Abstract
High dose corticosteroid therapy is widely used as attack therapy of inflammatory central nervous system disorders and can induce several adverse reactions. Bradycardia is an infrequent event after corticosteroids administration and is often asymptomatic. We report a case of a woman admitted to the neurological department of our hospital for paraesthesias of the lower limbs. She received adiagnosis of inflammatory myelitis and high dose corticosteroid therapy was prescribed. During the therapy she complained of chest tightness, dyspnoea, weakness and malaise. An electrocardiogram revealed sinus bradycardia. A significant increase in body weight, probably due to plasma volume expansion, was detected. Bradycardia and high blood pressure spontaneously resolved in few days. We provide a collection and a statistical analysis of literature data about steroid induced bradycardia. We found that higher total doses are associated with lower pulse rate and symptomatic bradycardia. Bradycardia is more frequent in older patients and those with underlying cardiac disease or with autonomic disturbance. However clinicians must be aware about the occurrence of symptomatic bradycardia in all patients who undergo high dose corticosteroid therapy, not only in those at risk, to early detect and treat this potentially dangerous condition.
Keywords: Corticosteroid, bradycardia, side effects, clinical practice guideline, myelitis
Introduction
Corticosteroids (CS) have a wide range of uses, mainly related to their strong anti-inflammatory and immune-modulating properties. Inflammatory myelitis is commonly treated with pulse corticosteroid therapy (PST) (high-dose of intravenous methylprednisolone at 1000 mg daily for 3-7 days).1 The most common side-effects of high-dose PST are hyperglycemia, gastrointestinal intolerance, minor infections, and psychiatric symptoms. Minor adverse effects can be considered as transient facial flushing, a brief disturbance of taste, distal paresthesia, insomnia, and mild weight gain. Overall, cardiac arrhythmias (atrial fibrillation/flutter, ventricular tachycardia, and sinus bradycardia) have been reported in 1% to 82%2,3 of patients undergoing high-dose corticosteroid therapy. Bradycardia is a rare adverse effect1 of PST and is often asymptomatic.4,5 In this case report, we describe an episode of severe and symptomatic sinus bradycardia, developed after 5 days of PST in a 48-year-old woman with a inflammatory myelitis. We also provide a statistical analysis of the published data regarding bradycardia associated with steroid treatment, to identify variables related to the occurrence of this side-effect.
Case Presentation
A 48-year-old white woman was admitted to the neurological department of the Careggi Hospital (Florence, Italy) for a 15-month history of paresthesias of the lower limbs, in the absence of motor deterioration. Her medical history was only significant for gastric ulcer (chronically treated with proton-pump inhibitors [PPIs]) and smoking (about 20 cigarettes). A spinal cord magnetic resonance imaging (MRI) showed signal abnormalities in cervical (C2-C6) and thoracic (D6) regions; none had contrast enhancement (Figure 1). A brain MRI revealed a 44-mm meningioma situated on the floor of left middle cranial fossa, without signs of raised intracranial pressure. A lumbar puncture was performed, demonstrating 2.05 g of total proteins, normal cell count, link index of 0.66, and oligoclonal immunoglobulin G (IgG) with a mirror pattern.
Figure 1.
