Abstract
Human cytomegalovirus (HCMV) remains a major public health burden domestically and abroad. Current approved therapies, including ganciclovir, are only moderately efficacious, with many transplant patients suffering from a variety of side effects. A major impediment to the efficacy of current anti-HCMV drugs is their antiviral effects are restricted to the lytic stage of viral replication. Consequently, the non-lytic stages of the viral lifecycle remain major sources of HCMV infection associated with transplant recipients and ultimately the cause morbidity and mortality. While work continues on new antivirals that block lytic replication, the dormant stages of HCMV’s unique lifecycle need to be concurrently assessed for new therapeutic interventions. In this review, we will examine the role that the PI3K/Akt/mTOR signaling axis plays during the different stages of HCMV’s lifecycle, and describe the advantages of targeting this cellular pathway as an antiviral strategy. In particular, we focus on the potential of exploiting the unique modifications HCMV imparts on the PI3K/Akt/mTOR pathway during quiescent infection of monocytes, which serve an essential role in the viral dissemination strategy of the virus.
Keywords: Cytomegalovirus, Monocytes, Protein kinase B, Apoptosis
1. Introduction
Human cytomegalovirus (HCMV) is a member of the betaherpesvirus family and a major worldwide public health burden. While primary infections in immunocompetent individuals are generally self-limiting, infections are associated with severe morbidity and mortality in immunocompromised individuals such as transplant and chemotherapy patients (Bissinger et al., 2002; Lawlor and Moss, 2010; Nerheim et al., 2004; Reinke et al., 1999; Staras et al., 2006). HCMV is also the most common infectious cause of newborn malformations in developed countries (Bristow et al., 2011; Dollard et al., 2007; Kenneson and Cannon, 2007; Manicklal et al., 2013). Despite the huge public health burden of HCMV, limited options for prophylactic or fulminant therapy exist today (Ahmed, 2011). Historically, replication inhibitors targeting viral proteins has been the first choice in developing new antiviral therapeutics as viral proteins mediates distinct evolutionary conserved functions during the virus lifecycle. Such therapies approved for HCMV are ganciclovir, valganciclovir, cidofovir, and foscarnet, which are synthetic nucleotide analogues that inhibit the viral polymerase UL97 to halt replication (Griffiths and Boeckh, 2007; Prichard and Kern, 2011). However, there are several drawbacks to this class of anti-HCMV drugs, including 1) The side effects from these inhibitors can be severe, particularly for transplant patients on long-term regimens; 2) The emergence of antiviral resistant strains; 3) The low efficacies of these drugs with up to 30% of transplant patients on ganciclovir therapy eventually developing disease and a significant proportion experiencing transplant rejection (Ahmed, 2011; Echenique et al., 2017; Griffiths and Boeckh, 2007). Overall, the poor efficacy of replication inhibitors is in large part due to the inability of these drugs to exert any effect on HCMV during the dormant (non-lytic replication) periods of the virus life cycle. Thus, current antivirals are inadequate to address the needs of patients, and new therapeutic strategies must consider the other stages of HCMV’s unique life cycle.
2. HCMV Life Cycle
HCMV infection can be divided into three stages: lytic, latent, and quiescent (Chan et al., 2010; Jean Beltran and Cristea, 2014; Sinzger and Jahn, 1996; Smith et al., 2004a; Yurochko and Huang, 1999). During a lytic infection HCMV expresses three temporal classes of viral genes: immediate early (IE), early (E), and late (L) genes (Crough and Khanna, 2009). Lytic gene expression allows for replication of the viral genome, packaging of nascent virions, and release from the host cell (Hamirally et al., 2009; Jean Beltran and Cristea, 2014; Milbradt et al., 2007; Milbradt et al., 2014). In contrast, during a latent infection HCMV transiently expresses a subset of viral latent genes in the absence of a fully productive viral infection (Cheng et al., 2017; Gatherer et al., 2011; Shnayder et al., 2018). Undifferentiated hematopoietic progenitor cells in the bone marrow are generally thought to be the major site of latent HCMV infection (Goodrum et al., 2012; Hahn et al., 1998; Mendelson et al., 1996; Movassagh et al., 1996; Taylor-Wiedeman et al., 1993; Taylor-Wiedeman et al., 1991b; von Laer et al., 1995). An external stimulus is required to differentiate these cells and reactivate lytic replication (Ibanez et al., 1991; Soderberg-Naucler et al., 1997b; Söderberg-Nauclér et al., 2001; Taylor-Wiedeman et al., 1994b). A “quiescent infection” is a state recently defined by our and the Yurochko group to occur within monocytes (Chan et al., 2012c; Smith et al., 2004b; Stevenson et al., 2014). While our understanding of a quiescent infection is largely incomplete, there is lack of lytic gene expression similar to latent infection (Ibanez et al., 1991; Sinclair and Sissons, 1996b; Smith et al., 2004a). However, a quiescent infection appears to differ from a latent infection by being able to “reactivate” and begin lytic replication without the need of an external activation signal (Chan et al., 2008; Smith et al., 2004a). In quiescently infected monocytes, spontaneous expression of lytic viral gene products and productive replication occurs 2–3 weeks after the initial infection (Chan et al., 2008; Smith et al., 2004a). However, while latency in CD34+ or other hematopoietic cell types and quiescence in monocytes are referred to as being separate and distinct in this review, the line differentiating these two types of HCMV infections is likely blurred with overlapping biological traits. Indeed, monocytes harboring episomal viral genome can be induced to reactivate with growth factor treatment prior to 2 weeks post infection (Sinclair and Sissons, 1996a, 2006; Soderberg-Naucler et al., 1997a; Soderberg-Naucler et al., 2001; Streblow and Nelson, 2003; Taylor-Wiedeman et al., 1991a; Taylor-Wiedeman et al., 1994a). Consequently, HCMV-infected monocytes have been widely used as a model of latency. Nonetheless, HCMV utilizes quiescent infections in monocytes as a key cog in its dissemination strategy during a primary infection in order to bridge the initial transient lytic infection with the establishment of a persistent latent infection.
3. HCMV Dissemination
HCMV was first detected in peripheral blood monocytes, which are the primary cell type in circulation harboring the viral genome, suggesting these blood sentinels may be important for the early dissemination of the virus despite not being permissive for lytic replication (Schrier et al., 1985; Taylor-Wiedeman et al., 1991b; von Laer et al., 1995). In support, leukocyte depletion eliminates HCMV transmission through blood donations, monocytes are carriers of the virus following organ transplantation, and monocyte-derived macrophages are the first cells to express viral antigen within infected organs (Adler et al.; Gnann Jr. et al., 1988; Larsson et al., 1998; Lipson et al., 2001; Mazeron, 2000; Sinzger et al., 1996). Ex vivo studies from naturally HCMV infected monocytes showed HCMV reactivation from monocytes that have been matured into macrophages using differentiation factors, supporting a role for monocytes in the HCMV dissemination strategy (Poole et al., 2015; Soderberg-Naucler et al., 1997b; Taylor-Wiedeman et al., 1994b). The importance of myelomononuclear cells during virus dissemination was further corroborated by in vivo murine CMV (MCMV) studies showing monocytes were the predominant cell type responsible for spread during an acute infection (Bale and O’Neil, 1989; Collins et al., 1993; Collins et al., 1994; Daley-Bauer et al., 2014; Stoddart et al., 1994). A recently developed humanized mouse model of HCMV infection that supported latent viral infection and dissemination also found the source of HCMV in the peripheral organs was from human macrophages derived from peripheral blood monocytes (Smith et al., 2010). In addition to facilitating spread to peripheral organs, HCMV-infected monocytes can travel into the bone marrow and transmit the virus to CD34+ bone marrow stem cells, the major site of HCMV latency. In these ways, monocytes represent a key link between acute and persistent infections as an initial lytic infection cannot propagate to distant organs, nor establish a latent infection within the bone marrow without monocyte-mediated dissemination. While effective therapies targeting all three stages of the viral lifecycle would be ideal, quiescently infected monocytes present new opportunities for specific interventions at a crucial stage linking both lytic and/or latent infections.
As current therapies are virally focused, resistance has rapidly developed through selective pressure. Rather than targeting viral replication and proteins, cellular pathways represent highly conserved and regulated processes. Signaling pathways are differentially regulated during HCMV infection in a multitude of cell types across all three life stages. Viral infection tends to usurp the activities of select cellular kinases important to the viral life cycle (Brinkmann and Schulz, 2006; Cheeran et al., 2005; Dawson et al., 2003; Herbein et al., 2010). Targeting these pathways may be key to not only suppressing virus replication, but also eliminating latent and/or quiescent viral reservoirs, which are highly dependent on cellular kinases to effect cellular change due to the limited expression of viral gene products. In particular, a number of prosurvival pathways have been recently identified to be specifically upregulated in HCMV-infected monocytes but not uninfected cells (Chan et al., 2010; Cojohari et al., 2016; Collins-McMillen et al., 2015; Peppenelli et al., 2016; Peppenelli et al., 2018; Stevenson et al., 2014). These pathways represent a unique opportunity for the design of new antivirals that target the virally infected cell rather than the virus itself. This review summarizes the current research on these signaling pathways during HCMV infection with a particular focus on the quiescent stage of the viral life cycle.
4. Cellular Receptors and HCMV
The first step in modifying cellular signaling pathways comes during viral entry. HCMV enters cells through the interaction of a number of its surface viral glycoproteins with a panoply of cell surface receptors. In monocytes these key glycoproteins include gB, gH, gL, gO, and UL128–131 (Chan et al., 2009; Isaacson and Compton, 2009; Nogalski et al., 2011; Nogalski et al., 2013; Smith et al., 2004b; Yurochko and Huang, 1999; Yurochko et al., 1997). Glycoprotein gB forms a trimer linked by disulfide bonds (Sharma et al., 2013). Glycoproteins gH and gL form a complex through disulfide bond interactions at the viral envelope, which then forms two separate complexes, the trimeric gH/gL/gO or the pentameric gH/gL/UL128–131, either through disulfide or covalent interactions (Huber and Compton, 1998, 1999; Yurochko et al., 1997). The trimeric complex is required for viral entry into fibroblasts, while the pentameric complex is essential for entry into endothelial, epithelial, monocytic, and dendritic cells (Adler et al., 2006; Liu et al., 2018; Straschewski et al., 2011; Wang and Shenk, 2005; Wille et al., 2013).
