Abstract
Introduction
Non-small-cell lung cancer (NSCLC) accounts for more than half of all lung cancer cases. Cytokines play an important role in NSCLC, including IL-27. IL-27 inhibits NSCLC progression; however, the mechanism is not clear. The purpose of this study is to investigate the effects of IL-27 on NSCLC cell proliferation and metastasis.
Materials and methods
NSCLC cells were treated with IL-27 or transfected with miR-935, and the cell proliferation was assayed by Cell Counting Kit-8 (CCK-8) and colony formation. Cell metastasis was analyzed by Transwell chamber system and wound healing assay. IL-27 protein in the medium was analyzed by ELISA. IL-27 mRNA expression was measured by quantitative reverse transcriptase-PCR.
Results
We found that IL-27 played an inhibiting role in NSCLC cell proliferation and metastasis. The molecular mechanism of the suppressing role of IL-27 in NSCLC was regulated by miR-935. IL-27 expression was negatively associated with miR-935 in the clinical NSCLC samples.
Conclusion
The study revealed that IL-27 decreased lung cancer cell proliferation and metastasis via miR-935.
Keywords: non-small cell lung cancer, IL-27, miR-935
Introduction
IL-27 is a cytokine and may share chain components in both cytokines and receptor chains.1–4 IL-27 plays important roles in tumor progression.1–4 IL-27 has been reported as a tumor suppressor gene and plays a prominent role in various cancers, such as inhibiting tumor growth, invasion, and metastasis and promoting cell death.1–4
Non-small-cell lung cancer (NSCLC) accounts for more than half of all lung cancer cases.5 Although the therapy of NSCLC is advanced, the incidence of recurrence and metastasis remains very high and the median survival period is <1 year.5 There are large research interests regarding the role of cytokines in NSCLC.1–4 IL-27 inhibits the proliferation and increases apoptosis of NSCLC cells.6–8 IL-27 not only downregulates stemness- and epithelial–mesenchymal transition (EMT)-related genes but also pushes intratumor myeloid cells to exert antitumor effects in xenotransplant models.9 The combined use of the COX-2 inhibitor Apricoxib and IL-27 inhibits EMT of NSCLC cells in a signal transducer and activator of transcription (STAT) 1-dependent manner.10 IL-27 suppresses EMT and the expression of proangiogenic factors such as vascular endothelial growth factor, (C–X–C motif) ligand 8, and (C–X–C motif) ligand 5 in NSCLC cells.11,12
miRNAs are non-coding RNAs with about 20 nucleotides, and there are binding sequences in the target genes.1 A recent study verifies the important regulatory roles of miRNAs in NSCLC cell proliferation and metastasis.13 Although the roles of IL-27 in NSCLC are reported, investigation on its regulation by miRNAs in NSCLC is lacking. This study is purposed to explore this. We aim to find in this study if IL-27 could suppress NSCLC cell proliferation and metastasis by miR-935. The results obtained indicate that IL-27 by way of suppressing miR-935 expression may constitute a potential therapy for NSCLC.
Materials and methods
NSCLC samples
Informed consent was provided by the patients, and the research procedures were approved by the ethics committee of Zhongnan Hospital of Wuhan University. It was confirmed that the written informed consent was obtained in the study. NSCLC tissues and their adjacent normal tissues as the controls were obtained from Zhongnan Hospital of Wuhan University (Wuhan, China). Twelve NSCLC patients were in II stage, 35 in III stage, and 29 NSCLC patients were in IV stage. The blood samples were collected when the patients were diagnosed with NSCLC. The serum of blood samples was used for analyzing IL-27 or miR-935 expression.
Cell culture
NSCLC cell lines (HCC827, H1299, H1650, A549, and H520) and normal lung cell line (BEAS-2B) were all ordered from Jingkang Biotech (Shanghai, China), and they were maintained in DMEM (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% FBS (HyClone, Logan, UT, USA) and 1% penicillin/streptomycin at 37°C, 5% CO2.
