INTRODUCTION
Decades of research have established a significant association between ambient particulate matter (PM) and increased risk of death (Dockery et al., 1993, Lelieveld et al., 2015). Additional research has assessed potential mechanisms underlying the PM-mortality association by investigating the effects of physical and chemical properties of PM, such as particle size and chemical components (Atkinson et al., 2015, Cassee et al., 2013, Kelly and Fussell, 2012). However, the properties of particles responsible for their toxicity are still not fully understood. While there is a general consensus that PM causes inflammation and oxidative stress, the exact mechanisms are still unknown (Brook et al., 2010, Landrigan et al., 2017). Improving our understanding of the toxigenic properties of PM is critical to developing cost-effective air quality regulations and ultimately protecting public health.
We propose a new hypothesis to explain the toxicity of ambient particles, suggesting that particle toxicity may be mediated by local radon concentrations. Radon, a naturally occurring gas, is a product of the radioactive decay of trace elements found in the soil. Once radon is emitted it migrates upwards, accumulates in homes, and decays to radioactive progeny. These freshly generated progeny react with water vapor and atmospheric gases to form highly mobile clusters, which then rapidly attach to airborne aerosols (Porstendörfer, 1994;2001). The fraction of attached progeny increases with increasing ambient aerosol concentrations (Porstendörfer, 1994). The attached fraction usually composes 90% or more of total radon progeny in a typical room (Porstendörfer, 1994, Reineking and Porstendörfer, 1990, Guo et al., 2016). Respirable air pollution particles then act as vectors of the attached radon progeny, which continue to decay and emit radiation after inhalation and deposition on the bronchial epithelium. Significant research has documented the cellular and biochemical damage induced by exposure to radon and alpha-emitting particles. The inhalation of radon gas and alpha-emitting radioisotopes has been demonstrated to cause pulmonary inflammation and oxidative damage in both animal models and human cells (Li and Tong, 2007, Nie et al., 2012, Narayanan et al., 1997, Chauhan et al., 2012). Once inhaled, radioisotopes may also be translocated into systemic circulation and cause systemic effects (Marsh and Bailey, 2013).
Exposure to radon is a well-documented cause of lung cancer at both occupational and environmental levels (Darby et al., 2005, Krewski et al., 2005, Tirmarche et al., 2010). There is also some limited evidence for non-cancer effects of radon and other low-level radiation exposures, including circulatory disease (Little et al., 2012), COPD mortality (Turner et al., 2012) and COPD hospital admissions (Barbosa-Lorenzo et al., 2017). However our hypothesis is unique in that it focuses on PM2.5 toxicity and its potential modification by radon. While we know of one study that considered traffic exhaust as a potential modifier of radon-associated cancer risk (Bräuner et al., 2010), we are not aware of any studies that have examined radon as a potential modifier of PM-associated risks. We previously studied the combined effects of ambient radiation and PM2.5 and found effects on increased blood pressure in an elderly community based cohort (Nyhan et al., 2018). This existing experimental and epidemiologic evidence provides the scientific premise for our current study. To test our hypothesis, we conducted a national epidemiological study investigating whether the acute effects of PM2.5 (PM with an aerodynamic diameter ≤ 2.5 μm) on total and cause-specific mortality varied by average local radon concentrations.
METHODS
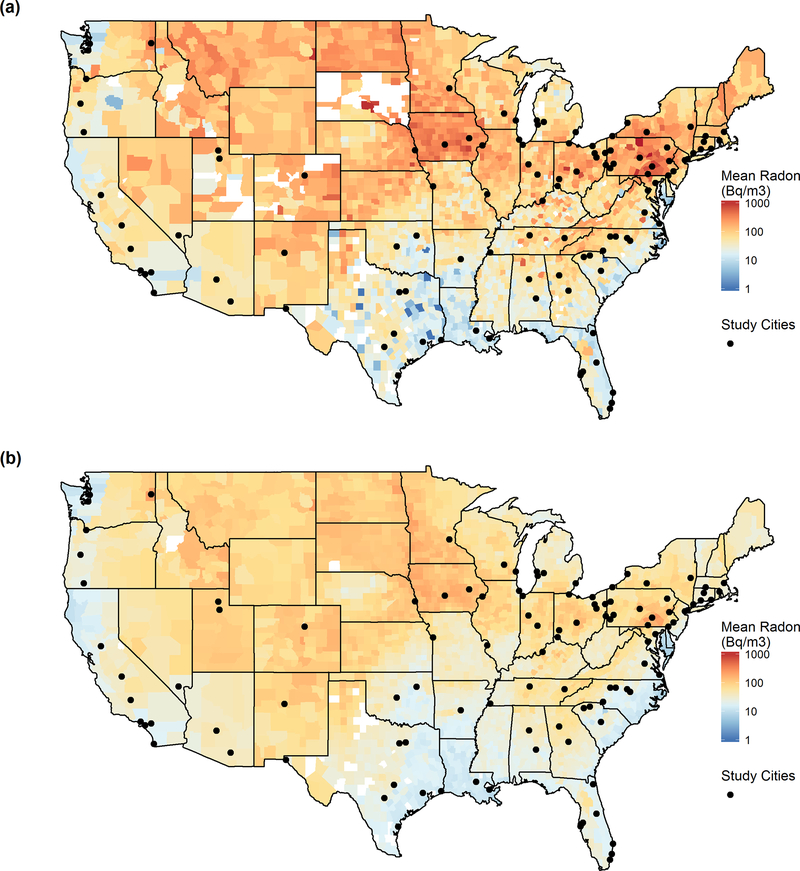
Our study encompasses daily data from 108 U.S. cities. Cities were included if they had at least 265 days of data per year (including PM2.5, weather and mortality data) for at least two consecutive years between 1999 and 2013. The geographic location of the cities is shown in Figure 1. A list of included cities and their respective years of data are included as Table S1 in the supplemental material.
