Supplemental Digital Content is available in the text
Keywords: intubated anesthesia, lung resection surgery, nonintubated anesthesia, postoperative pulmonary complications
Abstract
Background:
Since postoperative pulmonary complications are one of the main causes of morbidity and mortality in patients undergoing lung resection surgery, we performed a meta-analysis to compare the incidence of postoperative pulmonary complications and hospital death, and the length of hospital stay in patients who received nonintubated or intubated anesthesia during thoracoscopic surgery for lung resection and further explore the tricks in nonintubated anesthesia.
Methods:
PubMed, Embase, and Cochrane Library were searched from inception to September 2017. We included eligible research comparing nonintubated anesthesia with intubated anesthesia in thoracoscopic surgery for lung resection. The primary outcomes involved postoperative pulmonary complications, hospital death, and hospital stay. The rates and causes of conversion from nonintubated anesthesia to intubated anesthesia were also analyzed.
Results:
After screening through 754 potentially relevant articles, we included 3 randomized controlled trials and 7 observational studies with 1138 patients. There was no perioperative mortality in 2 groups. The nonintubated group revealed comparable postoperative pulmonary complications (OR = 0.57; P = .07; P for heterogeneity = .49, I2 = 0%) and shorter hospital stay (WMD = −1.10; P < .00001; P for heterogeneity = .84, I2 = 0%) in overall findings with little heterogeneity.
Conclusion:
Nonintubated anesthesia in thoracoscopic surgery for lung resection shortened the length of hospital stay compared with intubated anesthesia. However, the incidence of postoperative pulmonary complications was comparable between nonintubated and intubated group. Given the potential perioperative emergencies, such as persistent hypoxemia, carbon dioxide retention, or extensive pleural adhesions, nonintubated anesthesia in lung resection surgery requires extra vigilance to ensure the safety of the patients and the success of the surgery. Powerful randomized controlled trials in the future are essential to provide more certainty and address long-term effectiveness. Only when anesthesiologists and surgeons make efforts together can better clinical outcomes in lung resection surgery be achieved.
1. Introduction
Postoperative pulmonary complications (PPCs) have an important clinical impact associated with the increased observed rate of morbidity and mortality, and length of hospital stay.[1] Lung resection surgery (LRS) is the surgical removal of all or part of the lung, because of lung cancer, primary spontaneous pneumothorax, and other lung diseases. The extent ranges from lobar resection (lobectomy, bilobectomy, sleeve-lobectomy up to pneumonectomy), and sublobar resections (segmentectomy, wedge resection, bullectomy). Specifically, the incidence of PPCs in LRS reaches 15% to 37.5%, which is greater than in other major procedures.[2]
The pioneering application of video-assisted thoracoscopic surgery (VATS) has contributed to less invasiveness and faster recovery. In the current fashion of enhanced recovery and personalized medicine, nonintubated anesthesia—an old regime, has been reintroduced to further diminish complications caused by intubation, mechanical ventilation (MV), and general anesthesia (GA), which challenges standard anesthetic techniques under GA with a double-lumen tube and one-lung ventilation in VATS. Nonintubated video-assisted thoracoscopic surgery (NIVATS) is performed without GA and MV in spontaneously breathing patients.[3] Patients in NIVATS remain either fully alert or mildly sedated.[4] Encouraging outcomes of NIVATS in randomized trials have gained global popularity in different thoracic surgery.
In thoracic surgery, patients with intratracheal intubation or long-term MV predisposed to PPCs.[5] Moreover, Ueo et al have shown that epidural anesthesia without endotracheal intubation would reduce the occurrence of PPCs compared with general anesthesia under endotracheal intubation in abdominal surgery.[6] However, the studies cited were not designed to investigate PPCs in LRS comparing nonintubated and intubated anesthesia. Recent meta-analyses with high heterogeneity only explored general complications in various thoracoscopic surgery.[7,8]
Thus, the present meta-analysis focused on evaluating the incidence of PPCs and hospital death, and the length of hospital stay, and further analyzing the tricks in NIVATS for lung resection.
2. Methods
Ethical approval and patient consent are not required in a meta-analysis.
