Abstract
The functions and biochemical mechanisms of major classes of plant oxygenases are discussed, and their potential utility for plant synthetic biology is explored.
Plant-specialized metabolites account for arguably the largest and most diverse pool of natural products accessible to humans. Conferring the plants’ selective traits, such as UV defense, pathogen resistance, and enhanced nutrient uptake, these chemicals are crucial to a species’ viability. During biosynthesis of these compounds, a vast array of specialized enzymes catalyze diverse chemical modifications, with oxidation being one of the most predominant (Smanski et al., 2016; Dong et al., 2018). Considering these observations, it is not surprising that within plant genomes, two families of oxygenases are the most abundant: the cytochrome P450 monooxygenases (P450s) and the iron/2-oxoglutarate-dependent oxygenases (Fe/2OGs). These and other oxygenases represent the synthetic workhorses of plant-specialized metabolism and also play key roles in primary metabolism, cellular regulation, and fitness. Furthermore, the challenging and selective chemistry they catalyze cannot currently be matched by synthetic chemists. Since many plant natural products serve as valuable pharmaceuticals and commodity chemicals, plant oxygenases represent a promising toolset for synthetic biologists to manipulate plant traits or develop biocatalysts (Harvey et al., 2015). Here, we review families of plant oxygenases and their chemistry and suggest potential applications.
CYTOCHROME P450S
P450s (or CYPs) are hemoproteins that catalyze NADPH- and O2-dependent hydroxylation, demethylation, epoxidation, cyclization, and desaturation reactions, among others. P450s form a vast superfamily of proteins, the genes for which are found in bacteria, insects, fish, mammals, plants, and fungi. P450 nomenclature is based on amino acid sequence similarity, assigning proteins with greater than 40% sequence identity into the same family and proteins with greater than 55% sequence identity into the same subfamily (Nelson, 2006). Genomes of higher plants contain a large number of P450s. For example, the Arabidopsis (Arabidopsis thaliana) genome encodes 246 full-length P450s, accounting for approximately 1% of the Arabidopsis gene complement, and the Jatropha curcas (Barbados nut) genome is estimated to encode greater than 400 P450s (Schuler, 2015). Plant P450s have been shown to participate in a variety of pathways, including the biosynthesis of phenylpropanoids, alkaloids, terpenoids, lipids, cyanogenic glycosides, and glucosinolates, as well as plant growth regulators such as auxin, gibberellins, jasmonic acid, and brassinosteroids.
All eukaryotic P450s are strictly membrane bound and dependent upon partner flavin-dependent reductase proteins that provide reducing equivalents. Some also recruit cytochrome b5s as additional electron donors. These transmembrane domains make it difficult to obtain recombinant protein; therefore, it is challenging to obtain structural data for eukaryotic P450s. To date, a handful of human P450s and one plant member have been successfully crystallized by replacing the N-terminal signal peptide sequence to increase protein solubility. These structures are largely helical, with the heme buried in a solvent-exposed central cleft and an axial Cys residue additionally coordinating the iron cofactor (Fig. 1A). Oxygen activation generates the characteristic compound I intermediate (Fig. 1B) that catalyzes hydrogen atom transfer (HAT) from the substrate. While the mechanism of hydroxylation is largely understood (Fig. 1B; Box 1), control of the reaction outcome by nonhydroxylating members is a continuing field of study.
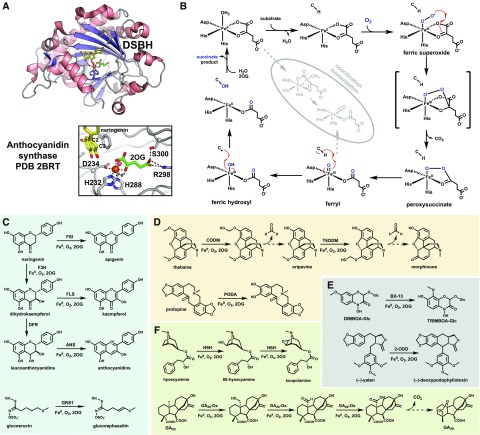
Figure 1.
A, 3D structure of the plant P450 allene oxide synthase and zoomed-in view of the active site (PDB 3DSI). The heme scaffold is shown as green sticks, the ferric ion as an orange sphere, and a hydroxylated substrate mimic as yellow sticks. An active-site Asn residue helps tether the substrate above the heme. B, The mechanism of hydroxylation by the P450s invoking the potent compound I oxidant. A detailed discussion can be found in Box 1. The faded inset depicts an alternative nonhydroxylating pathway utilizing compound II for a second HAT step. C to F, Select plant P450 reactions discussed in the text that catalyze promiscuous hydroxylations (C), rearrangement coupled with hydroxylation (D), phenolic C-C bond coupling (E), and a Baeyer-Villiger-like oxidation (F). Possible independent reaction outcomes catalyzed by CYP76s are depicted by different colors. Dashed arrows represent spontaneous reactions.
Many characterized plant P450s catalyze hydroxylation (Schuler, 2015) of sp3 or sp2 C-H bonds, but their arsenal of catalysis is continually expanding as new plant natural product chemistry is discovered. P450 hydroxylases typically display strong preference toward a selective C-H bond of their substrate, but many have been shown to exhibit a general promiscuity, catalyzing a small percentage of altered regiospecific outcomes and/or activity upon related chemical scaffolds. For example, CYP76C subfamily P450s from Arabidopsis can install multiple hydroxyls into monoterpenol substrates (Fig. 1C; Höfer et al., 2014). Other P450s have dual functionality, such as Avena sativa (oat) CYP51H10 that hydroxylates C16 and epoxidates the C12-C13 bond of β-amyrin (Fig. 1C; Geisler et al., 2013) or ent-kaurene oxidase (CYP701A) that catalyzes three consecutive oxidation reactions to produce the GA precursor ent-kaurenoic acid (Fig. 1C; Helliwell et al., 2001; Mafu et al., 2016). In the latter reaction, the second turnover generates a dihydroxylated carbon center that dehydrates to an aldehyde prior to a third oxidation. In a similar manner, P450-dependent demethylation likely proceeds through hydroxylation, followed by decomposition to formaldehyde and the demethylated product (Kellner et al., 2015a). Another spontaneous rearrangement following hydroxylase activity by a P450 is thought to occur in (+)-menthofuran production by Mentha spicata (mint) CYP71A32 (Fig. 1D), wherein the newly installed hydroxyl group acts as a nucleophile (Bertea et al., 2001). In hydroxylation-derived reactions such as the latter, it is likely that additional protein residues aid in rearrangement.
Other P450s favor radical rearrangement of their substrate immediately following HAT but prior to hydroxylation. The ability to slow -OH rebound in these systems is not well understood but gives rise to unique P450 catalysis, for example, the ring contraction of ent-7-OH-kaurenoic acid (Fig. 1D) by CYP88A (Helliwell et al., 2001) and the oxidative ring opening of loganin to secologanin (Fig. 1D) by CYP72A1 or secologanin synthase (Irmler et al., 2000). In both systems, it is likely that radical rearrangement post HAT leads to -OH rebound at a different C atom than initially targeted that is ultimately dehydrated to give the final product (Hakamatsuka and Hashim, 1991; Yamamoto et al., 2000). Whereas these P450s delay hydroxylation, others completely suppress it by utilizing compound II for a second HAT, generating two substrate radicals that undergo recombination to form a new bond (Fig. 1B, inset). This strategy is utilized by P450s for olefin installation and the intra/intermolecular cyclization of C-C or C-O bonds, the latter being used extensively during plant benzylisoquinoline alkaloid biosynthesis (Kellner et al., 2015a, 2015b; Kilgore et al., 2016). The intramolecular C-C coupling of (S)-reticuline and (R)-reticuline is catalyzed by Coptis japonica CYP80G2 and Papaver somniferum CYP719B1, respectively (Fig. 1E; Kellner et al., 2015b). In both systems, HAT from hydroxyl groups on opposite rings leads to C-C bond formation via radical rearrangement (Mizutani and Sato, 2011). Subsequent enolization then rearomatizes, a step possibly facilitated by active-site residues. Both of these P450s must bind the phenolic rings such that the hydroxyls are primed for HAT, but the altered chirality likely brings different C atoms within close proximity for radical coupling. A more recently identified P450, CYP96T1 from daffodil (Narcissus pseudonarcissus), catalyzes a similar C-C coupling of 4′-O-methylnorbelladine to noroxomaritidine but produces an enantiomeric mixture (Fig. 1E), thought to arise from flexibility of the C-C coupled product prior to spontaneous N-C ring closure (Kilgore et al., 2016). The continued research on properties of P450s that enable targeting of an O-H bond and/or utilization of compound II for a second HAT step will yield valuable information for the rational design of atypical P450 reactivity.
Much of the P450’s catalytic capability extends from its ability to generate high-valent iron species that allow it to target otherwise unreactive C-H bonds. However, the ferric-peroxo species (Fig. 1B) is also thought to act as a nucleophile during the Baeyer-Villiger oxidation catalyzed by CYP85A2 in brassinosteroid biosynthesis (Fig. 1F; Nomura et al., 2005). In this system, the peroxo intermediate likely attacks the steroid backbone ketone and undergoes a Criegee-like rearrangement to produce the expanded ester-containing ring of the brassinosteroids. The examples described above represent only a subset of known plant P450 reactions, a list that is likely to expand in the future.
Plant P450s have become a focal point of synthetic biology research due to their abundance and the challenging oxidative chemistry they catalyze. Total synthetic routes to many clinically used plant metabolites are crippled by efficiency, selectivity, and cost, often at steps that plant oxygenases catalyze with ease (King-Smith et al., 2018). Chemo-enzymatic approaches using plant P450s expressed in Escherichia coli and yeast hosts have overcome some of these bottlenecks, enabling more efficient production of highly complex compounds. Among others, semisynthesis of the antimalarial drug artemisinin is a prominent example (Paddon and Keasling, 2014). Nevertheless, expression of plant P450s in nonplant hosts can be challenging, as they require coexpression of partner reductases, need to be localized to the endoplasmic reticulum membrane, and may produce numerous undesired products (O’Reilly et al., 2011; O’Connor, 2015). Additionally, some P450s have been shown to preferentially operate synergistically with other P450s (Quinlan et al., 2012) or in multienzyme metabolon complexes (Jørgensen et al., 2005; Ralston and Yu, 2006; Bassard et al., 2017), which must be considered when designing synthetic systems involving P450s. These challenges can be alleviated through the use of a heterologous plant host for which new strategies have been devised to achieve improved activity. One promising approach is to synthetically target P450s to the thylakoid membranes of the chloroplasts in planta (Nielsen et al., 2013). This not only insulates the engineered pathway from other metabolic pathways of the hosts but also obviates the dependency upon a partner reductase, as photosynthesis can provide the reducing equivalents required for catalysis (Lassen et al., 2014). This strategy, first tested in 2013, has since been used to reconstitute the entire dhurrin biosynthetic pathway via CYP79A1 (Gnanasekaran et al., 2016) and was recently adapted to produce artemisinic acid, the precursor to artemisinin, in tobacco (Nicotiana tabacum) leaves (Fuentes et al., 2016).