Quantitative reverse transcriptase PCR (qRT-PCR)
Total RNA was isolated from the cell lines and tissues using Trizol (Thermo Fisher Scientific). The qRT-PCR results, which were recorded as threshold cycle numbers (Ct), were normalized against an internal control (U6 RNA), and the comparative threshold cycle method (2−ΔΔCt) was used to determine the levels of miRNA expression. The primers of IL-27 and miR-935 were purchased from Kangcheng Biotechnology (Shanghai, China). The primer sequences were as follows: IL-27: forward, 5′AGCTGGTGTCTGGGGATTCCCA3′; reverse, 5′AGGAGGCCCTCCAACTTAGG3′; miR-935: forward, 5′ACACTCCAGCTGGGCCCAGTTACCG CTTCCGC3′; reverse, 5′TGGTGTCGTGGAGTCG3′; GAPDH: forward, 5′-CAATGACCCCTTCATTGACC-3′; reverse 5′-GACAAGCTTCCCGTTCTCAG-3′; and U6 snRNA: forward, 5′CTCGCTTCGGCAGCACA3′; reverse 5′AACGCTTCACGAATTTGCGT3′.
Enzyme-linked immunosorbent assay
The blood samples of NSCLC patients or the cell conditioned medium of NSCLC cells with miR-935 transfection was collected for ELISA (Abcam, Cambridge, MA, USA). Standard ELISA methods as described by the manufacturer were used to measure IL-27 protein concentrations.
Cell Counting Kit-8 (CCK-8) assay
The proliferation of NSCLC cells was examined by CCK-8 (Beyond, Suzhou, China). Simply, 2×103 cells were seeded in a 96-well plate. At the indicated time, 10 μL CCK-8 was added to each well and the cells were cultured for 2 hours. The absorbance was measured using an ELISA reader at 450 nm.
Colony formation assay
Colony formation assay was performed to assess the ability of H1299 or A549 cells to grow into colonies in vitro. Briefly, after transfection with miR-935 or IL-27 treatment for 48 hours, 400 cells were seeded in six-well plates. The plates were incubated for 12 days at 37°C and then stained with 0.1% crystal violet. Colonies with greater than 50 cells were counted.
Wound healing analysis
The migration ability of NSCLC cells with miR-935 transfection or IL-27 treatment was evaluated using the scratch assay. After miR-935 transfection, cells were seeded in six-well plastic plates at a density of 3×105 cells/well. After 24 hours, the cells reached 90%–100% monolayer confluence. A straight scratch was artificially created in the cell monolayers with a sterile 100 μL pipette tip. Cell debris was removed with PBS. The cell growth medium was supplemented with fresh Roswell Park Memorial Institute-1640 medium and cultured for 48 hours at 37°C. Migration images were captured using an inverted microscope (Carl Zeiss, Oberkochen, Germany). Scratch wound widths were measured under the microscope, and the relative percentage of wound closure was determined by comparing to control cells.
Transwell invasion assay
The effect of miR-935 or IL-27 on NSCLC cell invasion was evaluated by Transwell invasion assay. Exactly 5×103 cells were transfected with miR-935 mimics or IL-27 for 48 hours and then plated into the Transwell upper chamber with Matrigel-coated (1 mg/mL; BD Matrigel™) polycarbonate membrane (8.0 μm; Corning Incorporated, Corning, NY, USA). A total of 600 μL medium containing 10% FBS was added to the lower Transwell chamber. After incubation for 6 hours, cells on the lower surface of the polycarbonate membrane were fixed with 4% paraformaldehyde and stained
Statistical analyses
All analyses were performed using the SPSS 16.0 statistical software package (SPSS Inc., Chicago, IL, USA) or Excel. Every experiment was completed independently at least three times. A P-value <0.05 was considered significant.
