Abstract
Tomato spotted wilt virus (TSWV) causes major losses of many crops worldwide. Several strategies have been attempted to control disease caused by TSWV. However, many challenges for the effective control of this disease remain. A promising approach is the use of abiotic or biotic inducers to enhance plant resistance to pathogens. We screened a diterpenoid compound from Wedelia trilobata, 3α-Angeloyloxy-9β-hydroxy-ent-kaur-16-en-19-oic acid (AHK), which had higher curative and protective effects against TSWV than the ningnanmycin control. The rapid initiation of the expression of all the TSWV genes was delayed by more than 1d in the curative assay, and the expression of the NSs, NSm and RdRp genes was inhibited. In addition, the replication of all TSWV genes in systemic leaves was inhibited in the protective assay, with an inhibition rate of more than 90%. The concentrations of jasmonic acid (JA) and jasmonic acid isoleucine (JA-ILE) in the AHK-treated and systemic leaves of the treated plants were significantly higher than those observed in the control. The results suggested that AHK can induce systemic resistance in treated plants. The transcription of the NtCOI1 gene, a key gene in the JA pathway, was significantly higher in both the inoculated and systemic leaves of the AHK-treated plants compared to the control. The AHK-induced resistance to TSWV in Nicotiana benthamiana could be eliminated by VIGS-mediated silencing of the NtCOI1 gene. These results indicated that AHK can activate the JA pathway and induce systemic resistance to TSWV infection.
Introduction
Tomato spotted wilt virus (TSWV) belongs to the genus Orthotospovirus and is transmitted by thrips (Thysanoptera) in a propagative manner1. TSWV is a destructive plant virus with a wide host range, infecting more than 1000 plants species from 90 families and is considered to be one of the top ten economically important plant viruses worldwide2,3. Despite developing many TSWV control measures, including insecticide application, the use of resistant cultivars, phytosanitation, and cultural control tactics, successful control is not readily achieved in many agricultural systems owing to the absence of resistant varieties or resistance breaking and the resistance of thrips to insecticides4.
The use of abiotic or biotic inducers is an effective way to enhance plant resistance to pathogens. Several natural or chemical products have been described as potential elicitors of induced resistance to diseases5,6. However, the antiviral efficacies of these compounds against systemic infections of plant viruses are unknown, with the exceptions of acibenzolar-S-methyl (BTH), eugenol, 3-acetonyl-3-hydroxyoxindole (AHO), and chitosan oligosaccharide (COS)7–10. These compounds have been shown to effectively enhance tobacco (Nicotiana xanthi NN, N. tabacum) and tomato resistance to systemic infection by TSWV and Tobacco mosaic virus (TMV), respectively, through induction of systemic acquired resistance (SAR). BTH is a signaling molecule that can enhance the expression of defense genes and elicits an increase in the concentration of H2O2 in treated leaves7. Defense activation by BTH, AHO, and COS in response to viral infection is supported by an induction of the salicylic acid (SA) signaling pathway, which is the primary biochemical marker of the systemic acquired resistance (SAR) transduction pathway7–10. Eugenol enhances the resistance of tomato plants to Tomato yellow leaf curl virus(TYLCV) by stimulating the production of endogenous nitric oxide (NO) and SA and by regulating the expression of SlPer1, a host R gene specific to TYLCV in tomato plants11,12. Phytohormones, such asjasmonic acid (JA), SA, and abscisic acid (ABA), can enhance resistance to pathogens during plant defense responses13–15.
Wedelia trilobata (L.) Hitchc. [syn. Sphagneticola trilobata (L.) Pruski] (creeping oxeye) is native to the tropics of Central America. Recently, this notoriously invasive weed has invaded India, South China, and Japan16. The results of some studies have suggested that compounds extracted from W. trilobata have pharmacological functions in medical treatments and biological activities in agriculture17,18, including anti-TMV19, insect toxin20, fungistatic, and bacterial inhibitory activities19,21. However, little is known regarding compounds from W. trilobata with anti-TSWV inhibition mechanisms. In this study, 28 diterpenoid compounds extracted from W. trilobata were screened using the lesion counting method. The results showed that 3α-Angeloyloxy-9β-hydroxy-ent-kaur-16-en-19-oic acid (AHK) had the highest activity against TSWV by eliciting the JA signaling pathway and elicited induced systemic resistance (ISR) in tobacco.This approach may be an effective and environmentally friendly way to control TSWV infections.
Results
Compound structural analysis
3α-Angeloyloxy-9β-hydroxy-ent-kaur-16-en-19-oic acid (10.4 mg), a white powder, was shown to have the molecular formula C25H36O5 as determined by the results of ESI-MS, 13C NMR and DEPT analyses. Comparison of the 1D NMR spectroscopic data of 1 with those of 3α-Angeloyloxy-9β-hydroxy-ent-kaur-16-en-19-oic acid revealed that they shared an identical structure that was determined to be 3α-angeloyloxy-9β- hydroxy-ent-kaur-16-en-19-oic acid22.
3α-Angeloyloxy-9β-hydroxy-ent-kaur-16-en-19-oic acid: white powder, C25H36O5; ESI-MS m/z: 439 [M + Na]+, 1H NMR (CDCl3, 400 MHz) δ (ppm): 6.01 (1 H, dd, J = 13.8, 6.8 Hz, H-3′), 4.75 (1 H, s, H-17a), 4.71 (1 H, s, H-17b), 4.58 (1 H, dd, J = 11.9, 4.1 Hz, H-3), 2.64 (1 H, d, J = 17.5 Hz, H-15a), 2.57 (1 H, s, H-13), 1.24 (3 H, s, H-18), 1.11 (3 H, s, H-20); 13C NMR (CDCl3, 100 MHz) δ (ppm): 180.6 (s, C-19), 167.6 (s, C-1′), 154.7 (s, C-16), 138.1 (d, C-3′), 127.9 (s, C-2′), 103.4 (t, C-17), 78.4 (d, C-3), 77.3 (s, C-9), 49.2 (d, C-5), 48.9 (s, C-8), 47.9 (s, C-4), 43.68 (s, C-10), 43.65 (t, C-15), 42.1 (d, C-13), 40.3 (t, C-14), 35.8 (t, C-7), 34.4 (t, C-12), 30.6 (t, C-1), 30.0 (t, C-11), 24.1 (q, C-18), 23.9 (t, C-2), 21.4 (t, C-6), 20.7 (q, C-5′), 17.2 (q, C-20), 15.7 (q, C-4′).