As HCMV has tropism for a wide range of cell types, there is a significant body of research into discovering entry receptors. Huang et al identified epidermal growth factor receptor (EGFR) bound with gB in order to mediate viral binding and entry in a number of cells (Wang et al., 2003). However, conflicting reports indicated that EGFR was not the receptor for gB and has no role in mediating viral entry (Isaacson et al., 2007). The relationship between gB and EGFR during viral entry remains unsettled across a variety of cell types, but in monocytes, gB was required for EGFR activation and subsequent entry into monocytes (Chan et al., 2009). Recently, the interaction of gB with PDGFR-α was described (Cobbs et al., 2014; Kabanova et al., 2016; Soroceanu et al., 2008; Wu et al., 2017); however, like with EGFR, there are conflicting reports about the importance of PDGFR-α with regard to both its ability to bind gB as well as function as a bona fide entry receptor (Vanarsdall et al., 2012). Moreover, monocytes do not express PDGFR-α as a surface receptor (Chan et al., 2009; Inaba et al., 1993; Krettek et al., 2001). Integrins were also found to be important in facilitating viral entry, with different integrins mediating entry in different cell types. In monocytes, β1 and β3 integrins bind glycoprotein complexes gH/gL/gO and gH/gL/UL128–131 in order to mediate viral entry into these cells (Feire et al., 2004; Nogalski et al., 2013; Wang et al., 2005a). Additional HCMV receptors, including Nrp1, CD147, CD90, and BST2 have emerged as potentially having key roles in mediating entry as well (Li et al., 2016; Li et al., 2015; Martinez-Martin et al., 2018; Vanarsdall et al., 2018; Viswanathan et al., 2011). Given the complexity of HCMV entry, targeting this step of infection as an antiviral strategy will be challenging as finding a “magic bullet” to prevent entry and signaling in all the different cell types infected by HCMV is doubtful. Moreover, cellular receptors such as EGFR are also involved in stages of the viral life cycle other than viral entry. Early inhibition of EGFR prevents viral entry and viral gene expression, which is likely due to decreased viral entry, while inhibition of EGFR post entry promotes viral replication, suggesting that late EGFR activity lowers virus yields in fibroblasts. These time dependent opposing effects on HCMV infection increase the unlikelihood targeting of HCMV entry receptors as an antiviral strategy.
5. Phosphatidyl Inositol 3 Kinase/ Protein Kinase B (PI3K/Akt) Pathway
Despite the multitude of receptors engaged by HCMV, a constant feature among several of the receptors is the activation of the same downstream targets critical to viral infection. In particular, EGFR, PDGFR-α, and integrins independently activate the PI3K/Akt signaling axis highlighting the possibility of intervention at this central signaling hub within the HCMV-induced signalsome (Alessi et al., 1996; Lemmon and Schlessinger, 2010; Liu et al., 2009; Soroceanu et al., 2008). Canonical Akt signaling involves an initial recruitment and activation of class 1 PI3Ks (compromised of a p85 regulatory and a p110 catalytic subunit) by receptor tyrosine kinases, such as EGFR, to mediate the phosphorylation of PI(4,5)-P2 into PI(3,4,5)-P3 (Manning and Cantley, 2007; Martini et al., 2014) (Fig. 1A). Isoforms of the p110 catalytic subunit include p110α, p110β, and p110δ, the latter of which is the predominant isoform found in monocytes (Papakonstanti et al., 2008). Production of PI(3,4,5)-P3 allows for the recruitment of Akt to the membrane through the binding of pleckstrin homology (PH) domain to PI(3,4,5)-P3. Akt is then phosphorylated by the combined actions of phosphoinositide-dependent kinase-1 (PDK1) at T308 and mammalian target of rapamycin complex 2 (mTORC2) at S473 (Scheid et al., 2002a; Scheid et al., 2002b). The production of PI(3,4,5)-P3, and thus Akt activation, is negatively regulated by the actions of phosphatase and tensin homolog (PTEN), which converts PI(3,4,5)-P3 back to PI(4,5)-P2, and SH-2 containing inositol 5’ polyphosphate (SHIP1), which dephosphorylates PI(3,4,5)-P3 into PI(3,4)-P2 (Manning and Cantley, 2007; Martini et al., 2014).
Fig. 1.
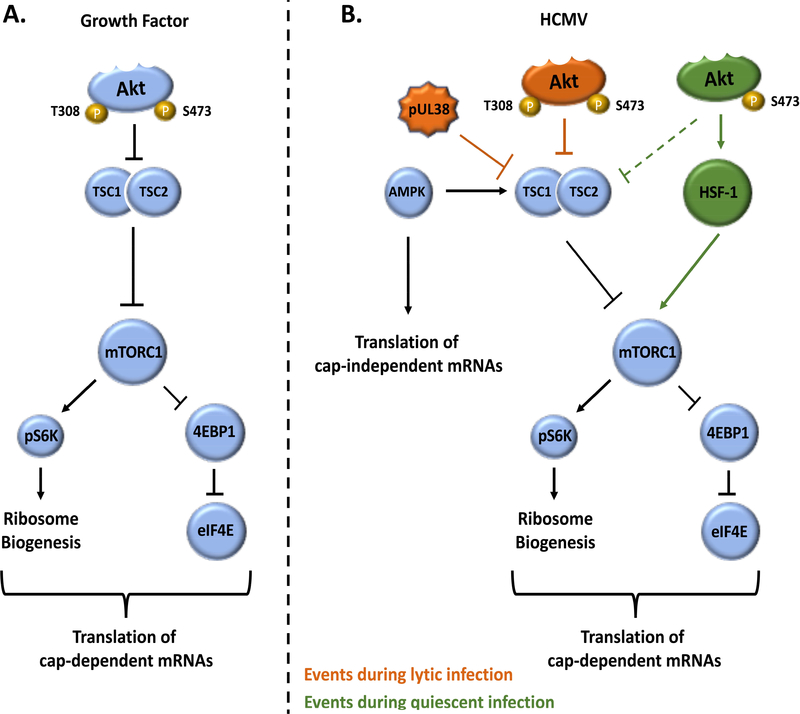
Model of differential activation of PI3K/Akt signaling by growth factors and HCMV infection. (A) Growth factors binding to their cognate receptors activate canonical PI3K signaling, leading to activation of Akt through phosphorylation at T308 and S473 by PDK1 and mTORC2, respectively. (B) During lytic infection, a biphasic activation of PI3K/Akt occurs with an early transient burst of activation mediated by PDGFR, EGFR, and/or integrin engagement followed by a sustained secondary activation mediated by viral proteins IE72 and IE86. Both T308 and S473 appear to be phosphorylated during lytic infection. (C) The atypical activation of PI3K/Akt during quiescent infection of monocytes is initiated by HCMV binding to EGFR and integrins. The coordinated action of PI3K’s catalytic isoform p110β, as opposed to the predominant p110δ isoform found in monocytes, and SHIP-1 leads to preferential phosphorylation of Akt at S473 by a yet to be identified mechanism. While not much is known about the PI3K activity during latency, EGFR is believed to be activated during HCMV entry in CD34+ cells and PI3K has been observed to be rapidly upregulated during early latent infection.
5.1. PI3K/Akt During HCMV Infection
The PI3K/Akt pathway is temporally and differentially regulated by HCMV during all three stages of infection. During lytic infection of fibroblasts, a biphasic activation of PI3K/Akt occurs with an early transient activation triggered by receptor signaling and a later sustained activation mediated by viral immediate early proteins IE72 and IE86 (Cobbs et al., 2008; Yu and Alwine, 2002) (Fig. 1B). The early activation of PI3K is required for efficient viral entry while the later sustained activation is needed for optimal viral gene expression and viral DNA replication (Cobbs et al., 2008; Johnson et al., 2001; McFarlane et al., 2011). However, the late activation of EGFR and PI3K represses lytic replication in fibroblasts, hinting at the possibility that EGFR and PI3K promote entry into and/or maintenance of latency (Buehler et al., 2016b). In support, pUL138 promotes latency by sustaining EGFR activity in CD34+ stem cells (Buehler et al., 2016b). Regardless, the early activation of PI3K during lytic replication underscores the importance of the PI3K/Akt signaling pathway to a productive HCMV infection. PI3K is also rapidly activated during the initial establishment of latency within CD34+ stem cells in order to promote viral entry and alter cellular gene expression that favors latency (Kim et al., 2017). Whether elevated PI3K activity is maintained throughout latency is unknown; however, the inhibition of PI3K enhances reactivation from latently infected CD34+ cells, suggesting a basal level of PI3K is at least required for the maintenance of latency (Buehler et al., 2016a). As with lytic and latent infection, recent reports have also demonstrated the importance of PI3K/Akt signaling during quiescent infection of monocytes. HCMV induces PI3K/Akt within 15 minutes post infection in monocytes and remains elevated through 48 hours (Chan et al., 2010; Cojohari et al., 2016; Smith et al., 2004b; Smith et al., 2007). Maintenance of PI3K/Akt signaling is critical to the long-term survival of HCMV-infected monocytes. Pharmacological targeting of the Akt pathway prevents quiescently infected monocytes from surviving through a 48-hour viability checkpoint when monocytes must differentiate towards macrophages or undergo cell death. Moreover, because of their increased dependency on Akt, infected monocytes had increased sensitivity to the effects of Akt inhibition when compared to uninfected monocytes. These data indicate the prosurvival function of PI3K/Akt signaling is essential to the progression of a quiescent infection by bridging the gap between initial infection and the expression of viral antiapoptotic proteins at 2–3 weeks post infection (Cline et al., 1978; Smith et al., 2004a; Whitelaw, 1966, 1972). Thus, deciphering the mechanism by which HCMV sustains the PI3K/Akt signaling during quiescent infection may provide critical insight to new therapeutic targets aimed at selectively eliminating infected monocytes.
