Abstract
Disturbances in the potassium homeostasis are common among patients with heart failure (HF) and negatively affect clinical outcome. Patients with HF have a higher prevalence of common risk factors related to hyperkalaemia, including diabetes mellitus, hypertension, and chronic kidney disease. Furthermore, the use of renin–angiotensin–aldosterone system (RAAS) inhibitors, is an important risk factor for developing hyperkalaemia. The association between hyperkalaemia and mortality is not unequivocal, depends on the study type (trial vs. real-world setting) and is often confounded. More importantly, hyperkalaemia is an important cause of discontinuation or failure to uptitrate to guideline recommended dosages of RAAS inhibitors, which in turn may negatively impact clinical outcomes. The goal of this review is to discuss the epidemiology, aetiology, and clinical consequences of potassium disturbances in HF.
Keywords: Potassium, Hyperkalaemia, Treatment response, Clinical outcomes
Introduction
Potassium levels are often routinely measured in patients with heart failure (HF) and HF guidelines recommend frequent measurement of potassium during hospitalization for HF.1 In the general population, disturbances in potassium homeostasis are associated with (insulin dependent) diabetes, chronic kidney disease, hypertension and use of renin–angiotensin–aldosterone system (RAAS) inhibitors, as well as diuretics.2–4 Hyperkalaemia is associated with worse outcomes in patients with HF as well as with discontinuation or a reduction of RAAS inhibitors, which may impact survival.5–8 Therefore, hyperkalaemia warrants specific attention. This is even more relevant due to the emergence of new treatment possibilities including novel potassium binding agents.9 This review summarizes the aetiology, epidemiology, and clinical consequences of hyperkalaemia in HF.
Clinical causes and aetiology of hyperkalaemia
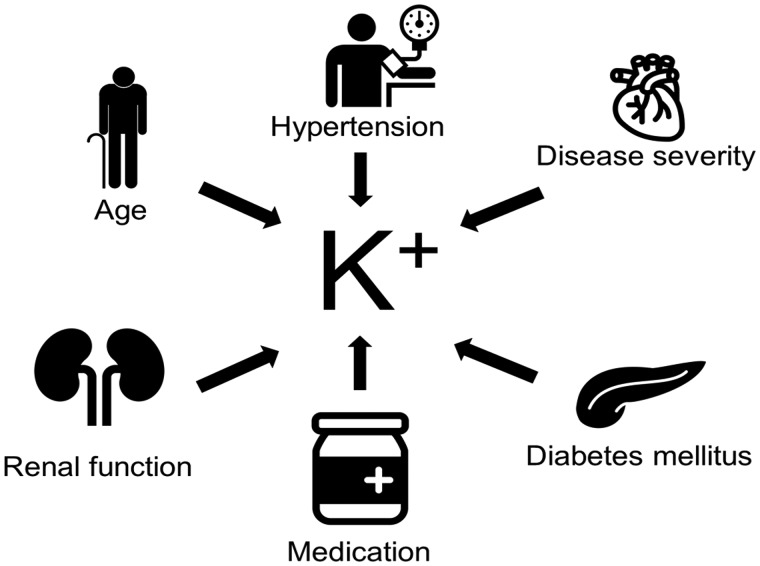
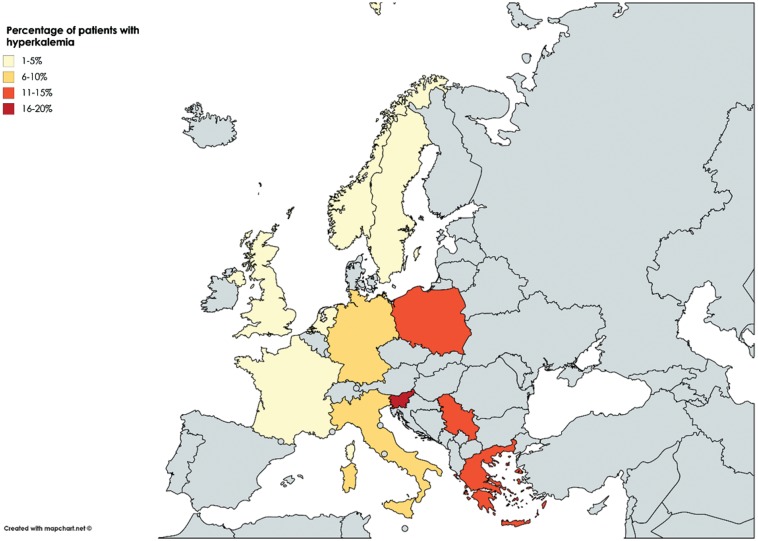
Hyperkalaemia is an often observed and potentially dangerous event in patients with HF. Yet, in patients with HF, volume overload and activation of the RAAS will lead to potassium excretion and sodium reabsorption in the proximal tubule of the kidney, which suggests that patients with HF have a tendency for lower potassium levels. Then why is hyperkalaemia an often-observed event in patients with HF? Hyperkalaemia is caused by a complex interplay of both environmental as well as physiological factors. Predictors of hyperkalaemia in the Candesartan in Heart Failure-Assessment of Reduction in Mortality and Morbidity (CHARM) program included age ≥75 years, male gender, diabetes, creatinine ≥2.0 mg/dL, and background use of angiotensin-converting enzyme (ACE) inhibitors or spironolactone. Furthermore, candesartan increased the risk of hyperkalaemia from 1.8% in placebo to 5.3%, however, in this post hoc analysis no strict threshold for hyperkalaemia was defined as the definition of hyperkalaemia as an adverse event was determined by the treating physician.7 These findings