Abstract
Background:
Novel prognostic markers and therapeutic targets for advanced cancer are urgently needed. This report with trial sequential analysis (TSA) was first conducted to provide robust estimates of the correlation between aldehyde dehydrogenase 1 (ALDH1) and Nestin and clinical outcomes of advanced cancer patients.
Methods:
Hazard ratios (HRs) with 95% confidence intervals (CIs) were summarized for overall survival (OS), disease-free survival (DFS), progression-free survival (PFS), cancer-specific survival (CSS), relapse/recurrence-free survival (RFS), and metastasis-free survival (MFS) from multivariable analysis. TSA was performed to control for random errors.
Results:
A total of 20 studies with 2050 patients (ALDH1: 15 studies with 1557 patients and Nestin: 5 studies with 493 patients) were identified. ALDH1 (HR = 2.28, p < 0.001) and Nestin (HR = 2.39, p < 0.001) were associated with a worse OS, as confirmed by TSA. Nestin positivity was linked to a poor PFS (HR = 2.08, p < 0.001), but ALDH1 was not linked to DFS, RFS, MFS, or PFS, and TSA showed that more studies were needed. Subgroup analysis by tumor type indicated that ALDH1 positivity may be associated with shorter OS in breast, head and neck cancers, but there was no association with colorectal cancer. Subgroup analysis by study source showed that ALDH1 positivity was correlated with a worse OS for Japanese (HR = 1.94, p = 0.002) and European patients (HR = 4.15, p < 0.001), but there was no association for Chinese patients. Subgroup analysis by survival rate showed that ALDH1 positivity correlated with poor OS at ⩾ 5 years (HR = 2.33, p < 0.001) or 10 years (HR = 1.76, p = 0.038).
Conclusions:
ALDH1 may be more valuable as an effective therapeutic target than Nestin for improving the long-term survival rate of advanced cancer. Additional prospective clinical trials are needed across different cancer types.
Keywords: advanced cancer, ALDH1, Nestin, prognosis, therapy
Introduction
Cancer is still one of the most threatening diseases in the world, with high morbidity and mortality rates.1 Although the therapeutic strategies (surgery, chemotherapy, radiotherapy, targeted molecular therapy, and immunotherapy) have greatly improved in recent years, effective therapeutic opportunities are still limited. Therefore, the 5-year survival rate of patients with liver, lung or lymph node metastases (advanced stage) remains disappointing.2–5 The addition of prognostic markers to better prognostic models could improve the outcomes of advanced or metastatic cancer patients and aid in the appropriate selection of treatment strategies as potential novel therapeutic targets.
Cancer stem cells (CSCs), a small subset of cancer cells, are responsible for the capability of self-renewal, uncontrolled proliferation and differentiation, tumor progression, and resistance to traditional therapy.6,7 CSCs have been identified in many tumors, for example, lung, breast, colorectal and cervical cancer.8–10 Nestin, namely, neural stem cell protein, a member of the class VI intermediate filament (IF) family, was first discovered as a neural stem and progenitor cells marker.11 Subsequent reports suggested that Nestin is observed in nonneural tissues12 and may play a role in CSC phenotypes.13 Nestin is involved in tumor angiogenesis, growth and cellular processes such as cell adhesion, proliferation, migration, and invasion.14–16 The aldehyde dehydrogenase 1 (ALDH1) gene has been mapped to chromosome 12q24.2 and is responsible for oxidation and detoxification functions.17 ALDH1 is involved in cellular differentiation and proliferation and in resistance to alkylating chemotherapeutic agents.18,19 ALDH1 has been identified in some tumors and acts as a promoter, inducing an epithelial-mesenchymal transition (EMT) in tumor cells.20,21 ALDH1 and Nestin are expressed in some malignant tumors.22–25 However, there are some uncertain conclusions for ALDH1 and Nestin in advanced cancer; for example, Dong and colleagues reported that ALDH1-positive expression was not correlated with overall survival (OS) in multivariable analysis in advanced breast cancer.26 However, ALDH1-positive expression was associated with worse overall survival in multivariable analysis in advanced breast cancer by Alamgeer and colleagues.27 Thus, further analysis is needed to elucidate the significance of ALDH1 and Nestin in advanced cancer.
To better understand the prognostic mechanisms of ALDH1 and Nestin in advanced or metastatic cancer, we first performed a systematic meta-analysis to evaluate the benefits of ALDH1 and Nestin using multivariable survival analysis. We also conducted trial sequential analysis (TSA) to correct for the increased risk of random errors and to determine if additional studies were needed. Our study’s results could assist in the selection of appropriate treatment strategies, suggest novel therapeutic targets for advanced cancer patients, and help stratify patients in future prospective clinical trials.
Materials and methods
Search strategy
This meta-analysis was performed based on the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) statement.28 We systematically searched the PubMed, EMBASE, EBSCO, Web of Science, and Cochrane Library databases for relevant studies published prior to April 2018. The search strategy was on the basis of the following key words and search terms: ‘aldehyde dehydrogenase 1 OR ALDH1 OR Nestin’, ‘metastatic OR advanced OR metastasized OR recurrent’, ‘cancer OR tumor OR carcinoma OR neoplasm’, ‘survival OR outcome OR prognosis’ (Table S1). The reference lists of all eligible articles were also manually reviewed to identify additional studies. Overall, three authors (SH, TH, and FH) independently assessed the publications, and discrepancies were resolved by consensus.
Eligibility criteria
Articles that met the following inclusion criteria were selected: (1) patients were diagnosed with advanced, metastatic, or recurrent cancer; (2) prospective or retrospective clinical studies that reported sufficient information for the prognostic estimation of ALDH1 and Nestin positivity; (3) studies reported available data regarding hazard ratio (HR) and 95% confidence interval (CI) using multivariable survival analysis for prognostic indicators of OS, disease-free survival (DFS), progression-free survival (PFS), cancer-specific survival (CSS), relapse/recurrence-free survival (RFS), or metastasis-free survival (MFS); (4) if studies did not record sufficient data on HR with 95% CI, HR and 95% CI values were calculated on the basis of the described method if possible,29,30 or we emailed the corresponding author to request useful information; (5) the eligible studies were limited to the English language. If the authors published multiple papers using overlapping sample data, only the study with the latest information or the largest number of patients was extracted to avoid repeated data. Studies that did not meet the above-described inclusion criteria were excluded.