HCMV triggers PI3K/Akt signaling in an EGFR-dependent manner during entry into monocytes (Chan et al., 2008; Chan et al., 2012a; Chan et al., 2009; Cojohari et al., 2016; Smith et al., 2004b; Smith et al., 2007). Yet, HCMV’s persistent induction is in contrast to EGF’s transient activation of PI3K, suggesting a virus-specific regulatory mechanism controlling PI3K/Akt signaling (Chan et al., 2010; Cojohari et al., 2016; Smith et al., 2004b). UV-inactivated virus stimulates a chronic PI3K/Akt activation similar to “live” virus, indicating that sustained PI3K activity is not mediated by de novo IE gene products as it is with lytic infection (Smith et al., 2004a; Yurochko and Huang, 1999). HCMV utilizes a multitude of glycoprotein complexes and putative cellular receptors during viral entry. Thus, while stimulation of EGFR alone with gB may lead to the canonical activation of PI3K, co-signaling from other glycoprotein and receptor interactions may be responsible for the persistent nature of PI3K/Akt signaling within infected monocytes. Indeed, binding of the virus particle to fibroblasts brings EGFR and integrins into close proximity leading to receptor clustering within lipid rafts and crosstalk between the two signaling cascades (Chan et al., 2012b; Kim et al., 2017; Wang et al., 2005b). Signaling by gB/EGFR and gH/integrins needs to occur within 5 minutes of each other in order to facilitate virus entry (Wang et al., 2005b). In monocytes crosstalk between the gB/EGFR and gH/αvβ3 axes is required for full Akt signal strength (Chan et al., 2012b). Together, these data indicate that the unique spatial and temporal kinetics of glycoprotein-initiated signaling lead to the formation of a HCMV-specific PI3K/Akt signalsome.
Monocytes express all class 1A PI3Ks including the p110α, p110β, and p110δ isoforms (Martini et al., 2014). Although highly homologous, PI3K isoforms have divergent, non-redundant biological functions, as well as differential effects on Akt activity (Thorpe et al., 2015). PI3K p110δ is the major isoform found in uninfected monocytes and is induced following M-CSF treatment to promote long-term survival (Cojohari et al., 2016; Voss et al., 2005). However, HCMV entry induces a switch from p110δ to p110β as the central PI3K isoform regulating the survival of infected monocytes and Akt activity (Cojohari et al., 2016) (Fig. 1C). Biologically, we speculate that the preferential usage of p110β is due to its lack of negative self-regulatory activity and decreased antiviral activity (Guo et al., 2008; Vanhaesebroeck et al., 1999). Simultaneous to the activation of PI3K, HCMV entry into monocytes modifies the activities of two major Akt negative regulators, PTEN and SHIP1 (Cojohari et al., 2016). PTEN directly reverses PI3K activity but is rapidly shutdown during HCMV infection allowing for maximum Akt activity (Cojohari et al., 2016). Alternatively, SHIP1 antagonizes Akt activation under homeostatic conditions via the conversion of PI(3,4,5)-P3 into PI(3,4)-P2 (Kerr, 2011). Yet, HCMV rapidly upregulates SHIP1 expression in contrast to normal myeloid growth factors. Inhibition of SHIP1 also reduces rather than enhance Akt activity (Cojohari et al., 2016). Accordingly, loss of SHIP1 activity prevents HCMV-infected monocytes from acquiring a prosurvival state (Cojohari et al., 2016). Interestingly, leukemia cells also overexpress SHIP1 and PI(3,4)-P2 to promote Akt-dependent cell survival (Kerr, 2011). With PI3K isoform specific inhibitors and SHIP1 inhibitors available, these data hint at the possibility of selectively eliminating infected monocytes by targeting the highly virus-specific changes made to the PI3K signaling pathway. Accordingly, inhibition of p110β and SHIP1 stimulates the death of infected monocytes, while having minimal effects on the viability of uninfected cells (Cojohari et al., 2016).
The functional output of PI3K/Akt signaling is largely governed by two main regulatory sites on Akt, T308 and S473, both of which are believed to be required for full Akt activity (Alessi et al., 1996). However, the diverse cellular effects of Akt appear to be dependent on the specific combination of targets activated and/or deactivated by Akt-mediated phosphorylation. Recent studies showed the ratio of S473 to T308 phosphorylation to modulate Akt target specificity (Yung et al., 2011). HCMV infection of monocytes induces a site-specific phosphorylation of Akt at S473 (Cojohari et al., 2016). In contrast, GM-CSF and M-CSF treatments stimulate both S473 and T308 phosphorylation (Baran et al., 2003; Goyal et al., 2002), indicating that the growth factor and HCMV-initiated PI signaling have distinct functional outputs. Indeed, global analysis revealed differential phosphorylation of downstream targets between HCMV- and growth factor-activated Akt (Peppenelli et al., 2018). One major downstream target differentially regulated was mTOR (Peppenelli et al., 2018), which is responsible for controlling protein translation. HCMV rapidly phosphorylated mTOR in an Akt-dependent manner following HCMV infection, while GM-CSF and M-CSF treatment had no effect on mTOR activity despite also activating Akt, suggesting that downstream targets may provide increased selectivity in terms of antivirals (Peppenelli et al., 2016).
6. mTOR Pathway
MTor is found in two complexes, mTORC1 and mTORC2 (Helliwell et al., 1994; Loewith et al., 2002). MTORC2 plays a central role in phosphorylating and activating Akt, as detailed previously. MTORC1’s major function is the control of protein synthesis through the regulation of protein translation (Kim et al., 2002; Thoreen, 2013) (Fig. 2A). MTORC1 is repressed by AMP-regulated protein kinase (AMPK) and the tuberous sclerosis complex (TSC) in times of nutrient stress (Gao and Pan, 2001; Inoki et al., 2003a). When resources are plentiful AMPK is not activated, while Akt phosphorylates and inhibits the TSC, leading to activation of mTORC1 and increased cap-dependent translation (Hardie et al., 2016; Inoki et al., 2003b; Manning et al., 2002). In times of stress, such as during viral infection, AMPK becomes activated and increases cap-independent translation through initiation of IRES-dependent translation (Mizrachy-Schwartz et al., 2011). MTORC1 regulates translation initiation through phosphorylation of the eIF4F complex and S6 kinase (S6K) (Faller et al., 2015; Shahbazian et al., 2006; Wang et al., 2001). EIF4E is normally bound in the hypophosphorylated state to eIF4E-binding protein 1 (4E-BP1), which prevents the formation of a functional translation initiation complex (Pause et al., 1994). MTORC1 phosphorylates 4E-BP1, reducing its affinity for eIF4E and allowing for eIF4F complex formation and increasing translation through direction of the ribosome to the 5’ cap on mRNA (Fadden et al., 1997). Overall levels eIF4F directly relate to levels of protein synthesis within a cell (Pestova et al., 2001; Vincent et al., 2016). Phosphorylation of S6K leads to activation of several other translation factors, which increase translational scanning (ze et al., 2011), repress translational inhibitors (Faller et al., 2015; Wang et al., 2001), and stimulate the addition of amino acids to nascent peptide chains (Redpath et al., 1996; Wang et al., 2000). Overall, mTORC1 serves as a master regulator of translation in the presence of growth factors and/or plentiful resources.
Fig. 2.
Model of regulation of cellular translation by growth factors and HCMV infection. (A) Growth factor activation of Akt leads to the inhibitory phosphorylation of TSC2, thereby relieving the suppressive effect of the TSC complex on mTORC1. Activation of mTORC1, in turn, stimulates translation of cap-dependent mRNAs through S6K and eIF4E. (B) MTORC1 is activated through distinct mechanisms during lytic and quiescent infections. Activated during times of cellular stress, such as HCMV infection, AMPK stabilizes the TSC complex through activating phosphorylation of TSC2, thereby inhibiting mTORC1 activation, even in the presence of activated Akt. Simultaneously, AMPK activation has also been associated with an upregulation of IRES-dependent, cap-independent translation. However, during lytic infection, TSC complex inhibition is achieved despite AMPK activation through a yet uncharacterized interaction with pUL38 in order to sustain cap-dependent translation. In contrast, the atypical activation of Akt that occurs during quiescent infection maintains mTORC1 activity and cap-dependent translation by circumventing the TSC complex via HSF1. Overall, HCMV infection leads to the simultaneous translation of cap- and IRES-dependent proteins during both quiescent and lytic infections.
6.1. mTOR During HCMV Infection
Multiple studies have established the importance of mTOR during lytic infection. Typically, during times of cellular stress, such as during a viral infection, mTOR activity is decreased. Although AMPK serves as an inhibitor of mTOR activation and is upregulated during HCMV infection, this relationship appears to be uncoupled in infected cells (Kudchodkar et al., 2007; McArdle et al., 2012; Terry et al., 2012). HCMV pUL38 disrupts the negative regulatory effects of AMPK by binding to TSC1/2, a mechanism distinct from Akt-mediated inhibition through phosphorylation (Moorman et al., 2008) (Fig. 2B). Consequently, increased phosphorylation and levels of eIF4E are maintained during lytic infection allowing for continued cap-dependent protein translation (Clippinger et al., 2011a, b; Vincent et al., 2016). Blocking eIF4F complex formation reduces viral replication and progeny production, underpinning the critical need to maintain mTOR activity during a productive HCMV infection (Kudchodkar et al., 2004; Lenarcic et al., 2014; Moorman and Shenk, 2010). There is little known about the role of mTOR during latency. MTOR has been found to phosphorylate KAP1, a transcriptional co-repressor that can force HCMV out of latency when phosphorylated, suggesting that suppression of mTOR may be required for the maintenance of latency (Rauwel et al., 2015). Other results suggest that mTOR does not play a role in reactivation from latency (Glover et al., 2014). During a quiescent infection of monocytes, HCMV stimulates mTOR activity in a PI3K/Akt dependent manner (Peppenelli et al., 2018). Despite increasing mTOR activity, lytic replication is not initiated indicating either a threshold level is needed to drive replication or additional factors are required in combination with mTOR to drive replication. Nonetheless, similarly to lytic infection, cellular stress appears to be uncoupled to decreasing mTOR activity; however, in the absence of pUL38 during a quiescent infection, the mechanism employed by HCMV to uncouple mTOR and stress is unclear.
