Abstract
Purpose:
Hyperactivation of AKT is common and associated with endocrine resistance in estrogen receptor–positive (ER+) breast cancer. The allosteric pan-AKT inhibitor MK-2206 induced apoptosis in PIK3CA-mutant ER+ breast cancer under estrogen-deprived condition in preclinical studies. This neoadjuvant phase II trial was therefore conducted to test the hypothesis that adding MK-2206 to anastrozole induces pathologic complete response (pCR) in PIK3CA mutant ER+ breast cancer.
Experimental Design:
Potential eligible patients with clinical stage II/III ER+/HER− breast cancer were preregistered and received anastrozole (goserelin if premenopausal) for 28 days in cycle 0 pending tumor PIK3CA sequencing. Patients positive for PIK3CA mutation in the tumor were eligible to start MK-2206 (150 mg orally weekly, with prophylactic prednisone) on cycle 1 day 2 (C1D2) and to receive a maximum of four 28-day cycles of combination therapy before surgery. Serial biopsies were collected at preregistration, C1D1 and C1D17.
Results:
Fifty-one patients preregistered and 16 of 22 with PIK3CA-mutant tumors received study drug. Three patients went off study due to C1D17 Ki67 >10% (n = 2) and toxicity (n = 1). Thirteen patients completed neoadjuvant therapy followed by surgery. No pCRs were observed. Rash was common. MK-2206 did not further suppress cell proliferation and did not induce apoptosis on C1D17 biopsies. Although AKT phosphorylation was reduced, PRAS40 phosphorylation at C1D17 after MK-2206 persisted. One patient acquired an ESR1 mutation at surgery.
Conclusions:
MK-2206 is unlikely to add to the efficacy of anastrozole alone in PIK3CA-mutant ER+ breast cancer and should not be studied further in the target patient population.
Introduction
Estrogen receptor–positive (ER+) and HER2− breast cancer represents approximately 70% of breast cancer diagnosis and is a major cause of cancer-related death in women (1). Although most patients present initially with early-stage disease and are treated with curative intent, over 20% of patients relapse and die from metastatic disease (2). The lack of apoptotic response of ER+ HER2− breast cancer to standard-of-care treatments has been well recognized (3, 4). For example, the pathologic complete response (pCR) rate is less than 10% to 15% in response to chemotherapy (5, 6) and is approximately 1% with endocrine therapy (7, 8). Approaches that promote cell death of ER+ HER2− breast cancer could potentially reduce recurrence.
AKT, also known as protein kinase B, consists of 3 isoforms, AKT1, 2 and 3, which are serine/threonine protein kinases that play key roles in multiple cellular processes including glucose metabolism, apoptosis, cell proliferation, transcription, and migration (9–11). Emerging evidence indicates that hyperactivation of PI3K/AKT signaling is a key survival mechanism mediating resistance to endocrine therapy (12–15), and AKT phosphorylation in tumor cells is associated with activation of ER and predicts relapse in patients with ER+ breast cancer treated with endocrine therapy (16–18). Importantly, inhibition of AKT in combination with endocrine therapy induced apoptosis in preclinical studies (19). We therefore hypothesized that the addition of an AKT inhibitor, MK-2206, to a standard aromatase inhibitor could induce a cell death response and induce pCR in the neoadjuvant setting.
MK-2206 is an allosteric inhibitor against AKT1 (IC50, 5 nmol/L), AKT2 (IC50, 12 nmol/L), and AKT3 (IC50, 65 nmol/L) and has greater than 100-fold selectivity for AKT, compared with 256 other kinases (20). In previous phase I trials, MK-2206 was well tolerated and showed evidence of AKT inhibition (21, 22). In breast cancer cell culture studies, MK-2206 was particularly effective in cell lines with PI3K pathway abnormalities, including PIK3CA mutation and PTEN loss (19, 23). As activating PIK3CA mutations occur in 30% to 50% of ER+ HER2− breast cancer (24, 25) and PIK3CA-mutant tumors have been shown to be growth dependent on PI3K signaling (26–28), we limited the study population to those with PIK3CA-mutant ER+ HER2− breast cancer, to increase the chance of detecting a positive signal. The dosing and schedule of MK-2206 (150 mg orally weekly with prophylactic prednisone) used in this trial was the MTD of MK-2206 when combined with anastrozole in our previous phase I trial for patients with metastatic breast cancer (19).
Materials and Methods
Patients
Patients with newly diagnosed ER+ HER2− breast cancer were first assessed for eligibility to preregister for central Clinical Laboratory Improvement Amendments (CLIA) tumor PIK3CA sequencing and to receive 28 days of anastrozole monotherapy (with goserelin if premenopausal) in cycle 0. Preregistration eligible patients were women 18 years of age or older with clinical stageT2-T4c, N0–3, M0 ER+ (Allred score of 6–8), HER2− (0 or 1+ by IHC or FISH negative for amplification) breast cancer. Additional criteria were the presence of a palpable lesion measuring more than 2 cm in at least one dimension by imaging or physical examination, mammogram and ultrasound of the breast, and axilla at most 42 days prior to preregistration, Eastern Cooperative Oncology Group (ECOG) performance status 0 to 2, adequate organ and marrow function, fasting glucose <120 mg/dL and HbA1c <8% if diabetic, willingness to provide samples for PIK3CA sequencing and, if of childbearing potential, a negative serum pregnancy test within 7 days of preregistration.
Preregistration exclusion criteria included prior treatment for invasive breast cancer, prior sentinel lymph node surgery, excisional breast biopsy, invasive cancer or ductal carcinoma in situ (DCIS) in the contralateral breast, inflammatory breast cancer, known metastatic disease, QTc prolongation or other significant ECG abnormalities, receiving any medications or substance that are strong inhibitors or inducers of CYP450 3A4 or increase the risk of torsade de pointes, receiving therapeutic anticoagulation therapy, HIV-positive receiving combination antiretroviral therapy, uncontrolled intercurrent illness, and any condition that impairs the ability to swallow a tablet. Pregnant and nursing women were not allowed to enroll onto this trial.
Registration criteria included detection of a PIK3CA mutation by central laboratory testing and, in premenopausal women, a serum estradiol level in the postmenopausal range on goserelin at registration.
This study was approved by the Institutional Review Board at all participating institutions; all patients provided signed informed consent. This trial is sponsored by the NCI (NCI Protocol #: 9170) and was conducted through the Mayo Phase 2 Consortium. The trial was registered at ClinicalTrials.gov (NCT01776008).