2.1. Search strategy
We applied the recommendations from the Cochrane Handbook for Systematic Reviews of Interventions statement, as well as Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (PRISMA). PubMed, EMBASE, and Cochrane Central Register of Controlled Trials (CENTRAL) were searched by the end of September 2017. Various relevant combinations, keywords and MeSH terms pertinent to NIVATS were performed, using “Thoracic Surgery, Video-Assisted” or “Videothoracoscopic Surgery” or “Video-Assisted Thoracoscopic Surgery” or “Video-Assisted Thoracic Surgery” or “VATS”; “Nonintubated Anesthesia” or “Epidural Anesthesia” or “Thoracic Epidural” or “Intercostal Block” or “Local Anesthesia” (A list of search strategies was presented in online supplementary appendix, eFigure 1); The reviewers conducted a secondary search in other sources manually to complete the searching.
2.2. Selection criteria
Study inclusion criteria went as follows: Population: adult patients (>18 years) scheduled to receive VATS for lung resection; Intervention: the nonintubated group under combined anesthesia with a facemask or a laryngeal mask; control: the intubated group under general anesthesia with a double-lumen tube; outcomes: PPCs, hospital death, and hospital stay; conversion rates, surgical pulmonary complications, cardiologic complications, blood gas results, anesthesia duration, and surgical duration. PPCs refer to atelectasis, suspected pulmonary infection, respiratory failure, bronchospasm, aspiration pneumonitis, pleural effusion, and pneumothorax.[2] Surgical pulmonary complications are defined as postoperative wound infection and prolonged pulmonary air leak lasting beyond postoperative day five. Cardiologic complications are classified as atrial fibrillation, cardiac failure, myocardial ischemia, and cardiac arrest. Conversion means that patients under nonintubated anesthesia were converted to receive intubated anesthesia during surgery when an emergency happened, such as persistent hypoxemia, carbon dioxide retention, or severe bleeding, etc. Qualified articles must state at least one of the primary outcomes, that is, PPCs, hospital stay, and hospital death. Design: randomized controlled trials (RCTs) or observational studies.
Meanwhile, the exclusion criteria were as follows: studies not available in English, research with the significant discrepancy in characteristics or different surgical procedures between intervention and control group.
2.3. Data extraction
Three authors independently extracted data from the included research using a standard form. The collected information went as follows: year of publication, first author, study design, sample size, surgical type, PPCs, hospital death, hospital stay, conversion rates, surgical pulmonary complications, cardiologic complications, intraoperative highest PaCO2 and lowest PaO2, anesthesia duration, and surgical duration. The causes of conversion were analyzed. Discrepancies were resolved via discussion among the 3 authors.
2.4. Validity assessment
Cochrane Collaboration risk of bias tool was used for quality assessment of the RCTs, which was related to the following factors: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases. Three authors independently evaluated observational articles according to the Newcastle–Ottawa scale (NOS). The NOS consisted of 3 parts: patient selections, comparability of the study groups, and assessment of outcomes. Each part possessed a score of 4, 2, and 3. An overall quality score of ≥ 7 was defined as a high-quality study.[9] Risk of bias analysis was conducted by using Review Manager Version 5.3 for Windows and STATA 14.0 package (Stata Corp, College Station, TX).
2.5. Quality of evidence
Quality of evidence was evaluated by GRADE (Grades of Recommendation, Assessment, Development, and Evaluation) system using GRADEpro Guideline Development Tool (Software). The GRADE Working Group classifies the quality of evidence in 1 of 4 levels: high, moderate, low, and very low. Meta-analysis based on RCTs starts as high-quality evidence, and that based on observational studies begins with a low-quality rating.[10]
2.6. Statistical analysis
For conversion, we calculated conversion rates in respective cites by the equation (conversion rates = conversion number/sample size in NIVATS × 100%). For dichotomous outcomes, we estimated the odds ratio (OR) with 95% confidence intervals (CIs). Results for continuous variables were expressed as the weighted mean difference (WMD) with 95% CIs. Synthesis of the data was performed using the random effects model. When the standard deviation (SD) was not provided in the study, it was calculated according to the recommendation of the Cochrane Collaboration.[11–14]
Homogeneity assumption was tested with I2 statistics. It was calculated as I2 = 100% × (Q − df)/Q, where Q was Cochran's heterogeneity statistic. Heterogeneity was suggested if P≤.10. I2 values of 0% to 24.9%, 25% to 49.9%, 50% to 74.9%, and 75% to 100% indicated none, low, moderate, and high thresholds for statistical heterogeneity. To further evaluate heterogeneity, subgroup analyses and sensitivity analyses were performed. We conducted subgroup analyses according to study type (RCTs and observational studies) and surgical type (lobectomy and wedge resection). Sensitivity analyses were carried out by sequential removal of each study.
