Abstract
We mainly aimed to preliminarily explore the prognostic values of nutrition-based prognostic scores in patients with advanced hilar cholangiocarcinoma (HCCA).
We retrospectively analyzed 73 cases of HCCA, who underwent percutaneous transhepatic biliary stenting (PTBS) combined with 125I seed intracavitary irradiation from November 2012 to April 2017 in our department. The postoperative changes of total bilirubin (TBIL), direct bilirubin (DBIL), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and albumin (ALB) were observed. The preoperative clinical data were collected to calculate the nutrition-based scores, including controlling nutritional status (CONUT), C-reactive protein/albumin ratio (CAR), and prognostic nutritional index (PNI). Kaplan–Meier curve and Cox regression model were used for overall survival (OS) analyses.
The serum levels of TBIL, DBIL, ALT, AST, and ALP significantly reduced, and ALB significantly increased at 1 month and 3 months postoperatively. The median survival time of the cohort was 12 months and the 1-year survival rate was 53.1%. Univariate analysis revealed that the statistically significant factors related to OS were CA19-9, TBIL, ALB, CONUT, and PNI. Multivariate analysis further identified CA19-9, CONUT, and PNI as independent prognostic factors.
Nutrition-based prognostic scores, CONUT and PNI in particular, can be used as predictors of survival in unresectable HCCA.
Keywords: 125I seed, biliary stent, controlling nutritional status, hilar cholangiocarcinoma, prognostic nutritional index
1. Introduction
Cholangiocarcinoma is a rare malignancy arising from bile duct epithelial cells, with an incidence of approximately 1 to 2 per 100,000 population.[1] Surgical resection and liver transplantation remain the only radical therapies for hilar cholangiocarcinoma (HCCA, also known as Klatskin tumors). Unfortunately, up to 80% of HCCA patients present with unresectable stage at the time of diagnosis.[2] Because of the vital location of tumor, the prognosis of unresectable HCCA remains dismal, with a median survival time of less than 1 year.[2]
To date, chemotherapy remains the preferred treatment for locally advanced, recurrent, or metastatic HCCA, while it has limited improvements in patients’ survival.[3] Biliary drainage, especially via the percutaneous transhepatic approach, has been proposed to relieve cholestasis and improve survival in HCCA.[4] Recently, percutaneous transhepatic biliary stenting (PTBS) has also been applied to patients with HCCA due to the effective relief of biliary obstruction.[4,5] However, as the ingrowth of tumor, the biliary stent alone is associated with a high risk of biliary reobstruction and a poor survival.[6] In contrast, the PTBS combined with 125I seed intracavitary irradiation shows a longer stent patency time and a better survival for patients with HCCA,[7,8] while further research is still needed to determine the efficacy of this technique and factors associated with outcome.
Malnutrition may result in wound healing delay, immune function disorder, and the dysfunction of neutrophils, macrophages, and lymphocytes.[9] It has been reported that malnutrition involves in the cancer progression, and is associated with an increased risk of postoperative complications and a prolonged hospital stay in cancer patients.[10,11] In addition, preoperative poor nutritional condition leads to an adverse survival in patients with malignant tumors.[9,12] On the basis of the above findings, in recent years, several nutrition-based scores have been identified as possible prognostic markers in various cancers. Details of these scores are all easily available from peripheral blood sample, including the controlling nutritional status (CONUT), C-reactive protein (CRP)-to-albumin (ALB) ratio (CAR), and prognostic nutritional index (PNI).[12–14] To our knowledge, the values of the 3 nutrition-based prognostic scores have rarely been investigated in unresectable HCCA patients. In this study, we preliminarily explored the prognostic significance of the above 3 nutritional scores in HCCA patients treated with PTBS combined with 125I seed intracavitary irradiation.
2. Materials and methods
2.1. Study population
We retrospectively reviewed the data of HCCA patients who undergone PTBS combined with 125I seed implantation from November 2012 to April 2017 in our department. Eligible patients were HCCA diagnosed by pathological or clinical method; unresectability or refusal to resection; and treated with PTBS followed by 125I seed implantation. Exclusion criteria were benign bile duct stricture; with a history of surgery, endoscopic biliary stenting, or chemotherapy for HCCA; intrahepatic or distal cholangiocarcinoma; and incomplete data or follow-up information. In total, 73 patients met the selection standard and were included. We reported the current study according to the Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD).[15] Our study was in compliance with the Declaration of Helsinki[16] and was approved by the Ethics Committee of the First Affiliated Hospital of Bengbu Medical College. In addition, written informed consent was given by all patients.
