Abstract
The antimicrobial peptide cathelicidin inhibits development of colitis-associated colon cancer. However, the role of cathelicidin in colon cancer metastasis remains unknown. We hypothesized that cathelicidin is effective in inhibiting colon cancer metastasis. Human colon cancer HT-29 cells were injected intravenously into nude mice. Control HA-tagged adeno-associated virus (HA-AAV) or cathelicidin-overexpressing AAV (CAMP-HA-AAV) were injected intravenously into nude mice on the same day. Four weeks later, the nude mice were assessed for lung and liver metastases. Human colon cancer SW620 cells were used to study the effect of cathelicidin on cell migration and cytoskeleton. Incubation of SW620 cells with cathelicidin dose-dependently reduced cell migration, disrupted cytoskeletal structure, and reduced βIII-tubulin (TUBB3) mRNA expression. The addition of the P2RX7 antagonist KN62, but not the FPRL1 antagonist WRW4, prevented the LL-37-mediated inhibition of cell migration and TUBB3 mRNA expression. The CAMP-HA-AAV-overexpressing group showed significantly reduced human CK20 protein (by 60%) and TUBB3 mRNA expression (by 40%) in the lungs and liver of the HT-29-loaded nude mice, compared to the HA-AAV control group. Intraperitoneal injection of KN62 reversed the CAMP-HA-AAV-mediated inhibition of human CK20 and TUBB3 expression in the lungs and liver of HT-29-loaded nude mice. In conclusion, cathelicidin inhibits colon cancer metastasis via a P2RX7-dependent pathway.
Keywords: colon cancer, metastasis, antimicrobial peptide
Introduction
Low survival rates of advanced colon cancer (stage IIIC 53% and stage IV 11% in 5 years; American Cancer Society) and high treatment resistance have driven discovery of novel drug targets and therapeutic approaches. Cathelicidin (human gene CAMP, human protein LL-37, mouse gene Camp, and mouse protein mCRAMP) is an antimicrobial peptide that possesses therapeutic effects in inflammatory bowel disease, Clostridium difficile infection, and obesity.1, 2, 3, 4 Recent studies have also shown that cathelicidin is involved in malignancies. The tumoral expression of endogenous cathelicidin varies among different types of cancer.5, 6 For example, cathelicidin expression is diminished in gastric and colon cancers,7, 8 while it is increased in breast, ovarian, and lung cancers.9, 10, 11 Endogenous cathelicidin modulates azoxymethane (AOM)-mediated colon cancer in mice.7 Cathelicidin suppresses gastric cancer cell proliferation via the bone morphogenetic protein-mediated pathway.8 The biological functions of cathelicidin are largely mediated by its receptors, which include FPRL1 and P2RX7.12
Cathelicidin and its analog FK-16 induce p53-dependent apoptosis in human colon cancer HCT116 cells.7, 13 Other cathelicidin analogs (FF/CAP18 and Ceragenin CSA13) inhibit HCT116 cell proliferation without relying on the p53-dependent mechanism in vitro.14, 15 Interestingly, cathelicidin does not inhibit the viability of colon cancer HT-29 cells directly, but inhibits tumor-associated fibroblasts (TAFs) through suppression of epithelial-mesenchymal transition (EMT) and disruption of the cytoskeleton.16 The inhibition of TAFs, in turn, reduces their support of colon cancer cell proliferation. All of the evidence suggests that cathelicidin is a potential target for colon cancer. However, the role of cathelicidin and its receptor in metastatic colon cancer is unknown.
Metastatic colon cancer cells often possess mesenchymal characteristics, as exemplified by βIII-tubulin expression, which is associated with increased cell migratory behavior.17, 18 We hypothesize that cathelicidin acts through receptor-dependent modulation of the cytoskeleton in the colon cancer cells, leading to a reduction of colon cancer cell migration. To address this hypothesis, a nude mouse model and various cell-based assays were included in this study.
Results
Systemic Overexpression of Cathelicidin Inhibited Colon Cancer Metastasis in Nude Mice
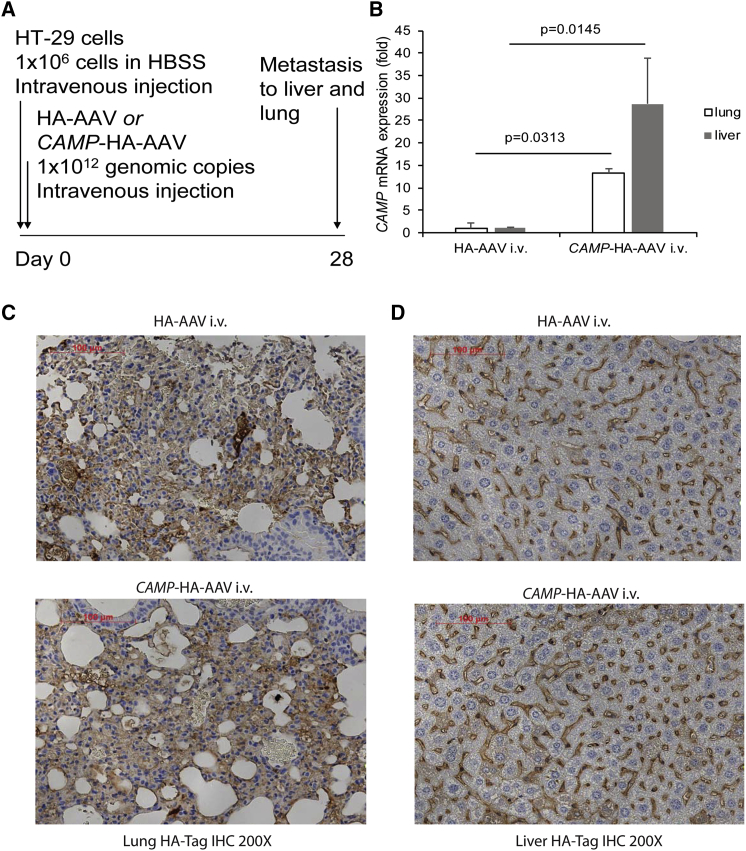
To determine whether cathelicidin inhibits colon cancer metastasis, the nude mice were injected intravenously with human colon cancer HT-29 cells (Figure 1A). Control hemagglutinin-expressing adeno-associated viruses (HA-AAVs) and human cathelicidin-overexpressing AAVs (CAMP-HA-AAVs) were injected into nude mice intravenously on the same day as the HT-29 injection, as described in a previous study.16
Figure 1.
Intravenous Cathelicidin-Expressing Adeno-Associated Virus Administration Expressed Cathelicidin mRNA and Viral Marker Protein in Lungs and Liver of HT-29-Loaded Nude Mice
(A) Experimental plan. (B) Human cathelicidin mRNA expression in lungs and liver. (C and D) HA-Tag Immunohistochemistry of (C) lung and (D) liver tissues. AAV-infected cells were stained with HA antibody and appeared brown. The extent of AAV infection was similar among tumors from the HA-AAV and CAMP-HA-AAV groups.