were confirmed in other randomized controlled trials including the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF) trial, the Trial of Intensified vs. Standard Medical Therapy in Elderly Patients with Congestive Heart Failure (TIME-CHF), in a retrospective analysis of the Studies of Left Ventricular Dysfunction (SOLVD) trials and in a more recent example of a real-world population from the BIOSTAT-CHF study, (Figure 1).8,10–12 Particularly in the BIOSTAT-CHF study, the prevalence of hyperkalaemia showed a distinct geographical distribution independent of risk factors for developing hyperkalaemia including age, sex, usage of RAAS inhibition, and the presence of diabetes mellitus and hypertension, suggesting possible differences in potassium monitoring as well as clinical practice across Europe (Figure 2).10
Figure 1.
Factors in patients with heart failure associated with hyperkalaemia.
Figure 2.
Differences in prevalence of hyperkalaemia across Europe (reproduced with permission from de Denus et al.12).
In physiological conditions, potassium is sequestrated from the plasma, creating an equilibrium between potassium intake and excretion. Two processes are key, first the Na+/K+ ATPase pump is important for exchanging intracellular Na+ for extracellular K+.5,13–15 This process is of particular importance in stress situations under the influence from the sympathetic nervous system through beta-2-receptors as well as insulin, where cellular uptake of potassium is increased following an increased potassium load.16 Following, potassium homeostasis is established mostly by excretion of potassium via urine. This also explains the key importance of renal function in potassium homeostasis. In physiological circumstances, potassium is filtered through the glomerular capillaries and excreted by the distal collecting duct. This process is highly dependent on adequate function of the RAAS and renal perfusion as well as sodium availability to the distal nephron.15 Hence, disturbance of the RAAS, reduction of renal perfusion and reduction in sodium availability are all risk factors for hyperkalaemia.
In the case of HF all these processes are disturbed: the RAAS is up-regulated, renal perfusion is reduced and sodium is often excreted due to usage of diuretics. Particularly in diabetics, the elderly, and patients on RAAS inhibition, aldosterone production is reduced.17,18 Additional age dependent reduction in the availability of nephrons further increases the risk for hyperkalaemia.19 In addition, beta-blockers are also associated with a decreased cellular uptake of potassium by inhibition of the sympathetic nervous system, which increases the risk for a hyperkalaemic event.16 The association between hypertension and hyperkalaemia is characterized by pseudohypoaldosteronism, renal tubular unresponsiveness to aldosterone, which leads to hyperkalaemia and metabolic acidosis.20 However, the association between hypertension and hyperkalaemia in HF is not unequivocal and previous reports might have been confounded by renal function and medication use.
