Immunotherapy treatment is promising for some patients; however, many patients are resistant. A synergistic effect of radiotherapy in combination with immunotherapy has been reported and is further explored in this article.
Keywords: Cancer, Radiotherapy, Immunotherapy, Abscopal effect, Radiologist
Abstract
Despite the promising efficacy of immunotherapy in some patients, many other patients are resistant. The synergistic effect of radiotherapy (RT) in combination with immunotherapy reported in case reports and clinical trials has piqued the interest of radiologists in investigating the underlying mechanisms and efficacy of the combination in preclinical and clinical trials. To date, the reported data are limited to small‐sized samples, trials lacking a comparison arm, and trials using diverse immunotherapies, various radiation doses, and fractionations. There are just a few studies comparing the efficacy of immunotherapy and radiotherapy to that of conventional therapies or different combinations. Radiologists should design and conduct clinical trials wisely to confirm the efficacy of the combination, particularly the abscopal effect, identify the best combination of various immunotherapeutic drugs and different radiation models for patients, identify the best sequence of the combination, determine the optimal timing of the combination, select the target site and volume, lower adverse effects, and explore predictive models to identify patients who may benefit from the combination therapy. We expect that these clinical trials performed by radiologists will offer definitive evidence for the wide use of the combination of RT and immunotherapy in clinical practice.
Implications for Practice.
This review will provide an update on the use of a combination of radiotherapy and immunotherapy, a cautious interpretation of preliminary results, and future directions for radiologists to perform well‐designed clinical trials.
摘要
尽管在一些患者身上免疫治疗的疗效颇具前景,但大量其他患者仍具有耐药性。根据病例报告和临床试验报告,放射治疗 (RT) 与免疫治疗联合使用的协同效应激发了放疗科医生在临床前试验和临床试验中研究该联合治疗潜在机制和疗效的兴致。迄今为止,报告的数据仅限于小型样本、无对照组的试验、以及采用多种免疫治疗、不同放射剂量和分割的试验。只有少数研究将免疫治疗和放射治疗的疗效与传统治疗或不同联合治疗的疗效进行了对比。放疗科医生应精心设计和实施临床试验,以确认联合治疗的疗效,特别是远端效应,确认患者各种免疫治疗药物和不同放射模型的最佳联合方案,确定联合治疗的最佳顺序,判断联合治疗的最佳时间,选择靶标部位和用量,减少不良反应,并探索预测模型,以确定有可能受益于联合治疗的患者。我们预计放疗科医生实施的这些临床试验将为在临床实践中广泛联合使用 RT 和免疫治疗提供坚实的基础。
实践意义:本篇综述将提供有关联合使用放射治疗和免疫治疗的最新信息,对初步结果的谨慎解释,以及放射科医生未来实施精心设计的临床试验的方向。
Introduction
Treatment with immunotherapy, particularly checkpoint blockades, induces durable responses in patients with various types of cancer. However, large proportions of patients are resistant to immunotherapy [1], [2], [3]. A treatment that synergizes with immunotherapy must be identified. In the well‐known case report published in 2012, the abscopal effect was observed on a female melanoma patient treated with radiotherapy and ipilimumab [4]. The report aroused the interest of radiologists to investigate the underlying mechanisms and effects of radiotherapy combined with immunotherapy in preclinical and clinical trials.
The Basis of Combining Radiotherapy with Immunotherapy
Proper localized radiotherapy (RT) with the appropriate doses and fractions may tip the balance in favor of antitumor immunity [5].
Radiation Requires an Immune Response
Mice with the same tumors received the same dose of radiation, and tumor regression occurred in wild type mice but not immunodeficient mice (nude) [6]. Using a highly aggressive B16 tumor model, significant tumor regression was observed in wild type mice treated with 20 Gy of radiation in one fraction [7]. Furthermore, the efficacy of ablative RT largely depended on the induction of type I interferon‐dependent immunity [5] and the production of CD8+ T cells [7].
In our previous study, tumor‐infiltrating lymphocytes (TILs) were screened in 62 patients with locally advanced rectal tumors by immunohistochemistry. Tumors with a high density of CD8+ TILs, CD4+ TILs, and low myeloid‐derived suppressor cells (MDSCs) were more sensitive to chemoradiotherapy (p = .022, .022, and .005, respectively) [8]. Based on these findings, the therapeutic effects of local radiation require an immune response in tumors.
The abscopal effect depends on the immune system. An abscopal (from the Latin word “ab scopus,” meaning away from the target) response describes the regression of metastatic tumors at sites distant to an irradiated field mediated by the immune response and is a rare event observed in patients with various types of metastatic tumors receiving palliative radiotherapy for a single metastasis [9], [10], [11]. Abscopal effects result from a cellular feedback mechanism involving effector T cell function, cytokine release, and MDSC deletion within the irradiated tumor microenvironment [12].
Radiation Influences Various Aspects of the Immune System
Radiation can enhance tumor antigen expression and antigen presentation. Radiation expands the intracellular peptide pool and enhances the surface expression of major histocompatibility complex (MHC) class I molecules to increase antigen presentation [13]. Radiation can stimulate the secretion of granulocyte‐macrophage colony‐stimulating factor (GM‐CSF), which can promote the MDSC migration and induce the MDSCs to differentiate into antigen‐presenting cells, then more antigen presentation occurs [14]. Radiation‐damaged dying and stressed cells increase the release of damage‐associated molecular patterns, including ATP, C‐type lectin receptors, and mobility group box 1 protein, to induce dendritic cells maturation [15].
According to the results of a gene expression array analysis, local RT increases the production of chemokines responsible for the enhanced recruitment of CD45+ hematopoietic cells into the tumor, including infiltrating dendritic cells with an enhanced functional capacity of tumor antigen cross‐presentation [5]. RT increases the release and recruitment of proinflammatory cytokines, such as reactive oxygen species, interleukin 1β, tumor necrosis factor, transforming growth factor β, interferon (IFN)‐γ, and fibroblast growth factor, that enhance antigen‐specific responses [13], [14]. RT can also increase the induction of many kinds of chemokines, such as CXC‐motif chemokines (CXCL9, 10, 11, and 16), resulting in chemotaxis of T cells into the tumor microenvironment [13], [14].
Radiation can change the tumor immune cell microenvironment. A single high dose of 30 Gy induces durable and complete remissions by altering the highly immunosuppressive microenvironment through an increase in CD8+ T cells and a loss of MDSCs in the stroma [16]. Reduced levels of circulating T cells observed before treatment in 21 patients with oligometastatic breast cancer compared to those of healthy controls were restored by the application of stereotactic body radiotherapy (SBRT) at a dose of 30 Gy in three fractions [17].
Radiation can function as an “in situ” vaccine. Cured mice harboring CT26 tumors that had been treated with 30 Gy of RT were challenged with a subcutaneous injection of 5.0 × 105 of the same tumor cells after 100–150 days, and 9 of 12 tumors were resolved after a brief increase in volume. Furthermore, T cells from those mice adoptively transferred the ability to effectively treat CT26 tumors, suggesting that the immune response generated by radiation possesses antitumor memory [16].