Human CAMP mRNA expression was low in the lungs and liver of the HA-AAV control group (threshold cycle [Ct] value, 38–40). Infection of CAMP-HA-AAVs significantly increased cathelicidin mRNA expression in the lungs and liver of the recipient mice (Figure 1B). All groups carried similar intensities of HA-tagged staining in the lungs and liver, indicating equal loading of AAV particles and expression of their gene products in nude mice (Figures 1C and 1D).
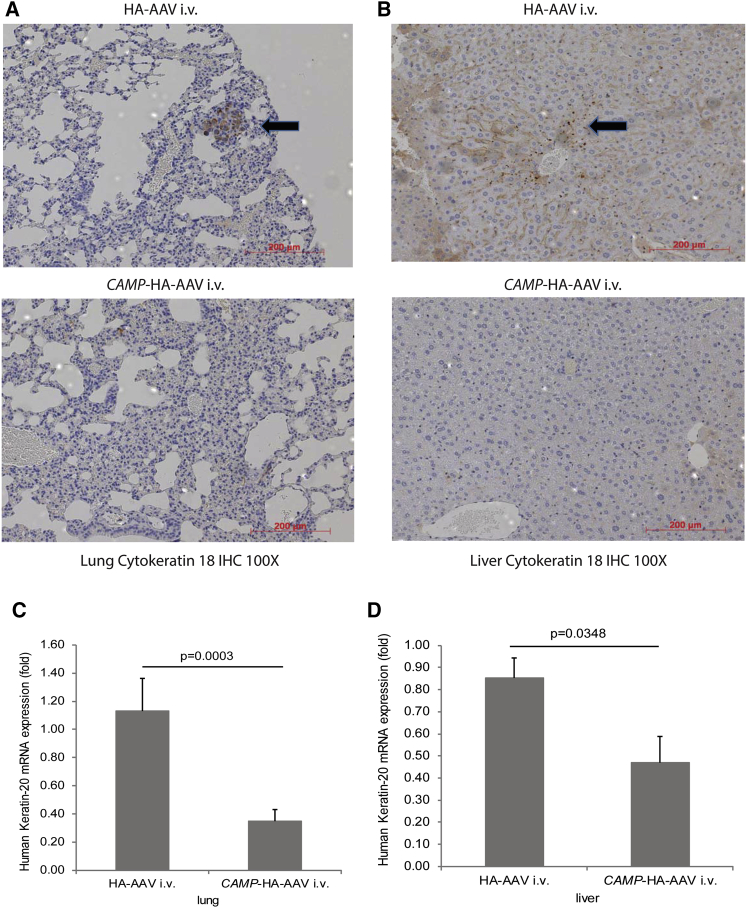
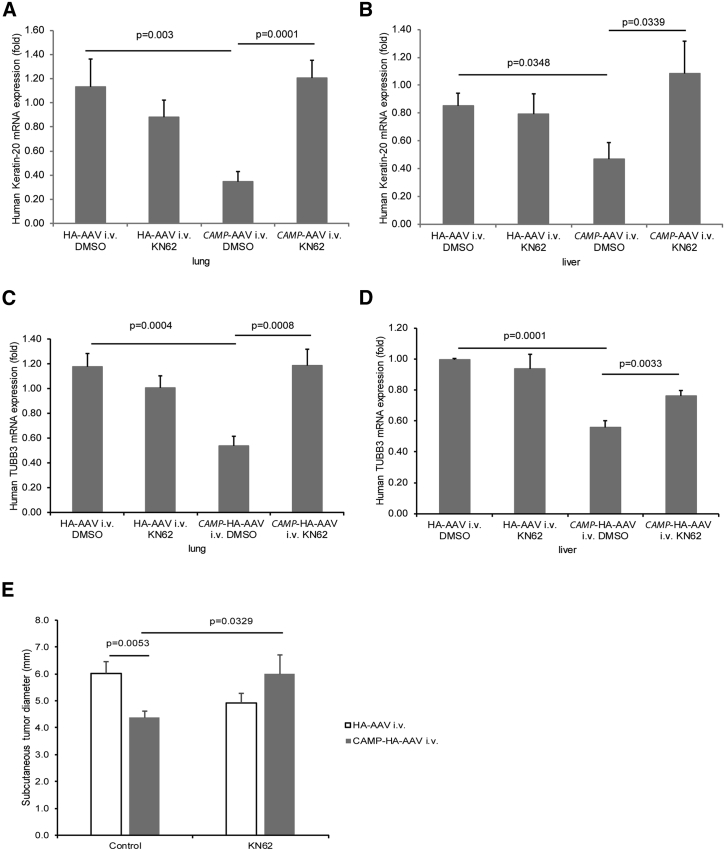
The injected nude mice developed human cytokeratin 18-positive tumor colonies in the lungs and liver, indicating colon cancer metastasis (Figures 2A and 2B). The lung and liver tissues in the cathelicidin-overexpressing group showed much less human-specific cytokeratin 18 staining than those in the control group. Cathelicidin overexpression significantly reduced human keratin-20 mRNA expression in the lungs and liver of HT-29-loaded nude mice (Figures 2C and 2D). Cytokeratin 18 and keratin 20 are epithelial colon cancer markers.19, 20 Both approaches indicated that cathelicidin overexpression inhibited colon cancer metastasis.
Figure 2.
Intravenous Cathelicidin-Expressing Adeno-Associated Virus Administration Reduced the Presence of Human Colon Cancer Cells in Lungs and Liver of HT-29-Loaded Nude Mice
(A and B) Human cytokeratin-18 expression (representing human colon cancer cells) in (A) lungs and (B) liver of nude mice was identified by brown color spots (indicated by arrows). Intravenous cathelicidin expressing AAVs reduced human cytokeratin 18 expression in lungs and liver of nude mice. (C and D) Human keratin 20 mRNA expression in (C) lungs and (D) liver of nude mice was significantly reduced by CAMP-HA-AAV.