Curative effect assay
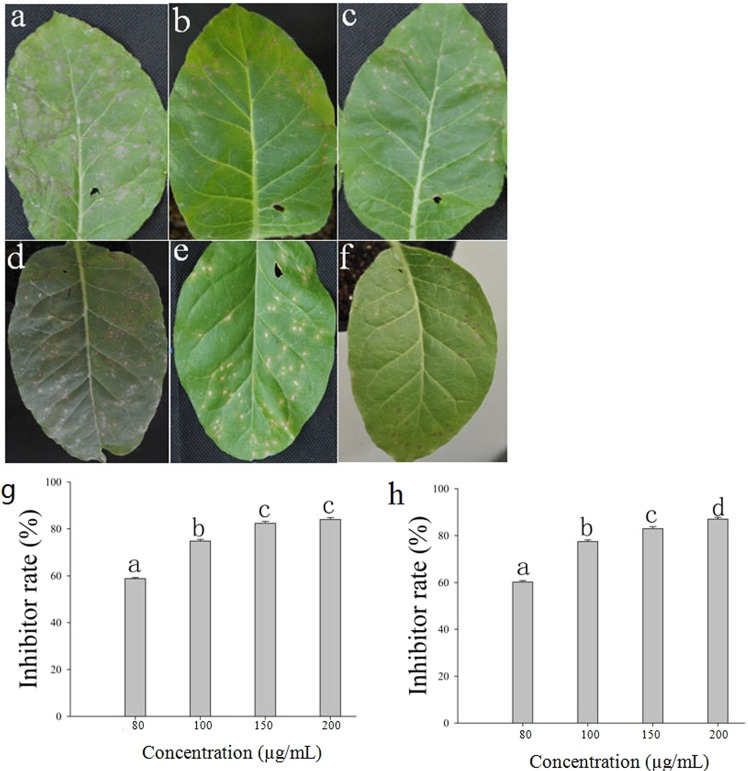
The activities of 28 diterpenoid compounds from W. trilobata against TSWV were tested by counting lesions on inoculated leaves at 3 days post inoculation (dpi). An examination of the inhibition rates revealed that 15 of these compounds had varying degrees of curative effects, whereas the other 13 compounds had no inhibitory effect. Compounds 8, 9, 13, 16, 20, 25, 27 and 28 had higher curative inhibition rates than that of the control ningnanmycin (52.48%) at a concentration of 100 μg/mL. Compound 20, 3α-Angeloyloxy-9β-hydroxy-ent-kaur-16-en-19-oic acid (AHK), had the highest inhibition rate (62.40%) at a concentration of 100 μg/mL (Fig. 1a–c and Table 1).
Figure 1.
Inhibitory effect of anti-TSWV with AHK different treatments. (a–c) The curative effect of AHK. Image (a) shows the positive control (treatment with TSWV and H2O), image (b) shows the ningnanmycin control (treatment with TSWV and ningnanmycin), and image (c) shows the AHK treatment (treatment with TSWV and AHK); (d–f) The protective effect of AHK. Image (d) shows the positive control (treatment with H2O and TSWV), image (e) shows the ningnanmycin control (treatment with ningnanmycin and TSWV), and image (f) shows the AHK treatment (treatment with AHK and TSWV); Image (g) shows the curative effect of AHK at different concentrations; Image (h) shows the protective effect of AHK at different concentrations. Different letters indicate significant differences among the compounds compared with ningnanmycin; differences are considered significant at P ≤ 0.05.
Table 1.
The inhibition rates of TSWV with treatment by different compounds.
| Compound name | Inhibitor rate (%) | Compound name | Inhibitor rate (%) | ||
|---|---|---|---|---|---|
| Protection | Curation | Protection | Curation | ||
| Compound1 | 56.50 ± 1.90b | 54.80 ± 0.70b | Compound16 | 66.30 ± 0.70b | 61.60 ± 0.60b |
| Compound2 | 53.30 ± 0.90b | 45.80 ± 0.30b | Compound18 | 57.20 ± 2.80b | 47.80 ± 3.10b |
| Compound3 | 58.10 ± 0.40b | 46.30 ± 0.20b | Compound20 | 76.50 ± 0.40b | 62.40 ± 1.70b |
| Compound6 | 58.80 ± 1.10a | 56.00 ± 0.70a | Compound22 | 60.50 ± 0.40a | 52.10 ± 1.20b |
| Compound7 | 54.80 ± 0.70b | 52.40 ± 1.60b | Compound25 | 63.20 ± 1.60a | 61.30 ± 2.10b |
| Compound8 | 54.40 ± 0.70b | 60.80 ± 1.30a | Compound27 | 67.10 ± 0.20b | 62.20 ± 0.20b |
| Compound9 | 50.00 ± 0.40b | 61.90 ± 1.60b | Compound28 | 69.50 ± 0.60b | 61.90 ± 0.30b |
| Compound13 | 58.4 ± 0.90a | 60.30 ± 1.10b | Ningnanmycin | 61.25 ± 0.70a | 58.80 ± 1.30a |
Different letters indicate significant differences for different compounds compared with ningnanmycin; differences are significant at P ≤ 0.05.
Protective effect assay
The results of this assay revealed that 15 of the 28 diterpenoid compounds assayed had varying degrees of activity against TSWV, whereas the other 13 compounds had no inhibitory effect. Compounds 20, 22, 25, 27 and 28 had higher protective inhibition rates than that of the control ningnanmycin (61.25%) at a concentration of 100 μg/mL. AHK had the highest inhibition rate (76.50%) (Fig. 1d–f and Table 1).
Concentration gradient assay of AHK
The results of a necrotic lesion inhibition assay demonstrated that the activity of AHK against TSWV in tobacco K326 increased with increasing concentrations of AHK (80, 100, 150 and 200 µg/mL). At a concentration of 200 μg/mL, AHK was observed to exhibit the highest in vivo curative and protective effects (84.0 and 87.0%, respectively) among the concentrations tested (Fig. 1g,h).
AHK inhibition of gene expression of TSWV in vivo
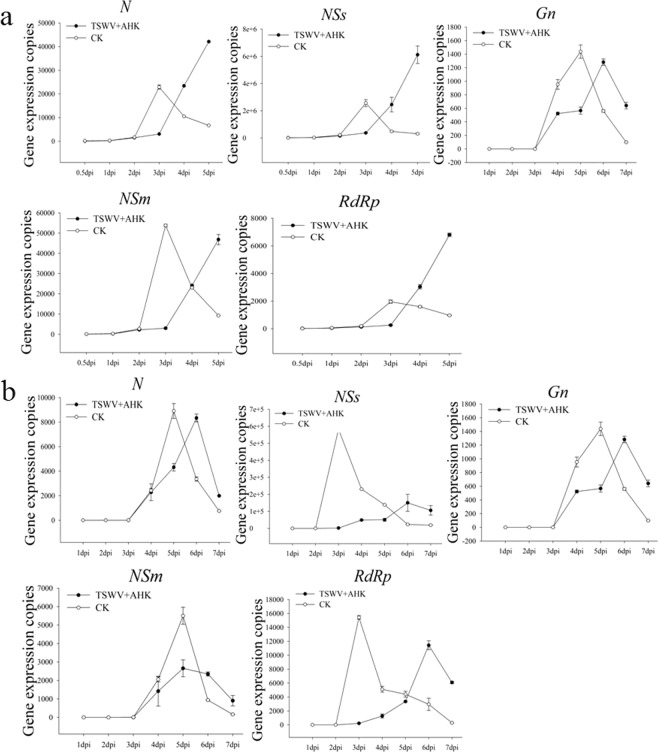
The transcription levels of the TSWV genes Gn, N, NSs, NSm and RdRp were detected by qRT-PCR in the AHK-treated and systemic upper leaves for the in vivo curative assay. Sampling time was determined by the development of symptoms, and sampling ceased when the degree of leaf necrosis was very serious or when the leaf withered. The results showed that the rapid initiation of expression of all TSWV genes was delayed by 1 day (postponed from 2 to 3 dpi) in the inoculated leaves of the AHK- treated plants. Compared with untreated control leaves, the peak expression of the N, Gn, NSs, and RdRp genes in the treatment leaves was delayed from 3 to 5 dpi (Fig. 2a). The degree of leaf necrosis was used to determine the viral titer, and as leaf withering increased, the replication of the viral genes also decreased (Table S4).
