Abstract
Background
Infantile colic is typically defined as full‐force crying for at least three hours per day, on at least three days per week, for at least three weeks. Colic appears to be more frequent in the first six weeks of life (prevalence range of 17% to 25%), depending on the specific location reported and definitions used, and usually resolves by three months. The aetiopathogenesis of infantile colic is unclear but most likely multifactorial. A number of psychological, behavioural and biological components (food hypersensitivity, allergy or both; gut microflora and dysmotility) are thought to contribute to it. The role of diet as a component in infantile colic remains controversial.
Objectives
To assess the effects of dietary modifications for reducing colic in infants less than four months of age.
Search methods
In July 2018 we searched CENTRAL, MEDLINE, Embase, 17 other databases and 2 trials registers. We also searched Google, checked references and contacted study authors.
Selection criteria
Randomised controlled trials (RCTs) and quasi‐RCTs evaluating the effects of dietary modifications, alone or in combination, for colicky infants younger than four months of age versus another intervention or placebo. We used specific definitions for colic, age of onset and the methods for performing the intervention. We defined 'modified diet' as any diet altered to include or exclude certain components.
Data collection and analysis
We used standard Cochrane methodological procedures. Our primary outcome was duration of crying, and secondary outcomes were response to intervention, frequency of crying episodes, parental/family quality of life, infant sleep duration, parental satisfaction and adverse effects.
Main results
We included 15 RCTs involving 1121 infants aged 2 to 16 weeks. All studies were small and at high risk of bias across multiple design factors (e.g. selection, attrition). The studies covered a wide range of dietary interventions, and there was no scope for meta‐analysis. Using GRADE, we assessed the quality of the evidence as very low.
No study reported on parental or family quality of life, infant sleep duration per 24 h, or parental satisfaction.
Low‐allergen maternal diet versus a diet containing potential allergens: one study (90 infants) found that 35/47 (74%) of infants responded (reduction in cry/fuss duration of 25%) to a low‐allergen maternal diet, compared with 16/43 (37%) of infants with a maternal diet containing potential allergens (37% difference; 95% confidence interval (CI) 18 to 56; P < 0.001).
Low‐allergen diet or soy milk formula versus standard diet or cow's milk formula and dicyclomine hydrochloride: one study (120 infants) found that 10/15 (66.6%) breastfed babies responded to dicyclomine hydrochloride and a normal diet, compared with 10/16 (62.5%) on a low‐allergen diet, while 24/45 (53.3%) standard formula‐fed babies taking dicyclomine hydrochloride improved compared with 29/44 (65.9%) on soy milk formula. Response was defined as a reduction of crying to less than one hour per day after 48 hours of treatment, with remission persisting for one month.
Hydrolysed formula versus standard formula: one study (43 infants) reported that the number of infants who responded to the intervention (cried for less than 3 hours per day on at least 3 days a week) was 8/23 in the whey hydrolysate group versus 5/20 in the standard formula group (χ2 using yate's correction = 0.20, P = 0.65).
The same study (43 infants) reported a greater reduction in crying time postintervention with hydrolysed formula (104 min/d, 95% CI 55 to 155) than with standard formula (3 min/d, 95% CI −63 to 67); difference = 101 min/d, 95% CI 25 to 179; P = 0.02).
The author confirmed there were no adverse effects.
Hydrolysed formula or dairy‐ and soy‐free maternal diet versus standard diet/formula and parental education or counselling: one study (21 infants) found that crying time decreased to 2.03 h/d (SD 1.03) in the hydrolysed or dairy‐ and soy‐free maternal diet group compared with 1.08 h/d (SD 0.7) in the parent education or counselling group, nine days postintervention.
Partially hydrolysed, lower lactose, whey‐based formulae containing oligosaccharide versus standard formula with simethicone: one study (267 infants) found both groups experienced decreased colic episodes after seven days (partially hydrolysed formula: from 5.99 episodes (SD 1.84) to 2.47 episodes (SD 1.94); standard formula: from 5.41 episodes (SD 1.88) to 3.72 episodes (SD 1.98)); 95% CI 95% −0.7 to −1.8; P < 0.001). This difference was significant after two weeks (partially hydrolysed: 1.76 episodes (SD 1.60); standard formula: 3.32 episodes (SD 2.06); P < 0.001). The study author confirmed there were no adverse effects.
Lactase enzyme supplementation versus placebo: three studies (138 infants) assessed this comparison, but they are cross‐over trials that did not report data from before washout. There were no adverse effects in any of the studies.
Extract of Foeniculum vulgare, Matricariae recutita, and Melissa officinalis versus placebo: one study (93 infants) found that average daily crying time was lower for infants given the extract (76.9 min/d (SD 23.5), than infants given placebo (169.9 min/d (SD 23.1) at the end of the one‐week study (95% CI −102.89 to −83.11; P < 0.01). There were no adverse effects.
Soy protein‐based formula versus standard cows' milk protein‐based formula: one study (19 infants) reported a mean crying time of 12.7 h/week (SD 16.4) in the soy formula group versus 17.3 h/week (SD 6.9) in the standard cows' milk group, and that 5/10 (50%) responded in the soy formula group versus 0/9 (0%) in the standard cows' milk group.
Soy protein formula with polysaccharide versus standard soy protein formula: one cross‐over study (27 infants) assessed this comparison but did not provide disaggregated data for the pre‐wash‐out data.
Authors' conclusions
Currently, evidence of the effectiveness of dietary modifications for the treatment of infantile colic is sparse and at significant risk of bias. The few available studies had small sample sizes, and most had serious limitations. There were insufficient studies, making the use of meta‐analysis unfeasable. Benefits reported for hydrolysed formulas were inconsistent.
Based on available evidence, we are unable to recommend any intervention. Future studies of single interventions, using clinically significant outcome measures, and appropriate design and power are needed.
Keywords: Female, Humans, Infant, Male, Infant Formula, Allergens, Colic, Colic/diet therapy, Crying, Diet Therapy, Diet Therapy/methods, Lactase, Lactase/administration & dosage, Randomized Controlled Trials as Topic, Soybean Proteins, Soybean Proteins/administration & dosage, Time Factors
Plain language summary
Diet changes for infant colic
Review question
Do colicky infants show an improvement when breastfeeding mothers follow a low‐allergen diet, or when formula‐fed infants are fed a special formula?
Background
Infantile colic is a common problem afflicting otherwise healthy infants in the first three months of life. It is characterised by episodes of inconsolable crying lasting for longer than three hours per day, for more than three days a week, for at least three weeks.
It can be very distressing for parents.
Dietary changes, such as removing cows' milk from a breastfeeding mother's diet or switching formula‐fed babies to a special soy‐based formula, might reduce the symptoms of colic.
Study characteristics
We found 15 randomised controlled trials, a type of study in which participants are randomly assigned to one of two or more treatment groups, involving a total of 1121 babies with colic. The evidence is current to July 2018.
Infants (balanced between boys and girls) were less than three months of age.
Key results
Most studies reported data on a combination of outcomes: duration of crying, number of responders in each group after treatment (i.e. those who experienced a decrease in daily crying), or frequency of crying episodes. We present these findings below. No study reported on parental or family quality of life, infant sleep or parental satisfaction. Six studies reported that there were no side effects as a result of the dietary changes.
Low‐allergen diet
One study (90 infants) found that more breastfed infants responded to a low‐allergen maternal diet than infants on a standard diet containing known potential allergens.
Another study (120 infants) found little difference in breastfed infants whose mothers were given a low‐allergen diet (10/16, 62.5%) and formula‐fed babies who were given soy milk (29/44, 65.9%), but the researchers did find that breastfed babies responded more to dicyclomine hydrochloride (a tablet for treating stomach spasms) than formula‐fed babies.
Hydrolysed formula milk
One study (43 infants) found no clear difference in resolving symptoms of colic between the hydrolysed (hypoallergenic) and standard cow's milk groups. They also reported a greater reduction in crying time at study end in infants who were given hydrolysed, and reported no adverse effects.
A third study (21 infants) reported that infants whose parents were given information and support experienced a more rapid reduction in crying time than infants who were given a hydrolysed formula or dairy‐ and soy‐free diet (within nine days).
A fourth study (267 infants) found that both partially hydrolysed formula with oligosaccharides (carbohydrates) and a standard formula with simethicone (a drug for treating symptoms of gas) reduced colic episodes after seven days, but effects were greater in the hydrolysed plus oligosaccharides group after two weeks. The study author confirmed there were no adverse effects.
Lactase enzyme supplementation
Three studies (138 infants) tested the effect of adding lactase (an enzyme which helps break down the lactose (sugar) in milk) to the infants' milk. There were no adverse effects in any of the studies.
Fennel, chamomile and lemon balm extract
One study (93 infants) found that average daily crying time in breastfed babies reduced within one week of treatment with a fennel, chamomile and lemon balm extract. There were no adverse effects.
Soy protein‐based formula
One study (19 infants) found that, compared with cows' milk formula, soy formula reduced crying time and increased the number of responders. However, international guidance does not support the use of soy milk due to concerns that they can impact hormones in babies, so these results are not relevant.
Quality of the evidence
Many of the studies included only small numbers of participants and were of poor quality. We did not find evidence of effectiveness for most dietary interventions. Where studies did report some benefit, this was not large enough to be meaningful.
Conclusions
Based on the available evidence, we are not able to recommend any of the dietary modifications assessed in this review.
Summary of findings
Summary of findings for the main comparison. Summary of findings: dietary interventions for infantile colic versus placebo or other interventions.
| Dietary interventions for infantile colic versus placebo or other interventions | |||
|
Patient or population: infants with colic defined by recognised criteria Settings: outpatient Intervention: any dietary intervention to treat infantile colic Comparison: placebo or any other intervention | |||
| Outcomes | Impacts | Number of studiesa | Quality of the evidence (GRADE) |
| Duration of crying | This was the most commonly reported outcome, but studies did so in an extremely heterogenous manner due to measurement tools used, as well as time and frequency of determination. There was no clear effect as regards the efficacy of any of the agents under study for reducing the duration of crying in affected infants. One study reported that the number of infants crying for less than 3 hours per day on at least 3 days a week following the intervention was 8 (out of 23) in the whey hydrolysate group versus 5 (out of 20) in the standard formula group (χ2 = 0.20, P = 0.65). Results from 3 individual studies found that a hydrolysed formula, herbal drops and soy protein‐based formula may reduce crying time at study end (continuous outcome). 1 study found no difference between 2 types of hydrolysed formulas. |
6 | ⊕⊝⊝⊝ Very lowb |
| Number of responders in each group after treatment | There were mixed effects as regards the efficacy of the agents under study for improving the number of responders. Results from 2 individual studies showed that a low‐allergen maternal diet and a soy protein‐based formula may increase the number of responders. However, another study found no evidence in favour of a low‐allergen diet or soy‐milk formula but did find that dicyclomine hydrochloride may increase the number of breastfed babies who respond. | 3 | ⊕⊝⊝⊝ Very lowb |
| Frequency of crying episodes per 24 h | Results from 2 individual studies showed that a hydrolysed or dairy‐ and soy‐free formula and a partially hydrolysed formula may reduce the frequency of crying episodes per 24 h. As this is very difficult to discern from normality and is not a key component of infantile colic diagnostic criteria or a necessary a goal of clinicians, the clinical relevance of this outcome is worth readers' consideration. | 2 | ⊕⊝⊝⊝ Very lowb |
| Parental or family quality of life, including measures of parental stress, anxiety or depression | No data | ||
| Infant sleep duration per 24 h at 7, 14 and 21 days from start of intervention | No data | ||
| Parental satisfaction | No data | ||
| Adverse effects to dietary modifications | This is a key outcome, given the population under study, which was poorly reported in many studies. 3 studies reported that there were no adverse effects. 3 authors (one of whom is an author on this review) of 3 other studies confirmed there were no adverse effects. The 9 remaining studies did not report on adverse effects. | 6 | ⊕⊝⊝⊝ Very lowb |
| GRADE Working Group – grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||
aParticipant numbers have not been included in the table as it contains multiple comparisons. bWe downgraded the quality of the evidence for all outcomes, across all studies, due to consistent issues with incomplete outcome data, selective reporting, the presence of extremely small sample sizes, drug and nutrition company involvement, and risk of bias. These issues were pervasive across the evidence base and must be considered when interpreting any of the reported findings.
Background
The most frequently cited definition of infantile colic is the rule of three: unexplained episodes of paroxysmal crying for longer than three hours per day, for three days per week, for at least three weeks (Wessel 1954). More recently, colic has been included under functional gastrointestinal disorders (Rome IV diagnostic criteria), and the definition has been expanded to include paroxysms of irritability and fussiness for at least one week in an infant that has no failure to thrive (Drossman 2016).
This condition appears to be more frequent in the first six weeks of life, occurring in 17% to 25% of newborns, depending on geography and definitions employed, with prevalence often peaking at that point. It is important to note that without any intervention, colic symptoms are usually below the threshold of such diagnostic criteria by three months of age (Reijneveld 2001; Vandenplas 2015; Wolke 2017).
Description of the condition
Paroxysms of inconsolable crying due to colic are often accompanied by flushing of the face, meteorism (excessive flatulence in the intestinal tract with distention of the abdomen), drawing‐up of the legs, and flatulence (Gupta 2007; Savino 2010). Symptoms typically start in the second week of life in both breastfed and formula‐fed infants and usually resolve by three months of age (Lucas 1998; Vandenplas 2017). Generally speaking, these symptoms are not indicative of disease and thus hospital admission for these infants is generally unnecessary, detrimental and not to be encouraged (Savino 2007). However, most understanding of the condition is based on research that includes a cohort of children whose parents have chosen to seek help, so it is worth noting that this may not reflect the whole population, and what separates colic from non‐colic may simply be the parents' decision to self‐seek care (further discussions on this point are outside of the scope of this review). Furthermore, five per cent of colicky crying infants do have a serious underlying medical problem (Freedman 2009; Savino 2005; Savino 2007). Thus, clinicians should assess all colicky infants to rule out underlying medical conditions that require investigation and treatment (Savino 2010).
The aetiopathogenesis of infantile colic remains undefined and is most likely multifactorial. Despite the common nature of the condition, there is a general paucity of evidence investigating this area. Different authors have suggested that a number of behavioural (psychological and social) and biological (food hypersensitivity or allergy (or both) components (Clifford 2002); gut microflora and dysmotility) factors can contribute to its manifestation (Camilleri 2017). These include the following.
First, lactose intolerance – due to a relative lactase deficiency – has been postulated as a possible causative factor in infant colic. Carbohydrate malabsorption leads to colonic fermentation of sugars and an increase in levels of hydrogen gas. The rapid production of hydrogen in the lower bowel distends the colon, sometimes causing pain, whereas the osmotic pressures generated by lactose and lactic acid in the colon cause an influx of water, leading to further distension of the bowel. Although studies evaluating the degree of hydrogen in the breath of colicky infants have produced inconsistent results, some studies have reported increases in breath hydrogen levels (Hyams 1989; Miller 1990; Moore 1988).
Second, the immunological model of colic focuses on possible allergens, such as cows' milk proteins in breast milk or infant formula, as the cause of colic. Intact proteins from the mother's diet are hypothesised to cross over into the breast milk and provoke an allergic response and symptoms of colic in some infants. Consequently, some authors have proposed a low‐allergen maternal diet as a form of treatment (Hill 2005; Schach 2002). Shannon 1921 was the first to raise the possibility that infantile colic could be related to allergens. Since then, a number of studies have evaluated the possible association between colic and food hypersensitivity (Campbell 1989; Estep 2000; Forsyth 1989; Hill 1995; Hill 2005; Iacono 1991; Jakobsson 1983; Lindberg 1999; Lothe 1987; Lucassen 2000). Articles in favour of this hypothesis report that about 25% of infants with moderate or severe symptoms might have cows' milk protein‐dependent colic (Axelsson 1986; Hill 2000; Lindberg 1999), which improves after some days of a hypoallergenic diet (Campbell 1989; Dupont 2010; Estep 2000; Iacono 1991; Iacono 2005; Jakobsson 1983; Jakobsson 2000; Savino 2001). For these infants, infantile colic has been identified as the first possible manifestation of atopic disease, and dietetic treatment should be the first therapeutic approach (Gupta 2007; Hall 2012; Perry 2011; Savino 2010). Indeed, dietary changes, such as eliminating cows' milk proteins, are particularly indicated in cases of suspected intolerance to cows' milk proteins (for example, in infants with a positive family history; atopic disease such as asthma, eczema and other immune disorders; onset after the first month of life; and colic associated with other gastrointestinal symptoms such as reflux, vomiting or diarrhoea) (Hill 1995; Hill 2005; Jakobsson 1983; Lucassen 2000).
Third, there is growing evidence that intestinal microbiota in colicky infants differ from those in healthy controls; research has shown higher levels of anaerobic bacteria, such as coliform and Escherichia coli, and a lower concentration of lactobacilli, in infants with colic (Savino 2010).
Advances in molecular technologies utilising 16S ribosomal RNA and ribosomal DNA created the opportunity for researchers to index the intestinal microbial composition to better understand its association with infantile colic. The researchers found that infants who manifested symptoms of colic were colonised with significantly higher levels of Proteobacteria and exhibited lower bacterial diversity when compared to their unaffected counterparts (Dubois 2016). Additionally, colonisation levels of Actinobacteria Bifidobacterium and Firmicute lactobacilli were inversely related to the amount of crying and fussiness in newborns. (de Weerth 2013).
A comparison of formula‐fed infants with and without colic revealed significant differences in total bacteria, Enterobacteriaceae and faecal ammonia (Savino 2017).
Human milk naturally contains these prebiotics, defined as indigestible oligosaccharides, which could selectively enhance the proliferation of certain probiotic bacteria in the colon, especially Bifidobacterium spp (Thomas 2010). Some studies have failed to find a protective effect of breastfeeding on the development of colic in breastfed infants (Clifford 2002). However, it is unclear whether these studies compared infants who were exclusively breastfed from birth versus infants who were exclusively formula‐fed from birth, so it is still unclear whether breastfeeding has some protective effect or whether artificial feeding compromises the infant gut microbiome in some way. Oligosaccharide prebiotics (a mixture of oligosaccharides (0.8 g/100 mL), comprising 90% galacto‐oligosaccharides and 10% fructo‐oligosaccharides), may be effective treatments for crying in formula‐fed infants with colic (Savino 2006; Vandenplas 2017; Vivatvakin 2010).
More recently, researchers exploring hypotheses and rationale for causes of infantile colic have proposed three hypothetical mechanisms that could potentially be involved in the aetiopathogenesis of infantile colic: immaturity of bile acid mechanisms that alter intraluminal and absorptive mechanisms, immaturity in motility, and alterations in the microbiome (Camilleri 2017).
Description of the intervention
Dietary modifications have often been suggested for both breastfed and formula‐fed infants with colic. We examined the following dietary interventions.
Dietary modifications for breastfed, colicky infants who are allergic to certain foods (cows' milk, wheat, eggs, soy, nuts, fish) involve modifying the mother's diet to exclude these components so the infant receives a low‐allergen maternal diet. A number of studies have demonstrated a reduction in colic when breastfeeding mothers consumed a hypoallergenic diet (Axelsson 1986; Clyne 1991; Jakobsson 1983; Lothe 1990). For example, Hill 2005 demonstrated that a monitored, low‐allergen maternal diet, which excludes cows' milk, eggs, peanuts, tree nuts, wheat, soy and fish, leads to a reduction in distressed behaviour. Estep 2000 also proposed a brief interruption of breastfeeding and a temporary substitution with an amino‐acid‐based formula; however, this intervention could have negative effects on maternal‐infant interaction and on the longer term continuation of breastfeeding and should only ever be considered as a last resort (Savino 2001; Savino 2007; Savino 2010).
For formula‐fed infants with colicky symptoms, dietary modifications involve decreasing or removing the intake of cows' milk from the infant's diet, or changing the type of milk formula from starting formulas to special formulas (hypoallergenic formula, soy milk formula, whey hydrolysed formula, casein hydrolysed formula, amino‐acid based formula, partially hydrolysed formula, low‐lactose milk formula, formula with prebiotic, etc). Some trials have used formulas containing partially hydrolysed whey proteins, low amounts of lactose, prebiotic oligosaccharides, and a high beta palmitic acid content (Oggero 1994; Osborn 2013; Savino 2005; Savino 2006). In formula‐fed babies, where an underlying allergy to cows' milk protein is hypothesised to affect the infant, extensively hydrolysed formulas, based on casein or whey, have been shown to reduce colic symptoms (Cohen‐Silver 2009; Forsyth 1989; Gupta 2007; Jakobsson 2000; Lucassen 2000). Other studies, hypothesising that malabsorption of lactose may lead to fussing and crying, have tested infant formulas with low‐lactose content on the basis that this may reduce excess intestinal gas (Hyams 1989; Infante 2011; Moore 1988; Savino 2003).
Soy formulas may also reduce symptoms of colic in some formula‐fed infants. However, the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) Committee on Nutrition stated recently that there is no evidence to support the use of soy formulas for managing colic. Additionally, due to concerns regarding a cross‐over allergy to cows' milk protein and their oestrogen content, such formulas should not be given to infants with a food allergy during the first six months of life (Agostoni 2006). As far back as 2004, the UK Chief Medical Officer advised against administering soy protein formula to infants under 12 months of age.
Given the clinical and methodological heterogeneity of studies on these interventions, the efficacy of these interventions in reducing infant colic remains inconclusive at present.
How the intervention might work
Managing gut‐related symptoms in infants can be challenging. Many factors need to be taken into consideration, including geographical, psychological, behavioural, social and family environments, as well as the dietary approach taken to relieve symptoms of infantile colic.
Many published studies have investigated dietary interventions for reducing colic (Campbell 1989; Clifford 2002; Clyne 1991), proposing a link between infant crying and the gastrointestinal tract, thereby implicating the role of nutritional factors such as lactose, lipids and cows' milk proteins (Feinle‐Bisset 2013; Jakobsson 1983; Jakobsson 2000; Lindberg 1999). Cows' milk whey protein elicits symptoms of infantile colic in colicky, formula‐fed infants (Lothe 1989). Intact proteins from the mother's diet are hypothesised to cross over into the breast milk and provoke an allergic response and symptoms of colic in some infants (Axelsson 1986; Clyne 1991).
There are several potential pathophysiological mechanisms which could constitute a rational basis for the therapeutic use of dietary interventions, including immunomodulatory and anti‐inflammatory actions, and effects on motility and pain perception (Drossman 2016; Gupta 2007; Hill 2000).
Different studies have proposed a possible role of nutrients in the development of infantile colic; Nocerino 2012 investigated potentially harmful metabolites, and Iacovou 2018 suggested that a maternal low FODMAP (fermentable oligo‐, di‐ and mono‐saccharides and polyols) diet may be associated with a reduction of infant colic symptoms.
When exploring the causes of colic, we have to consider the possibility that immaturity in hepatic synthesis, reduced levels of intraluminal bile acids and impaired ileal bile absorption of bile acids result in malabsorption of fat and other nutrients. Alternatively, the colonic flora may be abnormal, thereby resulting in increased nutrient fermentations with harmful metabolites, or immaturity of the enteric nervous system might lead to abnormal motility and sensory functions of the intestine and colon (Camilleri 2017).
The growing body of evidence of gut dysfunction support the possible role of nutrients and gut microbiota in the development of infantile colic due to hypersensitivity and abnormal motility (Gupta 2007; Heine 2008; Nocerino 2012). However, the exact mechanisms by which cows' milk and other food allergens induce gastrointestinal motility disorders need further investigation to understand the relationships of these symptoms to the diet (Camilleri 2017; Farré 2013; Heine 2006). Additional factors that could be at play include oversensitivity to stimuli, which may predispose some infants to irritability, fussing and increased crying (Farré 2013; Keefe 1998; Savino 2007).
It is important to note that when all other pathologies have been ruled out, the natural course of infantile colic is resolution; no intervention is necessary. However, parents are often extremely affected in a variety of ways by the symptoms of colic and seek interventions from multiple sources. This review is clearly situated within the context of utility for such families. Indeed, parents of infants with symptoms of colic who do not seek attention would not be recruited for the studies likely to be found in any review of treatments for infantile colic.