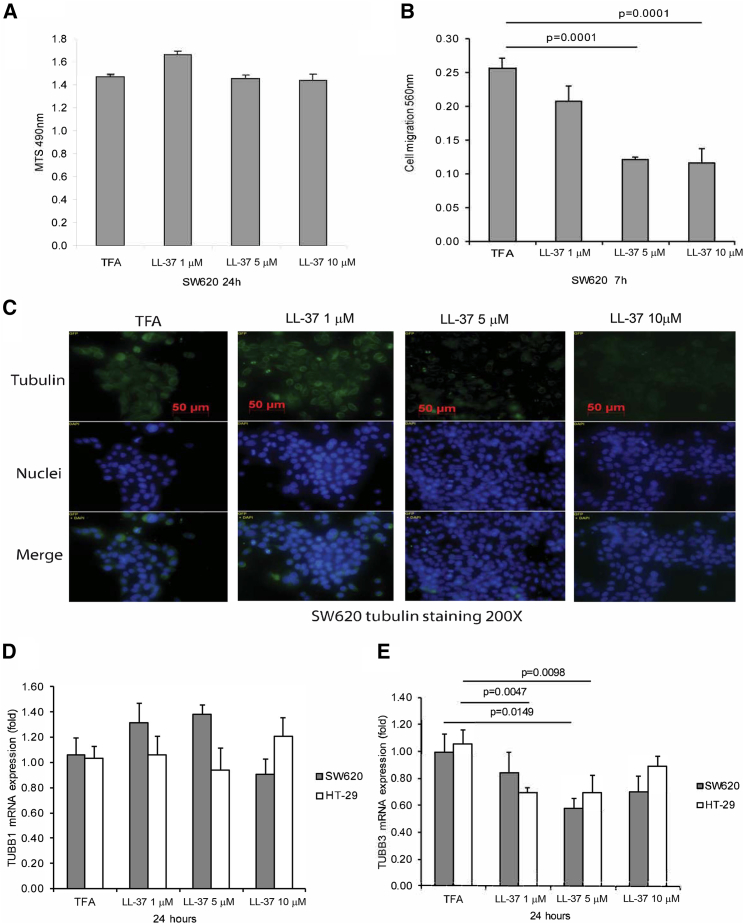
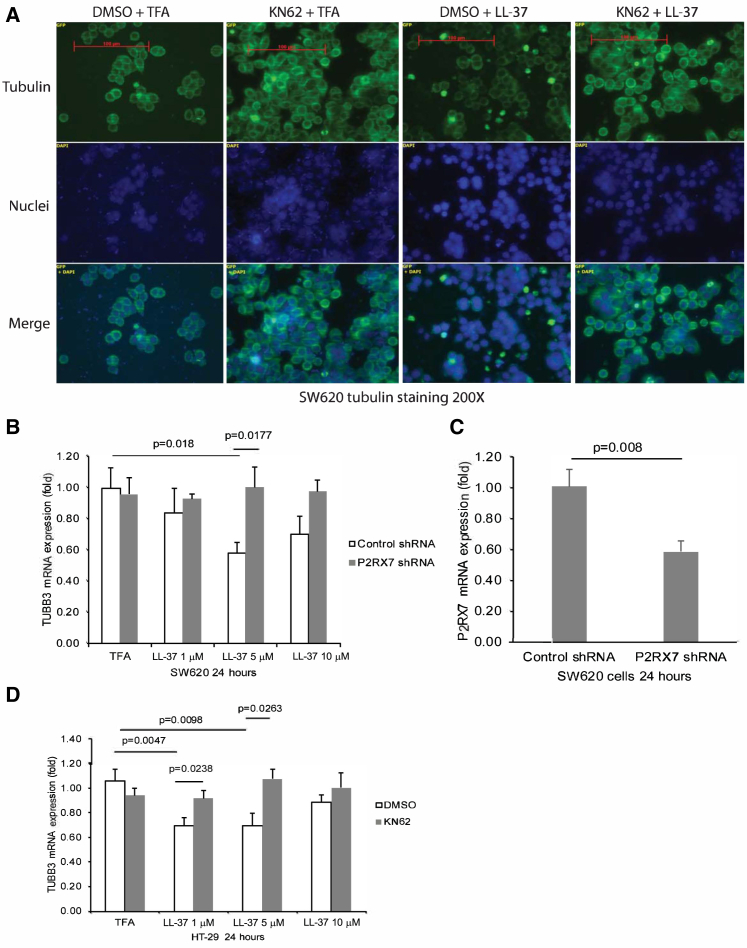
Cathelicidin Disrupted Tubulin Cytoskeleton and Inhibited Cell Migration of Colon Cancer Cells
Consistent with prior cell viability studies involving HT-29 colon cancer cells and CCD-18Co fibroblasts,16 cathelicidin peptide (LL-37) did not affect the viability of SW620 cells (Figure 3A). LL-37 (5–10 μM) inhibited migration of SW620 cells (Figure 3B), which reflected the inhibition of metastatic potential. Tumoral tubulin expression is associated with liver metastasis of colon cancer.21 Cathelicidin-mediated disruption of tubulin structure in HT-29 and CCD-18Co cells suggests the potential role of tubulin in the anti-metastatic effect of cathelicidin.16 Tubulin tracker staining demonstrated that incubation of human advanced colon cancer SW620 cells with LL-37 (5–10 μM) disrupted the tubulin structure in a dose-dependent manner (Figure 3C). Constitutive TUBB1 mRNA expression in SW620 and HT-29 cells was not affected by exposure to LL-37 (Figure 3D).
Figure 3.
Cathelicidin Inhibited Cell Migration and TUBB3 Expression
(A) Cell viability of SW620 cells. (B) Cell migration of SW620 cells. (C) Green tubulin tracker staining with blue nuclear staining in human cancer SW620 cells. LL-37 reduced tubulin expression in SW620 cells. (D) TUBB1 mRNA expression in SW620 and HT-29 cells. (E) TUBB3 mRNA expression in SW620 and HT-29 cells. Results were pooled from three independent experiments.
