Abstract
Kidney cysts can manifest as focal disease (simple and complex kidney cysts), affect a whole kidney (eg, multicystic dysplastic kidney or cystic dysplasia), or manifest as bilateral cystic disease (eg, autosomal recessive polycystic kidney disease [ARPKD] or autosomal dominant polycystic kidney disease [ADPKD]). In children, as opposed to adults, a larger proportion of kidney cysts are due to genetic diseases (eg, HNF1B nephropathy, various ciliopathies, and tuberous sclerosis complex), and fewer patients have simple cysts or acquired cystic kidney disease. The purpose of this consensus statement is to provide clinical guidance on standardization of imaging tests to evaluate kidney cysts in children. A committee of international experts in pediatric nephrology, pediatric radiology, pediatric US, and adult nephrology prepared systematic literature reviews and formulated recommendations at a consensus meeting. The final statement was endorsed by the European Society of Pediatric Radiology, the European Federation of Societies for Ultrasound in Medicine and Biology, the European Society of Pediatric Nephrology, and reviewed by the European Reference Network for Rare Kidney Diseases. Main recommendations are as follows: US is the method of choice when assessing pediatric kidney cysts, with selected indications for MRI and contrast-enhanced US. CT should be avoided whenever possible because of ionizing radiation. Renal US yields essential diagnostic information in many cases. In patients with ARPKD or other ciliopathies, abdominal US is needed for diagnosis and screening of portal hypertension. US is usually sufficient for follow-up kidney imaging, but MRI can be valuable for clinical trials in patients with ADPKD or in older children with tuberous sclerosis complex to evaluate both kidney cysts and angiomyolipomas.
© RSNA, 2018
Summary
This international consensus statement on imaging of cystic kidney diseases in children highlights US as the most useful imaging modality with which to diagnose a wide variety of underlying disorders and for clinical follow-up.
Implications for Patient Care
■ Consensus-based imaging criteria for diagnosis and follow-up aim to harmonize and improve standard of care in children with kidney cysts.
■ US is the preferred examination method in most children with a large variety of cystic kidney diseases or simple cysts.
■ The indications for MRI are limited (eg, for suspicion of malignancy [complex cysts], tuberous sclerosis complex with cysts and angiomyolipomas, clinical trials in autosomal dominant polycystic kidney disease measuring total kidney volume).
Introduction
Cystic kidney diseases are being diagnosed earlier and earlier (1) because of more accessible routine prenatal US and advances in US technology. However, differential diagnosis often remains a challenge because kidney cysts can arise in a large variety of illnesses, imaging patterns evolve over time, and extrarenal features of systemic diseases may not be present at a young age. Thus, imaging findings for a particular disease in newborns may be different from those in adolescents, and predicting prognosis or deciding on optimal follow-up intervals can be challenging. Imaging can contribute not only to accurate diagnosis and prognosis but also to rational management of cystic nephropathies. In adults with autosomal dominant polycystic kidney disease (ADPKD), for example, total kidney volume measured at MRI has emerged as a useful tool with which to identify patients who will benefit from treatment (2); however, transfer and adaptation of these findings to children is still an important research need.
The purpose of this consensus statement is to establish uniform standards for choosing the correct imaging modality and diagnostic criteria for the most common cystic kidney diseases in childhood and adolescence and to propose rational approaches to diagnosis and follow-up in important clinical settings. The spectrum of diseases covered here includes simple cysts; multicystic dysplastic kidneys (MCDKs); cystic dysplasia and HNF1B-associated disease; autosomal recessive polycystic kidney disease (ARPKD) and ADPKD; other ciliopathies, such as nephronophthisis and Bardet-Biedl syndrome; acquired cystic kidney disease (ACKD); and renal cysts in other diseases, such as tuberous sclerosis complex (TSC). Glomerulocystic disease is not covered separately here, as it is a purely descriptive diagnosis and a condition that can be caused by a variety of underlying diseases, such as autosomal dominant tubulointerstitial disease, HNF1B nephropathy, or early TSC, and therefore has little clinical value. Cystic tumors are covered briefly only in the context of complex cysts.
This consensus statement was endorsed by the European Society of Pediatric Radiology Task Force on Genitourinary Imaging, the European Federation of Societies for Ultrasound in Medicine and Biology, the European Society of Pediatric Nephrology (working group on inherited kidney disorders), and reviewed by the European Reference Network for Rare Kidney Diseases (working group on autosomal dominant structural kidney disorders).
Materials and Methods
The Network for Early Onset Cystic Kidney Disease (or NEOCYST) is a consortium of clinical, genetic, and translational researchers devoted to the study of early-onset cystic kidney diseases with a work package devoted to the development of guidelines and position papers (3). It is supported by the German Ministry of Education and Research, which did not influence the choice of topic or content of the position paper. In addition to pediatric nephrologists from the consortium, external experts in pediatric radiology, pediatric US, and kidney imaging, as well as adult nephrologists with specific expertise in cystic diseases, were invited to participate. The allocation of individual members of the guideline committee to the different working groups is given in Table E1 (online).
Six researchers (C.G., K.B., A.T., R.T, E.F.A., D.M.) prepared systematic literature reviews in advance of the consensus workshop held on September 26, 2017, in Hanover, Germany. Literature search strategies and ratings are explained in detail in Appendix E1 (online). Initial recommendations were developed during the workshop via iterative discussion in thematic workgroups and plenary sessions. Because of the lack of randomized trials for almost all questions discussed, formalized grading of evidence and recommendations was not performed. A first draft summarizing the consensus recommendations was compiled by two researchers (C.G., F.S.) and then reviewed by all members of the consensus group. After changes proposed by the academic societies were made, the final draft was reviewed again by all members. A list of suggestions for further research can be found in Appendix E1 (online).
Technical Issues and General Recommendations
Recommendations for Measuring and Describing Kidney Cysts and Polycystic Kidneys
Kidney cysts and cystic kidneys should be investigated by using US in the first instance, with detailed examination and description of the renal parenchyma, urinary tract, and cysts.—At US, kidney size should be measured by using maximal midsagittal length, as well as width and depth at the level of the hilum. Kidney volume should be estimated with the prolate ellipsoid formula or may optionally be determined by using manual contouring of the kidney and by applying the method of disc summation at three-dimensional imaging (4). All measurements should be compared with normal limits of locally applicable reference values (5–7). The examination should document cortical echogenicity (hypo-, iso-, or hyperechogenic when compared with liver) and whether corticomedullary differentiation is normal, absent, or reversed. Comparison with liver echogenicity may be difficult in patients with concomitant liver disease. Multiple very small cysts can also generate abnormal parenchymal echogenicity (eg, salt-and-pepper sign at ARPKD) or even solely increased echogenicity without visible cysts. When macroscopic cysts are present, the number of cysts (one, two to five, six to 10, or more than 10), laterality (uni- or bilateral), location (cortical, medullary, corticomedullary border, ubiquitous), and size (maximum diameter of the largest cyst) should be noted. The renal hilum and collecting system should be identified to avoid confusion of cysts with dilated calyces due to outflow obstruction or reflux. The complete urinary tract should be examined to identify associated disease. Care should be taken to examine for abnormal features of the cysts, such as signs of bleeding, infection, or malignancy (including, but not limited to, calcification, thickened wall, septations, echogenic and/or floating material within cysts, or hyperperfusion of septations or the cyst wall). However, except for complex cysts, neither Doppler US nor contrast material–enhanced US are systematically required for characterization of cysts (see also the Technical Recommendations for Standard and Contrast-enhanced US section).
CT or MRI is not recommended for routine assessment of kidney cysts.
US examination of the liver is recommended for initial evaluation of cystic kidney disease. Additionally, transabdominal genital system US should be performed in female patients at first examination.—The liver examination should focus on detection of liver cysts, signs of fibrosis (such as irregular liver margin, coarse echotexture, or patchy, uniform, or periportal hyperechogenicity), and signs that may indicate portal hypertension (such as splenomegaly and decreased or reversed portal Doppler flow patterns). When a disease with known liver involvement is confirmed, US elastography can be used to examine for liver fibrosis, with normal values for children becoming available only recently (8–12). In patients with ARPKD, liver US may also reveal mild biliary dilatation or cystic transformation of the intrahepatic bile ducts (Caroli syndrome).
Transabdominal US of the internal genitalia in female patients is generally recommended with urinary tract US (13) but is especially important in this setting, as HNF1B mutations and Bardet-Biedl syndrome are associated with Müllerian duct anomalies, such as uterus didelphys, which can lead to obstructed hemivagina at first menstruation.
Technical Recommendations for Standard and Contrast-enhanced US
US should be performed by an experienced examiner, with special training in pediatric US using the highest possible resolution transducers with settings optimized to the child.—A curvilinear transducer with a frequency of more than 7 MHz is recommended for examinations in the prone and supine positions, and a linear transducer with a frequency of more than 10 MHz is recommended for examination in the prone position. For children who are older, obese, or both, lower-frequency transducers may be needed. For optimal visualization, settings need to be adjusted to the age and weight of the patient. US devices capable of color Doppler imaging and, ideally, that are also capable of performing elastography measurements are recommended.