A funnel plot was used to estimate potential publication bias for analyses over 10 studies. Publication bias was further tested by Egger's test (significant publication bias: P < .1).
All analyses were performed using computer program including Review Manager (RevMan) V.5.3 and STATA 12.0 package (Stata Corp, College Station, TX). Statistical significance was set at a two-sided P value <.05.
3. Results
3.1. Literature identification and study characteristics
We initially identified 754 potentially relevant articles in the database searches. Selection flow of the literature was shown in Figure 1. After the removal of 211 duplicated publications, 454 articles were excluded based on titles and abstracts. Finally, 10 trials were included based on the full text.
Figure 1.
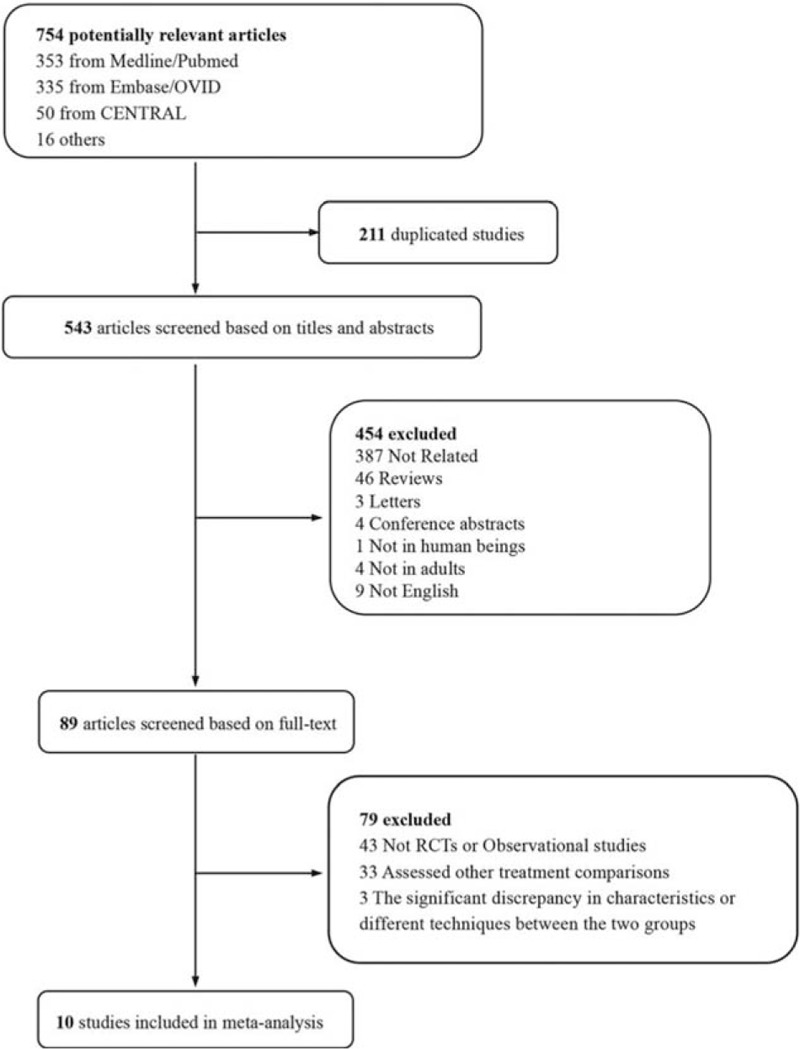
Flow chart of selecting process in this meta-analysis.
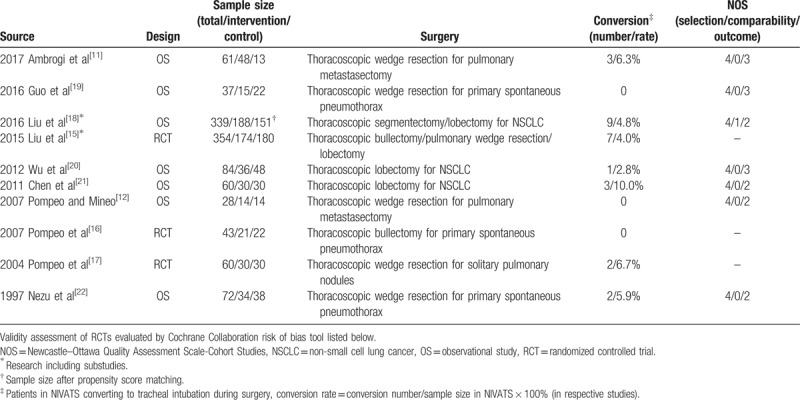
Characteristics of the qualified studies were described in Table 1. Eligible literature was published from inception to September 2017. Three RCTs[15–17] (457 patients) and 8 observational studies[11,12,18–22] (681 patients) were identified for final analyses. For patients in the nonintubated group, the intervention methods included combinations of thoracic epidural anesthesia (TEA), intrathoracic vagal block, intercostal block, or local anesthesia, with or without sedation, while for patients in the intubated group, general anesthesia (GA) or GA plus TEA was applied. There was research involving substudies according to different surgical procedures including bullae surgery, pulmonary wedge resection, segmentectomy, and lobectomy.[15,18]
Table 1.