Figure 2.
Levels of TSWV gene expression in AHK-treated leaves to assess the curative effect of AHK. Image (a) presents the level of TSWV gene expression with and without the AHK treatment in inoculated K326 leaves in the curative effect assay using qRT-PCR; Image (b) presents the level of TSWV gene expression with and without the AHK treatment in K326 systemic leaves in the curative effect assay using qRT-PCR; CK: positive control (TSWV-infected tobacco).
In contrast to the N, Gn, NSm, and RdRp genes, the expression of the NSs gene was lowest in the systemic leaves. The inhibition rate of NSs gene expression was as great as 74.15% and was higher than other genes (N, 6.40%; Gn, 10.79%; NSm, 57.33%; and RdRp, 25.88%). The rapid replication initiation for NSs and RdRp genes was delayed by 3 days from 2 to 5 dpi (Fig. 2b and Table S3). The results may demonstrate that systemic infection by TSWV was inhibited in the AHK-treated plants by inhibition of the expression of NSs.
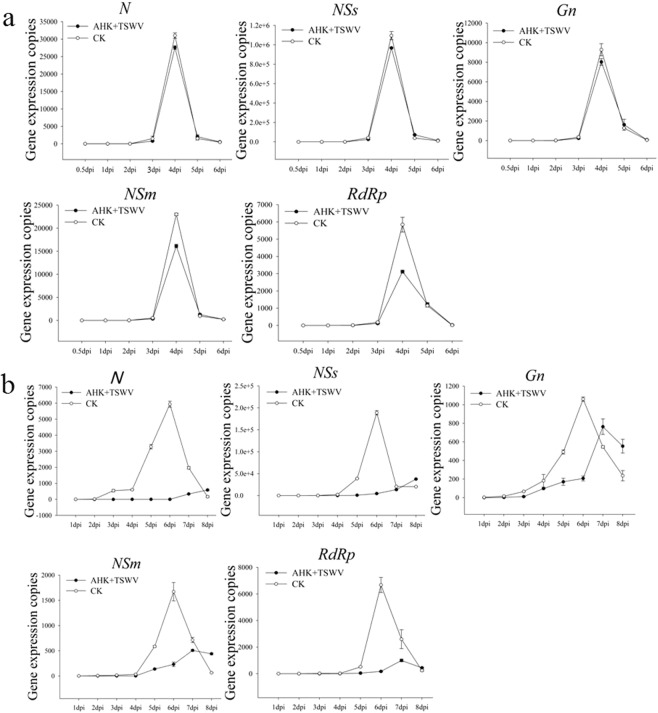
In addition, the transcription levels of TSWV genes Gn, N, NSs, NSm, and RdRp were detected by qRT-PCR in the AHK-treated leaves and in the systemic upper leaves for the protective assay in vivo. In the inoculated leaves, the results showed that the expression of all TSWV genes in the treatment and control plants had the same trend of a rapid initiation of expression at 2 dpi, peaking at 3 dpi and followed by an abrupt decrease. AHK treatment had no any effect on the expression of TSWV genes except for RdRp in the protective assay (Fig. 3a).
Figure 3.
Levels of TSWV gene expression in AHK-treated leaves to assess the protective effect of AHK. Image (a) presents the level of TSWV gene expression with and without the AHK treatment in inoculated K326 leaves assayed by qRT-PCR; Image (b) presents the level of TSWV gene expression with and without the AHK treatment in systemic K326 leaves in the protective effect assay using qRT-PCR; CK: positive control (TSWV-infected tobacco).
In the systemic upper leaves of the control plants, all TSWV genes exhibited rapid expression initiation at 4 dpi, peaking at 6 dpi and decreasing thereafter. However, the expression of all TSWV genes in the AHK-treated plants remained at a low level until 8 dpi, when the leaves of control plants exhibited a very serious degree of necrosis or were withered, and had inhibition rates from 69.61 to 90.14%, except for Gn (27.91%) (Fig. 3b and Table S3). These results demonstrated that systemic movement of the virus was inhibited in the protective assay.
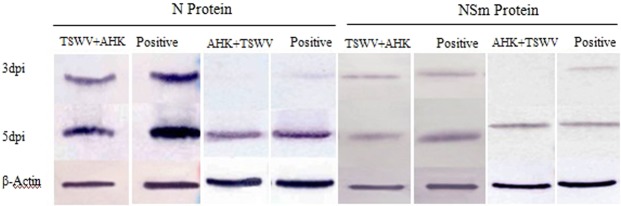
Effects of AHK on N and NSm protein expression
The expression of both N (a structural protein) and NSm (a nonstructural protein) was detected by western blot at 0.5, 1.5, 3, and 5 days post inoculation(dpi) in the inoculated leaves from the curative and protective assays (Fig. 4). The results showed that the expression of the N and NSm proteins were not detected at 0.5 dpi, 1.5 dpi, and the expression of the N protein in the curative and protective assays was lower than that observed in the positive control (TSWV-treated leaves) at 3 and 5 dpi, while the expression of the N protein at 5 dpi was higher than at 3 dpi. The expression of the NSm protein in the curative assay was lower than that of the positive control at 3 and 5 dpi, while the expression of the NSm protein in the protective effect assay was lower than that of the positive control on 3 dpi, but no difference was observed on 5 dpi. Taken together, these results show that the expression of the N protein was inhibited after AHK treatment in both the curative and protective assays, while the expression of the NSm protein was notably inhibited in the curative assay but not in the protective assay.
Figure 4.
Expression of the TSWV N and NSm proteins. The expression of the N and NSm proteins with the AHK treatment in the protective and curative effect assays on the inoculated leaves by western blot; positive control (TSWV-infected leaves).
