Blood-brain barrier (BBB) pericytes regulate several vital functions of the neurovascular unit. Bertrand et al. argue that BBB pericytes are a previously unrecognized HIV target and reservoir, with HIV infection of BBB pericytes confirmed in cell cultures, a mouse model of HIV infection, and in human HIV-infected brains.
Keywords: blood–brain barrier pericytes, HIV, HIV reservoirs, neuroinfection, HIV-associated neurocognitive disorder
Abstract
Pericytes are multifunctional cells wrapped around endothelial cells via cytoplasmic processes that extend along the abluminal surface of the endothelium. The interactions between endothelial cells and pericytes of the blood–brain barrier are necessary for proper formation, development, stabilization, and maintenance of the blood–brain barrier. Blood–brain barrier pericytes regulate paracellular flow between cells, transendothelial fluid transport, maintain optimal chemical composition of the surrounding microenvironment, and protect endothelial cells from potential harmful substances. Thus, dysfunction or loss of blood–brain barrier pericytes is an important factor in the pathogenesis of several diseases that are associated with microvascular instability. Importantly, recent research indicates that blood–brain barrier pericytes can be a target of HIV-1 infection able to support productive HIV-1 replication. In addition, blood–brain barrier pericytes are prone to establish a latent infection, which can be reactivated by a mixture of histone deacetylase inhibitors in combination with TNF. HIV-1 infection of blood–brain barrier pericytes has been confirmed in a mouse model of HIV-1 infection and in human post-mortem samples of HIV-1-infected brains. Overall, recent evidence indicates that blood–brain barrier pericytes can be a previously unrecognized HIV-1 target and reservoir in the brain.
Introduction
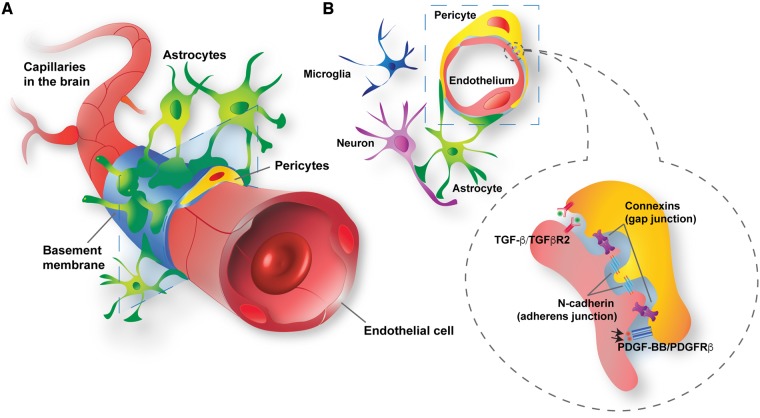
Pericytes are multifunctional cells wrapped around endothelial cells via cytoplasmic processes that extend along the abluminal surface of the endothelium. Most of the pericyte-endothelial interface is separated by the basement membrane (Armulik et al., 2011), which is actively remodelled by both pericytes and endothelial cells during angiogenesis (Kloc et al., 2015), development (Armulik et al., 2011), and tumorigenesis (Baluk et al., 2003). However, selected pericyte populations are also present with incomplete or absent basement membranes and in direct contact with endothelial cells (Armulik et al., 2011). Pericytes can transiently rearrange their contacts with endothelial cells via the peg-socket type of arrangements, and/or modulation of adherent junctions or gap junctions (Winkler et al., 2011) (Fig. 1).
Figure 1.
Structural and molecular blood–brain barrier pericyte connections within the neurovascular unit. (A) Blood–brain barrier pericytes (yellow) and endothelial cells (red) share the basement membrane (sky blue) or are in direct contacts. In addition, blood–brain barrier pericytes are surrounded by glial cells (astrocytes, green; microglia, dark blue) and neurons (purple). (B) Blood–brain barrier pericytes and endothelial cells communicate with each other by direct contact (gap and adherens junctions) or through signalling pathways, such as platelet-derived growth factor BB (PDGF-BB)/PDGFRβ and transforming growth factor-β (TGF-β)/type 2 TGF-β receptor (TGFβR2).
Pericytes display contractile, cytoskeletal, and surface proteins; however, no single or specific pericyte marker is currently available. Identification of pericytes is frequently achieved by determination of more than one marker (Fig. 2). Platelet-derived growth factor receptor-β (PDGFRβ) (Armulik et al., 2010), neuron-glial antigen 2 (NG2) (Trost et al., 2013), CD13 (Armulik et al., 2010), alpha-smooth muscle actin (αSMA) (Trost et al., 2016), regulator of G protein signaling 5 (RGS5) (Mitchell et al., 2008), and desmin (Bergers and Song, 2005) have been suggested to be useful for pericyte characterization based on their dynamics of expression related to developmental status and pathological conditions. For example, a contractile protein αSMA is differentially expressed in pericytes depending on the type of blood vessel.
Figure 2.
