Abstract
24 h exposure to 4 °C primes Arabidopsis thaliana in the pre-bolting rosette stage for several days against full cold activation of the ROS responsive genes ZAT10 and BAP1 and causes stronger cold-induction of pleiotropically stress-regulated genes. Transient over-expression of thylakoid ascorbate peroxidase (tAPX) at 20 °C mimicked and tAPX transcript silencing antagonized cold-priming of ZAT10 expression. The tAPX effect could not be replaced by over-expression of stromal ascorbate peroxidase (sAPX) demonstrating that priming is specific to regulation of tAPX availability and, consequently, regulated locally at the thylakoid membrane. Arabidopsis acquired cold primability in the early rosette stage between 2 and 4 weeks. During further rosette development, primability was widely maintained in the oldest leaves. Later formed and later maturing leaves were not primable demonstrating that priming is stronger regulated with plant age than with leaf age. In 4-week-old plants, which were strongest primable, the memory was fully erasable and lost seven days after priming. In summary, we conclude that cold-priming of chloroplast-to-nucleus ROS signalling by transient post-stress induction of tAPX transcription is a strategy to modify cell signalling for some time without affecting the alertness for activation of cold acclimation responses.
Introduction
Most plants of the temperate climate zones are adapted to annual and diurnal temperature variations. They can acclimate to slowly decreasing temperatures and to persisting cold1–3, but are harmed by sudden, short cold snaps4,5. Cold inhibits the Calvin-Benson-Cycle stronger than photosynthetic electron transport6,7. The imbalance between the two photosynthetic processes supports generation of reactive oxygen species (ROS) at the thylakoid membrane6–8. Additionally, cold decreases membrane fluidity, endangers membrane integrity and affects membrane protein function9–11. The impact of cold stress on plant growth and fitness can be severe. For example, three cold days in April 2017 (after a warm start into spring) destroyed up to 95% of the apple and cherry blossoms in Germany´s main fruit cultivation areas close to the Lake Constance, Hamburg (Altes Land) and Berlin (Havelland) and caused an average harvest loss of 46%12. Upon prolonged cold, acclimation processes re-establish photostasis, adjust membrane fluidity and accumulate protectants, such as osmolytes9,13–16. Regulation of metabolism and gene expression starts within minutes17,18, but it takes several days to establish full protection of the plants against chilling and freezing stress4. Induction and maintenance of acclimation are widely under control of the ICE1 (At3g26744)-CBF (C-repeat binding factor)-pathway19. It is costly to keep plants acclimated. Consequently, deacclimation starts as soon as the temperature increases and consumes cold-protective metabolites quickly20. For example, in Arabidopsis thaliana var. Col-0, about 90% of cold-induced carbohydrates are metabolized and gene expression is widely reset within 24 h at optimal growth temperatures21,22.
As shown recently, a single short cold period of 24 h at 4 °C primes Arabidopsis thaliana independent from activation of cold acclimation and modifies its response to future stresses23,24. In cold-primed plants, the pleiotropically stress regulated genes CHS (chalcone synthase; At5g13930) and PAL1 (phenylalanine ammonium lyase; At2g37040) were stronger activated by a second cold stimulus that was applied 5 days after the 24 h priming cold stimulus. During the lag-phase between the two stresses, the transcript levels of CHS and PAL1 were fully reset within the first 24 h at 18–20 °C. They were kept low, until the triggering cold stimulus reactivated their expression. Induction of the chloroplast ROS marker genes ZAT10 (C2H2 zinc finger transcription factor; At1g27730) and BAP1 (BON association protein 1; At3g61190) was almost entirely blocked in primed plants upon the second (=triggering) 24 h cold stress at 4 °C24. Such a modification of the response to a future stress depending on a previous stress over a stress-free period characterizes priming25.
Weakening of the priming effect in a sAPX (stromal ascorbate peroxidase; At4g08390) knock-out line of Arabidopsis thaliana and inversion of the effect in a tAPX (thylakoid ascorbate peroxidase; At1g77490) knock-out line pointed out a regulatory function of chloroplast ascorbate peroxidases in memorizing the priming stimulus24. The genes for ZAT10 and BAP1, which are part of the plant environmental stress control system24,26, respond to chloroplast superoxide and singlet oxygen signals27,28. The signal transduction is still under investigation. As shown in stomata, the SAL1 (At5g63980)-PAP (3′-phosphoadenosine-5′-phosphate)-pathway mediates ZAT10 induction in high-light29. Analysis in the genetic background of the protochlorophyllide accumulating flu1 mutant showed that BAP1 is under control of the EXECUTER pathway30. Besides ROS signalling, cold activates the HOS1 (At2g39810) and OST1 (At4g33950) controlled ICE1-CBF pathway and hormone and metabolic signalling, which cross-talk with ROS signalling19,26.
Priming phenomena have been described for the response to various biotic and abiotic stresses, including cold23,25,31,32. The examples have in common that the stress stimuli were too short or too weak to establish acclimation. Priming typically sets metabolic marks (elicitor factors) or chromatin modifications, which affect signal transduction and gene expression when the plants are triggered by a second stress stimulus23,25,33. Compared to acclimation, which binds large amounts of resources in protection (which could otherwise support growth), the metabolic costs of priming are assumed to be low21,23,34. But even priming can be costly, if it restricts the stress sensitivity or the reaction potentials35,36. Consequently, there is a necessity for “extinction” or at least for an option for “overwriting” of the stress memory required to re-establish stress responsiveness after some time and to avoid exhaustion by accumulative memory formation in response to multiple priming events35,36.
In our initial analysis of cold-priming in Arabidopsis thaliana24, we showed cold-priming of ROS-responsive genes in 4-week-old plants. The plants had formed several rosette leaves, but were still far from initiation of bolting under short-day conditions. If priming competes with growth for resources, the memory should be extinguished or at least weakened before bolting starts to avoid loss of reproductive fitness. In the present study, we analysed cold-priming and the priming stability in the seedling, the pre-bolting and the highly bolting activation-sensitive stage of 2-, 4- and 6-week-old Arabidopsis plants and in young, intermediate and old leaves of 6 week old plants. We show that the primability is regulated more by plant age than by leaf age and correlates with tAPX transcript abundance regulation in response to the priming cold stress. Priming analysis in inducible chloroplast APX over-expresser and silencing lines gave causal evidence that cold-priming is specifically regulated by post-cold regulation of tAPX expression.