Effects of AHK on phytohormone concentration in host leaves
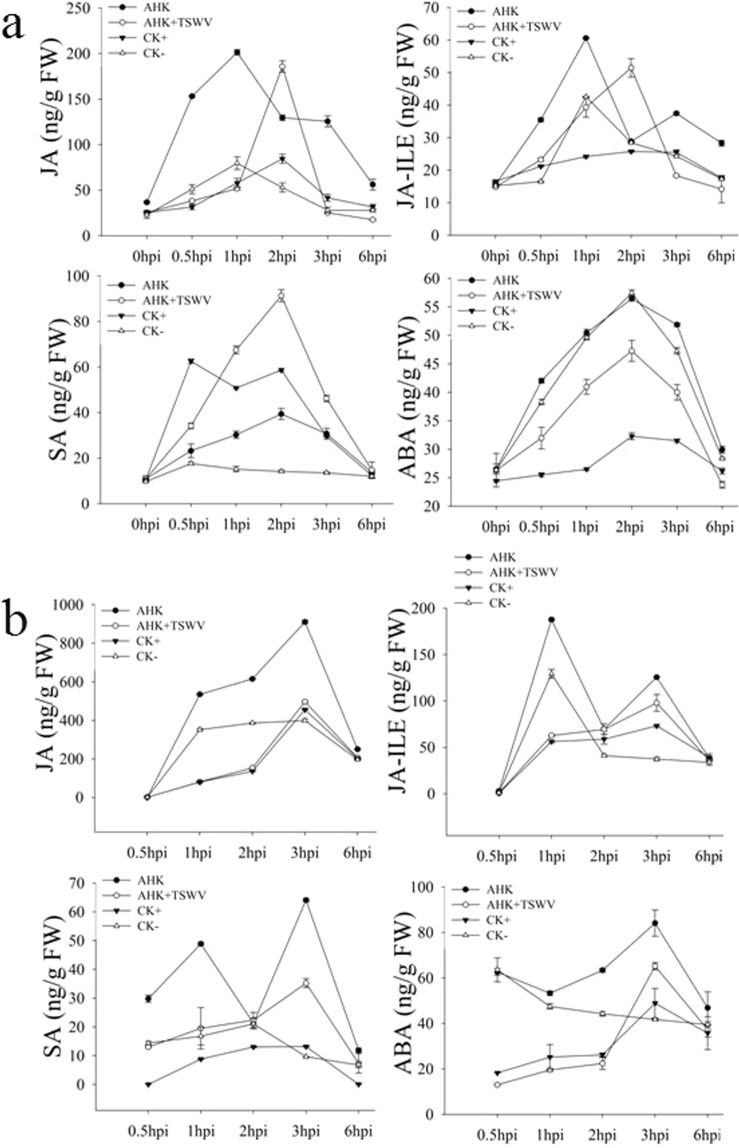
Plant phytohormones are closely associated with plant resistance to viral infections. The concentrations of JA, jasmonic acid isoleucine (JA-ILE), SA, and ABA in the inoculated leaves were determined to evaluate the defense-associated signaling pathway induced by AHK (Fig. 5a). The levels of both JA and JA-ILE in the AHK-treated inoculated leaves were generally higher than in the control leaves. The peak concentrations of JA and JA-ILE (AHK: 201.36 and 60.58 ng/g; AHK-TSWV: 185.80 and 51.47 ng/g, respectively) were 3 times higher than those observed in the control leaves. The concentration of SA in leaves from the AHK and AHK + TSWV treatments (AHK was smeared 6 h, after which TSWV was inoculated into the same leaves) peaked at 2 hpi and was higher than that observed in the control plant leaves (treated by DMSO), while the SA concentration in the AHK treatment leaves was lower than that observed in the leaves inoculated with TSWV. In contrast, the concentration of ABA in the treatment leaves did not increase compared with that observed in the control leaves. These results showed that AHK can significantly induce the production of JA and JA-ILE in the treated leaves. To confirm that the signals induced by AHK in the treated leaves were transferred into the systemic upper leaves, the concentrations of JA, JA-ILE, SA, and ABA in the systemic upper leaves were determined (Fig. 5b). The results showed that concentrations of JA and SA peaked at 3 hpi (910.49 and 64.08 ng/g, respectively) and were higher than those observed in the control plants (treated with TSWV or DMSO), and the concentration of JA was 14 times greater than that of SA. The concentration of ABA in the AHK-treated leaves was similar to that observed in the control leaves. Although the trends in the concentrations of JA, JA-ILE, SA, and ABA using the AHK + TSWV treatment were similar to the TSWV treatment, the concentrations of JA, JA-ILE, SA, and ABA in the AHK + TSWV-treated leaves were higher than in the TSWV treatment at 3 hpi, the same time that the JA concentrations peaked. These results indicated that the signals induced by AHK could be transferred into the systemic upper leaves, and for leaves pretreated with AHK, the pretreatment promoted a primed state of the plants23. Thus, when infected by TSWV, the plants could initiate the phytohormone-associated defense pathway to inhibit TSWV.
Figure 5.
Concentrations of hormones after AHK treatment. Image (a) shows the concentrations of phytohormones (JA, JA-ILE, SA, and ABA) in inoculated leaves at 0, 0.5, 1, 2, 3, and 6 hpi; image (b) shows the concentrations of phytohormones (JA, JA-ILE, SA, and ABA) in systemic upper leaves at 0.5, 1, 2, 3, and 6 hpi; AHK: treatment with AHK; AHK + TSWV: inoculation of TSWV at 6 h post-AHK treatment; CK+: positive (TSWV inoculated); CK-: negative (DMSO treatment).
Effects of AHK on the expression of key genes of different phytohormone signaling pathways
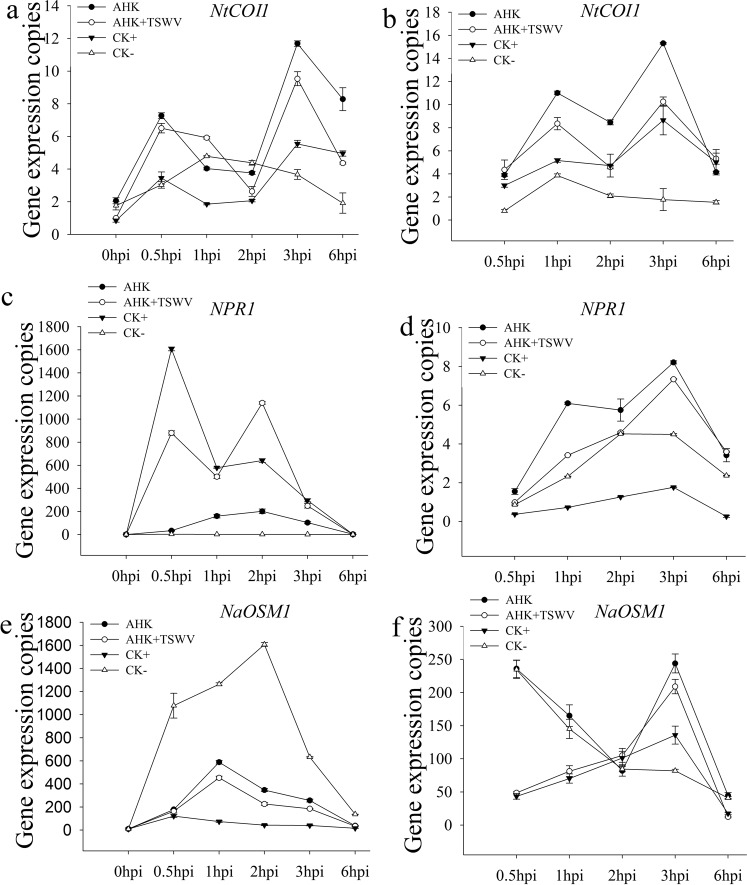
To further confirm the key signal pathways involved in the AHK-induced resistance to TSWV, we examined the transcription of the JA signal pathway marker gene NtCOI1, which is the key component of ubiquitin-ligase complexes (SCFCOI1)24–26, the transcription of the NPR1 gene of the SA signal pathway, and the transcription of the NaOSM1 gene of the ABA signal pathway. The qRT-PCR analysis showed that the AHK and AHK + TSWV treatments induced higher expression of the NtCOI1 gene in the inoculated leaves and in the systemic upper leaves than was observed in the control treatment. These results indicated that signal induced in the inoculated leaves was transferred to the upper leaves and was amplified. The AHK treatment had no effect on the transcription of the NPR1 and NaOSM1 genes. The TSWV infection caused the SA levels to increase at 0.5 hpi, although the signal was not transferred from the treated leaves to the upper leaves (Fig. 6). These results showed that the JA signaling pathway was activated by AHK and played a key role in the resistance to TSWV infection.