Data extraction and study assessment
Methodological assessment was performed based on the Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK) guidelines.31 REMARK criteria include 20 items (introduction: 1 item, materials and methods: 10 items, results: 7 items, and discussion: 2 items), having a maximum possible score of 40. An item had the following possible values: 2 if the item was clearly described, 1 if the item was incompletely described, and 0 if the item was not applicable or not fully defined. Additionally, multivariable survival analysis adjusted factors was considered more valuable than a study using univariable survival analysis. Thus, the meta-analysis only included the prognostic data from multivariable analyses. The REMARK scores are listed in Table S2. The following data were extracted from the eligible full-text papers: the surname of the first author, publication year, number of patients, demographic data (median or mean age), study source, median or mean follow-up time, cancer type, study design, testing method, therapy regimes, sample type, cut-off value, survival rate, adjusted variables, and clinical outcomes of multivariable analysis. Any disagreements were resolved by discussion until consensus was achieved.
Statistical analysis
Data with an HR >1 indicated worse survival, whereas an HR <1 indicated favorable survival. Pooled HR and 95% CI were calculated to evaluate the prognostic effect of ALDH1 and Nestin positivity using multivariable analysis for advanced cancer (OS, DFS, PFS, CSS, RFS, or MFS). Heterogeneity among the eligible studies was measured using the Cochran’s Q statistic and I2 test.32 According to the der Simonian–Laird method, the random-effects model was applied in the meta-analysis.33,34 Both I2 < 50% and p > 0.1 were considered as signifying low-level heterogeneity. For the results (> seven studies) with obvious heterogeneity, subgroup analyses by cancer type, study source, survival rate, age (years), detection method, and study center design, etc. were conducted to explain the possible sources of heterogeneity and the strength of the correlation among different subgroups. Sensitivity analyses were also performed to estimate the effect of each individual study on the HR value by removing one study from the analysis at a time. Publication bias was detected using Egger’s and Begg’s funnel plots.35,36 If the meta-analysis included a small sample size, random errors may cause spurious findings.37,38 TSA was performed to evaluate the required sample information.39 Alpha (type I error) and beta (type II error) levels of significance of 5% and 20% were used, respectively, and the optimal a priori anticipated information size (APIS) method was set. Monitoring boundaries were constructed to decide whether a study could be terminated early. When the cumulative Z-curve crossed the trial sequential monitoring boundary or required information size boundary, the evidence was considered to be conclusive, and further studies were deemed unlikely to change the results; otherwise, additional clinical studies were necessary. Meta-analysis was performed with Stata software, version 12.0 (Stata Corp., College Station, TX, USA) and R software, version 3.4.2 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Study characteristics
A flow diagram of the literature search procedure is presented in Figure 1. After applying the eligibility criteria, a total of 20 studies published from 2010 to 2018 were included in the meta-analysis,26,27,40–56 including 2050 patients with advanced cancer. These studies were evaluated against the REMARK criteria, with a mean REMARK score of 20. A total of nine studies were conducted in Europe, two in the USA, four in China, four in Japan, and the remaining one in Korea. Among the included studies, 15 studies involving 1557 advanced cancer patients evaluated the association between ALDH1 positivity and the prognosis using multivariable analysis for CSS, OS, DFS, RFS, MFS, and PFS.26,27,41,43,45–52,54,56 Overall, five studies involving 493 advanced cancer patients assessed the correlation between Nestin positivity and the prognosis using multivariable analysis for OS and PFS.40,42,44,53,55 The characteristics of the included studies are listed in Table 1 and Table S3.
Figure 1.
PRISMA diagram/flow chart.
PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Table 1.
Main characteristics of the eligible studies.
| First author | Study source | Age | Method | Cancer type | Study design | n | Therapy | Survival rate | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Charafe-Jauffret and colleagues56 | France | NA | IHC | Inflammatory breast cancer | Retrospective, multicenter | 109 | Surgery and adjuvant therapy | 5 years | CSS, MFS |
| Yoshioka and colleagues54 | Japan | NA | IHC | Node-positive breast cancer | Retrospective, single-center | 109 | Surgery and adjuvant therapy | 10 years | OS, RFS |
| Koukourakis and colleagues52 | Greece | 68 | IHC, blind | Advanced squamous cell head-neck cancer | Retrospective, multicenter | 74 | Surgery and adjuvant therapy | 5 years | RFS |
| Dong and colleagues26 | China | 49 | IHC, blind | Breast cancer with axillary lymph node metastasis | Retrospective, single-center | 161 | Adjuvant therapy | 5 years | OS, RFS |
| Alamgeer and colleagues27 | Australia | NA | IHC, blind | Advanced breast cancer | Prospective trial, multicenter | 119 | Surgery and adjuvant chemotherapy | 5 years | OS |
| Qian and colleagues51 | Germany | NA | IHC, blind | Advanced pharyngeal squamous cell carcinoma | Retrospective, multicenter | 56 | NA | > 5 years | OS |
| Qian and colleagues51 | Germany | NA | IHC, blind | Advanced head and neck squamous cell carcinoma | Retrospective, multicenter | 66 | NA | > 5 years | OS |
| Fitzgerald and colleagues50 | USA | NA | IHC | Metastatic colon cancer | Retrospective, single-center | 21 | Surgery | < 5 years | OS |
| Bednarz-Knoll and colleagues47 | Germany/ Poland | NA | IHC | Breast cancer with lymph node metastasis | Retrospective, multicenter | 102 | Surgery and adjuvant therapy | > 5 years | DFS, MFS |
| Ito and colleagues49 | Japan | 54 | IHC, blind | Breast cancer with axillary lymph node metastasis | Retrospective, single-center | 47 | Surgery and adjuvant therapy | > 5 years | DFS |
| Xie and colleagues48 | China | 43 | IHC | Advanced cervical cancer | Retrospective, single-center | 52 | Surgery and adjuvant chemotherapy | 5 years | OS, DFS |
| Ishiguro and colleagues46 | Japan | NA | IHC | Advanced serous ovarian carcinoma | Retrospective, multicenter | 90 | Surgery and adjuvant therapy | > 5 years | OS, PFS |
| Adamczyk and colleagues45 | Poland | 55.7 | IHC | Breast cancer with lymph node metastasis | Retrospective, single-center | 155 | Surgery and adjuvant therapy | 5 years | DFS |
| Miyoshi and colleagues43 | Japan | NA | IHC | Recurrent breast cancer | Retrospective, multicenter | 318 | Surgery and adjuvant therapy | 10 years | OS |
| Ning and colleagues41 | USA# | 57.5 | qRT-PCR | Metastatic colorectal cancer | Retrospective, multicenter | 78 | Chemotherapy | < 5 years | OS, PFS |
| Fusi and colleagues55 | Germany | 54 | FC analysis | Metastatic melanoma | Retrospective, single-center | 32 | Chemotherapy | 1 year | OS |
| Qin and colleagues53 | China | NA | IHC | Advanced serous ovarian cancer | Retrospective, multicenter | 123 | Chemotherapy | 5 years | OS |
| Kuk and colleagues44 | Korea | 58 | IHC | Advanced melanoma | Retrospective, single-center | 33 | Surgery and adjuvant therapy | 5 years | PFS |
| Zhang and colleagues42 | China | NA | IHC | Advanced hepatocellular carcinoma | Retrospective, single-center | 220 | Surgery and chemotherapy | < 3 years | OS, PFS |
| Onisim and colleagues40 | Romania | 56 | IHC | Advanced serous ovarian carcinoma | Retrospective, single-center | 85 | Surgery and chemotherapy | 5 years | OS, PFS |
Note: the studies from Charafe-Jauffret 2010 to Ning 2018 were of ALDH1 and the studies from Fusi 2011 to Onisim 2016 were of Nestin.