Radiation can induce the abscopal effect. The abscopal effect was observed in preclinical trials, case reports, and clinical trials [4], [9], [10], [11]. Molecular and cellular events generating the abscopal effect result from a cellular feedback mechanism involving effector T cells within the irradiated tumor microenvironment.
Radiation overcomes the resistance to programmed death‐1 (PD‐1)‐mediated blockade. Wang et al. observed the downregulation of the expression of the MHC complex and reduced infiltration and function of TILs in anti‐PD‐1‐resistant lung cancer. Localized RT at 36 Gy/3 fractions induced IFN‐β production, elevated MHC class I expression, and restored the responsiveness to anti‐PD‐1 therapy [18].
All the above‐mentioned data describe the positive effect of radiation on the immune response. However, radiation also activates a suppressive immune response by recruiting regulatory T cells, MDSCs, macrophages, and other stromal cells. Radiation‐induced tumor equilibrium has been reported [12]. Once the suppressive response dominates the tumor microenvironment, treatment resistance occurs. Thus, only immunotherapy that enhances the positive response or decreases the suppressive effect works synergistically with RT.
The Synergistic Effects of Combinations on Patients in Preclinical Trials
Despite the immune‐stimulating effects of local radiation, relapses often occur, and abscopal effects are rare, suggesting that radiation likely does not engage a sufficiently strong adaptive immune response to completely eliminate the tumor.
Synergistic effects of various combinations have been observed on different models. The combination of LIGHT, a tumor necrosis factor superfamily member that is homologous to lymphotoxin, with RT produces better control of metastasis [7]. In a lung metastatic cancer model, the combination of an anti‐PD‐1 treatment with radiation significantly reduced the growth of both the primary irradiated tumor and nonirradiated tumors and reduced spontaneous lung metastases [18]. Local radiotherapy increases the tumor cell expression of programmed death ligand 1 (PD‐L1) [19], [20]. Acquired resistance to radiation has been overcome by concurrent application of PD‐1/PD‐L1 blockers [19]. As shown in the study by Deng et al., a combination of ionizing radiation plus anti‐PD‐L1 treatments effectively controlled primary tumor growth and delayed the growth of distant tumors [20]. Combination treatments can generate a tumor‐specific memory immune response. Surviving mice exhibit complete tumor regression following treatment with radiotherapy and anti‐PD‐1/PD‐L1 therapy and inhibited tumor growth when rechallenged by the same tumor cells [18], even at a much higher dose [20]. Chimeric antigen receptor (CAR) T cell therapy is an emerging immunotherapy that has been approved for treating many kinds of tumors. In mice glioma models, natural killer group 2‐member D CAR T cells in combination with local RT produced stronger CAR T cell activity upon recognition of irradiated tumor cells and improved trafficking of CAR T cells to the tumor site, then resulted in synergistic antitumor activity [21].
In summary, preclinical studies highlight the potential of combination immunotherapy with radiation as a promising therapeutic approach against tumors. However, radiotherapy for humans is limited by many factors, the host immune system is much more complex in humans, and it is difficult to assess adverse effects in mice model. So the conversion of combination therapy into clinical use must be on the basis of clinical trials.
Preliminary Effect on Clinical Outcomes
To date, most reports about RT and checkpoint blockers have been retrospective studies or prospective studies of a small cohort (Table 1).
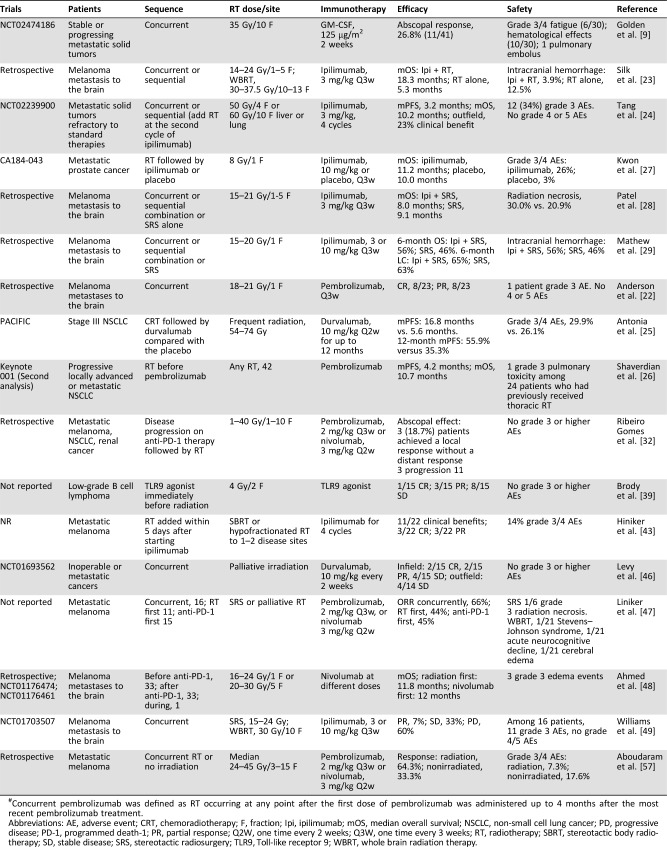
Table 1. Preliminary results from clinical trials.
Concurrent pembrolizumab was defined as RT occurring at any point after the first dose of pembrolizumab was administered up to 4 months after the most recent pembrolizumab treatment.
Abbreviations: AE, adverse event; CRT, chemoradiotherapy; F, fraction; Ipi, ipilimumab; mOS, median overall survival; NSCLC, non‐small cell lung cancer; PD, progressive disease; PD‐1, programmed death‐1; PR, partial response; Q2W, one time every 2 weeks; Q3W, one time every 3 weeks; RT, radiotherapy; SBRT, stereotactic body radiotherapy; SD, stable disease; SRS, stereotactic radiosurgery; TLR9, Toll‐like receptor 9; WBRT, whole brain radiation therapy.
According to a retrospective study, concurrent stereotactic radiosurgery (SRS) and the administration of pembrolizumab for melanoma brain metastases produces a much better response (8/23 with a complete response [CR] and 8/23 with a partial response [PR]) than SRS without concurrent immunotherapy (22%) [22]. The addition of ipilimumab to radiotherapy significantly prolongs the survival of patients with melanoma metastasis to the brain compared with RT alone (median overall survival: 18.3 months vs. 5.3 months) [23].
