., 2011). Here we examined whether the disrupted response of the left fusiform gyrus is present during the initial stages of learning to read, or whether it is instead a consequence of prolonged difficulty in learning to read.
Most studies of the neurocognitive basis for dyslexia examine brain differences in children or adults after years of struggling with reading, which makes it uncertain whether the brain differences reflect the etiology or cause of dyslexia versus the consequence of dyslexia (e.g., reduced reading experience). Studies examining the etiology of dyslexia have focused on pre-reading children before the formal teaching of reading (typically in middle of the kindergarten year in the U.S.). Because the diagnosis of dyslexia is based on assessment of reading and thus cannot be made prior to reading instruction, such studies have taken two approaches. Some studies have evaluated children born to families with a history of dyslexia (familial risk), because such children have an elevated risk for dyslexia, estimated near 50% (Pennington and Lefly, 2001; Puolakanaho et al., 2007; Torgesen, 2001). Other studies have examined behavioral risk factors for dyslexia, because weaknesses in several pre-reading skills have associated with future dyslexia (Catts et al., 2015; Snowling and Melby-Lervag, 2016).
A limitation of these studies of pre-reading children is that the characteristics used to identify children at risk for future dyslexia have limited specificity (Ozernov-Palchik & Gaab, 2016). Indeed, approximately half of pre-reading children with familial or behavioral risk become typical readers (Snowling et al., 2007). This lack of specificity may be due to several factors, including the instability of profiles over time, measurement error in assessment, variation in reading instruction and experience, and/or moderate heritability of dyslexia. Here, we overcame that concern by measuring brain functions in children prior to school-based reading instruction in kindergarten and then longitudinally assessing which behaviorally at-risk children did or did not develop reading impairment by the end of 2nd grade.
Reading is a complex ability that involves many processes including language, vision, and attention, but the best understood cause of reading disability is a weakness in phonological awareness (Bradley and Bryant, 1983; Elbro et al., 1994; Ramus et al., 2003; Swan and Goswami, 1997; Vellutino and Scanlon, 1987). Consequently, many studies have examined brain differences related to auditory language perception to understand the etiology of dyslexia. Differences have been identified in infants and young children at risk for dyslexia as measured by event-related potentials (ERPs) to language (Guttorm et al., 2001; Guttorm and Leppänen, 2003; Leppänen et al., 2010; Molfese, 2000), functional MRI activation for language sounds (Raschle et al., 2012), and the structure of the arcuate fasciculus, which connects anterior and posterior language regions (Saygin et al., 2013, Saygin et al., 2016; Vandermosten et al., 2012; Wang et al., 2017). These studies all support the idea that neural systems supporting auditory language processing are atypical in at-risk children prior to reading instruction, and therefore these brain differences appear to be related to the cause of dyslexia.
Far fewer studies have examined whether brain regions involved in the visual processing of print are typical or atypical in pre-reading children who will subsequently develop dyslexia. The VWFA is a small functionally defined region of the left fusiform gyrus that specializes for print over the course of reading acquisition (Baker et al., 2007; Cohen et al., 2002; Glezer and Riesenhuber, 2013; Hirshorn et al., 2016). Insults to this left fusiform region, via lesions or disruptive electrical stimulation to the cortex, impair reading, which indicates that this region is essential for the recognition of print (Cohen and Dehaene, 2004; Dejerine, 1892; Hirshorn et al., 2016). The degree of specificity for print (that is, greater response to print than other stimuli) in this region develops over a long trajectory, even into late adolescence (Centanni et al., 2017). In young pre-reading children who develop typical reading skills, white-matter connections between the future VWFA (region of cortex that will become the print-selective VWFA after reading acquisition) and other brain regions are already present (Saygin et al., 2016), as is sensitivity to letters in the left fusiform gyrus (Ben-Shachar et al., 2011; Centanni et al., 2018a). Such activation in the visual word form region was predictive of second grade reading when combined with behavioral assessment scores in a small sample of typically-developing German-speaking kindergartners (Bach et al., 2013).
Only two previous studies have examined functional responses to letters and other visual stimuli in pre-reading children with versus without risk for dyslexia. One study compared English-speaking children in early kindergarten who were at no risk (on track) in terms of literacy development (N = 7) to children who were at risk (in the lower 35% on standardized measures of letter knowledge and phonological awareness, N = 7) (Yamada et al., 2012). The no-risk group showed greater activation to letters than false font letters than the at-risk group in several regions related to reading, but no differences were observed in fusiform regions. The at-risk children received supplemental instruction over the first half of kindergarten, and did not subsequently differ significantly in behavioral measures from the no-risk group, so these children were unlikely to be later diagnosed with dyslexia. An fMRI study of children in early kindergarten in Norway compared children who were versus were not at risk for dyslexia based on a composite of several developmental and family factors (Specht et al., 2009). During both simple and complex word-reading tasks, the groups showed activation differences in multiple reading-related regions, but no differences were reported in fusiform regions.
These studies suggest that children who are at-risk for dyslexia do not have differential responses to print in the VWFA region of the left fusiform gyrus as compared to their typical peers, despite the frequent observation of reduced activation in older individuals with dyslexia. This discrepancy could be due to processes that unfold later, such as a reduction in reading experience in people with dyslexia over time, or this difference may be present but not detected by other studies. A critical limitation of the previous fMRI studies in pre-readers (Specht et al., 2009; Yamada et al., 2012), like earlier behavioral studies, is the grouping of participants based on risk category rather than reading outcome; thus, each group includes an unknown mixture of children who will or will not read poorly years later. Consequently, brain differences in children who will develop dyslexia may be obscured when averaged with a similar number of apparently at-risk children who will not develop dyslexia. The design of the present longitudinal study solves this problem by assessing reading ability at the end of second grade, and then retrospectively comparing the kindergarten measures between the at-risk children who did or did not develop reading impairment.
The aim of the current study was to determine whether hypoactivation to letters in fusiform gyrus is present before or early in kindergarten, before formal school reading instruction, in children who subsequently develop reading impairment. We collected behavioral and fMRI data in the spring before or the fall of kindergarten, before the typical start of reading instruction in later kindergarten, and subsequently determined longitudinally which children showed reading impairment at end of second grade. The children in our study were not yet formally evaluated for dyslexia at the second grade evaluation and so we refer to the children who exhibited poor reading in second grade as having reading impairment rather than having dyslexia. We then asked whether atypical responses to print in the VWFA region of the left fusiform gyrus occur in children who later develop reading impairment. Given the evidence from adults with dyslexia and children at risk for dyslexia, we predicted that the group of at-risk impaired readers would show significantly reduced activation to print in the left VWFA as compared to their typical-reading peers (regardless of kindergarten risk status). We also examined responses to print in the right fusiform gyrus because of evidence that responses to print are bilateral in beginning readers (Centanni et al., 2018a) before becoming left-lateralized in older, skilled readers (Dundas et al., 2013; Maurer et al., 2005, 2008).
2. Materials and methods
2.1. Participants, assessments, and group designation
Children were recruited through schools and participated as part of a larger longitudinal study on reading and literacy development (The READ Study; see also Centanni et al., 2018a; Ozernov-Palchik et al., 2016; Saygin et al., 2013, Saygin et al., 2016). The kindergarten wave of data collection took place at the end of the pre-kindergarten year or fall of the kindergarten year (hereafter both referred to as kindergarten).
All children met eligibility criteria including: being a native speaker of American English; born after at least 36 weeks gestation; no sensory or perceptual difficulties other than corrected vision; no history of head or brain injury or trauma; no neurological, neuropsychological, or developmental disorder diagnoses; no medications affecting the nervous system; standard scores >85 on measures of nonverbal IQ and verbal IQ at initial assessment (Peabody Picture Vocabulary Test/PPVT-4 Dunn and Dunn, 2007 and Kaufman Brief Intelligence Test/KBIT-2 Matrices subtest, Kaufman and Kaufman, 2004). This study was approved by the institutional review boards at the Massachusetts Institute of Technology and Boston Children's Hospital. Parents gave written consent and children provided verbal assent to participate.
A total of 161 children attempted the MRI in kindergarten. Of these, 12 were excluded because they did not complete the MRI session or did not complete both runs of the task; 11 were excluded due to excessive motion, falling asleep during the scan, or poor performance on the in-scanner task; 31 did not return for follow-up assessment at 2nd grade; 5 were excluded due to skipping or repeating a grade, or having a neurological disorder diagnosed before 2nd grade; and 3 had complete data, but did not meet criteria for our groups, as defined below. Thus, in the present analyses, we report findings from all 99 children who completed both fMRI in kindergarten and reading assessment at the end of second grade (average age at initial enrollment; 67.05 months, range 58–77, 46 female).
Children completed a short battery of standardized assessments focused on pre-reading skills known to be associated with risk for dyslexia in their schools at kindergarten. Assessments were administered by trained research assistants and were audio recorded and checked for accuracy of administration and scoring. Families also completed demographic questionnaires and a home literacy environment questionnaire. Home literacy environment score was calculated as a mean of the parent's response for 5 elements: the number of child's and parents' books in the home, how often the child was read to, how often the child read independently, and the engagement of the parent in letter and reading instruction. Parent responses were converted to a scale of 1–7 (higher scores indicate a more supportive home literacy environment) (Powers et al., 2016). In order to increase the number of children whose kindergarten pre-reading skills indicated they were at risk for future reading difficulty, approximately twice as many children with risk (defined as low scores on pre-reading measures and/or family history of dyslexia, see detailed characterization below) than without risk were invited to participate in MRI sessions.
