Abstract
Mitochondria play an important role in maintaining cardiac homeostasis by supplying the major energy required for cardiac excitation-contraction coupling as well as controlling the key intracellular survival and death pathways. Healthy mitochondria generate ATP molecules through an aerobic process known as oxidative phosphorylation (OXPHOS). Mitochondrial injury during myocardial infarction impairs OXPHOS and results in the excessive production of reactive oxygen species (ROS), bioenergetic insufficiency, and contributes to the development of cardiovascular diseases. Therefore, mitochondrial biogenesis along with proper mitochondrial quality control machinery, which removes unhealthy mitochondria is pivotal for mitochondrial homeostasis and cardiac health. Upon damage to the mitochondrial network, mitochondrial quality control components are recruited to segregate the unhealthy mitochondria and aberrant mitochondrial proteins for degradation and elimination. Impairment of mitochondrial quality control and accumulation of abnormal mitochondria have been reported in the pathogenesis of various cardiac disorders and heart failure. Here, we provide an overview of recent studies describing various mechanistic pathways underlying mitochondrial homeostasis with the main focus on cardiac cells. In addition, this review demonstrates the potential effects of mitochondrial quality control dysregulation in the development of cardiovascular disease.
Keywords: Mitochondrial quality control, Mitophagy, Proteasome, Cardiomyopathy, BAG3, Ischemia/Reperfusion Injury, Fission, Fusion
Introduction
Mitochondria play an important role in maintaining cellular homeostasis by generating energy and by regulating in part key signaling pathways involved in cell survival. Moreover, mitochondrial disorders have been associated with the development of many cardiovascular diseases such as atherosclerosis, ischemic heart disease, cardiac hypertrophy and heart failure [Chistiakov et al., 2017]. Mitochondria synthesize a large amount of energy via oxidation of carbohydrates, fats and amino acids through oxidative phosphorylation (OXPHOS) within the respiratory chain in which electron transport across the mitochondrial inner membrane results in the reduction of oxygen and subsequent production of ATP molecules [Narendra et al., 2011, Koene et al., 2009, Joncheere et al., 2012]. Patients with mitochondrial defects show deficiencies in ATP synthesis and energy metabolism [Koene et al., 2009]. Considering the high energy demand of the cardiac excitation-contraction cycle and the role of OXPHOS in supplying the cardiac muscle, patients with mitochondrial abnormalities are at a higher risk for developing heart disease, which is a leading cause of mortality among those patients [Holmgren et al., 2003, Rosca et al., 2013]. For example, hypertrophic cardiomyopathy was found in children with cytochrome c deficiency, which led to death before the age of 13 years [Holmgren et al., 2003].
Dysfunctional mitochondria have a diminished capacity for ATP synthesis and generate excessive amounts of reactive oxygen species (ROS). ROS are highly reactive by-products of mitochondrial respiration and its accumulation within cells damages cellular components such as DNA, carbohydrates, proteins and lipids leading to accelerated aging and cell death [Narendra et al., 2011, Koene et al., 2011]. Considering the high abundance of mitochondria in cardiac muscle, elevated levels of ROS result in chronic oxidative damage and contribute to the development of cardiovascular disease and potentially heart failure [Drummomd et al., 2011, Billia et al., 2011]. Impairment of mitochondrial quality control has been associated with contractile dysfunction and cardiovascular disease [Nakai et al., 2007]. In this regard, mitochondrial quality control machinery, which targets the unhealthy mitochondria or aberrant mitochondrial proteins for degradation and removal, plays a vital role in maintaining myocardial homeostasis [Chistiakov et al., 2017]. In fact, the accumulation of abnormal, enlarged mitochondria has been reported in myocardial tissue of patients with hypertrophic and dilated cardiomyopathy [Holmgren et al., 2003, Rosca et al., 2013].
Various intracellular pathways are involved in maintaining the quality of mitochondria. Degradation of damaged mitochondrial proteins usually occurs via the ubiquitin-proteasome system (UPS); whereas, under chronic pathological conditions, the entire mitochondrion is degraded by the autophagy-lysosomal pathway, termed mitophagy [Hammerling et al., 2014]. In these two pathways, substrates are covalently conjugated with a small protein called ubiquitin, in a ubiquitination process mediated by ubiquitin-handling enzymes. The ubiquitinated protein is then targeted for degradation and removal either by the UPS or mitophagy [Bragoszewski et al., 2017]. In this review, we describe recent discoveries regarding the mechanisms underlying the quality control of mitochondria in cardiac cells. Furthermore, we discuss how impairment in the functioning of mitochondrial quality control components may impact cardiac homeostasis leading to the development of cardiovascular complications.
1. Mitochondrial metabolism in heart
Oxidative phosphorylation is an aerobic metabolic process whereby electrons are transferred through an electron transport chain (ETC) across the mitochondrial inner membrane to reduce oxygen into water molecules. Simultaneously, protons are pumped into the mitochondrial intermembrane space against a concentration gradient (ΔpHm) [Perry et al., 2011, Koene et al., 2009]. The movement of electrons in the respiratory chain creates a negative charge inside the mitochondrial matrix termed the mitochondrial membrane potential (ΔΨm) [Logan et al., 2016]. Subsequently, the F1/F0 ATPase in complex V moves protons down the electrochemical gradient (ΔΨm and ΔpHm) into the matrix resulting in the synthesis of ATP molecules [Holmgren et al., 2003, Perry et al., 2011, Jonckeere et al., 2012]. Alterations in the mitochondrial respiratory chain have been associated with the development of cardiovascular disorders that can potentially advance to heart failure [Doenst et al., 2013]. For example, mitochondrial ETC defects have been implicated in the pathogenesis of diabetic cardiomyopathy in patients with diabetes mellitus [Berthiaume et al., 2017]. Furthermore, oxidative stress as a consequence of respiratory chain dysfunction is accompanied may be development of cardiac hypertrophy [Maulik et al., 2012].
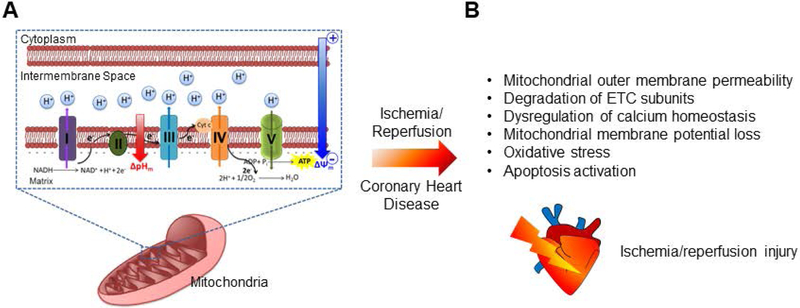
ΔΨm functions as a crucial driving force for ATP synthesis. Considering the abundance of mitochondria in the heart and the role of mitochondria in supplying ATP required for excitation-contraction coupling of cardiac cells [Griffiths et al., 2009], maintaining proper ΔΨm serves as an important indicator of cardiomyocyte health [Logan et al., 2016]. Damage to mitochondria by pathological stress results in ΔΨm collapse and energetic deficit leading to activation of cell death pathways and potential cardiovascular disorders [Perry et al., 2011, Jonckeere et al., 2012, Kuzmicic et al., 2011]. The extent of mitochondrial damage is suggested as a key factor when determining myocardial injury under ischemia-reperfusion toward progression to heart failure [Doenst et al., 2013, Niemann et al., 2017]. Ischemia-reperfusion injury as a consequence of coronary heart disease dramatically increases mitochondrial permeability leading to dissipation of electron and proton gradients, dysregulation of mitochondrial calcium homeostasis and release of superoxide and apoptosis, inducing factors which lead to myocardial cell death [Kang et al., 2017, Lesnefsky et al., 2017, Zhang et al., 2018]. Furthermore, mitochondrial super complexes lose their integrity as ETC subunits degrade under ischemia/reperfusion (IR), which leads to impairment of mitochondrial metabolism and enhanced ROS generation [Jang et al., 2017, Sepuri et al., 2017] (Figure 1).
Figure 1. Schematic of the mitochondrial oxidative phosphorylation process.