Activated during times of cellular stress, heat shock factor 1 (HSF1) transcription factor responsible for the expression of stress-associated proteins, which are generally independent of cap-mediated translation (Calderwood et al., 2010; Wu, 1995). HCMV rapidly phosphorylates HSF1 in an Akt-dependent fashion while myeloid growth factors have little effect on HSF1 activity (Peppenelli et al., 2018). A positive feedback loop from HSF1 to mTOR was found to exist in HCMV-infected monocytes where inhibition of HSF1 activity decreased mTOR activity (Figure 2B). Thus, HCMV-activated Akt specificity towards HSF1 provides a mechanism by which HCMV is able to activate mTOR during times of stress. Biologically, although generally a switch from cap-dependent to IRES-mediated translation occurs during cellular stress, HCMV appears to simultaneously drive the translation of both cap-dependent and independent survival proteins within infected monocytes in part due to the substrate specificity of Akt for HSF1.
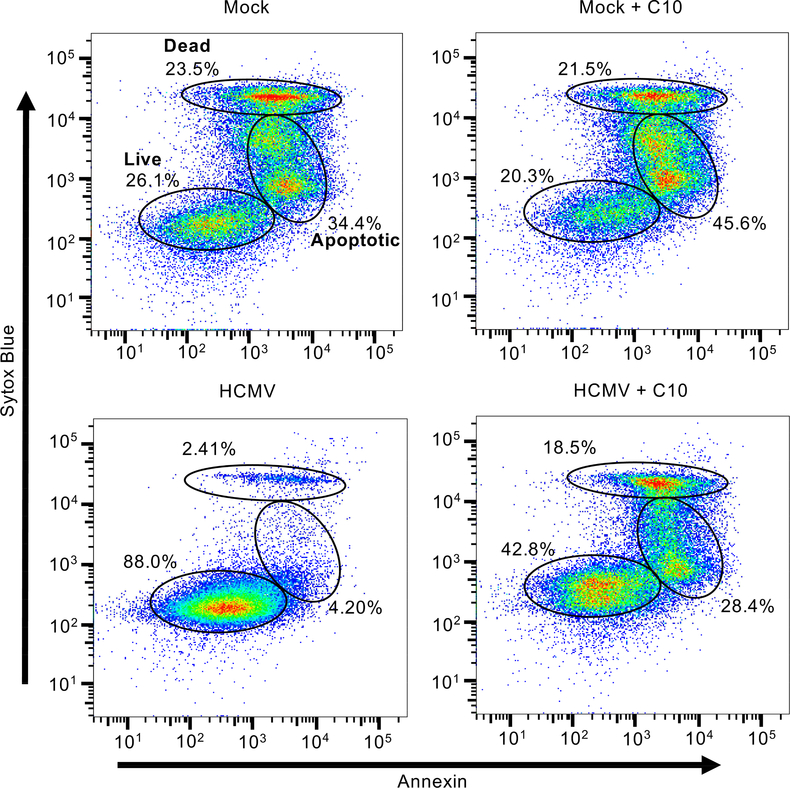
The unique interplay between mTOR and HSF1 during HCMV infection of monocytes stimulates the synthesis of a unique milieu of prosurvival proteins, including myeloid leukemia cell differentiation protein 1 (Mcl-1), X linked inhibitor of apoptosis (XIAP), and heat shock protein 27 (HSP27), that were not or marginally induced in growth factor-treated cells (Collins-McMillen et al., 2015; Peppenelli et al., 2016; Peppenelli et al., 2018). Consistent with our previous studies, we found that inhibition of Mcl-1 with C10 (a selective Mcl-1 small-molecule inhibitor (Abulwerdi et al., 2014)) led to significant induction of apoptosis and death of infected monocytes (6.6% in untreated versus 46.9 in treated) (Burrer et al., 2017; Chan et al., 2010; Peppenelli et al., 2018). Although uninfected cells are naturally programmed to undergo apoptosis, we now show that the loss of Mcl-1 has minimal effect on accelerating this process (57.9% in untreated versus 67.1% in treated), suggesting the possibility of selectively eliminating HCMV-infected monocytes while allowing uninfected monocytes to maintain normal immune surveillance functions (Fig. 3). Inhibition of XIAP with small molecule inhibitors also induces death of infected monocytes but had minimal effect on uninfected cells (Burrer et al., 2017; Chan et al., 2010; Peppenelli et al., 2018). Thus, this select pool of upregulated prosurvival proteins represents novel cellular antiviral targets aimed at selectively eliminating HCMV-infected monocytes while permitting uninfected monocytes to maintain their normal function. Interestingly, latently infected CD34+ stem cells are also highly dependent on Mcl-1 for viability (Reeves et al., 2012). Consequently, Mcl-1 inhibitors have the potential to eliminate quiescently and latently infected cells.
Fig. 3.
HCMV utilizes Mcl-1 to prevent apoptosis in infected monocytes. Primary human monocytes were mock or HCMV infected for 24 hours (h), after which infected cells were treated with Compound 10 (C10), an Mcl-1 inhibitor, for an additional 24 h. Viability was measured by flow cytometry using Sytox Blue (live/dead stain) and Annexin V (early apoptotic marker) staining. Gates represent live cells (Sytox Blue and Annexin V negative), apoptotic cells (Sytox Blue low and Annexin V high), and late apoptotic or dead cells (Sytox Blue and Annexin V positive).
7. Conclusions
The use of antiviral agents as prophylaxis to limit virus replication has significantly reduced the incidence of early infection to ≤10% in transplant patients (Fishman et al., 2007a; Sagedal et al., 2004). However, prophylactic treatment appears to have simply shifted the kinetics of HCMV infection to later after transplantation (≥100 days), and thus HCMV still remains a serious post-transplantation problem (Fishman et al., 2007b; Rubin and Colvin, 1986; Sagedal et al., 2004). Since therapies against HCMV are designed to block specific steps along the virus replication cycle, the delayed onset of disease indicates that current antivirals are ineffective at preventing viral spread mediated by quiescently infected monocytes. We advocate that the suppression of HCMV replication with current prophylactic treatments must be done in combination with drugs capable of directly eliminating infected monocytes. Inhibiting cellular factors crucial to the survival of HCMV infected cells provides an alternative strategy to the development of replication inhibitors as targeting host proteins has the advantage of also affecting the dormant phases of the viral life cycle. Moreover, targeting cellular factors would decrease the likelihood of drug resistance as host factors are highly conserved.
The PI3K/Akt/mTOR pathway has critical function during all three stages of HCMV infection by 1) ensuring metabolic requirement are met for optimal virus production during lytic infection, 2) altering cellular transcription that favors the establishment of latency, and 3) maintaining the survival of quiescently infected monocytes. However, PI3K plays a critical role to normal cellular function, thus inhibition may have significant bystander effects. HCMV-infected cells display increase dependency on this pathway often significantly altering the IC50 of the kinases within the signaling cascade. Ultimately, these virus-induced changes may provide a therapeutic window for the use of PI3K/Akt inhibitors for the treatment of HCMV infection. In addition, HCMV makes highly specific alterations to kinase activities during both lytic and quiescent infection. Targeting these unique changes may provide increased selectivity in eliminating the virus-infected cell populations. As a proof-of-concept, several drugs targeting the components of the PIK/Akt cascade led to death of infected monocytes while having little effect on uninfected cells (Burrer et al., 2017; Cojohari et al., 2016; Peppenelli et al., 2018). Consequently, inhibition of the PI3K/Akt signaling pathway is positioned to play a dual role in inhibiting HCMV replication and eliminating reservoirs of persistently infected cells, which is critical to the long-term prognosis of transplant patients.
Highlights.
Targeting cellular signaling pathways as a new anti-HCMV strategy.
HCMV induces the aberrant activation of the PI3K/Akt signaling network during HCMV infection.
Selectively inducing death of infected monocytes with small molecule inhibitors.
Acknowledgments
This work was supported by grants from the Carol M. Baldwin Breast Cancer Research Fund to G.C. Chan, National Institute of Allergy and Infectious Disease (R01AI141460) to G.C. Chan, National Heart, Lung, and Blood Institute (R01HL139824) to G.C. Chan, and National Cancer Institute (R01CA149442 and R01CA217141) to Z. Nikolovska-Coleska.