At kindergarten age, children were categorized into two groups based on dyslexia risk; children with no risk for dyslexia (N = 44) and children at risk for dyslexia (N = 55). Risk for dyslexia was defined as having familial risk (an immediate family member with a diagnosis of dyslexia or self-report of lifelong reading difficulties) and/or a score in the lower 25th percentile of our larger study's screening sample (N = 1433; see Ozernov-Palchik et al., 2017 for more details) on a composite score of phonological awareness (CTOPP, Elision and Blending Words subtests; Wagner et al., 1999), letter knowledge (WRMT-R/NU; Woodcock, 1998), and/or rapid automatized naming (RAN; RAN/RAS Tests, Objects and Colors subtests; Wolf and Denckla, 2005).
We further characterized children in terms of their reading outcomes at the end of second grade. Children with age-based standard scores of 90 or higher on all 4 standardized word reading subtests (TOWRE-2 Sight Word Efficiency, Phonemic Decoding Efficiency; WRMT-3 Word ID, Word Attack; Torgesen, Wagner, & Rashotte, 2012; Woodcock, 2011) were classified as typical readers. Children with standard scores of <90 on at least 2 of the 4 reading subtests were classified as having a reading impairment. This criterion of having two tests with a standard score below 90 (25th percentile) is typical in other studies (Centanni et al., 2018a; Christodoulou et al., 2014; Clark et al., 2014), and is consistent with criteria used for a diagnosis of dyslexia. We use the term “impaired reader” here because many of the children in our sample had not yet been formally tested for dyslexia. Among the children who were at risk in kindergarten (N = 55), 19 were classified as impaired readers (34.5%), and 36 were classified as typical readers (65.5%). Of the children who had no risk in kindergarten, 44 of 47 (93.6%) developed typical reading skills (standard scores ≥90 on all 4 subtests). Children who scored below 90 on only 1 of the 4 subtests at the second grade time point (N = 6) were not included in these analyses because such scores are ambiguous for categorizing a child as having impaired or unimpaired reading. Regarding the 3 children who were classified as no-risk but who had reading impairment in 2nd grade (false negatives), a small group showing this pattern is to be expected (e.g., 8.3% of no risk children who became poor readers in Maurer et al., 2007; 9.8% in Puolakanaho et al., 2007). Consistent with these and other previous studies (e.g., Lyytinen et al., 2006; Torppa et al., 2006), this group was not included in the current analyses because of the small sample size. Behavioral assessment scores and in-scanner performance for the groups included in the final sample are reported in Table 1.
Table 1.
Group characteristics and scores.
| No risk typical readers (N = 44) | At-risk typical readers (N = 36) | At-risk impaired readers (N = 19) | At-risk typical readers vs. at-risk impaired readers (t values) | |
|---|---|---|---|---|
| Kindergarten measures – children | ||||
| Age at kindergarten assessment (months) | 67.02 ± 3.87 | 66.11 ± 3.23 | 68.89 ± 3.97 | 2.80+ |
| Females (N) | 23 | 15 | 8 | |
| KBIT-2 Nonverbal IQ SS | 102.82 ± 9.60 | 99.50 ± 9.31 | 96.26 ± 9.53⁎ | 1.22 |
| WRMT-R Letter ID SS | 111.02 ± 7.13 | 108.36 ± 7.73 | 100.74 ± 9.95⁎ | 3.100+ |
| WRMT-R Word ID SS | 119.41 ± 27.93 | 101.39 ± 18.02⁎ | 96.21 ± 17.07⁎ | 0.97 |
| RAN Objects and Colors composite SS | 105.22 ± 9.58 | 95.46 ± 13.93⁎ | 87.22 ± 11.53⁎ | 2.140+ |
| CTOPP Elision and Blending Words composite SS | 10.96 ± 1.77 | 9.73 ± 2.01⁎ | 8.89 ± 1.90⁎ | 1.45 |
| In-scanner accuracy: face stimuli | 91.36 ± 9.41 | 92.08 ± 7.45 | 86.60 ± 10.90 | 2.220+ |
| In-scanner accuracy: letter stimuli | 90.13 ± 10.90 | 91.95 ± 7.49 | 89.14 ± 8.92 | 1.24 |
| In-scanner accuracy: false-font stimuli | 91.12 ± 8.53 | 92.20 ± 6.10 | 88.23 ± 9.71 | 1.86 |
| RAN risk (N) | 0 | 12 | 12 | |
| Letter knowledge risk (N) | 0 | 5 | 6 | |
| Phonological awareness risk (N) | 0 | 10 | 8 | |
| Kindergarten measures – parent report | ||||
| Home literacy environmenta | 3.53 ± 1.67 | 3.14 ± 1.54 | 3.42 ± 1.36 | 0.66 |
| Familial risk for dyslexia (N) | 0 | 14 | 8 | |
| 2nd grade outcome measures – children | ||||
| WRMT-3 Word ID SS | 112.86 ± 10.04 | 110.78 ± 8.70 | 87.44 ± 9.72⁎ | 7.750+ |
| WRMT-3 Word Attack SS | 109.34 ± 11.19 | 109.50 ± 7.67 | 85.89 ± 9.22⁎ | 13.000+ |
| TOWRE-SWE SS | 109.09 ± 9.09 | 107.83 ± 8.12 | 88.26 ± 11.42⁎ | 9.280+ |
| TOWRE-PDE SS | 105.61 ± 10.16 | 105.78 ± 7.60 | 79.53 ± 6.76⁎ | 10.270+ |
Values are presented as mean ± standard deviation. SS = standard score.
Home literacy environment score represents a mean of several items (possible range 1–7, higher scores indicate a more supportive home literacy environment).
Significant difference for at-risk impaired readers compared to the no risk group (p < .05).
Significant difference between the two at-risk groups (p < .05).
2.2. fMRI task and imaging acquisition
Participants completed a visual processing task in the scanner with three conditions: letters, false fonts, and faces (previously described in Centanni et al., 2018a). Participants were asked to watch stimuli presented one at a time in the middle of the screen, and press a button if any stimulus was repeated twice in a row (i.e., a one-back task). Ten unique stimuli were used in each condition. Letter stimuli included lowercase English letters (b, c, f, k, m, p, r, s, t, y). In order to control for visual complexity, false font stimuli were created by rearranging the components of the 10 individual letter stimuli. Faces were all of a neutral expression and forward gaze, half male and half female, all Caucasian (from Karolinska Directed Emotional Faces; Lundqvist et al., 1998). Example false font and face stimuli are presented in Fig. 1.
Fig. 1.
Example false font and face stimuli. False fonts were created by rearranging parts of real letters. Face stimuli were neutral faces from the Karolinska Directed Emotional faces (KDEF) set.
Blocks of 10 trials of the same stimulus type (condition) and resting fixation blocks were presented. Repeated stimuli occurred randomly 3 times in each block, and stimulus order was counterbalanced within the blocks and across runs. Order of the runs and the hand used to respond during the task were each counterbalanced across participants. Participants completed 6 blocks of each condition and 6 blocks of resting fixation, with the order of blocks pseudo-randomized so that no condition was presented twice in a row. In order to optimize performance in children, the task was divided into two runs lasting 4 min and 8 s each so as to provide a break between runs.
Imaging was performed using a Siemens 3 T MAGNETOM Trio, A Tim System, (Siemens Medical Solutions, Erlangen, Germany) and a commercial Siemens 32 channel head coil. Functional data were collected with 3 × 3 × 4 mm resolution, 2000 ms TR, 30 ms TE, 90° flip, 64 × 64 base resolution, and 32 slices approximately parallel to the AC/PC line with coverage of the entire cortex. Prior to each scan, four images were acquired and discarded to allow longitudinal magnetization to reach equilibrium. PACE, an online prospective motion correction algorithm (Thesen et al., 2000), was implemented to reduce the effect of motion artifacts on data quality.
A critical issue in developmental neuroimaging is the observation that head motion during fMRI is frequently correlated with age (Satterthwaite et al., 2012) and this increased motion can be especially troublesome when scanning young children. Therefore, proper care needs to be taken such that fMRI differences are neither manufactured nor masked by differences in head motion (Chai et al., 2014). In the current study, care was taken to acclimate participants to the scanner environment prior to the actual fMRI session. This practice session consisted of the researcher describing the parts of the scanner, introducing the participants to the sight, sound, and feel of the scanner using a custom built mock scanner setup, and children practicing staying as still as possible with feedback from the researcher. Children practiced a shortened run of the same experimental task using different stimuli, and researchers monitored performance during practice to ensure that children understood and could complete the task during fMRI. We further consider motion in our analyses, below.