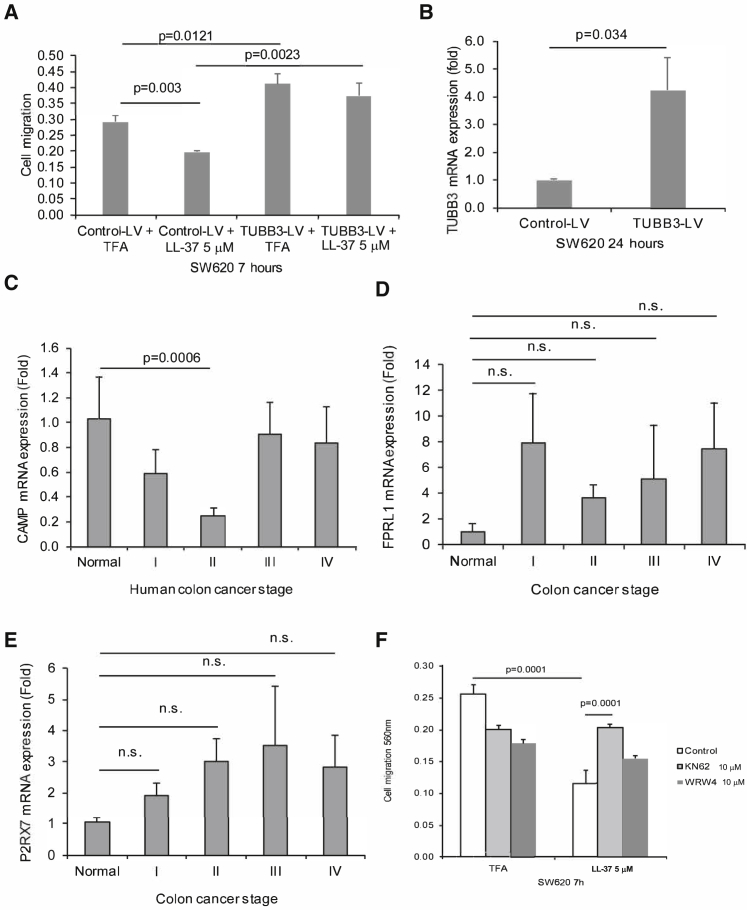
Cathelicidin Inhibited Colon Cancer Cell Migration via TUBB3 Inhibition
LL-37 (5 μM) significantly inhibited TUBB3 mRNA expression in both colon cancer cells (Figure 3E). Lentiviral overexpression of TUBB3 also led to increased colon cancer cell migration of SW620 cells, with or without exposure to LL-37 (Figure 4A). Infection of TUBB3-overexpressing lentivirus significantly increased human TUBB3 mRNA expression in SW620 cells (Figure 4B).
Figure 4.
Cathelicidin-Mediated Inhibition of Colon Cancer Cell Migration Was P2RX7 Dependent
(A) SW620 cells were transfected with control lentivirus or TUBB3-overexpressing lentivirus, followed by exposure to LL-37. Cell migration of SW620 cells. (B) SW620 cells were transiently transfected with control small interfering RNA (siRNA) or P2RX7 siRNA (80 pmol/mL), followed by exposure to LL-37. TUBB3 mRNA expression. (C) CAMP, (D) FPRL1, and (E) P2RX7 mRNA expression in human colon cancer PCR array plate. (F) Cell migration of SW620 cells. SW620 cells were treated with DMSO, KN62, and WRW4 for 30 min, followed by LL-37 for 7 h. Results were pooled from three independent experiments.
LL-37 Inhibited Colon Cancer Cell Migration and TUBB3 Expression via P2RX7
We used human colon cancer PCR arrays (Origene) and found that tumoral cathelicidin mRNA expression was reduced in stage II colonic tumors, but not in stage III and IV colonic tumors (Figure 4C). The finding was consistent with a previous report.7 Cathelicidin interacts with two putative receptors, i.e., FPRL1 and P2RX7,22, 23 which mediate downstream effects. Normal colonic tissues and colonic tumors of all stages had positive mRNA expression of P2RX7 and FPRL1 (Figures 4D and 4E).
To identify the involvement of cathelicidin receptors in the anti-metastatic effects of cathelicidin, we pretreated the colon cancer SW620 cells with FPRL1 antagonist WRW4 and P2RX7 antagonist KN62, followed by LL-37 exposure. The LL-37-mediated inhibition of cell migration was prevented by KN62, but not by WRW4 (Figure 4F). KN62 also prevented the LL-37-mediated disruption of tubulin structure in SW620 cells (Figure 5A). Exposure of SW620 cells to LL-37 (5 μM) significantly reduced TUBB3 mRNA expression (Figure 5B). This reduction was reversed by short hairpin RNA (shRNA) inhibition of P2RX7 (Figure 5B). Transient transfection of P2RX7 shRNA significantly reduced P2RX7 mRNA expression in SW620 cells (Figure 5C). Also, KN62 prevented the LL-37-mediated inhibition of TUBB3 mRNA expression in HT-29 cells (Figure 5D).
Figure 5.
Cathelicidin Inhibited TUBB3 Expression via P2RX7
(A) Green tubulin tracker staining with blue nuclear staining in human cancer SW620 cells. SW620 cells were pretreated with DMSO (10 μL/mL) or KN62 (10 μM) for 30 min, followed by exposure to LL-37 (5 μM) for 24 h. LL-37 reduced tubulin expression in SW620 cells that was prevented by KN62. (B) SW620 cells were transiently transfected with control small interfering RNA (siRNA) or P2RX7 shRNA (1 μg/mL), followed by exposure to LL-37. TUBB3 mRNA expression in SW620 cells. (C) P2RX7 mRNA expression in the transfected SW620 cells. (D) HT-29 cells were pretreated with DMSO (10 μL/mL) or KN62 (10 μM) for 30 min, followed by exposure to LL-37. TUBB3 mRNA expression. Results were pooled from three independent experiments.
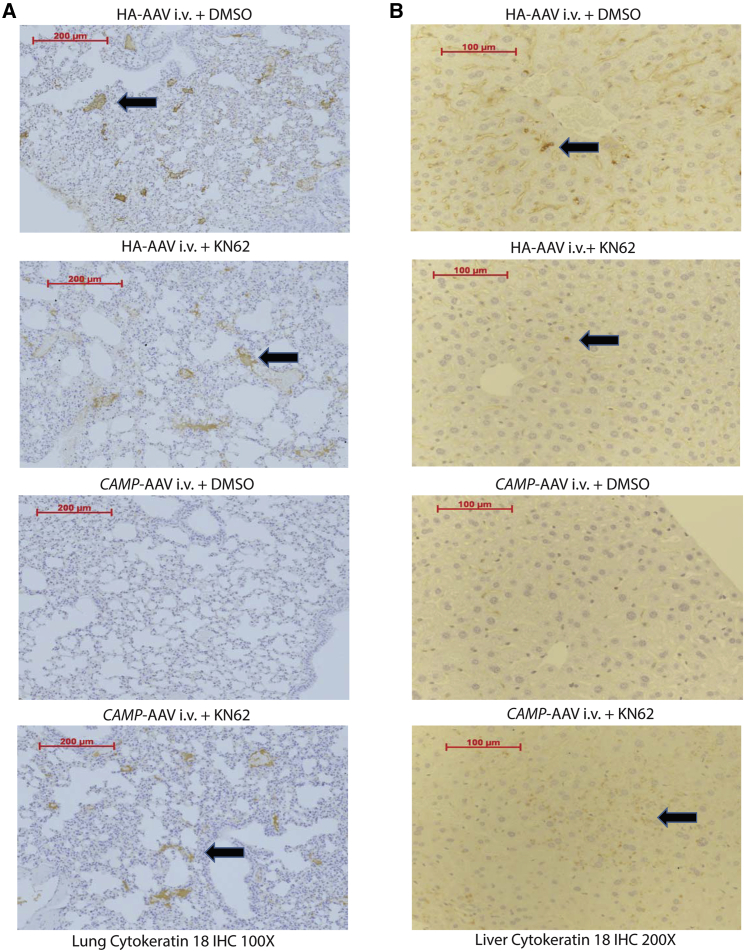
Cathelicidin Inhibited Colon Cancer Metastasis via P2RX7
To validate this receptor pathway in vivo, we injected KN62 into the HT-29-loaded nude mice. Injection of KN62 did not significantly affect colon cancer metastasis to lungs and liver in the control vector-expressing group, but reversed the colon cancer metastasis in the cathelicidin-expressing group (Figures 6A, 6B, 7A, and 7B). Therefore, cathelicidin inhibited colon cancer metastasis via P2RX7. Cathelicidin overexpression significantly reduced human TUBB3 mRNA expression in the lungs and liver of HT-29-loaded nude mice, which was reversed by KN62 administration (Figures 7C and 7D).
