Abstract
Purpose
To longitudinally monitor liver fat before and after bariatric surgery by using quantitative chemical shift-encoded (CSE) MRI and to compare with changes in body mass index (BMI), weight, and waist circumference (WC).
Materials and Methods
For this prospective study, which was approved by the internal review board, a total of 126 participants with obesity who were undergoing evaluation for bariatric surgery with preoperative very low calorie diet (VLCD) were recruited from June 27, 2010, through May 5, 2015. Written informed consent was obtained from all participants. Participants underwent CSE MRI measuring liver proton density fat fraction (PDFF) before VLCD (2–3 weeks before surgery), after VLCD (1–3 days before surgery), and 1, 3, and 6–10 months following surgery. Linear regression was used to estimate rates of change of PDFF (ΔPDFF) and body anthropometrics. Initial PDFF (PDFF0), initial anthropometrics, and anthropometric rates of change were evaluated as predictors of ΔPDFF. Mixed-effects regression was used to estimate time to normalization of PDFF.
Results
Fifty participants (mean age, 51.0 years; age range, 27–70 years), including 43 women (mean age, 50.8 years; age range, 27–70 years) and seven men (mean age, 51.7 years; age range, 36–62 years), with mean PDFF0 ± standard deviation of 18.1% ± 8.6 and mean BMI0 of 44.9 kg/m2 ± 6.5 completed the study. By 6–10 months following surgery, mean PDFF decreased to 4.9% ± 3.4 and mean BMI decreased to 34.5 kg/m2 ± 5.4. Mean estimated time to PDFF normalization was 22.5 weeks ± 11.5. PDFF0 was the only strong predictor for both ΔPDFF and time to PDFF normalization. No body anthropometric correlated with either outcome.
Conclusion
Average liver proton density fat fraction (PDFF) decreased to normal (< 5%) by 6–10 months following surgery, with mean time to normalization of approximately 5 months. Initial PDFF was a strong predictor of both rate of change of PDFF and time to normalization. Body anthropometrics did not predict either outcome.
Online supplemental material is available for this article.
© RSNA, 2018
Summary
Using chemical shift-encoded MRI, our study evaluated the longitudinal change in liver fat content in severely obese adults who underwent bariatric surgery with a preoperative very low calorie diet. Our results showed that decrease in liver fat content was strongly correlated with pretreatment liver fat content, but was weakly correlated with both starting weight and overall weight loss, suggesting that the greatest potential benefit is for patients with marked hepatic steatosis regardless of starting weight or weight loss.
Implications for Patient Care
■ Advanced quantitative chemical shift-encoded MRI techniques can be used to noninvasively monitor longitudinal changes in liver fat content in severely obese adults.
■ In patients with severe obesity and hepatic steatosis, decreases in liver fat content (measured as proton density fat fraction by quantitative chemical shift-encoded MRI) were strongly correlated with pretreatment liver fat content, suggesting proton density fat fraction can help inform bariatric surgery patient selection.
■ Decreases in liver fat content were only weakly correlated with starting weight and the amount of overall weight loss, suggesting possible utility in monitoring liver fat with MRI following bariatric surgery, independent of monitoring weight loss.
Introduction
Obesity is a major public health issue in the United States, with over two-thirds of American adults considered overweight or obese (1). Nonalcoholic fatty liver disease, widely considered the hepatic manifestation of metabolic syndrome (2), is an increasingly prevalent condition common in patients with obesity (3). Intracellular accumulation of triglycerides (hepatic steatosis) is the hallmark feature of nonalcoholic fatty liver disease, which can progress to nonalcoholic steatohepatitis and ultimately cirrhosis (4,5) while also increasing the risk of hepatocellular carcinoma (6). Bariatric surgery is an effective weight loss intervention in patients with obesity, reducing liver fat (7,8) and improving other health outcomes (9–11). Furthermore, use of a very low calorie diet (VLCD) prior to bariatric surgery facilitates surgery and may augment the degree of weight loss (12–14). However, the relationship between overall weight loss achieved by these treatments and decreases in liver fat content is not well understood, to our knowledge.
A barrier to understanding these relationships has been the lack of reproducible, noninvasive methods to quantify liver fat. Liver biopsy has long been the reference standard for liver fat quantification (15) but is invasive. Traditional imaging methods, including conventional MRI, MR spectroscopy (16), and US (17), are of limited utility due to poor accuracy or technical difficulty (18). Advanced complex-based chemical shift-encoded (CSE) MRI methods for quantifying proton density fat fraction (PDFF) as a fundamental biomarker of liver fat concentration (19) have been developed and validated across multiple vendors and field strengths (20–23). These advanced methods correct for a number of confounders that adversely affect the quantitative measurement of liver fat content, yielding noninvasive, accurate, and precise evaluation of liver fat across the entire biologic range of hepatic steatosis (18), and are valid even in patients with morbid obesity (24).
Our purpose was to perform longitudinal monitoring of PDFF as a biomarker of liver fat before and after bariatric surgery using quantitative CSE MRI and to compare PDFF with changes in body mass index (BMI), weight, and waist circumference (WC).
Materials and Methods
Participating Institutions and Compliance
Two U.S. academic medical centers (UC San Diego and the University of Wisconsin-Madison) participated in this prospective, observational study, which is compliant with the Health Insurance Portability and Accountability Act and was approved by our respective institutional review boards. Written informed consent was obtained from all study participants. Our study was supported by funding from the National Institutes of Health. We acknowledge that GE Healthcare provides research support to both participating centers but did not contribute directly to this project and had no input into this specific article.
Study Participant Population and Study Design
Over 66 months (June 27, 2010, through May 5, 2015), consecutive adult study participants with severe obesity undergoing surgical evaluation for possible bariatric surgery with preoperative VLCD were recruited with the intent to undergo pre- and postsurgery MRI with liver fat quantification at defined time points in conjunction with planned preoperative VLCD, bariatric surgery, and intraoperative biopsy. Potential participants were excluded for history elements precluding MRI (extreme claustrophobia, body habitus exceeding scanner weight limit or bore diameter, metal in the eyes, or noncompatible metallic medical devices), pregnancy, known liver disease other than nonalcoholic fatty liver disease, and hepatotoxic medications. Enrolled participants were excluded if bariatric surgery was ultimately not performed or if preoperative VLCD was deferred.