Results
IL-27 expression was lower in clinical NSCLC samples
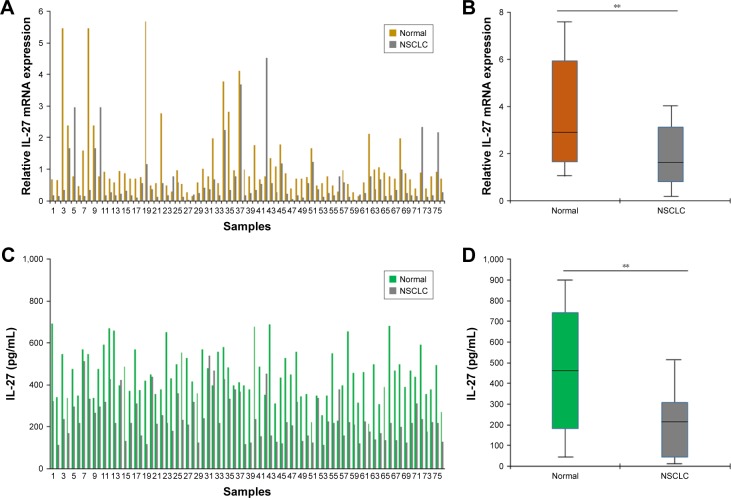
First, the expression of IL-27 in NSCLC samples was examined by real-time RT-PCR. The levels of IL-27 mRNA were lower in NSCLC tissues (n=76) than in normal samples (n=76; Figure 1A and B). The levels of IL-27 protein in NSCLC tissues were analyzed, and the result showed that the levels of IL-27 in the tumor tissues (n=76) were lower than in controls (n=76; Figure 1C and D). The low expression of IL-27 in NSCLC blood samples suggests that IL-27 may function as a tumor suppressor in NSCLC progression.
Figure 1.
IL-27 expression was lower in clinical NSCLC samples.
Notes: (A) IL-27 mRNA expression in NSCLC tissues (n=76) and the adjacent normal tissues (n=76). Real-time RT-PCR was used to examine IL-27 mRNA. (B) The average of IL-27 mRNA levels in NSCLC tissues. Data analysis from (A). (C) IL-27 expression in NSCLC blood samples (n=76) and pooled blood samples of normal volunteers (n=76). ELISA was used to examine IL-27 protein levels. (D) The average of IL-27 protein levels in NSCLC tissues. Data analysis from (C). **P<0.01.
Abbreviations: NSCLC, non-small-cell lung cancer; RT-PCR, reverse transcriptase PCR.
IL-27 decreased cellular growth in NSCLC cells
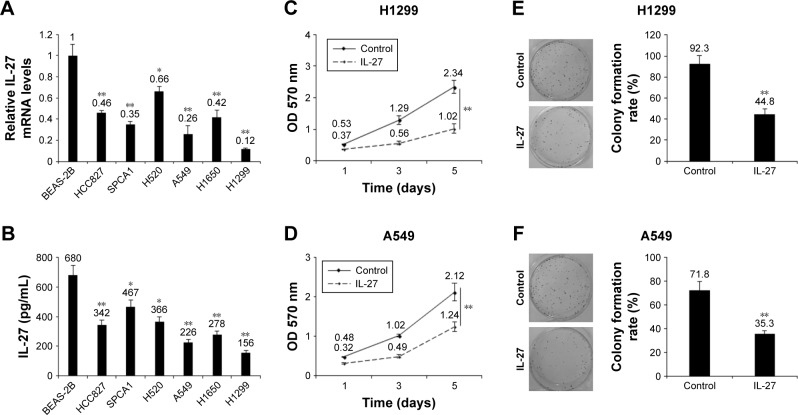
To explore the role of IL-27 in cellular growth, NSCLC cells and normal lung epithelial cells were used to examine IL-27 protein in the medium of the cells, and the result showed that IL-27 mRNA and protein levels in H1299 and A549 cells were relatively lower than the IL-27 mRNA and protein levels in other cells (Figure 2A and B). Next, H1299 and A549 cells were treated with IL-27 or the control for cell survival assays. CCK-8 assay was used to examine cell proliferation, and the data showed that IL-27 could inhibit cell growth in H1299 and A549 cells (Figure 2C and D). We used colony formation assay to evaluate cell proliferation, and the results displayed that IL-27 could significantly decrease colony formation rates in H1299 and A549 cells (Figure 2E and F).