There are insufficient data in children for the use of contrast-enhanced US in cystic kidney disease.—Contrast-enhanced US has demonstrated good specificity and sensitivity in the examination of complex renal cysts (and suspicious solid renal lesions) in adults (Table E2 [online]). However, although it may be promising for the future, it has not been evaluated for investigation of cystic kidney disease in adults or children. Also, there is little expected benefit of contrast-enhanced US in this setting, except for tumorous lesions such as fat-poor angiomyolipomas (AML), which are potentially better delineated (14). Because indications, standard of equipment, and training await systematic evaluation, its use is currently restricted to selected cases with complex cysts in experienced centers.
Technical Recommendations for MRI and CT
MRI is not routinely necessary and should be restricted to selected cases of pediatric kidney cysts. Examination techniques should be customized to the pediatric patient.—MRI usually requires sedation in smaller children. This poses extra risks, especially in patients with pulmonary problems resulting from gross nephromegaly. Because US yields adequate information in most children with kidney cysts, MRI should be reserved for specific indications, such as suspicion of malignancy in complex cysts, detection of AML in patients with TSC, and total kidney volume (TKV) measurements (eg, in clinical trials of ADPKD). MRI should be preferred to CT because of the added importance of avoiding ionizing radiation in children. Referral to a center that specializes in pediatric MRI may be necessary.
In patients with impaired renal function, only macrocyclic gadolinium chelates should be used if a contrast agent is required. Gadolinium-containing contrast agents are contraindicated if the estimated glomerular filtration rate is less than 30 mL/min · 1.73 m2 due to the risk of gadolinium-induced nephrogenic systemic fibrosis. In patients younger than 1 year or if the estimated glomerular filtration rate is less than 60 mL/min · 1.73 m2, gadolinium-based contrast agents should be used with caution and should not be used twice within 7 days (15). A history of allergic reactions should be obtained prior to contrast material administration.
T2-weighted sequences are best for use in the visualization of cystic involvement (including cyst volume measurement in cases of ADPKD), while T1- or T2-weighted sequences can be used to delineate the kidney outline and determine TKV. Contrast-enhanced T1-weighted sequences should only be performed to investigate hemorrhage and tumors, and fat-suppression techniques should be used to examine AML. Diffusion-weighted MRI may be useful in assessing suspected tumorous lesions. Further specific sequences may be necessary to image the liver parenchyma and changes associated with portal hypertension.
Assessment of TKV at MRI does not require use of a contrast medium; it can be measured with several different techniques that have only been validated in adults; planimetry is most accurate and should be preferred for clinical trials, while simplified methods (ellipsoid formula or the midsection method) are fast and useful to provide a rough estimate of kidney volume or to classify adult patients with ADPKD (16).
CT should not be routinely used for investigation of pediatric kidney cysts because US and MRI usually provide better contrast resolution without radiation exposure. It may be useful in a very small subset of children (eg, those with claustrophobia or when MRI is not available even with referral). Examination techniques should be customized to the pediatric patient.—Because kidney cysts are well visualized at US and MRI, there is usually no justification for exposing children to radiation during their investigation. CT should be avoided and is only acceptable if MRI is unavailable or is not possible (eg, a patient with TSC and an implanted vagus nerve stimulator) and suspected malignancy, bleeding, or complicated infection require urgent treatment decisions. If needed in an individual situation, CT protocols appropriate to the child should be applied by experienced radiologists and CT radiographers to optimize acquisition data relative to the child’s age and weight, and the radiologist or radiographer should limit the exploration field and radiation dose as much as possible. Kidney function and history of allergic reactions to contrast agents should be verified prior to their administration.
Prenatal Suspicion of Cystic Kidney Disease
Cystic kidney diseases often manifest only with hyperechogenicity and/or enlarged kidneys prenatally. Oligohydramnios and extrarenal features, such as congenital malformations and reduced lung volume, are important imaging findings because they affect prognosis. Regular monitoring and multidisciplinary care are required during pregnancy and postnatally.—Prenatal presentation of cystic kidney disease poses numerous challenges to diagnosis and management within a tight chronologic framework and requires the care of multiple health professionals, which this group has addressed in a previous clinical practice recommendation (17).
Disease-specific Recommendations
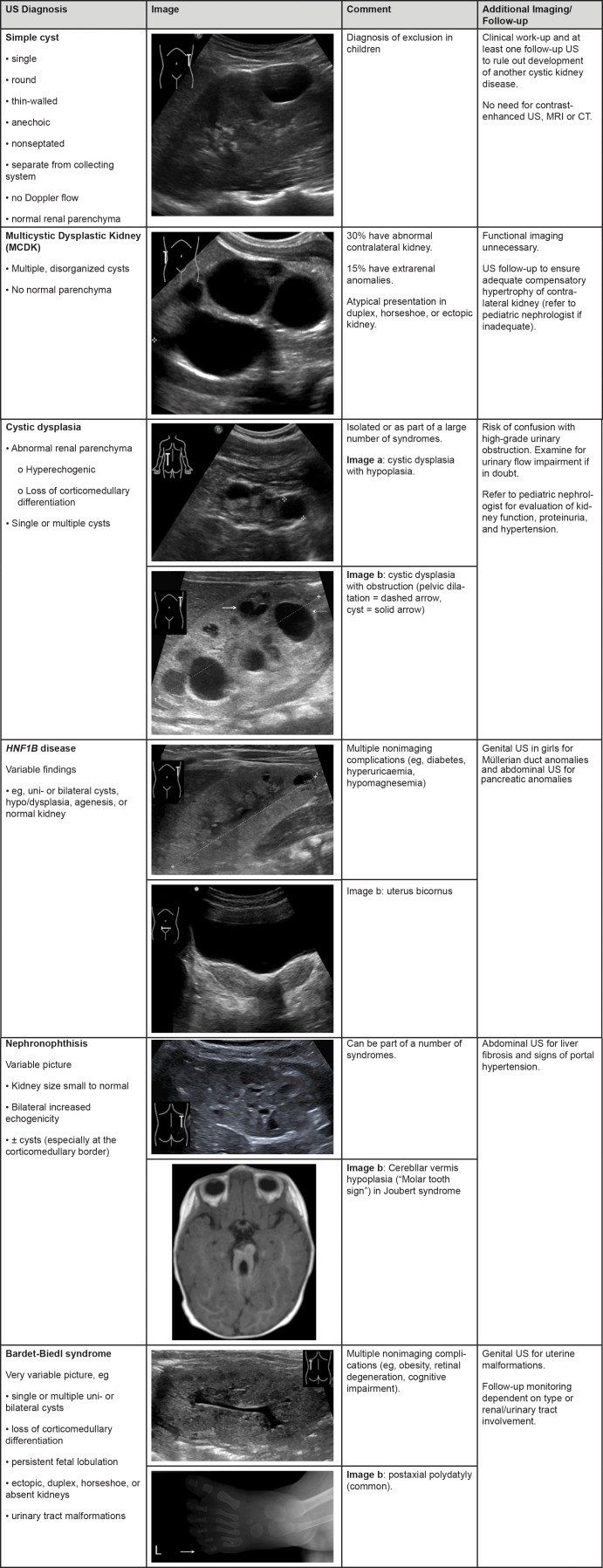
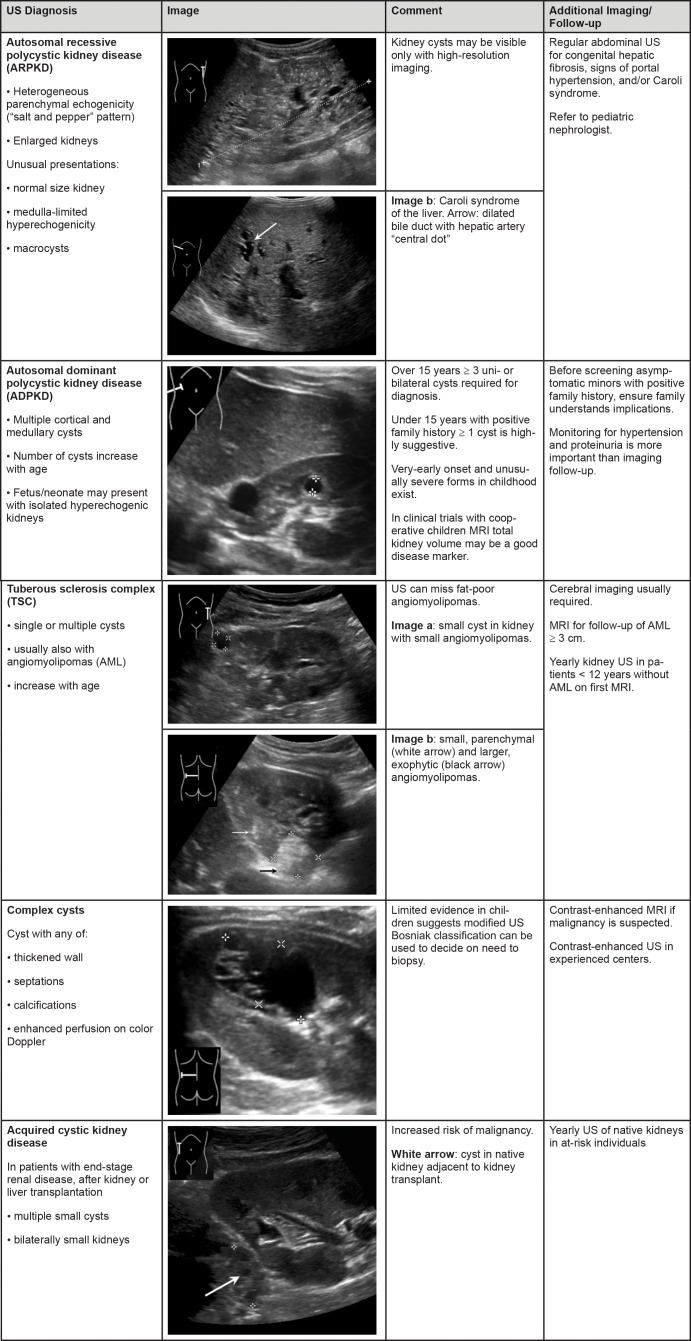
Disease-specific diagnostic imaging criteria and recommendations for further imaging are summarized in Figures 1 and 2.
