Abstract
Background
Return of normal gastrointestinal (GI) function is a critical determinant of recovery after colorectal surgery. The aim of this meta-analysis was to evaluate whether perioperative intravenous (IV) lidocaine benefits return of gastrointestinal function after colorectal resection.
Methods
A comprehensive search of Ovid Medline, PubMed, Embase, Cochrane library, and clinicaltrials.org was performed on 1st July 2018. A manual search of reference lists was also performed. Inclusion criteria were as follows: randomized controlled trials (RCTs) of intravenous (IV) lidocaine administered perioperatively compared to placebo (0.9% saline infusion) as part of a multimodal perioperative analgesic regimen, human adults (> 16 years), and open or laparoscopic colorectal resectional surgery. Exclusion criteria: non-colorectal surgery, non-placebo comparator, children, non-general anaesthetic, and pharmacokinetic studies. The primary endpoint was time to first bowel movement. Secondary endpoints were time to first passage of flatus, time to toleration of diet, nausea and vomiting, ileus, pain scores, opioid analgesia consumption, and length of stay.
Results
One hundred and ninety one studies were screened, with 9 RCTs meeting inclusion criteria (405 patients, four laparoscopic and five open surgery studies). IV lidocaine reduced time to first bowel movement compared to placebo [seven studies, 325 patients, mean weighted difference − 9.54 h, 95% CI 18.72–0.36, p = 0.04]. Ileus, pain scores, and length of stay were reduced with IV lidocaine compared with placebo.
Conclusions
Perioperative IV lidocaine may improve recovery of gastrointestinal function after colorectal surgery. Large-scale effectiveness studies to measure effect size and evaluate optimum dose/duration are warranted.
Electronic supplementary material
The online version of this article (10.1007/s10151-019-1927-1) contains supplementary material, which is available to authorized users.
Keywords: Intravenous lidocaine, Colorectal surgery, Ileus, Laparoscopic
Introduction
Colorectal resection causes an unavoidable cessation of normal gastrointestinal (GI) function in every patient; hence, the return of GI function is a critical determinant of recovery [1, 2]. Modern minimally invasive techniques and multimodal “enhanced recovery” programs have reduced the historically high prevalence of delayed return of GI function associated with open colorectal surgery [3, 4]. Despite this, return of GI function after colorectal resection can lag behind other aspects of recovery such as mobilization and pain control [5]. A prolonged delay in return of GI function (commonly known as postoperative ileus) is characterized by inability to resume normal diet, vomiting, abdominal distension, and absolute constipation, requires active supportive management [intravenous (IV) fluids, anti-emetics, nasogastric intubation], and results in longer hospital stay with a substantially poorer patient experience. Recovery of GI function is important to patients and surgeons alike and was identified as a key research focus in a recent research prioritization exercise undertaken jointly between patients and the Association of Coloproctology of Great Britain and Ireland [6, 7].
Perioperative IV lidocaine has well-established anti-inflammatory and opioid-sparing analgesic properties [8–10]. There are also data to suggest a beneficial effect on return of GI function following abdominal surgery. However, interpretation of the existing literature is challenging, as it includes a variety of operations, access techniques and perioperative management protocols [11, 12]. Furthermore, despite the existence of validated consensus-derived composite endpoint definitions of return of GI function (GI-2, GI-3) [2, 13, 14], many studies of perioperative IV lidocaine report a variety of sub-optimal univariate endpoints to measure GI recovery.
This study updates existing meta-analyses of perioperative IV lidocaine by inclusion of new data and seeks to limit study heterogeneity by focusing on return of GI function following colorectal surgery.
Materials and methods
Literature search
The study was placed prospectively on the International Prospective Register of Systematic Reviews (PROSPERO) register [CRD42016049847]. A comprehensive search of Ovid Medline, PubMed, Embase, Cochrane library, and clinicaltrials.org was completed on 5th September 2018. A manual search of reference lists was also performed. The following search strategy was used: (colorectal surgery OR colectomy OR colon OR colonic OR bowel) AND (intravenous lidocaine OR intravenous lignocaine OR lidocaine infusion OR lignocaine infusion OR IV lidocaine OR IV lignocaine OR I.V lidocaine).
Inclusion criteria
Randomized controlled trials (RCTs), human adults [> 16 years], open or laparoscopic colorectal resectional surgery.
Exclusion criteria
Non-colorectal surgery, non-placebo comparator, children, non-general anaesthetic, and pharmacokinetic studies.
Intervention and comparator
Intravenous lidocaine administered perioperatively was compared to placebo (0.9% saline infusion) as part of a multimodal perioperative analgesic regimen.
