Abstract
Quality specimens from biobanks are key resources to support reproducible research. Sustaining biobanks requires robust management. We recently published a pilot survey that indicated that over half the participating biobanks had business plans in place and another third were working on business planning. While the results provided a clue to the status of business planning in biobanking, it was concluded that a longer and more in-depth survey and analysis were required. In April 2017, an extended survey was distributed worldwide in English, French, Chinese, German, and Spanish, through multiple channels. The survey was built using the Survey Monkey tool. Our hypothesis was that those biobanks that already have a business plan also have a more professional management structure. The questions were designed to understand more details about each biobank's business operations and communications. A total of 276 biobanks participated (China 65, France 40, United States 34, Spain 27, Germany 24, Australia 23, and rest of the world 63). About two thirds of the biobanks were established in the last 10 years. The responses provided data on the size of biobanks answering the survey, their status of business planning, and how and through what mediums they are communicating with customers. Biobanks with a business plan or preparing to have one showed a clear trend of having a customer strategy for marketing the samples and communicating with customers. No trend could be seen regarding websites and activities in social media. We confirmed our hypothesis that biobanks that have or are in the process of preparing a business plan are showing a trend toward more professional structures. In the biobanking community, the business mind-set and use of the business plan as a management tool have not quite arrived.
Keywords: sustainability, business plan, biobanking, biobank management
Introduction
There is a clear imperative for the use of quality human biological samples and associated data in basic, preclinical, and clinical research, which has led to an increase in the reliance of biobanking infrastructures to support these research demands. Biobanks, which are often based on clinical and academic settings, are relied on as key infrastructures, which must meet ongoing and emerging needs for a range of quality specimen types and associated data for the stakeholders they serve. Consequently, biobanks must ensure ongoing sustainability through sound business planning with the ability to adapt to future market requirements.
Globally, biobanks have an increasing interest in sustainability, and are moving to develop their strategic and operating models and planning to ensure long-term success.1–4 However, due to the variability and diversity of biobanks5 in terms of size, sample type, specificity of research area, resource requirements, etc., the applicability and level of business planning may differ, and there is not a “one size fits all” model of sustainability. By considering the different pillars of sustainability, including operational, financial, and social aspects and learning from the experiences of others, biobanks can develop and adapt their model to support their stakeholder and organizational requirements.6,7
Relatively little reporting has been done on the level of business planning in biobanks8 and associated effectiveness (or not) in achieving sustainable practices. In this article, we provide insight on the move to business planning and sustainability in biobanking across the globe.
Early in 2017, we published the results of a pilot survey focused on the awareness and level of business planning in biobanks, which included the participants of the sustainability symposium at the 2016 Annual Meeting of the International Society of Biological and Environmental Repositories (ISBER) held in Berlin, Germany.6 The survey was delivered through a smartphone device during the symposium. While the pilot survey gave us some clues to the level of business planning and indications that biobanks are professionalizing, the participation was small and may not represent the wider biobanking community. Hence, we acknowledged that the results and any interpretation had limitations.
To achieve a greater sample size and to ensure better representation of the global community, we decided to extend the scope of the survey and do more extensive sampling of biobanks worldwide. In doing so, our aim was to gather more information about the types, size, and setup of the biobanks taking part, although we made it clear that the scope of the survey was to focus on sustainability of research biobanks. Hence, potential participants were informed that the purpose of the material and data stored in their biobank should be for biomedical research only and not for therapeutic or diagnostic purposes, and the survey should be answered with that in mind.
Following the analysis of the pilot survey results, we expanded the survey to clarify specific areas, and we continued to ask about (1) information on the biobanks they represent, (2) business planning practices, (3) level of utilization of the existing collections, (4) their users/customers, and (5) extent of marketing/advertising. This was done to collect data on levels of existing business planning, marketing activities, and sustainability. We extended the number of questions in each of these areas and added more specific questions on cost recovery, sources of income, and performance measures.