Figure 1:
Study city locations and mean county radon levels (a) measured by the EPA SRRS survey and (b) modeled by the Lawrence Berkeley Laboratory.
Environmental data
Daily PM2.5 concentrations were obtained from the U.S. EPA Air Quality System Technology Transfer Network (U.S. EPA, 2016). PM2.5 monitors for each city were selected based on the county(ies) of each city. For cities with more than one sampling site, daily values were calculated using a method previously described and explained here briefly (Zanobetti and Schwartz, 2009). For each city, we: 1) calculated daily deviations from the annual mean for each monitor; 2) standardized each monitor’s daily deviations by dividing by its annual standard deviation; 3) calculated mean daily standardized deviations for each city by averaging the daily standardized deviations for all monitors assigned to the city; 4) multiplied this mean daily deviation value by the standard deviation of all monitors within the city, and; 5) added back the annual mean of all monitors within the city. This method standardizes daily measurements for all monitors within a city boundary and prevents missing days from one monitor from adding false variability to the daily value.
Weather data, including daily temperature and dew point temperature, were obtained from the National Oceanic Atmospheric Administration’s (NOAA) National Climatic Data Center and were used to calculate relative humidity. Each city was assigned to a weather station within a 60 km radius using a weighted selection criteria, which was calculated as the number of days with weather data divided by the distance between the city center and weather station.
We used two measures of radon exposures in our analysis. The first, the State/EPA Residential Radon Survey (SRRS), collected over 63,000 measurements from 1987–1992 to estimate short-term county-level mean indoor radon levels (Phillips et al., 1992, U.S. EPA, 1993). The majority of state-level surveys were conducted by the EPA using short-term charcoal canister samplers in the winter. Canisters were exposed for approximately 7 days in the lowest livable level of each sampled residence (White et al., 1992). Eight states conducted independent surveys, which are also included in our analysis. Our second measure of radon concentrations were modeled by the Lawrence Berkeley National Laboratory (LBL), where Price and his colleagues estimated long-term average indoor living area radon concentrations using the SRRS short-term measurements, an additional survey of long-term U.S. indoor radon measurements, and additional regional characteristics including geologic and housing characteristics (Price, 1997, Price and Nero, 1996a;b). Each city included in this analysis was assigned its county mean radon concentration from both the SRRS and LBL datasets. County radon concentrations are shown in Figure 1.
Monthly long-term averages of the planetary boundary layer (PBL) height are available from NOAA’s National Center for Atmospheric Prediction at a resolution of 32 km (Mesinger et al., 2006, NOAA ESRL PSD, 2016). These values were used to calculate a long-term annual mean PBL heights. Each city was assigned to the PBL estimate closest to the city center.
Health and demographic data
Daily mortality data through 2006 were obtained from the National Center for Health Statistics (NCHS) and data after 2006 were acquired from individual state Departments of Public Health. We analyzed non-accidental deaths due to all causes and specific diseases among individuals who resided in the city where they died. Outcomes were classified by the International statistical Classification of Disease, 10th revision codes as follows: all causes (ICD-10, A00-R99), cardiovascular diseases (ICD-10, I01-I59), and respiratory diseases (ICD-10, J00-J99) (World Health Organization, 2004). The proportion of residents over age 65 was obtained from the 2014 American Community Survey (U.S. Census Bureau, 2016b), as was the percent of all people whose income is below the poverty level (U.S. Census Bureau, 2016a). Current cigarette use among adults was obtained from the 2016 Behavior Risk Factor Surveillance System (BRFSS) survey data for the year 2016 (CDC, 2018).
Statistical analysis
We used a two-stage statistical approach in our analysis. First, we estimated city-specific and season-stratified mortality risk from exposure to averaged same-day and previous-day PM2.5 concentrations, using a generalized additive model (GAM) with a quasi-Poisson link function to account for over-dispersion. We stratified the analysis by season because previous studies have found seasonal variation in effects of PM2.5 (Zanobetti and Schwartz, 2009). Seasons were defined as follows: winter (December-February), spring (March-May), summer (June-August), and fall (September-November). The model controlled for long-term time using a penalized spline (s) with 1.5 degrees of freedom (df) per season per year and for day of week as an indicator variable. We also controlled for potential confounding due to weather by including smooth functions of daily temperature (temp), lag-1 temperature (temp-lag1), and relative humidity (RH), each with 3 df per year. This model can be represented as:
| (1) |
where for each city and season, E(Yt) is the expected mortality count at day t, α is the intercept, PM2.5 t is the two-day averaged PM2.5 concentration, β is the main effect of PM2.5, s() is the penalized smoothing spline function, dowt is a vector of indicator variables reflecting the day of week at time t, and δ is its corresponding vector of coefficients. was estimated for each city and season and was standardized for a 10 μg/m3 increase in PM2.5 concentration.
In the second stage, we used a three-level mixed-effects meta-regression model to estimate the association between city-season specific PM2.5 mortality effect estimates and city-specific average radon levels for each season. In this model, random variation in the PM2.5 effect estimates are divided into three parts: (i) within-season uncertainty for a given city, (ii) between-season variation within a given city, and (iii) between-city variation. This properly accounts for potential correlation of seasonal effect estimates within each city. The model can be written as:
| (2) |
where is the estimated PM2.5 coefficient for city i in season s obtained in the first stage, βS is a vector of coefficients for each season indicator variable, and βR is a vector of season-specific coefficients for the natural-log of radon, where ln(radon) is centered at its median value. The city-specific random effects are represented by ui, which satisfies the season-specific random effects for each city are represented by vi,s, which satisfies and the random deviation in within-season city estimates are represented by ri,s, which satisfies (Van den Noortgate et al., 2015). We allowed the modifying effect of radon on PM2.5 to vary by season because this effect may vary due to weather, home ventilation, and other seasonal patterns. We used a log-transformation of radon because this provided the best fit for the observed exposure-response curve between PM2.5 slopes and radon, as judged by Akaike Information Criterion (AIC) values among several model alternatives.