Data extraction
Full-text randomized control trials meeting inclusion criteria were reviewed by two independent researchers (EK/CC). A proforma was used to extract relevant information: data presented as mean and standard deviation were extracted directly, whereas non-parametric results (median and interquartile range) were converted using previously described techniques. For skewed data, the median was used instead of the mean [15].
Primary outcome
Since none of the included studies reported the validated GI-2 or GI-3 definitions of GI function, the primary outcome was time (hours) to first bowel movement (various phrases “bowel function”, “defecation”, and “bowel motion” were used in the included studies and we have assumed them to mean the same thing, i.e., defecation).
Secondary outcomes
Return of GI function
Time to first passage of flatus (hours).
Time to toleration of diet (hours).
Incidence of postoperative ileus.
Incidence of nausea and vomiting.
Pain
Numerical pain score at rest at 24 h (score 0–10, 0 = no pain, 10 worst imaginable pain, alternative methods converted to 0–10 range).
Numerical pain score on movement at 24 h (0–10 as above).
Opioid consumption over first 24 h after surgery (milligrams and morphine equivalent doses).
Total opioid consumption (milligrams).
Other
Length of stay (hours).
Subgroup analyses
A predefined subgroup analysis was performed for open and laparoscopic surgery.
Bias and quality assessment
Overall quality and potential bias were assessed using a previously described 15-point scale adapted from criteria described by Chalmers and Jadad, with a threshold score of ≥ 12 for high quality (Table 1) [16, 17]. A sensitivity analysis was conducted for the primary endpoint by excluding each study.
Table 1.
Details of trials included in meta-analysis
| Author | Number of participants | Operation | Intervention | Protocol | Additional analgesia | Modified quality score |
|---|---|---|---|---|---|---|
| IV Lidocaine versus placebo as part of a multimodal analgesic regimen | ||||||
| Laparoscopic | ||||||
| Elhafz [18] | 18 |
Hemicolectomy n = 9 Total colectomy n = 4 Proctocolectomy n = 3 Sigmoid resection n = 1 |
IV lidocaine n = 9 | IV lidocaine infusion 2 mg/minute if > 70 kg, or 1 mg/minute if < 70 kg, stopped on return of bowel function or day 5 postoperatively | Recovery—fentanyl 15–30 micrograms every 15 min, or morphine 2 mg every 20 min PRN | 7 |
| Placebo n = 9 | IV saline infusion throughout, stopped on return of bowel function or day 5 postoperatively | PCA morphine 2 mg, lockout time 10 min | ||||
| Kaba [19] | 40 |
Right hemicolectomy n = 9 Left hemicolectomy n = 31 |
IV lidocaine n = 20 | Lidocaine 1.5 mg/kg i.v. bolus at induction THEN by 2 mg/kg/h i.v. infusion THEN 1.33 mg/kg/h i.v. infusion postoperatively for 24 h |
Paracetamol 1 g 30 min prior to end of surgery THEN every 6 h for first 24 h Ketorolac 30 mg IV every 8 h for first 24 h Piritramide [opioid] as rescue medication—1 mg, lockout 5 min After 24 h Paracetamol 1 g oral every 6 h Diclofenac 75 mg BD Tramadol 100 mg PRN |
14 |
| Placebo n = 20 | Normal saline i.v. bolus at induction THEN i.v. infusion of normal saline throughout procedure and postoperatively for 24 h | |||||
| Kim [20] | 68 |
Right hemicolectomy n = 14 Left hemicolectomy n = 4 Anterior resection n = 49 Subtotal colectomy n = 1 |
IV lidocaine n = 32 | IV bolus of lidocaine 1 mg/kg was given THEN IV infusion of lidocaine 1 mg/kg/h AND ketorolac 90 mg for 24 h | NSAIDs after ketorolac infusion stopped | 14 |
| Placebo n = 36 | 5 ml bolus of normal saline THEN 90 mg ketorolac in 240 ml saline for 24 h | |||||
| Tikuisis [21] | 60 | Laparoscopic anterior resection n = 60 | IV lidocaine n = 30 | IV bolus of lidocaine 1.5 mg/kg was given [maximum 100 mg] THEN IV infusion of lidocaine 2 mg/kg/h during the entire surgical procedure THEN 1 mg/kg/h in the postoperative anaesthesia care unit and continued for the first 24 h after surgery |
