Abstract
Approaches enabling chemoselective covalent modification of proteins in a site-specific manner has emerged as a powerful technology for a wide range of applications. The electron-rich unnatural amino acid 5-hydroxytryptophan was recently genetically encoded in both E. coli and eukaryotes, allowing its site-specific incorporation into virtually any recombinant protein. Here we report the chemoselective conjugation of various aromatic amines to full-length proteins under mild oxidative conditions targeting the site-specifically incorporated 5-hydroxytryptophan residue.
Keywords: bioconjugation, unnatural amino acid, genetic code expansion, site-specific protein modification
Graphical Abstract
The electron-rich unnatural amino acid 5-hydroxytryptophan was recently genetically encoded in both bacteria and eukaryotes. A chemoselective protein bioconjugation strategy is described here that labels site-specifically incorporated 5-hydroxytryptophan residues with aromatic amines under mild oxidative conditions.
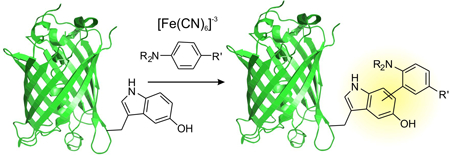
The ability to reliably label proteins at predefined sites has emerged as a powerful technology in chemical biology to both interrogate and engineer their structure and function. Over the last few decades, a variety of chemical strategies have been developed to label proteins at specific canonical amino acid residues, selectively at the termini, as well as at unnatural amino acid (UAA) residues.[1] UAAs can be co-translationally and site-specifically introduced into recombinant proteins using the genetic code expansion approach.[2] This technology uses a repurposed nonsense codon to specify the position of the desired UAA, which is suppressed by an engineered aminoacyl-tRNA synthetase (aaRS)/tRNA pair.[2] The ability to chemically modify a protein at predefined sites represents a major strength of the UAA-directed labeling approach. One particularly useful chemical strategy for protein bioconjugation has taken advantage of oxidative cross-coupling reactions between an electron rich aromatic amino acid and a suitable reaction partner. For example, tyrosine residues have been labeled under oxidative conditions by a variety of entities such as aromatic amines and phenols.[3] However, such labeling strategies targeted to canonical amino acids intrinsically lack site-selectivity, since the target residue may be present at multiple positions. Identification of electron-rich aromatic groups that could be chemoselectively functionalized under mild oxidative conditions, and the ability to site-specifically incorporate them into target proteins can create new approaches for site-selective protein labeling.[4] Here we show that the 5-hydroxytryptophan (5HTP) residue, which has been recently genetically encoded in both E. coli and eukaryotes,[5] can be selectively and efficiently labeled with a variety of aromatic amines under relatively mild oxidative conditions.
Recently, our group has developed a novel tryptophanyl-tRNA synthetase (EcTrpRS)/tRNA pair which can be used for UAA mutagenesis both in an engineered E. coli strain, ATMW1, as well as in eukaryotic cells.[5a] We further engineered this EcTrpRS/tRNA pair to achieve site-specific incorporation of a variety of tryptophan derivatives, including 5-hydroxytryptophan (5HTP, 1), with high fidelity and efficiency.[5a] 5HTP is an inexpensive and readily available unnatural amino acid with an electron rich 5-hydroxyindole functionality. We envisioned that it might be possible to develop novel chemoselective bioconjugation strategies taking advantage of this unusually electron-rich genetically encoded 5-hydroxyindole group. Indeed, we have recently demonstrated that 5HTP undergoes azo-coupling reaction significantly faster than canonical amino acids (e.g., tyrosine), providing a basis for chemoselective protein labeling.[6] 5-hydroxyindoles such as serotonins are also known to be prone to oxidation under relatively mild conditions, producing oligomeric products.[7] This provides an opportunity to develop new chemoselective protein modification strategies targeted to 5HTP through oxidative coupling reactions (Figure 1).
Figure 1.