Treatment administration and evaluation
Preregistration eligible patients received anastrozole 1 mg by mouth daily for 28 days in cycle 0, while waiting on the results of PIK3CA mutation analysis. Premenopausal women also received 3.6 mg of goserelin subcutaneously on day 1 prior to the start of anastrozole during cycle 0. Study participants with PIK3CA-mutant breast cancer were eligible to continue to study registration. Otherwise, study participation was discontinued.
Women with PIK3CA-mutant breast cancer received 4 cycles (each cycle is 28 days) of 1 mg anastrozole by mouth on days 1 to 28 with 150 mg MK-2206 by mouth on days 2, 9, 16, and 23. Premenopausal women received 3.6 mg of goserelin subcutaneously every 28 days after its first administration during the preregistration period regardless of when combination therapy began. Patients were required to complete a pill diary that was to be returned at the end of each cycle. To prevent skin rash due to MK-2206, prophylactic prednisone 20 mg was administered by mouth the day before, the day of, and the day following each MK-2206 administration (i.e., days 1–3, 8–10, 15–17, and 22–24 of each cycle). The prophylactic prednisone dose could be tapered every 4 or more weeks to the next lower dose level (10 mg, 5 mg, 0 mg) if there was not any grade 2 or higher rash. Prednisone could be increased to the highest dose level of 40 mg/day for grade 3 rash. If a patient experienced grade 3 rash despite the maximum dose of prophylactic prednisone (40 mg) and the lowest dose of MK-2206 (75 mg) allowed in the protocol, she was to go off study treatment. Patients were also to be taken off study if grade 4 rash was experienced. At most, two dose reductions of MK-2206 were allowed (150 mg to 100 mg to 75 mg) for severe toxicity before MK-2206 was to be discontinued. MK-2206 was omitted for grade 2–4 thrombocytopenia as well as grade 3–4 neutropenia, hyperglycemia, and nonhematologic adverse events (AE) until recovery to a platelet count of at least 100,000/mcL and absolute neutrophil counts of at least 1,000/mcL. If recovery took more than 7 days, MK-2206 was to be reduced by one dose level.
Neoadjuvant treatment was to be discontinued if the tumor Ki67 level on C1D17, after 2 weeks of combination therapy, was above 10%; if there was disease progression that was to be confirmed by ultrasound or mammogram; if the patient withdrew consent; or if the patient sought immediate surgery.
Within 14 days of preregistration, within 7 days of registration, and at the completion of each treatment cycle, patients underwent a complete physical examination; assessment of ECOG performance status; tumor assessment by tape, ruler, or caliper using WHO criteria to assess disease status; blood chemistries; and toxicity assessment. An EKG was to be performed within 14 days of preregistration. If tumor progression was suspected, ultrasound or mammogram was required for confirmation. Blood and biopsy specimens for correlative studies were collected at preregistration (before cycle 0), at registration (C1D1), and the day after day 16 MK-2206 dose (C1D17).
Statistical analysis
The primary endpoint was the pCR rate among the women with ER+ HER2−PIK3CA-mutant breast cancer. pCR was defined as no histologic evidence of invasive tumor cells in the surgical breast sample or lymph nodes. A two-stage phase II clinical trial design was chosen to assess whether the pCR rate with anastrozole in combination with MK-2206 (and goserelin if premenopausal) in women with stage II/III ER+ HER2−PIK3CA-mutant breast cancer is at most 1% against the alternative that the pCR rate is 15% or more. Setting the type I and type II error rate at 10% yielded the design described next. Sixteen patients were to be enrolled onto stage I. If none of these 16 patients met the criteria for pCR, the trial was to be closed to further enrollment, and it was to be concluded that the pCR rate was not 15% or more. If at least one of the 16 patients had a pCR, 13 patients were to be enrolled onto stage II. If at most 1 of the 29 patients met the criteria for pCR, it was to be concluded that the pCR is not 15% or more.
Secondary objectives included the toxicity profile, and correlative studies of serial tumor biopsies to assess target inhibition by MK-2206 and its effect on tumor cell proliferation and apoptosis. Exploratory objectives included assessment of the mutation profiles by an 83-gene next-generation sequencing (NGS) panel to explore potential resistance mechanisms. Wilcoxon signed rank test was performed to compare the levels of Ki67, pAKT, and pPRAS40 from different time points (C1D1 vs. preregistration; C1D17 vs. C1D1; surgery vs. C1D17).
CLIA PIK3CA sequencing
DNA was extracted from formalin-fixed paraffin-embedded (FFPE) baseline (preregistration) tumor biopsies. Exons 2, 5, 8, 10, and 21 and exon-intron splice junctions of the PIK3CA gene (ref seq# NM_006218) were PCR amplified and then sequenced at the CLIA-certified Washington University Genomic and Pathology Service using Sanger technology.
Tumor Ki67 IHC and quantification
Tumor Ki67 staining was performed centrally at the CAP/CLIA–certified AMP laboratory at Washington University (CLIA number 26D2013203) using the CONFIRM anti-Ki67 rabbit mAb (clone 30–9) as a prediluted reagent on the Ventana Benchmark XT platform according to the manufacturer’s instructions (29). Tonsil was used as the positive control. Ki67 slides were scanned and analyzed using the Ventana iScan Coreo Au scanner and the Virtuoso software. Areas of interest for Ki67 image analysis were selected at ×4 magnification by study pathologists to exclude stromal and DCIS components. If invasive cancer cells could not be distinguished from nonmalignant cells by image analysis, visual point counting of at least three representative fields was performed as described previously (8, 29). A minimum of 200 tumor cells were counted. Ki67 results are expressed as immune reactive over total numbers of tumor cells.
IHC assay and scoring for pPRAS40, pAKT, and cleaved PARP
IHC of pPRAS40 (Thr246), pAKT (Ser473), and cleaved PARP were conducted on 5-mm tissue sections of FFPE tumor biopsy cores as described previously using the EnVision + Single Reagents HRP-Rabbit (Dako, cat. no. K4003) and REAL substrate buffer (REAL DAB + chromogen, Dako, cat. no. K3468; ref. 30). The primary antibodies and dilutions are as follows: pAKT (Ser473; 1:200, Cell Signaling Technology, cat. no. 4060), pPRAS40 (Thr246; 1:200, Cell Signaling Technology, cat. no. 2997), and cleaved PARP (Asp214; 1:200, Cell Signaling Technology, cat. no. 9541). Abreast cancer xenograft model treated with either vehicle or MK-2206 was used as positive/negative control for staining. Allred score was used to quantify pAKT and pPRAS40 tumor staining (31). Briefly, based on reviewing the entire immunostained slide, a proportion score (PS) was assigned representing the estimated proportion of positive staining target cells (0 = none; 1 = ≤ 1%; 2 = 1%–10%; 3 = 11%–33%; 4 = 34%–66%; 5 = 67%–100%). Then, an intensity score (IS) was assigned representing the estimated average intensity of positive target cells (0 = none; 1 = weak; 2 = intermediate; 3 = strong). The PS and IS were then added to obtain a total score (TS, i.e., the Allred score), ranging from 0 to 8.