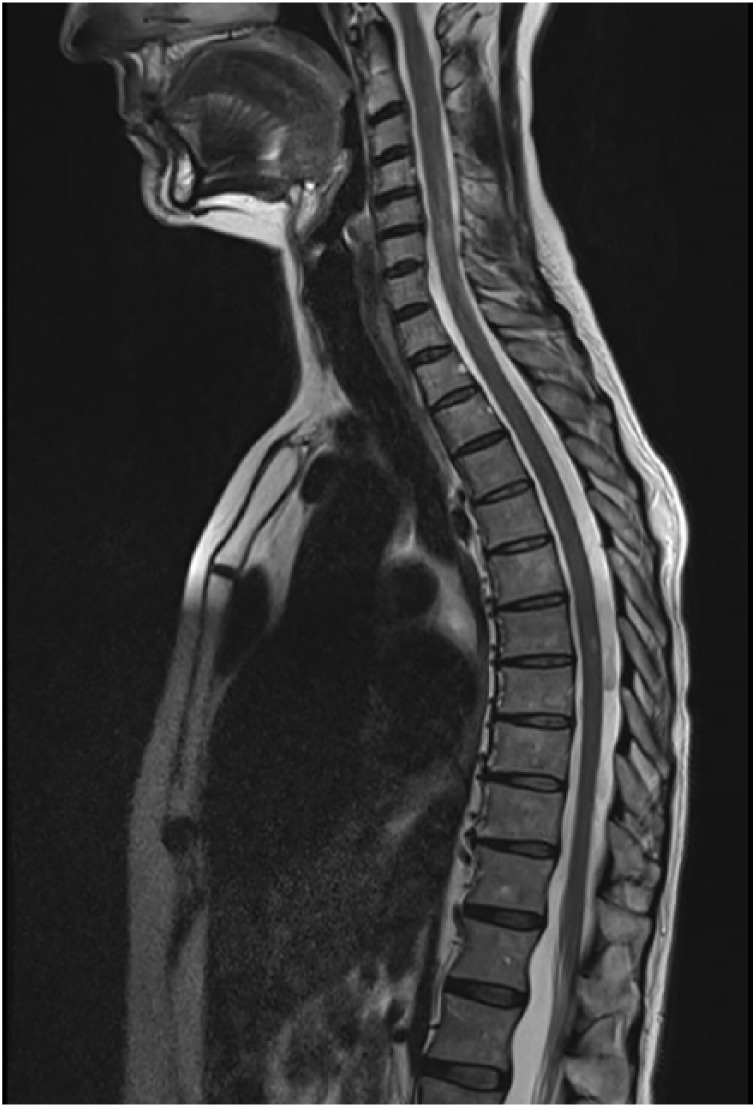
The patient spinal cord MRI showing signal abnormalities in cervical (C2-C6) and thoracic (D6) regions. MRI indicates magnetic resonance imaging.
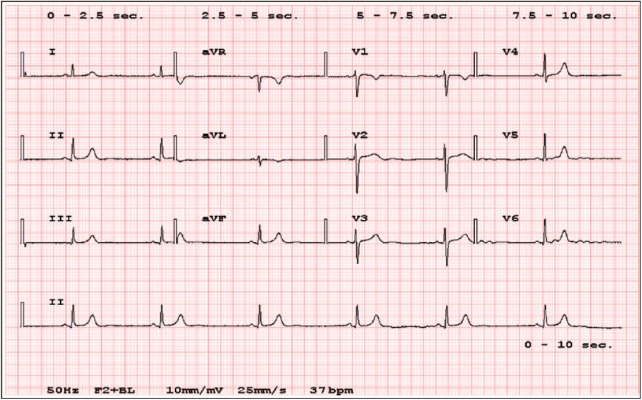
The patient received a diagnosis of inflammatory myelitis and then a 5-day course of intravenous methylprednisolone was prescribed. On Day 3 of PST, she complained of chest tightness, which worsened over the next 2 days. On Day 5, she also developed dyspnea, weakness, and malaise. Her blood pressure was 170/90 mm Hg and her pulse rate (PR) was ranging from 37 to 40 bpm. Pulse corticosteroid therapy was immediately suspended. An increase in body weight (5 kg) was detected compared with Day 1. A 12-lead electrocardiogram (ECG) revealed sinus bradycardia (37 bpm), without signs of acute myocardial infarction, acute coronary syndromes, atrio-ventricular block, or other forms of arrhythmias (Figure 2). A prior ECG, done a month earlier, showed normal sinus rhythm at 76 bpm. Cardiac biomarkers were normal and blood tests showed mild anemia, slight elevation of creatinine (0.95 mg/dL), potassium level of 3.9 mEq/L, sodium level of 141 mEq/L, calcium level of 8.4 mg/dL, and a mild decrease of total protein 5.6 g/dL. A normal left ventricular function was proved with echocardiography. A cranial computed tomography (CT) scan was performed, which did not show any significant modifications compared with prior brain MRI images. The patient did not receive any treatment, whereas high blood pressure and bradycardia spontaneously resolved in few hours and 4 days, respectively.
