Abstract
Bastadin-6–34-O-sulfate ester (8) was isolated from methanol extracts of Ianthella basta. The structure of 8 was characterized by analysis of MS and NMR data, and conversion through acid hydrolysis, to the parent compound, bastadin-6, which was identical by HpLC, MS and NMR with an authentic sample. An improved procedure for procurement of pure samples of bastadins-4, −5 and −6 is described.
Graphical Abstract
1. Introduction
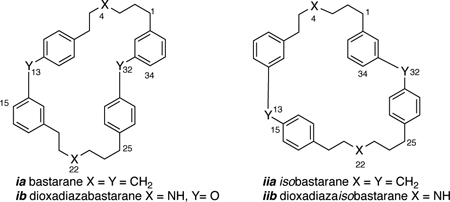
Bastadins are group of over 20 highly brominated natural products, formally derived from bromotyrosine (1), dibromotyrosine (2) or their corresponding tyramines, and classified by two hypothetical parent skeletons: ‘bastarane’ (ia)† and its constitutional isomer, ‘isobastarane’ (iia). The first seven bastadins including the ring opened symmetrical dimer, bastadin-3 (3), and macrodilactams, bastadins-4 (4), −5 (5) and −6 (6), were characterized over 35 years ago by Kazlauskus and coworkers from samples of the Australian marine sponge Ianthella basta, Pallas [1a,b] and identified with modest antibiotic properties. They gained new significance in 1995 with the finding that bastadin-5 (5) and bastadin-6 (6, Figure 1) are potent agonists for the release of Ca2+ ions from stores within the sarcoplasmic reticulum (SR) though modulation of heterotetrameric megaprotein (2.5 MDa) Ca2+ ion-channel, RyR-1.[2] The most potent agonist, bastadin-5 (5) (EC50 = 2.3 μM), was shown to promote release of Ca2+ through modulation of RyR-1[3,4] – the last stage of excitation-contraction coupling in mammalian striated muscle – through alteration of the gating kinetics of channel opening and closing.[2]
Figure 1.
Structures of bromotyrosines (1, 2), bastadins −3 (3), −4 (4), −5 (5), −6 (6), −19 (7) and bastadin-6-O-34-sulfate (8).
Although high-resolution cryo-EM structures of the RyR-1 complex have been reported recently,[5] the binding site of 5 is as yet unknown.
Two ‘channelopathies’ - malignant hyperthermia (MH)[6] and central core disease (CCD)[7] - are manifestations of different single-point autosomally dominant inherited mutations of the same gene, RYR1 in chromosome 19q13.1, that encodes RyR-1. MH is a ‘cryptic’ mutation that reveals itself during general anesthesia with certain inhalation anesthetics. The resulting condition ‒ runaway hyperthermia ‒ can be fatal to the patient if not treated immediately. CCD is congenital myopathy that is often debilitating and can lead to delayed ambulation and reduced vital capacity.
preliminary structure activity relationships of the bastadins have been described.[8] While 5 and 6 are almost equipotent RyR-1 agonists, bastadin-4 (4, 5,6-dehydrobastadin-5) is less potent by order of magnitude, while the constitutional isomer of 5 ‒ bastadin-19 (5) with an isobastarane skeleton - is essentially inactive (IC50 > 100 μM).[2,19] Nevertheless, 4 is valuable because it can be converted into 5 or the isotopically labeled probe, 5-d (and, ostensibly, 5-t) through selective cationic reduction (Et3SiH or Et3SiD, TFA) as we demonstrated earlier.[10]
Compounds 5 and 6, and synthetic analogs inspired by their structures,[8] may be useful probes in the study of RyR-1 mutations, expanding our understanding of the mechanism of RyR-1 channel gating and Ca2+ release, and the possible development of therapeutic agents for the treatment of Ca2+ channel-related myopathies.
However, contemporary investigations into the pharmacology of 5 and 6 are limited by supply; both compounds only occur as minor components along with complex mixtures of bastadins and their O-sulfate esters. The natural products are not separable on silica chromatography and are typically won through tedious, multistep reversed-phase chromatography.[11] Total syntheses of 5[12a] and 6[12b,c] have been reported, but they involve lengthy series of linear steps and the overall yields are typically low. Thus, practical sources of 5 and 6 are required.
in our investigations of sources of new bastadins from samples of I. basta collected at two locations in separated oceans ‒ Guam in the Pacific and Exmouth Gulf, Western Australia, in the Indian Ocean ‒we discovered a new member of the series: bastadin-6-O-sulfate ester (8) which is the subject of this report. In addition, we describe a streamlined procedure for rapid procurement of the two most useful RyR-1 modulators, 5 and 6 from I. basta, and an improved protocol for conversion of 4 to the highly valued 5.