Few prospective trials reporting clinical outcomes are available. Data from a phase I study data examining the effects of the combination of ipilimumab with stereotactic ablative radiation therapy (SABR) on patients with metastatic solid tumors have been reported [24]. The median progression‐free survival (mPFS) and median overall survival were 3.2 months and 10.2 months, respectively [24]. Durvalumab was used as consolidation therapy for patients with stage III non‐small cell lung cancer (NSCLC) who did not display disease progression after platinum‐based chemoradiotherapy (CRT) and achieved a significantly longer mPFS (16.8 months) than the placebo (5.6 months) [25]. The response rate was higher with durvalumab (28.4%) than with placebo (16.0%) [25].
In the phase I KEYNOTE‐001 trial, 98 patients with progressive locally advanced or metastatic NSCLC received pembrolizumab. The progression‐free survival (PFS) and overall survival (OS) were significantly longer in patients who had previously received RT than in patients without previous RT (hazard ratio [HR] = 0.56, p = .019 and HR = 0.58, p = .026, respectively) [26].
However, not all combinations produce positive results. Seven hundred ninety‐nine patients with prostate cancer who presented with at least one bone metastasis resistant to castration and docetaxel treatment were randomly assigned to receive bone‐directed RT followed by either ipilimumab or placebo (CA184‐043). No significant difference in OS was observed [27]. Compared with SRS alone, ipilimumab and SRS treatment of melanoma brain metastases did not show superior effects on local control and OS [28], [29].
We speculate the potential reasons for why no positive results were reached in the above studies. First, in CA184‐043 trial, these patients with castration‐resistant prostate cancer may be less amenable to inhibition of suppressive T cell responses or be less “immunocompetent” or have more exuberant inhibitory T cell responses than patients with melanoma and perhaps patients with less heavily treated prostate cancer [30]. Second, the trials of ipilimumab and SRS versus SRS in treating melanoma brain metastases have some limitations. For example, nonrandomized treatment cohorts and small sample size may contribute to selection bias [28].
Last and most important, treatment‐resistance to combination really exists in some patients. The response rate to checkpoint blockades is only about 20%. Radiation activates both a positive and a suppressive immune response. Once the suppressive response dominates the tumor microenvironment, treatment resistance occurs [12]. Thus, only immunotherapy that is able to enhance the positive response‐derived by radiation can achieve better response than single treatment.
Abscopal Effects
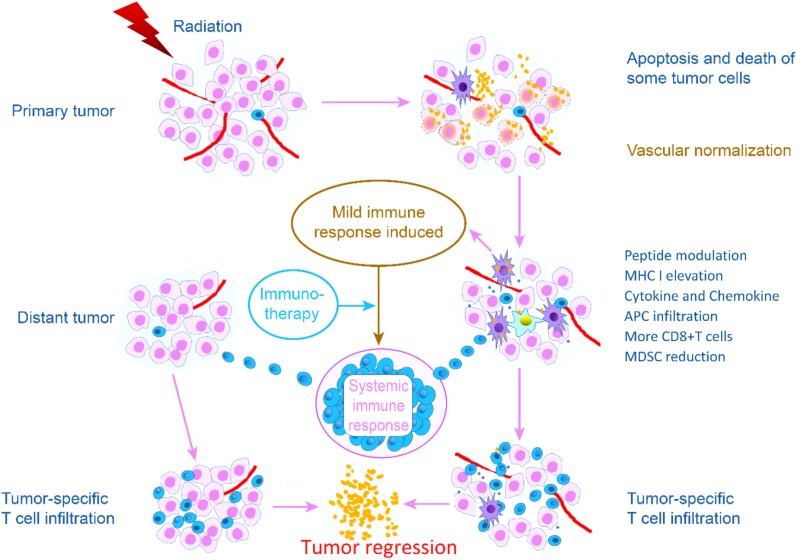
One main goal and challenge of radioimmunotherapy is to evoke abscopal effects [31]. The immunotherapy can enhance mild immune response initiated by RT. When a strong systemic immune response stimulated by the combination treatment inhibits the growth of tumor lesions in areas both targeted and not targeted by RT, an abscopal effect has occurred (Fig. 1).
Figure 1.
Radiotherapy (RT) initiates a mild antitumor immune response through various mechanisms. When immunotherapy induces a sufficiently strong response to inhibit the growth tumor lesions in areas targeted and not targeted by RT, an abscopal effect has occurred.Abbreviations: APC, antigen‐presenting cell; MDSC, myeloid‐derived suppressor cell; MHC I, major histocompatibility complex class I.
A female patient with melanoma had progressive enlargement of the pleural‐based paraspinal mass and new splenic lesions during ipilimumab treatment. She received palliative radiotherapy to the paraspinal mass at a dose of 28.5 Gy in three fractions (F). Abscopal effect was observed in lesions not targeted by radiotherapy [4]. Among 31 patients who received ipilimumab with SABR, three patients (10%) exhibited PR outside the radiation field and 7 (23%) experienced a clinical benefit [24]. For 12 patients with metastatic melanomas, 12 with NSCLC, and 2 with renal cancer, RT was added to anti‐PD‐1 therapy to treat disease progression. Three patients developed the abscopal effect [32].
In the murine model, vaccination with irradiated melanoma cells engineered to secrete GM‐CSF, a potent and powerful immune adjuvant to antigen‐presenting cell maturation, stimulated robust and long‐lasting antitumor immunity [33]. Forty‐one patients with stable or progressing metastatic solid tumors were treated with concurrent radiotherapy (35 Gy/10 F) at one metastatic site and GM‐CSF (125 μg/m2 daily for 2 weeks) [9]. Abscopal responses occurred in 11 (26.8%) of 41 patients. At our institute, a 67‐year‐old female patient with metastatic pancreatic cancer who was refractory to paclitaxel albumin treatment received 45 Gy/15 F delivered to the primary pancreatic tumor with concurrent GM‐CSF. She obtained an evident abscopal response following the administration of concurrent localized RT and GM‐CSF [34].
The Role of Radiologists in Applying the Combination of Radiation and Immunotherapy
Radioimmunotherapy has shown promising efficacy, particularly in the abscopal effect. However, most of the above reported results were obtained from case reports or small‐sized trials. Many questions remain to be addressed by radiologists to enable the combination to be widely used in clinical practice.
Rational Selection of the Radiotherapy Dose and Fractions
The use of radiation to promote antitumor immunity depends on the total dose and dose per fraction applied [35]. Diverse doses and fractions of radiotherapy have been employed in combination with immunotherapy.
Currently, SBRT with hypofractionated radiation is preferred in trials. According to the study by Filatenkov et al., single‐dose radiation (30 Gy) to mice with colon cancer decreases the number of MDSCs and increases the number of CD8+ T cells [16]. The administration of single 15‐ to 25‐Gy radiation doses in combination with immunotherapy exerts a synergistic effect on primary and distant tumors [11], [36]. Tyramide signal amplification mice with breast carcinoma received anti‐cytotoxic T‐lymphocyte‐associated protein‐4 antibody in combination with three distinct regimens of radiotherapy (20 Gy/1 F, 24 Gy/3 F, or 30 Gy/5 F). The highest effectiveness was obtained in the 8 Gy × three dose group [37]. Two patients with melanoma showed abscopal effects after treatment with radiation (28.5 Gy/3 F and 54 Gy/3 F) in combination with ipilimumab. Based on the aforementioned evidence, SBRT was recommended in combination with immunotherapy. A new concept even arose: immunotherapy and stereotactic ablative radiotherapy [38].