2.2. Instruments
The Innova 3100 digital subtraction angiography (DSA) device (General Electric, Fairfield, Connecticut) was used during the operation. Percutaneous transhepatic cholangial drainage (PTCD) was performed by using pigtail catheters (Guangzhou Leadgem Medical Devices Co., Ltd., China). The self-expandable metal stent was purchased from Micro-Tech Co., Ltd (Nanjing, China) and the catheter with various sizes (8 × 40 mm,8 × 60 mm, 8 × 80 mm, 8 × 100 mm, 10 × 40 mm, 10 × 60 mm, 10 × 80 mm, and 10 × 100 mm, as appropriate) was applied. The 125I brachytherapy particles (Beijing Atomic Hi-Tech Co., Ltd., China) were cylindrical, with the length of 4.5 mm, the diameter of 0.8 mm, the radioactivity of 33.3 MBq, and the half-life of 59.43 days.
2.3. Surgical procedures
About a week after PTCD, the PTBS and 125I seed implantation were performed under DSA. The process of PTBS has been described previously.[17] Briefly, cholangiography through the PTCD tube was first performed to observe the situation of biliary stricture and expansion. The guidewire was then inserted through PTCD tube, which was then replaced by catheter sheath. The cholangiographic catheter was further inserted pass through the guidewire and the direction was repeatedly adjusted so that the catheter can completely reach the duodenum. Subsequently, we measured the length and the diameter of the obstructed bile duct to select suitable stent. Under DSA guidance, the cholangiographic catheter was withdrawn and the biliary stent was slowly inserted through the guidewire.
The number of 125I particles was calculated according to the following formula: (length + width + height of tumor) (cm)/3 × 5÷activity per particle (mCi). The “P” type tube was used as the particles source applicator. The distribution of 125I particles in the “P” type tube was determined by the situation of biliary obstruction. The 125I particles were separated with the average particle space 0.6 to 1.0 cm. Under the guidance of the guidewire, the “P” type tube that contains 125I particles was inserted into the stent cavity of the obstructive location.
2.4. Data collection and follow-up
We reviewed the electronic medical records to collect the following clinical data: age, gender, and preoperative laboratory tests. Preoperative measurements included serum total bilirubin (TBIL), direct bilirubin (DBIL), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), ALB, total cholesterol, neutrophil count, lymphocyte count, CRP, carbohydrate antigen 19-9 (CA19-9), and carcinoembryonic antigen (CEA). The scores of CONUT, CAR, and PNI were calculated according to the following formulas:
-
a)
CONUT: ALB (g/L): ≥35 g/L = 0; 30–34.9 = 2; 25–29.9 = 4; <25 = 6. Lymphocyte count (109/L): >1.6 = 0; 1.2–1.6 = 1; 0.8–1.2 = 2; <0.8 = 3. Total cholesterol (mg/dL): >180 = 0; 140–179 = 1; 100–139 = 2; <100 = 3. The CONUT score is the sum of the above 3 variables and therefore is varied from 0 to 12.
-
b)
CAR: CRP (mg/L)/ALB (g/L).
-
c)
PNI: ALB (g/L) +5 × lymphocyte count (109/L).
Patients were followed up until September 2017 or until death. The follow-up contents included routine biochemical measurements and imaging examinations. The levels of TBIL, DBIL, ALT, AST, ALP, and ALB at 1 month and 3 months postoperatively were compared with the preoperative levels.
2.5. Statistical analysis
SPSS version 21.0 (IBM Corp., Armonk, New York) was used to collect and analyze the data. Continuous variables were described as median (range) for these with non-normal distribution and mean ± standard deviation for the normally distributed variables. Different subgroups were compared by using the t test, Wilcoxon test, or χ2 test, as appropriate. Paired-sample t test was used to evaluate the postoperative changes of liver function and bilirubin. We used the receiver operating characteristic (ROC) curve to determine the optimal cut-off point (the maximum of specificity and sensitivity) of nutrition-based scores for discriminating between deceased and living patients.
The primary outcome we observed was overall survival (OS), which was estimated by the Kaplan–Meier curve. Factors possibly affecting OS were assessed by COX regression. It was considered statistically significant provided P < .05.
3. Results
3.1. Characteristics of patients and the assessment of efficacy
Demographic data, laboratory tests, and evaluation of nutrition-based scores of the 73 patients are presented in Table 1. There were 44 men and 29 women, with the mean age of 64.7 ± 10.6 years. All patients successfully received the implantations of biliary stent and 125I seed.
Table 1.
Basic characteristics of the 73 HCCA patients.