2. Experimental
2.1. General methods
Optical rotations were measured on a JASCO P-2000 at the D-double emission line of Na°. UV-vis spectra were measured on a JASCO V-630 spectrometer. FTIR spectra were collected on thin-film samples using a JASCO FTIR-4100 fitted with an ATR accessory (ZnSe plate). 1D NMR and inverse-detected 2D NMR spectra were measured on a Bruker Avance II NMR spectrometer with a 1.7 mm1H{13C/15N} 600 MHz microcryoprobe. Other NMR spectra were measured on a JEOL ECA spectrometer equipped with a 5 mm1H{13C} 500 MHz room-temperature probe.13C NMR spectra were measured using a Varian NMR spectrometer equipped with a 5 mm Xsens13C{1H} 125 MHz cryoprobe. NMR spectra are referenced to residual solvent signals. High-resolution ESITOF analysis was carried out on an Agilent 1200 HPLC coupled to an Agilent 6230 TOFMS. Low-resolution MALDI MS measurements were made on a Bruker Biflex IV in a nitrobenzyl alcohol matrix. Low-resolution MS measurements were made on a Thermoelectron Accela UHPLC coupled to an MSQ single-quadrupole detector. Preparative, semipreparative, and analytical HPLC were performed on a JASCO PU-2086 Plus system consisting of a dynamic mixer (MX-2080–32) with UV-VIS detector (UV-2075) operating at λ 250 nm. Automated flash chromatography was carried out with a Teledyne-Isco CombiFlash system with UV (λ 250 nm) and RI detection.
2.2. Animal Material
Two collections of the sponge Ianthella basta were made using scuba ‒ one in 2009 from Guam (09-IBC, sample courtesy of Peter Schupp, University of Oldenburg) and a second in 1993 from Bennett Shoal, Exmouth Gulf, Western Australia (93-07-101). The samples were stored at −20 °C until required.
2.3. Expedient Purification of Bastadins-5 and −6.
A typical isolation procedure is described here for Ianthella basta. Frozen lyophilized sponge (93-07-101, 92 g, dry wt.) was cut into smaller pieces (~5–10 cm) and extracted by slow stirring with CH3OH at room temperature (2 × 600 mL, 12 h), and the combined extracts concentrated under reduced to half of the volume. The H2O content was adjusted to 1:9 H2O-CH3OH, and the extract repeatedly partitioned against hexanes (2 × 400 mL) to give, after removal of solvent, ‘fraction A’ (1.41 g). The water content was re-adjusted to 2:3 H2OCH3OH and the solution partitioned against CH2Cl2 (2 × 400 mL) to give, after removal of solvent, ‘fraction B’ (2.49 g). MeOH was removed from the remaining aqueous-MeOH partition, under reduced pressure, and the remaining H2O solution partitioned against n-BuOH (2 × 100 mL) to give ‘fraction C’ (600 mg). The residue of the aqueous phase constituted ‘fraction D’ (9.8 g).