PM2.5 effect estimates are presented as the percent change in mortality associated with a 10 μg/m3 increase in daily PM2.5 at the study’s median radon value. The effects of radon on mortality are presented as the predicted PM2.5 effects at the 10th and 90th percentile of radon across cities. We assessed the significance of any remaining heterogeneity in PM2.5 effect estimates among cities using the QE test for residual heterogeneity.
As a sensitivity analysis, we assessed whether effect modification by radon changed after adjusting for spatially-varying city characteristics. We ran a series of second-stage meta-regression models, each including our basic model and an additional term for a potential confounder. We adjusted for the following city-averaged variables: average annual temperature, average annual planetary boundary layer height, the percent of population over age 65, and the poverty rate. We also adjusted for state estimates of current tobacco smoking.
RESULTS
Our study included 108 cities and over 215,000 days with mortality and air pollution data. The mean daily total mortality rate was 27 deaths/day, with winter having the highest rate (30 deaths/day) and summer having the lowest (25 deaths/day). The mean 2-day averaged PM2.5 was 12.7 μg/m3, with the highest seasonal average levels in summer (14.4 μg/m3) and lowest in spring (11.3 μg/m3). Summary statistics are presented in Table 1.
Table 1:
Summary (mean, SD) of daily mortality counts and environmental parameters.
| Variable | Overall | Winter | Spring | Summer | Fall |
|---|---|---|---|---|---|
| Daily Mortality (deaths/day) | |||||
| Total | 27 (31) | 30 (34) | 28 (31) | 25 (29) | 26 (30) |
| Cardiovascular Disease | 9 (11) | 10 (13) | 9 (11) | 8 (10) | 8 (11) |
| Respiratory Disease | 3 (3) | 3 (4) | 3 (3) | 2 (3) | 2 (3) |
| Environmental Parameters | |||||
| Temperature (˚C) | 14.7 (9.7) | 4.7 (7.9) | 14.0 (7.5) | 24.1 (4.1) | 15.6 (7.3) |
| Relative Humidity (%) | 65.6 (16.6) | 67.4 (16.6) | 62.1 (17.3) | 65.8 (15.9) | 67.2 (16) |
| 2-day Average PM2.5 (μg/m3) | 12.7 (7.1) | 13.1 (7.4) | 11.3 (5.7) | 14.4 (7.7) | 12.3 (7.3) |
| Rn (Bq/m3), SRRS1 | 111.4 (125.9) | — | — | — | — |
| Rn (Bq/m3), LBL1 | 51.8 (42) |
In this paper we use the international units for radon concentration, Bq/m3. The U.S. EPA still uses the conventional units of pCi/L.
Radon levels varied significantly across the U.S., with the highest values found in the Northeast and Upper Midwest (Figure 1). Based on the SRRS data, the maximum radon concentration among the cities included in our analysis was 844 Bq/m3 (Harrisburg, PA) and the minimum concentration was 11.1 Bq/m3 (Jacksonville, FL; New Orleans, LA; Port Arthur, TX; and Riverside, CA). LBL radon concentrations were lower and had less variability than SRRS concentrations, but the two measures were strongly correlated (r = 0.87). A Spearman test for correlation found that radon concentrations did not correlate significantly with mean city PM2.5 concentrations (r = 0.11 and r = 0.08 for the SRRS and LBL data, respectively).
Table 2 presents the estimated percent increase in mortality for a 10-μg/m3 increase in PM2.5 calculated at the median radon level (74 Bq/m3 based on the SRRS data). We found significant associations (p<0.05) between PM2.5 and total, cardiovascular and respiratory mortality in both the spring and fall, as well as significant associations between PM2.5 and total mortality in the summer. For example, a 10 μg/m3 increase in PM2.5 at the median radon level was associated with a 2.86% (95% CI: 2.40, 3.33) increase in all-cause mortality in the spring, as compared to a 0.29% (95% CI: −0.08, 0.67) increase in the winter. Forest plots of city- and season-specific PM2.5 effect estimates are included in the supplemental material as Figure S1.
Table 2:
Estimated percent increase in mortality (95% CI) associated with a 10 μg/m3 increase in PM2.5 at the median SRRS radon level (74 Bq/m3).
| Total | Cardiovascular | Respiratory | |
|---|---|---|---|
| Winter | 0.29 (−0.08, 0.67) | −0.19 (−0.85, 0.47) | 0.32 (−0.79, 1.43) |
| Spring | 2.86 (2.40, 3.33) | 2.97 (2.16, 3.79) | 5.74 (4.30, 7.19) |
| Summer | 0.70 (0.30, 1.11) | 0.41 (−0.31, 1.15) | 1.27 (−0.04, 2.60) |
| Fall | 1.49 (1.11, 1.87) | 1.26 (0.57, 1.95) | 2.76 (1.53, 3.99) |
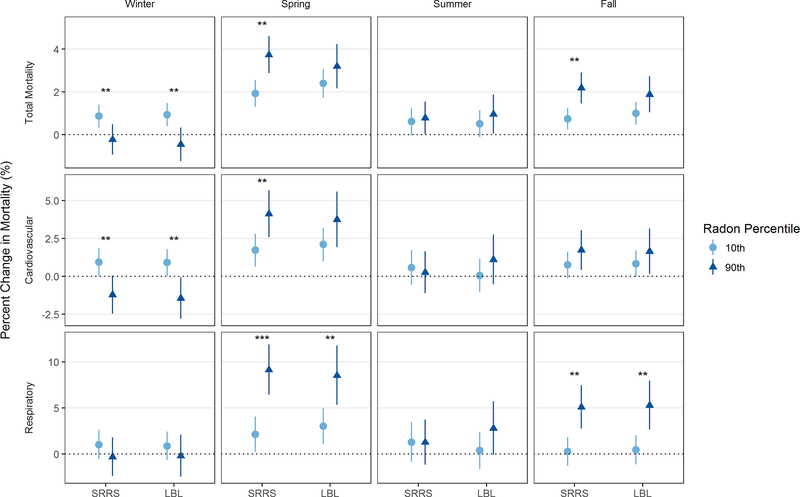
Radon modified the PM2.5 associations for total, cardiovascular, and respiratory deaths, with the strongest modification observed in the spring and fall. We estimated the PM2.5-related mortality risk by season at the 10th and 90th percentile of radon. The modifying effect of radon on PM2.5-associated mortality was most significant in the spring and fall, the two seasons where the PM2.5 effect was also the strongest. The greatest modification was seen in the spring. For example, using the SRRS data (based on short-term sampling), a 10 μg/m3 increase in PM2.5 in the spring at the 10th percentile of radon (21.1 Bq/m3) was associated with a 1.92% increase in total mortality (95% CI: 1.29, 2.55), while the same increase in PM2.5 at the 90th percentile of radon (234.2 Bq/m3) was associated with a 3.73% increase in total mortality (95% CI: 2.87, 4.59). Similarly, a 10 μg/m3 increase in PM2.5 in the spring at the 10th percentile of radon was associated with a 2.13% increase in respiratory mortality (95% CI: 0.24, 4.07), while a 10 μg/m3 increase in PM2.5 at the 90th percentile of radon was associated with an increase of 9.14% in respiratory mortality (95% CI: 6.45, 11.9). While the radon interaction term was significant for some outcomes in winter, the main association between PM2.5 and mortality in winter was small and not significant, reducing our ability to meaningfully assess effect modification. Conducting the second stage meta-regression using LBL radon concentrations (based on long-term living area estimates) yielded similar results for respiratory mortality that remained significant in the spring and fall. Results for total and cardiovascular mortality were similar but attenuated in magnitude and significance. Results for both radon exposures are shown in Figure 2 and in the supplemental material as Table S2.