Figure 6.
Cathelicidin Inhibited Colon Cancer Metastasis via P2RX7
(A and B) Human cytokeratin-18 expression (representing human colon cancer cells) in (A) lungs and (B) liver of nude mice was identified by brown color spots (indicated by arrows). Intravenous cathelicidin expressing AAV administration reduced human CK18 expression in lungs and liver of nude mice.
Figure 7.
Cathelicidin Inhibited TUBB3 mRNA Expression in Metastasized Tumors via P2RX7
(A and B) Human keratin 20 mRNA expression (human colon cancer cell marker) in (A) lungs and (B) liver of nude mice was significantly reduced by CAMP-HA-AAV. Intraperitoneal KN62 treatment increased the presence of cytokeratin 18 protein and keratin 20 mRNA expression in the CAMP-HA-AAV-treated nude mice. (C and D) Human TUBB3 mRNA expression in (C) lungs and (D) liver of nude mice was significantly reduced by CAMP-HA-AAV. Intraperitoneal KN62 treatment increased the presence of human TUBB3 mRNA expression in the CAMP-HA-AAV-treated nude mice. (E) Diameters of subcutaneous tumors. Intravenous CAMP-HA-AAV significantly reduced subcutaneous tumor diameters in nude mice that were partially reversed by KN62 treatment.
Cathelicidin Inhibited Colonic Tumor Growth via P2RX7
We also injected the HT-29 cells into the nude mice subcutaneously followed by intravenous CAMP-AAV injection and intraperitoneal KN62 injection. Consistent with our previous report,16 the cathelicidin-overexpressing group had significantly reduced subcutaneous tumor diameter (Figure 7E). This reduction was partially reversed by KN62 (Figure 7E). Thus, cathelicidin-mediated inhibition of colonic tumor growth is P2RX7 dependent.
Cathelicidin Did Not Affect Colon Cancer Cell Invasion
Cathelicidin did not affect SW620 cell invasion (Figure S1A). It altered the protein secretion of MMP1, TIMP1-2, TGF-β1, and VEGF in HT-29, SW620, and SW480 cells differently (Figure S1B). We found no consistent pattern of LL-37-dependent soluble cancer mediator secretion among the three cultured colon cancer cell lines. Therefore, metalloprotease activity cannot address the anti-metastatic effect of cathelicidin.24 Also, cathelicidin overexpression did not affect the endothelial cell marker von Willebrand factor (vWF) mRNA expression in lungs and liver of the HT-29-loaded nude mice (Figure S1C). LL-37 is unlikely to modulate tumoral angiogenesis in vivo.
Discussion
This study demonstrates that cathelicidin inhibits colon cancer metastasis via a P2RX7-dependent pathway. Cathelicidin does not inhibit the cell viability of colon cancer cells (Figure 3A) and normal colonic fibroblasts.16 Instead, cathelicidin inhibits colonic tumor growth via indirect inhibition of TAFs.16 Both metastatic colon cancer cells and TAFs possess mesenchymal cell properties.3 The cathelicidin-dependent inhibition of colon cancer development and metastasis shares a common characteristic: the disruption of tubulin cytoskeleton in cells with mesenchymal cell properties.
Cytoskeleton, including tubulin, mediates the metastatic potential of circulating tumor cells (CTCs) in the vasculature.25, 26 The adherent tumor cells extravasate to form new metastatic foci in distant organs. Although the biophysics of CTCs is complex and beyond our scope of investigation, cathelicidin-mediated disruption of the cytoskeleton is associated with the specific inhibition of TUBB3 mRNA expression (Figure 3D). βIII-tubulin (TUBB3) is associated with exacerbated liver metastasis in colon cancer patients and with increased colon cancer cell migration.18, 21 Overexpression of TUBB3 reversed the anti-migratory effect of cathelicidin because cytoskeleton mediates cell migration.18, 27
Consistent with our findings, deficiency of P2RX7 augmented colon cancer tumor development and metastasis in nude mice injected with mouse colon cancer CT26 cells.28 This report suggests that P2RX7 mediates anti-cancer effects. The role of cathelicidin receptor in cancer development may depend on cell type and disease condition. In colon cancer HCT116 cells, cathelicidin mediates its anti-tumoral effects via G-protein coupled receptor (GPCR)-dependent and FPRL1-independent pathways.7 Given the heterogeneity and complexity of cancer, our study cannot cover all aspects of colon cancer conditions. This study attempts to reveal the potential cathelicidin-P2RX7 pathway in colon cancer development, which is worth further investigation.
To understand how P2RX7 modulates TUBB3 expression, we profiled the microRNA (miRNA) expression in SW620 cells (Figure S2A). LL-37 significantly increased miR200c-3p expression in SW620 and HT-29 cells (Figures S2B and S2C). miR200c is associated with distant metastasis in colorectal cancer patients29 and is currently the only validated miRNA shown to target TUBB3.30 Inhibition of P2RX7 with shRNA did not affect basal miR200c-3p expression but abolished the LL-37-mediated increase of miR200c-3p expression (Figure S2B). Similarly, KN62 also inhibited LL-37-induced miR200c-3p expression in HT-29 cells (Figure S2C). Pretreatment of SW620 cells with miR200c-3p inhibitor reversed the LL-37-mediated inhibition of TUBB3 mRNA expression, cell migration, and disruption of tubulin structure (Figure S2D and S2F). Therefore, miR200c-mediated TUBB3 inhibition is relevant to cathelicidin-mediated inhibition of cell migration. We did not determine miR200c expression in the lung and liver tissues of HT-29-loaded nude mice, because the miR200c primers could not differentiate between the human and mouse miR200c.
Cathelicidin is a molecular target for colon cancer. Systemic infusion or oral administration of cathelicidin peptide is not feasible, because cathelicidin peptides degrade in body fluids or blood.31, 32 A non-peptide cathelicidin mimic Ceragenin CSA13 is effective in inducing cell cycle arrest and apoptosis in cultured colon cancer HCT116, HT-29, and DLD1 cells.14, 33 Development of clinically applicable cathelicidin-based strategies for colon cancer metastasis needs further investigation.