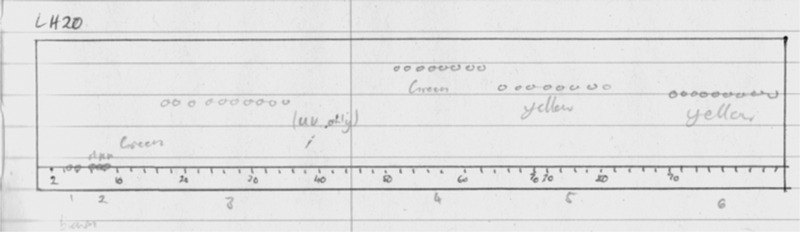
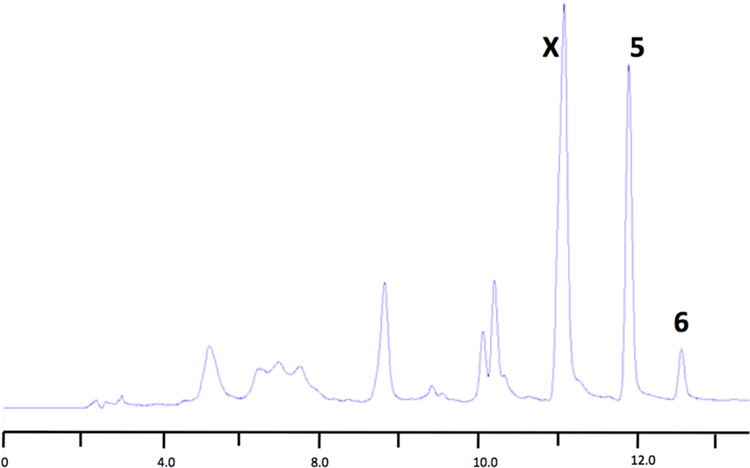
Fraction B was purified by size-exclusion chromatography (Sephadex LH-20) with elution by MeOH to give six fractions (F1–6). grouped by TLC (silica F254, developed with 1:9 MeOH-CH2Cl2, UV visualization and staining with vanillin-H2SO4-EtOH, Figure 2). TLC of F-4, containing 5 and 6 (LCMS), gave a spot that characteristically stained green while F-6, containing mostly 4, stained a yellow or orange yellow (Figure 2). Further purification of F-4 was achieved by reversed-phase preparative HPLC (Phenomenex Kinetex C18 column, 150 × 21.2 mm, 5μ, linear gradient, 50:50 H2O-0.1% TFA: CH3CN to 30:70 over 20 min, 13 mL/min flow rate, Figure 3). Pure bastadin-5 (5, 5.2 mg) eluted as a peak at 15.6 min, while pure bastadin-6 (6, 4.0 mg) eluted at 17.2 min. The peak eluting at 14.2 min, ‘X’, contained a mixture of bastadins-16[15] and −19[9] that could be separated under alternative HPLC conditions (RP C18). Bastadin-4 (4, 11.0 mg) was the major component of fraction 6. The identity of all compounds were confirmed by comparisons of their MS and1H NMR data with literature values.
Figure 2.
TLC of fractions F1–6 from Sephadex LH-20 of an extract of Ianthella basta (1:9 MeOH-CH2Cl2, visualization with vanillin-H2SO4). Fraction 4 contained 5 and 6. Fraction 6 (orange-yellow) largely pure 4.
Figure 3.
RP HPLC chromatogram of Sephadex LH-20 Fraction 4 from Ianthella basta (UV detection, λ 250 nm) ; See Experimental and Section 2.4 for conditions.). Retention times: X – a mixture of bastadins-16[15], and −19[9] (tR = 14.2 min), bastadin-5 (5, tR = 15.6 min), bastadin-6 (6, tR = 17.2 min).
2.4. Bastadin-6–34-O-sulfate Ester (8)
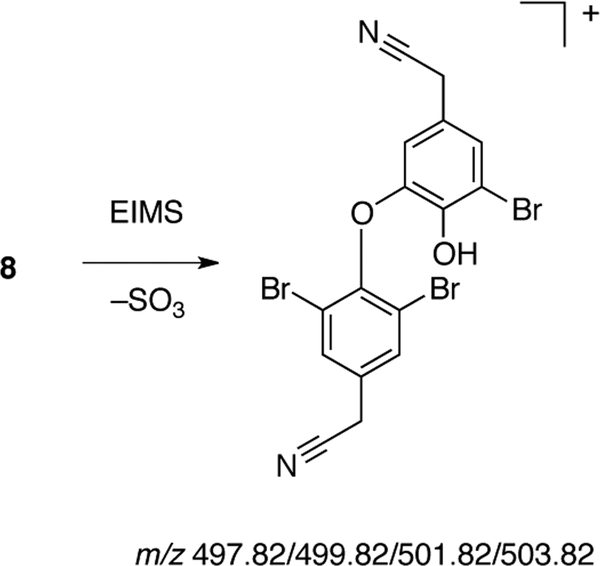
The MeOH extract of I. basta from Guam was solvent-partitioned and a portion of the n-BuOH-soluble partition (‘fraction C’) was separated by automated low-pressure chromatography (silica, gradient elution, 050% MeOH-CH2Cl2 over 60 min) to give nine fractions. Fraction 7 (385 mg) was further separated by HPLC (Phenomenex Luna 5μ Phenyl-hexyl, 21.2 × 250 mm, 8.5 mL.min−1, 70–100% MeOH-10 mM Na2S04 over 60 min). Individual HPLC fractions were freed of salts and recovered by capture on C18 solid-phase extraction cartridges, followed by washing with H2O and elution of the organic compound with MeOH to give bastadin-6 (6, 4.5 mg), bastadin-5 (5, 3.2 mg) bastadin-4 (4, 3.8 mg) and a mixed fraction containing 8. The latter was further purified by reversed phase HPLC (Phenyl-hexyl, 4.0 mL.min−1, 60–100% MeOH-H2O over 15 min) and a similar recovery procedure, to provide pure 8 (4.5 mg). Colorless powder; FTIR (ATR, ZnSe plate): ν 1676, 1437, 1203, 1133, 841, 801, 723 cm−1;1H and13C NMR (CD3OD), see Table 1 (see Supporting Information for NMR data in CD3CN). MALDI-TOF m/z 1222.87 [M+Na]+ (calcd for C34H2579Br381Br3N4Na2O11S 1222.61). EIMS m/z 497.8 (34%)/499.8 (99%)/501.8 (100)/503.8 (36), see Figure 4. HRESIMS m/z 1092.6943 [M−SO3+H]+ (calcd for C34H2779Br6N4O8 1092.6924).
