Abstract
Background
Hepatocellular carcinoma (HCC) is the fifth most common global cancer. When HCC is diagnosed early, interventions such as percutaneous ethanol injection (PEI), percutaneous acetic acid injection (PAI), or radiofrequency (thermal) ablation (RF(T)A) may have curative potential and represent less invasive alternatives to surgery.
Objectives
To evaluate the beneficial and harmful effects of PEI or PAI in adults with early HCC defined according to the Milan criteria, that is, one cancer nodule up to 5 cm in diameter or up to three cancer nodules up to 3 cm in diameter compared with no intervention, sham intervention, each other, other percutaneous interventions, or surgery.
Search methods
We searched the Cochrane Hepato‐Biliary Group Controlled Trials Register (July 2014), the Cochrane Central Register of Controlled Trials (CENTRAL) (2014, Issue 6), MEDLINE (1946 to July 2014), EMBASE (1976 to July 2014), and Science Citation Index Expanded (1900 to July 2014). We handsearched meeting abstracts of six oncological and hepatological societies and references of articles to July 2014. We contacted researchers in the field.
Selection criteria
We considered randomised clinical trials comparing PEI or PAI versus no intervention, sham intervention, each other, other percutaneous interventions, or surgery for the treatment of early HCC regardless of blinding, publication status, or language. We excluded studies comparing RFA or combination of different interventions as such interventions have been or will be addressed in other Cochrane Hepato‐Biliary Group systematic reviews.
Data collection and analysis
Two review authors independently selected trials for inclusion, and extracted and analysed data. We calculated the hazard ratios (HR) for median overall survival and recurrence‐free survival using the Cox regression model with Parmar's method. We reported type and number of adverse events descriptively. We assessed risk of bias by The Cochrane Collaboration domains to reduce systematic errors and risk of play of chance by trial sequential analysis to reduce random errors. We assessed the methodological quality with GRADE.
Main results
We identified three randomised trials with 261 participants for inclusion. The risk of bias was low in one and high in two trials.
Two of the randomised trials compared PEI versus PAI; we included 185 participants in the analysis. The overall survival (HR 1.47; 95% confidence interval (CI) 0.68 to 3.19) and recurrence‐free survival (HR 1.42; 95% CI 0.68 to 2.94) were not statistically significantly different between the intervention groups of the two trials. Trial sequential analysis for the comparison PEI versus PAI including two trials revealed that the number of participants that were included in the trials were insufficient in order to judge a relative risk reduction of 20%. Data on the duration of hospital stay were available from one trial for the comparison PEI versus PAI showing a significantly shorter hospital stay for the participants treated with PEI (mean 1.7 days; range 2 to 3 days) versus PAI (mean 2.2 days; range 2 to 5 days). Quality of life was not reported. There were only mild adverse events in participants treated with either PEI or PAI such as transient fever, flushing, and local pain.
One randomised trial compared PEI versus surgery; we included 76 participants in the analyses. There was no significant difference in the overall survival (HR 1.57; 95% CI 0.53 to 4.61) and recurrence‐free survival (HR 1.35; 95% CI 0.69 to 2.63). No serious adverse events were reported in the PEI group while three postoperative deaths occurred in the surgery group.
In addition to the three randomised trials, we identified one quasi‐randomised study comparing PEI versus PAI. Due to methodological flaws of the study, we extracted only the data on adverse events and presented them in a narrative way.
We found no randomised trials that compared PEI or PAI versus no intervention, best supportive care, sham intervention, or other percutaneous local ablative therapies excluding RFTA. We found also no randomised clinical trials that compared PAI versus other interventional treatments or surgery. We identified two ongoing randomised clinical trials. One of these two trials compares PEI versus surgery and the other PEI versus transarterial chemoembolization. To date, it is unclear whether the trials will be eligible for inclusion in this meta‐analysis as the data are not yet available. This review will not be updated until new randomised clinical trials are published and can be used for analysis.
Authors' conclusions
PEI versus PAI did not differ significantly regarding benefits and harms in people with early HCC, but the two included trials had only a limited number of participants and one trial was judged a high risk of bias. Thus, the current evidence precludes us from making any firm conclusions.
There was also insufficient evidence to determine whether PEI versus surgery (segmental liver resection) was more effective, because conclusions were based on a single randomised trial. While some data from this single trial suggested that PEI was safer, the high risk of bias and the lack of any confirmatory evidence make a reliable assessment impossible.
We found no trials assessing PEI or PAI versus no intervention, best supportive care, or sham intervention.
There is a need for more randomised clinical trials assessing interventions for people with early stage HCC. Such trials should be conducted with low risks of systematic errors and random errors.
Plain language summary
Percutaneous ethanol injection for the treatment of early liver cancer
Background
Liver cancer (hepatocellular carcinoma) is the fifth most common cancer worldwide. In the majority of people, liver cancer is diagnosed at advanced stages of the disease and is mostly accompanied by liver cirrhosis. In high‐income countries, about 30% of people present with the more favourable early liver cancer. For these people, percutaneous ablation techniques (destruction of the cancer cells by heat, cold, or chemical substances such as ethanol), surgical resection (removal of part of the liver), and liver transplantation (which is limited by organ donor shortage) are currently considered potentially curative treatments. We aimed to investigate the role of percutaneous injection of ethanol (PEI) and acetic acid (PAI) as compared with other treatments or no intervention for early liver cancer. This review excluded the effects of radiofrequency thermal ablation as this has been already addressed in a previous Cochrane Hepato‐Biliary Group systematic review.
Study characteristics
The review authors searched the medical literature in order to clarify the role of PEI and PAI for the treatment of liver cancer and to compare their benefits and harms with no treatment, with placebo (a pretend treatment), or with other treatments (such as laser, cryoablation, or microwave ablation; hepatic resection; and liver transplantation). We collected and analysed data from randomised clinical trials (where people were allocated at random to one of two or more treatments groups) of people with liver cancer who were able to receive PEI or PAI. Evidence is current to July 2014.
Key results and quality of evidence
The review authors only identified three randomised trials with 261 participants. The risk of bias was low in one and high in two trials. We found two trials that compared PEI versus PAI and one trial that compared PEI versus surgery. We found no trials that compared PEI or PAI versus sham (pretend) intervention, best supportive care, cryotherapy, laser‐induced thermotherapy, or high‐frequency ultrasound. We found no randomised trials that compared PAI versus surgery.
The review authors found low‐quality evidence suggesting that PEI yielded the same result as PAI regarding overall survival (the length of time that the person remains alive) and recurrence‐free survival (time that the person remains free of cancer). We calculated the number of participants that would be required to judge a relative risk reduction (relative risk is a comparison of the risk of an event happening for one treatment group compared with another treatment group) for survival of 20%. We found that for the comparisons PEI versus PAI, the number of participants was too low to reach valid conclusions. In both groups, participants reported the occurrence of mild side effects such as transient fever, flushing, and local pain. Based on one randomised trial with high risk of bias, there was very low quality evidence that surgical resection does not seem to be superior to PEI in people with early liver cancer. Of note, no severe side effects occurred in people treated with PEI while there were three postoperative deaths in people treated surgically. Again, too few participants were randomised to claim or reject important differences.
There is a need for more randomised clinical trials assessing interventions for people with early‐stage liver cancer. Such trials should be conducted with low risks of bias (systematic errors, that is overestimation of benefits and underestimation of harms) and of play of chance (random errors, that is errors due to too few participants and too few outcomes).
Summary of findings
Summary of findings for the main comparison. Percutaneous ethanol injection (PEI) or percutaneous acetic acid injection (PAI) for early hepatocellular carcinoma (HCC).
| PEI versus PAI or surgery for early HCC | |||||
|
Patient or population: hospitalised people with early HCC treated with PEI, PAI, or surgery. Settings: Taiwanese and Japanese university and adjunct teaching hospitals. Intervention: PEI. Comparison: PAI or surgery. | |||||
| Outcomes | Comparison |
No. of trials |
Hazard ratio (95% CI) | No. of participants | Quality of the evidence (GRADE) |
| PEI versus PAI | |||||
| Overall survival | PAI | 2 |
HR 1.47 (0.68 to 3.19) |
185 | ⊕⊕⊝⊝ low1 |
| Recurrence‐free survival | PAI | 2 |
HR 1.42 (0.68 to 2.94) |
185 | ⊕⊕⊝⊝ low1 |
| Number and type of adverse events | PAI | 2 | Pain reported in both groups. Data not extractable for HR or OR. | ||
| Duration of hospital stay | PAI | 1 |
Mean PAI: 2.2 days (range 2 to 5) versus PEI: 1.7 days (range 2 to 3) |
125 | ⊕⊝⊝⊝ very low2 |
| Quality of life | PAI | 2 | No data were provided. | ||
| PEI versus surgery | |||||
| Overall survival | Surgery | 1 |
HR 1.57 (0.53 to 4.61) |
76 | ⊕⊝⊝⊝ very low3 |
| Recurrence‐free survival | Surgery | 1 |
HR 1.35 (0.69 to 2.63) |
76 | ⊕⊝⊝⊝ very low3 |
| Number and type of adverse events | Surgery | 1 | Insufficient amount of data provided. | 76 | ⊕⊝⊝⊝ very low4 |
| Duration of hospital stay | Surgery | 1 | No data were provided. | ||
| Quality of life | Surgery | 1 | No data were provided. | ||
| GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate. CI: confidence interval; HR: hazard ratio, MD: mean difference. | |||||
1 Graded as low, due to the risk of bias. Only one trial with low risk of bias was available and trial sequential analysis of both the high and low risk of bias trial revealed an insufficient number of included participants. 2 Graded as very low, as only one trial with low risk of bias was available. 3 Graded as very low, as only one trial of high risk of bias was available. 4 Graded as very low, as only one trial of high risk of bias was available and data presentation was not sufficiently precise.
Background
Description of the condition
Epidemiology and risk factors
Hepatocellular carcinoma (HCC) currently ranks fifth in global cancer incidence and mortality (Parkin 2001; Parkin 2005). Highest occurrences of HCC are found in Eastern Asia and Middle Africa. In addition, one trial suggested a rising incidence of HCC in high‐income countries (El Serag 1999). Due to the large pool of hepatitis C virus (HCV) in these countries, the rise in cirrhosis and HCC incidence is expected to continue over the next decades (El Serag 2004a). Overall, 75% to 80% of primary liver cancers are attributable to persistent viral infections with either hepatitis B virus (HBV) (50% to 55%) or HCV (25% to 30%). High consumption of alcohol, non‐alcoholic fatty liver disease, cumulative amount of aflatoxins in the liver over time, haemochromatosis, and alpha‐1‐antitrypsin are further risk factors for HCC development (Sørensen 2003; Bosch 2004; EASL‐EORTC 2012). Smoking is an independent and a dose‐related risk factor contributing to HCC (Lee 2009). Associations of primary liver cancer with diabetes (El Serag 2004b), obesity (Calle 2003), and syndromes related to insulin resistance are subject of ongoing research activity, but their impacts are currently unclear.