Figure 1:
Summary of findings for a simple cyst and a multicystic dysplastic kidney. Summary of findings for cystic dysplasia and HNF1B disease. Summary of findings for nephronophthisis and Bardet-Biedl syndrome.
Figure 2:
Summary of findings for autosomal recessive polycystic kidney disease and autosomal dominant polycystic kidney disease. Summary of findings for tuberous sclerosis complex, complex cysts, and acquired cystic kidney disease.
Simple Cyst
By definition, a simple cyst occurs in a kidney with otherwise normal parenchyma and a normal contralateral kidney. It is round, thin-walled, anechoic, nonseptated, separate from the collecting system, and has no Doppler blood flow related to the cyst.—Simple cysts can occur anywhere within the parenchyma (18–20). They are much rarer in children than in adults, with incidences of less than 0.5% in children (19), more than 10% in adults aged 50 years or older (21), and more than 30% in adults older than 70 years (22). Cysts with additional features, such as solid components, septations, or thickened walls, should be considered complex cysts. Differential diagnosis includes the first manifestation of other cystic diseases, caliceal diverticulae (23), hydrocalyx, cystic dysplasia, and—very rarely—hydatid cysts. Posttraumatic kidney cysts have been described mainly in adults.
A child with a first-time diagnosis of a single kidney cyst requires a detailed medical and family history, thorough clinical examination, and at least one follow-up assessment.—Simple kidney cysts are rare in children and may be the first manifestation of an underlying cystic disorder, such as genetic cystic disease or, in rare cases, malignancy (18). Thus, simple cysts should be considered a diagnosis of exclusion. Clinical examination and at least one follow-up US examination should be performed to assess for development of additional cysts or changes in cyst size or imaging characteristics.
A simple cyst in a child does not need contrast-enhanced US, MRI, or CT imaging.—Because of the low risk of malignancy, standard US with color Doppler imaging is sufficient for diagnosis and follow-up, unless atypical features develop.
Multicystic Dysplastic Kidney
An MCDK is characterized by replacement of the whole kidney with multiple disorganized cysts lacking any normal surrounding parenchyma. If functional renal imaging is performed, no (or minimal) function is seen.—MCDK is characterized by a lack of renal parenchyma, although high-resolution probes may show a small amount of central echogenic dysplastic undifferentiated tissue. Bilateral MCDK is associated with severe oligohydramnios or anhydramnios before birth and usually leads to perinatal death because of inadequate lung development. Unilateral MCDK may be an isolated finding, but one in three children have additional urogenital tract anomalies, and 15% show signs of extrarenal anomalies (24). The contralateral kidney should be examined thoroughly for dysplasia and other congenital uropathies, as this affects overall renal prognosis (25). Atypical presentation can occur with MCDK in only one moiety of a duplex or horseshoe kidney or in an ectopic kidney (26).
In children with unilateral MCDK, renal functional imaging, such as dimercaptosuccinic acid (DMSA) or technetium 99m diethylenetriaminepentacetate (DTPA) scintigraphy is not recommended, particularly if the contralateral kidney is structurally normal at US with compensatory hypertrophy.—The concordance between US and nuclear medicine studies for MCDK is very high (27). Clinically, there is no benefit in showing lack of functional tissue, as nephrectomy is no longer routinely performed in most centers (28,29). Compensatory hypertrophy (kidney size more than two standard deviations above the mean for one kidney) can occur in utero but may take several years to develop (30,31).
In children with unilateral cystic dysplasia or MCDK, the contralateral kidney should be monitored with serial US to ensure continued appropriate compensatory hypertrophy.—Frequency of monitoring depends on the child’s age and the degree of compensatory hypertrophy. Local practices differ over imaging monitoring intervals. Observational data suggest that, of those children who develop compensatory hypertrophy, 95% will do so by 3 years of age. However, 10% still do not show compensatory hypertrophy by 10 years of age (30). Additional clinical follow-up for hypertension and proteinuria is warranted in all children with only one functioning kidney (25).
Children with bilateral cystic dysplasia or unilateral cystic dysplasia/MCDK without contralateral compensatory hypertrophy are at increased risk for progressive chronic kidney disease (32) and require follow-up by a pediatric nephrologist.—This will include monitoring of blood pressure, proteinuria and kidney function. Imaging follow-up is unlikely to have therapeutic consequences and yields less prognostic information than serial kidney function measurements. Thus, imaging does not need to be performed regularly unless there are other clinical indications, such as infections, pain, or bleeding.
There is no evidence for an increased risk of malignancy in MCDK in children and young adults; therefore, monitoring solely to exclude malignancy is unnecessary.—Despite case reports of various malignancies coinciding with MCDK (33–36), numerous cohort studies with follow-up during childhood have not shown an increased risk of malignancy (Table E3 [online]).
Cystic Dysplasia
Cystic dysplasia is characterized by at least one cyst within an abnormal kidney (eg, hyperechogenic parenchyma, loss of corticomedullary differentiation, small kidney).—Other urinary tract abnormalities (such as flow impairment [in ureteropelvic or vesicoureteral junction stenosis], vesicoureteral reflux, or posterior urethral valves) may coexist. Cystic dysplasia usually involves the whole kidney but can be localized as segmental dysplasia or in a moiety of a duplex kidney. It can occur in isolation or as part of many syndromes and chromosomal aberrations. Referral to pediatric nephrology is usually required to assess for renal function, proteinuria, or hypertension.
Dilated calyces in patients with high-grade obstruction or a urinoma may be misdiagnosed as kidney cysts. If there is any suspicion of urinary flow impairment, further urologic examination is recommended.—While dysplastic cysts are usually more subcapsular than dilated calyces, differentiation may be difficult even for experienced examiners. Obstruction should be ruled out (usually with MAG3 scintigraphy) if there is evidence of collecting system dilatation, such as an enlarged renal pelvis or megaureter, or if normal surrounding parenchyma can be demonstrated.
HNF1B-associated Disease
The diagnosis of HNF1B-associated disease cannot be made with kidney imaging alone and requires genetic confirmation.—Renal presentation of mutations in the HNF1B gene (also known as transcription factor 2) is variable and nonspecific (eg, uni- or bilateral cysts, hypo- or dysplasia, agenesis, or normal kidney imaging). HNF1B nephropathy is the most common cause of kidney hyperechogenicity at prenatal US and of kidney cysts in older children (37,38), and its US appearance can mimic that of ARPKD and other cystic nephropathies. Many patients have prenatal US kidney abnormalities, but other associated prenatal anomalies that may be detected with abdominal US include genital malformations (eg, vaginal and uterine anomalies) and pancreatic anomalies (eg, atrophy and partial agenesis) (Table E4 [online]). However, prenatal US is often not sensitive enough to routinely show these anomalies.
Because of the autosomal dominant inheritance, kidney US in parents (and grandparents, if available) may provide useful clues, but 50% of cases carry de novo mutations with a negative family history. A family history of maturity-onset diabetes of the young type 5 (renal cysts and diabetes syndrome), gestational diabetes, hyperuricemia, or concurrent hypomagnesemia also makes the diagnosis more likely. HNF1B deletions can also be part of chromosome 17q12 deletion syndrome.