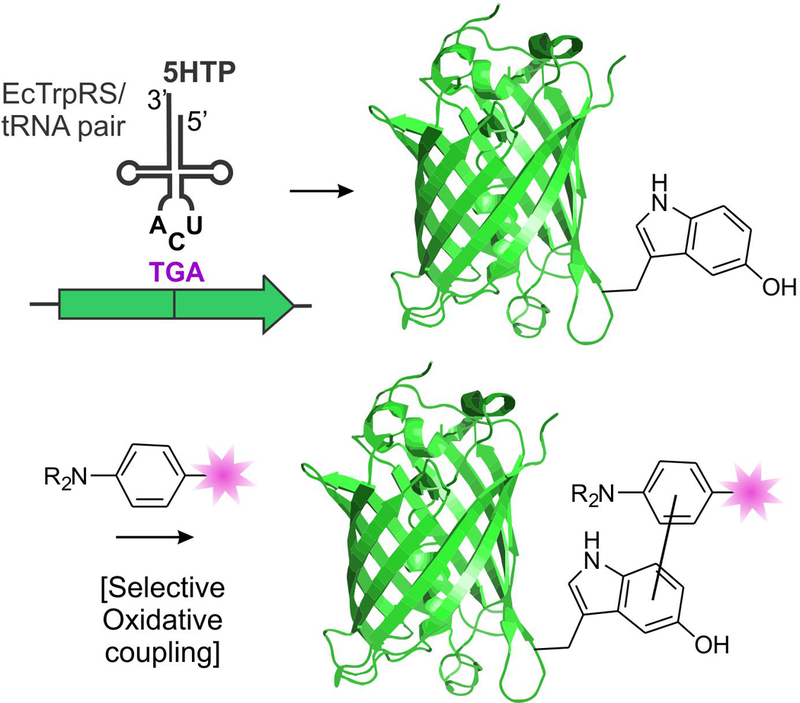
The genetic code expansion technology can be used to site-specifically incorporate the 5HTP residue into full-length proteins, which can be targeted for bioconjugation through the development of chemoselective oxidative coupling reactions.
To develop such a 5HTP-directed oxidative protein labeling reaction, it is necessary to have a mild oxidant that is compatible with proteins, and a coupling partner that would selectively react with 5HTP under these conditions. Aromatic amines have been used as coupling partners with much success in analogous oxidative coupling reactions targeted to electron-rich functionalities such as aminophenols.[4a-c] To trigger similar oxidative coupling reaction on proteins, relatively mild oxidants such as ferricyanide and periodate have been employed.[4] We noted that the treatment of free 5HTP with potassium ferricyanide led to its rapid conversion to a complex product mixture (Figure S1), indicating the potential suitability of this reagent to trigger its oxidative modification. It has been well-established that under oxidative conditions, 5-hydroxyindoles undergo self-coupling to generate a variety of oligomeric products.[7] To explore if aromatic amines can couple with 5HTP under oxidative conditions, we incubated a protected 5HTP (2; 0.1 mM) with an excess of p-toluidine (3; 1 mM) in methanolic phosphate buffer (pH 7) in the presence of 0.5 mM potassium ferricyanide. HPLC-coupled MS analysis of the reaction mixture showed a complex product mixture (Figure S2); we noted the presence of products whose masses are consistent with both self-oligomerization of 2 as well as its cross-coupling product with p-toluidine (Figure S2). Despite the complex outcome of this reaction, the presence of the desired cross-coupling product (Figure S2B) was encouraging, and indicated that further optimization may enable selective labeling of 5HTP residues on a protein with aromatic amines. Additionally, when incorporated into a protein, the site-isolated nature of the 5HTP residue should significantly suppress its self-coupling (the major undesired side-reaction), further promoting its cross-coupling with the aromatic amine – which may be used in excess. Indeed, similar electron-rich aromatic groups (e.g., aminophenols) on proteins have been previously subjected to efficient oxidative cross-coupling with aromatic amines.[4a-d]
To explore the feasibility of labeling proteins using this strategy, we generated a mutant superfolder green fluorescent protein harboring a 5HTP residue at a surface exposed site (sfGFP-151–5HTP). To this end, the sfGFP-151-TGA gene was expressed in our previously described ATMW1 strain in the presence of a TGA-suppressing TrpRS/tRNA pair, which can selectively charge 5HTP from the growth medium.