Figure 6.
Transcription levels of key genes of the JA, SA, and ABA signaling pathways. (a) Shows the transcription level of the NtCOI1 gene of the JA pathway in inoculated leaves; (b) shows the transcription level of the NtCOI1 gene of the JA pathway in systemic upper leaves; (c) shows the transcription level of the NPR1 gene of the SA pathway in inoculated leaves; (d) shows the transcription level of the NPR1 gene of the SA pathway in systemic upper leaves; (e) shows the transcription level of the NaOSM1 gene of the ABA pathway in inoculated leaves; (f) shows the transcription level of the NaOSM1 gene of the ABA pathway in systemic upper leaves. AHK: AHK treatment; AHK + TSWV: inoculation of TSWV at 6 h post-AHK treatment. CK+: positive (TSWV inoculated); CK-: negative (DMSO treatment).
Loss of AHK-induced resistance to TSWV after silencing the NtCOI1 gene
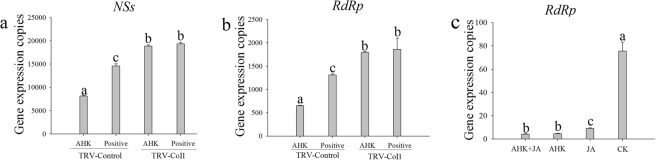
The transcription levels of the NSs gene in the curative assay and the RdRp gene in the protective assay were analyzed by qRT-PCR.The transcription levels of the NSs and RdRp genes were both significantly higher in the TRV-COI1 tobacco plants than in the TRV-Control tobacco plants (Fig. 7a,b). However, RdRp expression in the TRV-COI1 plants was significantly inhibited when the plants were sprayed with JA (Fig. 7c). Thus, the results indicated that AHK-induced resistance to TSWV depended on the JA signaling pathway.
Figure 7.
Effective anti-virus activity of AHK after silencing the COI1 gene. (a) Shows the replication level of the NSs gene in the curative effect assay in the TRV-Control and TRV-CoI1 tobacco; (b) shows the replication level of the RdRp gene in the protective effect assay in the TRV-Control and TRV-CoI1 tobacco; (c) shows the transcription level of the RdRp gene in the silenced tobacco and inoculated with TSWV 6 h after different treatments; AHK: AHK treatment; positive: samples only inoculated with TSWV; AHK + JA: silenced tobacco and treatment with AHK and JA; JA: silenced tobacco and treatment with JA; CK: Silenced tobacco, inoculated with TSWV JA. Differences are considered significant at P ≤ 0.05.
Discussion
Plant resistance to viruses can be induced by chemicals, such as benzothiadiazole, eugenol, 3-acetonyl-3-hydroxyoxindole (AHO), andsesquiterpenoids10,12,27,28. Induced resistance has been shown to be an alternative for managing viral infections of crops29. TSWV disease is one of the largest constraints on agricultural production because of the lack of pesticides against TSWV and the extensive adaptability of its vector. In this study, we used the tobacco-TSWV system to demonstrate that diterpenoid compounds from W. trilobata can induce systemic resistance to TSWV. The results of a lesion inhibition screening assay showed that of the 28 diterpenoid compounds isolated from W. trilobata, 100 µg/mL of AHK had a higher inhibitory activity than the control ningnanmycin, which has been widely used in agriculture to control TMV and other viral diseases30,31. In assays testing the curative and protective effects of AHK, the inhibition rates obtained using 100 and 200 µg/mL of AHK were 62.40 and 61.25% and 84.00 and 87.00%, respectively.
To complete a successful infection cycle in the host plant cell, a virus must enter into the cell of the host plant and undergo uncoating, translation and replication of the genome followed by encapsidation and multiplication before infecting neighboring cells and spreading systemically. Replication of the viral genome is one of the key steps in the completion of a successful infection cycle. We performed qRT-PCR to detect the replication level of five genes encoded by TSWV. Compared with the controls, the rapid initiation of the expression of all the assayed genes was delayed by 1 day in the inoculated leaves of the AHK-treated plants. In the systemic upper leaves, the expression of the NSs, NSm and RdRp genes was maintained at a lower level in the AHK-treated plants compared to the control, with gene expression inhibition rates of greater than 90% observed together with a delay in rapid expression initiation by 3 days. The NSs protein, a viral suppressor of RNA silencing, plays a key role in the replication and transcription of viral genes and is necessary for the establishment and maintenance of systemic infection in plants32,33. NSm, the movement protein, is also related to symptom development34–36, while RdRp is a replicase of viral RNAs37,38. These results indicated that AHK-treated plants could activate RNA silencing and block the movement and replication of viruses to defend against TSWV infection. RNA silencing is regarded as pattern-triggered immunity (PTI) against viruses. AHK had no effect on the replication level of the membrane glycoprotein (Gn/Gc) gene of TSWV. The results also demonstrated that the Gn/Gc protein, which is involved in viral transmission by thrips, did not play a key role in TSWV infection of tobacco plants. The observed inhibition rates of the replication of TSWV genes demonstrated that AHK had more of protective effect than curative effect.
The plant hormones SA, JA, ABA, and ethylene (ET) play key roles in regulating signaling networks associated with plant defense toward pathogens. SA is required for SAR activation in tissues distal from the site of infection, while jasmonate and ethylene are indispensable for ISR. SAR and ISR, two primary forms of plant defense, can be activated when plants are infected by pathogens, e.g., bacteria, fungi, and viruses, or by abiotic agents, e.g., elicitors, which may result in resistance against subsequent infections by pathogens13,39. For example, in TMV-infected tobacco, the transcript level of genes associated with the JA-mediated defense pathway was significantly upregulated after 1 day, whereas genes associated with the SA-mediated defense pathway were significantly upregulated after 5 days13. Some chemicals, including the cyclodipeptides cyclo (L-Pro-L-Pro) and cyclo (D-Pro-D-Pro), chitosan, schisanhenol derivatives, and a glycoprotein of BDP-30 inhibited TMV infection through activation of SA- or JA-induced systemic resistance9,40–43. In our study, after AHK treatment of tobacco leaves, the accumulation of JA and JA-ILE significantly increased in both the treated leaves and in the systemic upper leaves and was higher than that observed for SA and ABA. The level of SA accumulation in the TSWV-inoculated leaves was higher than that observed in the uninoculated leaves, and the SA signal was not transduced into the systemic upper leaves (Figs 5a,b-SA and 6c,d). The results of the qRT-PCR analysis showed that the transcription of NtCOI1, which plays a key role in the JA pathway, was higher in both inoculated and systemic leaves than was observed in the controls. AHK-induced resistance to TSWV could be eliminated by VIGS-mediated silencing of NtCOI1. Thus, the results indicated that AHK-induced systemic resistance to TSWV was dependent on the JA signaling pathway, not the SA signaling pathway. Although TSWV infection could induce the SA signaling pathway, the SA signal could be enhanced under AHK induction (Fig. 5a-SA). Additionally, TSWV is transmitted by thrips (order Thysanoptera), and the JA pathwayis required for resistance, predominantly against herbivorous insects44. Thus, the results of our study indicate that AHK, a diterpenoid compound from W. trilobata, is a strong candidate for the development of an environment-friendly pesticide for preventing TSWV diseases.