83-gene panel NGS
Tumor DNA extracted from fresh-frozen biopsies and matched leukocyte germline DNA were subjected to targeted Illumina NGS by 2 × 100 paired-end reads of an 83-gene panel and analyzed using the Genome Modeling System as described by Griffith and colleagues (32–34). Somatic variants detected and passing manual review, including single-nucleotide variants, small insertions and deletions, and structural variants, were visualized using a mutation waterfall plot. Somatic mutations were displayed using the bioconductor package GenVisR (1.4.4; ref. 35).
Results
Patient characteristics
Between March 1, 2013, and October 5, 2014, 51 women with ER+ HER2− breast cancer were preregistered onto the study (Supplementary Fig. S1). Of these 51 patients, 35 (68.6%) did not go on to register onto the study due to a nonmutated PIK3CA on tumor sequencing (n = 22), insufficient tissue to complete PIK3CA testing (n = 6), physician/patient decision (n = 5), ineligible due to presence of contralateral breast cancer (n = 1), and not specified (n = 1). Thus, 16 women found to have PIK3CA-mutated breast cancer by central laboratory testing registered onto the study. The distribution of the PIK3CA mutations enrolled in this trial is illustrated in Supplementary Fig. S2. Table 1 shows the patient and disease characteristics of these 16 patients. After 2 weeks of anastrozole in combination with MK-2206, breast biopsies were performed on cycle 1 day 17 (C1D17) for central Ki67 testing. Two patients had a 2-week Ki67 value >10% and as such discontinued treatment (per protocol). Another patient discontinued study therapy having developed grade 4 alanine aminotransferase (ALT) and aspartate aminotransferase (AST) elevation after 2 cycles of treatment. Thirteen patients completed the 4 cycles of combination therapy and surgery.
Table 1.
Patient and tumor characteristics (n = 16)
| Median age (range) | 57 (40–77) years |
|---|---|
| Menopausal status | |
| Premenopausal | 4 (25%) |
| Postmenopausal | 12 (75%) |
| Histology | |
| Ductal | 8 (50%) |
| Lobular | 8 (50%) |
| Histologic grade | |
| 1 | 3 (19%) |
| 2 | 11 (69%) |
| 3 | 2 (12%) |
| Clinical T and N stage | |
| T2N0 | 6 (38%) |
| T2N1 | 3 (19%) |
| T3N0 | 2 (13%) |
| T3N1 | 4 (25%) |
| Unknown | 1 (6%) |
| PIK3CA mutation | |
| E542K/V | 2 (13%) |
| E545K | 3 (19%) |
| H1047L/R | 8 (50%) |
| E418G, C420R | 1 (6%) |
| Del/ins | 2 (13%) |
AEs
All 16 patients were evaluable for AEs. Toxicities observed in the 2 patients who went off study early after C1D17 Ki67 >10% included grade 2 hyperglycemia (n = 1) and grade 1 maculopapular rash (n = 2). Of the remaining 14 patients, 13 completed all 4 cycles of MK-2206 but 5 of them required at least one dose omission due to grade 3 rash (n = 4) or grade 1 diarrhea (n = 1; Supplementary Table S1). The remaining patient discontinued after two cycles of treatment due to grade 4 ALT and AST (Table 2). The median total dose of MK-2206 administered was 2,325 mg (25th percentile = 1,675; 75th percentile = 2,400). Severe toxicities reported among these 14 patients included grade 4 ALT increase (n = 1), grade 4 AST increase (n = 1), grade 3 maculopapular rash (n = 4), and grade 3 pruritus (n = 2). Table 2 provides all toxicities reported among these 14 patients regardless of attribution. There were no hematologic toxicities.
Table 2.
Toxicities among the 14 patients who continued on study post-C1D17 Ki67 evaluation
| Grade | ||||||
|---|---|---|---|---|---|---|
| Toxicity | 1 | 2 | 3 | 4 | N (%) | |
| Fatigue | 10 (71.4%) | 3 (21.4%) | 0 | 0 | 13 (92.9%) | |
| Hyperglycemia | 10 (71.4%) | 3 (21.4%) | 0 | 0 | 13 (92.9%) | |
| Maculopapular rash | 4 (28.6%) | 2 (14.3%) | 4 (28.6%) | 0 | 10 (71.4%) | |
| Pruritus | 3 (21.4%) | 2 (14.3%) | 2 (14.3%) | 0 | 7 (50%) | |
| Dry skin | 5 (35.7%) | 2 (14.3%) | 0 | 0 | 7 (50%) | |
| Nausea | 4 (28.6%) | 1 (7.1%) | 0 | 0 | 5 (35.7%) | |
| ALT increase | 0 | 0 | 0 | 1 (7.1%) | 1 (7.1%) | |
| AST increase | 0 | 0 | 0 | 1 (7.1%) | 1 (7.1%) | |
| Angioedema | 0 | 1 (7.1%) | 0 | 0 | 1 (7.1%) | |
| Sinus bradycardia | 0 | 1 (7.1%) | 0 | 0 | 1 (7.1%) | |
| Vaginal dryness | 0 | 1 (7.1%) | 0 | 0 | 1 (7.1%) | |
Pathologic responses and type of breast surgery
All 13 patients who completed four cycles of combination therapy proceeded to surgery. Eight patients underwent breast-conserving surgery and 5 patients underwent mastectomy (Supplementary Table S2). Nodal procedures undertaken included sentinel lymph node surgery (n = 5), axillary lymph node dissection (n = 4), or both (n = 4). Residual disease was found in the breast of all 13 patients (Supplementary Table S2). The pCR rate among the 16 patients with PIK3CA-mutated breast cancer who began combination treatment was 0% (90% confidence interval, 0–17.1).