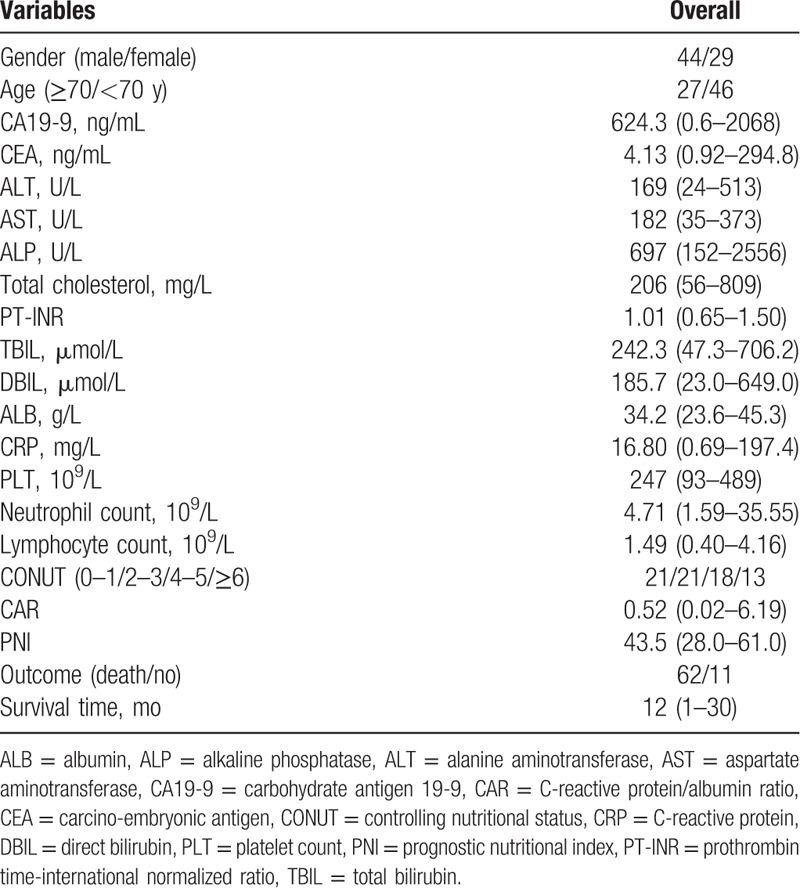
After the operation, the clinical symptoms such as jaundice and fever were significantly improved in all patients. The levels of TBIL, DBIL, ALT, AST, and ALP significantly reduced, and ALB significantly increased at 1 month and 3 months postoperatively, compared with the preoperative levels (Fig. 1, all P < .05).
Figure 1.
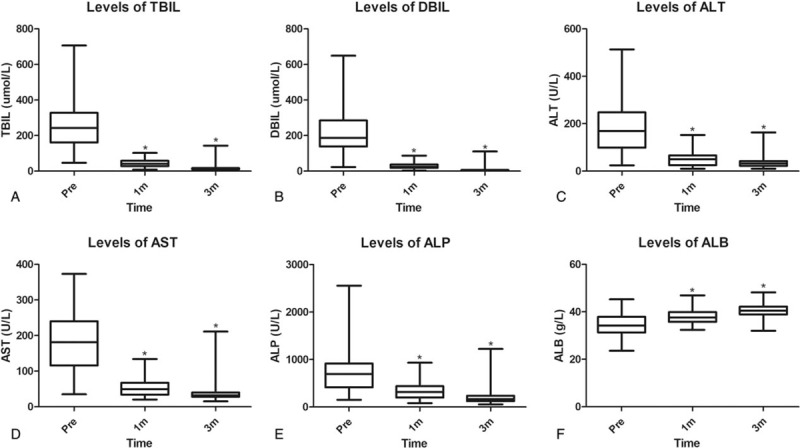
Comparison of liver function between preoperation and postoperation. Compared with the preoperative data, the levels of TBIL (A), DBIL (B), ALT (C), AST (D), ALP (E), and ALB (F) significantly improved at 1 month and 3 months postoperatively (∗P < .05).
3.2. Determination of the cut-off values for the CONUT, CAR, and PNI
The ROC curves of CONUT, CAR, and PNI revealed that 2, 0.31, and 47.4 were the optimal cut-off values, with 77.4% sensitivity and 63.6% specificity, 72.6% sensitivity and 54.5% specificity, and 54.5% sensitivity and 85.5% specificity, respectively. In particular, CONUT and PNI were significant models for determining dead from living cases, with the area under the curve (AUC) values 0.710 [95% confidence interval (95% CI): 0.526–0.893, P = .022] and 0.693 (95% CI: 0.521–0.864, P = .036), respectively.
3.3. Predictors of the OS
During the follow-up period, there were 62 deaths (84.9%). Figure 2A showed the Kaplan–Meier cumulative OS curve of the whole cohort. The median survival time was 12 months and the 1-year OS rate was 53.1%. Figure 2B to D further revealed the Kaplan–Meier OS curves of patients stratified according to nutrition-based prognostic scores. More specifically, patients with a higher level of CONUT (Fig. 2B, P < .05) and a lower level of PNI (Fig. 2D, P < .05) had significantly worse OS. Accordingly, the median survival time of patients with lower versus higher CONUT scores was 15 versus 10 months, and the 1-year OS rates were 71.4% versus 45.6%. With regard to PNI, the median survival time of patients with higher versus lower levels was 22 versus 11 months, and the 1-year OS rates were 66.7% versus 49.5%. However, no statistically significant association was demonstrated between CAR and OS in HCCA (Fig. 2C, P > .05).
Figure 2.
