Abstract
Fear conditioning is widely employed to study dysregulations of the fear system. The repeated presentation of a conditioned stimulus in the absence of a reinforcer leads to a decrease in fear responding—a phenomenon known as extinction. From a translational perspective, identifying whether an individual might respond well to extinction prior to intervention could prove important to treatment outcomes. Here, we test the hypothesis that CO2 reactivity predicts extinction phenotype in rats, and that variability in CO2 reactivity as well as extinction long-term memory (LTM) significantly predicts orexin activity in the lateral hypothalamus (LH). Our results validate a rat model of CO2 reactivity and show that subcomponents of behavioral reactivity following acute CO2 exposure explain a significant portion of the variance in extinction LTM. Furthermore, we show evidence that variability in CO2 reactivity is also significantly predictive of orexin activity in the LH, and that orexin activity, in turn, significantly accounts for LTM variance. Our findings open the possibility that we may be able to use CO2 reactivity as a screening tool to determine if individuals are good candidates for an extinction/exposure-based approach.
Keywords: Extinction phenotype, fear conditioning, fear extinction, CO2, individual differences
Introduction
In fear conditioning, an initially neutral conditional stimulus comes to elicit fear expression after its pairing to an uncondi- tional, aversive stimulus. The subsequent repeated presenta- tion of the conditioned stimulus in the absence of a reinforcer leads to a progressive decrease in fear responding—a phe- nomenon and paradigm known as extinction. Exposure ther- apy, a therapeutic approach employed in clinical settings, shares characteristics and mechanisms with extinction, and mounting evidence suggests that there are considerable differ- ences in individual responding to both exposure therapy and extinction (Shumake et al. 2014, 2018; Bush et al. 2007; Galatzer-Levy et al. 2013; Schwartze et al. 2017). From a translational perspective, identifying whether an individual might respond well to extinction prior to intervention could prove important to treatment outcomes. Effectively, if we could determine, prior to treatment, that an individual is not a good candidate for extinction/exposure therapy, they could be reasonably assigned to another treatment strategy.
In a recent study, Sharko et al. (2017) found that differences in orexin activity in the hypothalamus significantly account for individual differences in extinction phenotype in rats. Individual differences in extinction as well as CO2 exposure have been respectively found to activate orexin neurons in the lateral hypothalamus (Johnson et al. 2011). Orexin from the lateral hypothalamus (LH) modulates amygdala threat (fear) learning (Sears et al. 2013), and orexin receptor antagonism has been found to facilitate extinction from context and cued fear conditioning (Flores et al. 2014). Furthermore, antago- nism of orexin receptors increases the recruitment of basolateral amygdala (BLA) neurons that project to the infralimbic cortex during extinction (Flores et al. 2017). Those very same neurons (the IL projecting BLA neurons) are the ones found to be active during extinction (Senn et al. 2014), supporting the notion that individual differences in orexin activation in the hypothalamus could account for indi- vidual differences in extinction (Sharko et al. 2017). In humans, adults with anxiety disorders display heightened emotional reactivity to a single inhalation of 35% CO2; however, data investigating prospective linkages between emotional reactivity to CO2 and susceptibility are limited (Telch et al. 2012). We propose that CO2-reactivity might prove an important tool to identify extinction phenotype. Consistent with this idea, CO2 reactivity predicts the later development of PTSD symptom severity (Telch et al. 2012), and individuals with PTSD show deficits in extinction (Pitman et al. 2012), and dysregulation of HPA axis (Yehuda 2009; Michopoulos et al. 2017).
Here, we test the hypothesis that CO2 reactivity predicts extinction phenotype in rats, that variability in CO2 reactivity significantly predicts orexin activity in the LH, and that, in turn, orexin activity in the LH predicts extinction long-term memory (LTM).
Here, we test the hypothesis that CO2 reactivity predicts extinction phenotype in rats, that variability in CO2 reactivity significantly predicts orexin activity in the LH, and that, in turn, orexin activity in the LH predicts extinction long-term memory (LTM).
Methods
Procedures were conducted in compliance with the National Institutes of Health Guide for the Care and Use of Experimental Animals and were approved by the Institutional Animal Care and Use Committee of the University of Texas at Austin.
Husbandry
Throughout all experimental procedures, subjects were housed in pairs in temperature and humidity-controlled transparent polyethylene cages and were maintained on a 12h/12h light/dark cycle with food and water available ad libitum. Subjects consisted of 56 male Sprague Dawley rats (approximately 80 days of age), obtained from Envigo (Houston, TX, USA).
Apparatus
All experimental manipulations (fear conditioning, extinction, long-term memory) were administered in the same context (operant conditioning chambers; Coulbourn Instruments, Whitehall, PA). Each chamber was equipped with a stainless-steel rod flooring connected to a shock generator (Model H10–11R-TC-SF; Coulbourn Instruments, Whitehall, PA) and individually enclosed in a sound-insulated box (Isolation Cubicle, Model H10–24T; Coulbourn Instruments, Whitehall, PA). Chambers were illuminated with a red light. Behavior was recorded by infrared digital cameras (Panasonic, model wvBP334, Osaka, Japan) mounted on the ceiling of each unit. Stimulus presentation was automated using FreezeFrame2 software (Coulbourn Instruments, Whitehall, PA). Equipment was cleaned with Windex (SC Johnson, Racine, WI) between each session.
Experimental timeline
Rats were first screened for reactivity to CO2 (n=34) or Normoxic Air (n=22). Then, at least 5 days later, they were fear conditioned using 3 tone shock pairings. The next day, they received an extinction session (19 CSs). The day after extinction, they received a long-term memory test (LTM). At least 4 days after the LTM test, a subset of rats were tested in the elevated plus maze, and another subset were tested in the light-dark box. At least 6 days later, all rats received a CO2 challenge, and were sacrificed one hour later for later immunohistochemistry processing. See Figure 1.
Figure 1. Experimental timeline.
Rats were first screened for reactivity to CO2 (n=34) or Normoxic Air (n=22). Then,at least 5 days later, they were fear conditioned using 3 tone shock pairings. The next day, they received an extinction session (19 CSs). The day after extinction, they received a long-term memory test (LTM). At least 4 days after the LTM test, a subset of rats were tested in the elevated plus maze, and another subset were tested in the light-dark box. At least 6 days later, all rats received a CO2 challenge, and were sacrificed one hour later for later immunohistochemistry processing.