This article focuses on the status and effectiveness of biobank business planning in developing professional structures with associated successful marketing measures to enable broad and sustainable support for internal and external projects.
Material and Methods
The focus of the survey was to ask questions about biobanking and business practices in biobanks globally. To receive responses from the biobankers around the world, the survey was created and coded into the Survey Monkey online tool.a Our plan to reach biobankers in many areas of the world included translation into several languages. We found contacts that would verify the translation of the survey in Spanish, German, and French.
The survey was originally coded in English in April 2017 and included 37 questions (Supplementary Appendix S1). The questions were sent in an Excel file to biobank colleagues in France, Spain, and Germany to translate the questions into their native languages. Once it was translated, the translations were coded into Survey Monkey, and the link to the survey was sent back for a final verification to the translators and alternates to make sure the translations as coded were completely understandable. For the Chinese survey, we sent the 37 questions to a colleague in China to translate the survey and to put it within a tool that could be used to reach the biobanks in China.
The survey was initiated in English, Spanish, German, and French in late June 2017 and stayed open until January 2018, allowing for the Chinese survey to be initiated and closed at the same time as the other version. The English, Spanish, German, and French versions were advertised with three major campaigns by the authors and several organizations in the biobanking sector (ISBER, European, Middle Eastern & African Society for Biopreservation and Biobanking (ESBB), Biobanking and Biomolecular Resources Research Infrastructure (BBMRI) country nodes, and various connections throughout the community). The majority of the responses from the English, Spanish, and German surveys were received in July 2017. The bulk of the responses to the French survey were received in September. The Chinese survey was coded in local software and opened across China. Subsequently, the raw data were translated and delivered back to the authors. The raw data from the other translations were downloaded from Survey Monkey in three data sets, and then all data were combined to complete the analyses.
A descriptive analysis of the data was performed using Microsoft Access 2013. As is customary with these types of surveys, the data reported by the participants are assumed to be given, but responses are not always complete. A monitoring and data cleaning step usually does not take place. This, in turn, severely restricts the possibilities of statistical analysis, as the individual groups have unbalanced records.
Results
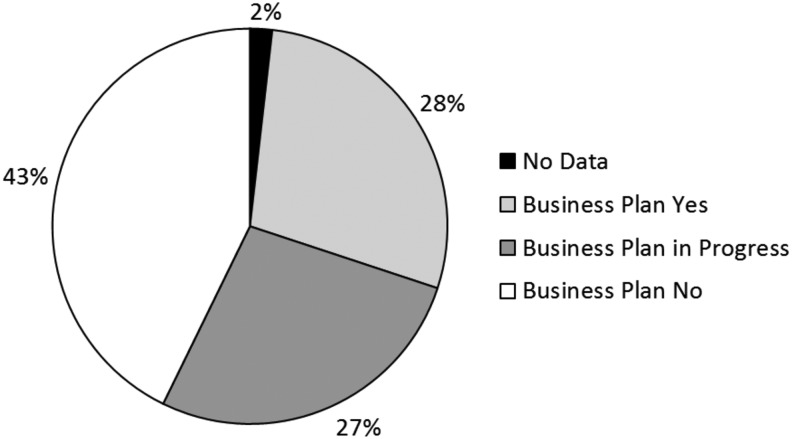
A total of 276 biobanks participated (China 65, France 40, United States 34, Spain 27, Germany 24, Australia 23, and rest 63) (Table 1). Each response came from an individual biobank, although it is possible that a biobank was also part of a multicenter network. More than half of the biobanks (51.4%) are academic biobanks, 21.7% hospital-based biobanks, and 8.7% governmental biobanks. Seventy-eight biobanks (28.3%) have a business plan (BP+), 75 biobanks (27.2%) have a business plan in progress (BP-IP), and 118 biobanks (42.8%) have no business plan (BP−); 5 biobanks (1.8%) gave no answer to this question (Fig. 1).
Table 1.
