Abstract
Objectives
While bile duct brush cytology during endoscopic retrograde cholangiopancreatography (ERCP) is a well-established procedure for detecting malignant biliary stricture, its sensitivity is reportedly low. We aimed to determine the pre-ERCP factors affecting brush cytology sensitivity.
Methods
We retrospectively analyzed 185 patients who underwent brush cytology during the first ERCP for undiagnosed biliary stricture at our institution between January 2014 and December 2016. We analyzed the relationship of age, sex, final diagnosis, stricture location, tumor size, stricture length, total bilirubin level, white blood cell count, and C-reactive protein level with brush cytology sensitivity.
Results
The following conditions were established as final diagnoses: benign disease, 19 cases (10.3%); intrahepatic cholangiocarcinoma, 10 cases (5.4%); hilar cholangiocarcinoma, 38 cases (20.5%); extrahepatic cholangiocarcinoma, 44 cases (23.8%); pancreatic cancer, 55 cases (29.7%); other malignant tumors, 19 cases (10.3%). The sensitivity and specificity of brush cytology were 60.8% and 94.7%, respectively. The stricture length, total bilirubin level, and white blood cell count in true-positive cases were significantly higher than those in false-negative cases. Furthermore, a stratified analysis of the bilirubin levels demonstrated that sensitivity was highest in patients with moderate jaundice (80% for a total bilirubin level of 10-20 mg/dL), but significantly lower in patients with severe jaundice (total bilirubin level ≥20 mg/dL).
Conclusion
While the sensitivity of brush cytology increases with bilirubin levels of up to 20 mg/dL, severe jaundice has a negative effect on sensitivity, warranting additional pathological examinations according to the pre-ERCP bilirubin level.
Keywords: bile duct brush cytology, biliary stricture, endoscopic retrograde cholangiopancreatography, jaundice, malignant disease
Introduction
It is often difficult to differentiate between benign and malignant biliary strictures. Endoscopic retrograde cholangiopancreatography (ERCP) is needed for the diagnosis of most patients with biliary stricture. Bile duct brush cytology is a well-established diagnostic modality for distinguishing malignant strictures during ERCP for biliary stricture (1). Although aspiration cytology and transpapillary bile duct biopsy are also suitable modalities for making a histological diagnosis, brush cytology is more practical and thus continues to play an important role in diagnosing and assessing benign and malignant strictures (2,3). However, brush cytology is known to have low sensitivity. While substantial efforts has been expended in improve the sensitivity of brush cytology, these attempts have so far been unsuccessful. The few reports focused on identifying factors that affect brush cytology sensitivity have reported that tumor size and stricture length are correlated with sensitivity (4). However, it is unclear whether the sensitivity of brush cytology is affected by jaundice or by inflammation of the bile duct epithelium, which commonly occur in patients with biliary stricture. Thus, we retrospectively investigated our hospital’s records of brush cytology procedures performed for undiagnosed biliary stricture, evaluated the factors potentially associated with brush cytology sensitivity, and analyzed how jaundice and inflammation affect sensitivity.
Materials and Methods
This study was approved by the institutional review board of the International Medical Center affiliated with Saitama Medical University (approval no. 17-008) and conformed to the provisions of the Declaration of Helsinki (as revised in Fortaleza, Brazil, in October 2013). We retrospectively analyzed 1,473 ERCP procedures performed at Saitama Medical University International Medical Center, which is a cancer center, between January 2014 and December 2016. We extracted the bile duct brush cytology data pertaining to the first ERCP procedure performed for undiagnosed biliary stricture in each patient managed during the study period. The collected data included the age, sex, final diagnosis, stricture location, tumor size, stricture length, pre-ERCP total bilirubin (T-bil) level, white blood cell (WBC) count, C-reactive protein (CRP) level, and whether the pathological examination was negative or positive for malignancy. The final diagnosis was defined as the diagnosis established based on the pathological findings of biopsy or surgery, or as the diagnosis considered appropriate after at least 6 months of follow-up. The size of the tumor obstructing the bile duct was measured on computed tomography scans, and the stricture length was measured on endoscopic retrograde cholangiography (ERC) images. The T-bil level, WBC count, and CRP level were measured on the day before ERCP or on the day of ERCP.