Girls with HNF1B mutations should be examined with transabdominal US of the genital tract.—The reported incidence of genital tract abnormalities varies widely between studies (Table E4 [online]), with a substantial proportion of female subjects having uterine abnormalities. Some of these may become clinically relevant at first menstruation, justifying examination of the uterus during abdominal US. Transvaginal US is not recommended in childhood since it is unlikely to yield information of therapeutic relevance.
Nephronophthisis
Since US findings of nephronophthisis are nonspecific, the diagnosis cannot be based on imaging findings alone. In addition, MRI of the kidneys does not help diagnosis or management.—Juvenile nephronophthisis usually manifests in the form of normal-sized or small kidneys and bilaterally increased echogenicity. Since cysts are not universally present, they are not mandatory for diagnosis. However, cysts at the corticomedullary junction are suggestive of a nephronophthisis-associated renal phenotype. Infantile nephronophthisis usually manifests as enlarged kidneys and cortical microcysts prenatally or in neonates.
Nephronophthisis can manifest as isolated renal disease or as part of a syndrome (eg, Joubert, Senior-Løken, Cogan, Jeune [asphyxiating thoracic dystrophy], Meckel-Gruber, Bardet-Biedl, or Sensenbrenner syndromes); thus, detailed clinical examination, including ophthalmologic assessment, is required. Differential diagnosis includes cystic dysplasia and autosomal dominant tubulointerstitial kidney disease (ADTKD), which can occasionally manifest as medullary cysts. (ADTKD is a group of diseases, including those previously known as medullary cystic kidney disease or familial juvenile hyperuricemic nephropathy, and may be due to UMOD, REN, HNF1b, or MUC1 mutations [39].)
Children suspected of having nephronophthisis should undergo abdominal US to look for signs of liver fibrosis and portal hypertension.—Liver fibrosis has been described in patients with NPHP3, NPHP11, TMEM67, ANKS6, and DCDC2 mutations. In the absence of a known genetic defect, or in case of mutations in one of the aforementioned genes, abdominal US is recommended to screen for liver fibrosis and portal hypertension.
In children suspected of having nephronophthisis and disability, developmental delay, or cerebral dysfunction, cranial MRI is suggested to look for cerebellar vermis hypoplasia.—This “molar tooth” sign (Fig 1) is found in patients with Joubert syndrome.
Bardet-Biedl Syndrome
Since imaging findings are nonspecific, the diagnosis of Bardet-Biedl syndrome cannot be made on the basis of imaging findings alone.—Kidney US in patients with Bardet-Biedl syndrome reveals a wide range of abnormalities, including single or multiple uni- or bilateral cysts; loss of corticomedullary differentiation; persistent fetal lobulation; horseshoe, ectopic, duplex, and absent kidneys; and urinary tract malformations (40). Prenatal US often shows bilaterally enlarged homogeneously hyperechoic kidneys without corticomedullary differentiation (41), while in later life the renal phenotype may resemble nephronophthisis. Thus, the diagnosis cannot be based on imaging findings alone but must include clinical findings and genetic analysis; however, imaging can be helpful because kidney findings may precede other features (eg, obesity, retinal degeneration, and cognitive impairment) and, when found in conjunction with polydactyly, are highly suggestive. While there are a range of extrarenal findings, only a few are seen solely at imaging (eg, uterine malformations). In later stages of the disease, US quality is sometimes hampered by obesity.
Monitoring Children with Nephronophthisis, Bardet-Biedl Syndrome, and HNF1B Disease
Regular kidney imaging is not required in children with nephronophthisis. However, monitoring for signs of hepatic fibrosis is recommended in children with genetic defects known to be associated with liver involvement and those with an unknown genotype. Monitoring in children with Bardet-Biedl syndrome and HNF1B-associated disease will depend on the type of renal and urinary tract involvement.—Children with Bardet-Biedl syndrome who have substantial structural kidney or urinary tract abnormalities are at risk for progression to end-stage renal disease in childhood (40). Therefore, regular screening for urinary tract involvement and voiding dysfunction is recommended.
Autosomal Recessive Polycystic Kidney Disease
The classic US appearance of ARPKD is bilaterally enlarged kidneys with heterogeneous parenchymal echogenicity with a salt-and-pepper pattern. This imaging appearance is caused by multiple tiny cysts smaller than or just approaching the size necessary for detection, which disrupt the echo pattern without being clearly distinguishable. However, the renal sonographic phenotype can be variable: kidney size may range from normal to massively enlarged, hyperechogenicity can be limited by the medulla or diffuse, and cysts can appear as ductal dilatation or as macrocysts of variable size, number, and location.—Prenatal US may also depict large hyperechogenic kidneys and oligohydramnios in severe cases, and these findings are markers that the patient is at risk for early need for dialysis (42). Liver involvement (congenital hepatic fibrosis, Caroli syndrome, or both) is variable (43) and is not tightly correlated with renal involvement (44). In classic cases, abdominal US reveals liver enlargement, increased or coarsely granulated echogenicity with periportal fibrosis (appearing as increased periportal echogenicity), intrahepatic bile duct dilatation, cysts, or a combination of findings. Signs of portal hypertension, such as decreased or reversed portal blood flow and splenomegaly, may be present. In addition, sonographic signs of Caroli syndrome (ie, intrahepatic cystic anechoic areas that may contain fibrovascular bundles [“central dot sign”], stones, or septa) should be assessed. The fibrovascular bundles are composed of portal vein and hepatic artery branches, which can be depicted by color Doppler US (45).
US is the most useful imaging tool with which to diagnose ARPKD, but further information is often necessary to confirm the diagnosis (eg, family history, US examination of the parents, clinical examination, and genetic analysis). MRI and MR cholangiopancreatography should be reserved for use in patients with clinical complications of liver disease. Differential diagnosis includes congenital hepatic fibrosis; atypical ADPKD; HNF1B disease; other ciliopathies, such as nephronophthisis, short rib-polydactyly, and Bardet-Biedl, Jeune, and Ivemark syndromes; as well as fatty acid oxidation defects (46,47). Antenatally, Meckel-Gruber syndrome may have a similar renal appearance, but it is always accompanied by severe anomalies of the central nervous system (46).
In children with known ARPKD, annual abdominal US is suggested to monitor for signs of portal hypertension. In severely affected infants with progressive disease, kidney size should be monitored according to clinical needs.—In patients with ARPKD, there is no clear prognostic value of kidney size on kidney function (48,49). Thus, kidney imaging is mainly useful for decision making prior to potential nephrectomy and transplantation. On the other hand, liver involvement is harder to quantify clinically than kidney involvement, and US should be used to look for signs of portal hypertension, such as splenomegaly or abnormal portal Doppler flow. In case of confirmed liver disease, it may be necessary to perform US (including color and duplex Doppler US of the portal vessels and collateral circulation) at shorter intervals. US elastography may also become a helpful tool to screen for liver fibrosis (50). Careful liver assessment is important prior to kidney transplantation, even if there are no clinical symptoms. Patients with substantial bile duct dilatation (Caroli syndrome) may be at increased risk for ascending cholangitis due to posttransplant immunosuppression. In addition, children with severe congenital hepatic fibrosis, portal hypertension, or recurrent cholangitis require systematic evaluation for combined kidney and liver transplantation (51,52).
Autosomal Dominant Polycystic Kidney Disease
ADPKD is characterized by the gradual development of multiple cortical and medullary cysts from early life. Initial presentation may be antenatal with subtle cortical hyperechogenicity and renal enlargement. Overt cyst development may then be detectable at any stage from before birth through childhood. The Pei Ravine criteria for US diagnosis of ADPKD (53) (three or more uni- or bilateral cysts) were derived from patients older than 15 years and have low sensitivity in younger children. In patients younger than 15 years with a positive family history, the presence of at least one kidney cyst, kidney enlargement above normal limits, or both should be considered highly suggestive of ADPKD.—US is the most useful imaging modality with which to diagnose ADPKD. While high-resolution US and MRI are able to depict more and smaller cysts than can standard US in adults, there are no diagnostic criteria for these imaging modalities in children (Table E5 [online]). In the absence of a known family history of ADPKD, parents and grandparents (especially if parents are younger than 40 years) of children with kidney cysts, nephromegaly, or both should undergo kidney US screening.
Very-early onset ADPKD can manifest as hyperechoic kidneys prenatally or in neonates, with a US appearance mimicking that of ARPKD or glomerulocystic disease (54). Liver involvement is very uncommon but may occur in patients with early onset ADPKD. In children with neurologic or cutaneous lesions suggestive of TSC, the TSC2/PKD1 contiguous gene deletion syndrome (CGS) should be considered. Differential diagnoses of ADPKD include ARPKD, autosomal dominant tubulointerstitial kidney disease, HNF1B-associated kidney disease, polycystic liver disease, von Hippel-Lindau disease (55), and X-linked dominant orofaciodigital syndrome type I in female patients. In patients with a negative family history or very-early onset ADPKD, the diagnosis should be confirmed with genetic testing.