Study participants underwent baseline preoperative MRI (hereafter, MRI0) with PDFF for liver fat quantification 2–3 weeks prior to surgery, with recording of initial anthropometrics (BMI, weight, and WC). VLCD consisted of 600–900 calories per day in the form of four to six sugar-free breakfast shakes daily (Carnation Breakfast Essentials Powder Drink Mix, one packet in 8 ounces of 1% milk, with substitutions in a small number of dairy-intolerant participants), in addition to at least 64 ounces of sugar-free liquids daily to maintain hydration (14). VLCD was initiated following MRI0 and continued until surgery, although in a small number of participants VLCD was initiated shortly before MRI0 due to appointment scheduling. A second preoperative MRI was performed near the conclusion of the VLCD period (hereafter, MRIVLCD), 1–3 days before bariatric surgery, with recording of anthropometrics.
Bariatric operations were performed by six bariatric surgeons (including L.M.F., G.M.C., J.A.G., G.J., and S.H.), with a range of five to 21 operations per surgeon. The specific operation performed (eg, gastric bypass, sleeve, band, or plication) was determined by the operating surgeon based on clinical considerations and participant's preferences. Following surgery, study participants were transitioned back to general diets at the surgeon’s discretion. At the time of surgery, liver biopsy was performed for research purposes to confirm the presence of hepatic steatosis (grade 1 or higher) by using a previously validated histologic scoring system for nonalcoholic fatty liver disease (15,25). Participants for whom liver biopsy could not be performed for technical or safety reasons and participants with pathology-confirmed absence of hepatic steatosis (grade 0 steatosis by consensus review of two hepatopathologists) were further excluded. Thus, only participants with histology-confirmed steatosis were analyzed.
Postsurgical CSE MRI examinations with PDFF quantification were obtained at 1 month, 3 months, and 6–10 months (hereafter, MRI1mo, MRI3mo, and MRI6–10mo, respectively) following surgery, with anthropometric measurements obtained at each study visit. This resulted in a maximum of five time points (MRI0, MRIVLCD, MRI1mo, MRI3mo, MRI6–10mo) for analysis. To be included in the final analysis, study participants were required to complete at least the MRI0, MRIVLCD, MRI1mo, and MRI3mo examinations.
CSE MRI Technique and Liver Fat Analysis
MRI examinations were performed by using a standardized protocol (24) on clinical MRI systems (GE Healthcare, Waukesha, Wis), encompassing both 3.0-T and 1.5-T field strengths and varying bore diameters to accommodate each study participant’s body habitus. Scanner models included Signa HDxt 3.0T, Signa HDxt 1.5T, Discovery MR750 3.0T, and Optima MR450w 1.5T.
Imaging was performed by using investigational versions of a complex-based quantitative CSE MRI method (IDEAL IQ, GE Healthcare) to quantify PDFF. Briefly, this method is a multiecho, three-dimensional, gradient-echo–based CSE MRI method that corrects or minimizes the factors that introduce errors in conventional MRI, including T1-related bias (26), noise bias (26), R2* signal decay (27), multifrequency interference effects of fat (28), and the effects of eddy currents (29). The sequence uses both magnitude and phase information from images acquired at multiple echo times to facilitate independent estimation of fat water signals. When these signals are corrected for the above confounders, the ratio of corrected fat signal to the total corrected signal of water and fat provides an accurate estimate of PDFF. The method automatically reconstructs water-only and fat-only images and PDFF maps (R2* maps were also generated, but were not used in our study). PDFF maps depict the spatial distribution of PDFF, pixel by pixel, throughout the scanned volume.
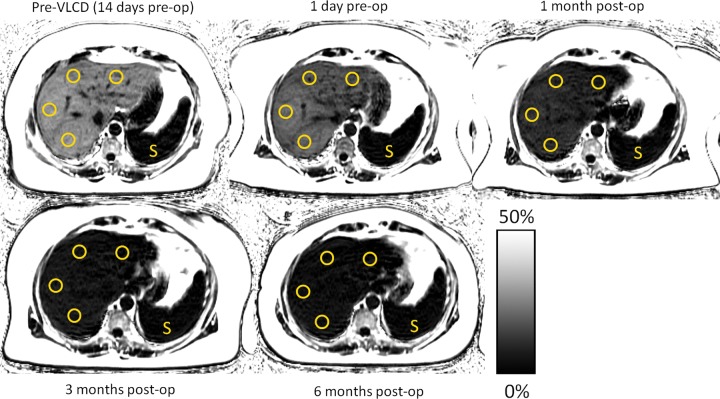
The reconstructed images were then transferred to a stand-alone workstation for analysis by trained research technologists (each with 1–3 years of experience) using OsiriX (Pixmeo SARL, Geneva, Switzerland). A circular region of interest with a 1-cm radius was placed manually in each of the nine Couinaud segments of the liver, so as to sample representative portions of the entire liver. To avoid potential information bias, regions of interest were placed on the water-only images (which have no quantitative information) and were propagated automatically onto the identical spatial locations on the PDFF maps. The PDFF in each of the nine regions of interest was averaged as a single composite hepatic PDFF value for every study participant and time point. Example PDFF maps with regions of interest are shown in Figure 1.
Figure 1:
Proton density fat fraction (PDFF, %) maps in a 55-year-old woman who underwent gastric bypass with preoperative very low calorie diet (VLCD) demonstrate progressive reduction in liver fat content. Regions of interest (circles) were obtained from each Couinaud segment of the liver, with care taken to avoid major vessels, and were averaged to obtain mean liver PDFF. Initial mean liver PDFF was 28.3% at the preoperative MRI (top left), which decreased to 18.9% following the preoperative VLCD period (top middle). Following gastric bypass, further decreases in PDFF were seen, measuring 9.0% at 1 month (top right), 5.8% at 3 months (bottom left), and finally normalizing to 4.1% at 6 months (bottom middle). A PDFF fat content scale is provided. As an internal reference, note that PDFF signal remains consistently low within the spleen (S), while the liver progressively darkens as fat content decreases. Also note the reduction in the quantity of subcutaneous adipose tissue. The woman’s body mass index at each time point was 43.7, 41.7, 38.7, 36.4, and 32.4 kg/m2, respectively.