(A) Movement of electrons through the complexes (I-V) embedded in the mitochondrial inner membrane creates an electrical gradient (ΔΨm) whereby protons are simultaneously pumped into the intermembrane space creating a proton gradient (ΔpHm). Electrical and proton gradients together create an electrochemical force leading to the synthesis of ATP by F1/F0 ATP-synthase. (B) Ischemia/reperfusion damages mitochondrial respiration machinery leading to myocardial injury. ETC, electron transport chain; NADH, reduced NAD+; NAD+, nicotinamide adenine dinucleotide; H+, hydrogen; O2, oxygen.
2. Mitochondrial dynamics in heart
Mitochondria account for over 30% of the cardiac cell mass [Chen et al., 2011]. In neonatal cardiomyocytes, mitochondria are located throughout the cytosol; whereas, in the adult heart, mitochondria fall into one of three subpopulations: subsarcolemmal, myofibrillar and perinuclear mitochondria [Ong et al., 2017]. Upon damage to the mitochondrial network, mitochondria undergo structural and functional remodeling to replenish the damaged mitochondrial DNA (mtDNA) and dysfunctional units [Gegg et al., 2011, Niemann et al., 2017]. To this end, mitochondrial fusion and fission allow for the efficient distribution of mitochondrial protein and DNA through the mitochondrial network in the cell. Fission is often followed by fission, Mitochondria undergo fission where they divide into two daughter mitochondria, one with increased ΔΨm and high fusion affinity and the other with diminished ΔΨm and low fusion affinity [Ashrafi et al., 2013]. The depolarized less functional mitochondrion is then targeted by mitochondrial quality control machinery for degradation and clearance, while the bioenergetically active mitochondrion undergoes further regeneration and repair to maintain membrane potential [Narendra et al., 2011, Song et al., 2015].
Proteins involved in fission and fusion function within a signaling network to maintain mitochondrial homeostasis and play important roles in regulating cardiac response under pathological conditions such as ischemia-reperfusion, cardiomyopathy and heart failure [Kuzmicic et al., 2011]. Alterations in mitochondrial dynamics play a key role in the pathophysiology of various cardiac diseases [Niemann et al., 2017]. For example, mice with deficiency of the fission protein, dynamin-related protein 1 (Drp1), exhibited lethal dilated cardiomyopathy with ventricular wall thinning and reduced ejection fraction [Song et al., 2015]. Drp1 is highly expressed in the heart, is upregulated during stress [Chan et al., 2011] and translocates from the cytosol to the outer mitochondrial membrane to regulate mitochondrial dynamics [Ikeda et al., 2015]. Drp1 deletion led to mitochondrial enlargement and excessive activation of mitochondrial autophag (mitophagy) machinery [Song et al., 2015, Song et al., 2015]; and Drp1-deficient mice developed heart failure 6 to 7 weeks following gene silencing [Song et al., 2015]. By promoting autophagic degradation of damaged mitochondria, mitochondrial fission plays a protective role, however, excessive fission leads to mitochondrial mass loss, ATP deficits and apoptotic pathway activation [Zhou et al., 2017, Jin et al., 2018]. Drp1 functions with the Bcl-2 family proteins, BAX and BAK, to promote mitochondrial fragmentation, mitochondrial outer membrane permeabilization and cytochrome c release in response to apoptosis stimulation [Große et al., 2016]. Inhibition of Drp1 maintains mitochondrial integrity and plays a cardioprotective role during cardiac stress circumstances such as IR and cardiac arrest in cells by hindering excessive fission at the onset of reperfusion in [Ong et al., 2010, Sharp et al., 2014], murine [Ong et al., 2010, Sharp et al., 2015] and rat models [Tian et al., 2017, Disatnik et al., 2013]. Furthermore, reducing the levels of the mitochondrial fission factor, Mff, under hypoxia/reoxygenation conditions resulted in enhanced mitochondrial homeostasis as potential loss of mitochondrial membrane potential was recovered and apoptosis was decreased [Jin et al., 2018].
Mitochondrial fusion proteins, Mfn1 and Mfn2, reside on the outer mitochondrial membrane and ensure health of the mitochondrial network by regulating the fusion process [Ikeda et al., 2015]. Considering the enormous amount of energy required for myocardial contractility, the presence of an intact mitochondrial fusion network is critical for maintaining proper cardiac functioning [Kuzmicic et al., 2011]. In neonatal cardiac cells, mitochondria are mobile; whereas, in adult cardiac cells, mitochondria are relatively static and have limited motility [Ong et al., 2017]. Fission and fusion dynamics in post-mitotic myocytes in adult heart remains controversial; however, fission/fusion proteins are essential for mitochondria to adjust their metabolism to meet the energy demands of cardiac muscle and disruption in these pathways can result in cardiovascular complications [Chen et al., 2011, Song et al., 2015]. Suppression of fusion proteins including in a Drosophila model led to dilated cardiomyopathy and contractile abnormality [Dorn et al., 2011]. Deletion of fusion proteins, Mfn1/Mfn2, in mouse heart resulted in abnormal mitochondrial morphology and mitochondrial fragmentation leading to ventricular wall thickening and increase in cardiac mass (>30%) accompanied by symptoms of eccentric hypertrophy [Song et al., 2015].
3. Mitochondrial quality control in heart
The autophagy-lysosome system and UPS are the two major proteolytic pathways that degrade intracellular substrates to maintain protein homeostasis [Minoia et al., 2014]. Deficiency or dysregulation of protein quality control pathways have been implicated in muscle degeneration in dystrophic patients [Wattin et al., 2018]. Clearance of misfolded proteins occurs in large part by the UPS, while protein aggregates and damaged organelles are mostly degraded by the autophagy-lysosomal pathway [Liu et al., 2013]. Damaged mitochondrial proteins are degraded via the UPS, while elimination of the entire mitochondrion under chronic stress selectively occurs via mitophagy [Hammerling et al., 2014]. Failure in the function of the mitochondrial quality control machinery and mitophagy have been reported in the pathogenesis of heart failure [Doenst et al., 2013].
3.1. Mitochondrial degradation via proteasome
Degradation of damaged mitochondrial proteins occurs via the proteasome. UPS components are localized on mitochondria and are key regulators of mitochondrial dynamics. Inhibition of proteasome activity can cause mitochondrial function abnormalities [Bragoszewski et al., 2017]. In this process, damaged proteins are tagged with ubiquitin proteins through the action of an E1 ubiquitin-activating enzyme, an E2 ubiquitin-conjugating enzyme, and an E3 ubiquitin ligase and targeted for elimination via the proteasome [Ciechanover et al., 2017]. Accumulation of polyubiquitinated substrates has been observed in heart tissue of patients with cardiac diseases such as cardiomyopathy and heart failure [Nishida et al., 2017]. Furthermore, impairment of UPS function has been detected in heart tissues of patients with hypertrophic cardiomyopathy and heart failure; while no changes in the protein content of the UPS subunits were observed [Predmore et al., 2009]. Enhancement of cardiac proteasome proteolytic function has been demonstrated to play a protective role against pathophysiology of proteinopathy and ischemiareperfusion injury in mice [Li et al., 2011].
Proteasome-dependent degradation is an important pathway for quality control of mitochondrial fission and fusion proteins [Bragoszewski et al., 2017]. In this regard, Parkin-mediated degradation of mitochondrial proteins via proteasomal activation has been reported in several research studies [Chan et al., 2011, Yoshii et al., 2011]. Upon mitochondrial membrane potential, Parkin translocates to mitochondria, ubiquitinates mitochondrial outer membrane proteins [Narendra et al., 2008, Bragoszewski et al., 2017], and by recruiting proteasome components to mitochondria activates UPS [Chan et al., 2011, Hammerling et al., 2014]. Also, the fission protein, Drp1 is ubiquitinated by Parkin and targeted for degradation through the proteasome in neurons. On the other hand, Parkin suppression resulted in the accumulation of Drp1 and mitochondrial fragmentation [Wang et al., 2011]. However, fewer studies have been performed investigating the role of proteasome in degradation of mitochondrial proteins in cardiac cells.
Autophagy-lysosome and UPS pathways are interrelated such that inhibition of proteasome activity leads to activation of the autophagy-lysosome pathway [Liu et al., 2013, Tahrir et al., 2017]. Under pressure overload, inhibition of proteasome activity by preventing cardiac remodeling played a protective role and inhibited progression of heart failure in a mouse model [Hedhi et al., 2009]. In addition, proteasome inhibition reduced the size of myocardial infarction after reperfusion injury [Pye et al., 2003]. However, whether protective effects of proteasome inhibition under stress conditions arise from activation of the autophagy-lysosomal system remains to be determined.