Characteristics of eligible literature in this meta-analysis.
3.2. Quality assessment
Quality assessments of included articles were shown in Table 1 and Figure 2. All RCTs were at high risk of bias in blinding of participants and personnel, while the detection bias was unclear. All RCTs presented a low risk of bias in random sequence generation and allocation concealment.[15–17] The attrition bias, reporting bias, and other biases were low in all RCTs. Quality assessments of observational studies showed 4 of them were ranked as publications with high quality,[11,18–20] while the others got a score of 6.[12,21,22]
Figure 2.
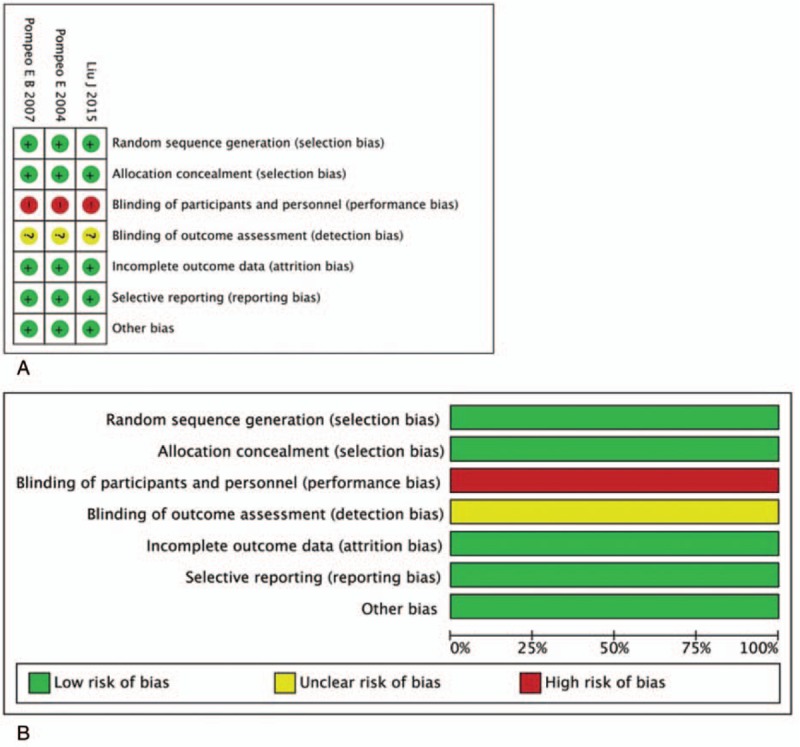
Assessment of risk bias for RCTs: (A) a summary of bias for each included study; (B) a graph with percentages for all included studies. RCT = randomized controlled trial.
3.3. Primary outcome
There was no perioperative mortality in all included studies. Eight trials recorded incidence of any postoperative complications including respiratory complications, cardiac complications, loco-regional anesthesia-related complications, endotracheal intubation-related complications, and others. As shown in Figure 3, there were comparable PPCs between the 2 groups (OR = 0.57; 95% CI 0.32 to 1.04; P = .07; P for heterogeneity = 0.49, I2 = 0%; Fig. 3). Moreover, data for hospital stay were available in 10 studies. Compared with control group, hospital stay in the nonintubated group was significantly shorter (WMD = −1.10; 95% CI −1.37 to −0.84; P < .00001; P for heterogeneity = .84, I2 = 0%; Fig. 4).
Figure 3.