Incidence and prevalence of hyperkalaemia in heart failure
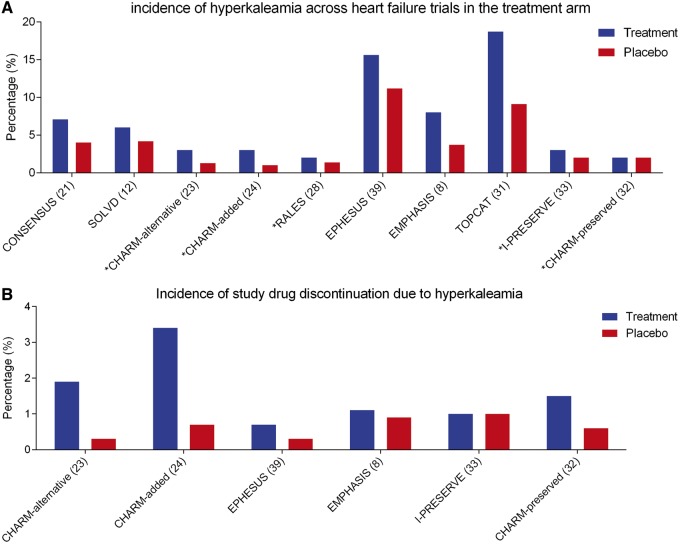
The overall reported incidence of hyperkalaemia differs depending on the study setting (trial vs. registry) and severity (acute vs. chronic) of HF. Among clinical trials involving RAAS inhibitors as a monotherapy, the incidence of hyperkalaemia ranges from 3% to 7% (Figure 3). In the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS), incidence rates of hyperkalaemia were almost double (7.1% vs. 4.0%) in the group treated with enalapril compared with placebo during a mean 188 days follow-up.21 In the SOLVD trials, where patients were also randomized to either placebo or enalapril, the incidence rates of hyperkalaemia during a mean follow-up of 2.7 years were 6% in the treatment group and 4.2% in the placebo group.12,22 When a definition of 6.0 mEq/L was used as a definition of hyperkalaemia, the incidence rate in the treatment group dropped to 1.1%.12,22 Nevertheless, the incidence in the SOLVD and CONSENSUS trials are largely underestimating contemporary rates of hyperkalaemia, as both trials were performed in an era where no other background RAAS inhibition was prescribed.22 In the results of Candesartan in Heart failure-Assessment of moRtality and Morbidity (CHARM) alternative trial, where 2258 patients intolerant to ACE inhibitors were randomized to candesartan or placebo, incidence hyperkalaemia (>6.0 mEq/L) was 3% compared with 1.3% in the placebo group during a median follow-up of 33.7 months. Here, hyperkalaemia was defined as a potassium level of >6.0 mEq/L.23 When candesartan was added to treatment with background ACE inhibition in the CHARM-Added trial, overall incidence rates of hyperkalaemia were 3% in the treatment arm compared with 1% in the placebo arm during a 41 months median follow-up time.24 The relatively lower incidence rates of hyperkalaemia in the treatment arm of the CHARM-added trial (3% in 41 months median follow-up) compared with the CHARM-alternative trial (3% in 33.7 months median follow-up), can potentially be explained by differences in the study population. Patients included in the CHARM-alternative trial had a higher prevalence of background treatment with spironolactone (25% in CHARM-alternative vs. 17% in CHARM-added), which based on previous results from a follow-up to the RALES trial is an important risk factor for developing hyperkalaemia when additional RAAS inhibition is added.23–25
Figure 3.
Prevalence of hyperkalaemia across heart failure trials (A). Discontinuation of study drug due to hyperkalaemic events across heart failure trials (B). Asterisk (*) denotes a definition of hyperkalaemia as >6.0 mEq/L.