3.2. Mitochondrial degradation via mitophagy
Autophagy is a catabolic process whereby the degradation of non-essential or dysfunctional cytoplasmic constituents preserves cellular ATP levels and allows cells to survive under adverse nutritional conditions [Yoshii et al., 2011, Zhang et al., 2014]. Under pathological conditions, cells protect themselves by activating survival pathways such as autophagy; however, chronic pathological conditions trigger apoptotic and necrotic pathways [Scherz-Shouval et al., 2007]. Autophagy upregulation in mouse atrial HL-1 cardiomyocytes and human AC16 cells under mitochondrial stress restored ΔΨm and mitochondrial respiration [Dutta et al., 2013]. During mitophagy, defective mitochondria are tagged with ubiquitin chains and engulfed into double-layered vesicles, autophagosomes, and ultimately delivered to the lysosome for degradation and removal [Padman et al., 2013, Yoshii et al., 2011, Matsuda et al., 2015]. Accumulation of damaged mitochondria due to mitophagy impairment is associated with oxidative stress, reduced respiration and activation of death pathways in cardiomyocytes [Tahrir et al., 2018]. Mitophagy- has been extensively studied in cardiac cells, suggesting the importance of mitophagy pathway in maintaining mitochondrial quality control in the heart [Song et al., 2015, Chen et al., 2013, McWilliams et al., 2018]. Various mechanisms of mitophagy in cardiac cells are reviewed below.
3.2.1. Parkin-mediated mitophagy
The parkin gene encodes an E3 ubiquitin ligase composed of 465 amino acids [Truban et al., 2017]. Parkin, through interaction with E2 ubiquitin-conjugating enzymes, promotes ubiquitination of mitochondrial proteins and targets them for degradation and removal [Chan et al., 2011, Lee et al., 2010]. Parkin translocation to the mitochondria is PINK1 (PTEN-induced putative kinase 1) dependent [Vives-Bauza et al., 2010], such that PINK1 phosphorylates Parkin and ubiquitin that is a signal for Parkin recruitment and subsequent ubiquitination [Chen et al., 2013, Lazarou et al., 2015, Kane et al., 2014, Kazlauskaite et al., 2014, Koyano et al., 2014] of other proteins including Mfn2. Ubiquitination then allows for binding of mitophagy proteins such as p62/SQSTM1 and LC3 followed by cargo sequestration within the autophagosome and elimination of dysfunctional mitochondria via the lysosome [Hammerling et al., 2014, Narendra et al., 2011, Gegg et al., 2011]. Parkin deficiency in mice does not alter mitochondrial respiration or cardiac function under basal condition, suggesting that Parkin-mediated mitophagy may not be essential for cardiac function [Kubli et al., 2013, Song et al., 2015]. However, mice deficient in Parkin-deficient in the hearts showed decreased cardiac function and larger infarct size compared to control mice after myocardial infarction [Kubli et al., 2013]. Parkin-mediated mitophagy maintained mitochondrial homeostasis and thereby prevented heart failure progression in mice with transverse aortic constriction [Wang et al., 2018] and in rats with injured hearts post MI [Qiao et al., 2018].
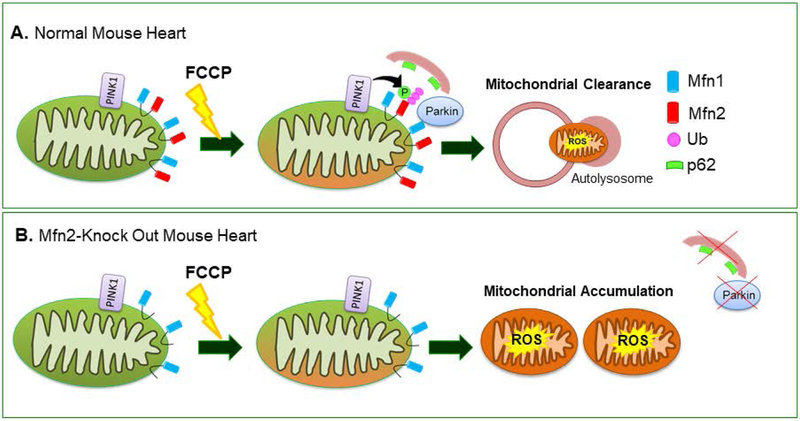
Mitochondrial outer membrane protein, Mfn2 functions as a substrate for PINK1 phosphorylation and phosphorylated Mfn2 is ubiquitinated by Parkin and subsequently mitophagy is activated [Chen et al., 2013]. Mutated Mfn2 lacking the PINK1 phosphorylation site inhibited recruitment of Parkin to depolarized mitochondria and subsequent activation of mitophagy in mouse hearts [Gong et al., 2015]. Mfn2 deletion in mouse hearts inhibited Parkin-mediated mitophagy [Song et al., 2014] and caused mitochondrial enlargement with impaired respiration [Chen et al., 2013] and higher ROS production, leading to heart failure [Song et al., 2014]. Mfn2 deficiency in mouse cardiac myocytes inhibited Parkin translocation to mitochondria and reduced ubiquitination when mitochondria were depolarized. Furthermore, mitophagy activation and p62 recruitment were reduced in Mfn2-deficient mouse cardiomyocytes in response to mitochondrial damage [Chen et al., 2013] (Figure 2). Additionally, Mfn1/Mfn2 deficiency in mouse hearts led to mitochondrial fragmentation and abnormal mitochondrial morphology without mitophagy activation [Song et al., 2015] followed by lethal cardiomyopathy and heart failure progression by 7 to 8 weeks of age [Chen et al., 2011]. However, Mfn1 deficiency did not impair Parkin translocation and mitochondrial ubiquitination [Chen et al., 2013].
Figure 2. Mfn2 functions as a substrate for Parkin ubiquitination in mitophagy process.
(A) Parkin ubiquitinates Mfn2 in a PINK1-dependent manner in depolarized cardiac myocytes. (B) Mfn2 ablation inhibited Parkin translocation and recruitment of p62 to depolarized mitochondria; leading to mitophagy impairment and accumulation of damaged mitochondria [Chen et al., 2013]. FCCP, carbonyl cyanide p-trifluoromethoxyphenylhydrazone; Mfn, mitofusin; ROS, reactive oxygen species;Ub, ubiquitin; p62, SQST1–1/p62.
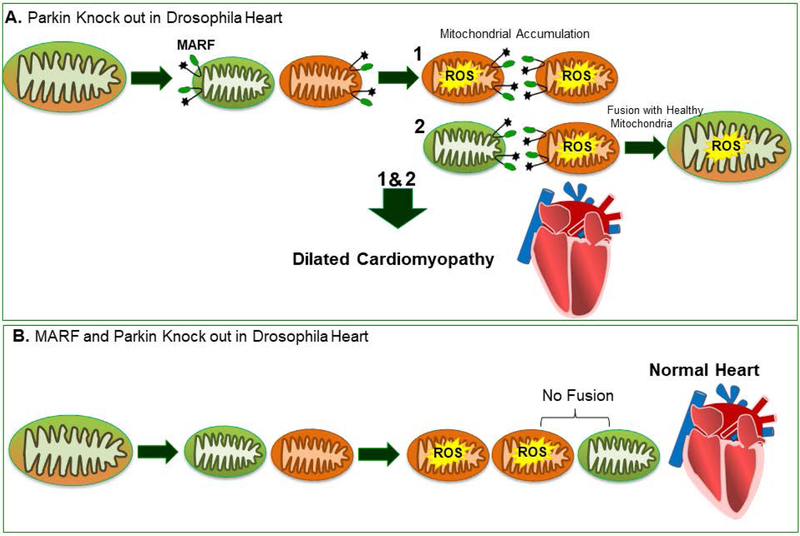
In a Drosophila model where Parkin was deleted in heart, the accumulation of enlarged, depolarized mitochondria with abnormal morphology and high ROS content were detected and led to the development of dilated cardiomyopathy [Bhandari et al., 2014] (Figure 3A). Furthermore, Mitofusin ortholog, MARF (mitochondrial assembly regulatory factor), deficiency in drosophila heart resulted in increased the levels of ROS and mitochondrial depolarization, leading to development of dilated cardiomyopathy [Bhandari et al., 2014]. Interestingly, MARF knockout in Parkin-deficient drosophila hearts rescued cardiomyopathy by correcting heart tube contraction and mitochondrial dysfunction; whereas, mitochondrial dysmorphology was not normalized [Bhandari et al., 2014] (Figure 3B). Parkin deficiency impairs mitophagy and the presence of fusion proteins may lead to an overabundance of unhealthy mitochondria; while, simultaneous suppression of Parkin and fusion proteins promote efficient fusion/fission with degradation of dysfunctional mitochondria [Bhandari et al., 2014].
