Summary
Conjugated polymers are emerging as promising organic photocatalysts for hydrogen evolution from water. However, it is still very challenging for conjugated polymers to realize highly efficient photocatalytic hydrogen evolution. Herein, we demonstrate an efficient strategy of hydrophilic side chain functionalization to boost the hydrogen evolution rates of conjugated polymers. By functionalizing conjugated polymers with hydrophilic oligo (ethylene glycol) monomethyl ether (OEG) side chains, a 90-fold improvement in hydrogen evolution rate has been achieved than that of alkyl-functionalized conjugated polymer. It is found that the OEG side chains interact robustly with Pt co-catalysts, resulting in more efficient charge transfer. Moreover, OEG side chains in conjugated polymers can adsorb H+ from water, resulting in significantly lowered energy levels on the surfaces of conjugated polymers, which enables cascade energy levels and enhances charge separation and photocatalytic performance. Our results indicate that rational side-chain engineering could facilitate the design of improved organic photocatalysts for hydrogen evolution.
Subject Areas: Polymer Chemistry, Organic Chemistry Methods, Electrochemical Energy Conversion, Polymers
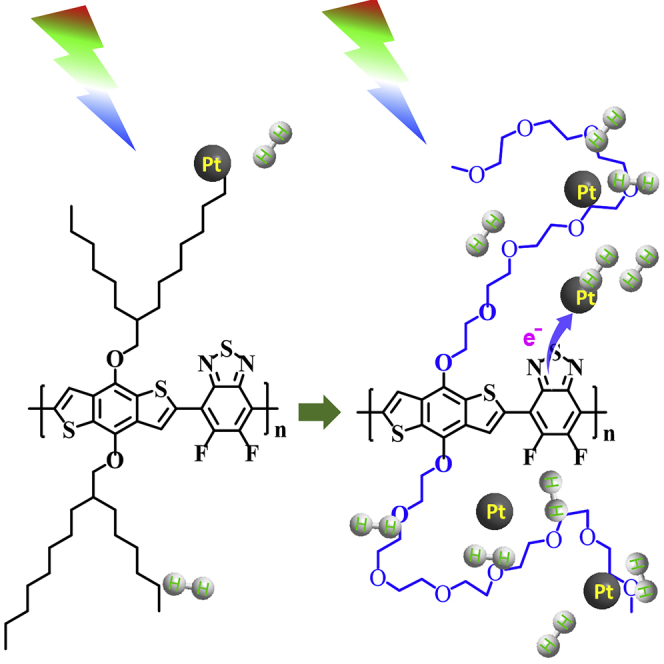
Graphical Abstract
Highlights
-
•
Conjugated polymers with oligoethylene glycol side chains are prepared
-
•
Oligoethylene glycol side chains improve photocatalytic hydrogen evolution rates
-
•
Oligoethylene glycol side chains interact robustly with Pt co-catalysts
-
•
Oligoethylene glycol side chains enable cascade energy levels
Polymer Chemistry; Organic Chemistry Methods; Electrochemical Energy Conversion; Polymers
Introduction
Photocatalytic hydrogen evolution from water is a promising technology to transfer solar energy into hydrogen energy with high-energy capacity and zero-emission features. Since the pioneering research of Honda and Fujishima, there has been much work on the development of semiconductors that enable efficient photocatalytic water splitting (Fujishima and Honda, 1972, Chen et al., 2017a, Ding et al., 2016, Ong et al., 2016). Organic photocatalysts for photocatalytic hydrogen evolution have received tremendous attention in the past several years (Ong et al., 2016, Vyas et al., 2016, Zhang et al., 2016, Fu et al., 2018, Wang et al., 2018a, Wang et al., 2018b, Yang et al., 2018, Lan et al., 2018, Ou et al., 2018) because of their unique feature of tunable electronic properties via molecular engineering (Zhang et al., 2017). The energy levels, absorption spectrum, and carrier mobility/type of organic photocatalysts can be easily tuned to realize efficient water reduction/oxidation. Consequently, various polymers with tailor-made chemical structures have been extensively studied. For example, g-C3N4 (Wang et al., 2009, Martin et al., 2014, Lau et al., 2016), porous conjugated polymers(Sprick et al., 2015, Li et al., 2016a, Yang et al., 2016, Wang et al., 2017), covalent conjugated polymers (Vyas et al., 2015, Pachfule et al., 2018), and linear conjugated polymers (Sprick et al., 2016, Li et al., 2016b, Lu et al., 2017, Woods et al., 2017, Pati et al., 2017, Tseng et al., 2018, Kosco et al., 2018) have been widely developed as organic photocatalysts for hydrogen evolution and have shown promising photocatalytic activity over the past few years. Moreover, through multiple modification strategies, including doping (Liu et al., 2010, Ran et al., 2015), hybridization (Du et al., 2012, Chen et al., 2017b, Yu et al., 2018), and copolymerization (Zhang et al., 2010) on organic photocatalysts, highly efficient hydrogen evolution can be realized. In addition, suitable metal co-catalysts have been used to lower the redox overpotential and improve charge transfer and separation, which has greatly improved the photocatalytic performance of organic photocatalysts (Wu et al., 2018).
To achieve high-performance hydrogen evolution, it is required that organic photocatalysts with strong light-harvesting capabilities and suitable energy levels be designed (Ong et al., 2016, Vyas et al., 2016, Zhang et al., 2016, Fu et al., 2018, Wang et al., 2018c, Yang et al., 2018). Furthermore, because of the short exciton diffusion length (Peumans et al., 2004) and low mobility of organic materials, the powder size of organic photocatalysts dispersed in water must be small to provide shorter distances for the separated charges emigrating to the edges of the organic photocatalysts, thus reducing the recombination inside the organic photocatalysts. Moreover, strong interactions between organic photocatalysts and metal co-catalysts are also encouraged to improve charge separation (Lau et al., 2016). However, it is challenging to realize all these characteristics because most reported organic materials/conjugated polymer-based photocatalysts sharing hydrophobic alkyl side chains show poor water dispersion (Li et al., 2016b) and lack binding points with metal co-catalysts.
Hydrophilic conjugated polymers share both semiconductive conjugated backbones and hydrophilic side chains (Duan et al., 2013, Chueh et al., 2015, Cui and Bazan, 2018). The hydrophilic side chains impart polymers with excellent dispersity/solubility in polar solvents and water, enabling the application of such polymers in biosensing and imaging applications (Traina et al., 2011). Moreover, hydrophilic side chains can robustly interact with metal substrates, resulting in well-modified metal surfaces that promote optoelectronic device performance (Duan et al., 2013, Chueh et al., 2015, Cui and Bazan, 2018). The advantages of hydrophilic conjugated polymers are identical to the requirements of organic photocatalysts for hydrogen evolution. However, despite the above-mentioned multiple potential advantages, hydrophilic conjugated polymers for highly efficient hydrogen evolution have rarely been reported.