However, abscopal responses were observed in a phase I/II study investigating the efficacy of 4‐Gy/2‐F irradiation in combination with the injection of a Toll‐like receptor 9 (TLR9) agonist as a treatment for patients with low‐grade B cell lymphoma [39]. In our previous study, the numbers of CD8+ and CD4+ TILs were significantly increased by chemoradiotherapy with a radiation dose of 45–50.4 Gy/1.8–2.0 Gy [8]. In the PACIFIC trial, PFS was significantly longer when durvalumab was used as a consolidation therapy for patients with stage III NSCLC after concurrent CRT (conventional low‐dose fraction) [25].
Current paradigms for radioimmunotherapy are based on results from animal models or case reports. The interaction of radiotherapy with the host immune response is much more complex in humans. SBRT may exert a better effect on initiating the immune response. Conventional curative fractionated radiotherapy is delivered in relatively low doses of 1.5–2.2 Gy per day for a total dose of 60–80 Gy to obviate toxicity to normal tissues [12]. The safety of treatments with different fractions is a major consideration. To date, no definite clinical evidence has revealed which fraction regimen is better in combination with immunotherapy. Many trials have investigated the radiation dose and fractions for patients with different histologies or tumor stages. The search for the optimal dose and fraction in combination with a certain immunotherapy for a given cancer is a time‐consuming process for radiologists.
Careful Selection of Target Site and Volume of Radiation
SBRT was recommended in combination with immunotherapy [38]. However, SBRT has to be delivered with precise contouring of target, positioning, and motion control. The delivery of SBRT was limited by the tumor anatomical location and specific organs at risk. In most SBRT trials, the maximum number of metastases treated simultaneously has been three, probably because of safety issues related to the large target volumes [15]. Therefore, the target site and volume of RT need to be carefully assessed by the radiologists, especially for patients with multisite metastatic tumors.
Previous study showed that increased tumor burden correlates with decreased efficacy of PD‐1 immunotherapy [40]. As we know, radiation can increase antigen release, antigen presentation, and function as an in situ vaccine. In the condition of same radiation dose, bigger target volume decreases more tumor burden and causes more death cells. It is unclear if bigger target volume causes stronger immune response or if partial tumor volume irradiation would be sufficient with fewer side effects. So it is interesting to make clear the role of target volume in the combination therapy. A phase I study was carried out to evaluate the safety of pembrolizumab combing with multisite SBRT treating 68 patients with metastatic solid tumors [41]. Seventeen (25%) of the 68 patients with at least one metastasis >65 ml were partially treated with SBRT. Median gross tumor volume for partial tumor irradiation was 116.6 ml versus 7.2 ml for the metastases treated with complete tumor irradiation (p = .0001). Control at 3 months was not significantly statistically different between patients with at least one tumor partially irradiated and patients with tumors that were completely irradiated [41].
We must understand the above results with caution. First, it is a study with a small sample size. Second, only control at 3 months was compared. The follow‐up time is too short to offer the information of survival. Third, the initial tumor burden was different between patients with tumors partially irradiated and patients with tumors completely irradiated. The influence of target volume on combination needs to be carefully investigated in a large randomized trial with similar baselines of tumor burden in different cohorts.
Identification of the Appropriate Time Window To Add Radiation to Immunotherapy
Despite early promising data, important caveats remain when combining radiotherapy with immunotherapy. The timing and sequencing of treatments are likely to impact the efficacy [42].
Immunotherapy Followed by RT.
Immunotherapy is delivered prior to the start of radiotherapy to enable immunotherapy to exert an effect and more infiltrated TILs at the time of irradiation [43] (Fig. 2). A patient with melanoma presented with progression to liver metastases after two cycles of ipilimumab. Radiotherapy with 54 Gy in three fractions was applied to two liver lesions, followed by another two cycles of ipilimumab. The patient achieved CR and long‐time clinical benefit [44]. Eleven of 22 metastatic melanoma patients (50.0%) showed a clinical benefit from radiotherapy at one or two disease sites within 5 days after starting ipilimumab, including 3 CRs and 3 PRs [43]. Among 99 patients with metastatic melanoma, patients who received SRS for new brain metastases within 5.5 months after ipilimumab therapy had better intracranial disease control than those who received SRS later [45]. When RT was added as a treatment for disease progression to patients on anti‐PD‐1 therapy, 3 of 18 patients developed abscopal effects, and three patients developed a local response [32].
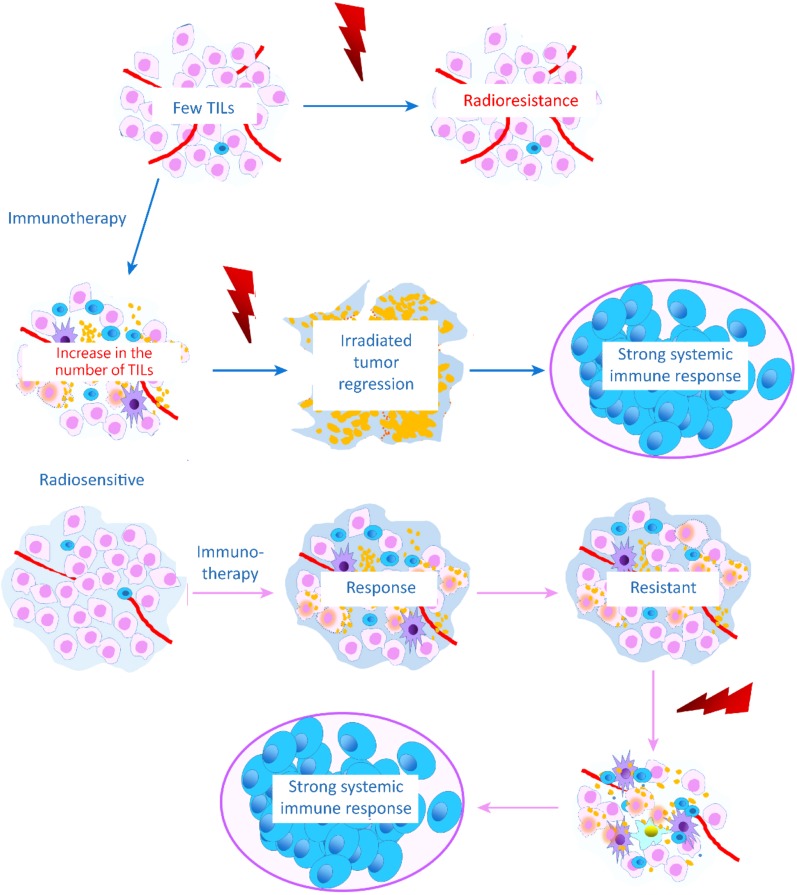
Figure 2.