There are important ethical controversies and psychosocial issues surrounding the use of presymptomatic imaging to diagnose ADPKD in minors, as therapeutic consequences may not arise until adulthood.—Systematic diagnostic screening with imaging of asymptomatic minors at risk for disease is controversial in current ADPKD guidelines (52,56,57). Treatable disease manifestations such as hypertension may arise in childhood (58). Therapeutic options to slow the progression of renal disease are becoming increasingly established in adults and may, in selected cases, be considered in children. Parents with ADPKD who request imaging of their asymptomatic children require genetic counseling. This group will publish recommendations on pediatric ADPKD addressing these issues in the near future.
In children suspected of having ADPKD without a genetic diagnosis or a clear family history, confirmatory US should be performed within 12 months after the initial screening. In children with multiple cysts, enlarged (2 or more standard deviations larger than the mean) kidneys, or both, serial renal US may be helpful but should not be performed more frequently than once per year unless patients have complications (eg, pain or hematuria). MRI should be restricted to rare cases when further imaging is needed and US is not feasible.—Imaging findings rarely have therapeutic consequences in pediatric patients with ADPKD; thus, clinical monitoring of blood pressure and microalbuminuria is more important, even though children with larger kidneys are at increased risk for hypertension (59–62). In patients older than 15 years, TKV at MRI has prognostic value for disease progression (63,64), which in adults is relevant for decisions about treatment with the vasopressin receptor inhibitor tolvaptan. Thus, these recommendations may change in the future if a licensed treatment becomes available in asymptomatic children, which might necessitate the identification of patients who are at risk for progression to end-stage renal disease.
In pediatric clinical trials in patients with ADPKD, total kidney volume determined with MRI is recommended to monitor progression in cooperative children.—During childhood, glomerular filtration rate is nearly always within the normal range or even increased due to hyperfiltration (58,65); thus, a decrease in estimated glomerular filtration rate is a suitable marker of disease progression only in the subgroup of children with very-early onset ADPKD (66). Height-adjusted TKV at MRI is the most established imaging parameter with which to monitor disease progression in adults in ADPKD trials (67). Correlation of MRI measurements to disease severity in children has been performed in only one study, which revealed a correlation with current hypertension status and a predictive value of cyst volume on development of hypertension (59).
US monitoring of kidney size and cyst number is preferable to MRI in uncooperative children with ADPKD.—In contrast to adults, kidneys in children with early ADPKD can usually be imaged within one US viewing field (maximum size, approximately 17 cm), which enables adequate measurements for volume calculation to be obtained with the ellipsoid formula. Although MRI measurements appear to be slightly larger than US kidney volumes in children with ADPKD, discrepancies arise mainly for larger kidneys (4,59). Correlation of hypertension to US kidney volume has been demonstrated in four pediatric studies (60–62,68). Three-dimensional US is a promising new tool for TKV measurement in children with ADPKD (4), especially for single kidney volumes less than 250–300 mL and in other pediatric kidney diseases (69), but it requires further validation. Because uncooperative children require sedation for MRI, this does not appear warranted in a research setting without direct benefit for the child. Use of MRI planimetry to measure TKV may be appropriate in adolescents in clinical trials or in children with very large kidneys.
Tuberous Sclerosis Complex
The diagnosis of TSC is based on diagnostic criteria, including clinical characteristics and imaging findings (mainly on cerebral MRI) (70). Kidney involvement in TSC can include AML or kidney cysts, which may be detected by using kidney US or MRI.—TSC is usually diagnosed based on clinical and cerebral imaging features before renal involvement occurs. AMLs are the most common renal manifestation of classic TSC and are considered a major clinical diagnostic feature (70). AMLs can often be detected with US, but US may not depict fat-poor AMLs. MRI may be needed for rapidly growing AMLs or for those with unusual features.
Kidney cysts occur in about 40% of children with classic TSC (Table E6 [online]), and the presence of multiple kidney cysts is considered a minor clinical diagnostic feature of TSC. In classic TSC, kidney cysts are usually small and scarce (thus, they may be unilateral and difficult to detect at US in early stages of disease) but may precede renal AMLs. Kidney size is initially normal. In the absence of an established diagnosis of TSC, differential diagnoses include HNF1B-associated disease, ARPKD, and ADPKD.
Patients with the TSC2/PKD1 CGS have a kidney phenotype reflecting concurrent severe ADPKD, with numerous bilateral large cysts (>2.5 cm) that appear early in life, replace the renal parenchyma, and lead to kidney enlargement (71–73). When numerous kidney cysts are present, AMLs may be difficult to detect with US, and MRI may be needed.
For observation of children with known TSC (classic and CGS), renal US is suggested until puberty, and MRI is recommended thereafter.—Kidney US can be used to screen for AMLs and to monitor cysts in children with classic TSC and TSC2/PKD1 CGS. However, since US can miss AMLs that are fat poor or that are in kidneys with numerous cysts, kidney MRI may be preferable in children at higher risk for AML (eg, older than 6 years of age) or with TSC2/PKD1 CGS. Use of a gadolinium-based contrast agent at MRI is not needed for baseline examination and should be reserved for cases of unusual presentation, such as rapid enlargement, or with clinical features indicating a potential complication. In patients undergoing brain MRI, the addition of kidney MRI can be considered every 1–3 years if it is feasible to perform in the same session. Potential risks, such as prolonged sedation or excessive examination time, must be considered when deciding to perform kidney MRI. In patients with AML 3 cm or larger, yearly repeat MRI has been recommended (74). In patients without AMLs at initial MRI, this group suggests annual US for follow up, with repeat MRI every 2–3 years. In patients older than 12 years, we recommend MRI every 1–3 years, depending on findings, which is analogous to recommendations for adults (75).
Complex Cysts and Cystic Tumors
Typical signs of malignancy at US are a unilateral single cystic mass with a thickened wall, septations, and possibly calcifications or enhanced perfusion at color Doppler imaging.—In addition to examining the cystic lesion itself, it is important to examine abdominal lymph nodes and evaluate potential tumor infiltration into the surrounding tissues or venous system by using standard and Doppler US.
The differential diagnosis of complex cysts includes cystic nephroma, mesoblastic nephroma, cystic Wilms tumor (nephroblastoma), rarer kidney tumors that are infrequently cystic (renal cell carcinoma, sarcoma, or rhabdoid tumors), and parasitic cysts. Cystic nephromas are morphologically very similar to cystic partially differentiated nephroblastoma and multilocular cystic renal cell carcinoma. Calcifications in the lesions or nodules in the septa would be suggestive of cystic partially differentiated nephroblastoma or cystic renal cell carcinoma, but neither US nor CT can be used to conclusively distinguish these entities (76). Kidney cysts and cystic tumors can occur in children with systemic overgrowth or tumor predisposition syndromes. For example, children with Beckwith-Wiedemann syndrome can have nephromegaly, kidney cysts, or Wilms tumor (77,78). Children with cystic nephroma often have germline DICER1 mutations, which also predispose them to several other benign and malignant tumors and require regular monitoring (79). Patients with von Hippel-Lindau syndrome can develop simple or complex kidney cysts and clear-cell renal cell carcinoma (80).
The Bosniak classification was originally developed in adults to classify kidney cysts as benign, intermediate, or likely malignant based on CT characteristics. In children, limited experience suggests that a modified Bosniak classification based on US appearance can be used to decide which lesions should be sampled for biopsy (81). There appears to be good agreement between US and CT classification of cysts (82) and good interobserver agreement of US Bosniak classification in children (83). This classification is less helpful in von Hippel-Lindau syndrome, as cystic lesions may contain microscopic tumors. On the other hand, renal cell carcinomas tend to be low grade and slow growing in this group (84).
US should be used in the initial assessment of complex cysts, with further imaging performed with contrast-enhanced MRI in cases of suspected malignancy. Contrast-enhanced US may be performed in experienced centers for further evaluation.—Contrast-enhanced US appears useful in the further characterization of complex cysts in adults (Table E2 [online]). Its role in children has not been evaluated; however, it may hold potential for the future. Thus, abdominal MRI (with diffusion-weighted imaging) should be used for further imaging, but abdominal CT is also acceptable (both should be used with contrast material; see the warnings already discussed). MRI (or in exceptional cases, CT) can also be used to look for small lesions or nephrogenic rests in the contralateral kidney, which may not have been visible at US. Unless a Wilms tumor is suspected (in which case chemotherapy or primary resection are performed), biopsy is usually required to diagnose complex cysts.
Acquired Cystic Kidney Disease
ACKD is characterized by multiple small cysts, usually occurring bilaterally in small kidneys, predominantly in the setting of end-stage renal disease, or in native kidneys after kidney or liver transplantation.—A limited number of older studies suggest that the incidence of ACKD in children at dialysis is 22%–46%, which is comparable to that in adults at dialysis (85–87). However, ACKD has also been described in children prior to the start of dialysis (88). In adults, incidence increases with duration of dialysis but is not associated with race, sex, or underlying renal diagnosis (89).