Results
Developmental regulation of cold-priming
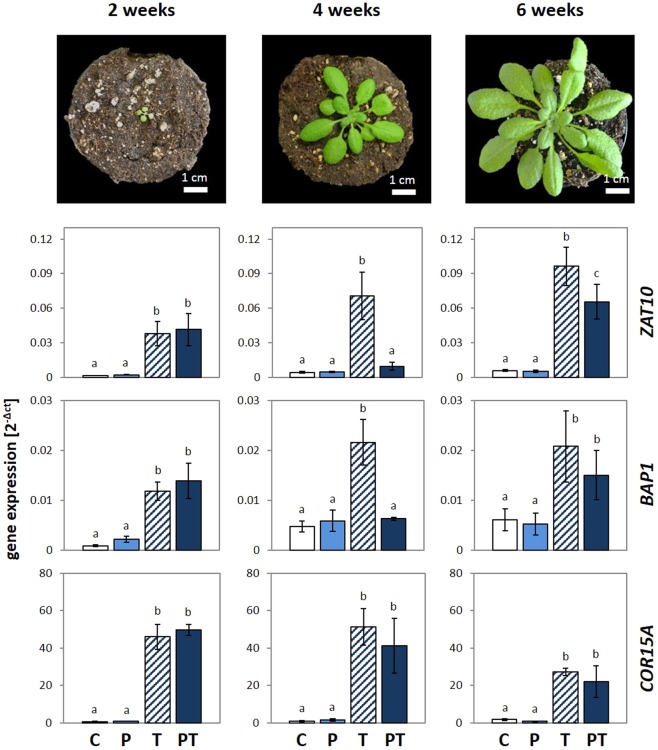
Cold-priming of ROS-signalling was previously analysed in 4-week-old Arabidopsis plants that are in the rosette stage with 12–15 leaves (stage 1.1437), of which more than 80% were still growing in length and width. To test if changes in chloroplast function and metabolism could affect the primability during leaf and plant development, we analysed 2-, 4- and 6-week-old Arabidopsis plants 5 days after cold-priming for priming effects on cold induction of ZAT10 expression. The 2-week-old plants were in the transition from the cotyledon stage to the rosette stage (stage 1.0237) and had just formed the first pair of primary leaves (Fig. 1). At this stage, the seed resources are widely consumed and growth depends on carbohydrate biosynthesis38–40. The 6-week-old plants were in stage 3.70 to 3.9037 (Fig. 1). The oldest leaves had reached their maximum size, while new leaves were still formed in the centre of the rosette (Fig. 1).
Figure 1.
The effect of plant age on priming of ZAT10 and BAP1. Arabidopsis thaliana var. Col-0 were primed at an age of 2, 4 and 6 weeks by a 24 h cold-treatment at 4 °C. The 24 h 4 °C triggering stress was applied 5 days after priming. The transcript abundance for the primable ROS marker genes ZAT10 and BAP1 was evaluated directly after triggering in primed and triggered (PT), only primed (P), only triggered (T) and in control plants (C) and normalized to the geometric mean of the transcript levels of two constitutively expressed genes. As control for monitoring the cold-responsiveness, the transcript levels of the non-primable cold marker gene COR15A were determined. The letters refer to distinct significance groups as determined by ANOVA (Tukey’s test, p < 0.05, n = 3 ± SD).
Prior to analysis of priming effects, the relative cold inducibility of the marker genes was analysed in naïve plants at the time primed plants were triggered (T-plants; Fig. 1). ZAT10 und BAP1 were, like the ICE1-CBF-controlled gene COR15A (cold-regulated gene 15A; At2g42540)41,42, cold-inducible in all three tested developmental stages, but the induction intensity varied with plant age in a gene-specific manner. For ZAT10, the intensity of cold induction steadily increased with age (Fig. 1). The cold responsiveness of BAP1 increased from the youngest to the medium old plants, but not further. The induction of COR15A, that encodes a chloroplast protein protecting the inner envelope membrane43 and served as a reference gene for the not-primable ICE1-CBF pathway24, was high in the youngest and in the medium old rosettes, but weak in the oldest plants.
In plants, which were primed for 24 h at 4 °C at an age of 2 weeks, neither ZAT10, representing the O2−/H2O2 signalling pathway44, nor BAP1, which responds to the singlet oxygen signals44, differed in their response between “triggered only” (T) and “primed and triggered” (PT) plants (Fig. 1). 6-weeks-old plants showed much weaker priming effects (PT/T = induction level in primed and triggered (PT) plants relative to only triggered ones (T)) on cold induction of ZAT10 than 4-week-old plants (Fig. 1). BAP1 did not show a priming effect in the 6-week-old plants. These regulation patterns demonstrated that cold-primability is established at an age between 2 and 4 weeks and fades out later during development. COR15A, which is transcriptionally induced by CBFs45,46, was not primable at any age.