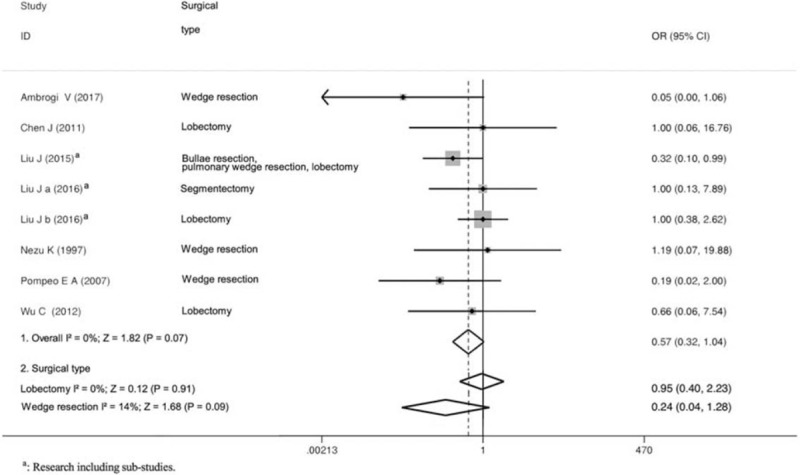
Forest plot showing the comparison of surgical pulmonary complications between the nonintubated group and the intubated group.
Figure 4.
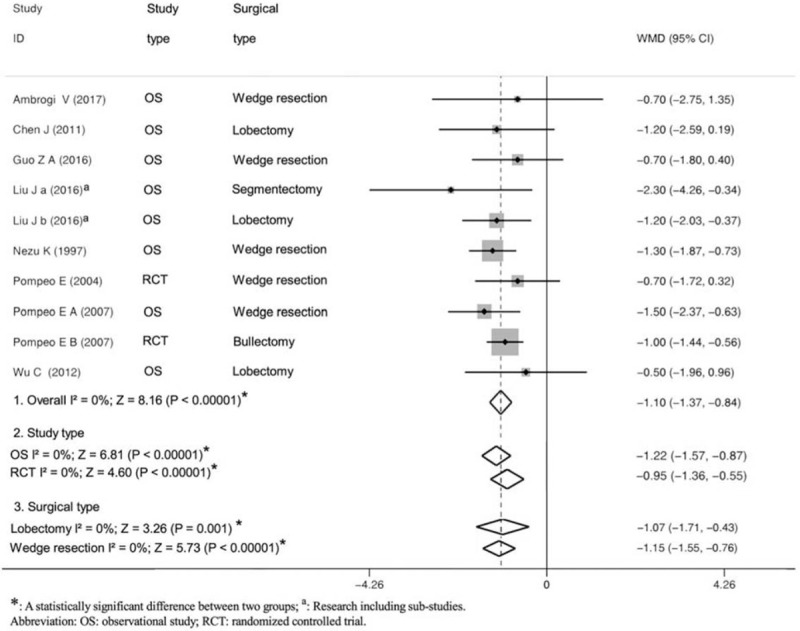
Forest plot showing the comparison of hospital stay after surgery between the nonintubated group and the intubated group.
To reduce heterogeneity derived from the difference of study design or surgical type, we performed subgroup analyses by stratifying trials into RCTs or observational studies, and/or lobectomy or wedge resection. Subgroup analyses turned out to be the same with overall analyses (Figs. 3 and 4).
3.4. Secondary outcome
Conversion to tracheal intubation during surgery was reported in 7 publications, and conversion rates ranged from 2.8% to 10% (Table 1). NIVATS group generally had comparable surgical pulmonary complications and cardiologic complications (eFigure 2,3). Intraoperative lowest PaO2 did not differ significantly, while the intraoperative highest PaCO2 in NIVATS group was significantly higher than in control group (eFigure 4). The overall analyses revealed that NIVATS suggested significantly shorter anesthesia duration and comparable surgical duration (eFigure 5, 6).
Similarly, subgroup analyses were conducted to balance heterogeneity (eFigure 2, 6). In the subgroup analyses of observational studies and lobectomy, the outcomes of anesthesia duration were comparable between the 2 groups. Other subgroup analyses were the same with overall analyses.
3.5. Sensitivity analysis and publication bias
In sensitivity analyses, the outcomes of PPCs and hospital stay had no change. The Egger's test suggested no publication based on the analysis of hospital stay (Egger's test: P = .985, eFigure 7A), and surgical duration (Egger's test: P = .619, eFigure 7B).
3.6. Quality of evidence
GRADE system grades of evidence were very low in observational studies and low in RCTs for primary outcomes (eFigure 8).