Fentanyl 24 h post op infusion 0.1 µg/kg/hr Ketorolac 30 mg PRN |
12 |
| Placebo n = 30 | IV bolus of normal saline THEN continuous infusion of normal saline during surgery and for 24 h after the operation | |||||
| Placebo n = 20 | Normal saline via IV infusion and epidural throughout procedure | |||||
| Open | ||||||
| Kuo [22] | 60 | ‘Elective surgery for colon cancer’, procedure not further detailed | IV lidocaine n = 20 | Lidocaine 2 mg/kg i.v. infusion over 10 min prior to induction THEN 3 mg/kg/hr i.v. infusion for duration of procedure |
Intraoperative: fentanyl 1 µg/kg Patient-controlled epidural analgesia [PCEA] postop with morphine 0.1 mg/ml in ropivacaine 0.2%. 4 ml bolus 15 min lockout |
13 |
| Placebo n = 20 | Normal saline via IV infusion and epidural throughout procedure | |||||
| Herroeder [23] | 60 |
Ileocaecal resection n = 2 Right hemicolectomy n = 10 Left hemicolectomy n = 5 Subtotal colectomy n = 1 Proctocolectomy n = 4 Sigmoid resection n = 20 Anterior resection n = 9 Other n = 9 |
IV lidocaine n = 31 | Lidocaine 1.5 mg/kg i.v. bolus at induction THEN 2 mg/min i.v. infusion during procedure terminated 4 h after skin closure |
IV piritramide 30 min prior to end of procedure PCA 2 mg piritramide PRN, lockout of 10 min 1 g metamizol or 1 g paracetamol 6-hourly |
15 |
| Placebo n = 29 | Saline i.v. bolus at induction THEN i.v. infusion during procedure terminated 4 h after skin closure | |||||
| Staikou [24] | 60 |
Right hemicolectomy n = 12 Left hemicolectomy n = 8 Sigmoidectomy n = 29 Anterior resection n = 5 Abdominoperineal resection n = 6 |
IV lidocaine n = 20 | Bolus of 1.5 mg/kg lidocaine IV THEN continuous IV infusion at 2 mg/kg/hr, stopped at end of procedure |
At start of skin suturing 20 mg ropivacaine and 1 mg morphine administered epidurally to all patients Intraoperative Remifentanil PCA with ropivacaine 2 mg/ml and morphine 0.1 mg/ml—released 4 ml per delivery, lockout of 20 min Up to 4 g paracetamol + 16 mg lornoxicam per day |
14 |
| Placebo n = 20 | Saline infusion IV and epidurally at volumes and rates as if containing lidocaine, stopped at end of procedure | |||||
| Harvey [11] | 22 | ‘Elective bowel surgery’, procedure not further detailed | IV lidocaine n = 11 | IV infusion postoperatively only of lidocaine 1 mg/min for 24 h | Morphine PCA | 12 |
| Placebo n = 11 | Saline infusion 10 ml/hr postoperatively for 24 h | |||||
| Ho [25] | 58 |
Stoma formation n = 28 Anterior resection n = 24 Right hemicolectomy n = 14 Left hemicolectomy n = 2 Total colectomy n = 5 Abdominoperineal resection n = 4 Proctocolectomy n = 4 Other n = 4 |
IV lidocaine n = 28 | 1.5 mg/kg over 5 min (lidocaine 2.5%, 0.06 ml/kg) immediately postinduction, followed by 1 mg/kg/h (lidocaine 2.5%, 0.04 ml/kg/h) for 48 h |
IV fentanyl PCA Other multimodal agents given at the discretion of the anaesthetist |
14 |
| Placebo n = 29 | Equal volume, rate and duration of infusion of normal saline | |||||
RCT randomized controlled trial, IV intravenous, PRN as required, IM intramuscular, PR per rectum, PO oral, PCA patient-controlled analgesia, NSAID non-steroidal anti-inflammatory drug, SC subcutaneous, IP intraperitoneal
Statistical analysis
Data were analyzed using the mean weighted difference (WMD) and pooled odds ratios for continuous variables and dichotomous data, respectively. A random effects model was selected on the basis of radial plots of the primary outcome. Statistical significance was set at p < 0.05. Heterogeneity was classified as low (< 33%), medium (33–66), or high (> 66%) using I2 estimated using the restricted maximum likelihood estimator function. Data were analyzed using the metafor package in R (version 3.4.2, R statistical programming, Vienna) [26].