Why it is important to do this review
A number of studies and reviews of the evidence suggest that dietary interventions may be effective in reducing the symptoms of colic in both breastfed and formula‐fed infants (Cohen‐Silver 2009; Garrison 2000; Hall 2012; Lucassen 2001; Perry 2011; Savino 2010). Potential interventions have included a low‐allergen diet for mothers of breastfed infants (Hill 2005), hydrolysed formulas (Forsyth 1989; Jakobsson 2000; Lucassen 2000), or low‐lactose content formulas for formula‐fed infants (Infante 2011; Savino 2003; Savino 2006). This systematic review examined the effectiveness and safety of dietary modifications for infantile colic, where possible distinguishing between breastfed and formula‐fed infants. Although there is a relatively recent systematic review on this topic (Iacovou 2012), the search took place in 2010 and excluded all unpublished and grey literature. We have also used a more recent review examining reported outcome measures within infantile colic, Steutel 2014, to ensure that our review examines an appropriate core outcome set (we consider this further within the Discussion). An up‐to‐date systematic review using Cochrane methodology was therefore required.
Objectives
To assess the effects of dietary modifications for reducing colic in infants less than four months of age.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐RCTs.
Types of participants
Infants younger than four months of age suffering from infantile colic (whether breastfed or formula‐fed), as defined by the study. Both breastfed and formula‐fed infants were eligible.
Types of interventions
This review sought to compare any one of the following dietary interventions, alone or in combination, versus another intervention(s) or placebo.
Breastfed infants
An educational intervention that supports and directs a specific dietary modification to modify the mother's diet by excluding certain components such as milk, yogurt, cheese and other foods
Low‐allergen maternal diet
Diet plan or dietary supplementation, regardless of duration of intervention
Formula fed infants
Soy‐based formula
Extensively hydrolysed formula based on whey or casein
Partially hydrolysed formula
Formula with low or no content of lactose
Amino‐acid based formula
Formula that includes prebiotics
We excluded studies involving probiotics. For further information on these interventions, please see Praveen 2014.
Types of outcome measures
Primary outcomes
Duration of crying* (postintervention versus baseline or postintevention). Data could have been continuous (for example, hours per day), or dichotomous (for example, reduction under a predefined threshold, as determined by the study authors). Data must have been collected prospectively, not through retrospective recollection at the end of the study period, using methods such as parent diaries, video or audio recordings, or actigraphy.
Secondary outcomes
Number of responders in each group after treatment*. Responders were defined as those who experienced a decrease in daily crying, as reported by the study authors (dichotomous outcome).
Frequency of crying episodes per 24 h* (postintervention versus baseline) (continuous outcome)
Parental or family quality of life, including measures of parental stress, anxiety or depression* (continuous outcome)
Infant sleep duration per 24 h at 7, 14, and 21 days* (postintervention versus baseline) (continuous outcome)
Parental satisfaction*, measured by Likert scales or a numeric rating scale (continuous outcome)
Adverse effects to dietary modifications: constipation*, vomiting*, diarrhoea, apnoea, apparent life‐threatening events and lethargy (dichotomous outcome). We analysed the frequency of all adverse effects in each study group.
We included outcomes evaluated after the completion of any treatment protocol (that is, any period, any number of treatments), and also at later follow‐up, when reported.
We used those outcomes indicated by an asterisk (*) to populate the 'Summary of findings' table for the main comparison, 'dietary interventions for infantile colic versus placebo or other interventions', where data permitted.
Search methods for identification of studies
Electronic searches
We searched the databases and trials registers listed below up to July 2018 using the strategies in Appendix 1. We imposed no restrictions on publication date or language.
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 6) in the Cochrane Library, which includes the Cochrane Developmental, Psychosocial and Learning Problems Specialised Register (searched 9 July 2018).
MEDLINE Ovid (1946 to June week 5 2018).
MEDLINE In‐Process & Other Non‐Indexed Citations Ovid (searched 9 July 2018).
MEDLINE Epub Ahead of Print Ovid (searched 9 July 2018).
Embase Ovid (1974 to 2018 week 28).
CINAHL Plus EBSCOhost (Cumulative Index to Nursing and Allied Health Literature; 1937 to 10 July 2018).
PsycINFO Ovid (1806 to July week 1 2018).
Science Citation Index Web of Science (SCI; 1970 to 10 July 2018).
Social Sciences Citation Index Web of Science (SSCI; 1970 to 10 July 2018).
Conference Proceedings Citation Index ‐ Science Web of Science (CPCI‐S; 1990 to 10 July 2018).
Conference Proceedings Citation Index ‐ Social Sciences & Humanities Web of Science (CPCI‐SS&H; 1990 to 10 July 2018).
Cochrane Database of Systematic Reviews (CDSR; 2018, Issue 7), part of the Cochrane Library (searched 9 July 2018 ).
Database of Abstracts of Reviews of Effects (DARE; 2015, Issue 2, Final issue), part of the Cochrane Library (searched 6 January 2016).
LILACS (Latin American and Caribbean Health Science Information database; search.bvsalud.org/portal/?lang=en; searched 10 July 2018).
IBECS (ibecs.isciii.es/cgi‐bin/wxislind.exe/iah/online/?IsisScript=iah/iah.xis&base=IBECS&lang=i&form=F; searched 10 July 2018).
HomeoIndex (bases.bireme.br/cgi‐bin/wxislind.exe/iah/online/?IsisScript=iah/iah.xis&base=HomeoIndex&lang=i&form=F; searched 10 July 2018).
Networked Digital Library of Theses and Dissertations (NDLTD; www.ndltd.org; searched 10 July 2018).
TROVE (limited to Australian theses; trove.nla.gov.au; searched 10 July 2018).
WorldCat (limited to theses; worldcat.org; searched 10 July 2018).
PubMed Dietary Supplement Subset (ods.od.nih.gov/Research/PubMed_Dietary_Supplement_Subset.aspx; searched 10 July 2018).
ClinicalTrials.gov (clinicaltrials.gov; searched 10 July 2018).
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; apps.who.int/trialsearch; searched 10 July 2018).
Searching other resources
We searched the bibliographies of included studies to identify any other potentially relevant studies. On 9 July 2018, we searched Google (www.google.com) for grey literature, using the terms 'Infantile colic AND (diet OR formula) AND randomised controlled trial'. We handsearched conference proceedings from the ESPGHAN annual scientific meetings from the past five years (from 2013 to 2018) to identify other potentially relevant studies that may not be published in full. Where we identified references to relevant unpublished or ongoing studies, we recorded them and made attempts to obtain sufficient information so as to incorporate them in the review. Where data were not complete, we contacted the study authors in order to verify the eligibility of the study.
Data collection and analysis
We were unable to use all pre‐planned methods, those not used have been summarised in 'Differences between protocol and review' section.
Selection of studies
Two reviewers (SB and MG) independently screened titles, abstracts, and full reports for eligibility against the inclusion criteria (see Criteria for considering studies for this review). Specifically, they:
merged search results using reference management software and removed duplicate records of the same report;
examined titles and abstracts to remove irrelevant reports;
retrieved full texts of potentially relevant reports;
linked together multiple reports of the same study;
examined full‐text reports for studies that met the eligibility criteria;
corresponded with investigators, when appropriate, to clarify study eligibility;
at all stages, noted reasons for inclusion and exclusion of reports, resolving any disagreements through consensus;
made final decisions on study inclusions and resolved any discrepancies through a process of consensus;
proceeded to data collection.
As Pitkin 1999 discusses, there are issues of the accuracy with which abstracts reflect the published report, so although we searched conference abstracts for possible studies to include, we excluded stand‐alone abstract publications from our review; that is, we only included abstract publications that related to a study for which we also had a full‐text report. See Differences between protocol and review.
We recorded the outcomes of our decisions in a PRISMA diagram (Moher 2009). See Figure 1.
1.

Study flow diagram.
Data extraction and management
We developed data extraction forms a priori, as per the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We then extracted, where possible, information on the following.
Characteristics of participants: source of participants, inclusion and exclusion criteria, total number at baseline, total number at completion, setting, definition of 'colic' applied, diagnostic criteria applied, type of feeding (breastfeeding, formula feeding), age at onset of colic, age at commencement of intervention, and potential effect modifiers such as sex.
Interventions and controls: number of groups, intervention(s) applied, frequency and duration of treatment, total number of treatments, and concomitant use of pacifier.
Methods: study design, duration, sequence generation, allocation concealment, blinding of outcome assessors, and evaluation of success of blinding.
Outcomes: list of outcomes assessed, definitions used, and values of means and standard deviations at baseline and at time points defined by the study protocol (or change from baseline measures, if given).
Results: outcome measures, follow‐up data (including means and standard deviations, standard errors, or confidence intervals (CI) for continuous data, and summary tables for dichotomous data), withdrawals, and losses to follow‐up.
Other: references to other relevant studies, points to follow‐up with study authors, comments from the study authors, key conclusions from the study (by the study authors), other comments from the review authors.
Two review authors (SB; MG) extracted the data independently using the data extraction form. A third review author (FS) resolved any persisting disagreements, which occurred on two occasions. We collated the data in the latest version of Review Manager 5 (RevMan 5) (RevMan 2014).
Assessment of risk of bias in included studies
Using the criteria recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017), two review authors (SB; MG) independently evaluated each study for risk of bias within each of the following domains: sequence generation; allocation concealment; blinding of parents and health professionals; blinding of outcome assessors; incomplete outcome data; selective outcome reporting; and other potential threats to validity, which included consideration of potential risks due to changing methods of data collection (such as different ways of recording crying). They judged each domain as being at low, high, or unclear risk of bias using the criteria described in Appendix 2, compared the judgments, and discussed and resolved any inconsistencies in their assessments. A third review author (FS) was available to resolve any persisting disagreements, had there been any.
Measures of treatment effect
Dichotomous data
We were unable to conduct any meta‐analyses and instead provided narrative descriptions of the results. For planned methods see Differences between protocol and review.
Continuous data
We were unable to conduct any meta‐analyses and instead provided narrative descriptions of the results. For planned methods see Differences between protocol and review.
Unit of analysis issues
For each included study, we determined whether the unit of analysis was appropriate for the unit of randomisation and the design of that study (that is, whether the number of observations matched the number of units that were randomised (Deeks 2017).
Studies with multiple treatment arms
We were unable to conduct any meta‐analyses and instead provided narrative descriptions of the results. For planned methods see Differences between protocol and review.
Cross‐over studies
We were unable to conduct any meta‐analyses and instead provided narrative descriptions of the results. For planned methods see Differences between protocol and review.
Dealing with missing data
Where data were missing, we contacted the corresponding authors of included studies, requesting them to supply any unreported data. Where it was not possible to obtain the missing data, we recorded this on the data collection form, reported it in the 'Risk of Bias' table, and discussed the extent to which the missing data might have altered the results and, hence, the conclusions of the review. For included studies, we noted levels of attrition.
Assessment of heterogeneity
We assessed clinical and methodological heterogeneity by comparing the distribution of important participant characteristics between trials (age or presence of prematurity, length of symptoms at recruitment) and trial characteristics (randomisation, concealment, blinding of outcome assessment, losses to follow‐up, treatment type, cointerventions).
Assessment of reporting biases
In order to minimise publication bias, we attempted to obtain the results of any unpublished studies, to compare the results extracted from published journal reports with the results obtained from other sources (including any correspondence).
Data synthesis
We were unable to conduct any meta‐analyses, for planned methods see Differences between protocol and review.
Subgroup analysis and investigation of heterogeneity
We were unable to conduct any meta‐analyses and thus subgroup analyses were not possible. For planned subgroup analyses see Differences between protocol and review.
Sensitivity analysis
We were unable to conduct any meta‐analyses and thus sensitivity analyses were not necessary. For planned methods see Differences between protocol and review.
Summary of findings table
We assessed the overall quality of the evidence for the following outcomes measured at postintervention using the GRADE approach (Guyatt 2008): duration of crying; number of responders in each group after treatment; frequency of crying episodes per 24 h; parental or family quality of life, including measures of parental stress, anxiety or depression; infant sleep duration per 24 h at 7, 14, and 21 days; parental satisfaction; and adverse effects to dietary modifications: constipation and vomiting. The GRADE approach appraises the quality of evidence based on the extent to which one can be confident that an estimate of effect, or association, reflects the item being assessed. RCTs start as high‐quality evidence, but may be downgraded due to: risk of bias (methodological quality), indirectness of evidence, unexplained heterogeneity, imprecision (sparse data), and publication bias. We determined the overall quality of the evidence for each outcome after considering each of these factors, and graded them as follows.
High quality: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
We reported our quality ratings in a 'Summary of findings' table, which we constructed using GRADEpro GDT (GRADEpro GDT 2015), for the comparison, 'dietary interventions for infantile colic versus placebo or other interventions'.
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies and Characteristics of ongoing studies.
Results of the search
Our electronic searches yielded 5486 records up to 10 July 2018; we found two additional records from searching other sources. After removing duplicates, two review authors independently screened 3575 titles and abstracts for relevance, excluding 3526. Of the 49 records brought forward for full‐text review, we excluded 29 records reporting on 28 studies (Characteristics of excluded studies), and included 17 records reporting on 15 studies (Characteristics of included studies). The three remaining reports relate to three ongoing studies (Characteristics of ongoing studies). See Figure 1.
Included studies
This review includes 15 studies involving a total of 1121 participants (Campbell 1989; Forsyth 1989; Hill 1995; Hill 2005; Jakobsson 2000; Kanabar 2001; Kearney 1998; Lothe 1987; Lucassen 2000; Miller 1990; Oggero 1994; Savino 2005; Savino 2006; Taubman 1988; Treem 1991). See Characteristics of included studies tables.
Study design
All 15 studies were RCTs, and of these, seven used a cross‐over design (Forsyth 1989; Jakobsson 2000; Kanabar 2001; Kearney 1998; Lothe 1987; Miller 1990; Treem 1991). In this review, we found both cross‐over trials that did not provide an adequate washout period and cross‐over trials that provided separate data for the first arm.
Setting
Three studies took place in Turin, Italy (Oggero 1994; Savino 2005; Savino 2006), while two apiece were in Connecticut, USA (Forsyth 1989; Treem 1991); Melbourne, Australia (Hill 1995; Hill 2005); and Malmö, Sweden (Jakobsson 2000; Lothe 1987). One study took place in Amsterdam, Netherlands (Lucassen 2000); Cork, Ireland (Kearney 1998); London, UK (Kanabar 2001); Pennsylvania, USA (Taubman 1988); Scotland, UK (Campbell 1989); and Sydney, Australia (Miller 1990).
Participants were recruited from outpatient services.
Participants
The age of participants ranged from 2 weeks in Hill 2005 to 16 weeks in Hill 1995.
Participants were diagnosed with colic on enrolment. The specific criteria for a diagnosis of colic varied between studies, as did the minimum length of symptoms required to make a diagnosis of infantile colic. Most studies (87.5%) used a definition of colic consistent with the Wessel criteria (Wessel 1954).
The studies excluded children with organic causes for their pathology (see Characteristics of included studies tables).
Interventions
The duration of initial dietary intervention varied from 4 days in Forsyth 1989 to 21 days in Savino 2005.
The dietary modifications included: changes to the maternal diet (Hill 1995; Hill 2005; Oggero 1994); extensively hydrolysed formula (Forsyth 1989; Hill 1995; Jakobsson 2000; Lucassen 2000; Taubman 1988); a partially hydrolysed, lower lactose, whey‐based formula with oligosaccharide (Savino 2006); the use of simethicone (Savino 2006); addition of lactase enzyme to the infant's standard milk (Kanabar 2001; Kearney 1998; Miller 1990); phytotherapeutic agents (Savino 2005); soy formula (Campbell 1989; Lothe 1987); or soy formula with polysaccharide (Treem 1991).
Outcomes
Below, we present the key outcomes that studies reported, as shown in Table 1.
Duration of crying (Campbell 1989; Forsyth 1989; Lucassen 2000; Jakobsson 2000; Oggero 1994; Savino 2005).
Number of responders in each group after treatment (Campbell 1989; Hill 2005; Oggero 1994).
Frequency of crying episodes per 24 h (Taubman 1988; Savino 2006).
Adverse effects to dietary modifications (Kanabar 2001; Kearney 1998; Lucassen 2000; Miller 1990; Savino 2005; Savino 2006).
Funding
Four studies reported public funding and stated that there had been no financial involvement with industry (Campbell 1989; Jakobsson 2000; Oggero 1994; Savino 2005). The manufacturing companies of the study intervention sponsored three studies, but the study authors confirmed via email that industry had no involvement in the conduct of the studies or the writing up of the results (Lucassen 2000; Miller 1990; Savino 2006). The manufacturers of the intervention supported eight studies in some way (Forsyth 1989; Hill 1995; Hill 2005; Kanabar 2001; Kearney 1998; Lothe 1987; Taubman 1988; Treem 1991).
Excluded studies
We excluded 28 studies for various reasons, as summarised below.
Nine studies were not RCTs or quasi‐RCTs (Arikan 2008; Bellaiche 2018; Evans 1981; Iacono 1991; Imanieh 2004; Jakobsson 1983; Nocerino 2012; Savilahti 1989; Xinias 2017).
Five studies were letters in journals or narrative review articles (Buchanan 1998; Gerrard 1984; Koonce 2011; Laws 1991; Sargsyan 2006).
Eleven studies did not select participating infants who were suffering colic before the study but included normal infants (Barr 1991; Berseth 2009; Campeotto 2011; Giovannini 2014; Iacovou 2018; Infante 2011; Rozé 2012; Savino 2003; Sherman 2015; Vandenplas 2017; Vivatvakin 2010). As this is a review of treating established colic, we excluded such studies.
Two studies were not of dietary modification, but of lactose and cows' milk protein allergy testing in colicky babies (Liebman 1981; Pärtty 2015).
One study was of probiotics (Dupont 2010).
Ongoing studies
There are three ongoing studies, which are all double‐blind RCTs.
NCT01721850 is in healthy infants of 35 to 42 weeks gestational age and 15 to 60 days old, with a Wessel diagnosis of colic (Wessel 1954). There will be a parallel assignment with three arms: control formula (standard formula), intervention formula one (infant formula with hydrolysed protein (type I) and pre‐ and probiotics), intervention formula two (infant formula with hydrolysed protein (type II) and pre‐ and probiotics).
NCT02813772 is enrolling full‐term infants with a diagnosis of "1C according to Rome III criteria" (quote). There will be a parallel assignment: a partially hydrolysed formula with reduced lactose content andLactobacillus reuteri versus a standard formula.
NCT03329222 is enrolling full‐term infants diagnosed with colic and comparing a standard formula versus hydrolysed formula.
Risk of bias in included studies
Please see the 'Risk of bias' tables, beneath the Characteristics of included studies tables, for more information of the risk of bias in the included studies. Please also see Figure 2 and Figure 3 for a graphic summary of the risk of bias in the included studies.
2.
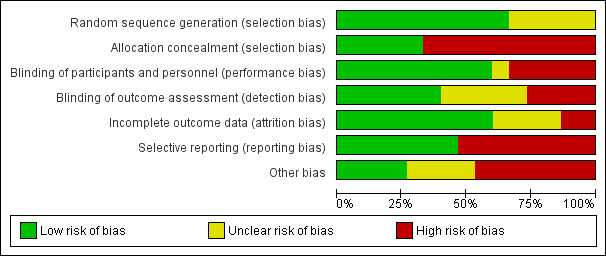
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
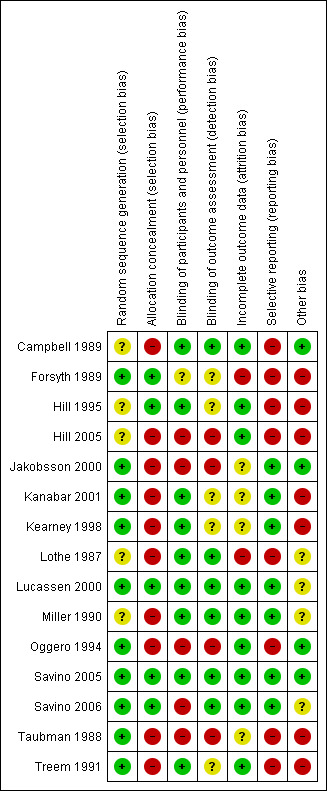
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
We considered nine studies to be at low risk of selection bias based on the published report (Forsyth 1989; Jakobsson 2000; Kanabar 2001; Kearney 1998; Oggero 1994; Savino 2005; Savino 2006; Taubman 1988; Treem 1991). In Lucassen 2000, the lead author responded to a request for more information and confirmed adequate sequence generation. The five remaining studies did not describe the method of randomisation, so we judged these studies to be at unclear risk of selection bias for this domain (Campbell 1989; Hill 1995; Hill 2005; Lothe 1987; Miller 1990).
Allocation concealment
We rated five studies at low risk of bias: four studies adequately described allocation (Forsyth 1989; Hill 1995; Savino 2005; Savino 2006), and the lead author of one study, Lucassen 2000, responded to a request for more information and confirmed adequate allocation concealment. We judged the 10 remaining studies to be at high risk of bias because the allocation concealment was not reported (Campbell 1989; Hill 2005; Jakobsson 2000; Kanabar 2001; Kearney 1998; Lothe 1987; Miller 1990; Oggero 1994; Taubman 1988; Treem 1991).
Blinding
Performance bias
We rated nine studies to be at low risk of performance bias (Campbell 1989; Hill 1995; Kanabar 2001; Kearney 1998; Lothe 1987; Miller 1990; Lucassen 2000; Savino 2005; Treem 1991). Authors of eight of these studies described adequate methods for blinding participants and personnel (Campbell 1989; Hill 1995; Kanabar 2001; Kearney 1998; Lothe 1987; Miller 1990; Savino 2005; Treem 1991), while for Lucassen 2000, again the lead author responded to a request for more information and confirmed adequate blinding of participants and personnel. One study reported the use of blinding but did not describe it clearly (Forsyth 1989), so we rated it at unclear risk of performance bias. We rated five studies at high risk of performance bias; four because they did not adequately describe blinding of participants and personnel (Hill 2005; Jakobsson 2000; Oggero 1994; Taubman 1988), and one, Savino 2006, because it was impossible to blind participants owing to the nature of the intervention and the control (simethicone had to be administered to the infant separately).
Detection bias
We rated six studies at low risk of detection bias (Campbell 1989; Lothe 1987; Lucassen 2000; Miller 1990; Savino 2005; Savino 2006). Five of these studies described adequate methods for blinding of outcome assessment (Campbell 1989; Lothe 1987; Miller 1990; Savino 2005; Savino 2006), while the lead author of Lucassen 2000 confirmed adequate blinding by correspondence. Five studies reported the use of blinding but did not describe it clearly, so we judged these studies to be at unclear risk of detection bias (Forsyth 1989; Hill 1995; Kanabar 2001; Kearney 1998; Treem 1991). We rated four studies at high risk of detection bias because they did not describe blinding of outcome assessment adequately (Hill 2005; Jakobsson 2000; Oggero 1994; Taubman 1988).
Incomplete outcome data
We judged two studies to be at high risk of attrition bias because the details of dropouts were not clear from the report, and there was only partial information as to which group they were from (Forsyth 1989; Lothe 1987). Whilst some inference for an intention‐to‐treat analysis is possible, further details were not available from the study authors. We rated four studies at unclear risk of attrition bias because they did not adequately describe dropouts (Jakobsson 2000; Kanabar 2001; Kearney 1998; Taubman 1988). We judged the remaining nine studies to be at low risk of attrition bias because dropouts were balanced across treatment groups, with similar reasons for withdrawal and few dropouts (Campbell 1989; Hill 1995; Hill 2005; Lucassen 2000; Miller 1990; Oggero 1994; Savino 2005; Savino 2006; Treem 1991).
Selective reporting
We rated eight studies as being of high risk of reporting bias, as the study authors did not report at all on adverse effects, did not have a protocol or did not supply us with the information (Campbell 1989; Forsyth 1989; Hill 1995; Hill 2005; Lothe 1987; Oggero 1994; Taubman 1988; Treem 1991). We judged the remaining seven studies to be at low risk of reporting bias, either because it was specifically stated in the report of the study that there were no adverse effects (Jakobsson 2000; Kanabar 2001; Kearney 1998; Savino 2005) or the study authors confirmed that this was the case through correspondence (Lucassen 2000; Miller 1990; Savino 2006).
Other potential sources of bias
Because of the nature of the evidence contained within these studies, and the claims for one product or intervention over another in such a vulnerable population, we considered any involvement by the companies supplying or manufacturing the intervention product in the conduct of the studies or the writing up of results to trigger a rating of high risk of other bias.