Herein, we demonstrate a highly efficient strategy to boost the photocatalytic hydrogen evolution of conjugated polymers by functionalizing conjugated backbones with hydrophilic oligo (ethylene glycol) monomethyl ether (OEG) side chains. With rational chemical design, benzodithiophene and difluorobenzothiadiazole moieties were copolymerized to yield conjugated polymeric backbones with a wide absorption spectrum of 300–720 nm. Moreover, hydrophilic tetra- and hepta-(ethylene glycol) monomethyl ether side chains were employed to modify the conjugated polymer backbones (PBDTBT-4EO/PBDTBT-7EO, Figure 1A), resulting in outstanding dispersion of conjugated polymers in water and high photocatalytic activity for hydrogen evolution. Compared with an alkyl-functionalized polymer (PBDTBT-C6C10, Figure 1A), the OEG side-chain-functionalized conjugated polymers exhibited a 90-fold improvement in hydrogen evolution rate, reaching to 40 μmol h−1. The OEG side chains interact robustly with Pt co-catalysts, resulting in better charge transfer from the conjugated polymers to the co-catalysts. The photocurrent response and electrochemical impedance spectroscopy results showed that OEG side chains improved the charge separation efficiency of the conjugated polymers when in contact with water. The Mott–Schottky plots and density functional theory (DFT) calculations revealed that the OEG side chains in conjugated polymers can adsorb H+ in water, resulting in lower energy bands of PBDTBT-4EO/-7EO film on the surface when in contact with water. This is the first report of conjugated polymers with hydrophilic side chains strongly interacting with water and improving charge separation, which paves the way for the development of hydrophilic conjugated polymers for highly efficient hydrogen evolution from water.
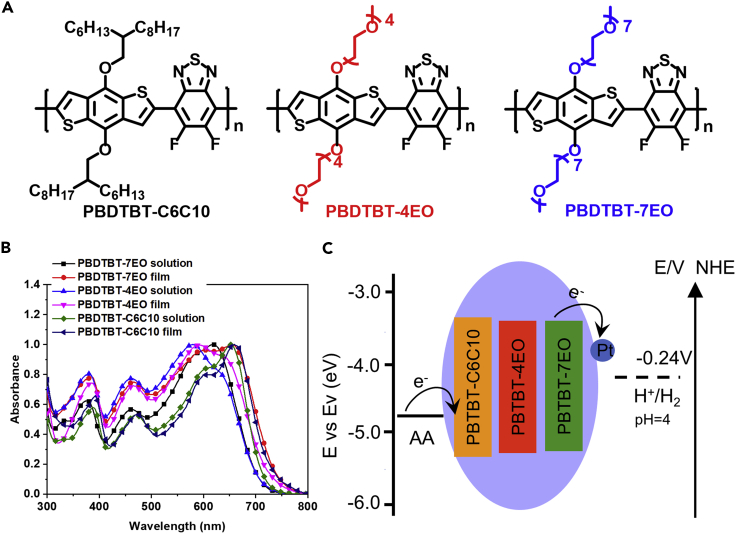
Figure 1.
Chemical Structures and Basic Properties of Conjugated Polymers
(A) Chemical structures of PBDTBT-C6C10, PBDTBT-4EO, and PBDTBT-7EO.
(B) UV-vis-NIR absorption spectra of PBDTBT-C6C10, PBDTBT-4EO, and PBDTBT-7EO.
(C) Energy levels of conjugated polymers and the energy transfers among photocatalysts, co-catalysts, scarifying agent, and water.
Results and Discussion
Conjugated polymers with OEG side chains (PBDTBT-4EO and PBDTBT-7EO, Figure 1A) were synthesized using Stille polymerization from OEG-functionalized benzodithiophene monomers and difluorobenzothiadiazole monomer (BT). For comparison, PBDTBT-C6C10 (Figure 1A) with alkyl side chains was also prepared. The synthetic details of these polymers can be found in the Transparent Methods section in Supplemental Information, and the chemical structures of these conjugated polymers were confirmed by 1H NMR, 13C NMR (Figure S1), and Fourier transform infrared spectroscopy (Figure S2). The average molecular weights of PBDTBT-C6C10, PBDTBT-4EO, and PBDTBT-7EO were estimated to be 20.1, 26.4, and 29.6 kDa, respectively. The poly(benzodithiophene-alt-difluorobenzthiadiazole) (PBDTBT) backbones possess multiple advantages for hydrogen evolution. First, the PBDTBT backbones show wide absorption spectra covering the visible range, which may drive efficient hydrogen evolution in visible light. Second, the nitrogen atoms in the BT units present a low hydrogen binding free energy (ΔGH) of about 0.632 eV (Figure S3), indicating the potential high-performance photocatalytic hydrogen evolution of PBDTBT (Pati et al., 2017). The side chain alternation on the conjugated polymers could further endow these polymers with different properties. Compared with PBDTBT-C6C10, which possesses hydrophobic side chains, PBDTBT-4EO and PBDTBT-7EO with OEG side chains exhibit much better dispersity in water because of the hydrogen bonding between the OEG side chains and water (Traina et al., 2011).
The absorption spectra of these conjugated polymers were investigated using UV-vis absorption spectroscopy. As shown in Figure 1B, the absorption spectra of PBDTBT-C6C10, PBDTBT-4EO, and PBDTBT-7EO cover the absorption range from 300 to 720 nm, implying excellent utilization of sunlight, especially in the visible range. PBDTBT-7EO exhibits a slightly broadened thin-film absorption spectrum in the wavelength from 680 to 750 nm than that of PBDTBT-C6C10, implying the formation of larger aggregation and better crystallinity (Chang et al., 2014), which can be evidenced by their X-ray powder diffraction (XRD) results (Figure S4). The optical band gaps of these polymers were calculated to be 1.71, 1.72, and 1.72 eV, respectively. The energy levels of these polymers were investigated using cyclic voltammetry analysis, and the results are presented in Figures 1C and S5. The highest occupied molecular orbit (HOMO) and lowest unoccupied molecular orbital (LUMO) energy levels of PBDTBT-C6C10 were calculated to be −5.41 and −3.36 eV, respectively. The HOMO energy levels of PBDTBT-4EO and PBDTBT-7EO were slightly up-shifted to about −5.29 and −5.27 eV, respectively, which can be attributed to the electron-donating effect of the OEG side chains. The LUMO energy levels were calculated to be −3.38 and −3.39 eV, respectively, similar to the level in PBDTBT-C6C10.