Abbreviations
- HCMV
human cytomegalovirus
- IE
immediate early
- E
early
- L
late
- MCMV
murine cytomegalovirus
- gB
glycoprotein B
- gH
glycoprotein H
- gL
glycoprotein L
- gO
glycoprotein O
- EGFR
epidermal growth factor receptor
- PDGFR-α
platelet-derived growth factor receptor α
- NRP1
neuropilin 1
- BST2
bone marrow stromal antigen 2 (tetherin
- PI3K
phosphoinositide 3-kinase
- Akt
protein kinase B
- PI(4,5)-P2
phosphatidylinositol (4,5)-bisphosphate
- PI(3,4,5)-P3
phosphatidylinositol (3,4,5)-triphosphate
- PH
pleckstrin homology
- PDK1
phosphoinositide-dependent kinase 1
- mTORC1
mammalian target of rapamycin complex 1
- mTORC2
mammalian target of rapamycin complex 2
- PTEN
phosphatase and tensin analog
- SHIP1
SH-2 containing inositol 5’ phosphatase 1
- UV
ultraviolet
- EGF
epidermal growth factor
- αvβ3
integrin αvβ3
- M-CSF
macrophage colony-stimulating factor
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- AMPK
adenosine monophosphate kinase
- TSC
tuberous sclerosis complex
- eIF4F
eukaryotic initiation factor 4F
- S6K
S6 kinase
- eIF4E
eukaryotic initiation factor 4E
- 4E-BP1
eIF4E-binding protein 1
- mTOR
mammalian target of rapamycin
- KAP1
KRAB-associated protein-1
- HSF1
heat shock factor 1
- IRES
internal ribosomal entry site
- Mcl-1
myeloid cell leukemia-1
- HSP27
heat shock protein 27
- XIAP
X-linked inhibitor of apoptosis protein
Footnotes
Conflicts of interest
All authors declare that they have no conflicts of interest with the contents of this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abulwerdi FA, Liao C, Mady AS, Gavin J, Shen C, Cierpicki T, Stuckey JA, Showalter HD, Nikolovska-Coleska Z, 2014. 3-Substituted-N-(4-hydroxynaphthalen-1-yl)arylsulfonamides as a novel class of selective Mcl-1 inhibitors: structure-based design, synthesis, SAR, and biological evaluation. J Med Chem 57, 4111–4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler B, Scrivano L, Ruzcics Z, Rupp B, Sinzger C, Koszinowski U, 2006. Role of human cytomegalovirus UL131A in cell type-specific virus entry and release. Journal of General Virology 87, 2451–2460. [DOI] [PubMed] [Google Scholar]
- Adler SP, Chandrika T, Lawrence L, Baggett J, Cytomegalovirus infections in neonates acquired by blood transfusions. Pediatric infectious disease 2, 114–118. [DOI] [PubMed] [Google Scholar]
- Ahmed A, 2011. Antiviral treatment of cytomegalovirus infection. Infectious disorders drug targets 11, 475–503. [DOI] [PubMed] [Google Scholar]
- Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA, 1996. Mechanism of activation of protein kinase B by insulin and IGF-1. The EMBO journal 15, 6541–6551. [PMC free article] [PubMed] [Google Scholar]
- Bale JF Jr., O’Neil ME, 1989. Detection of murine cytomegalovirus DNA in circulating leukocytes harvested during acute infection of mice. J Virol 63, 2667–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran CP, Tridandapani S, Helgason CD, Humphries RK, Krystal G, Marsh CB, 2003. The inositol 5’-phosphatase SHIP-1 and the Src kinase Lyn negatively regulate macrophage colony-stimulating factor-induced Akt activity. J Biol Chem 278, 38628–38636. [DOI] [PubMed] [Google Scholar]
- Bissinger AL, Sinzger C, Kaiserling E, Jahn G, 2002. Human cytomegalovirus as a direct pathogen: Correlation of multiorgan involvement and cell distribution with clinical and pathological findings in a case of congenital inclusion disease. Journal of Medical Virology 67, 200–206. [DOI] [PubMed] [Google Scholar]
- Brinkmann MM, Schulz TF, 2006. Regulation of intracellular signalling by the terminal membrane proteins of members of the Gammaherpesvirinae. Journal of General Virology 87, 1047–1074. [DOI] [PubMed] [Google Scholar]
- Bristow BN, O’Keefe KA, Shafir SC, Sorvillo FJ, 2011. Congenital Cytomegalovirus Mortality in the United States, 1990–2006. PLoS Neglected Tropical Diseases 5, e1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehler J, Zeltzer S, Reitsma J, Petrucelli A, Umashankar M, Rak M, Zagallo P, Schroeder J, Terhune S, Goodrum F, 2016a. Opposing Regulation of the EGF Receptor: A Molecular Switch Controlling Cytomegalovirus Latency and Replication. PLOS Pathogens 12, e1005655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehler J, Zeltzer S, Reitsma J, Petrucelli A, Umashankar M, Rak M, Zagallo P, Schroeder J, Terhune S, Goodrum F, 2016b. Opposing Regulation of the EGF Receptor: A Molecular Switch Controlling Cytomegalovirus Latency and Replication. PLoS Pathog 12, e1005655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrer CM, Auburn H, Wang X, Luo J, Abulwerdi FA, Nikolovska-Coleska Z, Chan GC, 2017. Mcl-1 small-molecule inhibitors encapsulated into nanoparticles exhibit increased killing efficacy towards HCMV-infected monocytes. Antiviral Res 138, 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood SK, Wang Y, Xie X, Khaleque MA, Chou SD, Murshid A, Prince T, Zhang Y, 2010. Signal Transduction Pathways Leading to Heat Shock Transcription. Signal Transduction Insights 2, STI.S3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan G, Bivins-Smith ER, Smith MS, Yurochko AD, 2008. Transcriptome Analysis of NF- B- and Phosphatidylinositol 3-Kinase-Regulated Genes in Human Cytomegalovirus-Infected Monocytes. Journal of Virology 82, 1040–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan G, Nogalski MT, Bentz GL, Smith MS, Parmater A, Yurochko AD, 2010. PI3K-Dependent Upregulation of Mcl-1 by Human Cytomegalovirus Is Mediated by Epidermal Growth Factor Receptor and Inhibits Apoptosis in Short-Lived Monocytes. The Journal of Immunology 184, 3213–3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan G, Nogalski MT, Stevenson EV, Yurochko AD, 2012a. Human cytomegalovirus induction of a unique signalsome during viral entry into monocytes mediates distinct functional changes: a strategy for viral dissemination, Journal of Leukocyte Biology, pp. 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan G, Nogalski MT, Stevenson EV, Yurochko AD, 2012b. Human cytomegalovirus induction of a unique signalsome during viral entry into monocytes mediates distinct functional changes: a strategy for viral dissemination. J Leukoc Biol 92, 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan G, Nogalski MT, Yurochko AD, 2009. Activation of EGFR on monocytes is required for human cytomegalovirus entry and mediates cellular motility. Proceedings of the National Academy of Sciences of the United States of America 106, 22369–22374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan G, Nogalski MT, Yurochko AD, 2012c. Human Cytomegalovirus Stimulates Monocyte-to-Macrophage Differentiation via the Temporal Regulation of Caspase 3. Journal of Virology 86, 10714–10723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeran MC-J, Hu S, Sheng WS, Rashid A, Peterson PK, Lokensgard JR, 2005. Differential responses of human brain cells to West Nile virus infection. Journal of Neurovirology 11, 512–524. [DOI] [PubMed] [Google Scholar]
- Cheng S, Caviness K, Buehler J, Smithey M, Nikolich-Zugich J, Goodrum F, 2017. Transcriptome-wide characterization of human cytomegalovirus in natural infection and experimental latency. Proc Natl Acad Sci U S A 114, E10586–E10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline MJ, Lehrer RI, Territo MC, Golde DW, 1978. UCLA Conference. Monocytes and macrophages: functions and diseases. Annals of internal medicine 88, 78–88. [DOI] [PubMed] [Google Scholar]
- Clippinger AJ, Maguire TG, Alwine JC, 2011a. The changing role of mTOR kinase in the maintenance of protein synthesis during human cytomegalovirus infection. Journal of virology 85, 3930–3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clippinger AJ, Maguire TG, Alwine JC, 2011b. Human cytomegalovirus infection maintains mTOR activity and its perinuclear localization during amino acid deprivation. Journal of virology 85, 9369–9376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbs C, Khan S, Matlaf L, McAllister S, Zider A, Yount G, Rahlin K, Harkins L, Bezrookove V, Singer E, Soroceanu L, 2014. HCMV glycoprotein B is expressed in primary glioblastomas and enhances growth and invasiveness via PDGFR-alpha activation. Oncotarget 5, 1091–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbs CS, Soroceanu L, Denham S, Zhang W, Kraus MH, 2008. Modulation of Oncogenic Phenotype in Human Glioma Cells by Cytomegalovirus IE1- Mediated Mitogenicity. Cancer Research 68, 724–730. [DOI] [PubMed] [Google Scholar]
- Cojohari O, Peppenelli MA, Chan GC, 2016. Human cytomegalovirus induces an atypical activation of Akt to stimulate the survival of short-lived monocytes. Journal of Virology 90, JVI.00214–00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins T, Pomeroy C, Jordan MC, 1993. Detection of latent cytomegalovirus DNA in diverse organs of mice. J Infect Dis 168, 725–729. [DOI] [PubMed] [Google Scholar]
- Collins TM, Quirk MR, Jordan MC, 1994. Biphasic viremia and viral gene expression in leukocytes during acute cytomegalovirus infection of mice. J Virol 68, 6305–6311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins-McMillen D, Kim JH, Nogalski MT, Stevenson EV, Chan GC, Caskey JR, Cieply SJ, Yurochko AD, 2015. Human Cytomegalovirus Promotes Survival of Infected Monocytes via a Distinct Temporal Regulation of Cellular Bcl-2 Family Proteins. Journal of virology 90, 2356–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crough T, Khanna R, 2009. Immunobiology of human cytomegalovirus: from bench to bedside. Clinical microbiology reviews 22, 76–98, Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley-Bauer LP, Roback LJ, Wynn GM, Mocarski ES, 2014. Cytomegalovirus hijacks CX3CR1(hi) patrolling monocytes as immune-privileged vehicles for dissemination in mice. Cell host & microbe 15, 351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson CW, Tramountanis G, Eliopoulos AG, Young LS, 2003. Epstein-Barr Virus Latent Membrane Protein 1 (LMP1) Activates the Phosphatidylinositol 3-Kinase/Akt Pathway to Promote Cell Survival and Induce Actin Filament Remodeling. Journal of Biological Chemistry 278, 3694–3704. [DOI] [PubMed] [Google Scholar]
- Dollard SC, Grosse SD, Ross DS, 2007. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Reviews in Medical Virology 17, 355–363. [DOI] [PubMed] [Google Scholar]