Lack of added effect on cell proliferation and apoptosis by MK-2206
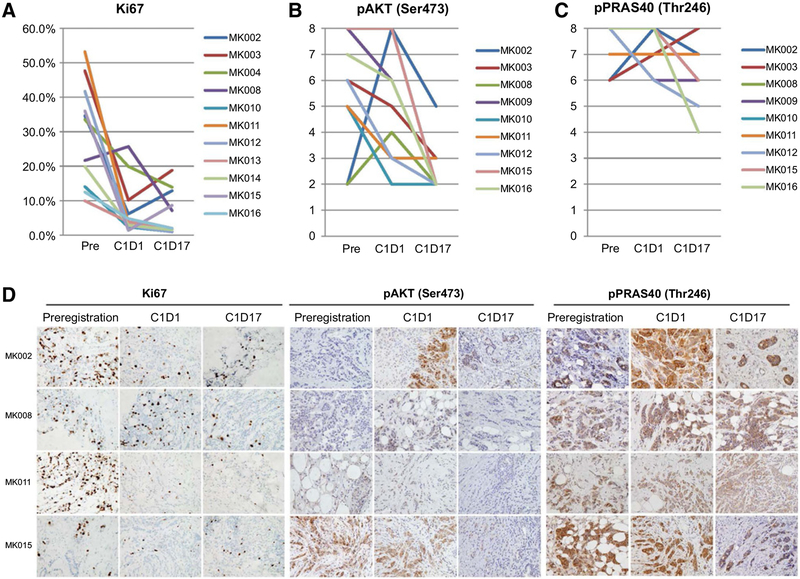
The effect of MK-2206 on cancer cell proliferation and apoptosis was assessed by IHC analysis of Ki67 and cleaved PARP, respectively, on serial tumor biopsies. When compared with the pretreatment samples, Ki67 was significantly reduced following cycle 0 anastrozole monotherapy on C1D1 (P = 0.002). However, the median Ki67 did not differ significantly between C1D17 and C1D1 (Table 3;Fig. 1AandD). No obvious induction of apoptosis was observed by either anastrozole alone on C1D1 biopsies or by the combination of MK-2206 and anastrozole on C1D17 biopsies or surgical specimens (Supplementary Fig. S3).
Table 3.
Changes in cell proliferation (Ki67) and AKT signaling during treatment
| C1D1 - preregistration | C1D17 - C1D1 | Surgery - C1D17 | |
|---|---|---|---|
| Biomarkers |
n
Median change (range) |
n
Median change (range) |
n
Median change (range) |
| Ki67 (%) | 11 −17.0% (−49.8%−4.1%) P = 0.0020 |
12 −1.5% (−18.6%−8.6%) P = 0.5186 |
12 3.8% (−6.9%−34.2%) P = 0.0640 |
| pAKT (Allred score) | 11 −2 (−3–6) P = 0.2031 |
10 −2 (−6–0) P = 0.0078 |
8 −1.5 (−3–5) P = 0.9375 |
| PPRAS40 (Allred score) | 10 0 (−2–2) P = 0.9063 |
9 −1 (−4–1) P = 0.1563 |
8 −0.5 (−6–3) P = 0.4922 |
Abbreviations: C1D1, biopsy after 28 days of anastrozole alone; C1D17, biopsy after 17 days on combination therapy.
Figure 1.
Biomarker response to anastrozole alone on C1D1 and to the addition of MK-2206 on C1D17 tumor biopsies. A–C, Percent Ki67 labeling index (A), Allred scores of pAKT (Ser473; B), and Allred scores of pPRAS40 (Thr246; C), by IHC of tumor biopsies collected at preregistration, C1D1 (28 days after single-agent anastrozole), and C1D17 (after 2 weeks of adding MK-2206) are shown for individual patients with serial samples available. Sample IDs are shown in the figure legends. Representative IHC pictures (magnification, ×400) for Ki67, pAKT (Ser473), and pPRAS40 (Thr246) from 4 patients are shown in D. Quantitative comparisons of levels between C1D1 and preregistration or between C1D17 and C1D1 of each biomarker are described in Table 3.
Effect of MK-2206 on AKT signaling
To examine the pharmacodynamics effect of MK-2206, changes in the levels of phosphorylated AKT (Ser473) and its substrate PRAS40 (Thr246) were examined by IHC on serial tumor biopsies collected at preregistration, C1D1, and then C1D17. No statistically significant reduction in pAKT or pPRAS40 was observed at C1D1 after anastrozole, but adding MK-2206 significantly reduced pAKT level at C1D17 (Fig. 1B and D; Table 3), consistent with MK-2206 target inhibition. However, the pAKT Allred score was above 2, indicating staining positive in more than 1% of tumor cells, in 3 cases (MK002, MK003, and MK011), 2 of which (MK002 and MK003) had Ki67 >10% on C1D17 and went off study. These data suggest incomplete inhibition of AKT in the resistant tumors. In addition, the effect of MK-2206 on pPRAS40 was less profound, and there was significant residual staining of pPRAS40 in all cases at C1D17 (Fig. 1C and D; Table 3). This result indicates that AKT pathway inhibition was potentially incomplete with the dosing and schedule of MK-2206 administered.
Genomic analysis of samples
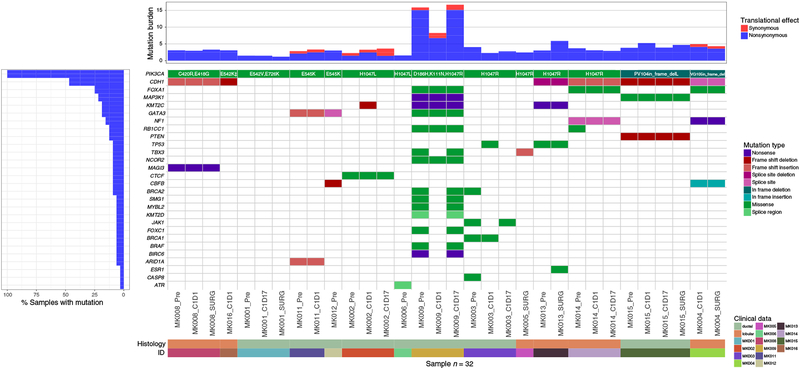
Sufficient tumor material was available for 32 samples collected over time from 14 patients enrolled in the trial for an 83-gene panel NGS analysis to explore somatic mutation patterns in an attempt to discover potential resistance mechanisms to anastrozole and MK-2206. The average number of mutations detected by the 83-gene panel sequencing for all individuals was 5 (range, 2–23). There was no association between variant load and clinical outcome or tumor stage. The PIK3CA sequencing data from the 83-gene panel included analysis of all of its exons, which confirmed the CLIA-diagnosed PIK3CA mutations in all 14 patients, but also diagnosed additional cooccurring PIK3CA mutations outside the CLIA sequencing range in 2 patients, including E726K in a patient with E542V-mutant tumor, and N1044K in a patient with E542K-mutant tumor (Supplementary Fig. S2). Other genes with mutations observed in more than 2 of the 14 patients included CDH1 (n = 6,42%), FOXA1 (n = 3), KMT2C (n = 3), GATA3 (n = 3), and in 2 patients each for MAP3K1, NF1, TP53, RB1CC1, TBX3, CBFB, and BRCA2 (Fig. 2). Five of the 6 patients with invasive lobular cancers carried CDH1 mutations (Supplementary Fig. S4), and 2 of the 3 cases with FOXA1 mutations were also observed in lobular cases, while all 3 cases with GATA3 mutations were in invasive ductal cancer (Fig. 2).