To examine the hydrophilic properties of PBDTBT-4EO/-7EO, we tested the water contact angles of these polymers as thin films. The water contact angles of PBDTBT-C6C10, PBDTBT-4EO, and PBDTBT-7EO were 101.2°, 33.9°, and 27.5°, respectively (Figure S6), indicating that the OEG side chains can effectively improve the hydrophilic properties of conjugated polymers. It has been shown that improved wettability of conjugated polymers will lead to better dispersity of conjugated polymers and better photocatalytic performance (Vyas et al., 2016). The much-reduced water contact angles of PBDTBT-4EO and PBDTBT-7EO indicates that the hydrophilic OEG side chains resulted in better interface wettability with water and balanced the short exciton diffusion length of the conjugated polymers by providing shorter paths for separated charges emigrating to the edges of the polymers, which is highly desirable for hydrogen evolution.
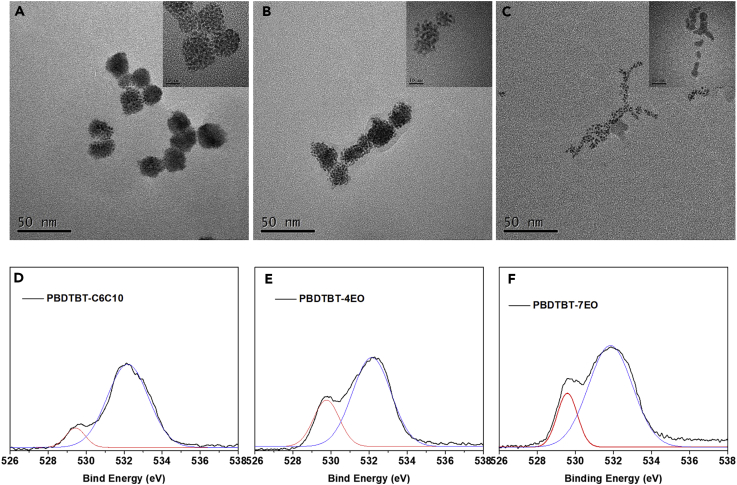
The transmission electron microscopy (TEM) images (Figures 2A–2C) of the dispersed conjugated polymers in water showed that these polymers can be dispersed well in water forming small-sized nanoparticles. Compared with PBDTBT-C6C10, which form small aggregates with average size and distribution of 24.1 ± 5.1 nm (Figure S7), PBDTBT-4EO and PBDTBT-7EO can form even smaller aggregates in water. Especially, PBDTBT-7EO showed the smallest aggregates and best dispersity in water with average size and distribution of 5.9 ± 1.1 nm (Figure S7). The much smaller size of PBDTBT-7EO aggregates will provide much more surface area for co-catalysts loading and shorter distances for the separated charges emigrating to the edges of the organic photocatalysts, both of which are highly desired for improving photocatalytic hydrogen evolution. The XRD analysis (Figure S4) of conjugated polymer powders indicates that the OEG functionalized polymers possess a π-π stacking distance closer than that of PBDTBT-C6C10. The closer stacking distance of OEG functionalized polymers could facilitate charge transporting along the conjugated backbones, which can reduce the charge recombination inside polymers and potentially improve photocatalytic activity.
Figure 2.
TEM Images and XPS O1s Spectra
(A–C) TEM images of PBDTBT-C6C10 (A), PBDTBT-4EO (B), and PBDTBT-7EO (C) dispersed in water (the scale bar in insets represents 10 nm).
(D–F) XPS O1s spectra for PBDTBT-C6C10 (D), PBDTBT-4EO (E), and PBDTBT-7EO (F) films upon Pt sheets.
X-ray photoelectron spectroscopy (XPS) was used to analyze the interaction between these polymers and Pt co-catalysts. We used thin Pt sheets instead of Pt nanoparticles, upon which thin layers of conjugated polymers (∼3 nm) were spin coated. It can be observed that a weak O1s peak corresponding to the O–Pt interaction at 529.6 eV (Rajumon et al., 1998) occurred in PBDTBT-C6C10 (Figure 2D). However, this signal became much more pronounced in PBDTBT-4EO and PBDTBT-7EO films (Figures 2E and 2F), indicating much stronger interactions between PBDTBT-4EO/-7EO and the Pt sheets. Thus, it can be easily deduced that the OEG side chains provide more intimate contact between conjugated polymers and Pt co-catalysts, resulting in better charge transfer (Li et al., 2017).
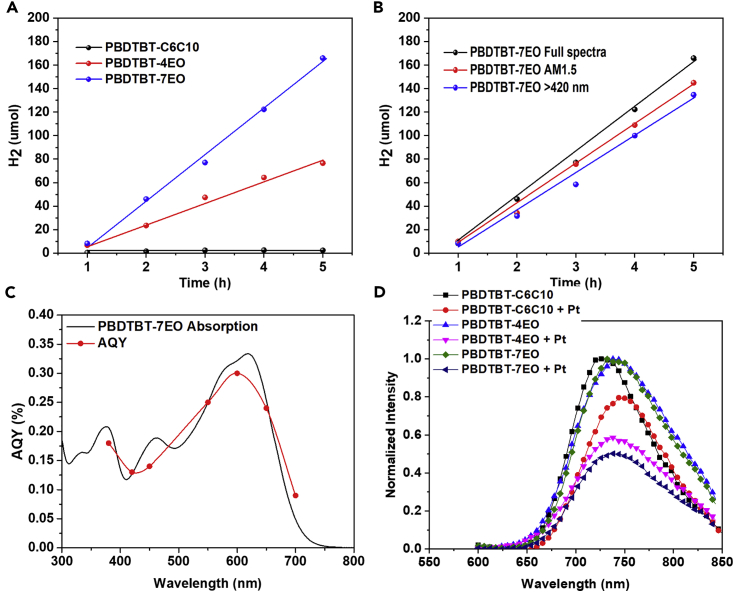
The photocatalytic hydrogen evolution of these polymers was evaluated using ascorbic acid (AA) as a scarifying agent and Pt (3 wt% of the polymers) as co-catalyst. These polymers were dispersed in water and ultrasonicated for 1 h before testing. The volumes of generated hydrogen as a function of time are presented in Figures 3A and 3B. It can be observed that hydrogen production increases linearly with increasing time. The hydrogen evolution rate (HER) of PBDTBT-C6C10 was calculated to be 0.45 μmol h−1 from the plot of produced H2 as a function of time. Surprisingly, PBDTBT-4EO with OEG side chains showed enhanced photocatalytic performance with a HER of 18.03 μmol h−1. The much-improved photocatalytic performance in PBDTBT-4EO indicates that the side chains of conjugated polymers play a key role in the photocatalytic process. Moreover, the HER of PBDTBT-7EO was further enhanced to 39.75 μmol h−1 compared with that of PBDTBT-4EO (a 90-fold enhancement), indicating that the longer hydrophilic side chains enable higher utilization of the conjugated polymers and higher photocatalytic activity. For comparison, PBDTBT without side chains were also prepared and tested. PBDTBT showed a lower HER of 5.2 μmol h−1 (Figure S8), which is much lower than that of PBDTBT-4EO and PBDTBT-7EO. Note that this performance is among the best of conjugated polymer-based photocatalysts (Tables S1 and S2). The photocatalytic activities of PBDTBT-7EO in different spectra were also investigated (Figure 3B). With an AM1.5 spectrum illumination, a HER of 34.5 μmol h−1 was obtained, whereas a HER of 32.0 μmol h−1 was achieved for testing with a 420-nm filter cut-off. The apparent quantum yield (AQY) as a function of light wavelength was also collected. As shown in Figure 3C, at 380, 420, 450, 550, 600, 650, and 700 nm, PBDTBT-7EO rendered AQY values of 0.18%, 0.13%, 0.14%, 0.25%, 0.30%, 0.24%, and 0.09%, respectively, indicating a broad photoresponse of PBDTBT-7EO for photocatalysis. The photocatalytic stability of OEG functionalized conjugated polymers was also tested (Figures S9 and S10). With illumination over 44 h (seven runs), PBDTBT-4EO delivered a HER of around 16.4 μmol h−1, which is 90% of the initial HER of PBDTBT-4EO (Figure S9).