Results
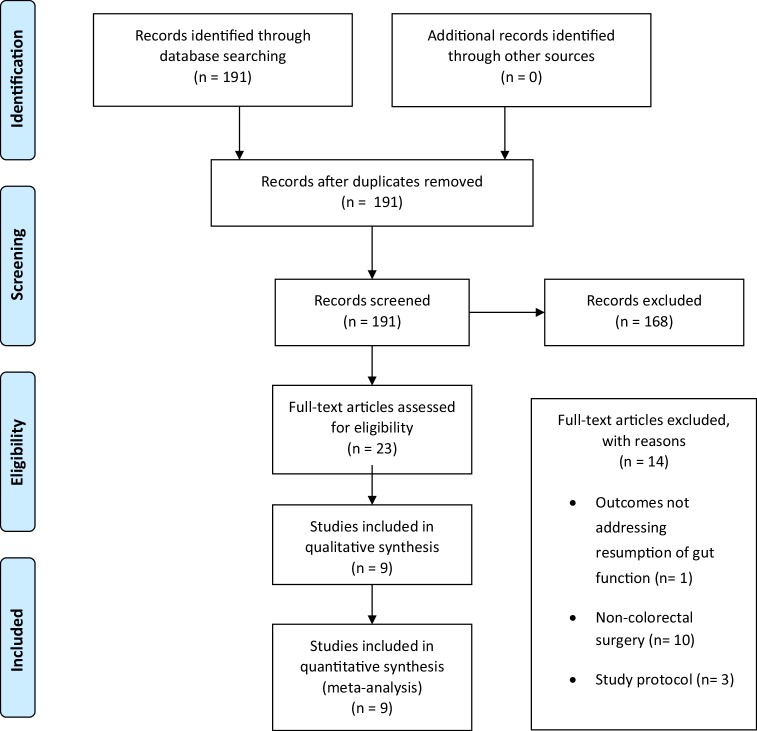
Nine RCTs were identified by the literature search strategy detailed in the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram in Fig. 1, with a total of 405 patients. All results and figures are presented in supplementary data.
Fig. 1.
PRISMA flow diagram of search strategy and included studies
Primary outcome: time to first bowel movement
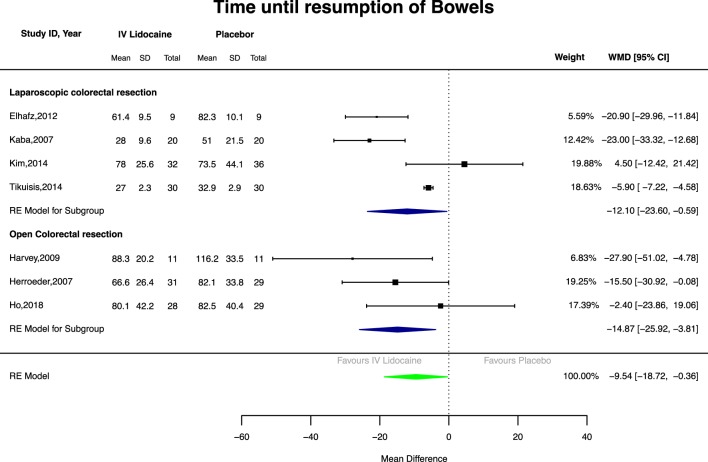
IV lidocaine was associated with a significantly reduced time to first bowel movement (Fig. 2) in pooled analysis compared with placebo (seven studies, 325 patients, WMD − 9.54 h, 95%CI 18.72 to − 0.36, p = 0.04). In subgroup analyses, IV lidocaine reduced time to first bowel movement in both open (three studies, 139 patients; WMD − 14.87 h, 95%CI 25.92 to − 3.81, p = 0.008) and laparoscopic surgery (four studies, 186 patients; WMD − 12.1 h, 95%CI 23.6 to − 0.59, p = 0.04).
Fig. 2.
Forest plot of time from operation to first bowel movement
Secondary outcomes
Time to first passage of flatus
Intravenous lidocaine did not significantly improve time to first passage of flatus in pooled analysis (8 studies, 345 patients; WMD − 3.42 h, 95%CI 10.41–3.58, p = 0.339). There was a significant decrease in the time to first passage of flatus in the open subgroup (five studies, 229 patients; WMD − 7.07 h, 95%CI 13.58 to − 0.57, p = 0.033) but not the laparoscopic subgroup (three studies, 126 patients; WMD − 4.58 h, 95%CI − 18.22 to 9.07, p = 0.511).
Time to resumption of diet
Only three studies reported this endpoint. IV lidocaine did not significantly hasten the time to toleration of diet in pooled analysis (three studies, 188 patients; WMD—10.93 h, 95%CI − 23.03 to 1.17, p = 0.077). IV lidocaine was associated with a shorter time to toleration of diet compared with placebo only in the laparoscopic subgroup (two studies, 128 patients; WMD − 5.97 h, 95%CI − 6.88 to − 5.09, p < 0.001). However, this was heavily weighted by a single study. Furthermore, resumption of diet is a less objective measurement than return of bowel function, as it greatly varies by individual practice.