stands for the mixed population.
CSS, cancer-specific survival; DFS, disease-free survival; FC, flow cytometry; IHC, immunohistochemistry; MFS, metastasis-free survival; N, number of cases; NA, not applicable; OS, overall survival; PFS, progression-free survival; qRT-PCR, real-time quantitative PCR; RFS, relapse/recurrence-free survival.
Association between ALDH1 positivity and the prognosis
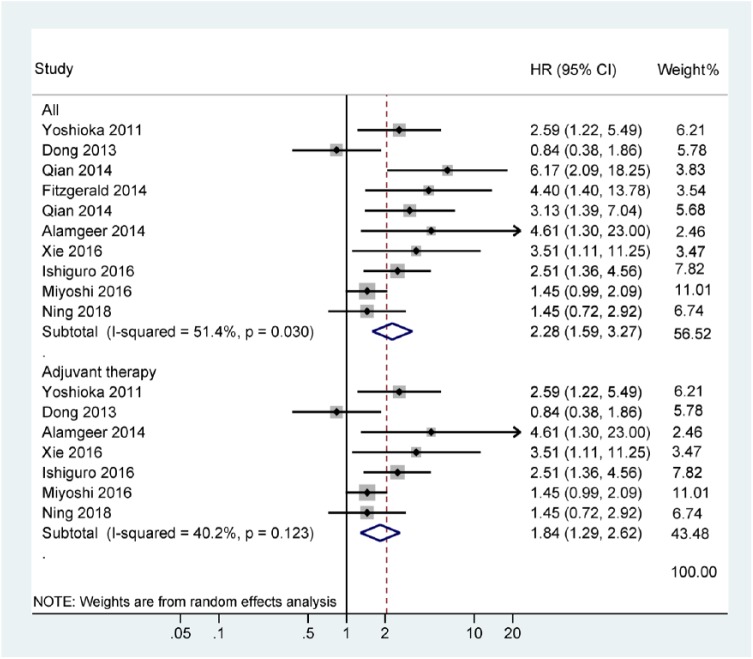
CSS was only available in one study with 77 cases that showed that ALDH1 positivity was correlated with a poor CSS (5-year CSS: HR = 2.7, 95% CI = 1.48–4.93; Figure 2). There was no statistically significant correlation with DFS (n = four studies with 333 cases, HR = 0.95, 95% CI = 0.38–2.36, p = 0.907), RFS (n = three studies with 344 cases, HR = 0.90, 95% CI = 0.41–1.99, p = 0.797), MFS (n = two studies with 112 cases, HR = 1.04, 95% CI = 0.16–6.70, p = 0.97), or PFS (n = two studies with 168 cases, HR = 1.32, 95% CI = 0.65–2.68, p = 0.445; Figure 2). Data from 10 studies indicated that ALDH1 positivity was significantly correlated with a negative effect on OS with an HR of 2.28 (95% CI: 1.59–3.27, p < 0.001, n = 1070 cases; Figure 3). Additionally, three studies did not report information about the use of adjuvant therapy, so we recalculated the OS results of the remaining seven studies using adjuvant therapy and found that ALDH1 positivity was still correlated with a worse OS (HR = 1.84, 95% CI = 1.29–2.62, p = 0.001; Figure 3).
Figure 2.
Forest plot of the association between ALDH1 positivity and DFS, RFS, MFS, PFS, and CSS.
ALDH1, aldehyde dehydrogenase 1; CSS, cancer-specific survival; DFS, disease-free survival; MFS, metastasis-free survival; PFS, progression-free survival; RFS, relapse/recurrence-free survival.
Figure 3.
Forest plot of the association between ALDH1 positivity and OS.
ALDH1, aldehyde dehydrogenase 1; OS, overall survival.
Subgroup and sensitivity analyses of ALDH1 positivity in OS
The results of subgroup analyses by the possible sources of heterogeneity for the OS results are listed in Table 2. Most subgroups were not significantly changed by the study factors for heterogeneity. The testing method and treatment regimens could reduce the level of statistical heterogeneity (testing method: blind, I2 = 72.2% and p = 0.013; NA, I2 = 30.3% and p = 0.208; treatment regimens: surgery with adjuvant therapy, I2 = 32.9% and p = 0.202; others, I2 = 68.0% and p = 0.014). In addition, the strength of the association among different subgroups was evaluated and there was a significant association for patients with head and neck carcinoma (n = 122 case, HR = 3.99, p < 0.001), cervical cancer (n = 52 case, HR = 3.51, p = 0.034), and ovarian carcinoma (n = 90 case, HR = 2.51, p = 0.003). Data from four studies with 707 cases showed a trend towards poor OS in breast cancer patients (HR = 1.67, 95% CI = 0.97–2.87, p = 0.062), but no association was found for colorectal cancer (two studies with 99 cases: HR = 2.30, 95% CI = 0.79–6.70, p = 0.129). Subgroup analysis by study source showed that ALDH1 positivity was linked to a shorter OS in Japanese (three studies with 517 cases: HR = 1.94, 95% CI = 1.28–2.92, p = 0.002) and Europeans (four studies with 262 cases: HR = 4.15, 95% CI = 2.46–7.03, p < 0.001), but not in Chinese (two studies with 213 cases: HR = 1.61, 95% CI = 0.40–6.53, p = 0.506). Stratified analysis based on survival rate indicated that ALDH1 positivity was correlated with a poor prognosis in terms of ⩾5-year OS (eight studies with 971 cases: HR = 2.33, 95% CI = 1.54–3.53, p < 0.001), and further pooled HR from two studies with 427 cases resulted in a decreased OS at 10 years (HR = 1.76, 95% CI = 1.03–3.00, p = 0.038),43,54 but no association at <5-year OS (two studies with 99 cases: HR = 2.30, 95% CI = 0.79–6.70, p = 0.129). Significant associations were noted between other subgroup analyses (testing method, study center design, and treatment regimens).