The efficacy of radiotherapy (RT) requires an immune response. Patients who lack TILs may be resistant to RT. If immunotherapy is administered first to increase the number of TILs in the tumor, patients may become radiosensitive to RT and acquire more benefits from the combination. Patients who respond to immunotherapy may be treated with RT until resistance occurs to avoid overtreatment.Abbreviation: TIL, tumor‐infiltrating lymphocyte.
RT Followed by Immunotherapy.
RT is delivered prior to the start of immunotherapy to induce a mild radiation‐mediated response, and immunotherapy can then maximize the systemic immune response to eliminate the tumors (Fig. 1). In the PACIFIC trial, the administration of durvalumab as a consolidation therapy produced a significantly longer mPFS, a higher 12‐month PFS rate, a higher response rate, and longer duration of response than the placebo [25]. In the Keynote 001 trial, the median PFS and OS in 42 (43%) of 97 patients received RT before the first cycle of pembrolizumab. The median PFS and OS were 4.2 months and 10.7 months respectively, significantly better than patients who were not previously treated with RT [26].
Concurrent Combination.
Twenty‐three patients with melanoma brain metastatic lesions were treated with concurrent SRS and pembrolizumab. Marked regression was observed (CR: 8/23 and PR: 8/23) [22]. Concurrent durvalumab and palliative irradiation were employed to treat inoperable or metastatic cancer [46]. Two of fifteen patients with infield lesions achieved a CR, and four patients with infield lesions achieved a PR without abscopal effects on outfield lesions [46].
The justification of which sequence is better is difficult, based on the limited amount of data. The sequence of treatment may have little impact on efficacy. In a study of a small sample, patients with metastatic melanoma were treated with extracranial RT or SRS in combination either sequentially (RT then anti‐PD‐1, n = 11) or concurrently (n = 16), or with salvage RT for lesions progressing while the patient was treated with anti‐PD‐1 therapy (anti‐PD‐1 then RT, n = 15). No significant difference in the response was observed between the concurrent and sequential cohorts [47]. Ahmed et al. retrospectively analyzed patients receiving nivolumab and SRS using two nivolumab protocols: NCT01176461 and NCT01176474. No significant differences in OS and local control were observed between the group receiving radiation administered before nivolumab and the group receiving radiation after nivolumab [48].
The combination of RT and immunotherapy may have a synergistic effect. However, no final conclusion has been reached. Immunotherapy as a single treatment option has been approved for systemically treating many kinds of cancer. Mild immune response initiated by RT delivered initially without immunotherapy was not strong enough to treat metastatic cancers. Salvage RT delivered when patient become resistant to immunotherapy, CR, and long‐time clinical benefit could be achieved [44]. So it is acceptable that salvage radiotherapy delivered when patient become resistant to immunotherapy unless the clinical trials offer definitive data about the efficacy of the combination and sequence of treatment.
Safety
The combination of immunotherapy with radiotherapy is well tolerated by patients in most studies (Table 1). No grade 3 or higher adverse effects (AEs) were observed in patients treated with anti‐PD‐1 therapy and radiation [32], concurrent durvalumab and palliative irradiation [46], and concurrent TLR9 agonists with low‐dose radiation [39]. However, grade 3 or higher toxicity has been noted in some reports. When combining ipilimumab with SABR, 12 (34%) patients experienced grade 3 or higher treatment‐related toxicity [24]. In the PACIFIC trial, discontinuation occurred for 15.4% of patients who received durvalumab and 9.8% of patients who received the placebo [25]. In a phase I study, 11 of 16 patients receiving ipilimumab and radiotherapy reported grade 3 toxicities [49]. Radiation‐induced necrosis was observed in patients receiving treatment for melanoma metastasis to the brain [47]. Moreover, two patients with NSCLC developed nivolumab‐induced radiation recall pneumonitis that exactly matched the irradiated field after the administration of 60 Gy of thoracic RT [50]. Among 30 patients treated by the radiation and GM‐CSF, 6 patients experienced grade 3–4 fatigue, 10 patients experienced grade 3–4 haematological AEs, and 1 patient was hospitalized for pulmonary emboli [9].
The follow‐up period of most reported trials is not of a sufficient length to confirm long‐term AEs. More AEs may be reported in the long follow‐up results. The underlying mechanism of AEs is complex. Exploring potential methods to lower risks is an important and challengeable direction that would require careful testing in clinical trials.
Identification of a Model or Marker To Guide Patient Selection
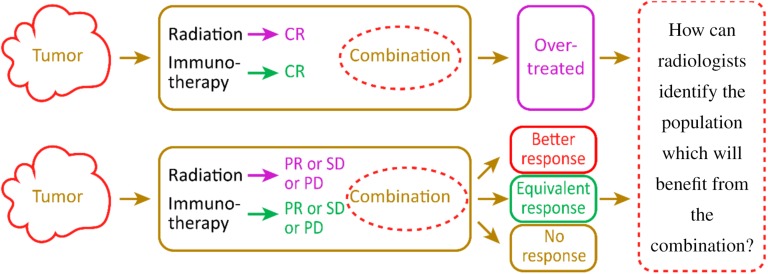
The combination of RT and immunotherapy may exert a synergistic effect. However, if patients can achieve CR by receiving only a single treatment with RT/immunotherapy, they may be overtreated by the combination at a substantial expense with a longer treatment time and more AEs (Fig. 3). Patients who achieved PR/stable disease/progressive disease after receiving single treatment may respond to the combination in three ways: a better response, equal response, or unequal response. Only some patients with a better response will achieve clinical benefits from the combination. A precise marker or model that can predict the response to immunotherapy or RT is still unavailable. Thus, the radiologist should attempt to identify a model that predicts the response to immunoradiotherapy.
Figure 3.
Only some patients who show a better response to the combination than treatment with a single agent will obtain clinical benefits from radioimmunotherapy. Most patients will be overtreated by the combination. A fundamental challenge is to identify a method to accurately identify patients who will derive a benefit from the combination.Abbreviations: CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
The clinical benefit of combining ipilimumab with radiation is associated with increases in the number of peripheral CD8+ T cells, the CD8+/CD4+ T cell ratio, and the proportion of CD8+ T cells expressing 4‐1BB and PD‐1 [24]. Following treatment with radiotherapy and GM‐CSF, patients with progressive disease and patients with stable disease showed different mean white blood cell counts (p = .033) and mean baseline neutrophil to lymphocyte ratios (p = .0062) [9]. Samples are conveniently acquired from peripheral blood cells. However, results obtained from these samples lack specificity, and the immune cells in the circulatory system do not accurately reflect the immune microenvironment in the tumor.