In two cohorts of children after liver transplantation, ACKD was observed in 11%–30% of children and was associated with reduced kidney function (90,91). ACKD was also reported in a case series of seven survivors of stage IV neuroblastoma who had undergone whole-body irradiation for bone marrow transplantation. All of these patients developed chronic kidney disease, two developed end-stage renal disease, and one developed renal cell carcinoma (92).
In patients on renal replacement therapy and after renal transplantation, yearly US follow-up of native kidneys is suggested to monitor for ACKD.—ACKD is an important risk factor for renal cell carcinoma in adults with end-stage renal disease (89). This can also occur during childhood (86), in which case it would warrant regular surveillance.
APPENDIX
Acknowledgments
Acknowledgments
We thank the following for review and constructive comments on behalf of their scientific societies: Damjana Kljucevsek, Michael Riccabona, Lil-Sofie Ording Müller, and Philippe Petit for the GU/GI Task Force of the European Society of Pediatric Radiology; Zoltan Harkanyi and Andreas Serra for the European Federation of Societies for Ultrasound in Medicine and Biology; and Laura Massella for the working group on autosomal dominant structural disorders of European Reference Network for Rare Kidney Diseases.
Supported by Bundesministerium für Forschung und Technologie (01GM1515D).
Disclosures of Conflicts of Interest: C.G. disclosed no relevant relationships. E.F.A. disclosed no relevant relationships. L.B. disclosed no relevant relationships. K.B. disclosed no relevant relationships. A.C. disclosed no relevant relationships. M.C. disclosed no relevant relationships. D.H. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a consultant for Amgen, Kyowa, Kirin, Sandoz, and Horizon; institution received grants from Amgen, Kyowa, Kirin, Sandoz, and Horizon; gave lectures for Amgen, Kyowa, Kirin, Sandoz, and Horizon. Other relationships: disclosed no relevant relationships. E.A.H. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a consultant for Kadmon; institution received grants from Kadmon. Other relationships: disclosed no relevant relationships. D.F. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: gave lectures for Bracco, Milano, and Pfizer; traveled to US congresses and meetings. Other relationships: disclosed no relevant relationships. J.K. disclosed no relevant relationships. M.C.L. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is on the Otsuka Pharma advisory board; gave lectures for Pfizer. Other relationships: disclosed no relevant relationships. D.M. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is on the Otsuka advisory board; is a consultant for Novartis; institution received grants from Novartis and Otsuka. Other relationships: disclosed no relevant relationships. A.C.M.O. disclosed no relevant relationships. L.P. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a consultant for Chiesi and Novartis; received grants from Chiesi and Novartis; gave lectures for Chiesi, Orphan Europe, Novartis, and Alexion; was reimbursed from travel expenses by Alexion and Astellas. Other relationships: disclosed no relevant relationships. A.T. disclosed no relevant relationships. R.T. disclosed no relevant relationships. P.J.D.W. disclosed no relevant relationships. F.S. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is on the Advisory Board on Tolvaptan Pediatric Trials Program (Otsuka). Other relationships: disclosed no relevant relationships.
Abbreviations:
- ACKD
- acquired cystic kidney disease
- ADPKD
- autosomal dominant polycystic kidney disease
- ARPKD
- autosomal recessive polycystic kidney disease
- AML
- angiomyolipoma
- CGS
- contiguous gene deletion syndrome
- MCDK
- multicystic dysplastic kidney disease
- TKV
- total kidney volume
- TSC
- tuberous sclerosis complex
References
- 1.Bakker MK, Bergman JEH, Fleurke-Rozema H, et al. Prenatal diagnosis of urinary tract anomalies, a cohort study in the Northern Netherlands. Prenat Diagn 2018;38(2):130–134. [DOI] [PubMed] [Google Scholar]
- 2.Alam A, Dahl NK, Lipschutz JH, et al. Total kidney volume in autosomal dominant polycystic kidney disease: a biomarker of disease progression and therapeutic efficacy. Am J Kidney Dis 2015;66(4):564–576. [DOI] [PubMed] [Google Scholar]
- 3.König JC, Titieni A, Konrad M; NEOCYST Consortium . Network for early onset cystic kidney diseases: a comprehensive multidisciplinary approach to hereditary cystic kidney diseases in childhood. Front Pediatr 2018;6:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breysem L, De Rechter S, De Keyzer F, et al. 3DUS as an alternative to MRI for measuring renal volume in children with autosomal dominant polycystic kidney disease. Pediatr Nephrol 2018;33(5):827–835. [DOI] [PubMed] [Google Scholar]
- 5.Dinkel E, Ertel M, Dittrich M, Peters H, Berres M, Schulte-Wissermann H. Kidney size in childhood: sonographical growth charts for kidney length and volume. Pediatr Radiol 1985;15(1):38–43. [DOI] [PubMed] [Google Scholar]
- 6.Kadioglu A. Renal measurements, including length, parenchymal thickness, and medullary pyramid thickness, in healthy children: what are the normative ultrasound values? AJR Am J Roentgenol 2010;194(2):509–515. [DOI] [PubMed] [Google Scholar]
- 7.Leung VY, Chu WC, Yeung CK, et al. Nomograms of total renal volume, urinary bladder volume and bladder wall thickness index in 3,376 children with a normal urinary tract. Pediatr Radiol 2007;37(2):181–188. [DOI] [PubMed] [Google Scholar]
- 8.Yoon HM, Cho YA, Kim JR, et al. Real-time two-dimensional shear-wave elastography for liver stiffness in children: interobserver variation and effect of breathing technique. Eur J Radiol 2017;97:53–58. [DOI] [PubMed] [Google Scholar]
- 9.Hong EK, Choi YH, Cheon JE, Kim WS, Kim IO, Kang SY. Accurate measurements of liver stiffness using shear wave elastography in children and young adults and the role of the stability index. Ultrasonography 2018;37(3):226–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamble R, Sodhi KS, Thapa BR, et al. Liver acoustic radiation force impulse (ARFI) in childhood obesity: comparison and correlation with biochemical markers. J Ultrasound 2016;20(1):33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailey SS, Youssfi M, Patel M, Hu HH, Shaibi GQ, Towbin RB. Shear-wave ultrasound elastography of the liver in normal-weight and obese children. Acta Radiol 2017;58(12):1511–1518. [DOI] [PubMed] [Google Scholar]
- 12.Garcovich M, Veraldi S, Di Stasio E, et al. Liver stiffness in pediatric patients with fatty liver disease: diagnostic accuracy and reproducibility of shear-wave elastography. Radiology 2017;283(3):820–827. [DOI] [PubMed] [Google Scholar]
- 13.Riccabona M, Avni FE, Blickman JG, et al. Imaging recommendations in paediatric uroradiology: minutes of the ESPR workgroup session on urinary tract infection, fetal hydronephrosis, urinary tract ultrasonography and voiding cystourethrography, Barcelona, Spain, June 2007. Pediatr Radiol 2008;38(2):138–145. [DOI] [PubMed] [Google Scholar]
- 14.Lu Q, Li CX, Huang BJ, Xue LY, Wang WP. Triphasic and epithelioid minimal fat renal angiomyolipoma and clear cell renal cell carcinoma: qualitative and quantitative CEUS characteristics and distinguishing features. Abdom Imaging 2015;40(2):333–342. [DOI] [PubMed] [Google Scholar]
- 15.Thomsen HS, Morcos SK, Almén T, et al. Nephrogenic systemic fibrosis and gadolinium-based contrast media: updated ESUR Contrast Medium Safety Committee guidelines. Eur Radiol 2013;23(2):307–318. [DOI] [PubMed] [Google Scholar]
- 16.Sharma K, Caroli A, Quach LV, et al. Kidney volume measurement methods for clinical studies on autosomal dominant polycystic kidney disease. PLoS One 2017;12(5):e0178488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gimpel C, Avni FE, Bergmann C, et al. Perinatal diagnosis, management, and follow-up of cystic renal diseases: a clinical practice recommendation with systematic literature reviews. JAMA Pediatr 2018;172(1):74–86. [DOI] [PubMed] [Google Scholar]
- 18.Ravine D, Gibson RN, Donlan J, Sheffield LJ. An ultrasound renal cyst prevalence survey: specificity data for inherited renal cystic diseases. Am J Kidney Dis 1993;22(6):803–807. [DOI] [PubMed] [Google Scholar]