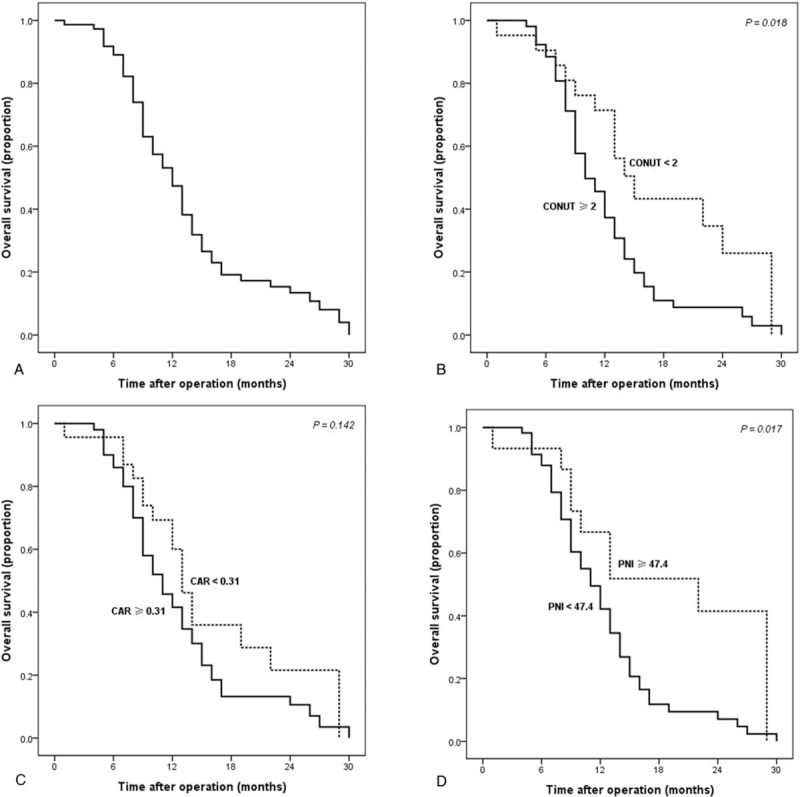
The survival curves of HCCA patients after the implantations of biliary stent and 125I seed. The survival curves of the whole cohort (A), and the survival curves stratified according to the CONUT (B), CAR (C), and PNI (D).
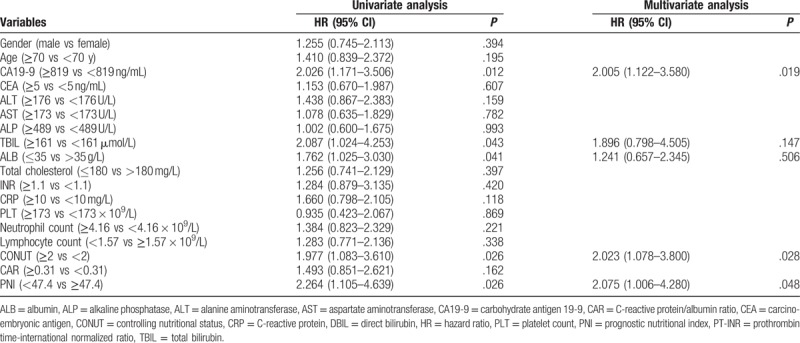
Preoperative CA19-9, TBIL, ALB, CONUT, and PNI were significant factors associated with OS in univariate analysis. Multivariate analysis was further performed, and CA19-9, CONUT [hazard ratio (HR) = 2.02, 95% CI: 1.08–3.80], and PNI (HR = 2.08, 95% CI: 1.01–4.28) were identified as independent prognostic markers of HCCA (Table 2).
Table 2.
Univariate and multivariate analyses of factors associated with OS of HCCA patients.
3.4. Associations between the nutrition-based scores and CA19-9, TBIL
According to the above-mentioned results, CA19-9 and TBIL were crucial prognostic markers for HCCA, which were in accordance with previous reports.[18–20] We subsequently investigated the associations between nutrition-based scores and CA19-9, TBIL. We found that patients with abnormal CONUT and PNI scores had significantly higher levels of CA19-9 and TBIL (Fig. 3).
Figure 3.