Diagnostic PD‐L1 assays were approved by the Food and Drug Administration to select patients for treatment with pembrolizumab [51]. PD‐L1 expression is correlated with a higher response to PD‐1/PD‐L1 checkpoint blockade [1], [2], [3], [52]. However, responses have also been observed in PD‐L1‐negative patients [1], [3], [53]. PD‐L1 expression before CRT is not correlated with a clinical benefit when durvalumab is used as a consolidation therapy after chemoradiotherapy for patients with stage III NSCLC [25]. Why does PD‐L1, the major mediator of the PD‐1/PD‐L1 pathway, fail to effectively predict the response to PD‐1/PD‐L1 blockade? One possible explanation is that PD‐L1 expression is dynamic. Patients lacking PD‐L1 expression who show a response in clinical trials may express PD‐L1 on tumor cells, but the levels are too low to be detected using modern tools.
Thus, the major barrier to predicting and monitoring the response to immunotherapy (checkpoint blockade) has been the lack of noninvasive tools to accurately assess dynamic checkpoint expression [54]. Mayer et al. [54] used small high‐affinity engineered protein scaffolds (64Cu‐radiolabeled high‐affinity consensus‐PD‐1 variants) to optimize noninvasive immuno‐positron emission tomography (PET) imaging of human PD‐L1 expression. Small high‐affinity engineered proteins used as immuno‐PET tracers exhibit favorable imaging and biodistribution properties in a preclinical model [54].
Our research team has performed several excellent studies using PET/computerized tomography (CT) to predict the response to different treatments, such as 11C‐PD153035 PET/CT to identify patients with refractory advanced NSCLC who are likely to respond to epidermal growth factor receptor tyrosine kinase inhibitors [55] and 18F‐RGD PET/CT to predict the sensitivity to concurrent CRT as early as 3 weeks after treatment initiation in patients with newly diagnosed glioblastoma [56]. We are exploring methods to predict the response to immunotherapy and immunoradiotherapy using special labeled radiotracer PET/CT methods.
Ongoing Clinical Trials
Several trials examining effects of immunoradiotherapy were searched and retrieved from www.clinicaltrials.gov (Table 2). The variety of immunotherapeutic agents and radiation doses/schedules produced many possible combinations. The checkpoint blockers ipilimumab, tremelimumab, pembrolizumab, nivolumab, durvalumab, and atezolizumab are the most frequently studied immunotherapeutic agents used in the clinic as a single or double treatment in combination with RT. One hundred forty‐six trials have investigated the effects and safety of checkpoint blockers in combination with radiation worldwide. Fifty‐seven trials have employed the combination of pembrolizumab with SBRT or frequent RT in patients with melanoma, NSCLC, head and neck cancer, cervical cancer, uterine cancer, sarcoma, lymphoma, and other solid cancers.
Table 2. Ongoing trials examining the effects of the combination of immunotherapy and radiotherapy on www.clinicaltrials.gov .
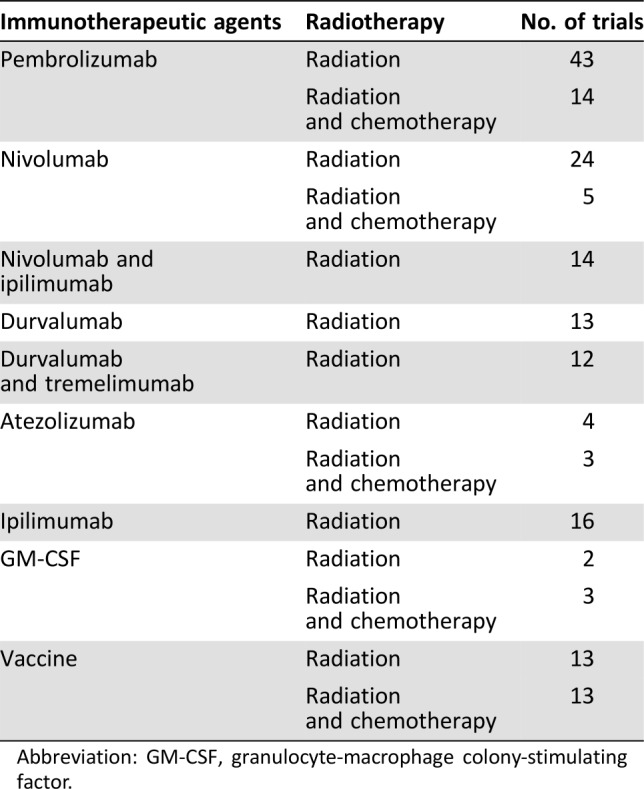
Abbreviation: GM‐CSF, granulocyte‐macrophage colony‐stimulating factor.
In China, four trials are currently recruiting participants, as registered on www.clinicaltrials.gov: a phase II trial of hypofractionated intensity‐modulated radiation therapy with temozolomide and GM‐CSF for patients with newly diagnosed glioblastoma multiforme (NCT02663440); a phase II trial to assess the efficacy and toxicity of hypofractionated carbon‐ion radiotherapy (40 Gy/5 fractions) with concurrent GM‐CSF for the treatment of hepatocellular carcinoma (NCT02946138); the observation of an abscopal effect of radiation (35 Gy/ 10 fractions) in combination with GM‐CSF on patients with metastatic non‐small cell lung cancer (NCT03113851); and a single‐arm multi‐center phase II study to assess the safety and abscopal effects of SBRT (50 Gy/5 fractions) in combination with GM‐CSF for patients with stage IV NSCLC who failed to respond to second‐line chemotherapy (NCT02623595).
Radiologists in China are positively designing trials combining immunotherapy with RT. We are planning to conduct two trials at multiple institutes: one observing the abscopal effect of hypofractionated radiation in combination with anti‐PD‐1 therapy on patients with oligometastatic solid tumors and a phase I study designed to assess the safety and efficacy of SBRT in combination with anti‐PD‐1 therapy for patients with stage I tumors.
To date, the reported data are limited to trials of small samples, a shorter follow‐up period, trials lacking a comparison arm, and trials using diverse immunotherapies, various radiation doses, and fractionations. Most studies have focused on metastatic melanoma, which is not prevalent in China. Conclusions based on evidence from case reports, retrospective analyses, or small‐sized prospective trials were not sufficiently strong to translate the combination of radiotherapy and immunotherapy into clinical practice or treatment guidelines. Randomized studies of large cohorts designed based on preliminary data from phase I/II studies are urgently needed to confirm the efficacy (abscopal effect) and safety of the combination in various tumors, particularly compared with conventional treatments. A fundamental challenge for radiologists is the rational selection of possible combinations with various types of immunotherapy [42] and diverse radiation doses and schedules. The designs of current clinical trials must support the implementation of combinations that have the potential to be translated into standard clinical care while contending with escalating financial costs and limited resources. Multicenter collaborations are strongly encouraged to avoid the duplication of efforts and cost.