Table 2.
Summary of ALDH1 by subgroup analyses in OS.
| Factors | Subgroups | Studies | HR with 95% CI | p value | Heterogeneity (I2; p) | Cases | TSA |
|---|---|---|---|---|---|---|---|
| Tumor type | Breast cancer | 4 | 1.67 (0.97–2.87) | 0.062 | 53.1%; 0.094 | 707 | More |
| Colorectal cancer | 2 | 2.30 (0.79–6.70) | 0.129 | 62.0%; 0.105 | 99 | More | |
| Head and neck carcinoma | 2 | 3.99 (2.08–7.64) | < 0.001 | 0.0%; 0.325 | 122 | More | |
| Cervical cancer | 1 | 3.51 (1.11–11.25) | 0.034 | NA | 52 | NA | |
| Ovarian carcinoma | 1 | 2.51 (1.36–4.56) | 0.003 | NA | 90 | NA | |
| Study source | Japanese | 3 | 1.94 (1.28–2.92) | 0.002 | 39.7%; 0.190 | 517 | More |
| Chinese | 2 | 1.61 (0.40–6.53) | 0.506 | 75.0%; 0.046 | 213 | More | |
| European | 4 | 4.15 (2.46–7.03) | < 0.001 | 0.0%; 0.798 | 262 | More | |
| Mixed population | 1 | 1.45 (0.72–2.92) | 0.298 | NA | 78 | NA | |
| Survival rate | ⩾5 years | 8 | 2.33 (1.54–3.53) | < 0.001 | 55.9%; 0.026 | 971 | No need |
| <5 years | 2 | 2.30 (0.79–6.70) | 0.129 | 62.0%; 0.105 | 99 | More | |
| Age (years) | ⩽60 | 3 | 1.47 (0.73–2.98) | 0.286 | 50.3%; 0.134 | 291 | More |
| NA | 7 | 2.71 (1.80–4.09) | < 0.001 | 48.8%; 0.068 | 779 | No need | |
| Testing method | Blind | 4 | 2.74 (1.07–7.01) | 0.036 | 72.2%; 0.013 | 402 | More |
| NA | 6 | 2.05 (1.46–2.88) | < 0.001 | 30.3%; 0.208 | 668 | More | |
| Study center design | Multicenter | 6 | 2.34 (1.51–3.62) | < 0.001 | 53.5%; 0.056 | 727 | More |
| Single-center | 4 | 2.24 (1.05–4.77) | 0.036 | 61.2%; 0.052 | 343 | More | |
| Treatment regimens | Surgery and adjuvant therapy | 5 | 2.18 (1.48–3.23) | < 0.001 | 32.9%; 0.202 | 688 | More |
| Others | 5 | 2.35 (1.17–4.72) | 0.017 | 68.0%; 0.014 | 382 | More |
ALDH1, aldehyde dehydrogenase 1; CI, confidence interval; HR, hazard ratio; NA, not applicable; OS, overall survival; TSA, trial sequential analysis.
Sensitivity analysis was conducted based on the omission of one study at a time, and when the studies of Dong and colleagues26 or Miyoshi and colleagues43 were removed, heterogeneity was lacking (I2 = 0.0% and p = 0.442), but the HR was not significantly changed (HR = 2.75, 95% CI = 2.03–3.73, p < 0.001).
Association between Nestin positivity and the prognosis
Data from four studies with 460 cases demonstrated that Nestin-positive expression was associated with significantly poorer OS (HR = 2.39, 95% CI = 1.86–3.05, p < 0.001, I2 = 0.0%; Figure 4) and Nestin-positive expression was also significantly linked to a worse PFS (three studies with 338 cases: HR = 2.08, 95% CI = 1.58–2.73, p < 0.001, I2 = 0.0%; Figure 4).
Figure 4.
Forest plot for the association between Nestin positivity and OS and PFS.
OS, overall survival; PFS, progression-free survival.
Publication bias
The funnel plots showed that a slight publication bias existed regarding OS and ALDH1 (Egger’s test: p = 0.037 < 0.05), but no publication bias existed regarding OS (Begg’s test: p = 0.074; Figure S1).
TSA
The relative risk reduction of 20%, type I error α (5%), and type II error β (20%; power of 80%) were used. The TSA showed that the cumulative Z-curve crossed the trial sequential monitoring boundary regarding OS of ALDH1 and Nestin positivity (Figures S2 and 3) and the subgroup of ALDH1 at ⩾5-year OS (Table 2), suggesting that further studies are unlikely to change these results. However, for most subgroups of ALDH1 (Table 2), the results of Nestin positivity for PFS (Figure S4), and the results of ALDH1 for DFS, RFS, MFS, or PFS (Table S4), showed that the cumulative Z-curve did not cross the trial sequential monitoring boundary, suggesting that additional studies are needed. For the above results from the sensitivity analysis, further TSA still showed that the cumulative Z-curve was more than the trial sequential monitoring boundary (Figure S5), further suggesting that additional studies are not needed.
Discussion
Epithelial tumors are generally heterogeneous cell populations with highly variable abilities of survival, growth, and metastasis.57 Emerging evidence suggests that the top of the cellular hierarchy is a CSC population that can self-renew, proliferate and differentiate into progeny cells, thus causing the cellular and functional heterogeneity within epithelial tumors.58,59 CSCs have been reported in many tumors, such as breast, lung, melanoma, and colorectal cancers.60 Wu and colleagues reported that Nestin-positive expression was correlated with worse survival in glioma.24 Shan and colleagues found that weak Nestin expression was associated with favorable survival in ampullary adenocarcinoma.61 Some studies reported that ALDH1-positive expression was linked to decreased survival outcomes in lung, bladder, and breast cancers,62–64 while other studies reported that loss of ALDH1 was correlated with shortened survival in pancreatic and lung cancers.65,66 However, the prognostic effect of ALDH1 and Nestin in patients with advanced or metastatic cancer remains unclear and the present meta-analysis was conducted to better clarify the characteristics of CSCs expressing ALDH1 and Nestin. This information added to the current prognostic models could guide the development of new therapeutic strategies for advanced or metastatic cancer by identifying them as effective therapeutic and prognostic targets in clinical practice.
