Abstract
Myotonic dystrophy type 1 (DM1) is the most common form of adult muscular dystrophy. It is an autosomal dominant inherited disease with multisystemic involvement. Respiratory function is often affected and respiratory failure is the most common cause of death. Pulmonary embolism is a rare cause of respiratory failure in DM1 patients, so that the best anticoagulation strategy in these patients is still unclear. We describe the case of pulmonary embolism in a DM1 patient, in which pulmonary thrombus was completely resolved with oral dabigatran etexilate therapy.
Key words: myotonic dystrophy, pulmonary embolism, dabigatran etexilate, pulmonary thrombus
Introduction
Myotonic dystrophy type 1 (DM1) is the most common muscular dystrophy in adults, affecting approximately 1 in 8000 people worldwide. It is a multisystem disorder with autosomal dominant inheritance. Clinical manifestations may vary from muscle symptoms such as myotonia, muscle weakness/atrophy and fatigue to cardiac arrhythmias, early cataracts, central/obstructive apnea, respiratory failure, insulin resistance, dysphagia and gastrointestinal dysmotility (1). Cardiac involvement is quite common, as in other muscular dystrophies (2), affecting about 80% of patients, and often precedes the skeletal muscle one. Cardiac involvement may include arrhythmias (3-6) atrioventricular block (7), ventricular premature contractions, atrial fibrillation (8-10), atrial flutter (11), right/left bundle branch block and non-sustained ventricular tachycardia (12) which often lead to needs of cardiac devices such as pacemakers or implantable cardiac defibrillator (13-16). Left ventricular systolic dysfunction has also been reported (17-22). Respiratory failure is a common feature in almost all muscular dystrophies and, together with cardiac involvement is the main cause of death (23-24). Respiratory failure in DM1 patients is often the consequence of a combination of respiratory muscle weakness and degeneration, and lung elastic properties alterations (25). Pulmonary embolism is a rare event in DM1 patients (26). Moreover, the best antithrombotic strategy in this subset of patients is still debated, and novel oral anticoagulants (NOAC) have never been systematically tested in DM1 patients (27). We described the case of a pulmonary embolism in a DM1 patient treated for the first time with dabigatran etexilate, obtaining a complete resolution of the pulmonary thrombus.
Case report
We report the case of a 62-year-old female patient affected by DM1. She had no other cardiovascular risk factors excepted for systemic arterial hypertension. She was admitted to the Emergency Department because of dyspnea arising 7 days before and worsening in the last 6 hours. The recent clinical history was not relevant, as only an unilateral knee pain was reported which limited her daily activities. At admission the patient was conscious; physical examination showed heart rate (HR) 124 bpm, blood pressure (BP) 110/60 mmHg, breathing rate (BR) 23/min, SpO2 85% (FiO2 21%). The ECG showed a new-onset incomplete right bundle branch block with S wave in DI, Q wave and inverted T wave in DIII (a combination known as S1Q3T3 pattern, suggestive for pulmonary embolism). The arterial blood gas analysis revealed hypoxemia with hypocapnia and respiratory alkalosis; the blood exams showed high levels of I-troponin and D-dimer. The echocardiogram showed normal structure and function of the left ventricle, while the right ventricle presented a reduced longitudinal contractile function (Tricuspid Annulus Plane Systolic Excursion, TAPSE 14 mm), with abnormal function of mid-basal free wall and apical hyper-contractility (Mc Connell’s sign). The systolic pulmonary artery pressure – derived from the echocardiographic measurement of the tricuspid regurgitant jet velocity – was 60 mmHg. The Wells score was 6. A parasternal short axis view revealed the presence of a large thrombus inside pulmonary artery just on the level of main pulmonary artery bifurcation (Fig. 1). As the suspicious for pulmonary embolism (PE) was high, Computed Thomography Pulmonary Angiography (CTPA) was performed. CTPA images, acquired with the maximum intensity of radio-opaque contrast in the pulmonary arteries, showed the presence of a large thrombus on the pulmonary artery bifurcation extended to the central part of the lumen of both branches (Fig. 2). In the absence of hemodynamic instability, the patient – according to the current ESC guidelines on acute pulmonary embolism management (28) – was treated with fondaparinux 7.5 mg, subcutaneously once daily. As after 5 days of medical therapy, the echocardiographic re-evaluation still showed the thrombus in pulmonary artery, the treatment was stopped and dabigatran (a direct thrombin inhibitor, DTI) was administered at a dosage according to the patient’s age and renal function (150 mg/bid). Seven days after, the cardiac ultrasound examination showed the complete resolution of the thrombus (Fig. 3). At the same time, the clinical and biochemical parameters returned within the normal ranges. Dabigatran was then prescribed as the long-life therapy according to the high thrombotic risk of the patient.