Nausea and vomiting
There was no significant difference in nausea and vomiting events when comparing IV lidocaine with placebo in pooled analysis (five studies, 271 patients, OR 0.54, 95%CI 0.21–1.41, p = 0.150). There was no significant difference in the laparoscopic and open subgroups.
Incidence of postoperative ileus
In pooled analysis, there was a significant reduction in the incidence of postoperative ileus in the IV lidocaine group (five studies, 256 patients, OR 0.32, 95%CI 0.15–0.71, p = 0.02). No differences in the incidence of postoperative ileus were seen in subgroup analyses.
Pain score at rest at 24 h
Intravenous lidocaine was associated with lower pain scores at rest at 24 h compared with placebo (seven studies, 280 patients, WMD − 0.72, 95%CI − 1.31 to − 0.13, p = 0.020). This benefit was seen in the open subgroup (four studies, 159 patients, WMD − 0.36, 95%CI − 0.66 to − 0.06, p = 0.02). There was no significant difference in the laparoscopic subgroup.
Pain score on movement at 24 h
Intravenous lidocaine was associated with lower pain scores on movement at 24 h compared with placebo (four studies, 133 patients, WMD − 1.02, CI − 1.89 to − 0.14, p = 0.020). This effect was seen in the laparoscopic (two studies, WMD − 1.70, 95%CI − 2.18 to − 1.22, p < 0.0001) but not the open subgroup (two studies, 80 patients, WMD − 0.38, 95%CI − 1.06 to 0.31, p = 0.28).
Opioid consumption during first 24 h after operation
There was no difference in opioid consumption in the first 24 h after operation in pooled or subgroup analyses (pooled analysis five studies, 205 patients; WMD − 4.24 mg, 95%CI − 9.86 to 1.38, p = 0.14).
Total opioid consumption
There was no significant difference in total opioid consumption in pooled or subgroup analyses (pooled analysis seven studies, 305 patients; WMD − 5.82 mg, 95%CI − 22.32 to 10.67, p = 0.49).
Length of stay
Intravenous lidocaine was associated with shorter length of stay in pooled analysis (seven studies, 347 patients; WMD − 17.84 h, 95%CI − 32.95 to − 2.74 h, p = 0.020). This was the case in both laparoscopic (three studies, 168 patients; WMD − 23.04 h, 95%CI − 32.52 to − 13.56 h, p < 0.0001) and open subgroups (four studies, 179 patients; WMD − 19.62 h, 95% CI − 36.66 to − 2.59 h, p = 0.020).
Forest plots for secondary outcomes are shown in Supplementary data 1.
Discussion
Previous meta-analyses of perioperative IV lidocaine have included a diverse range of operative procedures and focused on opioid analgesic consumption and pain scores [27, 28]. This meta-analysis examined the effect of IV lidocaine on return of GI function after major colorectal surgery, a critical determinant of recovery and discharge from hospital for this patient group. Time to first bowel movement was reduced by approximately 15 h in open and 12 h in laparoscopic surgery. Consistent with this finding was a substantially reduced risk of postoperative ileus (OR 0.32), reduced early pain scores, and reduced length of hospital stay of approximately 18 h (95% CI − 2.74 to − 32.95 h). If these findings were replicated in routine practice, perioperative IV lidocaine could hasten recovery, reduce postoperative ileus, and reduce length of stay for a significant proportion of patients. Given that colectomy is a common operation undertaken in every acute hospital in the western world, considerable cost savings could be achieved in reduced bed occupancy from this straightforward and inexpensive intervention. Although this analysis did not specifically study the safety of IV lidocaine, it is a familiar drug and the previous reviews suggest a low incidence of IV lidocaine-associated toxicity [29].
The mechanism of action of IV lidocaine in this setting remains uncertain. Pain scores were lower with IV lidocaine, but opioid consumption was not significantly different, suggesting that the faster return of gut function was not solely due to opiate sparing [30, 31]. IV lidocaine has a variety of analgesic and anti-inflammatory effects mediated through sodium channel receptors (recently summarized in detail [32]) and is known to reduce postoperative serum cytokine levels, suggesting that it acts centrally and peripherally to blunt the pro-inflammatory response to surgery [18, 33]. Postoperative ileus is multifactorial, and IV lidocaine probably acts via more than one mechanism.
Our study aimed to highlight potential benefits of perioperative IV lidocaine to colorectal surgeons, but has several limitations and its results need to be interpreted carefully. No study reported the consensus-derived, validated GI-2 or GI-3 composite endpoints of GI function and not all univariate endpoints were reported by all studies (for example, time to resumption of diet, integral to the GI2/GI-3 endpoint, was reported by only three studies). Although dose was consistent between studies (1–2 mg/kg/h), duration of infusion was not: most studies used 24 h, but ranged from operation only [22, 24] to 5 days (Elhafz et al. [18]). The latter study is, therefore, a methodological outlier, elimination of which in sensitivity analysis (Supplementary data 2) leads to a loss of statistical significance for the laparoscopic subgroup for the primary endpoint. This sensitivity analysis shows that our results are susceptible to removal of individual studies, reflecting study heterogeneity and the small total sample size, and is another reason for cautious interpretation.