4. Discussion
Overall analyses suggested that the use of nonintubated anesthesia during LRS suggested significantly shorter hospital stay. However, the incidence of PPCs was comparable in the nonintubated and intubated group. For the secondary outcomes, the overall findings indicated that nonintubated anesthesia significantly shortened the anesthesia duration. On the other hand, the nonintubated group achieved comparable surgical pulmonary complications, cardiologic complications, surgical duration, and higher but permissive intraoperative PaCO2.
Theoretically, shorter hospital stay length in nonintubated anesthesia stems from early oral intake and ambulation.[23] Moreover, NIVATS initiates reducing inflammatory response.[24] Therefore, nonintubated anesthesia helped to achieve faster recovery in VATS for lung resection. The previous meta-analyses increased the strength of our conclusion. For example, the meta-analysis by Ke et al[8] exhibited that thoracic epidural anesthesia acquired shorter operating time and postoperative hospital stay compared with GA. Meanwhile, the meta-analysis by Deng et al[7] revealed that NIVATS gained more advantages than equipollent procedures under GA for in-operating room time, hospital stay, as well as general postoperative complications in various thoracic surgery.
Despite the short-term benefits of hospital stay and anesthesia duration, it is still concerned about the safety of patients during NIVATS for lung resection. In NIVATS, some potential perioperative emergencies caused by cardiopulmonary disorders, patients’ discomfort, surgical difficulty and so on, would bring harm to the patients. To protect patients from these risks, conversions from nonintubated anesthesia to intubated anesthesia are essential. Thus, we further evaluated conversion rates and analyzed their causes (Table 2). Conversion to intubated anesthesia during surgery was reported in 7 publications,[11,15,17,18,20–22] and conversion rates ranged from 2.8% to 10% (Table 1). Data showed that persistent hypoxemia or carbon dioxide retention and extensive pleural adhesions were vital causes of conversion. Either accounted for 19% of total events. That is because NIVATS requires surgical pneumothorax directly associated with paradoxical respiration and mediastinal flutter, which may cause persistent hypoxia and hypercapnia.[23] Also, suppression of spontaneous ventilation created by artificial pneumothorax contributes to patients’ feeling of difficult breathing, which led to the panic attack and intolerance for NIVATS.[13] Thus, nonintubated approach required the conversion to general anesthesia for intolerance as well. In addition, pain from poor loco-regional anesthesia resulted in immediate conversion to intubated approach. If not, it was hard to keep patients being perfectly quiet and comfortable during the operation. Furthermore, patients were conversed due to severe bleeding. Otherwise, the contralateral lung in NIVATS is easy to be contaminated by massive bleeding because nonintubated anesthesia is less efficient than intubated anesthesia at lung isolation.[23] Moreover, unforeseen technical difficulties during the operation, such as extensive pleural adhesions, unsatisfactory lung collapse, conversion from wedge resection to lobectomy, tumor invasion, and diaphragmatic lifting, played important roles in the conversion to general anesthesia.
Table 2.
Cumulative analysis of conversion.
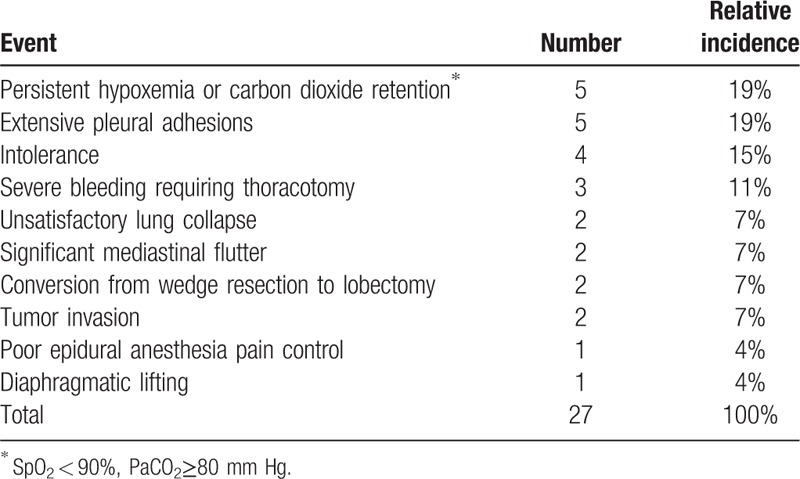
Consequently, to make the course of nonintubated anesthesia in LRS safe and smooth, it implied surgical teams including anesthesiologists and surgeons should be circumspect to prevent or handle these emergencies mentioned above when applying NIVATS. As for anesthesiologists, they should be proficient with indications, contraindications, anesthesia management, and urgent conversion related to nonanesthesia in LRS.[23] For surgeons, they should be skilled in challenging operations, such as lung resection, adhesiolysis, hemostasis and so on, which were conducted in a moving operative field due to spontaneous breathing.[4] In any case, the paramount trick in the successful application of NIVATS is close cooperation among patients, anesthesiologists, and surgeons.[25]
Our meta-analysis had several limitations. First, the overall analyses were mixed (including RCT and observational studies). Most of the patients came from observational studies in our analyses since related RCTs were limited. However, observational studies serving as real-world evidence may provide a comprehensive profile of the performance of NIVATS.[7,26] Also, we performed subgroup analyses based on the difference of study types to minimize biases and provide more information. Second, present evidence might not be strong enough since the quality of evidence evaluated by GRADE was from very low to low. That is because nonintubated anesthesia was hard to apply blinding method. Also, the available research was single center and small size. Third, short-term clinical data were focused on because limited cites provided survival analysis. Hopefully, this meta-analysis will inspire powered RCTs to provide long-term evidence with greater certainty.