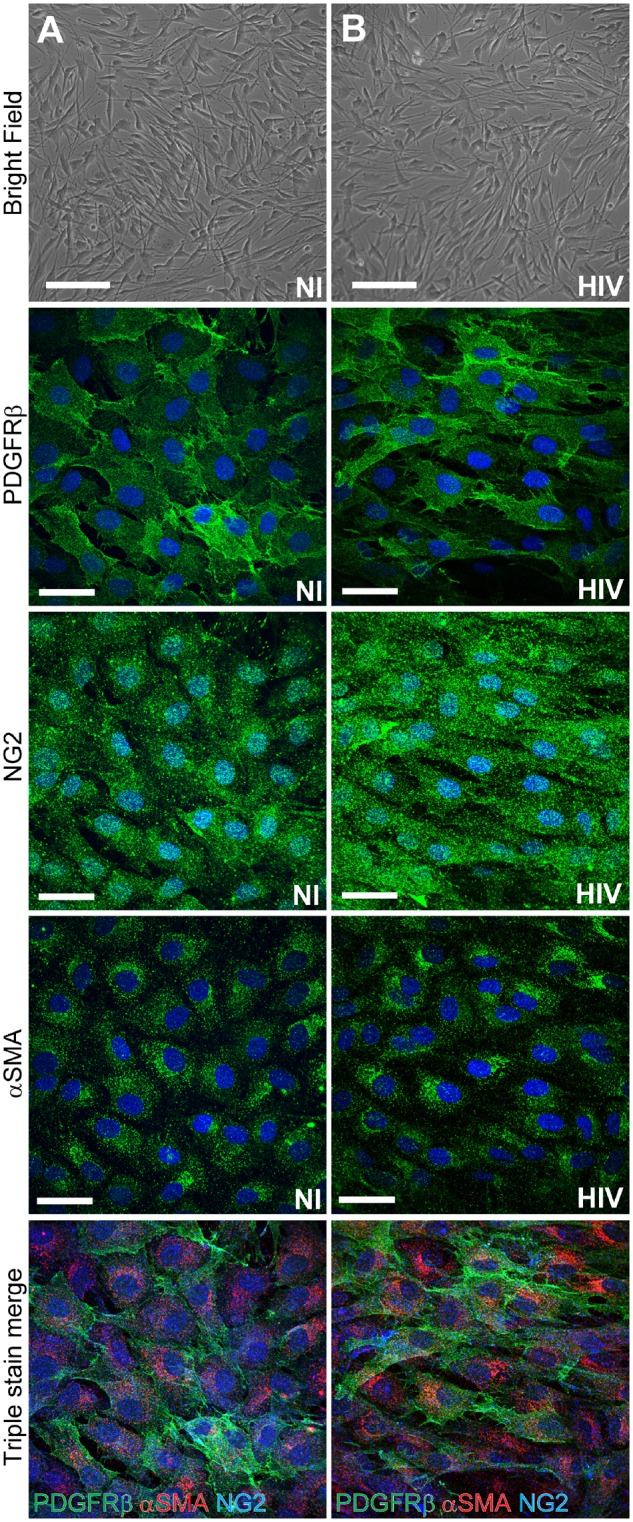
Blood–brain barrier pericyte markers. Standardized characterization of non-infected (A) and HIV-1-infected (B) pericytes. Infections were performed by incubating pericytes with HIV-1 (NL4-3 strain) in the amount of 60 ng p24/ml for 48 h. Top: Phase contrast imaging; middle three panels represent immunofluorescence staining for the three distinct pericyte markers; namely, PDGFRβ, NG2, and αSMA (all in green). Bottom: Merged images of triple stained pericytes for these markers (PDGFRβ, green; NG2, blue; and αSMA, red). Nuclei were stained with Hoechst (blue). Scale bars = 200 µm (brightfield) and 50 µm (PDGFRβ, NG2, and αSMA).
Given that αSMA, NG2, PDGFRβ, and desmin are also expressed by vascular smooth muscle cells (SMCs), a collective approach of well preserved tissue morphology, counter-labelling of endothelial cells, and expression of two or more pericyte markers has been advocated for pericyte identification in vivo (Trost et al., 2013; Yamamoto et al., 2017). Nevertheless, the precise structural and molecular identity of pericytes is still controversial. It was recently proposed that capillary pericytes are strictly defined and separated from other cell types by the lack of αSMA expression (Hill et al., 2015). However, the majority of literature reports acknowledge that pericytes exhibit heterogeneity with distinct morphologies, expression level of αSMA, and functions depending on their positioning within the vasculature (Hall et al., 2014; Attwell et al., 2016).
Although the origins of pericytes across all tissues are still not fully understood, peripheral pericytes, including those of the gut, lung, and liver, mostly develop from the mesoderm during embryogenesis via epithelial-to-mesenchymal transition, delamination, and migration (Wilm et al., 2005; Que et al., 2008; Asahina et al., 2011). Similarly, the epicardial mesothelium is thought to give rise to cardiac pericytes (Cai et al., 2008). While pericytes in the spinal cord and brainstem have developed from the mesoderm, the majority of blood–brain barrier pericytes in the CNS are more likely to be derived from the neural crest cells of the neuroectoderm (Korn et al., 2002; Simon et al., 2012). Moreover, a subset of cerebrovascular pericytes was shown to originate from mature macrophages in the early phase of CNS vascular development (Yamamoto et al., 2017).
While pericytes cover ~30% of the abluminal surface of peripheral endothelial cells (Mathiisen et al., 2010), this coverage is closer to 90% in the CNS (Sá-Pereira et al., 2012) and appears to be inversely correlated with endothelial cell proliferation (Díaz-Flores et al., 2009). By covering endothelial cells, pericytes play a critical role in regulation of capillary blood flow (Hamilton et al., 2010). Indeed, pericyte-mediated capillary contraction and dilation has been observed both in vivo and ex vivo (Attwell et al., 2010; Itoh and Suzuki, 2012). Recent evidence also suggests that interactions between endothelial cells and blood–brain barrier pericytes are necessary for the proper formation, development, and maintenance of the blood–brain barrier. Blood–brain barrier pericytes were shown to regulate paracellular flow between cells and transendothelial fluid transport, maintain optimal chemical composition of the surrounding microenvironment, and protect endothelial cells from potential harmful substances (Winkler et al., 2011). The intricate cross-talk and functional coupling between pericytes and endothelial cells have been implicated in vessel stabilization and development via several signal transduction cascades (Sweeney et al., 2016), including platelet-derived growth factor-BB (PDGFBB) (Winkler et al., 2010), transforming growth factor-β, (TGF-β) (Walshe et al., 2009), Notch (Hofmann and Iruela-Arispe, 2007), sphingosine-1 phosphate (Paik et al., 2004) and angiopoietin (Gaengel et al., 2009) signalling. Multiple ligand-receptor complexes mediate pericyte recruitment to the endothelium, such as PDGF-BB/PDGFβ (Tallquist et al., 2003), heparin-binding epidermal growth factor (HB-EGF)/EGF receptors (Stratman et al., 2010), and stromal-derived factor-1α, (SDF-1α)/CXCR4 (Song et al., 2009). Moreover, blood–brain barrier pericytes control the integrity of the blood–brain barrier by regulating endothelial tight and adherent junctions (Bell et al., 2010; Daneman et al., 2010).
Pericytes were demonstrated to actively participate in immune responses. Insult by inflammatory stimuli causes pericytes to release inflammatory mediators, including IL-1 β, IL-6, CCL2, and matric metalloproteinases (MMP2/9) (Nakagawa et al., 2012; Guijarro-Muñoz et al., 2014). Blood–brain barrier pericytes also control leucocyte adhesion and transmigration in the CNS (Olson and Soriano, 2011) and can perform macrophagic-like activities to clear tissue debris and cellular by-products in the CNS (Bergers and Song, 2005; Bell et al., 2010). Furthermore, pericytes were proposed to exhibit multipotent stem cell potential, capable of forming neurons, glia and vascular lineage cells (Nakagomi et al., 2015).