Figure 3.
Photocatalytic Performance of Conjugated Polymers
(A) Hydrogen evolution rates of the conjugated polymers with full spectra irradiation.
(B) Hydrogen evolution rates of PBDTBT-7EO at full, >420 nm, and AM1.5 irradiation (reaction conditions: 2.5 mg of polymer dispersed in 50 mL of deionized water containing 0.2 M AA, pH = 4.0).
(C) Absorption spectrum of PBDTBT-7EO and apparent quantum yield (AQY) values as a function of light wavelength.
(D) Photoluminescence emission spectra of PBDTBT-C6C10, PBDTBT-4EO, and PBDTBT-7EO with/without Pt co-catalysts.
Photoluminescence (PL) spectra of these polymers as thin films with/without Pt co-catalysts were obtained to investigate the charge transfer efficiency between conjugated polymer and Pt co-catalysts. It has been reported that modifying the energy communication between photocatalysts and co-catalysts can enhance the charge transfer from photocatalysts to co-catalysts and improve the photocatalytic performance (Li et al., 2017). In our case, the OEG side chains efficiently enhanced the interaction between the conjugated polymers and the Pt co-catalysts, resulting in improved charge transfer and better photocatalytic performance. As shown in Figure 3D, the PL spectra of these polymers were apparently quenched. For PBDTBT-4EO and PBDTBT-7EO, the PL quenching efficiency was higher than that of PBDTBT-C6C10 with Pt co-catalysts, indicating more efficient charge transfer from PBDTBT-4EO/-7EO to the Pt co-catalysts.
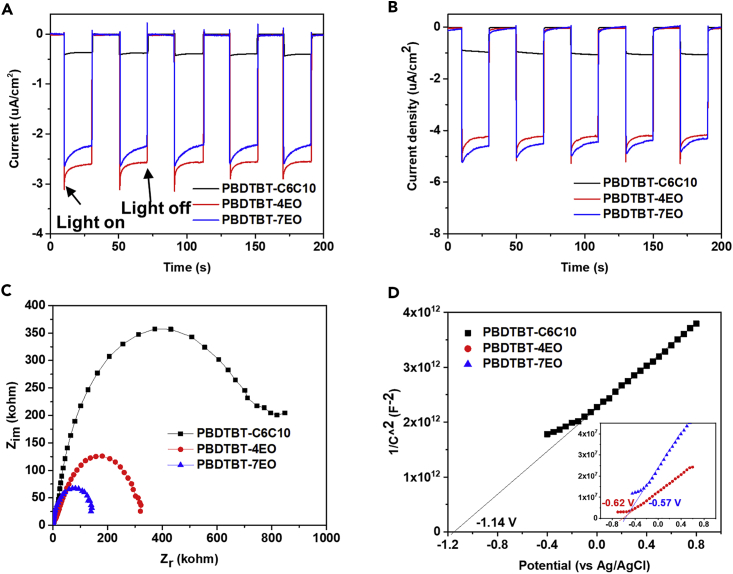
The photocurrent responses of these polymers as thin films were measured to further explore their electronic properties. The polymers were coated onto indium tin oxide electrodes and tested with no voltage or −0.2 V applied. As shown in Figure 4A, with no voltage applied, the PBDTBT-4EO/PBDTBT-7EO films showed apparently enhanced (about five times) current density responses compared with the PBDTBT-C6C10 film. A similar phenomenon was observed when −0.2 V (vs. Ag/AgCl) was applied to these films (Figure 4B), indicating that photo-excited charge carriers inside PBDTBT-4EO/-7EO are more strongly promoted than those in PBDTBT-C6C10 (Wang et al., 2018c). Electrochemical impedance spectroscopy (EIS) was also performed, and the semicircular Nyquist plots of these polymers are shown in Figure 4C. The Nyquist plot diameters of PBDTBT-4EO/-7EO are much less than that of PBDTBT-C6C10. In particular, the Nyquist plot diameters decreased with increasing length of the OEG side chains, indicating that the OEG side chains greatly improved the charge transfer properties in the polymer/water interface, which is beneficial for improved photo-excited charge separation (Zhang and Wang, 2014, Dutta and Ouyang, 2015).
Figure 4.
Photocurrent Response, EIS Nyquist Plots, and Mott–Schottky Plots
(A and B) Photocurrent response of the conjugated polymers at applied voltages of 0 V (A) and −0.2 V (B) (with 0.1 M Na2SO4 as the electrolyte).
(C) EIS Nyquist plots of conjugated polymers in the dark.
(D) Mott–Schottky plots of PBDTBT-C6C10, PDBDTBT-4EO, and PBDTBT-7EO.