Prognosis
Overall, liver cancer carries high mortality. In Europe and the USA, five‐year survival occurrences are below 10% (Coleman 2003; Ries 2007). Percutaneous techniques, surgical resection, and liver transplantation are currently classified as potentially curative treatments (Bruix 2001). In high‐income countries, only about 30% of people present with early HCC and are candidates for these potentially radical treatments.
Diagnosis
Due to the neovascularity of HCC and the ability to equate neovascularity with contrast enhancement on rapid‐sequence cross‐sectional imaging studies, the accurate diagnosis of HCC lesions that are bigger than 2 cm in diameter can be made non‐invasively in people with cirrhosis. According to the European Association for the Study of the Liver (Bruix 2001), non‐invasive diagnostic criteria for people with cirrhosis and HCC are:
radiological criteria: two coincident imaging techniques (the following imaging techniques are considered: ultrasonography, spiral computer tomography, magnetic resonance imaging, and angiography);
combined criteria: one imaging technique associated with alpha‐fetoprotein: focal lesion bigger than 2 cm with arterial hypervascularity and alpha‐fetoprotein levels higher than 400 ng/mL.
Image‐guided biopsies are common practice in case of indeterminate radiological findings (i.e., discordant findings on different imaging techniques) and lesions between 1 cm and 2 cm in diameter (Takamori 2000; Sersté 2012). If a curative surgical treatment is still possible, taking biopsies is frequently avoided due to its risks, especially bleeding and tumour cell dissemination.
Description of the intervention
Surgical resection of HCC is often not feasible due to the impairment of liver function associated with the prevalent underlying cirrhosis. HCC recurrences after surgical resection are about 50% at three years and 70% after five years (Arii 2000; Bismuth 2000; Llovet 2000; Torzilli 2013).
Percutaneous local ablative therapies are currently considered as the preferred option for early unresectable HCC. All local ablative techniques have the advantage of preserving the uninvolved liver parenchyma and avoid the morbidity and mortality of major hepatic surgery (Bruix 2001). Percutaneous ethanol injection (PEI) causes dehydration and necrosis of tumour cells (EASL‐EORTC 2012), accompanied by small vessel thrombosis, leading to tumour ischaemia and destruction (Livraghi 1995). PEI is usually carried out under ultrasound guidance with repeated injections of ethanol on separate days. Best results for PEI are achieved in single HCC lesions less than 3 cm in diameter, in which complete remission of about 70% of cancers can be expected. In larger or multinodular cancers (or both), total necrosis is less likely with PEI (Livraghi 1995; Lencioni 1997). Percutaneous acetic acid injection (PAI) has been used as an alternative for PEI (Ohnishi 1996a; Ohnishi 1998a). Contraindications for PEI and PAI are cirrhosis with poor liver function (Child C cirrhosis), complete portal vein thrombosis, and massive ascites. Radiofrequency thermal ablation (RFTA) makes use of frictional heat to induce cell death from coagulation necrosis. The major advantages of RFTA are the lower number of sessions necessary for tumour destruction compared to PEI or PAI, and the prolonged overall and recurrence‐free survival (Lencioni 2003; Galandi 2004; Lin 2004; Lin 2005; Shiina 2005; Brunello 2008). Furtherpercutaneous techniques, such as cryoablation or interstitial laser photocoagulation, are under clinical investigation.
Liver transplantation is an alternative approach for selected people with small HCC. However, the beneficial removal of liver cirrhosis as a predisposing factor for HCC is often counterbalanced by progression of the tumour while the patient is waiting for a new organ. Organ shortage is a major factor limiting the availability of this procedure.
How the intervention might work
Why it is important to do this review
Percutaneous techniques are used with increasing frequency as a bridge to liver transplantation and with a curative intention for small HCC. The present systematic review is an update of our previous review published in 2009 (Schoppmeyer 2009). We identified no other meta‐analyses or systematic reviews on PEI or PAI for people with early HCC. The aim of this new review was to investigate whether new trials have been published since the first review and whether the evidence has been improved or changed.
Objectives
To evaluate the beneficial and harmful effects of PEI or PAI in adults with early HCC defined according to the Milan criteria, that is, one cancer nodule up to 5 cm in diameter or up to three cancer nodules up to 3 cm in diameter compared with no intervention, sham intervention, each other, other percutaneous interventions, or surgery.
Methods
Criteria for considering studies for this review
Types of studies
Randomised clinical trials on percutaneous local ablative therapies, irrespective of blinding, publication status, or language. We included abstracts or unpublished data if sufficient information on study design, characteristics of participants, interventions, and outcomes was available and if full information was confirmed by contact with the study authors. We considered non‐randomised studies that were retrieved with the searches for randomised trials for harm data only.
Types of participants
People with early HCC, defined according to the Milan criteria as "one nodule up to five cm in diameter or up to three nodules up to three cm in diameter" (Mazzaferro 1996). The diagnosis of HCC may have been established either by histology or cytology, or a combination of radiological criteria and alpha‐fetoprotein as described in the Background section.
Types of interventions
We assessed the following comparisons.
Percutaneous local ablative therapies (PEI or PAI) versus no intervention or best supportive care or sham intervention.
PEI versus other percutaneous local ablative therapies excluding RFTA.
PAI versus other percutaneous local ablative therapies excluding RFTA.
Percutaneous local ablative therapies (PEI or PAI) versus surgery.
We did not consider in this review studies using combinations of percutaneous treatments and other approaches, such as transarterial chemoembolisation, RFTA, surgery, and cryotherapy, which are dealt with in other Cochrane Hepato‐Biliary Group reviews (Galandi 2004; Abrishami 2008; Awad 2009; Oliveri 2011; Weis 2013). We did not consider liver transplantation as a type of intervention in this review since organ shortage limits the availability of this treatment and living donor transplantation has inherent ethical issues that prevents it from being a treatment option in randomised clinical trials.
Types of outcome measures
Primary outcomes
Overall survival.
Secondary outcomes
Recurrence‐free survival.
Number and type of adverse events. We defined adverse event as any untoward medical occurrence that did not necessarily have a causal relationship with the treatment, but did result in a dose reduction, discontinuation of treatment, or registration of the event as an adverse event (ICH‐GCP 1997).
Duration of hospital stay.
Quality of life according to a validated questionnaire (Heffernan 2002).
Search methods for identification of studies
Electronic searches
We searched the Cochrane Hepato‐Biliary Group Controlled Trials Register (July 2014) (Gluud 2014), the Cochrane Central Register of Controlled Trials (CENTRAL) (2014, Issue 6), MEDLINE (1946 to July 2014), EMBASE (1980 to July 2014), and Science Citation Index Expanded (1900 to July 2014) (Royle 2003). Appendix 1 shows the search strategies with the time spans of the searches.
We searched the ClinicalTrials.gov database using the terms "percutaneous ethanol injection", "percutaneous acetic acid injection", "PEI", and "PAI".
Searching other resources
We handsearched meeting abstracts from the American Society for Clinical Oncology (ASCO) (1990 to 2014), the European Society of Medical Oncology (ESMO) (1990 to 2014, published in the Annals of Oncology), the European Council for Clinical Oncology (ECCO) (1990 to 2014, published in the European Journal of Cancer); the American Association for the Study of the Liver (AASLD) (1991 to 2014, published in Hepatology); the European Association for the Study of the Liver (EASL) (1985 to 2014, published in the Journal of Hepatology), and the Asian Pacific Association for the Study of the Liver (APASL) (1990 to 2014, published in the Journal of Gastroenterology and Hepatology).
We identified further trials from the reference lists of the identified studies, by contacting investigators as well as national and international experts, and by writing to companies selling equipment for percutaneous local ablative therapies.
Data collection and analysis
Selection of studies
Two review authors (KS, SW) independently scanned the title, abstract, and keywords of every record retrieved. Publications were taken into account for further assessment if the information given suggested that the trial might meet our inclusion criteria. We then listed these publications under included studies or excluded studies. We provided the reason for exclusion of excluded studies.
Data extraction and management
Two review authors independently extracted details of trial population, interventions, and outcomes using a standardised data extraction form. We resolved differences in data extraction by consensus with a third review author. We contacted all trial authors, as questions arose in all included trials. The data extraction form included the following items.
General information: title, authors, source, contact address, country, publication status, language, year of publication, and trial sponsors.
Trial characteristics: design, duration of follow‐up, number of participants randomised.
Participants: inclusion and exclusion criteria, sample size, baseline characteristics (Child score, aetiology of liver cirrhosis: unknown, HBV or HCV, others), withdrawals and losses to follow‐up, method of diagnosis of HCC.
Interventions: category of percutaneous therapy (percutaneous injection: ethanol, acetic acid; percutaneous thermal ablation, percutaneous cryoablation) and comparator intervention (sham, best supportive care, no intervention, other percutaneous intervention, surgery).
Outcomes: primary outcome: survival time, hazard ratios (HR) and their 95% confidence intervals (CI), log rank Chi2, log rank P values, number of events, number of participants per group, median survival in people with HCC. Secondary outcomes: recurrence‐free survival, number and type of adverse events, duration of hospital stay, and quality of life.
Assessment of risk of bias in included studies
Quality assessment of trials
Because of the risk of overestimation of treatment effects in randomised clinical trials with high risk of bias (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008; Lundh 2012; Savovic 2012a; Savovic 2012b), we assessed the impact of methodological quality according to the following criteria.
Allocation sequence generation
Low risk of bias: sequence generation was achieved using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards, and throwing dice were adequate if performed by an independent research assistant not otherwise involved in the trial.
Uncertain risk of bias: the method of sequence generation was not specified.
High risk of bias: the sequence generation method was not random.
Allocation concealment
Low risk of bias: the participant allocations could not have been foreseen in advance of, or during, enrolment. Allocation was controlled by a central and independent randomisation unit. The allocation sequence was unknown to the investigators (e.g., if the allocation sequence was hidden in sequentially numbered, opaque, and sealed envelopes).
Uncertain risk of bias: the method used to conceal the allocation was not described so that intervention allocations may have been foreseen in advance of, or during, enrolment.
High risk of bias: the allocation sequence was likely to be known to the investigators who assigned the participants.
Blinding of participants, personnel, and outcome assessors
Low risk of bias: blinding was performed adequately, or the assessment of outcomes was not likely to be influenced by lack of blinding.
Uncertain risk of bias: there was insufficient information to assess whether blinding was likely to induce bias on the results.
High risk of bias: no blinding or incomplete blinding, and the assessment of outcomes were likely to be influenced by lack of blinding.
Incomplete outcome data
Low risk of bias: missing data were unlikely to make treatment effects depart from plausible values. Sufficient methods, such as multiple imputation, have been employed to handle missing data.
Uncertain risk of bias: there was insufficient information to assess whether missing data in combination with the method used to handle missing data were likely to induce bias on the results.
High risk of bias: the results were likely to be biased due to missing data.