For the histological diagnosis, aspiration cytology, brush cytology, and transpapillary biopsy were selectively performed depending on the situation and the ERC findings. Additionally, endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) was performed on a separate occasion in almost all patients with suspected pancreatic cancer.
A JF260V or TJF260V duodenoscope (Olympus, Tokyo, Japan) was used for ERCP. After inserting a cannula into the bile duct with a guidewire (VisiGlide2; Olympus), bile was aspirated for aspiration cytology, and transpapillary biopsy was performed as necessary at the appropriate time. For brush cytology, cells were collected by moving the wire-guided cytology brush back and forth 10-20 times at the location of the stricture. Two types of brushes were used, namely the Cytomax II® Double Lumen Cytology Brush (3 mm ×2.5 cm; Cook Medical, Bloomington, USA) and the RX Cytology Brush (2.1 mm ×2 cm; Boston Scientific, Natick, USA). There were no specific indications for selecting the cytology brush that was used. Brush cytology was performed only once per ERCP session for each patient, by a trainee under the supervision of an expert. Because our institute does not perform rapid on-site evaluation, the brush cytology specimens were immediately applied to slides, fixed with 95% ethanol, and sent to the pathology laboratory. To establish the pathological diagnosis, Papanicolaou, Giemsa, and periodic-acid-Schiff staining were performed by two laboratory technicians and two pathologists. For the purpose of the present analysis, a pathological diagnosis of “Suspicious for malignancy” or higher was considered a positive result for malignancy.
The patients with malignant disease were stratified according to whether the brush cytology result concurred with the final diagnosis (true-positive vs. false-negative group). Two-group comparisons of age, tumor size, stricture length, T-bil level, WBC count, and CRP level were conducted using the Mann-Whitney test. Fisher's exact probability test was used to analyze sex-specific differences. The strength of the associations between brush cytology sensitivity and various factors (stricture length, T-bil level, and WBC count) was analyzed using receiver operating characteristic curves. The distributions of the final diagnosis and stricture locations were compared using the Fisher-Freeman-Halton test. The assumption of a monotonous increase was evaluated using the Cochran-Armitage trend test. The groups obtained by stratification according to the T-bil levels were compared using Fisher’s exact probability test. The correlation between the T-bil level and stricture length was analyzed via Spearman’s rank correlation coefficient. SAS JMP version 12.2.0 and SAS version 9.1.3 SP4 (SAS Institute, Cary, USA) were used to perform the statistical analyses. p values of <0.05 were considered to indicate statistical significance.
Results
During the study period, brush cytology was first performed in 185 patients with confirmed or suspected biliary stricture, among whom 116 were male (62.7%; median age, 72 years; age range, 42-85 years) and 69 were female (37.3%; median age, 70 years; age range, 42-90 years). The final diagnosis was benign disease in 19 cases (10.3%) and malignant disease in 166 cases (89.7%; intrahepatic cholangiocarcinoma, 10/185, 5.4%; hilar cholangiocarcinoma, 38/185, 20.5%; extrahepatic cholangiocarcinoma, 44/185, 23.8%; gallbladder cancer, 12/185, 6.5%; pancreatic cancer, 55/185, 29.7%; ampullary carcinoma, 1/185, 0.5%; and other malignant tumors, 6/185, 3.2%) (Table 1).
Table 1.
Overview of Diagnoses and Brush Cytology Findings of Malignancy.