[5a] The resulting sfGFP-151–5HTP protein was purified using a C-terminal poly-histidine tag by immobilized metal ion affinity chromatography with excellent yields (92 mg/L). Subsequent MS-analysis of the full-length protein revealed a mass consistent with successful incorporation of 5HTP at the desired site (Figure S3). We also purified the wild-type sfGFP protein as a control, which harbors a tyrosine residue at the 151 site. We incubated 10 µM sfGFP-151–5HTP on ice with varying concentrations of potassium ferricyanide and 2-(4-aminophenyl)ethan-1-ol (4, Figure 2A; model aromatic amine) in 100 mM phosphate buffer (pH 7), and monitored the fate of the reaction by HPLC-coupled mass-spectrometry (MS) analysis (Figure 2B-C). Through such efforts, we were able to find an optimal condition (10 µM protein, 50 µM ferricyanide and 2 mM aromatic amine in aqueous phosphate buffer, pH 7, on ice) that led to clean and complete functionalization of sfGFP-151–5HTP with the aromatic amine (Figure 2). Quenching the modification reaction with excess TCEP under optimized labeling conditions revealed near-complete labeling of sfGFP-151–5HTP within 30 minutes (Figure 2D). When the wild-type sfGFP was subjected to identical labeling conditions, no modification was observed, highlighting the chemoselective nature of this reaction (Figure S4). We also screened several other oxidants, including periodate, ceric ammonium nitrate and Fremy’s salt, but found only ferricyanide to facilitate efficient and clean protein labeling.
Figure 2.
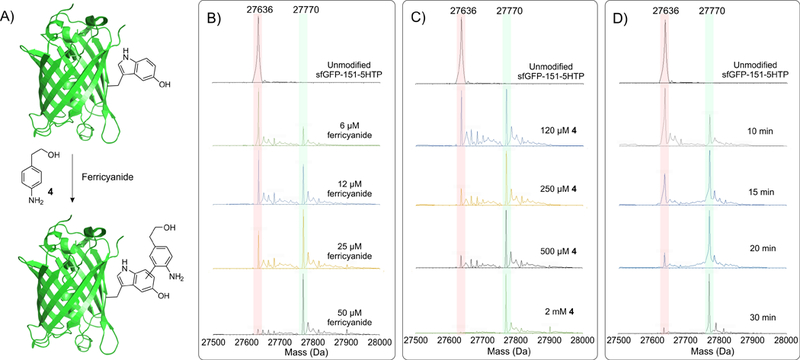
Optimization of the oxidative coupling of the aromatic amine 4 to sfGFP-151–5HTP. A) Scheme of the reaction. For all reactions, 10 µM protein in 100 mM phosphate buffer was incubated on ice with varying concentrations of potassium ferricyanide and the aromatic amine 4 for different lengths of time. Coupling of 4 to sfGFP-151–5HTP is monitored by whole-protein ESI-MS analysis; the expected peaks for the unmodified and the modified protein are highlighted. B) To find optimal ferricyanide concentration, it was systematically changed keeping the concentration of 4 constant at 2 mM. C) To find the optimal concentration of 4, it was systematically altered, keeping ferricyanide concentration constant at 50 µM. D) A conjugation reaction under the optimal conditions (50 µM ferricyanide, 2 mM 4) was quenched at different times with TCEP to reveal near-complete modification within 30 minutes.
Next, we investigated if other aromatic amines can also be attached to 5HTP residue using the optimized labeling condition. We were able to demonstrate successful labeling of sfGFP-151–5HTP with p-toluidine (3), p-aminophenylalanine (5) and N,N-dimethylaniline (6), while identical treatment of wild-type sfGFP did not result in a modification observable by HPLC-MS analysis (Figure 3). Chemoselective bioconjugation reactions are frequently used to site-specifically attach biophysical probes, such as fluorophores, onto target proteins. We wondered if our conjugation reaction can be used to attach the fluorescent probe aminofluorescein (7; Figure 4A), which harbors a built-in aromatic amine, onto 5HTP. Both wild-type sfGFP and sfGFP-151–5HTP were subjected to oxidative modification using aminofluorescein as the conjugation partner, and the resulting reaction was monitored using HPLC-MS, as well as fluorescence imaging following resolution of the protein by SDS-PAGE (Figure 4). Both analyses showed efficient labeling of sfGFP-151–5HTP but not wild-type sfGFP, further confirming the selectivity and versatility of this labeling reaction.