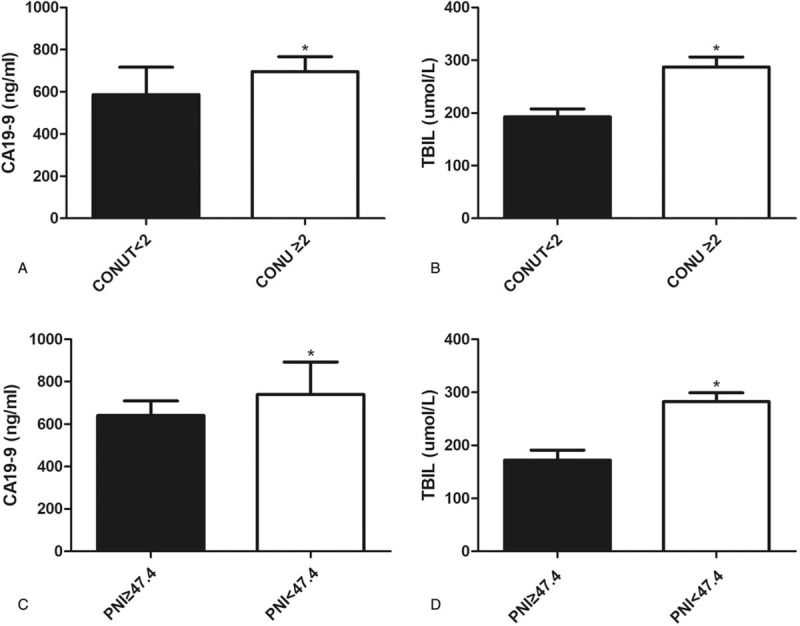
Associations between CONUT and CA19-9 (A), TBIL (B), and associations between PNI and CA19-9 (C), TBIL (D) (∗P < .05).
4. Discussion
HCCA is a devastating malignancy with a poor prognosis. To date, surgical resection and liver transplantation are the radical options for HCCA. However, the majority of patients lose the opportunity of surgery at the time of diagnosis. Biliary drainage has become one of the primary treatments in unresectable HCCA due to the alleviation of jaundice and the improvement of the quality of life.[21] Biliary stenting is also an effective palliative therapy, and is associated with a decreased risk of complications and a shorter hospital stay compared with surgery.[6] However, as a result of the ingrowth or overgrowth of tumor, biliary stent implantation alone leads to a high risk of stent occlusion and repeated procedures may be needed.[6] In contrast, biliary stenting combined with radiotherapy significantly relieves stent occlusion.[22,23] Moreover, compared with biliary stent alone, the combination of biliary stenting with 125I seed intracavitary irradiation significantly prolongs stent patency time and improves prognosis in patients with unresectable HCCA.[7,24–26] The implanted 125I seed has a direct effect on the local lesion, and the radioactivity inside the tumor is much higher than the surrounding normal tissues. Therefore, radiation-induced complications, such as gastrointestinal ulcer, hemorrhage, and enteritis, significantly decreased compared with external irradiation. We have adopted the combination of PTBS with 125I seed implantation in unresectable HCCA since 2012. In the current study, the combined therapy displayed significant benefits in the relief of symptoms and the improvement of liver function.
Identifying the potential prognostic factors is crucial not only for improving outcomes but also for stratifying patients for treatment. Unfortunately, the majority of previous studies have assessed prognostic factors only in patients who underwent surgical resection, while relevant data remain limited in unresectable HCCA.[27]
Malnutrition is highly prevalent in inpatients, especially in cancer patients. Increasing evidence indicates that postoperative complications and long-term outcomes of cancer patients are not only affected by the malignant features of the tumor cells but also by the preoperative nutritional status.[9,14,28] In recent years, several nutrition-based scores have been identified as predictors of postoperative complications and survival in various malignancies.[12,13,29] In the current study, we summarized the clinical data of 73 HCCA patients with the combination of PTBS and 125I seed implantation, and found that CONUT and PNI were independent predictors of survival. Our study further supports the view that the measurements of preoperative nutrition-based scores can predict outcomes in patients with HCCA.
The CONUT score is originally used as a tool to assess patients’ nutritional status.[30] A recent meta-analysis with 4 studies showed that patients with a higher preoperative CONUT score had a worse survival in several solid tumors.[31] In the current study, we first demonstrated that CONUT was an independent prognostic model in HCCA.
PNI has been recently advocated as a significant predictive indicator of postoperative morbidity and prognosis in some kinds of tumors.[32,33] However, with regard to HCCA, there was no significant association between PNI and prognosis in patients who underwent surgical resection.[34] Interestingly, however, we found that PNI was significantly correlated with the prognosis of HCCA patients who underwent PTBS combined with 125I seed implantation.
The mechanisms underlying the association between abnormal nutritional scores and poor outcomes in HCCA remain unclear, and several hypotheses have been proposed. First, poor nutritional status is associated with cancer progression, complications, infections, and thus may lead to delayed treatment.[35,36] Second, oxidative stress is involved in the development and progress of various chronic inflammations and malignancies.[11,37,38] It is reported that malnutrition can induce oxidative stress, augment a cascade of molecular reactions, and thus play a crucial role in carcinogenesis.[11] Third, the prognostic predictive value of nutrition-based scores in HCCA might be clarified by the roles of the 3 original variables, including serum ALB, total cholesterol, and lymphocyte count.
Hypoalbuminemia is one of the most commonly used methods to assess malnutrition and disease progress in cancer patients.[36] Activated pro-inflammatory cytokines, such as tumor necrosis factor and interleukin-6, can inhibit the production of ALB from hepatocytes.[39] In turn, low serum ALB is correlated with increased inflammatory response in cancer patients.[40] In addition, recent studies show that lower serum level of ALB is associated with worse survival in various malignancies.[29,41,42] Waghray et al[27] analyzed 116 cases of histologically confirmed HCCA, and demonstrated that low level of ALB implied a poor prognosis. In the current study, we also found that pretreatment level of ALB < 35 g/L was associated with worse survival in unresectable HCCA.