| Brush cytology result | ||||||
|---|---|---|---|---|---|---|
| Final diagnosis | Positive | Negative | Total | |||
| Benign disease | 1 | 18 | 19 | |||
| Autoimmune pancreatitis | 5 | |||||
| Benign stricture (e.g., postoperative inflammation) | 4 | |||||
| IgG4-related sclerosing cholangitis | 1 | 2 | ||||
| Tumor-forming pancreatitis | 2 | |||||
| Sphincter of Oddi dysfunction | 1 | |||||
| Primary sclerosing cholangitis | 1 | |||||
| Lemmel syndrome | 1 | |||||
| Mirizzi syndrome | 1 | |||||
| Chronic pancreatitis | 1 | |||||
| Malignancy | 101 | 65 | 166 | |||
| Intrahepatic cholangiocarcinoma | 7 | 3 | ||||
| Hilar cholangiocarcinoma | 18 | 20 | ||||
| Extrahepatic cholangiocarcinoma | 32 | 12 | ||||
| Gallbladder cancer | 9 | 3 | ||||
| Pancreatic cancer | 29 | 26 | ||||
| Ampullary carcinoma | 1 | |||||
| Hepatocellular carcinoma | 1 | |||||
| Metastatic cancer | ||||||
| Breast cancer (Hilar lymph node / Pancreas) | 3 (2 / 1) | |||||
| Colon cancer (Hilar lymph node) | 1 (1) | |||||
| Malignant lymphoma (Pancreas) | 1 | |||||
| Total | 102 | 83 | 185 | |||
Data represent number of endoscopic retrograde cholangiopancreatography reports analyzed (185 patients). Final diagnosis was defined as the diagnosis established via biopsy or surgery, or as the final diagnosis at the 6-month follow-up.
The sensitivity and specificity of brush cytology were 60.8% (101/166) and 94.7% (18/19), respectively. Further, the sensitivity of aspiration cytology and biopsy was 47.8% (76/159) and 53.5% (46/86), respectively (Table 2). Although the final diagnosis and background factors including age, sex, and stricture location were not significantly associated with the sensitivity of brush cytology, factors such as the T-bil level and WBC count were associated with sensitivity, and were significantly higher in the true-positive group than in the false-negative group (Table 3, Fig. 1). In our study, the stricture length was significantly longer in the true-positive group and was correlated with sensitivity; however, no difference in tumor size was observed between the true-positive and false-negative groups.
Table 2.
Diagnostic Performance Parameters of Pathological Examinations for the Detection of Biliary Strictures.
| Parameter | Brush cytology | Aspiration cytology | Biopsy | |||
|---|---|---|---|---|---|---|
| Number of examinations | 185 | 176 | 95 | |||
| Sensitivity | 60.8% | 47.8% | 53.5% | |||
| Specificity | 94.7% | 100% | 100% | |||
| Positive predictive value | 99.0% | 100% | 100% | |||
| Negative predictive value | 21.7% | 17.0% | 18.4% | |||
| Accuracy | 64.3% | 52.8% | 57.9% |
Table 3.
Patient Characteristics according to Brush Cytology Findings of Biliary Stricture.
| Characteristic | True-positive | False-negative | p value | |||||
|---|---|---|---|---|---|---|---|---|
| Number of patients | 101 | 65 | ||||||
| Age, years | Median (IQR) | 72 (66-78) | 68 (63-76) | 0.20 | ||||
| Sex | Male/Female | 67/34 | 35/30 | 0.14 | ||||
| Malignancy | 0.11 | |||||||
| Intrahepatic cholangiocarcinoma | 7 (6.9%) | 3 (4.6%) | ||||||
| Hilar cholangiocarcinoma | 18 (18.0%) | 20 (30.8%) | ||||||
| Extrahepatic cholangiocarcinoma | 32 (31.7%) | 12 (18.5%) | ||||||
| Gallbladder cancer | 9 (9.0%) | 3 (4.6%) | ||||||
| Pancreatic cancer | 29 (28.7%) | 26 (40.0%) | ||||||
| Ampullary carcinoma | 1 (1.0%) | 0 (0%) | ||||||
| Other | 5 (5.0%) | 1 (1.5%) | ||||||
| Stricture location | 0.41 | |||||||
| Intrahepatic | 3 (3.0%) | 1 (1.5%) | ||||||
| Hilar | 25 (24.8%) | 22 (33.8%) | ||||||
| Upper | 9 (8.9%) | 2 (3.1%) | ||||||
| Middle | 19 (18.8%) | 9 (13.8%) | ||||||
| Lower | 45 (44.6%) | 31 (47.7%) | ||||||
| Tumor size, cm | Median (IQR) | 3 (2-3) | 3 (2-4) | 0.77 | ||||
| Stricture length, cm | Median (IQR) | 3 (2-4) | 2 (2-3) | 0.046 | ||||
| T-bil levels, mg/dL | Median (IQR) | 10.5 (5.4-15) | 4.5 (1.2-13.9) | 0.01 | ||||
| WBC count, cells/μL | Median (IQR) | 6,330 (5,100-8,050) | 5,530 (4,110-7,000) | 0.007 | ||||
| CRP levels, mg/dL | Median (IQR) | 0.831 (0.361-2.379) | 0.654 (0.191-1.386) | 0.08 | ||||
Data shown as number (percentage) unless otherwise specified. A total of 166 patients with malignancy were included in this analysis. T-bil: total bilirubin, WBC: white blood cell, CRP: C-reactive protein, IQR: interquartile range
Figure 1.