- Echenique IA, Beltran D, Ramirez-Ruiz L, Najafian N, Agrawal N, 2017. Ganciclovir Dosing Strategies and Development of Cytomegalovirus Resistance in a Kidney Transplant Recipient: A Case Report. Transplant Proc 49, 1560–1564. [DOI] [PubMed] [Google Scholar]
- Fadden P, Haystead TA, Lawrence JC, 1997. Identification of phosphorylation sites in the translational regulator, PHAS-I, that are controlled by insulin and rapamycin in rat adipocytes. The Journal of biological chemistry 272, 10240–10247. [DOI] [PubMed] [Google Scholar]
- Faller WJ, Jackson TJ, Knight JRP, Ridgway RA, Jamieson T, Karim SA, Jones C, Radulescu S, Huels DJ, Myant KB, Dudek KM, Casey HA, Scopelliti A, Cordero JB, Vidal M, Pende M, Ryazanov AG, Sonenberg N, Meyuhas O, Hall MN, Bushell M, Willis AE, Sansom OJ, 2015. mTORC1-mediated translational elongation limits intestinal tumour initiation and growth. Nature 517, 497–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feire AL, Koss H, Compton T, 2004. Cellular integrins function as entry receptors for human cytomegalovirus via a highly conserved disintegrin-like domain. Proceedings of the National Academy of Sciences of the United States of America 101, 15470–15475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman JA, Emery V, Freeman R, Pascual M, Rostaing L, Schlitt HJ, Sgarabotto D, Torre-Cisneros J, Uknis ME, 2007a. Cytomegalovirus in transplantation - challenging the status quo. Clin Transplant 21, 149–158. [DOI] [PubMed] [Google Scholar]
- Fishman JA, Emery V, Freeman R, Pascual M, Rostaing L, Schlitt HJ, Sgarabotto D, Torre-Cisneros J, Uknis ME, 2007b. Cytomegalovirus in transplantation – challenging the status quo. Clinical Transplantation 21, 149–158. [DOI] [PubMed] [Google Scholar]
- Gao X, Pan D, 2001. TSC1 and TSC2 tumor suppressors antagonize insulin signaling in cell growth. Genes & Development 15, 1383–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatherer D, Seirafian S, Cunningham C, Holton M, Dargan DJ, Baluchova K, Hector RD, Galbraith J, Herzyk P, Wilkinson GW, Davison AJ, 2011. High-resolution human cytomegalovirus transcriptome. Proc Natl Acad Sci U S A 108, 19755–19760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover TE, Kew VG, Reeves MB, 2014. Rapamycin does not inhibit human cytomegalovirus reactivation from dendritic cells in vitro. J Gen Virol 95, 2260–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnann JW Jr., Ahlmen J, Svalander C, Olding L, Oldstone MB, Nelson JA, 1988. Inflammatory cells in transplanted kidneys are infected by human cytomegalovirus. Am J Pathol 132, 239–248. [PMC free article] [PubMed] [Google Scholar]
- Goodrum F, Caviness K, Zagallo P, 2012. Human cytomegalovirus persistence. Cellular Microbiology 14, 644–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal A, Wang Y, Graham MM, Doseff AI, Bhatt NY, Marsh CB, 2002. Monocyte survival factors induce Akt activation and suppress caspase-3. Am J Respir Cell Mol Biol 26, 224–230. [DOI] [PubMed] [Google Scholar]
- Griffiths PD, Boeckh M, 2007. Antiviral therapy for human cytomegalovirus. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. [Google Scholar]
- Guo H, Samarakoon A, Vanhaesebroeck B, Malarkannan S, 2008. The p110 delta of PI3K plays a critical role in NK cell terminal maturation and cytokine/chemokine generation. J Exp Med 205, 2419–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn G, Jores R, Mocarski ES, 1998. Cytomegalovirus remains latent in a common precursor of dendritic and myeloid cells. Proceedings of the National Academy of Sciences of the United States of America 95, 3937–3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamirally S, Kamil JP, Ndassa-Colday YM, Lin AJ, Jahng WJ, Baek M-C, Noton S, Silva LA, Simpson-Holley M, Knipe DM, Golan DE, Marto JA, Coen DM, 2009. Viral Mimicry of Cdc2/Cyclin-Dependent Kinase 1 Mediates Disruption of Nuclear Lamina during Human Cytomegalovirus Nuclear Egress. PLoS Pathogens 5, e1000275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG, Schaffer BE, Brunet A, 2016. AMPK: An Energy-Sensing Pathway with Multiple Inputs and Outputs. Trends in Cell Biology 26, 190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell SB, Wagner P, Kunz J, Deuter-Reinhard M, Henriquez R, Hall MN, 1994. TOR1 and TOR2 are structurally and functionally similar but not identical phosphatidylinositol kinase homologues in yeast. Molecular biology of the cell 5, 105–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbein G, Gras G, Khan K, Abbas W, 2010. Macrophage signaling in HIV-1 infection. Retrovirology 7, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber MT, Compton T, 1998. The human cytomegalovirus UL74 gene encodes the third component of the glycoprotein H-glycoprotein L-containing envelope complex. Journal of virology 72, 8191–8197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber MT, Compton T, 1999. Intracellular formation and processing of the heterotrimeric gH-gL-gO (gCIII) glycoprotein envelope complex of human cytomegalovirus. Journal of virology 73, 3886–3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez CE, Schrier R, Ghazal P, Wiley C, Nelson JA, 1991. Human cytomegalovirus productively infects primary differentiated macrophages. Journal of virology 65, 6581–6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba T, Shimano H, Gotoda T, Harada K, Shimada M, Ohsuga J, Watanabe Y, Kawamura M, Yazaki Y, Yamada N, 1993. Expression of platelet-derived growth factor beta receptor on human monocyte-derived macrophages and effects of platelet-derived growth factor BB dimer on the cellular function. The Journal of biological chemistry 268, 24353–24360. [PubMed] [Google Scholar]
- Inoki K, Li Y, Xu T, Guan K-L, 2003a. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes & Development 17, 1829–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan K-L, 2003b. TSC2 mediates cellular energy response to control cell growth and survival. Cell 115, 577–590. [DOI] [PubMed] [Google Scholar]
- Isaacson MK, Compton T, 2009. Human Cytomegalovirus Glycoprotein B Is Required for Virus Entry and Cell-to-Cell Spread but Not for Virion Attachment, Assembly, or Egress Journal of Virology, pp. 3891–3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson MK, Feire AL, Compton T, 2007. Epidermal Growth Factor Receptor Is Not Required for Human Cytomegalovirus Entry or Signaling. Journal of Virology 81, 6241–6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean Beltran PM, Cristea IM, 2014. The life cycle and pathogenesis of human cytomegalovirus infection: lessons from proteomics. Expert Rev Proteomics 11, 697–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RA, Wang X, Ma XL, Huong SM, Huang ES, 2001. Human cytomegalovirus up-regulates the phosphatidylinositol 3-kinase (PI3-K) pathway: inhibition of PI3-K activity inhibits viral replication and virus-induced signaling. Journal of virology 75, 6022–6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabanova A, Marcandalli J, Zhou T, Bianchi S, Baxa U, Tsybovsky Y, Lilleri D, Silacci-Fregni C, Foglierini M, Fernandez-Rodriguez BM, Druz A, Zhang B, Geiger R, Pagani M, Sallusto F, Kwong PD, Corti D, Lanzavecchia A, Perez L, 2016. Platelet-derived growth factor-α receptor is the cellular receptor for human cytomegalovirus gHgLgO trimer. Nature microbiology 1, 16082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenneson A, Cannon MJ, 2007. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Reviews in Medical Virology 17, 253–276. [DOI] [PubMed] [Google Scholar]
- Kerr WG, 2011. Inhibitor and activator: dual functions for SHIP in immunity and cancer. Annals of the New York Academy of Sciences 1217, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D-H, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM, 2002. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110, 163–175. [DOI] [PubMed] [Google Scholar]
- Kim JH, Collins-McMillen D, Buehler JC, Goodrum FD, Yurochko AD, 2017. Human Cytomegalovirus Requires Epidermal Growth Factor Receptor Signaling To Enter and Initiate the Early Steps in the Establishment of Latency in CD34+ Human Progenitor Cells. Journal of virology 91, e01206–01216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krettek A, Ostergren-Lundén G, Fager G, Rosmond C, Bondjers G, Lustig F, 2001. Expression of PDGF receptors and ligand-induced migration of partially differentiated human monocyte-derived macrophages. Influence of IFN-gamma and TGF-beta. Atherosclerosis 156, 267–275. [DOI] [PubMed] [Google Scholar]
- Kudchodkar SB, Del Prete GQ, Maguire TG, Alwine JC, 2007. AMPK-mediated inhibition of mTOR kinase is circumvented during immediate-early times of human cytomegalovirus infection. Journal of virology 81, 3649–3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudchodkar SB, Yu Y, Maguire TG, Alwine JC, 2004. Human cytomegalovirus infection induces rapamycin-insensitive phosphorylation of downstream effectors of mTOR kinase. Journal of virology 78, 11030–11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson S, Söderberg-Nauclér C, Wang FZ, Möller E, 1998. Cytomegalovirus DNA can be detected in peripheral blood mononuclear cells from all seropositive and most seronegative healthy blood donors over time. Transfusion 38, 271–278. [DOI] [PubMed] [Google Scholar]
- Lawlor G, Moss AC, 2010. Cytomegalovirus in inflammatory bowel disease: Pathogen or innocent bystander? Inflammatory Bowel Diseases 16, 1620–1627. [DOI] [PubMed] [Google Scholar]
- Lemmon MA, Schlessinger J, 2010. Cell signaling by receptor tyrosine kinases. Cell 141, 1117–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenarcic EM, Ziehr B, De Leon G, Mitchell D, Moorman NJ, 2014. Differential Role for Host Translation Factors in Host and Viral Protein Synthesis during Human Cytomegalovirus Infection. Journal of Virology 88, 1473–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Fischer E, Cohen JI, 2016. Cell Surface THY-1 Contributes to Human Cytomegalovirus Entry via a Macropinocytosis-Like Process. Journal of virology 90, 9766–9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Wilkie AR, Weller M, Liu X, Cohen JI, 2015. THY-1 Cell Surface Antigen (CD90) Has an Important Role in the Initial Stage of Human Cytomegalovirus Infection. PLoS pathogens 11, e1004999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipson SM, Shepp DH, Match ME, Axelrod FB, Whitbread JA, 2001. Cytomegalovirus Infectivity in Whole Blood Following Leukocyte Reduction by Filtration. American Journal of Clinical Pathology 116, 52–55. [DOI] [PubMed] [Google Scholar]