CO2 screening
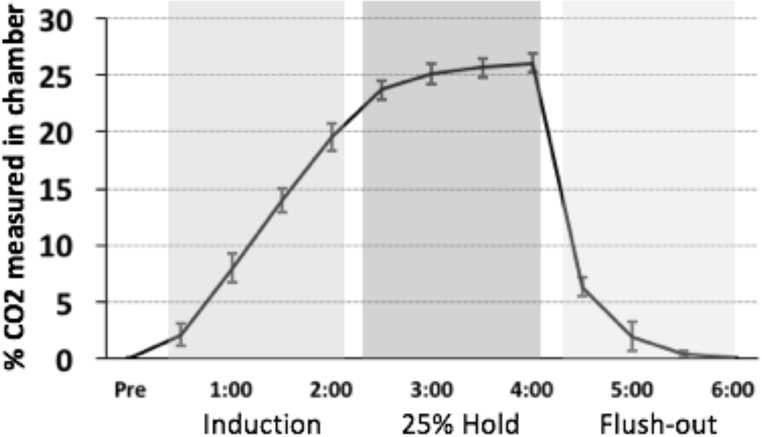
Flow cages (12” width X 12” height X 24” length) were custom built using plexi-glass. Gas flow was regulated using a two-stage regulator (Praxair, Inc., Danbury, CT, USA). An infusion hose was placed to allow air to enter the chamber. Infusion of hypercarbic gas blended with normoxic air (25% CO2) began 0.5 min after placement of the rat in the chamber, and continued for 2 min (Induction phase; shown in Figure 2A). After 2 min, the gas flow was held constant at 25% (25% CO2 Hold phase). After 2 additional minutes, the gas flow was terminated and the cage was flushed to allow rapid equilibration with atmospheric air (Flush-out phase). The rat was left in the chamber for an additional 4 min, and then was transferred to its original home cage. Tests of CO2 levels across the different stages of CO2 flow are shown in Figure 2. Additionally, as a control condition, a subset of rats were exposed to only normoxic air in the plexi-glass chambers for a similar 9 minute duration.
Figure 2.
Measurements of CO2 concentration in chamber during “induction”, “Hold”, and “Flush-out” phases. Mean (+/− SEM) percent CO2 concentrations in the CO2 chamber during the induction, 25% hold, and flush-out periods, as measured by a device located directly inside the chamber. Note that after 2 min and 30 seconds of CO2 infusion, the levels inside the chamber reach the desired concentration of 25%.
CO2 behavioral analysis
We developed a detailed scoring system to describe and quantify the behaviors observed in response to a CO2 challenge in rats. Each behavior was quantified at baseline (30 seconds), during CO2 induction (2 min), during the CO2 hold period (2 min), and during the flush-out period (4 min). For the analyses, the flush-out period is divided into 2, so that all the periods of interest are quantified for the same duration. Each session was videotaped from 2 different angles. Behaviors from each angle were scored and averaged for offline analyses. The following behaviors were quantified: Ambulation (A) (time spent moving around. Any displacement of paws). Grooming (G) (time spent grooming), Labored breathing (L) (deep and long breaths, usually noticeable from movements of the torso). Rearing (R) (number of times rat stands on rear legs). For coding purposes, Induction was referred to as Phase 1, 25% hold as Phase 2, and the first and second half of the Flush-out period as Phases 3 and 4. As such, ambulation recorded during the first half of the Flush-out phase will be reported as “A3”.
Fear conditioning
Subjects were placed in the conditioning chambers, allowed to habituate for 3 minutes, and then fear conditioned with 3, 20-s, 5 kHz, 80 dB tones (CS). Each co-terminated with a 0.5-s, 0.7 mA footshock (US). The interval between each CS was 120 s. After conditioning, each subject remained in the chamber for 3 minutes and was then returned to its home cage.
Extinction
The day after conditioning, subjects were returned to the conditioning chambers and allowed to habituate for 3 minutes. Subjects received 19 unreinforced CSs. The interval between CSs was variable, with an average of 180 sec. Upon completion of extinction, subjects remained in the conditioning chambers for 3 minutes.
Long-term memory test
The day after extinction, the rats were brought back to the conditioning chambers, allowed to acclimate for 3 min, then received 4 unreinforced CS presentations.
Behavioral scoring: freezing
Freezing was defined as the absence of all movement aside from breathing and ear twitching, not including sleeping or resting. Behavior was scored manually from videos by an experimenter blind to experimental conditions. The total amount of CS-induced freezing was expressed as a percentage of total time spent freezing during each 20-s CS. We opera- tionalized post-acquisition conditioned fear as mean freezing over the first 2 tone-alone trials 24 h after acquisition (i.e., the first 2 trials of extinction), end of extinction conditioned fear as mean freezing over the last 2 trials of extinction, and ex- tinction long-term memory as freezing over the first 2 trials of the LTM test, to minimize the likelihood of re-extinction, and provide a more accurate estimate of long-term memory post extinction.
Elevated Plus Maze
Rats were individually placed in the middle of the elevated plus maze (a maze that possesses 2 open arms, and 2 closed arms). They were allowed to move freely throughout the maze for a 5-minute period. A camera mounted on the ceiling allowed to record the rats’ behavior for later offline analysis. We analyzed time (in seconds) and percent time spent in the open arms and percent time spent in the closed arms.
Light-Dark Test
Rats were tested for 2 days in exploratory chambers that automatically measured horizontal (ambulatory) and vertical (rearing) activity under a light–dark condition. This test allowed us to measure activity in response to a relatively threatening environment (the light) versus a relatively safe environment (the dark box). We quantified the traditional metrics (latency to exit dark box and time spent in light box) of the light–dark test (Crawley and Goodwin 1980) in addition to the total activity metrics, which consisted of absolute horizontal and vertical activity (ambulatory distance and rearing counts, respectively) and time-normalized versions of the same metrics (velocity and rearing duration, respectively). Velocity reflects the vigor of movement (distance in cm covered per second of time), and rearing duration reflects the mean length of a single rear (seconds spent rearing per rearing count).
CO2 challenge and brain harvesting
At the end of the experiment, all rats received a CO2 challenge (as described above under CO2 screening). One hour after this CO2 challenge, rats received a lethal dose of sodium pentobarbital and were intra-cardially perfused with phosphate buffered saline followed by 4% buffered paraformaldehyde. Next, brains were extracted, stored in paraformaldehyde overnight, and then stored in cryoprotectant (30% phosphate buffered sucrose) until sectioning.
Orexin-cFos immunohistochemistry
Methodological details can be found in our previous publications (Lee et al., 2005, 2010, Jones et al., 2013; Jones et al., 2016; Cunha et al., 2010). Briefly, for each rat, the entire brain was sectioned at a thickness of 30 micrometers and 4 sets of serial floating sections stored in phosphate buffered saline at −4 °C. Tissue to be analyzed was rinsed, blocked and incubated in rabbit anti-cFos antibody (1:1000 dilution; Immunostar) for 72 hrs at 4 degrees, and mouse anti-orexin antibody (R&D Systems) for 24 hrs. Tissue was then incubated overnight at −4 °C with direct conjugate fluorescent secondary antibodies (Anti-mouse 568 Alexa Fluor; Anti-rabbit 488 Alexa Fluor, Abcam), the sections mounted on slides, and coverslipped with fluorescence mounting medium (Prolong® Gold Antifade Mountant, Invitrogen).
Orexin-cFos imaging and quantification
Cells were imaged and counted according to our previously described procedures (Lee et al., 2010; Jones et al., 2013). Briefly, sections from one series were visualized on a fluorescence microscope (Zeiss) with an objective magnification of 20X through an eyepiece with a magnification of 12.5X. The lateral hypothalamus region identified (defined according to the Paxinos & Watson brain atlas, and observed between −2.8 and −3.3 mm from Bregma), and a minimum of 3 sections from similar anterior-posterior planes per brain were photographed bilaterally from an experimenter blind to behavioral outcome. Imaging of each fluorofore was performed separately, and care was taken to ensure that there was no bleed through between emissions at the different excitation wavelengths. Cells from the lateral hypothalamus that expressed cFos, orexin A, or both were quantified offline by a different observer who was also blind to behavioral outcome. Since the brains were cut in 4 series, the sections from which cells were counted were not adjacent from one another (the sections that we sampled from were approximately 120 micrometers apart). All the cells from the sampled region that expressed cFos and/or orexin were counted using ImageJ software (NIH) for Mac. Care was taken to match sections for each brain and the regions were sampled from both the left and right hemispheres.