We considered four studies that stated no financial involvement with industry, whether by provision of experimental product or direct financial support for the work, to be at low risk of bias (Campbell 1989; Jakobsson 2000; Oggero 1994; Savino 2005). We judged a further three studies to be at unclear risk of bias as they were sponsored by the manufacturing companies of the study intervention, but we received confirmation that industry had no involvement in the conduct of the studies or the writing up of the results (Lucassen 2000; Miller 1990; Savino 2006). We rated the remaining eight studies at high risk of bias as they stated that they were supported in some way by the manufacturers of the intervention or related products (Forsyth 1989; Hill 1995; Hill 2005; Kanabar 2001; Kearney 1998; Lothe 1987; Taubman 1988; Treem 1991).
None of the studies appeared to have any other potential sources of bias other than industry funding.
Effects of interventions
See: Table 1
Below, we present the results for each combination of dietary regimen and comparison, by assessed outcome and colic type, with the exception of those studies for which we could not extract data. Despite the significant number of studies, there was no opportunity to complete a meta‐analysis due to a combination of heterogenous outcome measures, grouping of different populations in reports of results, and lack of reporting on key outcomes (in particular, adverse effects) and summary outcome statistics. Thus, we provide a narrative description of the results. We report exact P values, where available, from the primary studies; where these were not available, we reported the figure given. We report the GRADE rating throughout. However, as the same two key issues affect all studies (imprecision due to very small sample sizes and risk of bias across all criteria), we do not make special mention of these within each comparison. See Table 1.
1. Low‐allergen maternal diet versus a diet containing known potential allergens
Two studies (205 infants) by the same team in Australia examined the effect of modifying breastfeeding mothers' diets to control for proteins or other substances that might be triggering symptoms of colic (Hill 1995; Hill 2005). However, we were unable to combine the data from these studies in a meta‐analysis because Hill 1995 grouped together breastfed babies whose mothers' diets were modified and formula‐fed babies whose own diet was modified to remove the proteins. Authors did not report separate data for breastfeeding mothers only, and the study authors did not respond to our request for these data.
Primary outcome: duration of crying
Neither study reported data on this outcome (Hill 1995; Hill 2005).
Secondary outcomes: number of responders in each group after treatment
Hill 2005 (90 infants) reported a significant difference (37% difference; 95% CI 18 to 56; P < 0.001) in responders (i.e. reduction in cry/fuss duration of 25%). This occurred in the low‐allergen group in 35/47 babies, compared with 16/43 babies in the control group. The low‐allergen diet excluded all dairy products, soy, wheat, eggs, peanuts, tree nuts and fish, and included a rice milk drink, meat, vegetables, fruit, corn and rice, as well as a calcium supplement and rice‐based bread. We rated the quality of this evidence as very low due to risk of bias and imprecision (Table 1).
Neither study assessed our other secondary outcomes: frequency of crying episodes per 24 h, parental or family quality of life, infant sleep duration per 24 h, parental satisfaction or adverse effects to the dietary modifications.
2. Low‐allergen diet or soy milk formula versus standard diet or cow's milk formula and dicyclomine hydrochloride
Only one study (120 infants) contributed data to this comparison (Oggero 1994). It compared a restricted, low‐allergen diet in breastfeeding mothers or soy milk in formula‐fed babies versus the addition of dicyclomine hydrochloride (a pharmacological treatment for stomach spasms) for both breastfed and formula‐fed infants in the treatment group who also had normal diet, over a period of 30 days.
Primary outcome: duration of crying
Authors did not specifically report duration of crying at end of intervention.
Secondary outcomes: number of responders in each group after treatment
Oggero 1994 reported data on this outcome, but investigators used stricter rules for classifying 'improvement' compared to our protocol (Savino 2014), stating "A positive result was defined as a reduction of crying to less than one hour per day after 48 hours of treatment, with remission persisting for one month". The study found that 10/15 (66.6%) breastfed babies responded to dicyclomine hydrochloride and a normal diet, compared with 10/16 (62.5%) on a low‐allergen diet, while 24/45 (53.3%) formula‐fed babies on dicyclomine improved compared with 29/44 (65.9%) on a low allergen formula.
The study did not assess our other secondary outcomes: frequency of crying episodes per 24 h, parental or family quality of life, infant sleep duration per 24 h, parental satisfaction or adverse effects.
3. Hydrolysed formula versus standard formula
Three studies (185 infants) compared an extensively hydrolysed cows' milk formula with standard cows' milk formula (Forsyth 1989; Hill 1995; Lucassen 2000). The Hill 1995 study did not separate the results for breastfed and formula‐fed infants and the study author provided no further details in response to our request. The Forsyth 1989 study (number of infants?) used a cross‐over design. However, it was not clear from the reportwhether the results were from the first period alone or from the first and second cross‐over periods combined, and the study authors did not respond to a request for further information, possibly due to the age of the study. Given this uncertainty, the Cochrane Developmental, Psychosocial and Learning Problems editorial team advised against combining these data in a meta‐analysis, hence we provide a narrative description of the results of this study below.
Primary outcome: duration of crying
One study (43 participants), Lucassen 2000, reported that the number of infants crying for less than 3 hours per day on at least 3 days a week following the intervention was 8/23 in the whey hydrolysate group versus 5/20 in the standard formula group (χ2= 0.20, P = 0.65).
The same study reported continuous data on this outcome (Lucassen 2000), demonstrating a greater reduction in crying time postintervention with the hydrolysed formula (104 min/d, 95% CI 55 to 155) than with the standard formula (3 min/d, 95% CI −63 to 67); difference = 101 min/d 95% CI = 25 to 179; P = 0.02). We rated the quality of the evidence as very low (Table 1).
Secondary outcomes: adverse effects
Lucassen 2000 did not report on adverse effects, but we received further information from the study author indicating that there were no adverse effects. The other two studies did not report on adverse effects either, so it was not possible to conduct an analysis of any adverse effects or causes of dropouts from the studies from use of the hydrolysed formulas (Forsyth 1989; Hill 1995). We rated the quality of the evidence as very low (Table 1).
None of the three studies assessed our other secondary outcomes: number of responders in each group after treatment, frequency of crying episodes per 24 h, parental or family quality of life, infant sleep duration per 24 h or parental satisfaction.
4. Hydrolysed formula versus another hydrolysed formula
One study (22 infants) was designed as a cross‐over trial, with each infant receiving both types of formula for a week (Jakobsson 2000). In this study, 10 infants were randomised to receive Alimentum (manufactured by Abbott) and 12 infants to receive Nutramigen (manufactured by Mead Johnson).
Primary outcome: duration of crying
The study authors concluded that both hydrolysed formulas were equally effective in resolving symptoms for babies in the trial who were started on standard formula. However, no separate data from the period before the cross‐over period are provided by the authors, therefore we could not use this outcome data. We rated the quality of this evidence as very low (Table 1).
Secondary outcomes: adverse effects
The study authors monitored a number of possible adverse effects, including vomiting and stool consistency. Two patients were withdrawn from the Nutramigen group; one due to vomiting and another due to loose stools. Stool consistency did not differ between the two study periods; however, infants experienced a significant increase in incidence of liquid stools from baseline only when fed Nutramigen.
The study did not assess any of our secondary outcomes: number of responders in each group after treatment, frequency of crying episodes per 24 h, parental or family quality of life, infant sleep duration per 24 h, parental satisfaction.
5. Hydrolysed formula or dairy‐ and soy‐free maternal diet versus standard diet/formula and parental education or counselling
One study (21 participants) reported data on this comparison (Taubman 1988). One group received education and training with normal diet and thea second received no training, but either hydrolysed formula or maternal milk‐free diet.
Primary outcome: duration of crying
Taubman 1988 found that duration of crying per 24 h in the hydrolysed or dairy‐ and soy‐free group (N = 10) decreased to 2.03 h (SD 1.03) by nine days into the intervention (P = 0.01). In the parent education or counselling group (N = 10), the crying of babies per 24 h decreased to 1.08 h (SD 0.7) after nine days (P = 0.001). We rated the quality of this evidence as very low (Table 1).
Secondary outcomes
The study did not assess any other of our secondary outcomes: number of responders in each group after treatment, frequency of crying episodes per 24 hours, parental or family quality of life, infant sleep duration per 24 h, parental satisfaction or adverse effects.
6. Partially hydrolysed, lower lactose, whey‐based formula containing oligosaccharide versus standard formula with simethicone
One study (267 infants) analysed the effectiveness of a partially hydrolysed, whey‐based formula containing a mixture of oligosacharides, low lactose level, modified vegetable oil and starch versus a standard formula (as used by parents) with simethicone for infantile colic (Savino 2006).
Primary outcome: duration of crying time
Savino 2006 did not report data on this outcome.
Secondary outcomes
Frequency of crying episodes per 24 h
Savino 2006 found that infants (N = 130) receiving the partially hydrolysed formula had a significant decrease (95% CI −0.7 to −1.8) in colic episodes after one week (2.47 episodes (SD 1.94) at day 7 versus 5.99 episodes (SD 1.84) at study entry) compared with infants (N = 137) receiving the standard formula (3.72 episodes (SD 1.98) at day 7 versus 5.41 episodes (SD 1.88) at study entry). After two weeks, episodes of crying were significantly different (P < 0.001) between the two groups of infants (partially hydrolysed formula: 1.76 episodes (SD 1.60) versus standard formula: 3.32 episodes (SD 2.06)). We rated the quality of the evidence as very low (Table 1).
Adverse effects
Savino 2006 did not report data on adverse effects, but the lead author confirmed that these were assessed and recorded as part of the protocol, and that no infants experienced them.
The study did not report data on any of our other secondary outcomes: number of responders in each group after treatment, parental or family quality of life, infant sleep duration per 24 h or parental satisfaction.
7. Lactase enzyme supplementation versus placebo
Three studies (138 participants) investigated the addition of lactase enzyme to infant milk (Kanabar 2001; Kearney 1998; Miller 1990). Once again, due to significant heterogeneity of outcome reporting and limited data within the reports, as well as all three studies being cross‐over trials that did not report data from before washout, we were unable to conduct a meta‐analysis.
Primary outcome: duration of crying time
None of the three studies reported data on this outcome (Kanabar 2001; Kearney 1998; Miller 1990).
Secondary outcomes: adverse effects
Two studies reported that there were no adverse effects (Kanabar 2001; Kearney 1998), while the author of Miller 1990 confirmed via personal correspondence that this was the case.
The study did not report on our other secondary outcomes: number of responders in each group after treatment, frequency of crying episodes per 24 h, parental or family quality of life, infant sleep duration per 24 h or parental satisfaction.
8. Extract of Foeniculum vulgare, Matricariae recutita, and Melissa officinalis versus placebo
One study (93 infants), Savino 2005, assessed the effectiveness and side effects of a phytotherapeutic agent versus placebo, both of which were administered twice a day for one week, in the treatment of infantile colic. The phytotherapeutic agent was a liquid containing extract of Foeniculum vulgare (fennel), Matricariae recutita (camomile) and Melissa officinalis (lemon balm), with vitamins B1, B5 and B6.
Primary outcome: duration of crying
Savino 2005 found that the average daily crying time of infants given the phytotherapeutic agent (N = 41) was 76.9 min/d at the end of the 1‐week study (SD 23.5), compared with an average daily crying time of 169.9 min/d (SD 23.1) in infants given placebo (N = 47) (95% CI −102.89 to −83.11; P < 0.005). We rated the quality of the evidence as very low (Table 1).
Secondary outcomes: adverse effects
Savino 2005 reported that there were no serious adverse effects. They also reported episodes of vomiting (intervention = 8, placebo = 2; 95% CI 0.02 to 0.28; P = 0.06) and constipation (intervention = 4, placebo = 5; 95% CI −0.13 to 0.13; P = 0.72) for babies in the intervention (n = 41) and placebo (n = 47) groups. We rated the quality of this evidence as very low (Table 1).
Savino 2005 did not assess any of our other secondary outcomes: number of responders in each group after treatment, frequency of crying episodes per 24 h, parental or family quality of life, infant sleep duration per 24 h or parental satisfaction.
9. Soy protein‐based formula versus standard cows' milk protein‐based formula
Two studies (84 infants) compared a soy protein‐based formula with standard cows' milk protein‐based formula (Campbell 1989; Lothe 1987). We were unable to conduct a meta‐analysis as the outcomes were extremely heterogeneous.
In the cross‐over study (65 infants) by Lothe 1987, study authors reported only aggregated results and did not respond to our request for data from the first arm only. Therefore, we are unable to consider the results any further.
Campbell 1989 (19 infants) compared duration of symptoms of colic following a single week on either a standard casein‐based cows' milk protein formula (as control) or the same company's (Cow and Gate) soy formula. The study was run as a cross‐over with all babies receiving both formulas over the space of two weeks. However, the data from the first phase were not presented separately and so was not included in this review.
Primary outcome: duration of crying
In Campbell 1989, mean crying time was lower in the soy protein‐based formula group (12.7 h/week (SD 16.4); N = 10) than in the standard cows' milk protein‐based formula group (17.3 h/week (SD 6.9); N = 9). We rated the quality of the evidence as very low (Table 1).
Secondary outcomes: number of responders in each group after treatment
Campbell 1989 reported 0/9 responders (0%) in the control group after therapy and 5/10 responders (50%) in the intervention group. We rated the quality of the evidence as very low (Table 1).
The study did not assess our other secondary outcomes: frequency of crying episodes per 24 h, parental or family quality of life, infant sleep duration per 24 h, parental satisfaction or adverse effects.
10. Soy protein formula with polysaccharide versus standard soy protein formula
One study (27 infants) assessed this comparison (Treem 1991). Twelve babies received standard soy protein formula, and 15 babies received a formula supplemented with polysaccharide. As we did not receive a response from the study author to our request for pre‐washout‐phase data, we are unable to analyse these results.
Primary outcomes: duration of crying
Treem 1991 did not report data on this outcome.
Secondary outcomes
Treem 1991 did not present pre‐washout‐phase data to allow us to assess our secondary outcomes: number of responders in each group after treatment, frequency of crying episodes per 24 h, parental or family quality of life, infant sleep duration per 24 h, parental satisfaction or adverse effects.
Discussion
Summary of main results
This review includes 15 studies, with a total of 1121 enrolled infants, that evaluated the effects of dietary modifications for treating infant colic. We were not able to perform any meta‐analyses due to the heterogeneity of the studies and the outcomes that they measured.
The studies did not routinely report adverse effects, although a small number of study authors provided these data on request. There were also no studies reporting data on quality of life outcomes, which are of great interest to parents.
There is insufficient evidence to support the claims that soy protein benefits infants with fussiness and crying, in keeping with international guidelines (ESPGHAN) (Agostoni 2006), and that suggested soy milk formulas should not be used (see section Quality of the evidence below).
In sum, dietary modifications may or may not be useful or detrimental.
Overall completeness and applicability of evidence
The results of this review rest upon trials which, in general, were poorly designed, conducted and reported. Even though the studies were conducted in both university clinics and primary care hospitals in different countries, the applicability of the evidence to clinical practice is limited. Most dietary modifications explored by the trials, such as soy‐based formula, were outdated, and clinical outcomes and data, such as adverse effects, were limited. Moreover, heterogeneity was evident among definitions of colic. Studies were most often based on a small sample from a single centre, with no replication.
The number of infants included in the comparisons was low, ranging from 13 for lactase drops versus placebo, to 267 for partially hydrolysed, low‐lactose, modified‐oil, whey‐based formula containing oligosaccharide and starch versus a standard formula with simethicone.
The small sample sizes do not reflect the large scale of the issue with infantile colic in our populations. In over 30 years of research included in this review, the studies we found are not robust enough to provide definitive answers regarding which – if any – dietary modification works. Nor do they shed any light on what colic is, a clearly symbiotically linked problem that also requires study. Most research apparently concentrates on testing specific products rather than the individual ingredients and their efficacy.
The outcome measures used are also of concern. As crying is a very subjective concept, objective methods of recording crying would be preferable. However, recording in diaries was the most common method, and this is a significant weakness in the utility of the evidence base. The use of 'treatment success' or 'responders' was also reported in a very heterogenous manner, and many of the specific thresholds reported bear little utility to parents or clinicians in real‐life clinical situations.
Unfortunately, the included studies did not evaluate the impact of interventions on the quality of family life with the colicky infant. Validated questionnaires are available for parents (Sung 2014), and, in many ways, this is the most important set of measures for a self‐limiting problem that does not necessarily require treatment but is treated to enhance outcomes for families. However, to date, investigators have failed to recognise the impact that symptoms of colic can have on parents' emotional state (Landgren 2010). This is a very sensitive issue because this clinical situation could damage the future parent‐child relationship (Pauli‐Pott 2000). In a recent systematic review of outcome measures reported in trials of infant colic, Steutel 2014 suggested a core set of measures that would address such issues. As the natural history of the condition is improvement, outcomes that measure the impact of symptoms whilst present are, in many ways, the most relevant, and the lack of reporting limits the applicability of the evidence.
In 2004 the UK's Chief Medical Officer, Sir Liam Donaldson, stated that soy‐based formulas should be avoided for infants because of the "high phytoestrogen content, which could pose a risk to the long‐term reproductive health of infants" (Donaldson 2004). This recommendation was based on "a 2003 report from the Committee on Toxicity (COT), an independent scientific committee that advises the Department of Health and other government agencies". It is unlikely, therefore, that studies would now consider soy formula at all and instead would go straight to extensively hydrolysed formula as the dietary modification for formula‐fed babies (see Forsyth 1989; Hill 1995 and Lucassen 2000 above, and to some extent Taubman 1988 also). This limits the applicability of these earlier studies to current practice.
Quality of the evidence
We judged the quality of the evidence on the effectiveness of dietary modifications for infantile colic to be very low. This was particularly impacted by the high risk of bias in the design and conduct of studies, and a particular concern of publication bias linked to small study sizes, possibly associated with nutrition company sponsorship. Additionally, sample sizes were universally very small, with no power calculations, further downgrading quality for imprecision.
The lack of signalled adverse effects in the studies raises serious doubts about the quality, accountability and transparency of these trials. In any medical research, a researcher's first priority is to be accountable for exploring the effectiveness of different interventions while protecting the safety of patients. It is obviously preferable for researchers to contribute to improvements in the safety of healthcare interventions by recording all potential adverse effects. Collecting data on adverse effects in small children might require additional efforts, as researchers would often need to train caregivers to recognise and record potential adverse effects.
Given what has been said above, and the consequent low quality of the evidence, readers should exercise caution when interpreting the available data. Even if some results look positive (e.g. for hydrolysed protein formulas), it is possible that the advantages mentioned could simply be due to bias or chance. Based on the evidence presented, we cannot recommend hydrolysed protein formulas or other dietary modifications, and an allergen exclusion diet is no better than standard diet or placebo.
Potential biases in the review process
We conducted comprehensive searches, including extensive searches of the grey literature, to identify all relevant studies.
To avoid bias, two review authors (MG; SB) independently evaluated study eligibility, extracted data, assessed risk of bias and rated the quality of the evidence. On two occasions we resolved initial disagreements about inclusion or exclusion with another member of the team (FS). For the three studies in which one review author (FS) was involved (Oggero 1994; Savino 2006; Savino 2005), two other review authors (MG; SB) who did not participate in these studies evaluated study eligibility, extracted data, assessed risk of bias and rated the quality of the evidence.
As stated above, the quality of study reports in terms of detail, clarity, study flow, outcome reporting and availability of protocols, amongst many other factors, was variable and sporadic. In a number of circumstances, when study authors did not respond to our requests for further data, the reviewers had to interpret the data to the best of their ability. This may have introduced bias into the process.
Agreements and disagreements with other studies or reviews
We found three earlier reviews evaluating dietary modifications for infantile colic (Cohen‐Silver 2009; Hall 2012; Perry 2011).
Cohen‐Silver 2009 concluded that physicians can suggest a change to formulas containing whey hydrolysate for formula‐fed infants, as well as maternal dietary modifications for breastfed infants. In our view, however, the conclusions appear to over‐interpret the results of primary studies, not taking into account the poor quality of the evidence. Although Cohen‐Silver 2009 underlines that exclusive hypoallergenic formula feeds should be reserved only for infants with a true allergy to cows' milk protein, change to hydrolysed formulas cannot generally be advised to parents as routine clinical practice.
Hall 2012 is a systematic review of studies of several nutritional interventions such as high‐fibre formula, low‐allergenic formula, lower allergenic maternal diet or the addition of lactase. Hall 2012 agreed with our review in that the quality of the research in this field must be recognised as a priority across different independent research groups.
Perry 2011 published a broad overview of all complementary and alternative medicines and nutritional supplements for the treatment of infantile colic. While we considered some of the trials in Perry 2011, they did not focus on dietary approaches with infant formulas or dietary modifications for breastfeeding mothers, so their scope was very different from ours. Also, their team concluded that there was no evidence to support clinical recommendations of any studied dietary intervention.
Authors' conclusions
Implications for practice.
We have concluded the following.
It is not possible to draw any robust conclusions on the effectiveness of dietary modifications for infantile colic because the evidence is scant and prone to bias; presently, only a few trials are available, most of them outdated and at serious risk of bias.
Only one unreplicated study, with high risk of bias in several areas, found that parental education or counselling achieves a significant decrease in crying within the first three days compared to a dairy‐free maternal diet or extensively hydrolysed formula.
It is not possible to draw any conclusions on the efficacy of dairy‐free diets, soy‐free diets, lactase enzyme supplementation of infant milk, or the addition of soy polysaccharide to standard soy protein formula.
Amongst studies examining the effect of an allergen‐exclusion diet in breastfeeding mothers, we found a significant difference between responders in the low‐allergen group versus the control group. However, because the quality of these studies is very poor, and the extent of the benefit observed is variable, readers should interpret these results with caution.
Available data show no difference between hydrolysed and standard infant formulas for colic; however, one study in our review stated that the two different hydrolysed protein formulas studied are equally effective in resolving symptoms.
Moreover, two different studies reported a reduction in symptoms of infantile colic with hydrolysed formulas in other comparisons: in the first study, a partially hydrolysed, low‐lactose, modified‐oil, whey‐based formula containing oligosaccharide and starch showed a significant decrease in colic episodes compared with standard formula with simethicone. In the second study, hydrolysed formula relieved symptoms of colic after soy‐based formula.
Given these interventions are not classified as medicinal products by regulatory bodies, there is a risk that an interventional dietary approach can be marketed to the public with a poor evidence base that would not occur for prescribed medicinal products. Thus, it is important to generate high‐quality evidence to investigate such new milk formulas and, in the meantime, ensure the results of this review are used to guide current clinical practice and advice.
The implication for practice from this systematic review is that dietary modifications can be neither recommended nor excluded from therapies, given their unknown benefit, and that new research must address key methodological issues, some of which are context specific.
Implications for research.
Current evidence on the effectiveness of dietary approaches for infantile colic is based mainly on old studies, which are usually affected by methodological limitations.
Future trials must align with clinically relevant outcomes about which there is a consensus regarding measurement methods and relevant time points. Reporting the resolution of colic, defined using up‐to‐date criteria for diagnosis, should be mandatory. Regarding crying time, objective measures using the host of audiovisual and other technologies that are cheaply available, should be considered essential to allow such assessment.
Other outcomes of importance to parents, such as crying time per day, parental quality of life and sleeping time should be reported, together with the data needed for synthesis. Adverse effects must be reported in a manner that recognises the different forms of such events (i.e. serious, minor and those requiring withdrawal of therapy). Given the natural history of the condition, a major challenge is to design trials that intervene early in the development of colic. Alternatively, subgroups will permit consideration of different patients based on age, for example, since a population pre‐six weeks of age is, in many ways, very different from one that is post‐six weeks of age.
More rudimentary methodological issues must also be addressed. Power calculations based on existing primary and secondary studies should be completed, as many of the studies we found were underpowered and of little value. Long‐term outcomes should be considered, as this may allow economic evaluation in the future, given that many of the interventions being studied in this review are likely to be purchased directly by parents, and failure to address colic can lead to extra visits with medical staff or requirement for future medication.
Standardised tools for measuring outcomes that allow comparison and pooling of results across studies are needed for all outcomes, particularly given the new Rome IV criteria that refer to several elements in defining colic that are too subjective on their own to enable performance of sensible experimental studies (Drossman 2016). We would advise all future researchers to read this review in detail to identify primary works that may support study design. A recent published analysis of the existing tools being employed underlines the need to design and validate new assessment devices or scales for this clinical condition (García Marqués 2017), and this analysis should consider this existing evidence base in its entirety.