Statistical Analysis
Data collation and analysis were performed by B.D.P., T.W., and A.C.G. Data were collated by using Microsoft Excel (2010 version; Microsoft, Redmond, Wash), and statistical calculations were performed either in Excel or R (version 3.3.1, 2016; R Foundation for Statistical Computing, Vienna, Austria). Cohort flow, reasons for exclusion, and demographics of the final sample were summarized. Participants who had gastric bypass versus gastric sleeve procedures were compared based on demographic and baseline characteristics (age, sex, liver PDFF, BMI, weight, and WC) by using Wilcoxon-Mann-Whitney tests and based on sex by using the Fisher exact test. Liver PDFF, BMI, weight, and WC were summarized at baseline and every subsequent visit. Because the overall change over time was not linear, the longitudinal record was divided into two periods: short-term change spanning approximately the first 6 weeks of our study (MRI0 through MRI1mo) and longer term change spanning the remaining months (MRI1mo through MRI6–10mo). Linear regression analysis was used to compute rates of change (linear slopes in time, one for each study participant in each time period) and each of the four measures of interest (PDFF, BMI, weight, and WC). Declines in all metrics were represented by negative values. These rates of change were then used in subsequent analyses as derived data.
One-sample t tests were used to assess significance of slopes for all measures in both time periods. Paired t tests were used to compare slopes between the short-term and longer term periods for each of the four measures. Pearson correlation was used to separately examine the relationship between slopes of PDFF, BMI, weight, and WC for the short-term and longer term periods. Univariate relationships between short-term and longer term slopes and baseline characteristics (including type of surgery) were evaluated by using Wilcoxon-Mann-Whitney tests or Pearson correlation analysis, as appropriate. Stepwise linear regression based on Bayesian information criterion was used to examine the effect of baseline characteristics (age, sex, initial PDFF, BMI, weight, WC, and type of weight loss surgery) on PDFF slopes in the short-term and longer term time periods. For this analysis, three participants with band or plication procedures were excluded. Finally, with the upper limit for normal liver fat previously defined as 5% (3,30), we used mixed-effects linear regression modeling log-transformed PDFF as a function of time to estimate time to normalization for each study participant and used these estimates as derived data. A summary of normalization times was presented, and baseline predictors of time to normalization were examined by using Bayesian information criterion–based stepwise linear regression. Piece-wise Bonferroni correction for multiple comparisons was applied to each related group of significance tests. A P value less than .05 (or family-wise P value < .05 after the Bonferroni correction) was considered indicative of statistical significance.
For simplicity, initial liver PDFF, BMI, weight, and WC are hereafter designated PDFF0, BMI0, weight0, and WC0, respectively, while rates of change in these variables are designated ΔPDFF, ΔBMI, Δweight, and ΔWC.
Results
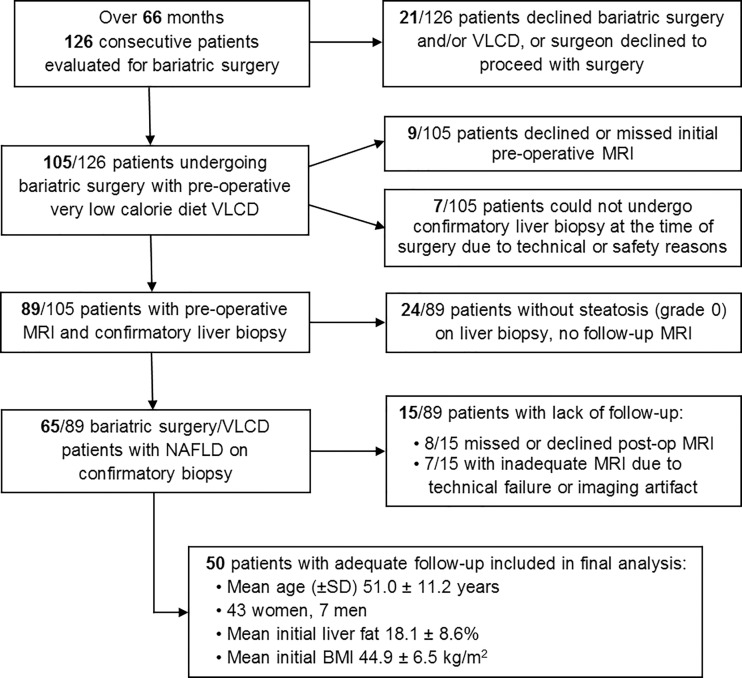
Of the 126 potentially eligible study participants recruited (center 1, n = 49; center 2, n = 77), 89 underwent MRI0 at a mean (± standard deviation) of 3.0 weeks ± 1.4 prior to bariatric surgery. VLCD was initiated at a mean of 2.6 weeks ± 1.2 prior to surgery. A total of 76 participants were excluded for this study for the reasons mentioned in Figure 2.
Figure 2:
Flow diagram of study cohort. VLCD = very low calorie diet, NAFLD = nonalcoholic fatty liver disease, BMI = body mass index, SD = standard deviation.
Among the 50 participants included in the final analysis, all completed MRI examinations at the MRI0, MRIVLCD, MRI1mo, and MRI3mo time points, with 40 also completing the final MRI6–10mo visit. All had liver histology findings demonstrating at least grade 1 steatosis. At MRI0, the mean age of this final cohort was 51.0 years ± 11.2 (age range, 27–70 years); for the 43 women in this cohort, the mean age was 50.8 years (age range, 27–70 years), and for the seven men, 51.7 years (age range, 36–62 years). Specific bariatric operations included gastric band (n = 2), gastric bypass (n = 28), gastric sleeve (n = 19), and gastric plication (n = 1). Complete individual study participant demographics and initial anthropometrics are provided in Table E1 [online]. Participants who underwent gastric bypass versus gastric sleeve procedures were not different in terms of age (P = .69), sex (P = .67), PDFF0 (P = .51), BMI0 (P = .40), weight0 (P = 42), or WC0 (P = .28).