The P450 scaffold itself also holds great potential for engineering novel catalysts, as demonstrated by pioneering work of the Arnold lab and others. Through high-throughput directed evolution approaches, bacterial P450-BM3 and others have been evolved extensively for a wide range of oxidations on a diverse set of substrates (Jung et al., 2011; Renata et al., 2015; Arnold, 2018). P450-BM3 mutants are being used in the chemo-enzymatic synthesis of plant metabolites such as nigaelladine A (Loskot et al., 2017), a norditerpenoid alkaloid from Nigella glandulifera (Chen et al., 2014). The P450-BM3 system was targeted because it is naturally fused to its partner reductase, a trait not observed for any known plant P450. However, successful fusion of plant P450s to partner reductases has enabled an increase in their catalytic efficiency (Didierjean et al., 2002; Schückel et al., 2012; Sadeghi and Gilardi, 2013). Directed evolution of P450s has also yielded novel activities and even some previously unobserved for any natural catalyst, such as carbene/nitrene insertions to generate cyclopropyl/butyl groups, aziridination, and sulfimidation, greatly expanding the utility of this enzyme scaffold (Coelho et al., 2013; Wang et al., 2014; Brandenberg et al., 2017; Chen et al., 2018a). Such exhaustive evolution of any plant P450 has yet to be explored, owing to their difficulty in heterologous expression, but their native promiscuity could yield improved access to a plethora of plant secondary metabolites. For example, researchers observed that only three amino acid variations were needed to efficiently alter P450 activity in carnosic acid biosynthesis (Scheler et al., 2016). Significant advancement in P450 structure and mechanistic control are necessary to expand such efforts in the future.
FE/2OGS
The second largest oxygenase family in plants is the Fe/2OGs, represented by ∼130 genes in Arabidopsis (Hagel and Facchini, 2018). The Fe/2OGs participate in many of the same plant pathways as P450s and also function in histone modification and DNA repair, roles conserved in all domains of life. These oxygenases utilize a nonheme ferrous cofactor and a 2OG cosubstrate for O2 activation to functionalize unreactive C-H bonds, concomitant with the production of CO2 and succinate coproducts. Plant Fe/2OGs mainly catalyze hydroxylation reactions, and other reactivities include C/N/O demethylation, olefin installation, C-/O,C bond coupling, and methoxy migration (Hagel and Facchini, 2018). Furthermore, bacterial Fe/2OGs catalyze C-N cyclization, epoxidation, halogenation, azidation, epimerization, and endoperoxdiation (Vaillancourt et al., 2005; Steffan et al., 2009; Chang et al., 2014, 2016; Matthews et al., 2014; Dunham et al., 2018). The wide range of catalytic functions, their cytosolic nature, and the absence of a required partner reductase make Fe/2OGs a valuable source of novel biocatalysts.
The mechanism and structure/function relationships of Fe/2OGs have been studied extensively and are largely understood for the common hydroxylation outcome (Fig. 2, A and B; Box 2; Bollinger et al., 2015). Structurally, they are defined by a conserved cupin or double-stranded β-helix, and the ferrous cofactor is coordinated by a conserved 2His-1Glu/Asp (HxD/ExnH) facial triad with the 2OG cosubstrate coordinated via its C1 carboxylate and C2 carbonyl (Fig. 2A). The substrate binds in the general vicinity such that its target carbon is positioned closest to the iron center. No plant Fe/2OG has been characterized biophysically in great depth, and only four x-ray crystal structures have been obtained (Wilmouth et al., 2002; Sun et al., 2015; Kluza et al., 2018). Besides a histone demethylase, the three other plant Fe/2OG structures are involved in plant specialized metabolism and share a highly similar secondary structure (∼2.5 Å root-mean-square deviation), despite disparity in substrates and reactivity. This conservation may extend to most specialized plant Fe/2OGs, suggesting these scaffolds could be more tractable for engineering purposes than their bacterial counterparts.
Figure 2.
A, Overall double-stranded β-helix (DSBH) fold and active site of the plant Fe/2OG anthocyanidin synthase bound to substrate analog naringenin (PDB 2BRT). Naringenin is shown as yellow sticks, the 2OG cosubstrate as green sticks, the ferrous cofactor as an orange sphere, a water molecule as a red sphere, and the iron-binding facial triad residues and 2OG-stabilizing residues as gray sticks. B, The standing mechanistic proposal for Fe/2OG-dependent hydroxylation. A detailed discussion can be found in Box 2. The faded inset depicts possible routes to coordination relocation of the key ferryl intermediate to possibly suppress the canonical hydroxylation reactivity. C to F, Select plant Fe/2OGs described in the text that participate in flavonoid biosynthesis and catalyze olefin installation (C), catalyze demethylation or dealkylation (D), catalyze unique rearrangements (E), and catalyze iterative turnovers upon the same substrate (F). Dashed arrows represent spontaneous reactions. ANS, Anthocyanidin synthase; CODM, codeine O-demethylase; FLS, flavonol synthase; FSI, flavone synthase I; GA20ox, GA 20-oxidase; GRS1, glucoraphasatin synthase1; H6H, hyoscyamine-6β-hydroxylase; PODA, protopine O-dealkylase; T6ODM, thebaine-6 O-demethylase; DIMBOA, 2-(2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one)-b-D-glucopyranose; TRIBOA, 2-(2,4,7- trihydroxy-8-methoxy-1,4-benzoxazin-3-one)-b-D-glucopyranose; 2-ODD, Phex30848.
The specialization of plant Fe/2OGs is prominently displayed by those involved in flavonoid biosynthesis (Fig. 2C). The flavonoid precursor naringenin acts as the substrate for both flavanone-3-hydroxylase (F3H) and flavone synthase I; the former hydroxylates C3, whereas the latter desaturates along the C2-C3 bond to yield apigenin. The F3H reaction product dihydrokaempferol serves as the substrate for flavonol synthase (FLS) that also desaturates the C2-C3 bond to yield kaempferol. Alternatively, the ketone of dihydrokaempferol can be reduced by the NADPH-dependent reductase DFR to give various leucoanthocyanidins that are desaturated once again along the C2-C3 bond by a fourth Fe/2OG, anthocyanidin synthase (Saito et al., 1999). This desaturation triggers spontaneous dehydration of C4 to produce the oxinium-containing anthocyanidins leading to the vibrant pigments of many flowering plants. These four Fe/2OGs are an excellent group of related enzymes from which structural features dictating substrate promiscuity and specificity can be learned. Structures of anthocyanidin synthase bound to narigenin or dihydrokaempferol (Wilmouth et al., 2002; Welford et al., 2005) suggest that these Fe/2OGs, assuming a roughly similar binding mode, initially target C3. While this is in line with hydroxylation by F3H, desaturation via initial targeting of C3 would not correlate to standing and recently supported mechanistic proposals of either an adjacent-heteroatom-assisted or dual HAT pathway (Dunham et al., 2018), the latter of which would require the C2-H bond to be directed toward the iron center. Further analysis of these Fe/2OGs may reveal a third desaturation strategy. It is worth noting that an other known plant Fe/2OG catalyzing desaturation, glucoraphasatin synthase1 from Raphanus raphanistrum subsp. sativus (radish; Fig. 2C), installs an alkene adjacent to an S atom of its glucoerucin substrate, consistent with the proposed heteroatom-assisted mechanism (Kakizaki et al., 2017; Dunham et al., 2018).
Other plant Fe/2OGs have repurposed their hydroxylase activity to trigger rearrangement similar to P450 reactions. For the N-Lys demethylases involved in histone regulation, these rearrangements occur spontaneously, as the charged amino group is an inherently good leaving group (Tsukada et al., 2006; Chen et al., 2011). In other cases, such as codeine/thebaine-6 O-demethylase and protopine O-dealkylase (Fig. 2D; Farrow and Facchini, 2013), assistance from surrounding active-site residues for proton donation is likely. These Fe/2OGs are the first members shown to catalyze O-demethylation, and the dealkylation activity of protopine O-dealkylase was previously observed only for P450s (Farrow and Facchini, 2013). Other rearrangements are catalyzed by the Fe/2OGs Phex30848 (or 2-ODD) and BX13 that catalyze the C-C bond formation of (−)-yatein (Lau and Sattely, 2015) and an odd methoxy migration in benzoxazinoid biosynthesis (Handrick et al., 2016), respectively (Fig. 2E). The exact mechanisms remain unclear but could invoke rearrangement of thecarbon radical post HAT instead of an -OH-modified product. Other Fe/2OGs can catalyze additional oxidations on their initially hydroxylated products. Identified as the main contributor to the famous dwarf phenotype characterized by reduced levels of the growth hormone GA in an Oryza sativa (rice) mutant (Sasaki et al., 2002), GA 20-oxidase catalyzes three consecutive oxidations of the C20 methyl of GA53 to produce GA20 (Fig. 2F). GA 20-oxidase converts this methyl group to a hydroxyl, formal, and carboxyl group consecutively, of which the final reaction triggers rearrangement, installing the characteristic carboxyl bridge. Another dual-function Fe/2OG is the epoxide-forming hyoscyamine-6β-hydroxylase in Solanaceae plants that first hydroxylates the C6 position prior to C-O ring closure with C7 in two independent turnovers (Ushimaru et al., 2018; Fig. 2F). Hyoscyamine-6β-hydroxylase differs from other epoxidizing Fe/2OGs that directly target alkenes in a single turnover (Chang et al., 2016) and is more similar to other C-O-cyclizing members such as clavaminate synthase (Zhou et al., 2001) and the loline oxidase LolO (Pan et al., 2018). Additional studies are required to fully understand the intricacies of the reaction outcome in this enzyme family.
Although not as heavily studied or utilized as biocatalysts compared with P450s, Fe/2OGs could serve as valuable synthetic biology tools for engineering purposes. Their involvement in numerous plant hormone and defense pathways makes them good targets for altering certain plant traits, as demonstrated by the generation of pathogen resistance Solanum tuberosum (potato) through CRISPR targeting of the DOWNY MILDEW RESISTANCE6 gene, an Fe/2OG involved in plant defense signaling (Zeilmaker et al., 2015). Specialized plant Fe/2OGs in particular could be useful due to the wide array of substrate chemical scaffolds they accommodate and a potentially conserved secondary structure. For example, a recent review proposed a plausible chemo-enzymatic synthesis for natural and modified vinblastine/vincristine alkaloids through the use of desacetoxyvindoline-4-hydroxylase from Catharanthus roseus (Madagascar periwinkle; King-Smith et al., 2018). Such approaches using bacterial Fe/2OGs for natural product synthesis have already been demonstrated (Zhang et al., 2018; Zwick and Renata, 2018). Moreover, Fe/2OGs could be engineered to produce novel halogenated natural products in planta, a strategy exemplified by engineering chlorinated monoterpene indole alkaloid production in Madagascar periwinkle using bacterial FAD-dependent halogenases (Runguphan et al., 2010). It was recently demonstrated that a bacterial Fe/2OG amino acid hydroxylase could be modified to catalyze halogenation of its native substrate, albeit at reduced efficiency (Mitchell et al., 2017a). Theoretically, plant Fe/2OG hydroxylases could be engineered to produce novel halogenated compounds as well. Researchers have also recently developed a promising chemical handle upon Fe/2OG activity in vivo through the use of a modified or bumped N-oxalylglycine (NOG) chemical inhibitor (Sudhamalla et al., 2018). NOG is a structural analog of 2OG that nonspecifically inhibits Fe/2OG activity. By expanding the active site of an Fe/2OG protein involved in nucleotide repair via rational design, it was demonstrated that NOG harboring small aliphatic groups at the C4 position could selectively inhibit only the engineered enzyme while wild-type activity was unaffected. This approach could be applied to transgenic plants to selectively regulate artificially engineered Fe/2OG-gated pathways.