As indicated above, in planning new clinical trials, researchers should adopt a standard definition of infantile colic, such as the definition proposed by the Rome IV Committee (Benninga 2016; Drossman 2016; Wolke 2017), which includes the following diagnostic criteria for infantile colic: all of the following in infants from birth to four months of age: paroxysms of irritability, fussing or crying that start and stop without obvious cause; episodes lasting three or more hours per day and occurring at least three days per week for at least one week; and no failure to thrive.
Given the growing evidence around the impact of infants' balance of gut microbiota on colic and other symptoms (Dubois 2016; Savino 2017), clear sample populations should include birth circumstances (i.e. vaginal versus caesarean), and feeding method (i.e. exclusive breastfeeding from birth versus exclusive formula feeding from birth). In addition, the populations for future trials should be separated, perhaps, into infants with a pre‐existing family history of allergy to a certain dietary component (e.g. soy or cows' milk protein) versus those who do not.
What's new
| Date | Event | Description |
|---|---|---|
| 28 February 2019 | Amended | This review has been revised to clarify reporting of data in summary versions. |
| 1 November 2018 | Amended | Typo corrected in Plain Language Summary. |
Acknowledgements
This review was produced within Cochrane Developmental, Psychosocial and Learning Problems (CDPLP).
Many thanks to: Margaret Anderson, Information Specialist of CDPLP, for invaluable assistance in refining the search strategy; Geraldine Macdonald, Co‐ordinating Editor of CDPLP, for assistance in preparing the manuscript; Joanne Duffield, Managing Editor of CDPLP, for support and guidance provided throughout the review process; and Laura MacDonald, former Managing Editor of CDPLP, for support and guidance provided throughout the development of the protocol (Savino 2014).
We would also like to recognise the contribution of our peer reviewers.
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library
#1[mh Colic] #2colic* #3((stomach or abdominal or abdomen*) near/3 (spasm* or pain* or cramp*)) #4((gastric or gastro*) near/3 (spasm* or pain* or cramp*)) #5[mh Crying] #6(cry or crying or cries) #7{or #1‐#6} #8[mh Infant] #9(baby or babies or child* or infant* or newborn* or neonate*) #10{or #8‐#9} #11[mh Milk] #12[mh "Milk, Human"] #13[mh "Breast Feeding"] #14(breastfeed* or "breast feed*" or breastfed or "breast fed" or breastmilk* or "breast milk*" or milk*) #15[mh ^Hypersensitivity] #16[mh "Food Hypersensitivity"] #17[mh ^Allergens] #18[mh "Lactose Intolerance"] #19(allerg* or hypoallerg* or hypo next allerg* or hyperallerg* or hyper next allerg* or hypersensitiv* or hyper next sensitiv* or intoleran* or non next allerg* or nonallerg* or sensitiv*) #20[mh "Infant Food"] #21(formula* or bottle next fed* or bottlefed* or bottlefeed* or bottle next feed*) #22[mh Hydrolysis] #23(hydrolys* or hydrolyz*) #24[mh Prebiotics] #25[mh ^"Amino acids"] 1 #26(amino next acid* or aminoacid* or casein* or fibre* or fiber* or prebiotic* or pre next biotic* or soy* or whey*) #27[mh "Dietary Proteins"] #28[mh "diet therapy"/FS] #29diet* #30[mh "Dairy Products"] #31[mh Fishes] #32[mh Eggs] #33[mh Gluten] #34[mh Nuts] #35(cheese* or dairy or egg* or fish* or gluten* or wheat* or nut* or peanut* or lactose* or yogurt* or yoghurt*) #36{or #11‐#35} #37#7 and #10 and #36 in Trials
MEDLINE Ovid
1 Colic/ 2 colic$.tw. 3 ((stomach or abdominal or abdomen$) adj3 (spasm$ or pain$ or cramp$)).tw. 4 ((gastric or gastro$) adj3 (spasm$ or pain$ or cramp$)).tw. 5 crying/ 6 (cry or crying or cries).tw. 7 or/1‐6 8 exp Infant/ 9 (baby or babies or child$ or infant$ or newborn$ or neonate$).tw. 10 8 or 9 11 7 and 10 12 Milk/ 13 Milk, Human/ 14 Breast Feeding/ 15 (breastfe?d$ or breast fe?d$ or breastmilk$ or breast‐milk$ or milk$).tw. 16 Hypersensitivity/ 17 exp Food Hypersensitivity/ 18 Allergens/ 19 Lactose Intolerance/ 20 (allerg$ or hypoallerg$ or hypo‐allerg$ or hyperallerg$ or hyper‐allerg$ or hypersensitiv$ or hyper‐sensitiv$ or intoleran$ or non‐allerg$ or nonallerg$ or sensitiv$).tw. 21 exp Infant Food/ 22 (formula$ or bottle fed$ or bottlefed$ or bottlefeed$ or bottle feed$).tw. 23 Hydrolysis/ 24 (hydrolys$ or hydrolyz$).tw. 25 Prebiotics/ 26 Amino acids/) 27 (amino acid$ or aminoacid$ or casein$ or fibre$ or fiber$ or prebiotic$ or pre‐biotic$ or soy$ or whey$).tw. 28 exp Dietary Proteins/ 29 diet therapy.fs. 30 diet$.tw. 31 exp Dairy Products/ 32 exp Eggs/ 33 Fishes/ 34 Gluten/ 35 Nuts/ 36 (cheese$ or dairy or egg$ or fish$ or gluten$ or wheat$ or nut$ or peanut$ or lactose$ or yog?urt$).tw. 37 or/12‐36 38 11 and 37 39 randomized controlled trial.pt. 40 controlled clinical trial.pt. 41 randomi#ed.ab. 42 placebo$.ab. 43 drug therapy.fs. 44 randomly.ab. 45 trial.ab. 46 groups.ab. 47 or/39‐46 48 exp Animals/ not Humans.sh. 49 47 not 48 50 38 and 49
MEDLINE In‐Process & Other Non‐Indexed Citations Ovid
1 colic$.mp. 2 ((stomach or abdominal or abdomen$) adj3 (spasm$ or pain$ or cramp$)).mp. 3 ((gastric or gastro$) adj3 (spasm$ or pain$ or cramp$)).mp. 4 (cry or crying or cries).mp. 5 or/1‐4 6 (baby or babies or child$ or infant$ or newborn$ or neonat$).mp. 7 5 and 6 8 (breastfe?d$ or breast fe?d$ or breastmilk$ or breast‐milk$ or milk$).mp. 9 (allerg$ or hypoallerg$ or hypo‐allerg$ or hyperallerg$ or hyper‐allerg$ or hypersensitiv$ or hyper‐sensitiv$ or intoleran$ or non‐allerg$ or nonallerg$ or sensitiv$).mp. 10 (formula$ or bottle fed$ or bottlefed$ or bottlefeed$ or bottle feed$).mp. 11 (hydrolys$ or hydrolyz$).mp. 12 (amino acid$ or aminoacid$ or casein$ or fibre$ or fiber$ or prebiotic$ or pre biotic$ or soy$ or whey$).mp. 13 diet$.mp. 14 (cheese$ or dairy or egg$ or fish$ or gluten$ or wheat$ or nut$ or peanut$ or lactose$ or yog?urt$).mp. 15 or/8‐14 16 7 and 15
MEDLINE EPub Ahead of Print Ovid
1 colic$.mp. 2 ((stomach or abdominal or abdomen$) adj3 (spasm$ or pain$ or cramp$)).mp. 3 ((gastric or gastro$) adj3 (spasm$ or pain$ or cramp$)).mp. 4 (cry or crying or cries).mp. 5 or/1‐4 6 (baby or babies or child$ or infant$ or newborn$ or neonat$).mp. 7 5 and 6 8 (breastfe?d$ or breast fe?d$ or breastmilk$ or breast‐milk$ or milk$).mp. 9 (allerg$ or hypoallerg$ or hypo‐allerg$ or hyperallerg$ or hyper‐allerg$ or hypersensitiv$ or hyper‐sensitiv$ or intoleran$ or non‐allerg$ or nonallerg$ or sensitiv$).mp. 10 (formula$ or bottle fed$ or bottlefed$ or bottlefeed$ or bottle feed$).mp. 11 (hydrolys$ or hydrolyz$).mp. 12 (amino acid$ or aminoacid$ or casein$ or fibre$ or fiber$ or prebiotic$ or pre biotic$ or soy$ or whey$).mp. 13 diet$.mp. 14 (cheese$ or dairy or egg$ or fish$ or gluten$ or wheat$ or nut$ or peanut$ or lactose$ or yog?urt$).mp. 15 or/8‐14 16 7 and 15
Embase Ovid
1 colic/ 2 colic$.tw. 3 ((gastric or gastro$) adj3 (spasm$ or pain$ or cramp$)).tw. 4 crying/ 5 (cry or crying or cries).tw. 6 or/1‐5 7 exp infant/ 8 (baby or babies or child$ or infant$ or newborn$ or neonate$).tw. 9 7 or 8 10 6 and 9 11 infantile colic/ 12 10 or 11 13 milk/ 14 breast milk/ 15 breast feeding/ 16 (breastfe?d$ or breast fe?d$ or breastmilk$ or breast‐milk$ or milk$).tw. 17 hypersensitivity/ 18 exp nutritional intolerance/ 19 exp allergen/ 20 lactose intolerance/ 21 (allerg$ or hypoallerg$ or hypo‐allerg$ or hyperallerg$ or hyper‐allerg$ or hypersensitiv$ or hyper‐sensitiv$ or intoleran$ or non‐allerg$ or nonallerg$ or sensitiv$).tw. 22 exp baby food/ 23 (formula$ or bottle fed$ or bottlefed$ or bottlefeed$ or bottle feed$).tw. 24 hydrolysis/ 25 prebiotic agent/ 26 amino acid/ 27 (amino acid$ or aminoacid$ or casein$ or fibre$ or fiber$ or prebiotic$ or pre‐biotic$ or soy$ or whey$).tw. 28 exp dairy product/ 29 protein intake/ 30 diet$.tw. 31 egg/ 32 fish/ 33 exp nut/ 34 gluten/ 35 gluten free diet/ 36 (cheese$ or dairy or egg$ or fish$ or gluten$ or wheat$ or nut$ or peanut$ or lactose$ or yog?urt$).tw. 37 or/13‐36 38 12 and 37 39 Randomized controlled trial/ 40 controlled clinical trial/ 41 Single blind procedure/ 42 Double blind procedure/ 43 triple blind procedure/ 44 Crossover procedure/ 45 (crossover or cross‐over).tw. 46 ((singl$ or doubl$ or tripl$ or trebl$) adj1 (blind$ or mask$)).tw. 47 Placebo/ 48 placebo.tw. 49 prospective.tw. 50 factorial$.tw. 51 random$.tw. 52 assign$.ab. 53 allocat$.tw. 54 volunteer$.ab. 55 or/39‐54 56 38 and 55
CINAHL Plus EBSCOhost (Cumulative Index to Nursing and Allied Health Literature)
S36 S13 AND S35 S35 S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 S34 (cheese* or dairy or egg* or fish* or gluten* or wheat* or nut* or peanut* or lactose* or yog#urt*) S33 (MH "Nuts+") S32 (MH "Gluten") OR (MH "Diet, Gluten‐Free") S31 (MH "Fish") S30 (MH "Eggs") S29 (MH "Dairy Products+") S28 diet* S27 (MH "Dietary Proteins+") S26 ("amino acid*" or aminoacid* or casein* or fibre* or fiber* or prebiotic* or "pre‐biotic*" or soy* or whey*) S25 (MH "Amino Acids") S24 (MH "Prebiotics") S23 (hydrolys* or hydrolyz*) S22 (formula* or bottle‐fe#d* or bottlefe#d* S21 (MH "Infant Food+") S20 (allerg* or hypoallerg* or hypo‐allerg* or hyperallerg* or hyper‐allerg* or hypersensitiv* or hyper‐sensitiv* or intoleran* or non‐allerg* or nonallerg* or sensitiv *) S19 (MH "Allergens") S18 (MH "Food Hypersensitivity") S17 (MH "Milk Hypersensitivity") S16 (breastfe#d* OR breast‐fe#d* or breastmilk or breast‐milk or milk) S15 (MH "Breast Feeding") S14 (MH "Milk, Human") S13 S11 OR S12 S12 (MH "Infant Colic") S11 S7 AND S10 S10 S8 OR S9 S9 (MH "Infant+") S8 TI(baby or babies or child* or infant* or newborn* or neonate*) OR AB(baby or babies or child* or infant* or newborn* or neonate*) S7 S1 OR S2 OR S3 OR S4 OR S5 OR S6 S6 (cry or crying or cries) S5 (MH "Crying") S4 ((gastric or gastro*) N3 (spasm* or pain* or cramp*)) S3 ((stomach or abdominal or abdomen*) N3 (spasm* or pain* or cramp*)) S2 colic* S1 (MH "Colic")
PsycINFO Ovid
1 crying/ 2 colic$.tw. 3 (cry or crying or cries).tw. 4 ((gastric or gastro$) adj3 (spasm$ or pain$ or cramp$)).tw. 5 ((stomach or abdominal or abdomen$) adj3 (spasm$ or pain$ or cramp$)).tw. 6 or/1‐5 7 (baby or babies or child$ or infant$ or newborn$ or neonate$).tw. 8 (infancy 2 23 mo or neonatal birth 1 mo).ag. 9 7 or 8 10 breast feeding/ 11 (breastfe?d$ or breast fe?d$ or breastmilk$ or breast‐milk$ or milk$).tw. 12 bottle feeding/ 13 Food Allergies/ 14 antigens/ 15 (allerg$ or hypoallerg$ or hypo‐allerg$ or hyperallerg$ or hyper‐allerg$ or hypersensitiv$ or hyper‐sensitiv$ or intoleran$ or non‐allerg$ or nonallerg$ or sensitiv$).tw. 16 (formula$ or bottle fed$ or bottlefed$ or bottlefeed$ or bottle feed$).tw. 17 (hydrolys$ or hydrolyz$).tw. 18 dietary supplements/ 19 (amino acid$ or aminoacid$ or casein$ or fibre$ or fiber$ or prebiotic$ or pre‐biotic$ or soy$ or whey$).tw. 20 Amino acids/ 21 diet$.tw. 22 (cheese$ or dairy or egg$ or fish$ or gluten$ or wheat$ or nut$ or peanut$ or lactose$ or yog?urt$).tw. 23 or/10‐22 24 6 and 9 and 23 25 clinical trials/ 26 random$.tw. 27 (allocat$ or assign$).tw. 28 ((clinic$ or control$) adj trial$).tw. 29 ((control$ or experiment$ or intervention$) adj3 group$).tw. 30 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 31 (crossover$ or "cross over$").tw. 32 random sampling/ 33 Experiment Controls/ 34 Placebo/ 35 placebo$.tw. 36 exp program evaluation/ 37 treatment effectiveness evaluation/ 38 ((effectiveness or evaluat$) adj3 (stud$ or research$)).tw. 39 or/25‐38 40 24 and 39
Science Citation Index (SCI) and Social Sciences Citation Index (SSCI); Web of Science
# 12 #11 AND #10 # 11 TS=(random* or control* or trial* or placebo* or group* or blind* or double‐blind*) . # 10 #9 AND #1 # 9 #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 # 8 TS= (cheese* or dairy or egg* or fish* or gluten* or wheat* or nut* or peanut* or lactose* or yog*urt*) # 7 TS=(hydrolys* or hydrolyz*) # 6 TS=("amino acid*" or aminoacid* or casein* or fibre* or fiber* or prebiotic* or pre‐biotic* or soy* or whey*) # 5 TS= (allerg* or hypoallerg* or hypo‐allerg* or hyperallerg* or hyper‐allerg* or hypersensitiv* or hyper‐sensitiv* or intoleran* or non‐allerg* or nonallerg* or sensitiv *) # 4 TS=(formula* or bottle‐fed or bottle‐feed* or bottlefeed* or bottlefed) # 3 TS=(breastfed or breast‐fed or breastfeed* or breast‐feed* ) # 2 TS=(diet*) #1 TS=((colic* or crying or cries or cry) Near/10 (infant* or baby or babies or child*))
Conference Proceedings Citation Index ‐ Science (CPCI‐S) and Conference Proceedings Citation Index ‐ Social Science & Humanities (CPCI‐SS&H); Web of Science
#10 #9 AND #1 #9 #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 #8 TS= (cheese* or dairy or egg* or fish* or gluten* or wheat* or nut* or peanut* or lactose* or yog*urt*) #7 TS=(hydrolys* or hydrolyz*) #6 TS=("amino acid*" or aminoacid* or casein* or fibre* or fiber* or prebiotic* or pre‐biotic* or soy* or whey*) #5 TS= (allerg* or hypoallerg* or hypo‐allerg* or hyperallerg* or hyper‐allerg* or hypersensitiv* or hyper‐sensitiv* or intoleran* or non‐allerg* or nonallerg* or sensitiv *) #4 TS=(formula* or bottle‐fed or bottle‐feed* or bottlefeed* or bottlefed) #3 TS=(breastfed or breast‐fed or breastfeed* or breast‐feed* ) #2 TS=(diet*) #1 TS=((colic* or crying or cries or cry) Near/10 (infant* or baby or babies or child*))
Cochrane Database of Systematic Reviews (CDSR), part of the Cochrane Library
#1[mh Colic] #2colic*:ti,ab #3[mh Crying] #4(cry or crying or cries):ti,ab #5{or #1‐#4} #6[mh Infant] #7(baby or babies or child* or infant* or newborn* or neonate*):ti,ab #8{or #6‐#7} #9#5 and #8 #10[mh Milk] #11[mh "Milk, Human"] #12[mh "Breast Feeding"] #13(breastfeed* or "breast feed*" or breastfed or "breast fed" or breastmilk* or "breast milk*" or milk*):ti,ab #14[mh ^Hypersensitivity] #15[mh "Food Hypersensitivity"] #16[mh ^Allergens] #17[mh "Lactose Intolerance"] #18(allerg* or hypoallerg* or hypo next allerg* or hyperallerg* or hyper next allerg* or hypersensitiv* or hyper next sensitiv* or intoleran* or non next allerg* or nonallerg* or sensitiv*):ti,ab #19[mh "Infant Food"] #20(formula* or bottle next fed* or bottlefed* or bottlefeed* or bottle next feed*):ti,ab #21[mh Hydrolysis] #22(hydrolys* or hydrolyz*):ti,ab #23[mh Prebiotics] #24[mh ^"Amino acids"] #25(amino next acid* or aminoacid* or casein* or fibre* or fiber* or prebiotic* or pre next biotic* or soy* or whey*):ti,ab #26[mh "Dietary Proteins"] #27[mh "diet therapy"/FS] #28diet*:ti,ab #29[mh "Dairy Products"] #30[mh Fishes] #31[mh Eggs] #32[mh Gluten] #33[mh Nuts] #34(cheese* or dairy or egg* or fish* or gluten* or wheat* or nut* or peanut* or lactose* or yogurt* or yoghurt*):ti,ab #35{or #10‐#34} #36 #9 and #35 in Cochrane Reviews (Reviews and Protocols)
Database of Abstracts of Reviews of Effects (DARE), part of the Cochrane Library
#1[mh Colic] #2colic*:ti,ab #3[mh Crying] #4(cry or crying or cries):ti,ab #5{or #1‐#4} #6[mh Infant] #7(baby or babies or child* or infant* or newborn* or neonate*):ti,ab #8{or #6‐#7} #9#5 and #8 #10[mh Milk] #11[mh "Milk, Human"] #12[mh "Breast Feeding"] #13(breastfeed* or "breast feed*" or breastfed or "breast fed" or breastmilk* or "breast milk*" or milk*):ti,ab #14[mh ^Hypersensitivity] #15[mh "Food Hypersensitivity"] #16[mh ^Allergens] #17[mh "Lactose Intolerance"] #18(allerg* or hypoallerg* or hypo next allerg* or hyperallerg* or hyper next allerg* or hypersensitiv* or hyper next sensitiv* or intoleran* or non next allerg* or nonallerg* or sensitiv*):ti,ab #19[mh "Infant Food"] #20(formula* or bottle next fed* or bottlefed* or bottlefeed* or bottle next feed*):ti,ab #21[mh Hydrolysis] #22(hydrolys* or hydrolyz*):ti,ab #23[mh Prebiotics] #24[mh ^"Amino acids"] #25(amino next acid* or aminoacid* or casein* or fibre* or fiber* or prebiotic* or pre next biotic* or soy* or whey*):ti,ab #26[mh "Dietary Proteins"] #27[mh "diet therapy"/FS] #28diet*:ti,ab #29[mh "Dairy Products"] #30[mh Fishes] #31[mh Eggs] #32[mh Gluten] #33[mh Nuts] #34(cheese* or dairy or egg* or fish* or gluten* or wheat* or nut* or peanut* or lactose* or yogurt* or yoghurt*):ti,ab #35{or #10‐#34} #36#9 and #35 in Other Reviews
LILACS (Latin American and Caribbean Health Science Information Database; search.bvsalud.org/portal/?lang=en)
tw:((colic* OR cries OR crying OR cry) AND (infant* OR newborn* OR neonate* OR baby OR babies OR child*)) AND (instance:"regional") AND ( db:("LILACS") AND type_of_study:("clinical_trials"))
IBECS (search.bvsalud.org/portal/?lang=en)
(tw:(colic* OR cries OR crying OR cry)) AND (tw:(infant* OR newborn* OR neonate* OR baby OR babies OR child*)) AND (instance:"regional") AND ( db:("IBECS")) AND (instance:"regional") AND ( type_of_study: ("clinical_trials"))
HomeoIndex (bases.bireme.br/cgi‐bin/wxislind.exe/iah/online)
Search on : colic$ or cry$ or cries [Words] and infant$ [Words]
Networked Digital Library of Theses and Dissertations (NDLTD; www.ndltd.org)
infant* AND colic AND random*
TROVE (limited to Australian theses; trove.nla.gov.au)
Keyword: Any of the words: infant* babies Keyword: Any of the words: colic* crying Keyword: Any of the words: random* placebo* control* blind* group*
Limited to Books Format: Thesis
WorldCat OCLC (limited to theses; www.worldcat.org)
(ti:infant* OR babies) AND (kw:colic* OR crying) AND (kw:random* OR trial* OR control* OR blind*)
PubMed Diet Supplement subgroup (ods.od.nih.gov/Research/PubMed_Dietary_Supplement_Subset.aspx)
#26 (#22 and #25) Filters: Dietary Supplements #25 (#23 or #24) Filters: Dietary Supplements #24 (baby [tiab] or babies [tiab] or child* [tiab] or infant [tiab] or newborn [tiab] or neonate [tiab]) Filters: Dietary Supplements #23 infant [mh] Filters: Dietary Supplements #22 (#9 and #21) Filters: Dietary Supplements #21 (#18 NOT #20) Filters: Dietary Supplements #20 ((animals [mh] NOT humans [mh])) Filters: Dietary Supplements #18 (#10 or #11 or #12 or #13 or #14 or #15 or #16 or #17) Filters: Dietary Supplements #17 groups [tiab] Filters: Dietary Supplements #16 trial [tiab] Filters: Dietary Supplements #15 randomly [tiab] Filters: Dietary Supplements #14 drug therapy [sh] Filters: Dietary Supplements #13 placebo [tiab] Filters: Dietary Supplements #12 randomized [tiab] Filters: Dietary Supplements #11 controlled clinical trial [pt] Filters: Dietary Supplements #10 randomized controlled trial [pt] Filters: Dietary Supplements #9 (#1 or #2 or #5 or #6 or #7 or #8) Filters: Dietary Supplements #8 (crying[tiab] or cry [tiab] or cries [tiab]) Filters: Dietary Supplements #7 crying [mh] Filters: Dietary Supplements #6 ((spasm* [tiab] or pain* [tiab] or cramp* [tiab])) AND (gastro* [tiab] or gastric[tiab]) Filters: Dietary Supplements #5 (((spasm* [tiab] or pain* [tiab] or cramp* [tiab]))) AND ((stomach[tiab] or abdominal [tiab] or abdomen* [tiab]) Filters: Dietary Supplements #2 colic* [tiab] Filters: Dietary Supplements #1 colic [mh] Filters: Dietary Supplements
ClinicalTrials.gov (clinicaltrials.gov)
infant colic AND Study type= intervention
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; apps.who.int/trialsearch)
Basic search infant* AND crying OR infant* AND cries OR infant* AND colic*
Appendix 2. 'Risk of bias' criteria and judgements
Sequence generation for randomisation
We included only RCTs or quasi‐RCTs in this review. We assessed randomisation at low risk of bias if the procedure of sequence generation was explicitly described. Examples include computer‐generated random numbers, a random numbers table or coin‐tossing. Where no description was given, we contacted the study authors for further information, and where we failed to receive a response, we assigned a judgment of unclear risk of bias. We considered studies to be at high risk of bias when reporting methods that were not random.