Selective outcome reporting
Low risk of bias: all outcomes were pre‐defined (e.g., in a published protocol) and reported, or all clinically relevant and reasonably expected outcomes were reported. We considered the following outcome measures as clinically relevant: survival, recurrence‐free survival, and adverse events.
Uncertain risk of bias: it was unclear whether all pre‐defined and clinically relevant and reasonably expected outcomes were reported.
High risk of bias: one or more clinically relevant and reasonably expected outcomes were not reported, and data on these outcomes were likely to have been recorded.
Academic bias
Low risk of bias: if authors had not performed a trial on the same topic before.
Uncertain risk of bias: if it could not be made clear if one of the authors was involved in a trial on the same topic before.
High risk of bias: if authors had performed a trial on the same topic before.
Other risks of bias
Low risk of bias: the trial appeared free of other components that could put it at risk of bias.
Uncertain risk of bias: it was unclear whether the trial was free of other components that could put it at risk of bias.
High risk of bias: there were other factors in the trial that could put it at risk of bias (e.g. for‐profit involvement).
Overall risk of bias assessment
We considered a trial to have low risk of bias if all of the above specified domains of risk assessment were considered low risk of bias. We considered a trial with high risk of bias if one or more of the specific domains of risk assessment were unclear or with high risk of bias.
Measures of treatment effect
We calculated the HR and 95% CI for median overall survival and recurrence‐free survival using the Cox regression model with Parmar's method (Parmar 1998). We reported type and number of adverse events descriptively.
Unit of analysis issues
We included trials with three or more treatment groups if pair‐wise comparison of a single intervention to a percutaneous local ablative therapy was possible and if inclusion criteria of both groups fulfilled our inclusion criteria. In order to exclude analysis bias by multiple counting of the shared intervention group, we split the shared intervention group into a corresponding number of subgroups with smaller sample size. We analysed and included each pair‐wise comparison separately. We measured two survival outcomes. We analysed recurrence‐free survival and overall survival separately.
Dealing with missing data
In the case of missing data, we contacted trial authors and asked them to provide us with the missing data. We assessed missing data in the judgement of selective and incomplete reporting bias. We obtained HR and standard deviations or Kaplan Meier survival plots for the survival outcomes if the required data (HR and 95% CI) were not available from the original publications.
Assessment of heterogeneity
We performed Cochrane's Q‐test (with a significance threshold of P value = 0.1) in order to look for statistical heterogeneity between the trials. In addition, we calculated the I2 statistic (Higgins 2002). We considered the bias risk of the trials a potential source of heterogeneity.
Assessment of reporting biases
We planned to prepare a funnel plot if we included 10 or more trials for visual assessment of whether treatment estimates were associated with trial size. To detect bias, we planned to use a method that has a good trade‐off in sensitivity and specificity for the trials included in our review (Begg 1994; Egger 1997; Macaskill 2001).
Data synthesis
Meta‐analysis
We performed the meta‐analyses according to the recommendations of The Cochrane Collaboration (Higgins 2011), and the Cochrane Hepato‐Biliary Group Module (Gluud 2014). We extracted HRs as relevant effect measures for survival time and recurrence‐free survival with 95% CI. We estimated HR (under some assumptions) from log rank Chi2, from log rank P values, from observed to expected event ratios, or Kaplan‐Meier curves with participants at a given risk, using methods presented by Machin 1997 (Machin 1997) and Parmar 1998 (Parmar 1998) We conducted random‐effects model and fixed‐effect model meta‐analyses and reported both results if they differed. We performed statistical analyses with Review Manager 5 (RevMan 2012).
Trial sequential analysis
We applied trial sequential analysis (CTU 2011; Thorlund 2011) because cumulative meta‐analyses are at risk of producing random errors due to sparse data and repetitive testing of the accumulating data (Wetterslev 2008). To minimise random errors, we calculated the required information size (i.e., the number of participants needed in a meta‐analysis to detect or reject a certain intervention effect) (Wetterslev 2008). The required information size calculation should also account for the diversity present in the meta‐analysis (Wetterslev 2008; Wetterslev 2009). In our meta‐analysis, the required information size was based on the event proportion in the control group; assumption of a plausible relative risk reduction of 20%; a risk of type I error of 5%; a risk of type II error of 20%; and the assumed diversity of the meta‐analysis (Brok 2008; Wetterslev 2008; Brok 2009; Thorlund 2009; Wetterslev 2009; Thorlund 2010).
The underlying assumption of trial sequential analysis is that testing for significance may be performed each time a new trial is added to a meta‐analysis. We added the trials according to the year of publication, and if more than one trial was published in one year, we added trials alphabetically according to the last name of the first author. On the basis of the diversity‐adjusted required information size, we constructed trial sequential monitoring boundaries (Brok 2008; Wetterslev 2008; Brok 2009; Thorlund 2009; Wetterslev 2009; Thorlund 2010). These boundaries determine the statistical inference one may draw regarding the cumulative meta‐analysis that has not reached the required information size. If the trial sequential monitoring boundary for benefit or harm is crossed by the cumulative Z‐curve before the required information size is reached, firm evidence may perhaps be established and further trials may turn out to be superfluous. In contrast, if the boundary is not surpassed, it is most probably necessary to continue doing trials in order to detect or reject a certain intervention effect if the trial sequential monitoring boundaries for futility are not crossed. If the latter case, futility is declared (Brok 2008; Wetterslev 2008; Brok 2009; Thorlund 2009; Wetterslev 2009; Thorlund 2010; Thorlund 2011).
Subgroup analysis and investigation of heterogeneity
In the case of sufficient data, we had planned subgroup analyses in order to make a distinction between the aetiology of liver cirrhosis and the diagnosis of HCC.
Trials with low risk of bias compared to trials with high risk of bias.
Cirrhosis of unknown cause compared to cirrhosis caused by HBV and compared to cirrhosis caused by HCV.
People with HCC diagnosed by histology or cytology compared to people with HCC diagnosed by radiological or combined criteria.
Sensitivity analysis
We planned to conduct 'best‐worst' and 'worst‐best' case scenario analyses to test for sensitivity to attrition bias (Higgins 2011; Gluud 2014).
'Summary of findings' table
We summarised the evidence in Table 1 using GRADEpro (ims.cochrane.org/revman/other‐resources/gradepro) in order to present the findings of the review in a simple tabular format (Higgins 2011), and using the GRADE classification to assess the quality of the evidence (Guyatt 2008).
Results
Description of studies
We identified 2379 publications by electronic database searches.
Results of the search
Electronic literature searches revealed 57 hits in the Cochrane Hepato‐Biliary Group Controlled Trials Register, 300 hits in the Cochrane Central Register of Controlled Trials (CENTRAL), 563 hits in MEDLINE, 747 hits in EMBASE, and 712 hits in Science Citation Index Expanded. Appendix 1 shows the search strategies. The handsearches of meeting abstracts found no additional studies. We found two new trials in the US trial registry (www.clinicaltrial.gov) that were just completed and from which results or data were not yet available (NCT00357422; NCT00357474). We classified them as ongoing trials. Two review authors (KS and SW) independently screened the results of the literature searches and found 41 studies suitable for further analysis; we excluded 23 of these due to duplication. We retrieved the entire manuscripts of the remaining 18 studies. Of these, we excluded 13 studies. Four publications on trials fulfilled the inclusion criteria, of which one was published twice (Ohnishi 1998b). We excluded one quasi‐randomised study (Huo 2003) (Figure 1), which was initially considered for a subgroup analysis in the first published version of this review (Schoppmeyer 2009), from the primary analysis in the present updated review as trials with inadequate randomisation methodology tend to increase the risk of false‐positive findings (Gluud 2005; Wood 2008; Savovic 2012a; Savovic 2012b). We did not include one trial due to insufficient available information and classified it as 'study awaiting classification' (Tsai 2008).
1.
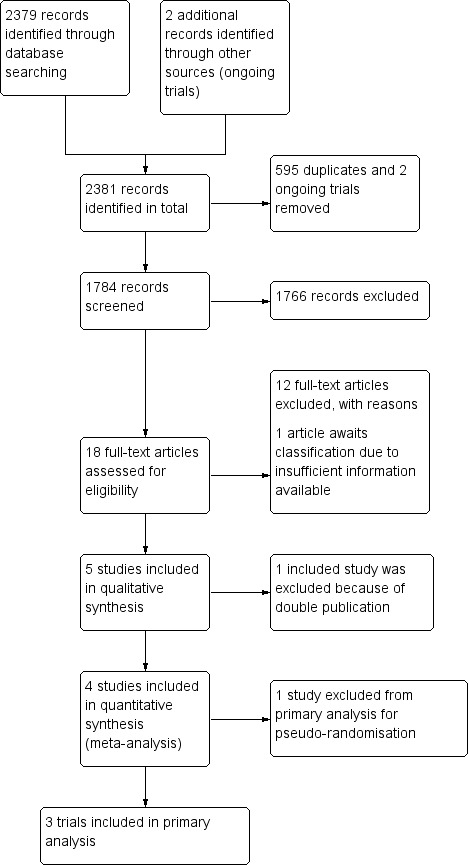
Study flow diagram.
Included studies
We included three randomised trials presented in four publications in this systematic review with 261 participants. One of these three trials compared PEI versus PAI and was published twice (Ohnishi 1998b). The 'three' arm trial compared RFA versus PEI versus PAI (Lin 2005). We excluded 62 participants who had received RFA from the analysis (Lin 2005). Ninety‐one participants in both trials were allocated to PEI versus 94 participants to PAI (Ohnishi 1998b; Lin 2005) and 182/185 participants had underlying liver cirrhosis. The third trial compared 76 people with early HCC, of whom 60 had underlying liver cirrhosis, who received PEI versus surgical resection (Huang 2005). One study is awaiting classification as there are currently insufficient data available to judge whether it can be included (Tsai 2008). We contacted the authors twice but they did not reply.
Excluded studies
We excluded 13 studies: one was published as a meeting abstract and it did not contain sufficient data. We were unsuccessful in contacting the corresponding author (Bottelli 1997). Five studies were not adequately randomised (Orlando 1997; Huo 2002; Huo 2003; Huo 2004; Dettmer 2006). One of them (Dettmer 2006), as well as two additional studies (Sarin 1994; Bruix 1998), investigated large instead of early HCC. Five studies were case series or retrospective studies (Danila 2009; Kirikoshi 2009; Lencioni 2010; Peng 2010; Pompili 2011).
Risk of bias in included studies
One trial had a low risk of bias (Lin 2005), while two trials had high risk of bias (Ohnishi 1998b; Huang 2005) (Figure 2; Figure 3).
2.