FLAVIN MONOOXYGENASES
Flavin-dependent monooxygenases (FMOs) utilize an organic FAD cofactor and O2 for oxidation. Their reactions are also dependent on reducing equivalents from a NADPH cosubstrate, and, in contrast to metalloenzymes capable of accessing high-valent oxo species to target unreactive C-H bonds, FMO activity is restricted to the targeting of nucleophilic or electrophilic substrates. Currently, only three sets of plant FMOs are known, the FMO1s, glucosinolate-S-oxidases (GS-Oxs), and YUCCAs, all with highly specific biochemical functions (Schlaich, 2007). This is in stark contrast to homologous human FMOs that lack specificity and play a general role in xenobiotic metabolism (Lattard et al., 2004). Bacterial FMOs are vastly more diversified than both aforementioned sets, with known members capable of halogenation, oxidative rearrangements, and more (Huijbers et al., 2014). The term FMO can be applied across many classes of FAD/O2-utilizing enzymes, most of which have not been identified in plant genomes. Plant FMOs are defined by a double Rossmann fold that simultaneously binds FAD and NADPH via conserved motifs in the N/C termini (Fig. 3A; Huijbers et al., 2014). Reduction of the FAD cofactor by NADPH allows for the activation of O2 to produce the common hydroperoxyflavin or C4α(OOH)FAD intermediate for catalysis, observed in all characterized FMOs (Fig. 3B). Structures of bacterial FMOs have also suggested a second role of the bound NADP+ in stabilizing the reactive intermediate, which needs to be investigated further (Alfieri et al., 2008).
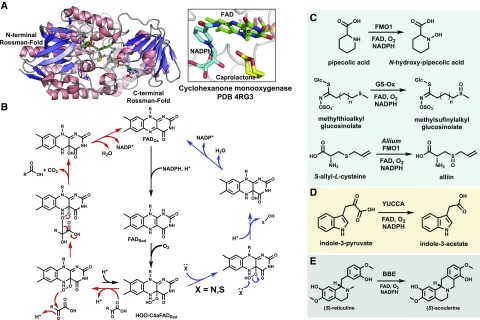
Figure 3.
A, The double Rossmann fold and active site of a bacterial cyclohexanone monooxygenase bound to its caprolactone product (PDB 4RG3). No plant FMO has been crystallized, but this bacterial Baeyer-Villiger monooxygenase (BVMO) is predicted to share an overall secondary structure. The FAD cofactor is shown as green sticks, the NADPH cosubstrate as cyan sticks, the product as yellow sticks, and the C4α atom that reacts with O2 is labeled. B, The proposed mechanisms for plant FMO activity utilizing the C4α(OOH)FAD intermediate as an electrophile (blue path) or nucleophile (red path). C to E, Select plant FMO reactions discussed in the text that catalyze hetero-atom oxidation (C), oxidative decarboxylation (D), and a C-C coupling reaction catalyzed by the plant FAD-dependent oxidase BBE (E).
Among the three classes of plant FMOs, two catalyze heteroatom oxidation and the other catalyzes oxidative decarboxylation. Arabidopsis FMO1 was first identified as an enzyme linked to plant systemic acquired resistance (Mishina and Zeier, 2006), but its function was only resolved recently. Two groups independently discovered that FMO1 is responsible for the N-hydroxylation of pipecolic acid (Fig. 3C), producing the mobile signal molecule of the systemic acquired resistance pathway (Chen et al., 2018b; Hartmann et al., 2018). This reaction likely proceeds via nucleophilic attack upon the C4α(OOH)FAD intermediate and is shared by the GS-Oxs (Hansen et al., 2007; Kong et al., 2016). GS-Oxs catalyze the S-oxidation of thiol-glucosinolates to their respective sulfinyls, with homologs participating in alliin biosynthesis in Allium sativum (garlic; Fig. 3C; Yoshimoto et al., 2015). The GS-Oxs are highly selective toward glycosylated substrates (Hansen et al., 2007), further highlighting the distinction compared with the promiscuous human FMOs. The YUCCA family FMOs catalyze the oxidative decarboxylation of indole pyruvate to indole-3-acetic acid (IAA; Fig. 3D), the primary plant growth hormone (Mashiguchi et al., 2011). YUCCAs were initially proposed to catalyze the N-hydroxylation reaction earlier in the IAA pathway (Zhao et al., 2001), but follow-up studies clarified their bona fide activity (Mashiguchi et al., 2011; Stepanova et al., 2011). This reaction proceeds via nucleophilic attack by the C4α(OOH)FAD on the α-keto group and subsequent decarboxylation with O-O bond cleavage to produce IAA (Dai et al., 2013). Detection of a short-lived C4α(OOH)FAD species is consistent with its nucleophilic role compared with other FMOs (Dai et al., 2013). The YUCCAs are chemically similar to the BVMOs found in bacteria that oxidize linear or cyclic ketones to new ester compounds via rearrangement of a Criegee intermediate between the substrate and peroxy-flavin (Mascotti et al., 2015). No BVMO has been identified in plants, but these bacterial enzymes share conserved sequence and secondary structure features with plant and human FMOs, suggesting that plant FMOs could potentially be engineered to BVMO-like enzymes (Huijbers et al., 2014). In fact, a human FMO was recently shown to catalyze a BVMO-like reaction (Fiorentini et al., 2017). There are also Baeyer-Villiger-like oxidations of plant metabolites with unknown biosynthetic routes, such as the degradation of (+)-camphor in Salvia officinalis (sage; Croteau et al., 1984). The BVMOs have been used in numerous chemo-enzymatic applications, and their selectivity has also been successfully engineered (van Beek et al., 2012; Li et al., 2018a; Woo et al., 2018).
Other notable classes of FAD-dependent oxidases found in plants include the berberine-bridging enzymes (BBEs) and FAD-epoxidases. BBE, or (s)-reticuline oxidase from Eschscholzia californica (California poppy), catalyzes a C-C coupling reaction (Fig. 3E), producing H2O2 as a by-product, and utilizes a bicovalently tethered FAD cofactor (Daniel et al., 2017). Other members participate in similar cyclizations during cannabinoid metabolism (Daniel et al., 2017). FAD epoxidases specifically target alkenes of carotenoids or the triterpenoid precursor squalene and have a different source of reducing equivalents from other FMOs (Büch et al., 1995; Han et al., 2010). It is also worth mentioning the bacterial flavo-halogenases that generate a reactive hypohalous ion to chlorinate and brominate electron-rich substrates (van Pée and Patallo, 2006; Payne et al., 2016). Improvement of the aforementioned in planta halogenation of indole alkaloids (Runguphan et al., 2010) could lead to promising new therapeutics, as it is estimated that one-third of the drugs in clinical trials are halogenated (Fraley and Sherman, 2018), and the presence of halogens can significantly enhance drug potency (Imai et al., 2008; Gillis et al., 2015).
CAROTENOID CLEAVAGE DIOXYGENASES
First identified in Zea mays (maize; Schwartz et al., 1997), the carotenoid cleavage dioxygenases (CCDs) are a specialized class of non-heme-iron-dependent enzymes that cleave the electron-rich alkene chain of various carotenoids and apocarotenoids. While β-carotenes are important antioxidants and pigments themselves, CCD activity produces the precursors for the important signaling hormones strigolactone and abscisic acid, along with many notable fragrant compounds. The identification of plant CCDs has since revealed mammalian homologs involved in retinal production and bacterial homologs capable of metabolizing noncarotenoid substrates. In plants, CCD1, CCD4, CCD7, and CCD8 and the neoxanthin cleavage enzymes are universally conserved (Auldridge et al., 2006; Harrison and Bugg, 2014), the latter of which specifically target epoxidized carotenoids during the biosynthesis of abscisic acid, a key regulator of drought tolerance and seed dormancy (Fig. 4C). A number of specialized plant CCDs have also been identified, such as CCD2 from Crocus sativus, which cleaves zeaxanthin during corcin biosynthesis (Frusciante et al., 2014), and lycopene cleavage dioxygenase from Bixa orellana, which is capable of cleaving the β-carotenoid precursor (Satpathy et al., 2010).
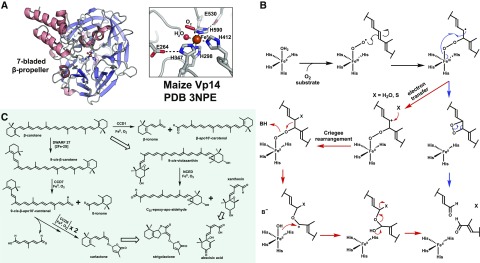
Figure 4.
A, The seven-bladed β-propeller structure and active site of iron-bound Vp14, a plant CCD from maize. The four iron-coordinating His and the conserved second-sphere Glu residues are shown as gray sticks, the ferrous cofactor as an orange sphere, and a water molecule as a red sphere. An end-on coordinating O2 is modeled and shown as a red stick, but the accuracy of this model is suspect due to low resolution of the structure (∼3.1 Å). Structures of bacterial CCDs (sub-2 Å resolution) contain clear water molecules modeled at said location. B, Mechanistic proposals for CCD dioxygenation invoking either a dioxetane (blue path) or Criegee-like (red path) rearrangement. These two possibilities are explained further in Box 3. C, Schematic of conserved CCD reactions catalyzed in higher plants. Hollow arrows represent multiple and/or non-CCD enzyme reactions. DWARF27 is a 2Fe-2S-cluster-dependent carotenoid isomerase. NCED, Neoxanthin cleavage enzyme.
The cleavage activity of CCDs is catalyzed by a single ferrous cofactor and O2 molecule, drawing all four reducing equivalents from the primary substrate. Structural characterization and sequence comparison of CCDs revealed a conserved seven-bladed β-propeller architecture housing a 4-His coordination motif (Fig. 4A; Messing et al., 2010; Sui et al., 2013), also present in the bacterial lignostilbene dioxygenases that catalyze similar oxidative cleavage reactions (Bugg et al., 2011). Three second-sphere Glu residues that form hydrogen bonds with the His residues are also conserved (Sui et al., 2013; Priya and Siva, 2015). The exact mechanism for CCD-catalyzed cleavage remains in question, but the current proposal invokes the action of a Fe(III)-superoxide species (Fig. 4B; Box 3). Limited biochemical and structural studies of the plant CCDs have been reported to date.
The specialization of plant CCDs is exemplified by members CCD7 and CCD8 that act sequentially to produce carlactone, the direct precursor to strigolactone, a more recently identified plant hormone that controls root and shoot branching. Similar to CCD1 and CCD4 (Harrison and Bugg, 2014), CCD7 catalyzes the selective 9/10 cleavage of β-carotene; however, it specifically recognizes 9-cis-β-carotene (Fig. 4C) and displays no activity upon the more abundant trans-conformer (Bruno et al., 2014). Its 9-cis-β-apo-10′-carotenal product then serves as the substrate for CCD8, the most chemically unique plant CCD, catalyzing sequential dioxygenations to produce carlactone (Fig. 4C; Harrison et al., 2015). The first cleavage reaction of CCD8 is likely akin to others, but the second results in a unique rearrangement to produce the enol ether of carlactone. CCD8 seems to not release its initial cleavage product, an observation consistent with the recent proposal involving a covalent enzyme-product complex (Harrison et al., 2015). Alternatively, this could be achieved via direct coordination to the iron cofactor. Pre-steady-state kinetic analysis of CCD8 under submolar O2 concentrations and anaerobic crystallographic experiments would help decipher this mechanism. For other CCDs, substrate positioning seems to be the major factor dictating reaction outcome. Engineering of CCD activity could be used for the production of high-value rose (Rosa spp.) ketones, such as ionones and damascenones, used in fragrances and oils, but it will require improved structural and mechanistic knowledge to efficiently alter reactivity.