Statistical Analyses
All statistical analyses were performed using R (R Core Team, 2018) using the following packages: beset (Shumake, 2018) and car (Fox & Weisberg, 2011). Fear acquisition, extinction, and long-term memory were compared between CO2-exposed and Air-exposed rats using separate repeated measures ANOVA for each behavior.
To determine which combinations of CO2-reactivity be- haviors accounted for the most important portion of the vari- ance in the extinction long-term memory freezing, we used a modified version of the “best subset” approach to linear re- gression. Linear regression models are generally easy to fit and interpret, and can provide a good degree of accuracy, since they take into account the whole range of values, and information is thus not lost (which can often occur when var- iables are categorized into subgroups). It is often the case, however, that not all the variables included into a linear re- gression model contribute their fair share to the variance ex- plained. In such cases, it is best to weigh the contribution of different components vs. their cost to the analysis (in other words, make sure the cost/benefit analysis makes them worth being included, since including irrelevant variables leads to unnecessary complexity). The best subset approach fits a dif- ferent linear model for every possible combination of predic- tor variables. We then used resampling (k-fold cross-valida- tion where k = 10) to estimate how well each model would predict new samples in terms of mean squared error. Each model was repeatedly refit to random subsamples of the data and tested for how well it could predict the remainder of the data that it was not fit to. This results in a sampling distribution of prediction errors. The “best” model was then chosen as the simplest model (the one with the fewest predictors) that was within one standard error of the model that was, on average, best at predicting new data. A detailed explanation of how the beset package implements the best-subsets approach is freely available (Shumake 2018). In addition, since cross-validation estimates of prediction error tend to be overly optimistic if they are used to select the best model (a concept referred to as selection bias), we also ran a nested cross-validation (Cawley and Talbot 2010; Taylor and Tibshirani 2015). This procedure nests the cross-validation used to select the best model within a cross-validation used to estimate test error (prediction error on a new sample), such that test error is evaluated on holdout examples neither used to fit the models nor to select the best models, thus providing a fairer estimate of the model’s generalizability.
Results
We analyzed the following behaviors observed in response to a CO2 challenge in rats: Ambulation (A), Grooming (G), Labored Breathing (L), and Rearing (R) across 4 different phases: CO2 induction (phase 1), CO2 hold (phase 2), and flush-out period (phases 3 and 4). As such, Ambulation re- corded during the first half of the Flush-out phase, for example, is reported as “A3.” Three R2 statistics will be re- ported for each best model: the full-sample R2 (the unadjusted R2 obtained by fitting the model to the full data set), the CV- selection R2 (the R2 obtained by predicting random holdout examples and used to select the best model), and the CV-test R2 (the R2 obtained by predicting holdout examples that were not used for model fitting or model selection).
CO2 reactivity is a significant predictor of extinction long term memory
We operationally defined post-extinction LTM as the mean freezing of the first 2 trials of LTM. For our initial data anal- ysis on post-extinction long-term memory (LTM), we used the best-subset approach to estimate our best model that is within 1 standard error, with the lowest cross-validation error, and with the fewest number of predictors. With those parameters, the model suggests that the best predictive effect of CO2 reac- tivity is from predictor A3. A3 alone yields an full-sample R2 of 0.19, with a CV-selection R2 of .08, and a CV-test R2 of − .19. Thus, the CO2 reactivity predictor A3 accounts for 19% of the variance in post extinction LTM in the observed sample, and is expected to account for between − 19 and 8% of the variance in post extinction LTM in new samples (cross-vali- dation analysis). We also analyzed how sensitive the selection outcome was to the randomized assignments of observations to cross-validation folds and found that the A3 model was selected 52 times out of 100. If we allow the model to run unconstrained (that is, if we remove the 1 standard error rule, and allow more predictors), the best model generated includes R3, G3, and L2, and the full sample yields an R2 of 0.4, with a CV-selection R2 of 0.27, and a CV-test R2 of − .173. If we query the best subset analysis for the best generated model for 2 predictors, the best 2-predictor solution was A3 + G3 (full-sample R2= 0.31, CV-selection R2 = 0.18, CV-test R2 = − .267). We also examined whether the best-subset analysis could generate the best model for the end of extinction, and found that it did not (full sample R2 = 0). When testing the best subset analysis on the rats that received normoxic air rather than CO2, the full sample R2 was 0. When specifically testing our best predictor (A3) for the CO2 sample on the rats that were exposed to normoxic air, the full sample R2 was .01, suggesting that CO2 reactivity, and not simply baseline ambu- lation, specifically explained variance in post-extinction LTM. We separately ran our analysis on the mean of all 4 LTM trials, since it could provide a more stable estimate of post-extinction LTM (albeit at the risk of re-engaging extinction mechanisms) (see Supplementary Materials).
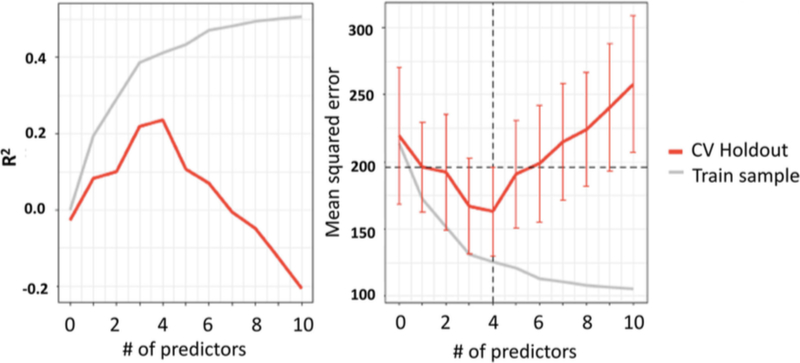
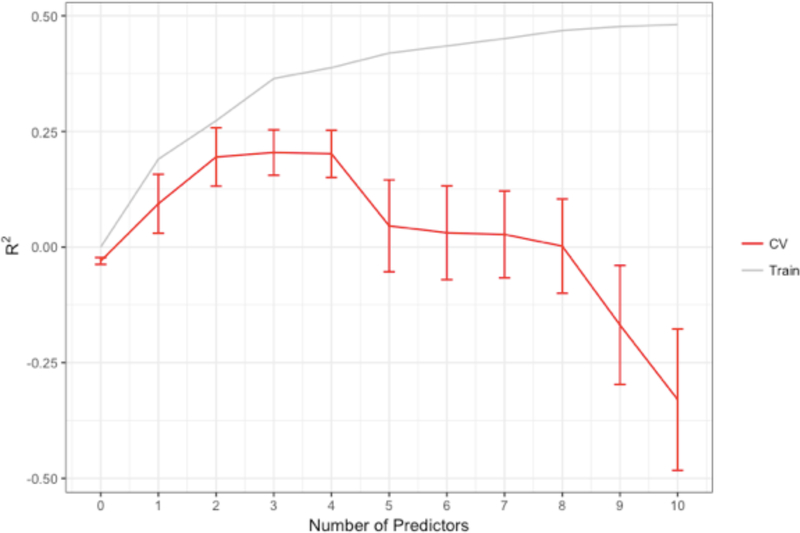
In looking at the R2 and the standard error of the estimate for the training sample and the cross-validation as a function of the number of predictors, it is apparent that while adding more predictors explains a larger portion of the variance in the training sample, it yields diminishing returns for the cross- validation (Fig. 3 and 4). In fact, not only are there diminishing returns, more than 4 predictors was actively det- rimental to finding a reproducible solution. Figure 3 also conveys that there was severe overfitting when too many pre- dictors were included—the predictive R2 becomes negative, which indicates that the model is predicting noise, and that the predictions are actually worse than simply predicting the mean for everyone. A negative predictive R2 was also observed for even the single-predictor model for the test cross-validation, which provides a meta-estimate of how well models selected using this procedure on this sample can predict new observa- tions. This statistic principally reflects that the current sample size may be too small to both fit and select a model that will reliably generalize to future samples.