- 19.McHugh K, Stringer DA, Hebert D, Babiak CA. Simple renal cysts in children: diagnosis and follow-up with US. Radiology 1991;178(2):383–385. [DOI] [PubMed] [Google Scholar]
- 20.Bayram MT, Alaygut D, Soylu A, Serdaroğlu E, Cakmakçı H, Kavukçu S. Clinical and radiological course of simple renal cysts in children. Urology 2014;83(2):433–437. [DOI] [PubMed] [Google Scholar]
- 21.Choi JD. Clinical characteristics and long-term observation of simple renal cysts in a healthy Korean population. Int Urol Nephrol 2016;48(3):319–324. [DOI] [PubMed] [Google Scholar]
- 22.Terada N, Ichioka K, Matsuta Y, Okubo K, Yoshimura K, Arai Y. The natural history of simple renal cysts. J Urol 2002;167(1):21–23. [PubMed] [Google Scholar]
- 23.Karmazyn B, Kaefer M, Jennings SG, Nirmala R, Raske ME. Caliceal diverticulum in pediatric patients: the spectrum of imaging findings. Pediatr Radiol 2011;41(11):1369–1373. [DOI] [PubMed] [Google Scholar]
- 24.Schreuder MF, Westland R, van Wijk JA. Unilateral multicystic dysplastic kidney: a meta-analysis of observational studies on the incidence, associated urinary tract malformations and the contralateral kidney. Nephrol Dial Transplant 2009;24(6):1810–1818. [DOI] [PubMed] [Google Scholar]
- 25.Westland R, Schreuder MF, Bökenkamp A, Spreeuwenberg MD, van Wijk JAE. Renal injury in children with a solitary functioning kidney: the KIMONO study. Nephrol Dial Transplant 2011;26(5):1533–1541. [DOI] [PubMed] [Google Scholar]
- 26.Han JH, Lee YS, Kim MJ, et al. Conservative management of segmental multicystic dysplastic kidney in children. Urology 2015;86(5):1013–1018. [DOI] [PubMed] [Google Scholar]
- 27.Whittam BM, Calaway A, Szymanski KM, et al. Ultrasound diagnosis of multicystic dysplastic kidney: is a confirmatory nuclear medicine scan necessary? J Pediatr Urol 2014;10(6):1059–1062. [DOI] [PubMed] [Google Scholar]
- 28.Mattioli G, Pini-Prato A, Costanzo S, et al. Nephrectomy for multicystic dysplastic kidney and renal hypodysplasia in children: where do we stand? Pediatr Surg Int 2010;26(5):523–528. [DOI] [PubMed] [Google Scholar]
- 29.Eickmeyer AB, Casanova NF, He C, et al. The natural history of the multicystic dysplastic kidney: is limited follow-up warranted? J Pediatr Urol 2014;10(4):655–661. [DOI] [PubMed] [Google Scholar]
- 30.Gaither TW, Patel A, Patel C, Chuang KW, Cohen RA, Baskin LS. Natural history of contralateral hypertrophy in patients with multicystic dysplastic kidneys. J Urol 2018;199(1):280–286. [DOI] [PubMed] [Google Scholar]
- 31.Abidari JM, Park KH, Kennedy WA, Shortliffe LD. Serial followup of the contralateral renal size in children with multicystic dysplastic kidney. J Urol 2002;168(4 Pt 2):1821–1825; discussion 1825. [DOI] [PubMed] [Google Scholar]
- 32.Aslam M, Watson AR; Trent & Anglia MCDK Study Group . Unilateral multicystic dysplastic kidney: long term outcomes. Arch Dis Child 2006;91(10):820–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Truong LD, Choi YJ, Shen SS, Ayala G, Amato R, Krishnan B. Renal cystic neoplasms and renal neoplasms associated with cystic renal diseases: pathogenetic and molecular links. Adv Anat Pathol 2003;10(3):135–159. [DOI] [PubMed] [Google Scholar]
- 34.Cuda SP, Brand TC, Thibault GP, Sutherland RS. Collecting duct carcinoma arising from multicystic dysplastic kidney disease. J Pediatr Urol 2006;2(5):500–502. [DOI] [PubMed] [Google Scholar]
- 35.Akkad T, Sergi C, Gozzi C, et al. Metastasizing renal cell carcinoma developing in a congenital ectopic and dysplastic kidney. Urol Int 2008;81(4):477–479. [DOI] [PubMed] [Google Scholar]
- 36.Cui Y, Eom M, Jung SH, Kim KJ, Jung WH. Malignant rhabdoid tumor of the kidney combined with multicystic dysplasia in a 5-year-old child. J Korean Med Sci 2010;25(5):785–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Decramer S, Parant O, Beaufils S, et al. Anomalies of the TCF2 gene are the main cause of fetal bilateral hyperechogenic kidneys. J Am Soc Nephrol 2007;18(3):923–933. [DOI] [PubMed] [Google Scholar]
- 38.Weber S, Moriniere V, Knüppel T, et al. Prevalence of mutations in renal developmental genes in children with renal hypodysplasia: results of the ESCAPE study. J Am Soc Nephrol 2006;17(10):2864–2870. [DOI] [PubMed] [Google Scholar]
- 39.Eckardt KU, Alper SL, Antignac C, et al. Autosomal dominant tubulointerstitial kidney disease: diagnosis, classification, and management—a KDIGO consensus report. Kidney Int 2015;88(4):676–683. [DOI] [PubMed] [Google Scholar]
- 40.Forsythe E, Sparks K, Best S, et al. Risk factors for severe renal disease in Bardet-Biedl syndrome. J Am Soc Nephrol 2017;28(3):963–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cassart M, Eurin D, Didier F, Guibaud L, Avni EF. Antenatal renal sonographic anomalies and postnatal follow-up of renal involvement in Bardet-Biedl syndrome. Ultrasound Obstet Gynecol 2004;24(1):51–54. [DOI] [PubMed] [Google Scholar]
- 42.Burgmaier K, Kunzmann K, Ariceta G, et al. Risk factors for early dialysis dependency in autosomal recessive polycystic kidney disease. J Pediatr 2018;199:22–28.e6. [DOI] [PubMed] [Google Scholar]
- 43.Guay-Woodford LM, Desmond RA. Autosomal recessive polycystic kidney disease: the clinical experience in North America. Pediatrics 2003;111(5 Pt 1):1072–1080. [DOI] [PubMed] [Google Scholar]
- 44.Luoto TT, Pakarinen MP, Jahnukainen T, Jalanko H. Liver disease in autosomal recessive polycystic kidney disease: clinical characteristics and management in relation to renal failure. J Pediatr Gastroenterol Nutr 2014;59(2):190–196. [DOI] [PubMed] [Google Scholar]
- 45.Yonem O, Bayraktar Y. Clinical characteristics of Caroli’s syndrome. World J Gastroenterol 2007;13(13):1934–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Erger F, Brüchle NO, Gembruch U, Zerres K. Prenatal ultrasound, genotype, and outcome in a large cohort of prenatally affected patients with autosomal-recessive polycystic kidney disease and other hereditary cystic kidney diseases. Arch Gynecol Obstet 2017;295(4):897–906. [DOI] [PubMed] [Google Scholar]
- 47.Hackl A, Mehler K, Gottschalk I, et al. Disorders of fatty acid oxidation and autosomal recessive polycystic kidney disease: different clinical entities and comparable perinatal renal abnormalities. Pediatr Nephrol 2017;32(5):791–800. [DOI] [PubMed] [Google Scholar]
- 48.Gunay-Aygun M, Font-Montgomery E, Lukose L, et al. Correlation of kidney function, volume and imaging findings, and PKHD1 mutations in 73 patients with autosomal recessive polycystic kidney disease. Clin J Am Soc Nephrol 2010;5(6):972–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adeva M, El-Youssef M, Rossetti S, et al. Clinical and molecular characterization defines a broadened spectrum of autosomal recessive polycystic kidney disease (ARPKD). Medicine (Baltimore) 2006;85(1):1–21. [DOI] [PubMed] [Google Scholar]
- 50.Kummer S, Sagir A, Pandey S, et al. Liver fibrosis in recessive multicystic kidney diseases: transient elastography for early detection. Pediatr Nephrol 2011;26(5):725–731. [DOI] [PubMed] [Google Scholar]
- 51.Telega G, Cronin D, Avner ED. New approaches to the autosomal recessive polycystic kidney disease patient with dual kidney-liver complications. Pediatr Transplant 2013;17(4):328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guay-Woodford LM, Bissler JJ, Braun MC, et al. Consensus expert recommendations for the diagnosis and management of autosomal recessive polycystic kidney disease: report of an international conference. J Pediatr 2014;165(3):611–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pei Y, Obaji J, Dupuis A, et al. Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol 2009;20(1):205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bergmann C. ARPKD and early manifestations of ADPKD: the original polycystic kidney disease and phenocopies. Pediatr Nephrol 2015;30(1):15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Granata A, Sessa A, Righetti M, et al. Juvenile renal cell carcinoma as first manifestation of von Hippel-Lindau disease. J Nephrol 2004;17(2):306–310. [PubMed] [Google Scholar]