2.3. fMRI preprocessing and analysis
Preprocessing and data analyses were performed using Nipype, a Python-based framework for integrating neuroimaging analysis packages (Gorgolewski et al., 2011). The software packages used in this analysis pipeline included FMRIB Software Library (FSL 5.0.8), FreeSurfer (5.1.0), Advanced Normalization Tools (ANTS), and Nipype's implementation of Artifact Detection Tools (ART).
FreeSurfer was used to generate cortical surfaces and subcortical segmentations from each participant's anatomical image; surfaces were visually inspected for quality and manually edited. Functional images were realigned using FSL's MCFLIRT, with the first volume of the first run used as the reference volume. We spatially smoothed the functional data with a 6 mm FWHM Gaussian kernel, and applied a high-pass filter of 1/128 Hz. ART was used to identify outlier volumes based on motion and to calculate the number of motion outliers that coincided with stimulus presentation (reported as the correlation coefficient). The median functional image for each run was averaged across the two runs for each participant. This average median was then coregistered to the structural scan using FreeSurfer's bbregister. ANTS was used to register the structural image to MNI space (Oasis-30 Atropos template in MNI152, 2 mm version) and an adult template was used (for reasons described in Centanni et al., 2018a).
First-level analyses were performed using a general linear model approach. Regressors in the design matrix included the three task conditions (letters, false fonts, and faces) convolved with a double gamma hemodynamic response function. The six rigid-body realignment parameters (3 translations, 3 rotations) and the motion outliers detected by ART were included in the model as nuisance regressors to account for any degree of motion during the scan. Outliers were defined as any image where head placement deviated from the previous image by >1 mm or whose average signal intensity differed from the series average by >3 standard deviations. No participants had >20% of the acquired images flagged as outliers. A fixed effects analysis was performed to combine contrast images across runs, and a composite transform (bbregister and ANTS transformations) was used to normalize the resulting contrast images to MNI space in a single interpolation step.
2.4. VWFA identification and analysis approach
We used a combination of functional contrasts and anatomical landmarks to define each participant's regions of interest (ROIs). The VWFA is usually defined by overlapping activation in an area of normalized space across many individuals (Cohen et al., 2002; Dehaene et al., 2010; Olulade et al., 2013) or as a customized individual area of activation in native space (Baker et al., 2007; Ben-Shachar et al., 2011; Glezer and Riesenhuber, 2013; Saygin et al., 2016). There is some evidence in adults that individually defined VWFAs are more sensitive for defining that region than group averages or a location defined by the literature because the VWFA is a relatively small functional region and its precise location within the left fusiform region varies somewhat across individuals (Glezer and Riesenhuber, 2013). If the VWFA develops with reading experience, then its size may be smaller in children who are beginning readers.
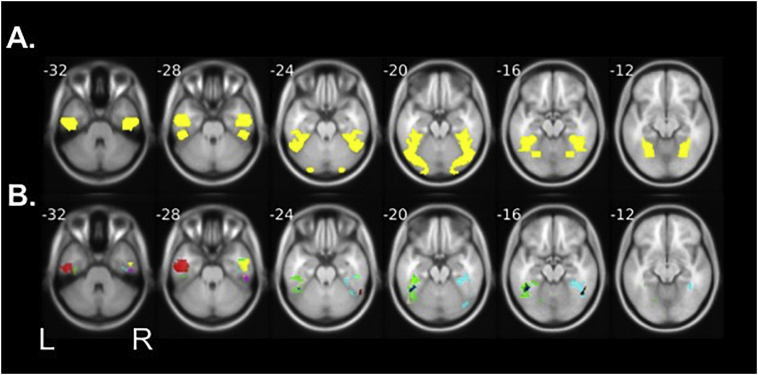
To address this issue in the current study, we analyzed findings using individually defined regions of interest (ROIs). Analyses were anatomically limited to left and right fusiform gyrus using a mask image created in WFU pickatlas (http://fmri.wfubmc.edu). We used this broad search space to accommodate any age-related anatomical differences between our participants and the MRI template used (Fig. 2). To identify a letter-sensitive region, each participant's response to the letters > faces contrast was thresholded to include only voxels with a z-value >2 (equivalent to p < .0455). Mean percent signal change values were then extracted for each participant's VWFA ROIs in response to letters, and to false fonts (both in comparison to a fixation rest condition).
Fig. 2.
ROI definition and locations. Numbers indicate slice (z coordinate, MNI), displayed on structural average brains. (A) Boundaries of fusiform gyrus in the left and right hemispheres are shown in yellow. Regions of interest for each participant were contained within the boundaries of this search space. (B) Representative regions of interest from 6 individual participants. Each color represents a different participant. All regions of interest analyzed contained a minimum of 10 contiguous voxels.
2.5. VWFA ROI descriptive statistics and analysis plan
For left hemisphere analyses, data were analyzed from children with a left fusiform region of interest that was 10 or more voxels (no-risk typical reader, N = 34; at risk-typical reader, N = 29, at-risk impaired reader, N = 15). There was no effect of group on the size of the included left hemisphere ROIs (F (2, 75) = 0.62, p = .54). For right hemisphere analyses, data were analyzed from children with a right fusiform region of interest containing 10 or more voxels (no risk typical reader, N = 30; at-risk typical reader, N = 25, at-risk impaired reader, N = 15). There was again no effect of group on the size of the included right hemisphere ROIs (F (2, 67) = 0.02, p = .98).
Repeated measures ANOVA (3 groups as repeated measures X 2 conditions) and post hoc t-tests were used to compare activation across groups and stimulus conditions. Other follow-up t-tests were paired when comparing condition within groups, or unpaired when comparing across groups, and were two-tailed unless otherwise indicated. In addition, we conducted the same analysis in a subset of children from each group who were matched for age and nonverbal IQ, in order to ensure that such differences did not drive the effect in the fMRI data. These matched subgroups were created by removing participants with the highest scores.
3. Results
Group characteristics and scores are reported in Table 1. In order to compare in-scanner behavioral performance, we conducted a 3 (group: no risk typical reader, at-risk typical reader, at-risk impaired reader) × 3 (stimulus: letters, faces, false fonts) repeated measures ANOVA. There were no main effects of stimulus (F (2, 192) = 0.16, p = .85) or group (F (2, 192) = 1.63, p = .20), and no interaction between stimulus and group (F (4, 192) = 1.17, p = .32). The at-risk impaired reader group did exhibit lower accuracies relative to both other groups, but this trend was similar for all three stimulus types.
3.1. Left fusiform activations across groups
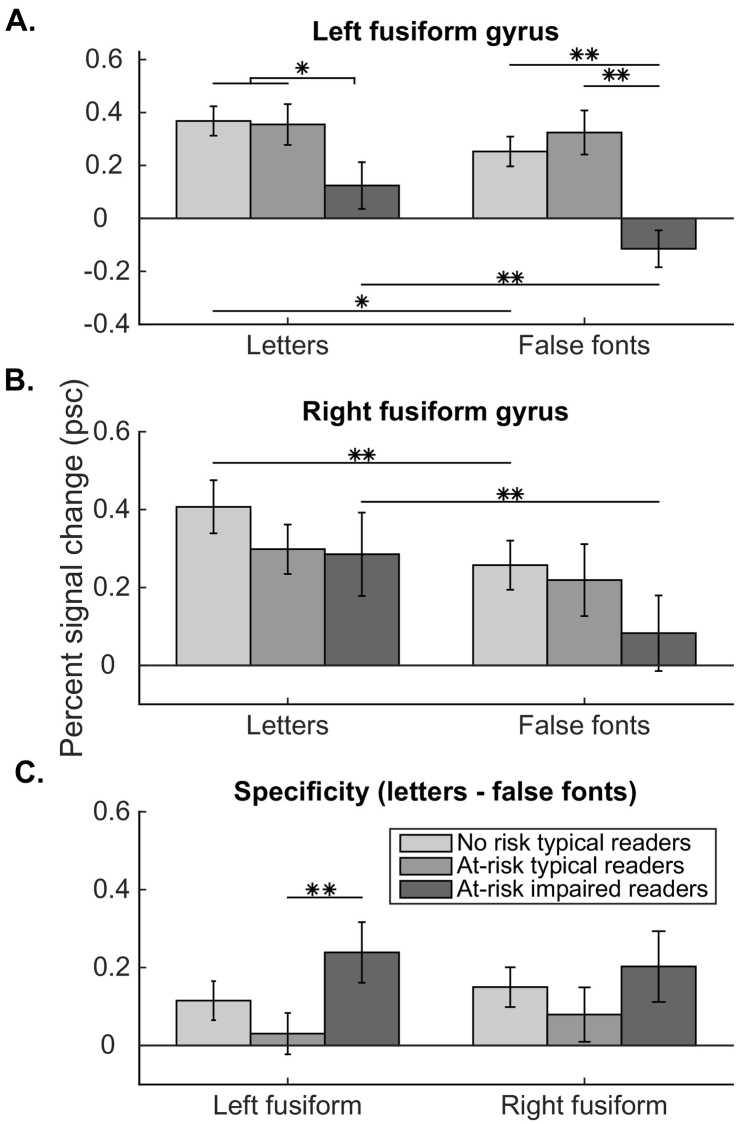
We compared percent signal change in each participant's left fusiform gyrus ROI for letters > fixation and false fonts > fixation across groups. Group activations are plotted in Fig. 3A. There was a main effect of group (F(2, 75) = 5.75, p = .005), with both typical reader groups (no-risk and at-risk) showing greater responses than the at-risk impaired reader group. There was also a main effect of condition (F(1, 75) = 11.02, p = .001), with a higher percent signal change to letters than false fonts. In addition, there was a trend in the interaction between group and stimulus (F(2, 75) = 2.65, p = .08; Fig. 3A), reflecting that both the no-risk typical reader group and the at-risk impaired reader groups showed greater activation for letters than false fonts, but the at-risk typical reader group showed no difference between letters and false fonts.