The Mott–Schottky plots of these polymer films are shown in Figures 4D, S11, and S12. The positive slopes of the linear plots of these polymers imply the n-type characteristics of these polymers. The flat-band potentials (Efb) of these polymers were then determined (details are shown in Supplemental Information) from the linear plots by intercepting with voltage axis. The Efb values of PBDTBT-C6C10, PBDTBT-4EO, and PBDTBT-7EO were derived to be −1.14, −0.62, and −0.57 V vs. Ag/AgCl, respectively, indicating that the Efb values of PBDTBT-4EO/-7EO were much more positive than those of PBDTBT-C6C10, even though these three polymers possess same backbones. It should be noted that the Efb values of PBDTBT-4EO/-7EO were very close to the redox potential of the electrolyte (−0.60 V vs. Ag/AgCl for 0.1 M Na2SO4). The obvious change in Efb values in these polymers implied stronger interactions between PBDTBT-4EO/-7EO and water. Generally, Efb indicates the energy band position (approximately equal to the Fermi level [EF] [Li et al., 2016c]) of the semiconductor regarding to the redox potentials of the electroactive species in the electrolyte. Here, the changed Efb values in the PBDTBT-4EO/-7EO films indicate that the EF values of PBDTBT-4EO/-7EO down-shifted when in contact with water. Considering that the only difference in these polymers was their side chains, it can be deduced that the OEG side chains and their interaction with water were the determining factors that led to such an EF shift.
Before studying the interaction between these polymers and water, we first conducted UV photoelectron spectroscopy (UPS) to determine the pristine relative EF and ionization potentials (IPs) of these polymers. As shown in Figure S13, from the high binding-energy onsets, the EF values of PBDTBT-C6C10, PBDTBT-4EO, and PBDTBT-7EO were determined to be 3.88, 3.82, and 3.74 eV, respectively. The IPs of PBDTBT-C6C10, PBDTBT-4EO, and PBDTBT-7EO were 5.59, 5.53, and 5.43 eV, determined from their low binding-energy onsets of UPS spectra. The band gaps calculated from IP and EF are 1.71, 1.71, and 1.69 eV for PBDTBT-C6C10, PBDTBT-4EO, and PBDTBT-7EO, respectively, values that are very close to their band gaps obtained from UV-vis absorption spectra. These results indicated that OEG side chains had a limited effect on the EF values and energy levels of the pristine conjugated polymers. However, after coming into contact with water, the estimated EF values in the surfaces of the PBDTBT-4EO and PBDTBT-7EO films down-shifted greatly in comparison with that of the PBDTBT-C6C10 film (Figure S14), approaching the redox potential of the electrolyte. These results indicate that OEG side chains interacted with water more strongly than the -C6C10 side chains. More specifically, the oxygen atom in OEG side chains acted as a medium to improve the interface contact of the conjugated backbones with water, resulting in large changes in the energy levels of the conjugated polymers (Hu et al., 2017).
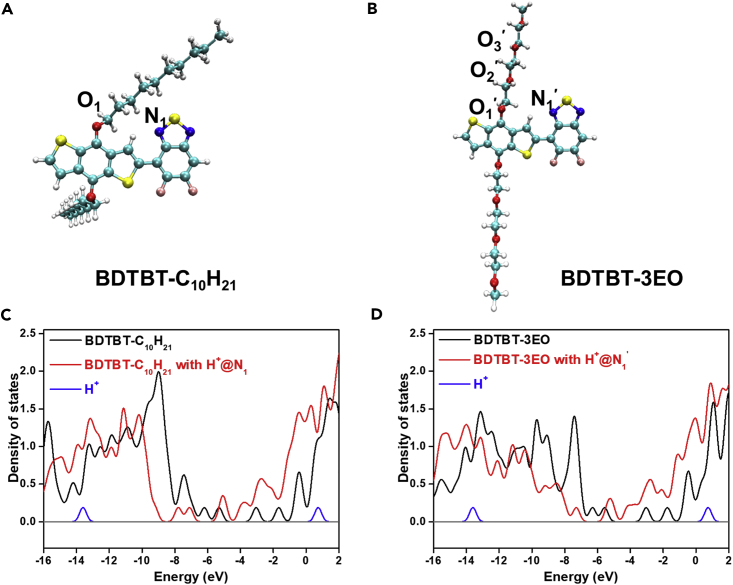
Heteroatoms in conjugated polymers are regarded as active sites that can interact with proton and catalyze hydrogen evolution reaction (Pati et al., 2017, Pei et al., 2017). To examine the effect of OEG side chains for free H+ binding, the model oligomers with two -C10H21 and tri-(ethylene glycol) monomethyl ether side chains (BDTBT-C10H21 and BDTBT-3EO) were submitted for DFT calculations at the level of B3LYP-D3(BJ)/def2-TZVP (Goerigk and Grimme, 2011). The optimized geometries of BDTBT-C10H21 and BDTBT-3EO are presented in Figures 5A and 5B. The binding energy between the side chains and H+ was carefully examined by scanning the contact distance for H+ at different oxygen sites on the side chains of BDTBT-C10H21 and BDTBT-3EO. As shown in Figure S15, the optimized minimum contact distances were around 1 Å, and thus the minimum binding energy was obtained. As shown in Table 1, the minimum binding energy between the O1 site (in BDTBT-C10H21) and H+ was calculated to be −8.568 eV, whereas those between the O3′, O2′, and O1′ sites (in BDTBT-3EO) and H+ were calculated to be −8.164, −8.202, and −8.426 eV in gradient, respectively, suggesting that the OEG side chains act more like H+ transport channels, enabling closer contact between H+ and the conjugated backbones. Moreover, the minimum binding energies at N1 and N1′ of the backbone were much lower (−9.531 and −9.500eV) than those at the oxygen site, indicating that the N atoms are potential active sites for final H+ reduction (Pati et al., 2017). These results indicate that the OEG side chains provide many more adsorption sites for H+ loading, which could potentially accelerate the photocatalytic progress.
Figure 5.
DFT Calculation
(A and B) Optimized geometry of oligomers BDTBT-C10H21 (A) and BDTBT-3EO (B).
(C and D) DOS of BDTBT-C10H21 (C) and BDTBT-3EO (D) with H+ adsorbed at nitrogen sites (the case of one hydrogen atom).
Table 1.
Binding Energy between H+ and Oxygen/Nitrogen Sites in BDTBT-C10H21 and BDTBT-3EO, Calculated LUMO and HOMO Energies of BDTBT-C10H21 and BDTBT-3EO with Adsorbed H+ (the Case of One Hydrogen Atom)
| Binding Energya (eV) | LUMO+1 (eV) | LUMO (eV) | HOMO (eV) | HOMO-1 (eV) | ||
|---|---|---|---|---|---|---|
| BDTBT-C10H21 | −1.66 | −3.04 | −5.32 | −6.20 | ||
| O1 | −8.57 | −4.72 | −5.59 | −8.73 | −9.17 | |
| N1 | −9.53 | −5.15 | −7.10 | −7.79 | −9.22 | |
| BDTBT-3EO | −1.74 | −3.03 | −5.57 | −6.31 | ||
| O3′ | −8.16 | −4.54 | −5.30 | −7.11 | −7.81 | |
| O2′ | −8.20 | −5.02 | −5.16 | −7.79 | −8.13 | |
| O1′ | −8.43 | −4.81 | −5.60 | −8.32 | −8.70 | |
| N1′ | −9.50 | −5.36 | −7.29 | −8.03 | −8.32 | |
The minimum binding energy between H+ and O/N sites in BDTBT-C10H21 and BDTBT-3EO.
