Abstract
Objective:
To compare self-reported with objective measurements of energy intake changes (∆EI) during a 1-year weight loss intervention with subjects randomized to low-carbohydrate versus low-fat diets.
Methods:
We used repeated body weight measurements as inputs to an objective mathematical model to calculate ∆EIModel to compare with self-reported energy intake changes assessed by repeated 24-hr recalls (∆EIRecall).
Results:
∆EIRecall indicated a relatively persistent state of calorie restriction of ~500–600 kcal/d at 3, 6, and 12 months with no significant differences between the diets. ∆EIModel demonstrated large early decreases in calorie intake >800 kcal/d followed by an exponential return to ~100 kcal/d below baseline at the end of the year. Accounting for self-reported physical activities did not materially affect the results. Discrepancies between ∆EIModel and ∆EIRecall became progressively greater over time. The low-carbohydrate diet resulted in ∆EIModel that was 162±53 kcal/d lower than the low-fat diet over the first 3 months (p=0.002), but no significant diet differences were found thereafter.
Conclusions:
Self-reported ∆EI measurements were inaccurate. Model-based calculations of ∆EI found that instructions to follow the low-carbohydrate diet resulted in greater calorie restriction than the low-fat diet in the early phases of the intervention, but these diet differences were not sustained.
Keywords: Energy intake, diet composition
Introduction
Diet assessment instruments that rely on self-report, such as 24-hr recall, are known to substantially underestimate energy intake (1). However, repeated self-reported measurements could possibly track changes in energy intake accurately if the measurement bias is roughly constant for each subject. For example, if a person habitually eats a weight maintenance diet of 2500 kcal/d then their 24-hr recall might under-report eating only 1900 kcal/d. If they consistently underestimated their energy intake, then after starting a weight loss diet program they might report eating 1400 kcal/d whereas they actually consumed 2000 kcal/d. Their reported absolute energy intake would still be 600 kcal/d too low, but the self-reported change in energy intake of 500 kcal/d would be accurate. It is presently unknown whether people can accurately report changes in energy intake during a weight loss intervention.
We recently validated an objective mathematical method for calculating energy intake changes over time using only information about age, sex, height, and repeated measurements of body weight (2). Here, we applied this method to data from the Diet Intervention Examining The Factors Interacting with Treatment Success (DIETFIITS) randomized weight loss trial (3) and compared the model-calculated energy intake changes with self-reported values determined by repeated 24hr recalls.
Methods
As previously described (3, 4), participants were randomized to the low-carbohydrate or low-fat diet groups and were instructed reduce intake of total fat or digestible carbohydrates to 20 g/d during the first 8 weeks and then slowly add fats or carbohydrates back to their diets in increments of 5 to 15 g/d per week until they reached the lowest level of intake they believed could be maintained indefinitely. No instructions were provided regarding calorie restriction.
As previously described (3, 4), self-reported dietary intake was assessed using 3 unannounced 24-hour multiple-pass recall interviews (2 on weekdays and 1 on a weekend day) administered before the intervention and again after approximately 3, 6 and 12 months. Data were collected using Nutrition Data System for Research (NDSR), a computer-based software application developed at the University of Minnesota Nutrition Coordinating Center (http://www.ncc.umn.edu/products/). Dietary recalls were collected in a standardized fashion using a multiple-pass interview approach consisting of five steps to ensure completeness and accuracy. Throughout the recall, the NDSR software searched for foods and brand name products by name and prompted the data collectors with requests for additional detailed information. In addition, the interviewers entered recipes or ingredients for homemade, restaurant, and other items not included in the software. All data collectors were trained by NDSR certified lead staff and were blinded to the assigned diets. The lead dietary assessment nutritionist conducted a quality check for each cohort after data collection at each study collection point. This involved an in-depth review of both individual and composite reports for completeness and errors.