Dysfunction or loss of blood–brain barrier pericytes leads to blood–brain barrier breakdown (Bell et al., 2010; Winkler et al., 2012), contributing to the pathogenesis of several diseases that are associated with microvascular instability and blood vessel ruptures (Armulik et al., 2011). Dysfunctions of signalling pathways, such as PDGF-BB/PDGFRβ, TGF-β/TGFβR2, Notch, and Ang-1/Tie-2, within pericytes or between pericytes and neighbouring cells were shown to be involved in CNS diseases, such as stroke (Li et al., 2011; Arnold et al., 2014), Alzheimer’s disease (Sagare et al., 2013), amyotrophic lateral sclerosis (Winkler et al., 2013), and brain tumours (Bergers et al., 2003; Zhang et al., 2003).
Infection of blood–brain barrier pericytes by HIV-1
Compelling evidence demonstrates that HIV-1 infection of cells of the CNS, resulting in production of toxic viral proteins, is a critical factor in the development of HIV-associated neurocognitive disorder (HAND). The majority of HIV-1 replication in the brain occurs in microglial cells and perivascular macrophages (Kim et al., 2005; Joseph et al., 2015; Chen et al., 2017). It has also been established that astrocytes can be productively infected and play a role as a HIV-1 reservoir, given their longevity and ability to support HIV-1 reactivation (Li et al., 2016). In addition, our group demonstrated for the first time that blood–brain barrier pericytes can be productively infected by HIV-1 (Nakagawa et al., 2012). These findings were confirmed in subsequent studies by us and others (Persidsky et al., 2016; Cho et al., 2017).
HIV-1 entry in blood–brain barrier pericytes
Canonical HIV-1 virus entry uses both the main HIV-1 receptor, CD4, and the co-receptors, primarily CXCR4 and CCR5. Pericytes express high levels of HIV-1 co-receptors, CCR5 and CXCR4, and also CD4, albeit at a low level (Nakagawa et al., 2012). Consistent with high expression of CCR5 and CXCR4, blood–brain barrier pericytes can be infected by both X4 and R5 tropic HIV-1 strains. The importance of these co-receptors in the viral entry to blood–brain barrier pericytes was demonstrated by the successful inhibition of infection with maraviroc, a clinically approved inhibitor of CCR5, and AMD3100, a specific inhibitor of CXCR4 (Nakagawa et al., 2012). The exact mechanism of entry for HIV-1 to blood–brain barrier pericytes remains to be elucidated; however, data from myeloid cells show that even with low CD4 levels, receptor mediated fusion can occur (Joseph et al., 2014). In addition, astrocytes, which do not express CD4, can still be infected by HIV-1 (Liu et al., 2004; Chauhan et al., 2014; Li et al., 2015). Given that in vitro infection of blood–brain barrier pericytes with HIV-1 results in a relatively low number of infected cells, it is highly probable that infection could be enhanced by cell-to-cell transmission, as it was demonstrated for astrocytes (Li et al., 2015). This is especially relevant given the high degree of cell-cell contact present within the neurovascular unit. In addition, the extensive coverage of the brain perivascular environment by blood–brain barrier pericytes increases the possibility of direct viral contact or of cell-to-cell transmission with transmigrating peripheral infected cells, such as T cells and/or macrophages (Armulik et al., 2010; Daneman et al., 2010).
A controversial issue is transmission of HIV-1 to blood–brain barrier pericytes from brain endothelial cells. While endothelial cells can be infected with HIV-1 in vitro (Moses et al., 1993), no productive infection of these cells was demonstrated in vivo. However, blood–brain barrier pericytes, when co-cultured with brain endothelial cells, enhance the lipopolysaccharide-mediated transendothelial transfer of HIV-1 free virus. This effect occurs without altering transendothelial electrical resistance, suggesting that pericytes affect the transcytotic component of HIV-1 permeation (Dohgu and Banks, 2013).
Productive HIV-1 infection in blood–brain barrier pericytes
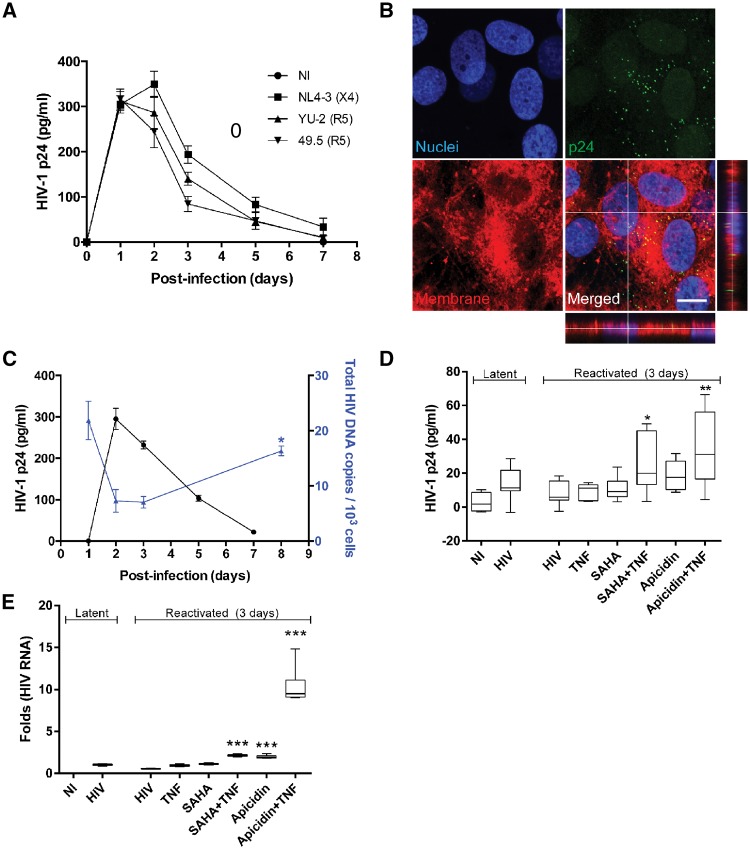
While virus entry is an essential step in infection, it does not necessarily signify that cells can be productively infected. Indeed, several additional factors come into play to enable successful HIV-1 integration and viral replication. By measuring extracellular p24 levels produced by infected cells and reverse transcriptase (RT) activity, we demonstrated that blood–brain barrier pericytes support HIV-1 replication, leading to a peak of extracellular virus production 2–3 days post-infection, followed by a gradual decline to the baseline 7–10 days post-infection (Castro et al., 2016; Cho et al., 2017). Further demonstration of competent replication was obtained using UV-inactivated virus (Cho et al., 2017). Blood–brain barrier pericytes are resistant to HIV-1-induced cytopathic effects, continue to proliferate, and do not display any apparent changes in morphology after infection. A typical experiment demonstrating HIV-1 infection of cultured human pericytes is shown in Fig. 3A and B.