Figure 1.
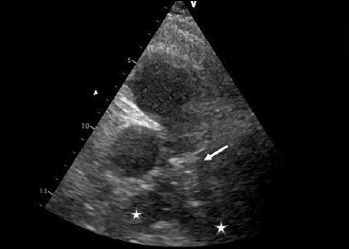
Parasternal short axis view of the pulmonary artery. The stars indicate the right and left pulmonary artery. The white arrow indicates the thrombus lying on the bifurcation of the pulmonary artery.
Figure 2.
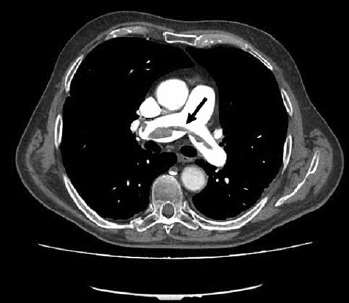
Computed thomography pulmonary angiography (CTPA) demonstrating saddle pulmonary embolism partially obstructing both main pulmonary arteries; the white area above the center is the pulmonary artery, opacified by radiocontrast; the black arrow indicates the pulmonary thrombus.
Figure 3.
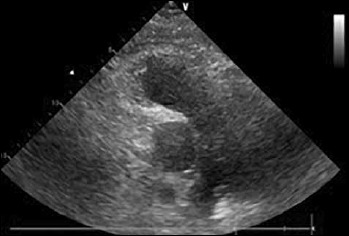
Parasternal short axis view of the pulmonary artery showing the complete resolution of the thrombus on the bifurcation of the pulmonary artery.
Discussion
Myotonic dystrophy type 1 is mainly characterized by skeletal muscle involvement, anyway cardiac involvement is quite common (29-31). Respiratory system is also affected with diaphragmatic weakness and/or recurrent pulmonary infections, which can lead to respiratory failure.
Recently it has been observed that dystrophic patients may have high thrombotic risk due to some predisposing factors. Firstly, they are affected by a myopathy that leads to mobility restriction and a sedentary lifestyle, which may increase their thromboembolic risk. The increasing age and a personal history of venous thrombo-embolism seem to be other predisposing factors. Compared with other inherited myopathies, patients with DM1 have a higher thromboembolic risk (32). Moreover, muscle degeneration can enhance coagulation and fibrinolysis processes as described in other forms of muscular dystrophies such as Duchenne muscular dystrophy (DMD), Becker muscular dystrophy (BMD) and Fukuyama congenital muscular dystrophy (FCMD). Hyper-coagulability state could be another predisposing factor for recurrent thrombotic events; however little is still known about the mechanisms underlying hypercoagulability state in these patients (33). In DMD and BMD a protein known as utrophin (a dystrophin-related protein), could play a role in hypercoagulability and recurrent thrombosis events. It seems that the upregulation of utrophin observed in these patients, leads to a lower expression of thrombomodulin, resulting in hypercoagulability state (34). However, none of these evidences is quite strong and referred to DM1. New perspective about incidence of venous thromboembolism and predicting factors of recurrent thrombosis specifically in the subset of DM1 patients may will rise from the results of a retrospective cohort study by Whabi et al. (NCT number: NCT03141749). What could be the best anticoagulation strategy in these patients is still debated. To date, Warfarin is still considered the first line treatment and the standard of care for DM1 patients requiring anticoagulation, as its safety and effectiveness has been established over the last decades (35). However, the difficulties in achieving an optimal anticoagulation with conventional warfarin therapy, likely related to several factors such as the slow onset of action, the variable pharmacologic effects, the interaction with several food and drug and the needs of periodic closely target INR monitoring, make the therapeutic management in clinical practice it difficult and reduce the real-life DM1 patient’s compliance. All these challenges have prompted an extensive research on the use of NOACs in a subset of patients (36, 37). Unfortunately, none of the trials evaluating the use of NOACs (38) in clinical practice, included DM1 patients (39-41). Dabigatran etexilato is a thrombin direct inhibitor whose safety and effectiveness in the treatment of venous thrombo-embolism and pulmonary embolism (PE) was tested in RE-COVER (42) and RE-COVER II (43) trials. Its efficacy was non-inferior to warfarin, with no significant differences in minor and major bleedings. No data are available about the use of this drug for the management of PE in DM1 patients. This case report is the first to report the use of Dabigatran etexilato for PE in a DM1 patient; using this drug the complete resolution of the pulmonary thrombus after an episode of pulmonary embolism was achieved. No major or minor bleedings were observed during treatment, and clinical and biochemical parameters returned within normal range 7 days after therapy.