Body weight was measured by digital scale at the Stanford Clinical Translational Research Unit. Self-reported body weight was also recorded when subjects participated in the 22 instructional sessions over the course of the year. We used data from 414 subjects in the DIETFITS study (209 subjects randomized to the low-carbohydrate diet and 205 subjects randomized to the low-fat diet) with complete body weight data at all clinic visits. Of the subjects with complete clinic weight data, only one was missing baseline self-reported energy intake and 3, 11, and 13 were missing self-reported energy intake at 3, 6, and 12 months, respectively.
As previously described (2), we used a linearized mathematical model of body weight dynamics that was solved for the change in energy intake averaged over each time interval i as compared to a weight-maintaining baseline diet, ΔEIModel, as a function of body weight and its rate of change as follows:
where ρ is an effective energy density associated with the BW change:
and εi is a parameter that defines how energy expenditure depends on BW:
The parameters γFFM and γFM are the regression coefficients relating resting metabolic rate to fat-free mass (FFM) and fat mass (FM), respectively. Parameters ρFM and ρFFM are the energy densities associated with changes in FM and FFM, respectively. Physical activity energy expenditure is proportional to body weight, where δ0 represents the baseline level of physical activity and Δδi is the change in physical activity from baseline over each time period. The parameter β accounts for the adaptation of energy expenditure during a diet perturbation, ΔEI. Parameters ηFM and ηFFM account for the biochemical cost of tissue deposition and turnover assuming that the change of FFM is primarily accounted for by body protein and its associated water. The parameter α represents the relationship between changes of fat-free and fat mass: α≡dFFM/dFM = CFM where C = 10.4 kg is the Forbes parameter. For modest weight changes, α can be considered to be approximately constant with FM fixed at its initial value FM0. The larger the initial fat mass, FM0, the smaller the parameter α.
The model parameters are listed in Supplementary Table 1 and we used the initial age, sex, and height to calculate the parameter α for each subject. For the main analysis, we assumed that the baseline physical activity parameter was δ0 = 10 kcal/kg/d corresponding to an initial free-living physical activity level (PAL) ~1.6. Therefore, the average linearized model parameters were (mean ± SE) ρ = 10036 ± 21 kcal/kg and ε = 23 ± 0.05 kcal/kg/d assuming no physical activity changes (i.e. Δδi = 0). We also conducted an analysis of the subset of subjects (N=338) with self-reported physical activities at baseline and 12 months (5) to define individual values for δ0 and Δδi where we linearly interpolated between the times when Δδi was measured. The mean values were δ0 = 8.8 ± 0.1 kcal/kg/d, Δδi = 0.4 ± 0.1 kcal/kg/d at both i = 3 and 6 months, and Δδi = 0.5 ± 0.1 kcal/kg/d at i = 12 months.
The change of mean body weight versus baseline over each interval, , and the moving average of the measured body weight time course was used to calculate the rate of change of body weight over each interval, dBWi/dt. The interval length was t = (N-1)*T, where N was the number of body weight measurements per interval and T was the number of days between measurements. When clinic weights were used, N=2 for all periods and T=90 days for the first and second 3-month periods and T=180 days for the final 6 months. When self-reported weights were used, we specified the interval lengths of t=30 days, t=60 days, and t=90 days to calculate the average ΔEIModel and the values for N and T were calculated using the available data on each subject in the corresponding time interval. In the figures, ΔEIModel values were plotted at the midpoint time of each averaging interval.
Exponential time courses were fit to ΔEIModel values using Berkeley Madonna software (version 8.3) with equal weight given to the values determined by clinic and self-reported weights since they all appeared to lie on the same curve. Statistical analysis was performed using SAS (version 9.4) and a paired, two-sided t-test with significance declared at the p<0.05 threshold. The data are reported as mean±SE.