Figure 2:
Estimated percent change in mortality associated with a 10 μg/m3 increase in PM2.5 at the 10th and 90th percentile of radon (SRRS: 21.1 and 234.2 Bq/m3; LBL: 18.1 and 108.0 Bq/m3).
Significance for the ln(radon) term: * p<0.1; ** p<0.05; *** p<0.001
We found significant remaining heterogeneity in our meta-regression models. Meta-regression models that controlled for both SRRS radon and season instead of just season reduced total remaining heterogeneity by 52%, 42%, and 32% in models for total, respiratory, and cardiovascular mortality, respectively. Meta-regression models that controlled for both LBL radon and season reduced total remaining heterogeneity by 19%, 19%, and 13% in models for total, respiratory, and cardiovascular mortality.
Our results were robust to adjustment for spatially-varying city characteristics. Radon-season interaction terms remained significant and relatively unchanged after adjustment for the city-specific percent of the population over age 65, percent population below the poverty line, and mean planetary boundary height, as well as state-wide smoking rates. Results also remained similar after adjustment for city-mean temperature, although significance for the interaction term between radon and winter in the model of PM2.5-associated cardiovascular mortality changed from significant to marginally significant. A presentation of the meta-regression results with and without adjustment for these additional spatial characteristics is included as Figure S2 in the supplemental material.
DISCUSSION
It is well established that radon and its decay products cause lung cancer (Tirmarche et al., 2010, Darby et al., 2005). However, little is known about the non-cancer effects of radon exposure, especially in non-occupational environments. In this large national study with almost six million deaths, we found that city-specific estimates of average indoor radon were associated with PM2.5-related total, cardiovascular and respiratory mortality risk.
The estimated PM2.5 health effects from our study are similar in magnitude to previous national studies. For example, a national study based on 112 U.S. cities estimated the percent increase in all-cause mortality associated with a 10 μg/m3 increase in two-day averaged PM2.5 to be 2.57 (95% CI: 1.96, 3.19), 0.25 (−0.13, 0.63), 0.95 (0.56, 1.34) and 0.56 (0.17, 0.94) in the spring, summer, fall, and winter, respectively (Zanobetti and Schwartz, 2009). While our estimated PM2.5 effects are somewhat higher in the fall than this earlier study, they follow a similar pattern with greatest effects seen in the spring and fall. This increased risk in the spring and fall may be due to an increased indoor-outdoor air exchange rates during the milder months, where windows are more likely to be open.
We observed stronger mortality effects of PM2.5 in cities with high average indoor radon concentrations, with the most significant effects in the spring and fall. This radon-season interaction may reflect seasonal variation in radon levels, as both indoor and outdoor radon levels vary seasonally (Miles and Algar, 1988). While most studies have found indoor radon levels to be highest in the winter, many factors can influence seasonal trends (Miles and Algar, 1988). These factors include soil moisture, indoor and outdoor temperature, wind speed, and building characteristics. These characteristics vary regionally and can cause regional differences in seasonal radon trends (Arvela et al., 2016). In addition, some studies have found an association between heating type and radon concentrations, and one EPA study found that heating in the winter lowers indoor concentrations of radon progeny (Hans and Lyon, 1986). Additional research is needed to determine whether temporal trends in concentrations of radon and its progeny could explain the observed seasonal variation in our interaction effect estimates.
When we used the modeled radon concentrations obtained from the Lawrence Berkeley lab rather than the measured EPA SRRS concentrations, our interaction effects for radon remained consistent for respiratory mortality but were of decreased magnitude and significance for total and cardiovascular mortality. This change is not unexpected. The two radon datasets are estimates of exposures during different timeframes (short-term versus long-term) and locations in the home (lowest living area versus a living-area average), and the LBL estimates have significantly less variation across cities. In addition, the LBL estimates use sparse long-term measurements from 125 counties to predict long-term radon concentrations for all U.S. counties (Price and Nero 1996). As has been demonstrated in other studies of spatially misaligned environmental exposure estimates, this can induce both Berkson and classical measurement errors and may introduce bias when used in our model (Szpiro et al., 2011, Peng and Bell, 2010, Gryparis et al., 2008). Any model misspecification in the LBL models may cause additional downward bias in our effect estimates (Alexeeff et al., 2016). It must be noted that the SRRS radon measurements are also subject to both classical and Berkson errors (Heid et al., 2004). Although EPA has based its approach to mitigating cancer risk on short-term lower living area measurements, these measurements of radon do not always reflect true long-term means and may not be a good surrogate for overall radon concentrations (Lubin et al., 1990). However, in light of the limitations posed by both sets of radon concentrations, we are encouraged by the close similarities in our results.