Figure 3.
HIV-1 replication and reactivation in human primary blood–brain barrier pericytes. (A) Quantification of p24 release into culture media at the indicated days post-infection with different HIV-1 strains, namely NL4-3, YU-2, or 49.5, in the amount of 60 ng p24/ml. The tropism (X4 or R5) of these strains is indicated on the graph. Day 0 indicates the start of infection. Data represent mean ± standard error of the mean (SEM) from three independent experiments, (3 × 105 pericytes per replicate, total n = 9 per group). No p24 levels were detected in the non-infected (NI) group. (B) Representative images of p24 immunoreactivity at Day 2 post-infection with HIV-1 NL4-3 (60 ng p24/ml; orthogonal view in the merged image of HIV-1 NL4-3 group). No p24 levels were detected in NI group. Nuclei (blue, Hoechst staining), p24 (green, HIV-1 marker) and membranes (red, DiI staining). Scale bar = 10 µm. (C) Blood–brain barrier pericytes were infected with HIV-1 NL4-3 as in Fig. 3A (3 × 105, 60 ng p24/ml), washed and incubated for 7 days. HIV-1 p24 release from HIV-1-infected pericytes and HIV-1 DNA integration into their genome as quantified by droplet digital PCR (ddPCR). Note that a decrease in active production of p24 is associated with elevated integration of the HIV-1 genome into the host genome. *P < 0.05 versus Day 3 post-infection. (D and E) On Day 8 post-infection, 3 × 105 pericytes were exposed to the indicated factors for 3 days and then assayed for either (D) HIV-1 p24 by ELISA or (E) HIV-1 RNA using RT-qPCR. Results are reflected by minimum and maximum box and whisker plots. The HIV-1 reactivation factors were used at the following concentrations: TNF, 100 U/ml; SAHA, 10 µM; apicidin, 1 µg/ml. *P < 0.05 versus HIV-1; **P < 0.01 versus HIV-1; ***P < 0.001 versus HIV-1. A–C were adapted from Cho et al. (2017).
The initial peak of HIV-1 virus production followed by a gradual decline in p24 production, and an increase in integrated HIV-1 genome (Fig. 3C) suggest a potential for the establishment of a latent infection. To confirm these findings, we performed HIV-1 reactivation studies using histone modifiers. Specifically, HIV-1-infected blood–brain barrier pericytes in the latent stage were exposed to mixtures of histone deacetylase (HDAC) inhibitors vorinostat (suberoylanilide hydroxamic acid, SAHA) and apicidin, as well as tumour necrosis factor (TNF) for 3 days. Treatments using HDAC inhibitors in combination with TNF resulted in a significant increase in p24 production and HIV-1 RNA (Fig. 3D and E, respectively). Overall, the results from in vitro studies indicate that pericytes can be a target for a productive HIV-1 infection, which can thereafter enter a latent phase and be reactivated, acting as a potential reservoir.
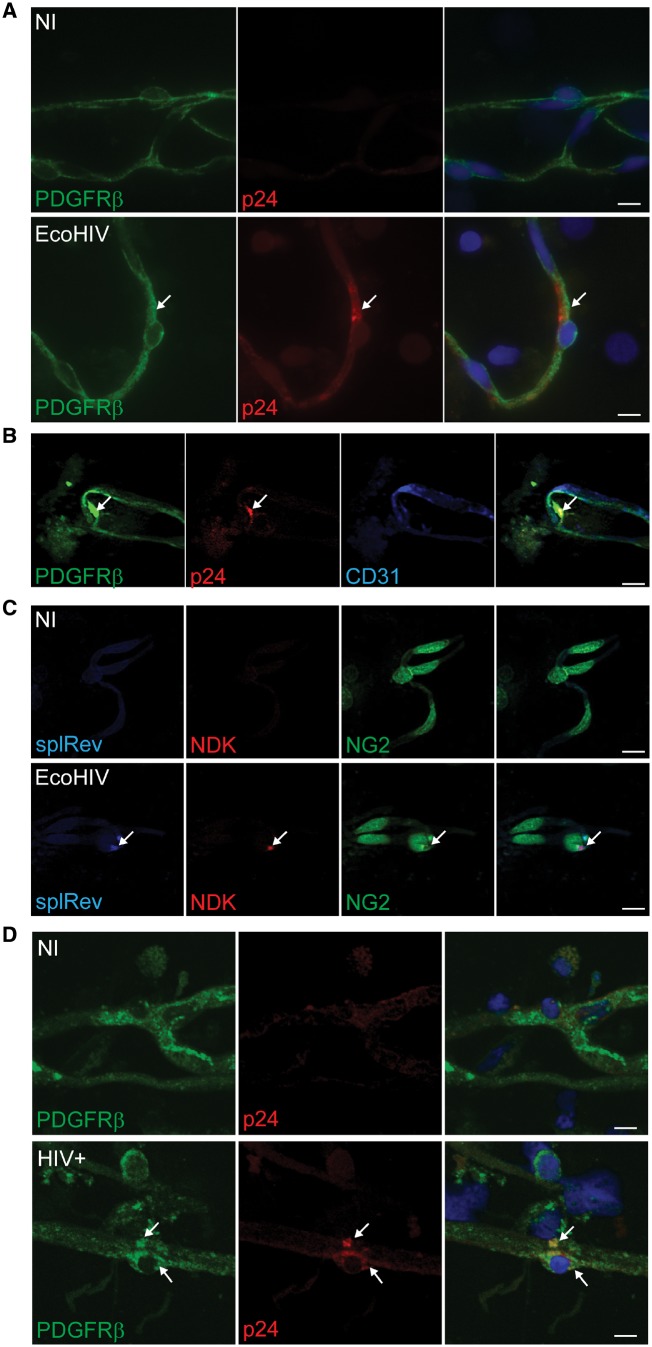
Initial in vivo demonstration of HIV-1 infection in blood–brain barrier pericytes was obtained in mice infected with a chimeric HIV-1 strain called EcoHIV-1, which was derived by replacing gp120 with gp80 of murine leukaemia virus (Potash et al., 2005). This change altered the species specificity; thus, EcoHIV-1 infects mice, but not humans. Brain infections with EcoHIV-1 exhibit neuroinvasion, microglial activation, astrocytosis, and increased expression of genes coding for inflammatory cytokines and chemokines typical of human HIV-1 brain disease. We standardized the EcoHIV-1 brain inoculation technique by infusion of 1 µg EcoHIV-1 p24 into the internal carotid artery (Bertrand et al., 2016a). This approach mimics blood-borne HIV-1 trafficking into the brain in peak viraemia (Stephens et al., 2003; Wright et al., 2015). Mice were sacrificed 1 week post-infection, and brain microvessels were isolated and stained for NG2 (marker of pericytes) and p24 (marker of active HIV-1 replication). Pericytes positive for HIV-1 were detected on isolated brain microvessels derived from infected animals (Cho et al., 2017), as evidenced by the co-localization of the pericyte markers PDGFRβ and HIV-1 protein p24, but not endothelial marker CD31 (Fig. 4A and B). Importantly, pericytes positive for both HIV-1 gag and spliced Rev mRNA were observed using in situ PCR (Fig. 4C), indicating active transcription in vivo.