Country of Origin of Participating Biobanks
| n | % | |
|---|---|---|
| China | 65 | 24 |
| United States | 34 | 12 |
| France | 40 | 14 |
| Spain | 27 | 10 |
| Australia | 23 | 8 |
| Germany | 24 | 9 |
| The Netherlands | 11 | 4 |
| United Kingdom | 15 | 5 |
| Canada | 9 | 3 |
| Rest of the world | 28 | 10 |
| Total | 276 | 100 |
FIG. 1.
Availability of biobanking business plan.
Approximately two-thirds of BP+ biobanks plan to regularly update their business plan, and another quarter plan to revise it as requested by their organizations. In BP-IP biobanks, one-quarter plan to periodically update their BP, and one-half plan to revise it as requested by their organizations. In academic biobanks, 28.2% have BP+, 26.8% BP-IP, and 41.5% BP−; hospital-based biobanks have 18.3% BP+, 40.0% BP-IP, and 41.7% BP−; and governmental biobanks have 29.2% BP+, 16.7% BP-IP, and 54.2% BP−.
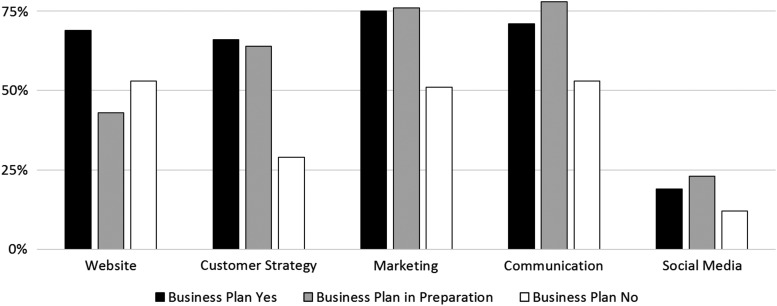
Biobanks, classified as BP+ and BP-IP, respectively, report that they more actively market their samples (75.4%/76.3%) than BP− biobanks (51.1%) (Fig. 2). They also communicate more regularly with their customers (BP+/BP-IP/BP−: 71.0%/78.3%/52.7%) and have a strategy to approach their customers (66.2%/63.9%/28.6%). About one of five biobanks surveyed (BP +19.1%; BP-IP 23.0%) are active on social media. BP− biobanks are less active (one of eight; 12.1%). There are no obvious differences between BP+, BP-IP, or BP− biobanks regarding repeated users/customers (89.9%/88.5%/84.8%) or in terms of a strategy to proactively collect specimens for specific customers (65.7%/70.5%/66.3%). Just >69% of BP+ biobanks (69.1%) have a website, whereas 43.3% of BP-IP biobanks and 53.3% of BP− biobanks have one.
FIG. 2.
Measure of professional communication practices (% positive responses) in Biobanks with (BP+) or in Preparation (BP-IP) of a Business Plan.
Seventy-two percent of BP+ biobanks, 67.8% of BP-IP biobanks, and 44.2% of BP− biobanks report that they have established performance metrics.
Three of four biobanks that participated in this survey were established after 2003, and one-quarter of these were established after 2012. BP+ biobanks are slightly older (about one-third were established before 2004), and BP-IP biobanks are more recently formed (62.7% established after 2010). One-third of the academic and governmental biobanks were established before 2004, whereas 11.7% of hospital-based biobanks were established at this time.
The number of samples stored in the participating biobanks ranges from 0 to 11 million with a mean of 457,273 and a median of 64,837. The first quartile is from 0 to 5000 samples, and the fourth quartile is ≥200,000 samples. The median in BP+ biobanks is 90,000, in BP-IP 60,000 samples, and in BP− 50,000 samples.
Discussion
The global move toward precision medicine and advanced methods of molecular interrogation of samples for disease research has expanded the demand for large numbers of quality biospecimens and data to support these efforts. Our business practices survey has a larger reach across biobanking organizations in the world, especially as we translated the originally English survey into several languages (French, Spanish, German, and Chinese). We realize that our survey reached only a small portion of biobanks that we know to exist across the world, but the information gleaned from participants is representative of the more advanced operations. This is based on our earlier smaller survey, a review of the literature, and personal communications.8 We are cautious in speculating all causes of the move toward business planning, but we discuss several drivers of professionalization in biobanking.