- Liu J, Jardetzky TS, Chin AL, Johnson DC, Vanarsdall AL, 2018. The human cytomegalovirus trimer and pentamer promote sequential steps in entry into epithelial and endothelial cells at cell surfaces and endosomes. Journal of Virology, JVI.013360–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Cheng H, Roberts TM, Zhao JJ, 2009. Targeting the phosphoinositide 3-kinase pathway in cancer. Nature reviews. Drug discovery 8, 627–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, Oppliger W, Jenoe P, Hall MN, 2002. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Molecular cell 10, 457–468. [DOI] [PubMed] [Google Scholar]
- Manicklal S, Emery VC, Lazzarotto T, Boppana SB, Gupta RK, 2013. The “Silent” Global Burden of Congenital Cytomegalovirus, Clinical Microbiology Reviews, pp. 86–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Cantley LC, 2007. AKT/PKB signaling: navigating downstream. Cell 129, 1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC, 2002. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Molecular cell 10, 151–162. [DOI] [PubMed] [Google Scholar]
- Martinez-Martin N, Marcandalli J, Huang CS, Arthur CP, Perotti M, Foglierini M, Ho H, Dosey AM, Shriver S, Payandeh J, Leitner A, Lanzavecchia A, Perez L, Ciferri C, 2018. An Unbiased Screen for Human Cytomegalovirus Identifies Neuropilin-2 as a Central Viral Receptor. Cell 174, 1158–1171.e1119. [DOI] [PubMed] [Google Scholar]
- Martini M, De Santis MC, Braccini L, Gulluni F, Hirsch E, 2014. PI3K/AKT signaling pathway and cancer: an updated review. Annals of medicine 46, 372–383. [DOI] [PubMed] [Google Scholar]
- Mazeron MC, 2000. [Leukocyte depletion and infection by cytomegalovirus]. Transfusion clinique et biologique : journal de la Societe francaise de transfusion sanguine 7 Suppl 1, 31s–35s. [DOI] [PubMed] [Google Scholar]
- McArdle J, Moorman NJ, Munger J, 2012. HCMV Targets the Metabolic Stress Response through Activation of AMPK Whose Activity Is Important for Viral Replication. PLoS Pathogens 8, e1002502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane S, Nicholl MJ, Sutherland JS, Preston CM, 2011. Interaction of the human cytomegalovirus particle with the host cell induces hypoxia-inducible factor 1 alpha. Virology 414, 83–90. [DOI] [PubMed] [Google Scholar]
- Mendelson M, Monard S, Sissons P, Sinclair J, 1996. Detection of endogenous human cytomegalovirus in CD34+ bone marrow progenitors. Journal of General Virology 77, 3099–3102. [DOI] [PubMed] [Google Scholar]
- Milbradt J, Auerochs S, Marschall M, 2007. Cytomegaloviral proteins pUL50 and pUL53 are associated with the nuclear lamina and interact with cellular protein kinase C. Journal of General Virology 88, 2642–2650. [DOI] [PubMed] [Google Scholar]
- Milbradt J, Kraut A, Hutterer C, Sonntag E, Schmeiser C, Ferro M, Wagner S, Lenac T, Claus C, Pinkert S, Hamilton ST, Rawlinson WD, Sticht H, Couté Y, Marschall M, 2014. Proteomic Analysis of the Multimeric Nuclear Egress Complex of Human Cytomegalovirus. Molecular & Cellular Proteomics 13, 2132–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrachy-Schwartz S, Cohen N, Klein S, Kravchenko-Balasha N, Levitzki A, 2011. Up-regulation of AMP-activated protein kinase in cancer cell lines is mediated through c-Src activation. J Biol Chem 286, 15268–15277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman NJ, Cristea IM, Terhune SS, Rout MP, Chait BT, Shenk T, 2008. Human Cytomegalovirus Protein UL38 Inhibits Host Cell Stress Responses by Antagonizing the Tuberous Sclerosis Protein Complex. Cell Host & Microbe 3, 253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman NJ, Shenk T, 2010. Rapamycin-Resistant mTORC1 Kinase Activity Is Required for Herpesvirus Replication. Journal of Virology 84, 5260–5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movassagh M, Gozlan J, Senechal B, Baillou C, Petit JC, Lemoine FM, 1996. Direct infection of CD34+ progenitor cells by human cytomegalovirus: evidence for inhibition of hematopoiesis and viral replication. Blood 88, 1277–1283. [PubMed] [Google Scholar]
- Nerheim PL, Meier JL, Vasef MA, Li WG, Hu L, Rice JB, Gavrila D, Richenbacher WE, Weintraub NL, 2004. Enhanced Cytomegalovirus Infection in Atherosclerotic Human Blood Vessels. American Journal of Pathology 164, 589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogalski MT, Chan G, Stevenson EV, Gray S, Yurochko AD, 2011. Human Cytomegalovirus-Regulated Paxillin in Monocytes Links Cellular Pathogenic Motility to the Process of Viral Entry. Journal of Virology 85, 1360–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogalski MT, Chan GCT, Stevenson EV, Collins-McMillen DK, Yurochko AD, 2013. The HCMV gH/gL/UL128–131 complex triggers the specific cellular activation required for efficient viral internalization into target monocytes. PLoS pathogens 9, e1003463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papakonstanti EA, Zwaenepoel O, Bilancio A, Burns E, Nock GE, Houseman B, Shokat K, Ridley AJ, Vanhaesebroeck B, 2008. Distinct roles of class IA PI3K isoforms in primary and immortalised macrophages. Journal of Cell Science 121, 4124–4133. [DOI] [PubMed] [Google Scholar]
- Pause A, Belsham GJ, Gingras A-C, Donzé O, Lin T-A, Lawrence JC, Sonenberg N, 1994. Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5’-cap function. Nature 371, 762–767. [DOI] [PubMed] [Google Scholar]
- Peppenelli MA, Arend KC, Cojohari O, Moorman NJ, Chan GC, 2016. Human Cytomegalovirus Stimulates the Synthesis of Select Akt-Dependent Antiapoptotic Proteins during Viral Entry To Promote Survival of Infected Monocytes. Journal of Virology 90, 3138–3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppenelli MA, Miller MJ, Altman AM, Cojohari O, Chan GC, 2018. Aberrant regulation of the Akt signaling network by human cytomegalovirus allows for targeting of infected monocytes. Antiviral Research 158, 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestova TV, Kolupaeva VG, Lomakin IB, Pilipenko EV, Shatsky IN, Agol VI, Hellen CUT, 2001. Molecular mechanisms of translation initiation in eukaryotes. Proceedings of the National Academy of Sciences 98, 7029–7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole E, Juss JK, Krishna B, Herre J, Chilvers ER, Sinclair J, 2015. Alveolar Macrophages Isolated Directly From Human Cytomegalovirus (HCMV)–Seropositive Individuals Are Sites of HCMV Reactivation In Vivo. Journal of Infectious Diseases 211, 1936–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichard MN, Kern ER, 2011. The search for new therapies for human cytomegalovirus infections. Virus research 157, 212–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauwel B, Jang SM, Cassano M, Kapopoulou A, Barde I, Trono D, 2015. Release of human cytomegalovirus from latency by a KAP1/TRIM28 phosphorylation switch. eLife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redpath NT, Foulstone EJ, Proud CG, 1996. Regulation of translation elongation factor-2 by insulin via a rapamycin-sensitive signalling pathway. The EMBO journal 15, 2291–2297. [PMC free article] [PubMed] [Google Scholar]
- Reeves MB, Breidenstein A, Compton T, 2012. Human cytomegalovirus activation of ERK and myeloid cell leukemia-1 protein correlates with survival of latently infected cells. Proceedings of the National Academy of Sciences of the United States of America 109, 588–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke P, Prösch S, Kern F, Volk H-D, 1999. Mechanisms of human cytomegalovirus (HCMV) (re)activation and its impact on organ transplant patients. Transplant Infectious Disease 1, 157–164. [DOI] [PubMed] [Google Scholar]
- Rubin R, Colvin RB, 1986. Cytomegalovirus infection in renal transplantation: clinical importance and control., in: Williams GM, Burdick JF, Solez K (Eds.), Kidney Transplant Rejection: Diagnosis and Treatment. Dekker, New York, pp. 283–304. [Google Scholar]
- Sagedal S, Hartmann A, Nordal KP, Osnes K, Leivestad T, Foss A, Degre M, Fauchald P, Rollag H, 2004. Impact of early cytomegalovirus infection and disease on long-term recipient and kidney graft survival. Kidney Int 66, 329–337. [DOI] [PubMed] [Google Scholar]
- Scheid MP, Huber M, Damen JE, Hughes M, Kang V, Neilsen P, Prestwich GD, Krystal G, Duronio V, 2002a. Phosphatidylinositol (3,4,5)P3 is essential but not sufficient for protein kinase B (PKB) activation; phosphatidylinositol (3,4)P2 is required for PKB phosphorylation at Ser-473: studies using cells from SH2-containing inositol-5-phosphatase knockout mice. The Journal of biological chemistry 277, 9027–9035. [DOI] [PubMed] [Google Scholar]
- Scheid MP, Marignani PA, Woodgett JR, 2002b. Multiple Phosphoinositide 3-Kinase-Dependent Steps in Activation of Protein Kinase B, Molecular and Cellular Biology, pp. 6247–6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier RD, Nelson JA, Oldstone MB, 1985. Detection of human cytomegalovirus in peripheral blood lymphocytes in a natural infection. Science (New York, N.Y.) 230, 1048–1051. [DOI] [PubMed] [Google Scholar]
- Shahbazian D, Roux PP, Mieulet V, Cohen MS, Raught B, Taunton J, Hershey JWB, Blenis J, Pende M, Sonenberg N, 2006. The mTOR/PI3K and MAPK pathways converge on eIF4B to control its phosphorylation and activity. The EMBO Journal 25, 2781–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Wisner TW, Johnson DC, Heldwein EE, 2013. HCMV gB shares structural and functional properties with gB proteins from other herpesviruses. Virology 435, 239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shnayder M, Nachshon A, Krishna B, Poole E, Boshkov A, Binyamin A, Maza I, Sinclair J, Schwartz M, Stern-Ginossar N, 2018. Defining the Transcriptional Landscape during Cytomegalovirus Latency with Single-Cell RNA Sequencing. MBio 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair J, Sissons P, 1996a. Latent and persistent infections of monocytes and macrophages. Intervirology 39, 293–301. [DOI] [PubMed] [Google Scholar]
- Sinclair J, Sissons P, 1996b. Latent and persistent infections of monocytes and macrophages. Intervirology 39, 293–301. [DOI] [PubMed] [Google Scholar]
- Sinclair J, Sissons P, 2006. Latency and reactivation of human cytomegalovirus. J Gen Virol 87, 1763–1779. [DOI] [PubMed] [Google Scholar]