Fig. 3.
Percent signal change (PSC) by group in regions of interest. (A) PSC to letters and false font letters compared to fixation in left fusiform gyrus. (B) PSC to letters and false font letters compared to fixation in right fusiform gyrus. (C) Specificity to PSC to (letters – fixation) – (false fonts – fixation) in each hemisphere ROI. Note: * = p < .05, ** = p < .01.
Post hoc t-tests were used to further specify the observed main effects and interactions in left fusiform gyrus. In regard to group differences, there were no significant differences in percent signal change to letters > fixation between the groups of children who became typical readers, whether they were no-risk (0.37 ± 0.06) or at-risk (0.35 ± 0.08) (unpaired t-test, t (61) = 0.14, p = .89). Similarly, there was no significant difference in the two typical reader groups in response to false font letters > fixation (no-risk, 0.25 ± 0.06 and at-risk, 0.32 ± 0.08; unpaired t-test, t (61) = 0.74, p = .46). The two typical reader groups differed significantly from the at-risk impaired reader group in response to letters (impaired reader percent signal change = 0.12 ± 0.09; a priori one-tailed, unpaired t-test, t (76) = 2.34, p = .02) and to false fonts (−0.12 ± 0.07; t (76) = 3.86, p < .0001). The at-risk typical reader group did not differ significantly from the no-risk typical reader group in specificity for letters (t (61) = 1.18, p = .24), as both had higher activation for letters > fixation than false fonts > fixation. The at-risk typical reader group exhibited significantly less specificity than the at-risk impaired reader group (t (42) = 2.31, p = .026), reflecting the de-activation to false fonts in the at-risk impaired reader group (Fig. 3C). There were significant correlations between greater activation to letters and greater activation to false fonts in the left fusiform in the no-risk typical reader group (r = 0.60, p = .0002), the at-risk typical reader group (r = 0.78, p < .0001), and the at-risk impaired reader group (r = 0.54, p = .039).
3.2. Right fusiform activations across groups
In the right fusiform gyrus, the same analyses were performed (group activations plotted in Fig. 3B). Here, there was no significant main effect of group (F (2, 67) = 1.01, p = .37). There was a main effect of condition, with a significantly greater percent signal change for letters than false fonts (F (1, 67) = 13.09, p = .0006), but no interaction between group and stimulus (F (2, 67) = 0.77, p = .47). There were significant correlations between greater activation to letters and greater activation to false fonts in right hemisphere in the no-risk group (r = 0.70, p < .0001), the at-risk typical reader group (r = 0.65, p = .0004), and the at-risk impaired reader group (r = 0.61, p = .016).
3.3. Relation between activation and task performance
We evaluated whether activation in fusiform gyrus for a given task was associated with accuracy on the in-scanner task. Because most of the in-scanner performance values were above 80% and the distribution was thus skewed, we used Spearman correlations. For the whole-sample (all groups combined), there were no significant correlations between false font performance and fusiform ROI activation to false fonts in either hemisphere (ps > 0.45). There were also no relationships for letter performance and letter activation in VWFA in either hemisphere (ps > 0.25).
3.4. Print-specificity of group differences
To address the possibility that the at-risk impaired reader group exhibited hypoactivation broadly, we evaluated activation to faces > fixation in the left and right fusiform ROIs. At-risk impaired readers exhibited significantly less activation (or significantly more hypoactivation) to faces compared to both groups of typical readers (main effect of group in left hemisphere (F (2, 75) = 4.28, p = .017). There was no difference among the groups in the right fusiform (main effect of group in right hemisphere: F (2, 66) = 2.12, p = .13). Of note, the letters > faces contrast was used to select these ROIs and thus, the regions of interest were not face-specific (i.e., these are not analyses of the fusiform face area or FFA).
3.5. Relations between brain measures and future reading ability
Activation to letters and false fonts in left VWFA was also related to future reading outcomes. Using a composite reading score (calculated as the average of all 4 s grade reading measures, see Table 1), there were significant positive correlations between this composite measure and activation to letters (r = 0.27, p = .016) and to false fonts (r = 0.40, p = .0002). There was a trend in the negative relationship between this composite measure and specificity for letters (letters > false fonts; r = −0.20, p = .07).
3.6. Analyses of effects of age, IQ, and attention
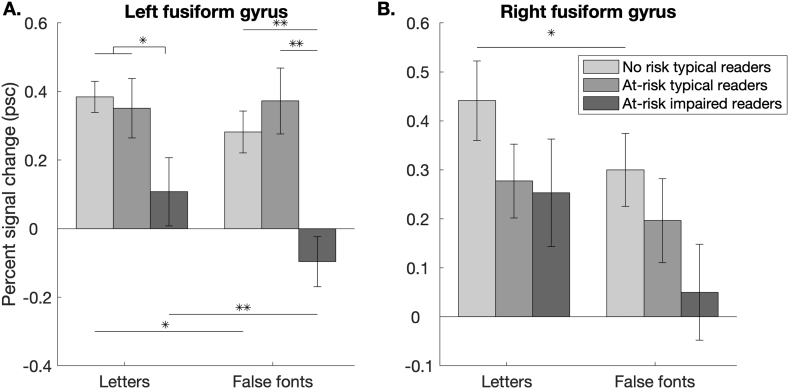
Because the three groups were not matched on age or IQ, we evaluated a smaller controlled sample to ensure that these two variables did not impact these results. A total of 79 children were included in this smaller sample (no risk N = 32; at-risk typical reader N = 30; at-risk impaired reader N = 17). These smaller groups did not differ significantly on age (F (2, 76) = 1.13, p = .33) or nonverbal IQ (F (2, 76) = 0.66, p = .52), and were created by excluding children who had outlying values. Of these children, 24 no-risk children had left FFG ROIs with 10 voxels or more along with 24 at-risk typical readers and 13 at-risk impaired readers. In right hemisphere, 24 no-risk children had ROIs with 10 or more voxels along with 19 at-risk typical readers and 14 at-risk impaired readers. Importantly, the pattern of results did not change when age and IQ were strictly controlled across groups, suggesting that age and IQ did not drive these findings (Fig. 4). Alhough the general pattern of findings was similar to that from the overall sample, the difference between letters vs. false fonts was no longer significant when comparing the age- and IQ-matched subsamples at-risk typical readers vs. at-risk impaired readers (t (36) = 1.60, p = .12), perhaps due to the smaller sample size. The comparison between the two typical reader groups remained non-significant (t (46) = 0.19, p = .85).
Fig. 4.
Percent signal change to letters and false font letters compared to fixation in regions of interest in a smaller sample matched for age- and IQ- across risk and reading outcomes. (A) Left fusiform gyrus. (B) Right fusiform gyrus. Note: * = p < .05, ** = p < .01.
Finally, to ensure that attention deficits did not influence these findings, we evaluated the results after removal of children whose parents reported that they had a diagnosis of ADHD in second grade, which included 3 no risk typical readers and 4 at-risk impaired readers (resulting groups: no risk typical readers N = 32, at-risk typical readers; N = 29; at-risk impaired readers N = 12). Patterns were similar to those observed in the larger groups; there were no differences between no-risk and at-risk typical readers in left fusiform activation to letters (t (59) = 0.28, p = .78) or false fonts (t (59) = 0.68, p = .50). There were significant differences between no-risk typical readers and at-risk impaired readers in left fusiform in response to false font (t (42) = 3.07, p = .004) and a trend in response to letters (t (42) = 1.95, p = .058). There was a trend level of difference between at-risk typical readers and at-risk impaired readers in the left fusiform activation to letters (t (71) = 1.67, p = .10) and a significant difference to false fonts (t (71) = 2.96, p = .004). There were no differences between letters (ps > 0.21) or false fonts (ps > 0.29) across groups in the right fusiform.
4. Discussion
These findings indicate, for the first time, that kindergarten children who will progress to reading impairment (consistent with dyslexia) have reduced responses to print, for both familiar letters and novel letter-like false fonts, in the VWFA of the left fusiform gyrus. The kindergarten children who subsequently developed reading impairment exhibited reduced activations to both kinds of print in the left fusiform, but there were no differences among the groups of children in right fusiform responses. Among children who appeared to be at risk based on family history or low scores on pre-reading assessments, only the group of children who developed reading impairment exhibited the reduced VWFA responses. Thus, reduced responses to print in the VWFA region, which have been regularly observed in older children and adults with dyslexia, are not only a consequence of long-term reading difficulty, but are present prior to school instruction in reading. Further, brain responses to letters and false fonts accounted for unique and significance variance in future reading outcomes beyond typical behavioral measures. These findings are unlikely to be attributable to age, IQ, or attention deficits, as analyses of subgroups matched on those measures showed nearly identical patterns to the full sample. It is unknown whether the reduced responses in children who go on to be impaired readers are due to genetics, early experiences, or both, but these findings indicate that reduced responses to print in the VWFA region may contribute to reading impairment at the outset of learning to read in school.