Figure 3.
R2 and mean squared error for the training sample and the cross- validation as a function of the number of predictors. It is apparent that while adding more predictors explains a larger portion of the variance in the training sample, it yields diminishing returns for the cross-validation. In fact, not only are there diminishing returns, more than 4 predictors is actively detrimental to finding a reproducible solution. Our data also suggest that there is severe overfitting when too many predictors are included—the predictive R2 is negative, which indicates that the model is predicting noise, and that the predictions are actually worse than simply predicting the mean for everyone. Similarly, as the mean square error continually decreases for the training sample, the error decreases for up to 4 predictors in the CV Holdout, and then increases
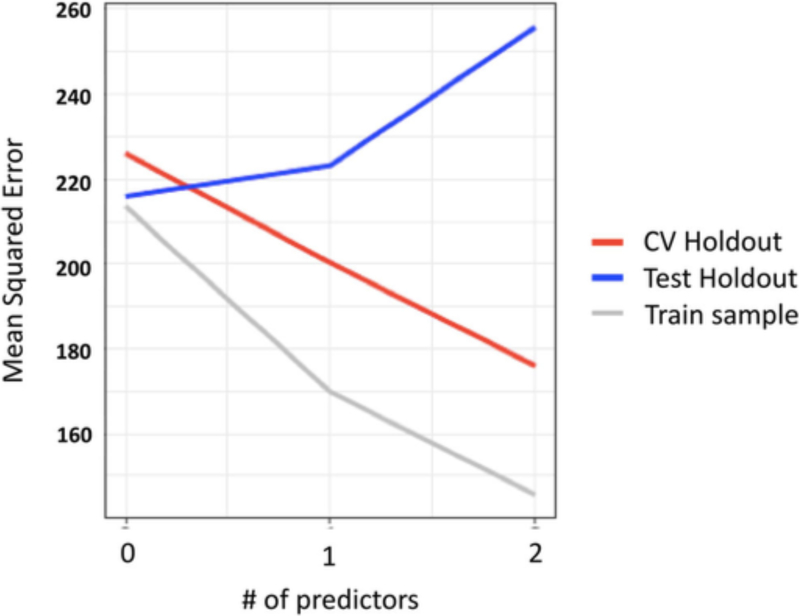
Figure 4.
Mean squared error for the training sample and the inner and outer cross-validation as a function of the number of predictors. It is apparent that as the number of predictors increase from 1 to 2, the standard error of estimate decreases for our training sample and the inner cross-validation (CV Holdout); however, under the same circumstances, the standard error of estimate increases for the outer-cross validation (Test Holdout)
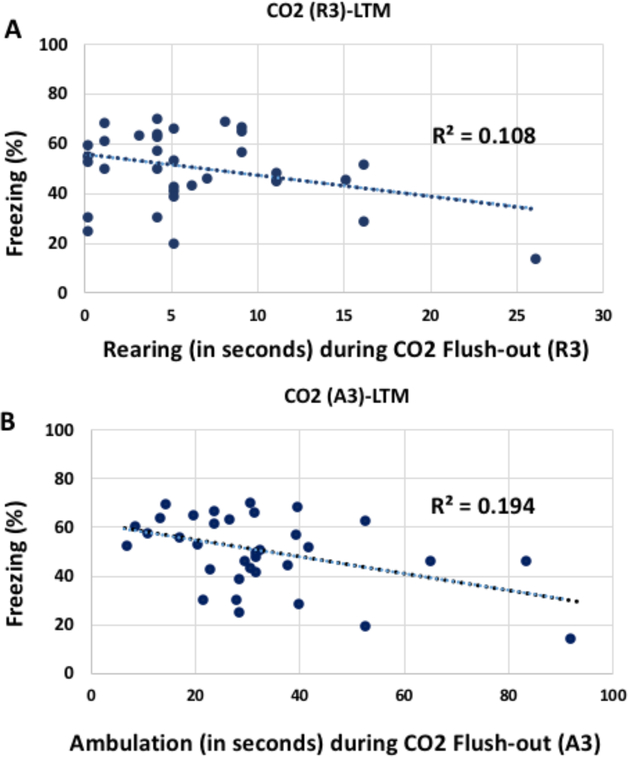
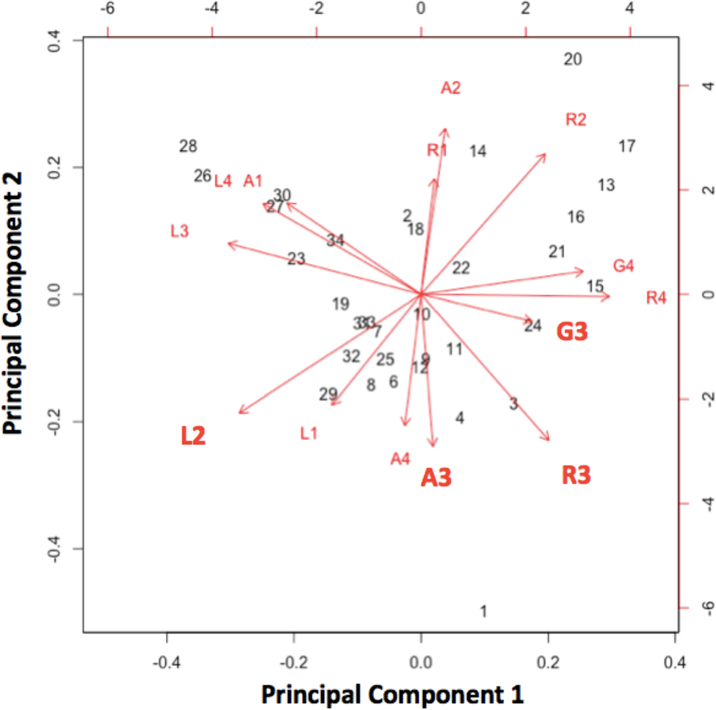
Two of the predictors’ (R3 and A3; Rearing and ambula- tion during the flush-Out phase of CO2 challenge) relation- ship with LTM are shown in Fig. 5, with R3 individually being the weakest and A3 the strongest of the 4 best predictors. The differential predictive effect of R3 and A3 may reflect an adaptive behavioral response: increased movement and ex- ploratory movement might maximize the opportunity to flee when a threat is no longer present see Fig. 6. That the same 4 behaviors (A3, R3, G3, and L2) were found in various com- binations across the best predictive scenarios suggests that some might be tapping into similar predictive concepts, and as such, might also be highly exchangeable. To examine the possibility that some of these behaviors might be conceptually related, we ran a principal component analysis (PCA) on all the predictor variables. Our results revealed that the first 2 principal components accounted for ~ 50% of the overall var- iance, and the first four principal components accounted for nearly 80% of the variance. A correlation matrix for A3, R3, G3, and L2 with the first 4 principal components is found in Table 1. In Fig. 7, we show the relationship between the vec- tors for all the predictor variables, which conveys that A3 was predominantly associated with principal component 2, G3 was predominantly associated with principle component 1, and R3 and L2 were associated with a combination of the two.