Table 1–
1H and13C NMR data for 8 (CD3OD, 23 °C)
| Atom |
δ13ca 6 |
δ13c’b 8 |
δ1H (mult, J, integ.)c 8 |
HMBC 8 (13C–>1H) |
|---|---|---|---|---|
| 1 | 27.3 | 27.2 | 3.70 (s, 2H) | 36,38 |
| 2 | 151.5 | 150.9 | 1 | |
| 3 | 163.0 | 164.0 | 1,5 | |
| 4 | ||||
| 5 | 38.4 | 40.5 | 3.45 (m, 2H) | 6 |
| 6 | 33.9 | 33.9 | 2.79 (t, J=7.5 Hz, 2H) | 5 |
| 7 | 140.1 | 140.0 | 5,6 | |
| 8 | 133.6 | 133.4 | 7.59 (s, 1H) | 6,10 |
| 9 | 117.4 | 117.5 | 8 | |
| 10 | 146.1 | 147.0 | 8,10 | |
| 11 | 117.6 | 117.5 | 10 | |
| 12 | 133.6 | 133.4 | 7.59 (s, 1H) | 6,8 |
| 13 | – | |||
| 14 | 144.6 | 144.8 | 19 | |
| 15 | 141.6 | 142.0 | 17,19 | |
| 16 | 110.2 | 110.2 | 17 | |
| 17 | 126.2 | 126.0 | 7.06 (d, J=1.9 Hz, 1H) | 19,20 |
| 18 | 130.7 | 130.8 | 20,21 | |
| 19 | 111.7 | 112.0 | 6.30 (d, J=1.9 Hz, 1H) | 17,20 |
| 20 | 32.7 | 33.5 | 2.7 (t, J=7.2 Hz, 2H) | 17,19,21 |
| 21 | 40.4 | 39.1 | 3.40 (m, 2H) | 20 |
| 22 | ||||
| 23 | 163.3 | 164.4 | 21,25 | |
| 24 | 150.4 | 150.4 | 25 | |
| 25 | 28.7 | 28.3 | 3.80 (s, 2H) | 27,31 |
| 26 | 137.6 | 137.2 | 25 | |
| 27 | 133.2 | 133.2 | 7.55 (s, 1H) | 25,31 |
| 28 | 117.1 | 117.6 | 27 | |
| 29 | 146.1 | 147.9 | 27,31 | |
| 30 | 117.1 | 117.6 | 31 | |
| 31 | 133.2 | 133.2 | 7.55 (s, 1H) | 25,27 |
| 32 | ||||
| 33 | 144.8 | 150.5 | 38 | |
| 34 | 141.8 | 137.2 | 36,38 | |
| 35 | 109.8 | 119.0 | 36 | |
| 36 | 126.8 | 135.2 | 7.21 (d, J=1.9 Hz. 1H) | 1,38 |
| 37 | 128.1 | 127.0 | 1 | |
| 38 | 112.6 | 113.8 | 6.30 (d, J=1.9 Hz, 1H) | 1,36 |
Figure 4.