These results provide evidence even earlier in development than in previous studies that the VWFA plays an important role in learning to read. The two previous studies that examined print responses (letters or words) in young children at risk for dyslexia reported no differences in the VWFA region relative to typically developing children (Specht et al., 2009; Yamada et al., 2012). This could be because of those studies' approach of comparing no-risk to at-risk groups without separating them by subsequent reading outcomes. Those studies also used different risk criteria; for example, the composite risk index used by Specht et al., included handedness and motor development, which have not been linked as closely with reading as our proximal reading-related measures.
The present results show that kindergarten children who develop impaired reading exhibit reduced VWFA responses to print even before formal reading instruction, but it is not yet known as to whether the reduced VWFA response is secondary to atypical language processes or reflects an independent developmental difference arising from visual cortex. Skilled reading, even in the early stages of instruction, is not only associated with visual activation of the left fusiform gyrus for print, but also auditory activation of the left fusiform gyrus during auditory phoneme processing tasks (Blau et al., 2010; Desroches et al., 2010; Wang et al., 2018). Inconsistent neural responses to auditory are correlated with reading ability in children with dyslexia (Centanni et al., 2018b; Hornickel and Kraus, 2013; Neef et al., 2017), also suggesting that there is a link between speech sound processing and reading ability. Further, pre-reading children with familial risk for dyslexia have exhibited reduced left fusiform activation to auditory stimuli in tasks requiring phonemic awareness in both transparent (Dębska et al., 2016) and opaque orthographies (Powers et al., 2016) (although these studies did not evaluate subsequent reading outcomes in their samples). These findings raise the possibility that initial differences in auditory processes (for speech sound processing) lead to differences in fusiform gyrus function even before formal reading instruction. This may be due to the existing connections between auditory areas and the fusiform gyrus in pre-reading children (Saygin et al., 2016), and supports evidence of deficits in letter-sound integration in dyslexia (Blau et al., 2009; Froyen et al., 2011; Mittag et al., 2013).
Alternatively, children who will subsequently develop reading impairment or dyslexia may exhibit early (pre-reading) parallel developmental differences in both auditory and visual neocortices that both compromise learning to read. Pre-reading children without risk for dyslexia develop occipito-temporal responses to printed words prior to the start of formal reading instruction (Centanni et al., 2018a) as well as after just 8 weeks of training on letter-sound correspondences (Brem et al., 2010), demonstrating that even brief instructional exposure to print drives measurable plasticity in the brain. This rapid plasticity is also seen in adults learning to read for the first time (Dehaene et al., 2010) or learning to read in a new orthography over training periods as short as 2 days (Hashimoto and Sakai, 2004; Mei et al., 2013; Perrone-Bertolotti et al., 2014). Such early plasticity may be critical for future reading success. The apparently atypical responses observed in at-risk impaired readers at kindergarten age supports the hypothesis that differences in early plasticity may predispose them to impaired reading outcomes. Future research using longitudinal imaging datasets are needed to fully address this hypothesis.
Two aspects of these findings are particularly noteworthy. First, relative to children who progressed to typical reading, pre-reading children who became impaired readers had differences only in the left fusiform gyrus, ipsilateral to left-hemisphere language regions, and not in the right visual cortex. These findings support that idea that the developmental differences in fusiform response to print that lead to dyslexia are predominantly or exclusively left-lateralized. Second, the at-risk impaired-outcome children had reduced VWFA responses to all stimuli in our study, including real letters and false fonts.
The at-risk impaired reader group and the no-risk typical readers both exhibited a greater response to real letters than false fonts, whereas the at-risk typical-outcome children failed to exhibit any difference in response to real letters versus false fonts. A question of interest is whether the reduced activations in response to letters and false fonts could have been related to reduced familiarity with letters in the at-risk impaired-outcome group. This group of children had significantly lower Letter ID scores compared to both typical-outcome groups, but the lower scores were in the average range. Also, the at-risk impaired-outcome children were not impaired on either the letter or false-font one-back tasks performed during neuroimaging. Overall, the behavioral findings suggest that reduced activations in the at-risk impaired-outcome were not simply the consequence of substantially reduced familiarity with letters. Though sensitivity for print is present early in reading acquisition (Centanni et al., 2018a), specificity for letters over letter-like stimuli such as false fonts is more protracted and requires additional training and experience with print (Centanni et al., 2017; Dehaene et al., 2010). Such additional experience drives plasticity in the VWFA, as has been demonstrated even in previously illiterate adults learning to read (Dehaene et al., 2010). Experience with letters also leads to an organized gradient in the VWFA such that false fonts are processed posterior to real words (Olulade et al., 2013) and this organization is lacking in children with dyslexia (Olulade et al., 2015). The fact that at-risk impaired readers demonstrated greater activation in both left and right fusiform gyri to real letters than false fonts indicates that the experience of seeing real letters may have induced at least limited plasticity in VWFA regions of bilateral fusiform gyri.
It appears that responses to both letters and false fonts were driven by a shared mechanism because there were strong correlations across children in the magnitudes of activation is response to both letters and false fonts in all three groups of children and in both hemispheres. Perhaps experience with real letters drove the weaker response to false fonts, which were letter-like but novel variants of real letters (in order to ensure that the two kinds of stimuli shared similar features). The strong correlation between activations to letters and false fonts precludes distinguishing among three alternative interpretations of reduced responses in the at-risk reading-impaired group. One possibility is that experience with letters (perhaps in relation to spoken language) drove the weaker response to letter-like false fonts. When a child is first exposed to print, a parent or caregiver often provides the name or sound of the letter or word to facilitate learning. Therefore, from the earliest stages of reading acquisition, spoken language is inextricably linked to the corresponding visual print. This early reliance on auditory feedback may drive fusiform gyrus responses to letters and false fonts in typical readers, as described above. For those children who advance to reading impairment, poor auditory processing of speech sounds may influence early abnormal fusiform responses to the corresponding letters (Blau et al., 2010; Centanni et al., 2018b; Desroches et al., 2010; Hornickel and Kraus, 2013; Wang et al., 2018). Even individuals who learned to read in adulthood exhibit reduced top-down activation in the VWFA during an auditory lexical decision task (Dehaene et al., 2010), demonstrating this important link between speech sound and print processing.
Alternatively, there could have been an underlying difference in the visual processing of all kinds of letter-like stimuli independent of associated spoken language that extended to both real letters and false fonts. A third possibility is that the left fusiform gyrus is broadly hypoactive in dyslexia, as the at-risk impaired reader group exhibited significantly reduced activation to faces in addition to hypoactivation to letters and false fonts. Adults with dyslexia exhibit behavioral deficits in face recognition and matching tasks (Gabay et al., 2017; Sigurdardottir et al., 2015) as well as reduced habituation to non-linguistic stimuli such as faces (Perrachione et al., 2016), suggesting a more general deficit in visual processing of complex stimuli. Our results suggests that there may be a specific deficit in left VWFA or fusiform function in children who go on to impaired reading outcomes because those same children exhibited typical responses to letters, false fonts, and faces in the right fusiform fgyrus.
The findings from children who were at risk in kindergarten but who progressed to typical reading by second grade are also informative about early identification of children at risk for dyslexia. A consistent finding is that assessments of pre-reading skills (such as phonological awareness, rapid naming, and letter knowledge) can have high sensitivity in identification of children who will progress to dyslexia, but limited specificity, such that for every child identified as at-risk who progress to dyslexia there is another child identified as at-risk who progresses to typical reading (Johnson et al., 2009; Snowling et al., 2007). Indeed, this pattern was evident in our sample at rates similar to previous studies, indicating that our sample is similar to what is observed in other research settings and classrooms.
What underlies these false positives? One critical issue is the definition of risk. We used a relatively liberal definition (poor performance on any one of three measures or family history), which increases sensitivity for identifying truly at-risk children but does so at the cost of decreasing specificity. Another possibility is that some individual assessment scores for such young children may simply involve large error variance. A young child may have had a poor night of sleep prior to testing or have his or her attention wander during a test. Testing can involve error at any age, but the possibility is greater in young children (Bracken, 2007). An alternative (and not mutually exclusive) possibility is that there is a real difference between children without versus with identified risk who progress to typical reading. These children who screen as at risk but become typical readers, could, for example, be somewhat slower in development, or be more responsive to school or home instruction in reading.