Oral anticoagulation is an important issue in this subset of patients because they present a high prevalence of atrial fibrillation (44-47) requiring long-term anticoagulation to reduce the risk of thromboembolic events (48, 49).
Conclusions
The present case is the first to report the complete resolution of a pulmonary thrombus in a DM1 patient with pulmonary embolism, by using dabigatran etexilato. The use of dabigatran etexilate as anticoagulation treatment could be particularly useful in this subset of patients, for their variable cognitive impairment and consequent poor compliance with periodic INR monitoring.
References
- 1.Smith CA, Gutmann L. Myotonic dystrophy type 1 management and therapeutics. Curr Treat Options Neurol 2016;18:52. [DOI] [PubMed] [Google Scholar]
- 2.Nigro G, Papa AA, Politano L. The heart and cardiac pacing in Steinert disease. Acta Myol 2012;31:110-6. [PMC free article] [PubMed] [Google Scholar]
- 3.Nigro G, Russo V, Politano L, et al. Right atrial appendage versus Bachmann’s bundle stimulation: a two-year comparative study of electrical parameters in myotonic dystrophy type-1 patients. Pacing Clin Electrophysiol 2009;32:1191-6. [DOI] [PubMed] [Google Scholar]
- 4.Russo V, Papa AA, Rago A, et al. Arrhythmic risk evaluation in myotonic dystrophy: the importance of selection criteria and methodological approach. Clin Auton Res 2017;27:203-4. [DOI] [PubMed] [Google Scholar]
- 5.Russo V, Rago A, Ciardiello C, et al. the role of the atrial electromechanical delay in predicting atrial fibrillation in myotonic dystrophy type 1 patients. J Cardiovasc Electrophysiol 2016;27:65-72. [DOI] [PubMed] [Google Scholar]
- 6.Russo V, Papa AA, Rago A, et al. Increased heterogeneity of ventricular repolarization in myotonic dystrophy type 1 population. Acta Myol 2016;35:100-6. [PMC free article] [PubMed] [Google Scholar]
- 7.Nigro G, Russo V, Vergara P, et al. Optimal site for atrial lead implantation in myotonic dystrophy patients: the role of Bachmann’s Bundle stimulation. Pacing Clin Electrophysiol 2008;31:1463-6. [DOI] [PubMed] [Google Scholar]
- 8.Russo V, Rago A, Politano L, et al. The effect of atrial preference pacing on paroxysmal atrial fibrillation incidence in myotonic dystrophy type 1 patients: a prospective, randomized, single-bind cross-over study. Europace 2012;14:486-9. [DOI] [PubMed] [Google Scholar]
- 9.Russo V, Di Meo F, Rago A, et al. Paroxysmal atrial fibrillation in myotonic dystrophy type 1 patients: P wave duration and dispersion analysis. Eur Rev Med Pharmacol Sci 2015;19:1241-8. [PubMed] [Google Scholar]
- 10.Russo V, Papa AA, Rago A, et al. Which is the true epidemiology of atrial fibrillation in myotonic dystrophy type 1 patients? Pacing Clin Electrophysiol 2016;39:1418-9. [DOI] [PubMed] [Google Scholar]
- 11.Russo V, Rago A, Papa AA, et al. Voltage-directed cavo-tricuspid isthmus ablation using a novel ablation catheter mapping technology in a myotonic dystrophy type I patient. Acta Myol 2016;35:109-13. [PMC free article] [PubMed] [Google Scholar]
- 12.Russo V, Rago A, Di Meo F, et al. Ventricular fibrillation induced by coagulating mode bipolar electrocautery during pacemaker implantation in myotonic dystrophy type 1 patient. Acta Myol 2014;33:149-51. [PMC free article] [PubMed] [Google Scholar]
- 13.Proietti R, Labos C, Davis M, et al. A systematic review and meta-analysis of the association between implantable cardioverter-defibrillator shocks and long-term mortality. Can J Cardiol 2015;31:270-7. [DOI] [PubMed] [Google Scholar]
- 14.Russo V, Nigro G, Politano L. Role of electrophysiological evaluation for the best device choice to prevent sudden cardiac death in patients with myotonic dystrophy type 1 and Emery Dreifuss muscular dystrophy. Trends Cardiovasc Med 2017. pii: S1050-1738(17)30013-0. [DOI] [PubMed] [Google Scholar]