ESIMS fragmentation of 8
2.5. Acid Hydrolysis of 8
A mixture of 8 (0.5 mg) and 2M HCl (0.5 mL) was heated in a sealed tube at 50 °C for 30 minutes. The sample was cooled, dried under a stream of nitrogen and taken up in CD3OD. The1H NMR (500 MHz) of the solution was identical to that of authentic bastadin-6 (6). Analytical HPLC of 8 and authentic 6 (Dynamax Microsorb C18 column, 10 × 250 mm, 4.0 mL.min−1, gradient: 60–100% MeOH-H2O over 15 min, then isocratic) gave retention times of 9.9 min and 15.2 min, respectively. HPLC of the hydrolysate of 8 also gave a single peak with retention time of 15.2 min, consistent with 6.
2.6. Cationic Reduction of Bastadin-4 to Bastadin-5.
Cationic reduction of bastadin-4 (4) to bastadin-5 (5) was carried out by an improved modification of our earlier reported procedure.[10] To a solution of 4 (3.8 mg, 3.7 μmol, 1.0 equiv) in CF3COOH (0.5 mL) under an atmosphere of dry N2 was added a solution of triethylsilane (5.5 μL, 37 μmol, 10 equiv) in CH2Cl2 (0.5 mL). The mixture was stirred vigorously for 30 min, dried under a stream of dry N2, and the residue purified by reversed phase HPLC (Microsorb C18, 3.0 mL.min−1, 80:20 MeOH-H2O+0.1% TFA) to afford recovered 4 (0.5 mg, 13%) and 5 (1.5 mg, 40%; 65% based on recovered starting material) identical with an authentic sample by MS and1H NMR.
3. Results and Discussion
3.1. Structure Elucidation of 8
Compound 8 was purified from the Guamanian sample of Ianthella basta using a variation of the conventional protocols we have used in the past and reported elsewhere.[9] The formula of 8 C34H25Br6N4NaO11S was assigned from mass spectrometric measurements [MALDI m/z 1222.87 [M+Na]+ calcd. 1222.61 for C34H2579Br381Br3N4Na2O11S; HRESITOFMS m/z 1092.6943 [MSO3+H]+). The isotope pattern reveals the presence of Br6, the highest number of Br atoms that can be accommodated in a bastadin skeleton (e.g. 6). While the79Br381Br3 isotopomer of 6 is expected to show m/z 1120.67 [M+Na]+ the higher mass measured for 8 is reconciled by the difference ΔM= 101.94 [SO3+Na-H]; therefore, 8 is an O-sulfate half-ester of 6.
The1H NMR spectrum of 8 (Table 1) showed characteristic two-proton aryl signals for each of the symmetrical 3,5-dibromotyrosine-like spin systems (δ 7.59, s, 2H, H-8/H-12; δ 7.55, s, 2H, H-27/H-31). These data could only be accommodated by the aryl-ring substitution pattern found in the hexabromo-substituted, bastadin-6 (6), or its unreported iso-bastarane (iia) constitutional isomer. Assignment of the catechol ether linkages between the eastern and western hemispheres of structure 8 was secured by observation of two sets of HMBC correlations ((Table 1, Figure 2) recorded in different solvents (600 MHz), CD3OD (Table 1) and CD3CN (see Supporting Information) to resolve equivocal assignments from overlapping signals. Cross peaks were observed between H-21 (δ 3.40, m, 2H) and H-25 (δ 3.80, s, 2H) to the C-23 amide carbonyl at (δ 164.4, s), and between H-1 (δ 3.70, s, 2H) and H-5 (δ 3.45, m, 2H) and C-3 (d, 164.0, s).
The foregoing data for 8 support the same constitution as 6. The locations of Br substituents in the carbon skeleton of 8 were verified by electron-impact mass spectrometry (EIMS) that revealed loss of SO3 and a major Br3-containing fragment (m/z 497.82/499.8/501.82/503.82) arising from the previously noted [1] and characteristic double-heterolytic cleavage between the amide carbonyl and ketoxime C=N double bond (Figure 4). Additional fragment ions from sequential homolytic losses of Br (m/z 417.8/419.8/421.8 and 339.9/341.9) were also observed in the EI mass spectrum of 8. Therefore, 8 is a member of the ‘bastarane’ series, ia.
Confirmation of the structure of 8 followed from its acid hydrolysis (2M HCl aqueous MeOH, 50 °C, 30 min) which gave a compound that was identical with authentic 6 by1H NMR[1] and HPLC retention time.