Over the past two decades, biobanking has progressively become part of the core services operations of academic, industrial, government, and philanthropic research organizations. Inherent in the setup and operations of biobanking around the world is the understanding that biobanks are very heterogeneous units, based on the goals of the research organization, the stakeholders of the biobank, as well as the size and diversity of collections to support the current customer/user base.
Business planning is a required component for those biobanks that are considering their marketability with industrial partners. The need to harmonize and sustain biobanks within multicentered networks could also be drivers toward business planning and professionalization. The lack of networking information for our respondents is a limitation of our data set, and it means that we are unable to confirm this correlation. Quality biobanking is a long-term and expensive institutional commitment. Thus, sustaining the operations of biobanks financially, operationally, and socially requires flexible solutions over time, based on the type of unit established.9–12
Underlying the operations of a modern and professionalized biobank is the need for strong business practices, with business planning at its core. Our global survey suggests that organizations that support biobanks have increasingly recognized the need to professionalize their management, staffing, and operations.
Advanced degrees in biobanking have been established at several universities across the world,13–15 and classes in business management for core operations are being conducted as stand-alone training and tools (16, add SBA on side note) or included in conferences.3,4 Biobankers are now taking the opportunity to be trained to develop business plans,17 which includes training in analysis of cost of collection, processing, operations, staffing, training, quality control, governance, marketing, stakeholder engagement, cost-recovery and disaster-recovery planning. There are also tools18 and case examples19–29 on cost-recovery and business planning from the existing biobanks to aid others in this area.
It is good news that biobanks around the world have established, or are beginning the process to establish, business plans to support their biobanking operations. It is clear that research organizations are still in the early “wave” of becoming professionalized, as we see that 44% of respondents in our survey have not yet established their business plans.
Based on the responses, no one sector can be singled out as leading the charge in developing business plans for their biobanking operations. The larger the total collection size, the higher the percentage of biobanks that have established business plans or are in the process of developing them. This suggests that the burden of cost and maintaining quality of large collections have been recognized as a driver for the organization to become more professionalized.
It might be an interesting follow-up to collect additional survey data to correlate the level of core biobank funding with the collection size and the level of professionalization, including staffing numbers of each biobank. One might speculate finding more developed staffing matrices and professionalization of operations (business planning) in larger and more well-funded biobanks versus those that are less well funded, but this could be likely an oversimplification without the available data.
We can also speculate that it is possible that larger biomedical organizations, such as government or industry, may have centralized business capacities that are not being considered as part of the biobanks' operations role and response. A reported lack of a business plan in this scenario could be their misinterpretation because the business planning is being managed outside of the biobank.
The majority of the responders from hospital-based biobanks are fairly new, being established in the last 15 years. This may explain the higher percentage of hospital-based biobanks with business plans in development versus those already established. We expect that for those with business plans in place, this has been a relatively recent endeavor; thus, the absence of a clear plan to periodically update the plans. More experience in monitoring the metrics of the plans against the “real world” in the biobank operations should guide the need for updates and revisions to the business plans. To be most effective, the business plan must be a “living document” that guides the biobank toward the measurement of metrics that leads to sustainability and relevance for their organizational needs.
Once an organization has initiated the process of gathering their data to build a customized business plan for their biobank, the key elements of the plan, including stakeholder engagement and marketing, become increasingly relevant. Publicity of the biobank is important to bring new users and increase utilization of collections in storage. In turn, the use of the specimens builds a “user history,” and shows the value of the collections and the biobank to the organization.