Figure 3.
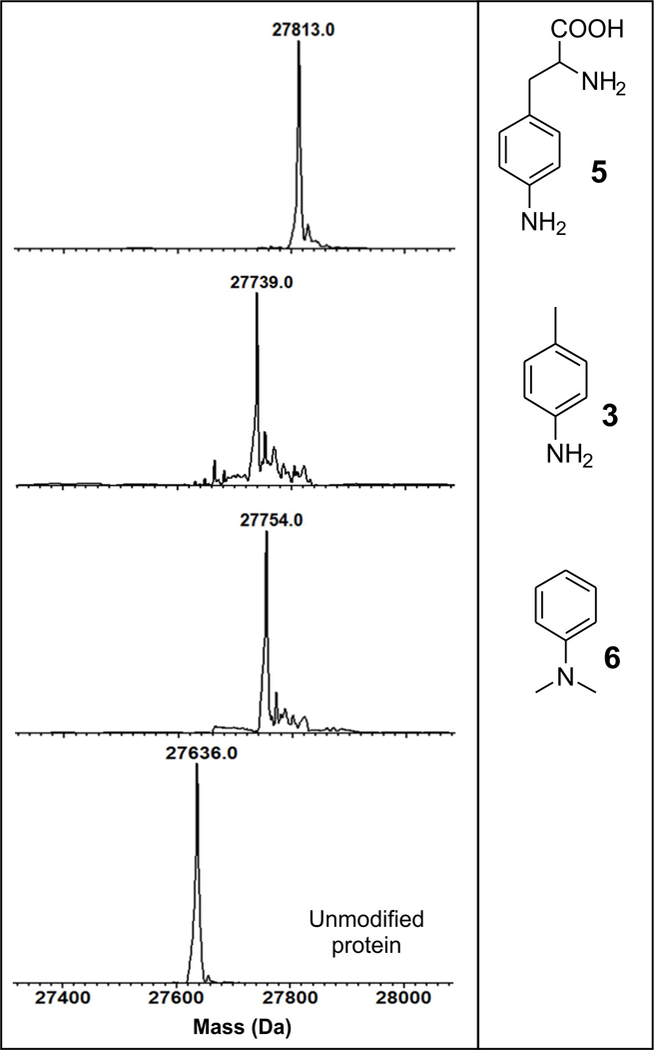
Conjugation of different aromatic amines to sfGFP-151–5HTP under optimized labeling conditions (Figure 2).
Figure 4.
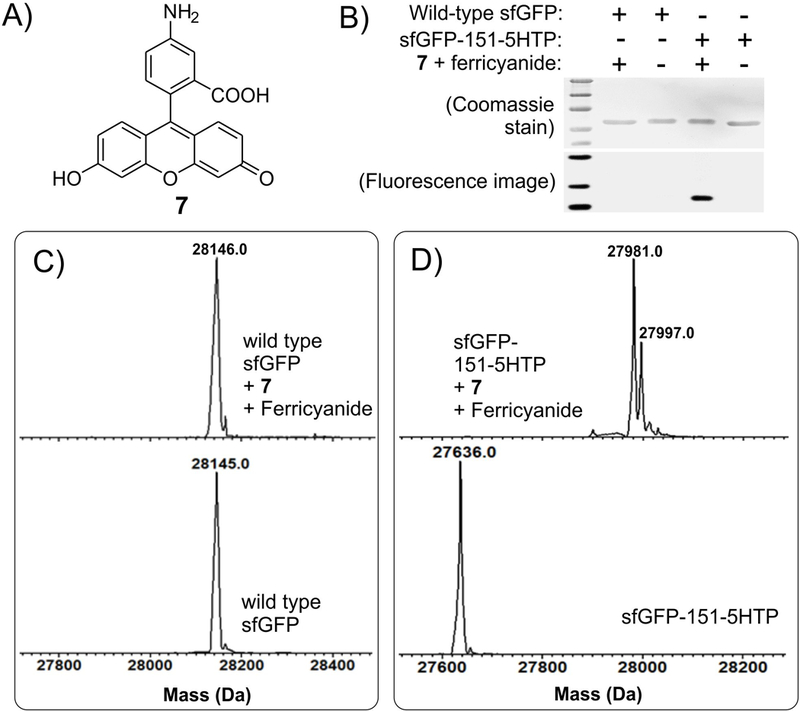
Labeling sfGFP-151–5HTP with aminofluorescein (7) under optimized conditions. A) Structure of aminofluorescein. Treatment of sfGFP-151–5HTP and wild-type sfGFP under optimized oxidative coupling reaction to 7 results in the modification of the former but not the latter, as shown by SDS-PAGE followed by fluorescence imaging and Coomassie staining (panel B), as well as by whole-protein ESI-MS analysis (panels C, D). The 27997 Da peak is likely an oxidation product.