Figure 4.
Infection of blood–brain barrier pericytes by HIV in vivo. (A) Male C57BL/6 mice were infected with EcoHIV-1/NDK (1 µg of p24) via infusion through the internal carotid artery, while control animals (non-infected, NI) received saline. Mice were sacrificed at Day 7 post-infection. Brain microvessels were isolated and immunostained for PDGFRβ (green, arrow, marker of pericytes) and HIV-1 protein p24 (red, arrow, marker of active HIV-1 infection). Scale bars = 20 µm. (B) Triple staining of infected microvessels from (A) for PDGFRβ (green, arrow), p24 (red, arrow), and CD31 (blue, marker for microvessel endothelial cells). Arrows denote area of co-localization solely between p24 and PDGFRβ but not with CD31, indicating that blood–brain barrier pericytes but not brain endothelial cells, are infected. (C) In situ RT-PCR assay using fluorescently-labelled primers against spliced HIV-1 Rev mRNA (blue, arrow), HIV-1 gag (NDK, red, arrow), and NG2 spliced mRNA (green, pericyte marker). Focal signal indicates area of cDNA and DNA amplification. No signal for spliced Rev and NDK were observed in non-infected mice. (D) Brain samples (frontal cortex; 0.5 cm3 each) from three healthy (non-infected, NI) and three HIV-1-infected patients with HIV encephalopathy were processed to isolate microvessels. Microvessels from each brain sample were spread on around 30 slides, each slide containing ~100–150 microvessels of varying sizes and number of associated pericytes. Samples were then immunostained for PDGFRβ (green, marker of pericytes) and HIV-1 protein p24 (red, marker of active HIV-1 infection). Approximately five infected pericytes were clearly detected on each slide containing microvessels from infected brains. Arrows indicate area of co-localization of p24 and PDGFRβ. Scale bars = 10 µm. Arrow indicates area of co-localization.
To confirm these results in human samples, we examined brain samples from patients with HAND. Human brain microvessels were isolated using the same approach as for mouse brains and the microvessels were stained for the pericyte markers PDGFRβ and HIV-1 p24. Figure 4D shows the first evidence that blood–brain barrier pericytes are infected in the brains of HIV-1-positive patients. Specifically, immunofluorescence analysis of the microvessels from HIV-1 infected brains demonstrates the presence of the protein p24 associated with blood–brain barrier pericytes. Details of these analyses are provided in the legend to Fig. 4D. At least five infected pericytes were clearly detected per slide that contained on average 100–150 microvessels.
Blood–brain barrier pericytes as potential HIV-1 reservoir cells
Mounting evidence indicates that secluded HIV-1 populations and reservoirs in the CNS can perpetuate and become distinct from the periphery (Blankson et al., 2002; Strain et al., 2005). Restrictions of treatment efficacy due to the blood–brain barrier, which limits brain entry of several antiretroviral drugs, can result in their suboptimal concentrations, leading to the selection of antiretroviral-resistant mutants (Lambotte et al., 2003; Letendre et al., 2008; Bertrand et al., 2016b). Importantly, these resistant viruses can re-enter the periphery, leading to treatment failure (Hellmuth et al., 2015; Joseph et al., 2016). We propose that blood–brain barrier pericytes may play a pivotal role as CNS reservoirs. Indeed, these cells have a ubiquitous presence on the CNS vasculature (Armulik et al., 2010; Daneman et al., 2010) and they can self-renew. While the pericyte turnover rate in the neurovascular unit is not clearly defined, they may have a potentially long half-life, given the low angiogenesis plasticity in the CNS. The location of pericytes in the neurovascular unit could also facilitate the role of shuttling HIV-1 between the CNS and the periphery. As demonstrated in Fig. 3C–E, pericytes can harbour latent HIV-1, evidenced by the increase in DNA genome copies, decrease in HIV-1 p24 production and subsequent ability to reactivate. The switch from productive to latent steps and back of the viral cycle observed in pericytes suggests their potential as a new and previously unrecognized HIV-1 CNS reservoir.
Impact of HIV-1 infection on functions of blood–brain barrier pericytes
As described earlier, blood–brain barrier pericytes play a primordial role in the maintenance of the blood–brain barrier (Armulik et al., 2010). Through their contractile ability they can regulate cerebral blood flow and by paracrine signalling they can influence the tightness of the blood–brain barrier. This delicate balance can be altered by pathological conditions and its disruption can affect progression of several CNS disorders (Persidsky et al., 2016; Ferland-McCollough et al., 2017; Iadecola, 2017). Alterations of pericyte morphology and functions in HIV-1 infected patients have been observed in several recent reports. For example, it was demonstrated that patients infected with HIV-1, with or without antiretroviral treatment, have a significantly lower pericyte coverage of blood–brain barrier capillaries as shown by co-staining for CD13 with endothelial marker CD31 (Persidsky et al., 2016). The results presented in the present study (Fig. 2) indicate that infection with HIV-1 does not result in any apparent decrease in the expression of pericyte markers, suggesting that true loss of blood–brain barrier pericytes, and not merely a loss of marker expression, occurs in HIV-1 infection.