In conclusion, cathelicidin inhibits colon cancer metastasis via a P2RX7 pathway.
Materials and Methods
Cell Culture
Human colon cancer HT-29, SW480, and SW620 cells were cultured in DMEM (10564, Thermo Fisher Scientific) containing 10% fetal calf serum (Thermo Fisher Scientific) and 1% penicillin-streptomycin (Thermo Fisher Scientific). All cultured cells were purchased from American Type Culture Collection (ATCC).
Cells were seeded in 12-well plates and cultured to 80% confluence. HT-29 cells were transfected with 1 × 104 infectious units per well of control lentivirus (PS100064V, Origene) and TUBB3 lentivirus (RC200755L1V, Origene) in 2 μg/mL Polybrene (sc-134220, Santa Cruz Biotechnology). Some groups were pretreated with control inhibitor (YI00199006-DFA, QIAGEN) or miR200c-3p inhibitor (YI04101122-DFA, QIAGEN) overnight before exposure to cathelicidin peptide (LL-37).
Intravenous Injection of Colon Cancer Cells into Nude Mice
HT-29 cells (1 × 106 cells) in Hanks’ balanced salt solution (100 μL) were injected intravenously into 8-week-old male and female nude mice (stock number 002019, Jackson Laboratory) for the determination of colon cancer metastasis. The same number of cells was injected subcutaneously into the left and right flanks of the nude mice for the determination of local tumor growth. The injected nude mice were housed in the UCLA animal facility under standard conditions, as described previously.16 All animal experiments were approved by the UCLA Animal Research Committee (#2007-116).
The human CAMP-HA-AAV and control HA-AAV were generated by Vector Laboratories, as described previously.16 HA-AAV and CAMP-HA-AAV (1 × 1012 genomic copies in 100 μL) were injected intravenously via tail veins into nude mice under transient isoflurane anesthesia. Some of the nude mice were injected intraperitoneally with P2RX7 antagonist KN62 (5 mg/kg, Tocris) or control DMSO solution (50 μL per mouse) daily from day 21 to day 28. Lung and liver tissues were collected for analyses on day 28. Each group consisted of 10 mice per group in two separate experiments.
Tail vein intravenous injection, but not orthotopic transplantation, was used because the intravenous injection model simulates how cathelicidin affects migration of CTCs to the distant organs.34, 35 SW620 cells were not used in this study because intravenous injection of these cells fails to develop metastatic tumors in the lungs and liver of nude mic.36
Immunohistochemistry
Immunohistochemistry was assisted by UCLA Translational Pathology Core Laboratory (TPCL), as described previously.16 In short, paraffin-embedded lung and liver tissue sections were stained with a rabbit monoclonal anti-LL-37 antibody (ab207758, Abcam, 1:50 dilution), mouse polyclonal anti-cytokeratin 18 antibody (sc-51583, Santa Cruz Biotechnology, 1:50 dilution), or anti-HA-tagged antibody (3724, Cell Signaling Technology, 1:50 dilution). Images were recorded with a Zeiss AX10 microscope.
Cell Viability MTS Assay
SW620 cells in 96-well plates (106 cells/plate) were treated with trifluoroacetic acid (TFA) 0.1% or cathelicidin peptide (LL-37). Cell viability was measured with MTS solution (G3580, Promega), as described previously.16
Cell Migration and Invasion Assays
Cell migration and invasion assays of SW620 cells were performed using a modified Boyden chamber approach (ECM508 and ECM554, EMD Millipore), as we described previously.37 Briefly, TFA and cathelicidin peptide (LL-37) were added to the lower chamber. Control-LV- and TUBB3-LV-infected SW620 (2.5 × 104) cells were seeded into the upper chamber and incubated for 7 h (for cell migration) and 24 h (for cell invasion) at 37°C. The FPRL1 antagonist WRW4 and the P2RX7 antagonist KN62 were added to both upper and lower chambers at the beginning of the cell migration experiments. Some of the SW620 cells were pretreated with control inhibitor (YI00199006, QIAGEN) and miR200c-3p inhibitor (YI04100915, QIAGEN) 24 h before the cell migration assay began. The migrated colon cancer cells through the membrane were stained and determined by absorbance at 650 nm.
Real-Time RT-PCR
The total RNA from lung and liver tissues were extracted with an RNeasy kit (74106, QIAGEN). The RNA was converted to cDNA by iScript cDNA Synthesis kit (170-8891, Bio-Rad). The mRNA expression was determined with a Bio-Rad CFX384 PCR system, using Thermo Fisher Scientific’s inventoried primers (Table 1) and iTaq Universal Probe Supermix (172-5135, Bio-Rad). Human colon cancer PCR arrays (HCRT101) were purchased from Origene. Relative mRNA expression was normalized to human 18S or mouse GAPDH mRNA expression. miRNAs were converted to cDNA by miRCURY LNA RT kit (339340, QIAGEN). PCR reactions were determined using miRCURY LNA SYBR Green PCR kit (339346, QIAGEN). Relative miRNA expression was normalized to RNU1A1 expression. Results were expressed as fold induction compared to their respective controls, as we described previously.16
Table 1.
Primers for Real-Time RT-PCR
| Gene | Assay Number |
|---|---|
| Human CAMP | Hs00189038_m1 |
| Human KRT20 | Hs00300643_m1 |
| Human TUBB1 | Hs00917771_g1 |
| Human TUBB3 | Hs00801390_s1 |
| Human FPR2 | Hs02759175_s1 |
| Human P2RX7 | Hs00175721_m1 |
| Human 18S | Hs99999901_s1 |
| Mouse GAPDH | Mm99999915_g1 |
| miR200c-3p | YP00204482 |
| RNU1A1 | YP00203909 |
| Unisp6 | YP00203954 |
Inventoried primer list of RT-PCR reactions.
Tubulin Tracker Staining
SW620 cells (2 × 105 cells/well) were seeded onto chamber slides and were exposed to TFA 0.1% or cathelicidin peptide (LL-37) for 24 h. The cells were incubated with tubulin-tracker (T34075, Invitrogen) for 45 min, followed by Hoechst 33342 (H3570, Invitrogen), and viewed under a Zeiss AX10 confocal microscope, as described previously.16
ELISA
The cells were serum starved overnight, followed by incubation with TFA and LL-37 for 24 h. Soluble mediators in the conditioned media were determined by R&D Systems DuoSet ELISA assays: MMP1 (DY901B), TIMP1 (DY970), TIMP2 (DY971), TGF-β1 (DY240), and VEGF (DY293B).