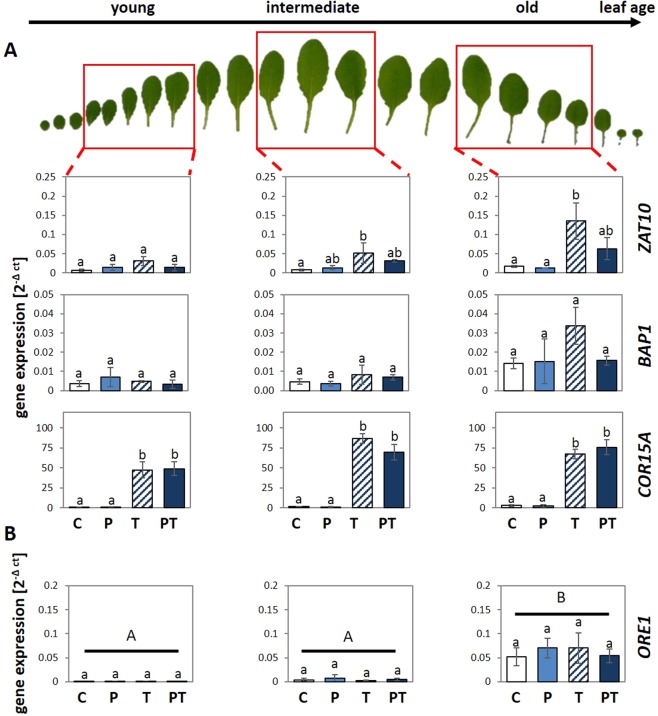
Age-dependent priming regulation within the rosette of 6-week-old plants
To analyse if the weaker primability in 6-week-old plants is linked to specific leaves or characteristic for the entire rosette, the priming effects on ZAT10 and BAP1 transcript levels were compared in young, intermediate and old leaves of 6-week-old Arabidopsis plants (Fig. 2). The oldest leaves expanded between the 2nd and 3rd week of growth (in stage 1.02–1.03) and formed the main leaf biomass in 4-week-old plants. The medium old ones formed the centre of the rosette of 4-week-old plants and were at this stage of development less than 8 mm long, similar to the youngest leaves of 6-week-old-plants. Although the ZAT10 and BAP1 transcript levels did not differ significantly between PT and T plants in the three leaf sets, they showed in all biological replicates a trend towards stronger primability in the oldest leaves of the 6-week-old-plants (Fig. 2A). COR15A was strongly cold-inducible in all leaves, but not sensitive to 24 h cold-priming in any of the three leaf groups (Fig. 2A).
Figure 2.
Priming of leaves in different developmental stages of a 6-week-old rosette. Six week old plants were primed and triggered according to the experimental design (Fig. 7) and harvested after the triggering stimulus was applied. (A) The transcript abundance was measured for the primable ROS marker genes ZAT10 and BAP1 and normalized to the geometric mean of two constitutively expressed genes. Additionally the non-primable cold marker gene COR15A was analysed. (B) Transcript levels of the early senescence gene ORE1 were determined in the same samples as quantitative measure for the onset of senescence. An ANOVA (Tukey’s test, p < 0.05, n = 3 ± SD) was performed. The small letters refer to significance groups with leaf sets of the same age and different capital letters show significant differences between different age groups.
Regulation of onset of senescence and sugar distribution
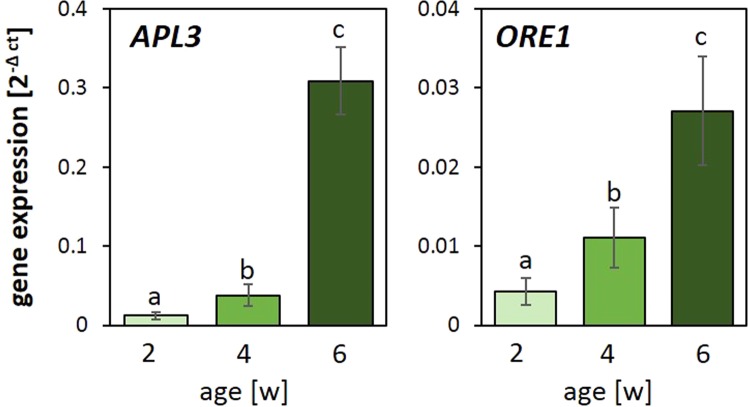
The transcript levels of APL3 and ORE1 were analysed as markers for the physiological status of the plant material (Fig. 3). APL3 (At4g39210) encodes the large subunit of ADP-glucose pyrophosphorylase and supports synthesis of transitory starch in chloroplasts in the feast status47,48. Its expression is induced in the leaf blade upon excess carbohydrate availability and characterizes the carbohydrate storage status of leaves48,49. In our study, APL3 expression was low in 2-week-old plants, slightly higher in 4-week-old ones and strongly elevated in 6-week-old plants (Fig. 3 left) demonstrating that 2-week-old plants were still in the sink-status, 4-week-olds were just in the process of accumulating excess starch and 6-week-olds had a strong carbohydrate storage setting.
Figure 3.
Normalized transcript abundance of APL3 and ORE1 in 2-, 4- and 6-week-old rosettes. The transcript abundance of the carbohydrate sensitive gene APL3 and senescence marker gene ORE1 were determined in 2-, 4- and 6-week-old rosettes and normalized to the transcript levels of two constitutively expressed genes. The letters refer to distinct significance groups as determined by ANOVA (Tukey’s test, p < 0.05, n = 3 ± SD).
Activation of ORESARA 1 (ORE1, ANAC092; At5g39610) marks the onset of senescence prior to the occurrence of visible phenotypes, like chlorosis50–52. The transition process involves an increase in the sensitivity to chloroplast ROS53 and chlorophyll degradation54. In the youngest plants, ORE1 transcript levels were almost not detectable (Fig. 3 right). Consistent with the work by Kim and co-workers55, ORE1 transcripts started to accumulate in 4-week-old plants (Fig. 3). Within the next two weeks, the transcript level more than doubled demonstrating manifestation of the transition.
ORE1 expression was very low in the youngest leaves of 6-week-old plants, only weakly expressed in the intermediate old leaves and activated in the oldest leaves of 6-week-old plants (Fig. 2B). The transcript abundance patterns of APL3 and ORE1 relative to leaf age resembled that of 2-, 4- and 6-week-old plants, demonstrating comparability of the two experimental set-ups of our study (Figs 1 and 2) with respect to carbohydrate and senescence regulation.
ORE1 transcript abundance regulation was not cold-sensitive. Comparison of the transcript levels in T- and PT-plants gave also no indication that the gene is cold-primable (Fig. 2B). The similarity of the ORE1 transcript levels in C, P, T and PT-plants (Fig. 2B) demonstrated that the 24 h 4 °C priming stimulus did not induce or accelerate aging.