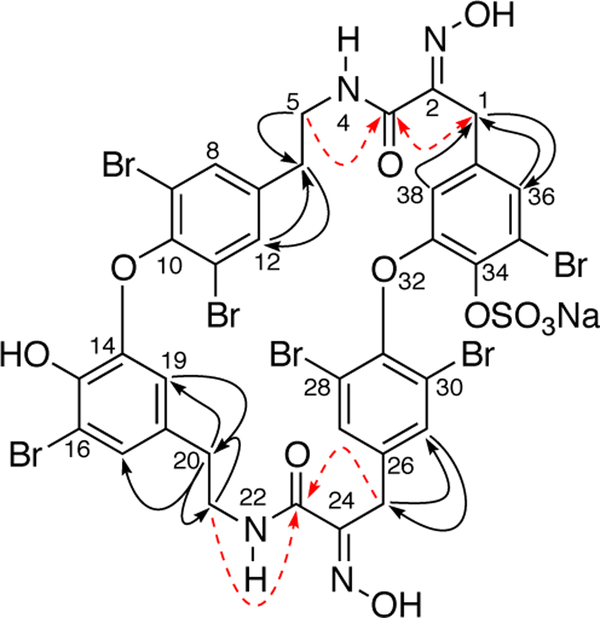
The structures of known bastadin sulfate half-esters have O-sulfate groups placed at either C-15 or C-34, or both.[9,13] In order to ascertain the position of the O -sulfate in 8, the13C NMR chemical shifts of the latter were compared with those of 6.[1,] As noted earlier by Ragan [14], Wright and co-workers[13] and others,[9] sulfation of a phenoxyl group leads to an upfield shift of the ipso13C signal by approximately Δδ 5 ppm and downfield shifts of ortho and para13C signals. The 13C NMR chemical shifts of bastadin-6 (6) were assigned by HMBC and HSQC and compared with those of 8 (Table 1 and Figure 5), and subjected to differential analysis. The C-34 quaternary carbon signal of 1 (δ 137.2, s) was shifted upfield (Δδ −4.6 ppm), while C-33 and C-35 appeared downfield (Δδ 5.7 and 9.2 ppm, respectively); therefore, the O-sulfate half-ester in 8 is positioned at C-34.
Figure 5.
HMBC data (500 MHz) of 8. Correlations (1H −>13C) observed in CD3CN (red dashed lines, obscured in CD3OD), and those observed both CD3OD and CD3CN (solid lines).
3.2. Optimized Procurement of Bastadins-4–6
Given the lengthy and tedious procedure required to obtain 4–6 and 8 from the Guamanian sample of I. basta, we invested time to optimize the purification of the former desirable compounds and reduce the number of HPLC purification steps. A sample of I. basta collected in 1993 in Exmouth Gulf, Western Australia, was found to be devoid of 4 but contained 5 and 6 (LCMS). Since the former complicates the separations of the latter by preparative reversed phase HPLC, we investigated high-loading purification of MeoH extracts of this sponge. In the event, gel filtration (Sephadex LH-20, MeOH elution) of the solvent-partitioned ‘fraction-B’, with monitoring by LCMS, delivered a single late-eluting fraction containing 5 and 6. The latter fraction was separated in single HPLC step (reversed phase Phenyl-hexyl column, Phenomenex, H2O-CH3CN gradient) giving pure samples of 5 and 6.
Although 4 is the 5,6-dehydro-derivative of 5, conversion of 4 into 5 by catalytic hydrogenation is complicated by over-reduction and loss of Br through hydrogenolysis. Attempted homogenous catalytic hydrogenation of 4 with Wilkinson’s catalyst ((Ph3P)3RhCl, H2 >60 atm) returned only starting material.[10] Eventually, we refined an optimized procedure for procurement of high-value 5; cationic reduction of 4 (Figure 6, Et3SiH, CH2Cl2-CF3COOH, vigorous stirring under nitrogen) gave 5 in 65% yield (based on recovered 4) after HPLC purification (Figure 5), which significantly improves over our earlier protocol.[10]
Figure 6.