Statistical Analyses
Results were analyzed using Prism professional statistics software program (GraphPad). Unpaired Student’s t tests were used for intergroup comparisons. Quantitative results were expressed with error bars as means ± SEM. Only p values of statistically significant differences (p < 0.05) are shown in the figures.
Author Contributions
J.W., M.C., I.K.M.L., C.O., and M.S. contributed the animal study and cell culture data. H.W.K. supervised the study, drafted the manuscript, and approved the submitted final version. All authors reviewed and approved the final version of the manuscript.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This work was supported by an NIH grant (K01-DK084256) to H.W.K. and a student research fellowship from the Crohn's and Colitis Foundation of America (287244) to M.C. The funding sponsors were not involved in the design of the study or the collection, analysis, and interpretation of the data. We thank Prof. Charalabos Pothoulakis for financial assistance and Samantha Ho for technical assistance in the experiments. The data and materials described in this manuscript are available for sharing. Please contact H.W.K.
Footnotes
Supplemental information includes two figures and can be found with this article online at https://doi.org/10.1016/j.omto.2019.01.004.
Contributor Information
Mingjun Sun, Email: smjmw@sina.com.
Hon Wai Koon, Email: hkoon@mednet.ucla.edu.
Supplemental Information
References
- 1.Hing T.C., Ho S., Shih D.Q., Ichikawa R., Cheng M., Chen J., Chen X., Law I., Najarian R., Kelly C.P. The antimicrobial peptide cathelicidin modulates Clostridium difficile-associated colitis and toxin A-mediated enteritis in mice. Gut. 2013;62:1295–1305. doi: 10.1136/gutjnl-2012-302180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koon H.W., Shih D.Q., Chen J., Bakirtzi K., Hing T.C., Law I., Ho S., Ichikawa R., Zhao D., Xu H. Cathelicidin signaling via the Toll-like receptor protects against colitis in mice. Gastroenterology. 2011;141:1852–1863.e1–3. doi: 10.1053/j.gastro.2011.06.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoo J.H., Ho S., Tran D.H., Cheng M., Bakirtzi K., Kukota Y., Ichikawa R., Su B., Tran D.H., Hing T.C., Chang I. Anti-fibrogenic effects of the anti-microbial peptide cathelicidin in murine colitis-associated fibrosis. Cell Mol. Gastroenterol. Hepatol. 2015;1:55–74.e51. doi: 10.1016/j.jcmgh.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoang-Yen Tran D., Hoang-Ngoc Tran D., Mattai S.A., Sallam T., Ortiz C., Lee E.C., Robbins L., Ho S., Lee J.E., Fisseha E. Cathelicidin suppresses lipid accumulation and hepatic steatosis by inhibition of the CD36 receptor. Int. J. Obes. 2016;40:1424–1434. doi: 10.1038/ijo.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu W.K., Sung J.J., Cheng A.S., Chan F.K., Ng S.S., To K.F., Wang X.J., Zhang L., Wong S.H., Yu J., Cho C.H. The Janus face of cathelicidin in tumorigenesis. Curr. Med. Chem. 2014;21:2392–2400. doi: 10.2174/0929867321666140205135351. [DOI] [PubMed] [Google Scholar]
- 6.Wu W.K., Wang G., Coffelt S.B., Betancourt A.M., Lee C.W., Fan D., Wu K., Yu J., Sung J.J., Cho C.H. Emerging roles of the host defense peptide LL-37 in human cancer and its potential therapeutic applications. Int. J. Cancer. 2010;127:1741–1747. doi: 10.1002/ijc.25489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ren S.X., Cheng A.S., To K.F., Tong J.H., Li M.S., Shen J., Wong C.C., Zhang L., Chan R.L., Wang X.J. Host immune defense peptide LL-37 activates caspase-independent apoptosis and suppresses colon cancer. Cancer Res. 2012;72:6512–6523. doi: 10.1158/0008-5472.CAN-12-2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu W.K., Sung J.J., To K.F., Yu L., Li H.T., Li Z.J., Chu K.M., Yu J., Cho C.H. The host defense peptide LL-37 activates the tumor-suppressing bone morphogenetic protein signaling via inhibition of proteasome in gastric cancer cells. J. Cell. Physiol. 2010;223:178–186. doi: 10.1002/jcp.22026. [DOI] [PubMed] [Google Scholar]
- 9.Heilborn J.D., Nilsson M.F., Jimenez C.I., Sandstedt B., Borregaard N., Tham E., Sørensen O.E., Weber G., Ståhle M. Antimicrobial protein hCAP18/LL-37 is highly expressed in breast cancer and is a putative growth factor for epithelial cells. Int. J. Cancer. 2005;114:713–719. doi: 10.1002/ijc.20795. [DOI] [PubMed] [Google Scholar]
- 10.Coffelt S.B., Waterman R.S., Florez L., Höner zu Bentrup K., Zwezdaryk K.J., Tomchuck S.L., LaMarca H.L., Danka E.S., Morris C.A., Scandurro A.B. Ovarian cancers overexpress the antimicrobial protein hCAP-18 and its derivative LL-37 increases ovarian cancer cell proliferation and invasion. Int. J. Cancer. 2008;122:1030–1039. doi: 10.1002/ijc.23186. [DOI] [PubMed] [Google Scholar]
- 11.von Haussen J., Koczulla R., Shaykhiev R., Herr C., Pinkenburg O., Reimer D., Wiewrodt R., Biesterfeld S., Aigner A., Czubayko F., Bals R. The host defence peptide LL-37/hCAP-18 is a growth factor for lung cancer cells. Lung Cancer. 2008;59:12–23. doi: 10.1016/j.lungcan.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 12.Verjans E.T., Zels S., Luyten W., Landuyt B., Schoofs L. Molecular mechanisms of LL-37-induced receptor activation: An overview. Peptides. 2016;85:16–26. doi: 10.1016/j.peptides.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Ren S.X., Shen J., Cheng A.S., Lu L., Chan R.L., Li Z.J., Wang X.J., Wong C.C., Zhang L., Ng S.S. FK-16 derived from the anticancer peptide LL-37 induces caspase-independent apoptosis and autophagic cell death in colon cancer cells. PLoS ONE. 2013;8:e63641. doi: 10.1371/journal.pone.0063641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuroda K., Fukuda T., Okumura K., Yoneyama H., Isogai H., Savage P.B., Isogai E. Ceragenin CSA-13 induces cell cycle arrest and antiproliferative effects in wild-type and p53 null mutant HCT116 colon cancer cells. Anticancer Drugs. 2013;24:826–834. doi: 10.1097/CAD.0b013e3283634dd0. [DOI] [PubMed] [Google Scholar]