Specificity of tAPX regulation in cold-priming of ROS signalling
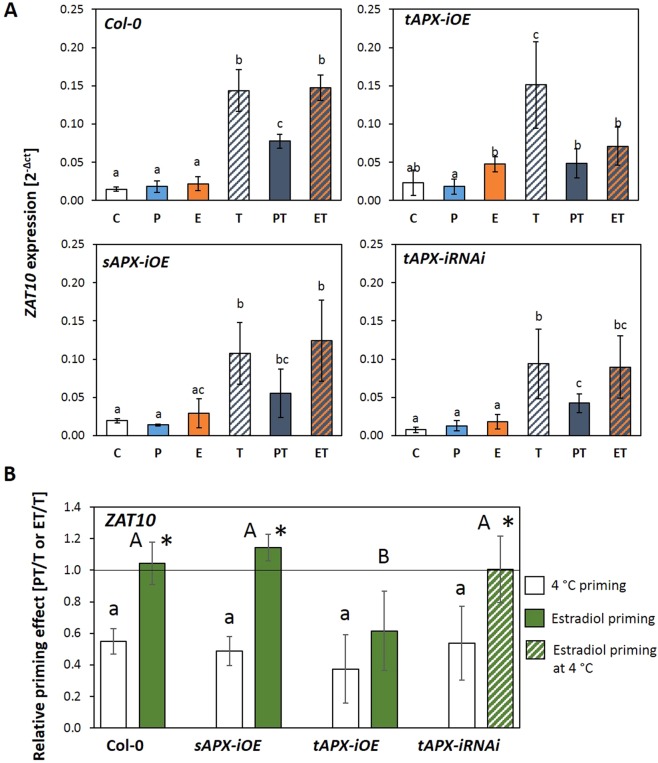
In cold-primed plants, tAPX transcripts and proteins accumulated in the post-stress phase24. The priming effect of 24 h of cold (4 °C) on ZAT10 and BAP1 expression was inverted in tAPX knock-out lines and weakened in sAPX knock-out lines pointing out that tAPX is of stronger importance for setting and maintenance of the priming memory than sAPX24. The catalytic subunits of the two chloroplast APX isoforms are highly conserved56 As shown in sAPX and tAPX-knockout lines, the two enzymes can compensate for the loss of the respective other one under low stress conditions57–61. To differentiate the functions of the closely related genes for tAPX and sAPX in mediating priming, we tested whether the priming effect of a 24 h 4 °C cold pre-treatment can be mimicked in absence of cold solely by transient tAPX over-expression or also by sAPX over-expression (Fig. 4). We induced expression of tAPX and sAPX full-length constructs at 20 °C in 4-week-old Arabidopsis thaliana using an estradiol-inducible system62. As an inverse approach, cold-induced accumulation of tAPX transcript levels was antagonized by transient silencing of tAPX expression in an estradiol-responsive tAPX-RNAi (RNA interference) line60 after a 24 h 4 °C cold stimulus. tAPX and sAPX transcript levels were monitored by qPCR prior to application of the cold trigger (Suppl. 1 and 2) and ZAT10 transcript abundances were analysed before and after triggering (Fig. 4).
Figure 4.
The effect of cold or deregulation of plastidic ascorbate peroxidases on a subsequent cold trigger. (A) ZAT10 transcript levels in control plants (C), only cold-primed (P), only estradiol treated (E), only cold triggered (T) and cold-primed and cold-triggered (PT) and estradiol-treated and cold-triggered (ET) Col-0, sAPX-iOE, tAPX-iOE and tAPX-iRNAi plants of the same age. The tAPX-iRNAi ET plants were cold primed and sprayed with estradiol. The letters refer to distinct significance groups as determined by ANOVA (Tukey’s test, p < 0.05, n = 4 ± SD). (B) Priming effect. ZAT10 transcript abundance in cold (white) or by estradiol spraying (green) primed Col-0, sAPX-iOE and tAPX-iOE and tAPX-iRNAi lines after 24 h cold triggering (PT and ET, respectively) normalized on the transcript abundance in triggered only plants (T-plants). The tAPX-iRNAi plants were cold-primed and sprayed with estradiol (green-white striped). The crude data are identical to those in section A. for calculation of the means, standard deviations and the statistical analysis (one-sided t-Test p < 0.05; n = 4) the PT/T- and ET/T-ratios, respectively, were calculated independently for each biological replicate first. Different small letters show significance of difference in cold primability, different capital letters difference in the cold response after estradiol spraying. The asterisks label significantly different results between cold- and estradiol-priming.
All APX transgenes were strongly inducible by estradiol (Suppl. 1 and 2). Western-Blots demonstrated that induction of the sAPX- and tAPX-iOE (induced overexpresser) constructs increased the APX protein levels (Suppl. 1). The apparent molecular size of the sAPX and tAPX proteins corresponded to that of the mature chloroplast forms reflecting processing of the import signal and, consequently, translocation of the proteins into chloroplasts (Suppl. 1). The estradiol-treatment itself did not affect cold-induction of ZAT10 in Col-0 plants (Fig. 4A; comparison of only cold-treated T- and estradiol- and cold-treated ET-plants). In all three transgenic lines, the gene constructs were (slightly) active in absence of estradiol (Suppl. 1 and 2). The construct leakiness did not affect ZAT10 transcript levels under control conditions in any of the lines (Fig. 4A; C-plants), but the ZAT10 transcript levels were slightly (but not significantly) increased in estradiol-treated plants (Fig. 4A; E-plants). The ZAT10 transcript levels were decreased in estradiol-treated tAPX-iOE plants to similar levels as in cold-primed plants of the same line. Confirming the regulatory function of tAPX expression in mediating priming, the ZAT10 transcript levels were not decreased in the tAPX-iRNAi line after cold pretretament and cold triggering (Fig. 4A; comparison of ET- and PT-plants). Normalization of the PT- and ET-values on the T-value in each independently cultivated and treated biological replicate and calculation of the means and standard derivation between the biological replicates eliminates part of the unspecific background variation (Fig. 4B). The normalized data confirmed that the cold-priming effect on ZAT10 regulation can be mimicked by transient induction of tAPX (tAPX-iOE-line) and blocked by transient silencing of tAPX (tAPX-iRNAi-line). Estradiol-induced sAPX overexpression, which is the stromal counterpart of tAPX with a conserved catalytic domain56, had no effect on the cold response of ZAT10.