Figure 2.
The patient ECG showing isolated sinus bradycardia (37 bpm). ECG indicates electrocardiogram.
Statistical Analysis and Review of the Literature
Considering cases already reported in the literature and the one described here, we performed a correlation study of demographic, clinic, and laboratory data (Table 1). Depending on the distribution of data, we used t-test and non-parametric Mann–Whitney U tests for between groups’ comparisons and non-parametric Spearman ρ (rho) to evaluate correlations between groups’ numeric measures. We used chi-square test to compare categorical data. We included in this analysis patients whose data were available: 34 cases, including 27 women and 7 men.
Table 1.
Cases of bradycardia associated with steroids administration included in our analysis.
| Reference | Patients | Disease | Daily dose* | No. of doses | Route | Symptomatic |
|---|---|---|---|---|---|---|
| Tvede et al.6 | 5 patients: 4 female (21-52 years) and 1 male (53 years) | Rheumatoid arthritis | 1250 mg | 2-3 | IV | 1 patient |
| Guillén et al.7 | 73-year-old female | Pulmonary-renal syndrome | 375 mg | 1 | IV | Yes |
| Küçükosmanoğlu et al.8 | 14-year-old male | Glomerulonephritis | 1875 mg | 1 | IV | Yes |
| Pudil and Hrncir9 | 2 female (14-28 years) | Rheumatoid arthritis, polyarticular arthritis syndrome | 156.3 mg | 4-5 | IV | No |
| Jain et al.10 | 10 patients: 9 female (29-58 years), 1 male (39 years) | Pemphigus vulgaris | 933.3 mg | 1 | IV | No |
| Al Shibli et al.11 | 14-year-old female | Steroid-sensitive nephrotic syndrome | 80 mg | 7 | Oral | No |
| Taylor and Gaco5 | 45-year-old female | Multiple sclerosis | 1250 mg | 5 | Oral | No |
| Kundu and Fitzgibbons12 | 48-year-old female | Multiple sclerosis | 1250 mg | 4 | IV | Yes |
| Domínguez-Pinilla et al.13 | 15-year-old male | Juvenile idiopathic arthritis | 312.5 mg | 3 | IV | No |
| John et al.14 | 58-year-old male | Laryngeal edema | 50 mg | 2 | IV | Yes |
| Beyan et al.15 | 2 patients: 1 female (24 years) and 1 male (25 years) | Behçet and LES | 1250 and 150 mg | 3-4 | IV | 1 patient |
| Dashore et al.16 | 5 female patients (34-67 years) | Pemphigus vulgaris | 125 mg | 2-3 | IV | 2 patients |
| Marinov et al.17 | 51-year-old female | Postoperative nausea and vomiting | 25 mg | 1 | IV | Yes |
| Hasan and Al-Khazraji18 | 54-year-old female | Adrenal insufficiency | 100 mg | 1 | Oral | Yes |
Abbreviation: IV, intravenous.
steroids daily dose.
In Table 2, mean and SD for age, number of doses, daily dose, total dose, systolic blood pressure before CS (pre-sBP), diastolic blood pressure before CS (pre-dBP), pulse rate before CS (pre-PR), and minimal pulse rate after CS (min-PR) are reported. Most subjects developed bradycardia at the first day (46.7%) and at the third day (10%) of administration. According to the development of symptoms of bradycardia, we divided the whole sample in 2 groups, asymptomatic subjects (n = 23) and symptomatic subjects (n = 11). These 2 groups had comparable sex, number of doses, pre-PR, pre-sBP, and pre-dBP. As expected, symptomatic patients reached a lower min-PR (P = 0.001). Total dose, but not daily dose, was different between the 2 groups, being significantly lower in patients w developed symptoms (P < 0.034). There were no differences in age, pre-PR, pre-sBP, pre-dBP, and min-PR between female and male patients. Symptomatic patients were older than asymptomatic patients, although this difference was not statistically significant (P = 0.07).
Table 2.