Figure 3. Parkin-mediated mitophagy in drosophila heart.
(A) Parkin deficiency in drosophila heart impaired mitophagy and led to development of dilated cardiomyopathy. (B) Simultaneous deletion of MARF and Parkin rescued cardiomyopathy [Bhandari et al., 2014]. MARF, mitochondrial assembly regulatory factor.
In mice undergoing transverse aortic constriction causing pressure overload, mitophagy was upregulated in a Drp1-dependent manner. Prolonged pressure overload led to suppression of mitophagy and development of heart failure [Shirakabe et al., 2016]. Deficiency of cardiac Drp1 impaired mitochondrial degradation and resulted in the accumulation of enlarged mitochondria surroundeding vacuolar compartments, impaired mitochondrial respiration and development of lethal cardiomyopathy in mice [Kageyama et al., 2014]. Parkin deletion led to increased levels of Drp1 and simultaneous deficiency of Drp1 and Parkin exacerbated cardiomyopathy [Kageyama et al., 2014]. Another group demonstrated however that Parkin deficiency rescued the effects of Drp1 ablation in adult mouse hearts by improving cardiac function and survival [Song et al., 2015]. They demonstrated that Drp1 deficiency led to Parkin upregulation and elevated mitophagy resulting in mitochondrial mass loss and lethal cardiomyopathy. Therefore, simultaneous deficiency of Parkin and Drp1 reduced mitophagy and ventricular remodeling and improved cardiac contractility and diminished cardiac myocyte necrosis [Song et al., 2015].
However, whether dysregulation of cardiac function under pathological conditions such as ischemia/reperfusion occurs via direct effects on cardiomyocytes or by impaired functioning of neighboring cells such as endothelial cells contributes to pathogenesis of cardiomyocyte complications remains elusive. A recent study in mouse hearts reported that excessive Drp1-mediated fission and subsequent Parkin-mediated mitophagy triggers endothelial cell death pathways leading to microvascular injury and increased barrier permeability under reperfusion injury [Zhou et al., 2017]. Suppression of Drp1-mediated mitochondrial fission inhibited apoptosis and protected cardiac microvascular system [Zhou et al., 2017].
3.2.2. Receptor-mediated mitophagy
Mitophagy receptors such as Bnip3l, BCL2/adenovirus E1B, also known as Nix (Nip3-like protein X), and FUNDC1 (FUN14 domain containing 1) reside on the mitochondrial outer membrane and by regulating the mitophagy pathway, play an important role in acute ischemia/reperfusion (IR) [Hammerling et al., 2014, Nishida et al., 2017, Yuan et al., 2017]. FUNDC1 is highly expressed in the heart and mediates mitochondrial fragmentation and mitophagy under hypoxia [Liu et al., 2012, Zhang et al., 2017]. FUNDC1 interacts with LC3 on the autophagosomal membrane and inhibiiton of the LC3-interacting domain of FUNDC1 impairs mitophagy [Liu et al., 2012]. Under cardiac IR, activation of FUNDC1-mediated mitophagy during ischemia blocks apoptosis; whereas, in the reperfusion phase, FUNDC1-mediated mitophagy is impaired which leads to apoptosis activation [Zhou et al., 2017]. Cardiomyocytes have been reported as major targets of IR injury, however; recent findings have demonstrated that circulating platelets play a crucial role in determining cardiac dysfunction as a consequence of IR injury [Zhang et al., 2018]. Mice lacking FUNDC1 in their platelets had smaller infarcts with improved cardiac function compared to control mice under IR [Zhang et al., 2017]. Mitophagy improves energy metabolism of platelets, therefore, activated platelets form thrombi and occlude the coronary artery leading to secondary ischemia and contribute to cardiac IR injury [Zhang et al., 2018]. In this regard, pharmacological or genetic manipulation of FUNDC1-mediated mitophagy in platelets, which suppresses their hyperactivity, can protect the heart against IR injury [Zhang et al., 2017, Zhou et al., 2017, Zhang et al., 2016].
3.2.3. BAG3-mediated mitophagy
Bcl-2-associated athanogene 3 (BAG3) protein is highly expressed in skeletal and cardiac muscles [Homma et al., 2006], maintains myofibrillar organization and prevents z-disk disruption and muscle degeneration [Hishiya et al., 2010]. BAG3 gene disruption in mice led to myofibrillar degeneration and development of lethal myopathy followed by death 4 weeks post disruption [Homma et al., 2006]. BAG3 also functions as a chaperone protein and targets misfolded proteins for lysosomal degradation via the autophagy pathway [Minoia et al., 2014, Zhang et al., 2014, Gamerdinger et al., 2010] and mutations in BAG3 have led to proteotoxic stress and severe cardiomyopathy [Schänzer et al., 2018]. Alterations in mtDNA, mitochondrial respiration and mitochondrial biogenesis have been reported in patients with myofibrillar myopathies [Vincet et al., 2016]. Therefore, recent studies have reported the role of BAG3 in regulating mitochondrial dynamic and mitochondrial quality control in cardiac cells, however, the exact underlying mechanisms remain elusive [Tahrir et al., 2017, Quintana et al., 2016]. In neonatal cardiomyocytes, BAG3 levels were significantly increased in the mitochondrial fraction under mitochondrial stress. Moreover, suppression of BAG3 reduced mitophagy and impaired clearance of damaged mitochondria leading to apoptosis induction and significant cardiomyocyte death [Tahrir et al., 2017]. Furthermore, BAG3 mutation resulted in mitochondrial fragmentation and alterations of mitochondrial fission (Drp1) and fusion (Opa1) (cellular optic atrophy-1 protein) proteins in mouse hearts leading to the development of progressive heart failure [Quintana et al., 2016].
4. Mitophagy during cardiac development
During development, mitochondrial dynamics and metabolism changes [Neary et al., 2014, Gong et al., 2015]. Fetal cardiomyocytes have metabolic tolerance to hypoxia; whereas, after birth, oxygen encountered by the neonate acts as a driving force for mitochondrial remodeling and biogenesis toward aerobic metabolism to meet the energy demand of adult myocardium [Neary et al., 2014, Papanicolaou et al., 2012, Dorn et al., 2015]. In mouse fetal cardiomyocytes, mitochondria undergo mitophagy mediated by the PINK1-Mfn2-Parkin complex to be replaced with mature adult mitochondria within the first 3 weeks of life. Parkin deficiency in adult cardiomyocytes did not lead to cardiomyopathy; whereas, Parkin ablation in fetal mice led to impaired mitochondrial maturation and lethal cardiomyopathy [Gong et al., 2015]. Ablation of mitofusins in mouse embryonic myocardium was lethal [Chen et al., 2011] and perinatal expression of mutated Mfn2 lacking the PINK1 phosphorylation site inhibited postnatal mitochondrial maturation thereby leading to metabolic arrest followed by lethal cardiomyopathy by 7 to 8 weeks of age [Gong et al., 2015]. Furthermore, double deficiency of Mfn1/Mfn2 in midgestational cardiomyocytes impaired mitochondrial remodeling, mitochondrial biogenesis and metabolic switching in postnatal cardiac cells and led to heart failure by day of 7, followed by death at 16 days of age [Papanicolaou et al., 2012].