Allocation concealment
We assessed concealment of treatment allocation at low risk of bias if the procedure was explicitly described and adequate efforts were made to ensure that intervention allocations could not have been foreseen in advance of, or during, enrolment. Examples include centralised randomisation, numbered or coded containers, or sealed envelopes. We considered the following procedures to have a high risk of bias: alternation, or reference to case record numbers or dates of birth. Where no description was given, we contacted the study authors and, if we did not receive a response, we assigned a judgment of unclear risk of bias.
Blinding of parents and health professionals
In this context, the intervention is administered by parents and so, in effect, we considered them to be the target of the blinding procedures. Indeed, as the participants were less than four months of age by the defined inclusion criteria (Criteria for considering studies for this review), we deemed that this item was not applicable to them. Furthermore, parents often act as outcome assessors. We primarily assessed the risk of bias associated with the blinding of participants based on the likelihood that such blinding was sufficient to ensure that parents had no knowledge as to which intervention the infant received. We assessed blinding at low risk of bias where it was explicitly described how the parents could have no knowledge of which intervention the infant was receiving, and at high risk of bias where it was clear that parents were aware of which intervention the infant was receiving. We assessed blinding at unclear risk of bias where we did not have enough information to make an assessment of either high or low risk of bias, based on the information provided in the papers, or after contacting the study authors.
Blinding of outcome assessment
For each included study we described the methods used, if any, to blind the outcome assessors from knowledge of which intervention a participant received. We judged studies at low risk of bias if they were blinded, or if we considered that the lack of blinding could not have affected the results. If blinding was not possible because of the nature of intervention, we judged the study at high risk of bias since it is possible that the lack of blinding influenced the results. We noted the blinding of health professionals where reported. Where no description was given, we contacted the study authors for more information, and where we did not receive a response, we assigned a judgment of unclear risk of bias.
Incomplete outcome data
Incomplete outcome data essentially include attrition, exclusions, and missing data.
We assigned a judgment of low risk of bias if:
participants included in the analysis were exactly those who were randomized into the trial, if missing outcome data were balanced in terms of numbers across intervention groups, with similar reasons for missing data across groups, or if there were no missing outcome data;
for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk was not sufficient to have a clinically relevant impact on the intervention effect estimate;
for continuous outcome data, the plausible effect size (SMD) among missing outcomes was not sufficient to have a clinically relevant impact on observed effect size; or
missing data were imputed using appropriate methods.
We assigned a judgment of high risk of bias:
when reasons for missing outcome data were likely to be related to the true outcome, with either an imbalance in numbers or reasons for missing data across intervention groups;
for dichotomous outcome data, when the proportion of missing outcomes compared with observed event risk was sufficient to induce clinically relevant bias in the intervention effect estimate;
for continuous outcome data, when the plausible effect size (SMD) among missing outcomes was sufficient to induce clinically relevant bias in the observed effect size;
when an 'as‐treated' analysis was carried out in cases where there was substantial departure of the intervention received from that assigned at randomisation; or
when there was a potentially inappropriate application of simple imputation.
We assigned a judgment of unclear risk of bias:
when there was insufficient reporting of attrition or exclusions (or both) to permit a judgment of low or high risk of bias;
if the study reported incomplete outcome data; or
if the numbers randomized to intervention and control groups were not clearly reported.
Selective outcome reporting
We assessed the reporting of outcomes at low risk of bias if all study outcomes declared in the Methods section were reported in the Results section. We also evaluated whether different reports of the study were available, including protocols, and examined them to ensure there was no suggestion of selective outcome reporting. Where no description was given, we contacted the study authors for more information, and where no response was received, we assigned a judgment of unclear risk of bias. Where there was evidence of selective outcome reporting, we assigned a judgment of high risk of bias.
Other potential threats to validity
Where a study was at risk of other sources of bias, we assessed it at high risk of bias. For instance, sources of sponsorship or funding constitute one common example of a factor which may pose a risk of bias. We assessed each study at low risk of bias if it appeared to be free from such threats to validity. Where the risk of bias was unclear from the published information, we attempted to contact the study authors for clarification. Where this information was not forthcoming, we assessed these studies at unclear risk of bias.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Campbell 1989.
| Methods | Single‐centre, double‐blind, randomised controlled trial with 2 treatment groups | |
| Participants |
Sample size: 19 infants diagnosed with colic (0 dropped out) Setting: recruited in a single town (Livingston, West Lothian in Scotland, UK) Sex: 11 boys (58%), 8 girls (42%) Mean age: 7 weeks (SD not reported, range 3 to 14) Mean weight: not reported Mean duration of colic (baseline): not reported Mean crying (baseline): not reported Feeding: formula fed (100%) Birth order: 4 first born, 7 second born, 8 third to sixth born Inclusion criteria: formula‐fed infants with a clinical diagnosis were included in the study if they met the Wessel 1954 criteria Exclusion criteria: spontaneous remission in the observation week, colic not severe or already improving during baseline week. Does not specify that babies were 'otherwise well'; however, referral was via GP or HV who considered the baby to have infant colic |
|
| Interventions |
Intervention (n = 10): soy formula Control (n = 9): standard formula Duration: 1 week |
|
| Outcomes | Mothers asked to complete a record sheet noting the amount of time of baby's colic symptoms each day. Record sheets scored by totaling all the periods of colic, to the nearest half hour, for 6 days of the week, omitting the first day of the week to allow for transition from previous milk | |
| Notes |
Study start and end dates: not recorded; however it was a 2‐year study period and published in 1989 COIs: none stated Funding source: formula provided by Cow & Gate. Author was a GP, doing a research fellowship funded by the Health Service Research Committee of Scottish Home and Health Department Adverse effects: not reported Comments: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "on the basis of random assignment" Comment: no further details given. Wrote to study author but received no response |
| Allocation concealment (selection bias) | High risk | Comment: no details given. Wrote to study author but received no response |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "control and intervention were packaged in identical coded tins" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "and the code of each pair of milks was not broken until the end of the ... period so that the observations would be double blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all data recorded. Accounted for all patients |
| Selective reporting (reporting bias) | High risk | Comment: no specific mention or reporting of adverse effects |
| Other bias | Low risk | Comment: not apparent |
Forsyth 1989.
| Methods | Double‐blind, randomised control trial with 2 treatment groups (cross‐over study but we are using just the first trial) | |
| Participants |
Sample size: 32 infants (40 referred but 8 did not satisfy eligibility criteria) diagnosed with colic enrolled (15 dropped out: 6 did not begin taking the formula, 5 did not complete the diary and 4 discontinued the study after beginning it – specific groups not described) Setting: private practitioners and Department of Pediatrics, and Yale Child Study Center, New Haven, Connecticut, USA Sex: 11 boys (65%) Mean age: 5.38 weeks (SD 1.54, range 4 to 7) Mean weight: not reported Mean duration of colic (baseline): not reported Mean crying (baseline): not reported Feeding: formula fed (100%) Birth order: not reported Inclusion criteria: aged 8 weeks or less at the time of enrolment, formula fed, crying reported 3 or more h/d, parents' subjective description of colic Exclusion criteria: any other causes of excessive crying |
|
| Interventions |
Intervention (n = 9): Nutramigen casein hydrolysate Control (n = 8): ''a cow milk formula" (quote); 1 part Enfamil (standard formula) and 2 parts Nutramigen, to ensure blinding or alternative as per cross‐over trial Duration: 4‐day period and then crossed over |
|
| Outcomes | Mothers recorded crying episodes in diaries and indicated which episodes they considered to have been caused by colic. Crying quantified in h/d | |
| Notes |
Study start and end dates: not reported, although this paper was written in early 1989 COIs: none specifically reported; however, study funded by manufacturers of the product that was used as the intervention Funding source: supported by a grant from Mead Johnson, whose products were used Adverse effects: not reported Comments: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random assignment table of random numbers" |
| Allocation concealment (selection bias) | Low risk | Comment: assignment by central pharmacy |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: described as double‐blind, but no details given and none received from study author |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: described as double‐blind, but no details given and none received from study author |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: only partial details given on dropouts and which group they were from. Whilst some inference can be made for an intention‐to‐treat analysis, further details were not available from the study author |
| Selective reporting (reporting bias) | High risk | Comment: no specific mention or reporting of adverse effects. No response received from study author |
| Other bias | High risk | Comment: report says that cans of formula were prepared by Mead Johnson. No further details of involvement and no response received from study author |
Hill 1995.
| Methods | Double‐blind, randomised, placebo‐controlled trial over 1 week with 4 treatment groups | |
| Participants |
Sample size: 38 formula‐fed and 77 breastfed infants (36 dropped out – 18 in each group) Setting: metropolitan, community‐based, well‐infant centres, Melbourne, Australia Sex: not reported Mean age: not reported (SD not reported) Mean weight: not reported Mean duration of colic (baseline): 5.1 h/d (SD 3.0) intervention, 5.9 h/d (SD 3.1) control Mean crying (baseline): The mean day 1 total distress score for the intervention diet group was 330.5 minutes, and for the control diet group, 268.0 minutes (P = 0.12) Feeding: formula fed (33%; n = 38) Inclusion criteria: aged 4‐16 weeks, uncomplicated pregnancy of more than 37 weeks' duration, uneventful perinatal period, colic definition 'rule of three', and otherwise healthy except for colic. Also included those on medication for colic, as long as medications continued throughout the trial Exclusion criteria: not reported |
|
| Interventions | Intervention (n = 54): mothers of breastfed babies given a hypoallergenic or anti‐oligogenic diet (excluding food dyes, additives, preservatives, milk, egg, wheat or nuts), and formula‐fed babies provided with a casein based hydrolysate formula (Pregestamil) Control (n = 61): mothers of breastfed babies given a standard oligoantigenic diet (avoiding food dyes, additives, preservatives), and formula‐fed babies given standard formula (Enfamil Reduced Iron) Duration: 1 week | |
| Outcomes | Parents instructed in the use of a 24‐h distress score chart, which they were asked to complete on day 1 and day 8 of the trial, with distress marked in min/h | |
| Notes |
Study start and end dates: not reported; however, paper does report that the original study was intended to be 12 months, but that it was harder to recruit than they had expected and the study was finally closed after 3 years COIs: none reported Funding source: supported by a grant from Mead Johnson Adverse effects: none reported Comments: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: says it is randomised. Wrote to study author but received no response |
| Allocation concealment (selection bias) | Low risk | Comment: sealed envelopes for assignment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: double‐blind – identical sealed tins |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: as above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all patient outcomes described |
| Selective reporting (reporting bias) | High risk | Comment: no specific mention or reporting of adverse effects. No response received from study author |
| Other bias | High risk | Comment: supported by a grant from Mead Johnson. No further details of involvement and no response received from study author |
Hill 2005.
| Methods | Randomised controlled trial with 2 treatment groups | |
| Participants |
Sample size: 107 infants (17 dropped out – intervention = 6, control = 11) Setting: metropolitan, community‐based, well‐infant centres, Melbourne, Australia Sex: 54 boys (50%) 53 girls (50%) Mean age: 5.7 weeks (SD 1.1, range 2.9 to 8.6) Mean weight: not reported Mean duration of colic (baseline): Mean distress duration at start of diet, in weeks intervention = 3.2 (range = 1 to 7), control = 3.4 (range = 1 to 6.0) Mean crying (baseline): not reported Feeding: breastfed (100%) Birth order: not reported Inclusion criteria: < 6 weeks of age, breastfed, well‐term infants (gestational age of > 37 weeks), normal singleton pregnancy, otherwise uneventful perinatal history and no perinatal morbidity other than distress, and presence of 'rule of three' crying in the week before presentation Exclusion criteria: mothers who were vegan, babies who were formula fed, spontaneous improvement |
|
| Interventions | Intervention (n = 47): low‐allergen diet without milk, soy, nuts, eggs, wheat and soy, but including a rice drink every day and rice bread Control (n = 43): diet must have included milk, peanuts, egg, wheat, fish, tree nuts and soy every day Duration: 1 week | |
| Outcomes | Detailed food diary by mothers, and recording of infant crying or fussing behaviour on a pre‐validated chart, for 48 h on days 1, 2, 8 and 9 | |
| Notes |
Study start and end dates: 2000 (start) to 2002 (end) COIs: not reported but see funding source directly below Funding source: financed by the Rice Growers' Co‐operative, Australia. Intervention was rice milk and rice bread Adverse effects: none reported Comments: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "referred to the Department of Allergy for diet randomisation" Quote: "assigned to one of the diets by the research dietician on the basis of a randomisation schedule provided by the statistician" Comment: no further details. Wrote to study author but received no response |
| Allocation concealment (selection bias) | High risk | Comment: no details. Wrote to study author but received no response |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not possible to blind participant mothers as diets were different |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: not possible to blind participant mothers as diets were different |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: participant enrolment/study progress diagram included in paper. Accounted for all participants |
| Selective reporting (reporting bias) | High risk | Comment: no specific mention or reporting of adverse effects. No response received from study author |
| Other bias | High risk | Comment: funded by Rice Grower's Co‐operative, and intervention includes a rice drink and rice bread daily. No further details of involvement and no response received from study author |
Jakobsson 2000.
| Methods | Randomised, cross‐over‐style, baseline‐control trial with 2 treatment groups | |
| Participants |
Sample size: 22 enrolled infants (7 dropped out – 3 from CH1 and 4 from CH2) Setting: outpatient clinic of a hospital. Recruited from well‐baby clinics in Malmö, Sweden Sex: 7 boys (47%) 8 girls (53%) Mean age: not reported (SD not reported, range 2 to 8 weeks) Mean weight: not reported Mean duration of colic (baseline): not reported Mean crying (baseline): 7.36 h/d (SD 1.32) Feeding: formula fed (100%) Birth order: not reported Inclusion criteria: severe colic: "crying for many times per day for at least 4 days in a week, and continuing for one week or more with each episode lasting 30 minutes to 2 hours, totaling > 3 hours per day" (quote). Symptoms not resolved after parenting and feeding advice given to mother. Anti‐spasmodic and anti‐cholinergic drugs to manage the colic may have been tried prior to enrolment Exclusion criteria: spontaneous resolution, removed for vomiting, refused to follow the protocol, families must not have previously used hydrolysed formula for their babies |
|
| Interventions |
Intervention (n = 22): Alimentum hydrolysate formula with iron (Abbott Laboratories, CH1) or Nutramigen hydrolysate formula with iron (Mead Johnson Nutritionals, CH2) Control (n = 22): baseline infant's standard formula Duration: 1 week |
|
| Outcomes | Parents recorded daily crying time in h/d, crying intensity, and other colicky behaviour (summarised as a percentage of the days a particular feature was observed). Formula intake and stool consistency also recorded | |
| Notes |
Study start and end dates: not reported COIs: not reported Funding source: not reported Adverse effects: none reported Comments: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "at enrolment, infants were randomised to one of two feeding sequences: CH1 for a week then CH2, or CH2 for a week then CH1 in crossover style" |
| Allocation concealment (selection bias) | High risk | Comment: no details. Wrote to study author but no received response |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no details. Wrote to study author but received no response |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no details. Wrote to study author but received no response |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: some data missing after death of one of the investigators |
| Selective reporting (reporting bias) | Low risk | Comment: mention of clear methods and adverse effects no protocol. |
| Other bias | Low risk | Comment: none noted |
Kanabar 2001.
| Methods | Double‐blind, randomised, placebo‐controlled cross‐over trial with 2 treatment groups | |
| Participants |
Sample size: 53 infants (7 dropped out – groups not clear) Setting: Guy & St Thomas Hospital London, UK Sex: not reported Mean age: not reported (SD not reported, range 3 to 13 weeks). Mothers given trial literature in the recovery room after birth and asked to get in touch if their babies had colic Mean weight: not reported Mean duration of colic (baseline): not reported Mean crying (baseline): not reported Feeding: not reported Birth order: not reported Inclusion criteria: visit from community midwife at home to establish if baby meets trial criteria. Colic according to Wessel criteria adapted to 14 days of symptoms, does not specify that infants were otherwise well, but recruitment was via general population and supervised by a midwife Exclusion criteria: changes of address, and a failure to understand the dosage instructions |
|
| Interventions |
Intervention (n = not stated): Colief lactase drops. For formula‐fed babies, 2 drops added to every made‐up formula bottle then bottle refrigerated for 4 h before use. For breastfed babies, mother to express 'foremilk' onto a spoon, add 4 drops of lactase, and then give this at the end of a feed (having tested this method of short incubation with small volume of milk in vitro, using 15 mL Aptamil – not breast milk) Control (n = not stated): heat inactivated placebo of the lactase obtained from the manufacturer, in identical packaging, delivered in the same way Duration: treatment for each feed for 10 days |
|
| Outcomes | Parents noted total daily crying time in minutes over 10 days, and breath hydrogen testing done before and after a single feed on day 10 to assess whether treatment produced 45% less crying time | |
| Notes |
Study start and end dates: not reported COIs: Treatment products – both intervention and control – provided by Crosscare Ltd, the manufacturer of Colief, which was being tested in this study Funding source: not reported but see COIs directly above Adverse effects: none reported Comments: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned by use of a predetermined computer‐generated randomisation schedule, to verum or placebo arm for 10 days" |
| Allocation concealment (selection bias) | High risk | Comment: no details. Wrote to study author but received no response |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: double‐blind, identical packaging of intervention and control |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: says double‐blind but paper does not make clear whether outcome assessors were aware of treatment arm when analysing parents' records. Wrote to study author but received no response |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: paper states that 46 (out of 53) participants were available for "cry time analysis" (quote) and the reasons for non‐availability included changes of address and failure to understand dosage instructions. A significant proportion (14/46) were found to be non‐compliant with the usage of the lactase or placebo (judged by the amount that was gone from the bottle) |
| Selective reporting (reporting bias) | Low risk |
Comment: paper reports on per‐protocol and intention‐to‐treat data but the results from the two cross‐over arms are lumped together so meta‐analysis not possible Adverse effect data given |
| Other bias | High risk |
Comment: this is a cross‐over study but the data is lumped together so we could not, as preferred, refer only to data from the first arm of the study. Wrote to study author for separate data but received no response Treatment products – both intervention and control – provided by Crosscare Ltd, the manufacturer of Colief, which was being tested in this study |
Kearney 1998.
| Methods | Randomised, double‐blind, cross‐over trial with 2 treatment groups | |
| Participants |
Sample size: 13 infants (0 dropped out) Setting: general practices and paediatric clinics in Cork, Ireland Sex: 9 boys (69%) 4 girls (31%) Mean age: 53.5 days (SD 26.2, range 23 to 113 days) Mean weight: not reported Mean duration of colic (baseline): not reported Mean crying (baseline): not reported Feeding: formula fed (100%) Birth order: not reported Inclusion criteria: formula‐fed infants with colic symptoms using a modified Wessel's criteria: full force crying for ≥ 3 h/d for ≥ 3 d/week. Their definition did not require crying of 3 weeks or more Exclusion criteria: 'otherwise well' (quote) |
|
| Interventions |
Intervention (n = not reported): lactase (Lactaid) drops; 3 drops added to each feed, which was then refrigerated for 24 h before feeding to the baby Control (n = not reported): placebo supplied by manufacturer for lactase (Lactaid) drops; 3 drops added to each feed, which was then refrigerated for 24 h before feeding to the baby Duration: 1 week |
|
| Outcomes | Parents asked to keep a diary containing information about baby's crying time, stool habit and details of volumes, strength and type of formula | |
| Notes |
Study start and end dates: not known COIs: none reported Funding source: Mediplan and Myplan, who make the lactase product Lactaid, which was used as the intervention Adverse effects: none reported Comments: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random permutated blocks of size four to ensure that the numbers of babies assigned to the two treatment orders were fairly even" |
| Allocation concealment (selection bias) | High risk | Comment: no details. Wrote to study author but received no response |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "preparations given in bottles marked week 1 and week 2 to ensure the double blind nature of the trial" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no details at all. Wrote to study author but received no response |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk |
Quote: "13 babies completed the trial" Comment: does not say how many began the trial |
| Selective reporting (reporting bias) | Low risk |
Comment: results from each cross‐over arm are lumped together in the data reports so meta‐analysis not possible Adverse effect data given |
| Other bias | High risk | Comment: funded by industry and no response received from study author to confirm level of involvement |
Lothe 1987.
| Methods | Double‐blind, cross‐over trial with 1 treatment group | |
| Participants |
Sample size: 65 infants (5 dropped out) Setting: children's hospital in Malmö, Sweden Sex: 23 boys (38%) 37 girls (62%) Mean age: 6.38 weeks (SD 2.50, range 3 to 13) Mean weight: not reported Mean duration of colic (baseline): not reported Mean crying (baseline): not reported Feeding: predominantly or totally formula‐fed infants Birth order: not reported Inclusion criteria: severe colic (paroxysmal abdominal pain, severe crying for several hours per day especially between 5 pm and 10 pm, abdomen distended by gas, and the wish to suck often). Infants receiving dimeticonum, methylscopolaminum and dicycloverine‐chloride with no effect were able to participate. Exclusion criteria: urinary infections |
|
| Interventions |
Intervention (n = not clear): one can of ProSobee (soy protein‐based formula) for 1 week Control (n = not clear): Enfamil (standard cows' milk protein‐based formula) for 1 week Duration: observation period of 1 week followed by a week of either cows' milk protein‐based formula or soy protein‐based formula. The infants were then swapped onto the other formula and the results of the 2 cross‐over arms' were pooled for reporting in the paper |
|
| Outcomes | Parents given standardised protocols and asked to record the length of the crying period and any changes in stools and vomit | |
| Notes |
Study start and end dates: 1980 (start); end date not reported COIs: not reported Funding source: control and intervention products manufactured and provided free by Mead Johnson Adverse effects: not reported Comments: formulas were provided and coded by Mead Johnson; any babies still symptomatic on soy formula were then given cows' milk protein hydrolysate formula Nutramigen, also provided by Mead Johnson |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: says double‐blind cross‐over. Does not say randomised anywhere |
| Allocation concealment (selection bias) | High risk | Comment: no details. Wrote to study author but received no response |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: says double‐blind cross‐over. Cans were labelled by Mead Johnson |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "at the end of the test period ... the protocols were evaluated and the code was broken" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 60 participants (out of 65 at outset) completed the trial. No explanation of the other 5 |
| Selective reporting (reporting bias) | High risk | Comment: reported on a per‐protocol rather than an intention‐to‐treat basis, no specific adverse effect data given |
| Other bias | Unclear risk | Comment: cans were labelled by Mead Johnson |
Lucassen 2000.