Figure 5.
Our data suggest the best predictive effect of CO2 reactivity is from the combination of predictors A2, A3, G4, and L4. Together, they yield an adjusted R square of 0.43. Thus, the combined CO2 reactivity predictors account for 43% of the variance in post extinction LTM. Predictors R3 and A3’s relationship with LTM are shown in A and B, respectively.
Figure 6. R-squared for the training sample and the cross-validation as a function of the number of predictors.
It is apparent that while adding more predictors explains a larger portion of the variance in the training sample, it yields diminishing returns for the cross-validation. In fact, not only are there diminishing returns, more than 4 predictors is actively detrimental to finding a reproducible solution. Our data also suggest that there is severe overfitting when too many predictors are included—the predictive R-squared is negative, which indicates that the model is predicting noise, and that the predictions are actually worse than simply predicting the mean for everyone.
Table 1. Correlations between A3, R3, G3, and L2 and the 4 first principal components.
Our results revealed that the first 2 principal components accounted for ~ 50% of the overall variance,and the first four principal components accounted for nearly 80% of the variance. A3 was predominantly associated with principal components 2 and 3, G3 was predominantly associated with principle components 1 and 4, and R3 and L2 were associated with a combination of principal components 1 and 2.
| PC 1 | PC 2 | PC 3 | PC 4 | |
|---|---|---|---|---|
| A3 | 0.025 | −0.369 | −0.457 | 0.179 |
| R3 | 0.267 | −0.354 | −0.237 | −0.031 |
| G3 | 0.232 | −0.065 | −0.021 | −0.567 |
| L2 | −0.380 | −0.288 | 0.109 | 0.027 |
Figure 7. Principal component analysis vectors for A3, R3, G3, and L2.
Our results revealed that the first 2 principal components accounted for ~ 50% of the overall variance, and the first four principal components accounted for nearly 80% of the variance. Here, we show the relationship between the vectors for all the predictor variables, which conveys that A3 was predominantly associated with principal component 2, G3 was predominantly associated with principle component 1, and R3 and L2 were associated with a combination of the two.
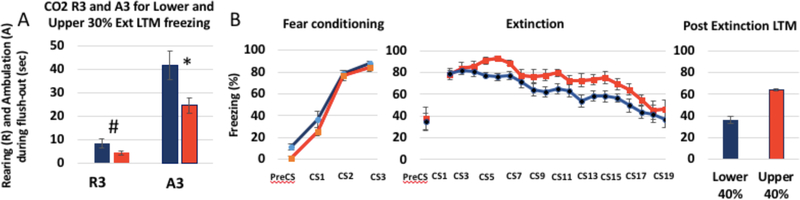
Figure 8 shows the effect in good and poor extinguishers (lower and upper 40% of the LTM freezing distribution). The predictive potential of R3 and A3 seems to be highly specific to extinction, as evidenced by the data shown in Fig. 8. A between subjects t test revealed that there was a significant difference between the lower and upper 40% LTM freezers for the post-extinction LTM (p < 0.05), but not during fear acqui- sition (p > 0.2). Furthermore, there was a significant difference between the lower and upper groups on their ambulation dur- ing the flush-out period (A3), p = 0.019, and their rearing be- havior during that same time period approached significance, p = 0.068. Additional correlations between A3 and preCS freezing for the conditioning, extinction, and post extinction LTM sessions are shown in the Supplementary Materials. In teasing apart elements of CO2 reactivity that account for ex- tinction phenotype, it is important to remember that the current steps are exploratory, and that this study is a first step in determining which elements of CO2 reactivity might reveal to be most important.
Figure 8. CO2 reactivity, fear conditioning, Extinction, and post-extinction LTM for the rats in the Lower (Blue) and Upper (Red) 40% range of LTM freezing.
CO2 reactivity is shown here using 2 of the most important predictors of extinction phenotype: Rearing and Ambulation during the Flush-out periods (R3 and A3). The CO2 reactivity measures (A) between the Lower and Upper 40% freezers approached significance, and there were significant differences between the Lower and Upper 40% freezers during extinction, and at the post-extinction LTM test, but not during Fear conditioning, preCS freezing, or freezing during the first 3 trials of extinction (B), suggesting that CO2 reactivity may be specific to differences in extinction rather than fear learning. N=22, *= p<.05, #= p< .1, and p greater than .2 for all other comparisons.
CO2 reactivity predicts cFos+Orexin co-labeling in the lateral hypothalamus
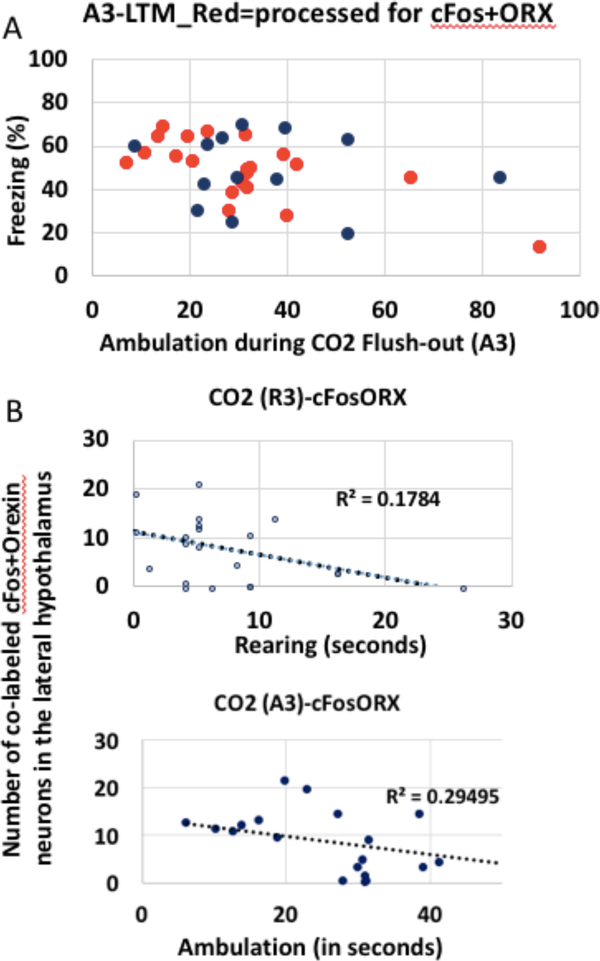
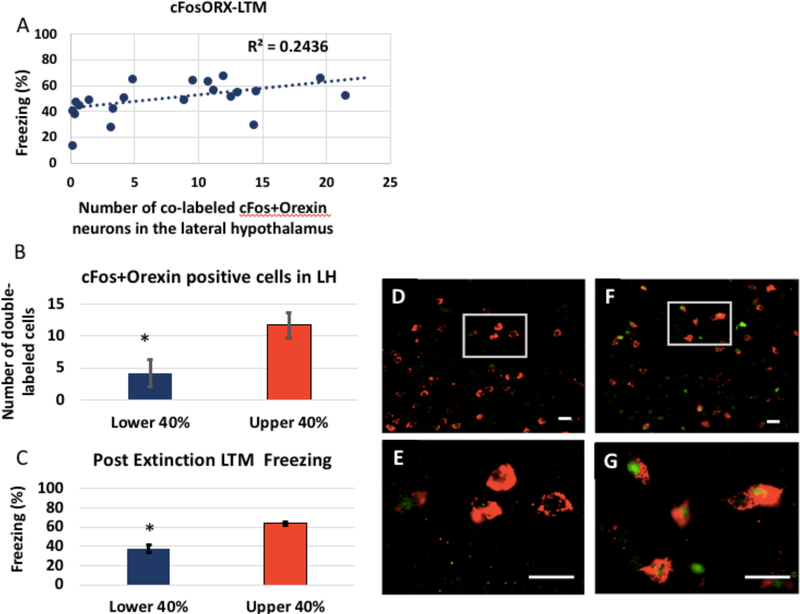
A subset of rats from the CO2 reactivity experiment were processed and quantified for cFos and orexin immunohisto- chemistry (n = 21) (see Fig. 9). We found that there was a significant predictive relationship of CO2 reactivity on cFos+Orexin co-labeled cells in the lateral hypothalamus. Our previous analysis identified A3 as the single best predic- tor of extinction LTM. Here, A3 gives us a significant predic- tion of cFos+Orexin, with an R2 of 0.25, F(1, 19) = 6.452, p = 0.01.