- 15.Russo V, Rago A, Papa AA, et al. Bachmann bundle pacing reduces atrial electromechanical delay in type 1 myotonic dystrophy patients. J Interv Card Electrophysiol 2018;51:229-36. [DOI] [PubMed] [Google Scholar]
- 16.Muto C, Solimene F, Russo V, et al. Optimal left ventricular lead placement for cardiac resynchronization therapy in postmyocardial infarction patients. Future Cardiol 2018;14:215-24. [DOI] [PubMed] [Google Scholar]
- 17.Petri H, Witting N, Ersbøll MK, et al. High prevalence of cardiac involvement in patients with myotonic dystrophy type 1: a cross-sectional study. Int J Cardiol 2014;174:31-6. [DOI] [PubMed] [Google Scholar]
- 18.Russo V, Di Meo F, Rago A, et al. Atrial electromechanical delay in myotonic dystrophy type 1 patients. Eur Rev Med Pharmacol Sci 2015;19:3991-2. [PubMed] [Google Scholar]
- 19.Russo V, Rago A, D’Andrea A, et al. Early onset “electrical” heart failure in myotonic dystrophy type 1 patient: the role of ICD biventricular pacing. Anadolu Kardiyol Derg 2012;12:517-9. [DOI] [PubMed] [Google Scholar]
- 20.Russo V, Papa AA, Nigro G. The controversial epidemiology of left ventricular dysfunction in patients with myotonic dystrophy type 1. JAMA Cardiol 2017;2:1044. [DOI] [PubMed] [Google Scholar]
- 21.Russo V, Rago A, Papa AA, et al. Which is the true epidemiology of left ventricular dysfunction in patients with myotonic dystrophy type 1? J Chin Med Assoc 2017;80:740-1. [DOI] [PubMed] [Google Scholar]
- 22.Russo V, Papa AA, Williams EA, et al. ACE inhibition to slow progression of myocardial fibrosis in muscular dystrophies. Trends Cardiovasc Med 2018;28:330-7. [DOI] [PubMed] [Google Scholar]
- 23.Nigro G, Russo V, Politano L, et al. Does Bachmann’s bundle pacing prevent atrial fibrillation in myotonic dystrophy type 1 patients? A 12 months follow-up study. Europace 2010;12:1219-23. [DOI] [PubMed] [Google Scholar]
- 24.Nigro G, Russo V, Rago A, et al. Right atrial preference pacing algorithm in the prevention of paroxysmal atrial fibrillation in myotonic dystrophy type 1 patients: a long term follow-up study. Acta Myol 2012;31:139-43. [PMC free article] [PubMed] [Google Scholar]
- 25.Lo Mauro A, Aliverti A. Physiology of respiratory disturbances in muscular dystrophies. Breathe (Sheff) 2016;12:318-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho JY, Seo SY, Cho YJ, et al. Pulmonary thromboembolism in a patient with myotonic dystrophy type 1. Ann Indian Acad Neurol 2012;15:317-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rago A, Papa AA, Arena G, et al. Complete resolution of left atrial appendage thrombosis with oral dabigatran etexilate in a patient with myotonic dystrophy type 1 and atrial fibrillation. Acta Myol 2017;36:218-22. [PMC free article] [PubMed] [Google Scholar]
- 28.Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014;35:3033-69, 69a-69k. [DOI] [PubMed] [Google Scholar]
- 29.Russo V, Rago A, Papa AA, et al. Cardiac resynchronization improves heart failure in one patient with myotonic dystrophy type 1. A case report. Acta Myol 2012;31:154-5. [PMC free article] [PubMed] [Google Scholar]
- 30.Russo V, Nigro G, Rago A, et al. Atrial fibrillation burden in myotonic dystrophy type 1 patients implanted with dual chamber pacemaker: the efficacy of the overdrive atrial algorithm at 2 year follow-up. Acta Myol 2013;32:142-7. [PMC free article] [PubMed] [Google Scholar]
- 31.Russo V, Rago A, Nigro G. Sudden cardiac death in neuromuscolar disorders: time to establish shared protocols for cardiac pacing. Int J Cardiol 2016;207:284-5. [DOI] [PubMed] [Google Scholar]
- 32.Wahbi K, Sochala M, Porcher R, et al. Venous thromboembolism in adult patients with inherited myopathies: a high-risk in myotonic dystrophy. Neuromuscular Disorders 2017;27:S179. [Google Scholar]
- 33.Saito Y, Komiya T, Kawai M. Hypercoagulable state in Duchenne muscular dystrophy. Rinsho Shinkeigaku 1997;37:374-8. [PubMed] [Google Scholar]