Total cholesterol, an essential component of the cell membrane, has also been identified as a useful biomarker of malnutrition.[36] The activity of low-density lipoprotein receptor is reported to be elevated in cholangiocarcinoma cells, indicating that hypocholesteremia may result from excessive uptake of cholesterol by tumor cells.[43] In addition, chronic depletion of cholesterol can induce the activation of NF-κB and promote the proliferation of tumor cells.[44] It is also reported that hypocholesterolemia is significantly associated with fewer circulating total T cells and CD8+ T cells.[45] Therefore, low total cholesterol may not only affect intracellular signaling but also impair immune system. Recently, several epidemiological studies further demonstrated that a lower level of serum total cholesterol was associated with worse prognosis in various cancers.[28,46,47]
In contrast, as a crucial component of immune system, lymphocyte can eradicate tumor cells by regulating cytokines secretion and inducing cytotoxic cell death.[48] A low lymphocyte count contributes to a weakened defense against tumor, an advanced stage, and an unfavorable outcome in cancer patients.[49,50]
There are several limitations in the current study. First, it was designed as a single-center, relatively small sample size, and retrospective cohort study. The level of evidence of retrospective study is relatively low, and confounding bias, and missing values may be inevitable. Therefore, further multicenter, larger randomized controlled trials or prospective studies are required to validate our findings. Second, the results of the current study can be influenced by several potential factors, such as tumor stage, tumor size, performance status, postoperative adjuvant therapy, and so forth. In addition, concomitant nutritional diseases, preoperative use of anti-infective medications, blood transfusion, and ALB infusion can affect the estimation of nutrition-based scores. Third, due to the lack of the relative information, several other nutrition-based markers, such as body mass index,[36] were not assessed in our study.
In summary, the preoperative nutrition-based scores that can be obtained from routine laboratory examinations, CONUT and PNI in particular, were independent prognostic tools for patients with unresectable HCCA. These simple and inexpensive models may not only contribute to evaluate the outcomes but also help to formulate an individual treatment strategy for HCCA.
Author contributions
Data curation: Yong Wang, Zhen Qian, Wei Wang, Song Yang.
Formal analysis: Qing Pang.
Funding acquisition: Hao Jin, Huichun Liu.
Investigation: Peiyuan Cui, Zhen Qian, Wei Wang, Song Yang.
Methodology: Qing Pang, Zhongran Man.
Project administration: Zongkuang Li, Lei Zhou, Hao Jin, Huichun Liu.
Resources: Wei Wang, Zongkuang Li.
Software: Qing Pang, Zhongran Man.
Supervision: Lei Zhou, Hao Jin, Huichun Liu.
Validation: Peiyuan Cui, Lei Zhou.
Writing – original draft: Yong Wang.
Writing – review & editing: Peiyuan Cui, Qing Pang, Xiaosi Hu, Huichun Liu.
Footnotes
Abbreviations: ALB = albumin, ALP = alkaline phosphatase, ALT = alanine aminotransferase, AST = aspartate aminotransferase, CA19-9 = carbohydrate antigen 19-9, CAR = C-reactive protein/albumin ratio, CEA = carcino-embryonic antigen, CONUT = controlling nutritional status, CRP = C-reactive protein, DBIL = direct bilirubin, DSA = digital subtraction angiography, HCCA = hilar cholangiocarcinoma, OS = overall survival, PLT = platelet count, PNI = prognostic nutritional index, PTBS = percutaneous transhepatic biliary stenting, PTCD = percutaneous transhepatic cholangial drainage, TBIL = total bilirubin.
PC, QP, YW, and ZQ contributed equally to this work.
Funding/support: This study was supported by the Science and Technology Innovation Fund project of Anhui Province (grant no. 1501041155) and the Science and Technology Development Fund project of Bengbu Medical Collage (grant no. BYKF1404).
The authors have no conflicts of interest to disclose.