5. Conclusions
Nonintubated anesthesia in VATS for lung resection achieved comparable PPCs and faster recovery compared with intubated anesthesia. Since it is concerned about the safety of patients during NIVATS for lung resection, anesthesiologists, and surgeons should be circumspect when pursuing minimal invasiveness and enhanced recovery. Only when the whole surgical team makes efforts together can better clinical outcomes in LRS be achieved.
Acknowledgments
The authors thank Haidan Lan, MD and Yabing Zhang, MD, Department of Anesthesiology, West China Hospital of Sichuan University, Chengdu, PR China for valuable guidance on data analysis. The authors appreciate the help of Ang Li for language modification.
Author contributions
Conceptualization: Yu Shi, Hong Yu, Lili Huang, Siyang Wang, Bin Liu.
Data curation: Yu Shi.
Formal analysis: Yu Shi.
Methodology: Yu Shi, Hong Yu.
Project administration: Yu Shi.
Resources: Yu Shi.
Software: Yu Shi, Dongmei Chi.
Supervision: Yu Shi, Chan Chen, Bin Liu.
Validation: Yu Shi.
Writing – original draft: Yu Shi, Hong Yu, Lili Huang.
Writing – review & editing: Yu Shi, Hong Yu, Lili Huang, Dongmei Chi, Siyang Wang, Chan Chen, Bin Liu.
Supplementary Material
Footnotes
Abbreviations: CENTRAL = Cochrane Central Register of Controlled Trials, GA = general anesthesia, GRADE = Grades of Recommendation, Assessment, Development, and Evaluation, MV = mechanical ventilation, NIVATS = nonintubated video-assisted thoracoscopic surgery, NOS = Newcastle–Ottawa scale, NSCLC = non-small cell lung cancer, OS = observational studies, PPCs = postoperative pulmonary complications, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement, RCTs = randomized controlled trials, SD = standard deviation, TEA = thoracic epidural anesthesia, VATS = video-assisted thoracoscopic surgery, WMD = weighted mean difference.
YS, HY, and LH contributed equally to the article.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Agostini P, Cieslik H, Rathinam S, et al. Postoperative pulmonary complications following thoracic surgery: are there any modifiable risk factors? Thorax 2010;65:815–8. [DOI] [PubMed] [Google Scholar]
- [2].de la Gala F, Piñeiro P, Reyes A, et al. Postoperative pulmonary complications, pulmonary and systemic inflammatory responses after lung resection surgery with prolonged one-lung ventilation. Randomized controlled trial comparing intravenous and inhalational anaesthesia. Br J Anaesth 2017;119:655–63. [DOI] [PubMed] [Google Scholar]
- [3].Tacconi F, Pompeo E. Non-intubated video-assisted thoracic surgery: where does evidence stand? J Thorac Dis 2016;8(suppl 4):S364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mineo TC, Tacconi F. From “awake” to “monitored anesthesia care” thoracic surgery: a 15 year evolution. Thorac Cancer 2014;5:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jiang SP, Li ZY, Huang LW, et al. Risk factors for postoperative pulmonary complications after gastroduodenal operation. Zhonghua Wei Chang Wai Ke Za Zhi 2005;8:425–8. [PubMed] [Google Scholar]
- [6].Ueo H, Takeuchi H, Arinaga S, et al. The feasibility of epidural anesthesia without endotracheal intubation for abdominal surgery in patients over 80 years of age. Int Surg 1994;79:158–62. [PubMed] [Google Scholar]
- [7].Deng HY, Zhu ZJ, Wang YC, et al. Non-intubated video-assisted thoracoscopic surgery under loco-regional anaesthesia for thoracic surgery: a meta-analysis. Interact Cardiovasc Thorac Surg 2016;23:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ke JD, Hou HJ, Wang M, et al. The comparison of anesthesia effect of lung surgery through video-assisted thoracic surgery: a meta-analysis. J Cancer Res Ther 2015;11(suppl):C265–270. [DOI] [PubMed] [Google Scholar]