The correlations between brush cytology sensitivity and the stricture length, total bilirubin level, and white blood cell (WBC) count. 95%CI: 95% confidence interval, AUC: area under the curve
Because the pre-ERCP T-bil level was correlated with the sensitivity of brush cytology, we divided the patients into six strata according to their T-bil level (<2 mg/dL; 2-5 mg/dL; 5-10 mg/dL; 10-15 mg/dL; 15-20 mg/dL; and ≥20 mg/dL) to further examine the relationship between jaundice and brush cytology sensitivity. The Cochran-Armitage trend test indicated that sensitivity showed a significant monotonous increase at T-bil levels of up to 20 mg/dL, with the highest sensitivity of 81.8% observed at T-bil levels of 15-20 mg/dL. However, while there were no significant differences in background factors between the true-positive and false-negative subgroups with T-bil levels of ≥20 mg/dL (Table 4), the sensitivity in this sub-group was significantly decreased to a remarkably low value of 42.9% (Fig. 2).
Table 4.
Characteristics of Patients with Severe Jaundice (T-bil Levels ≥20 Mg/dL), Stratified according to Brush Cytology Findings of Biliary Stricture.
| Characteristic | True-positive | False-negative | p value | |||||
|---|---|---|---|---|---|---|---|---|
| Number of patients | 9 | 12 | ||||||
| Age, years | Median (IQR) | 65 (60-78) | 68 (60-76) | 0.54 | ||||
| Sex | Male/Female | 6/3 | 8/4 | >0.99 | ||||
| Malignancy | 0.56 | |||||||
| Intrahepatic cholangiocarcinoma | 1 (11.1%) | 0 (0%) | ||||||
| Hilar cholangiocarcinoma | 3 (33.3%) | 5 (42%) | ||||||
| Extrahepatic cholangiocarcinoma | 2 (22.2%) | 1 (8.3%) | ||||||
| Gallbladder cancer | 1 (11.1%) | 0 (0%) | ||||||
| Pancreatic cancer | 2 (22.2%) | 5 (42%) | ||||||
| Ampullary carcinoma | 0 (0%) | 0 (0%) | ||||||
| Malignant lymphoma | 0 (0%) | 1 (8.3%) | ||||||
| Stricture location | >0.99 | |||||||
| Intrahepatic | 0 (0%) | 0 (0%) | ||||||
| Hilar | 4 (44.4%) | 4 (33%) | ||||||
| Upper | 1 (11.1%) | 1 (8.3%) | ||||||
| Middle | 1 (11.1%) | 2 (16.7%) | ||||||
| Lower | 3 (33.3%) | 5 (42%) | ||||||
| Tumor size, cm | Median (IQR) | 3 (2-4) | 3 (2-5) | 0.49 | ||||
| Stricture length, cm | Median (IQR) | 2 (2-3) | 3 (2-4) | 0.29 | ||||
| T-bil levels, mg/dL | Median (IQR) | 22.9 (21.2-27.2) | 21 (20.7-22.2) | 0.13 | ||||
| WBC count, cells/μL | Median (IQR) | 7,116 (5,760-10,010) | 7,000 (5,410-8,560) | 0.83 | ||||
| CRP levels, mg/dL | Median (IQR) | 2.764 (0.705-3.565) | 2.434 (0.927-5.516) | 0.52 | ||||
Data shown as number (percentage) unless otherwise specified. A total of 21 patients with malignancy and severe jaundice were included in this analysis. T-bil: total bilirubin, WBC: white blood cell, CRP: C-reactive protein, IQR: interquartile range
Figure 2.