To further investigate the effect of H+ adsorption on the energy levels of these conjugated polymers, we calculated the densities of state (DOS) of BDTBT-C10H21 and BDTBT-3EO with H+ adsorbed at the oxygen and nitrogen sites. As shown in Figures 5C, 5D, and S16, the calculated DOS indicate that the total energy levels can be efficiently lowered, with H+ adsorbed both in oxygen and nitrogen sites. For BDTBT-C10H21, the calculated LUMO and HOMO decreased from −3.04 and −5.32 eV to −7.10 and −7.79 eV, respectively, when the H+ adsorbed in N1. Similar observations were found for BDTBT-3EO. Surprisingly, BDT-3EO with adsorbed H+ in the OEG chains also showed lower total energy levels (with smaller magnitudes, Table 1), indicating that the adsorbed H+ can significantly lower the energy levels of conjugated polymers.
The much-lowered energy levels of the conjugated polymers with adsorbed H+ is consistent with the observation of the Efb changes in PBDTBT-4EO/-7EO. In PBDTBT-C6C10, the hydrophobic alkyl side chain hindered the transport of H+ approaching the conjugated backbones, leading to a negligible energy level shift in PBDTBT-C6C10 films when in contact with water (Figure S17). However, in PBDTBT-4EO/7EO, the hydrophilic OEG side chains can adsorb H+, resulting in intimate contact between H+ and the conjugated backbones and apparently down-shifted energy levels (Figure S17). The lowered energy bands on the surface of PBDTBT-4EO/-7EO led to a cascade energy distribution inside PBDTBT-4EO/-7EO, significantly improving charge separation, as also demonstrated by the photocurrent and EIS results. The improved charge separation thus promotes photocatalytic performance of PBDTBT-4EO/-7EO successfully.
In summary, we have presented hydrophilic side-chain-functionalized conjugated polymers for successful application as organic photocatalysts for hydrogen evolution. The OEG side-chain-functionalized conjugated polymers can render a 90-fold improvement in photocatalytic performance over that of alkyl-functionalized conjugated polymers. The OEG side chains interacted robustly with the Pt co-catalysts, resulting in better charge transfer from the polymer to the Pt co-catalysts. Moreover, our findings indicate that the OEG side chains can adsorb H+ in water, resulting in lowered Fermi level of PBDTBT-4EO/-7EO in the surface in contact with water, which was evidenced by down-shifted Efb of PBDTBT-4EO/-7EO in the polymer/water interface. As a result, the OEG-functionalized conjugated polymers showed improved charge transfer and separation efficiency and resulted in a much higher photocatalytic performance. Our results show that rational side chain engineering can significantly improve the photocatalytic performance of conjugated polymers, facilitating the novel design of organic photocatalysts with highly efficient hydrogen evolution.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Limitations of Study
In our study, the longer OEG side chains of conjugated polymer enable better dispersion in water and improve the photocatalytic activity. We believe that further optimizing the length of OEG side chains may further enhance the photocatalytic activity of conjugated polymers. However, the relationships between the length of OEG side chains and the HER performance have not been fully studied. More investigation on the optimization of side chains is needed to further improve the HER of conjugated polymers.
Acknowledgments
This work was financially supported by the Natural Science Foundation of China (No. 21634004, and 21490573) and the Foundation of Guangzhou Science and Technology Project (No. 201707020019). Z.H. thanks the support from the China Postdoctoral Science Foundation (No. 2017M622684).
Author Contributions
Z.H., F.H., and Y.C. developed the idea, designed the experiments, and drafted the manuscript. Z.H. synthesized the polymers and performed the photocatalytic experiments. Z.W. and H.T. performed the DFT calculation. X.Z., and X.L. performed the polymer characterization. All the members discussed the results and analyzed the data.
Declaration of Interests
The authors declare no competing interests.
Published: March 29, 2019
Footnotes
Supplemental Information includes Transparent Methods, 17 figures, and 2 tables and can be found with this article online at https://doi.org/10.1016/j.isci.2019.02.007.
Supplemental Information
References
- Chang W.-H., Gao J., Dou L., Chen C.-C., Liu Y., Yang Y. Side-chain tunability via triple component random copolymerization for better photovoltaic polymers. Adv. Energy Mater. 2014;4:1300864. [Google Scholar]
- Chen S., Takata T., Domen K. Particulate photocatalysts for overall water splitting. Nat. Rev. Mater. 2017;2:17050. [Google Scholar]
- Chen J., Dong C.L., Zhao D., Huang Y.C., Wang X., Samad L., Dang L., Shearer M., Shen S., Guo L. Molecular design of polymer heterojunctions for efficient solar–hydrogen conversion. Adv. Mater. 2017;29:1606198. doi: 10.1002/adma.201606198. [DOI] [PubMed] [Google Scholar]
- Chueh C.-C., Li C.-Z., Jen A.K.-Y. Recent progress and perspective in solution-processed Interfacial materials for efficient and stable polymer and organometal perovskite solar cells. Energy Environ. Sci. 2015;8:1160–1189. [Google Scholar]
- Cui Q., Bazan G.C. Narrow band gap conjugated polyelectrolytes. Acc. Chem. Res. 2018;51:202–211. doi: 10.1021/acs.accounts.7b00501. [DOI] [PubMed] [Google Scholar]
- Ding C., Shi J., Wang Z., Li C. Photoelectrocatalytic water splitting: significance of cocatalysts, electrolyte, and interfaces. ACS Catal. 2016;7:675–688. [Google Scholar]