Regulation of tAPX promoter activity
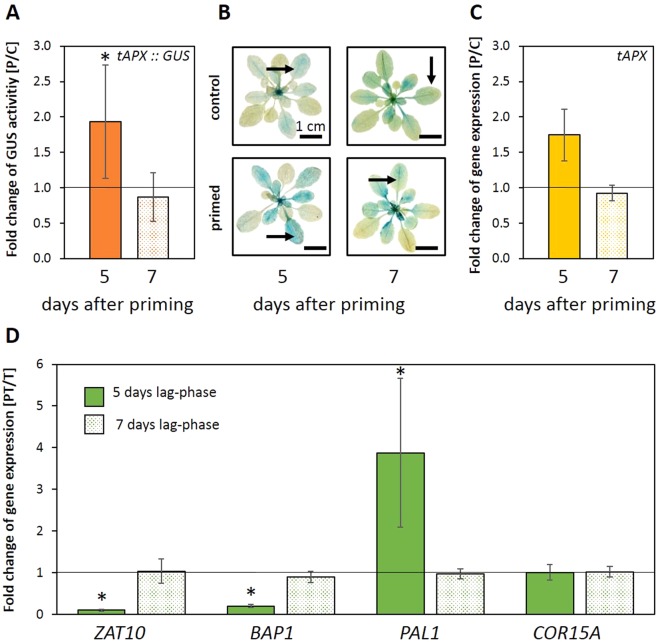
The catalytic site of tAPX, like that of other chloroplast antioxidant enzymes, e.g. sAPX and peroxiredoxins, is sensitive to inactivation upon stressful conditions63,64. Consequently, de novo tAPX synthesis is required to maintain the enzyme activity level in chloroplasts65. To test if stronger transcription of the nuclear located tAPX gene is involved in the priming response we performed full factorial priming assays with C, P, T and PT-plants24 in 4-week-old plants of a reporter gene line expressing a fusion protein of GFP (green-fluorescent protein) and GUS (glucuronidase) under the control of the tAPX promoter (tAPXprom::GFP-GUS). 5 days after priming, higher GUS activities were observed in cold primed plants (Fig. 5A). After priming, the tAPX promoter was strongest activated in the medium old leaves by priming (arrow in Fig. 5B), although the background transcription activity was highest in the youngest leaves.
Figure 5.
The effect of a prolonged lag-phase of 7 days on primable genes and tAPX expression. (A) Quantification of GUS activity in 4-week-old rosettes 5 (orange bar) or 7 (dotted bar) days after priming, respectively. The graph depicts the specific activity in primed plants at the end of the lag-phase relative to the specific activity in control plants (n = 10; mean ± SD, * t-Test p < 0.05. (B) Representative GUS staining pattern of tAPXprom::GUS plants (n = 10) 5 or 7 days after cold-priming and in control plants. The arrows marks the leaf stage that was used for the quantification of GUS activity. (C) Comparison of the normalized tAPX transcript abundance 5 and 7 days after priming relative to the transcript levels in control plants (n = 3; mean ± SD, * one-sided t-Test p < 0.05). (D) Normalized transcript levels of ZAT10, BAP1, PAL1 and COR15A in PT-plants relative to T-plants at the time-point directly after the end of the triggering stimulus after a lag-phase length of either 5 days (green bar) or 7 days (dotted bar) (n = 3; mean ± SD, * one-sided t-Test p < 0.05).
Stability of the priming memory in wildtype plants
7 days after priming, tAPX transcript levels and tAPX promoter activity were (almost) back to the levels detected in naïve plants of the same age (Fig. 5A,C). Consistent with the decrease in tAPX promoter activity, the priming effects were lost for ZAT10 and BAP1 (Fig. 5D). In the original publication on cold-priming of genes in 4-week-old Arabidopsis plants24, we also reported stronger cold activation of pleiotropically stress regulated genes, such as PAL1, 5 days after the priming treatment. Like ZAT10 priming, the expression promoting priming effect on PAL1 was lost 7 days after the cold pre-treatment (Fig. 5D). COR15A expression showed no priming response after a lag-phase of 7 days, as after a lag-phase of 5 days (Fig. 5D).
The analysis of tAPX expression and memory stability regulation was extended to 2- and 6-week-old plants (Fig. 6). In these younger and older plants, tAPX promoter activity (analysed as GUS activity) was not increased 5 days after cold-priming (Fig. 6A). In 2-week-old plants, comparison of tAPX transcript level regulation (Fig. 6B) demonstrated that the tAPX transcript level decreased during 24 h at 4 °C to less than half of the level of naïve plants. Within the next 24 h, the transcript levels in cold-treated plants (P-plants) were indistinguishable from that in control plants (C-Plants). In 6-week-old plants, the tAPX transcripts accumulated on the first day of the post-stress phase to even higher levels than in 4-week-old plants, but declined to levels similar to that in naïve plants within 5 days. The comparison demonstrated, consistent with the GUS-staining patterns (reporting tAPX promoter activity) that 2-week-old plants did not activate tAPX expression after 24 h exposure to 4 °C to levels higher than prior to the priming cold stimulus and that 6-week-old plants lost the tAPX induction effect faster.
Figure 6.