The mechanisms of HIV-1-induced loss of pericytes may be related to the exposure to viral proteins, such as Tat protein (Niu et al., 2014, 2015). Specifically, HIV-1 Tat was shown to induce PDGF-BB expression in pericytes via stimulation of the MAPK/NF-κB pathway. Secreted PDGF-BB resulted in autocrine activation of the PDGF-BB/PDGFRβ signalling, culminating into increased pericyte migration (Niu et al., 2014, 2015). PDGF-BB that is essential for pericyte generation, in high concentrations can lead to pericyte loss, the effect that was also implicated in HIV-1 Tat-mediated blood–brain barrier pericyte loss (Niu et al., 2014, 2015). In addition, PDGF-BB has been shown to induce changes to pericyte secretome, increasing production of growth factors and proinflammatory cytokine IL-6 (Gaceb et al., 2018).
Loss of pericyte coverage is exemplified by a decrease in blood–brain barrier integrity (Persidsky et al., 2016). Indeed, our studies indicate that exposure of brain endothelial cells to conditioned media obtained from HIV-1-infected pericytes results in disturbances in barrier integrity (Nakagawa et al., 2012). Part of this effect may be mediated by increased production of IL-6 (Nakagawa et al., 2012), which can induce proinflammatory responses and decrease integrity of the neurovascular unit (Blecharz-Lang et al., 2018). An increase in pericyte-produced proinflammatory cytokines, along with dysregulation of autophagy, was also observed in response to cocaine exposure (Sil et al., 2018). Loss of pericyte coverage on the brain endothelium can also lead to a reduction of connexin expression, leading to compromised cell-cell communication. Indeed, we demonstrated that HIV-1 infection of pericytes results in an increase in connexin 43 (Cho et al., 2017). While the role of these processes in HIV-1 infection remains to be elucidated, gap junction inhibitors, such as carbenoxolone, resulted in a drastic reduction in virus production by infected pericytes (Cho et al., 2017).
In summary, recent results from in vitro, in vivo, and human samples clearly indicate that blood–brain barrier pericytes can be productively infected with HIV-1 and may constitute a previously unrecognized HIV-1 reservoir in the CNS. The role of these processes in the pathogenesis of HAND and/or HIV-1 escape from the CNS remain to be elucidated.
Future perspectives
The present report indicates the need for more research on the role of pericytes not only in the brain infection by HIV-1 but also in brain physiology, the blood–brain barrier development, and the pathology of other neurodegenerative diseases and neuroinfections. The current controversy on defining blood–brain barrier pericytes highlights the critical gaps of knowledge related to these cells. The crucial role of pericytes in the maintenance of a healthy blood–brain barrier determines the paramount necessity to better understand their involvement in disease processes. Such knowledge is important because a high degree of plasticity of pericytes widens their range of responses to disruption of the neurovascular unit. While the data obtained from patients harbouring HIV-1 might potentially be extrapolated to other conditions affecting the blood–brain barrier, little is known about the involvement of pericytes in other neuroinfections. Emerging experimental and clinical evidence indicate a crucial role of pericytes in accelerated ageing, Alzheimer’s disease, cerebrovascular diseases, and neurocognitive disorders (Meir-Shafrir and Pollack, 2012; Gross et al., 2016; Rajasuriar et al., 2017).
Funding
Supported by the National Institutes of Health (NIH), grants HL126559, DA039576, MH098891, MH072567, DA040537, and DA044579. L.B. was supported in part by a postdoctoral fellowship from the American Heart Association (16POST31170002).
Competing interests
The authors report no competing interests.
References
- Armulik A, Genové G, Betsholtz C.. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 2011; 21: 193–215. [DOI] [PubMed] [Google Scholar]
- Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, et al. Pericytes regulate the blood-brain barrier. Nature 2010; 468: 557–61. [DOI] [PubMed] [Google Scholar]
- Arnold TD, Niaudet C, Pang MF, Siegenthaler J, Gaengel K, Jung B, et al. Excessive vascular sprouting underlies cerebral hemorrhage in mice lacking αVβ8-TGFβ signaling in the brain. Development 2014; 141: 4489–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahina K, Zhou B, Pu WT, Tsukamoto H.. Septum transversum-derived mesothelium gives rise to hepatic stellate cells and perivascular mesenchymal cells in developing mouse liver. Hepatology 2011; 53: 983–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature 2010; 468: 232–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Mishra A, Hall CN, O’Farrell FM, Dalkara T.. What is a pericyte? J Cereb Blood Flow Metab 2016; 36: 451–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluk P, Morikawa S, Haskell A, Mancuso M, McDonald DM. Abnormalities of basement membrane on blood vessels and endothelial sprouts in tumors. Am J Pathol 2003; 163: 1801–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 2010; 68: 409–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Song S.. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol 2005; 7: 452–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D.. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest 2003; 111: 1287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand L, Dygert L, Toborek M.. Antiretroviral treatment with efavirenz disrupts the blood-brain barrier integrity and increases stroke severity. Sci Rep 2016a; 6: 39738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand L, Nair M, Toborek M.. Solving the blood-brain barrier challenge for the effective treatment of HIV replication in the central nervous system. Curr Pharm Des 2016b; 22: 5477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankson JN, Persaud D, Siliciano RF. The challenge of viral reservoirs in HIV-1 infection. Annu Rev Med 2002; 53: 557–93. [DOI] [PubMed] [Google Scholar]
- Blecharz-Lang KG, Wagner J, Fries A, Nieminen-Kelhä M, Rösner J, Schneider UC, et al. Interleukin 6-mediated endothelial barrier disturbances can be attenuated by blockade of the IL6 receptor expressed in brain microvascular endothelial cells. Transl Stroke Res 2018; 9: 631–42. [DOI] [PubMed] [Google Scholar]