Demographic characteristics and steroid dosage of patients in analysis.
| Whole sample (n = 34) | Asymptomatic (n = 23) | Symptomatic (n = 11) | P-value | |
|---|---|---|---|---|
| Age (±SD) years | 42.20 (±15.09) | 38.81 (±12.93) | 47.55 (±17.53) | 0.077 |
| Sex (women/men) | 27/7 | 19/4 | 8/3 | 0.505 |
| No. of doses (±SD) | 2.23 (±1.50) | 2.43 (±1.67) | 2.36 (±1.20) | 0.856 |
| Pre-sBP (±SD) | 119.27 (±15.25) | 118.05 (±9.63) | 122.11 (±24.46) | 0.859 |
| Pre-dBP (±SD) | 73.90 (±9.32) | 73.05 (±7.76) | 75.89 (±12.56) | 0.449 |
| Pre-PR (±SD) | 80.43 (±12.08) | 77.86 (±11.95) | 84.80 (±11.33) | 0.135 |
| Min-PR (±SD) | 46.70 (±71.73) | 49.68 (±6.00) | 38.18 (±8.01) | <0.001 |
| Daily dose (±SD) | 687.15 (±524.98) | 778.83 (±443.85) | 495.45 (±645.25) | 0.106 |
| Total dose (±SD) | 1435.65 (±1542.83) | 1559.23 (±1.49) | 1177.27 (±1.68) | 0.034 |
Abbreviations: min-PR, minimal pulse rate after CS; pre-dBP, diastolic blood pressure before CS; pre-PR, pulse rate before CS; pre-sBP, systolic blood pressure before CS.
Values quoted in the table are mean (±SD); age is expressed in years; Pre-sBP and Pre-dBP are expressed in mm Hg; Pre-PR and Minimal PR are expressed in beats per minute; daily dose and total dose are expressed as prednisone equivalent in mg.
P-value indicates level of significance for comparison between asymptomatic and symptomatic (significant differences at P < 0.05, in bold characters).
Discussion
High-dose corticosteroid therapy is widely used as attack therapy of inflammatory central nervous system (CNS) disorders and their relapses. Tachyarrhythmia and sinus bradycardia have been both reported as adverse effects; however, bradycardia is often asymptomatic.4,5 Bradycardia is generally associated with high-dose intravenous corticosteroid administration, but some cases after low-dose intravenous and oral corticosteroid therapy have been reported.11,15,17,18 In a review of the literature by Stroeder et al.,19 93 cases of bradycardia attributed to PST have been reported, from 1970 to 2014. Other cases, not included in this work, have been reported12-14,17,18 reaching a total amount of 105 cases of bradycardia consequently to corticosteroids administration (11 case reports, 6 case series, 2 prospective studies, and 1 retrospective study).
Nowadays, the pathogenetic mechanism of CS-associated bradycardia remains unclear. An animal study suggests that a single large dose of methylprednisolone may cause a depression of alpha- and beta-1-receptor sensitivity in myocardial cells.20 In humans, CS work on kidneys’ mineralocorticoid receptors inducing an excretion of potassium and reabsorption of sodium, leading to an expansion in extracellular volume and rise in blood pressure.21 Sudden changes in serum potassium levels may alter potassium flux across the cardiomiocyte’s cellular membrane, causing an alteration in the cardiac rhythms.22,23
It is possible that expansion of plasma volume induces a reflex bradycardia by activation of atrial baroreceptors.24 Indeed, our patient showed a fluid accumulation attested by a 5-kg weight gain during the steroid therapy, which could explain the increase in the blood pressure and the bradycardia described in our report.