Radon effects remained significant even after adjusting for other spatially-varying potential confounders. PBL height was considered as a potential confounder because ground-level radon concentrations are known to change due to changes in PBL height (Sesana et al., 2003). Other potential confounders, including temperature, smoking and population age, could indicate greater regional susceptibility to PM2.5 effects. None of these potential confounders were significant when they were included in the meta-regression, and their inclusion did not impact the significance of interaction effects between radon and PM2.5 effect estimates. This suggests that the observed interaction effect is robust to spatially-varying variables.
An interaction between environmental radon levels and PM toxicity is biologically plausible. Particles may serve as vectors for radon progeny, which continue to emit alpha, beta, and gamma radiation after inhalation and deposition in the lungs. Alpha radiation causes considerably more biological damage than equivalent activities of beta and gamma radiation but cannot penetrate the epidermis. Therefore, inhalation of PM is a critical route of exposure to alpha radiation (UNSCEAR, 2012). Occupational and environmental health studies have shown that chronic inhalation of radionuclides emitting alpha radiation, primarily radon and its progeny, is an important risk factor for lung cancer even at the levels typically found in residential housing (Tirmarche et al., 2010, Darby et al., 2005). In addition, there is limited epidemiological evidence for non-cancer effects of low-level radiation. Using the LBL estimates, radon exposure was found to be associated with chronic COPD mortality in the American Cancer Society Prevention Study II (Turner et al., 2012). An ecological study in Galicia, Spain found a positive association between municipal residential radon levels and COPD hospital admissions (Barbosa-Lorenzo et al., 2017). Some epidemiological studies have suggested excess relative risk for circulatory disease at low-levels of ionizing radiation (Little et al., 2012), and a recent study found that environmental particle radioactivity measured as beta radiation on ambient air pollution samples was associated with an increase in both diastolic and systolic blood pressure in the Normative Aging Study cohort (Nyhan et al., 2018). Finally, there is support for acute effects from low-dose radiation exposure in both animal models and in human cells. Inhalation of radon by rats induced bronchoalveolar fluid (BALF) inflammation and IL-6 mRNA expression in both BALF and peripheral white blood cells (Li and Tong, 2007). There is also evidence that radon causes oxidative damage, as radon exposure in rats resulted in a dose-dependent increase in 8-OHdG levels in lung tissue and an increase in reactive oxygen species (ROS) in BALF (Nie et al., 2012). Human fibroblasts exposed to alpha-emitting particles had increases in ROS production (Narayanan et al., 1997), and human pulmonary epithelial cells exposed to alpha-emitting particles demonstrated up-regulation of gene pathways that included those associated with inflammatory and respiratory diseases (Chauhan et al., 2012). However, it is important to stress that all previous studies have examined environmental radiation independently of PM exposures, whereas our analysis focuses primarily on PM2.5 toxicity and its modification by radon. Toxicological studies could further investigate the presence of interaction between PM2.5 and radon exposures by conducting a two-factorial experiment in mice and assessing known biomarkers.
Our findings, if true, have important scientific and regulatory implications. First, regional differences in radon levels may partially explain the spatial variability in PM2.5 effect estimates across the U.S. found in many previous studies (Dominici et al., 2003, Dominici et al., 2006, Bell et al., 2008). Second, considering that the amount of attached radionuclides is expected to be a function of PM surface area, our hypothesis provides an explanation for why PM mass (or volume) is the most reproducible predictor of PM toxicity compared to PM components (HEI, 2013). Third, under typical atmospheric air pollution conditions most of the freshly generated ultrafine radon progeny attach to the PM accumulation mode, which has an approximate size of 0.1–1 μm. Thus our hypothesis may explain why PM2.5 is, in general, more toxic than coarse particles (Peng et al., 2008). Finally, the radon effect on PM2.5 risk justifies the lack of threshold in the PM exposure-response relationship (Di et al., 2017), as even low levels of PM can deliver sufficient alpha-emitting radionuclides into the human body.
Strengths and Limitations
Our analysis utilizes data from a large number of cities and applies well-established statistical methods. In addition, our hypothesis about modification of PM toxicity is biologically plausible, since the adverse effects of alpha radiation have been previously demonstrated. However, our analysis has several limitations. A major limitation of our study is that it relies on county-level radon estimates and does not include any information on seasonal variability in radon, which may be driving some of the patterns seen in our results. Both sets of radon concentrations are subject to potential measurement error and spatial misalignment, as discussed above. In addition, the radon concentrations used in this study were collected before the study period began. However, because long-term average indoor radon concentrations depend largely on geologic parameters and housing characteristics (Nazaroff, 1992), we expect long-term radon to remain relatively consistent. We would expect individual, current estimates of radon exposure based on measurements of particle-bound radon progeny to strengthen the observed associations. In addition, our ecological study design uses PM2.5 concentrations measured at one or several central sites in each city instead of individual measurements of PM2.5 exposures. This assumes that the monitor value for each city represents the true average ambient concentration for all individuals within a study. Any resulting measurement error may reduce efficiency and induce some downward bias on our estimated effect of PM2.5 (Zeger et al., 2000, Dominici et al., 2000). Future studies could use a prospective cohort design with individual measurements of radon and air pollution exposures to help overcome these limitations. Finally, we assume that there is no unmeasured confounding that could explain the effect modification of PM2.5 on mortality by radon. Causality still needs to be demonstrated.