- Du A., Sanvito S., Li Z., Wang D., Jiao Y., Liao T., Sun Q., Ng Y.H., Zhu Z., Amal R., Smith S.C. Hybrid graphene and graphitic carbon nitride nanocomposite: gap opening, electron–hole puddle, interfacial charge transfer, and enhanced visible light response. J. Am. Chem. Soc. 2012;134:4393–4397. doi: 10.1021/ja211637p. [DOI] [PubMed] [Google Scholar]
- Duan C., Zhang K., Zhong C., Huang F., Cao Y. Recent advances in water/alcohol-soluble π-conjugated materials: new materials and growing applications in solar cells. Chem. Soc. Rev. 2013;42:9071–9104. doi: 10.1039/c3cs60200a. [DOI] [PubMed] [Google Scholar]
- Dutta A., Ouyang J. Ternary NiAuPt nanoparticles on reduced graphene oxide as catalysts toward the electrochemical oxidation reaction of ethanol. ACS Catal. 2015;5:1371–1380. [Google Scholar]
- Fu J., Yu J., Jiang C., Cheng B. g-C3N4-based heterostructured photocatalysts. Adv. Energy Mater. 2018;8:1701503. [Google Scholar]
- Fujishima A., Honda K. Electrochemical photolysis of water at a semiconductor electrode. Nature. 1972;238:37–38. doi: 10.1038/238037a0. [DOI] [PubMed] [Google Scholar]
- Goerigk L., Grimme S. A thorough benchmark of density functional methods for general main group thermochemistry, kinetics, and noncovalent interactions. Phys. Chem. Chem. Phys. 2011;13:6670–6688. doi: 10.1039/c0cp02984j. [DOI] [PubMed] [Google Scholar]
- Hu Z., Li Q., Lei B., Zhou Q., Xiang D., Lyu Z., Hu F., Wang J., Ren T., Guo R. Water-catalyzed oxidation of few-layer black phosphorous in a dark environment. Angew. Chem. Int. Ed. 2017;56:9131–9135. doi: 10.1002/anie.201705012. [DOI] [PubMed] [Google Scholar]
- Kosco J., Sachs M., Godin R., Kirkus M., Francas L., Bidwell M., Qureshi M., Anjum D., Durrant J.R., McCulloch I. The effect of residual palladium catalyst contamination on the photocatalytic hydrogen evolution activity of conjugated polymers. Adv. Energy Mater. 2018 [Google Scholar]
- Lan Z.-A., Fang Y., Zhang Y., Wang X. Photocatalytic oxygen evolution from functional triazine-based polymers with tunable band structure. Angew. Chem. Int. Ed. 2018;57:470–474. doi: 10.1002/anie.201711155. [DOI] [PubMed] [Google Scholar]
- Lau V.W.-H., Moudrakovski I., Botari T., Weinberger S., Mesch M.B., Duppel V., Senker J., Blum V., Lotsch B.V. Rational design of carbon nitride photocatalysts by identification of cyanamide defects as catalytically relevant sites. Nat. Commun. 2016;7:12165. doi: 10.1038/ncomms12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Cai Z., Wu Q., Lo W.Y., Zhang N., Chen L.X., Yu L. Rational design of porous conjugated polymers and roles of residual palladium for photocatalytic hydrogen production. J. Am. Chem. Soc. 2016;138:7681–7686. doi: 10.1021/jacs.6b03472. [DOI] [PubMed] [Google Scholar]
- Li L., Hadt R.G., Yao S., Lo W.-Y., Cai Z., Wu Q., Pandit B., Chen L.X., Yu L. Photocatalysts based on cobalt-chelating conjugated polymers for hydrogen evolution from water. Chem. Mater. 2016;28:5394–5399. [Google Scholar]
- Li H., Yu H., Quan X., Chen S., Zhang Y. Self-assembled framework enhances electronic communication of ultrasmall-sized nanoparticles for exceptional solar hydrogen evolution. ACS Appl. Mater. Interfaces. 2016;8:2111. doi: 10.1021/jacs.6b12976. [DOI] [PubMed] [Google Scholar]
- Li X.B., Gao Y.J., Wang Y., Zhan F., Zhang X.Y., Kong Q.Y., Zhao N.J., Guo Q., Wu H.L., Li Z.J. Self-assembled framework enhances electronic communication of ultrasmall-sized nanoparticles for exceptional solar hydrogen evolution. J. Am. Chem. Soc. 2017;139:4789–4796. doi: 10.1021/jacs.6b12976. [DOI] [PubMed] [Google Scholar]
- Liu G., Niu P., Sun C., Smith S.C., Chen Z., Lu G.Q., Cheng H.M. Unique electronic structure induced high photoreactivity of sulfur-doped graphitic C3N4. J. Am. Chem. Soc. 2010;132:11642–11648. doi: 10.1021/ja103798k. [DOI] [PubMed] [Google Scholar]
- Lu H., Hu R., Bai H., Chen H., Lv F., Liu L., Wang S., Tian H. Efficient conjugated polymer–methyl viologen electron transfer system for controlled photo-driven hydrogen evolution. ACS Appl. Mater. Interfaces. 2017;9:10355–10359. doi: 10.1021/acsami.7b00069. [DOI] [PubMed] [Google Scholar]
- Martin D.J., Reardon P.J.T., Moniz S.J.A., Tang J. Visible light-driven pure water splitting by a nature-inspired organic semiconductor-based system. J. Am. Chem. Soc. 2014;136:12568–12571. doi: 10.1021/ja506386e. [DOI] [PubMed] [Google Scholar]
- Ong W.-J., Tan L.-L., Ng Y.H., Yong S.-T., Chai S.-P. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: are we a step closer to achieving sustainability? Chem. Rev. 2016;116:7159–7329. doi: 10.1021/acs.chemrev.6b00075. [DOI] [PubMed] [Google Scholar]
- Ou H., Chen X., Lin L., Fang Y., Wang X. Biomimetic donor-acceptor motifs in conjugated polymers for promoting exciton splitting and charge separation. Angew. Chem. Int. Ed. 2018;57:8729–8733. doi: 10.1002/anie.201803863. [DOI] [PubMed] [Google Scholar]
- Pachfule P., Acharjya A., Roeser J., Langenhahn T., Schwarze M., Schomacker R., Thomas A., Schmidt J. Diacetylene functionalized covalent organic framework (COF) for photocatalytic hydrogen generation. J. Am. Chem. Soc. 2018;140:1423–1427. doi: 10.1021/jacs.7b11255. [DOI] [PubMed] [Google Scholar]
- Pati P.B., Damas G., Tian L., Fernandes D.L.A., Zhang L., Pehlivan I.B., Edvinsson T., Araujo C.M., Tian H. An experimental and theoretical study of an efficient polymer nano-photocatalyst for hydrogen evolution. Energy Environ. Sci. 2017;10:1372–1376. [Google Scholar]