In trials involving aldosterone inhibition, overall incidence rates of hyperkalaemia were on average higher. In the EMPHASIS-HF study, where 2737 patients were either randomized to the MRA eplerenone or placebo, incident hyperkalaemia (>5.5 mEq/L) was 8.0% in the treatment group compared with 3.7% in the placebo group.8,26 However, the incidence rate of hyperkalaemia in this study is probably underestimated as patients with potassium levels >5.0 mEq/L were excluded at study inclusion.26 In the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS), 6632 patients with acute myocardial infarction complicated by left ventricular dysfunction and HF, incidence rates of hyperkalaemia were 15.6% in the patient group treated with eplerenone vs. 11.2% in the placebo group during a 16 month mean follow-up, but again similar to the EMPHASIS-HF trial, patients with potassium of >5.0 mEq/L were excluded.27 In contrast to the EMPHASIS-HF and EPHESUS trials, the Randomized Aldactone Evaluation Study (RALES) reported rates of hyperkalaemia (>6.0 mEq/L) with 2% in the study group treated with spironolactone vs. 1.4% in the placebo group during a follow-up of 24 months.28 The overall incident rates of hyperkalaemia seemed lower in the RALES trial due to the stricter definition of hyperkalaemia (>6.0 mEq/L) and the lower prevalence of background RAAS inhibition by ACE inhibitors/angiotensin II receptor blockers (ARB).26–28 The fact that this considerably influenced incidence rates of hyperkalaemia is further supported by a follow-up study, which showed that after the introduction of spironolactone on top of treatment with ACE inhibitors, the rate of hospitalization for hyperkalaemia rose from 2.4 per 1000 patients in 1994 to 11.0 per 1000 patients in 2001.25
A more recent study is the Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) study, which saw 8399 patients randomized to either entresto or enalapril in addition to recommended therapy. Incidence rates were considerably higher in this trial compared with previous trials involving RAAS inhibition, incidence rates of hyperkalaemia were similar between patients treated with entresto (16.1%) and enalapril (17.3). When using a more stringent definition of >6.0 mEq/L, patients treated with entresto (4.3%) had lower incidence rates (P = 0.007) of hyperkalaemia compared with patients treated with enalapril (5.6%).29 The fact that concomitant usage of entresto on top of background therapy can reduce the risk for incident hyperkalaemia, particularly in patients treated with background MRA, is further supported by a recent post hoc analysis of the PARADIGM-HF trial.30
In trials with HF patients with preserved ejection fraction (HFpEF) incidence rates of hyperkalaemia are arguably lower (Figure 3).31–33 In the CHARM-preserved trial, where patients with HFpEF were randomized to candesartan or placebo, incidence rates of hyperkalaemia (defined as >6.0 mEq/L) were 2% in both groups during a median follow-up of 36.6 months.32 In the Irbesartan in HF patients with a preserved ejection fraction (I-PRESERVE) trial, incidence rates of hyperkalaemia (defined as >6.0 mEq/L) were slightly higher in the treatment group (3%) vs. the placebo group (2%, P = 0.01), however, this did not lead to more drug discontinuation.33 In the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial, patients from the Americas with HFpEF treated with spironolactone had higher incidence rates of hyperkalaemia (18.7%) compared with placebo (9.1%) during a follow-up of 3.3 years.31,34 Although also here, similar to the Mineralocorticoid receptor antagonist (MRA) trials in patients with HF with reduced ejection fraction (HFrEF), patients with HFpEF and a potassium of ≥5.5 mEq/L within the 6 months prior or ≥5.0 mEq/L in 2 weeks prior to randomization were excluded.31
Among patients with acute HF, hyperkalaemia occurred in 7.8% of patients treated with tolvaptan and 6.6% of patients treated with placebo in the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) trial.35 In a cross-sectional post hoc analysis from the Patients Hospitalized with acute heart failure and Volume Overload to Assess Treatment Effect on Congestion and Renal FuncTion (PROTECT) trial, hyperkalaemia occurred only in 1% of overall study participants with no difference between study drug and placebo.36 However, patients with potassium <3.0 mEq/L at admission were excluded from this study, so the overall prevalence could be lower in a real-world setting. Similarly, only 3% of patients in the Coordinating Study Evaluating Outcomes of Advising and Counseling Failure (COACH) trial showed potassium levels >5.5 mEq/L.36 However, in both these studies only cross-sectional measurements of potassium were taken into account.
Clinical consequences of hyperkalaemia
Hyperkalaemia has two important clinical consequences. The first one is a direct effect on clinical outcomes by causing possible fatal arrhythmias. The second clinical consequence of hyperkalaemia is discontinuation or down titration of key HF drugs, which may indirectly affect clinical outcomes.