5. Cardiac complications as a result of mitophagy dysregulation
Mitophagy has been reported to play a dual role during cardiac stress; mitophagy can be either protective or detrimental [Zhou et al., 2017, Shirakabe et al., 2016, Zhang et al., 2018]. By eliminating malfunctioning mitochondria, mitophagy avoids release of pro-apoptotic proteins into the cytosol and activation of apoptosis. On the other hand, disruption of mitophagy causes intracellular accumulation of ROS and increases cell vulnerability to oxidative damage [Zhou et al., 2017, Wild et al., 2010]. ROS are byproducts of OXPHOS and have been reported in cardiovascular complications particularly in cardiac hypertrophy [Dutta et al., 2013, Liu et al., 2012]. Manipulating the metabolic activity of mitochondria by attenuating myocardial oxidative stress ameliorated cardiomyopathy after myocardial infarction [Engberding et al., 2004, Liu et al., 2012]. Autophagy induction in aortic-banded mice attenuated oxidative stress, reduced myocardial infract size and improved cardiac function in hypertrophic myocardium [Ma et al., 2018]. On the other hand, excessive mitophagy is followed by mitochondrial mass loss and myocardial ATP deficiency [Jin et al., 2018]. Therefore, a balance between mitochondrial degradation and mitochondrial biogenesis is of great importance toward myocardial protection under stress. Atrial biopsy of patients undergoing cardiopulmonary bypass during cardiac surgery indicated that both mitophagy and mitochondrial biogenesis were upregulated after heart surgery [Andres et al., 2017].
Mutations of Parkin and PINK1 genes have been associated with the development of degenerative disorders [Barodia et al., 2017]. Parkin-deficient mice had morphologically normal hearts [Piquereau et al., 2013, Song et al., 2015], however, mitochondrial functional abnormalities were observed [Piquereau et al., 2013]. Under stress conditions, the protective role of Parkin-mediated mitophagy has been reported in several research studies [Song et al., 2015]. Loss of Parkin was accompanied by mitophagy reduction and accumulation of damaged mitochondria after myocardial infarction [Kubli et al., 2013]. Parkin deficiency exacerbated cardiac and mitochondrial dysfunction during sepsis [Piquereau et al., 2013]. A substantial reduction in the level of PINK1 protein was detected in end-stage heart failure patients [Billia et al., 2011]. PINK1 deficiency in mouse hearts resulted in elevated levels of oxidative stress and induction of apoptosis leading to left ventricular dysfunction and cardiac hypertrophy [Billia et al., 2011]. In addition, PINK1 deficiency increased cardiac susceptibility to IR injury in mice and overexpression of PINK1 protected against IR injury in cardiac cells [Sidall et al., 2013].
6. Mitochondrial homeostasis as a potential therapeutic target against cardiovascular complications
The excitation-contraction activity of the myocardium highly relies on the energy supplied by mitochondria via OXPHOS. However, intracellular accumulation of excess ROS that are generated during mitochondrial metabolism, and the lack of their removal can induce oxidative stress that becomes a major contributor toward the pathophysiology of myocardial infarction and cardiac senescence [Davalli et al., 2016]. Furthermore, poor quality of mitochondrial DNA during aging can also lead to the overproduction of ROS [Bielas et al., 2018] an event that may contribute to the development of cardiovascular diseases in aged individuals especially when they are exposed to stresses [Dutta et al., 2012]. Emerging evidence has suggested mitochondrial ROS may play a role in sudden cardiac death and heart failure. Studies in a pig model of heart failure revealed that elevated mitochondrial ROS promotes chronic proteome remodeling, altering protein expression and phosphorylation, and mitigates electrophysiological stability of the myocardium leading to the progression of sudden cardiac death and heart failure [Dey et al., 2018]. Considering the nature of cardiomyocytes as terminally differentiated post-mitotic cells which could not undergo further division and subsequent cell replacement, maintaining mitochondrial homeostasis as a balance between clearance of dysfunctional mitochondria via mitophagy and biogenesis of healthy mitochondria is vital to preserve cardiac health and prevent heart aging [Dutta et al., 2012, Shiattarella et al., 2016]. Accordingly, dysregulation of autophagy has been observed in various human cardiovascular diseases such as in patients with dilated cardiomyopathy and heart failure with reduced ejection fraction [Shiattarella et al., 2016], leading to speculation that autophagy may serve as a promising target for development of clinical interventions to impair cardiac aging and rescue cardiac function under disease pathogenesis. Rapamycin by inhibiting mTOR pathway stimulates autophagy and selective degradation of mitochondria [Li et al., 2014]. Treatment of mice under pathological stress caused by Lamin A deficiency enhanced clearance of accumulated proteins via autophagy pathway and ameliorated cardiac function and mice survival [Ramos et al., 2012]. Further, Rapamycin treatment enhanced the quality of mitochondrial DNA and ETC in aging mice [Bielas et al., 2018] and under mitochondrial stress condition, rapamycin by regulating mitochondrial oxidative metabolism created resistance against senescence in human cardiac fibroblasts [Nacarelli et al., 2018]. Combined treatment of simvastatin and rapamycin mitigated cardiac allograft rejection and histopathological damage post cardiac transplantation and prolonged survival of the recipient rats [Liu et al., 2018]. Induction of autophagy for the removal of long-lived proteins and damaged organelles exerts protective function; however, excessive autophagy has been associated with activation of cell death pathways and implicated in the pathogenesis of heart failure. These findings suggest that precise modulation of autophagy and the key molecular players involved in this process could be a potential therapeutic strategy in cardiac complications.
Conclusions
Mitochondria produce over 90% of the ATP required by the high energy demands of cardiac tissues. Mitochondrial disorders have been associated with the development of many cardiovascular diseases including atherosclerosis, ischemic heart disease, cardiac hypertrophy and heart failure. In fact, considering the high energy demand of proper cardiac excitation-contraction coupling and the role of OXPHOS in supplying the cardiac muscle, patients with mitochondrial abnormalities are at a higher risk for developing heart disease, which is a leading cause of mortality among those patients. In this context, maintaining mitochondrial fitness is critical to cardiac health. This process is controlled in part by a balance of mitochondrial fusion and fission, whereby healthy mitochondria, normal mitochondrial proteins and intact DNA are distributed throughout the mitochondrial network in the cell. Many intracellular pathways regulate mitochondrial protein quality control and the elimination of dysfunctional mitochondria. Disturbance of either the UPS or mitophagy results in the accumulation of abnormal mitochondrial proteins or dysfunctional mitochondria. Given that heart failure is a bioenergetic disease an increased understanding of how to address energy deficit in cardiac tissue is required for improving new therapeutics to treat or prevent mitochondrial failure in heart disease.
Acknowledgments
The authors wish to thank past and present members of the Department of Neuroscience and Center for Neurovirology for sharing of ideas and reagents. We also thank C. Papaleo for editorial assistance. This work was made possible by grants (R01HL123093 and P30MH092177) awarded by the NIH to KK.
Footnotes
Conflict of Interests
The authors declare that there are no competing conflicts.