Conclusion
Based on promising preliminary data, the combination of RT and immunotherapy is predicted to be an important treatment model in the future. To date, the reported data are limited to trials with a small sample, a shorter follow‐up period, a lack of a comparison arm, a use of diverse immunotherapies, and various radiation doses and fractionations. A comparison of the efficacy with conventional therapy or with different combinations is difficult. The radiologist should adequately address several questions before the combination is widely used in clinical practice.
First, clinical trials should be conducted using a wise design. Second, the best combination for patients should be chosen from various immunotherapeutic drugs and different radiation models. Third, the best sequence of the combination should be identified to which radiation will be added. Fourth, the prediction model should be established to determine the response to the combination. Fifth, the patient should be assisted in choosing the best and most appropriate treatment from options such as surgery, immunotherapy, chemotherapy, targeted therapy, chemoradiotherapy, and radioimmunotherapy.
Many trials are ongoing or in the design stage. Those well‐designed trials are expected to offer definite evidence that patients will acquire a real benefit from the combination treatment, with fewer adverse effects and at a lower cost.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (No. 81572970) and National Key R&D Program of China (2017YFC0107502).
Contributor Information
Ligang Xing, Email: xinglg@medmail.com.cn.
Jinming Yu, Email: sdyujinming@126.com.
Author Contributions
Conception/design: Xiangjiao Meng, Rui Feng, Lian Yang, Ligang Xing, Jinming Yu
Manuscript writing: Xiangjiao Meng, Rui Feng, Lian Yang, Ligang Xing, Jinming Yu
Final approval of manuscript: Xiangjiao Meng, Rui Feng, Lian Yang, Ligang Xing, Jinming Yu
Disclosures
The authors indicated no financial relationships.
References
- 1.Borghaei H, Paz‐Ares L, Horn L et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015;373:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herbst RS, Baas P, Kim DW et al. Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): A randomised controlled trial. Lancet 2016;387:1540–1550. [DOI] [PubMed] [Google Scholar]
- 3.Fehrenbacher L, Spira A, Ballinger M et al. Atezolizumab versus docetaxel for patients with previously treated non‐small‐cell lung cancer (POPLAR): A multicentre, open‐label, phase 2 randomised controlled trial. Lancet 2016;387:1837–1846. [DOI] [PubMed] [Google Scholar]
- 4.Postow MA, Callahan MK, Barker CA et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012;366:925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnette BC, Liang H, Lee Y et al. The efficacy of radiotherapy relies upon induction of type I interferon‐dependent innate and adaptive immunity. Cancer Res 2011;71:2488–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apetoh L, Tesniere A, Ghiringhelli F et al. Molecular interactions between dying tumor cells and the innate immune system determine the efficacy of conventional anticancer therapies. Cancer Res 2008;68:4026–4030. [DOI] [PubMed] [Google Scholar]
- 7.Lee Y, Auh SL, Wang Y et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: Changing strategies for cancer treatment. Blood 2009;114:589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teng F, Meng X, Kong L et al. Tumor‐infiltrating lymphocytes, forkhead box P3, programmed death ligand‐1, and cytotoxic T lymphocyte‐associated antigen‐4 expressions before and after neoadjuvant chemoradiation in rectal cancer. Transl Res 2015;166:721–732.e721. [DOI] [PubMed] [Google Scholar]
- 9.Golden EB, Chhabra A, Chachoua A et al. Local radiotherapy and granulocyte‐macrophage colony‐stimulating factor to generate abscopal responses in patients with metastatic solid tumours: A proof‐of‐principle trial. Lancet Oncol 2015;16:795–803. [DOI] [PubMed] [Google Scholar]
- 10.Demaria S, Ng B, Devitt ML et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys 2004;58:862–870. [DOI] [PubMed] [Google Scholar]
- 11.Kingsley DP. An interesting case of possible abscopal effect in malignant melanoma. Br J Radiol 1975;48:863–866. [DOI] [PubMed] [Google Scholar]
- 12.Weichselbaum RR, Liang H, Deng L et al. Radiotherapy and immunotherapy: A beneficial liaison? Nat Rev Clin Oncol 2017;14:365–379. [DOI] [PubMed] [Google Scholar]
- 13.Reits EA, Hodge JW, Herberts CA et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 2006;203:1259–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chajon E, Castelli J, Marsiglia, et al. The synergistic effect of radiotherapy and immunotherapy: A promising but not simple partnership. Crit Rev Oncol Hematol. 2017. Mar;111:124–132. [DOI] [PubMed] [Google Scholar]
- 15.Herrera FG, Bourhis J, Coukos G. Radiotherapy combination opportunities leveraging immunity for the next oncology practice. CA Cancer J Clin. 2017;67:65–85. [DOI] [PubMed] [Google Scholar]
- 16.Filatenkov A, Baker J, Mueller AM et al. Ablative tumor radiation can change the tumor immune cell microenvironment to induce durable complete remissions. Clin Cancer Res 2015;21:3727–3739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muraro E, Furlan C, Avanzo M et al. Local high‐dose radiotherapy induces systemic immunomodulating effects of potential therapeutic relevance in oligometastatic breast cancer. Front Immunol 2017;8:1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Schoenhals JE, Li A et al. Suppression of type I IFN signaling in tumors mediates resistance to anti‐PD‐1 treatment that can be overcome by radiotherapy. Cancer Res 2017;77:839–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dovedi SJ, Adlard AL, Lipowska‐Bhalla G et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD‐L1 blockade. Cancer Res 2014;74:5458–5468. [DOI] [PubMed] [Google Scholar]
- 20.Deng L, Liang H, Burnette B et al. Irradiation and anti‐PD‐L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014;124:687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiss T, Weller M, Guckenberger M et al. NKG2D‐based CAR T cells and radiotherapy exert synergistic efficacy in glioblastoma. Cancer Res 2018;78:1031–1043. [DOI] [PubMed] [Google Scholar]
- 22.Anderson ES, Postow MA, Wolchok JD et al. Melanoma brain metastases treated with stereotactic radiosurgery and concurrent pembrolizumab display marked regression; efficacy and safety of combined treatment. J Immunother Cancer 2017;5:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silk AW, Bassetti MF, West BT et al. Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med 2013;2:899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang C, Welsh JW, de Groot P et al. Ipilimumab with stereotactic ablative radiation therapy: Phase I results and immunologic correlates from peripheral T cells. Clin Cancer Res 2017;23:1388–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antonia SJ, Villegas A, Daniel D et al.; PACIFIC Investigators . Durvalumab after chemoradiotherapy in stage III non‐small‐cell lung cancer. N Engl J Med 2017;377:1919–1929. [DOI] [PubMed] [Google Scholar]