EXTRADIOL-CLEAVING DIOXYGENASES
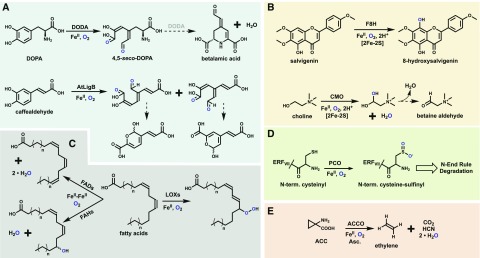
Similar in reactivity to the CCDs, the extradiol-cleaving dioxygenases (ECDs) are iron-dependent enzymes that catalyze oxidative cleavage of C-C bonds. However, ECDs selectively target catechols to cleave the adjacent phenolic bonds, hence the name extradiol (Lipscomb, 2008). These enzymes contain a single ferrous cofactor coordinated via two His and one Asp residues, with their diol substrates occupying two other coordination sites (Lipscomb, 2008). In plants, there are a number of annotated ECDs, but few members have been functionally characterized. DOPA 4,5-dioxygenase (DODA) is responsible for the production of betalamic acid, the betalain pigment precursor, via cleavage of l-DOPA (Fig. 5A; Christinet et al., 2004; Chung et al., 2015), LigB from Arabidopsis cleaves caffealdehyde (Fig. 5A; Weng et al., 2012), and a third is proposed to be involved in stizolob(in)ic acid biosynthesis (Saito and Komamine, 1976). The 4,5 cleavage reaction of DODA produces 4,5-seco-DOPA that spontaneously rearranges to betalamic acid with high enantiomeric selectivity in both in planta and in vitro assays, suggesting that DODA may also play an additional role (Christinet et al., 2004). Bacterial ECDs catalyze a wide range of catechol cleavage reactions, some of which have been expressed in Medicago sativa (alfalfa) for bioremediation (Wang et al., 2015). It is possible that some of the uncharacterized plant ECDs have additional biosynthetic roles.
Figure 5.
The representative reactions catalyzed by plant ECDs (A), RSOs (B), the LOXs and FaD/FaHs (C), PCOs (D), and ACCO (E). The role of DODA during the spontaneous formation of betalamic acid remains in question. Molecular oxygen atoms are shown in blue for tracing purposes. Dashed arrows represent spontaneous rearrangements, and hollow arrows represent multistep pathways. CMO, Choline monooxygenase.
RIESKE-TYPE OXYGENASES
Rieske-type oxygenases (RSOs) represent a small family of non-heme-iron-dependent oxygenases in plants. These enzymes contain separate ferrous- and 2Fe-2S-cluster-binding domains, the latter of which transfers electrons to the iron center via a bridging Asp residue during the catalytic mechanism for oxygen activation (Ferraro et al., 2005; Barry and Challis, 2013). Crystallized RSOs from bacteria revealed functional trimers with these domains juxtaposed. All higher plants possess a set of RSOs involved in chlorophyll degradation (Gray et al., 2004), and the only other known plant RSO is choline monooxygenase (Rathinasabapathi et al., 1997), which produces the osmoprotectant Gly-betaine (Fig. 5B; Hibino et al., 2002). RSOs are more abundant in bacterial genomes catalyzing known monohydroxylations and dihydroxylations, demethylations, N oxidation, and even C-C bond coupling (Barry and Challis, 2013), highlighting the synthetic potential of the RSO scaffold. A recent study identified a novel RSO from Ocimum basilicum (basil) trichomes that functions as a flavone-8-hydroxylase with strong selectivity toward salvigenin (Fig. 5B; Berim et al., 2014). Additional uncharacterized RSO homologs found in plant genomes may be proven to operate in specialized metabolism as well.
FATTY ACID OXYGENASES
The oxygenation of fatty acids is utilized by plants for the production of signaling and defense compounds. All higher plants possess two classes of oxygenases that target polysaturated or unsaturated fatty acids. The lipoxygenases (LOXs) are mononuclear nonheme enzymes that catalyze the insertion of molecular oxygen into unsaturated bonds to form hydroperoxo units (Fig. 5C; Baysal and Demirdöven, 2007). These compounds are then cleaved during production of various plant volatiles and oxylipins, such as the hormone jasmonic acid (Vick and Zimmerman, 1984; Fuller et al., 2001; Baysal and Demirdöven, 2007). LOXs are abundant in both plants and animals, utilizing three His residues and their C-terminal carboxyl group to coordinate an iron cofactor. This assembly differs from the di-iron cofactor used by the fatty acid hydroxylases (FaHs; Broun et al., 1998). Di-iron enzymes constitute an abundant superfamily that operates in critical biological processes in bacteria, such as ribonucleotide reduction and methane oxygenation, and catalyzes many monooxygenation or dioxygenation reactions such as N-hydroxylation in chloramphenicol biosynthesis (Jasniewski and Que, 2018; Komor et al., 2018). FaHs have only been found in select plant species but are closely related to the fatty acid desaturases (FaDs) present in nearly all higher plants (Fig. 5C; Broadwater et al., 2002). Sequence comparison of FaDs and FaHs revealed only seven class-specific variations (Broun et al., 1998). Subsequent mutagenesis studies showed that the desaturation:hydroxylation activity partition of Arabidopsis FAD2 and several FaHs could be manipulated with as few as a single amino acid change (Broun et al., 1998; Broadwater et al., 2002). Manipulation of plant LOXs and FaD/FaHs could be used to gain access to a range of high-value fatty acid derivatives and to manipulate plant growth and development.
CYS DIOXYGENASES
Cys dioxygenases (CDOs) are mononuclear non-heme-iron-dependent enzymes that couple the reduction of O2 to the oxidation of a Cys thiol to sulfinic acid. Bacterial and animal CDOs target free Cys in taurine biosynthesis (Dominy et al., 2006; Stipanuk et al., 2009), but plant CDOs (PCOs) target N-terminal cysteinyl residues of select plant peptides (Weits et al., 2014). As these sequences vary significantly, it remains to be determined whether PCOs utilize a similar active-site architecture to their mammalian and bacterial homologs. Briefly, CDOs utilize a cupin fold with a three-His iron motif to which the l-Cys substrate coordinates via its thiol and amino groups (McCoy et al., 2006; Simmons et al., 2006). Mammalian CDOs also contain a conserved Cys(S)-Tyr(meta-C) cross-link in the active site (McCoy et al., 2006; Li et al., 2018b), believed to help stabilize reactive Fe-oxo species (Ye et al., 2007). PCOs, first identified in Arabidopsis, are linked to the regulation of hypoxic response and were recently shown to specifically oxidize the N termini of class VII Ethylene Response Factors (ERF-VIIs; Fig. 5D; Weits et al., 2014; White et al., 2017). During periods of low cellular oxygen, such as flooding, the ERF-VIIs trigger up-regulation of anaerobic genes, enabling the plant to enter a generally anaerobic state (Bailey-Serres et al., 2012). Under normal O2 levels, the ERF-VIIs are oxidized by PCOs, which initiates their degradation via the N-end rule pathway, suggestingthat the PCOs act as the primary O2 sensor in vivo (Licausi et al., 2011; White et al., 2017). Flashman and coworkers recently provided support for this notion through the kinetic characterization of all five Arabidopsis PCOs, which displayed apparent Km values toward O2 within the physiological range (White et al., 2018). These findings and the in vivo role of PCOs are analogous to the regulation of the hypoxia-inducible transcription factors in animals by a set of Fe/2OGs, the hypoxia-inducible transcription factor hydroxylases, or PHDs, which display an even stronger O2 dependence (Ehrismann et al., 2007; Tarhonskaya et al., 2014). Arabidopsis PCOs were also shown to possess unique substrate preferences, varied enzymatic rates, and O2 dependence, suggesting nonredundant roles in vivo (White et al., 2018). Suppression of PCO activity could be used to increase the stability and abundance of ERF-VIIs, leading to improved flood tolerance and crop recovery (Licausi et al., 2010; Gibbs et al., 2011).
AMINOCYCLOPROPANECARBOXYLATE OXIDASE
Found in all higher plants, aminocyclopropanecarboxylate oxidase (ACCO) catalyzes the final step of ethylene biosynthesis via the Yang cycle (Fig. 5E; McKeon et al., 1995). Produced in nearly all plant tissues, ethylene is a major plant hormone controlling fruit ripening, senescence, and other aspects of plant growth (Iqbal et al., 2017). Manipulation of ethylene levels, both exogenously and within the plant, is routinely exercised to hasten ripening and extend shelf life. Tissue-specific regulation of ACCO activity is a powerful strategy to modulate many aspects of plant physiology (Tonutti et al., 1997; Ben-Amor et al., 1999; Lemus et al., 2007). ACCO utilizes a ferrous cofactor and O2 to produce one equivalent of ethylene, CO2, and cyanide from ACC (Rocklin et al., 2004). This reaction is also dependent upon ascorbate, presumably providing reducing equivalents, and an apparent requirement for CO2 or bicarbonate that remains to be understood. ACCO is structurally related to the Fe/2OGs, but its mechanism is not 2OG dependent (Hagel and Facchini, 2018). ACC binds in a pocket analogous to that of 2OG and coordinates in a bidentate manner via its amino and carboxyl groups. HAT from C2 by an Fe(IV)-oxo species is believed to initiate breakdown to ethylene and cyanoformate, the latter of which is shunted away from the active site before it decomposes (Murphy et al., 2014). Interestingly, bacteria also use an Fe/2OG-like enzyme for ethylene production, known as the ethylene-forming enzyme, which directly oxidizes the iron-bound 2OG molecule to ethylene and two molecules of CO2 (Martinez and Hausinger, 2016; Martinez et al., 2017; Zhang et al., 2017). Ethylene-forming enzymes have garnered much interest as a sustainable source for bioethylene production, as their 2OG substrate is cheap and it does not produce a toxic by-product like cyanide compared with ACCO.
CONCLUSION
Although the enzyme families discussed here do not include all plant oxygenases, those chosen provide a representative picture of the diverse chemistry and catalytic strategies utilized by oxygenases in plant metabolism. While many of the reaction outcomes are chemically redundant (i.e. hydroxylation), each system has its own advantages regarding their adaptability, selectivity, and/or accessibility as potential synthetic biocatalysts. P450s have garnered much of the attention due to their abundance, plasticity, and established engineering efforts. However, the influx of plant genomic data has helped illuminate the activity and role of numerous other, less studied oxygenases in plant metabolism. Many of these oxygenase families have proven to be useful biocatalysts in bacterial systems, highlighting their synthetic potential. Similar endeavors using plant catalysts have presented many difficulties, as many plant enzymes are unstable in vitro, harbor suboptimal activity, and/or require additional partnering enzymes. Establishment of in vivo optimization techniques, such as directed evolution, in plant cells may ultimately circumvent this issue and allow the rapid generation of novel plant catalysts. A continued effort to enhance basic structural and biochemical knowledge of each oxygenase family should be emphasized to lay the foundation for future rational design of enzyme activity. If such strategies can be routinely accessed, the presence of oxygenases throughout plant genomes will give synthetic biologists access to nearly all facets of plant biochemistry and physiology.
Footnotes
This work was supported by grants from the Edward N. and Della L. Thome Memorial Foundation, the Family Larsson-Rosenquist Foundation, and the National Science Foundation (grant no. 1709616). J.-K.W. was supported by the Arnold and Mabel Beckman Foundation, Pew Charitable Trusts (grant no. 27345), and the Kinship Foundation (grant no. 15-SSP-162).