Conventional chemotherapy and radiation therapy are two common useful strategies to eliminate cancer cells and shrink tumors. However, chemotherapy/radiotherapy resistance and disease recurrence are major challenges for the long-term survival of cancer patients.67–69 Thus, understanding the mechanisms of chemoresistance and radioresistance are important for improving chemotherapy and radiation therapy. The reasons for cancer drug resistance are very complex and affected by many factors, which also affect therapy efficacy.70–72 Importantly, recent studies have suggested that CSCs are resistant to traditional chemotherapy and radiotherapy.73,74 Some mechanisms, such as important signaling pathways (e.g. PI3K/Akt/mammalian target of rapamycin, vascular endothelial growth factor, Notch, and Wnt/β-catenin pathways) are closely correlated with CSCs.75 Therefore, targeting CSCs may be a promising approach to curing cancer patients.76 Based on the recommended adjustment of survival analysis for known prognostic factors, a comprehensive analysis of published studies (ALDH1: 15 studies with 1557 cases and Nestin: 5 studies with 493 patients with advanced cancer who received adjuvant chemotherapy/radiotherapy) was performed. We found that ALDH1 positivity was significantly associated with a shorter OS (HR = 2.28, p < 0.001) but was not linked to DFS, RFS, MFS, or PFS. Additionally, evidence from some previously published studies is consistent with our analyses, where ALDH1 positivity was found to be correlated with worse OS in advanced cancers.27,46,48,50,51,54 A possible explanation for the results for the other outcomes might be different definitions of the various non-OS outcome measures.77,78 OS is almost always defined as the time from study enrollment to the date of death from any cause or the last follow up. OS is the most common standard endpoint because it is easy to measure and interpret and it is completely objective and unbiased.79 We also found that the sample sizes in our analyses regarding the other outcome measurements (DFS, RFS, MFS, and PFS) were small (<350 patients per outcome measurement), which was confirmed by TSA. Further analysis of only the seven studies with 927 advanced cancer patients treated with adjuvant therapy also showed a significant association between ALDH1 positivity and poor OS (HR = 1.84, p = 0.001). Nestin-positive expression was also correlated with a significantly shorter OS (HR = 2.39, p < 0.001) and PFS (HR = 2.08, p < 0.001). These findings were verified by TSA and the results suggested that additional clinical studies are not needed to confirm these conclusions regarding OS of ALDH1 and Nestin. Our analyses suggest that ALDH1 and Nestin may be effective therapeutic targets to eliminate the subpopulation of CSCs and this may improve the treatment outcome of patients with advanced cancer.
Stratified analysis by cancer type suggested that ALDH1 positivity may be correlated with inferior OS in breast (HR = 1.67, p = 0.062), head and neck (HR = 3.99, p < 0.001), cervical (HR = 3.51, p = 0.034), and ovarian carcinomas (HR = 2.51, p = 0.003) but was not associated with OS in colorectal cancer (p = 0.129), suggesting that ALDH1 may play different roles in different cancer types and could become a potential target for the treatment of advanced breast, head and neck, cervical, and ovarian cancers. Moreover, evidence from some of the previously published studies on these specific tumor types is consistent with ours, such as colorectal cancer,41 breast cancer,27,54 and head and neck carcinoma.51 Stratified analysis by study source indicated that ALDH1 positivity was associated with a shorter OS for Japanese (HR = 1.94, p = 0.002) and Europeans (HR = 4.15, p < 0.001), but there was no association for Chinese (p = 0.506), which suggested that ALDH1 might only play a role in Japanese and European patients with advanced cancer and this may have potential implications to help stratify patients for treatment and prognostication. It is also possible that early detection, treatment schedules, and cancer-related lifestyles might to some extent differ in China compared with the rest of the world,80,81 which might have contributed to the lack of an association of the ALDH1 marker with the OS of Chinese patients. Stratified analysis by survival rate demonstrated that ALDH1 positivity was linked to an unfavorable OS at ⩾5 years (HR = 2.33, p < 0.001), and further analysis showed a reduced long-term OS rate at 10 years (HR = 1.76, p = 0.038). However, the eligible studies did not report long-term follow-up results, such as 10 years in terms of Nestin. According to the present analyses, ALDH1 might be a more important and meaningful marker than Nestin in advanced cancer. The above results of the subgroups were further confirmed by TSA, which suggested that more clinical studies were necessary to confirm these conclusions except for the subgroup of ⩾5-year OS.
Some limitations should be acknowledged. First, although we systematically searched the relevant databases as completely as possible, a slight publication bias was noted for the OS of ALDH1 data. Potential explanations for this are: (1) only final eligible studies published in English were selected. Studies of other types, such as unpublished papers or conference abstracts, were excluded because of incomplete information; (2) papers with positive results are more easily published than papers with negative results found in survival analyses, which are therefore lacking. Second, although the results were confirmed by TSA, and TSA suggested that only the results for OS were firm, most of these studies were retrospective in design. Third, the testing method and treatment regimens may impact the current findings of the subgroup analyses in this meta-analysis. Cancer is a heterogeneous disease and different cancer types may impact overall effect estimates; however, we did not find this factor to be a potential source of heterogeneity, possibly because the number of the eligible cancer types was relatively small. Fourth, we found that most eligible studies did not report that the assessors of the Nestin and ALDH1 tests were blinded to clinical findings. Finally, the REMARK guidelines only evaluate the reporting aspect of each study and they are not a tool for study quality assessment, because reporting quality and study quality are not necessarily interchangeable. For example, a prospective study design is more valuable and more easily achieved in a clinic in the future than any retrospective study design.
To conclude, our meta-analysis and TSA indicated that ALDH1 and Nestin were associated with shorter OS, and these two results were reliable. Nestin positivity may be correlated with a worse PFS, but ALDH1 positivity was not linked to DFS, RFS, MFS, or PFS. Subgroup analysis by cancer type showed that ALDH1 positivity may be correlated with poor OS in breast, head and neck, cervical, and ovarian cancers, but there was no association in colorectal cancer. Subgroup analysis by study source showed that ALDH1 positivity was linked to a worse OS for Japanese and European patients, but there was no correlation for Chinese patients. Subgroup analysis by survival rate demonstrated that ALDH1 positivity was associated with an unfavorable OS at 10 years. ALDH1 may be valuable as a potential effective therapeutic target for the improvement of treatment of advanced cancer in clinical practice. Additional high-quality, prospective, randomized clinical trials are needed to provide more conclusive evidence in different types of advanced cancer.