- 26.Shaverdian N, Lisberg AE, Bornazyan K et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non‐small‐cell lung cancer: A secondary analysis of the KEYNOTE‐001 phase 1 trial. Lancet Oncol 2017;18:895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwon ED, Drake CG, Scher HI et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration‐resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184‐043): A multicentre, randomised, double‐blind, phase 3 trial. Lancet Oncol 2014;15:700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel KR, Shoukat S, Oliver DE et al. Ipilimumab and stereotactic radiosurgery versus stereotactic radiosurgery alone for newly diagnosed melanoma brain metastases. Am J Clin Oncol 2017;40:444–450. [DOI] [PubMed] [Google Scholar]
- 29.Mathew M, Tam M, Ott PA et al. Ipilimumab in melanoma with limited brain metastases treated with stereotactic radiosurgery. Melanoma Res 2013;23:191–195. [DOI] [PubMed] [Google Scholar]
- 30.Schrand B, Verma B, Levay A et al. Radiation‐induced enhancement of antitumor T‐cell immunity by VEGF‐targeted 4‐1BB costimulation. Cancer Res 2017;77:1310–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trump D. Commentary on: “Ipilimumab versus placebo after radiotherapy in patients with metastatic castration‐resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184‐043): A multicentre, randomised, double‐blind, phase 3 trial.” Urol Oncol 2016;34:249–250.25907621 [Google Scholar]
- 32.Ribeiro Gomes J, Schmerling RA, Haddad CK et al. Analysis of the abscopal effect with anti‐PD1 therapy in patients with metastatic solid tumors. J Immunother 2016;39:367–372. [DOI] [PubMed] [Google Scholar]
- 33.Dranoff G, Jaffee E, Lazenby A et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte‐macrophage colony‐stimulating factor stimulates potent, specific, and long‐lasting anti‐tumor immunity. Proc Natl Acad Sci USA 1993;90:3539–3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi F, Wang X, Teng F et al. Abscopal effect of metastatic pancreatic cancer after local radiotherapy and granulocyte‐macrophage colony‐stimulating factor therapy. Cancer Biol Ther 2017;18:137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demaria S, Formenti SC. Radiation as an immunological adjuvant: Current evidence on dose and fractionation. Front Oncol 2012;2:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verbrugge I, Hagekyriakou J, Sharp LL et al. Radiotherapy increases the permissiveness of established mammary tumors to rejection by immunomodulatory antibodies. Cancer Res 2012;72:3163–3174. [DOI] [PubMed] [Google Scholar]
- 37.Dewan MZ, Galloway AE, Kawashima N et al. Fractionated but not single‐dose radiotherapy induces an immune‐mediated abscopal effect when combined with anti‐CTLA‐4 antibody. Clin Cancer Res 2009;15:5379–5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernstein MB, Krishnan S, Hodge JW et al. Immunotherapy and stereotactic ablative radiotherapy (ISABR): A curative approach? Nat Rev Clin Oncol 2016;13:516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brody JD, Ai WZ, Czerwinski DK et al. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: A phase I/II study. J Clin Oncol 2010;28:4324–4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang AC, Postow MA, Orlowski RJ, et al. T‐cell invigoration to tumour burden ratio associated with anti‐PD‐1 response. Nature 2017;545:60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luke JJ, Lemons JM, Karrison TG et al. Safety and clinical activity of pembrolizumab and multisite stereotactic body radiotherapy in patients with advanced solid tumors. J Clin Oncol. 2018;36:1611–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Day D, Monjazeb AM, Sharon E et al. From famine to feast: Developing early‐phase combination immunotherapy trials wisely. Clin Cancer Res 2017;23:4980–4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hiniker SM, Reddy SA, Maecker HT et al. A prospective clinical trial combining radiation therapy with systemic immunotherapy in metastatic melanoma. Int J Radiat Oncol Biol Phys 2016;96:578–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hiniker SM, Chen DS, Reddy S et al. A systemic complete response of metastatic melanoma to local radiation and immunotherapy. Transl Oncol 2012;5:404–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.An Y, Jiang W, Kim BYS et al. Stereotactic radiosurgery of early melanoma brain metastases after initiation of anti‐CTLA‐4 treatment is associated with improved intracranial control. Radiother Oncol 2017;125:80–88. [DOI] [PubMed] [Google Scholar]
- 46.Levy A, Massard C, Soria JC et al.Concurrent irradiation with the anti‐programmed cell death ligand‐1 immune checkpoint blocker durvalumab: Single centre subset analysis from a phase 1/2 trial. Eur J Cancer 2016;68:156–162. [DOI] [PubMed] [Google Scholar]
- 47.Liniker E, Menzies AM, Kong BY et al. Activity and safety of radiotherapy with anti‐PD‐1 drug therapy in patients with metastatic melanoma. Oncoimmunology 2016;5:e1214788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahmed KA, Stallworth DG, Kim Y et al. Clinical outcomes of melanoma brain metastases treated with stereotactic radiation and anti‐PD‐1 therapy. Ann Oncol 2016;27:434–441. [DOI] [PubMed] [Google Scholar]
- 49.Williams NL, Wuthrick EJ, Kim H et al. Phase 1 study of ipilimumab combined with whole brain radiation therapy or radiosurgery for melanoma patients with brain metastases. Int J Radiat Oncol Biol Phys 2017;99:22–30. [DOI] [PubMed] [Google Scholar]
- 50.Shibaki R, Akamatsu H, Fujimoto M et al. Nivolumab induced radiation recall pneumonitis after two years of radiotherapy. Ann Oncol 2017;28:1404–1405. [DOI] [PubMed] [Google Scholar]
- 51.Sul J, Blumenthal GM, Jiang X et al. FDA approval summary: Pembrolizumab for the treatment of patients with metastatic non‐small cell lung cancer whose tumors express programmed death‐ligand 1. The Oncologist 2016;21:643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reck M, Rodríguez‐Abreu D, Robinson AG et al. Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med 2016;375:1823–1833. [DOI] [PubMed] [Google Scholar]
- 53.Brahmer J, Reckamp KL, Baas P et al. Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med 2015;373:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mayer AT, Natarajan A, Gordon SR et al. Practical immuno‐PET radiotracer design considerations for human immune checkpoint imaging. J Nucl Med 2017;58:538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meng X, Loo BW, Jr., Ma L et al. Molecular imaging with 11C‐PD153035 PET/CT predicts survival in non‐small cell lung cancer treated with EGFR‐TKI: A pilot study. J Nucl Med 2011;52:1573–1579. [DOI] [PubMed] [Google Scholar]
- 56.Zhang H, Liu N, Gao S et al. Can an 18F‐ALF‐NOTA‐PRGD2 PET/CT scan predict treatment sensitivity to concurrent chemoradiotherapy in patients with newly diagnosed glioblastoma? J Nucl Med 2016;57:524–529. [DOI] [PubMed] [Google Scholar]
- 57.Aboudaram A, Modesto A, Chaltiel L et al. Concurrent radiotherapy for patients with metastatic melanoma and receiving anti‐programmed‐death 1 therapy: A safe and effective combination. Melanoma Res 2017;27:485–491. [DOI] [PubMed] [Google Scholar]