Whether potassium is an independent risk factor for outcome or a consequence of other risk factor remains unclear.5,35–38 A follow-up study after publication of the RALES trial reported an increase in hyperkalaemia related mortality.25 In acute HF, post hoc analyses from the PROTECT, COACH, and EVEREST trials potassium levels at admission or a change of potassium levels during hospitalization did not show a significant association with post-discharge survival.35,36 Similarly, hyperkalaemia was not associated with increases in mortality in both the EPHESUS and EMPHASIS-HF trials.8,39 The fact that hyperkalaemia was not associated with increased mortality in many of these trials can potentially be explained by the controlled settings in which these trials took place. Indeed, potassium was routinely monitored in these trials and overall tightly controlled, hence very high potassium levels (>6.0 mEq/L) were relatively rare in these trials. Further proof for this is provided by two recent real-world studies, which showed a significant association between hyperkalaemia and an increased mortality.40,41 In the first study, Núñez et al.40 showed that among 2164 patients with a total of 16 116 potassium observation measured at every physician-patient encounter (including hospital admissions and ambulatory settings) hyperkalaemia (>5.0 mEq/L) was associated with an increased mortality. Similarly, in a study by Aldahl et al.41 among 19 549 patients with HF, hyperkalaemia was associated with increase mortality rates. In a very recent individual-level data meta-analysis of 27 international cohorts from the general population, both hypo- and hyperkalaemia was associated with more adverse outcomes.42 In these real-world studies the overall distribution of potassium levels was wider compared with trial, which suggests an increased mortality risk of hyperkalaemia potentially occurs at higher potassium levels compared with the conventional >5.0 mEq/L or >5.5 mEq/L threshold.41
The second and perhaps most important clinical consequence of hyperkalaemia is discontinuation of lifesaving medication for HF. Indeed, in both the CHARM-alternative as well as the CHARM added trials, the study drugs were discontinued in respectively 1.9% and 3.4% of participants, which were higher rates compared with the placebo arm.23,24 Also, in the EPHESUS and EMPHASIS studies, eplerenone was discontinued in 0.7% and 1.1% of patients.26,27 Perhaps most importantly, the occurrence of hyperkalaemia did not affect the survival benefit of RAAS inhibitors.6–8,39 Further proof for this was provided in a real-world study from the BIOSTAT-CHF study group.10,43 In this study, potassium levels were measured as part of the study protocol in 1666 patients with HF and a reduced ejection fraction. Patients were sub-optimally treated at baseline. The authors showed that higher levels of baseline potassium were independently associated with lower uptitration rates of RAAS inhibition after 3 months of the study. Furthermore, we showed that there was no significant interaction between potassium or a change in potassium and the beneficial effects of uptitration to guideline recommended levels of RAAS inhibition.10
Conclusion
Concluding, a combination of a higher prevalence of risk factors including diabetes mellitus, older age, renal failure, hypertension, and usage of medication that increases potassium levels, put patients with HF at considerable risk for hyperkalaemia. Hyperkalaemia is potentially associated with adverse clinical outcomes and might lead to reductions or even discontinuation of RAAS inhibitors. Taken together, this warrants closer monitoring of potassium levels. Furthermore, novel potassium binding agents might be useful in patients with HF and may allow for more adequate uptitration of RAAS inhibitors, which in turn can have an impact on clinical outcomes in these patients.9,44–46
Conflict of interest: P.v.d.M: Consultancy for Vifor Pharma, AstraZeneca and Novartis. Unrestricted grant from Vifor Pharma and AstraZeneca.