According to our analysis, we can suggest that lower total doses may be associated with lower PR and with symptomatic bradycardia. This could be explained by the sudden treatment discontinuation that happens when symptoms appear, leading to a lower total dosage of steroids in these patients, if compared with asymptomatic group. Daily dose mean values instead do not differ significantly in the two groups. Other authors showed that bradycardia is more frequent in older patients and those with underlying cardiac disease25 or with autonomic disturbance like sphincter dysfunction.26 In the absence of these conditions, corticosteroid bradycardia seems to be well tolerated.4,25
Considering the analysis of the literature, as most cases of bradycardia due to PST are asymptomatic, it is probable that this condition is underdiagnosed. It could be appropriate to identify patients with risk factors for cardiac arrhythmias before the first administration of PST. These patients should require close monitoring, recording continuously or at least in 3 phases: at the beginning, in the middle, and at the end of the daily steroid treatment. An earlier detection of bradycardia could lead to a prompt interruption of the steroid therapy, possibly avoiding that the arrhythmic condition became symptomatic. Moreover, early identification, and eventually treatment of bradycardia, is crucial, especially in older patients, to reduce the risk of falls and to avoid the consequent injuries and hospitalization.
Old age and the presence of underlying cardiac disease or autonomic disturbance are neither necessary nor sufficient to early identify patients who will develop steroid-induced bradycardia, as demonstrated in the case we report. It could also be useful to monitor steroid-induced fluid retention by daily weight measurement and serum electrolytes variations, to identify patients at risk for this adverse event.
Conclusions
CS therapy is a wide-ranging therapy. In many inflammatory diseases of CNS, high-dose corticosteroid is used as attack therapy of relapse of the disease. Many different adverse effects are frequent in chronic and in PST, but only few cases of bradycardia are reported in the literature. Bradycardia may happen both after intravenous and oral administration and is asymptomatic in most of cases; moreover, PR generally normalizes spontaneously in few days after discontinuation of the therapy. However, severe bradycardia is an uncomfortable experience and potentially dangerous, as described in our case. Clinicians should be aware of this adverse effect and consider that some patients have a higher risk.
Footnotes
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author Contributions: AS, S Mazzeo, MP and FP initiated the project. AS and S Mazzeo were responsible for design and methodology. AS, S Mazzeo, MS and MP wrote the final draft. S Matà, VB and SS supervised project and reviewed the final draft.
ORCID iD: Alessandro Sodero  https://orcid.org/0000-0003-4356-6912
https://orcid.org/0000-0003-4356-6912
References
- 1. West TW. Transverse myelitis—a review of the presentation, diagnosis, and initial management. Discov Med. 2013;16:167–177. [PubMed] [Google Scholar]
- 2. Klein-Gitelman MS, Pachman LM. Intravenous corticosteroids: adverse reactions are more variable than expected in children. J Rheumatol. 1998;25:1995–2002. [PubMed] [Google Scholar]
- 3. Miura M, Ohki H, Yoshiba S, et al. Adverse effects of methylprednisolone pulse therapy in refractory Kawasaki disease. Arch Dis Child. 2005;90:1096–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Akikusa JD, Feldman BM, Gross GJ, et al. Sinus bradycardia after intravenous pulse methylprednisolone. Pediatrics. 2007;119:e778–e782. [DOI] [PubMed] [Google Scholar]
- 5. Taylor MR, Gaco D. Symptomatic sinus bradycardia after a treatment course of high-dose oral prednisone. J Emerg Med. 2013;45:e55–e58. [DOI] [PubMed] [Google Scholar]
- 6. Tvede N, Nielsen LP, Andersen V. Bradycardia after high-dose intravenous methylprednisolone therapy. Scand J Rheumatol. 1986;15:302–304. [DOI] [PubMed] [Google Scholar]