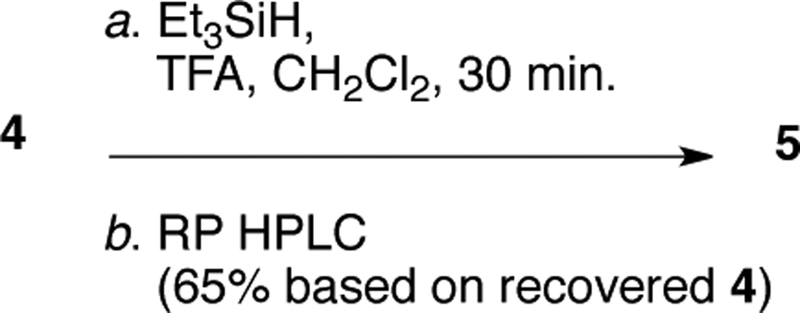
Cationic reduction of 4 to 5.
3.3. Conclusions
The new compound, bastadin-6-34-0-sulfate ester (8) was isolated from a specimen of Ianthella basta collected in Guam. Refinement of a new purification protocol gave pure samples of highly-value bastadins-5 (5) and −6 (6) in two steps from a polar solvent-partitioned fraction. An improved conversion of 4 to 5 by cationic reduction facilitates access to this most potent RyR-1 agonist. Compound 8 undergoes acid hydrolysis to provide 6. Thus, these reactions of 4 and 8 follow convergent paths to deliver more of the high-value analogs 5 and 6, respectively.
Supplementary Material
Acknowledgements
We thank Jenny Kwan and Brandon Morinaka (UCSD) for preliminary preparative isolation of 4, 5, 6 and other known bastadins. This work was supported by funding from the National institutes of Health (AI100776).
Footnotes
The parent names ‘bastarane’ for ia, or more informatively 13,32-dioxa-4,22 diazabastarane, ib, were proposed to unify the family of compounds and avoid awkward nomenclature. See Ref. 1a for a discussion. The name ‘isobastarane’ skeleton was coined later by Capon to describe bastadin-13, the first member with an alternate catechol ether linkage (see Ref. 1c), but it also brings order to numbering the schemes.
References
- 1.(a) Kazlauskas R; Lidgard RO; Murphy PT; Wells RJ;Blount JF, Brominated Tyrosine-Derived Metabolites from the Sponge Ianthella basta. Aust. J. Chem 1981, 34, 765–786. [Google Scholar]; (b) Kazlauskas R; Lidgard RO; Murphy PT; Wells RJ, Brominated Tyrosine Metabolites from the Sponge Ianthella basta.Tetrahedron Lett. 1980, 21, 2277–2280. [Google Scholar]; (c) Butler MS; Lim TK; Capon RJ; Hammond LS, The bastadins revisited: New Chemistry from the Australian Marine Sponge Ianthella basta. J. Nat. Prod 1991, 44, 287–96. [Google Scholar]
- 2.Mack M; Molinski TF; Buck ED; Pessah IN, Novel Modulators of Skeletal Muscle FKBP12/Calcium Channel Complex from Ianthella basta. Role of FKBP12 in Channel Gating. J. Biol. Chem 1994, 269, 23236–23349. [PubMed] [Google Scholar]
- 3.(a) Clarke OB; Hendrickson WA Curr. Opin. Struct. Biol 2016, 39,144–152; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Santulli G; Marks AR Curr. Mol. Pharmacol 2015, 8, 206–222. [DOI] [PubMed] [Google Scholar]; (c) Pessah IN; Cherednichenko G; Lein PJ Pharmacol. Ther 2010, 125, 260–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rebbeck RT; Karunasekara Y; Board PG; Beard NA; Casarotto MG; Dulhunty AF Int. J. Biochem. Cell Biol 2014, 48, 28–38. [DOI] [PubMed] [Google Scholar]
- 5.(a) Efremov RG; Leitner A; Aebersold R; Raunser S Architecture and conformational switch mechanism of the ryanodine receptor. Nature 2015, 517, 39–43. [DOI] [PubMed] [Google Scholar]; (b) Zalk R Clarke OB; des Georges A; Grassucci RA; Reiken S; Mancia F; Hendrickson WA; Frank J; Marks AR Structure of a mammalian ryanodine receptor. Nature 2015, 517, 44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Yan Z; Bai X-C; Yan C; Wu J; Li Z; Xie T; Peng W; Yin C-C; Li X; Scheres SHW; Shi Y; Yan N Structure of the rabbit ryanodine receptor RyR1 at near-atomic resolution. Nature 2015, 517, 50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Pessah IN; Allen PD, Malignant hyperthermia. Best Pract. Res. Clin. Anaesth 2001, 15, 