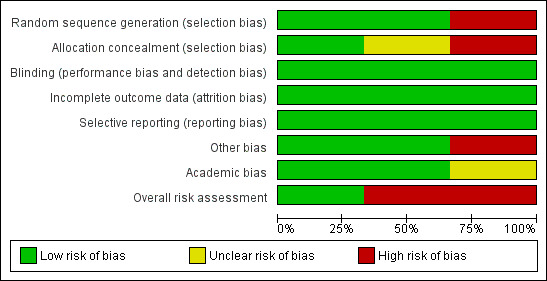
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies (survival outcomes).
3.
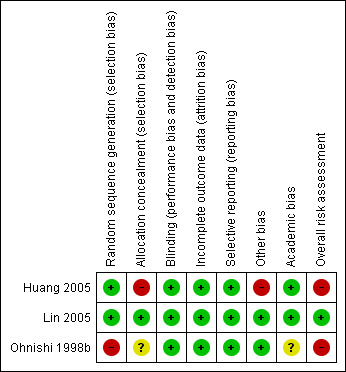
Methodological quality summary: review authors' judgements about each methodological quality item for each included study (survival outcomes).
Allocation
Random sequence generation
Generation of the allocation sequence was done by computer randomisation in Lin 2005, by random table in Huang 2005, and was unclearly reported in Ohnishi 1998b.
Allocation concealment
Allocation concealment was adequate in Lin 2005, inadequate in Huang 2005, and unclear in Ohnishi 1998b. In one trial, treatment groups were equalised in number of participants before the end of randomisation, but the number of participants was not stated (Huang 2005). In addition, six participants discontinued their foreseen therapy and were replaced by the next recruited participant without randomisation.
Blinding
We assumed that lack of blinding is unlikely to influence the assessment of objective efficacy outcomes such as survival. Moreover, as treatment modalities such as PEI and hepatic resection differ substantially concerning the degree of invasiveness, procedure performance, etc., reasonable blinding to the treatment of an investigator or an informed patient is unlikely. Regarding survival, all of the trials were therefore considered to have a low risk of bias. In contrast, quality events or outcomes such as tumour recurrence and adverse events were not blinded in any of the trials. Accordingly, we considered all trials to have high risk of bias regarding such outcomes.
Incomplete outcome data
One trial gave reasons for withdrawals (Ohnishi 1998b), but not the other two trials. Two trials performed intention‐to‐treat analysis for all outcomes except for adverse events (Ohnishi 1998b; Lin 2005), and one trial did not reported it (Huang 2005).
Selective reporting
We judged that the included trials were not biased by selective reporting. We retrieved relevant data for overall survival and recurrence‐free survival from all trials.
Other potential sources of bias
We judged academic bias low in two trials (Huang 2005; Lin 2005). Risk of academic bias was unclear in one trial (Ohnishi 1998b). Ohnishi et al. have published two retrospective trials on PEI for HCC previously (Ohnishi 1996a; Ohnishi 1996b). Lin et al. performed two randomised clinical trials during the same time period. People were assigned to one of the trials according to their hospital admission week (Lin 2004; Lin 2005). However, we judged this low risk of bias.
Moreover, two trials included only treatment‐naive participants (Ohnishi 1998b; Lin 2005). Only two trials performed a sample size calculation (Ohnishi 1998b; Lin 2005), whereas one trial mentioned no such calculation (Huang 2005).
Effects of interventions
See: Table 1
We found no eligible studies that compared PEI or PAI versus sham intervention, best supportive care, cryotherapy, laser‐induced thermotherapy, or high‐frequency ultrasound. We found no eligible trials that compared PAI with surgery.
Percutaneous ethanol injection versus percutaneous acetic acid injection
Comparison for efficacy outcomes
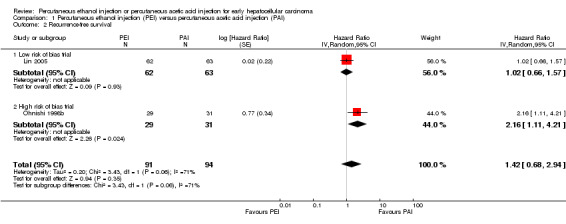
Two randomised trials compared PEI versus PAI and found that 31/91 participants treated with PEI and 19/94 participants treated with PAI died. There was no statistically significant difference between the two groups in overall survival (HR 1.47; 95% CI 0.68 to 3.19; Analysis 1.1) or recurrence‐free survival (HR 1.42; 95% CI 0.68 to 2.94; Analysis 1.2) (Ohnishi 1998b; Lin 2005). There were more deaths in the PEI group in the Ohnishi 1998b trial than in the Lin 2005 trial. There was heterogeneity for the trials (overall survival: Chi2 = 1.61 (P value = 0.21); I2 = 38%; recurrence‐free survival: Chi2 = 3.43 (P value = 0.06); I2 = 71%).
1.1. Analysis.
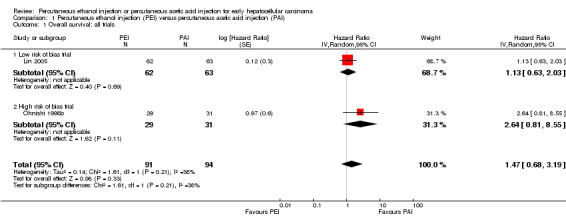
Comparison 1 Percutaneous ethanol injection (PEI) versus percutaneous acetic acid injection (PAI), Outcome 1 Overall survival: all trials.
1.2. Analysis.
Comparison 1 Percutaneous ethanol injection (PEI) versus percutaneous acetic acid injection (PAI), Outcome 2 Recurrence‐free survival.
Subgroup analyses
We performed a subgroup analysis for the comparison PEI versus PAI that included the only low risk of bias trial (Lin 2005). There was no statistically significant difference for outcome overall survival (HR 1.13; 95% CI 0.63 to 2.03) (Analysis 1.1) or recurrence‐free survival (HR 1.02; 95% CI 0.66 to 1.57) (Analysis 1.2).
Due to missing data, we could not perform subgroup analyses for the aetiology of liver cirrhosis or the diagnosis of HCC.
Sensitivity analysis
We did not conduct 'best‐worst' and 'worst‐best' sensitivity analyses due to paucity of data.
Comparison for safety outcomes
Pain was the most frequent adverse event for people treated with PEI and PAI. The Lin 2005 trial reported it as severe pain in 1/55 participants treated with PEI and in 3/58 participants treated with PAI. The Ohnishi 1998b trial reported it as "pain" in '"most patients" in both groups.
Comparison for economic outcomes
There were no data for economic outcomes. Only one trial reported the length of hospital stay (Lin 2005). Lin 2005 reported a significantly shorter hospital stay from the beginning of therapy to dismissal of the participants treated with PEI (mean 1.7 days (range 2 to 3 days) versus PAI (mean 2.2 days (range 2 to 5 days); P value < 0.01).
Comparison for quality of live
None of the included trials collected data on quality of life.
Risk of random error
We calculated the diversity‐adjusted required information size (DARIS) based upon a proportion of deaths of 23% in the PEI group; a relative risk reduction (RRR) of 20%; an alpha of 5% (α) and a beta of 20% (ß). Trial sequential analysis with data from two trials that compared PEI with PAI found that (only) 185/2896 (6.3%) participants of the DARIS were accrued. The cumulative Z‐score did not cross the monitoring boundary and did not reached the monitoring boundary for the area of futility (Figure 4).
4.
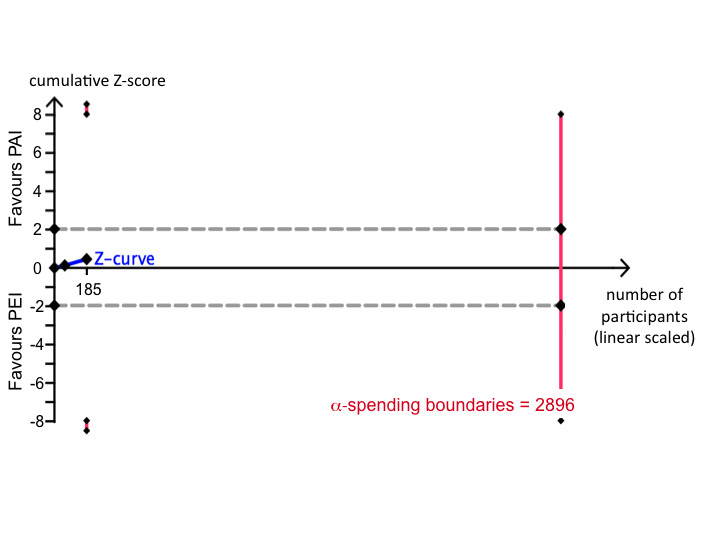
Trial sequential analysis for the comparison PEI versus PAI on survival in people with early hepatocellular carcinoma. The diversity‐adjusted required information size (DARIS) was calculated based upon a proportion of deaths of 23% in the percutaneous ethanol injection (PEI) group; a relative risk reduction (RRR) of 20%; an alpha of 5% and a beta of 20%. The cumulative Z‐score did not cross the monitoring boundary and did not reach the monitoring boundary for futility. Therefore, further randomised trials are needed.
Risk of bias
We judged risk of bias low in one trial (Lin 2005), and high in the other trial due to the unclear random sequence generation (Ohnishi 1998b) (Figure 2). We did not create a funnel plot, as there were only two trials in the meta‐analysis. We also did not perform a linear regression model analysis to determine funnel plot asymmetry for the same reason.
GRADE assessment
Evidence as evaluated by the GRADE approach (Guyatt 2008) was low for the comparison of PEI versus PAI for overall survival, recurrence‐free survival, and hospital stay (Table 1).
Percutaneous ethanol injection versus surgery
Comparison for efficacy outcomes
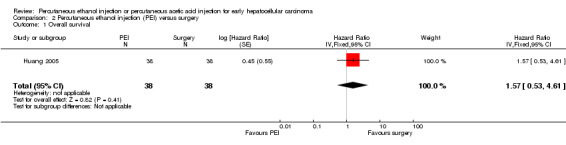
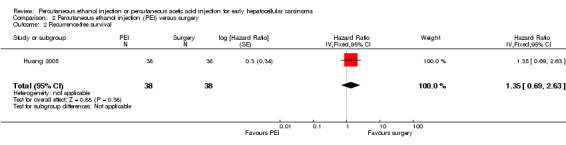
One trial compared PEI with surgery for early HCC (Huang 2005). Three/38 participants treated with PEI and 5/38 participants treated with surgical resection died. Tumour recurrence occurred in 18/38 participants in the PEI group and 15/38 participants in the surgery group. There was no significant difference in overall survival (HR 1.57; 95% CI 0.53 to 4.61; Analysis 2.1) or recurrence‐free survival (HR 1.35; 95% CI 0.69 to 2.63; Analysis 2.2).
2.1. Analysis.
Comparison 2 Percutaneous ethanol injection (PEI) versus surgery, Outcome 1 Overall survival.
2.2. Analysis.
Comparison 2 Percutaneous ethanol injection (PEI) versus surgery, Outcome 2 Recurrence‐free survival.