Even though the conjugation of aromatic amines to a 5HTP residue on a protein occurs cleanly and efficiently under the optimized conditions described above, attempts at reconstituting this reaction using free 5HTP yields complex mixtures (e.g., Figure S2). As mentioned earlier, this could be due to the site-isolated nature of the 5HTP residue on a protein that suppresses unwanted side-reactions involving another 5HTP. Indeed, under our optimized conditions, we see no evidence of protein dimerization from potential coupling between two 5HTP residues. However, the difficulty of cleanly reconstituting this reaction using free 5HTP has so far precluded a thorough structural characterization of the resulting conjugate and the mechanism of its formation. It is possible that 5HTP can couple to the aromatic amine from a radical intermediate following a single electron oxidation, or from a further oxidized intermediate through the nucleophilic addition of the amine (Figure S5). The fact that N,N-dimethylaniline was able to efficiently couple to sfGFP-151–5HTP argues in favor of the former. We propose a mechanism involving bond formation between the 4-position of the 5-hydroxyindole ring and the 2-position of the aromatic amine, which is consistent with these observations and the established reactivity of 3-substituted 5-hydroxyindoles to generate 4–4´self-coupling products when subjected to oxidation.[7] Efforts are currently under way to unambiguously characterize the structure of the coupling product of this reaction.
In summary, here we show that the electron-rich 5-hydroxyindole residue of 5HTP can be chemoselectively labeled with a variety of aromatic amines under mild conditions using an oxidative coupling reaction. This relatively small and inexpensive UAA can be readily incorporated into recombinant proteins expressed in both E. coli and eukaryotic expression systems. It is also likely that this conjugation strategy would be compatible with other established reactions commonly used for protein labeling, which is currently under investigation.
Supplementary Material
Acknowledgements
We thank the National Institute of Health (R01GM124319 to A.C.) for financial support.
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- [1] a).Stephanopoulos N, Francis MB, Nat. Chem. Biol 2011, 7, 876–884; [DOI] [PubMed] [Google Scholar]; b) deGruyter JN, Malins LR, Baran PS, Biochemistry 2017, 56, 3863–3873; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Koniev O, Wagner A, Chem. Soc. Rev 2015, 44, 5495–5551; [DOI] [PubMed] [Google Scholar]; d) Boutureira O, Bernardes G. a. J., Chem. Rev 2015, 115, 2174–2195; [DOI] [PubMed] [Google Scholar]; e) Lin S, Yang X, Jia S, Weeks AM, Hornsby M, Lee PS, Nichiporuk RV, Iavarone AT, Wells JA, Toste FD, Science 2017, 355, 597–602; [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Spicer CD, Davis BG, Nat. Commun 2014, 5, ncomms5740; [DOI] [PubMed] [Google Scholar]; g) Lang K, Chin JW, Chem. Rev 2014, 114, 4764–4806; [DOI] [PubMed] [Google Scholar]; h) Lang K, Chin JW, ACS Chem. Biol 2014, 9, 16–20; [DOI] [PubMed] [Google Scholar]; i) Bloom S, Liu C, Kölmel DK, Qiao JX, Zhang Y, Poss MA, Ewing WR, MacMillan DW, Nat. Chem 