- Cai CL, Martin JC, Sun Y, Cui L, Wang L, Ouyang K, et al. A myocardial lineage derives from Tbx18 epicardial cells. Nature 2008; 454: 104–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro V, Bertrand L, Luethen M, Dabrowski S, Lombardi J, Morgan L, et al. Occludin controls HIV transcription in brain pericytes via regulation of SIRT-1 activation. FASEB J 2016; 30: 1234–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan A, Mehla R, Vijayakumar TS, Handy I.. Endocytosis-mediated HIV-1 entry and its significance in the elusive behavior of the virus in astrocytes. Virology 2014; 456–457: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen NC, Partridge AT, Sell C, Torres C, Martín-García J.. Fate of microglia during HIV-1 infection: From activation to senescence? Glia 2017; 65: 431–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HJ, Kuo AM, Bertrand L, Toborek M.. HIV alters gap junction-mediated intercellular communication in human brain pericytes. Front Mol Neurosci 2017; 10: 410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 2010; 468: 562–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Flores L, Gutiérrez R, Madrid JF, Varela H, Valladares F, Acosta E, et al. Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol 2009; 24: 909–69. [DOI] [PubMed] [Google Scholar]
- Dohgu S, Banks WA. Brain pericytes increase the lipopolysaccharide-enhanced transcytosis of HIV-1 free virus across the in vitro blood-brain barrier: evidence for cytokine-mediated pericyte-endothelial cell crosstalk. Fluids Barriers CNS 2013; 10: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferland-McCollough D, Slater S, Richard J, Reni C, Mangialardi G.. Pericytes, an overlooked player in vascular pathobiology. Pharmacol Ther 2017; 171: 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaceb A, Özen I, Padel T, Barbariga M, Paul G.. Pericytes secrete pro-regenerative molecules in response to platelet-derived growth factor-BB. J Cereb Blood Flow Metab 2018; 38: 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaengel K, Genové G, Armulik A, Betsholtz C.. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol 2009; 29: 630–8. [DOI] [PubMed] [Google Scholar]
- Gross AM, Jaeger PA, Kreisberg JF, Licon K, Jepsen KL, Khosroheidari M, et al. Methylome-wide analysis of chronic HIV infection reveals five-year increase in biological age and epigenetic targeting of HLA. Mol Cell 2016; 62: 157–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guijarro-Muñoz I, Compte M, Álvarez-Cienfuegos A, Álvarez-Vallina L, Sanz L.. Lipopolysaccharide activates Toll-like receptor 4 (TLR4)-mediated NF-κB signaling pathway and proinflammatory response in human pericytes. J Biol Chem 2014; 289: 2457–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014; 508: 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton NB, Attwell D, Hall CN. Pericyte-mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Front Neuroenergetics 2010; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmuth J, Valcour V, Spudich S.. CNS reservoirs for HIV: implications for eradication. J Virus Erad 2015; 1: 67–71. [PMC free article] [PubMed] [Google Scholar]
- Hill RA, Tong L, Yuan P, Murikinati S, Gupta S, Grutzendler J.. Regional blood flow in the normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes. Neuron 2015; 87: 95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann JJ, Iruela-Arispe ML. Notch signaling in blood vessels: who is talking to whom about what? Circ Res 2007; 100: 1556–68. [DOI] [PubMed] [Google Scholar]
- Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron 2017; 96: 17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y, Suzuki N.. Control of brain capillary blood flow. J Cereb Blood Flow Metab 2012; 32: 1167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph J, Cinque P, Colosi D, Dravid A, Ene L, Fox H, et al. Highlights of the global HIV-1 CSF escape consortium meeting, 9 June 2016, Bethesda, MD, USA. J Virus Erad 2016; 2: 243–50. [PMC free article] [PubMed] [Google Scholar]
- Joseph SB, Arrildt KT, Sturdevant CB, Swanstrom R.. HIV-1 target cells in the CNS. J Neurovirol 2015; 21: 276–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph SB, Arrildt KT, Swanstrom AE, Schnell G, Lee B, Hoxie JA, et al. Quantification of entry phenotypes of macrophage-tropic HIV-1 across a wide range of CD4 densities. J Virol 2014; 88: 1858–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WK, Avarez X, Williams K.. The role of monocytes and perivascular macrophages in HIV and SIV neuropathogenesis: information from non-human primate models. Neurotox Res 2005; 8: 107–15. [DOI] [PubMed] [Google Scholar]
- Kloc M, Kubiak JZ, Li XC, Ghobrial RM. Pericytes, microvasular dysfunction, and chronic rejection. Transplantation 2015; 99: 658–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn J, Christ B, Kurz H.. Neuroectodermal origin of brain pericytes and vascular smooth muscle cells. J Comp Neurol 2002; 442: 78–88. [DOI] [PubMed] [Google Scholar]
- Lambotte O, Deiva K, Tardieu M.. HIV-1 persistence, viral reservoir, and the central nervous system in the HAART era. Brain Pathol 2003; 13: 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, et al. Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol 2008; 65: 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Lan Y, Wang Y, Wang J, Yang G, Meng F, et al. Endothelial Smad4 maintains cerebrovascular integrity by activating N-cadherin through cooperation with Notch. Dev Cell 2011; 20: 291–302. [DOI] [PubMed] [Google Scholar]
- Li GH, Anderson C, Jaeger L, Do T, Major EO, Nath A.. Cell-to-cell contact facilitates HIV transmission from lymphocytes to astrocytes via CXCR4. AIDS 2015; 29: 755–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GH, Henderson L, Nath A.. Astrocytes as an HIV reservoir: mechanism of HIV infection. Curr HIV Res 2016; 14: 373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Liu H, Kim BO, Gattone VH, Li J, Nath A, et al. CD4-independent infection of astrocytes by human immunodeficiency virus type 1: requirement for the human mannose receptor. J Virol 2004; 78: 4120–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia 2010; 58: 1094–103. [DOI] [PubMed] [Google Scholar]
- Meir-Shafrir K, Pollack S.. Accelerated aging in HIV patients. Rambam Maimonides Med J 2012; 3: e0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell TS, Bradley J, Robinson GS, Shima DT, Ng YS. RGS5 expression is a quantitative measure of pericyte coverage of blood vessels. Angiogenesis 2008; 11: 141–51. [DOI] [PubMed] [Google Scholar]
- Moses AV, Bloom FE, Pauza CD, Nelson JA. Human immunodeficiency virus infection of human brain capillary endothelial cells occurs via a CD4/galactosylceramide-independent mechanism. Proc Natl Acad Sci USA 1993; 90: 10474–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Castro V, Toborek M.. Infection of human pericytes by HIV-1 disrupts the integrity of the blood-brain barrier. J Cell Mol Med 2012; 16: 2950–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagomi T, Kubo S, Nakano-Doi A, Sakuma R, Lu S, Narita A, et al. Brain vascular pericytes following ischemia have multipotential stem cell activity to differentiate into neural and vascular lineage cells. Stem Cells 2015; 33: 1962–74. [DOI] [PubMed] [Google Scholar]