References
- Andres AM, et al. (2017). Mitophagy and mitochondrial biogenesis in atrial tissue of patients undergoing heart surgery with cardiopulmonary bypass.JCI insight, 2, e89303. doi: 10.1172/jci.insight.89303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafi G, & Schwarz TL (2013). The pathways of mitophagy for quality control and clearance of mitochondria. Cell death and differentiation, 20, 31–42. doi: 10.1038/cdd.2012.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barodia SK, Creed RB, & Goldberg MS (2017). Parkin and PINK1 functions in oxidative stress and neurodegeneration. Brain research bulletin, 133, 51–59. doi: 10.1016/j.brainresbull.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthiaume JM, Kurdys JG, Muntean DM, & Rosca MG (2017). Mitochondrial NAD+/NADH redox state and diabetic cardiomyopathy. Antioxid Redox Signaling, doi: 10.1089/ars.2017.7415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari P, Song M, Chen Y, Burelle Y, & Dorn GW (2014). Mitochondrial contagion induced by Parkin deficiency in Drosophila hearts and its containment by suppressing mitofusin. Circ Re, 114, 257–265. doi: 10.1161/CIRCRESAHA.114.302734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielas J, et al. (2018). Long term rapamycin treatment improves mitochondrial DNA quality in aging mice. Exp Gerontol, 106, 125–131. doi: 10.1016/j.exger.2018.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billia F, Hauck L, Konecny F, Rao V, Shen J, & Mak TW (2011). PTEN-inducible kinase 1 (PINK1)/Park6 is indispensable for normal heart function. Proc Natl Acad Sciences USA, 108, 9572–9577. doi: 10.1073/pnas.1106291108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragoszewski P, Turek M, & Chacinska A (2017). Control of mitochondrial biogenesis and function by the ubiquitin–proteasome system. Open biology, 7, pii: 170007. doi: 10.1098/rsob.170007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan NC, et al. (2011). Broad activation of the ubiquitin–proteasome system by Parkin is critical for mitophagy. Hum Mol Genet, 20, 1726–1737. doi: 10.1093/hmg/ddr048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Liu Y, & Dorn GW (2011). Mitochondrial fusion is essential for organelle function and cardiac homeostasis. Circ Res, 109, 1327–1331. doi: 10.1161/CIRCRESAHA.111.258723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, & Dorn GW (2013). PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science, 340, 471–475. doi: 10.1126/science.1231031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chistiakov DA, Shkurat TP, Melnichenko AA, Grechko AV, & Orekhov AN (2018). The role of mitochondrial dysfunction in cardiovascular disease: a brief review. Ann Med, 50, 121–127. doi: 10.1080/07853890.2017.1417631 [DOI] [PubMed] [Google Scholar]
- Ciechanover A (2017). Intracellular protein degradation: from a vague idea thru the lysosome and the ubiquitin-proteasome system and onto human diseases and drug targeting. Best Prac Res Clin Haematol, 30, 341–355. doi: 10.1002/anie.200501428 [DOI] [PubMed] [Google Scholar]
- Davalli P, Mitic T, Caporali A, Lauriola A, & D’Arca D (2016). ROS, cell senescence, and novel molecular mechanisms in aging and age-related diseases. Oxid Med Cell Longev, doi: 10.1155/2016/3565127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey S, DeMazumder D, Sidor A, Foster DB, & O’Rourke B (2018). Mitochondrial ROS Drive Sudden Cardiac Death and Chronic Proteome Remodeling in Heart Failure. Circ Res,123, 356–371. doi: 10.1161/CIRCRESAHA.118.312708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disatnik MH, et al. (2013). Acute inhibition of excessive mitochondrial fission after myocardial infarction prevents long-term cardiac dysfunction. J Am Heart Assoc, 2, e000461. doi: 10.1161/JAHA.113.000461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn GW, Vega RB, & Kelly DP (2015). Mitochondrial biogenesis and dynamics in the developing and diseased heart. Genes Dev, 29, 1981–1991. doi: 10.1101/gad.269894.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn GW, et al. (2011). MARF and Opa1 control mitochondrial and cardiac function in Drosophila. Circ Res, 108, 12–17. doi: 10.1161/CIRCRESAHA.110.236745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doenst T, Nguyen TD, & Abel ED (2013). Cardiac metabolism in heart failure: implications beyond ATP production. Circ Res, 113, 709–724. doi: 10.1161/CIRCRESAHA.113.300376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond GR, Selemidis S, Griendling KK, & Sobey CG (2011). Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov, 10, 453–471. doi: 10.1038/nrd3403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta D, Xu J, Kim JS, Dunn WA Jr, & Leeuwenburgh C (2013). Upregulated autophagy protects cardiomyocytes from oxidative stress-induced toxicity. Autophagy, 9, 328–344. doi: 10.4161/auto.22971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta D, Calvani R, Bernabei R, Leeuwenburgh C, & Marzetti E (2012). Contribution of impaired mitochondrial autophagy to cardiac aging: mechanisms and therapeutic opportunities. Circ Res, 110, 1125–1138. doi: 10.1161/CIRCRESAHA.111.246108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberding N, et al. (2004). Allopurinol attenuates left ventricular remodeling and dysfunction after experimental myocardial infarction: a new action for an old drug?.Circulation, 110, 2175–2179. doi: 10.1161/01.CIR.0000144303.24894.1C [DOI] [PubMed] [Google Scholar]
- Gamerdinger M, Kaya AM, Wolfrum U, Clement AM, & Behl C (2011). BAG3 mediates chaperone-based aggresome-targeting and selective autophagy of misfolded proteins. EMBO Rep, 12, 149–156. doi: 10.1038/embor.2010.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegg ME, & Schapira AH (2011). PINK1-parkin-dependent mitophagy involves ubiquitination of mitofusins 1 and 2: implications for Parkinson disease pathogenesis. Autophagy, 7, 243–245. doi: 10.4161/auto.7.2.14332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths EJ, & Rutter GA (2009). Mitochondrial calcium as a key regulator of mitochondrial ATP production in mammalian cells. Biochim Biophys Acta -Bioenergetics, 1787, 1324–1333. doi: 10.1016/j.bbabio.2009.01.019 [DOI] [PubMed] [Google Scholar]
- Große L, Wurm CA, Brüser C, Neumann D, Jans DC, & Jakobs S (2016). Bax assembles into large ring-like structures remodeling the mitochondrial outer membrane in apoptosis. EMBO J, 35, 402–413. doi: 10.15252/embj.201592789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong G, Song M, Csordas G, Kelly DP, Matkovich SJ, & Dorn GW (2015). Parkin-mediated mitophagy directs perinatal cardiac metabolic maturation in mice.Science, 350, aad2459. doi: 10.1126/science.aad2459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerling BC, & Gustafsson ÅB (2014). Mitochondrial quality control in the myocardium: cooperation between protein degradation and mitophagy.J Mol Cell Cardiol, 75, 122–130. doi: 10.1016/j.yjmcc.2014.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedhli N, et al. (2008). Proteasome inhibition decreases cardiac remodeling after initiation of pressure overload.Am J Physiol Heart Circ Physiol, 295, H1385–H1393. doi: 10.1152/ajpheart.00532.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hishiya A, Kitazawa T, & Takayama S (2010). BAG3 and Hsc70 Interact With Actin Capping Protein CapZ to Maintain Myofibrillar Integrity Under Mechanical Stress. Circ Res, 107, 1220–1231. doi: 10.1161/CIRCRESAHA.110.225649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren D, Wahlander H, Eriksson BO, Oldfors A, Holme E, & Tulinius M (2003). Cardiomyopathy in children with mitochondrial disease: Clinical course and cardiological findings.Eur HeartJ, 24, 280–288. doi: 10.1016/S0195-668X(02)00387-1 [DOI] [PubMed] [Google Scholar]