Comparison for safety outcomes
In the PEI versus surgery trial), 3/38 participants in the surgical group died within one, two, and seven months postoperatively (Huang 2005). These participants died from acute renal failure (at one month), sudden death (at two months), and uraemia (at seven months) (personal communication). In the PEI group, 2/38 participants had pain, and 1/38 participants had transient arterial hypotension (Huang 2005). The studies that were excluded for inadequate randomisation provided no additional report on adverse events.
Comparison for economic outcome
Huang 2005 did not report any data on duration of hospital stay for either group.
Comparison for quality of live
Huang 2005 did not report any data on quality of life.
Risk of random error
As there was only one trial available, we could not apply trial sequential analysis. The trial did not perform a sample size calculation; therefore, we assumed a substantial risk of random error.
Risk of bias
We judged the overall risk of bias of the one trial high due to the risk of pseudo‐randomisation (Huang 2005).
GRADE assessment
We assessed the evidence to be very low for the comparison of PEI versus surgery, as there was only one trial with a high risk of bias (Huang 2005) (Table 1).
Discussion
Percutaneous techniques are used with increasing frequency as a bridge to liver transplantation and with a curative intention for small HCC. We performed our first meta‐analysis in 2009 in order to identify current evidence for the treatment of early HCC with PEI or PAI as compared to other treatment modalities (Schoppmeyer 2009). Since this first review, no new trials were published. We found two new randomised trials on the ClinicalTrials.gov database. One trial compared PEI with surgical resection for early HCC (NCT00357474). The other trial compared PEI with transarterial chemoembolisation for multiple small HCCs (NCT00357422). Despite that these two trials are finalised, no results are published yet.
Summary of main results
We found no significant difference regarding beneficial or harmful effects on median and recurrence‐free survival of people with early‐stage HCC, neither for treatment with PEI compared with PAI nor for treatment with PEI compared with surgical resection.
Resection, liver transplantation, and percutaneous treatments compete as first‐line treatment options in people with early‐stage HCC, defined according to the Milan criteria (Mazzaferro 1996), and well‐preserved liver function. In these people, surgery yields a five‐year‐survival of 50% (Arii 2000; Bismuth 2000; Llovet 2000). However, liver dysfunction as a consequence of underlying liver cirrhosis often limits surgical resection. Liver transplantation is hampered by the shortage of organ donors, which in turn can lead to progression of HCC while waiting for a new organ. Results of liver transplantation decline steeply with application of broader selection criteria due to high recurrence (Llovet 2000). The most suitable candidates for transplantation are people with a single HCC nodule of 5 cm or less or up to three nodules of 3 cm or less who achieve low recurrence rates and a five‐year survival of 70% (Mazzaferro 1996; Llovet 2000). These so‐called Milan criteria have been modestly expanded to a single nodule of 6.5 cm or less or up to three nodules of 4.5 cm or less, and a total tumour diameter of 8 cm or less (Yao 2002). The expanded criteria predict the survival after liver transplantation comparable to the more stringent Milan criteria (Yao 2002).
Local ablative therapies in early HCC have been developed since the 1980s. Treatment criteria for local ablative treatment resemble those in liver transplantation: one nodule of 5 cm or less or up to three nodules of 3 cm or less (Bruix 2001). Image‐guided, repeated PEI and PAI are procedures that can cause necrosis in small HCC but are safe and effective treatment modalities (Livraghi 1995; Lencioni 1997; Ohnishi 1998b).
We identified three trials that fulfilled the inclusion criteria of this systematic review. Only people with tumours of 3 cm or less were included in the three trials and most participants (242/261 participants) had liver cirrhosis. Two trials compared PEI versus PAI in early HCC (Ohnishi 1998b; Lin 2005), and one trial assigned participants to undergo PEI versus surgical resection (Huang 2005). Two trials had a high risk of bias (Ohnishi 1998b; Huang 2005), while one trial had a low risk of bias (Lin 2005). We found no randomised trials that compared local ablative therapy to no intervention or best supportive care or transplantation.
In people treated with PEI versus PAI, the median survival was not significantly different. There were no significant differences in recurrence‐free survival. Both trials reported well‐balanced participant groups with no striking differences in participants' characteristics between the trials except for aetiology of cirrhosis. Whereas 82% of participants had HCV‐induced cirrhosis in the Ohnishi 1998b trial, most participants had HBV‐induced cirrhosis, and only 32% had HCV‐induced cirrhosis in the Lin 2005 trial. Both PEI and PAI were well tolerated. No severe adverse events were reported. The most frequent adverse event in both treatment modalities was pain, which was easily controlled with analgesics in most participants. Transient fever after both PEI and PAI and facial flushing following PEI were also observed. Duration of hospital stay and number of hospital admissions per year were each reported once (Ohnishi 1998b; Lin 2005); hence, no firm conclusion can be drawn. However, the number of treatment sessions per tumour was consistently less with PAI than with PEI. Neither trial addressed quality of life.
For the comparison of PEI with surgical resection, the single retrieved trial found no significant difference regarding median survival and recurrence‐free survival (Huang 2005). Pain was reported rarely in participants undergoing PEI and led to treatment discontinuation once. The authors specified no adverse events for surgical resection; however, three participants died postoperatively and were excluded from the authors' analysis of the trial. This and the flawed concealment procedure (equalising of participant number per trial group before the end of recruitment, non‐randomised inclusion following exclusion of another participant) limits the value of this trial. The results of this trial concur with those of uncontrolled trials showing similar survival for participants treated with PEI compared to participants treated with segmental liver resection (Yamamoto 2001). In clinical practice, the less invasive percutaneous treatment may be considered the preferable option.
RF(T)A is another well‐established local ablative technique in early HCC. Available data from randomised clinical trials suggest that RFA is superior to injection therapies in terms of number of treatment sessions, recurrence‐free survival, and overall survival (Lencioni 2003; Lin 2004; Lin 2005; Shiina 2005; Brunello 2008). This is supported by one Cochrane systematic review from the Cochrane Hepato‐Biliary Group (Weis 2013). However, RFA is associated with rare, but severe complications such as haemothorax and gastrointestinal perforation. Therefore, certain restrictions concerning location of the tumour in the liver apply to RFA. The practice guidelines of the European Association for the Study of the Liver (EASL) and the European Organisation for Research and Treatment of Cancer (EORTC) from 2012 recommend PEI only in people with early HCC in which RFA is technically not feasible. It is stated that it is unclear whether in people with very early HCC (tumours less than 2 cm, Barcelona Clinic Liver‐Cancer (BCLC) 0) any of the two techniques can be used (EASL‐EORTC 2012).
Overall completeness and applicability of evidence
What is the role that local ablative injection techniques may play in early HCC in the future? Because RFA is superior as compared with PEI regarding overall survival and tumour recurrence (Bouza 2009; Cho 2009; Weis 2013), RFA should be preferentially used wherever it is available and small tumours are located within the range of that technique(i.e., not in the vicinity of larger vessels, the gallbladder, or the liver surface). In contrast, injection techniques may be used for their simplicity, safety, inexpensiveness, and wide availability in other instances.
Quality of the evidence
Due to the small number of trials and participants included, the quality of evidence was low to very low. We conclude that the evidence does not allow a robust conclusion regarding the effects of PEI or PAI as compared to other interventions for early HCC. Moreover, we judged two of the three identified trials to have a high risk of bias.
Potential biases in the review process
We could not identify any potential bias in the review process.
Agreements and disagreements with other studies or reviews
Apart from our previous review (Schoppmeyer 2009), we did not identify any reviews or meta‐analyses that compared PEI versus PAI with other interventions excluding RFA for early HCC.
From a global perspective, HCC is a predominant cancer in the developing world with a much higher incidence than in industrialised countries. This is another potential setting for an effective and easy‐to‐manage local injection technique.
Authors' conclusions
Implications for practice.
On the basis of the limited evidence from the randomised clinical trials that we identified and included in this analysis, percutaneous ethanol injection (PEI) did not seem to differ from percutaneous acetic acid injection (PAI) regarding benefits and harms in people with one to three small (3 cm or less) HCC nodules. However, this evidence from two randomised trials was insufficient to guide the choice between PEI and PAI. This is also the case for the evidence for the comparison of PEI versus surgery for which we included only one trial.
Implications for research.
Due to the risk of systematic errors (bias) and risk of random errors ('play of chance') as well as the limited number of participants studied, randomised clinical trials to assess the benefits and harms of local ablative interventions for people with small HCC should be conducted.
Furthermore, in intermediately progressed HCC (i.e., single nodule larger than 4 cm, more than four small nodules), no standard treatment has been sufficiently assessed. In particular, the role of combination therapies (e.g., transarterial chemoembolisation plus percutaneous local ablative therapy) in these tumours should be investigated in randomised trials. In addition, transarterial chemoembolisation itself is often applied in clinical practice, but we still lack large randomised trials or a conclusive meta‐analysis that confirmed its effectiveness. Future trials should also address other clinically relevant aspects, such as number of treatment sessions, adverse events, and quality of life. The agreement on one optimal prognostically relevant staging system is important in order to make studies comparable. Another therapeutic interface that has not been well studied is the therapy to bridge the waiting time for people with HCC on the liver transplantation list. Randomised trials in this field are most awaited. Randomised trials ought to be designed according to the SPIRIT guidelines (Standard Protocol Items: Recommendations for Interventional Trials; www.spirit‐statement.org) and reported according to the CONSORT guidelines (CONsolidated Standards of Reporting Trials; www.consort‐statement.org).
What's new
| Date | Event | Description |
|---|---|---|
| 28 February 2019 | Review declared as stable | The interventions studied in this review are no longer used. Newer and better type of interventions for early hepatocellular carcinoma have been developed. |
History
Protocol first published: Issue 3, 2007 Review first published: Issue 3, 2009
| Date | Event | Description |
|---|---|---|
| 7 July 2014 | New citation required but conclusions have not changed | The conclusions have not changed as no new trials were added. This review will not be updated every second year unless new trials are published. |
| 7 July 2014 | New search has been performed | Literature search updated. A quasi‐randomised study was removed from assessment of benefits (Huo 2003). |
| 21 August 2013 | Amended | Peer reviewers comments included. Risk of bias assessment changed. |
| 31 October 2012 | New search has been performed | First author changed and new authors joined the review team. Definitions of bias risk domains, used to assess the quality of trials, were up‐dated. A section on quality of evidence was added. A study flow diagram was added. The improved format of the RevMan software necessitated improvement of the structure of the review text. |
Notes
Büchner‐Steudel P, Behl S, and Fleig WE were the authors of the published protocol for the systematic review in Issue 3, 2002 of The Cochrane Library. Since the review could not be prepared on time, its protocol had to be withdrawn. The author team changed again for the preparation of this and the last update. Authors are as follows: Weis S, Franke A, Berg T, Mössner J, Fleig WE, and Schoppmeyer K.
Acknowledgements
We thank Hannelore Tenckhoff from the Kompetenznetz Hepatitis Ostdeutschland for her help with the bibliographic research and Dimitrinka Nikolova, Sarah Klingenberg, and Christian Gluud from the Cochrane Hepato‐Biliary Group for their patience and ongoing support.