Supplemental Material
Supplemental material, Supplementary_file_(1) for Prognostic value of ALDH1 and Nestin in advanced cancer: a systematic meta-analysis with trial sequential analysis by Susu Han, Tao Huang, Xing Wu, Xiyu Wang, Wen Li, Shanshan Liu, Wei Yang, Qi Shi, Hongjia Li, Kunhe Shi and Fenggang Hou in Therapeutic Advances in Medical Oncology
Footnotes
Author Contributions: Susu Han, Tao Huang, Fenggang Hou, and Kunhe Shi contributed to the conception and design of this research. Susu Han, Wen Li, Shanshan Liu, Wei Yang, Qi Shi, Hongjia Li, Xing Wu, and Xiyu Wang contributed to the drafting of the article and final approval of the submitted version. Susu Han, Tao Huang, Xing Wu, Xiyu Wang, Wen Li, Shanshan Liu, Wei Yang, Qi Shi, Hongjia Li, Kunhe Shi, and Fenggang Hou contributed to data analyses and interpretation and completion of the figures and tables. All authors read and approved the final manuscript.
Funding: This research was supported by grants from Shanghai Science and Technology Innovation Action Plan Project (NO. 16401970500-3). The sponsor had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Supplemental material: Supplemental material for this article is available online.
ORCID iDs: Susu Han  https://orcid.org/0000-0002-3999-0078
https://orcid.org/0000-0002-3999-0078
Tao Huang  https://orcid.org/0000-0002-9198-2868
https://orcid.org/0000-0002-9198-2868
Contributor Information
Susu Han, Shanghai Municipal Hospital of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, 274 Zhijiang Road, Shanghai 200071, People’s Republic of China anyasue@163.com.
Tao Huang , The Affiliated Hospital of Ningbo University, Ningbo, Zhejiang, 315020, People’s Republic of China.
Xing Wu, Shanghai Municipal Hospital of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, People’s Republic of China.
Xiyu Wang, Shanghai Municipal Hospital of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, People’s Republic of China.
Wen Li, Shanghai Municipal Hospital of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, People’s Republic of China.
Shanshan Liu, Shanghai Municipal Hospital of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, People’s Republic of China.
Wei Yang, Shanghai Municipal Hospital of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, People’s Republic of China.
Qi Shi, Shanghai Municipal Hospital of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, People’s Republic of China.
Hongjia Li, Shanghai Municipal Hospital of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, People’s Republic of China.
Kunhe Shi, Shanghai Municipal Hospital of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, People’s Republic of China.
Fenggang Hou, Shanghai Municipal Hospital of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, 274 Zhijiang Road, Shanghai 200071, People’s Republic of China fghou555@126.com.
References
- 1. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 2. McQuade JL, Daniel CR, Hess KR, et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol 2018; 19: 310–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017; 67: 7–30. [DOI] [PubMed] [Google Scholar]
- 4. Farkona S, Diamandis EP, Blasutig IM. Cancer immunotherapy: the beginning of the end of cancer? BMC Med 2016; 14: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cousins SE, Tempest E, Feuer DJ. Surgery for the resolution of symptoms in malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. Cochrane Database Syst Rev 2016; 1: CD002764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liau BB, Sievers C, Donohue LK, et al. Adaptive chromatin remodeling drives glioblastoma stem cell plasticity and drug tolerance. Cell Stem Cell 2017; 20: 233–246.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. Identification and expansion of human colon-cancer-initiating cells. Nature 2007; 445: 111–115. [DOI] [PubMed] [Google Scholar]
- 8. Han S, Yang W, Zong S, et al. Clinicopathological, prognostic and predictive value of CD166 expression in colorectal cancer: a meta-analysis. Oncotarget 2017; 8: 64373–64384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Han S, Zong S, Shi Q, et al. Is Ep-CAM expression a diagnostic and prognostic biomarker for colorectal cancer? A systematic meta-analysis. EBioMedicine 2017; 20: 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hurt EM, Kawasaki BT, Klarmann GJ, et al. CD44+ CD24(-) prostate cells are early cancer progenitor/stem cells that provide a model for patients with poor prognosis. Br J Cancer 2008; 98: 756–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell 1990; 60: 585–595. [DOI] [PubMed] [Google Scholar]
- 12. Krupkova O, Jr, Loja T, Zambo I, et al. Nestin expression in human tumors and tumor cell lines. Neoplasma 2010; 57: 291–298. [DOI] [PubMed] [Google Scholar]
- 13. Neradil J, Veselska R. Nestin as a marker of cancer stem cells. Cancer Sci 2015; 106: 803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tampaki EC, Nakopoulou L, Tampakis A, et al. Nestin involvement in tissue injury and cancer–a potential tumor marker? Cell Oncol (Dordr) 2014; 37: 305–315. [DOI] [PubMed] [Google Scholar]
- 15. Narita K, Matsuda Y, Seike M, et al. Nestin regulates proliferation, migration, invasion and stemness of lung adenocarcinoma. Int J Oncol 2014; 44: 1118–1130. [DOI] [PubMed] [Google Scholar]
- 16. Michalczyk K, Ziman M. Nestin structure and predicted function in cellular cytoskeletal organisation. Histol Histopathol 2005; 20: 665–671. [DOI] [PubMed] [Google Scholar]
- 17. Chute JP, Muramoto GG, Whitesides J, et al. Inhibition of aldehyde dehydrogenase and retinoid signaling induces the expansion of human hematopoietic stem cells. Proc Natl Acad Sci U S A 2006; 103: 11707–11712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Singh S, Arcaroli J, Thompson DC, et al. Acetaldehyde and retinaldehyde-metabolizing enzymes in colon and pancreatic cancers. Adv Exp Med Biol 2015; 815: 281–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang EH, Hynes MJ, Zhang T, et al. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res 2009; 69: 3382–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Charafe-Jauffret E, Ginestier C, Bertucci F, et al. ALDH1-positive cancer stem cells predict engraftment of primary breast tumors and are governed by a common stem cell program. Cancer Res 2013; 73: 7290–7300. [DOI] [PubMed] [Google Scholar]