- Homma S, Iwasaki M, Shelton GD, Engvall E, Reed JC, & Takayama S (2006). BAG3 deficiency results in fulminant myopathy and early lethality. Am J Pathol, 169, 761–773. doi: 10.2353/ajpath.2006.060250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, Shirakabe A, Brady C, Zablocki D, Ohishi M, & Sadoshima J (2015). Molecular mechanisms mediating mitochondrial dynamics and mitophagy and their functional roles in the cardiovascular system. J Mol Cell Cardiol, 78, 116–122. doi: 10.1016/j.yjmcc.2014.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S, et al. (2017). Elucidating mitochondrial electron transport chain supercomplexes in the heart during ischemia–reperfusion. Antioxid Redox Signal, 27, 57–69. doi: 10.1089/ars.2016.6635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Q, et al. (2018). DUSP1 alleviates cardiac ischemia/reperfusion injury by suppressing the Mff-required mitochondrial fission and Bnip3-related mitophagy via the JNK pathways. Redox Biol, 14, 576–587. doi: 10.1016/j.redox.2017.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonckheere AI, Smeitink JA, & Rodenburg RJ (2012). Mitochondrial ATP synthase: architecture, function and pathology. J Inherit Metab Dis, 35, 211–225. doi: 10.1007/s10545-011-9382-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama Y, et al. (2014). Parkin-independent mitophagy requires Drp1 and maintains the integrity of mammalian heart and brain. EMBO J, 33, 2798–2813. doi: 10.15252/embj.201488658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane LA, et al. (2014). PINK1 phosphorylates ubiquitin to activate Parkin E3 ubiquitin ligase activity.J Cell Biol, 205, 143–153. doi: 10.1083/jcb.201402104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang PT, Chen CL, Lin P, Chilian WM, & Chen YR (2017). Impairment of pH gradient and membrane potential mediates redox dysfunction in the mitochondria of the post-ischemic heart. Basic Res Cardiol, 112, 36. doi: 10.1007/s00395-017-0626-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazlauskaite A, et al. (2014). Parkin is activated by PINK1-dependent phosphorylation of ubiquitin at Ser65.Biochem J,460, 127–141. doi: 10.1042/BJ20140334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koene S, & Smeitink J (2009). Mitochondrial medicine: entering the era of treatment. J Intern Medi, 265, 193–209. doi: 10.1111/j.1365-2796.2008.02058.x [DOI] [PubMed] [Google Scholar]
- Koene S, & Smeitink J (2011). Metabolic manipulators: a well founded strategy to combat mitochondrial dysfunction. J Inherit Metab Dis, 34, 315–325. doi: 10.1007/s10545-010-9162-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyano F, et al. (2014). Ubiquitin is phosphorylated by PINK1 to activate parkin.Nature, 510, 162–166. doi: 10.1038/nature13392 [DOI] [PubMed] [Google Scholar]
- Kubli DA, et al. (2013). Parkin protein deficiency exacerbates cardiac injury and reduces survival following myocardial infarction.J Biol Chem, 288, 915–926. doi: 10.1074/jbc.M112.411363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmicic J, et al. (2011). Mitochondrial dynamics: a potential new therapeutic target for heart failure. Re Esp Cardiol (English Edition), 64, 916–923. doi: 10.1016/j.recesp.2011.05.018 [DOI] [PubMed] [Google Scholar]
- Lazarou M, et al. (2015). The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature, 524, 309–314. doi: 10.1038/nature14893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Nagano Y, Taylor JP, Lim KL, & Yao TP (2010). Disease-causing mutations in parkin impair mitochondrial ubiquitination, aggregation, and HDAC6-dependent mitophagy. J Cell Biol, 189, 671–679. doi: 10.1083/jcb.201001039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesnefsky EJ, Chen Q, Tandler B, & Hoppel CL (2017). Mitochondrial dysfunction and myocardial ischemia-reperfusion: implications for novel therapies. Ann Rev Pharmacol Toxicol, 57, 535–565. doi: 10.1146/annurev-pharmtox-010715-103335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, et al. (2014). BAG3 promoted starvation-induced apoptosis of thyroid cancer cells via attenuation of autophagy. J Clin Endocrinol Metab, 99, 2298–2307. doi: 10.1210/jc.2014-1779 [DOI] [PubMed] [Google Scholar]
- Li Q, et al. (2014). Rapamycin attenuates mitochondrial dysfunction via activation of mitophagy in experimental ischemic stroke. Biochem Biophys Res Commun, 444, 182–188. doi: 10.1016/j.bbrc.2014.01.032 [DOI] [PubMed] [Google Scholar]
- Li J, Horak KM, Su H, Sanbe A, Robbins J, & Wang X (2011). Enhancement of proteasomal function protects against cardiac proteinopathy and ischemia/reperfusion injury in mice. J Clin Invest, 121, 3689–3700. doi: 10.1172/JCI45709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, et al. (2018). Combined treatment with simvastatin and rapamycin attenuates cardiac allograft rejection through the regulation of T helper 17 and regulatory T cells. Exp Ther Med, 15, 1941–1949. doi: 10.3892/etm.2017.5635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu BQ, et al. (2013). BAG3-dependent noncanonical autophagy induced by proteasome inhibition in HepG2 cells.Autophagy, 9, 905–916. doi: 10.4161/auto.24292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, et al. (2012). Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat Cell Biol, 14, 177–185. doi: 10.1038/ncb2422 [DOI] [PubMed] [Google Scholar]
- Liu X, et al. (2012). Ablation of ALCAT1 mitigates hypertrophic cardiomyopathy through effects on oxidative stress and mitophagy.Mol Cell Biol, 32, 4493–4504. doi: 10.1128/MCB.01092-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan A, et al. (2016). Assessing the mitochondrial membrane potential in cells and in vivo using targeted click chemistry and mass spectrometry.Cell Metab, 23, 379–385. doi: 10.1016/j.cmet.2015.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma LL, et al. (2018). Mammalian target of rapamycin inhibition attenuates myocardial ischaemia–reperfusion injury in hypertrophic heart. J Cell Mol Med, 22, 1708–1719. doi: 10.1111/jcmm.13451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda N, & Tanaka K (2015). Cell biology: Tagged tags engage disposal. Nature, 524, 294–295. doi: 10.1038/nature15199 [DOI] [PubMed] [Google Scholar]
- Maulik SK, & Kumar S (2012). Oxidative stress and cardiac hypertrophy: a review. Toxicol Mech Methods, 22, 359–366. doi: 10.3109/15376516.2012.666650 [DOI] [PubMed] [Google Scholar]
- McWilliams TG, et al. (2018). Basal nitophagy occurs independently of PINK1 in mouse tissues of high metabolic demand. Cell Metab, 27, 439–449. doi: 10.1016/j.cmet.2017.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoia M, et al. (2014). BAG3 induces the sequestration of proteasomal clients into cytoplasmic puncta: implications for a proteasome-to-autophagy switch. Autophagy, 10, 1603–1621. doi: 10.4161/auto.29409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai A, et al. (2007). The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nature Med, 13, 619–624. doi: 10.1038/nm1574 [DOI] [PubMed] [Google Scholar]
- Nacarelli T, Azar A, Altinok O, Orynbayeva Z, Sell C (2018). Rapamycin increases oxidative metabolism and enhances metabolic flexibility in human cardiac fibroblasts. Geroscience, 1–14. doi: 10.1007/s11357-018-0030-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra DP, & Youle RJ (2011). Targeting mitochondrial dysfunction: role for PINK1 and Parkin in mitochondrial quality control. Antioxid Redox Signal, 14, 1929–1938. doi: 10.1089/ars.2010.3799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra D, Tanaka A, Suen DF, & Youle RJ (2008). Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol, 183, 795–803. doi: 10.1083/jcb.200809125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary MT, et al. (2014). Hypoxia signaling controls postnatal changes in cardiac mitochondrial morphology and function. J Mol Cell Cardiol, 74, 340–352. doi: 10.1016/j.yjmcc.2014.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann B, Schwarzer M, & Rohrbach S (2017). Heart and mitochondria: pathophysiology and Implications for cardiac surgeons. Thorac Cardiovasc Surg, 66, 11–19. doi: 10.1055/s-0037-1615263 [DOI] [PubMed] [Google Scholar]
- Nishida K, & Otsu K (2017). Sterile inflammation and degradation systems in heart failure. Circ J, 81, 622–628. doi: 10.1253/circj.CJ-17-0261 [DOI] [PubMed] [Google Scholar]
- Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, & Hausenloy DJ (2010). Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation, 121, 2012–2022. doi: 10.1161/CIRCULATIONAHA.109.906610 [DOI] [PubMed] [Google Scholar]
- Ong SB, Kalkhoran SB, Hernández-Reséndiz S, Samangouei P, Ong SG, & Hausenloy DJ (2017). Mitochondrial-shaping proteins in cardiac health and disease–the long and the short of it!. Cardiovasc Drugs Ther, 31, 87–107. doi: 10.1007/s10557-016-6710-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padman BS, Bach M, Lucarelli G, Prescott M, & Ramm G (2013). The protonophore CCCP interferes with lysosomal degradation of autophagic cargo in yeast and mammalian cells. Autophagy, 9, 1862–1875. doi: 10.4161/auto.26557 [DOI] [PubMed] [Google Scholar]
- Papanicolaou KN, et al. (2012). Mitofusins 1 and 2 are essential for postnatal metabolic remodeling in heart. Circ Res, 111, 1012–1026. doi: 10.1161/CIRCRESAHA.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry SW, Norman JP, Barbieri J, Brown EB, & Gelbard HA (2011). Mitochondrial membrane potential probes and the proton gradient: a practical usage guide. Biotechniques, 50, 98–115. doi: 10.2144/000113610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piquereau J, et al. (2013). Protective role of PARK2/Parkin in sepsis-induced cardiac contractile and mitochondrial dysfunction. Autophagy, 9, 1837–1851. doi: 10.4161/auto.26502 [DOI] [PubMed] [Google Scholar]