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P.. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2016;37:2129–2200.27206819 [Google Scholar]
- 2. Kieneker LM, Eisenga MF, Joosten MM, de Boer RA, Gansevoort RT, Kootstra-Ros JE, Navis G, Bakker SJL.. Plasma potassium, diuretic use and risk of developing chronic kidney disease in a predominantly White population. PLoS One 2017;12:e0174686.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Foley RN, Wang C, Ishani A, Collins AJ.. NHANES III: influence of race on GFR thresholds and detection of metabolic abnormalities. J Am Soc Nephrol 2007;18:2575–2582. [DOI] [PubMed] [Google Scholar]
- 4. Flynn MA, Nolph GB, Baker AS, Martin WM, Krause G.. Total body potassium in aging humans: a longitudinal study. Am J Clin Nutr 1989;50:713–717. [DOI] [PubMed] [Google Scholar]
- 5. Desai AS. Hyperkalemia in patients with heart failure: incidence, prevalence, and management. Curr Heart Fail Rep 2009;6:272–280. [DOI] [PubMed] [Google Scholar]
- 6. Vardeny O, Claggett B, Anand I, Rossignol P, Desai AS, Zannad F, Pitt B, Solomon SD.. Incidence, predictors, and outcomes related to hypo- and hyperkalemia in patients with severe heart failure treated with a mineralocorticoid receptor antagonist. Circ Heart Fail 2014;7:573–579. [DOI] [PubMed] [Google Scholar]
- 7. Desai AS, Swedberg K, McMurray JJV, Granger CB, Yusuf S, Young JB, Dunlap ME, Solomon SD, Hainer JW, Olofsson B, Michelson EL, Pfeffer MA.. Incidence and predictors of hyperkalemia in patients with heart failure: an analysis of the CHARM Program. J Am Coll Cardiol 2007;50:1959–1966. [DOI] [PubMed] [Google Scholar]
- 8. Rossignol P, Dobre D, McMurray JJV, Swedberg K, Krum H, van Veldhuisen DJ, Shi H, Messig M, Vincent J, Girerd N, Bakris G, Pitt B, Zannad F.. Incidence, determinants, and prognostic significance of hyperkalemia and worsening renal function in patients with heart failure receiving the mineralocorticoid receptor antagonist eplerenone or placebo in addition to optimal medical therapy: results from the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF). Circ Heart Fail 2014;7:51–58. [DOI] [PubMed] [Google Scholar]
- 9. Pitt B, Bakris GL, Bushinsky DA, Garza D, Mayo MR, Stasiv Y, Christ-Schmidt H, Berman L, Weir MR.. Effect of patiromer on reducing serum potassium and preventing recurrent hyperkalaemia in patients with heart failure and chronic kidney disease on RAAS inhibitors. Eur J Heart Fail 2015;17:1057–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beusekamp JC, Tromp J, van der Wal HH, Anker SD, Cleland JG, Dickstein K, Filippatos G, van der Harst P, Hillege HL, Lang CC, Metra M, Ng LL, Ponikowski P, Samani NJ, van Veldhuisen DJ, Zwinderman AH, Rossignol P, Zannad F, Voors AA, van der Meer P.. Potassium and the use of renin-angiotensin-aldosterone system inhibitors in heart failure with reduced ejection fraction: data from BIOSTAT-CHF. Eur J Heart Fail 2018;20:923.. [DOI] [PubMed] [Google Scholar]
- 11. Muzzarelli S, Maeder MT, Toggweiler S, Rickli H, Nietlispach F, Julius B, Burkard T, Pfisterer ME, Brunner-La Rocca HP.. Frequency and predictors of hyperkalemia in patients ≥60 years of age with heart failure undergoing intense medical therapy. Am J Cardiol 2012;109:693–698. [DOI] [PubMed] [Google Scholar]
- 12. de Denus S, Tardif JC, White M, Bourassa MG, Racine N, Levesque S, Ducharme A.. Quantification of the risk and predictors of hyperkalemia in patients with left ventricular dysfunction. Am Heart J 2006;152:705–712. [DOI] [PubMed] [Google Scholar]
- 13. Weir MR, Rolfe M.. Potassium homeostasis and renin-angiotensin-aldosterone system inhibitors. Clin J Am Soc Nephrol 2010;5:531–548. [DOI] [PubMed] [Google Scholar]
- 14. Buxbaum JN, Tagoe C, Gallo G, Walker JR, Kurian S, Salomon DR.. Why are some amyloidoses systemic? Does hepatic “chaperoning at a distance” prevent cardiac deposition in a transgenic model of human senile systemic (transthyretin) amyloidosis? FASEB J 2012;26:2283–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Khuri R, Strieder W, Giebisch G.. Effects of flow rate and potassium intake on distal tubular potassium transfer. Am J Physiol 1975;228:1249–1261. [DOI] [PubMed] [Google Scholar]