References
- [1].Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology 2001;33:1353–7. [DOI] [PubMed] [Google Scholar]
- [2].Xiang S, Lau WY, Chen XP. Hilar cholangiocarcinoma: controversies on the extent of surgical resection aiming at cure. Int J Colorectal Dis 2015;30:159–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mansour JC, Aloia TA, Crane CH, et al. Hilar cholangiocarcinoma: expert consensus statement. HPB (Oxford) 2015;17:691–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tang Z, Yang Y, Meng W, et al. Best option for preoperative biliary drainage in Klatskin tumor: a systematic review and meta-analysis. Medicine (Baltimore) 2017;96:e8372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Walter T, Ho CS, Horgan AM, et al. Endoscopic or percutaneous biliary drainage for Klatskin tumors? J Vasc Interv Radiol 2013;24:113–21. [DOI] [PubMed] [Google Scholar]
- [6].Indar AA, Lobo DN, Gilliam AD, et al. Percutaneous biliary metal wall stenting in malignant obstructive jaundice. Eur J Gastroenterol Hepatol 2003;15:915–9. [DOI] [PubMed] [Google Scholar]
- [7].Wang T, Liu S, Zheng YB, et al. Clinical study on using 125I seeds articles combined with biliary stent implantation in the treatment of malignant obstructive jaundice. Anticancer Res 2017;37:4649–53. [DOI] [PubMed] [Google Scholar]
- [8].Tan Y, Zhu JY, Qiu BA, et al. Percutaneous biliary stenting combined with radiotherapy as a treatment for unresectable hilar cholangiocarcinoma. Oncol Lett 2015;10:2537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Schwegler I, von Holzen A, Gutzwiller JP, et al. Nutritional risk is a clinical predictor of postoperative mortality and morbidity in surgery for colorectal cancer. Br J Surg 2010;97:92–7. [DOI] [PubMed] [Google Scholar]
- [10].Beier-Holgersen R, Boesby S. Influence of postoperative enteral nutrition on postsurgical infections. Gut 1996;39:833–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Saha SK, Lee SB, Won J, et al. Correlation between oxidative stress, nutrition, and cancer initiation. Int J Mol Sci 2017;18:pii: E1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhang W, Ye B, Liang W, et al. Preoperative prognostic nutritional index is a powerful predictor of prognosis in patients with stage III ovarian cancer. Sci Rep 2017;7:9548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Xu HJ, Ma Y, Deng F, et al. The prognostic value of C-reactive protein/albumin ratio in human malignancies: an updated meta-analysis. Onco Targets Ther 2017;10:3059–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Miyata T, Yamashita YI, Higashi T, et al. The prognostic impact of controlling nutritional status (CONUT) in intrahepatic cholangiocarcinoma following curative hepatectomy: a retrospective single institution study. World J Surg 2018;42:1085–91. [DOI] [PubMed] [Google Scholar]
- [15].Collins GS, Reitsma JB, Altman DG, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med 2015;162:55–63. [DOI] [PubMed] [Google Scholar]
- [16].World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191–4. [DOI] [PubMed] [Google Scholar]
- [17].Jin H, Pang Q, Liu H, et al. Prognostic value of inflammation-based markers in patients with recurrent malignant obstructive jaundice treated by reimplantation of biliary metal stents: a retrospective observational study. Medicine (Baltimore) 2017;96:e5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chen P, Li B, Zhu Y, et al. Establishment and validation of a prognostic nomogram for patients with resectable perihilar cholangiocarcinoma. Oncotarget 2016;7:37319–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cai WK, Lin JJ, He GH, et al. Preoperative serum CA19-9 levels is an independent prognostic factor in patients with resected hilar cholangiocarcinoma. Int J Clin Exp Pathol 2014;7:7890–8. [PMC free article] [PubMed] [Google Scholar]
- [20].Ou Yang Q, Zhang S, Cheng QB, et al. Dynamic change of total bilirubin after portal vein embolization is predictive of major complications and posthepatectomy mortality in patients with hilar cholangiocarcinoma. J Gastrointest Surg 2016;20:960–9. [DOI] [PubMed] [Google Scholar]
- [21].Squadroni M, Tondulli L, Gatta G, et al. Cholangiocarcinoma. Crit Rev Oncol Hematol 2017;116:11–31. [DOI] [PubMed] [Google Scholar]
- [22].Boulay BR, Birg A. Malignant biliary obstruction: from palliation to treatment. World J Gastrointest Oncol 2016;8:498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Barkun AN, Adam V, Martel M, et al. Partially covered self-expandable metal stents versus polyethylene stents for malignant biliary obstruction: a cost-effectiveness analysis. Can J Gastroenterol Hepatol 2015;29:377–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhu HD, Guo JH, Zhu GY, et al. A novel biliary stent loaded with (125)I seeds in patients with malignant biliary obstruction: preliminary results versus a conventional biliary stent. J Hepatol 2012;56:1104–11. [DOI] [PubMed] [Google Scholar]
- [25].Shinohara ET, Guo M, Mitra N, et al. Brachytherapy in the treatment of cholangiocarcinoma. Int J Radiat Oncol Biol Phys 2010;78:722–8. [DOI] [PubMed] [Google Scholar]
- [26].Nag S, DeHaan M, Scruggs G, et al. Long-term follow-up of patients of intrahepatic malignancies treated with iodine-125 brachytherapy. Int J Radiat Oncol Biol Phys 2006;64:736–44. [DOI] [PubMed] [Google Scholar]