Conclusions
In summary, this is the first report describing the use of 3α-Angeloyloxy-9β-hydroxy-ent-kaur-16-en-19-oic acid (AHK), a diterpenoid compound isolated and screened from extracts of Wedelia trilobata, to inhibit TSWV infection. In AHK-treated tobacco leaves, the rapid initiation TSWV gene expression was delayed by at least 1 day, the expression of the NSs, NSm and RdRp genes was inhibited, and the concentrations of jasmonic acid (JA) and jasmonic acid isoleucine (JA-ILE) increased significantly. The level of NtCOI1 transcription in the JA signaling pathway increased in the AHK-treated plants. After NtCOI1 gene silencing, AHK-induced systemic resistance to TSWV was lost. Our results indicated that AHK activates the JA pathway and induces systemic resistance to inhibit the expression of TSWV genes to inhibit infection of the plant by TSWV.
Materials and Methods
Plant material preparation
An entire Wedelia trilobata plant was collected in Xishuangbanna, Yunnan Province, China, on August, 2012. The specimen was identified by Yu Chen of Kunming Institute of Botany (KIB), Chinese Academy of Sciences (CAS). A voucher specimen (H20121005) has been deposited in the State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany.
Materials
The TSWV YN-Chili isolate was maintained and cultured in Nicotiana tabacum cv.K326. N. tabacum cv. K326 and N. benthamiana seeds were provided by theYunnan Academy of Tobacco Agricultural Science. Ningnanmycin was purchased from Heilongjiang DeQiang Biology Co., Ltd. The molecular structures of the pTV00 vector and the compounds are shown in Table S1.
Compound separation and preparation
Dried powder of the whole Wedelia trilobata plant (22 kg) was extracted with MeOH (three times under reflux for 4, 4 and 3 h). Subsequently, the solvent was removed under reduced pressure to give a residue (2500 g, 11.4%), which was suspended with water and then successively extracted with petroleum ether, chloroform, and EtOAc. The extracts were evaporated under vacuum to produce the corresponding extracts of petroleum ether (903 g), chloroform (179 g), and EtOAc (70 g).
The petroleum ether extract was separated into 13 fractions(A-M) by silica gel column chromatography (100–200 mesh, 15 × 120 cm, 3000 g) and eluted with petroleum ether/EtOAc (v/v = 10:0, 9:1, 8:2, 7:3, 6:4, 5:5, and 0:10, 40 L each).
Fraction G (48 g) was separated on a silica gel G column(200–300 mesh, 10 × 100 cm, 1.65 kg) and eluted with petroleum ether/acetone (v/v = 19:1, 9:1,8:2, 7:3, and 6:4, 20 L each) to produce five fractions (1–5). Fraction 2 (7.18 g) was subjected to RP-18 silica gel column chromatography (20–45 µm, 30 × 460 mm, 150 g) and eluted with MeOH-H2O (v/v = 3:7, 5:5, 7:3, 8:2, and 9:1, 10 L each) and Sephadex LH-20 (CHCl3- MeOH, 1:1, 1.8 × 120 cm) to produce the compound AHK (20.4 mg) and other compounds.
Curative assay in vivo
Twenty-eight diterpenoid compounds were extracted from W. trilobata and tested using the lesion counting method. The curative effect treatments were initiated with the inoculation of TSWV (100 μg/mL) onto whole N. tabacum cv. K326 leaves using cotton swabs. After 24 h, the diterpenoid compound solutions were spread onto the same area of the leaf that had been previously inoculated with TSWV. Two positive controls were used and involved the inoculation of tobacco leaves with TSWV (100 μg/mL) followed by the application of a ningnanmycin solution (80 µg/mL) or DMSO to the same leaf after 24 h, while leaves treated with DMSO alone were used as a negative control. The number of local lesions was recorded 3–4 days after inoculation, and three replicates were conducted for each sample. The compounds were dissolved in DMSO at a final concentration of 10 mg/mL and were diluted to 80, 100, 150 and 200 µg/mL with ddH2O.
The TSWV inhibition rates of the compounds were calculated according to the formula: inhibition rate (%) = [(T−C)/T] × 100%, where T is the average number of local lesions for the positive control and C is the average number of local lesions for the treatment.
Protective assay in vivo
Twenty-eight diterpenoid compounds were tested using the lesion counting method. To examine protective effects of the compounds, solutions of the compounds were applied to whole N. tabacum cv. K326 leaves using cotton swabs. After 6 h, TSWV (100 μg/mL) was inoculated onto one leaf that had a compound applied previously. Two positive controls were included and involved the application of a ningnanmycin solution or DMSO to a leaf followed by inoculation with TSWV (100 μg/mL) onto the same leaf after 6 h, while leaves treated with DMSO alone were used as a negative control. The number of local lesions was recorded 3–4 days after inoculation, and three replicates were conducted for each sample.
Quantitative RT-PCR
qRT-PCR was conducted to determine the number of copies of transcribed TSWV genes. Total RNA was extracted from tobacco leaves (0.2 g, fresh weight) using TriPure Isolation Reagent (Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer’s instructions. Five replicates were performed for each sample. First-strand cDNA was synthesized using a One-Step gDNA Removal kit (Transgen, Beijing, China) followed by qRT-PCR using FastStart Universal SYBR Green Master (Rox) (Trans, Beijing, China) on an Applied Biosystems Stepone Plus instrument (Applied Biosystems, Foster City, CA, USA). The primer sequences used in this study are listed in Table S2.
A standard curve was established using plasmid DNA containing the corresponding gene sequence. The plasmid DNA was diluted for the standard samples with a dilution series (10−2, 10−3, 10−4, 10−5 and 10−6).
Western blotting
The levels of the TSWV proteins N and NSm were analyzed by western blotting. Total protein was extracted from tobacco leaves (0.2 g, fresh weight) using TriPure Isolation Reagent (Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer’s instructions. Equal sample volumes (20 μL) were loaded on a 12.5% polyacrylamide gel, and proteins were separated by electrophoresis at 100 V for 90 min. After being transferred to a PVDF membrane, the N and NSm proteins were detected using a primary antibody (1: 4,000) and were subsequently probed with AP-coupled goat anti-rabbit IgG (1:8,000; Sigma, Santa clara, USA). The signals on the membrane were visualized using a ready-for-use 5-bromo-4-chloro-3-indolylphosphate/nitroblue tetrazolium (BCIP/NBT) solution (Sangon Biotech, Shanghai, China).
Phytohormone measurements
Phytohormones were extracted and quantified by LC-MS/MS as described previously45. Samples (approximately 150 mg) were ground to a fine powder in liquid nitrogen and briefly crushed, then 1 mL of ethyl acetate spiked with labeled internal standards (100 ng each of 13C2-JA, 13C6-JA-Ile, D4-SA, and D6-ABA) was added to each sample. After centrifugation at 13 000 g for 10 min at 4 °C, the supernatants were transferred to fresh tubes and evaporated to dryness using a vacuum concentrator (Concentrator plus, Eppendorf, Germany). Each residue was resuspended in 0.5 mL 70% (v/v) methanol and centrifuged (15 min, 13 000 g, 4 °C) to remove particles. The supernatants were analyzed on an HPLC-tandem mass spectrometry (1200 L LC-MS system, Varian, American). Five replicate leaf samples were used for each treatment.