Several of the biobanks are establishing websites for their operations, although it is unclear if the main driver of these sites is an organizational requirement, providing data to existing users or to market to new users. It would be interesting to further analyze the types of data provided and uses of biobank websites across our global community. Websites can be a key factor in discoverability of biobanks and also offer one avenue to provide updates on use of samples to patients and volunteers; these can be a key factor in supporting both financial and social sustainability. Some of the marketing of biobank collections and services is being done by social media, although it is in its infancy, per the survey results. A future analysis of a variety of biobank websites may yield some interesting clues toward successful best practices in biobank marketing.
Biobankers around the world are worried about long-term continuity of their quality specimen collections and sustainability of their operations. Professionalizing the activities within a business plan is a critical way to analyze the operational goals, costs, stakeholders, management processes, and revenue streams to work toward financial, social, and operational sustainability. Business planning for biobanking organizations is beginning to build momentum across the world and across a variety of types and sizes of biobanks.
While we feel that business planning is at the core of professionalism of biobanking, currently, it is not yet at the point where it has become a mandatory activity. Our survey provided some insights into the status and some aspects of business planning and stakeholder engagement in biobanking; however, understanding all of the drivers underlying the choices taken by the biobanks in their business planning and stakeholder engagement will require additional research.
In this article, we have focused on the primary goals of the survey, although in the future there may be opportunities to conduct further analyses for ancillary questions from the initial data set, combined with additional sources, as noted. A companion paper discusses the metrics being established within reported biobank business plans and further expands on global utilization rates.
Supplementary Material
Acknowledgments
We are indebted to our colleagues, Jeanne-Hélène Di Donate and the Club 3C-R, France; Johanna Dungl, Austria/BBMRI-ERIC; Eoin Gaffney, Ireland; Catherine Kennedy, Australia; Manuel Morente, Spain; Phil Quinlan, United Kingdom; Peter Riegman, the Netherlands; Roman Siddiqui, Germany; Peter Watson, Canada; Andy Zaayenga, US/ISBER Weekly News Digest; and Xuexun Zhou, China, who took the effort to translate the English survey to their own native languages and/or for the promotion of the survey in the multiple campaigns that were launched across the world.
Disclaimer
The authors do not have any institutional or commercial affiliations that might pose a conflict of interest regarding the publication of this article.
This research was conducted after review and exemption of the NCI Special Studies Institutional Review Board.
Author Disclosure Statement
No conflicting financial interests exist.
Supplementary Material
References
- 1. Henderson MK, Simeon-Dubach D, Zaayenga A. When bad things happen: Lessons learned from effective and not so effective disaster and recovery planning for biobanks. Biopreserv Biobank 2013;11:193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Simeon-Dubach D, Henderson MK. Sustainability in biobanking. Biopreserv Biobank 2014;12:287–291 [DOI] [PubMed] [Google Scholar]
- 3. Henderson MK, Simeon-Dubach D, Albert M. Finding the path to biobank sustainability through sound business planning. Biopreserv Biobank 2015;13:385–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Henderson MK, Goldring K, Simeon-Dubach D. Achieving and maintaining sustainability in biobanking through business planning, marketing, and access. Biopreserv Biobank 2017;15:1–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chalmers D, Nicol D, Kaye J, et al. Has the biobank bubble burst? Withstanding the challenges for sustainable biobanking in the digital era. BMC Med Ethics 2016;17:39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cadigan RJ, Edwards TP, Lassiter D, et al. “Forward-thinking” in U.S. biobanking. Genet Test Mol Biomarkers 2017;21:148–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Simeon-Dubach D, Watson P. Biobanking 3.0: Evidence based and customer focused biobanking. Clin Biochem 2014;47:300–308 [DOI] [PubMed] [Google Scholar]