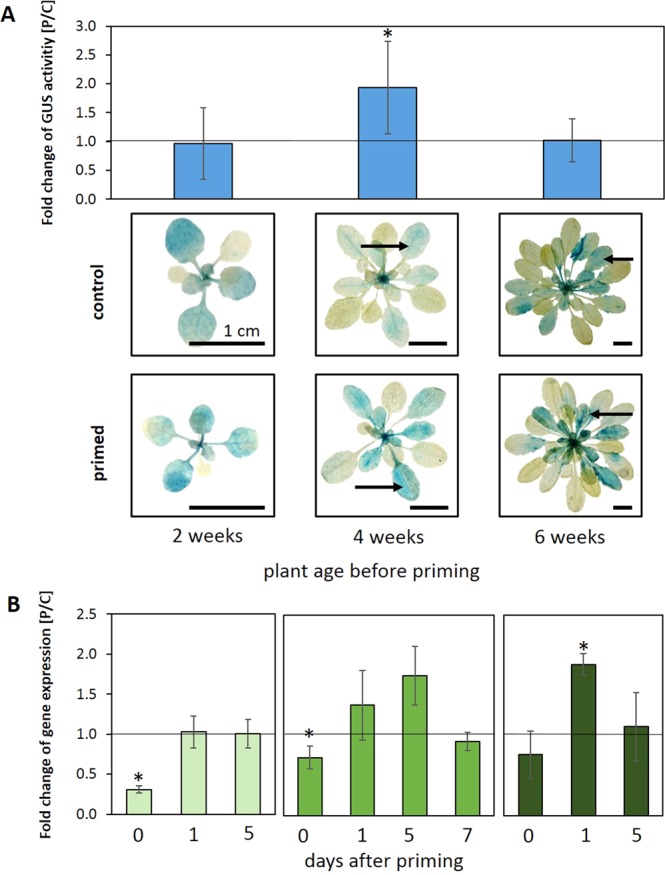
tAPX regulation in response to the priming stimulus. (A) The GUS activity in 2-, 4- and 6-week-old rosettes of primed tAPXprom::GUS reporter gene plants 5 days after priming relative to the activity in untreated control plants of the same line (n = 10; mean ± SD, * t-Test p < 0.05). (B) GUS staining patterns of representative plants out of 10 individuals 5 days after priming (bottom) and un-treated controls (top) at different ages (2-, 4- and 6-week-old). The arrow indicates the developmental stage of leaves used for the GUS activity measurements. (B) tAPx transcript levels 0, 1 and 5 days (and 7 days additionally for 4-week-old plants) after priming relative to the levels of parallel cultivated untreated plants (n = 3; mean ± SD, * one-sided t-Test p < 0.05).
Discussion
Throughout development, cold-priming of ZAT10 regulation correlated with post-cold induction of tAPX expression in Arabidopsis wildtype plants (Figs 5 and 6). Furthermore, overexpression of tAPX at 20 °C mimicked and tAPX silencing (after a 4 °C treatment) antagonized cold-priming of ZAT10 expression regulation (Fig. 4). The tAPX effect could not be replaced by stronger sAPX expression (Fig. 4), although the catalytic domains of sAPX and tAPX are highly conserved56. From these observations, we conclude that cold-priming is causally and specifically regulated by APX availability at the thylakoid membrane. Due to the large stromal domain, tAPX is located in the unstacked areas of thylakoids, where also photosystem I (PS-I) is placed66,67. From various studies, tAPX is known to be the main enzyme detoxifying H2O2 generated at the stromal site of PS-I67–70. However, it´s catalytic centre is sensitive to inactivation by ROS71,72. Recovery takes place by de-novo synthesis and depends on chloroplast-to-nucleus signalling, cytosolic translation and protein import into chloroplasts56. Additionally, tAPX accumulation decreases ZAT10 induction upon stress, but does not antagonize ZAT10 expression per se, as the comparison of C- and E-plants of the tAPX-iOE line at 20 °C and the comparison of C-plants of Col-0 and tAPX-iOE demonstrated (Fig. 4A). If tAPX availably is insufficient upon stress, H2O2 can escape from the thylakoid membrane60,61, accumulate in the stroma, diffuse into the cytosol and, finally, trigger extra-plastidic signalling cascades73,74. The primable genes ZAT10 and BAP1 sensitively respond to chloroplast ROS and are key regulators of plant stress signalling pathways24,28,74,75. They control vitally important stress responses like effector triggered immunity and induction of high light protection26,74,76,77. Attenuating ZAT10 induction by priming specifies ROS-signalling and enables stronger cold induction of pleiotropically stress regulated genes, such as CHS and PAL1, after cold-priming23,24,78,79.
In our study, the cold-priming effect on ZAT10 expression was independent of the metabolite status or senescence regulation, but regulated during rosette developmental (Figs 1–6). We think that the strong primability in the pre-bolting stage evolved in the context of the natural life cycle pattern. Arabidopsis thaliana var. Col-0 typically follows a winter annual growth regime80. Temperatures above 15 °C can shift the life history towards a rapid cycling one80–82. Consitently, the shoot apical meristem of Arabidopsis thaliana forms more than 40 leaves under short day conditions under optimal, stress free lab conditions83,84. Long-day conditions promote bolting. However, the shoot apical meristem is arrested in the vegetative stage even under bolting-promoting long-day conditions up to around 4 weeks37,85. In non-vernalized plants, the lengths of the juvenile and transition phase are genetically fixed86. Prior to bolting, Arabidopsis rather invests in growth and protection of already existing leaves than in formation of new leaves37,87,88 to support habitat occupation and to acquire resources and stability for inflorescence and fruit formation89,90. Arabidopsis bolts in spring after a series of unpredictable cold snaps. In the diversity of vitality promoting mechanisms, priming is assumed to be the least cost intensive one23,25. As shown for COR15A, it does not affect cold induction of canonically cold-regulated cold accumulation processes1 (Figs 1–3), but adjusts cell signalling in a very specific, developmentally controlled and temporally restricted manner.
Conclusion
Cold-priming of chloroplast-to-nucleus ROS signalling is mediated by transcriptional regulation of tAPX availability in a developmentally controlled and erasable manner (Figs 1–6). We have postulated that cold-priming evolved as a specific strategy to manage stress signalling, when stresses occur in an unpredictable pattern and are too short to activate acclimation24. The catalytic site of the main regulator of the cold-priming memory, tAPX, is highly sensitive to inactivation by H2O2/ROS63,64. The “instability” of chloroplast APX against ROS characterizes tAPX as an ideal target for priming regulation: Firstly, the priming setting can be quickly erased upon severe stress by inactivation of tAPX, which avoids fixation into an inappropriate setting35,36. Secondly, the lability of tAPX makes priming depended on de-novo synthesis of tAPX. Transcription in the nucleus, translation in the cytosol, protein import into chloroplasts and embedding of tAPX into the thylakoid membrane enable fine-tuning and cross-talk with other signalling processes38,39,65,91–93. Thirdly, tAPX controls an electron dissipation pathway subordinated to thioredoxin and NADP+ reduction94,95. The tAPX-dependent water-water-cycle is of minor importance at low stress levels59, but has a key function in relaxing the electron pressure in the photosynthetic electron transport chain upon severe imbalances from photostasis96. In addition to chloroplast and cellular ROS levels, tAPX activity also controls electron flux into cyclic photosynthetic electron transport, non-photochemical quenching and plastoquinone reduction28,57,97–99. In our opinion, tAPX is a predetermined breaking point in the centre of the plant stress signalling network. The lability of the catalytic site of tAPX enables plants to switch upon prolonged cold periods from attenuating chloroplast ROS signalling and activating pleotropic stress responses to activation of canonical cold acclimation1. With the onset of acclimation, down-regulation of tAPX expression intensity24,100 can manifest the switch.