Figure 2.
Somatic mutation profiles for 32 tumor samples from 14 patients obtained at indicated time points are shown. Histology subtype (invasive ductal vs. lobular) of the baseline or preregistration sample for each patient is also indicated. The mutation burden (mutations/Mb) was calculated for each sample as the total number of exonic mutations over the number of bases covered to ≥30× depth * 1000000. For situations in which there are multiple mutations in the same gene/sample combination, the most deleterious mutation is plotted following the order of the mutation type legend. Note that silent mutations are included for the mutation burden calculation. Specific amino acid changes in PIK3CA are indicated for each patient. All PIK3CA mutations remained present and are the same at all time points tested for each patient, except E726K, which was not detected at surgery time point for patient MK001. †, indicates the presence of a concurrent PIK3CA N1044K mutation in MK016.
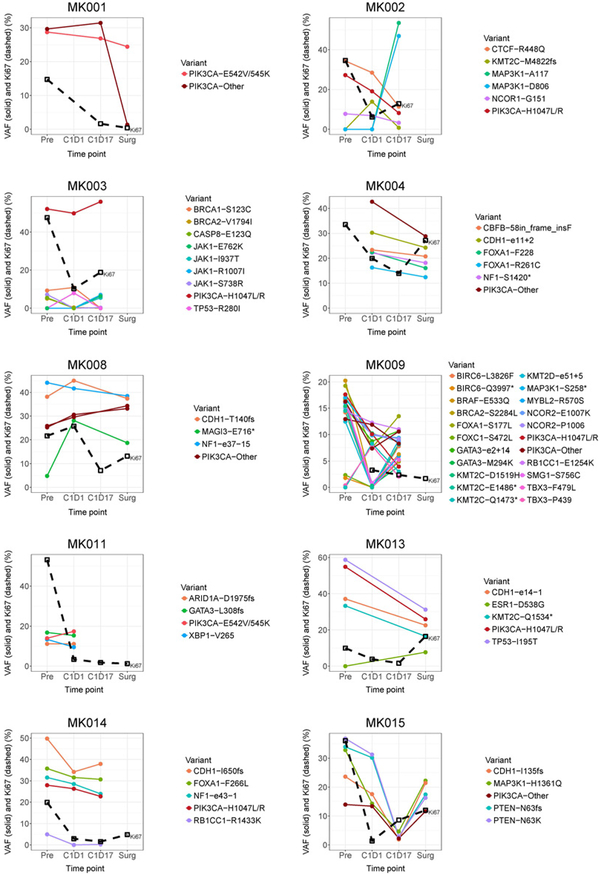
The PIK3CA variant allele frequency (VAF) of the 14 patients ranged from approximately 15% to 55% at preregistration, which did not change significantly over the course of therapy for any individuals evaluated (Fig. 3). Interestingly, patient MK009, which had the highest number of somatic mutations (n = 23), showed two subclones, both of which demonstrated a VAF response at time C1D1 but one rebounded by time C1D17, although the Ki67 remained below 10% (Fig. 3). Two other tumors likely possessed subclones, MK002 and MK003, which showed a rise in the VAF for MAP3K1 and JAK1 mutations, respectively, at C1D17 compared with other genes, both of which showed treatment resistance at C1D17 (Ki67 > 10%; Fig. 3). Patient MK013 acquired an ESR1 D538G mutation at the surgery time point, which coincided with an elevated Ki67 (Fig. 3; Supplementary Fig. S5). The ESR1 D538G is one of the well-recognized ESR1 ligand-binding domain hotspot mutations associated with constitutive activation of ER and aromatase inhibitor resistance (36–39). Although patient MK015 showed a potential response to therapy at time C1D17, which is visualized by a decrease in VAF of all mutations (Fig. 3), this response, however, could be attributed to low tumor purity, as Ki67 was increased from 1.4% at C1D1 to 8.6% at C1D17 (Fig. 3).
Figure 3.
VAFs and Ki67 levels tracked over clinical time points. VAFs of somatic mutations by 83-gene panel sequencing, along with Ki67 level, were plotted over time for each of the 10 patients who have sequencing data available for at least two time points.
Discussion
In this single-arm phase II trial of MK-2206 in combination with anastrozole in patients with newly diagnosed clinical stage II to III ER-rich, HER2− breast cancer, none of the 16 patients enrolled in the first stage of the trial achieved a pCR despite limiting to the PIK3CA-mutant population. The study was therefore closed to further accrual, based on failure to meet the predefined pCR criteria. The lack of pCR response was accompanied by the inability to induce apoptosis (assessed by cleaved PARP) from the addition of MK-2206 to anastrozole and the failure of MK-2206 to further suppress tumor cell proliferation (Ki67) above that achieved by anastrozole alone. We conclude from this trial that the combination of MK-2206 and anastrozole is unlikely to be more efficacious than anastrozole alone in patients with the newly diagnosed endocrine-naive patient population.
The lack of pCR and effects on cell proliferation and apoptosis by adding MK-2206 to anastrozole was supported by the pharmacodynamic studies of pAKT and pPRAS40 on serially collected tumor biopsies from patients enrolled in this trial. Although AKT phosphorylation was reduced in the majority of the cases, the 2 patients who had high Ki67 had persistent AKT phosphorylation and higher levels of pPRAS40 24 hours after the third dose of MK-2206. Interestingly, we did not observe a clear reduction in the phosphorylation of the AKT substrate PRAS40 in all specimens. The modest degree of target inhibition observed in this trial suggests that the dosing and schedule of MK-2206 were likely suboptimal or alternatively, AKT-independent activation of downstream signaling was present.