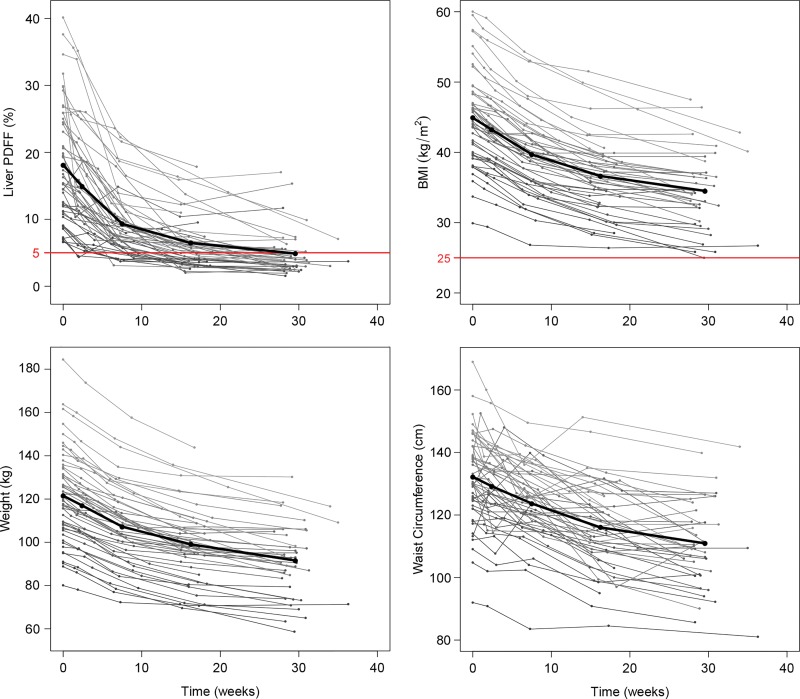
Mean liver PDFF0 was 18.1% ± 8.6 (range, 6.6%–40.1%), with mean BMI0 of 44.9 kg/m2 ± 6.5 (range, 29.9–60.0 kg/m2), mean weight0 of 121.5 kg ± 21.1 (range, 80.0–184.4 kg), and mean WC0 of 132.2 cm ± 14.3 (range, 92.0–169.0 cm). Mean intervals from MRI0 were 2.4 weeks ± 0.8 for MRIVLCD, 7.5 weeks ± 1.4 for MRI1mo, 16.2 weeks ± 1.5 for MRI3mo, and 29.5 weeks ± 1.9 for MRI6–10mo. By the conclusion of our study, mean liver PDFF had decreased to 4.9% ± 3.4 (range, 1.5%–17.0%; mean absolute decrease of 13.2%), with final mean BMI of 34.5 kg/m2 ± 5.4 (range, 25.0–47.5 kg/m2; mean decrease, 10.4 kg/m2), final mean weight of 91.6 kg ± 16.4 (range, 58.6–130.2 kg; mean decrease, 29.9 kg), and final mean WC of 110.9 cm ± 13.8 (range, 81.0–141.8 cm; mean decrease, 21.3 cm). All decreases were statistically significant (P < .0001). A summary of liver PDFF, BMI, weight, and WC by visit is provided in Table 1, with longitudinal trajectories of these variables for each study participant presented in Figure 3.
Table 1:
Summary of Liver Proton Density Fat Fraction and Anthropometrics by Visit
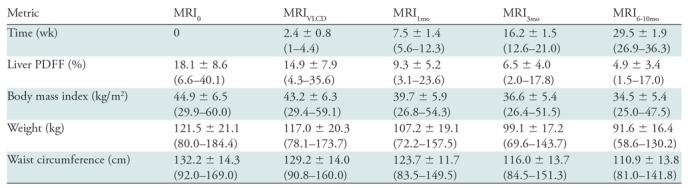
Note.—Data are mean ± standard deviation. Data in parentheses are ranges. PDFF = proton density fat fraction, VLCD = very low calorie diet.
Figure 3:
Liver proton density fat fraction (PDFF), body mass index (BMI), weight, and waist circumference trajectories over time for each study participant. The thick trajectory on each plot represents sample averages at average visit times. Note the relatively rapid decrease in both liver PDFF and body anthropometrics over the first 6–8 weeks (time spanning initiation of very low calorie diet [VLCD] through the 1-month postoperative MRI: visits MRI0, MRIVLCD, MRI1mo), compared with the remainder of the study period (time spanning the 1-month postoperative MRI through the 6-month postoperative MRI: visits MRI1mo, MRI3mo, MRI6–10mo). Upper bounds for normal liver PDFF (5%) and normal BMI (25 kg/m2) are represented on the corresponding plots with red lines.
Short-term and longer term ΔPDFF, ΔBMI, Δweight, and ΔWC are summarized in Table 2. For each measure, the observed decline was statistically significant (P < .001) in each time period, and the rate of decline in the short-term period was statistically significantly higher than in the longer term period (ΔPDFF, ΔBMI, and Δweight: P < .001; ΔWC: P = .01). All comparisons were statistically significant after Bonferroni correction.
Table 2:
Short-term and Longer Term Rates of Change of Proton Density Fat Fraction and Anthropometrics
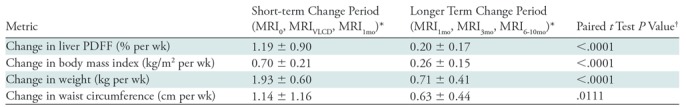
Note.—PDFF = proton density fat fraction, VLCD = very low calorie diet.
*Data are mean ± standard deviation. All means represent decrease per week and are significant (one-sample t test, P < .0001) for both time periods.
†All comparisons are statistically significant after the Bonferroni correction.