Results
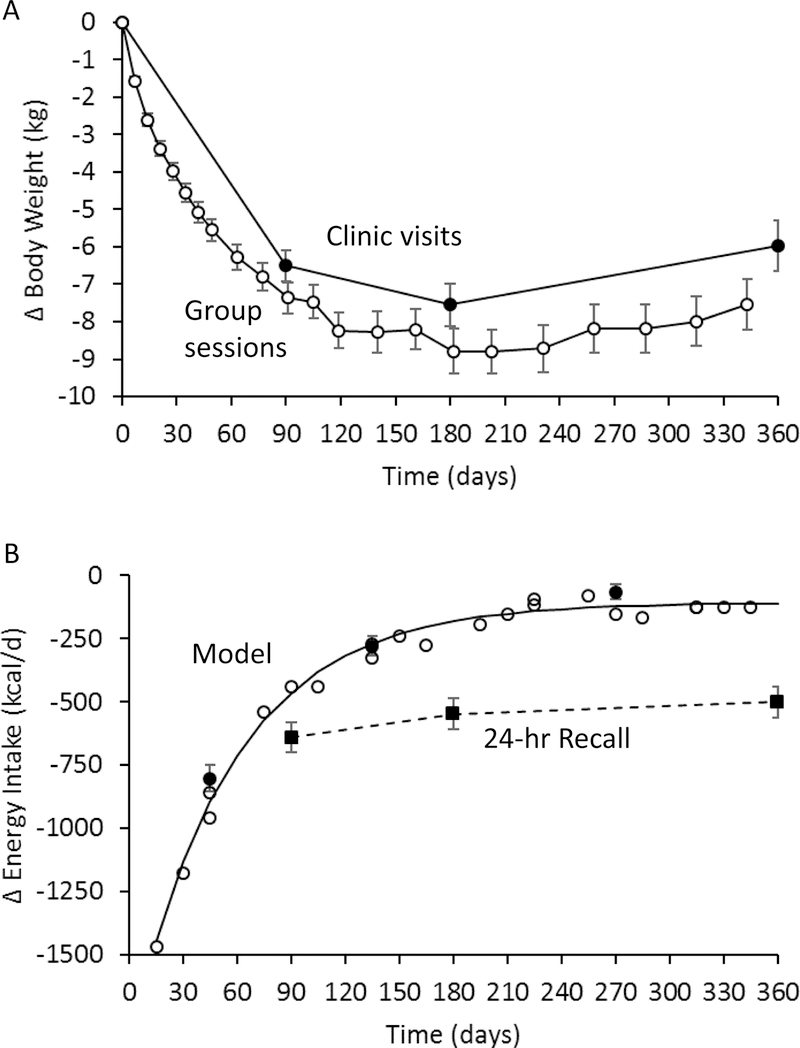
Figure 1A shows the mean weight changes measured at the clinic visits as well as those recorded at group counseling sessions where participants reported their weights as somewhat lower than could be documented in the clinic. Figure 1B illustrates the model-based measurements as well as the self-reported measurements of energy intake change. After 3 months of the intervention, ∆EIRecall =−641±31 kcal/d which was significantly lower than ∆EIRecall=−547±32 kcal/d at 6 months (p<0.0001). At 12 months, ∆EIRecall=−500±31 kcal/d and was similar to the value at 6 months (p=0.05) indicating a relatively persistent and substantial reduction of energy intake.
Figure 1.
A) Mean body weight changes measured during the DIETFITS trial clinic visits (●) or self-reported by subjects at group counseling sessions (○) for all 414 subjects with complete clinic weight data. B) Mean self-reported energy intake changes (■) indicated a relatively persistent reduction in energy intake whereas the model-based measurements (○ from self-reported weights and ● from clinic weights) followed an exponential time course (solid curve). Error bars indicate 95% CI.