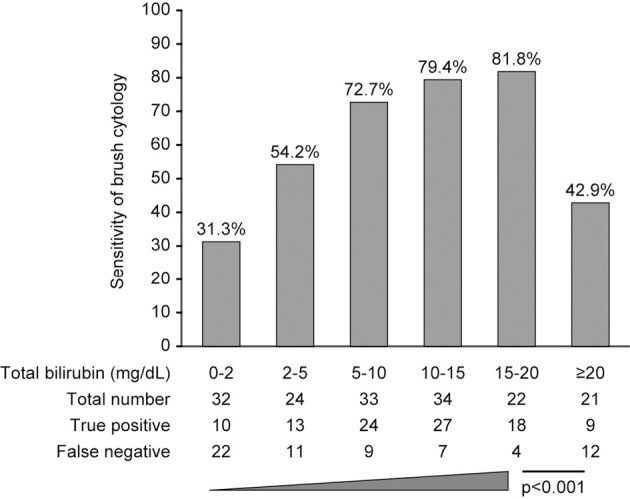
The relationship between brush cytology sensitivity and the total bilirubin levels.
Discussion
In this retrospective, single-center study evaluating the factors affecting the sensitivity of bile duct brush cytology in the diagnosis of undiagnosed biliary stricture, we found that brush cytology had the highest sensitivity among the three modalities evaluated, which was in agreement with previous reports. While brush cytology cannot be used on its own, we believe that it is the most practical modality for diagnosing malignant bile duct stricture.
Our hospital is a high-volume cancer center for patients with malignant biliary strictures. The present data reflect a single technique that was performed at a single institution using the same devices, with pathological results assessed by a single pathology facility. Thus, our findings are expected to correctly reflect the factors affecting the sensitivity of brush cytology.
Bile duct brush cytology has been widely adopted in clinical practice because it can be performed at the same time as endoscopic drainage for obstructive jaundice and because it is associated with few complications. However, many studies have reported that the sensitivity of brush cytology is 20-70% (5-14). In our patients the sensitivity was 61.2%, despite the fact that brush cytology was performed by a trainee. This is similar to the sensitivity reported by a previous retrospective investigation with a relatively large sample size (approximately 60%) (5). This indicates that the sensitivity of brush cytology is largely independent of the operator’s skill level.
Fogel et al. reported that using a device with a long brush did not have a significant effect on the sensitivity (7). Thus, although we did not investigate this particular aspect, we believe that the brush characteristics would have a negligible impact on the sensitivity. Advanced staining techniques such as p53 immunostaining and fluorescence in situ hybridization using various probes have been reported to increase the sensitivity (12,13,15), but the observed increase was negligible in comparison to the difficulties associated with applying these techniques in clinical practice. Performing EUS-FNA in patients in whom brush cytology is negative for malignant bile duct stricture was reported as a strategy to improve the sensitivity of histological examinations (14,16). However, considering the technical difficulty of EUS-FNA and the risks of complications and dissemination, this technique can only be used in a limited number of institutions and cases.
Mahmoudi et al. reported that the sensitivity of brush cytology increased with tumor size and stricture length (4). Parsi et al. reported an association between brush cytology sensitivity and advanced age, high T-bil levels, and the presence of a mass on cross-sectional imaging (11). In our study, we not only examined the effect of these previously reported factors, but also investigated the effect of inflammation. We found no significant differences between the true-positive and false-negative groups with regard to age or tumor size; however, stricture length, the T-bil level, and the WBC count were significantly higher in the true-positive group, confirming the association between these factors and the sensitivity of brush cytology (Fig. 1). Furthermore, although it seems reasonable that the diagnostic sensitivity of bile duct brush cytology would be higher in patients with cholangiocarcinoma than in those with pancreatic cancer or gallbladder cancer, we found no significant difference between the true-positive and false-negative groups regarding the distribution of malignant disease. The accumulation of further cases may be required to elucidate the reason for this result.
It is generally believed that a pathological diagnosis is more difficult to make in patients with inflammation because of the atypical changes caused by inflammation in the bile duct epithelium. However, in the present study, a high WBC count was associated with increased brush cytology sensitivity, and the CRP level also tended to affect sensitivity. While the WBC count and CRP level may not be directly related to inflammation of the bile duct epithelium, we hypothesize that tissue fragility caused by inflammation made it easier to collect more tissue. In addition, the presence of a progressive tumor that has deeply invaded the bile duct wall may cause an increased WBC count. Since a progressive tumor also causes more pronounced bile duct obstruction, this may explain why the sensitivity was higher in patients with a high WBC count. Although we did not note any procedure-related complications, brush cytology should be performed very carefully in patients with severe inflammation, in order to avoid aggravating the inflammation or causing perforation.