| Methods | Randomised, double‐blind, parallel trial with 2 treatment groups | |
| Participants |
Sample size: 38 infants (5 dropouts: 2 from illness, 2 from non‐compliance, and 1 referred because of worsening symptoms – 3 intervention, 2 control) Setting: infants recruited from community‐based, well‐child clinics in 6 regions of an area of Holland with 7500 births annually, co‐ordinated by the Academic Hospital of Vrije University, Amsterdam, The Netherlands Sex: 19 boys (50%) Mean age: 7.7 weeks (SD not reported, range 6.4 to 8.9) intervention; 8.3 weeks (SD not reported, range 6.6 to 10.1) control Mean weight: 4.5 kg (4.2 to 4.8) intervention; 4.9 kg (4.3 to 5.6) control Mean duration of colic (baseline): 403 min/day (341 to 466) intervention; 328 min/day (291 to 366) control Mean crying (baseline): 299 min/day (251 to 347) intervention; 267 min/day (226 to 307) control Feeding: fully formula fed or fed with mix of breast and formula, but the paper neither specifies how many babies were also receiving breast milk, nor treated the receipt of breast milk as a confounding factor Birth order: not reported Inclusion criteria: infants with good medical condition on examination by clinic doctor, thriving, formula fed (at least 1 formula feed per day), good feeding technique, < 6 months old, crying > 3 h/d on at least 3 d/week Exclusion criteria: history of anaphylaxis to cows' milk, previous trial of hypoallergenic feeding, refusal to give informed consent, communication problems, referred to paediatrician, no crying problem anymore, other illness, other advice from own doctor, refusal to keep diary |
|
| Interventions |
Intervention (n = 23): Nutricia whey hydrolysate formula Control (n = 20): Nutricia standard formula Duration: 1‐week study after baseline for one week |
|
| Outcomes | Parents instructed on the use of a 24‐h diary and questionnaire to assess minutes of crying per 24 h, which included the question, "Do you know which formula your infant has been using this week?" (quote) to test for adequate blinding. Evaluation of results to include proportion of infants who, after the intervention, would no longer meet the inclusion criteria | |
| Notes |
Study start and end dates: August 1994 (start) to October 1996 (end) COIs: Nutricia provided the formula Funding source: Praeventie Fonds – the Dutch National Preventative Fund Adverse effects: none reported Comments: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: randomised to intervention or control group by SPSS Inc, Chicago |
| Allocation concealment (selection bias) | Low risk | Comment: response from study author as follows (quote), "a box containing the formula was sent to each participating centre. Each box contained 16 cans of formula of which 8 contained hypo‐allergenic formula and 8 normal formula (of which taste and smell were changed in the direction of hypoallergenic formula). All cans were identical and contained a sticker with a number. Each set of two cans had the same number (so it was impossible to provide one can with hypoallergenic formula and one can with control formula to the same infant). The local centre distributed the cans, two for each infant. The persons at the local centre were completely unaware of the codes. The codes were generated with a random numbers list" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: study author responded. Blinding of parents was made possible by changing the taste and smell of the control formula in the direction of taste and smell of the hypoallergenic formula |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: study author responded. Blinding of the parents was made possible by changing the taste and smell of the control formula in the direction of taste and smell of the hypoallergenic formula |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: accounted for all participants |
| Selective reporting (reporting bias) | Low risk | Comment: study author contacted and we were informed that there were no adverse effects |
| Other bias | Unclear risk | Comment: Nutricia provided the formula. Contact from the study author tells us that Nutricia did not play any role except in the provision of the formulas; they were not involved in writing or checking the manuscript |
Miller 1990.
| Methods | Double‐blind, cross‐over trial with 2 treatment groups | |
| Participants |
Sample size: 15 (0 dropped out) Setting: infants recruited from a family care centre serving the northern suburbs of Sydney, Australia Sex: 5 boys (33%) 10 girls (67%) Mean age: 6.5 weeks (SD 2.2, range 3 to 9) Mean weight: not reported Mean duration of colic (baseline): not reported Mean crying (baseline): not reported Feeding: breastfed (100%) Birth order: not reported Inclusion criteria: total duration of crying and fussing of at least 3 h in 2 consecutive 24‐h periods; crying and fussing behaviour not responding to mother‐craft skills, and no apparent cause for the crying and fussing Exclusion criteria: hydrogen concentration in breath of over 20 ppm |
|
| Interventions |
Intervention (n = not reported): lactase (Lactaid) in glycerol, 6 drops into baby's mouth within 5 min of commencing feed Control (n = not reported): glycerol with water and caramel, 6 drops into baby's mouth within 5 min of commencing feed Duration: 7 days in each treatment arm |
|
| Outcomes | Mothers instructed on how to complete a 24‐h recording form detailing durations of infant behaviours such as sleeping, being awake and content, crying, fussing and feeding, to determine the mean number of minutes of crying or fussing in 24 h. Also, measurement of H2 concentrations in pre‐and post‐prandial breath tests, to determine the effect of yeast lactase on the mean value of each infants' breath hydrogen | |
| Notes |
Study start and end dates: July 1987 (start) to June 1988 (end) COIs: none reported Funding source: Sharpe Laboratories, which seemed to have produced the active and placebo preparations, but no details available as to any further extent of their involvement Adverse effects: none reported Comments: cross‐over study but we reported on the first arm only. Based on PhD Thesis by John Miller |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "infants were randomly allocated" Comment: we contacted the study author who was unable to confirm how the random list was generated Quote: "Dr D Shaw, Principal Consultant, Siromath, Sydney, performed the sample size calculation and I recall that he also provided a randomisation schema for allocation of the treatments to the breast fed, and to the formula‐fed infants. I don't remember how these randomisation schema were derived" |
| Allocation concealment (selection bias) | High risk | Comment: no details. Wrote to study author who was unable to confirm whether this was done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: described as double‐blind but method not specified. Wrote to study author who responded stating, "The active & placebo enzyme preparations were aseptically filled into plastic squeeze bottles, capped, packed into tamper‐proof plain cardboard cartons and labelled with a code by Sharpe Laboratories, Sydney. The packaged preparations were indistinguishable except for the labelled code." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: no details. Wrote to study author who responded stating, "The active & placebo enzyme preparations were aseptically filled into plastic squeeze bottles, capped, packed into tamper‐proof plain cardboard cartons and labelled with a code by Sharpe Laboratories, Sydney. The packaged preparations were indistinguishable except for the labelled code." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 15 entered the study, but only 12 completed the study and no reasons for non‐completion are provided in the paper. We contacted the study author who replied stating, "Of these infants, 2 ‘dropped out' – 1 on active treatment, the other on placebo. In the former case, active treatment was discontinued after 48 hours because the parents considered the treatment worsened crying and fussing behaviour. In the latter case, the mother stopped using the placebo after 4 days because of lack of effect. One infant was withdrawn from the study. In this case, the mother was admitted to hospital with a breast abscess. The infant was temporarily weaned. However, at the mother's request, active treatment was continued." |
| Selective reporting (reporting bias) | Low risk | Comment: results are based on per protocol, not intention‐to‐treat, but all major outcomes are reported, with adverse effect data given by study author |
| Other bias | Unclear risk | Comment: designed as cross‐over trial but we reported on the first arm only. Sharpe Laboratories were the study sponsor and seemed to have produced the active and placebo preparations but no details were available as to any further extent of their involvement. The study author confirmed no involvement. |
Oggero 1994.
| Methods | Randomised controlled trial with 3 treatment groups | |
| Participants |
Sample size: 120 infants (0 dropped out) Setting: Department of Pediatrics at the University of Turin, Children Hospital Regina Margherita, Turin, Italy Sex: not reported Mean age: 6.2 weeks (SD not reported, range 3‐12 weeks) Mean weight: not reported Mean duration of colic (baseline): not reported Mean crying (baseline): not reported Feeding: formula fed (n = 89), and breastfed (n = 31). No mention of any of the babies being mixed fed Birth order: not reported Inclusion criteria: infants aged 3‐12 weeks suffering from severe colic, symptoms lasting for at least 2 weeks, presence of inconsolable crying, closed fists and meteorism, presence of sleep disorders, crying for more than a total of 3 h/d, no response to common consolation procedures (pacifier, rocking, dull continuous background noise, hot water bottle on abdomen) Exclusion criteria: known organic causes of abdominal pain |
|
| Interventions |
Intervention (n = 60): hypoallergenic dietary regimen. Breastfed babies' mothers were given a diet containing no milk, eggs or fish; formula‐fed babies were given soy milk (If symptoms continued the symptomatic, formula‐fed babies were moved onto Nutramigen for 15 days – these data are not included in our analyses) Control (n = 60): gastrointestinal antispastic drugs. All infants given dicyclomine hydrocholoride (3 mg/kg/day divided into 3 doses per day), and no dietary modifications were made Duration: 15 days |
|
| Outcomes | Parents asked to note the beginning and end of unexplained crying spells and to note the beginning and end of unexplained periods of fussiness or irritability. The evaluation of treatment results based on this information gathered by parents and written in a diary | |
| Notes |
Study start and end dates: not reported in the text. October 1991 (start) to January 1993 (end) COIs: none reported Funding source: none reported Adverse effects: none reported Comments: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly divided" |
| Allocation concealment (selection bias) | High risk | Comment: participants were randomly assigned to the different groups but the type of treatment was known (not blinded) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not possible to blind |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: not a blinded study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: accounted for all participants |
| Selective reporting (reporting bias) | High risk | Comment: study authors reported results for all outcomes declared in the Methods section, except adverse effects which are not reported. |
| Other bias | Low risk | Comment: no significant differences between groups at baseline were reported |
Savino 2005.
| Methods | Randomised, double‐blind, placebo‐controlled trial with 2 treatment groups | |
| Participants |
Sample size: 93 colicky infants (5 dropped out (2 = intervention, 3 = control); 2 did not come to the second visit, 3 were excluded because of fever. Nobody withdrew because of problems related to the trial and therefore the study population may be considered homogeneous) Setting: recruited from patients seen at the Department of Pediatrics, Regina Margherita Children's Hospital, University of Turin, Italy Mean age: 4.2 weeks (SD 1.4, range not reported) intervention; 4.4 weeks (SD 1.6, range not reported) control Sex: 41 (46.6%) boys (18 intervention, 23 control); 47 (53.4%) girls (23 intervention, 24 control) Mean weight: 3420 g (SD 390) intervention; 3510 g (SD 330) control Mean duration of colic: not reported Mean crying: 201.2 min (SD 18.3) intervention; 198.7 min (SD 16.9) control Feeding: not specified Birth order: not specified Inclusion criteria: colic according to Wessel criteria, breastfed, healthy infants with regular growth, 21 to 60 days old, born at term (gestational age 38 to 42 weeks), birth weight between 2500 g and 4000 g, no clinical evidence of gastroenterological disease, and Apgar (appearance, pulse, grimace, activity, respiration) score > 7 at 5 min after birth Exclusion criteria: infants receiving any medication, such as antibiotics or probiotics, which could affect abdominal symptoms |
|
| Interventions |
Intervention 1 (n = 41): phytotherapeutic agent (extracts of Foeniculum vulgare (fennel), Matricaria recutita (chamomile), and Melissa officinalis (lemon balm)). Each dose of herbal agent consisted of 1 bottle, with tank cap, containing Foeniculum vulgare miller var. dulce (164.29 mg), Matricaria recutita L. (177.69 mg), Melissa officinalis L. (96.89 mg), vitamin B1 (0.85 mg), calcium pantothenate (3.24 mg), vitamin B6 (1.20 mg), maltodextrin (dose not specified) and syloid 244 FP (dose not specified) (ColiMil, Milte‐Milan, Italy). At the administered dosage, the herbal agent provided Foeniculum vulgare miller var. dulce 65.71 mg/kg/d, Matricaria recutita L. 71.10 mg/kg/d, and Melissa officinalis L. 38.75 mg/kg/d Control (n = 47): placebo looking like the phytotherapeutic agent with regard to colour, smell, taste and package, but containing only vitamins. Each dose of placebo consisted of 1 identical bottle, with tank cap, containing water obtained by inverted osmosis, fructose, pineapple flavour, citric acid and sorbate potassium. Administration: both herbal agent and placebo were administered twice a day at 5 pm and 8 pm, some minutes before feeding, at a dosage of 2 ml/kg/d. Infants had to take treatment consecutively for 7 days Duration: 21 days |
|
| Outcomes | Parents wrote a daily, structured diary, recording (1) the start of crying time – when the medication was administered, (2) the end of crying time, and (3) any side effects (vomiting, sleepiness, restlessness, appetite, cutaneous reactions, constipation, diarrhoea) they observed for the 7 days of therapy and until day 21 from enrolment. Before starting treatment, parents were invited to record data on daily crying time for 3 days (days 0, 1, and 2). At days 1 and 7, infants were seen in the department, and parents gave the diary to researchers. At day 21, after baseline, mothers were asked to complete a questionnaire about crying time during the observation period. To ensure that all parents noted crying time in a uniform way, and to ensure that infants were given medication correctly, a researcher was always available by phone to help parents. Therapy was considered effective if crying time was reduced by ≥ 50% per day; responders were infants who showed such a reduction in crying time | |
| Notes |
Study start and end dates: March 2001 (start) to March 2003 (end) COIs: none reported Funding source: funded, in part, by Milte who provided the study products but had no other role in the study; they were not involved in writing or checking the manuscript Adverse effects: no adverse effects Comments: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: this was performed by computer |
| Allocation concealment (selection bias) | Low risk | Comment: conducted by a statistician not involved with the study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: placebo looked like the phytotherapeutic agent with regard to colour, smell, taste and packaging Quote: "Neither doctors nor parents knew whether the infants received treatment or not" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Neither doctors nor parents knew whether the infants received treatment or not" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 5 infants (2 from the intervention group and 3 from the placebo group) dropped out: 2 did not come to the second visit, and 3 were excluded because of fever. Nobody withdrew because of problems related to the trial. |
| Selective reporting (reporting bias) | Low risk | Comment: study authors reported results for all outcomes declared in the Methods section |
| Other bias | Low risk | Comment: no significant differences between groups at baseline were reported |
Savino 2006.
| Methods | Prospective, randomised controlled trial with 2 treatment groups | |
| Participants |
Sample size: 267 recruited (68 withdrawn (34 intervention, 34 control); 45 (20 intervention, 25 control) did not meet inclusion criteria by the time the study began, and 23 (14 intervention, 9 control) excluded during the study due to missing data) Setting: 78 general paediatricians and the Department of Pediatrics based at the University of Turin, Children Hospital Regina Margherita, Turin, Italy Sex: 50 (52.1%) boys intervention, 49 (47.6%) boys control; 47.9% girls intervention, 52.4% girls control Mean age: 1.39 months (SD 0.84, range not reported) intervention; 1.29 months (SD 0.77, range not reported) control; less than 4 months at age of entry into study Mean weight: not reported Mean duration of colic (baseline): 5.99 colic episodes per day (SD 1.84) intervention, 5.41 colic episodes per day (SD 1.88) control Mean crying (baseline): not reported Feeding: formula fed (100%) Birth order: not reported Inclusion criteria: infants aged less than 4 months, with infantile colic according to Wessel definition, gestational age between 37 and 42 weeks, normal birth weight (> 2500 g), regular weight gain (more than 150 g/week) and normal physical examination Exclusion criteria: neonatal problems, consumption of any kind of medication during the week before the beginning of the study and during the study period |
|
| Interventions |
Intervention (n = 130): Numic oOmneo Comfort (100% whey protein, low lactose, contains GOS/FOS)
Control (n = 137): Numico Nutrilon Standard 1 formula plus simethicone (rationale being that simethicone* is as effective as placebo (Lucassen 1998), so used as placebo)
Duration: 2 weeks *Simethicone is an anti‐foaming agent designed to reduce the surface tension of bubbles of gas trapped in liquid, so they group together and can be passed more easily. As such, this is not a dietary modification and its addition to the standard formula was intended, in this case, to assess the superiority of the intervention formula, which contained oligosaccharides thought to improve the balance of the infant's microbiota, over the common recommendation to administer simethicone to infants with colicky symptoms, alongside their regular milk. The oligosaccharide blend used in this study formula was reported as being 90% galacto‐oligosaccharide and 10% fructo‐oligosaccharide (known as GOS/FOS), and the formula also had a 100% whey base rather than standard formula's 60:40 whey:casein ratio, and a lower lactose level than standard formula (with additional maltodextrin and starch to thicken the milk), in addition to the cows milk proteins having been partially hydrolysed. This study was a larger scale comparison with 103 of the infants who completed the trial randomised to the control with standard casein‐based formula plus simethicone, and 96 randomised to the intervention of a partially hydrolysed, lower lactose, whey‐based formula with starches, and supplemented with oligosaccharide |
|
| Outcomes | Questionnaire given to parents to monitor symptoms, frequency and feeding volume. On days 1, 7 and 14, infants examined by paediatricians. Feeding frequency and feeding volume was decided by the family and not by the study protocol. The number of significant colic episodes (over 40 min in duration) was recorded by parents daily. Study measured number of colic episodes per day (multivariate analysis between intervention or control pairs adjusted for variables) | |
| Notes |
Study start and end dates: August 2002 (start) to January 2003 (end) COIs: none reported Funding source: Numico, Italy, who provided the intervention formula Adverse effects: not reported, but we contacted the author, who is also an author on this review, who confirmed these were recorded and none were experienced Comments: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: effective randomisation |
| Allocation concealment (selection bias) | Low risk | Comment: sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not possible because of simethicone |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: blinding of outcome assessment performed by statistician |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 68/267 excluded at the end of the trial for non‐adherence to intervention or control, or because lost to follow‐up – see above under 'Participants'. Clarified by study author |
| Selective reporting (reporting bias) | Low risk | Comment: accounted for all participants. Study authors confirmed no adverse effects experienced |
| Other bias | Unclear risk | Comment: study supported by funds from Numico, Italy. They were not involved in writing or checking the manuscript. |
Taubman 1988.
| Methods | Randomised controlled trial with 2 treatment groups. For our purposes, we have labelled 'maternal dairy‐free diet' and 'parental counselling plus usual diet', as 'intervention' and 'control' respectively. | |
| Participants |
Sample size: 11 (1 dropout) in group 1; 10 (0 dropouts) in group 2 Setting: private practice and the gastroenterology clinic at the Children's Hospital of Philadelphia, Pennsylvania, USA Sex: not reported Mean age: 5.4 weeks (SD 2.2, range not reported) intervention; 6.5 weeks (SD 1.8, range not reported) control Mean weight: not reported Mean duration of colic (baseline): 3.75 weeks (SD 5.8) intervention; 4.85 weeks (SD 4.12) control Mean crying (baseline): 3.21 h/d (SD 1.10) intervention; 3.19 h/d (SD 0.69) control Feeding: not reported Birth order: 3 first born in each group (30%) Inclusion criteria: infants crying for more than 2 h/d, younger than 3 months of age, normal growth and development, normal physical findings, no history of diarrhoea or vomiting, and receiving enough milk Exclusion criteria: babies already receiving Nutramigen hydrolysed milk or breastfed babies whose mothers are already eating dairy‐free |
|
| Interventions |
Intervention* (n = 11): maternal dairy‐free diet if baby breastfed, or Nutramigen hydrolysed casein formula if baby formula fed
Control (n = 10): parental counselling plus usual diet
Duration: 9 days duration in each treatment arm of study *The intervention group went on to have counselling along with return to usual diet. This is not included in our analysis as is the second treatment arm, and we only used data from first treatment arm. |
|
| Outcomes | Parents kept a diary of the infants behaviour, in the prescribed manner, during both phases of the study and returned them to the investigator every 2 or 3 days | |
| Notes |
Study start and end dates: not reported COIs: none reported, but see funding source directly below Funding source: funded, in part, by Mead Johnson, who provided the intervention formula Adverse effects: not recorded Comments: this was a 2‐phased, randomised study with 11 infants in the counselling group (group 1) and 10 in the 'dairy and soy free' group (group 2), although the first group lost 1 participant from the study in the first few days and did not include that infant's data in the results. 3 infants in the counselling group were breastfed and 4 infants in the dairy and soy free group were breastfed. This study was designed to show the effectiveness of counselling by using dietary changes as a comparison. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: effective randomisation. Investigator had no knowledge of the allocation until after consent was given |
| Allocation concealment (selection bias) | High risk | Comment: no details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not possible given the nature of the trial |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no details. Wrote to study author but received no response |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: accounted for all participants, though paper reports per protocol, not intention‐to‐treat, results after one participant dropped out of the control group |
| Selective reporting (reporting bias) | High risk | Comment: paper reports per protocol, not intention‐to‐treat, results after one participant dropped out of the control group. Adverse effects not reported |
| Other bias | High risk | Comment: funded, in part, by Mead Johnson, who provided the intervention formula |
Treem 1991.
| Methods | Double‐blind, randomised, 2‐period, cross‐over trial with 2 treatment groups | |
| Participants |
Sample size: 33 infants (6 dropped out – sequence group not clear) Setting: paediatricians in the greater Hartford community, Connecticut, USA Sex: 13 boys (48%) 14 girls (52%) Mean age: 34 days (SD not reported, range 10 to 54) Mean weight: not reported Mean duration of colic (baseline): not reported Mean crying (baseline): not reported Feeding: formula fed (100%) Birth order: 15 first born Inclusion criteria: crying as if in pain, crying suddenly, crying continuously for more than 15 min at a time, and difficult or impossible to console during these crying spells, with colic defined as more than 3 h crying or fussing per day on at least 3 days out of 6 successive days, birth weight > 2500 g, normal gestational age, absence of neonatal problems, normal weight gain (> 150 g/week), normal physical examination Exclusion criteria: infants on medications during the first week before or during the study |
|
| Interventions |
Intervention (n = 12): Isomil with soy polysaccharide added to increase dietary fibre (mean values 14.1 g dietary fibre per litre) Control (n = 15): Isomil with nothing added (mean values 3.1 g dietary fibre per litre) Duration: baseline for 1 week before beginning study. Cross‐over study, including 3‐day washout between 2 × 9‐day‐long arms of study. Patients seen 5 times during the study: at the beginning of the baseline period, at each of the 2 × 9‐day study periods, at the end of the last 9‐day period, and at the 30‐ to 35‐day follow‐up period. Parents also contacted by telephone at least once during each of the 2 × 9‐day study periods |
|
| Outcomes | Daily behaviour, feeding, and stool diaries completed for 27 days. At the end of this time, parents asked to indicate during which study period the symptoms of colic were most alleviated and whether the infant's symptoms were alleviated during one of the study periods more than during any time before the study. We did not include these data in our analysis as we were looking only at the first arm. Results for crying and fussing in min per 24 h, but aggregated cross‐over data |
|
| Notes |
Study start and end dates not reported COIs: none reported Funding source: supported by grants from Ross Laboratories Adverse effects: not reported Comments: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: randomised |
| Allocation concealment (selection bias) | High risk | Comment: no details. Wrote to study author but received no response |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: double‐blind and disguised |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no details. Wrote to study author but received no response |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: accounted for all participants |
| Selective reporting (reporting bias) | High risk | Comment: accounted for all participants, adverse effects not reported |
| Other bias | High risk | Comment: supported by grants from Ross Laboratories who make Isomil. No further details of involvement were available from the study author |
COIs: conflicts of interest; GP: general practitioner; HV: health visitor; SD: standard deviation; SPSS: Statistical Package for the Social Sciences.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arikan 2008 | Appears to be randomised, but study authors state that in order to prevent discontinuation of breastfeeding, only participants who were formula fed went into the group receiving hydrolysed formula, so, in fact, not random |
| Barr 1991 | Not suffering with colic on entry to study |
| Bellaiche 2018 | Not an RCT |
| Berseth 2009 | No definition of colic: "parent‐identified as very fussy or extremely fussy in the baseline tolerance evaluation" (quote) |
| Buchanan 1998 | Letter to Editor of BMJ |
| Campeotto 2011 | Not suffering with colic on entry to study |
| Dupont 2010 | Using probiotics to treat so not dietary modification |
| Evans 1981 | Not an RCT – no control |
| Gerrard 1984 | Narative review only |
| Giovannini 2014 | Not suffering with colic on entry to study |
| Iacono 1991 | Not an RCT – no control |
| Iacovou 2018 | Recruited infants did not match infantile colic criteria reported in their own Methods |
| Imanieh 2004 | Study looking for skin prick test as predictor of cows' milk protein allergy in colicky infants |
| Infante 2011 | Not colic by our definition: only 35% (7 out of 20) infants included in analysis cried for more than 3 h/d at baseline |
| Jakobsson 1983 | Not an RCT or quasi‐RCT |
| Koonce 2011 | Narrative review only |
| Laws 1991 | Letter to Editor of Journal of Pediatrics |
| Liebman 1981 | Not an RCT or quasi‐RCT |
| Nocerino 2012 | Conference abstract; not randomised |
| Pärtty 2015 | Using probiotics to treat so not dietary modification |
| Rozé 2012 | Not suffering with colic on entry to study |
| Sargsyan 2006 | Letter to Editor of European Journal of Pediatrics |
| Savilahti 1989 | Not an RCT or quasi‐RCT |
| Savino 2003 | Not colic by our definition |
| Sherman 2015 | Not suffering with colic on entry to study |
| Vandenplas 2017 | Not suffering with colic on entry to study |
| Vivatvakin 2010 | Not suffering with colic on entry to study |
| Xinias 2017 | Not an RCT – consecutive entry |
RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
NCT01721850.
| Trial name or title |
Title: Evaluation of safety and efficacy of new infant formula in infantile colic (Coco) Official title: Evaluation of the safety and efficacy of new infant formula and its effects on the gastrointestinal tolerance (crying time) in infantile colic: a double‐blind, randomised, controlled intervention study |
| Methods | Double‐blind, randomised‐controlled intervention study |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions | Parallel assignment with 3 arms:
|
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | December 2011 |
| Contact information |
Name: Christina Hecht Email: Christina.hecht@hipp.de Telephone: 00498441757855 |
| Notes |
Status: recruiting Funding source: HiPP GmbH & Co. Vertrieb KG |
NCT02813772.
| Trial name or title |
Title: Efficacy of a partially hydrolyzed formula, containing Lactobacillus reuteri, for infant colic Official title: Efficacy of a partially hydrolyzed formula, containingLactobacillus reuteri, for infant colic: a double blind, randomised‐controlled trial |
| Methods | Double‐blind, randomised controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions | Parallel assignment: a partially hydrolysed formula with reduced lactose content and L reuteri versus a standard formula |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | November 2015 |
| Contact information |
Name: Annamaria Staiano Email: staiano@unina.it Telephone: none provided |
| Notes |
Status: recruiting Funding source: Federico II University |
NCT03329222.
| Trial name or title |
Title: An infant formula trial on dietary management of infantile colic Official title: A randomised, double‐blind, controlled, multi‐centre study to assess the efficacy of an infant formula in the dietary management of infantile colic |
| Methods | Double‐blind, randomised controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions | An infant formula that contains specific hydrolysed proteins with a fat‐blend, prebiotic mixture, starch and reduced lactose versus standard cows' milk with prebiotic mixture |
| Outcomes |
Primary outcome
Secondary outcome
|
| Starting date | 27 October 2017 |
| Contact information |
Name: Wan Wen Tee; Anneke Ravensbergen Email: wanwen.tee@danone.com; anneke.ravensbergen@danone.com Telephone: +65 68309466; +65 6830 9419 |
| Notes |
Status: recruiting Funding source: Danone Asia Pacific Holdings Pte, Ltd |
Differences between protocol and review
Please see our protocol (Savino 2014).