CONCLUSIONS
Radon is a ubiquitous natural pollutant, with over 7 million U.S. homes having radon concentrations exceeding the EPA mitigation level of 148 Bq/m3 (4 pCi/L) (Angell, 2008). This level has been established based on lung cancer risk. However, our research suggests that radon, through its interaction with PM, is also associated with all-cause, cardiovascular and respiratory mortality. Future efforts should focus on investigating the non-cancer effects of radon. Furthermore, elucidating mechanisms responsible for PM toxicity, especially those related to a pollutant of natural origin such as radon, is of paramount importance to environmental and public health policies. Specifically, our findings suggest that it may be more effective to develop regional rather than national PM air quality standards. Given the significant regional variation in radon, a national PM2.5 standard may not adequately protect individuals living in areas with high radon exposures.
Supplementary Material
Implications.
In this large national study, city-averaged indoor radon concentration was a significant effect modifier of PM2.5-associated total, cardiovascular and respiratory mortality risk in the spring and fall. These results suggest that radon may enhance PM2.5-associated mortality. In addition, local radon concentrations partially explain the significant variability in PM2.5 effect estimates across U.S. cities, noted in this and previous studies. While the concept of PM as a vector for radon progeny is feasible, additional research is needed on the non-cancer health effects of radon and its potential interaction with PM. Future air quality regulations may need to consider the increased risk for particle mortality in cities with higher radon levels.
Acknowledgments
FUNDING
This work was supported by the Office of the Director of the NIH under Award Number DP5OD021412; NIEHS R01 ES019853; NIH/NIEHS 3P30ES000002-53S3; and U.S. EPA grant numbers RD-834798 and RD-835872. Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the U.S. EPA. Further, the U.S. EPA does not endorse the purchase of any commercial products or services mentioned in the publication.
REFERENCES
- Alexeeff SE, Carroll RJ, and Coull B. 2016. “Spatial measurement error and correction by spatial SIMEX in linear regression models when using predicted air pollution exposures.” Biostatistics (Oxford, England) 17 (2):377–389. doi: 10.1093/biostatistics/kxv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angell WJ 2008. “The U.S. radon problem, policy, program and industry: achievements, challenges and strategies.” Radiat Prot Dosimetry 130 (1):8–13. doi: 10.1093/rpd/ncn105. [DOI] [PubMed] [Google Scholar]
- Arvela H, Holmgren O, and Hanninen P. 2016. “Effect of soil moisture on seasonal variation in indoor radon concentration: modelling and measurements in 326 Finnish houses.” Radiat Prot Dosimetry 168 (2):277–90. doi: 10.1093/rpd/ncv182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson RW, Mills IC, Walton HA, and Anderson HR. 2015. “Fine particle components and health--a systematic review and meta-analysis of epidemiological time series studies of daily mortality and hospital admissions.” J Expo Sci Environ Epidemiol 25 (2):208–14. doi: 10.1038/jes.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa-Lorenzo R, Ruano-Ravina A, Ramis R, Aragones N, Kelsey KT, Carballeira-Roca C, Fernandez-Villar A, Lopez-Abente G, and Barros-Dios JM. 2017. “Residential radon and COPD. An ecological study in Galicia, Spain.” Int J Radiat Biol 93 (2):222–230. doi: 10.1080/09553002.2017.1238526. [DOI] [PubMed] [Google Scholar]
- Bell ML, Ebisu K, Peng RD, Walker J, Samet JM, Zeger SL, and Dominici F. 2008. “Seasonal and Regional Short-term Effects of Fine Particles on Hospital Admissions in 202 US Counties, 1999–2005.” American Journal of Epidemiology 168 (11):1301–1310. doi: 10.1093/aje/kwn252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bräuner EV, Andersen CE, Andersen HP, Gravesen P, Lind M, Ulbak K, Hertel O, Schüz J, and Raaschou-Nielsen O. 2010. “Is there any interaction between domestic radon exposure and air pollution from traffic in relation to childhood leukemia risk?” Cancer Causes & Control 21 (11):1961–1964. doi: 10.1007/s10552-010-9608-4. [DOI] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC Jr., Whitsel L, Kaufman JD, American E Heart Association Council on, C.o.t.K.i.C.D. Prevention, P.A. Council on Nutrition, and Metabolism. 2010. “Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association.” Circulation 121 (21):2331–78. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Cassee FR, Heroux ME, Gerlofs-Nijland ME, and Kelly FJ. 2013. “Particulate matter beyond mass: recent health evidence on the role of fractions, chemical constituents and sources of emission.” Inhal Toxicol 25 (14):802–12. doi: 10.3109/08958378.2013.850127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 2018. Behavioral Risk Factor Data: Tobacco Use (2011 to present). In Behavior Risk Factor Surveillance System. Atlanta, GA. [Google Scholar]
- Chauhan V, Howland M, Mendenhall A, O’Hara S, Stocki TJ, McNamee JP, and Wilkins RC. 2012. “Effects of alpha particle radiation on gene expression in human pulmonary epithelial cells.” International journal of hygiene and environmental health 215 (5):522–535. [DOI] [PubMed] [Google Scholar]
- Darby S, Hill D, Auvinen A, Barros-Dios J, Baysson H, Bochicchio F, Deo H, Falk R, Forastiere F, and Hakama M. 2005. “Radon in homes and risk of lung cancer: collaborative analysis of individual data from 13 European case-control studies.” Bmj 330 (7485):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Q, Wang Y, Zanobetti A, Wang Y, Koutrakis P, Choirat C, Dominici F, and Schwartz JD. 2017. “Air Pollution and Mortality in the Medicare Population.” New England Journal of Medicine 376 (26):2513–2522. doi: 10.1056/NEJMoa1702747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockery DW, Pope CA 3rd, Xu X, Spengler JD, Ware JH, Fay ME, Ferris BG Jr., and Speizer FE. 1993. “An association between air pollution and mortality in six U.S. cities.” N Engl J Med 329 (24):1753–9. doi: 10.1056/nejm199312093292401. [DOI] [PubMed] [Google Scholar]