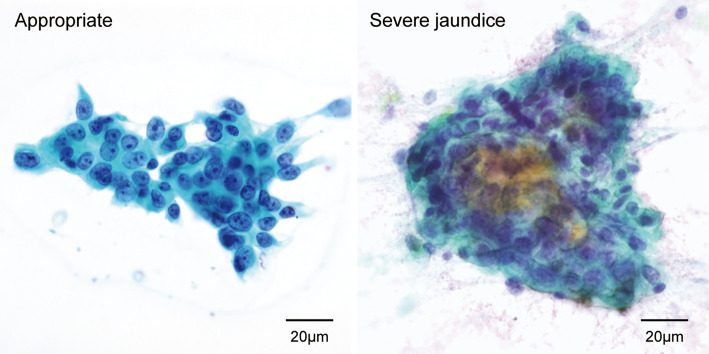
Longer strictures provide a larger area of contact with the brush and thus result in a more stable procedure, which may be why longer strictures were associated with a higher positive rate in patients who underwent brush cytology. We found a statistically significant but weak correlation between the T-bil level and stricture length (Fig. 3), suggesting that the T-bil level may not be a good marker of stricture length. Furthermore, in comparison to stricture length, the T-bil level showed a stronger correlation with brush cytology sensitivity (Fig. 1), which likely reflects the fact that the T-bil level is a better markers of bile obstruction and stricture duration. We found a monotonous increase in diagnostic sensitivity with increasing T-bil levels, with a surprising sensitivity of 80% at T-bil levels of up to 20 mg/dL, but with significantly lower sensitivity at T-bil levels of ≥20 mg/dL (severe jaundice). As advanced cell degeneration is more frequently observed in patients with severe obstructive jaundice (Fig. 4), this phenomenon may partially explain the difficulty in establishing a diagnosis of malignancy in these patients.
Figure 3.

A diagram of the correlations with stricture length and the total bilirubin level.
Figure 4.
Representative brush cytology specimens from patients with severe jaundice. Left: An adequate brush cytology specimen indicating adenocarcinoma. Cells with atypical nuclei of different sizes, disturbance in polarity, and clear nucleoli are easily observed. Right: An inadequate specimen obtained from a patient with malignant stricture and severe jaundice. Cells with large nuclei are seen as an aggregate, but the nuclei of the peripheral cells appeared condensed due to damage. Although the findings suggest malignancy, the diagnosis is unclear because the nuclei could not be accurately assessed.
The present study is associated with several limitations. In particular, this was a retrospective study, and thus the decision to perform brush cytology during ERCP may have been affected by the pre-treatment imaging results, ERC findings, the clinical course of the patient, or blood test results. Moreover, factors related to the brush cytology procedure, such as stroke length and any technical difficulties, could not be extracted from the clinical records. These aspects may have influenced the sensitivity of brush cytology and should be examined more precisely through prospective case-control studies. Furthermore, although we conducted a multiple logistic regression analysis to determine the factors affecting the sensitivity of brush cytology, we may have missed some clinically significant differences because of the limited sample size. Specifically, because of the small number of patients with high T-bil levels, it is possible that significant differences between the true-positive and false-positive groups could not be confirmed at T-bil levels of ≥20 mg/dL (Table 4). New findings may be obtained with the accumulation of data from more cases.
Based on our examination of factors affecting the sensitivity of bile duct brush cytology, we found that sensitivity increases with stricture length, the T-bil level, and the WBC count. Importantly, sensitivity increases with T-bil levels of up to 20 mg/dL, reaching a maximum of 80%, but decreases substantially once the T-bil level increases above 20 mg/dL (severe jaundice). These characteristics of brush cytology sensitivity manifested independently of malignancy type. Thus, if malignant disease is highly suspected in a patient with severe jaundice, it is strongly recommended pathological examinations, such as repeated aspiration cytology using an endoscopic nasobiliary catheter, be performed in addition to routine examinations such as brush cytology, which has the highest sensitivity among ERCP-based histological examinations. Otherwise, close monitoring is required.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We wish to express our gratitude to Dr. Ami Kawamoto for her kind support.