In contrast, the model-based calculations demonstrated that energy intake changes followed an exponential time course shown in Figure 1B. Using the clinic weights, ∆EIModel was −804 ±27 kcal/d averaged over the first 3 months. Over the next 3 months, ∆EIModel = −279 ±20 kcal/d indicating a substantial relaxation of calorie restriction (p<0.0001) which was again relaxed to ∆EIModel= −65 ±14 kcal/d between 6 and 12 months (p<0.0001). In a subset of 307 subjects with self-reported physical activity measurements over the course of the intervention, we found that the ∆EIModel results were within 60 kcal/d at 3 and 6 months, and within 70 kcal/d at 12 months, of the corresponding values calculated assuming that physical activity was 10 kcal/kg/d throughout the intervention (Supplementary Table 2).
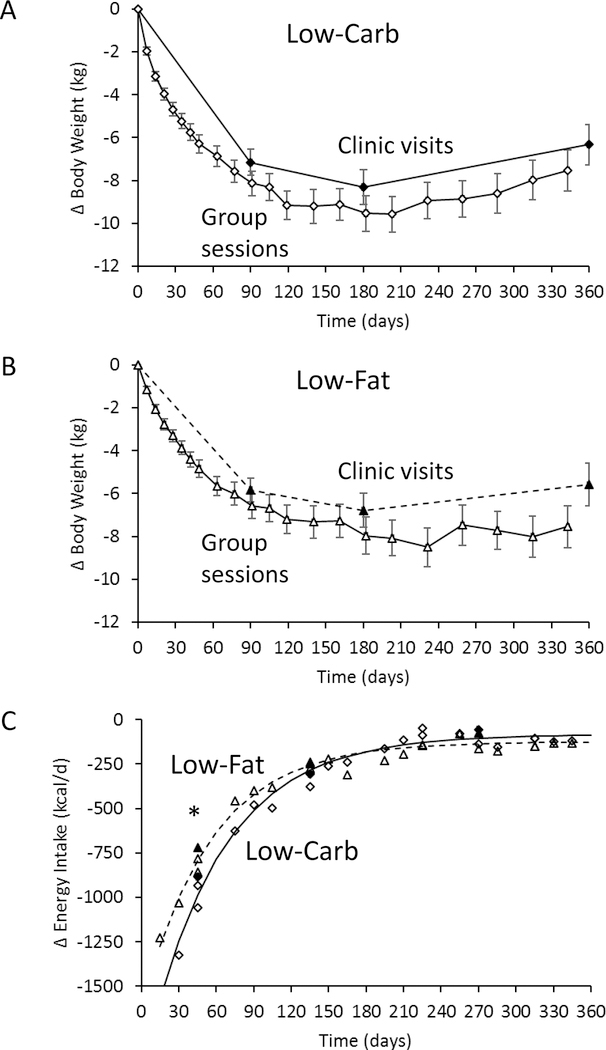
Figures 2A and 2B show the mean clinic weight changes in the low-carbohydrate and low-fat diet groups, respectively, which were significantly different at 3 months (p=0.002) and 6 months (p=0.001), but not at 12 months (p=0.29). Weights reported at the group counseling sessions indicated similar degrees of underreporting in each diet group. Self-reported energy intake was not significantly different between low-carbohydrate and low-fat diet groups at any time point (Table 1). However, model-based calculations using the clinic weights found that energy intake decreased over the first 3 months by 162±53 kcal/d more with the low-carbohydrate diet group as compared to the low-fat diet (p=0.002), but there were no significant differences at later times. Figure 2B shows that ∆EIModel followed a similar exponential pattern regardless of diet, but the low-carbohydrate diet led to larger early reductions in calorie intake that were not sustained.
Figure 2.
A) Mean body weight changes for the 209 subjects in the low-carbohydrate diet group (♦ clinic and ◊ self-reported) and B) the 205 subjects in the low-fat (▲clinic and Δ self-reported) diet group. C) Mean model-based measurements of energy intake changes in the low-carbohydrate diet group (▲ from clinic weights and Δ from self-reported weights ) and the low-fat diet group (◊ from self-reported weights and ♦ from clinic weights) both followed an exponential time courses (solid curve and dashed curve for low-carbohydrate and low-fat diets, respectively). * indicates p<0.05 between diet groups and the error bars indicate 95% CI.