The present findings support the idea that there are real differences, on average, between children identified inaccurately as being at risk versus both children identified correctly as being not at risk and also children identified correctly as being at risk and that these differences are especially obvious in the brain. If the only issue were invalid measurement, the false-positive (at-risk typical reader) group's brain responses should have been similar to the children who were at no risk (and who became typical readers). This was, however, not the case. The at-risk typical reader group was the only group to show no difference between real letters and false fonts in both the left fusiform gyrus and the right fusiform gyrus. It may be the case that among this group, having increased false font responses in the left fusiform is a protective factor, supporting typical reading development despite the children having other types of risk such as poor phonological awareness. These findings provide the first brain evidence that the frequent over-identification of risk for impaired reading in pre-reading children is not simply a matter of mismeasurement, but instead reflects true neurodevelopmental diversity in children who will progress to typical reading skill.
There are several limitations of the present study. First, the functional localizations reported here reflect particular analytic strategies. We used a constant, a priori threshold of z > 2 (or p < .0455) to threshold functional activation across all analyses (Centanni et al., 2018a), but findings could vary with more or less conservative thresholds. Second, because young children vary in their knowledge of printed words, with many having almost no measureable knowledge of printed words, we used individual letters that were known by all the children. The precise relation between left fusiform regions responding to individual letters versus words is complex in adults (Centanni et al., 2017; Flowers et al., 2004; James et al., 2005; Vinckier et al., 2007). It is clear from the present study that atypically reduced responses to individual letters in the left fusiform are associated with subsequent reading impairment. Precise linkage between left fusiform responses to letters in pre-reading children with left fusiform responses to words in older children who can read will require a longitudinal imaging study. Third, we can not draw conclusions about the children who were classified as no risk, but who became impaired readers due to the very small proportion of children with this profile. Fourth, we used a 1-back task for both letters and false fonts so that we could measure performance and attention in the children, and this task involves some working memory demands. The absence of a significant difference in performance across groups, however, suggests that ant potential differences in working memory ability were unlikely to have had a substantial influence on the activations.
In sum, hypoactivation of a region of left fusiform gyrus in response to both letters and false fonts occurred only in the group of kindergarten children who developed reading impairment over the next three years (from around the fall of kindergarten to the summer following second grade). The root cause of this hypoactivation is unknown as to whether it is a primary factor in dyslexia or secondary to auditory-language disabilities and as to whether it is genetic, environmental (experience), or both. The fact that it precedes the beginnings of school-based instruction for learning to read suggests that the hypoactivation of the VWFA that is characteristic of older children and adults is not simply a consequence of prolonged difficulty in learning to read, but may be present in the very early stages of school and thus may contribute to the initial impairment in learning to read. The present findings also suggest that interventions that include some support for visual processing of print may be helpful to beginning readers at risk for poor reading.
Acknowledgments
Acknowledgments
The authors thank Abigail Cyr, Keri-Lee Garel, Sydney Robinson, Candice Coulter, and Andrew Peach for assistance with assessment and MRI data collection. We thank the Athinoula A. Martinos Imaging Center at the McGovern Institute for Brain Research at MIT and its staff. We also thank our READ Study research testers, school coordinators and principals, and participating families. Participating schools are listed at http://gablab.mit.edu/index.php/READstudy. This work was supported by grants from NIH/NICHD (R01HD067312) to JDEG and NG (5R01HD065762) and from the Chan Zuckerberg Foundation to JDEG.
Declarations of interest
None.
References
- Bach S., Richardson U., Brandeis D., Martin E., Brem S. Print-specific multimodal brain activation in kindergarten improves prediction of reading skills in second grade. NeuroImage. 2013;82:605–615. doi: 10.1016/j.neuroimage.2013.05.062. [DOI] [PubMed] [Google Scholar]
- Baker C., Liu J., Wald L. Visual word processing and experiential origins of functional selectivity in human extrastriate cortex. Proc. Natl. Acad. Sci. 2007;104(21):9087–9092. doi: 10.1073/pnas.0703300104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shachar M., Dougherty R., Deutsch G., Wandell B. The development of cortical sensitivity to visual word forms. J. Cogn. Neurosci. 2011;23(9):2387–2399. doi: 10.1162/jocn.2011.21615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau V., Van Atteveldt N., Ekkebus M., Goebel R., Blomert L. Reduced neural integration of letters and speech sounds links phonological and reading deficits in adult dyslexia. Curr. Biol. 2009;19(6):503–508. doi: 10.1016/j.cub.2009.01.065. [DOI] [PubMed] [Google Scholar]
- Blau V., Reithler J., van Atteveldt N., Seitz J., Gerretsen P., Goebel R., Blomert L. Deviant processing of letters and speech sounds as proximate cause of reading failure: a functional megnetic resonance imaging study of dyslexia children. Brain. 2010;133(3):868–879. doi: 10.1093/brain/awp308. [DOI] [PubMed] [Google Scholar]
- Bracken B.A. Creating the optimal preschool testing situation. In: Bracken B.A., Nagle R.J., editors. Psychoeducational Assessment of Preschool Children. 4th ed. Lawrence Earlbaum Associates; Mahwah, NJ: 2007. pp. 137–153. [Google Scholar]
- Bradley L., Bryant P. Categorizing sounds and learning to read – a causal connection. Nature. 1983;310:419–421. [Google Scholar]
- Brem S., Bach S., Kucian K., Guttorm T.K., Martin E., Lyytinen H., Brandeis D., Richardson U. Brain sensitivity to print emerges when children learn letter-speech sound correspondences. Proc. Natl. Acad. Sci. 2010;107(17):7939–7944. doi: 10.1073/pnas.0904402107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catts H.W., Nielsen D.C., Bridges M.S., Liu Y.S., Bontempo D.E. Early identification of reading disabilities within an RTI framework. J. Learn. Disabil. 2015;48(3):281–297. doi: 10.1177/0022219413498115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centanni T., King L., Eddy M., Whitfield-Gabrieli S., Gabrieli J. Development of sensitivity versus specificity for print in the visual word form area. Brain Lang. 2017;170:62–70. doi: 10.1016/j.bandl.2017.03.009. [DOI] [PubMed] [Google Scholar]
- Centanni T.M., Norton E.S., Park A., Beach S.D., Halverson K., Ozernov-Palchik O.…Gabrieli J. Early development of letter specialization in left fusiform is associated with better word reading and smaller fusiform face area. Dev. Sci. 2018 doi: 10.1111/desc.12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centanni T., Pantazis D., Truong D., Gruen J., Gabrieli J., Hogan T. Increased variability of stimulus-driven cortical responses is associated with genetic variability in children with and without dyslexia. Dev. Cogn. Neurosci. 2018 doi: 10.1016/j.dcn.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai X., Ofen N., Gabrieli J., Whitfield-Gabrieli S. Selective development of anticorrelated networks in the intrinsic functional organization of the human brain. J. Cogn. Neurosci. 2014;26(3):501–513. doi: 10.1162/jocn_a_00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christodoulou J., Del Tufo S., Lymberis J. Brain bases of reading fluency in typical reading and impaired fluency in dyslexia. PLoS One. 2014;9(7) doi: 10.1371/journal.pone.0100552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark K.A., Helland T., Specht K., Narr K.L., Manis F.R., Toga A.W., Hugdahl K. Neuroanatomical precursors of dyslexia identified from pre-reading through to age 11. Brain. 2014;137(12):3136–3141. doi: 10.1093/brain/awu229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L., Dehaene S. Specialization within the ventral stream: the case for the visual word form area. NeuroImage. 2004;22:466–476. doi: 10.1016/j.neuroimage.2003.12.049. [DOI] [PubMed] [Google Scholar]
- Cohen L., Lehéricy S., Chochon F., Lemer C., Rivaud S., Dehaene S. Language-specific tuning of visual cortex? Functional properties of the visual word form area. Brain J. Neurol. 2002;125:1054–1069. doi: 10.1093/brain/awf094. Pt 5. [DOI] [PubMed] [Google Scholar]