2018, 10, 205; [DOI] [PMC free article] [PubMed] [Google Scholar]; j) Rosen CB, Francis MB, Nat. Chem. Biol 2017, 13, 697–705; [DOI] [PubMed] [Google Scholar]; k) Kim CH, Axup JY, Schultz PG, Curr. Opin. Chem. Biol 2013, 17, 412–419; [DOI] [PMC free article] [PubMed] [Google Scholar]; l) Shih HW, Kamber DN, Prescher JA, Curr. Opin. Chem. Biol 2014, 21, 103–111; [DOI] [PubMed] [Google Scholar]; m) Agarwal P, Bertozzi CR, Bioconjugate Chem 2015, 26, 176–192; [DOI] [PMC free article] [PubMed] [Google Scholar]; n) Sletten EM, Bertozzi CR, Angew. Chem. Int. Ed 2009, 48, 6974–6998; [DOI] [PMC free article] [PubMed] [Google Scholar]; o) Basle E, Joubert N, Pucheault M, Chem. Biol 2010, 17, 213–227. [DOI] [PubMed] [Google Scholar]
- [2] a).Chin JW, Nature 2017, 550, 53; [DOI] [PubMed] [Google Scholar]; b) Young DD, Schultz PG, ACS Chem. Biol 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Italia JS, Zheng Y, Kelemen RE, Erickson SB, Addy PS, Chatterjee A, Biochem. Soc. Trans 2017, 45, 555–562; [DOI] [PubMed] [Google Scholar]; d) Mukai T, Lajoie MJ, Englert M, Söll D, Annu. Rev. Microbiol 2017, 71, 557–577; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Dumas A, Lercher L, Spicer CD, Davis BG, Chem. Sci 2015, 6, 50–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3] a).Kodadek T, Duroux-Richard I, Bonnafous J-C, Trends Pharmacol. Sci 2005, 26, 210–217; [DOI] [PubMed] [Google Scholar]; b) Sato S, Nakamura H, Angew. Chem. Int. Ed 2013, 52, 8681–8684; [DOI] [PubMed] [Google Scholar]; c) Seim KL, Obermeyer AC, Francis MB, J. Am. Chem. Soc 2011, 133, 16970–16976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4] a).Carrico ZM, Romanini DW, Mehl RA, Francis MB, Chem. Commun 2008, 1205–1207; [DOI] [PubMed] [Google Scholar]; b) Hooker JM, Esser-Kahn AP, Francis MB, J. Am. Chem. Soc 2006, 128, 15558–15559; [DOI] [PubMed] [Google Scholar]; c) Obermeyer AC, Jarman JB, Netirojjanakul C, El Muslemany K, Francis MB, Angew. Chem. Int. Ed 2014, 53, 1057–1061; [DOI] [PubMed] [Google Scholar]; d) Stephanopoulos N, Tong GJ, Hsiao SC, Francis MB, ACS nano 2010, 4, 6014–6020; [DOI] [PubMed] [Google Scholar]; e) Burdine L, Gillette TG, Lin H-J, Kodadek T, J. Am. Chem. Soc 2004, 126, 11442–11443. [DOI] [PubMed] [Google Scholar]
- [5] a).Italia JS, Addy PS, Wrobel CJ, Crawford LA, Lajoie MJ, Zheng Y, Chatterjee A, Nat. Chem. Biol 2017, 13, 446–450; [DOI] [PubMed] [Google Scholar]; b) Ellefson JW, Meyer AJ, Hughes RA, Cannon JR, Brodbelt JS, Ellington AD, Nat. Biotech 2014, 32, 97. [DOI] [PubMed] [Google Scholar]
- [6].Addy PS, Erickson SB, Italia JS, Chatterjee A, J. Am. Chem. Soc 2017, 139, 11670–11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7] a).Napolitano A, d’Ischia M, Prota G, Tetrahedron 1988, 44, 7265–7270; [Google Scholar]; b) Wrona MZ, Dryhurst G, Chem. Res. Toxicol 1998, 11, 639–650; [DOI] [PubMed] [Google Scholar]; c) Huether G, Reimer A, Schmidt F, Schuff-Werner P, Brudny M, in Amine Oxidases and Their Impact on Neurobiology, Springer, 1990, pp. 249–257; [Google Scholar]; d) Akeel T, J. Chem. Soc., Perkin Trans 2 1992, 657–661. [Google Scholar]; Anden NE, Magnusson T, Acta Physiol 1967, 69, 87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