- 21. Ueda K, Ogasawara S, Akiba J, et al. Aldehyde dehydrogenase 1 identifies cells with cancer stem cell-like properties in a human renal cell carcinoma cell line. PLoS One 2013; 8: e75463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dong Y, Ochsenreither S, Cai C, et al. Aldehyde dehydrogenase 1 isoenzyme expression as a marker of cancer stem cells correlates to histopathological features in head and neck cancer: a meta-analysis. PLoS One 2017; 12: e0187615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lv D, Lu L, Hu Z, et al. Nestin expression is associated with poor clinicopathological features and prognosis in glioma patients: an association study and meta-analysis. Mol Neurobiol 2017; 54: 727–735. [DOI] [PubMed] [Google Scholar]
- 24. Wu B, Sun C, Feng F, et al. Do relevant markers of cancer stem cells CD133 and Nestin indicate a poor prognosis in glioma patients? A systematic review and meta-analysis. J Exp Clin Cancer Res 2015; 34: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu Y, Lv DL, Duan JJ, et al. ALDH1A1 expression correlates with clinicopathologic features and poor prognosis of breast cancer patients: a systematic review and meta-analysis. BMC Cancer 2014; 14: 444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dong Y, Bi LR, Xu N, et al. The expression of aldehyde dehydrogenase 1 in invasive primary breast tumors and axillary lymph node metastases is associated with poor clinical prognosis. Pathol Res Pract 2013; 209: 555–561. [DOI] [PubMed] [Google Scholar]
- 27. Alamgeer M, Ganju V, Kumar B, et al. Changes in aldehyde dehydrogenase-1 expression during neoadjuvant chemotherapy predict outcome in locally advanced breast cancer. Breast Cancer Res 2014; 16: R44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6: e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Altman DG, Bland JM. How to obtain the confidence interval from a P value. BMJ 2011; 343: d2090. [DOI] [PubMed] [Google Scholar]
- 30. Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007; 8: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McShane LM, Altman DG, Sauerbrei W, et al. Reporting recommendations for tumor marker prognostic studies (REMARK). J Natl Cancer Inst 2005; 97: 1180–1184. [DOI] [PubMed] [Google Scholar]
- 32. Zintzaras E, Ioannidis JP. HEGESMA: genome search meta-analysis and heterogeneity testing. Bioinformatics 2005; 21: 3672–3673. [DOI] [PubMed] [Google Scholar]
- 33. Evangelou E, Ioannidis JP. Meta-analysis methods for genome-wide association studies and beyond. Nat Rev Genet 2013; 14: 379–389. [DOI] [PubMed] [Google Scholar]
- 34. DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials 2007; 28: 105–114. [DOI] [PubMed] [Google Scholar]
- 35. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088–1101. [PubMed] [Google Scholar]
- 37. Miladinovic B, Mhaskar R, Hozo I, et al. Optimal information size in trial sequential analysis of time-to-event outcomes reveals potentially inconclusive results because of the risk of random error. J Clin Epidemiol 2013; 66: 654–659. [DOI] [PubMed] [Google Scholar]
- 38. Thorlund K, Devereaux PJ, Wetterslev J, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta-analyses? Int J Epidemiol 2009; 38: 276–286. [DOI] [PubMed] [Google Scholar]
- 39. Brok J, Thorlund K, Gluud C, et al. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. J Clin Epidemiol 2008; 61: 763–769. [DOI] [PubMed] [Google Scholar]
- 40. Onisim A, Iancu M, Vlad C, et al. Expression of Nestin and CD133 in serous ovarian carcinoma. J BUON 2016; 21: 1168–1175. [PubMed] [Google Scholar]
- 41. Ning Y, Zhang W, Hanna DL, et al. Clinical relevance of EMT and stem-like gene expression in circulating tumor cells of metastatic colorectal cancer patients. Pharmacogenomics J 2018; 18: 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang Y, Zeng S, Ma J, et al. Nestin overexpression in hepatocellular carcinoma associates with epithelial-mesenchymal transition and chemoresistance. J Exp Clin Cancer Res 2016; 35: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Miyoshi Y, Shien T, Ogiya A, et al. Differences in expression of the cancer stem cell marker aldehyde dehydrogenase 1 among estrogen receptor-positive/human epidermal growth factor receptor type 2-negative breast cancer cases with early, late, and no recurrence. Breast Cancer Res 2016; 18: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kuk SK, Won CH, Lee WJ, et al. Prognostic significance of nestin in primary malignant melanoma of the oral cavity. Melanoma Res 2016; 26: 457–463. [DOI] [PubMed] [Google Scholar]
- 45. Adamczyk A, Niemiec J, Ambicka A, et al. Survival of breast cancer patients according to changes in expression of selected markers between primary tumor and lymph node metastases. Biomark Med 2016; 10: 219–228. [DOI] [PubMed] [Google Scholar]
- 46. Ishiguro T, Sato A, Ohata H, et al. Establishment and characterization of an in vitro model of ovarian cancer stem-like cells with an enhanced proliferative capacity. Cancer Res 2016; 76: 150–160. [DOI] [PubMed] [Google Scholar]
- 47. Bednarz-Knoll N, Nastaly P, Zaczek A, et al. Stromal expression of ALDH1 in human breast carcinomas indicates reduced tumor progression. Oncotarget 2015; 6: 26789–26803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xie Q, Liang J, Rao Q, et al. Aldehyde dehydrogenase 1 expression predicts chemoresistance and poor clinical outcomes in patients with locally advanced cervical cancer treated with neoadjuvant chemotherapy prior to radical hysterectomy. Ann Surg Oncol 2016; 23: 163–170. [DOI] [PubMed] [Google Scholar]
- 49. Ito M, Shien T, Omori M, et al. Evaluation of aldehyde dehydrogenase 1 and transcription factors in both primary breast cancer and axillary lymph node metastases as a prognostic factor. Breast Cancer 2016; 23: 437–444. [DOI] [PubMed] [Google Scholar]
- 50. Fitzgerald TL, Rangan S, Dobbs L, et al. The impact of Aldehyde dehydrogenase 1 expression on prognosis for metastatic colon cancer. J Surg Res 2014; 192: 82–89. [DOI] [PubMed] [Google Scholar]
- 51. Qian X, Wagner S, Ma C, et al. Prognostic significance of ALDH1A1-positive cancer stem cells in patients with locally advanced, metastasized head and neck squamous cell carcinoma. J Cancer Res Clin Oncol 2014; 140: 1151–1158. [DOI] [PubMed] [Google Scholar]