References
- 1.Osnes M, Serck-Hnsses A, Myren J. Endoscopic retrograde brush cytology (ERBC) of the biliary and pancreatic ducts. Scand J Gastroenterol 10: 829-831, 1975. [PubMed] [Google Scholar]
- 2.Anderson MA, Appalaneni V, Ben-Menachem T, et al. ; American Society for Gastrointestinal Endoscopy (ASGE) Standards of Practice Committee.. The role of endoscopy in the evaluation and treatment of patients with biliary neoplasia. Gastrointest Endosc 77: 167-174, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Eloubeidi MA, Decker GA, Chandrasekhara V, et al. ; American Society for Gastrointestinal Endoscopy (ASGE) Standards of Practice Committee.. The role of endoscopy in the evaluation and management of patients with solid pancreatic neoplasia. Gastrointest Endosc 83: 17-28, 2016. [DOI] [PubMed] [Google Scholar]
- 4.Mahmoudi N, Enns R, Amar J, et al. . Biliary brush cytology: factors associated with positive yields on biliary brush cytology. World J Gastroenterol 14: 569-573, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stewart CJ, Mills PR, Carter R, et al. . Brush cytology in the assessment of pancreatico-biliary strictures: a review of 406 cases. J Clin Pathol 54: 449-455, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harewood GC, Baron TH, Stadheim LM, Kipp BR, Sebo TJ, Salomao DR. Prospective, blinded assessment of factors influencing the accuracy of biliary cytology interpretation. Am J Gastroenterol 99: 1464-1469, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Fogel EL, deBellis M, McHenry L, et al. . Effectiveness of a new long cytology brush in the evaluation of malignant biliary obstruction: a prospective study. Gastrointest Endosc 63: 71-77, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Moreno Luna LE, Kipp B, Halling KC, et al. . Advanced cytologic techniques for the detection of malignant pancreatobiliary strictures. Gastroenterology 131: 1064-1072, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitajima Y, Ohara H, Nakazawa T, et al. . Usefulness of transpapillary bile duct brushing cytology and forceps biopsy for improved diagnosis in patients with biliary strictures. J Gastroenterol Hepatol 22: 1615-1620, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Levy MJ, Baron TH, Clayton AC, et al. . Prospective evaluation of advanced molecular markers and imaging techniques in patients with indeterminate bile duct strictures. Am J Gastroenterol 103: 1263-1273, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parsi MA, Deepinder F, Lopez R, Stevens T, Dodig M, Zuccaro G. Factors affecting the yield of brush cytology for the diagnosis of pancreatic and biliary cancers. Pancreas 40: 52-54, 2011. [DOI] [PubMed] [Google Scholar]
- 12.Smoczynski M, Jablonska A, Matyskiel A, et al. . Routine brush cytology and fluorescence in situ hybridization for assessment of pancreatobiliary strictures. Gastrointest Endosc 75: 65-73, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Gonda TA, Glick MP, Sethi A, et al. . Polysomy and p16 deletion by fluorescence in situ hybridization in the diagnosis of indeterminate biliary strictures. Gastrointest Endosc 75: 74-79, 2012. [DOI] [PubMed] [Google Scholar]
- 14.Weilert F, Bhat YM, Binmoeller KF, et al. . EUS-FNA is superior to ERCP-based tissue sampling in suspected malignant biliary obstruction: results of a prospective, single-blind, comparative study. Gastrointest Endosc 80: 97-104, 2014. [DOI] [PubMed] [Google Scholar]
- 15.Barr Fritcher EG, Voss JS, Brankley SM, et al. . An optimized set of fluorescence in situ hybridization probes for detection of pancreatobiliary tract cancer in cytology brush samples. Gastroenterology 149: 1813-1824.e1, 2015. [DOI] [PubMed] [Google Scholar]
- 16.Fritscher-Ravens A, Broering DC, Knoefel WT, et al. . EUS-guided fine-needle aspiration of suspected hilar cholangiocarcinoma in potentially operable patients with negative brush cytology. Am J Gastroenterol 99: 45-51, 2004. [DOI] [PubMed] [Google Scholar]