- [9].Wells GA, Shea B, O’Connell D, et al. The Newcastle–Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed September 30, 2017. [Google Scholar]
- [10].Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ambrogi V, Sellitri F, Perroni G, et al. Uniportal video-assisted thoracic surgery colorectal lung metastasectomy in non-intubated anesthesia. J Thorac Dis 2017;9:254–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pompeo E, Mineo TC. Awake pulmonary metastasectomy. J Thorac Cardiovasc Surg 2007;133:960–6. [DOI] [PubMed] [Google Scholar]
- [13].Pompeo E, Tacconi F, Mineo TC. Comparative results of non-resectional lung volume reduction performed by awake or non-awake anesthesia. Eur J Cardiothorac Surg 2011;39:e51–58. [DOI] [PubMed] [Google Scholar]
- [14].Vanni G, Tacconi F, Sellitri F, et al. Impact of awake videothoracoscopic surgery on postoperative lymphocyte responses. Ann Thorac Surg 2010;90:973–8. [DOI] [PubMed] [Google Scholar]
- [15].Liu J, Cui F, Li S, et al. Nonintubated video-assisted thoracoscopic surgery under epidural anesthesia compared with conventional anesthetic option: a randomized control study. Surg Innov 2015;22:123–30. [DOI] [PubMed] [Google Scholar]
- [16].Pompeo E, Tacconi F, Mineo D, et al. The role of awake video-assisted thoracoscopic surgery in spontaneous pneumothorax. J Thorac Cardiovasc Surg 2007;133:786–90. [DOI] [PubMed] [Google Scholar]
- [17].Pompeo E, Mineo D, Rogliani P, et al. Feasibility and results of awake thoracoscopic resection of solitary pulmonary nodules. Ann Thorac Surg 2004;78:1761–8. [DOI] [PubMed] [Google Scholar]
- [18].Liu J, Cui F, Pompeo E, et al. The impact of non-intubated versus intubated anaesthesia on early outcomes of video-assisted thoracoscopic anatomical resection in non-small-cell lung cancer: a propensity score matching analysis. Eur J Cardiothorac Surg 2016;50:920–5. [DOI] [PubMed] [Google Scholar]
- [19].Guo Z, Yin W, Zhang X, et al. Primary spontaneous pneumothorax: simultaneous treatment by bilateral non-intubated videothoracoscopy. Interact Cardiovasc Thorac Surg 2016;23:196–201. [DOI] [PubMed] [Google Scholar]
- [20].Wu CY, Chen JS, Lin YS, et al. Feasibility and safety of nonintubated thoracoscopic lobectomy for geriatric lung cancer patients. Ann Thorac Surg 2013;95:405–11. [DOI] [PubMed] [Google Scholar]
- [21].Chen JS, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lobectomy for lung cancer. Ann Surg 2011;254:1038–43. [DOI] [PubMed] [Google Scholar]
- [22].Nezu K, Kushibe K, Tojo T, et al. Thoracoscopic wedge resection of blebs under local anesthesia with sedation for treatment of a spontaneous pneumothorax. Chest 1997;111:230–5. [DOI] [PubMed] [Google Scholar]
- [23].Kao MC, Lan CH, Huang CJ. Anesthesia for awake video-assisted thoracic surgery. Acta Anaesthesiol Taiwan 2012;50:126–30. [DOI] [PubMed] [Google Scholar]
- [24].Mineo TC, Sellitri F, Vanni G, et al. Immunological and inflammatory impact of non-intubated lung metastasectomy. Int J Mol Sci 2017;18:pii: E1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cheng Y-J. Role, benefits and limitations of non-intubated anesthesia in thoracic surgery. Video-Assisted Thorac Surg 2017;2:57–157. [Google Scholar]
- [26].Shrier I, Boivin JF, Steele RJ, et al. Should meta-analyses of interventions include observational studies in addition to randomized controlled trials? A critical examination of underlying principles. Am J Epidemiol 2007;166:1203–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