Currently, the ‘correct’ duration of infusion is unknown. The intraoperative period is probably the most important; thereafter, continuation of the infusion depends on availability of cardiac monitoring beyond the theatre suite, which may be dictated by local resources. Plasma accumulation, and hence risk of toxicity, is unlikely with less than 24 h continuous infusion [32]. The authors’ local practice is a 12-h infusion, and the UK ALLEGRO trial of perioperative IV lidocaine will compare outcomes from 6-h and 12-h infusion [34].
Finally, few studies reported a perioperative protocol consistent with modern enhanced recovery principles. Notably, those that did had short lengths of stay (median 3–4 days) and showed a clear benefit from IV lidocaine [19, 21]. In contrast, where enhanced recovery protocols were not used/reported and length of stay was longer (median 8–9 days), no benefit was shown [20, 25]. This suggests that IV lidocaine exerted the greatest benefit on early recovery and was most effective within a modern patient care protocol; conversely, where length of stay was long (outdated care pathways, complex case mix, or high complication rates), a benefit was more difficult to detect.
Conclusions
Although this analysis reduces heterogeneity by including colorectal surgery only, most studies were small, set in contrasting perioperative care protocols and reported sub-optimal endpoints to assess postoperative GI function. Nevertheless, an intriguing signal of benefit from IV lidocaine was seen consistently across the reported outcomes, suggesting that perioperative IV lidocaine could have a clinically meaningful effect on return of GI function, and hence, length of stay after colorectal surgery. IV lidocaine is inexpensive, straightforward to administer within existing evidence-based perioperative care protocols, and appears safe. Large-scale pragmatic effectiveness trials embedded within modern perioperative protocols are warranted to confirm or refute these findings and optimize dose and duration of infusion.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
CC: conception, literature searches, data synthesis, data analysis, and write up. EDK: literature searches, data synthesis, and approval of final manuscript. SN: conception, editing, and approval of final manuscript. IF: conception, editing, and approval of final manuscript. DS: conception, editing, and approval of final manuscript. HMP: conception, editing, and approval of final manuscript. NTV: conception, data analysis, write-up, editing, and approval of final manuscript.
Funding
No funding obtained for this study.
Compliance with ethical standards
Conflict of interest
HMP, DS, SN, and IF are lead investigators in a UK National Institute for Health Research Health Technology Assessment (NIHR-HTA)-funded UK multicenter randomized controlled trial of perioperative intravenous lidocaine in colorectal surgery (ALLEGRO: a placebo-controlled rAndomized trial of intravenous Lidocaine in acceLErating Gastrointestinal Recovery after cOlorectal surgery; EudraCT No. 2017-003835-12), which opened in August 2018 and will randomize 562 patients undergoing laparoscopic colorectal resection, measuring GI-3 return of GI function as the primary endpoint. Further details are available at: https://w3.abdn.ac.uk/hsru/ALLEGRO/Public/Public/index.cshtml. None of the authors have any commercial interest in perioperative IV lidocaine.
Declaration of originality
This manuscript has not been submitted elsewhere. An abstract based on this data was presented as a poster at the 2017 annual meeting of the Association of ColoProctologists of Great Britain and Ireland in Bournemouth, UK.
Ethical approval
This article does not contain any studies with human participants or animals performed by the authors.