- 56.Ars E, Bernis C, Fraga G, et al. Spanish guidelines for the management of autosomal dominant polycystic kidney disease. Nephrol Dial Transplant 2014;29(Suppl 4):iv95–iv105. [DOI] [PubMed] [Google Scholar]
- 57.Rangan GK, Alexander SI, Campbell KL, et al. KHA-CARI guideline recommendations for the diagnosis and management of autosomal dominant polycystic kidney disease. Nephrology (Carlton) 2016;21(8):705–716. [DOI] [PubMed] [Google Scholar]
- 58.Marlais M, Cuthell O, Langan D, Dudley J, Sinha MD, Winyard PJD. Hypertension in autosomal dominant polycystic kidney disease: a meta-analysis. Arch Dis Child 2016;101(12):1142–1147. [DOI] [PubMed] [Google Scholar]
- 59.Cadnapaphornchai MA, Masoumi A, Strain JD, McFann K, Schrier RW. Magnetic resonance imaging of kidney and cyst volume in children with ADPKD. Clin J Am Soc Nephrol 2011;6(2):369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cadnapaphornchai MA, McFann K, Strain JD, Masoumi A, Schrier RW. Increased left ventricular mass in children with autosomal dominant polycystic kidney disease and borderline hypertension. Kidney Int 2008;74(9):1192–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seeman T, Dusek J, Vondrichová H, et al. Ambulatory blood pressure correlates with renal volume and number of renal cysts in children with autosomal dominant polycystic kidney disease. Blood Press Monit 2003;8(3):107–110. [DOI] [PubMed] [Google Scholar]
- 62.Fick-Brosnahan GM, Tran ZV, Johnson AM, Strain JD, Gabow PA. Progression of autosomal-dominant polycystic kidney disease in children. Kidney Int 2001;59(5):1654–1662. [DOI] [PubMed] [Google Scholar]
- 63.Irazabal MV, Rangel LJ, Bergstralh EJ, et al. Imaging classification of autosomal dominant polycystic kidney disease: a simple model for selecting patients for clinical trials. J Am Soc Nephrol 2015;26(1):160–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu ASL, Shen C, Landsittel DP, et al. Baseline total kidney volume and the rate of kidney growth are associated with chronic kidney disease progression in autosomal dominant polycystic kidney disease. Kidney Int 2018;93(3):691–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fick GM, Duley IT, Johnson AM, Strain JD, Manco-Johnson ML, Gabow PA. The spectrum of autosomal dominant polycystic kidney disease in children. J Am Soc Nephrol 1994;4(9):1654–1660. [DOI] [PubMed] [Google Scholar]
- 66.Nowak KL, Cadnapaphornchai MA, Chonchol MB, Schrier RW, Gitomer B. Long-term outcomes in patients with very-early onset autosomal dominant polycystic kidney disease. Am J Nephrol 2016;44(3):171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jo WR, Kim SH, Kim KW, et al. Correlations between renal function and the total kidney volume measured on imaging for autosomal dominant polycystic kidney disease: a systematic review and meta-analysis. Eur J Radiol 2017;95(Suppl C):56–65. [DOI] [PubMed] [Google Scholar]
- 68.Massella L, Mekahli D, Paripović D, et al. Prevalence of hypertension in children with early-stage ADPKD. Clin J Am Soc Nephrol 2018;13(6):874–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Riccabona M, Fritz GA, Schöllnast H, Schwarz T, Deutschmann MJ, Mache CJ. Hydronephrotic kidney: pediatric three-dimensional US for relative renal size assessment—initial experience. Radiology 2005;236(1):276–283. [DOI] [PubMed] [Google Scholar]
- 70.Northrup H, Krueger DA; International Tuberous Sclerosis Complex Consensus Group . Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol 2013;49(4):243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Back SJ, Andronikou S, Kilborn T, Kaplan BS, Darge K. Imaging features of tuberous sclerosis complex with autosomal-dominant polycystic kidney disease: a contiguous gene syndrome. Pediatr Radiol 2015;45(3):386–395. [DOI] [PubMed] [Google Scholar]
- 72.Castagnetti M, Vezzù B, Laverda A, Zampieri S, Rigamonti W. Urological counseling and follow-up in pediatric tuberous sclerosis complex. J Urol 2007;178(5):2155–2159. [DOI] [PubMed] [Google Scholar]
- 73.Sampson JR, Maheshwar MM, Aspinwall R, et al. Renal cystic disease in tuberous sclerosis: role of the polycystic kidney disease 1 gene. Am J Hum Genet 1997;61(4):843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Buj Pradilla MJ, Martí Ballesté T, Torra R, Villacampa Aubá F. Recommendations for imaging-based diagnosis and management of renal angiomyolipoma associated with tuberous sclerosis complex. Clin Kidney J 2017;10(6):728–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Krueger DA, Northrup H; International Tuberous Sclerosis Complex Consensus Group . Tuberous sclerosis complex surveillance and management: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol 2013;49(4):255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Greco F, Faiella E, Santucci D, et al. Ultrasound imaging of cystic nephroma. J Kidney Cancer VHL 2017;4(3):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goldman M, Smith A, Shuman C, et al. Renal abnormalities in beckwith-wiedemann syndrome are associated with 11p15.5 uniparental disomy. J Am Soc Nephrol 2002;13(8):2077–2084. [DOI] [PubMed] [Google Scholar]
- 78.Mussa A, Peruzzi L, Chiesa N, et al. Nephrological findings and genotype-phenotype correlation in Beckwith-Wiedemann syndrome. Pediatr Nephrol 2012;27(3):397–406. [DOI] [PubMed] [Google Scholar]
- 79.Schultz KAP, Williams GM, Kamihara J, et al. DICER1 and associated conditions: identification of at-risk individuals and recommended surveillance strategies. Clin Cancer Res 2018;24(10):2251–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Launbjerg K, Bache I, Galanakis M, Bisgaard ML, Binderup MLM. von Hippel-Lindau development in children and adolescents. Am J Med Genet A 2017;173(9):2381–2394. [DOI] [PubMed] [Google Scholar]
- 81.Wallis MC, Lorenzo AJ, Farhat WA, Bägli DJ, Khoury AE, Pippi Salle JL. Risk assessment of incidentally detected complex renal cysts in children: potential role for a modification of the Bosniak classification. J Urol 2008;180(1):317–321. [DOI] [PubMed] [Google Scholar]
- 82.Peng Y, Jia L, Sun N, et al. Assessment of cystic renal masses in children: comparison of multislice computed tomography and ultrasound imaging using the Bosniak classification system. Eur J Radiol 2010;75(3):287–292. [DOI] [PubMed] [Google Scholar]
- 83.Karmazyn B, Tawadros A, Delaney LR, et al. Ultrasound classification of solitary renal cysts in children. J Pediatr Urol 2015;11(3):149.e1–149.e6. [DOI] [PubMed] [Google Scholar]
- 84.Meister M, Choyke P, Anderson C, Patel U. Radiological evaluation, management, and surveillance of renal masses in Von Hippel-Lindau disease. Clin Radiol 2009;64(6):589–600. [DOI] [PubMed] [Google Scholar]
- 85.Sieniawska M, Roszkowska-Blaim M, Welc-Dobies J. Acquired cystic kidney disease in renal insufficiency. Child Nephrol Urol 1991;11(1):20–24. [PubMed] [Google Scholar]
- 86.Querfeld U, Schneble F, Wradzidlo W, Waldherr R, Tröger J, Schärer K. Acquired cystic kidney disease before and after renal transplantation. J Pediatr 1992;121(1):61–64. [DOI] [PubMed] [Google Scholar]
- 87.Mattoo TK, Greifer I, Geva P, Spitzer A. Acquired renal cystic disease in children and young adults on maintenance dialysis. Pediatr Nephrol 1997;11(4):447–450. [DOI] [PubMed] [Google Scholar]
- 88.Hogg RJ. Acquired renal cystic disease in children prior to the start of dialysis. Pediatr Nephrol 1992;6(2):176–178. [DOI] [PubMed] [Google Scholar]
- 89.Matson MA, Cohen EP. Acquired cystic kidney disease: occurrence, prevalence, and renal cancers. Medicine (Baltimore) 1990;69(4):217–226. [DOI] [PubMed] [Google Scholar]
- 90.Franchi-Abella S, Mourier O, Pariente D, Frank-Soltysiak M, Bernard O, Debray D. Acquired renal cystic disease after liver transplantation in children. Transplant Proc 2007;39(8):2601–2602. [DOI] [PubMed] [Google Scholar]
- 91.Calvo-Garcia MA, Campbell KM, O’Hara SM, Khoury P, Mitsnefes MM, Strife CF. Acquired renal cysts after pediatric liver transplantation: association with cyclosporine and renal dysfunction. Pediatr Transplant 2008;12(6):666–671. [DOI] [PubMed] [Google Scholar]
- 92.Moodalbail DG, Apple LZ, Meyers KE, Ginsberg JP, Kaplan BS, Bellah R. Acquired multiple cysts of the kidney in neuroblastoma survivors. Am J Kidney Dis 2016;68(1):134–137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.