The ability to modify the sensitivity of selective stress signalling cascades24 in the trade-off between cold-acclimation, pleiotropic stress protection and growth after short or weak stresses, without blocking the responsiveness of signalling cascades mediating acclimation responses upon prolonged stress (Figs 1 and 2), characterizes priming and explains, in our opinion, manifestation of the process during evolution.
Methods
Plant material and growth conditions
Arabidopsis thaliana var. Col-0 wildtype plants and transgenic lines were grown on Arabidopsis soil [70 volumes “Topferde” (Einheitserde, Sinntal-Altengronau, Germany), 70 volumes “Pikiererde” (Einheitserde, Sinntal-Altengronau, Germany), 25 volumes Perligran Classic (Knauf, Germany)] supplied with 0.5 g l−1 dolomite lime (Deutsche Raiffeisen-Warenzentrale, Germany) and 0,5 g l−1 Axoris Insekten-frei (COMPO, Münster, Germany). After stratification at 4 °C, the plants were cultivated for 2, 4 and 6 weeks in a growth chamber at 10 h light (20 °C, 95–110 μmol photons*m−2*s−1)/14 h dark (18 °C) cycles and a humidity of 60% ± 5% prior to priming (Fig. 7). All cold treatments were performed at 4 °C at the same light intensity and with the same fluorescent stripes (L36W/840 Lumilux Cool White; Osram, Munich, Germany), like in the previous study24. Biological replicates were cultivated and cold-treated independently.
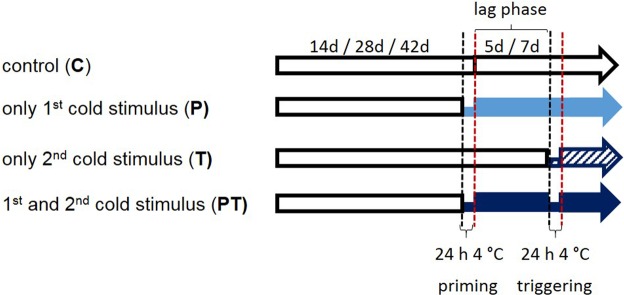
Figure 7.
Outline of the priming experiments. Plants were either grown for 2, 4 or 6 weeks under control conditions, before half of the plants were cold-treated for 24 h at 4 °C (primed, P). Afterwards, the plants were transferred back to the standard growth conditions. Five or seven days later (lag-phase) half of the plants of each group was treated for 24 h at 4 °C (trigger, T). Twice cold-treated plants are referred to as “primed and triggered” (PT), once treated as “only primed” (only the earlier cold treatment) (P) or “only triggered” (only the later cold treatment) (T) and not cold-treated plants as controls (C).
The priming treatments were started 2.5 h after the onset of light and terminated exactly 24 h later to avoid circadian effects. After priming, the plants were transferred back into the 20 °C/18 °C growth regime. Plant material was harvested in primed and parallel grown control plants immediately after priming or 1, 5 or 7 days later. If primed or naïve plants were triggered, the 24 h 4 °C triggering stress was started 5 or 7 days after the end of the priming stimulus 2.5 h after the onset of light. Primed and triggered (PT), only primed (P), only triggered (T) and control plants (C) were harvested at the same time after the 24 h cold stimulus at 4 °C (Fig. 7).
tAPXprom::GUS reporter gene line: construction and analysis
Using the primers CACGTACGGTGGCGAAACG and CACCTCATCAGTTACAAGTGC, a 1468 bp long genomic fragment of Arabidopsis thaliana starting 3 bp upstream of the translation start codon of tAPX (At1g77490), was amplified by PCR and cloned into the GATEWAY vector pENTR D/TOPO (Invitrogen, Carlsbad, U.S.A.) and transferred with LR-Clonase (Invitrogen, Carlsbad, U.S.A.) into the Gateway site of the vector pHGWFS7.0101 upstream of the fused cDNAs for GFP and GUS. Following confirmation of the cloning steps by sequencing, Arabidopsis thaliana var. Col-0 was transformed with the T-DNA using the Agrobacterium tumefaciens strain GV3101 (pMP90). Primary transformants were selected on kanamycin and tested fluorometrically for GFP activity102. Lines were isolated that segregated for single T-DNA insertions in the T2 generation. GUS histochemistry and quantitative GUS activity analysis were performed with homozygous lines according to standard protocols103,104.