Peer reviewers: Ronald L. Koretz, USA; Alain Braillon, France. Contact editor: Christian Gluud, Denmark.
Appendices
Appendix 1. Search strategies
| Database | Searched | Search strategy |
| The Cochrane Hepato‐Biliary Group Controlled Trials Register | July 2014 | (((ethanol or alcohol or acetic acid or vinegar) and (inject* or ablati*)) or PEI or PAI or PAAI) AND (((liver or hepatic or hepatocellular or hepato‐cellular) and (carcinom* or cancer* or neoplasm* or malign* or tumo*)) or HCC) |
| Cochrane Central Register of Controlled Trials (CENTRAL) | Issue 6 of 12, 2014 | #1 MeSH descriptor: [Ethanol] explode all trees and with qualifier(s): [Administration & dosage ‐ AD, Therapeutic use ‐ TU] #2 MeSH descriptor: [Acetic Acid] explode all trees #3 MeSH descriptor: [Injections] explode all trees #4 (((ethanol or alcohol or acetic acid or vinegar) and (inject* or ablati*)) or PEI or PAI or PAAI) #5 #1 or #2 or #3 or #4 #6 MeSH descriptor: [Liver Neoplasms] explode all trees #7 (((liver or hepatic or hepatocellular or hepato‐cellular) and (carcinom* or cancer* or neoplasm* or malign* or tumo*)) or HCC) #8 #6 or #7 #9 #5 and #8 |
| MEDLINE (Ovid SP) | 1946 to July 2014 | #1 exp Ethanol/ad, tu [Administration & Dosage, Therapeutic Use] #2 exp Acetic Acid/ad, tu [Administration & Dosage, Therapeutic Use] #3 exp Injections/ #4 (((ethanol or alcohol or acetic acid or vinegar) and (inject* or ablati*)) or PEI or PAI or PAAI).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] #5 #1 or #2 or #3 or #4 #6 exp Liver Neoplasms/ #7 (((liver or hepatic or hepatocellular or hepato‐cellular) and (carcinom* or cancer* or neoplasm* or malign* or tumo*)) or HCC).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] #8 #6 or #7 #9 #5 and #8 #10 (random* or blind* or placebo* or meta‐analys*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] #11 #9 and #10 |
| EMBASE (Ovid SP) | 1974 to July 2014 | #1 exp alcohol/ad, do, th [Drug Administration, Drug Dose, Therapy] #2 exp acetic acid/ad, do, dt [Drug Administration, Drug Dose, Drug Therapy] #3 exp injection/ #4 (((ethanol or alcohol or acetic acid or vinegar) and (inject* or ablati*)) or PEI or PAI or PAAI).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] #5 #1 or #2 or #3 or #4 #6 exp liver tumor/ #7 (((liver or hepatic or hepatocellular or hepato‐cellular) and (carcinom* or cancer* or neoplasm* or malign* or tumo*)) or HCC).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] #8 #6 or #7 #9 #5 and #8 #10 (random* or blind* or placebo* or meta‐analys*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] #11 #9 and #10 |
| Science Citation Index Expanded | 1900 to July 2014 | #1 TS=(((ethanol or alcohol or acetic acid or vinegar) and (inject* or ablati*)) or PEI or PAI or PAAI) #2 TS=(((liver or hepatic or hepatocellular or hepato‐cellular) and (carcinom* or cancer* or neoplasm* or malign* or tumo*)) or HCC) #3 #2 AND #1 #4 TS=(random* or blind* or placebo* or meta‐analys*) #5 #4 AND #3 |
Data and analyses
Comparison 1. Percutaneous ethanol injection (PEI) versus percutaneous acetic acid injection (PAI).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival: all trials | 2 | 185 | Hazard Ratio (Random, 95% CI) | 1.47 [0.68, 3.19] |
| 1.1 Low risk of bias trial | 1 | 125 | Hazard Ratio (Random, 95% CI) | 1.13 [0.63, 2.03] |
| 1.2 High risk of bias trial | 1 | 60 | Hazard Ratio (Random, 95% CI) | 2.64 [0.81, 8.55] |
| 2 Recurrence‐free survival | 2 | 185 | Hazard Ratio (Random, 95% CI) | 1.42 [0.68, 2.94] |
| 2.1 Low risk of bias trial | 1 | 125 | Hazard Ratio (Random, 95% CI) | 1.02 [0.66, 1.57] |
| 2.2 High risk of bias trial | 1 | 60 | Hazard Ratio (Random, 95% CI) | 2.16 [1.11, 4.21] |
Comparison 2. Percutaneous ethanol injection (PEI) versus surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 1 | 76 | Hazard Ratio (Fixed, 95% CI) | 1.57 [0.53, 4.61] |
| 2 Recurrence‐free survival | 1 | 76 | Hazard Ratio (Fixed, 95% CI) | 1.35 [0.69, 2.63] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Huang 2005.
| Methods | Randomised clinical trial. Recruitment: August 1998 to December 2002. Country: Taiwan. |
|
| Participants | 76 participants: 38 PEI, 38 surgery. No cirrhosis/Child A/Child B: PEI 6/29/3, surgery 10/28/0. |
|
| Interventions | PEI vs. surgery. | |
| Outcomes | Primary outcome: tumour recurrence. Secondary outcome: overall survival. |
|
| Inclusion criteria |
|
|
| Exclusion criteria |
|
|
| Follow‐up | 12 to 59 months. | |
| Notes | There were 2 participants switching from PEI to surgery despite the randomisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random table. |
| Allocation concealment (selection bias) | High risk | Risk of pseudo‐randomisation. The next enrolled participant who fulfilled inclusion criteria was assigned to the group from which a participant had just been excluded. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Not described. Survival outcomes may not be significantly influenced by a lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All drop‐outs were properly reported and not included in the analysis. |
| Selective reporting (reporting bias) | Low risk | We judged this trial free of selective reporting. |
| Other bias | High risk | The authors stated that prior to the enrolment, participants and doctors understood the protocol and suggested that this could have affected the person's decision to participate in the trial. In our opinion, understanding of the protocol is crucial for participation in all clinical trials for both participants and doctors. People with tumour recurrence were treated by additional treatments, which could have approximated potential survival differences by the 2 compared modalities. Groups were equalised in number before closure of randomisation. It was not stated how many participants were affected by this non‐randomised measure. Participants that did not complete their allocated treatment were displaced by the next recruited participant without randomisation (6 participants). |
| Academic bias | Low risk | There were no randomised clinical trials on this subject performed by this group. |
| Overall risk assessment | High risk | Due to the issues in other bias, we judged this trial high risk of bias. |
Lin 2005.
| Methods | Randomised clinical trial. Recruitment: April 2000 to June 2002. Country: Taiwan. |
|
| Participants | 187 participants: 62 RFTA, 62 PEI, 63 PAI. Child A/Child B: RFTA 46/16, PEI 47/15, PAI 45/18. |
|
| Interventions | RFTA versus PEI versus PAI. | |
| Outcomes | Primary outcome: local recurrence. Secondary outcome: overall survival, cancer‐free survival. |
|
| Inclusion criteria |
|
|
| Exclusion criteria |
|
|
| Follow‐up | 4 to 44 months. | |
| Notes | 4.8% major complications in RFTA, 0% in the PAI and PEI groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation. |
| Allocation concealment (selection bias) | Low risk | List not available for treating physicians. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Not described. Survival outcomes may not be significantly influences by a lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no drop‐outs. |
| Selective reporting (reporting bias) | Low risk | We judged this trial free of selective reporting. |
| Other bias | Low risk | We did not detect any other potential bias. |
| Academic bias | Low risk | The same group performed another randomised clinical trial at the same time. Participants were assigned to 1 of the parallel trials depending on hospital admission during alternative weeks (Lin 2004). Previous retrospective trial on PEI for HCC. |
| Overall risk assessment | Low risk | Low risk of bias. |
Ohnishi 1998b.
| Methods | Randomised clinical trial. Recruitment: August 1993 to September 1995. Country: Japan. |
|
| Participants | 60 participants: PEI 29, PAI 31. Child A/Child B: PEI 21/8, PAI 20/11. 3 of 60 participants had chronic hepatitis without cirrhosis. |
|
| Interventions | Total ethanol versus 50% acetic acid under US guidance. | |
| Outcomes | Primary outcome: local recurrence. Secondary outcome: overall survival, hospital admission, non‐local recurrence, 1‐ and 2‐year mortality, 1‐ and 2‐year recurrence, 1‐ and 2‐year cancer‐free survival. |
|
| Inclusion criteria |
|
|
| Exclusion criteria |
|
|
| Follow‐up | 13 to 37 months. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes, but no information was given whether the envelopes were serially numbered. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Not described. Survival outcomes may not be significantly influenced by a lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no incomplete outcome data, no participant was withdrawn. |
| Selective reporting (reporting bias) | Low risk | We judged this trial free of selective reporting. |
| Other bias | Low risk | We did not detect any other potential bias. |
| Academic bias | Unclear risk | The first author published at least 2 retrospective PAI analysis previously (Ohnishi 1996a; Ohnishi 1996b). |
| Overall risk assessment | High risk | Due to the unclear random sequence generation, we judged this trial high risk of bias. |
HCC: hepatocellular carcinoma; PAI: percutaneous acetic acid injection; PEI: percutaneous ethanol injection; PTT: partial thromboplastin time; RFTA: radiofrequency thermal ablation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bottelli 1997 | Contact with the author was unsuccessful, not enough data retrievable. |
| Bruix 1998 | Advanced, not early HCC. |
| Danila 2009 | Case series. |
| Dettmer 2006 | Stratification in an interdisciplinary tumour‐board instead of randomisation. |
| Huo 2002 | Prospective, but not randomised trial. |
| Huo 2003 | Quasi‐randomised study. |
| Huo 2004 | Prospective survey, not randomised clinical trial. |
| Kirikoshi 2009 | Retrospective study. |
| Lencioni 2010 | Not a randomised clinical trial. |
| Orlando 1997 | Case‐control study instead of prospective randomised trial. |
| Peng 2010 | Not a randomised clinical trial. |
| Pompili 2011 | Not a randomised clinical trial. |
| Sarin 1994 | Investigation of large HCC. |
HCC: hepatocellular carcinoma.
Characteristics of studies awaiting assessment [ordered by study ID]
Tsai 2008.
| Methods | Prospective randomised trial. Recruitment: July 1998 to July 2004. Country: Japan. |
| Participants | 125 participants: 55 PEI, 70 PAI. Child A/Child B‐C: PEI 39/16 PAI 56/14. |
| Interventions | PEI versus PAI. |
| Outcomes | Local tumour recurrence, new tumour recurrence, overall survival, cancer‐free survival. |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Follow‐up | 2 to 110 months. |
| Notes | Randomisation was unclear. The authors reported a "...somewhat random manner by the operator...". Size of treatment groups differed strongly. People who were unresectable or did not agree to surgery were enrolled. No primary and secondary outcomes were determined, only outcomes in general. Contacting with the author was unsuccessful. |
HCC: hepatocellular carcinoma; PEI: percutaneous ethanol injection; INR: International Normalized Ratio; PAI: percutaneous acetic acid injection.