- Dominici F, McDermott A, Zeger SL, and Samet JM. 2003. “National maps of the effects of particulate matter on mortality: exploring geographical variation.” Environmental Health Perspectives 111 (1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, and Samet JM. 2006. “Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases.” Jama 295 (10):1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici F, Zeger SL, and Samet JM. 2000. “A measurement error model for time-series studies of air pollution and mortality.” Biostatistics 1 (2):157–175. doi: 10.1093/biostatistics/1.2.157. [DOI] [PubMed] [Google Scholar]
- Gryparis A, Paciorek CJ, Zeka A, Schwartz J, and Coull BA. 2008. “Measurement error caused by spatial misalignment in environmental epidemiology.” Biostatistics 10 (2):258–274. doi: 10.1093/biostatistics/kxn033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Zhang L, and Guo Q. 2016. “Variation of the unattached fraction of radon progeny and its contribution to radon exposure.” Journal of Radiological Protection 36 (2):N34. [DOI] [PubMed] [Google Scholar]
- Hans JM, and Lyon RJ. 1986. Seasonal variations of radon and radon decay product concentrations in single family homes edited by United States Environmental Protection Agency. Las Vegas. Nevada: United States Environmental Protection Agency,. [Google Scholar]
- Health Effects Institute. 2013. “HEI’s National Particle Component Toxicity (NPACT) Initiative Executive Summary.” Research Reports 177 and 178. [PubMed]
- Heid I, Küchenhoff H, Miles J, Kreienbrock L, and Wichmann H. 2004. “Two dimensions of measurement error: classical and Berkson error in residential radon exposure assessment.” Journal of Exposure Science and Environmental Epidemiology 14 (5):365. doi: 10.1038/sj.jea.7500332. [DOI] [PubMed] [Google Scholar]
- Kelly FJ, and Fussell JC. 2012. “Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter.” Atmospheric Environment 60:504–526. doi: 10.1016/j.atmosenv.2012.06.039. [DOI] [Google Scholar]
- Krewski D, Lubin JH, Zielinski JM, Alavanja M, Catalan VS, Field RW, Klotz JB, Létourneau EG, Lynch CF, and Lyon JI. 2005. “Residential radon and risk of lung cancer: a combined analysis of 7 North American case-control studies.” Epidemiology 16 (2):137–145. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ, Fuller R, Acosta NJR, Adeyi O, Arnold R, Basu N, Baldé AB, Bertollini R, Bose-O’Reilly S, Boufford JI, Breysse PN, Chiles T, Mahidol C, Coll-Seck AM, Cropper ML, Fobil J, Fuster V, Greenstone M, Haines A, Hanrahan D, Hunter D, Khare M, Krupnick A, Lanphear B, Lohani B, Martin K, Mathiasen KV, McTeer MA, Murray CJL, Ndahimananjara JD, Perera F, Potočnik J, Preker AS, Ramesh J, Rockström J, Salinas C, Samson LD, Sandilya K, Sly PD, Smith KR, Steiner A, Stewart RB, Suk WA, van Schayck OCP, Yadama GN, Yumkella K, and Zhong M. 2017. “The Lancet Commission on pollution and health.” The Lancet. doi: 10.1016/s0140-6736(17)32345-0. [DOI] [PubMed] [Google Scholar]
- Lelieveld J, Evans JS, Fnais M, Giannadaki D, and Pozzer A. 2015. “The contribution of outdoor air pollution sources to premature mortality on a global scale.” Nature 525 (7569):367–71. doi: 10.1038/nature15371. [DOI] [PubMed] [Google Scholar]
- Li B-Y, and Tong J. 2007. “Adverse effects attributed to long-term radon inhalation in rats.” Journal of Toxicology and Environmental Health, Part A 70 (11):925–930. [DOI] [PubMed] [Google Scholar]
- Little MP, Azizova TV, Bazyka D, Bouffler SD, Cardis E, Chekin S, Chumak VV, Cucinotta FA, de Vathaire F, Hall P, Harrison JD, Hildebrandt G, Ivanov V, Kashcheev VV, Klymenko SV, Kreuzer M, Laurent O, Ozasa K, Schneider T, Tapio S, Taylor AM, Tzoulaki I, Vandoolaeghe WL, Wakeford R, Zablotska LB, Zhang W, and Lipshultz SE. 2012. “Systematic review and meta-analysis of circulatory disease from exposure to low-level ionizing radiation and estimates of potential population mortality risks.” Environ Health Perspect 120 (11):1503–11. doi: 10.1289/ehp.1204982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin JH, Samet JM, and Weinberg C. 1990. “Design issues in epidemiologic studies of indoor exposure to Rn and risk of lung cancer.” Health Physics 59 (6):807–817. [DOI] [PubMed] [Google Scholar]
- Marsh JW, and Bailey MR. 2013. “A review of lung-to-blood absorption rates for radon progeny.” Radiat Prot Dosimetry 157 (4):499–514. doi: 10.1093/rpd/nct179. [DOI] [PubMed] [Google Scholar]
- Mesinger F, DiMego G, Kalnay E, Mitchell K, Shafran PC, Ebisuzaki W, Jović D, Woollen J, Rogers E, and Berbery EH. 2006. “North American regional reanalysis.” Bulletin of the American Meteorological Society 87 (3):343–360. [Google Scholar]
- Miles JHC, and Algar RA. 1988. “Variations in radon-222 concentrations.” J. Radiol. Prot 8 (2):103–105. doi: 10.1088/0952-4746/8/2/005. [DOI] [Google Scholar]
- Narayanan P, Goodwin EH, and Lehnert B. 1997. “α particles initiate biological production of superoxide anions and hydrogen peroxide in human cells.” Cancer research 57 (18):3963–3971. [PubMed] [Google Scholar]
- Nazaroff WW 1992. “Radon transport from soil to air.” Reviews of geophysics 30 (2):137–160. [Google Scholar]
- Nie J-H, Chen Z-H, Liu X, Wu Y-W, Li J-X, Cao Y, Hei TK, and Tong J. 2012. “Oxidative damage in various tissues of rats exposed to radon.” Journal of Toxicology and Environmental Health, Part A 75 (12):694–699. [DOI] [PubMed] [Google Scholar]
- NOAA Earth System Research Laboratory Physical Sciences Division. 2016. NCEP North American Regional Reanalysis: NARR.