277–288. [Google Scholar]; (b) Eltit JM; Bannister RA; Moua O; Altamirano F; Hopkins PM; Pessah IN; Molinski TF; López JR; Beam KG; Allen PD, Malignant hyperthermia susceptibility arising from altered resting coupling between the skeletal muscle L-type Ca2+ channel and the type 1 ryanodine receptor. Proc. Natl. Acad. Sci. USA 2012, 109, 7923–7928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Quinlivan RM; Muller CR; Davis M; Laing NG; Evans GA; Dwyer J; Dove J; Roberts AP Sewry, C. A. Central core disease: clinical, pathological, and genetic features. Arch. Dis. Child 2003, 88, 1051–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Magee KR, Shy GM (1956). A new congenital non-progressive myopathy. Brain. 1956, 79, 610–21 [DOI] [PubMed] [Google Scholar]
- 8.Masuno MN; Pessah IN; Olmstead MM; Molinski TF, Simplified Cyclic Analogues of Bastadin-5. Structure-Activity Relationships for Modulation of the RyR1/FKBP12 Ca2+ Channel Complex. J. Med. Chem 2006, 49, 4497–4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franklin MA; Penn SG; Lebrilla CB; Lam TH; Pessah IN; Molinski TF, Bastadin 20 and Bastadin O-Sulfate esters from Ianthella basta: Novel Modulators of the RyR1 FKBP12 Receptor Complex. J. Nat. Prod 1996, 59, 1121–1127. [DOI] [PubMed] [Google Scholar]
- 10.Masuno MN; Molinski TF, Cationic reduction of bastadin-4 to bastadin-5. Preparation of 5-[2H]-bastadin-5 by site-specific isotopic labeling. J. Nat. Prod 2003, 66, 112–114. [DOI] [PubMed] [Google Scholar]
- 11.Calcul L; Inman WD; Morris AA; Tenney K; Ratnam J; McKerrow JH; Valeriote FA; Crews P, Additional Insights on the Bastadins: Isolation of Analogues from the Sponge Ianthella cf. reticulata and Exploration of the Oxime Configurations. J. Nat. Prod 2010, 73, 365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Couladouros EA; Pitsinos EN; Moutsos VI; Sarakinos G A General Method for the Synthesis of Bastaranes and Isobastaranes: First Total Synthesis of Bastadins 5, 10, 12, 16, 20, and 21. Chem. Eur. J 2005, 11, 406–421. [DOI] [PubMed] [Google Scholar]; (b) Kotoku N; Tsujita H; Hiramatsu A; Mori C; Koizumi N; Kobayashi M, Efficient total synthesis of bastadin 6, an anti-angiogenic brominated tyrosine-derived metabolite from marine sponge. Tetrahedron 2005, 61 (30), 7211–7218. [Google Scholar]; (c) Guo Z; Machiya K; Salamonczyk GM; Sih CJ, Total Synthesis of Bastadins 2,3 and 6. J. Org. Chem 1998, 63, 4269–4276. [Google Scholar]
- 13.(a) Gulavita NK; Wright AE; McCarthy PJ; Pomponi SA; Kelly-Borges M; Chin M; Sills MA, Isolation and Structure Elucidation of 34-Sulfatobastadin 13, an Inhibitor of the Endothelin A Receptor, from a Marine Sponge of the Genus Ianthella. J. Nat. Prod 1993, 56, 1613–1617. [DOI] [PubMed] [Google Scholar]; (b) Masuno MN; Hoepker AC; Pessah IN; Molinski TF 1-O-Sulfatobastadins-1 and −2 from Ianthella basta(Pallas). Antagonists of the RyR1-FKBP12 Ca2+ Channel . Mar. Drugs 2004, 2, 176–184. [Google Scholar]
- 14.Ragan MA Phenol Sulfate Esters: Ultraviolet, Infrared, Proton and Carbon-13 Nuclear Magnetic Resonance Spectroscopic Investigation. Can. J. Chem 1978, 56, 2681–2685. [Google Scholar]
- 15.Park SK; Jurek J; Carney JR; Scheuer PJ, Two More Bastadins, 16 and 17, from an Indonesian Sponge Ianthella basta. J. Nat. Prod 1994, 57 (3), 407–410. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.