Characteristics of ongoing studies [ordered by study ID]
NCT00357422.
| Trial name or title | Multicenter, Randomized, Controlled Trial of the Effective Therapy for Multiple, Small HCCs: Comparing Transarterial Chemoembolization with Percutaneous Ethanol Injection Therapy. |
| Methods | Prospective randomised trial. Country: Korea. |
| Participants | 284 participants. |
| Interventions | PEI versus TACE. |
| Outcomes | Primary outcome: overall survival. Secondary outcome: disease‐free survival, recurrence. |
| Starting date | October 2005. |
| Contact information | Yoon JH. Seoul National University Hospital. |
| Notes | Completed. ClinicalTrials.gov identifier: NCT00357474. |
NCT00357474.
| Trial name or title | Prospective Randomized Trial of the Effective Therapy for Small, Solitary HCC Comparing Operation and Percutaneous Ethanol Injection Therapy. |
| Methods | Prospective randomised trial. Country: Korea. |
| Participants | Approximately 206 participants. |
| Interventions | PEI versus surgery. |
| Outcomes | Primary outcome: overall survival. Secondary outcome: disease ‐free survival, recurrence. |
| Starting date | October 2005 |
| Contact information | Yoon JH. Seoul National University Hospital. |
| Notes | Completed. ClinicalTrials.gov identifier: NCT00357422. |
HCC: hepatocellular carcinoma; PEI: percutaneous ethanol injection; TACE: transarterial chemoembolisation.
Differences between protocol and review
We did not search CancerLit as we did not expect to find further information. First studies for PEI safety appeared in 1989. Therefore, meeting abstracts were searched starting 1989. We changed the secondary outcomes: after obtaining statistical advice, we deleted the one‐, two‐, three‐, and five‐year survival and one‐, two‐, three‐, and five‐year recurrence‐free‐rates, as we did not expect to find any additional information.
Contributions of authors
Konrad Schoppmeyer and Sebastian Weis searched the literature, analysed the studies, prepared the protocol for the first meta‐analysis and the update. Annegret Franke advised and controlled the study analysis and updated the risk assessment. Annegret Franke also performed the GRADE evaluation and judged the quality of evidence. Thomas Berg reviewed the literature, included the ongoing trials and with Joachim Mössner critically reviewed and updated the manuscript. Wolfgang E. Fleig suggested the topic, accompanied the group during the whole reviewing and updating process, and reviewed the manuscript.
Sources of support
Internal sources
Kompetenznetz Hepatitis (Hep‐Net) Ostdeutschland, Germany.
Division of Gastroenterology, Rheumatology, Department of Internal Medicine, Dermatology and Neurology, University Hospital Leipzig, Germany.
External sources
No sources of support supplied
Declarations of interest
None known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Huang 2005 {published data only}
- Huang GT, Lee PH, Tsang YM, Lai MY, Yang PM, Hu RH, et al. Percutaneous ethanol injection versus surgical resection for the treatment of small hepatocellular carcinoma: a prospective study. Annals of Surgery 2005;242(1):36‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin 2005 {published data only}
- Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut 2005;54(8):1151‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ohnishi 1998b {published data only}
- Ohnishi K. Comparison of percutaneous acetic acid injection and percutaneous ethanol injection for small hepatocellular carcinoma. Hepato‐Gastroenterology 1998;45:1254‐8. [PubMed] [Google Scholar]
- Ohnishi K, Yoshioka H, Ito S, Fujiwara K. Prospective randomized controlled trial comparing percutaneous acetic acid and percutaneous ethanol injection for small hepatocellular carcinoma. Hepatology 1998;27(1):67‐72. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bottelli 1997 {published data only}
- Bottelli R, Iamoni G, Gelosa F, Caspani B, Cecconi P, Zavaglia C, et al. Interstitial laser photocoagulation (ILP) vs percutaneous ethanol injection (PEI) for treatment of hepatocellular carcinoma (HCC): preliminary results of a randomized pilot study [EASL abstract]. Journal of Hepatology 1997;26:S141. [Google Scholar]
Bruix 1998 {published data only}
- Bruix J, Llovet JM, Castells A, Montañá X, Brú C, Ayuso MC, et al. Transarterial embolization versus symptomatic treatment in patients with advanced hepatocellular carcinoma: results of a randomized, controlled trial in a single institution. Hepatology 1998;27(6):1578‐83. [DOI] [PubMed] [Google Scholar]
Danila 2009 {published data only}
- Danila M, Sporea I, Sirli R, Popescu A. Percutaneous ethanol injection therapy in the treatment of hepatocarcinoma ‐ results obtained from a series of 88 cases. Journal of Gastrointestinal and Liver Diseases 2009;18(3):317‐22. [PubMed] [Google Scholar]
Dettmer 2006 {published data only}
- Dettmer A, Kirchhoff TD, Gebel M, Zender L, Malek NP, Panning B, et al. Combination of repeated single‐session percutaneous ethanol injection and transarterial chemoembolisation compared to repeated single‐session percutaneous ethanol injection in patients with non‐resectable hepatocellular carcinoma. World Journal of Gastroenterology 2006;12(23):3707‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huo 2002 {published data only}
- Huo TL, Huang YH, Wu JC, Lee PC, Chang FY, Lee SD. Survival benefit of cirrhotic patients with hepatocellular carcinoma treated by percutaneous ethanol injection as a salvage therapy. Scandinavian Journal of Gastroenterology 2002;37(3):350‐5. [DOI] [PubMed] [Google Scholar]
Huo 2003 {published data only}
- Huo TI, Huang YH, Wu JC, Lee PC, Chang FY, Lee SD. Comparison of percutaneous acetic acid injection and percutaneous ethanol injection for hepatocellular carcinoma in cirrhotic patients: a prospective study. Scandinavian Journal of Gastroenterology 2003;38(7):770‐8. [DOI] [PubMed] [Google Scholar]
Huo 2004 {published data only}
- Huo T, Huang YH, Wu JC, Chiang JH, Lee PC, Chang FY, et al. Comparison of transarterial chemoembolization and percutaneous acetic acid injection as the primary loco‐regional therapy for unresectable hepatocellular carcinoma: a prospective survey. Alimentary Pharmacology and Therapeutics 2004;19(12):1301‐8. [DOI] [PubMed] [Google Scholar]
Kirikoshi 2009 {published data only}
- Kirikoshi H, Saito S, Yoneda M, Fujita K, Mawatari H, Uchiyama T, et al. Outcome of transarterial chemoembolization monotherapy, and in combination with percutaneous ethanol injection, or radiofrequency ablation therapy for hepatocellular carcinoma. Hepatology Research 2009;39(6):553‐62. [DOI] [PubMed] [Google Scholar]
Lencioni 2010 {published data only}
- Lencioni R, Crocetti L, Cioni D, Della Pina C, Oliveri F, Simone P, et al. Single‐session percutaneous ethanol ablation of early‐stage hepatocellular carcinoma with a multipronged injection needle: results of a pilot clinical study. Journal of Vascular and Interventional Radiology 2010;21(10):1533‐8. [DOI] [PubMed] [Google Scholar]
Orlando 1997 {published data only}
- Orlando A, Cottone M, Virdone R, Parisi P, Sciarrino E, Maringhini A, et al. Treatment of small hepatocellular carcinoma associated with cirrhosis by percutaneous ethanol injection: a trial with a comparison group. Scandinavian Journal of Gastroenterology 1997;32(6):598‐603. [DOI] [PubMed] [Google Scholar]
Peng 2010 {published data only}
- Peng ZW, Chen MS, Liang HH, Gao HJ, Zhang YJ, Li JQ, et al. A case‐control study comparing percutaneous radiofrequency ablation alone or combined with transcatheter arterial chemoembolization for hepatocellular carcinoma. European Journal of Surgical Oncology 2010;36(3):257‐63. [DOI] [PubMed] [Google Scholar]
Pompili 2011 {published data only}
- Pompili M, Nicolardi E, Abbate V, Miele L, Riccardi L, Covino M, et al. Ethanol injection is highly effective for hepatocellular carcinoma smaller than 2 cm. World Journal of Gastroenterology 2011;17(26):3126‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sarin 1994 {published data only}
- Sarin SK, Sreenivas DV, Saraya A, Mehta S, Lahoti D, Malhotra V. Improved survival with percutaneous ethanol injection in patients with large hepatocellular carcinoma. European Journal of Gastroenterology and Hepatology 1994;6(11):999‐1004. [Google Scholar]
References to studies awaiting assessment
Tsai 2008 {published data only}
- Tsai WL, Cheng JS, Lai KH, Lin CP, Lo GH, Hsu PI, et al. Clinical trial: percutaneous acetic acid injection vs. percutaneous ethanol injection for small hepatocellular carcinoma ‐ a long‐term follow‐up study. Alimentary Pharmacology and Therapeutics 2008;28(3):304‐11. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT00357422 {published data only}
- NCT00357422. Prospective randomized trial of the effective therapy for small, solitary HCC comparing operation and percutaneous ethanol injection therapy. clinicaltrials.gov/ct2/show/NCT00357422 (accessed 7 August 2014).
NCT00357474 {published data only}
- NCT00357474. A multicenter, randomized, controlled trial of the effective therapy for multiple, small hepatocellular carcinomas: comparing transarterial chemoembolization with percutaneous ethanol injection therapy. clinicaltrials.gov/ct2/show/NCT00357474 (accessed 7 August 2014).