The weekly dosing of MK-2206 was developed because of its long half-life of 60 to 80 hours (21, 22) and results from the initial phase I study that compared the weekly administration with every other day dosing of MK-2206 in patients with advanced solid tumors, which demonstrated that the weekly dosing was similarly tolerated and induced similar degrees of target inhibition (22). The recommended phase II dose for MK-2206 single agent was 200 mg weekly. Analysis of paired tumor biopsies from 5 patients who received 200 mg weekly MK-2206 in the phase I study indicated a reduction of pAKT to 50% (range, 37.5%−60%) of baseline levels after 2 weeks of therapy (22), similar to the degree of pAKT suppression observed on C1D17 in this trial. In the prior phase I study, the lowest level of pAKT was observed 24 hours following MK-2206 administration, which gradually recovered to baseline at 96 hours (22); therefore, the timing of the tumor biopsy on C1D17, 24 hours after day 16 dose of MK-2206 in this trial, appeared to be optimal. Interestingly, similarly less impressive reduction in PRAS40 phosphorylation at Thr246 was observed in the initial phase I study (22). These data are consistent with preclinical cell culture studies, in which MK-2206 inhibited pAKT more dramatically than the AKT substrate pPRAS40; a higher dose of MK-2206 was required for more complete downstream target inhibition (23). The dosing of MK-2206 (150 mg weekly) in this trial was based on the phase I study of this agent when combined with hormonal therapy, including anastrozole in patients with metastatic hormone receptor–positive (HR+) breast cancer, in which rash was dose limiting and MK-2206 dosed at 200 mg was intolerable (19). In this trial, despite the use of prophylactic prednisone, dose omissions of MK-2206 were observed in close to 50% of patients in cycle 2 and beyond, commonly as a result of rash, rarely due to diarrhea. One patient discontinued study due to grade 4 LFT elevations that recovered. The reduced drug exposure due to treatment-related AEs as well as the modest AKT downstream signaling inhibition likely contributed to the lack of a pCR response observed in this trial and the lack of antitumor activity beyond hormonal therapy alone in the previous study of MK-2206 in combination with hormonal therapy in patients with metastatic HR+ breast cancer (19). In addition, an adverse effect of prophylactic steroid on the activity of MK-2206 cannot be ruled out.
The lack of activity of MK-2206 in ER+ breast cancer observed in this trial is also consistent with recent I-SPY2 trial results. Although the addition of weekly MK-2206 to weekly paclitaxel followed by adriamycin and cyclophosphamide improved the pCR rate in patients with triple-negative breast cancer [40% with the addition of MK-2206 (n = 93) vs. 22% without MK-2206 (n = 59)], an improvement in pCR was not observed in the HR+ population (40).
An 83-gene sequencing panel was accomplished for 14 of the 16 patients enrolled in the study, and for serial tumor samples obtained from at least 2 time points for 10 patients. In this PIK3CA-mutant patient population, we observed a 43% incidence of CDH1 mutation, and a 21% incidence of FOXA1 mutation, consistent with the high proportion of invasive lobular cancers (50%) in this study, as CDH1 and FOXA1 mutations are more commonly observed in invasive lobular cancer compared with invasive ductal cancer (41). Comparing the VAFs of mutations over time, the presence of subclones with different responsiveness to AKT inhibition was observed in at least 2 cases, reflecting the intratumoral heterogeneity of ER+ breast cancer. Interestingly, we also observed an acquired ESR1 ligand-binding domain mutation at surgery time point after 5 months of anastrozole and 4 months of MK-2206. Our data are consistent with the previous observation that even the short duration of neoadjuvant aromatase inhibitor therapy was sufficient to induce marked remodeling of clonal architecture of ER+ breast cancer and acquisition of ESR1 mutation (33).
Our study is limited as a single-arm trial with a small sample size. However, the sequential treatment approach of anastrozole followed by the addition of MK-2206 allowed us to obtain serial biopsies to discern the effects of anastrozole alone versus the combination of MK-2206 and anastrozole for individual tumors enrolled in the study. Also, the large representation of invasive lobular cancers in 50% of the study population may confound study conclusions due to the potential biological differences between lobular versus ductal cancer. In addition, the restriction of PIK3CA-mutant tumors excluded AKT1 or other PI3K pathway driver mutations, as they are often mutually exclusive (24, 25). As AKT inhibition has shown promise in AKT1 mutated solid tumor malignancies, the absence of these tumors in this trial potentially adversely impacted the study results, although overall AKT1 mutations are rare, and only a dedicated study could resolve this issue (42, 43).
In addition to MK-2206, other AKT inhibitors being developed in clinical trials include ATP-competitive inhibitors, including AZD5363, which has shown clear antitumor activity in a phase I trial that enrolled patients with solid tumors that harbor the AKT1 E17K mutation, which included breast cancer patients (44). In preclinical studies, ATP-competitive inhibitors of AKT have potent downstream target inhibition. AZD5363 also potently inhibits p70S6K (IC50, 6nmol/L), although the activity profile in cell lines was similar to MK-2206 (45), and AZD5363 synergizes with hormonal therapy in preclinical studies of ER+ cancers sensitive or resistant to estrogen deprivation (46). It remains to be seen whether the ATP-competitive AKT inhibitors may differ from allosteric AKT inhibitors, such as MK-2206, in regards to the antitumor activities in the clinical setting.
In conclusion, although the sample size was small, our proof-of-concept design allowed a definitive conclusion that an MK-2206 anastrozole combination should not be further pursued in early-stage ER+ HER2− PIK3CA-mutant breast cancer. The frequent dose-limiting toxicity of skin rash despite prophylactic prednisone is also an issue with further development of this combination. Aspects of this investigation, including sequential biopsies, careful patient selection, and pharmacodynamic biomarker analysis could be considered when investigating other endocrine therapy combinations with signal transduction inhibitors to avoid accrual to larger studies of combinations that ultimately prove ineffective.
Disclosure of Potential Conflicts of Interest
C.X. Ma reports receiving commercial research grants from Merck, Novartis, and Pfizer and is a consultant/advisory board member for AstraZeneca, Eli Lilly, Merck, Novartis, and Pfizer. M.P. Goetz reports receiving commercial research grants from Eli Lilly and Pfizer and is a consultant/advisory board member for Biotheranostics, Eli Lilly, Myriad, and RNA diagnostics. M.E. Burkard reports receiving other commercial research support from Abbvie, Bristol-Myers Squibb, and Genentech and is a consultant/advisory board member for Point-care Genomics. M.J. Naughton reports receiving speakers bureau honoraria from Amgen, Astra Zeneca, Biotheranostics, Celgene, Genentech, Novartis, and Pfizer. S. Sanati is a consultant/advisory board member for Genentech. M.J. Ellis reports receiving other commercial research support from Bioclassifier/Prosigna/Nanostring, holds ownership interest (including patents) in Bioclassifier/Prosigna, and is a consultant/advisory board member for AstraZenica, Nano-string, Novartis, and Pfizer. No potential conflicts of interest were disclosed by the other authors.