Table 1.
Changes in body weight, self-reported energy intake, and model-claculated energy intake during the DIETFITS intervention (mean ± SE).
| Variable | Both Diets (N=414) | Low-Carbohydrate (LC; N=209) | Low-Fat (LF; N=205) | P-value LC vs LF |
|---|---|---|---|---|
| ΔBW3 months | −6.5±0.2 kg | −7.2±0.3 kg | −5.8±0.3 kg | 0.002 |
| ΔBW6 months | −7.6±0.3 kg | −8.3±0.4 kg | −6.8±0.4 kg | 0.01 |
| ΔBW12 months | −5.9±0.3 kg | −6.3±0.5 kg | −5.6±0.5 kg | 0.29 |
| 3 month ΔEIRecall1 | −641±31 kcal/d | −628±43 kcal/d | −653±44 kcal/d | 0.68 |
| 6 month ΔEIRecall2 | −547±32 kcal/d | −552±45 kcal/d | −542±45 kcal/d | 0.87 |
| 12 month ΔEIRecall3 | −500±31 kcal/d | −532±44 kcal/d | −467±44 kcal/d | 0.30 |
| 0–3 month ΔEIModel | −804±27 kcal/d | −884±39 kcal/d | −722±36 kcal/d | 0.002 |
| 3–6 month ΔEIModel | −279±20 kcal/d | −307±29 kcal/d | −251±27 kcal/d | 0.16 |
| 6–12 month ΔEIModel | −65±28 kcal/d | −56±18 kcal/d | −75±22 kcal/d | 0.49 |
missing 1 LC value and 2 LF values.
missing 4 LC values and 7 LF values.
missing 8 LC values and 5 LF values.
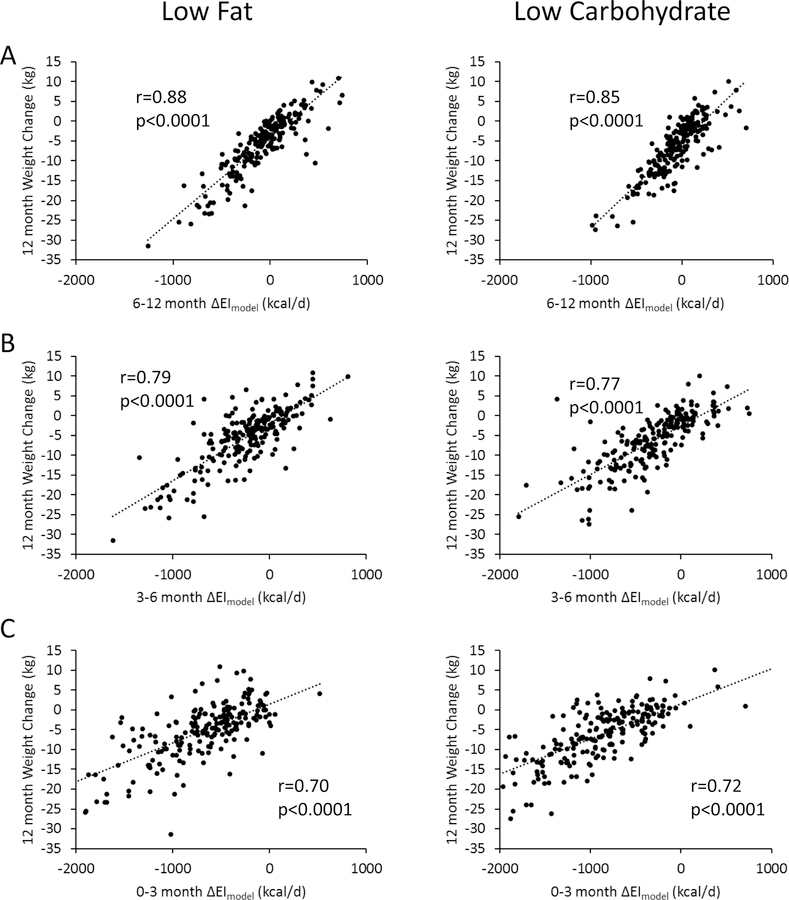
Figure 3 depicts individual 12 month clinic weight change data for both the low-fat (left column) and low-carbohydrate (right column) diets as a function of the ∆EIModel calculated using clinic weights averaged over the periods 6–12 months (panel A), 3–6 months (panel B), and 0–3 months (panel C). For the low-fat diet, weight loss at 12 months was correlated with ∆EIModel averaged over 6–12 months (r=0.88; p<0.0001), 3–6 months (r=0.79; p<0.0001), and 0–3 months (r=0.70; p<0.0001). Weight change at 6 months was correlated with ∆EIModel averaged over 3–6 months (r=0.88; p<0.0001), and 0–3 months (r=0.90; p<0.0001) and weight change at 3 months was correlated with ∆EIModel averaged over 0–3 months (r=1; p<0.0001) (not shown). For the low-carbohydrate diet, weight loss at 12 months was correlated with ∆EIModel averaged over 6–12 months (r=0.85; p<0.0001), 3–6 months (r=0.77; p<0.0001), and 0–3 months (r=0.70; p<0.0001). Weight change at 6 months was correlated with ∆EIModel averaged over 3–6 months (r=0.85; p<0.0001), and 0–3 months (r=0.87; p<0.0001) and weight change at 3 months was correlated with ∆EIModel averaged over 0–3 months (r=1; p<0.0001) (not shown). In contrast, ∆EIRecall was only weakly correlated with contemporaneous weight losses at 3-months (r=0.18; p=0.01) and 12-months (r=0.18; p=0.01) and only for the low-fat diet.
Figure 3.
Individual weight changes at 12 months for subjects assigned to the low-fat diet (left column) and low-carbohydrate diet (right column) were significantly correlated with model-calculated changes in energy intake averaged over A) 6–12 months; B) 3–6 months; and C) 0–3 months.