Additional references
Abrishami 2008
- Abrishami A, Nasseri‐Moghaddam S, Eghtesad B, Sherman M. Surgical resection for hepatocellular carcinoma. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD006935] [DOI] [PubMed] [Google Scholar]
Arii 2000
- Arii S, Yamaoka Y, Futagawa S, Inoue K, Kobayashi K, Kojiro M, et al. Results of surgical and nonsurgical treatment for hepatocellular carcinomas: a retrospective and nationwide survey in Japan. Hepatology 2000;32:1224‐9. [DOI] [PubMed] [Google Scholar]
Awad 2009
- Awad T, Thorlund K, Gluud C. Cryotherapy for hepatocellular carcinoma. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007611.pub2] [DOI] [PubMed] [Google Scholar]
Begg 1994
- Begg CB, Mazumar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50(4):1088‐101. [PubMed] [Google Scholar]
Bismuth 2000
- Bismuth H, Majno PE. Hepatobiliary surgery. Journal of Hepatology 2000;32:208‐24. [DOI] [PubMed] [Google Scholar]
Bosch 2004
- Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology 2004;127:S5‐16. [DOI] [PubMed] [Google Scholar]
Bouza 2009
- Bouza C, López‐Cuadrado T, Alcázar R, Saz‐Parkinson Z, Amate JM. Meta‐analysis of percutaneous radiofrequency ablation versus ethanol injection in hepatocellular carcinoma. BMC Gastroenterology 2009;11(9):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61:763‐9. [DOI] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive ‐ trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [DOI] [PubMed] [Google Scholar]
Bruix 2001
- Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona‐2000 EASL Conference. Journal of Hepatology 2001;35:421‐30. [DOI] [PubMed] [Google Scholar]
Brunello 2008
- Brunello F, Veltri A, Carucci P, Pagano E, Ciccone G, Moretto P, et al. Radiofrequency ablation versus ethanol injection for early hepatocellular carcinoma: a randomized controlled trial. Scandinavian Journal of Gastroenterology 2008;43(6):717‐35. [DOI] [PubMed] [Google Scholar]
Calle 2003
- Calle EE, Rodriguez C, Walker‐Thurmond K, Thun MJ. Overweight, obesity, and mortality of cancer in a prospectively studied cohort of U.S. adults. New England Journal of Medicine 2003;348:1625‐38. [DOI] [PubMed] [Google Scholar]
Cho 2009
- Cho YK, Kim JK, Kim MY, Rhim H, Han JK. Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology 2009;49(2):453‐9. [DOI] [PubMed] [Google Scholar]
Coleman 2003
- Coleman MP, Gatta G, Verdeccia A, Esteve J, Sant M, Storm H, et al. EUROCARE‐3 summary: cancer survival in Europe at the end of the 20th century. Annals of Oncology 2003;14(Suppl 5):V128‐49. [DOI] [PubMed] [Google Scholar]
CTU 2011
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis. ctu.dk/tsa/ 2011 (accessed 27 November 2014).
EASL‐EORTC 2012
- European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer. EASL‐EORTC clinical practice guidelines: management of hepatocellular carcinoma. Journal of Hepatology 2012;56(4):908‐43. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analyses detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
El Serag 1999
- Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. New England Journal of Medicine 1999;340:745‐50. [DOI] [PubMed] [Google Scholar]
El Serag 2004a
- El‐Serag H. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology 2004;127:S27‐34. [DOI] [PubMed] [Google Scholar]
El Serag 2004b
- Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology 2004;126:460‐8. [DOI] [PubMed] [Google Scholar]
Galandi 2004
- Galandi D, Antes G. Radiofrequency thermal ablation versus other interventions for hepatocellular carcinoma. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD003046.pub2] [DOI] [PubMed] [Google Scholar]
Gluud 2005
- Gluud LL. Bias in Clinical Intervention Research [doctoral dissertation]. Copenhagen: Eget forlag, 2005. [Google Scholar]
Gluud 2014
- Gluud C, Nikolova D, Klingenberg SL, Alexakis N, Als‐Nielsen B, Colli A, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2014, Issue 6. Art. No.: LIVER.
Guyatt 2008
- Guyatt GH, Oxman AD, Vist G, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. Rating quality of evidence and strength of recommendations GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clinical Research Ed.) 2008;336:924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heffernan 2002
- Heffernan N, Webster K, Odom L, Martone M, Passik S, Bookbinder M, et al. Measuring health‐related quality of life in patients with hepatobiliary cancers: the functional assessment of cancer therapy ‐ hepatobiliary questionnaire. Journal of Clinical Oncology 2002;20(9):2229‐39. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice CFR & ICH Guidelines. Vol. 1, PA 19063‐2043, USA: Barnett International/PAREXEL, 1997. [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Lee 2009
- Lee YC, Cohet C, Yang YC, Stayner L, Hashibe M, Straif K. Meta‐analysis of epidemiologic studies on cigarette smoking and liver cancer. International Journal of Epidemiology 2009;38:1497‐511. [DOI] [PubMed] [Google Scholar]
Lencioni 1997
- Lencioni R, Pinto F, Bassi AM, Moretti M, Giulio M, Marchi S, et al. Long‐term results of percutaneous ethanol injection therapy for hepatocellular carcinoma in cirrhosis: an European experience. European Radiology 1997;7:514‐9. [DOI] [PubMed] [Google Scholar]
Lencioni 2003
- Lencioni RA, Allgaier HP, Cioni D, Olschewski M, Deibert P, Crocetti L, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio‐frequency thermal ablation versus percutaneous ethanol injection. Radiology 2003;228(1):235‐40. [DOI] [PubMed] [Google Scholar]
Lin 2004
- Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or =4 cm. Gastroenterology 2004;127(6):1714‐23. [DOI] [PubMed] [Google Scholar]
Livraghi 1995
- Livraghi T, Giorgio A, Marin G, Salmi A, Sio I, Bolodi L, et al. Hepatocellular carcinoma and cirrhosis in 746 patients: long‐term results of percutaneous ethanol injection. Radiology 1995;197(1):101‐8. [DOI] [PubMed] [Google Scholar]
Llovet 2000
- Llovet JM, Sala M, Castells L, Suarez Y, Vilana R, Bianchi L, et al. Randomized controlled trial of interferon treatment for advanced hepatocellular carcinoma. Hepatology 2000;31:54‐8. [DOI] [PubMed] [Google Scholar]
Lundh 2012
- Lundh A, Sismondo S, Lexchin J, Busuioc OA, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.MR000033.pub2] [DOI] [PubMed] [Google Scholar]
Macaskill 2001
- Macaskill P, Walter SD, Irwing L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20:641‐54. [DOI] [PubMed] [Google Scholar]
Machin 1997
- Machin D, Stenning SP, Parmar MK, Fayers PM, Girling DJ, Stephens RJ, et al. Thirty years of Medical Research Council randomized trials in solid tumours. Clinical Oncology 1997;9:100‐14. [DOI] [PubMed] [Google Scholar]
Mazzaferro 1996
- Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. New England Journal of Medicine 1996;334:693‐9. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
Ohnishi 1996a
- Ohnishi K, Yoshioka H, Ito S, Fujiwara K. Treatment of nodular hepatocellular carcinoma larger than 3 cm with ultrasound‐guided percutaneous acetic acid injection. Hepatology 1996;24:1379‐85. [DOI] [PubMed] [Google Scholar]
Ohnishi 1996b
- Ohnishi K, Nomura F, Ito S, Fujiwara K. Prognosis of small hepatocellular carcinoma (less than 3 cm) after percutaneous acetic acid injection: study of 91 cases. Hepatology 1996;23:994‐1002. [DOI] [PubMed] [Google Scholar]
Ohnishi 1998a
- Ohnishi K, Yoshioka H, Ito S, Fujiwara K. Prospective randomized controlled trial comparing percutaneous acetic acid and percutaneous ethanol injection for small hepatocellular carcinoma. Hepatology 1998;27(1):67‐72. [DOI] [PubMed] [Google Scholar]
Oliveri 2011
- Oliveri RS, Wetterslev J, Gluud C. Transarterial (chemo)embolisation for unresectable hepatocellular carcinoma. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD004787.pub2] [DOI] [PubMed] [Google Scholar]
Parkin 2001
- Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: GLOBOCAN 2000. International Journal of Cancer 2001;94:153‐6. [DOI] [PubMed] [Google Scholar]
Parkin 2005
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA: A Cancer Journal for Clinicians 2005;55(2):74‐108. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815‐34. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Ries 2007
- Ries LAG, Eisner MP, Kosary CL, Hankey BF, Miller BA, Clegg L, et al. SEER cancer statistics, 2007. seer.cancer.gov/csr/1975_2001/ (accessed 14 July 2014).
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Savovic 2012a
- Savovic J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Health Technology Assessment 2012;16(35):1‐82. [DOI] [PubMed] [Google Scholar]
Savovic 2012b
- Savovic J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Sersté 2012
- Sersté T, Barrau V, Ozenne V, Vullierme MP, Bedossa P, Farges O, et al. Accuracy and disagreement of computed tomography and magnetic resonance imaging for the diagnosis of small hepatocellular carcinoma and dysplastic nodules: role of biopsy. Hepatology 2012;55:800‐6. [DOI] [PubMed] [Google Scholar]
Shiina 2005
- Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology 2005;129(1):122‐30. [DOI] [PubMed] [Google Scholar]
Sørensen 2003
- Sørensen HT, Mellemkjær L, Jepsen P, Thulstrup AM, Baron J, Olsen JH, et al. Risk of cancer in patients hospitalized with fatty liver: a Danish cohort study. Journal of Clinical Gastroenterology 2003;36:356‐9. [DOI] [PubMed] [Google Scholar]
Takamori 2000
- Takamori R, Wong LL, Dang C, Wong L. Needle‐tract implantation from hepatocellular cancer: is needle biopsy of the liver always necessary?. Liver Transplantation 2000;6:67‐72. [DOI] [PubMed] [Google Scholar]
Thorlund 2009
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses. International Journal of Epidemiology 2009;38(1):276‐86. [DOI] [PubMed] [Google Scholar]
Thorlund 2010
- Thorlund K, Anema A, Mills E. Interpreting meta‐analysis according to the adequacy of sample size. An example using isoniazid chemoprophylaxis for tuberculosis in purified protein derivative negative HIV‐infected individuals. Clinical Epidemiology 2010;2:57‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorlund 2011
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA), 2011. ctu.dk/tsa/files/tsa_manual.pdf 2011 (accessed 14 July 2014).
Torzilli 2013
- Torzilli G, Belghiti J, Kokudo N, Takayama T, Capussotti L, Nuzzo G, et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations? An observational study of the HCC East‐West study group. Annals of Surgery 2013;257:929‐37. [DOI] [PubMed] [Google Scholar]
Weis 2013
- Weis S, Franke A, Mössner J, Jakobsen JC, Schoppmeyer K. Radiofrequency (thermal) ablation versus no intervention or other interventions for hepatocellular carcinoma. Cochrane Database of Systematic Reviews 2013, Issue 12. [DOI: 10.1002/14651858.CD003046.pub3] [DOI] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in a random‐effects meta‐analysis. BMC Medical Research Methodology 2009;9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman GD, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed.) 2008;336:601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yamamoto 2001
- Yamamoto J, Okada S, Shimada K, Okusaka T, Yamasaki S, Ueno H, et al. Treatment strategy for small hepatocellular carcinoma: comparison of long‐term results after percutaneous ethanol injection therapy and surgical resection. Hepatology 2001;34:707‐13. [DOI] [PubMed] [Google Scholar]
Yao 2002
- Yao FY, Ferrell L, Bass NM, Bacchetti P, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: comparison of the proposed UCSF criteria with the Milan criteria and the Pittsburgh modified TNM criteria. Liver Transplantation 2002;8(9):765‐74. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Schoppmeyer 2009
- Schoppmeyer K, Weis S, Mössner J, Fleig WE. Percutaneous ethanol injection or percutaneous acetic acid injection for early hepatocellular carcinoma. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD006745.pub2] [DOI] [PubMed] [Google Scholar]