Supplementary Material
Translational Relevance.
Inability to induce tumor cell death is a fundamental problem for endocrine therapy in treating estrogen receptor–positive (ER+) breast cancer. In preclinical studies, MK-2206, a pan-AKT inhibitor, induced apoptosis of ER+ breast cancer under estrogen-deprived conditions. This single-arm phase II trial therefore tested whether the addition of MK-2206 to anastrozole could induce pathologic complete responses (pCR) for PIK3CA-mutant ER+ breast cancer. No pCR was observed in 16 patients enrolled in the first stage of the study. IHC for pAKT and pPRAS40 on serial tumor biopsies indicated incomplete target inhibition, accompanied by a lack of further Ki67 suppression upon MK-2206 treatment. The study demonstrates that MK-2206 with anastrozole is unlikely to be more effective than anastrozole alone in PIK3CA-mutant ER+ breast cancer and illustrates the importance of detailed proof-of-concept studies in the investigation of endocrine therapy combinations in early breast cancer.
Acknowledgments
We wish to thank the patients and their families for participation in this study. We would like to acknowledge the McDonnell Genome Institute for DNA sequencing and analysis, Shana Thomas for editorial assistance of the manuscript, and Jing Han for specimen collection, and clinical research and regulatory coordinators. We would like to thank the patient advocate members of our project team, Mary Lynn Donovan and Judy Johnson, for their input in the study design and the development of the patient brochure.
Grant Support
This study was funded in part by the Siteman Cancer Center, Fashion Footwear Association of New York, the Mayo PIIC (N01-CM-2011–00099), UWCCC CCSG (P30 CA014520), Saint Louis Men’s Group Against Cancer, and Cancer Clinical Investigator Team Leadership Award awarded by the NCI through a supplement to P30CA091842 (to C. Ma). A Komen Promise Grant PG12220321 to M.J. Ellis also supported the study and the McNair Medical Institute and the Cancer Prevention Institute of Texas also support M.J. Ellis.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Prior presentation: This work was presented in part at the 2015 San Antonio Breast Cancer Symposium.
References
- 1.Howlader N, Altekruse SF, Li CI, Chen VW, Clarke CA, Ries LAG, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst 2014;106:pii:dju055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists’ Collaborative Group. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. The Lancet 2011;378:771–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Houssami N, Macaskill P, von Minckwitz G, Marinovich ML, Mamounas E. Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur J Cancer 2012; 48:3342–54. [DOI] [PubMed] [Google Scholar]
- 4.Dowsett M, Smith IE, Ebbs SR, Dixon JM, Skene A, Griffith C, et al. Proliferation and apoptosis as markers of benefit in neoadjuvant endocrine therapy of breast cancer. Clin Cancer Res 2006;12:1024s–30s. [DOI] [PubMed] [Google Scholar]
- 5.Bonnefoi H, Litière S, Piccart M, MacGrogan G, Fumoleau P, Brain E, et al. Pathological complete response after neoadjuvant chemotherapy is an independent predictive factor irrespective of simplified breast cancer intrinsic subtypes: a landmark and two-step approach analyses from the EORTC 10994/BIG 1–00 phase III trial. Ann Oncol 2014;25:1128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 2012;30:1796–804. [DOI] [PubMed] [Google Scholar]
- 7.Eiermann W, Paepke S, Appfelstaedt J, Llombart-Cussac A, Eremin J, Vinholes J, et al. Preoperative treatment of postmenopausal breast cancer patients with letrozole: A randomized double-blind multicenter study. Ann Oncol 2001;12:1527–32. [DOI] [PubMed] [Google Scholar]
- 8.Ellis MJ, Suman VJ, Hoog J, Lin L, Snider J, Prat A, et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype-ACOSOG Z1031. J Clin Oncol 2011;29:2342–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev 1999;13:2905–27. [DOI] [PubMed] [Google Scholar]
- 10.Staal SP. Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: amplification of AKT1 in a primary human gastric adenocarcinoma. Proc Natl Acad Sci U S A 1987;84:5034–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 1999;96:857–68. [DOI] [PubMed] [Google Scholar]
- 12.Cantley LC. The Phosphoinositide 3-Kinase Pathway. Science 2002;296: 1655–7. [DOI] [PubMed] [Google Scholar]
- 13.Ma CX, Crowder RJ, Ellis MJ. Importance of PI3-kinase pathway in response/resistance to aromatase inhibitors. Steroids 2011;76:750–2. [DOI] [PubMed] [Google Scholar]
- 14.Ciruelos Gil EM. Targeting the PI3K/AKT/mTOR pathway in estrogen receptor-positive breast cancer. Cancer Treat Rev 2014;40:862–71. [DOI] [PubMed] [Google Scholar]
- 15.Ma CX, Reinert T, Chmielewska I, Ellis MJ. Mechanisms of aromatase inhibitor resistance. Nat Rev Cancer 2015;15:261–75. [DOI] [PubMed] [Google Scholar]
- 16.Kirkegaard T, Witton CJ, McGlynn LM, Tovey SM, Dunne B, Lyon A, et al. AKT activation predicts outcome in breast cancer patients treated with tamoxifen. J Pathol 2005;207:139–46. [DOI] [PubMed] [Google Scholar]
- 17.Bostner J, Karlsson E, Pandiyan MJ, Westman H, Skoog L, Fornander T, et al. Activation of Akt, mTOR, and the estrogen receptor as a signature to predict tamoxifen treatment benefit. Breast Cancer Res Treat 2013;137:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perez-Tenorio G, Stal O. Activation of AKT/PKB in breast cancer predicts a worse outcome among endocrine treated patients. Br J Cancer 2002;86: 540–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma CX, Sanchez C, Gao F, Crowder R, Naughton M, Pluard T, et al. A phase I study of the AKT inhibitor MK-2206 in combination with hormonal therapy in postmenopausal women with estrogen receptor–positive metastatic breast cancer. Clin Cancer Res 2016;22:2650–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan L Abstract #DDT01–1: MK-2206: a potent oral allosteric AKT inhibitor In: Proceedings of the 100th Annual Meeting of the American Association for Cancer Research; 2009 Apr 18–22; Denver, CO. Philadelphia (PA): AACR; 2009. Abstract nr DDT01–1. [Google Scholar]
- 21.Yap TA, Yan L, Patnaik A, Fearen I, Olmos D, Papadopoulos K, et al. First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J Clin Oncol 2011;29:4688–95. [DOI] [PubMed] [Google Scholar]