- Sinzger C, Jahn G, 1996. Human Cytomegalovirus Cell Tropism and Pathogenesis. Intervirology 39, 302–319. [DOI] [PubMed] [Google Scholar]
- Sinzger C, Plachter B, Grefte A, The TH, Jahn G, 1996. Tissue macrophages are infected by human cytomegalovirus in vivo. The Journal of infectious diseases 173, 240–245. [DOI] [PubMed] [Google Scholar]
- Smith MS, Bentz GL, Alexander JS, Yurochko AD, 2004a. Human cytomegalovirus induces monocyte differentiation and migration as a strategy for dissemination and persistence. Journal of virology 78, 4444–4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MS, Bentz GL, Smith PM, Bivins ER, Yurochko AD, 2004b. HCMV activates PI(3)K in monocytes and promotes monocyte motility and transendothelial migration in a PI(3)K-dependent manner. Journal of leukocyte biology 76, 65–76. [DOI] [PubMed] [Google Scholar]
- Smith MS, Bivins-Smith ER, Tilley AM, Bentz GL, Chan G, Minard J, Yurochko AD, 2007. Roles of phosphatidylinositol 3-kinase and NF-kappaB in human cytomegalovirus-mediated monocyte diapedesis and adhesion: strategy for viral persistence. Journal of virology 81, 7683–7694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MS, Goldman DC, Bailey AS, Pfaffle DL, Kreklywich CN, Spencer DB, Othieno FA, Streblow DN, Garcia JV, Fleming WH, Nelson JA, 2010. Granulocyte-colony stimulating factor reactivates human cytomegalovirus in a latently infected humanized mouse model. Cell host & microbe 8, 284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderberg-Naucler C, Fish KN, Nelson JA, 1997a. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell 91, 119–126. [DOI] [PubMed] [Google Scholar]
- Soderberg-Naucler C, Fish KN, Nelson JA, 1997b. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell 91, 119–126. [DOI] [PubMed] [Google Scholar]
- Soderberg-Naucler C, Streblow DN, Fish KN, Allan-Yorke J, Smith PP, Nelson JA, 2001. Reactivation of latent human cytomegalovirus in CD14(+) monocytes is differentiation dependent. J Virol 75, 7543–7554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderberg-Nauclér C, Streblow DN, Fish KN, Allan-Yorke J, Smith PP, Nelson JA, 2001. Reactivation of latent human cytomegalovirus in CD14(+) monocytes is differentiation dependent. Journal of virology 75, 7543–7554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soroceanu L, Akhavan A, Cobbs CS, 2008. Platelet-derived growth factor-α receptor activation is required for human cytomegalovirus infection. Nature 455, 391–395. [DOI] [PubMed] [Google Scholar]
- Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ, 2006. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin.Infect.Dis. 43, 1143–1151. [DOI] [PubMed] [Google Scholar]
- Stevenson EV, Collins-McMillen D, Kim JH, Cieply SJ, Bentz GL, Yurochko AD, 2014. HCMV Reprogramming of Infected Monocyte Survival and Differentiation: A Goldilocks Phenomenon, Viruses, pp. 782–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddart CA, Cardin RD, Boname JM, Manning WC, Abenes GB, Mocarski ES, 1994. Peripheral blood mononuclear phagocytes mediate dissemination of murine cytomegalovirus. J Virol 68, 6243–6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straschewski S, Patrone M, Walther P, Gallina A, Mertens T, Frascaroli G, 2011. Protein pUL128 of human cytomegalovirus is necessary for monocyte infection and blocking of migration. Journal of virology 85, 5150–5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streblow DN, Nelson JA, 2003. Models of HCMV latency and reactivation. Trends Microbiol 11, 293–295. [DOI] [PubMed] [Google Scholar]
- Taylor-Wiedeman J, Hayhurst GP, Sissons JGP, Sinclair JH, 1993. Polymorphonuclear cells are not sites of persistence of human cytomegalovirus in healthy individuals. Journal of General Virology 74, 265–268. [DOI] [PubMed] [Google Scholar]
- Taylor-Wiedeman J, Sissons JG, Borysiewicz LK, Sinclair JH, 1991a. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J Gen Virol 72 ( Pt 9), 2059–2064. [DOI] [PubMed] [Google Scholar]
- Taylor-Wiedeman J, Sissons JGP, Borysiewicz LK, Sinclair JH, 1991b. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. Journal of General Virology 72, 2059–2064. [DOI] [PubMed] [Google Scholar]
- Taylor-Wiedeman J, Sissons P, Sinclair J, 1994a. Induction of endogenous human cytomegalovirus gene expression after differentiation of monocytes from healthy carriers. J Virol 68, 1597–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Wiedeman J, Sissons P, Sinclair J, 1994b. Induction of endogenous human cytomegalovirus gene expression after differentiation of monocytes from healthy carriers. Journal of virology 68, 1597–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry LJ, Vastag L, Rabinowitz JD, Shenk T, 2012. Human kinome profiling identifies a requirement for AMP-activated protein kinase during human cytomegalovirus infection. Proceedings of the National Academy of Sciences 109, 3071–3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreen Carson C., 2013. Many roads from mTOR to eIF4F. Biochemical Society Transactions 41, 913–916. [DOI] [PubMed] [Google Scholar]
- Thorpe LM, Yuzugullu H, Zhao JJ, 2015. PI3K in cancer: divergent roles of isoforms, modes of activation and therapeutic targeting. Nature reviews. Cancer 15, 7–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanarsdall AL, Pritchard SR, Wisner TW, Liu J, Jardetzky TS, Johnson DC, 2018. CD147 Promotes Entry of Pentamer-Expressing Human Cytomegalovirus into Epithelial and Endothelial Cells. mBio 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanarsdall AL, Wisner TW, Lei H, Kazlauskas A, Johnson DC, 2012. PDGF Receptor-α Does Not Promote HCMV Entry into Epithelial and Endothelial Cells but Increased Quantities Stimulate Entry by an Abnormal Pathway. PLoS Pathogens 8, e1002905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Higashi K, Raven C, Welham M, Anderson S, Brennan P, Ward SG, Waterfield MD, 1999. Autophosphorylation of p110delta phosphoinositide 3-kinase: a new paradigm for the regulation of lipid kinases in vitro and in vivo. EMBO J 18, 1292–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent HA, Ziehr B, Moorman NJ, 2016. Human Cytomegalovirus Strategies to Maintain and Promote mRNA Translation. Viruses 8, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan K, Smith MS, Malouli D, Mansouri M, Nelson JA, Früh K, 2011. BST2/Tetherin Enhances Entry of Human Cytomegalovirus. PLoS Pathogens 7, e1002332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Laer D, Meyer-Koenig U, Serr A, Finke J, Kanz L, Fauser AA, Neumann-Haefelin D, Brugger W, Hufert FT, 1995. Detection of cytomegalovirus DNA in CD34+ cells from blood and bone marrow. Blood 86, 4086–4090. [PubMed] [Google Scholar]
- Voss OH, Kim S, Wewers MD, Doseff AI, 2005. Regulation of monocyte apoptosis by the protein kinase Cdelta-dependent phosphorylation of caspase-3. J Biol Chem 280, 17371–17379. [DOI] [PubMed] [Google Scholar]
- Wang D, Shenk T, 2005. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc Natl Acad Sci U S A 102, 18153–18158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Wang X, Proud CG, 2000. Activation of mRNA translation in rat cardiac myocytes by insulin involves multiple rapamycin-sensitive steps. American Journal of Physiology-Heart and Circulatory Physiology 278, H1056–H1068. [DOI] [PubMed] [Google Scholar]
- Wang X, Huang DY, Huong S-M, Huang E-S, 2005a. Integrin alphavbeta3 is a coreceptor for human cytomegalovirus. Nature medicine 11, 515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Huang DY, Huong SM, Huang ES, 2005b. Integrin ab3 is a coreceptor for human cytomegalovirus. Nat Med 11, 515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Huong SM, Chiu ML, Raab-Traub N, Huang ES, 2003. Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature 424, 456–461. [DOI] [PubMed] [Google Scholar]
- Wang X, Li W, Williams M, Terada N, Alessi DR, Proud CG, 2001. Regulation of elongation factor 2 kinase by p90RSK1 and p70 S6 kinase. The EMBO Journal 20, 4370–4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw DM, 1966. The intravascular lifespan of monocytes. Blood 28, 455–464. [PubMed] [Google Scholar]
- Whitelaw DM, 1972. OBSERVATIONS ON HUMAN MONOCYTE KINETICS AFTER PULSE LABELING. Cell Proliferation 5, 311–317. [DOI] [PubMed] [Google Scholar]
- Wille PT, Wisner TW, Ryckman B, Johnson DC, 2013. Human cytomegalovirus (HCMV) glycoprotein gB promotes virus entry in trans acting as the viral fusion protein rather than as a receptor-binding protein. mBio 4, e00332–00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, 1995. Heat Shock Transcription Factors: Structure and Regulation. Annual Review of Cell and Developmental Biology 11, 441–469. [DOI] [PubMed] [Google Scholar]
- Wu Y, Prager A, Boos S, Resch M, Brizic I, Mach M, Wildner S, Scrivano L, Adler B, 2017. Human cytomegalovirus glycoprotein complex gH/gL/gO uses PDGFR-α as a key for entry. PLOS Pathogens 13, e1006281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Alwine JC, 2002. Human cytomegalovirus major immediate-early proteins and simian virus 40 large T antigen can inhibit apoptosis through activation of the phosphatidylinositide 3’-OH kinase pathway and the cellular kinase Akt. Journal of virology 76, 3731–3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung HW, Charnock-Jones DS, Burton GJ, 2011. Regulation of AKT phosphorylation at Ser473 and Thr308 by endoplasmic reticulum stress modulates substrate specificity in a severity dependent manner. PLoS One 6, e17894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurochko AD, Huang ES, 1999. Human cytomegalovirus binding to human monocytes induces immunoregulatory gene expression. Journal of immunology (Baltimore, Md. : 1950) 162, 4806–4816. [PubMed] [Google Scholar]
- Yurochko AD, Hwang E-S, Rasmussen L, Keay S, Pereira L, Huang E-S, 1997. The Human Cytomegalovirus UL55 (gB) and UL75 (gH) Glycoprotein Ligands Initiate the Rapid Activation of Sp1 and NF-B during Infection. Journal of Virology 71, 5051–5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ze AR, Feoktistova K, Avanzino BC, Fraser CS, 2011. Duplex Unwinding and ATPase Activities of the DEAD-Box Helicase eIF4A Are Coupled by eIF4G and eIF4B. Journal of Molecular Biology 412, 674–687. [DOI] [PMC free article] [PubMed] [Google Scholar]