- 52. Koukourakis MI, Giatromanolaki A, Tsakmaki V, et al. Cancer stem cell phenotype relates to radio-chemotherapy outcome in locally advanced squamous cell head-neck cancer. Br J Cancer 2012; 106: 846–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Qin Q, Sun Y, Fei M, et al. Expression of putative stem marker nestin and CD133 in advanced serous ovarian cancer. Neoplasma 2012; 59: 310–315. [DOI] [PubMed] [Google Scholar]
- 54. Yoshioka T, Umekita Y, Ohi Y, et al. Aldehyde dehydrogenase 1 expression is a predictor of poor prognosis in node-positive breast cancers: a long-term follow-up study. Histopathology 2011; 58: 608–616. [DOI] [PubMed] [Google Scholar]
- 55. Fusi A, Reichelt U, Busse A, et al. Expression of the stem cell markers nestin and CD133 on circulating melanoma cells. J Invest Dermatol 2011; 131: 487–494. [DOI] [PubMed] [Google Scholar]
- 56. Charafe-Jauffret E, Ginestier C, Iovino F, et al. Aldehyde dehydrogenase 1-positive cancer stem cells mediate metastasis and poor clinical outcome in inflammatory breast cancer. Clin Cancer Res 2010; 16: 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Caswell DR, Swanton C. The role of tumour heterogeneity and clonal cooperativity in metastasis, immune evasion and clinical outcome. BMC Med 2017; 15: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Talmadge JE, Fidler IJ. AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res 2010; 70: 5649–5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Polyak K, Hahn WC. Roots and stems: stem cells in cancer. Nat Med 2006; 12: 296–300. [DOI] [PubMed] [Google Scholar]
- 60. Dawood S, Austin L, Cristofanilli M. Cancer stem cells: implications for cancer therapy. Oncology (Williston Park) 2014; 28: 1101–1107, 10. [PubMed] [Google Scholar]
- 61. Shan YS, Chen YL, Lai MD, et al. Nestin predicts a favorable prognosis in early ampullary adenocarcinoma and functions as a promoter of metastasis in advanced cancer. Oncol Rep 2015; 33: 40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kida K, Ishikawa T, Yamada A, et al. Effect of ALDH1 on prognosis and chemoresistance by breast cancer subtype. Breast Cancer Res Treat 2016; 156: 261–269. [DOI] [PubMed] [Google Scholar]
- 63. Xu N, Shao MM, Zhang HT, et al. Aldehyde dehydrogenase 1 (ALDH1) expression is associated with a poor prognosis of bladder cancer. Cancer Epidemiol 2015; 39: 375–381. [DOI] [PubMed] [Google Scholar]
- 64. Sullivan JP, Spinola M, Dodge M, et al. Aldehyde dehydrogenase activity selects for lung adenocarcinoma stem cells dependent on notch signaling. Cancer Res 2010; 70: 9937–9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dimou A, Neumeister V, Agarwal S, et al. Measurement of aldehyde dehydrogenase 1 expression defines a group with better prognosis in patients with non-small cell lung cancer. Am J Pathol 2012; 181: 1436–1442. [DOI] [PubMed] [Google Scholar]
- 66. Kahlert C, Bergmann F, Beck J, et al. Low expression of aldehyde dehydrogenase 1A1 (ALDH1A1) is a prognostic marker for poor survival in pancreatic cancer. BMC Cancer 2011; 11: 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nurgali K, Jagoe RT, Abalo R. Editorial: adverse effects of cancer chemotherapy: anything new to improve tolerance and reduce sequelae? Front Pharmacol 2018; 9: 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Viale PH, Grande C, Moore S. Efficacy and cost: avoiding undertreatment of chemotherapy-induced nausea and vomiting. Clin J Oncol Nurs 2012; 16: E133– E141. [DOI] [PubMed] [Google Scholar]
- 69. Delaney G, Jacob S, Featherstone C, et al. The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer 2005; 104: 1129–1137. [DOI] [PubMed] [Google Scholar]
- 70. Willers H, Azzoli CG, Santivasi WL, et al. Basic mechanisms of therapeutic resistance to radiation and chemotherapy in lung cancer. Cancer J 2013; 19: 200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Frosina G. DNA repair and resistance of gliomas to chemotherapy and radiotherapy. Mol Cancer Res 2009; 7: 989–999. [DOI] [PubMed] [Google Scholar]
- 72. Luqmani YA. Mechanisms of drug resistance in cancer chemotherapy. Med Princ Pract 2005; 14(Suppl. 1): 35–48. [DOI] [PubMed] [Google Scholar]
- 73. Kharkar PS. Cancer stem cell (CSC) inhibitors: a review of recent patents (2012–2015). Expert Opin Ther Pat 2017; 27: 753–761. [DOI] [PubMed] [Google Scholar]
- 74. Morrison R, Schleicher SM, Sun Y, et al. Targeting the mechanisms of resistance to chemotherapy and radiotherapy with the cancer stem cell hypothesis. J Oncol 2011; 2011: 941876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chang L, Graham P, Hao J, et al. Cancer stem cells and signaling pathways in radioresistance. Oncotarget 2016; 7: 11002–11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hu Y, Fu L. Targeting cancer stem cells: a new therapy to cure cancer patients. Am J Cancer Res 2012; 2: 340–356. [PMC free article] [PubMed] [Google Scholar]
- 77. Bellera CA, Penel N, Ouali M, et al. Guidelines for time-to-event end point definitions in sarcomas and gastrointestinal stromal tumors (GIST) trials: results of the DATECAN initiative (Definition for the Assessment of Time-to-event Endpoints in CANcer trials). Ann Oncol 2015; 26: 865–872. [DOI] [PubMed] [Google Scholar]
- 78. Punt CJ, Buyse M, Kohne CH, et al. Endpoints in adjuvant treatment trials: a systematic review of the literature in colon cancer and proposed definitions for future trials. J Natl Cancer Inst 2007; 99: 998–1003. [DOI] [PubMed] [Google Scholar]
- 79. Mauguen A, Pignon JP, Burdett S, et al. Surrogate endpoints for overall survival in chemotherapy and radiotherapy trials in operable and locally advanced lung cancer: a re-analysis of meta-analyses of individual patients’ data. Lancet Oncol 2013; 14: 619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zeng H, Chen W, Zheng R, et al. Changing cancer survival in China during 2003–15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health 2018; 6: e555–e567. [DOI] [PubMed] [Google Scholar]
- 81. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66: 115–132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_file_(1) for Prognostic value of ALDH1 and Nestin in advanced cancer: a systematic meta-analysis with trial sequential analysis by Susu Han, Tao Huang, Xing Wu, Xiyu Wang, Wen Li, Shanshan Liu, Wei Yang, Qi Shi, Hongjia Li, Kunhe Shi and Fenggang Hou in Therapeutic Advances in Medical Oncology