- 22.Yap TA, Yan L, Patnaik A, Tunariu N, Biondo A, Fearen I, et al. Interrogating two schedules of the AKT inhibitor MK-2206 in patients with advanced solid tumors incorporating novel pharmacodynamic and functional imaging biomarkers. Clin Cancer Res 2014;20:5672–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sangai T, Akcakanat A, Chen H, Tarco E, Wu Y, Do KA, et al. Biomarkers of response to Akt inhibitor MK-2206 in breast cancer. Clin Cancer Res 2012;18:5816–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saal LH, Holm K, Maurer M, Memeo L, Su T, Wang X, et al. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res 2005;65:2554–9. [DOI] [PubMed] [Google Scholar]
- 25.The Cancer Genome Atlas Research Network. Comprehensive molecular portraits of human breast tumours. Nature 2012;490:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao JJ, Cheng H, Jia S, Wang L, Gjoerup OV, Mikami A, et al. The p110alpha isoform of PI3K is essential for proper growth factor signaling and oncogenic transformation. Proc Natl Acad Sci U S A 2006;103:16296–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao JJ, Liu Z, Wang L, Shin E, Loda MF, Roberts TM. The oncogenic properties of mutant p110alpha and p110beta phosphatidylinositol 3-kinases in human mammary epithelial cells. Proc Natl Acad Sci U S A 2005;102:18443–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crowder RJ, Phommaly C, Tao Y, Hoog J, Luo J, Perou CM, et al. PIK3CA and PIK3CB inhibition produce synthetic lethality when combined with estrogen deprivation in estrogen receptor-positive breast cancer. CancerRes 2009;69:3955–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellis MJ, Suman VJ, Hoog J, Goncalves R, Sanati S, Creighton CJ, et al. Ki67 proliferation index as a tool for chemotherapy decisions during and after neoadjuvant aromatase inhibitor treatment of breast cancer: results from the American College of Surgeons Oncology Group Z1031 Trial (Alliance). J Clin Oncol 2017;35:1061–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma CX, Cai S, Li S, Ryan CE, Guo Z, Schaiff WT, et al. Targeting Chk1 in p53-deficient triple-negative breast cancer is therapeutically beneficial in human-in-mouse tumor models. J Clin Invest 2012;122:1541–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qureshi A, Pervez S. Allred scoring for ER reporting and it’s impact in clearly distinguishing ER negative from ER positive breast cancers. J Pak Med Assoc 2010;60:350–3. [PubMed] [Google Scholar]
- 32.Ma CX, Luo J, Naughton M, Ademuyiwa F, Suresh R, Griffith M, et al. A Phase I Trial of BKM120 (Buparlisib) in Combination with Fulvestrant in Postmenopausal Women with Estrogen Receptor-positive Metastatic Breast Cancer. Clin Cancer Res 2016;22:1583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller CA, Gindin Y, Lu C, Griffith OL, Griffith M, Shen D, et al. Aromatase inhibition remodels the clonal architecture of estrogen-receptor-positive breast cancers. Nat Commun 2016;7:12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Griffith M, Griffith OL, Smith SM, Ramu A, Callaway MB, Brummett AM, et al. Genome modeling system: a knowledge management platform for genomics. PLoS Comput Biol 2015;11:e1004274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skidmore ZL, Wagner AH, Lesurf R, Campbell KM, Kunisaki J, Griffith OL, et al. GenVisR: Genomic Visualizations in R. Bioinformatics 2016;32: 3012–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson DR, Wu YM, Vats P, Su F, Lonigro RJ, Cao X, et al. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat Genet 2013;45:1446–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toy W, Shen Y, Won H, Green B, Sakr RA, Will M, et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat Genet 2013;45:1439–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Merenbakh-Lamin K, Ben-Baruch N, Yeheskel A, Dvir A, Soussan-Gutman L, Jeselsohn R, et al. D538G mutation in estrogen receptor-alpha: a novel mechanism for acquired endocrine resistance in breast cancer. Cancer Res 2013;73:6856–64. [DOI] [PubMed] [Google Scholar]
- 39.Jeselsohn R, Yelensky R, Buchwalter G, Frampton G, Meric-Bernstam F, Gonzalez-Angulo AM, et al. Emergence of constitutively active estrogen receptor-alpha mutations in pretreated advanced estrogen receptor-positive breast cancer. Clin Cancer Res 2014;20:1757–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tripathy D, Chien AJ, Hylton N, Buxton MB, Ewing CA, Wallace AM, et al. Adaptively randomized trial of neoadjuvant chemotherapy with or without the Akt inhibitor MK-2206: Graduation results from the I-SPY 2 Trial. J Clin Oncol 33, 2015 (suppl; abstr 524) 2015. [Google Scholar]
- 41.Desmedt C, Zoppoli G, Gundem G, Pruneri G, Larsimont D, Fornili M, et al. Genomic characterization of primary invasive lobular breast cancer. J Clin Oncol 2016;34:1872–81. [DOI] [PubMed] [Google Scholar]
- 42.Jansen VM, Mayer IA, Arteaga CL. Is There a Future for AKT Inhibitors in the Treatment of Cancer? Clin Cancer Res 2016;22:2599–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hyman DM, Smyth L, Bedard PL, Oza A, Dean E, Armstrong A, et al. Abstract B109: AZD5363, a catalytic pan-Akt inhibitor, in Akt1E17K mutation positive advanced solid tumors. Mol Cancer Ther 2015;14:B109–B. [Google Scholar]
- 44.Hyman DM, Smyth L, Bedard PL, Oza A, Dean E, Armstrong A, et al. AZD5363, a catalytic pan-Akt inhibitor, in Akt1 E17K mutation positive advanced solid tumors Proceedings of the AACR-NCI-EORTC International Conference: Molecular Targets and Cancer Therapeutics; 2015 Nov 5–9; Boston, MA. Philadelphia (PA): AACR; 2015. Abstract nrB109 2015. [Google Scholar]
- 45.Davies BR, Greenwood H, Dudley P, Crafter C, Yu DH, Zhang J, et al. Preclinical pharmacology of AZD5363, an inhibitor of AKT: pharmacodynamics, antitumor activity, and correlation of monotherapy activity with genetic background. Mol Cancer Ther 2012;11:873–87. [DOI] [PubMed] [Google Scholar]
- 46.Ribas R, Pancholi S, Guest SK, Marangoni E, Gao Q, Thuleau A, et al. AKT Antagonist AZD5363 Influences Estrogen Receptor Function in Endocrine-Resistant Breast Cancer and Synergizes with Fulvestrant (ICI182780) In Vivo. Mol Cancer Ther 2015;14:2035–48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.