- 16. Rosa RM, Silva P, Young JB, Landsberg L, Brown RS, Rowe JW, Epstein FH.. Adrenergic modulation of extrarenal potassium disposal. N Engl J Med 1980;302:431–434. [DOI] [PubMed] [Google Scholar]
- 17. McFarlane SI, Sowers JR.. Aldosterone function in diabetes mellitus: effects on cardiovascular and renal disease. J Clin Endocrinol Metab 2003;88:516–523. [DOI] [PubMed] [Google Scholar]
- 18. Hegstad R, Brown RD, Jiang NS, Kao P, Weinshilboum RM, Strong C, Wisgerhof M.. Aging and aldosterone. Am J Med 1983;74:442–448. [DOI] [PubMed] [Google Scholar]
- 19. Denic A, Lieske JC, Chakkera HA, Poggio ED, Alexander MP, Singh P, Kremers WK, Lerman LO, Rule AD.. The substantial loss of nephrons in healthy human kidneys with aging. J Am Soc Nephrol 2017;28:313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Klemm SA, Gordon RD, Tunny TJ, Thompson RE.. The syndrome of hypertension and hyperkalemia with normal GFR (Gordon’s syndrome): is there increased proximal sodium reabsorption? Clin Invest Med 1991;14:551–558. [PubMed] [Google Scholar]
- 21.CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. N Engl J Med 1987;316:1429–1435. [DOI] [PubMed] [Google Scholar]
- 22.SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991;325:293–302. [DOI] [PubMed] [Google Scholar]
- 23. Granger CB, McMurray JJV, Yusuf S, Held P, Michelson EL, Olofsson B, Ostergren J, Pfeffer MA, Swedberg K.. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet 2003;362:772–776. [DOI] [PubMed] [Google Scholar]
- 24. McMurray JJV, Ostergren J, Swedberg K, Granger CB, Held P, Michelson EL, Olofsson B, Yusuf S, Pfeffer MA.. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet 2003;362:767–771. [DOI] [PubMed] [Google Scholar]
- 25. Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A, Redelmeier DA.. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med 2004;351:543–551. [DOI] [PubMed] [Google Scholar]
- 26. Zannad F, McMurray JJV, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B.. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011;364:11–21. [DOI] [PubMed] [Google Scholar]
- 27. Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M.. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003;348:1309–1321. [DOI] [PubMed] [Google Scholar]
- 28. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J.. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999;341:709–717. [DOI] [PubMed] [Google Scholar]
- 29. McMurray JJV, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR.. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 30. Desai AS, Vardeny O, Claggett B, McMurray JJV, Packer M, Swedberg K, Rouleau JL, Zile MR, Lefkowitz M, Shi V, Solomon SD.. Reduced risk of hyperkalemia during treatment of heart failure with mineralocorticoid receptor antagonists by use of sacubitril/valsartan compared with enalapril. JAMA Cardiol 2017;2:79.. [DOI] [PubMed] [Google Scholar]
- 31. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O'Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM.. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014;370:1383–1392. [DOI] [PubMed] [Google Scholar]
- 32. Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJV, Michelson EL, Olofsson B, Ostergren J.. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved trial. Lancet 2003;362:777–781. [DOI] [PubMed] [Google Scholar]
- 33. Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A.. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008;359:2456–2467. [DOI] [PubMed] [Google Scholar]
- 34. Desai AS, Liu J, Pfeffer MA, Claggett B, Fleg J, Lewis EF, McKinlay S, O'Meara E, Shah SJ, Sweitzer NK, Solomon S, Pitt B.. Incident hyperkalemia, hypokalemia, and clinical outcomes during spironolactone treatment of heart failure with preserved ejection fraction: analysis of the TOPCAT trial. J Card Fail 2018;24:313–320. [DOI] [PubMed] [Google Scholar]