- Niu F, Yao H, Liao K, Buch S.. HIV Tat 101-mediated loss of pericytes at the blood-brain barrier involves PDGF-BB. Ther Targets Neurol Dis 2015; 2: e471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu F, Yao H, Zhang W, Sutliff RL, Buch S.. Tat 101-mediated enhancement of brain pericyte migration involves platelet-derived growth factor subunit B homodimer: implications for human immunodeficiency virus-associated neurocognitive disorders. J Neurosci 2014; 34: 11812–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson LE, Soriano P.. PDGFRβ signaling regulates mural cell plasticity and inhibits fat development. Dev Cell 2011; 20: 815–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik JH, Skoura A, Chae SS, Cowan AE, Han DK, Proia RL, et al. Sphingosine 1-phosphate receptor regulation of N-cadherin mediates vascular stabilization. Genes Dev 2004; 18: 2392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persidsky Y, Hill J, Zhang M, Dykstra H, Winfield M, Reichenbach NL, et al. Dysfunction of brain pericytes in chronic neuroinflammation. J Cereb Blood Flow Metab 2016; 36: 794–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potash MJ, Chao W, Bentsman G, Paris N, Saini M, Nitkiewicz J, et al. A mouse model for study of systemic HIV-1 infection, antiviral immune responses, and neuroinvasiveness. Proc Natl Acad Sci USA 2005; 102: 3760–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que J, Wilm B, Hasegawa H, Wang F, Bader D, Hogan BL. Mesothelium contributes to vascular smooth muscle and mesenchyme during lung development. Proc Natl Acad Sci USA 2008; 105: 16626–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasuriar R, Chong ML, Ahmad Bashah NS, Abdul Aziz SA, McStea M, Lee ECY, et al. Major health impact of accelerated aging in young HIV-infected individuals on antiretroviral therapy. AIDS 2017; 31: 1393–403. [DOI] [PubMed] [Google Scholar]
- Sagare AP, Bell RD, Zhao Z, Ma Q, Winkler EA, Ramanathan A, et al. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat Commun 2013; 4: 2932. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sil S, Niu F, Tom E, Liao K, Periyasamy P, Buch S. Cocaine mediated neuroinflammation: role of dysregulated autophagy in pericytes. Mol Neurobiol 2018. doi: 10.1007/s12035-018-1325-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon C, Lickert H, Götz M, Dimou L.. Sox10-iCreERT2: a mouse line to inducibly trace the neural crest and oligodendrocyte lineage. Genesis 2012; 50: 506–15. [DOI] [PubMed] [Google Scholar]
- Song N, Huang Y, Shi H, Yuan S, Ding Y, Song X, et al. Overexpression of platelet-derived growth factor-BB increases tumor pericyte content via stromal-derived factor-1α/CXCR4 axis. Cancer Res 2009; 69: 6057–64. [DOI] [PubMed] [Google Scholar]
- Stephens EB, Singh DK, Kohler ME, Jackson M, Pacyniak E, Berman NE. The primary phase of infection by pathogenic simian-human immunodeficiency virus results in disruption of the blood-brain barrier. AIDS Res Hum Retroviruses 2003; 19: 837–46. [DOI] [PubMed] [Google Scholar]
- Strain MC, Letendre S, Pillai SK, Russell T, Ignacio CC, Günthard HF, et al. Genetic composition of human immunodeficiency virus type 1 in cerebrospinal fluid and blood without treatment and during failing antiretroviral therapy. J Virol 2005; 79: 1772–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratman AN, Schwindt AE, Malotte KM, Davis GE. Endothelial-derived PDGF-BB and HB-EGF coordinately regulate pericyte recruitment during vasculogenic tube assembly and stabilization. Blood 2010; 116: 4720–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney MD, Ayyadurai S, Zlokovic BV. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat Neurosci 2016; 19: 771–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sá-Pereira I, Brites D, Brito MA. Neurovascular unit: a focus on pericytes. Mol Neurobiol 2012; 45: 327–47. [DOI] [PubMed] [Google Scholar]
- Tallquist MD, French WJ, Soriano P.. Additive effects of PDGF receptor β signaling pathways in vascular smooth muscle cell development. PLoS Biol 2003; 1: E52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost A, Lange S, Schroedl F, Bruckner D, Motloch KA, Bogner B, et al. Brain and retinal pericytes: origin, function and role. Front Cell Neurosci 2016; 10: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost A, Schroedl F, Lange S, Rivera FJ, Tempfer H, Korntner S, et al. Neural crest origin of retinal and choroidal pericytes. Invest Ophthalmol Vis Sci 2013; 54: 7910–21. [DOI] [PubMed] [Google Scholar]
- Walshe TE, Saint-Geniez M, Maharaj AS, Sekiyama E, Maldonado AE, D’Amore PA. TGF-β is required for vascular barrier function, endothelial survival and homeostasis of the adult microvasculature. PLoS One 2009; 4: e5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilm B, Ipenberg A, Hastie ND, Burch JB, Bader DM. The serosal mesothelium is a major source of smooth muscle cells of the gut vasculature. Development 2005; 132: 5317–28. [DOI] [PubMed] [Google Scholar]
- Winkler EA, Bell RD, Zlokovic BV. Pericyte-specific expression of PDGF beta receptor in mouse models with normal and deficient PDGF beta receptor signaling. Mol Neurodegener 2010; 5: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci 2011; 14: 1398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler EA, Sengillo JD, Bell RD, Wang J, Zlokovic BV. Blood-spinal cord barrier pericyte reductions contribute to increased capillary permeability. J Cereb Blood Flow Metab 2012; 32: 1841–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler EA, Sengillo JD, Sullivan JS, Henkel JS, Appel SH, Zlokovic BV. Blood-spinal cord barrier breakdown and pericyte reductions in amyotrophic lateral sclerosis. Acta Neuropathol 2013; 125: 111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright PW, Vaida FF, Fernández RJ, Rutlin J, Price RW, Lee E, et al. Cerebral white matter integrity during primary HIV infection. AIDS 2015; 29: 433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Muramatsu M, Azuma E, Ikutani M, Nagai Y, Sagara H, et al. A subset of cerebrovascular pericytes originates from mature macrophages in the very early phase of vascular development in CNS. Sci Rep 2017; 7: 3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Yang N, Park JW, Katsaros D, Fracchioli S, Cao G, et al. Tumor-derived vascular endothelial growth factor up-regulates angiopoietin-2 in host endothelium and destabilizes host vasculature, supporting angiogenesis in ovarian cancer. Cancer Res 2003; 63: 3403–12. [PubMed] [Google Scholar]