Discussion
This study demonstrates that the energy intake bias calculated by self-reported 24hr recall was not constant over time in subjects participating in a low-fat versus low-carbohydrate diet intervention for weight loss. Rather, biases in self-reported energy intake become progressively larger such that early assessments of ∆EIRecall were closer to ∆EIModel as compared to later measurements. Whereas the ∆EIRecall measurements suggested a relatively persistent change in energy intake over time, the calculated average ∆EIModel exhibited a large initial reduction in energy intake that exponentially decayed towards baseline over time. Incorporating self-reported measurements of physical activity throughout the intervention did not materially affect the ∆EIModel results. The low-carbohydrate diet resulted in significantly greater early reductions in model-calculated energy intake, with correspondingly greater early weight losses as compared to the low-fat diet, but these diet differences were not sustained.
The early reductions in ∆EIModel after the onset of the intervention indicated that subjects dramatically cut their calorie intake despite instructions that did not focus on calorie restriction. Rather, the diet instructions emphasized avoiding highly processed foods and reducing dietary carbohydrate or fat to very low levels at the start of the intervention. The model-calculated reductions in energy intake may have been slightly exaggerated at the start of the intervention because they relied on weight losses reported by the subjects at the group counseling sessions which were somewhat greater than could be corroborated at the clinic visits. Also, water losses that typically occur at the onset of a weight loss intervention may have amplified the early reductions in energy intake, especially during the initial stages of the low-carbohydrate diet where participants were instructed to reduce digestible carbohydrates to <20 g/d for the first 8 weeks and slowly add back carbohydrates to the minimum sustainable level (3). In this early time period, there was a greater reduction in model-calculated energy intake compared to the low-fat diet which is consistent with greater water losses but may also indicated that very low carbohydrate diets suppress appetite by inducing nutritional ketosis (6). Nevertheless, short-term reductions in appetite did not result in sustained reductions in energy intake with the low-carbohydrate diet and long-term average weight losses were not significantly different between the diets.