VIGS assays
A 217-bp fragment of the COI1 cDNA sequence was amplified and cloned into pTV0046, and Agrobacterium tumefaciens (strain GV3101) carrying this construct was injected into N. benthamiana, generating COI1-silenced plants (VIGS COI1). To monitor the progress of VIGS, phytoene desaturase (PDS) was silenced, which eventually results in the visible bleaching of green tissues45,47 three weeks after injection. When the leaves of the PDS-silenced plants began to bleach and bolt, the youngest leaves of the VIGS COI1 and empty vector-inoculated plants were selected for curative and protective experiments. The samples used for qRT-PCR were collected after an additional 3 d. Five biological replicates per genotype were used for the experiments.
Supplementary information
Acknowledgements
This project was partially supported by grants from Yunnan Provincial Top Scientists Input Program (2013HA028), National Natural Science Foundation of China (No. 31470427, 31371919), Yunnan Provincial Natural Science Foundation (2017FA019, 2012CH007), Yunling Scholar Program (2015 Zhangzhongkai).
Author Contributions
J.D. and Z.Z. designed the study. L.Z. and Z.H. carried out the experiments. L.Z. and X.S. analyzed the data. L.Z. and J.D. prepared the manuscript. S.L., X.Z., J. L. and L.Z. participated in the discussion.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhongkai Zhang, Email: zhongkai99@sina.com.
Jiahong Dong, Email: dongjhn@126.com.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-019-39247-6.
References
- 1.Adams MJ, et al. Changes to taxonomy and the international code of virus classification and nomenclature ratified by the International Committee on Taxonomy of Viruses. Arch Virol. 2017;162:2505–2538. doi: 10.1007/s00705-017-3358-5. [DOI] [PubMed] [Google Scholar]
- 2.Parrella G, Gognalons P, Gebre-Selassiè K, Vovlas C, Marchous G. An update of the host range of Tomato spotted wilt virus. Journal of Plant Pathology. 2003;85:227–264. [Google Scholar]
- 3.Rybicki EP. A top ten list for economically important plant viruses. Archives Virology. 2015;160:17–20. doi: 10.1007/s00705-014-2295-9. [DOI] [PubMed] [Google Scholar]
- 4.Pappu HR, Jones RA, Jain RK. Global status of Tospovirus epidemics in diverse cropping systems: successes achieved and challenges ahead. Virus Research. 2009;141:219–236. doi: 10.1016/j.virusres.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Faoro F, Gozzo F. Is modulating virus virulence by induced systemic resistance realistic? Plant Science. 2015;234:1–13. doi: 10.1016/j.plantsci.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Oostendorp M, Kunz W, Dietrich B, Staub T. Induced disease resistance in plants by chemicals. European Journal of Plant Pathology. 2001;107:19–28. doi: 10.1023/A:1008760518772. [DOI] [Google Scholar]
- 7.Friedrich L, et al. A benzothiadiazole derivative induces systemic acquired resistance in tobacco. Plant Journal. 1996;10:61–70. doi: 10.1046/j.1365-313X.1996.10010061.x. [DOI] [PubMed] [Google Scholar]
- 8.Li YM, et al. 3-acetonyl-3-hydroxyoxindol: a new inducer of systemic acquired resistance in plants. Plant Biotechnology Journal. 2008;6:301–308. doi: 10.1111/j.1467-7652.2008.00322.x. [DOI] [PubMed] [Google Scholar]
- 9.Jia XC, Qing SM, Zeng H, Wang W, Yin H. Chitosan oligosaccharide induces resistance to Tobacco mosaic virus in Arabidopsis via the salicylic acid-mediated signalling pathway. Scientific Reports. 2016;10:1038–1050. doi: 10.1038/srep26144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen YD, et al. Integrating transcriptome and microRNA analysis identifies genes and microRNAs for AHO-induced systemic acquired resistance in N. tabacum. Scientific Report. 2017;7:12504. doi: 10.1038/s41598-017-12249-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang CM, Fan YJ. Eugenol enhances the resistance of tomato against Tomato yellow leaf curl virus. Journal of Science and Food Agricutural. 2013;94:677–682. doi: 10.1002/jsfa.6304. [DOI] [PubMed] [Google Scholar]
- 12.Sun WJ, et al. Eugenol confers resistance to tomato yellow leaf curl virus (TYLCV) by regulating the expression of SlPer1 in tomato plants. New Biotechnology. 2016;33:345–354. doi: 10.1016/j.nbt.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Zhu F, et al. Salicylic acid and jasmonic acid are essential for systemic resistance against Tobacco mosaic virus in Nicotiana benthamiana. Molecular Plant Microbe. 2014;27:567–577. doi: 10.1094/MPMI-11-13-0349-R. [DOI] [PubMed] [Google Scholar]
- 14.Alazem M, Lin NS. Roles of plant hormones in the regulation of host-virus interactions. Molecular Plant Pathology. 2015;16:529–540. doi: 10.1111/mpp.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patkar RN, Naqvi N. Fungal manipulation of hormone-regulated plant defense. Plospathogen. 2017;13:e1006334. doi: 10.1371/journal.ppat.1006334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Usda, G. Sphagneticola trilobata (L.) Pruski. GRIN taxonmy for plants U.S. department of agriculture germplasm resources, www. arsfrin.gov/cgi-bin/npgs/html/taxon.pl (2008).