- 35. Khan SS, Campia U, Chioncel O, Zannad F, Rossignol P, Maggioni AP, Swedberg K, Konstam MA, Senni M, Nodari S, Vaduganathan M, Subacius H, Butler J, Gheorghiade M.. Changes in serum potassium levels during hospitalization in patients with worsening heart failure and reduced ejection fraction (from the EVEREST trial). Am J Cardiol 2015;115:790–796. [DOI] [PubMed] [Google Scholar]
- 36. Tromp J, ter Maaten JM, Damman K, O'Connor CM, Metra M, Dittrich HC, Ponikowski P, Teerlink JR, Cotter G, Davison B, Cleland JGF, Givertz MM, Bloomfield DM, van der Wal MHL, Jaarsma T, van Veldhuisen DJ, Hillege HL, Voors AA, van der Meer P.. Serum potassium levels and outcome in acute heart failure (data from the PROTECT and COACH Trials). Am J Cardiol 2017;119:290.. [DOI] [PubMed] [Google Scholar]
- 37. Bowling CB, Pitt B, Ahmed MI, Aban IB, Sanders PW, Mujib M, Campbell RC, Love TE, Aronow WS, Allman RM, Bakris GL, Ahmed A.. Hypokalemia and outcomes in patients with chronic heart failure and chronic kidney disease: findings from propensity-matched studies. Circ Heart Fail 2010;3:253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jain N, Kotla S, Little BB, Weideman RA, Brilakis ES, Reilly RF, Banerjee S.. Predictors of hyperkalemia and death in patients with cardiac and renal disease. Am J Cardiol 2012;109:1510–1513. [DOI] [PubMed] [Google Scholar]
- 39. Pitt B, Bakris G, Ruilope LM, DiCarlo L, Mukherjee R; EPHESUS Investigators. Serum potassium and clinical outcomes in the eplerenone post-acute myocardial infarction heart failure efficacy and survival study (EPHESUS). Circulation 2008;118:1643–1650. [DOI] [PubMed] [Google Scholar]
- 40. Núñez J, Bayés-Genís A, Zannad F, Rossignol P, Núñez E, Bodí V, Miñana G, Santas E, Chorro FJ, Mollar A, Carratalá A, Navarro J, Górriz JL, Lupón J, Husser O, Metra M, Sanchis J.. Long-term potassium monitoring and dynamics in heart failure and risk of mortality. Circulation 2018;137:1320–1330. [DOI] [PubMed] [Google Scholar]
- 41. Aldahl M, Jensen A-SC, Davidsen L, Eriksen MA, Møller Hansen S, Nielsen BJ, Krogager ML, Køber L, Torp-Pedersen C, Søgaard P.. Associations of serum potassium levels with mortality in chronic heart failure patients. Eur Heart J 2017;38:2890–2896. [DOI] [PubMed] [Google Scholar]
- 42. Kovesdy CP, Matsushita K, Sang Y, Brunskill NJ, Carrero JJ, Chodick G, Hasegawa T, Heerspink HL, Hirayama A, Landman GWD, Levin A, Nitsch D, Wheeler DC, Coresh J, Hallan SI, Shalev V, Grams ME.. Serum potassium and adverse outcomes across the range of kidney function: a CKD Prognosis Consortium meta-analysis. Eur Heart J 2018;39:1535–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Voors AA, Anker SD, Cleland JG, Dickstein K, Filippatos G, van der Harst P, Hillege HL, Lang CC, ter Maaten JM, Ng L, Ponikowski P, Samani NJ, van Veldhuisen DJ, Zannad F, Zwinderman AH, Metra M.. A systems BIOlogy Study to TAilored Treatment in Chronic Heart Failure: rationale, design, and baseline characteristics of BIOSTAT-CHF. Eur J Heart Fail 2016;18:716–726. [DOI] [PubMed] [Google Scholar]
- 44. Packham DK, Rasmussen HS, Lavin PT, El-Shahawy MA, Roger SD, Block G, Qunibi W, Pergola P, Singh B.. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med 2015;372:222–231. [DOI] [PubMed] [Google Scholar]
- 45. Weir MR, Bakris GL, Bushinsky DA, Mayo MR, Garza D, Stasiv Y, Wittes J, Christ-Schmidt H, Berman L, Pitt B.. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med 2015;372:211–221. [DOI] [PubMed] [Google Scholar]
- 46. Anker SD, Kosiborod M, Zannad F, Piña IL, McCullough PA, Filippatos G, van der Meer P, Ponikowski P, Rasmussen HS, Lavin PT, Singh B, Yang A, Deedwania P.. Maintenance of serum potassium with sodium zirconium cyclosilicate (ZS-9) in heart failure patients: results from a phase 3 randomized, double-blind, placebo-controlled trial. Eur J Heart Fail 2015;17:1050–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]