Informed consent
For this type of study informed consent is not required.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Delaney CP, Marcello PW, Sonoda T, Wise P, Bauer J, Techner L. Gastrointestinal recovery after laparoscopic colectomy: results of a prospective, observational, multicenter study. Surg Endosc. 2010;24:653–661. doi: 10.1007/s00464-009-0652-7. [DOI] [PubMed] [Google Scholar]
- 2.Delaney CP, Kehlet H, Senagore A, Bauer A, Beart R, Billingham R. Postoperative ileus: profiles, risk factors, and definitions: a framework for optimizing surgical outcomes in patients undergoing major abdominal colorectal surgery. Clinical consensus update in general surgery. Roswell (GA): Pharmacolecture LLC; 2006. [Google Scholar]
- 3.Khoury W, Dakwar A, Sivkovits K, Mahajna A. Fast-track rehabilitation accelerates recovery after laparoscopic colorectal surgery. J Soc Laparoendosc Surg. 2014;18(4):e2014.00076. doi: 10.4293/JSLS.2014.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Artinyan A, Nunoo-Mensah JW, Balasubramaniam S, Gauderman J, Essani R, Gonzalez-Ruiz C, et al. Prolonged postoperative ileus-definition, risk factors, and predictors after surgery. World J Surg. 2008;32:1495–1500. doi: 10.1007/s00268-008-9491-2. [DOI] [PubMed] [Google Scholar]
- 5.Creamer F, Balfour A, Nimmo S, Foo I, Norrie JD, Williams LJ, et al. Randomized open-label phase II study comparing oxycodone-naloxone with oxycodone in early return of gastrointestinal function after laparoscopic colorectal surgery. Br J Surg. 2017;104:42–51. doi: 10.1002/bjs.10322. [DOI] [PubMed] [Google Scholar]
- 6.McNair AG, Heywood N, Tiernan J, Verjee A, Bach SP, Fearnhead NS, et al. A national patient and public colorectal research agenda: integration of consumer perspectives in bowel disease through early consultation. Colorectal Dis. 2017;19:O75–O85. doi: 10.1111/codi.13564. [DOI] [PubMed] [Google Scholar]
- 7.Tiernan J, Cook A, Geh I, George B, Magill L, Northover J, et al. Use of a modified Delphi approach to develop research priorities for the association of coloproctology of Great Britain and Ireland. Colorectal Dis. 2014;16:965–970. doi: 10.1111/codi.12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marret E, Rolin M, Beaussier M, Bonnet F. Meta-analysis of intravenous lidocaine and postoperative recovery after abdominal surgery. Br J Surg. 2008;95:1331–1338. doi: 10.1002/bjs.6375. [DOI] [PubMed] [Google Scholar]
- 9.Vigneault L, Turgeon AF, Cote D, Lauzier F, Zarychanski R, Moore L, et al. Perioperative intravenous lidocaine infusion for postoperative pain control: a meta-analysis of randomized controlled trials. Can J Anaesth. 2011;58:22–37. doi: 10.1007/s12630-010-9407-0. [DOI] [PubMed] [Google Scholar]
- 10.Hollmann MW, Durieux ME. Local anesthetics and the inflammatory response: a new therapeutic indication? Anesthesiology. 2000;93:858–875. doi: 10.1097/00000542-200009000-00038. [DOI] [PubMed] [Google Scholar]
- 11.Harvey KP, Adair JD, Isho M, Robinson R. Can intravenous lidocaine decrease postsurgical ileus and shorten hospital stay in elective bowel surgery? A pilot study and literature review. Am J Surg. 2009;198:231–236. doi: 10.1016/j.amjsurg.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Ventham NT, Kennedy ED, Brady RR, Paterson HM, Speake D, Foo I, et al. Efficacy of intravenous lidocaine for postoperative analgesia following laparoscopic surgery: a meta-analysis. World J Surg. 2015;39:2220–2234. doi: 10.1007/s00268-015-3105-6. [DOI] [PubMed] [Google Scholar]
- 13.Delaney CP, Weese JL, Hyman NH, Bauer J, Techner L, Gabriel K, et al. Phase III trial of alvimopan, a novel, peripherally acting, mu opioid antagonist, for postoperative ileus after major abdominal surgery. Dis Colon Rectum. 2005;48:1114–1125. doi: 10.1007/s10350-005-0035-7. [DOI] [PubMed] [Google Scholar]
- 14.Ludwig K, Enker WE, Delaney CP, Wolff BG, Du W, Fort JG, et al. Gastrointestinal tract recovery in patients undergoing bowel resection: results of a randomized trial of alvimopan and placebo with a standardized accelerated postoperative care pathway. Arch Surg. 2008;143:1098–1105. doi: 10.1001/archsurg.143.11.1098. [DOI] [PubMed] [Google Scholar]
- 15.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chalmers TC, Smith H, Jr, Blackburn B, Silverman B, Schroeder B, Reitman D, et al. A method for assessing the quality of a randomized control trial. Control Clin Trials. 1981;2:31–49. doi: 10.1016/0197-2456(81)90056-8. [DOI] [PubMed] [Google Scholar]