Figure 9. CO2 reactivity predicts cFos+Orexin co-labeling in the lateral hypothalamus.
A. In red, rats that were processed and quantified for cFos and orexin immunohistochemistry, and were exposed to CO2 (21 out of 34). B. The combination of A3+L2 gives us a significant prediction, with a combined multiple R squared of 0.31, F(2,18)=3.95,p=.03. Shown here are 2 of the highest individual predictors (R3 and A3).
cFos+Orexin co-labeling in the lateral hypothalamus predict extinction LTM freezing
We first confirmed that our best subset model (A3) accounted for a significant portion of the variance in LTM freezing for just the subset of rats that we processed for cFos+orexin (21 rats that were processed for immunohistochemistry and had received a CO2). We found that it was the case, with an R2 of.5. For that subgroup, A3 accounted for 50% of the observed variance in LTM freezing, F(1, 19) = 19.44, p < 0.001. cFos+ orexin co-labeling in the LH was found to account for a sig- nificant portion of the variance in LTM freezing, with an R2 of 0.24, F(1,19) = 5.925, p = 0.024 (See Fig. 8). Additionally, there was a significant difference in the number of cells that expressed cFos and orexin between those that were the lower and upper 40% freezing, p = 0.02. There was also a significant difference between those sub-groups for LTM freezing, p < 0.0001 (See Fig. 10). Additional correlations of the 4 best predictors and orexin with cFos cell counts and extinction LTM freezing are shown in the Supplementary Materials.
Figure 10. cFos+Orexin predict extinction LTM freezing.
A. cFos+orexin co-labeling in the LH was found to account for a significant portion of the variance in LTM freezing, with an R-squared of 0.24, F(1,19)= 5.925, p=0.024. B. cFos+Orexin positive cells in the lateral hypothalamus in the Lower and Upper 40% of post-extinction LTM freezing. There was a significant difference in the number of cells that expressed cFos and orexin between those that were the Lower and Upper 40% freezing, p=0.02. C. Lower and Upper 40% Post-extinction LTM freezing in rats processed for cFos and Orexin (N=12). Lower and Upper groups were significantly different, p<0.0001. Orexin (red) and cFos (green) immunohistochemistry in LH of Good (D, E) and Poor (F, G) extinguisher following CO2 reactivity. There appears to be more cFos labeled orexin cells in the Poor extinguisher, in support of our hypothesis. Scale bar=20 μm.
Predictive effects of CO2 reactivity are specific to extinction phenotype
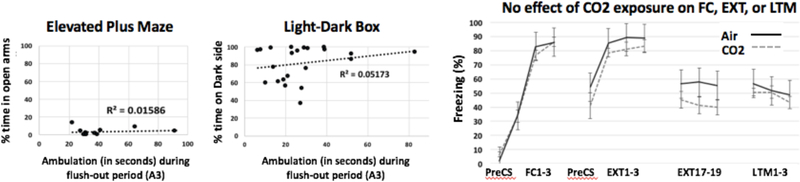
There was no predictive effect of CO2 reactivity (A3) in the elevated plus maze (Fig. 11a) or light dark box (Fig. 11b) R2 = 0.0159, F(1,10) = .1612, p = 0.695 and R2 = 0.052, F(1,20) = 1.091, p = 0.309, respectively. Importantly, there was no interaction effect of prior CO2 exposure (compared to prior normoxic air exposure) on fear conditioning, preCS freezing, early extinction, or post-extinction long-term mem- ory (Fig. 11c). There was an overall main effect of CO2 group (p < .05). Follow-up tests revealed that while there were no differences between CO2 and normoxic air on fear condition- ing, preCS freezing, early extinction, or post-extinction LTM (all comparisons, p > 0.10); there was a significant difference between the groups for the end of extinction (p < 0.05). Additional correlations between the light-dark box and elevat- ed plus maze data with the extinction LTM freezing are shown in the Supplementary Materials.
Figure 11. Predictive effects of CO2 reactivity are specific to extinction phenotype.
There is no predictive effect in elevated plus maze (A) or light dark box (B). Importantly, there is no effect of prior CO2 exposure (compared to prior control Air exposure) on fear conditioning, preCS freezing, early extinction, or post-extinction long-term memory (C). All comparisons, p>.1.
Discussion
We set out to test whether CO2 reactivity might be a predictor of extinction phenotype in rats. Here, we validated a rat model of CO2 reactivity and showed that subcomponents of behav- ioral reactivity following acute CO2 exposure explained a sig- nificant portion of the variance in extinction LTM. Furthermore, we showed evidence that variability in CO2 re- activity was also significantly predictive of orexin activity in the LH, and that orexin activity in the LH significantly pre- dicted extinction LTM freezing.