Authors
Valentina Tarasco left the review team and was replaced by Shel Banks and Simone Ceratto.
Morris Gordon took over as lead author.
Types of outcome measures
We reworded the outcomes of 'reduction in the duration of crying' and 'reduction in frequency of crying episodes per 24 h, to the following more neutral formulations, to reflect the fact that we are assessing the variable rather than a reduction in the variable: 'duration of crying' and 'frequency of crying episodes per 24 h'.
We planned on defining responders as a 50% reduction in crying. However, the peer reviewers raised questions as to the external basis for this decision, so we decided to revert to a more standard definition of 'responders', as defined by primary studies. As no study reported outcomes in this area with utility for analysis, this did not impact the analysis or findings.
Originally, we planned on reporting only change scores for the primary outcome; however, many studies reported only postintervention data, and so we decided to include these too.
We planned further analyses according to each specific adverse effect when the primary studies provided sufficient data. However, we did not complete this analysis as such data were not presented, with adverse effects rarely encountered.
In addition, we included the outcome 'parental satisfaction' in Table 1, but this was not in our protocol (Savino 2014). We modified the Methods sections accordingly.
Search methods for identification of studies
In order to ensure our search was as up‐to‐date as possible, we searched two additional databases, which are updated daily (Ovid MEDLINE In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE Epub Ahead of Print). See Electronic searches.
Selection of studies
We clarified that we excluded stand‐alone abstracts from this review. Given the extreme complexity of the studies found, including issues of heterogeneity of specific interventions, patient characteristics and outcomes reported, as well as the poor quality of the abstracts found within the search, the team decided not to consider abstracts unless they related to a study for which we also had a full‐text report. See Selection of studies.
Measures of treatment effect:
Dichotomous data
We planned to present dichotomous outcome data as risk ratios (RR), since the effects of the RR are readily understood (Walter 2000). We report all outcome data with their associated 95% CIs and P values (where possible). Using control event risks from the included trials, we planned to calculate the number needed to treat to benefit (NNTB) and its associated 95% CI for statistically significant dichotomous outcomes.
Continuous data
If all studies used the same measurement scale, we intended to calculate the mean difference (MD) for change scores. Where studies used different scales, we planned to calculate the standardised mean difference (SMD) using Hedges' g. Where necessary, we calculated effect estimates from P values, t statistics, analysis of variance (ANOVA) tables or other statistics, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017).
To analyse continuous data, we planned to use, according to need, either change scores or final values without combining them. However, no such analysis was possible due to a lack of data.
Had both continuous and dichotomous data been available for an outcome, we planned to include only the continuous outcome in the primary analysis. We planned that when studies had reported an outcome as a dichotomous measure, and others had used a continuous measure of the same construct, to convert the results for the former from the dichotomous measure to a standardised mean difference, provided that we could assume the underlying continuous measure had an approximately normal or logistic distribution (otherwise we would have carried out two separate analyses). However, no such analysis was possible due to a lack of data. See Measures of treatment effect.
Unit of analysis issues
Studies with multiple treatment arms
Had our strategy to combine data to make single, pair‐wise comparisons prevented investigation of potential sources of heterogeneity, we planned to analyse each formula separately (against a common control group – placebo), but divide the sample size for common comparator arms proportionately across each comparison (Higgins 2011b). This simple approach allows the use of standard software (including RevMan 2014), and prevents the inappropriate double‐counting of individuals; however, it was not needed due to a lack of data. Additionally, concerns with the length of a reasonable washout period existed and further limited scope for such analysis.
Cross‐over studies
For cross‐over trials, we planned to used the mean and standard error of the paired analysis for the meta‐analysis; however, this was not possible due to a lack of data. In future updates of this review, should we have sufficient data, we will include both parallel and cross‐over studies with an adequate washout period in a meta‐analysis using the inverse variance method, as recommended by Elbourne 2002; in the meta‐analysis, the weight of each study is inversely proportional to the variance (one over the square of the standard error) (Deeks 2017). We will include cross‐over studies with an inadequate washout period in a meta‐analysis using the data from the first arm only. Even though this method excludes some of the data, it avoids the inappropriate consideration of correlated information.
Dealing with missing data
For all outcomes in all studies, we planned to carry out analyses as far as possible on an intention‐to‐treat basis; that is, we planned to attempt to include all participants randomised to each group in the analyses, and we analysed all participants in the group to which they were allocated regardless of whether or not they received the allocated intervention. For missing continuous data, we intended to estimate standard deviations from other available data, such as standard errors, or impute them using the methods suggested in Higgins 2011b. For loss to follow‐up for continuous data, we planned to base analyses on those participants completing the trial, essentially assuming that outcome was the same in the missing participants and the observed participants. Where there was a discrepancy between the number randomised and the number analysed in each treatment group, we intended to calculate and reported the percentage lost to follow‐up in each group. We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by conducting sensitivity analyses (see Sensitivity analysis).
Data synthesis
Where interventions were similar in terms of type of dietary modification, type of outcome assessed and type of colic, we planned to group studies and synthesised their results in a meta‐analysis. Because we assumed that clinical heterogeneity was very likely to impact on our results, given the wide breadth and types of interventions included, we intended to combine studies using a random‐effects model, irrespective of statistical evidence of heterogeneity. We planned to calculate all overall effects using inverse variance methods and carried out statistical analysis using RevMan 5 (RevMan 2014).We included a new section on 'Assessment of the quality of the evidence' at the request of the editorial base. See Data synthesis.
Assessment of heterogeneity
We planned to assess statistical heterogeneity by examining the I2 statistic (Deeks 2017), a quantity that describes the proportion of variation in point estimates that is due to variability across studies rather than sampling error. We interpreted the I2 statistic as suggested in the latest version of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017).
0% to 40%: might not be important.
30% to 60%: may represent moderate heterogeneity.
50% to 90%: may represent substantial heterogeneity.
75% to 100%: considerable heterogeneity.
We also evaluated the CI for the I2 statistic. In addition, we employed a Chi2 test of homogeneity, with a 10% level of significance, to determine the strength of the evidence that the heterogeneity was genuine.
Subgroup analysis and investigation of heterogeneity
Large numbers of subgroup analyses may lead to misleading conclusions (Oxman 1992; Yusuf 1991). We planned to conduct the following subgroup analyses,
Age of mother at time of birth (21 years and younger versus older than 21 years).
Type of feeding (formula‐fed versus breastfed).
Atopy (lower versus higher risk of atopy).
Follow‐up (less than four weeks of treatment versus more than four weeks of treatment).
Trial quality (low quality (lack of allocation concealment or lack of blinding) versus high quality (allocation concealment and blinding)).
Sensitivity analysis
We planned to conduct sensitivity analyses to determine whether findings were sensitive to restricting the analyses to studies judged to be at low risk of bias for blinded assessment of the primary outcome. In addition, we planned to assess the sensitivity of findings to any imputed data, by calculating the treatment effect including and excluding the imputed data, to see whether this altered the outcome of the analysis. We planned to investigate the effect of dropouts and exclusions by conducting worst‐ versus best‐case scenario analyses. We analysed the effect of using the stringent Wessel 1954 definition of infant colic, the more recent definition given by Drossman 2016 and other variants.
Additional references
We updated our references to the latest versions of Chapters 8, Higgins 2017, and 9, Deeks 2017, in theCochrane Handbook for Systematic Reviews of Interventions.
Contributions of authors
Morris Gordon wrote the protocol (Savino 2014), organised retrieval of papers, wrote to study authors for additional information, supported the search, extracted and analysed data, wrote the core text of the review, and led revisions of the draft. Elena Biagioli wrote the protocol and reviewed the Results section of the review. Miriam Sorrenti wrote the protocol and the Discussion section of the review. Carla Lingua added references and supported the search. Lorenzo Moja provided statistical advice and revised key sections of the review. Shel Banks organised retrieval of papers, wrote to study authors for additional information, supported the search, extracted and analysed data, and wrote the review. Simone Ceratto wrote the Discussion section and contributed to the write‐up of the Results section of the review. Francesco Savino has primary responsibility for the review. He co‐ordinated the efforts of the review authors. He wrote the protocol and contributed to the write‐up of the Results and Discussion sections of the review.
Sources of support
Internal sources
-
Department of Pediatrics, Regina Margherita Children's Hospital, Città della Salute e della Scienza, Turin, Italy.
Logistical support
External sources
None, Other.
Declarations of interest
Morris Gordon (MG) received travel grants from a number of companies during the three years leading up to publication, including from Allergan, Ferring Pharmaceuticals, Biogaia, Synergy and Tillots, to attend conferences (Advances in Inflammatory Bowel Disease, Digestive Disease Week, World Congress of Gastroentrology) and present the results of other Cochrane Reviews. These companies have had no input or involvement in any aspect of the review process during this, or previous systematic reviews carried out by MG. MG currently holds a National Institute for Health Research (NIHR) Cochrane Programme Grant, although this did not support this work. MG is a paediatrician with an interest in gastroenterology. This involves seeing patients referred with infantile colic and managing their condition in line with current, accepted, evidence‐based practice. MG has no other interests to declare.
Elena Biagioli – none known.
Miriam Sorrenti – none known.
Carla Lingua – none known.
Lorenzo Moja – none known.
Shel Banks (SB) is self‐employed as an Internationally Board Certified Lactation Consultant in private practice, delivering expertise in infant feeding evidence base, writing briefing papers and newsletters, etc., and delivering workshops across the northwest of England – largely for the Local Infant Feeding Information Board (LIFIB), but also the Sudden Unexpected Death of a Child (SUDC) prevention team, Lanacshire. Until March 2016, the SUDC consultant post was funded by the local authority (Lancashire County Council) via breastfeeding voluntary sector charity (Breastfeeding Network) and SB was paid by the hour. SB is also self‐employed as a trainer and webinar presenter ad hoc and delivers Babyem's Open College Network Level Three and Four Maternity Nurse Training courses. Since 2009, SB has worked part‐time as a Baby Friendly Co‐ordinator, and she was paid for 12 months by Blackpool Teaching Hospitals NHS Foundation Trust as a Research Assistant for her work on this review. SB is Chair of LIFIB, Chair of the Communications Team, Chair of the Breastfeeding Festival, Committee Member of the Lactation Consultants of Great Britain, and a Trustee of the UK Association of Milk Banking, all of which are voluntary positions, although SB's travel expenses to meetings are paid. SB received travel expenses from Manchester City Council for speaking at their event 'Make Manchester a Breastfeeding Friendly City' in June 2018. SB has sat as a lay member on the Guideline Development Committee of three NICE Guidelines (Milk Banking, Postnatal Care Quality Standards and Faltering Growth), and was paid an honorarium by NICE (National Institute for Health and Care Excellence) for her services. SB declares that neither she personally, nor any of the entities she represents, take funding of any kind from any commercial interests in infant feeding or early years, and that she works completely within the professional code of ethics as an Internationally Board Certified Lactation Consultant. SB has no other interests to declare.
Simone Ceratto (SC) received travel grants from Nóos S.r.l and Nestlé, to attend scientific meetings. SC declares that he attended 'Campus Angelini 2017', which was sponsored by Angelini.
Francesco Savino (FS) received payment from: Danone Nutricia Middle‐East DMCC, Dubai, United Arab Emirates, and Innova Pharma SpA Recordati SpA, Milan, Italy, for talks at symposiums; Società Italiana di Pediatria, Milan, Italy, for speaking at the XX Congresso Nazionale SIAIP, sponsored by Biomedia srl, Milan; HiPP GmbH & Co, Vertrieb KG, Pfaffenhofen, Germany, for consultancy work; and from Aretrè srl, Milan, Italy, for development of educational materials, outside the submitted work. FS also received a travel grant from Noos Roma, Italy, to attend ESPGHAN's 51st Annual Meeting, Geneva, and receives royalties from Springer Verlag, Milan, Italy, for the book Nutrizione Parenterale in Pediatria. FS declares that these organizations have had no input or involvement in any aspect of the review process during this, or previous systematic review that he has carried out. FS has no other interests to declare.
Disclaimer: the views contained herein are those of the authors and not necessarily those of the Department of Health, NHS NICE or NIHR.
Edited (no change to conclusions)
References
References to studies included in this review
Campbell 1989 {published data only}
- Campbell JPM. Dietary treatment of infant colic: a double‐blind study. Journal of the Royal College of General Practitioners 1989;39(318):11‐4. [CENTRAL: PMC1711543; PMC1711543; PUBMED: 2553940] [PMC free article] [PubMed] [Google Scholar]
Forsyth 1989 {published data only}
- Forsyth BW. Colic and the effect of changing formulas: a double‐blind, multiple‐crossover study. Journal of Pediatrics 1989;115(4):521‐6. [PUBMED: 2677292] [DOI] [PubMed] [Google Scholar]
Hill 1995 {published data only}
- Hill DJ, Hudson IL, Sheffiled LJ, Shelton MJ, Menahem S, Hosking CS. A low allergen diet is a significant intervention in infantile colic: results of a community‐based study. Journal of Allergy and Clinical Immunology 1995;96(6 Pt 1):886‐92. [PUBMED: 8543745] [DOI] [PubMed] [Google Scholar]
Hill 2005 {published data only}
- Hill DJ, Hosking CS, Roy N, Carlin JB, Francis DEM, Brown J, et al. Colic in breast fed infants is due to hypersensitivity to dietary proteins excreted in breast milk. Journal of Allergy and Clinical Immunology 2004;113(2 Suppl):S338. [DOI: 10.1016/j.jaci.2004.01.724] [DOI] [Google Scholar]
- Hill DJ, Roy N, Heine RG, Hosking CS, Francis DE, Brown J, et al. Effect of a low‐allergen maternal diet on colic among breastfed infants: a randomized, controlled trial. Pediatrics 2005;116(5):e709‐15. [DOI: 10.1542/peds.2005-0147; PUBMED: 16263986] [DOI] [PubMed] [Google Scholar]
Jakobsson 2000 {published data only}
- Jakobsson I, Lothe L, Ley D, Borschel MW. Effectiveness of casein hydrolysate feedings in infants with colic. Acta Paediatrica 2000;89(1):18‐21. [DOI: 10.1111/j.1651-2227.2000.tb01180.x; PUBMED: 10677051] [DOI] [PubMed] [Google Scholar]
Kanabar 2001 {published data only}
- Kanabar D, Randhawa M, Clayton P. Improvement of symptoms in infant colic following reduction of lactose load with lactase. Journal of Human Nutrition and Dietetics 2001;14(5):359‐63. [PUBMED: 11906576] [DOI] [PubMed] [Google Scholar]
Kearney 1998 {published data only}
- Kearney PJ, Malone AJ, Hayes T, Cole M, Hyland M. A trial of lactase in the management of infant colic. Journal of Human Nutrition and Dietetics 1998;11(4):281‐5. [DOI: 10.1046/j.1365-277X.1998.00108.x] [DOI] [Google Scholar]
Lothe 1987 {published data only}
- Lothe L, Lindberg T. Cow's milk (CM) whey proteins as a cause of infantile colic in formula fed infants: a double‐blind cross‐over study. Pediatric Research 1987;22:108. [DOI: 10.1203/00006450-198707000-00095] [DOI] [PubMed] [Google Scholar]
- Lothe L, Lindberg T, Jakobsson I. Cow's milk formula as a cause of infantile colic: a double‐blind study. Pediatrics 1982;70(1):7‐10. [PUBMED: 7088636] [PubMed] [Google Scholar]
Lucassen 2000 {published data only}
- Lucassen P. Cochrane dietary modifications for colic review [personal communication]. Email to: M Gordon 8 November 2016.
- Lucassen PL, Assendelft WJ, Gubbels JW, Eijk JT, Douwes AC. Infantile colic: crying time reduction with a whey hydrolysate: a double‐blind, randomized, placebo‐controlled trial. Pediatrics 2000;106(6):1349‐54. [PUBMED: 11099588] [DOI] [PubMed] [Google Scholar]
Miller 1990 {published data only}
- Miller J. Details for our Cochrane review [personal communication]. Email to: S Banks 8 November 2016.
- Miller JJ, McVeagh P, Fleet GH, Petocz, P, Brand JC. Effect of yeast lactase enzyme on "colic" in infants fed human milk. Journal of Pediatrics 1990;117(2 Pt 1):261‐3. [PUBMED: 2116510] [DOI] [PubMed] [Google Scholar]
Oggero 1994 {published data only}
- Oggero R, Garbo G, Savino F, Mostert M. Dietary modifications versus dicyclomine hydrochloride in the treatment of severe infantile colics. Acta Paediatrica 1994;83(2):222‐5. [PUBMED: 8193507] [DOI] [PubMed] [Google Scholar]
Savino 2005 {published data only}
- Savino F, Cresi F, Castagno E, Silvestro L, Oggero R. A randomized double‐blind placebo‐controlled trial of a standardized extract of matricariae recutita, foeniculum vulgare and melissa officinalis (ColiMil) in the treatment of breastfed colicky infants. Phytotherapy Research 2005;19(4):335‐40. [DOI: 10.1002/ptr.1668; PUBMED: 16041731] [DOI] [PubMed] [Google Scholar]
Savino 2006 {published data only}
- Savino F. Confirmation of details from your review [personal communication]. Email to: M Gordon 15 December 2016.