Figure 2.
IL-27 decreased cellular growth in NSCLC cells.
Notes: (A) IL-27 mRNA levels in NSCLC cells and normal lung epithelial cells. NSCLC cells were cultured for total RNA extraction and then IL-27 mRNA was tested using qRT-PCR. (B) IL-27 protein levels in NSCLC cells and normal lung epithelial cells. NSCLC cells were cultured for the medium collection and then IL-27 protein was tested using ELISA kit. (C and D) IL-27 inhibited H1299 and A549 cell proliferation. H1299 and A549 cells were treated with IL-27 (10 ng/mL). (E and F) IL-27 inhibited H1299 and A549 cell colony formation. H1299 and A549 cells were treated with IL-27 (10 ng/mL) and seeded into six-well plates. *P<0.05; **P<0.01.
Abbreviations: NSCLC, non-small-cell lung cancer; qRT-PCR, quantitative reverse transcriptase-PCR.
IL-27 inhibited NSCLC cell metastasis
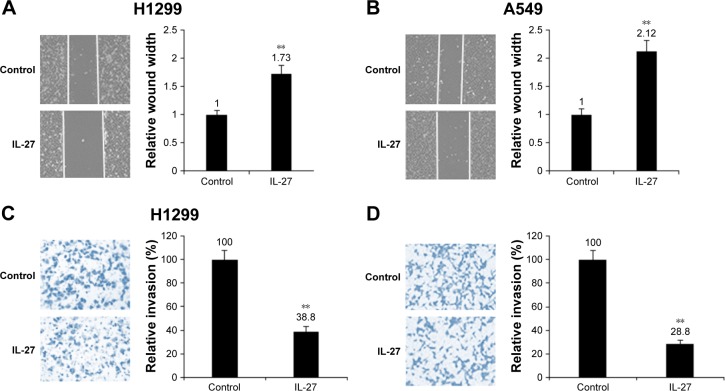
In order to find whether IL-27 is related to the cancer metastasis in NSCLC cells, H1299 and A549 cells were seeded in the upper chamber and IL-27 was added to the growth medium for cell migration assay. The results indicated that IL-27 decreased the migration of H1299 and A549 cells, respectively (Figure 3A and B). It was also demonstrated that IL-27 inhibited invasion of H1299 and A549 cells (Figure 3C and D). The data indicated that IL-27 suppressed NSCLC metastasis.
Figure 3.
IL-27 suppressed cell metastasis of NSCLC cells.
Notes: (A and B) IL-27 inhibited H1299 and A549 cell migration. H1299 and A549 cells were treated with IL-27 (10 ng/mL) and then cell migration was assayed by Transwell chamber. (C and D) IL-27 inhibited H1299 and A549 cell invasion ability. H1299 and A549 cells were treated with IL-27 (10 ng/mL) and seeded in upper chamber, and cell migration was assayed by Transwell chamber. **P<0.01.
Abbreviation: NSCLC, non-small-cell lung cancer.