Articles can be viewed without a subscription.
References
- Alfieri A, Malito E, Orru R, Fraaije MW, Mattevi A (2008) Revealing the moonlighting role of NADP in the structure of a flavin-containing monooxygenase. Proc Natl Acad Sci USA 105: 6572–6577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold FH. (2018) Directed evolution: Bringing new chemistry to life. Angew Chem Int Ed Engl 57: 4143–4148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auldridge ME, McCarty DR, Klee HJ (2006) Plant carotenoid cleavage oxygenases and their apocarotenoid products. Curr Opin Plant Biol 9: 315–321 [DOI] [PubMed] [Google Scholar]
- Bailey-Serres J, Fukao T, Gibbs DJ, Holdsworth MJ, Lee SC, Licausi F, Perata P, Voesenek LACJ, van Dongen JT (2012) Making sense of low oxygen sensing. Trends Plant Sci 17: 129–138 [DOI] [PubMed] [Google Scholar]
- Barry SM, Challis GL (2013) Mechanism and catalytic diversity of Rieske non-heme iron-dependent oxygenases. ACS Catal 3: 2362–2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassard JE, Møller BL, Laursen T (2017) Assembly of dynamic P450-mediated metabolons: Order versus chaos. Curr Mol Biol Rep 3: 37–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baysal T, Demirdöven A (2007) Lipoxygenase in fruits and vegetables: A review. Enzyme Microb Technol 40: 491–496 [Google Scholar]
- Behan RK, Hoffart LM, Stone KL, Krebs C, Green MT (2006) Evidence for basic ferryls in cytochromes P450. J Am Chem Soc 128: 11471–11474 [DOI] [PubMed] [Google Scholar]
- Ben-Amor M, Flores B, Latché A, Bouzayen M, Pech JC, Fomojaro F (1999) Inhibition of ethylene biosynthesis by antisense ACC oxidase RNA prevents chilling injury in Charentais cantaloupe melons. Plant Cell Environ 22: 1579–1586 [Google Scholar]
- Berim A, Park JJ, Gang DR (2014) Unexpected roles for ancient proteins: Flavone 8-hydroxylase in sweet basil trichomes is a Rieske-type, PAO-family oxygenase. Plant J 80: 385–395 [DOI] [PubMed] [Google Scholar]
- Bertea CM, Schalk M, Karp F, Maffei M, Croteau R (2001) Demonstration that menthofuran synthase of mint (Mentha) is a cytochrome P450 monooxygenase: Cloning, functional expression, and characterization of the responsible gene. Arch Biochem Biophys 390: 279–286 [DOI] [PubMed] [Google Scholar]
- Blasiak LC, Vaillancourt FH, Walsh CT, Drennan CL (2006) Crystal structure of the non-haem iron halogenase SyrB2 in syringomycin biosynthesis. Nature 440: 368–371 [DOI] [PubMed] [Google Scholar]
- Bollinger JM Jr, Chang WC, Matthews ML, Martinie RJ, Boal AK, Krebs C (2015) Mechanisms of 2-oxoglutarate-dependent oxygenases: The hydroxylation paradigm and beyond. In Schofield C, Hausinger R, eds, 2-Oxoglutarate-Dependent Oxygenases. Royal Society of Chemistry, Cambridge, UK, pp 95–122 [Google Scholar]
- Borowski T, Blomberg MRA, Siegbahn PEM (2008) Reaction mechanism of apocarotenoid oxygenase (ACO): A DFT study. Chemistry 14: 2264–2276 [DOI] [PubMed] [Google Scholar]
- Brandenberg OF, Fasan R, Arnold FH (2017) Exploiting and engineering hemoproteins for abiological carbene and nitrene transfer reactions. Curr Opin Biotechnol 47: 102–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwater JA, Whittle E, Shanklin J (2002) Desaturation and hydroxylation: Residues 148 and 324 of Arabidopsis FAD2, in addition to substrate chain length, exert a major influence in partitioning of catalytic specificity. J Biol Chem 277: 15613–15620 [DOI] [PubMed] [Google Scholar]
- Broun P, Shanklin J, Whittle E, Somerville C (1998) Catalytic plasticity of fatty acid modification enzymes underlying chemical diversity of plant lipids. Science 282: 1315–1317 [DOI] [PubMed] [Google Scholar]
- Bruno M, Hofmann M, Vermathen M, Alder A, Beyer P, Al-Babili S (2014) On the substrate- and stereospecificity of the plant carotenoid cleavage dioxygenase 7. FEBS Lett 588: 1802–1807 [DOI] [PubMed] [Google Scholar]
- Büch K, Stransky H, Hager A (1995) FAD is a further essential cofactor of the NAD(P)H and O2-dependent zeaxanthin-epoxidase. FEBS Lett 376: 45–48 [DOI] [PubMed] [Google Scholar]
- Bugg TDH, Ahmad M, Hardiman EM, Rahmanpour R (2011) Pathways for degradation of lignin in bacteria and fungi. Nat Prod Rep 28: 1883–1896 [DOI] [PubMed] [Google Scholar]
- Chang WC, Guo Y, Wang C, Butch SE, Rosenzweig AC, Boal AK, Krebs C, Bollinger JM Jr (2014) Mechanism of the C5 stereoinversion reaction in the biosynthesis of carbapenem antibiotics. Science 343: 1140–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WC, Li J, Lee JL, Cronican AA, Guo Y (2016) Mechanistic investigation of a non-heme iron enzyme catalyzed epoxidation in (−)-4′-methoxycyclopenin biosynthesis. J Am Chem Soc 138: 10390–10393 [DOI] [PubMed] [Google Scholar]
- Chen K, Huang X, Kan SBJ, Zhang RK, Arnold FH (2018a) Enzymatic construction of highly strained carbocycles. Science 360: 71–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QB, Xin XL, Yang Y, Lee SS, Aisa HA (2014) Highly conjugated norditerpenoid and pyrroloquinoline alkaloids with potent PTP1B inhibitory activity from Nigella glandulifera. J Nat Prod 77: 807–812 [DOI] [PubMed] [Google Scholar]
- Chen X, Hu Y, Zhou DX (2011) Epigenetic gene regulation by plant Jumonji group of histone demethylase. Biochim Biophys Acta 1809: 421–426 [DOI] [PubMed] [Google Scholar]
- Chen YC, Holmes EC, Rajniak J, Kim JG, Tang S, Fischer CR, Mudgett MB, Sattely ES (2018b) N-Hydroxy-pipecolic acid is a mobile metabolite that induces systemic disease resistance in Arabidopsis. Proc Natl Acad Sci USA 115: E4920–E4929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christinet L, Burdet FX, Zaiko M, Hinz U, Zrÿd JP (2004) Characterization and functional identification of a novel plant 4,5-extradiol dioxygenase involved in betalain pigment biosynthesis in Portulaca grandiflora. Plant Physiol 134: 265–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HH, Schwinn KE, Ngo HM, Lewis DH, Massey B, Calcott KE, Crowhurst R, Joyce DC, Gould KS, Davies KM, et al. (2015) Characterisation of betalain biosynthesis in Parakeelya flowers identifies the key biosynthetic gene DOD as belonging to an expanded LigB gene family that is conserved in betalain-producing species. Front Plant Sci 6: 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho PS, Brustad EM, Kannan A, Arnold FH (2013) Olefin cyclopropanation via carbene transfer catalyzed by engineered cytochrome P450 enzymes. Science 339: 307–310 [DOI] [PubMed] [Google Scholar]
- Croteau R, El-Bialy H, El-Hindawi S (1984) Metabolism of monoterpenes: Lactonization of (+)-camphor and conversion of the corresponding hydroxy acid to the glucoside-glucose ester in sage (Salvia officinalis). Arch Biochem Biophys 228: 667–680 [DOI] [PubMed] [Google Scholar]
- Dai X, Mashiguchi K, Chen Q, Kasahara H, Kamiya Y, Ojha S, DuBois J, Ballou D, Zhao Y (2013) The biochemical mechanism of auxin biosynthesis by an Arabidopsis YUCCA flavin-containing monooxygenase. J Biol Chem 288: 1448–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel B, Konrad B, Toplak M, Lahham M, Messenlehner J, Winkler A, Macheroux P (2017) The family of berberine bridge enzyme-like enzymes: A treasure-trove of oxidative reactions. Arch Biochem Biophys 632: 88–103 [DOI] [PubMed] [Google Scholar]
- Didierjean L, Gondet L, Perkins R, Lau SMC, Schaller H, O’Keefe DP, Werck-Reichhart D (2002) Engineering herbicide metabolism in tobacco and Arabidopsis with CYP76B1, a cytochrome P450 enzyme from Jerusalem artichoke. Plant Physiol 130: 179–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominy JE Jr, Simmons CR, Karplus PA, Gehring AM, Stipanuk MH (2006) Identification and characterization of bacterial cysteine dioxygenases: A new route of cysteine degradation for eubacteria. J Bacteriol 188: 5561–5569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Fernández-Fueyo E, Hollmann F, Paul CE, Pesic M, Schmidt S, Wang Y, Younes S, Zhang W (2018) Biocatalytic oxidation reactions: A chemist’s perspective. Angew Chem Int Ed Engl 57: 9238–9261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham NP, Chang WC, Mitchell AJ, Martinie RJ, Zhang B, Bergman JA, Rajakovich LJ, Wang B, Silakov A, Krebs C, et al. (2018) Two distinct mechanisms for C-C desaturation by iron(II)- and 2-(oxo)glutarate-dependent oxygenases: Importance of α-heteroatom assistance. J Am Chem Soc 140: 7116–7126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrismann D, Flashman E, Genn DN, Mathioudakis N, Hewitson KS, Ratcliffe PJ, Schofield CJ (2007) Studies on the activity of the hypoxia-inducible-factor hydroxylases using an oxygen consumption assay. Biochem J 401: 227–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrow SC, Facchini PJ (2013) Dioxygenases catalyze O-demethylation and O,O-demethylenation with widespread roles in benzylisoquinoline alkaloid metabolism in opium poppy. J Biol Chem 288: 28997–29012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro DJ, Gakhar L, Ramaswamy S (2005) Rieske business: Structure-function of Rieske non-heme oxygenases. Biochem Biophys Res Commun 338: 175–190 [DOI] [PubMed] [Google Scholar]
- Fiorentini F, Romero E, Fraaije MW, Faber K, Hall M, Mattevi A (2017) Baeyer-Villiger monooxygenase FMO5 as entry point in drug metabolism. ACS Chem Biol 12: 2379–2387 [DOI] [PubMed] [Google Scholar]
- Fraley AE, Sherman DH (2018) Halogenase engineering and its utility in medicinal chemistry. Bioorg Med Chem Lett 28: 1992–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frusciante S, Diretto G, Bruno M, Ferrante P, Pietrella M, Prado-Cabrero A, Rubio-Moraga A, Beyer P, Gomez-Gomez L, Al-Babili S, et al. (2014) Novel carotenoid cleavage dioxygenase catalyzes the first dedicated step in saffron crocin biosynthesis. Proc Natl Acad Sci USA 111: 12246–12251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes P, Zhou F, Erban A, Karcher D, Kopka J, Bock R (2016) A new synthetic biology approach allows transfer of an entire metabolic pathway from a medicinal plant to a biomass crop. eLife 5: e13664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller MA, Weichert H, Fischer AM, Feussner I, Grimes HD (2001) Activity of soybean lipoxygenase isoforms against esterified fatty acids indicates functional specificity. Arch Biochem Biophys 388: 146–154 [DOI] [PubMed] [Google Scholar]