- Savino F, Palumeri E, Castagno E, Cresi F, Dalmasso P, Cavallo F, et al. Reduction of crying episodes owing to infantile colic: a randomized controlled study on the efficacy of a new infant formula. European Journal of Clinical Nutrition 2006;60(11):1304‐10. [DOI: 10.1038/sj.ejcn.1602457; PUBMED: 16736065] [DOI] [PubMed] [Google Scholar]
Taubman 1988 {published data only}
- Taubman B. Parental counseling compared with elimination of cow's milk or soy milk protein for the treatment of infant colic syndrome: a randomized trial. Pediatrics 1988;81(6):756‐61. [PUBMED: 3285312] [PubMed] [Google Scholar]
Treem 1991 {published data only}
- Treem WR, Hyams JS, Blankschen E, Etienne N, Paule CL, Borschel MW. Evaluation of the effect of a fiber‐enriched formula on infant colic. Journal of Pediatrics 1991;119(5):695‐701. [PUBMED: 1658281] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Arikan 2008 {published data only}
- Arikan D, Alp H, Gözüm S, Orbak Z, Cifçi EK. Effectiveness of massage, sucrose solution, herbal tea or hydrolysed formula in the treatment of infantile colic. Journal of Clinical Nursing 2008;17(13):1754‐61. [PUBMED: 18592627] [DOI] [PubMed] [Google Scholar]
Barr 1991 {published data only}
- Barr RG, Wooldridge J, Hanley J. Effects of formula change on intestinal hydrogen production and crying and fussing behavior. Journal of Developmental and Behavioral Pediatrics 1991;12(4):248‐53. [PUBMED: 1939680] [PubMed] [Google Scholar]
- Barr RG, Wooldridge JA, Hanley J, Jajko L, Boisjoly C. Formula change reduces crying behavior: evidence for role of intraintestinal gas. Pediatric Research 1984;18:101A. [DOI: 10.1203/00006450-198404001-00047] [DOI] [Google Scholar]
Bellaiche 2018 {published data only}
- Bellaiche M, Oozeer R, Gerardi‐Temporel G, Faure C, Vandenplas Y. Multiple functional gastrointestinal disorders are frequent in formula‐fed infants and decrease their quality of life. Acta Paediatrica 2018;107(7):1276‐82. [DOI: 10.1111/apa.14348; MEDLINE: ; PMC6055647; PUBMED: 29604128] [DOI] [PMC free article] [PubMed] [Google Scholar]
Berseth 2009 {published data only}
- Berseth CL, Johnston WH, Stolz SI, Harris CL, Mitmesser SH. Clinical response to 2 commonly used switch formulas occurs within 1 day. Clinical Pediatrics 2009;48(1):58‐65. [DOI: 10.1177/0009922808321897; PUBMED: 18832532] [DOI] [PubMed] [Google Scholar]
Buchanan 1998 {published data only}
- Buchanan P. Effectiveness of treatments for infantile colic. Trial of hypoallergenic milk is not supported by strong enough evidence. BMJ 1998;317(7170):1451‐2. [PUBMED: 10232901] [PubMed] [Google Scholar]
Campeotto 2011 {published data only}
- Campeotto F, Suau A, Kapel N, Magne F, Viallon V, Ferraris L, et al. A fermented formula in pre‐term infants: clinical tolerance, gut microbiota, down‐regulation of faecal calprotectin and up‐regulation of faecal secretory IgA. British Journal of Nutrition 2011;105(12):1843‐51. [DOI: 10.1017/S0007114510005702; PUBMED: 21426607] [DOI] [PubMed] [Google Scholar]
Dupont 2010 {published data only}
- Dupont C, Rivero M, Grillon C, Belaroussi N, Kalindjian A, Marin V. Alpha‐lactalbumin‐enriched and probiotic‐supplemented infant formula in infants with colic: growth and gastrointestinal tolerance. European Journal of Clinical Nutrition 2010;64(7):765‐7. [DOI: 10.1038/ejcn.2010.81; PUBMED: 20517331] [DOI] [PubMed] [Google Scholar]
Evans 1981 {published data only}
- Evans RW, Fergusson DM, Allardyce RA, Taylor B. Maternal diet and infantile colic in breast‐fed infants. Lancet 1981;1(8234):1340‐2. [PUBMED: 6113313] [DOI] [PubMed] [Google Scholar]
Gerrard 1984 {published data only}
- Gerrard JW. Allergies in breastfed babies to foods ingested by the mother. Clinical Reviews in Allergy 1984;2(2):143‐9. [DOI: 10.1007/BF02991062; PUBMED: 6428733] [DOI] [PubMed] [Google Scholar]
Giovannini 2014 {published data only}
- Giovannini M, Verduci E, Gregori D, Ballali S, Soldi S, Ghisleni D, et al. Prebiotic effect of an infant formula supplemented with galacto‐oligosaccharides: randomized multicenter trial. Journal of the American College of Nutrition 2014;33(5):385‐93. [DOI: 10.1080/07315724.2013.878232; PUBMED: 25302927] [DOI] [PubMed] [Google Scholar]
Iacono 1991 {published data only}
- Iacono G, Carroccio A, Montalto G, Cavataio F, Bragion E, Lorello D, et al. Severe infantile colic and food intolerance: a long‐term prospective study. Journal of Pediatric Gastroenterology and Nutrition 1991;12(3):332‐5. [PUBMED: 2072224] [DOI] [PubMed] [Google Scholar]
Iacovou 2018 {published data only}
- Iacovou M, Mulcahy EC, Truby H, Barrett JS, Gibson PR, Muir JG. Reducing the maternal dietary intake of indigestible and slowly absorbed short‐chain carbohydrates is associated with improved infantile colic: a proof‐of‐concept study. Journal of Human Nutrition and Dietetics 2018;31(2):256‐65. [DOI: 10.1111/jhn.12488; PUBMED: 28631347] [DOI] [PubMed] [Google Scholar]
Imanieh 2004 {published data only}
- Imanieh MH, Moravej H, Kashef S, Handjani F, Eghtedari F, Kamali Sarvestani E. Cows' milk allergy in infantile colic. Armaghane Danesh 2004;9(3):39‐47. [armaghanj.yums.ac.ir/article‐1‐816‐en.html] [Google Scholar]
Infante 2011 {published data only}
- Infante D, Segarra O, Luyer B. Dietary treatment of colic caused by excess gas in infants: biochemical evidence. World Journal of Gastroenterology 2011;17(16):2104‐8. [DOI: 10.3748/wjg.v17.i16.2104; PMC3084395; PUBMED: 21547129] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jakobsson 1983 {published data only}
- Jakobsson I, Lindberg T. Cow's milk proteins cause infantile colic in breast‐fed infants: a double‐blind crossover study. Pediatrics 1983;71(2):268‐71. [PUBMED: 6823433] [PubMed] [Google Scholar]
Koonce 2011 {published data only}
- Koonce T, Mounsey A, Rowland K. Colicky baby? Here's a surprising remedy. Journal of Family Practice 2011;60(1):34‐6. [CENTRAL: PMC3183958; PMC3183958; PUBMED: 21209977] [PMC free article] [PubMed] [Google Scholar]
Laws 1991 {published data only}
- Laws HF 2nd. Effect of lactase on infantile colic. Journal of Pediatrics 1991;118(6):993‐4. [DOI: 10.1016/S0022-3476(05)82228-0; PUBMED: 1904092] [DOI] [PubMed] [Google Scholar]
Liebman 1981 {published data only}
- Liebman WM. Infantile colic: association with lactose and milk intolerance. JAMA 1981;245(7):732‐3. [PUBMED: 7193254] [DOI] [PubMed] [Google Scholar]
Nocerino 2012 {published data only}
- Nocerino R, Marco G, Cosenza L, Pezzella V, Passariello A, Terrin G, et al. Efficacy of a new partially hydrolyzed whey formula on infant colic: a randomized controlled trial. Digestive and Liver Disease 2012;44(Suppl 4):S272. [DOI: 10.1016/S1590-8658(12)60696-3; P032] [DOI] [Google Scholar]
Pärtty 2015 {published data only}
- Pärtty A, Lehtonen L, Kalliomäki M, Salminen S, Isolauri E. Probiotic lactobacillus rhamnosus GG therapy andmicrobiological programming in infantile colic: a randomized, controlled trial. Pediatric Research 2015;78(4):470‐5. [DOI: 10.1038/pr.2015.127; NCT00167700; PUBMED: 26151493] [DOI] [PubMed] [Google Scholar]
Rozé 2012 {published data only}
- Rozé JC, Barbarot S, Butel MJ, Kapel N, Waligora‐Dupriet AJ, Montgolfier I, et al. An α‐lactalbumin‐enriched and symbiotic‐supplemented v a standard infant formula: a multicentre, double‐blind, randomised trial. British Journal of Nutrition 2012;107(11):1616–22. [DOI: 10.1017/S000711451100479X; NCT00920166; PUBMED: 22079177] [DOI] [PubMed] [Google Scholar]
Sargsyan 2006 {published data only}
- Sargsyan K, Burmucie K, Dunitz‐Scheer M, Hauer AC. Effects of nutritional and psychosupportive interventions on the course of infantile colic ‐ first results of a prospective clinical study. European Journal of Pediatrics 2006;165(Suppl 1):360. [DOI: 10.1007/s00431-006-0349-z] [DOI] [Google Scholar]
Savilahti 1989 {published data only}
- Savilahti E, Ståhlberg MR. Lactose tolerance in colicky infants. Journal of Pediatrics 1989;115(2):333‐4. [DOI: 10.1007/BF02722300; PUBMED: 2754567] [DOI] [PubMed] [Google Scholar]
Savino 2003 {published data only}
- Savino F, Cresi F, Maccario S, Cavallo F, Dalmasso P, Fanaro S, et al. "Minor" feeding problems during the first months of life: effect of a partially hydrolysed milk formula containing fructo‐ and galacto oligosaccharides. Acta Pediatrica 2003;91(441 Suppl):86‐90. [PUBMED: 14599049] [DOI] [PubMed] [Google Scholar]
Sherman 2015 {published data only}
- Sherman AL, Anderson J, Rudolph CD, Walker LS. Lactose‐free milk or soy‐based formulas do not improve caregivers' distress or perceptions of difficult infant behavior. Journal of Pediatric Gastroenterology and Nutrition 2015;61(1):119‐24. [DOI: 10.1097/MPG.0000000000000743; 25643020; NCT01934257] [DOI] [PubMed] [Google Scholar]
Vandenplas 2017 {published data only}
- Vandenplas Y, Ludwig T, Bouritius H, Alliet P, Forde D, Peeters S, et al. Randomised controlled trial demonstrates that fermented infant formula with short‐chain galacto‐oligosaccharides and long‐chain fructo‐oligosaccharides reduces the incidence of infantile colic. Acta Paediatrica 2017;106(7):1150‐8. [DOI: 10.1111/apa.13844; PMC5485044; PUBMED: 28328065] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vivatvakin 2010 {published data only}
- Vivatvakin B, Mahayosnond A, Theamboonlers A, Steenhout PG, Conus NJ. Effect of a whey‐predominant starter formula containing LCPUFAs and oligosaccharides (FOS/GOS) on gastrointestinal comfort in infants. Asia Pacific Journal of Clinical Nutrition 2010;19(4):473‐80. [PUBMED: 21147707] [PubMed] [Google Scholar]
Xinias 2017 {published data only}
- Xinias I, Analitis A, Mavroudi A, Roilides I, Lykogeorgou M, Delivoria V, et al. Innovative dietary intervention answers to baby colic. Pediatric Gastroenterology, Hepatology & Nutrition 2017;20(2):100‐6. [DOI: 10.5223/pghn.2017.20.2.100; PMC5517376; PUBMED: 28730134] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
NCT01721850 {unpublished data only}
- NCT01721850. Evaluation of safety and efficacy of new infant formula in infantile colic (Coco) [Evaluation of the safety and efficacy of new infant formula and its effects on the gastrointestinal tolerance (crying time) in infantile colic: a double‐blind, randomized, controlled intervention study]. clinicaltrials.gov/ct2/show/NCT01721850 (first received 6 November 2012).
NCT02813772 {unpublished data only}
- NCT02813772. Efficacy of a partially hydrolyzed formula, containing Lactobacillus reuteri, for infant colic [Efficacy of a partially hydrolyzed formula, containing Lactobacillus reuteri, for infant colic: a double blind, randomized‐controlled trial]. clinicaltrials.gov/ct2/show/NCT02813772 (first received 27 June 2016).
NCT03329222 {unpublished data only}
- NCT03329222. An infant formula trial on dietary management of infantile colic [A randomised, double blind, controlled, multi‐centre study to assess the efficacy of an infant formula in the dietary management of infantile colic]. clinicaltrials.gov/ct2/show/NCT03329222?term=NCT03329222&rank=1 (first received 1 November 2017).
Additional references
Agostoni 2006
- ESPGHAN Committee on Nutrition, Agostoni C, Axelsson I, Goulet O, Koletzko B, Michaelsen KF, et al. Soy protein infant formulae and follow‐on formulae: a commentary by the ESPGHAN Committee on Nutrition. Journal of Pediatric Gastroenterology and Nutrition 2006;42(4):352‐61. [DOI: 10.1097/01.mpg.0000189358.38427.cd; PUBMED: 16641572] [DOI] [PubMed] [Google Scholar]
Axelsson 1986
- Axelsson I, Jakobsson I, Lindberg T, Benediktsson B. Bovine beta‐lactoglobulin in the human milk. A longitudinal study during the whole lactation period. Acta Paediatrica Scandinavica 1986;75(5):702‐7. [PUBMED: 3564937] [DOI] [PubMed] [Google Scholar]
Benninga 2016
- Benninga MA, Faure C, Hyman PE, James Roberts I, Schechter NL, Nurko S. Childhood functional gastrointestinal disorders: neonate/toddler. Gastroenterology 2016;150(6):1443‐55.e2. [DOI: 10.1053/j.gastro.2016.02.016; PUBMED: 27144631] [DOI] [PubMed] [Google Scholar]
Camilleri 2017
- Camilleri M, Park SY, Scarpato E, Staiano A. Exploring hypotheses and rationale for causes of infantile colic. Neurogastroenterology and Motility 2017;29(2):1‐11. [DOI: 10.1111/nmo.12943; NIHMS812612; PMC5276723; PUBMED: 27647578] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clifford 2002
- Clifford TJ, Campbell MK, Speechley KN, Gorodzinsky F. Infant colic: empirical evidence of the absence of an association with source of early infant nutrition. Archives of Pediatrics & Adolescent Medicine 2002;156(11):1123‐8. [PUBMED: 12413341] [DOI] [PubMed] [Google Scholar]
Clyne 1991
- Clyne PS, Kulczycki A Jr. Human breast milk contains bovine IgG. Relationship to infant colic?. Pediatrics 1991;87(4):439‐44. [PUBMED: 2011419] [PubMed] [Google Scholar]
Cohen‐Silver 2009
- Cohen‐Silver J, Ratnapalan S. Management of infantile colic: a review. Clinical Pediatrics 2009;48(1):14‐7. [DOI: 10.1177/0009922808323116; PUBMED: 18832537] [DOI] [PubMed] [Google Scholar]
de Weerth 2013
- Weerth C, Fuentes S, Puylaert P, Vos WM. Intestinal microbiota of infants with colic: development and specific signatures. Pediatrics 2013;131(2):e550‐8. [DOI: 10.1542/peds.2012-1449; PUBMED: 23319531] [DOI] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JPT, Altman DG, editor(s). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Donaldson 2004
- Donaldson L. Advice issued on soya‐based infant formulas. CMO Update 2004; Vol. 37:2. [www.second‐opinions.co.uk/soy‐online‐service/04070176.pdf]
Drossman 2016
- Drossman DA, Hasler WL. Rome IV‐functional GI disorders: disorders of gut‐brain interaction. Gastroenterology 2016;150(6):1257‐61. [DOI: 10.1053/j.gastro.2016.03.035; PUBMED: 27147121] [DOI] [PubMed] [Google Scholar]
Dubois 2016
- Dubois NE, Gregory KE. Characterizing the intestinal microbiome in infantile colic: findings based on an integrative review of the literature. Biological Research for Nursing 2016;18(3):307‐15. [DOI: 10.1177/1099800415620840; PUBMED: 26721871] [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI: 10.1093/ije/31.1.140] [DOI] [PubMed] [Google Scholar]
Estep 2000
- Estep DC, Kulczycki A Jr. Treatment of infant colic with amino acid‐based infant formula: a preliminary study. Acta Paediatrica 2000;89(1):22‐7. [PUBMED: 10677052] [DOI] [PubMed] [Google Scholar]
Farré 2013
- Farré R, Tack J. Food and symptom generation in functional gastrointestinal disorders: physiological aspects. American Journal of Gastroenterology 2013;108(5):698‐706. [DOI: 10.1038/ajg.2013.24; PUBMED: 23458851] [DOI] [PubMed] [Google Scholar]
Feinle‐Bisset 2013
- Feinle‐Bisset C, Azpiroz F. Dietary lipids and functional gastrointestinal disorders. American Journal of Gastroenterology 2013;108(5):737‐47. [DOI: 10.1038/ajg.2013.76; PUBMED: 23567355] [DOI] [PubMed] [Google Scholar]
Freedman 2009
- Freedman SB, Al‐Harthy N, Thull‐Freedman J. The crying infant: diagnostic testing and frequency of serious underlying disease. Pediatrics 2009;123(3):841‐8. [DOI: 10.1542/peds.2008-0113; PUBMED: 19255012] [DOI] [PubMed] [Google Scholar]
García Marqués 2017
- García Marqués S, Chillón Martínez R, González Zapata S, Rebollo Salas M, Jiménez Rejano JJ. Tools assessment and diagnosis to infant colic: a systematic review. Child: Care, Health and Development 2017;43(4):481‐8. [DOI: 10.1111/cch.12454; PUBMED: 28261843] [DOI] [PubMed] [Google Scholar]
Garrison 2000
- Garrison MM, Christakis DA. A systematic review of treatments for infant colic. Pediatrics 2000;106(1 Pt 2):184‐90. [PUBMED: 10888690] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed prior to 30 July 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Gupta 2007
- Gupta SK. Update on infantile colic and management options. Current Opinion in Investigational Drugs 2007;8(11):921‐6. [PUBMED: 17979025] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI: 10.1136/bmj.39489.470347.AD; PMC2335261; PUBMED: 18436948] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 2012
- Hall B, Chesters J, Robinson A. Infantile colic: a systematic review of medical and conventional therapies. Journal of Paediatrics and Child Health 2012;48(2):128‐37. [DOI: 10.1111/j.1440-1754.2011.02061.x; PUBMED: 21470331] [DOI] [PubMed] [Google Scholar]
Heine 2006
- Heine RG. Gastroesophageal reflux disease, colic and constipation in infants with food allergy. Current Opinion in Allergy and Clinical Immunology 2006;6(3):220‐5. [DOI: 10.1097/01.all.0000225164.06016.5d; PUBMED: 16670518] [DOI] [PubMed] [Google Scholar]
Heine 2008
- Heine RG. Allergic gastrointestinal motility disorders in infancy and early childhood. Pediatric Allergy and Immunology 2008;19(5):383‐91. [DOI: 10.1111/j.1399-3038.2008.00785.x; PUBMED: 18713339] [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Deeks JJ, editor(s). Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG, editor(s). Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2017
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Hill 2000
- Hill DJ, Hosking CS. Infantile colic and food hypersensitivity. Journal of Pediatric Gastroenterology and Nutrition 2000;30 Suppl:S67‐76. [PUBMED: 10634302] [DOI] [PubMed] [Google Scholar]
Hyams 1989
- Hyams JS, Geertsma MA, Etienne NL, Treem WR. Colonic hydrogen production in infants with colic. Journal of Pediatrics 1989;115(4):592‐4. [PUBMED: 2795353] [DOI] [PubMed] [Google Scholar]
Iacono 2005
- Iacono G, Merolla R, D'Amico D, Bonci E, Cavataio F, Prima L, et al. Gastrointestinal symptoms in infancy: a population‐based prospective study. Digestive and Liver Disease 2005;37(6):432‐8. [DOI: 10.1016/j.dld.2005.01.009; PUBMED: 15893282] [DOI] [PubMed] [Google Scholar]
Iacovou 2012
- Iacovou M, Ralston RA, Muir J, Walker KZ, Truby H. Dietary management of infantile colic: a systematic review. Maternal and Child Health Journal 2012;16(6):1319‐31. [DOI: 10.1007/s10995-011-0842-5; PUBMED: 21710185] [DOI] [PubMed] [Google Scholar]
Keefe 1998
- Keefe MR, Froese‐Fretz A, Kotzer AM. Newborn predictors of infant irritability. Journal of Obstetric, Gynecologic, and Neonatal Nursing 1998;27(5):513‐20. [PUBMED: 9773363] [DOI] [PubMed] [Google Scholar]
Landgren 2010
- Landgren K, Hallström I. Parents' experience of living with a baby with infantile colic ‐‐ a phenomenological hermeneutic study. Scandinavian Journal of Caring Sciences 2011;25(2):317‐24. [DOI: 10.1111/j.1471-6712.2010.00829.x; PUBMED: 20723153] [DOI] [PubMed] [Google Scholar]
Lindberg 1999
- Lindberg T. Infantile colic and small intestinal function: a nutritional problem?. Acta Paediatrica 1999;88(430):58‐60. [PUBMED: 10569224] [DOI] [PubMed] [Google Scholar]
Lothe 1989
- Lothe L, Lindberg T. Cow's milk whey protein elicits symptoms of infantile colic in colicky formula‐fed infants: a double‐blind crossover study. Pediatrics 1989;83(2):262‐6. [PUBMED: 2913556] [PubMed] [Google Scholar]
Lothe 1990
- Lothe L, Ivarsson SA, Ekman R, Lindberg T. Motilin and infantile colic. A prospective study. Acta Paediatrica Scandinavica 1990;79(4):410‐6. [PUBMED: 2349877] [DOI] [PubMed] [Google Scholar]
Lucas 1998
- Lucas A, James‐Roberts I. Crying, fussing and colic behaviour in breast‐ and bottle‐fed infants. Early Human Development 1998;53(1):9‐18. [PUBMED: 10193923] [DOI] [PubMed] [Google Scholar]
Lucassen 1998
- Lacassen PLBJ, Assendelft WJJ, Gubbels JW, Eijk JTM, Geldrop WJ, Knuistingh Neven A. Effectiveness of treatments for infantile colic: systematic review. BMJ 1998;316(7144):1563‐9. [DOI: 10.1136/bmj.316.7144.1563; PMC28556; PUBMED: 9596593] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lucassen 2001
- Lucassen PL, Assendelft WJ. Systematic review of treatments for infant colic. Pediatrics 2001;108(4):1047‐8. [DOI: 10.1542/peds.108.4.1047; PUBMED: 11589211] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLOS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097; PMC2707599; PUBMED: 19621072] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 1988
- Moore DJ, Robb TA, Davidson GP. Breath hydrogen response to milk containing lactose in colicky and noncolicky infants. Journal of Pediatrics 1988;113(6):979‐84. [PUBMED: 3193321] [DOI] [PubMed] [Google Scholar]
Osborn 2013
- Osborn DA, Sinn JK. Prebiotics in infants for prevention of allergy. Cochrane Database of Systematic Reviews 2013, Issue 3. [DOI: 10.1002/14651858.CD006474.pub3] [DOI] [PubMed] [Google Scholar]
Oxman 1992
- Oxman AD, Guyatt GH. A consumer's guide to subgroup analyses. Annals of Internal Medicine 1992;116(1):78‐84. [DOI: 10.7326/0003-4819-116-1-78; PUBMED: 1530753] [DOI] [PubMed] [Google Scholar]
Pauli‐Pott 2000
- Pauli‐Potta U, Becker K, Mertesacker T, Beckmann D. Infants with "colic" ‐ mothers' perspectives on the crying problem. Journal of Psychosomatic Research 2000;48(2):125‐32. [PUBMED: 10719128] [DOI] [PubMed] [Google Scholar]
Perry 2011
- Perry R, Hunt K, Ernst E. Nutritional supplements and other complementary medicines for infantile colic: a systematic review. Pediatrics 2011;127(4):720‐33. [DOI: 10.1542/peds.2010-2098; PUBMED: 21444591] [DOI] [PubMed] [Google Scholar]
Pitkin 1999
- Pitkin RM, Branagan MA, Burmeister LF. Accuracy of data in abstracts of published research articles. JAMA 1999;281(12):1110‐1. [PUBMED: 10188662] [DOI] [PubMed] [Google Scholar]
Praveen 2014
- Praveen V, Praveen S, Deshpande G, Patole SK. Oral probiotics for infantile colic. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD010986] [DOI] [Google Scholar]
Reijneveld 2001
- Reijneveld SA, Brugman E, Hirasing RA. Excessive infant crying: the impact of varying definitions. Pediatrics 2001;108(4):893‐7. [PUBMED: 11581441] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaberation. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaberation, 2014.
Savino 2001
- Savino F, Cresi F, Silvestro L, Oggero R. Use of an amino‐acid formula in the treatment of colicky breastfed infants. Acta Paediatrica 2001;90(3):359‐60. [PUBMED: 11332183] [PubMed] [Google Scholar]
Savino 2007
- Savino F. Focus on infantile colic. Acta Paediatrica 2007;96(9):1259‐64. [DOI: 10.1111/j.1651-2227.2007.00428.x; PUBMED: 17718777] [DOI] [PubMed] [Google Scholar]
Savino 2010
- Savino F, Tarasco V. New treatments for infant colic. Current Opinion in Pediatrics 2010;22(6):791‐7. [DOI: 10.1097/MOP.0b013e32833fac24; PUBMED: 20859207] [DOI] [PubMed] [Google Scholar]
Savino 2017
- Savino F, Quartieri A, Marco A, Garro M, Amaretti A, Raimondi S, et al. Comparison of formula‐fed infants with and without colic revealed significant differences in total bacteria, enterobacteriaceae and faecal ammonia. Acta Paediatrica 2017;106(4):573‐8. [DOI: 10.1111/apa.13642; PUBMED: 27763733] [DOI] [PubMed] [Google Scholar]
Schach 2002
- Schach B, Haight M. Colic and food allergy in the breastfed infant: is it possible for an exclusively breastfed infant to suffer from food allergy?. Journal of Human Lactation 2002;18(1):50‐2. [DOI: 10.1177/089033440201800108; PUBMED: 11845737] [DOI] [PubMed] [Google Scholar]
Shannon 1921
- Shannon WR. Colic in breast fed infants as a result of sensitization to foods in the mother's diet. Archives of Pediatrics 1921;38:756‐61. [Google Scholar]
Steutel 2014
- Steutel NF, Benninga MA, Langendam MW, de KruijffI, Tabbers MM. Reporting outcome measures in trials of infant colic. Journal of Paediatric Gastroenterology and Nutrition 2014;59(3):341‐6. [DOI: 10.1097/MPG.0000000000000412; PUBMED: 24796800] [DOI] [PubMed] [Google Scholar]
Sung 2014
- Sung V, Hiscock H, Tang MLK, Mensah FK, Nation ML, Satzke C, et al. Treating infant colic with the probiotic Lactobacillus reuteri: double blind, placebo controlled randomised trial. BMJ 2014;348:g2107. [DOI: 10.1136/bmj.g2107] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomas 2010
- Thomas DW, Greer FR, American Academy of Pediatrics Committee on Nutrition, America Academy of Paediatrics Section on Gastroenterology, Hepatology, Nutrition. Probiotics and prebiotics in pediatrics. Pediatrics 2010;126(6):1217‐31. [DOI: 10.1542/peds.2010-2548; PUBMED: 21115585] [DOI] [PubMed] [Google Scholar]
Vandenplas 2015
- Vandenplas Y, Abkari A, Bellaiche M, Benninga M, Chouraqui JP, Çokura F, et al. Prevalence and health outcomes of functional gastrointestinal symptoms in infants from birth to 12 months of age. Journal of Pediatric Gastroenterology and Nutrition 2015;61(5):531‐7. [DOI: 10.1097/MPG.0000000000000949; PMC4631121; PUBMED: 26308317] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walter 2000
- Walter SD. Choice of effect measure for epidemiological data. Journal of Clinical Epidemiology 2000;53(9):931‐9. [PUBMED: 11004419] [DOI] [PubMed] [Google Scholar]
Wessel 1954
- Wessel MA, Cobb JC, Jackson EB, Harris GS Jr, Detwiler AC. Paroxysmal fussing in infancy, sometimes called colic. Pediatrics 1954;14(5):421‐35. [PUBMED: 13214956] [PubMed] [Google Scholar]
Wolke 2017
- Wolke D, Bilgin A, Samara M. Systematic review and meta‐analysis: fussing and crying durations and prevalence of colic in infants. Journal of Pediatrics 2017;185:55‐61.e4. [DOI: 10.1016/j.jpeds.2017.02.020; PUBMED: 28385295] [DOI] [PubMed] [Google Scholar]
Yusuf 1991
- Yusuf S, Wittes J, Probstfield J, Tyroler HA. Analysis and interpretation of treatment effects in subgroups of patients in randomized clinical trials. JAMA 1991;266(1):93–8. [PUBMED: 2046134] [PubMed] [Google Scholar]
References to other published versions of this review
Savino 2014
- Savino F, Tarasco V, Sorrenti M, Lingua C, Moja L, Gordon M, Biagioli E. Dietary modifications for infantile colic. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD011029] [DOI] [PMC free article] [PubMed] [Google Scholar]