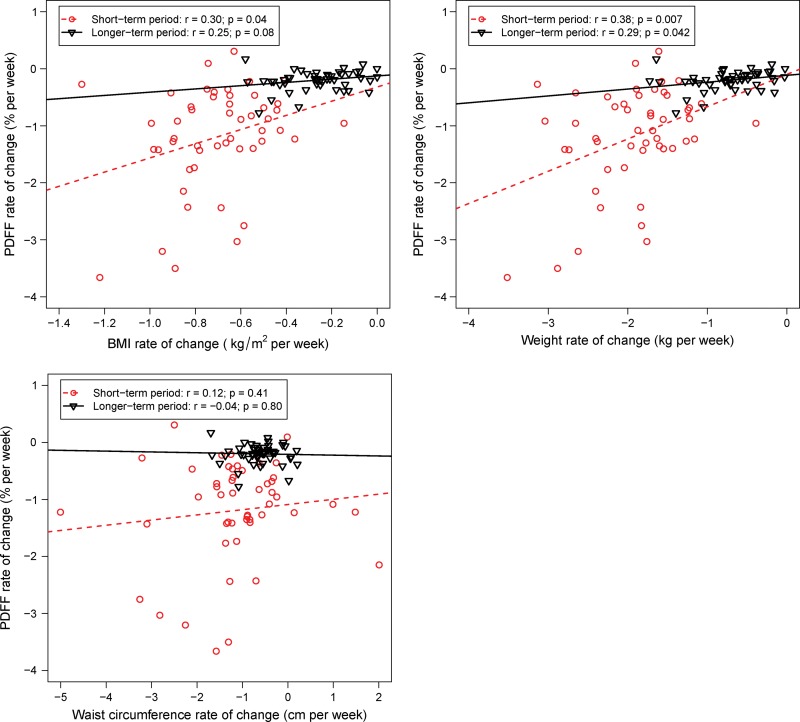
Correlations between ΔPDFF and ΔBMI, Δweight, and ΔWC are presented in Figure 4. The short-term period was characterized by greater variability in rates of change among study participants, although the relationships in the short-term period were informally stronger than those in the longer term period. During the short-term period, the correlations were as follows: for ΔBMI, r = 0.30, P = .04; for Δweight, r = 0.38, P = .007; for ΔWC, r = 0.12, P = .41. During the longer term periods, the correlations were as follows: for ΔBMI, r = 0.25, P = .08; for Δweight, r = 0.29, P = .04; for ΔWC, r = −0.04, P = .80. Only the short-term period correlations for ΔBMI and Δweight remained statistically significant after Bonferroni correction.
Figure 4:
Graphs show the rate of change in liver proton density fat fraction (PDFF) versus rates of change in other body anthropometrics: body mass index (BMI), weight, and waist circumference. The red circles and dashed line represent the short-term period (time spanning from initiation of very low calorie diet through the 1-month postoperative MRI). The black triangles and solid line represent the longer term period (time spanning from the 1-month postoperative MRI through the 6-month postoperative MRI). The relationships are stronger in the acute period. Changes in waist circumference were not significantly correlated with changes in PDFF.
Correlations were computed between ΔPDFF and PDFF0, BMI0, weight0, and WC0 during both short-term and longer term time periods. A strong negative correlation was noted between PDFF0 and ΔPDFF during both short-term and longer term time periods (r = −0.81 [P < .001] and r = −0.45 [P = .001], respectively, both significant after Bonferroni correction). BMI0, weight0, and WC0 were not statistically significantly correlated with ΔPDFF during either time period. In the short-term period: for BMI0, r = −0.23, P = .10; for weight0, r = −0.25, P = .09; and for WC0, r = −0.17, P = .24. In the longer term period: for BMI0, r = −0.13, P = .37; for weight0, r = −0.21, P = .14; and for WC0, r = −0.02, P = .89. Additionally, there was no statistically significant difference in ΔPDFF between subsets of participants who underwent gastric bypass versus gastric sleeve procedures in either time period (short-term P = .25, longer term P = .47, Wilcoxon-Mann-Whitney test). Bayesian information criterion–based multivariable regression selected only PDFF0 as a baseline predictor of ΔPDFF in both time periods (coefficient P value < .001 and P = .001, respectively, equivalent to those of the correlations). Surgery type (gastric bypass vs gastric sleeve) was one of the potential predictors but not selected into the final model for either time period.
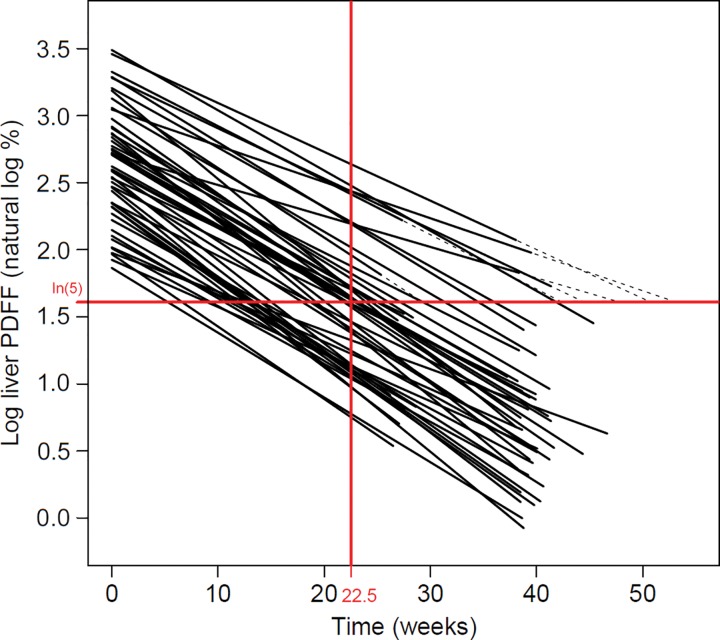
Overall, for 64% of study participants (32 of 50), liver fat was normalized to a PDFF less than 5%. Estimated time to normalization in the participant sample was a mean of 22.5 weeks ± 11.5 (Fig 5), with 90% of participants estimated to achieve normalized PDFF by 42 weeks. Bayesian information criterion–based multivariable regression selected only PDFF0 as a predictor of time to normalization, showing strong correlation (participants with lower PDFF0 normalized liver fat more quickly, r = 0.72, P < .001).
Figure 5:
Estimation of time to proton density fat fraction (PDFF) normalization, with the upper limit of normal PDFF for liver defined as 5%. Time 0 corresponds to baseline visit. Mixed-effects linear regression modeling of natural log-transformed PDFF (y-axis) as a function of time (x-axis) demonstrates a mean time to normalization (the point where the natural log-transformed trajectory crosses the ln [5%] = 1.6% line) of 22.5 weeks. Trajectories have been extended (dashed lines) for 13 participants for whom the estimated crossing time occurs outside the observation window. Of note, in this sample it was estimated that 90% of study participants achieved normal PDFF of 5% by 42 weeks.