The relatively constant self-reported energy intake changes gives the impression that the slowing and plateauing of weight loss was primarily due to reductions in energy expenditure which are known to occur with weight loss (7). However, energy expenditure reductions are quantitatively insufficient to account for the observed body weight trajectory given an approximately constant reduction in energy intake. Thus, energy intake must have risen after its early reduction at the start of the intervention (8). The body weight trajectories observed in the DIETFITS study conform to the ubiquitous slowing of weight loss and subsequent weight plateau after 6–8 months (9) corresponding to exponentially increasing energy intake time course (10, 11).
In contrast to the objective measurements of energy intake that exponentially increase over time after the start of the intervention, why do the subjects report a relatively constant reduction in energy intake that progressively deviates from the objective values over time? Perhaps the constant self-reported calorie restriction reflects that the subjects were exerting a persistent effort to adhere to the diet intervention in the face of progressively increasing appetite in proportion to lost weight (11, 12). The creeping upwards of actual energy intake over time may have been due to subconscious increases in portion sizes or snacking episodes that failed to register in the repeated 24-hour recalls.
At the end of the 12-month DIETFITS trial, there was a large interindividual variability in weight loss that was associated with the model-calculated energy intake changes at all stages of the intervention. Due to the long time-scale for human body weight to equilibrate to a constant energy intake (8), weight changes over periods of less than a few years are expected to be related to not only current energy intake, but the history of intake changes in the past year or more. Here, we observed that much of the 12-month weight loss variability was associated with energy intake changes occurring in the first few months as well as at later time points. Thus, studies designed to understand weight loss variability need to account for the dynamic nature of human weight loss.
The major limitation of this study was that we did not use doubly labeled water to measure free-living energy intake changes by the gold-standard intake-balance method (13). However, our mathematical method has been validated against the intake-balance method in a two-year human calorie restriction study (2) that also exhibited a consistent exponential pattern of energy intake changes over time (14). However, this previous validation study did not compare different diets and did not include subjects with obesity (15), so we cannot be certain that the model-based calculations of energy intake were valid in the present study population.
In summary, repeated self-reported measurements of energy intake changes during the DIETFITS weight loss intervention were not accurate. Model-based calculations demonstrated an exponential pattern of energy intake change whereby large early calorie reductions decayed back towards baseline over time. Instructions to adhere to a low-carbohydrate diet resulted in greater calorie restriction compared to a low-fat diet in the early phases of the DIETFITS intervention, but these diet differences were not sustained.
Supplementary Material
What is already known about this subject?
Diet assessments that rely on self-report, such as 24hr dietary recall, are known to underestimate actual energy intake as measured by doubly labeled water. However, it is possible that repeated self-reported measurements could accurately detect changes in energy intake over time if the absolute bias of self-reported of measurements was approximately constant for each subject.
What this study adds:
We compared energy intake changes measured using repeated 24hr dietary recall measurements collected over the course of the 1-year Diet Intervention Examining The Factors Interacting with Treatment Success (DIETFITS) trial versus energy intake changes calculated using repeated body weight measurements as inputs to a validated mathematical model.
Whereas self-reported measurements indicated a relatively persistent state of calorie restriction, objective model-based measurements demonstrated a large early calorie restriction followed by an exponential rise in energy intake towards the pre-intervention baseline.
Model-based calculations, but not self-reported measurements, found that low-carbohydrate diets led to significantly greater early decreases in energy intake compared to low-fat diets, but long-term energy intake changes were not significantly different.
Acknowledgements
KDH and JG were supported by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. JLR and CG were supported by Grant 1R01DK091831 from the NIDDK, Grant 1K12GM088033 from the NIH (CTSA) and the Nutrition Science Initiative. The clinical study protocol and deidentified individual data described in this manuscript will be made available for download on the Open Science Framework website (https://osf.io/) within 6 months of publication for any purpose by anyone.