There is strong evidence suggesting that CO2 exposure directly engages the fear system. In humans, adults with anx- iety disorders display heightened emotional reactivity to a single inhalation of 35% CO2, and Telch et al. (2012) reported that emotional reactivity to a single inhalation of 35% carbon dioxide was significantly predictive of later symptoms of post- traumatic stress disorder and anxiety in soldiers deployed to Iraq. In rats, exposure to moderate concentrations of creases in sympathetic activity (Elam et al. 1981), blood pres- sure (Johnson et al. 2012; Walker and Brizzee 1990), and anxiety-like behaviors (e.g., increases in panic/escape/flight- associated locomotor responses) (Johnson et al. 2012; Cuccheddu et al. 1995; Johnson et al. 2011). Furthermore, exposing healthy rats to 20% CO2 concentrations selectively increases cellular activity in panic and anxiety networks, such as the perifornical hypothalamus and dorsal periaqueductal gray (Johnson et al. 2011). When stimulated, those same areas are known to induce panic-associated symptoms in humans (Nashold et al. 1969; Wilent et al. 2011) and panic-associated behaviors and cardio-excitation in rats (Samuels et al. 2002; Shekhar and Katner 1995). Laboratory provocation of panic symptoms has been widely used as a means of investigating the pathogenesis of anxiety-related disorders (Margraf et al. 1986; McNally 1999); yet, to date, not one had tested whether emotional reactivity to a single CO2 challenge might be pre- dictive of fear reduction outcomes in response to standard extinction treatments. Rats, much like humans albeit to a less- er degree, show individual differences in responding to fear extinction. Individuals with PTSD show deficits in extinction (Pitman et al. 2012), and dysregulation of HPA axis (Yehuda 2009; Michopoulos et al. 2017). Individual differences in ex- tinction as well as CO2 exposure have been respectively found to activate orexin neurons in the lateral hypothalamus (Johnson et al. 2011). Orexin from the lateral hypothalamus modulates amygdala threat (fear) learning (Sears et al. 2013), and orexin receptor antagonism has been found to facilitate extinction from context and cued fear conditioning (Flores et al. 2014). Furthermore, antagonism of orexin receptors in- creases the recruitment of BLA neurons that project to the infralimbic cortex during extinction (Flores et al. 2017). Those very same neurons (the IL projecting BLA neurons) are the ones found to be active during extinction (Senn et al. 2014), supporting the notion that individual differences in orexin activation in the LH could account for individual dif- ferences in extinction (Sharko et al. 2017), and that those differences could be estimated, in vivo, through CO2 reactiv- ity. The findings from the present study show that this may indeed be the case.
The effect of CO2 reactivity appears to be specific to ex- tinction—the variability in CO2 reactivity did not account for significant variability in either elevated plus maze or light- dark box behavior. Our findings further show that CO2 expo- sure per se does not differentially affect fear acquisition, early extinction, or extinction long-term memory relative to normoxic air.
CO2 exposure is inexpensive and can be safely administered in humans (e.g., Telch et al. 2010, 2012). Together, the results of our study open the possibility that we may be able to use CO2 reactivity as a screening tool to determine if individuals are good candidates for an extinction/exposure-based approach. If they are not, other treatment avenues could be considered that may be better suited for these particular individuals. Evidence suggests that reconsolidation-based approaches may engage mechanisms that differ from those engaged during extinction (e.g., Tedesco et al. 2014; Lee et al. 2016; Monfils et al. 2009; Schiller et al. 2014). If CO2 reactivity is reflective of individual differences in susceptibility to respond to extinction treatment, it could also indicate a differential engagement of networks during extinction, which may make a candidate better suitable for an approach other than extinction (such as reconsolidation blockade or updating). Indeed, this idea would also be very much in line with a recent article showing that inhaling CO2 enhances the ability of retrieval to render an aversive memory labile (Du et al. 2017). These findings appear in support of a reconsolidation-based hypothesis.
Our study illustrates that CO2 reactivity is significantly predictive of Orexin activity in the LH. In recent years, a number of studies have found a relationship between Orexin activity and fear extinction. Our findings suggest the possibil- ity that CO2 reactivity could serve as a proxy to examine Orexin activity in an effective, rapid, and inexpensive way.
Overall, although CO2 reactivity accounted for a signifi- cant portion of the variance in long-term memory after extinc- tion, it remains to be seen whether we can extrapolate the results to a different subsample. Our cross-validation esti- mates from random sub-samples within our dataset combined with the positive test in our orexin sub-sample, as well as the fact that the same predictors were found to be of importance across a number of different validation estimates, increase our confidence that the selected model will generalize to a new sample. Still, our nested cross-validation suggests that models developed on this small data set may not yet generalize to new samples. Ultimately, it will be important to assess whether CO2’s predictive power translates into meaningful and practi- cal predictive significance (i.e., improvement in overall treat- ment outcomes within a subsample).
Supplementary Material
Footnotes
We have no conflicts of interest to disclose.
References
- Bush DE, Sotres‐Bayon F, LeDoux JE (2007) Individual differences in fear: isolating fear reactivity and fear recovery phenotypes. Journal of traumatic stress, 20:413–422. doi: 10.1002/jts.20261 [DOI] [PubMed] [Google Scholar]
- Cuccheddu T, Floris S, Serra M, Porceddu ML, Sanna E, Biggio G (1995) Proconflict effect of carbon dioxide inhalation in rats. Life Sci. 56:L321–L324. doi: 10.1016/0024-3205(95)00093-3 [DOI] [PubMed] [Google Scholar]
- Crawley J, Goodwin FK (1980) Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav, 13(2):167–70. doi: 10.1016/0091-3057(80)90067-2 [DOI] [PubMed] [Google Scholar]
- Cunha C, Monfils MH, LeDoux JE (2010) GABAC receptors in the lateral amygdala: a possible novel target for the treatment of fear and anxiety disorders? Frontiers in Behavioral Neuroscience, 4:6. doi: 10.3389/neuro.08.006.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Price MP, Taugher RJ, Grigsby D, Ash JJ, Stark AC, Hossain Saad MZ, Singh K, Mandal J, Wemmie JA, Welsh MJ (2017) Transient acidosis while retrieving a fear-related memory enhances its lability. eLife, 6, pii: e22564. doi: 10.7554/eLife.22564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elam M, Yao T, Thore P, Svensson TH (1981) Hypercapnia and hypoxia: chemoreceptor-mediated control of locus coeruleus neurons and splanchnic, sympathetic nerves. Brain Res. 222(2):373–381. doi: 10.1016/0006-8993(81)91040-4 [DOI] [PubMed] [Google Scholar]
- Flores Á, Valls-Comamala V, Costa G, Saravia R, Maldonado R, Berrendero F (2014) The hypocretin/orexin system mediates the extinction of fear memories. Neuropsychopharmacology, 39(12):2732–41. doi: 10.1038/npp.2014.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores Á, Herry C, Maldonado R, Berrendero F (2017) Facilitation of contextual fear extinction by orexin-1 receptor antagonism is associated with the activation of specific amygdala cell subpopulations. Int J Neuropsychopharmacol., 20(8):654–659. doi: 10.1093/ijnp/pyx029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J, Weisberg S (2011) An {R} Companion to Applied Regression, Second Edition. Thousand Oaks CA: Sage; URL: http://socserv.socsci.mcmaster.ca/jfox/Books/Companion [Google Scholar]