Discussion
Using a quantitative, complex-based CSE MRI method, we evaluated the longitudinal change in liver fat content in a cohort of study participants with obesity undergoing bariatric surgery and preoperative VLCD, with comparison to changes in body anthropometrics.
Our results support prior MRI studies (largely MR spectroscopy) showing that a reduction of 5% or more in BMI is associated with decreases in liver fat (31–34), as well as other studies with similar conclusions relying on other measures of liver fat, including serum biomarkers (35) and US (17). However, using a noninvasive, accurate CSE MRI technique (18,24), our study, to our knowledge, is the first longitudinal analysis of changes in liver fat over time in a bariatric surgery population. In addition to demonstrating the clinical utility and feasibility that CSE MRI provides in the monitoring of hepatic PDFF over time, our analysis provided a number of insights into reductions in liver fat content following bariatric surgery.
While several prior studies report decreases in liver fat content accompanying overall weight loss (17,31–35), our study provided several additional observations. All but one reviewed prior study comprised only two time points, with the final time point generally occurring 3–6 months following the initiation of treatment (32–34), and another study following up patients at 1 year (17). One study followed patients at 6 months, with limited follow-up occurring at 3 months (35). Our study methodology allowed us to evaluate liver fat content at five time points over a 6-month period, allowing for more complete characterization of change in liver fat content over time. Additionally, cohorts used in prior studies generally had a lower average BMI (range, 30.1–35.4 kg/m2) (31,32,34,35) than the 44.9 kg/m2 observed in our study. These studies used either low calorie diet (32,34,35) or medication (colesevalam) (31) to achieve weight loss, and the degree of weight loss recorded in these studies was comparatively small, ranging from a 1.5- to 3.5-kg/m2 decrease in BMI, compared with the 10.4-kg/m2 decrease in our study. Our cohort was similar to those of prior studies using bariatric surgery as the method of weight loss in terms of initial BMI (range, 39.0–43.1 kg/m2) (17,33) and similar in overall weight loss to the study by Engl et al (BMI decrease of 10.2 kg/m2), which followed participants at 1 year after surgery (17).
Furthermore, the PDFF values obtained with our CSE MRI method were a quantitative, highly accurate measurement of liver whole-liver fat content, clearly expressed as a percentage, and derived from an average of multiple regions of interest across all segments of the liver to give the best possible global evaluation of liver fat content for an individual patient. Prior studies assessing changes in liver fat content in association with weight loss have largely relied mainly on MR spectroscopy (33,34), which, while accurate, is generally performed as a single-voxel measurement from one point in the liver and which consequently may incorrectly estimate the fat content of the liver as a whole. Further, MR spectroscopy has greater variability with repeated measurements due to the need to place an MR spectroscopy voxel prospectively and has limited clinical availability. Some imaging-based studies rely on in- and out-of-phase MRI (32) or US (17), neither of which provide as reliable a measurement of liver fat content, and both of which are subject to error from multiple sources, including hepatic iron overload, edema, and fibrosis. Still other studies rely on laboratory data, including liver function tests (aspartate aminotransferase, alanine aminotransferase, and gamma-glutamyl transferase), lipid profile, glucose, insulin, and inflammatory markers, as indirect biomarkers of liver fat content (33,35).
Our observations suggest that reductions in liver fat relative to anthropometrics occur most rapidly, but more variably, during the first 6 weeks of the treatment course (short term) comprising the VLCD and early postsurgery periods. Following this short-term period of rapid loss, the decline slowed but persisted at a relatively steady rate through the final 6-month postsurgery mark (longer term), with a mean time to normalization of 22.5 weeks (approximately 5 months). We did not observe any difference between gastric sleeve and gastric bypass with regard to PDFF decline. The comparatively greater variation among study participants in changes in liver PDFF and anthropometrics during the short-term period may be attributable, at least in part, to baseline heterogeneity in both BMI and liver fat content in our participant population. The rapid correction of liver fat content early in the VLCD and surgery treatment course suggests the possibility that metabolic derangements leading to nonalcoholic fatty liver disease may begin to correct rapidly upon initiation of caloric deficit induced by VLCD, benefitting patients in advance of substantial overall weight loss. The phenomenon of rapid mobilization of intrahepatic fat stores has been suggested previously in the bariatric surgery literature (8), with the additional caveat that such rapid change may contribute to mild hepatitis in patients undergoing marked weight loss quickly. It has further been suggested that mild hepatic inflammatory changes may be exacerbated by protein deficiency, as well as other factors, including hepatotoxic drugs, alcohol intake, and viral infection, during the period of weight loss (8).
In evaluating predictors of ΔPDFF, we examined correlations with PDFF0, initial body anthropometrics, and rates of change of body anthropometrics. Somewhat surprisingly, the only strong predictor of ΔPDFF was PDFF0 (greater initial PDFF portends a more rapid decrease), with weaker and less statistically significant correlations seen between ΔPDFF and both body anthropometric starting values and rates of change. A similar phenomenon was observed regarding time to normalization, with only PDFF0—and not any of the body anthropometrics—identified as predictive. These paired observations have possible patient care implications, as bariatric patients with marked hepatic steatosis may see substantial improvement in liver PDFF regardless of starting anthropometrics or degree of weight loss following surgery. Consequently, it may be reasonable to consider the severity of hepatic steatosis—independent of weight or BMI—when deciding to enroll a patient for bariatric surgery. Furthermore, if liver PDFF is of interest to the clinician, it may be reasonable to follow PDFF with CSE MRI in addition to or instead of other anthropometric measurements.