- Geisler K, Hughes RK, Sainsbury F, Lomonossoff GP, Rejzek M, Fairhurst S, Olsen CE, Motawia MS, Melton RE, Hemmings AM, et al. (2013) Biochemical analysis of a multifunctional cytochrome P450 (CYP51) enzyme required for synthesis of antimicrobial triterpenes in plants. Proc Natl Acad Sci USA 110: E3360–E3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs DJ, Lee SC, Isa NM, Gramuglia S, Fukao T, Bassel GW, Correia CS, Corbineau F, Theodoulou FL, Bailey-Serres J, et al. (2011) Homeostatic response to hypoxia is regulated by the N-end rule pathway in plants. Nature 479: 415–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis EP, Eastman KJ, Hill MD, Donnelly DJ, Meanwell NA (2015) Applications of fluorine in medicinal chemistry. J Med Chem 58: 8315–8359 [DOI] [PubMed] [Google Scholar]
- Gnanasekaran T, Karcher D, Nielsen AZ, Martens HJ, Ruf S, Kroop X, Olsen CE, Motawie MS, Pribil M, Møller BL, et al. (2016) Transfer of the cytochrome P450-dependent dhurrin pathway from Sorghum bicolor into Nicotiana tabacum chloroplasts for light-driven synthesis. J Exp Bot 67: 2495–2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J, Wardzala E, Yang M, Reinbothe S, Haller S, Pauli F (2004) A small family of LLS1-related non-heme oxygenases in plants with an origin amongst oxygenic photosynthesizers. Plant Mol Biol 54: 39–54 [DOI] [PubMed] [Google Scholar]
- Green MT, Dawson JH, Gray HB (2004) Oxoiron(IV) in chloroperoxidase compound II is basic: Implications for P450 chemistry. Science 304: 1653–1656 [DOI] [PubMed] [Google Scholar]
- Hagel JM, Facchini PJ (2018) Expanding the roles for 2-oxoglutarate-dependent oxygenases in plant metabolism. Nat Prod Rep 35: 721–734 [DOI] [PubMed] [Google Scholar]
- Hakamatsuka T, Hashim F (1991) P-450-dependent oxidative rearrangement in isoflavone biosynthesis: Reconstitution of P-450 and NADPH:P-450 reductase. Tetrahedron 47: 5969–5978 [Google Scholar]
- Han JY, In JG, Kwon YS, Choi YE (2010) Regulation of ginsenoside and phytosterol biosynthesis by RNA interferences of squalene epoxidase gene in Panax ginseng. Phytochemistry 71: 36–46 [DOI] [PubMed] [Google Scholar]
- Handrick V, Robert CAM, Ahern KR, Zhou S, Machado RAR, Maag D, Glauser G, Fernandez-Penny FE, Chandran JN, Rodgers-Melnik E, et al. (2016) Biosynthesis of 8-O-methylated benzoxazinoid defense compounds in maize. Plant Cell 28: 1682–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen BG, Kliebenstein DJ, Halkier BA (2007) Identification of a flavin-monooxygenase as the S-oxygenating enzyme in aliphatic glucosinolate biosynthesis in Arabidopsis. Plant J 50: 902–910 [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Bugg TDH (2014) Enzymology of the carotenoid cleavage dioxygenases: Reaction mechanisms, inhibition and biochemical roles. Arch Biochem Biophys 544: 105–111 [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Newgas SA, Descombes F, Shepherd SA, Thompson AJ, Bugg TDH (2015) Biochemical characterization and selective inhibition of β-carotene cis-trans isomerase D27 and carotenoid cleavage dioxygenase CCD8 on the strigolactone biosynthetic pathway. FEBS J 282: 3986–4000 [DOI] [PubMed] [Google Scholar]
- Hartmann M, Zeier T, Bernsdorff F, Reichel-Deland V, Kim D, Hohmann M, Scholten N, Schuck S, Bräutigam A, Hölzel T, et al. (2018) Flavin monooxygenase-generated N-hydroxypipecolic acid is a critical element of plant systemic immunity. Cell 173: 456–469.e16 [DOI] [PubMed] [Google Scholar]
- Harvey AL, Edrada-Ebel R, Quinn RJ (2015) The re-emergence of natural products for drug discovery in the genomics era. Nat Rev Drug Discov 14: 111–129 [DOI] [PubMed] [Google Scholar]
- Helliwell CA, Chandler PM, Poole A, Dennis ES, Peacock WJ (2001) The CYP88A cytochrome P450, ent-kaurenoic acid oxidase, catalyzes three steps of the gibberellin biosynthesis pathway. Proc Natl Acad Sci USA 98: 2065–2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibino T, Waditee R, Araki E, Ishikawa H, Aoki K, Tanaka Y, Takabe T (2002) Functional characterization of choline monooxygenase, an enzyme for betaine synthesis in plants. J Biol Chem 277: 41352–41360 [DOI] [PubMed] [Google Scholar]
- Höfer R, Boachon B, Renault H, Gavira C, Miesch L, Iglesias J, Ginglinger JF, Allouche L, Miesch M, Grec S, et al. (2014) Dual function of the cytochrome P450 CYP76 family from Arabidopsis thaliana in the metabolism of monoterpenols and phenylurea herbicides. Plant Physiol 166: 1149–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbers MME, Montersino S, Westphal AH, Tischler D, van Berkel WJH (2014) Flavin dependent monooxygenases. Arch Biochem Biophys 544: 2–17 [DOI] [PubMed] [Google Scholar]
- Imai YN, Inoue Y, Nakanishi I, Kitaura K (2008) Cl-π interactions in protein-ligand complexes. Protein Sci 17: 1129–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal N, Khan NA, Ferrante A, Trivellini A, Francini A, Khan MIR (2017) Ethylene role in plant growth, development and senescence: Interaction with other phytohormones. Front Plant Sci 8: 475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irmler S, Schröder G, St-Pierre B, Crouch NP, Hotze M, Schmidt J, Strack D, Matern U, Schröder J (2000) Indole alkaloid biosynthesis in Catharanthus roseus: New enzyme activities and identification of cytochrome P450 CYP72A1 as secologanin synthase. Plant J 24: 797–804 [DOI] [PubMed] [Google Scholar]
- Jasniewski AJ, Que L Jr (2018) Dioxygen activation by nonheme diiron enzymes: Diverse dioxygen adducts, high-valent intermediates, and related model complexes. Chem Rev 118: 2554–2592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen K, Rasmussen AV, Morant M, Nielsen AH, Bjarnholt N, Zagrobelny M, Bak S, Møller BL (2005) Metabolon formation and metabolic channeling in the biosynthesis of plant natural products. Curr Opin Plant Biol 8: 280–291 [DOI] [PubMed] [Google Scholar]
- Jung ST, Lauchli R, Arnold FH (2011) Cytochrome P450: Taming a wild type enzyme. Curr Opin Biotechnol 22: 809–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakizaki T, Kitashiba H, Zou Z, Li F, Fukino N, Ohara T, Nishio T, Ishida M (2017) A 2-oxoglutarate-dependent dioxygenase mediates the biosynthesis of glucoraphasatin in radish. Plant Physiol 173: 1583–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner F, Geu-Flores F, Sherden NH, Brown S, Foureau E, Courdavault V, O’Connor SE (2015a) Discovery of a P450-catalyzed step in vindoline biosynthesis: A link between the aspidosperma and eburnamine alkaloids. Chem Commun (Camb) 51: 7626–7628 [DOI] [PubMed] [Google Scholar]
- Kellner F, Kim J, Clavijo BJ, Hamilton JP, Childs KL, Vaillancourt B, Cepela J, Habermann M, Steuernagel B, Clissold L, et al. (2015b) Genome-guided investigation of plant natural product biosynthesis. Plant J 82: 680–692 [DOI] [PubMed] [Google Scholar]
- Kilgore MB, Augustin MM, May GD, Crow JA, Kutchan TM (2016) CYP96T1 of Narcissus sp. aff. pseudonarcissus catalyzes formation of the para-para’ C-C phenol couple in the Amaryllidaceae alkaloids. Front Plant Sci 7: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Smith E, Zwick CR III, Renata H (2018) Applications of oxygenases in the chemoenzymatic total synthesis of complex natural products. Biochemistry 57: 403–412 [DOI] [PubMed] [Google Scholar]
- Kluza A, Niedzialkowska E, Kurpiewska K, Wojdyla Z, Quesne M, Kot E, Porebski PJ, Borowski T (2018) Crystal structure of thebaine 6-O-demethylase from the morphine biosynthesis pathway. J Struct Biol 202: 229–235 [DOI] [PubMed] [Google Scholar]
- Komor AJ, Jasniewski AJ, Que L, Lipscomb JD (2018) Diiron monooxygenases in natural product biosynthesis. Nat Prod Rep 35: 646–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong W, Li J, Yu Q, Cang W, Xu R, Wang Y, Ji W (2016) Two novel flavin-containing monooxygenases involved in biosynthesis of aliphatic glucosinolates. Front Plant Sci 7: 1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krest CM, Onderko EL, Yosca TH, Calixto JC, Karp RF, Livada J, Rittle J, Green MT (2013) Reactive intermediates in cytochrome P450 catalysis. J Biol Chem 288: 17074–17081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen LM, Nielsen AZ, Ziersen B, Gnanasekaran T, Møller BL, Jensen PE (2014) Redirecting photosynthetic electron flow into light-driven synthesis of alternative products including high-value bioactive natural compounds. ACS Synth Biol 3: 1–12 [DOI] [PubMed] [Google Scholar]
- Lattard V, Zhang J, Cashman JR (2004) Alternative processing events in human FMO genes. Mol Pharmacol 65: 1517–1525 [DOI] [PubMed] [Google Scholar]
- Lau W, Sattely ES (2015) Six enzymes from mayapple that complete the biosynthetic pathway to the etoposide aglycone. Science 349: 1224–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemus G, González C, Retamales J (2007) Control of pistillate flower abortion in ‘Serr’ walnuts in Chile by inhibiting ethylene biosynthesis with AVG. In Ramina A, Chang C, Giovannoni J, Klee H, Perata P, Woltering E, eds, Advances in Plant Ethylene Research. Springer, Dordrecht, The Netherlands, pp 305–307 [Google Scholar]
- Li G, Garcia-Borràs M, Fürst MJLJ, Ilie A, Fraaije MW, Houk KN, Reetz MT (2018a) Overriding traditional electronic effects in biocatalytic Baeyer-Villiger reactions by directed evolution. J Am Chem Soc 140: 10464–10472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Griffith WP, Davis I, Shin I, Wang J, Li F, Wang Y, Wherritt DJ, Liu A (2018b) Cleavage of a carbon-fluorine bond by an engineered cysteine dioxygenase. Nat Chem Biol 14: 853–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licausi F, van Dongen JT, Giuntoli B, Novi G, Santaniello A, Geigenberger P, Perata P (2010) HRE1 and HRE2, two hypoxia-inducible ethylene response factors, affect anaerobic responses in Arabidopsis thaliana. Plant J 62: 302–315 [DOI] [PubMed] [Google Scholar]
- Licausi F, Kosmacz M, Weits DA, Giuntoli B, Giorgi FM, Voesenek LACJ, Perata P, van Dongen JT (2011) Oxygen sensing in plants is mediated by an N-end rule pathway for protein destabilization. Nature 479: 419–422 [DOI] [PubMed] [Google Scholar]