- Dębska A., Łuniewska M., Chyl K., Banaszkiewicz A., Zelechowska A., Wypych M., Marchewka A.…Jednorog K. Neural basis of phonological awareness in beginning readers with familial risk of dyslexia—results from shallow orthography. NeuroImage. 2016;132:406–416. doi: 10.1016/j.neuroimage.2016.02.063. [DOI] [PubMed] [Google Scholar]
- Dehaene S., Pegado F., Braga L., Ventura P., Nunes Filho G., Jobert A.…Cohen L. How learning to read changes the cortical networks for vision and language. Science. 2010;330(6009):1359–1364. doi: 10.1126/science.1194140. [DOI] [PubMed] [Google Scholar]
- Déjerine J. Vol. 4. Mémoires de la Société de Biologie; 1892. Contribution à l’étude anatomopathologique et clinique des différents variétés de cécité verbale; pp. 61–90. [Google Scholar]
- Desroches A.S., Cone N.E., Bolger D.J., Bitan T., Burman D.D., Booth J.R. Children with reading difficulties show differences in brain regions associated with orthographic processing during spoken language processing. Brain Res. 2010;1356:73–84. doi: 10.1016/j.brainres.2010.07.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dundas E.M., Plaut D.C., Behrmann M. The joint development of hemispheric lateralization for words and faces. J. Exp. Psychol. Gen. 2013;142(2):348–358. doi: 10.1037/a0029503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn L., Dunn D. Pearson Assessments; San Antonio, TX: 2007. Peabody Picture Vocabulary Test: PPVT 4. [Google Scholar]
- Eicher J.D., Gruen J.R. Imaging-genetics in dyslexia: connecting risk genetic variants to brain neuroimaging and ultimately to reading impairments. Mol. Genet. Metab. 2013;110(3):201–212. doi: 10.1016/j.ymgme.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbro C., Nielsen I., Petersen D.K. Dyslexia in adults: evidence for deficits in non-word reading and in the phonological representation of lexical items. Ann. Dyslexia. 1994;44(1):203–226. doi: 10.1007/BF02648162. [DOI] [PubMed] [Google Scholar]
- Flowers D.L., Jones K., Noble K., VanMeter J., Zeffiro T.A., Wood F.B., Eden G.F. Attention to single letters activates left extrastriate cortex. NeuroImage. 2004;21:829–839. doi: 10.1016/j.neuroimage.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Froyen D., Willems G., Blomert L. Evidence for a specific cross-modal association deficit in dyslexia: an electrophysiological study of letter–speech sound processing. Dev. Sci. 2011;14(4):635–648. doi: 10.1111/j.1467-7687.2010.01007.x. [DOI] [PubMed] [Google Scholar]
- Gabay Y., Dundas E., Plaut D., Behrmann M. Atypical perceptual processing of faces in developmental dyslexia. Brain Lang. 2017;173:41–51. doi: 10.1016/j.bandl.2017.06.004. [DOI] [PubMed] [Google Scholar]
- Galaburda A.M., LoTurco J., Ramus F., Fitch R.H., Rosen G.D. From genes to behavior in developmental dyslexia. Nat. Neurosci. 2006;9(10):1213–1217. doi: 10.1038/nn1772. [DOI] [PubMed] [Google Scholar]
- Glezer L., Riesenhuber M. Individual variability in location impacts orthographic selectivity in the “visual word form area”. J. Neurosci. 2013;33(27):11221–11226. doi: 10.1523/JNEUROSCI.5002-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski K., Burns C., Madison C., Clark D., Halchenko Y., Waskom M., Ghosh S. Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in python. Front. Neuroinform. 2011;5 doi: 10.3389/fninf.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttorm T., Leppänen P. Event-related potentials in newborns with and without familial risk for dyslexia: principal component analysis reveals differences between the groups. J. Neural Transm. 2003;110(9):1059–1074. doi: 10.1007/s00702-003-0014-x. [DOI] [PubMed] [Google Scholar]
- Guttorm T., Leppänen P.H.T., Richardson U., Lyytinen H. Event-related potentials and consonant differentiation in newborns with familial risk for dyslexia. J. Learn. Disabil. 2001;34(6):534–544. doi: 10.1177/002221940103400606. [DOI] [PubMed] [Google Scholar]
- Hashimoto R., Sakai K.L. Learning letters in adulthood: direct visualization of cortical plasticity for forming a new link between orthography and phonology. Neuron. 2004;42(2):311–322. doi: 10.1016/s0896-6273(04)00196-5. [DOI] [PubMed] [Google Scholar]
- Hirshorn E.A., Li Y., Ward M.J., Richardson R.M., Fiez J.A., Ghuman A.S. Decoding and disrupting left midfusiform gyrus activity during word reading. Proc. Natl. Acad. Sci. 2016;113(29):8162–8167. doi: 10.1073/pnas.1604126113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F., Meyler A., Hernandez A., Juel C., Taylor-Hill H., Martindale J.L.…Gabrieli J.D.E. Functional and morphometric brain dissociation between dyslexia and reading ability. Proc. Natl. Acad. Sci. 2007;104(10):4234–4239. doi: 10.1073/pnas.0609399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornickel J., Kraus N. Unstable representation of sound: a biological marker of dyslexia. J. Neurosci. 2013;33(8):3500–3504. doi: 10.1523/JNEUROSCI.4205-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James K.H., James T.W., Jobard G., Wong A.C., Gauthier I. Letter processing in the visual system: different activation patterns for single letters and strings. Cogn. Affect. Behav. Neurosci. 2005;5:452–466. doi: 10.3758/cabn.5.4.452. [DOI] [PubMed] [Google Scholar]
- Johnson E.S., Jenkins J.R., Petscher Y., Catts H.W. How can we improve the accuracy of screening instruments? Learning Disabilities Research & Practice. 2009;24(4):174–185. [Google Scholar]
- Katzir T., Shaul S., Breznitz Z., Wolf M. The universal and the unique in dyslexia: a cross-linguistic investigation of reading and reading fluency in Hebrew-and English-speaking children with reading. Read. Writ. 2004;17(7–8):739–768. [Google Scholar]
- Kaufman A., Kaufman N. 2nd edition (KBIT-2) AGS, American Guidance Service; Circle Pines, MN: 2004. Kaufman Brief Intelligence Test. [Google Scholar]
- Leppänen P.H., Hämäläinen J.A., Salminen H.K., Eklund K.M., Guttorm T.K., Lohvansuu K.…Lyytinen H. Newborn brain event-related potentials revealing atypical processing of sound frequency and the subsequent association with later literacy skills in children with familial dyslexia. Cortex. 2010;46:1362–1376. doi: 10.1016/j.cortex.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Lundqvist D., Flykt A., Ohman A. Department of Clinical Neuroscience, psychology section, Karolinska Institutet; 1998. The Karolinska Directed Emotional Faces (KDEF) ISBN 91–630–7164-9. [Google Scholar]
- Lyytinen H., Erskine J., Tolvanen A., Torppa M., Poikkeus A.M., Lyytinen P. Trajectories of reading development: a follow-up from birth to school age of children with and without risk for dyslexia. Merrill-Palmer Q. 2006:514–546. [Google Scholar]
- Maisog J., Einbinder E., Flowers D., Turkeltaub P., Eden G. A meta-analysis of functional neuroimaging studies of dyslexia. Ann. N. Y. Acad. Sci. 2008;1145:237–259. doi: 10.1196/annals.1416.024. [DOI] [PubMed] [Google Scholar]
- Mascheretti S., De Luca A., Trezzi V., Peruzzo D., Marino C., Arrigoni F. Neurogenetics of developmental dyslexia: from genes to behavior through brain neuroimaging and cognitive and sensorial mechanisms. Transl. Psychiatry. 2017;(12) doi: 10.1038/tp.2016.240. (e987–15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer U., Brandeis D., McCandliss B.D. Fast, visual specialization for reading in English revealed by the topography of the N170 ERP response. Behav. Brain Funct. 2005;1(1):13. doi: 10.1186/1744-9081-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer U., Brem S., Bucher K., Kranz F., Benz R., Steinhausen H.C., Brandeis D. Impaired tuning of a fast occipito-temporal response for print in dyslexic children learning to read. Brain. 2007;130(12):3200–3210. doi: 10.1093/brain/awm193. [DOI] [PubMed] [Google Scholar]
- Maurer U., Zevin J.D., McCandliss B.D. Left-lateralized N170 effects of visual expertise in reading: evidence from Japanese syllabic and logographic scripts. J. Cogn. Neurosci. 2008;20(10):1878–1891. doi: 10.1162/jocn.2008.20125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei L., Xue G., Lu Z., He Q., Zhang M., Xue F., Chen C., Dong Q. Orthographic transparency modulates the functional asymmetry in the fusiform cortex: an artificial language training study. Brain Lang. 2013;125(2):165–172. doi: 10.1016/j.bandl.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittag M., Thesleff P., Laasonen M., Kujala T. The neurophysiological basis of the integration of written and heard syllables in dyslexic adults. Clin. Neurophysiol. 2013;124:315–326. doi: 10.1016/j.clinph.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Molfese D. Predicting dyslexia at 8 years of age using neonatal brain responses. Brain Lang. 2000;72:238–245. doi: 10.1006/brln.2000.2287. [DOI] [PubMed] [Google Scholar]