Our study had some limitations. We did not offer MRI surveillance to study participants in whom liver biopsy was negative for hepatic steatosis. This may have led to an underestimation of the effect of preoperative VLCD on liver fat, as participants resolving hepatic steatosis with VLCD alone would have been excluded. Furthermore, for inclusion in the final analysis, we required participants to participate in all but the final (6-month postsurgical) MRI visit, leading to a relatively high number of excluded participants. This was deemed necessary to ensure an adequate number of data points for each participant, particularly given our focus on trends over time. Finally, we acknowledge variation among participants in some demographic and clinical parameters, including male-to-female ratio, specific bariatric operation, and exact timing of VLCD initiation with respect to initial imaging and surgery, as well as other lifestyle parameters potentially affecting liver fat metabolism, including activity level, preprogram diet, and medication regimens. However, variations in our cohort are representative of the inherent baseline heterogeneity and individualized treatment typical of bariatric surgery populations.
In summary, in our study, bariatric surgery with preoperative VLCD substantially improved hepatic steatosis in participants with obesity without a strong correlation with starting weight or overall weight loss, and with the greatest potential benefit observed in participants with marked hepatic steatosis. Given the weak correlation between reductions in liver fat content and overall weight loss, it may be reasonable to monitor liver PDFF in these participants using advanced quantitative MRI.
SUPPLEMENTAL TABLES
Supported by the NIH (R01 DK088925, R01 DK083380, R01 DK100651, K24 DK102595). GE Healthcare provided research support to the University of Wisconsin and the University of California, San Diego, but did not specifically support this study. Effort on this study and manuscript was made possible by a VA Career Development Award to Dr Funk (CDA 15-060).
The views represented in this article represent those of the authors and not those of the DVA or the U.S. Government.
Disclosures of Conflicts of Interest: B.D.P. Activities related to the present article: disclosed an institutional grant by the National Institutes of Health. Activities not related to the present article: disclosed receipt of payment by InnoVenn for a one-time consultation fee. Other relationships: disclosed no relevant relationships. C.N.W. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed employment with Synaptive Medical. Other relationships: disclosed no relevant relationships. A.M. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed grants to institution by GE Healthcare and receipt of payment by GE Healthcare for travel/accommodations/meeting expenses incurred as a speaker at the GE PET/MR Masters Summit 2018. Other relationships: disclosed no relevant relationships. N.S.A. Activities related to the present article: disclosed that an institutional grant by NIH helped pay his salary when he worked on this study (part of an RO1-funded study) between 2010 and 2014. Activities not related to the present article: disclosed no relevant relationships. Other relationships: disclosed that he has a patent related to fat/water imaging in general, but has not received royalties from commercial use and did not use the patent idea for this study; however, he did receive royalty money from the Wisconsin Alumni Research Foundation when they submitted his invention for this patent, and they own the rights. A.S. disclosed no relevant relationships. Y.C. disclosed no relevant relationships. J.H. disclosed no relevant relationships. J.B.S. Activities related to the present article: disclosed an institutional grant from NIDDK. Activities not related to the present article: disclosed an institutional grant from Galmed. Other relationships: disclosed no relevant relationships. L.M.F. Activities related to the present article: disclosed that he was a co-investigator on the NIH R-01 grant that supported this work and that he and the institution received payment. Activities not related to the present article: disclosed no relevant relationships. Other relationships: disclosed no relevant relationships. G.M.C. disclosed no relevant relationships. J.A.G. disclosed no relevant relationships. G.J. disclosed no relevant relationships. S.H. disclosed no relevant relationships. T.W. disclosed no relevant relationships. A.C.G. disclosed no relevant relationships. C.B.S. Activities related to the present article: disclosed an institutional grant by NIH paid to UC Regents, and support (paid to UC Regents) from an NIH grant for travel to meetings for the study or other purposes. Activities not related to the present article: disclosed payment to institution by AMRA, Guerbet, and VirtualScopics for membership on the advisory board as representative of UC Regents; payment to institution by GE Healthcare, Bayer, Boehringer Ingelheim, AMRA, Fulcrum Therapeutics, IBM, and Exact Sciences for consultancy as representative of UC Regents; grants to institution by Gilead, GE Healthcare, Siemens, GE MRI, Bayer, GE Digital, GE US, ACR Innovation, Philips, and Celgene; and payment to institution (deposited to UC Regents) by GE Healthcare for speaking services; payment to institution (money deposited to UC Regents) by Medscape and Resoundant for development of educational presentations; payment (lab service agreements) to institution by Enanta, ICON Medical Imaging, Gilead, Shire, VirtualScopics, Intercept, Synageva, Takeda, Genzyme, Janssen, and NuSirt; and payment received from Epigenomics and Arterys for independent consulting contracts. Other relationships: research contracts from GE, Siemens, Guerbet; advisory board for Bracco; service or non-disclosure agreements with Alexion, AstraZeneca, Bioclinica, BMS, Fibrogen, Galmed, Genentech, Isis, Perspectum, Pfizer, Profil, Sanofi, Tobira. S.B.R. Activities related to the present article: disclosed NIH grant to institution for this study; disclosed that GE Healthcare provides research support to UW-Madison, but no funding specifically for this study. Activities not related to the present article: disclosed no relevant relationships. Other relationships: disclosed royalties received by institution from Wisconsin Alumni Research Foundation (WARF) and Stanford University, both which own patents for related inventions by S.B.R. (details of licensing agreements between WARF, Stanford, and licensees are unknown to S.B.R.); ownership interests in Cellectar Biosciences, and Elucent Medical, and is a founder of Calimetrix, LLC.