miR-935 downregulated IL-27 expression in NSCLC cells
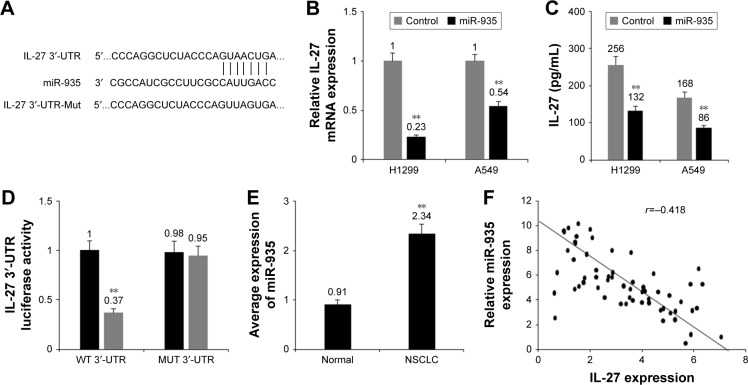
As predicted by PicTar, has-miR-935 (miR-935) was bound to IL-27 3′-UTR (Figure 4A). When we studied whether miR-935 regulated IL-27, it was found that miR-935 overex-pression reduced the mRNA levels of IL-27 in NSCLC cells (Figure 4B). miR-935 overexpression reduced the protein levels of IL-27 in H1299 and A549 cells (Figure 4C). miR-935 overexpression remarkably reduced luciferase activity of a reporter gene with wild-type, but not mutant IL-27 3′-UTR (Figure 4D). The above mentioned data verified that miR-935 was directly bound to IL-27 3′-UTR and it downregulated IL-27 expression in NSCLC cells. miR-935 expression in NSCLC tissues was measured and the result demonstrated that miR-935 was higher in the NSCLC tissues than in adjacent normal tissues (Figure 4E). The relationship between miR-935 and IL-27 levels in NSCLC was negative (Figure 4F). These data suggest that miR-935 negatively regulates IL-27 expression in NSCLC cells.
Figure 4.
miR-935 downregulated IL-27 expression in NSCLC cells.
Notes: (A) IL-27 was predicted as a target gene of miR-935 by PicTar and TargetScan. (B) miR-935 overexpression reduced the mRNA levels of IL-27 in H1299 and A549 cells. (C) miR-935 overexpression reduced the protein levels of IL-27 in H1299 and A549 cells. (D) miR-935 suppressed the luciferase activity of IL-27 3′-UTR in H1299 and A549 cells. (E) miR-935 was overexpressed in NSCLC samples. (F) miR-935 expression was negatively associated with IL-27 protein levels in NSCLC tissues. **P<0.01.
Abbreviation: NSCLC, non-small-cell lung cancer.
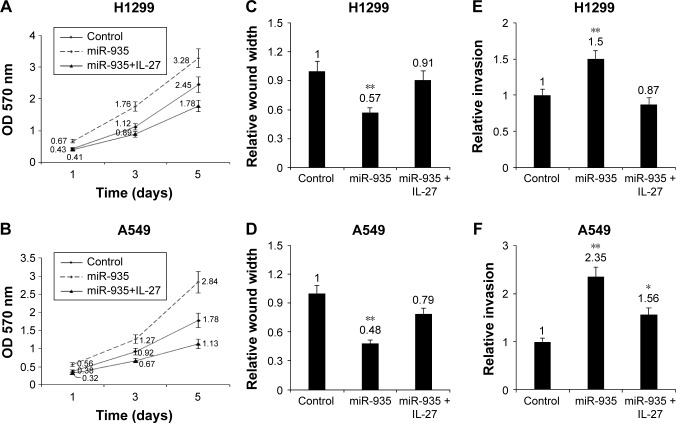
miR-935 targeting IL-27 promoted NSCLC cell proliferation and metastasis
To explore the role of IL-27 in cellular growth, NSCLC cells were transfected with miR-935 or treated with IL-27 for cell survival assays. MTT assay was used to examine cell proliferation, and the data showed that IL-27 could inhibit cell growth in H1299 and A549 cells (Figure 5A and B). In order to elucidate whether IL-27 is related to cancer metastasis by miR-935, NSCLC cells were transfected with miR-935 or treated with IL-27 and the cell migration and metastasis were assayed by Transwell chambers. The results indicated that miR-935 reversed the migration inhibition induced by IL-27 in H1299 and A549 cells (Figure 5C and D). They also demonstrated that miR-935 reversed the invasion inhibition induced by IL-27 in H1299 and A549 cells (Figure 5E and F). The result suggests that miR-935 promotes NSCLC cell proliferation and metastasis by IL-27.