- 7. Guillén EL, Ruiz AM, Bugallo JB. Hypotension, bradycardia, and asystole after high-dose intravenous methylprednisolone in a monitored patient. Am J Kidney Dis. 1998;32:e41–e43. [DOI] [PubMed] [Google Scholar]
- 8. Küçükosmanoğlu O, Karabay A, Ozbarlas N, et al. Marked bradycardia due to pulsed and oral methylprednisolone therapy in a patient with rapidly progressive glomerulonephritis. Nephron. 1998;80:484. [DOI] [PubMed] [Google Scholar]
- 9. Pudil R, Hrncir Z. Severe bradycardia after a methylprednisolone “minipulse” treatment. Arch Intern Med. 2001;161:1778–1779. [DOI] [PubMed] [Google Scholar]
- 10. Jain R, Bali H, Sharma VK, et al. Cardiovascular effects of corticosteroid pulse therapy: a prospective controlled study on pemphigus patients. Int J Dermatol. 2005;44:285–288. [DOI] [PubMed] [Google Scholar]
- 11. Al Shibli A, Al Attrach I, Hamdan MA. Bradycardia following oral corticosteroid use: case report and literature review. Arab J Nephrol Transplant. 2012;5:47–49. [PubMed] [Google Scholar]
- 12. Kundu A, Fitzgibbons TP. Acute symptomatic sinus bradycardia in a woman treated with pulse dose steroids for multiple sclerosis: a case report. J Med Case Reports. 2015;9:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Domínguez-Pinilla N, del Fresno-Valencia MR, de Inocencio Arocena J, et al. Bradicardia sinusal secundaria al uso de corticoides en bolo. An Pediatría. 2014;80:331–332. [DOI] [PubMed] [Google Scholar]
- 14. John PR, Khaladj-Ghom A, Still KL. Bradycardia associated with steroid use for laryngeal edema in an adult: a case report and literature review. Case Rep Cardiol. 2016;2016:e9785467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Beyan E, Ürün Y, Uzuner A. Bradycardia due to methylprednisolone therapy. J Clin Rheumatol. 2004;10:204 http://journals.lww.com/jclinrheum/Fulltext/2004/08000/Bradycardia_due_to_Methylprednisolone_Therapy.16.aspx. Accessed February 20, 2017. [DOI] [PubMed] [Google Scholar]
- 16. Dashore S, Pande S, Borkar M, et al. Late onset bradycardia: an unusual side-effect of high dose dexamethasone pulse therapy in patients of pemphigus vulgaris: a case series of five patients. Indian J Drugs Dermatol. 2015;1:23–26. [Google Scholar]
- 17. Marinov M, Fuessel M-U, Unterrainer AF. Bradycardia after dexamethasone for postoperative nausea and vomiting prophylaxis during induction of anaesthesia. Br J Anaesth. 2013;111:1025–1026. [DOI] [PubMed] [Google Scholar]
- 18. Hasan AQ, Al-Khazraji A. Corticosteroids-induced bradycardia: a case report & literature review. J Med Sci Clin Res. 4:64. [Google Scholar]
- 19. Stroeder J, Evans C, Mansell H. Corticosteroid-induced bradycardia. Can Pharm J CPJ. 2015;148:235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hall ED, Plaster M, Braughler JM. Acute cardiovascular response to a single large intravenous dose of methylprednisolone and its effects on the responses to norepinephrine and isoproterenol. Proc Soc Exp Biol Med. 1983;173:338–343. [DOI] [PubMed] [Google Scholar]
- 21. Hellal-Levy C, Couette B, Fagart J, et al. Specific hydroxylations determine selective corticosteroid recognition by human glucocorticoid and mineralocorticoid receptors. FEBS Lett. 1999;464:9–13. [DOI] [PubMed] [Google Scholar]
- 22. Parham WA, Mehdirad AA, Biermann KM, et al. Hyperkalemia revisited. Tex Heart Inst J. 2006;33:40–47. [PMC free article] [PubMed] [Google Scholar]
- 23. Fujimoto S, Kondoh H, Yamamoto Y, et al. Holter electrocardiogram monitoring in nephrotic patients during methylprednisolone pulse therapy. Am J Nephrol. 1990;10:231–236. [DOI] [PubMed] [Google Scholar]
- 24. Anzai Y, Nishikawa T. Heart rate responses to body tilt during spinal anesthesia. Anesth Analg. 1991;73:385–390. [DOI] [PubMed] [Google Scholar]
- 25. Hsu DT. Steroids and bradycardia: how slow can you go? J Pediatr Hematol Oncol. 2008;30:119–120. [DOI] [PubMed] [Google Scholar]
- 26. Vasheghani-Farahani A, Sahraian MA, Darabi L, et al. Incidence of various cardiac arrhythmias and conduction disturbances due to high dose intravenous methylprednisolone in patients with multiple sclerosis. J Neurol Sci. 2011;309:75–78. [DOI] [PubMed] [Google Scholar]