- Nyhan MM, Coull BA, Blomberg AJ, Vieira CL, Garshick E, Aba A, Vokonas P, Gold DR, Schwartz J, and Koutrakis P. 2018. “Associations Between Ambient Particle Radioactivity and Blood Pressure: The NAS (Normative Aging Study).” Journal of the American Heart Association 7 (6):e008245. doi: 10.1161/JAHA.117.008245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng RD, and Bell ML. 2010. “Spatial misalignment in time series studies of air pollution and health data.” Biostatistics 11 (4):720–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng RD, Chang RD, Bell ML, McDermott A, Zeger SL, Samet JM, and Dominici F. 2008. “Coarse particulate matter air pollution and hospitalization admissions for cardiovascular and respiratory diseases among medicare patients.” J Am Med Assoc 299 (18):2172–2179. doi: 10.1001/jama.299.18.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J, Marcinowski F, and Maconaughey K. 1992. “Two EPA approaches to radon surveys.” Journal of radioanalytical and nuclear chemistry 161 (1):273–282. doi: 10.1007/BF02034901. [DOI] [Google Scholar]
- Porstendörfer J 1994. “Properties and behaviour of radon and thoron and their decay products in the air.” Journal of Aerosol Science 25 (2):219–263. [Google Scholar]
- Porstendörfer J 2001. “Physical parameters and dose factors of the radon and thoron decay products.” Radiation Protection Dosimetry 94 (4):365–373. [DOI] [PubMed] [Google Scholar]
- Price P 1997. “Predictions and maps of county mean indoor radon concentrations in the mid-Atlantic states.” Health physics 72 (6):893–906. doi: 10.1097/00004032-199706000-00008. [DOI] [PubMed] [Google Scholar]
- Price P, and Nero A. 1996a. “Joint analysis of long-and short-term radon monitoring data from the Northern US.” Environment International 22:699–714. doi: 10.1016/S0160-4120(96)00173-0. [DOI] [Google Scholar]
- Price P, and Nero A. 1996b. “MAPPING OF MEAN RADON CONCENTRATIONS, USING SURVEY DATA AND COVARIATES.” 1996 International Radon Symposium, Lawrence Berkeley National Laboratory, Berkeley, Ca. [Google Scholar]
- Reineking A, and Porstendörfer J. 1990. ““ Unattached” fraction of short-lived Rn decay products in indoor and outdoor environments: an improved single-screen method and results.” Health physics 58 (6):715–727. [DOI] [PubMed] [Google Scholar]
- Sesana L, Caprioli E, and Marcazzan GM. 2003. “Long period study of outdoor radon concentration in Milan and correlation between its temporal variations and dispersion properties of atmosphere.” Journal of Environmental Radioactivity 65 (2):147–160. doi: 10.1016/s0265-931x(02)00093-0. [DOI] [PubMed] [Google Scholar]
- Szpiro AA, Sheppard L, and Lumley T. 2011. “Efficient measurement error correction with spatially misaligned data.” Biostatistics 12 (4):610–623. doi: 10.1093/biostatistics/kxq083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirmarche M, Harrison JD, Laurier D, Paquet F, Blanchardon E, Marsh JW, and International P Commission on Radiological. 2010. “ICRP Publication 115. Lung cancer risk from radon and progeny and statement on radon.” Ann ICRP 40 (1):1–64. doi: 10.1016/j.icrp.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Turner MC, Krewski D, Chen Y, Pope CA, Gapstur SM, and Thun MJ. 2012. “Radon and COPD mortality in the American Cancer Society Cohort.” Eur Respir J 39 (5):1113–9. doi: 10.1183/09031936.00058211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Census Bureau. 2016a. DP03 - selected economic characteristics In 2010–2014 American Community Survey 5-Year Estimates. American Fact Finder. [Google Scholar]
- U.S. Census Bureau. 2016b. S0101 - age and sex In 2010–2014 American Community Survey 5-Year Estimates. American Fact Finder. [Google Scholar]
- U.S. Environmental Protection Agency. 1993. State/EPA Residential Radon Survey. In National Radon Database, edited by Air and Radiation. Washington, DC. [Google Scholar]
- U.S. Environmental Protection Agency. 2016. Air Data: Air Quality Data Collected at Outdoor Monitors Across the US.
- United Nations Scientific Committee on the Effects of Atomic Radiaton. 2012. Biological Mechanisms of Radiation Actions at Low Doses. New York: United Nations. [Google Scholar]
- Van den Noortgate W, López-López JA, Marín-Martínez F, and Sánchez-Meca J. 2015. “Meta-analysis of multiple outcomes: a multilevel approach.” Behavior research methods 47 (4):1274–1294. [DOI] [PubMed] [Google Scholar]
- White SB, Bergsten JW, Alexander BV, Rodman NF, and Phillips JL. 1992. “Indoor 222Rn concentrations in a probability sample of 43,000 houses across 30 states.” Health Phys 62 (1):41–50. doi: 10.1097/00004032-199201000-00005. [DOI] [PubMed] [Google Scholar]
- World Health Organization. 2004. International statistical classification of diseases and related health problems. Vol. 1: World Health Organization. [Google Scholar]
- Zanobetti A, and Schwartz J. 2009. “The effect of fine and coarse particulate air pollution on mortality: a national analysis.” Environ Health Perspect 117 (6):898–903. doi: 10.1289/ehp.0800108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger SL, Thomas D, Dominici F, Samet JM, Schwartz J, Dockery D, and Cohen A. 2000. “Exposure measurement error in time-series studies of air pollution: concepts and consequences.” Environmental health perspectives 108 (5):419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.