- 17.Balekar N, Katkam NG, Nakpheng T, Jehtae K, Srichana T. Evaluation of the wound healing potential of Wedelia trilobata (L.) leaves. Journal of Ethnopharmacology. 2012;141:817–824. doi: 10.1016/j.jep.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 18.Nie CR, et al. Allelopathic potentials of Wedelia trilobata L. on rice. Acta AgronomicaSinica. 2004;9:942–946. [Google Scholar]
- 19.Li YT, et al. Eudesmanolides from Wedelia trilobata (L.) Hitchc. as potential inducers of plant systemic acquired resistance. Journal of Agricultural and Food Chemistry. 2013;61:3884–3890. doi: 10.1021/jf400390e. [DOI] [PubMed] [Google Scholar]
- 20.Junhirun P, Pluempanupat W, Bullangpoti V. Toxicological study of Wedelia trilobata (Asteraceae) extracts as alternative control strategies for Plutellaxylostella(Lepidoptera: Plutellidae) Communications in Agriculture and Applied Biology Science. 2012;77:721–725. [PubMed] [Google Scholar]
- 21.Chethan J, et al. Evaluation of antioxidant and antibacterial activities of methanolic flower extract of Wedelia trilobata (L.) Hitch. Frican. Journal of Biotechnology. 2012;11:9829–9834. [Google Scholar]
- 22.Li SF, Ding JY, Li YT, Hao XJ, Li SL. Antimicrobial diterpenoids of Wedelia trilobata (L.) Hitchc. Molecules. 2016;21:457. doi: 10.3390/molecules21040457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conrath U, et al. Priming: Getting Ready for Battle. Molecular Plant-Microbe Interactions. 2006;19:1062–1071. doi: 10.1094/MPMI-19-1062. [DOI] [PubMed] [Google Scholar]
- 24.Feys BF, Benedetti CE, Penfold CN, Turner JG. Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell. 1994;6:751–759. doi: 10.1105/tpc.6.5.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie DX, Feys BF, James S, Nieto-Rostro M, Turner JG. COI1: An Arabidopsis gene required for jasmonate regulated defense and fertility. Science. 1998;280:1091–1094. doi: 10.1126/science.280.5366.1091. [DOI] [PubMed] [Google Scholar]
- 26.Xu LH, et al. The SCFCOI1 Ubiquitin-Ligase complexes are required for jasmonate response in Arabidopsis. The Plant Cell. 2002;14:1919–1935. doi: 10.1105/tpc.003368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trejo-Saavedra DL, García-Neria MA, Rivera-Bustamante RFB. Enzothiadiazole(BTH) induces resistance to Pepper golden mosaic virus(PepGMV) in pepper (Capsicum annuum L.) Biology Research. 2013;46:333–40. doi: 10.4067/S0716-97602013000400004. [DOI] [PubMed] [Google Scholar]
- 28.Zhao LH, et al. Anti-TMV activity and functional mechanisms of two sesquiterpenoids isolated from Tithoniadiversifolia. Pesticide Biochemistry and Physiology. 2017;140:24–29. doi: 10.1016/j.pestbp.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Gao QM, Kachroo A, Kachroo P. Chemical inducers of systemic immunity in plants. Journal of Experiment Botany. 2014;65:1849–55. doi: 10.1093/jxb/eru010. [DOI] [PubMed] [Google Scholar]
- 30.Qin SR, et al. Effects of TMV or ningnanmycin treatment on protein composition in leaves and thylakoid membranes of tobacco. Chin Journal in Applied Environment Biology. 2014;10:158–161. [Google Scholar]
- 31.Yan XH, et al. Anti-Tobacco mosaic virus (TMV) quassinoids from Bruceajavanica (L.) merr. Journal of Agriculture and Food Chemistry. 2010;58:1572–1577. doi: 10.1021/jf903434h. [DOI] [PubMed] [Google Scholar]
- 32.Oliveira VC, Bartasson L, Castro ME, Ribeiro BM, Resende RO. A silencing suppressor protein (NSs) of a Tospovirusenhances baculovirus replication in permissive and semipermissive insect cell lines. Virus Research. 2011;155:259–67. doi: 10.1016/j.virusres.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 33.Margaria P, et al. The NSs protein of Tomato spotted wilt virus is required for persistent infection and transmission by Frankliniellaoccidentalis. Journal of Virology. 2014;88:5788–5802. doi: 10.1128/JVI.00079-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bridgen A, Friedemann W, Fazakerley JK. Bunyamwera bunyavirus nonstructural protein NSs is a nonessential gene product that contributes to viral pathogenesis. Proceedings of the National Academy of Sciences. 2001;98:664–669. doi: 10.1073/pnas.98.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cortez I, Aires A, Pereira AM, Kormelink R. Genetic organization of Iris yellow spot virus mRNA: indications for functional homology between the Gc glycoproteins of Tospoviruses and animal-infecting bunyaviruses. Archives of Virology. 2002;147:2313–2325. doi: 10.1007/s00705-002-0885-4. [DOI] [PubMed] [Google Scholar]
- 36.Leastro MO, Pallásb V, Resendea RO, Sánchez-Navarro JA. The functional analysis of distinct Tospovirus movement proteins (NSm) reveals different capabilities in tubule formation, cell-to-cell and systemic virus movement among the tospovirus species. Virus Research. 2017;227:57–68. doi: 10.1016/j.virusres.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 37.De Haan P, et al. Tomato spotted wilt virusLRNA encodes a putative RNA polymerase. Journal of General Virology. 1991;72:2207–2216. doi: 10.1099/0022-1317-72-9-2207. [DOI] [PubMed] [Google Scholar]
- 38.Van Knippenberg I, Goldbach R, Kormelink R. Purified Tomato spotted wilt virus particles support both genome replication and transcription in vitro. Virology. 2002;303:278–286. doi: 10.1006/viro.2002.1632. [DOI] [PubMed] [Google Scholar]
- 39.Schuhegger R, et al. Induction of systemic resistance in tomato by N-acyl-Lhomoserine lactone-producing rhizosphere bacteria. Plant Cell Environment. 2006;29:909–918. doi: 10.1111/j.1365-3040.2005.01471.x. [DOI] [PubMed] [Google Scholar]
- 40.Srivastava S, Verma HN, Srivastava A, Prasad V. BDP-30, a systemic resistance inducer from Boerhaaviadiffusa L., suppresses TMV infection, and displays homology with ribosome-inactivating proteins. Journal of Biology Science. 2015;40:125–35. doi: 10.1007/s12038-014-9494-0. [DOI] [PubMed] [Google Scholar]
- 41.Wang QY, et al. Schisanhenol derivatives and their biological evaluation against Tobacco mosaic virus (TMV) Fitoterapia. 2015;101:117–124. doi: 10.1016/j.fitote.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 42.Wu LM, Wu HJ, Chen L, Zhang H, Gao X. Induction of systemic disease resistance in Nicotiana benthamiana by the cyclo dipeptides cyclo (L-Pro-L-Pro) and cyclo (D-Pro-D-Pro) Molecular Plant Pathology. 2017;18:67–74. doi: 10.1111/mpp.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chizuru S, Yoshiya S, Kensuke N, Matsuura H. Kinetics of the accumulation of jasmonic acid and its derivatives in systemic leaves of tobacco (Nicotiana tabacumcv. Xanthi nc) and translocation of deuterium-labeled jasmonic acid from the wounding site to the systemic site.Bioscience. Biotechnology, and Biochemistry. 2014;73:1962–1970. doi: 10.1271/bbb.90119. [DOI] [PubMed] [Google Scholar]
- 44.Hiroshi A, et al. Antagonistic Plant defense system regulated by phytohormones assists interactions among vector insect, thrips and a Tospovirus. Plant Cell Physiol. 2012;53:204–212. doi: 10.1093/pcp/pcr173. [DOI] [PubMed] [Google Scholar]
- 45.Wu J, Wang L, Baldwin IT. Methyl jasmonate-elicited herbivore resistance: does MeJA function as a signal without being hydrolyzed to JA? Planta. 2008;227:1161–1168. doi: 10.1007/s00425-008-0690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ratcliff F, Martin Hernandez AM, Baulcombe DC. Technical advance. Tobacco rattle virus as a vector for analysis of gene function by silencing. Plant Journal. 2001;25:237–245. doi: 10.1046/j.0960-7412.2000.00942.x. [DOI] [PubMed] [Google Scholar]
- 47.Saedler R, Baldwin IT. Virus-induced gene silencing of jasmonate-induced direct defenses, nicotine and trypsin proteinase-inhibitors in Nicotiana attenuata. Journal of Experiment Botany. 2004;2855:151–157. doi: 10.1093/jxb/erh004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.