- Predmore JM, et al. (2010). Ubiquitin proteasome dysfunction in human hypertrophic and dilated cardiomyopathies. Circulation, 121, 997–1004. doi: 10.1161/CIRCULATIONAHA.109.904557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pye J, et al. (2003). Proteasome inhibition ablates activation of NF-κB in myocardial reperfusion and reduces reperfusion injury. Am J Physiol Heart Circ Physiol, 284, 919–926. doi: 10.1152/ajpheart.00851.2002 [DOI] [PubMed] [Google Scholar]
- Qiao H, Ren H, Du H, Zhang M, Xiong X, & Lv R (2018). Liraglutide repairs the infarcted heart: The role of the SIRT1/Parkin/mitophagy pathway. Mol Medi Rep, 17, 3722–3734. doi: 10.3892/mmr.2018.8371 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Quintana MT, et al. (2016). Cardiomyocyte-specific human Bcl2-associated anthanogene 3 P209L expression induces mitochondrial fragmentation, Bcl2-associated anthanogene 3 haploinsufficiency, and activates p38 signaling. Am J Pathol, 186, 1989–2007. doi: 10.1016/j.ajpath.2016.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos FJ, et al. (2012). Rapamycin reverses elevated mTORC1 signaling in lamin A/C–deficient mice, rescues cardiac and skeletal muscle function, and extends survival. Sci Transl Med, 4, 144ra103. doi: 10.1126/scitranslmed.3003802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosca MG, & Hoppel CL (2013). Mitochondrial dysfunction in heart failure. Heart Fail Rev, 18, 607–622. doi: 10.1007/s10741-012-9340-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepuri NB, et al. (2017). Mitochondrial LON protease-dependent degradation of cytochrome c oxidase subunits under hypoxia and myocardial ischemia. Biochim BiophysActa Bioenerget, 1858, 519–528. doi: 10.1016/j.bbabio.2017.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schänzer A, et al. (2018). Dysregulated autophagy in restrictive cardiomyopathy due to Pro209Leu mutation in BAG3. Mol Genet Metab, 123, 388–399. doi: 10.1016/j.ymgme.2018.01.001 [DOI] [PubMed] [Google Scholar]
- Scherz-Shouval R, & Elazar Z (2007). ROS, mitochondria and the regulation of autophagy. Trends Cell Biol, 17, 422–427. doi: 10.1016/j.tcb.2007.07.009 [DOI] [PubMed] [Google Scholar]
- Schiattarella GG, & Hill JA (2016). Therapeutic targeting of autophagy in cardiovascular disease. J Mol Cell Cardiol, 95, 86–93. doi: 10.1016/j.yjmcc.2015.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp WW, et al. (2015). Inhibition of the mitochondrial fission protein Drp1 improves survival in a murine cardiac arrest model. Crit Care Med, 43, 38–47. doi: 10.1097/CCM.0000000000000817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp WW, et al. (2014). Dynamin-related protein 1 (Drp1)-mediated diastolic dysfunction in myocardial ischemia-reperfusion injury: therapeutic benefits of Drp1 inhibition to reduce mitochondrial fission. FASEB J, 28, 316–326. doi: 10.1096/fj.12-226225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakabe A, et al. (2016). Drp1-dependent mitochondrial autophagy plays a protective role against pressure-overload-induced mitochondrial dysfunction and heart failure. Circulation, 133, 1249–1263. doi: 10.1161/CIRCULATIONAHA.115.020502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddall HK, et al. (2013). Loss of PINK1 increases the heart’s vulnerability to ischemiareperfusion injury. PloS One, 8, e62400. doi: 10.1371/journal.pone.0062400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, et al. (2015). Interdependence of Parkin-mediated mitophagy and mitochondrial fission in adult mouse hearts. Circ Res, 117, 346–351. doi: 10.1161/CIRCRESAHA.117.306859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Mihara K, Chen Y, Scorrano L, & Dorn GW (2015). Mitochondrial fission and fusion factors reciprocally orchestrate mitophagic culling in mouse hearts and cultured fibroblasts. Cell Metab, 21, 273–285. doi: 10.1016/j.cmet.2014.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Chen Y, Gong G, Murphy E, Rabinovitch PS, & Dorn GW (2014). Super-Suppression of mitochondrial reactive ixygen species signaling impairs compensatory autophagy in primary mitophagic cardiomyopathy. Circ Res, 115, 348–353. doi: 10.1161/CIRCRESAHA.115.304384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahrir FG, et al. (2018). Dysregulation of mitochondrial bioenergetics and quality control by HIV-1 Tat in cardiomyocytes. J Cell Physiol, 233, 748–758. doi: 10.1002/jcp.26002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahrir FG, et al. (2017). Evidence for the role of BAG3 in mitochondrial quality control in cardiomyocytes. J Cell Physiol, 232, 797–805. doi: 10.1002/jcp.25476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, et al. (2017). Ischemia-induced Drp1 and Fis1-mediated mitochondrial fission and right ventricular dysfunction in pulmonary hypertension. J Mol Med, 95, 381–393. doi: 10.1007/s00109-017-1522-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truban D, Hou X, Caulfield TR, Fiesel FC, & Springer W (2017). PINK1, parkin, and mitochondrial quality control: what can we learn about Parkinson’s disease pathobiology?. J Parkinsons Dis,7, 13–29. doi: 10.3233/JPD-160989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vives-Bauza C, et al. (2010). PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci USA, 107, 378–383. doi: 10.1073/pnas.0911187107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent AE, et al. (2016). Mitochondrial dysfunction in myofibrillar myopathy. Neuromuscul Disord, 26, 691–701. doi: 10.1016/j.nmd.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, et al. (2011). Parkin ubiquitinates Drp1 for proteasome-dependent degradation implication of dysregulated mitochondrial dynamics in parkinson disease. J Biol Chem, 286, 11649–11658. doi: 10.1074/jbc.M110.144238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, et al. (2017). AMPKα2 protects against the development of heart failure by enhancing nitophagy via PINK1 phosphorylation. Circ Res, 122, 712–729. doi: 10.1161/CIRCRESAHA.117.312317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattin M, et al. (2018). Modulation of protein quality control and proteasome to autophagy switch in immortalized myoblasts from Duchenne muscular dystrophy patients. In J Mol Sci, 19, pii: E178. doi: 10.3390/ijms19010178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild P, & Dikic I (2010). Mitochondria get a Parkin’ticket. Nat Cell Biol, 12, 104–106. doi: 10.1038/ncb0210-104 [DOI] [PubMed] [Google Scholar]
- Yoshii SR, Kishi C, Ishihara N, & Mizushima N (2011). Parkin mediates proteasome-dependent protein degradation and rupture of the outer mitochondrial membrane. Journal of Biological Chemistry, 286, 19630–19640. doi: 10.1074/jbc.M110.209338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, et al. (2017). BNIP3L/NIX-mediated mitophagy protects against ischemic brain injury independent of PARK2. Autophagy, 13, 1754–1766. doi: 10.1080/15548627.2017.1357792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Siraj S, Zhang R, & Chen Q (2017). Mitophagy receptor FUNDC1 regulates mitochondrial homeostasis and protects the heart from I/R injury. Autophagy, 13, 1080–1081. doi: 10.1080/15548627.2017.1300224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Chen C, Wang J, Liu L, He Y, & Chen Q (2018). Mitophagy in cardiomyocytes and in platelets: a major mechanism of cardioprotection against ischemia/reperfusion injury. Physiology, 33, 86–98. doi: 10.1152/physiol.00030.2017 [DOI] [PubMed] [Google Scholar]
- Zhang W, et al. (2016). Hypoxic mitophagy regulates mitochondrial quality and platelet activation and determines severity of I/R heart injury. Elife, 5 pii: e21407. doi: 10.7554/eLife.21407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, et al. (2018). AMPK activation serves a critical role in mitochondria quality control via modulating mitophagy in the heart under chronic hypoxia. Int J Mol Med, 41, 69–76. doi: 10.3892/ijmm.2017.3213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, et al. (2017). Ripk3 induces mitochondrial apoptosis via inhibition of FUNDC1 mitophagy in cardiac IR injury. Redox Biol, 13, 498–507. doi: 10.1016/j.redox.2017.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, et al. (2017). Melatonin suppresses platelet activation and function against cardiac ischemia/reperfusion injury via PPARγ/FUNDC1/mitophagy pathways. J Pineal Res, 63, doi: 10.1111/jpi.12438 [DOI] [PubMed] [Google Scholar]
- Zhou H, et al. (2017). Melatonin protects cardiac microvasculature against ischemia/reperfusion injury via suppression of mitochondrial fission-VDAC1-HK2-mPTP-mitophagy axis. J Pineal Res, 63, doi: 10.1111/jpi.12413 [DOI] [PMC free article] [PubMed] [Google Scholar]