- [27].Waghray A, Sobotka A, Marrero CR, et al. Serum albumin predicts survival in patients with hilar cholangiocarcinoma. Gastroenterol Rep (Oxf) 2017;5:62–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kang HW, Seo SP, Kim WT, et al. Low preoperative serum cholesterol level is associated with aggressive pathologic features and poor cancer-specific survival in patients with surgically treated renal cell carcinoma. Int J Clin Oncol 2018;23:142–50. [DOI] [PubMed] [Google Scholar]
- [29].Tokunaga R, Sakamoto Y, Nakagawa S, et al. Prognostic nutritional index predicts severe complications, recurrence, and poor prognosis in patients with colorectal cancer undergoing primary tumor resection. Dis Colon Rectum 2015;58:1048–57. [DOI] [PubMed] [Google Scholar]
- [30].Ignacio de Ulibarri J, Gonzalez-Madrono A, de Villar NG, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp 2005;20:38–45. [PubMed] [Google Scholar]
- [31].Liang RF, Li JH, Li M, et al. The prognostic role of controlling nutritional status scores in patients with solid tumors. Clin Chim Acta 2017;474:155–8. [DOI] [PubMed] [Google Scholar]
- [32].Zhao Y, Xu P, Kang H, et al. Prognostic nutritional index as a prognostic biomarker for survival in digestive system carcinomas. Oncotarget 2016;7:86573–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sun K, Chen S, Xu J, et al. The prognostic significance of the prognostic nutritional index in cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol 2014;140:1537–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Okuno M, Ebata T, Yokoyama Y, et al. Evaluation of inflammation-based prognostic scores in patients undergoing hepatobiliary resection for perihilar cholangiocarcinoma. J Gastroenterol 2016;51:153–61. [DOI] [PubMed] [Google Scholar]
- [35].Yang L, Xia L, Wang Y, et al. Low prognostic nutritional index (PNI) predicts unfavorable distant metastasis-free survival in nasopharyngeal carcinoma: a propensity score-matched analysis. PLoS One 2016;11:e0158853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zhang Z, Pereira SL, Luo M, et al. Evaluation of blood biomarkers associated with risk of malnutrition in older adults: a systematic review and meta-analysis. Nutrients 2017;9:pii: E829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Saed GM, Diamond MP, Fletcher NM. Updates of the role of oxidative stress in the pathogenesis of ovarian cancer. Gynecol Oncol 2017;145:595–602. [DOI] [PubMed] [Google Scholar]
- [38].Thanan R, Techasen A, Hou B, et al. Development and characterization of a hydrogen peroxide-resistant cholangiocyte cell line: a novel model of oxidative stress-related cholangiocarcinoma genesis. Biochem Biophys Res Commun 2015;464:182–8. [DOI] [PubMed] [Google Scholar]
- [39].McMillan DC, Watson WS, O’Gorman P, et al. Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr Cancer 2001;39:210–3. [DOI] [PubMed] [Google Scholar]
- [40].Liu J, Dai Y, Zhou F, et al. The prognostic role of preoperative serum albumin/globulin ratio in patients with bladder urothelial carcinoma undergoing radical cystectomy. Urol Oncol 2016;34: 484.e1–484.e8. [DOI] [PubMed] [Google Scholar]
- [41].Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J 2010;9:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Chen Z, Shao Y, Wang K, et al. Prognostic role of pretreatment serum albumin in renal cell carcinoma: a systematic review and meta-analysis. Onco Targets Ther 2016;9:6701–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Terai K, Jiang M, Tokuyama W, et al. Levels of soluble LR11/SorLA are highly increased in the bile of patients with biliary tract and pancreatic cancers. Clin Chim Acta 2016;457:130–6. [DOI] [PubMed] [Google Scholar]
- [44].Calleros L, Lasa M, Toro MJ, et al. Low cell cholesterol levels increase NFkappaB activity through a p38 MAPK-dependent mechanism. Cell Signal 2006;18:2292–301. [DOI] [PubMed] [Google Scholar]
- [45].Muldoon MF, Marsland A, Flory JD, et al. Immune system differences in men with hypo- or hypercholesterolemia. Clin Immunol Immunopathol 1997;84:145–9. [DOI] [PubMed] [Google Scholar]
- [46].Ko K, Park YH, Lee JW, et al. Influence of nutritional deficiency on prognosis of renal cell carcinoma (RCC). BJU Int 2013;112:775–80. [DOI] [PubMed] [Google Scholar]
- [47].Toyokawa T, Kubo N, Tamura T, et al. The pretreatment Controlling Nutritional Status (CONUT) score is an independent prognostic factor in patients with resectable thoracic esophageal squamous cell carcinoma: results from a retrospective study. BMC Cancer 2016;16:722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Man YG, Stojadinovic A, Mason J, et al. Tumor-infiltrating immune cells promoting tumor invasion and metastasis: existing theories. J Cancer 2013;4:84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Iwase R, Shiba H, Haruki K, et al. Post-operative lymphocyte count may predict the outcome of radical resection for gallbladder carcinoma. Anticancer Res 2013;33:3439–44. [PubMed] [Google Scholar]
- [50].Gooden MJ, de Bock GH, Leffers N, et al. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer 2011;105:93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]