- Pei Z., Gu J., Wang Y., Tang Z., Liu Z., Huang Y., Huang Y., Zhao J., Chen Z., Zhi C. Component matters: paving the roadmap toward enhanced electrocatalytic performance of graphitic C3N4-based catalysts via atomic tuning. ACS Nano. 2017;11:6004–6014. doi: 10.1021/acsnano.7b01908. [DOI] [PubMed] [Google Scholar]
- Peumans P., Yakimov A., Forrest S.R. Small molecular weight organic thin-film photodetectors and solar cells. J. Appl. Phys. 2004;93:3693. [Google Scholar]
- Rajumon M.K., Roberts M.W., Wang F., Wells P.B. Chemisorption of ethanol at Pt(111) and Pt(111)–O surfaces. J. Chem. Soc. Faraday Trans. 1998;94:3699–3703. [Google Scholar]
- Ran J., Ma T.Y., Gao G., Du X.-W., Qiao S.Z. Porous p-doped graphitic carbon nitride nanosheets for synergistically enhanced visible-light photocatalytic H2 production. Energy Environ. Sci. 2015;8:3708–3717. [Google Scholar]
- Sprick R.S., Jiang J.X., Bonillo B., Ren S., Ratvijitvech T., Guiglion P., Zwijnenburg M.A., Adams D.J., Cooper A.I. Tunable organic photocatalysts for visible-light-driven hydrogen evolution. J. Am. Chem. Soc. 2015;137:3265–3270. doi: 10.1021/ja511552k. [DOI] [PubMed] [Google Scholar]
- Sprick R.S., Bonillo B., Clowes R., Guiglion P., Brownbill N.J., Slater B.J., Blanc F., Zwijnenburg M.A., Adams D.J., Cooper A.I. Visible-light-driven hydrogen evolution using planarized conjugated polymer photocatalysts. Angew. Chem. Int. Ed. 2016;55:1792–1796. doi: 10.1002/anie.201510542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traina C.A., Bakus R.C., II, Bazan G.C. Design and synthesis of monofunctionalized, water-soluble conjugated polymers for biosensing and imaging applications. J. Am. Chem. Soc. 2011;133:12600–12607. doi: 10.1021/ja202877q. [DOI] [PubMed] [Google Scholar]
- Tseng P.-J., Chang C.-L., Chan Y.-H., Ting L.-Y., Chen P.-Y., Liao C.-H., Tsai M.-L., Chou H.-H. Design and synthesis of cycloplatinated polymer dots as photocatalysts for visible-light-driven hydrogen evolution. ACS Catal. 2018;8:7766–7772. [Google Scholar]
- Vyas V.S., Haase F., Stegbauer L., Savasci G., Podjaski F., Ochsenfeld C., Lotsch B.V. A tunable azine covalent organic framework platform for visible light-induced hydrogen generation. Nat. Commun. 2015;6:8508. doi: 10.1038/ncomms9508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas V.S., Lau V.W.-H., Lotsch B.V. Soft photocatalysis: organic polymers for solar fuel production. Chem. Mater. 2016;28:5191–5204. [Google Scholar]
- Wang X., Maeda K., Thomas A., Takanabe K., Xin G., Carlsson J.M., Domen K., Antonietti M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009;8:76–80. doi: 10.1038/nmat2317. [DOI] [PubMed] [Google Scholar]
- Wang L., Wan Y., Ding Y., Wu S., Zhang Y., Zhang X., Zhang G., Xiong Y., Wu X., Yang J., Xu H. Conjugated microporous polymer nanosheets for overall water splitting using visible light. Adv. Mater. 2017;29 doi: 10.1002/adma.201702428. [DOI] [PubMed] [Google Scholar]
- Wang H., Zhang X., Xie Y. Photoresponsive polymeric carbon nitride-based materials: design and application. Mater. Today. 2018 [Google Scholar]
- Wang L., Zhang Y., Chen L., Xu H., Xiong Y. 2D Polymers as emerging materials for photocatalytic overall water splitting. Adv. Mater. 2018;30:1801955. doi: 10.1002/adma.201801955. [DOI] [PubMed] [Google Scholar]
- Wang Y., Silveri F., Bayazit M.K., Ruan Q., Li Y., Xie J., Catlow C.R.A., Tang J. Bandgap engineering of organic semiconductors for highly efficient photocatalytic water splitting. Adv. Energy Mater. 2018;8:1801084. [Google Scholar]
- Woods D.J., Sprick R.S., Smith C.L., Cowan A.J., Cooper A.I. A Solution-processable polymer photocatalyst for hydrogen evolution from water. Adv. Energy Mater. 2017;7:1700479. [Google Scholar]
- Wu H.-L., Li X.-B., Tung C.-H., Wu L.-Z. Recent advances in sensitized photocathodes: from molecular dyes to semiconducting quantum dots. Adv. Sci. (Weinh) 2018;5:1700684. doi: 10.1002/advs.201700684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Ma B.C., Zhang L., Lin S., Ghasimi S., Landfester K., Zhang K.A.I., Wang X. Molecular engineering of conjugated polybenzothiadiazoles for enhanced hydrogen production by photosynthesis. Angew. Chem. Int. Ed. 2016;55:9202–9206. doi: 10.1002/anie.201603532. [DOI] [PubMed] [Google Scholar]
- Yang C., Huang W., da Silva L.C., Zhang K.A.I., Wang X. Functional conjugated polymers for CO2 reduction using visible light. Chem. Eur. J. 2018;44:17454–17458. doi: 10.1002/chem.201804496. [DOI] [PubMed] [Google Scholar]
- Yu F., Wang Z., Zhang S., Ye H., Kong K., Gong X., Hua J., Tian H. Molecular engineering of donor–acceptor conjugated polymer/g-c3n4 heterostructures for significantly enhanced hydrogen evolution under visible-light irradiation. Adv. Funct. Mater. 2018 [Google Scholar]
- Zhang M., Wang X. Two dimensional conjugated polymers with enhanced optical absorption and charge separation for photocatalytic hydrogen evolution. Energy Environ. Sci. 2014;7:1902–1906. [Google Scholar]
- Zhang J., Chen X., Takanabe K., Maeda K., Domen K., Epping J.D., Fu X., Antonietti M., Wang X. Synthesis of a carbon nitride structure for visible-light catalysis by copolymerization. Angew. Chem. Int. Ed. 2010;49:441–444. doi: 10.1002/anie.200903886. [DOI] [PubMed] [Google Scholar]
- Zhang G., Lan Z.A., Wang X. Conjugated polymers: catalysts for photocatalytic hydrogen evolution. Angew. Chem. Int. Ed. 2016;55:15712–15727. doi: 10.1002/anie.201607375. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Kelly M.A., Bauer N., You W. The curious case of fluorination of conjugated polymers for solar cells. Acc. Chem. Res. 2017;50:2401–2409. doi: 10.1021/acs.accounts.7b00326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.