- 8. Simeon-Dubach D, Goldring K, Henderson MK. Trends in biobanking business planning: initial results of a survey of biobankers. Biopreserv Biobank 2017;15:72–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Doucet M, Yuille M, Georghiou L, et al. Biobank sustainability: Current status and future prospects. J Biorepository Sci Appl Med 2017;51:7 [Google Scholar]
- 10. Macheiner T, Huppertz B, Bayer M, et al. Challenges and driving forces for business plans in biobanking. Biopreserv Biobank 2017;15:121–125 [DOI] [PubMed] [Google Scholar]
- 11. Ciaburri M, Napolitano M, Bravo E. Business planning in biobanking: How to implement a tool for sustainability. Biopreserv Biobank 2017;15:46–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yuille MM, Feller PI, Georghiou L, et al. Financial sustainability of biobanks: From theory to practice. Biopreserv Biobank 2017;15:85–92 [DOI] [PubMed] [Google Scholar]
- 13. Gormally E, Hardy I, Caboux E, et al. Training the next generation of biobankers: A two-year master's course in the management of biobanks. Biopreserv Biobank 2017;15:438–450 [DOI] [PubMed] [Google Scholar]
- 14. Postgraduate School. Master of Science Biobanking. 2018. Available at: https://postgraduate-school.medunigraz.at/universitaetslehrgaenge/masterlehrgaenge/master-of-science-biobanking/ (Accessed July9, 2018)
- 15. IBBL. University Biobanking Certificate. 2018. Available at: https://www.ibbl.lu/ibbl-bioservices/university-biobanking-certificate/ (Accessed July9, 2018)
- 16. ISBER. Biobanking Education Opportunities. 2018. Available at: https://www.isber.org/default.aspx?page=BiobankEduOpp (Accessed July9, 2018)
- 17. Sba.gov. Create a Business Plan | The U.S. Small Business Administration |. 2018. Available at: https://www.sba.gov/tools/business-plan/1 (Accessed July9, 2018)
- 18. Odeh H, Miranda L, Rao A, et al. The Biobank Economic Modeling Tool (BEMT): Online financial planning to facilitate biobank sustainability. Biopreserv Biobank 2015;13:421–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Parry-Jones A. Assessing the financial, operational, and social sustainability of a biobank: The Wales Cancer Bank case study. Biopreserv Biobank 2014;12:381–388 [DOI] [PubMed] [Google Scholar]
- 20. Sargsyan K, Macheiner T, Story P, et al. Sustainability in Biobanking: Model of Biobank Graz. Biopreserv Biobank 2015;13:410–420 [DOI] [PubMed] [Google Scholar]
- 21. Seiler CY, Eschbacher J, Bowser R, et al. Sustainability in a hospital-based biobank and university-based DNA biorepository: Strategic roadmaps. Biopreserv Biobank 2015;13:401–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brown T, Kelly DD, Vercauteren SM, et al. How biobanks are assessing and measuring their financial sustainability. Biopreserv Biobank 2017;15:65–71 [DOI] [PubMed] [Google Scholar]
- 23. Tarling TE, Lasser F, Carter C, et al. Business planning for a campus-wide biobank. Biopreserv Biobank 2017;15:37–45 [DOI] [PubMed] [Google Scholar]
- 24. Kelly SM, Wiehagen LT, Schumacher PE, et al. Methods to improve sustainability of a large academic biorepository. Biopreserv Biobank 2017;15:31–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Warth R, Perren A. Construction of a business model to assure financial sustainability of biobanks. Biopreserv Biobank 2014;12:389–394 [DOI] [PubMed] [Google Scholar]
- 26. Uzarski D, Burke J, Turner B, et al. Plan for academic biobank solvency-leveraging resources and applying business processes to improve sustainability. Clin Transl Sci 2015;8:553–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carpenter JE, Clarke CL. Biobanking sustainability—experiences of the Australian Breast Cancer Tissue Bank (ABCTB). Biopreserv Biobank 2014;12:395–401 [DOI] [PubMed] [Google Scholar]
- 28. Albert M, Bartlett J, Johnston RN, et al. Biobank bootstrapping: Is biobank sustainability possible through cost recovery? Biopreserv Biobank 2014;12:374–380 [DOI] [PubMed] [Google Scholar]
- 29. De Souza YG. Sustainability of biobanks in the future. Adv Exp Med Biol 2015;864:29–35 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.