- Lipscomb JD. (2008) Mechanism of extradiol aromatic ring-cleaving dioxygenases. Curr Opin Struct Biol 18: 644–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loskot SA, Romney DK, Arnold FH, Stoltz BM (2017) Enantioselective total synthesis of nigelladine A via late-stage C-H oxidation enabled by an engineered P450 enzyme. J Am Chem Soc 139: 10196–10199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mafu S, Jia M, Zi J, Morrone D, Wu Y, Xu M, Hillwig ML, Peters RJ (2016) Probing the promiscuity of ent-kaurene oxidases via combinatorial biosynthesis. Proc Natl Acad Sci USA 113: 2526–2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majamaa K, Hanauske-Abel HM, Günzler V, Kivirikko KI (1984) The 2-oxoglutarate binding site of prolyl 4-hydroxylase: Identification of distinct subsites and evidence for 2-oxoglutarate decarboxylation in a ligand reaction at the enzyme-bound ferrous ion. Eur J Biochem 138: 239–245 [DOI] [PubMed] [Google Scholar]
- Martinez S, Hausinger RP (2016) Biochemical and spectroscopic characterization of the non-heme Fe(II)- and 2-oxoglutarate-dependent ethylene-forming enzyme from Pseudomonas syringae pv. phaseolicola PK2. Biochemistry 55: 5989–5999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez S, Fellner M, Herr CQ, Ritchie A, Hu J, Hausinger RP (2017) Structures and mechanisms of the non-heme Fe(II)- and 2-oxoglutarate-dependent ethylene-forming enzyme: Substrate binding creates a twist. J Am Chem Soc 139: 11980–11988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinie RJ, Pollock CJ, Matthews ML, Bollinger JM Jr, Krebs C, Silakov A (2017) Vanadyl as a stable structural mimic of reactive ferryl intermediates in mononuclear nonheme-iron enzymes. Inorg Chem 56: 13382–13389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascotti ML, Lapadula WJ, Juri Ayub M (2015) The origin and evolution of Baeyer-Villiger monooxygenases (BVMOs): An ancestral family of flavin monooxygenases. PLoS ONE 10: e0132689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashiguchi K, Tanaka K, Sakai T, Sugawara S, Kawaide H, Natsume M, Hanada A, Yaeno T, Shirasu K, Yao H, et al. (2011) The main auxin biosynthesis pathway in Arabidopsis. Proc Natl Acad Sci USA 108: 18512–18517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews ML, Chang WC, Layne AP, Miles LA, Krebs C, Bollinger JM Jr (2014) Direct nitration and azidation of aliphatic carbons by an iron-dependent halogenase. Nat Chem Biol 10: 209–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy JG, Bailey LJ, Bitto E, Bingman CA, Aceti DJ, Fox BG, Phillips GN Jr (2006) Structure nd mechanism of mouse cysteine dioxygenase. Proc Natl Acad Sci USA 103: 3084–3089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon TA, Fernández-Maculet JC, Yang SF (1995) Biosynthesis and metabolism of ethylene. In Davies P, ed, Plant Hormones: Physiology, Biochemistry and Molecular Biology. Springer, Dordrecht, The Netherlands, pp 118–139 [Google Scholar]
- Messing SAJ, Gabelli SB, Echeverria I, Vogel JT, Guan JC, Tan BC, Klee HJ, McCarty DR, Amzel LM (2010) Structural insights into maize viviparous14, a key enzyme in the biosynthesis of the phytohormone abscisic acid. Plant Cell 22: 2970–2980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier B, de Visser SP, Shaik S (2004) Mechanism of oxidation reactions catalyzed by cytochrome p450 enzymes. Chem Rev 104: 3947–3980 [DOI] [PubMed] [Google Scholar]
- Mishina TE, Zeier J (2006) The Arabidopsis flavin-dependent monooxygenase FMO1 is an essential component of biologically induced systemic acquired resistance. Plant Physiol 141: 1666–1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AJ, Zhu Q, Maggiolo AO, Ananth NR, Hillwig ML, Liu X, Boal AK (2016) Structural basis for halogenation by iron- and 2-oxo-glutarate-dependent enzyme WelO5. Nat Chem Biol 12: 636–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AJ, Dunham NP, Bergman JA, Wang B, Zhu Q, Chang WC, Liu X, Boal AK (2017a) Structure-guided reprogramming of a hydroxylase to halogenate its small molecule substrate. Biochemistry 56: 441–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AJ, Dunham NP, Martinie RJ, Bergman JA, Pollock CJ, Hu K, Allen BD, Chang WC, Silakov A, Bollinger JM Jr, et al. (2017b) Visualizing the reaction cycle in an iron(II)- and 2-(oxo)-glutarate-dependent hydroxylase. J Am Chem Soc 139: 13830–13836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani M, Sato F (2011) Unusual P450 reactions in plant secondary metabolism. Arch Biochem Biophys 507: 194–203 [DOI] [PubMed] [Google Scholar]
- Murphy LJ, Robertson KN, Harroun SG, Brosseau CL, Werner-Zwanziger U, Moilanen J, Tuononen HM, Clyburne JAC (2014) A simple complex on the verge of breakdown: Isolation of the elusive cyanoformate ion. Science 344: 75–78 [DOI] [PubMed] [Google Scholar]
- Nelson DR. (2006) Cytochrome P450 nomenclature, 2004. Methods Mol Biol 320: 1–10 [DOI] [PubMed] [Google Scholar]
- Nielsen AZ, Ziersen B, Jensen K, Lassen LM, Olsen CE, Møller BL, Jensen PE (2013) Redirecting photosynthetic reducing power toward bioactive natural product synthesis. ACS Synth Biol 2: 308–315 [DOI] [PubMed] [Google Scholar]
- Nomura T, Kushiro T, Yokota T, Kamiya Y, Bishop GJ, Yamaguchi S (2005) The last reaction producing brassinolide is catalyzed by cytochrome P-450s, CYP85A3 in tomato and CYP85A2 in Arabidopsis. J Biol Chem 280: 17873–17879 [DOI] [PubMed] [Google Scholar]
- O’Connor SE. (2015) Engineering of secondary metabolism. Annu Rev Genet 49: 71–94 [DOI] [PubMed] [Google Scholar]
- O’Reilly E, Köhler V, Flitsch SL, Turner NJ (2011) Cytochromes P450 as useful biocatalysts: Addressing the limitations. Chem Commun (Camb) 47: 2490–2501 [DOI] [PubMed] [Google Scholar]
- Paddon CJ, Keasling JD (2014) Semi-synthetic artemisinin: A model for the use of synthetic biology in pharmaceutical development. Nat Rev Microbiol 12: 355–367 [DOI] [PubMed] [Google Scholar]
- Pan J, Bhardwaj M, Zhang B, Chang WC, Schardl CL, Krebs C, Grossman RB, Bollinger JM Jr (2018) Installation of the ether bridge of lolines by the iron- and 2-oxoglutarate-dependent oxygenase, LolO: Regio- and stereochemistry of sequential hydroxylation and oxacyclization reactions. Biochemistry 57: 2074–2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JT, Andorfer MC, Lewis JC (2016) Engineering flavin-dependent halogenases. Methods Enzymol 575: 93–126 [DOI] [PubMed] [Google Scholar]
- Price JC, Barr EW, Glass TE, Krebs C, Bollinger JM Jr (2003a) Evidence for hydrogen abstraction from C1 of taurine by the high-spin Fe(IV) intermediate detected during oxygen activation by taurine:α-ketoglutarate dioxygenase (TauD). J Am Chem Soc 125: 13008–13009 [DOI] [PubMed] [Google Scholar]
- Price JC, Barr EW, Tirupati B, Bollinger JM Jr, Krebs C (2003b) The first direct characterization of a high-valent iron intermediate in the reaction of an α-ketoglutarate-dependent dioxygenase: A high-spin FeIV complex in taurine/α-ketoglutarate dioxygenase (TauD) from Escherichia coli. Biochemistry 42: 7497–7508 [DOI] [PubMed] [Google Scholar]
- Priya R, Siva R (2015) Analysis of phylogenetic and functional diverge in plant nine-cis epoxycarotenoid dioxygenase gene family. J Plant Res 128: 519–534 [DOI] [PubMed] [Google Scholar]
- Proshlyakov DA, McCracken J, Hausinger RP (2017) Spectroscopic analyses of 2-oxoglutarate-dependent oxygenases: TauD as a case study. J Biol Inorg Chem 22: 367–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan RF, Shumskaya M, Bradbury LMT, Beltrán J, Ma C, Kennelly EJ, Wurtzel ET (2012) Synergistic interactions between carotene ring hydroxylases drive lutein formation in plant carotenoid biosynthesis. Plant Physiol 160: 204–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston L, Yu O (2006) Metabolons involving plant cytochrome P450s. Phytochem Rev 5: 459 [Google Scholar]
- Rathinasabapathi B, Burnet M, Russell BL, Gage DA, Liao PC, Nye GJ, Scott P, Golbeck JH, Hanson AD (1997) Choline monooxygenase, an unusual iron-sulfur enzyme catalyzing the first step of glycine betaine synthesis in plants: Prosthetic group characterization and cDNA cloning. Proc Natl Acad Sci USA 94: 3454–3458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renata H, Wang ZJ, Arnold FH (2015) Expanding the enzyme universe: Accessing non-natural reactions by mechanism-guided directed evolution. Angew Chem Int Ed Engl 54: 3351–3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittle J, Green MT (2010) Cytochrome P450 compound I: Capture, characterization, and C-H bond activation kinetics. Science 330: 933–937 [DOI] [PubMed] [Google Scholar]
- Rocklin AM, Kato K, Liu HW, Que L Jr, Lipscomb JD (2004) Mechanistic studies of 1-aminocyclopropane-1-carboxylic acid oxidase: Single turnover reaction. J Biol Inorg Chem 9: 171–182 [DOI] [PubMed] [Google Scholar]
- Runguphan W, Qu X, O’Connor SE (2010) Integrating carbon-halogen bond formation into medicinal plant metabolism. Nature 468: 461–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi SJ, Gilardi G (2013) Chimeric P450 enzymes: Activity of artificial redox fusions driven by different reductases for biotechnological applications. Biotechnol Appl Biochem 60: 102–110 [DOI] [PubMed] [Google Scholar]
- Saito K, Komamine A (1976) Biosynthesis of stizolobinic acid and stizolobic acid in higher plants: An enzyme system(s) catalyzing the conversion of dihydroxyphenylalanine into stizolobinic acid and stizolobic acid from etiolated seedlings of Stizolobium hassjoo. Eur J Biochem 68: 237–243 [DOI] [PubMed] [Google Scholar]
- Saito K, Kobayashi M, Gong Z, Tanaka Y, Yamazaki M (1999) Direct evidence for anthocyanidin synthase as a 2-oxoglutarate-dependent oxygenase: Molecular cloning and functional expression of cDNA from a red forma of Perilla frutescens. Plant J 17: 181–189 [DOI] [PubMed] [Google Scholar]
- Sasaki A, Ashikari M, Ueguchi-Tanaka M, Itoh H, Nishimura A, Swapan D, Ishiyama K, Saito T, Kobayashi M, Khush GS, et al. (2002) Green revolution: A mutant gibberellin-synthesis gene in rice. Nature 416: 701–702 [DOI] [PubMed] [Google Scholar]
- Satpathy R, Guru RK, Behera R, Priyadarshini A (2010) Homology modelling of lycopene cleavage oxygenase: The key enzyme of bixin production. J Comput Sci Syst Biol 3: 59–61 [Google Scholar]