Funding: KDH and JG were supported by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. JLR and CG were supported by Grant 1R01DK091831 from the NIDDK, Grant 1K12GM088033 from the NIH (CTSA) and the Nutrition Science Initiative.
ClinicalTrials.gov Identifier: NCT01826591
Footnotes
Conflict of interest disclosure statement: None of the authors have conflicts of interest
References
- 1.Schoeller DA. How accurate is self-reported dietary energy intake? Nutr Rev 1990;48: 373–379. [DOI] [PubMed] [Google Scholar]
- 2.Sanghvi A, Redman LA, Martin CK, Ravussin E, Hall KD. Validation of an inexpensive and accurate mathematical method to measure long-term changes in free-living energy intake. Am J Clin Nutr 2015;102: 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gardner CD, Trepanowski JF, Del Gobbo LC, et al. Effect of low-fat vs low-carbohydrate diet on 12-month weight loss in overweight adults and the association with genotype pattern or insulin secretion: The dietfits randomized clinical trial. JAMA 2018;319: 667–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stanton MV, Robinson JL, Kirkpatrick SM, Farzinkhou S, Avery EC, Rigdon J, et al. DIETFITS study (diet intervention examining the factors interacting with treatment success) - Study design and methods. Contemporary clinical trials 2017;53: 151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sallis JF, Haskell WL, Wood PD, Fortmann SP, Rogers T, Blair SN, et al. Physical activity assessment methodology in the Five-City Project. American journal of epidemiology 1985;121: 91–106. [DOI] [PubMed] [Google Scholar]
- 6.Gibson AA, Seimon RV, Lee CM, Ayre J, Franklin J, Markovic TP, et al. Do ketogenic diets really suppress appetite? A systematic review and meta-analysis. Obes Rev 2015;16: 64–76. [DOI] [PubMed] [Google Scholar]
- 7.Rosenbaum M, Leibel RL. Adaptive thermogenesis in humans. Int J Obes (Lond) 2010;34 Suppl 1: S47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall KD, Sacks G, Chandramohan D, Chow CC, Wang YC, Gortmaker SL, et al. Quantification of the effect of energy imbalance on bodyweight. Lancet 2011;378: 826837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franz MJ, VanWormer JJ, Crain AL, Boucher JL, Histon T, Caplan W, et al. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc 2007;107: 1755–1767. [DOI] [PubMed] [Google Scholar]
- 10.Gobel B, Sanghvi A, Hall KD. Quantifying energy intake changes during obesity pharmacotherapy. Obesity (Silver Spring) 2014;22: 2105–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polidori D, Sanghvi A, Seeley RJ, Hall KD. How Strongly Does Appetite Counter Weight Loss? Quantification of the Feedback Control of Human Energy Intake. Obesity (Silver Spring) 2016;24: 2289–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall KD, Schoeller DA, Brown AW. Reducing Calories to Lose Weight. Jama 2018;319: 2336–2337. [DOI] [PubMed] [Google Scholar]
- 13.Racette SB, Das SK, Bhapkar M, Hadley EC, Roberts SB, Ravussin E, et al. Approaches for quantifying energy intake and %calorie restriction during calorie restriction interventions in humans: the multicenter CALERIE study. Am J Physiol Endocrinol Metab 2012;302: E441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo J, Brager DC, Hall KD. Simulating long-term human weight-loss dynamics in response to calorie restriction. Am J Clin Nutr 2018;107: 558–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ravussin E, Redman LM, Rochon J, Das SK, Fontana L, Kraus WE, et al. A 2-Year Randomized Controlled Trial of Human Caloric Restriction: Feasibility and Effects on Predictors of Health Span and Longevity. J Gerontol A Biol Sci Med Sci 2015;70: 10971104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.