- Galatzer-Levy IR, Ankri Y, Freedman S, Israeli-Shalev Y, Roitman P, Gilad M, Shalev AY (2013) Early PTSD symptom trajectories: persistence, recovery, and response to treatment: results from the Jerusalem Trauma Outreach and Prevention Study (J-TOPS). PloS one, 8(8):e70084. doi: 10.1371/journal.pone.0070084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PL, Samuels BC, Fitz SD, Lightman SL, Lowry CA, Shekhar A (2012) Activation of the orexin 1 receptor is a critical component of CO2-mediated anxiety and hypertension but not bradycardia. Neuropsychopharmacology, 37:1911–1922. doi: 10.1038/npp.2012.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PL, Fitz SD, Hollis JH, Moratalla R, Lightman SL, Shekhar A, Lowry CA (2011) Induction of c-fos in “panic/defense”-related brain circuits following brief hypercarbic gas exposure. J. Psychopharmacol 25:26–36. doi: 10.1177/0269881109353464 [DOI] [PubMed] [Google Scholar]
- Jones CE, Ringuet S, Monfils MH (2013) Learned together, extinguished apart: reducing fear to complex stimuli. Learning and Memory, 20(12): 674–85. doi: 10.1101/lm.031740.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CE, Monfils MH(2016) Dominance Status Predicts Social Fear Transmission in Laboratory Rats. Animal Cognition, 19(6):1051–69. doi: 10.1007/s10071-016-1013-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Groshek F, Petrovich GD, Cantalini JP, Gallagher M, Holland PC (2005) Role of amygdalo-nigral circuitry in conditioning of a visual stimulus paired with food. Journal of Neuroscience, 25(15):3881–8. doi: 10.1523/JNEUROSCI.0416-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Gallagher M, Holland PC (2010) The central amygdala projection to the substantia nigra reflects prediction error information in appetitive conditioning. Learning and Memory, 17(10):531–8. doi: 10.1101/lm.1889510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Haberman RP, Roquet RF, Monfils MH (2016) Extinction and retrieval+ extinction of conditioned fear differentially activate medial prefrontal cortex and amygdala in rats. Frontiers in Behavioral Neuroscience, 9:369. doi: 10.3389/fnbeh.2015.00369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margraf J, Ehlers A, Roth WT (1986) Biological models of panic disorder and agoraphobia--a review. Behav Res Ther., 24(5):553–67. doi: 10.1016/0005-7967(86)90036-7 [DOI] [PubMed] [Google Scholar]
- McNally RJ (1999) On the experimental induction of panic. Behavior Therapy, 30(2):331–339. doi: 10.1016/S0005-7894(99)80012-4 [DOI] [Google Scholar]
- Michopoulos V, Powers A, Gillespie CF, Ressler KJ, Jovanovic T (2017) Inflammation in fear-and anxiety-based disorders: PTSD, GAD, and beyond. Neuropsychopharmacology, 42(1):254–270. doi: 10.1038/npp.2016.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monfils MH, Cowansage KK, Klann E, LeDoux JE (2009) Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science, 324(5929):951–5. doi: 10.1126/science.1167975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashold BS Jr., Wilson WP, Slaughter DG (1969) Sensations evoked by stimulation in the midbrain of man. J. Neurosurg 30:14–24. doi: 10.3171/jns.1969.30.1.0014 [DOI] [PubMed] [Google Scholar]
- Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, Milad MR, Liberzon I (2012) Biological studies of post-traumatic stress disorder. Nat Rev Neurosci, 13(11):769–87. doi: 10.1038/nrn3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2016) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: URL https://www.R-project.org/. [Google Scholar]
- Samuels BC, Zaretsky DV, DiMicco JA (2002) Tachycardia evoked by disinhibition of the dorsomedial hypothalamus in rats is mediated through medullary raphe. J. Physiol 538:941–946. doi: 10.1113/jphysiol.2001.013302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Kanen JW, LeDoux JE, Monfils MH, Phelps EA. (2014) Extinction during reconsolidation of threat memory diminishes prefrontal cortex involvement. Proc Natl Acad Sci USA, 110(50):20040–5. doi: 10.1073/pnas.1320322110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartze D, Barkowski S, Strauss B, Knaevelsrud C, Rosendahl J (2017) Efficacy of group psychotherapy for posttraumatic stress disorder: Systematic review and meta-analysis of randomized controlled trials. Psychother Res., 1–17. doi: 10.1080/10503307.2017.1405168 [DOI] [PubMed] [Google Scholar]
- Sears RM, Fink AE, Wigestrand MB, Farb CR, de Lecea L, Ledoux JE (2013) Orexin/hypocretin system modulates amygdala-dependent threat learning through the locus coeruleus. Proc Natl Acad Sci USA, 110(50):20260–5. doi: 10.1073/pnas.1320325110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senn V, Wolff SB, Herry C, Grenier F, Ehrlich I, Gründemann J, Fadok JP, Müller C, Letzkus JJ, Lüthi A (2014) Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron, 81(2):428–37. doi: 10.1016/j.neuron.2013.11.006 [DOI] [PubMed] [Google Scholar]
- Sharko AC, Fadel JR, Kaigler KF, Wilson MA (2017) Activation of orexin/hypocretin neurons is associated with individual differences in cued fear extinction. Physiology & behavior, 178:93–102. doi: 10.1016/j.physbeh.2016.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumake J, Furgeson-Moreira S, Monfils MH (2014) Predictability and heritability of individual differences in fear learning. Anim Cogn., 17(5):1207–21. doi: 10.1007/s10071-014-0752-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumake J, Jones CE, Auchter A, Monfils MH (2018) Data-driven criteria to assess fear remission and phenotypic variability of extinction in rats. Phil Trans R Soc B, 373(1742):20170035. doi: 10.1098/rstb.2017.0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar A, Katner JS (1995) Dorsomedial hypothalamic gaba regulates anxiety in the social interaction test. Pharmacol. Biochem. Behav 50:253–258. doi: 10.1016/0091-3057(94)00307-5 [DOI] [PubMed] [Google Scholar]
- Tedesco V, Roquet RF, DeMis J, Chiamulera C, Monfils MH (2014) Extinction, applied after retrieval of auditory fear memory, selectively increases zinc-finger protein 268 and phosphorylated ribosomal protein S6 expression in prefrontal cortex and lateral amygdala. Neurobiol Learn Mem., pii: S1074–7427(14). doi: 10.1016/j.nlm.2014.08.015 [DOI] [PubMed] [Google Scholar]
- Telch MJ, Rosenfield D, Lee HJ, Pai A (2012) Emotional reactivity to a single inhalation of 35% carbon dioxide and its association with later symptoms of posttraumatic stress disorder and anxiety in soldiers deployed to Iraq. Archives of General Psychiatry, 69(11):1161–68. doi: 10.1001/archgenpsychiatry.2012.8 [DOI] [PubMed] [Google Scholar]
- Telch MJ, Smits JAJ, Brown M, Dement M, Powers MB, Lee HJ, Pai A (2010) Effects of threat context and cardiac sensitivity on fear responding to a 35% CO2 challenge: a test of the context-sensitivity panic vulnerability model. Journal of Behavior Therapy and Experimental Psychiatry, 41: 365–72. doi: 10.1016/j.jbtep.2010.03.008 [DOI] [PubMed] [Google Scholar]
- Walker BR, Brizzee BL (1990) Cardiovascular responses to hypoxia and hypercapnia in barodenervated rats. J. Appl. Physiol 68:678–686. doi: 10.1152/jappl.1990.68.2.678 [DOI] [PubMed] [Google Scholar]
- Wilent WB, Oh MY, Buetefisch C, Bailes JE, Cantella D, Angle C, Whiting DM (2011) Mapping of microstimulation evoked responses and unit activity patterns in the lateral hypothalamic area recorded in awake humans. Technical note. J. Neurosurg 115(2):295–300. doi: 10.3171/2011.3.JNS101574 [DOI] [PubMed] [Google Scholar]
- Yehuda R (2009) Status of Glucocorticoid Alterations in Post‐traumatic Stress Disorder. Annals of the New York Academy of Sciences, 1179(1):56–69. doi: 10.1111/j.1749-6632.2009.04979.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.