Figure 5.
miR-935 targeting IL-27 promoted NSCLC cell proliferation and metastasis.
Notes: (A and B) Inhibition of miR-935 suppressed H1299 and A549 cell proliferation. H1299 and A549 cells were transfected with miR-935 or treated with IL-27 (10 ng/mL), and then cell survival rates were analyzed by MTT assay. (C and D) Inhibition of miR-935 decreased the migration ability of H1299 and A549 cells. H1299 and A549 cells were transfected with miR-935 inhibitors or treated with IL-27 (10 ng/mL), and then cell migration was assayed by Transwell chamber. (E and F) Inhibition of miR-935 decreased the invasion ability of H1299 and A549 cells. H1299 and A549 cells were transfected with miR-935 inhibitors or treated with IL-27 (10 ng/mL), and then cell invasion was assayed by Transwell chamber. *P<0.05; **P<0.01.
Abbreviation: NSCLC, non-small-cell lung cancer.
Discussion
IL-27 is one of the cytokines which plays a suppressing role in cancer, and its regulation by miRNA is not clear. In this study, we found that IL-27 was regulated by miRNAs in NSCLC cells. The results indicated that IL-27 suppressed NSCLC cell proliferation, migration, and invasion. Interestingly, we found that miR-935 regulated IL-27 expression in NSCLC cells. The functional relationship between IL-27 and miR-935 indicated that the upregulation of miR-935 could promote NSCLC cell survival ability and metastasis by directly targeting IL-27 expression.
IL-27 is a pleiotropic, two-chain cytokine and is composed of EBI3 and IL-27p28 subunits. IL-27 functions as a heterodimer receptor consisting of IL-27Rα (WSX1) and gp130 chains through STAT1 and STAT3 predominantly. IL-27 usually acts as a tumor suppressor in cancer.1 IL-27 is an immune-enhancing cytokine, which supports CD4+ T proliferation, T helper type 1 cell differentiation, and IFN-γ production, acting in concert with IL-12. IL-27 has a potential antitumor activity, related not only to the induction of tumor-specific T helper type 1 and cytotoxic T lymphocyte responses but also to the direct inhibitory effects on tumor cell proliferation, survival, invasiveness, and angiogenic potential.1 In this study, we found that IL-27 suppressed NSCLC cell proliferation and metastasis.
Studies involved in evaluating the role of miRNAs showed dysregulation of miRNA expression in NSCLC and postulated its important roles toward malignancy. These miRNAs serve as either oncogenes or tumor suppressors. It was observed that the expression of miR-10a, miR-200, miR-224, miR-410, miR-27a, and miR-320 is downregulated in NSCLC and the miRNAs can suppress cell growth and invasion.13–18 Others, such as miR-144, miR-30c, and miR-193-3 p, are upregulated in human NSCLC and promote cellular growth and invasion.19–21 miR-935 is upregulated in bladder cancer tissues through the miRNA array.22 Upregulation of miR-935 promotes the malignant behaviors of pancreatic carcinoma PANC-1 cells via targeting INPP4A.23 miR-935 promotes liver cancer cell proliferation and migration by targeting SOX7.24 miR-935 promotes gastric cancer cell proliferation by targeting SOX7.25 However, miR-935 inhibits cell proliferation, migration, and invasion by targeting Notch1 in gastric signet ring cell carcinoma.26 The reported data indicated that miR-935 has double roles in cancer, dependent on the origin of cancer. In our research, we found that miR-935 was increased in NSCLC cells and tissues. miR-935 promoted NSCLC cell proliferation and metastasis by targeting IL-27 expression.