- 15.Kuroda K., Fukuda T., Yoneyama H., Katayama M., Isogai H., Okumura K., Isogai E. Anti-proliferative effect of an analogue of the LL-37 peptide in the colon cancer derived cell line HCT116 p53+/+ and p53-/- Oncol. Rep. 2012;28:829–834. doi: 10.3892/or.2012.1876. [DOI] [PubMed] [Google Scholar]
- 16.Cheng M., Ho S., Yoo J.H., Tran D.H., Bakirtzi K., Su B., Tran D.H., Kubota Y., Ichikawa R., Koon H.W. Cathelicidin suppresses colon cancer development by inhibition of cancer associated fibroblasts. Clin. Exp. Gastroenterol. 2014;8:13–29. doi: 10.2147/CEG.S70906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vu T., Datta P.K. Regulation of EMT in Colorectal Cancer: A Culprit in Metastasis. Cancers (Basel) 2017;9:E171. doi: 10.3390/cancers9120171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sobierajska K., Wieczorek K., Ciszewski W.M., Sacewicz-Hofman I., Wawro M.E., Wiktorska M., Boncela J., Papiewska-Pajak I., Kwasniak P., Wyroba E. β-III tubulin modulates the behavior of Snail overexpressed during the epithelial-to-mesenchymal transition in colon cancer cells. Biochim. Biophys. Acta. 2016;1863:2221–2233. doi: 10.1016/j.bbamcr.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Scott L.C., Evans T.R., Cassidy J., Harden S., Paul J., Ullah R., O’Brien V., Brown R. Cytokeratin 18 in plasma of patients with gastrointestinal adenocarcinoma as a biomarker of tumour response. Br. J. Cancer. 2009;101:410–417. doi: 10.1038/sj.bjc.6605175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bayrak R., Yenidünya S., Haltas H. Cytokeratin 7 and cytokeratin 20 expression in colorectal adenocarcinomas. Pathol. Res. Pract. 2011;207:156–160. doi: 10.1016/j.prp.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Tóth C., Sükösd F., Valicsek E., Herpel E., Schirmacher P., Renner M., Mader C., Tiszlavicz L., Kriegsmann J. Expression of ERCC1, RRM1, TUBB3 in correlation with apoptosis repressor ARC, DNA mismatch repair proteins and p53 in liver metastasis of colorectal cancer. Int. J. Mol. Med. 2017;40:1457–1465. doi: 10.3892/ijmm.2017.3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho S., Pothoulakis C., Koon H.W. Antimicrobial peptides and colitis. Curr. Pharm. Des. 2013;19:40–47. doi: 10.2174/13816128130108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagaoka I., Tamura H., Hirata M. An antimicrobial cathelicidin peptide, human CAP18/LL-37, suppresses neutrophil apoptosis via the activation of formyl-peptide receptor-like 1 and P2X7. J. Immunol. 2006;176:3044–3052. doi: 10.4049/jimmunol.176.5.3044. [DOI] [PubMed] [Google Scholar]
- 24.Said A.H., Raufman J.P., Xie G. The role of matrix metalloproteinases in colorectal cancer. Cancers (Basel) 2014;6:366–375. doi: 10.3390/cancers6010366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rejniak K.A. Investigating dynamical deformations of tumor cells in circulation: predictions from a theoretical model. Front. Oncol. 2012;2:111. doi: 10.3389/fonc.2012.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matrone M.A., Whipple R.A., Balzer E.M., Martin S.S. Microtentacles tip the balance of cytoskeletal forces in circulating tumor cells. Cancer Res. 2010;70:7737–7741. doi: 10.1158/0008-5472.CAN-10-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miao Z., Ali A., Hu L., Zhao F., Yin C., Chen C., Yang T., Qian A. Microtubule actin cross-linking factor 1, a novel potential target in cancer. Cancer Sci. 2017;108:1953–1958. doi: 10.1111/cas.13344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adinolfi E., Capece M., Franceschini A., Falzoni S., Giuliani A.L., Rotondo A., Sarti A.C., Bonora M., Syberg S., Corigliano D. Accelerated tumor progression in mice lacking the ATP receptor P2X7. Cancer Res. 2015;75:635–644. doi: 10.1158/0008-5472.CAN-14-1259. [DOI] [PubMed] [Google Scholar]
- 29.Chen J., Wang W., Zhang Y., Chen Y., Hu T. Predicting distant metastasis and chemoresistance using plasma miRNAs. Med. Oncol. 2014;31:799. doi: 10.1007/s12032-013-0799-x. [DOI] [PubMed] [Google Scholar]
- 30.Prislei S., Martinelli E., Mariani M., Raspaglio G., Sieber S., Ferrandina G., Shahabi S., Scambia G., Ferlini C. MiR-200c and HuR in ovarian cancer. BMC Cancer. 2013;13:72. doi: 10.1186/1471-2407-13-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bucki R., Namiot D.B., Namiot Z., Savage P.B., Janmey P.A. Salivary mucins inhibit antibacterial activity of the cathelicidin-derived LL-37 peptide but not the cationic steroid CSA-13. J. Antimicrob. Chemother. 2008;62:329–335. doi: 10.1093/jac/dkn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leszczyńska K., Namiot A., Fein D.E., Wen Q., Namiot Z., Savage P.B., Diamond S., Janmey P.A., Bucki R. Bactericidal activities of the cationic steroid CSA-13 and the cathelicidin peptide LL-37 against Helicobacter pylori in simulated gastric juice. BMC Microbiol. 2009;9:187. doi: 10.1186/1471-2180-9-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niemirowicz K., Prokop I., Wilczewska A.Z., Wnorowska U., Piktel E., Wątek M., Savage P.B., Bucki R. Magnetic nanoparticles enhance the anticancer activity of cathelicidin LL-37 peptide against colon cancer cells. Int. J. Nanomedicine. 2015;10:3843–3853. doi: 10.2147/IJN.S76104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Timmons J.J., Cohessy S., Wong E.T. Injection of Syngeneic Murine Melanoma Cells to Determine Their Metastatic Potential in the Lungs. J. Vis. Exp. 2016 doi: 10.3791/54039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sasportas L.S., Gambhir S.S. Imaging circulating tumor cells in freely moving awake small animals using a miniaturized intravital microscope. PLoS ONE. 2014;9:e86759. doi: 10.1371/journal.pone.0086759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zirvi K.A., Najjar T.A., Slomiany B.L. Sensitivity of human colon tumor metastases to anticancer drugs in athymic (nude) mice. Cancer Lett. 1993;72:39–44. doi: 10.1016/0304-3835(93)90008-w. [DOI] [PubMed] [Google Scholar]
- 37.Koon H.W., Shih D., Karagiannides I., Zhao D., Fazelbhoy Z., Hing T., Xu H., Lu B., Gerard N., Pothoulakis C. Substance P modulates colitis-associated fibrosis. Am. J. Pathol. 2010;177:2300–2309. doi: 10.2353/ajpath.2010.100314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.