- 34.Higuchi I, Niiyama T, Uchida Y, et al. Multiple episodes of thrombosis in a patient with Becker muscular dystrophy with marked expression of utrophin on the muscle cell membrane. Acta Neuropathol 1999;98:313-6. [DOI] [PubMed] [Google Scholar]
- 35.Bushby K, Muntoni F, Bourke JP. 107th ENMC International Workshop: the management of cardiac involvement in muscular dystrophy and myotonic dystrophy. 7th-9th June 2002, Naarden, the Netherlands. Neuromuscular Disorders. 2003;13:166-72. [DOI] [PubMed] [Google Scholar]
- 36.Russo V, Bottino R, Rago A, et al. Atrial fibrillation and malignancy: the clinical performance of non-vitamin k oral anticoagulants-a systematic review. Semin Thromb Hemost 2018. Aug 17. doi: 10.1055/s-0038-1661386. [DOI] [PubMed] [Google Scholar]
- 37.Russo V, Attena E, Mazzone C, et al. Nonvitamin k antagonist oral anticoagulants use in patients with atrial fibrillation and bioprosthetic heart valves/prior surgical valve repair: a multicenter clinical practice experience. Semin Thromb Hemost 2018;44:364-9. [DOI] [PubMed] [Google Scholar]
- 38.Russo V, Rago A, Papa AA, et al. Use of non-vitamin k antagonist oral anticoagulants in atrial fibrillation patients with malignancy: clinical practice experience in a single institution and literature review. Semin Thromb Hemost 2018;44:370-6. [DOI] [PubMed] [Google Scholar]
- 39.Russo V, Di Napoli L, Bianchi V, et al. A new integrated strategy for direct current cardioversion in non-valvular atrial fibrillation patients using short term rivaroxaban administration: the MonaldiVert real life experience. Int J Cardiol 2016;224:454-5. [DOI] [PubMed] [Google Scholar]
- 40.Russo V, Rago A, D’Onofrio A, et al. The clinical performance of dabigatran in the Italian real-life experience. J Cardiovasc Med (Hagerstown) 2017;18:922-3. [DOI] [PubMed] [Google Scholar]
- 41.Russo V, Rago A, Papa AA, et al. Budget impact analysis of rivaroxaban vs. warfarin anticoagulation strategy for direct current cardioversion in non-valvular atrial fibrillation patients: the MonaldiVert Economic Study. Minerva Cardioangiol 2018;66:1-5. [DOI] [PubMed] [Google Scholar]
- 42.Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009;361:2342-52. [DOI] [PubMed] [Google Scholar]
- 43.Schulman S, Kakkar AK, Goldhaber SZ, et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation 2014;129:764-72. [DOI] [PubMed] [Google Scholar]
- 44.Russo V, Papa AA, Rago A, et al. Interatrial block to predict atrial fibrillation in myotonic dystrophy type 1. Neuromuscul Disord 2018;28:327-33. [DOI] [PubMed] [Google Scholar]
- 45.Russo V, Rago A, Papa AA, et al. Does a high percentage of right ventricular pacing influence the incidence of paroxysmal atrial fibrillation in myotonic dystrophy type 1 patients? Kardiol Pol 2013;71:1147-53. [DOI] [PubMed] [Google Scholar]
- 46.Russo V, Papa AA, Rago A, et al. Effect of dual-chamber minimal ventricular pacing on paroxysmal atrial fibrillation incidence in myotonic dystrophy type 1 patients: a prospective, randomized, single-blind, crossover study. Heart Rhythm 2018;15:962-8. [DOI] [PubMed] [Google Scholar]
- 47.Proietti R, Gonzini L, Pizzimenti G, et al. Glomerular filtration rate: a prognostic marker in atrial fibrillation. A Sub-analysis of the ata-af. Clin Cardiol 2018. Aug 24. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Russo V, Rago A, Papa AA, et al. Efficacy and safety of dabigatran in patients with atrial fibrillation scheduled for transoesophageal echocardiogram-guided direct electrical current cardioversion: a prospective propensity score-matched cohort study. J Thromb Thrombolysis 2018;45:206-12. [DOI] [PubMed] [Google Scholar]
- 49.Bertaglia E, Anselmino M, Zorzi A, et al. NOACs and atrial fibrillation: Incidence and predictors of left atrial thrombus in the real world. Int J Cardiol 2017;249:179-83. [DOI] [PubMed] [Google Scholar]