In summary, the data presented in this study show that miR-935 acts as an onco-miRNA by targeting IL-27 in NSCLC. It is possible that inhibition of miR-935 could be a possible therapeutic target. We hope that the combination of IL-27 and inhibition of miR-935 may be a good means of treating NSCLC in the future. More studies such as miRNA array are needed to be carried out to investigate IL-27 regulation by miRNAs.
Conclusion
The present research verified that IL-27 was downregulated in NSCLC and that its expression, cellular survival, and metastasis were regulated by miR-935 in NSCLC cells. It suggests that miR-935 might be a therapeutic target for NSCLC patients.
Acknowledgments
The authors would like to thank Dr Feng Gao for the assistance in data analysis and Dr Lili Wang for giving good suggestions for the study. This work was supported by National Nature Science Foundation of China (81670024 to Yang J).
Footnotes
Author contributions
TW and JY conceived and designed the whole experiment and the manuscript. TW and YC performed the major work of the assay. HN, YZ, and YH collected and analyzed the data. TW and JY wrote the manuscript. All authors contributed toward data analysis, drafting and revising the paper, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Fabbi M, Carbotti G, Ferrini S. Dual roles of IL-27 in cancer biology and immunotherapy. Mediators Inflamm. 2017;2017(42):3958069. doi: 10.1155/2017/3958069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwasaki Y, Fujio K, Okamura T, Yamamoto K. Interleukin-27 in T cell immunity. Int J Mol Sci. 2015;16(2):2851–2863. doi: 10.3390/ijms16022851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behzadi P, Behzadi E, Ranjbar R. IL-12 family cytokines: general characteristics, pathogenic microorganisms, receptors, and signalling pathways. Acta Microbiol Immunol Hung. 2016;63(1):1–25. doi: 10.1556/030.63.2016.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Friedman A, Liao KL. The role of the cytokines IL-27 and IL-35 in cancer. Math Biosci Eng. 2015;12(6):1203–1217. doi: 10.3934/mbe.2015.12.1203. [DOI] [PubMed] [Google Scholar]
- 5.Blandin Knight S, Crosbie PA, Balata H, Chudziak J, Hussell T, Dive C. Progress and prospects of early detection in lung cancer. Open Biol. 2017;7170070(9):170070. doi: 10.1098/rsob.170070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kachroo P, Lee MH, Zhang L, et al. IL-27 inhibits epithelial-mesenchymal transition and angiogenic factor production in a STAT1-dominant pathway in human non-small cell lung cancer. J Exp Clin Cancer Res. 2013;32:97. doi: 10.1186/1756-9966-32-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Airoldi I, Tupone MG, Esposito S, et al. Interleukin-27 re-educates intratumoral myeloid cells and down-regulates stemness genes in non-small cell lung cancer. Oncotarget. 2015;6(6):3694–3708. doi: 10.18632/oncotarget.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho MY, Leu SJ, Sun GH, Tao MH, Tang SJ, Sun KH. IL-27 directly restrains lung tumorigenicity by suppressing cyclooxygenase-2-mediated activities. J Immunol. 2009;183(10):6217–6226. doi: 10.4049/jimmunol.0901272. [DOI] [PubMed] [Google Scholar]
- 9.Duan M, Ning Z, Fu Z, et al. Decreased IL-27 negatively correlated with Th17 cells in non-small-cell lung cancer patients. Mediators Inflamm. 2015;2015:802939. doi: 10.1155/2015/802939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee MH, Kachroo P, Pagano PC, et al. Combination treatment with apricoxib and IL-27 enhances inhibition of epithelial-mesenchymal transition in human lung cancer cells through a STAT1 dominant pathway. J Cancer Sci Ther. 2014;6(11):468–477. doi: 10.4172/1948-5956.1000310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pastorkova Z, Skarda J, Andel J. The role of microRNA in metastatic processes of non-small cell lung carcinoma. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2016;160(3):343–357. doi: 10.5507/bp.2016.021. [DOI] [PubMed] [Google Scholar]