- 17.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 18.Elhafz AA, Elgebaly AS, Bassuoni AS, El Dabaa AA. Is lidocaine patch as effective as intravenous lidocaine in pain and illus reduction after laparoscopic colorectal surgery? A randomized clinical trial. Anesth Essays Res. 2012;6:140–146. doi: 10.4103/0259-1162.108291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaba A, Laurent S, Detroz B, Sessler D, Durieux M, Lamy M, et al. Intravenous lidocaine infusion facilitates acute rehabilitation after laparoscopic colectomy. Anesthesiology. 2007;106:11–18. doi: 10.1097/00000542-200701000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Kim HO, Lee SR, Choi WJ, Kim H. Early oral feeding following laparoscopic colorectal cancer surgery. ANZ J Surg. 2014;84:539–544. doi: 10.1111/ans.12550. [DOI] [PubMed] [Google Scholar]
- 21.Tikuisis R, Miliauskas P, Samalavicius N. Intravenous lidocaine for post-operative pain relief after hand-assisted laparoscopic colon surgery: a randomized, placebo-controlled clinical trial. Tech Coloproctol. 2014;18:373–380. doi: 10.1007/s10151-013-1065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuo CP, Jao SW, Chen KM, Wong CS, Yeh CC, Sheen MJ, et al. Comparison of the effects of thoracic epidural analgesia and i.v. infusion with lidocaine on cytokine response, postoperative pain and bowel function in patients undergoing colonic surgery. Br J Anaesth. 2006;97:640–646. doi: 10.1093/bja/ael217. [DOI] [PubMed] [Google Scholar]
- 23.Herroeder S, Pecher S, Schonherr ME, Kaulitz G, Hahnenkamp K, Friess H, et al. Systemic lidocaine shortens length of hospital stay after colorectal surgery: a double-blinded, randomized, placebo-controlled trial. Ann Surg. 2007;246:192–200. doi: 10.1097/SLA.0b013e31805dac11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Staikou C, Avramidou A, Ayiomamitis GD, Vrakas S, Argyra E. Effects of intravenous versus epidural lidocaine infusion on pain intensity and bowel function after major large bowel surgery: a double-blind randomized controlled trial. J Gastrointest Surg. 2014;18:2155–2162. doi: 10.1007/s11605-014-2659-1. [DOI] [PubMed] [Google Scholar]
- 25.Ho MLJ, Kerr SJ, Stevens J. Intravenous lidocaine infusions for 48 hours in open colorectal surgery: a prospective, randomized, double-blinded, placebo-controlled trial. Korean J Anesthesiol. 2018;71:57–65. doi: 10.4097/kjae.2018.71.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48. doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 27.Sun Y, Li T, Wang N, Yun Y, Gan T. Perioperative systemic lidocaine for postoperative analgesia and recovery after abdominal surgery: a meta-analysis of randomized controlled trials. Dis Colon Rectum. 2012;55:1183–1194. doi: 10.1097/DCR.0b013e318259bcd8. [DOI] [PubMed] [Google Scholar]
- 28.Tremont-Lukats IW, Challapalli V, McNicol ED, Lau J, Carr DB. Systemic administration of local anesthetics to relieve neuropathic pain: a systematic review and meta-analysis. Anesth Analg. 2005;101:1738–1749. doi: 10.1213/01.ANE.0000186348.86792.38. [DOI] [PubMed] [Google Scholar]
- 29.Weibel S, Jelting Y, Pace NL, Helf A, Eberhart LH, Hahnenkamp K, et al. Continuous intravenous perioperative lidocaine infusion for postoperative pain and recovery in adults. Cochrane Database Syst Rev. 2018;6:CD009642. doi: 10.1002/14651858.CD009642.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurz A, Sessler DI. Opioid-induced bowel dysfunction: pathophysiology and potential new therapies. Drugs. 2003;63:649–671. doi: 10.2165/00003495-200363070-00003. [DOI] [PubMed] [Google Scholar]
- 31.Bauer AJ, Boeckxstaens GE. Mechanisms of postoperative ileus. Neurogastroenterol Motil. 2004;16(Suppl 2):54–60. doi: 10.1111/j.1743-3150.2004.00558.x. [DOI] [PubMed] [Google Scholar]
- 32.Beaussier M, Delbos A, Maurice-Szamburski A, Ecoffey C, Mercadal L. Perioperative use of intravenous lidocaine. Drugs. 2018;78:1229–1246. doi: 10.1007/s40265-018-0955-x. [DOI] [PubMed] [Google Scholar]
- 33.Song X, Sun Y, Zhang X, Li T, Yang B. Effect of perioperative intravenous lidocaine infusion on postoperative recovery following laparoscopic cholecystectomy-a randomized controlled trial. Int J Surg. 2017;45:8–13. doi: 10.1016/j.ijsu.2017.07.042. [DOI] [PubMed] [Google Scholar]
- 34.ALLEGRO: A placebo controlled randomised trial of intravenous lidocaine in accelerating gastrointestinal recovery after colorectal surgery. https://w3.abdn.ac.uk/hsru/ALLEGRO/Public/Public/index.cshtml [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.