Abbreviations:
- BMI
- body mass index
- CSE
- chemical shift encoded
- PDFF
- proton density fat fraction
- VLCD
- very low calorie diet
- WC
- waist circumference
References
- 1.Wang Y, Beydoun MA. The obesity epidemic in the United States: gender, age, socioeconomic, racial/ethnic, and geographic characteristics–a systematic review and meta-regression analysis. Epidemiol Rev 2007;29(1):6–28. [DOI] [PubMed] [Google Scholar]
- 2.Adams LA, Waters OR, Knuiman MW, Elliott RR, Olynyk JK. NAFLD as a risk factor for the development of diabetes and the metabolic syndrome: an eleven-year follow-up study. Am J Gastroenterol 2009;104(4):861–867. [DOI] [PubMed] [Google Scholar]
- 3.Szczepaniak LS, Nurenberg P, Leonard D, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab 2005;288(2):E462–E468. [DOI] [PubMed] [Google Scholar]
- 4.Gramlich T, Kleiner DE, McCullough AJ, Matteoni CA, Boparai N, Younossi ZM. Pathologic features associated with fibrosis in nonalcoholic fatty liver disease. Hum Pathol 2004;35(2):196–199. [DOI] [PubMed] [Google Scholar]
- 5.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 1999;116(6):1413–1419. [DOI] [PubMed] [Google Scholar]
- 6.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 2005;129(1):113–121. [DOI] [PubMed] [Google Scholar]
- 7.Dixon JB, Bhathal PS, Hughes NR, O’Brien PE. Nonalcoholic fatty liver disease: improvement in liver histological analysis with weight loss. Hepatology 2004;39(6):1647–1654. [DOI] [PubMed] [Google Scholar]
- 8.Luyckx FH, Desaive C, Thiry A, et al. Liver abnormalities in severely obese subjects: effect of drastic weight loss after gastroplasty. Int J Obes Relat Metab Disord 1998;22(3):222–226. [DOI] [PubMed] [Google Scholar]
- 9.Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 2009;122(3):248–256.e5. [DOI] [PubMed] [Google Scholar]
- 10.Maggard MA, Shugarman LR, Suttorp M, et al. Meta-analysis: surgical treatment of obesity. Ann Intern Med 2005;142(7):547–559. [DOI] [PubMed] [Google Scholar]
- 11.Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012;366(17):1567–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faria SL, Faria OP, de Almeida Cardeal M, Ito MK. Effects of a very low calorie diet in the preoperative stage of bariatric surgery: a randomized trial. Surg Obes Relat Dis 2015;11(1):230–237. [DOI] [PubMed] [Google Scholar]
- 13.Van Nieuwenhove Y, Dambrauskas Z, Campillo-Soto A, et al. Preoperative very low-calorie diet and operative outcome after laparoscopic gastric bypass: a randomized multicenter study. Arch Surg 2011;146(11):1300–1305. [DOI] [PubMed] [Google Scholar]
- 14.van Wissen J, Bakker N, Doodeman HJ, Jansma EP, Bonjer HJ, Houdijk APJ. Preoperative methods to reduce liver volume in bariatric surgery: a systematic review. Obes Surg 2016;26(2):251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41(6):1313–1321. [DOI] [PubMed] [Google Scholar]
- 16.Cowin GJ, Jonsson JR, Bauer JD, et al. Magnetic resonance imaging and spectroscopy for monitoring liver steatosis. J Magn Reson Imaging 2008;28(4):937–945. [DOI] [PubMed] [Google Scholar]
- 17.Engl J, Sturm W, Sandhofer A, et al. Effect of pronounced weight loss on visceral fat, liver steatosis and adiponectin isoforms. Eur J Clin Invest 2008;38(4):238–244. [DOI] [PubMed] [Google Scholar]
- 18.Reeder SB, Cruite I, Hamilton G, Sirlin CB. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J Magn Reson Imaging 2011;34(4):729–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reeder SB, Hu HH, Sirlin CB. Proton density fat-fraction: a standardized MR-based biomarker of tissue fat concentration. J Magn Reson Imaging 2012;36(5):1011–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bannas P, Kramer H, Hernando D, et al. Quantitative magnetic resonance imaging of hepatic steatosis: validation in ex vivo human livers. Hepatology 2015;62(5):1444–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hines CDG, Yu H, Shimakawa A, et al. Quantification of hepatic steatosis with 3-T MR imaging: validation in ob/ob mice. Radiology 2010;254(1):119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Idilman IS, Aniktar H, Idilman R, et al. Hepatic steatosis: quantification by proton density fat fraction with MR imaging versus liver biopsy. Radiology 2013;267(3):767–775. [DOI] [PubMed] [Google Scholar]
- 23.Hernando D, Sharma SD, Aliyari Ghasabeh M, et al. Multisite, multivendor validation of the accuracy and reproducibility of proton-density fat-fraction quantification at 1.5T and 3T using a fat-water phantom. Magn Reson Med 2017;77(4):1516–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Artz NS, Haufe WM, Hooker CA, et al. Reproducibility of MR-based liver fat quantification across field strength: same-day comparison between 1.5T and 3T in obese subjects. J Magn Reson Imaging 2015;42(3):811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 1999;94(9):2467–2474. [DOI] [PubMed] [Google Scholar]
- 26.Liu CY, McKenzie CA, Yu H, Brittain JH, Reeder SB. Fat quantification with IDEAL gradient echo imaging: correction of bias from T(1) and noise. Magn Reson Med 2007;58(2):354–364. [DOI] [PubMed] [Google Scholar]
- 27.Yu H, McKenzie CA, Shimakawa A, et al. Multiecho reconstruction for simultaneous water-fat decomposition and T2* estimation. J Magn Reson Imaging 2007;26(4):1153–1161. [DOI] [PubMed] [Google Scholar]
- 28.Yu H, Shimakawa A, McKenzie CA, Brodsky E, Brittain JH, Reeder SB. Multiecho water-fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling. Magn Reson Med 2008;60(5):1122–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu H, Shimakawa A, Hines CDG, et al. Combination of complex-based and magnitude-based multiecho water-fat separation for accurate quantification of fat-fraction. Magn Reson Med 2011;66(1):199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang A, Tan J, Sun M, et al. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology 2013;267(2):422–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel NS, Doycheva I, Peterson MR, et al. Effect of weight loss on magnetic resonance imaging estimation of liver fat and volume in patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 2015;13(3):561–568.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rossi AP, Fantin F, Zamboni GA, et al. Effect of moderate weight loss on hepatic, pancreatic and visceral lipids in obese subjects. Nutr Diabetes 2012;2(3):e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phillips ML, Boase S, Wahlroos S, et al. Associates of change in liver fat content in the morbidly obese after laparoscopic gastric banding surgery. Diabetes Obes Metab 2008;10(8):661–667. [DOI] [PubMed] [Google Scholar]
- 34.Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes 2005;54(3):603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghaemi A, Taleban FA, Hekmatdoost A, et al. How much weight loss is effective on nonalcoholic fatty liver disease? Hepat Mon 2013;13(12):e15227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.