- 12.Taverna S, Giallombardo M, Gil-Bazo I, et al. Exosomes isolation and characterization in serum is feasible in non-small cell lung cancer patients: critical analysis of evidence and potential role in clinical practice. Oncotarget. 2016;7(19):28748–28760. doi: 10.18632/oncotarget.7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu T, Liu L, Li J, et al. miRNA-10a is upregulated in NSCLC and may promote cancer by targeting PTEN. Oncotarget. 2015;6(30):30239–30250. doi: 10.18632/oncotarget.4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JS, Kurie JM, Ahn YH. BMP4 depletion by miR-200 inhibits tumorigenesis and metastasis of lung adenocarcinoma cells. Mol Cancer. 2015;14:173. doi: 10.1186/s12943-015-0441-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui R, Meng W, Sun HL, et al. MicroRNA-224 promotes tumor progression in nonsmall cell lung cancer. Proc Natl Acad Sci U S A. 2015;112(31):E4288–E4297. doi: 10.1073/pnas.1502068112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li D, Yang Y, Zhu G, et al. MicroRNA-410 promotes cell proliferation by targeting BRD7 in non-small cell lung cancer. FEBS Lett. 2015;589(17):2218–2223. doi: 10.1016/j.febslet.2015.06.031. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Wang Y, Song Y, Fu Z, Yu W. miR-27a regulates cisplatin resistance and metastasis by targeting RKIP in human lung adenocarcinoma cells. Mol Cancer. 2014;13:193. doi: 10.1186/1476-4598-13-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang H, Lee M, Sharpe O, et al. Oxidative stress-responsive microRNA-320 regulates glycolysis in diverse biological systems. FASEB J. 2012;26(11):4710–4721. doi: 10.1096/fj.11-197467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan HL, Wen ZS, Huang YC, et al. Down-regulation of microRNA-144 in air pollution-related lung cancer. Sci Rep. 2015;5:14331. doi: 10.1038/srep14331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia Y, Chen Q, Zhong Z, et al. Down-regulation of miR-30c promotes the invasion of non-small cell lung cancer by targeting MTA1. Cell Physiol Biochem. 2013;32(2):476–485. doi: 10.1159/000354452. [DOI] [PubMed] [Google Scholar]
- 21.Liang H, Liu M, Yan X, et al. miR-193a-3p functions as a tumor suppressor in lung cancer by down-regulating ERBB4. J Biol Chem. 2015;290(2):926–940. doi: 10.1074/jbc.M114.621409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei Y, He R, Wu Y, et al. Comprehensive investigation of aberrant microRNA profiling in bladder cancer tissues. Tumour Biol. 2016;37(9):12555–12569. doi: 10.1007/s13277-016-5121-z. [DOI] [PubMed] [Google Scholar]
- 23.Wang C, Feng Z, Jiang K, Zuo X. Upregulation of microRNA-935 Promotes the malignant behaviors of pancreatic carcinoma PANC-1 cells via targeting inositol polyphosphate 4-phosphatase type I Gene (INPP4A) Oncol Res. 2017;25(4):559–569. doi: 10.3727/096504016X14759554689565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu X, Li J, Yu Z, Li J, Sun R, Kan Q. miR-935 promotes liver cancer cell proliferation and migration by targeting SOX7. Oncol Res. 2017;25(3):427–435. doi: 10.3727/096504016X14747300207374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang M, Cui G, Ding M, et al. miR-935 promotes gastric cancer cell proliferation by targeting SOX7. Biomed Pharmacother. 2016;79:153–158. doi: 10.1016/j.biopha.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 26.Yan C, Yu J, Kang W, Liu Y, Ma Z, Zhou L. miR-935 suppresses gastric signet ring cell carcinoma tumorigenesis by targeting Notch1 expression. Biochem Biophys Res Commun. 2016;470(1):68–74. doi: 10.1016/j.bbrc.2015.12.116. [DOI] [PubMed] [Google Scholar]