- Neef Nicole E., Müller Bent, Liebig Johanna, Schaadt Gesa, Grigutsch Maren, Gunter Thomas C., Wilcke Arndt. Dyslexia risk gene relates to representation of sound in the auditory brainstem. Developmental cognitive neuroscience. 2017;24:63–71. doi: 10.1016/j.dcn.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton E.S., Beach S.D., Gabrieli J.D.E. Neurobiology of dyslexia. Curr. Opin. Neurobiol. 2015;30:73–78. doi: 10.1016/j.conb.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olulade O., Flowers D., Napoliello E., Eden G. Developmental differences for word processing in the ventral stream. Brain Lang. 2013;125(2):134–145. doi: 10.1016/j.bandl.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olulade O.A., Flowers D.L., Napoliello E.M., Eden G.F. Dyslexic children lack word selectivity gradients in occipito-temporal and inferior frontal cortex. NeuroImage Clinical. 2015;7:742–754. doi: 10.1016/j.nicl.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozernov‐Palchik O., Gaab N. Tackling the ‘dyslexia paradox’: reading brain and behavior for early markers of developmental dyslexia. Wiley Interdisciplinary Reviews: Cognitive Science. 2016;7(2):156–176. doi: 10.1002/wcs.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozernov‐Palchik O., Norton E.S., Sideridis G., Beach S.D., Wolf M., Gabrieli J.D., Gaab N. Longitudinal stability of pre‐reading skill profiles of kindergarten children: implications for early screening and theories of reading. Developmental science. 2017;20(5) doi: 10.1111/desc.12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington B.F., Lefly D.L. Early reading development in children at family risk for dyslexia. Child Dev. 2001;72(3):816–833. doi: 10.1111/1467-8624.00317. [DOI] [PubMed] [Google Scholar]
- Perrachione T.K., Del Tufo S., Winter R., Murtagh J., Cyr A., Chang P., Halverson K., Ghosh S., Christodoulou J., Gabrieli J.D.E. Dysfunction of rapud neural adaptation in dyslexia. Neuron. 2016;92:1383–1397. doi: 10.1016/j.neuron.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone-Bertolotti M., Vidal J.R., de Palma L., Hamame C.M., Ossandon T., Kahane P., Minotti L., Bertrand O., Lachaux J.-P. Turning visual shapes into sounds: early stages of reading acquisition revealed in the ventral occipitotemporal cortex. NeuroImage. 2014;90:298–307. doi: 10.1016/j.neuroimage.2013.12.027. [DOI] [PubMed] [Google Scholar]
- Peterson R.L., Pennington B.F. Developmental dyslexia. Ann. Rev. Clin. Psychol. 2015;1:283–307. doi: 10.1146/annurev-clinpsy-032814-112842. [DOI] [PubMed] [Google Scholar]
- Powers S.J., Wang Y., Beach S.D., Sideridis G.D., Gaab N. Examining the relationship between home literacy environment and neural correlates of phonological processing in beginning readers with and without a familial risk for dyslexia: an fMRI study. Annals of Dyslexia. 2016;66(3):337–360. doi: 10.1007/s11881-016-0134-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puolakanaho A., Ahonen T., Aro M., Eklund K., Leppänen P.H.T., Poikkeus A.-M.…Lyytinen H. Very early phonological and language skills: estimating individual risk of reading disability. J. Child Psychol. Psychiatry. 2007;48:923–931. doi: 10.1111/j.1469-7610.2007.01763.x. [DOI] [PubMed] [Google Scholar]
- Ramus F., Rosen S., Dakin S., Day B. Theories of developmental dyslexia: insights from a multiple case study of dyslexic adults. Brain. 2003;126(4):841–865. doi: 10.1093/brain/awg076. [DOI] [PubMed] [Google Scholar]
- Raschle N.M., Zuk J., Gaab N. Functional characteristics of developmental dyslexia in left-hemispheric posterior brain regions predate reading onset. Proc. Natl. Acad. Sci. 2012;109(6):2156–2161. doi: 10.1073/pnas.1107721109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards T., Aylward E. Individual fMRI activation in orthographic mapping and morpheme mapping after orthographic or morphological spelling treatment in child dyslexics. J. Neurolinguistics. 2006;19(1):56–86. [Google Scholar]
- Richlan F. Developmental dyslexia: dysfunction of a left hemisphere reading network. Front. Hum. Neurosci. 2012;6(120) doi: 10.3389/fnhum.2012.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F., Kronbichler M., Wimmer H. Functional abnormalities in the dyslexic brain: a quantitative meta-analysis of neuroimaging studies. Hum. Brain Mapp. 2009;30:3299–3308. doi: 10.1002/hbm.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F., Kronbichler M., Wimmer H. Meta-analyzing brain dysfunctions in dyslexic children and adults. NeuroImage. 2011;56(3):1735–1742. doi: 10.1016/j.neuroimage.2011.02.040. [DOI] [PubMed] [Google Scholar]
- Satterthwaite T., Wolf D., Loughead J. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. NeuroImage. 2012;60(1):623–632. doi: 10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saygin Z., Norton E., Osher D.E., Beach S.D., Cyr A.B., Ozernov-Palchik O.…Gabrieli J. Tracking the roots of reading ability: white matter volume and integrity correlate with phonological awareness in prereading and early-reading kindergarten children. J. Neurosci. 2013;33(33):13251–13258. doi: 10.1523/JNEUROSCI.4383-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saygin Z., Osher D., Norton E., Youssoufian D., Beach S., Feather J.…Kanwisher N. Connectivity precedes function in the development of the visual word form area. Nat. Neurosci. 2016;19(9):1250–1255. doi: 10.1038/nn.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdardottir H.M., Ivarsson E., Kristinsdottir K., Kristjansson A. Impaired recognition of faces and objects in dyslexia: evidence for ventral stream dysfunction? Neuropsychology. 2015;29(5):739–750. doi: 10.1037/neu0000188. [DOI] [PubMed] [Google Scholar]
- Snowling M.J., Melby-Lervag M. Oral language deficits in familial dyslexia: a meta-analysis and review. Psychol. Bull. 2016;142:498–545. doi: 10.1037/bul0000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowling M.J., Muter V., Carroll J. Children at family risk of dyslexia: a follow-up in early adolescence. J. Child Psychol. Psychiatry. 2007;48(6):609–618. doi: 10.1111/j.1469-7610.2006.01725.x. [DOI] [PubMed] [Google Scholar]
- Specht K., Hugdahl K., Ofte S., NygÅrd M., BjØrnerud A., Plante E., Helland T. Brain activation on pre-reading tasks reveals at-risk status for dyslexia in 6-year-old children: health and disability. Scand. J. Psychol. 2009;50(1):79–91. doi: 10.1111/j.1467-9450.2008.00688.x. [DOI] [PubMed] [Google Scholar]
- Swan D., Goswami U. Phonological awareness deficits in developmental dyslexia and the phonological representations hypothesis. J. Exp. Child Psychol. 1997;66(1):18–41. doi: 10.1006/jecp.1997.2375. [DOI] [PubMed] [Google Scholar]
- Thesen S., Heid O., Mueller E., Schad L.R. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magn. Reson. Med. 2000;44(3):457–465. doi: 10.1002/1522-2594(200009)44:3<457::aid-mrm17>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Torgesen J.K. Vol. 185. 2001. The theory and practice of intervention: comparing outcomes from prevention and remediation studies. Dyslexia: Theory and good practice; p. 202. [Google Scholar]
- Torgesen J.K., Wagner R.K., Rashotte C.A. Pro-Ed; Austin, TX: 2012. TOWRE-2 Examiner’s Manual. [Google Scholar]
- Torppa M., Poikkeus A.M., Laakso M.L., Eklund K., Lyytinen H. Predicting delayed letter knowledge development and its relation to grade 1 reading achievement among children with and without familial risk for dyslexia. Dev. Psychol. 2006;42(6):1128. doi: 10.1037/0012-1649.42.6.1128. [DOI] [PubMed] [Google Scholar]
- Van der Mark S., Bucher K., Maurer U., Schulz E. Children with dyslexia lack multiple specializations along the visual word-form (VWF) system. NeuroImage. 2009;47(4):1940–1949. doi: 10.1016/j.neuroimage.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Vandermosten M., Boets B., Wouters J., Ghesquiere P. A qualitative and quantitative review of diffusion tensor imaging studies in reading and dyslexia. Neurosci. Biobehav. Rev. 2012;36(6):1532–1552. doi: 10.1016/j.neubiorev.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Vellutino F., Scanlon D. Phonological coding, phonological awareness, and reading ability: evidence from a longitudinal and experimental study. Merrill-Palmer Q. 1987;33:321–363. [Google Scholar]
- Vinckier F., Dehaene S., Jobert A., Dubus J., Sigman M., Cohen L. Hierarchical coding of letter strings in the ventral stream: dissecting the inner organization of the visual word-form system. Neuron. 2007;55:143–156. doi: 10.1016/j.neuron.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Wagner R., Torgesen J., Rashotte C. Pro-Ed; Austin, TX: 1999. Comprehensive Test of Phonological Processing. [Google Scholar]
- Wang Y., Mauer M.V., Raney T., Peysakhovich B., Becker B.L.C., Silva D.D., Gaab N. Development of tract-specific white matter pathways during early reading development in at-risk children and typical controls. Cereb. Cortex. 2017;27(4):2469–2485. doi: 10.1093/cercor/bhw095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Joanisse M.F., Booth J.R. Reading skill related to left ventral occipitotemporal cortex during a phonological awareness task in 5-6 year old children. Dev. Cogn. Neurosci. 2018;30:116–122. doi: 10.1016/j.dcn.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M., Denckla M. Pro-Ed; Austin, TX: 2005. Rapid Automatized Naming and Rapid Alternating Stimulus Tests (RAN/RAS) [Google Scholar]
- Woodcock R.W., editor. Woodcock Reading Mastery Tests-R/NU. Pearson; San Antonio, TX: 1998. [Google Scholar]
- Woodcock R.W. Pearson; 2011. Woodcock Reading Mastery Tests: WRMT-III. [Google Scholar]
- Yamada Y., Stevens C., Dow M., Harn B., Chard D.J., Neville H.J. Emergence of the neural network for reading in five-year old beginning readers of different levels of pre-literacy abilities: an fMRI study. NeuroImage. 2012;57(3):704–713. doi: 10.1016/j.neuroimage.2010.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]