- Scheler U, Brandt W, Porzel A, Rothe K, Manzano D, Božić D, Papaefthimiou D, Balcke GU, Henning A, Lohse S, et al. (2016) Elucidation of the biosynthesis of carnosic acid and its reconstitution in yeast. Nat Commun 7: 12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaich NL. (2007) Flavin-containing monooxygenases in plants: Looking beyond detox. Trends Plant Sci 12: 412–418 [DOI] [PubMed] [Google Scholar]
- Schückel J, Rylott EL, Grogan G, Bruce NC (2012) A gene-fusion approach to enabling plant cytochromes P450 for biocatalysis. ChemBioChem 13: 2758–2763 [DOI] [PubMed] [Google Scholar]
- Schuler MA. (2015) P450s in plants, insects, and their fungal pathogens. In Ortiz de Montellano P, ed, Cytochrome P450: Structure, Mechanism, and Biochemistry. Springer International Publishing, Cham, Switzerland, pp 409–449 [Google Scholar]
- Schwartz SH, Tan BC, Gage DA, Zeevaart JA, McCarty DR (1997) Specific oxidative cleavage of carotenoids by VP14 of maize. Science 276: 1872–1874 [DOI] [PubMed] [Google Scholar]
- Simmons CR, Liu Q, Huang Q, Hao Q, Begley TP, Karplus PA, Stipanuk MH (2006) Crystal structure of mammalian cysteine dioxygenase: A novel mononuclear iron center for cysteine thiol oxidation. J Biol Chem 281: 18723–18733 [DOI] [PubMed] [Google Scholar]
- Smanski MJ, Zhou H, Claesen J, Shen B, Fischbach MA, Voigt CA (2016) Synthetic biology to access and expand nature’s chemical diversity. Nat Rev Microbiol 14: 135–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srnec M, Wong SD, Matthews ML, Krebs C, Bollinger JM Jr, Solomon EI (2016) Electronic structure of the ferryl intermediate in the α-ketoglutarate dependent non-heme iron halogenase SyrB2: Contributions to H atom abstraction reactivity. J Am Chem Soc 138: 5110–5122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffan N, Grundmann A, Afiyatullov S, Ruan H, Li SM (2009) FtmOx1, a non-heme Fe(II) and α-ketoglutarate-dependent dioxygenase, catalyses the endoperoxide formation of verruculogen in Aspergillus fumigatus. Org Biomol Chem 7: 4082–4087 [DOI] [PubMed] [Google Scholar]
- Stepanova AN, Yun J, Robles LM, Novak O, He W, Guo H, Ljung K, Alonso JM (2011) The Arabidopsis YUCCA1 flavin monooxygenase functions in the indole-3-pyruvic acid branch of auxin biosynthesis. Plant Cell 23: 3961–3973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stipanuk MH, Ueki I, Dominy JE Jr, Simmons CR, Hirschberger LL (2009) Cysteine dioxygenase: A robust system for regulation of cellular cysteine levels. Amino Acids 37: 55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhamalla B, Wang S, Snyder V, Kavoosi S, Arora S, Islam K (2018) Complementary steric engineering at the protein-ligand interface for analogue-sensitive TET oxygenases. J Am Chem Soc 140: 10263–10269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui X, Kiser PD, von Lintig J, Palczewski K (2013) Structural basis of carotenoid cleavage: From bacteria to mammals. Arch Biochem Biophys 539: 203–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Zhou D, Kandavelu P, Zhang H, Yuan Q, Wang BC, Rose J, Yan Y (2015) Structural insights into substrate specificity of feruloyl-CoA 6′-hydroxylase from Arabidopsis thaliana. Sci Rep 5: 10355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarhonskaya H, Chowdhury R, Leung IKH, Loik ND, McCullagh JSO, Claridge TDW, Schofield CJ, Flashman E (2014) Investigating the contribution of the active site environment to the slow reaction of hypoxia-inducible factor prolyl hydroxylase domain 2 with oxygen. Biochem J 463: 363–372 [DOI] [PubMed] [Google Scholar]
- Tonutti P, Bonghi C, Ruperti B, Ramina A (1997) The modulation of ethylene biosynthesis and ACC oxidase gene expression during peach fruit development and fruitlet abscission. In Kanellis AK, Chang C, Kende H, Grierson D, eds, Biology and Biotechnology of the Plant Hormone Ethylene. Springer, Dordrecht, The Netherlands, pp 149–153 [Google Scholar]
- Tsukada Y, Fang J, Erdjument-Bromage H, Warren ME, Borchers CH, Tempst P, Zhang Y (2006) Histone demethylation by a family of JmjC domain-containing proteins. Nature 439: 811–816 [DOI] [PubMed] [Google Scholar]
- Ushimaru R, Ruszczycky MW, Chang WC, Yan F, Liu YN, Liu HW (2018) Substrate conformation correlates with the outcome of hyoscyamine 6β-hydroxylase catalyzed oxidation reactions. J Am Chem Soc 140: 7433–7436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillancourt FH, Yin J, Walsh CT (2005) SyrB2 in syringomycin E biosynthesis is a nonheme FeII α-ketoglutarate- and O2-dependent halogenase. Proc Natl Acad Sci USA 102: 10111–10116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beek HL, de Gonzalo G, Fraaije MW (2012) Blending Baeyer-Villiger monooxygenases: Using a robust BVMO as a scaffold for creating chimeric enzymes with novel catalytic properties. Chem Commun (Camb) 48: 3288–3290 [DOI] [PubMed] [Google Scholar]
- van Pée KH, Patallo EP (2006) Flavin-dependent halogenases involved in secondary metabolism in bacteria. Appl Microbiol Biotechnol 70: 631–641 [DOI] [PubMed] [Google Scholar]
- Vick BA, Zimmerman DC (1984) Biosynthesis of jasmonic acid by several plant species. Plant Physiol 75: 458–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ren H, Pan H, Liu J, Zhang L (2015) Enhanced tolerance and remediation to mixed contaminates of PCBs and 2,4-DCP by transgenic alfalfa plants expressing the 2,3-dihydroxybiphenyl-1,2-dioxygenase. J Hazard Mater 286: 269–275 [DOI] [PubMed] [Google Scholar]
- Wang ZJ, Peck NE, Renata H, Arnold FH (2014) Cytochrome P450-catalyzed insertion of carbenoids into N-H bonds. Chem Sci (Camb) 5: 598–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weits DA, Giuntoli B, Kosmacz M, Parlanti S, Hubberten HM, Riegler H, Hoefgen R, Perata P, van Dongen JT, Licausi F (2014) Plant cysteine oxidases control the oxygen-dependent branch of the N-end-rule pathway. Nat Commun 5: 3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welford RWD, Clifton IJ, Turnbull JJ, Wilson SC, Schofield CJ (2005) Structural and mechanistic studies on anthocyanidin synthase catalysed oxidation of flavanone substrates: The effect of C-2 stereochemistry on product selectivity and mechanism. Org Biomol Chem 3: 3117–3126 [DOI] [PubMed] [Google Scholar]
- Weng JK, Li Y, Mo H, Chapple C (2012) Assembly of an evolutionarily new pathway for α-pyrone biosynthesis in Arabidopsis. Science 337: 960–964 [DOI] [PubMed] [Google Scholar]
- White MD, Klecker M, Hopkinson RJ, Weits DA, Mueller C, Naumann C, O’Neill R, Wickens J, Yang J, Brooks-Bartlett JC, et al. (2017) Plant cysteine oxidases are dioxygenases that directly enable arginyl transferase-catalysed arginylation of N-end rule targets. Nat Commun 8: 14690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MD, Kamps JJAG, East S, Taylor Kearney LJ, Flashman E (2018) The plant cysteine oxidases from Arabidopsis thaliana are kinetically tailored to act as oxygen sensors. J Biol Chem 293: 11786–11795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmouth RC, Turnbull JJ, Welford RWD, Clifton IJ, Prescott AG, Schofield CJ (2002) Structure and mechanism of anthocyanidin synthase from Arabidopsis thaliana. Structure 10: 93–103 [DOI] [PubMed] [Google Scholar]
- Woo JM, Jeon EY, Seo EJ, Seo JH, Lee DY, Yeon YJ, Park JB (2018) Improving catalytic activity of the Baeyer-Villiger monooxygenase-based Escherichia coli biocatalysts for the overproduction of (Z)-11-(heptanoyloxy)undec-9-enoic acid from ricinoleic acid. Sci Rep 8: 10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Katano N, Ooi A, Inoue K (2000) Secologanin synthase which catalyzes the oxidative cleavage of loganin into secologanin is a cytochrome P450. Phytochemistry 53: 7–12 [DOI] [PubMed] [Google Scholar]
- Ye S, Wu X, Wei L, Tang D, Sun P, Bartlam M, Rao Z (2007) An insight into the mechanism of human cysteine dioxygenase: Key roles of the thioether-bonded tyrosine-cysteine cofactor. J Biol Chem 282: 3391–3402 [DOI] [PubMed] [Google Scholar]
- Ye S, Riplinger C, Hansen A, Krebs C, Bollinger JM Jr, Neese F (2012) Electronic structure analysis of the oxygen-activation mechanism by Fe(II)- and α-ketoglutarate (αKG)-dependent dioxygenases. Chemistry 18: 6555–6567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosca TH, Rittle J, Krest CM, Onderko EL, Silakov A, Calixto JC, Behan RK, Green MT (2013) Iron(IV)hydroxide pKa and the role of thiolate ligation in C-H bond activation by cytochrome P450. Science 342: 825–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto N, Onuma M, Mizuno S, Sugino Y, Nakabayashi R, Imai S, Tsuneyoshi T, Sumi S, Saito K (2015) Identification of a flavin-containing S-oxygenating monooxygenase involved in alliin biosynthesis in garlic. Plant J 83: 941–951 [DOI] [PubMed] [Google Scholar]
- Zeilmaker T, Ludwig NR, Elberse J, Seidl MF, Berke L, Van Doorn A, Schuurink RC, Snel B, Van den Ackerveken G (2015) DOWNY MILDEW RESISTANT 6 and DMR6-LIKE OXYGENASE 1 are partially redundant but distinct suppressors of immunity in Arabidopsis. Plant J 81: 210–222 [DOI] [PubMed] [Google Scholar]
- Zhang X, King-Smith E, Renata H (2018) Total synthesis of tambromycin by combining chemocatalytic and biocatalytic C-H functionalization. Angew Chem Int Ed Engl 57: 5037–5041 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Smart TJ, Choi H, Hardy F, Lohans CT, Abboud MI, Richardson MSW, Paton RS, McDonough MA, Schofield CJ (2017) Structural and stereoelectronic insights into oxygenase-catalyzed formation of ethylene from 2-oxoglutarate. Proc Natl Acad Sci USA 114: 4667–4672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Christensen SK, Fankhauser C, Cashman JR, Cohen JD, Weigel D, Chory J (2001) A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 291: 306–309 [DOI] [PubMed] [Google Scholar]
- Zhou J, Kelly WL, Bachmann BO, Gunsior M, Townsend CA, Solomon EI (2001) Spectroscopic studies of substrate interactions with clavaminate synthase 2, a multifunctional α-KG-dependent non-heme iron enzyme: Correlation with mechanisms and reactivities. J Am Chem Soc 123: 7388–7398 [DOI] [PubMed] [Google Scholar]
- Zwick CR III, Renata H (2018) Evolution of biocatalytic and chemocatalytic C-H functionalization strategy in the synthesis of manzacidin C. J Org Chem 83: 7407–7415 [DOI] [PubMed] [Google Scholar]