Generation, testing and analysis of estradiol-inducible tAPX and sAPX overexpression and silencing lines
Inducible tAPX silencing plants (tAPX-iRNAi) were kindly provided by Shigeru Shigeoka60. The inducible lines overexpressing sAPX and tAPX were generated by amplifying the full length open reading frames (ORF) for sAPX and tAPX by PCR with gene specific primers (forward: GTTGATCAACAATTAAACACAAAAAC, reverse: ACAAAACCAAGGGTGTGTAGTTATA for sAPX; forward: TCAGCTGATAGAAATCATTATCCA, reverse: AAGAAACTCACACTAATCTCAAAATTCT for tAPX) from genomic DNA by ligating the PCR products into the pCR8/GW/TOPO vector (Thermo Fisher Scientific, Germany). After control by sequencing, the APX encoding constructs were cloned into the vector pMDC7105 downstream of an XVE-inducible promoter based on the GATEWAY cloning technique (LR-reaction; Invitrogen, Carlsbad, U.S.A.). The plasmids, additionally harboring an ORF for the chimeric XVE transcription factor were transferred into Agrobacterium tumefaciens GV3101 (pMP90). XVE is activated by estradiol, which leads to the activation of the APX promoter105. Arabidopsis thaliana Col-0 plants were transformed using the floral dip technique106. Transgenic seedlings (T1) were selected on MS agar plates containing 15 µg/ml Hygromycin-B107. For confirmation of the T-DNA identity, the lines were tested for the T-DNA insertions by PCR using a forward primer binding to the vector directly upstream of the insert (GGACACGCTGAAGCTAGT) and gene specific reverse primers (same as used for PCR amplification of open reading frames). T2-seedlings were re-tested on Hygromycin B and analyzed for homozygosity by scoring the survival on Hygromycin-B media in the T3-generation.
For activation of the transgene, soil-grown T3 plants were sprayed with 100 µM β-estradiol (dissolved in 1 ml DSMO and diluted 1:125 in H2O plus 0.1% (v/v) Tween-20). The transgenic lines for the experiments were selected for strong XVE expression by qPCR with XVE specific primers (Table 1). tAPX and sAPX transcript levels were recorded with gene specific primers (Table 1) and tAPX and sAPX proteins were detected by Western Blot analysis as described before24.
Table 1.
List of primers.
| Annotation | AGI code | forward | reverse |
|---|---|---|---|
| ACT2 | At3g18780 | AATCACAGCACTTGCACCAAGC | CCTTGGAGATCCACATCTGCTG |
| APL3 | At4G39210 | AAACCGAGAAGTGCCGGATTG | GTTGGATGCTGCATTCTCCCAAG |
| BAP1 | At3G61190 | ATCGGATCCCACCAGAGATTACGG | AATCTCGGCCTCCACAAACCAG |
| COR15A | At2G42540 | AACGAGGCCACAAAGAAAGC | CAGCTTCTTTACCCAATGTATCTGC |
| ORE1 | At5G39610 | CTTACCATGGAAGGCTAAGATGGG | TTCCAATAACCGGCTTCTGTCG |
| PAL1 | At2G37040 | GCAGTGCTACCGAAAGAAGTGG | TGTTCGGGATAGCCGATGTTCC |
| sAPX | At4g08390 | AGAATGGGATTAGATGACAAGGAC | TCCTTCTTTCGTGTACTTCGT |
| tAPX | At1G77490 | GCTAGTGCCACAGCAATAGAGGAG | TGATCAGCTGGTGAAGGAGGTC |
| YLS8 | At5G08290 | TTACTGTTTCGGTTGTTCTCCATTT | CACTGAATCATGTTCGAAGCAAGT |
| ZAT10 | At1G27730 | TCACAAGGCAAGCCACCGTAAG | TTGTCGCCGACGAGGTTGAATG |
| XVE | non plant | AGATCACAGACACTTTGATCCACC | GAGAGGATGAGGAGGAGCTGG |
For the priming (mimicking) experiments, the plants were grown on soil under the standard growth and priming regimes. Col-0 and the transgenic lines were sprayed with estradiol at the time the priming treatment ended in cold-priming experiments (Fig. 7). To stabilize overexpression and the knock-down effect, the plants were re-treated with estradiol after 3 days.
Quantitative real-time PCR
RNA extraction, cDNA synthesis, contamination controls, qPCR, standardization and quality control were performed as described before24. Each sample was analyzed in triplicates and represents gene expression data from one out of 3–5 independently cultivated biological replicates. The primers used for the qPCR analyses were designed using the QUANTPRIME tool108 and are listed in Table 1.
Statistical analyses
For analysis of variance (ANOVA), Tukey tests (p < 0.05) and Student’s t-Tests (p < 0.05) were performed using the SPSS23 software package (IBM; New York, U.S.A.) or R (www.r-project.org).
Primary data
Primary data can be accessed on PrimeDB (https://primedb.mpimp-golm.mpg.de/?sid=reviewer&pid=79721b8c879ec3e00d0a27f966d340fa).
Supplementary information
Acknowledgements
We thank the German research foundation (CRC973-C4) and the FU Berlin for funding, Shigeru Shigeoka for kindly providing the tAPX-iRNAi line, Thomas Griebel, Elena Reifschneider, Britt Schaffranietz and Ulrike Ellersiek for critical reading and/or technical assistance and Dirk Walter and Rostyslav Braginets for the PrimeDB support. The work was funded by the German Research Foundation (CRC973/C4) and by the FU Berlin.
Author Contributions
J.V.B. performed all experiments with wildtype Arabidopsis and drafted the manuscript and the figures. A.P. generated and selected the inducible APX overexpression lines, performed the pre-tests and the experiments with them and pre-drafted Fig. 4 and the supplements. M.B. supervised the project and finalized the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-39838-3.
References
- 1.Gilmour SJ, Hajela RK, Thomashow MF. Cold-acclimation in Arabidopsis thaliana. Plant Physiology. 1988;87:745–750. doi: 10.1104/pp.87.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guy CL, Niemi KJ, Brambl R. Altered gene-expression during cold-acclimation of spinach. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:3673–3677. doi: 10.1073/pnas.82.11.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gray GR, Chauvin LP, Sarhan F, Huner NPA. Cold acclimation and freezing tolerance - A complex interaction of light and temperature. Plant Physiology. 1997;114:467–474. doi: 10.1104/pp.114.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomashow MF. Plant cold acclimation: Freezing tolerance genes and regulatory mechanisms. Annual Review of Plant Physiology and Plant Molecular Biology. 1999;50:571–599. doi: 10.1146/annurev.arplant.50.1.571. [DOI] [PubMed] [Google Scholar]
- 5.Huner NPA, et al. Photosynthesis, photoinhibition and low-temperature acclimation in cold tolerant plants. Photosynthesis Research. 1993;37:19–39. doi: 10.1007/BF02185436. [DOI] [PubMed] [Google Scholar]
- 6.Hurry V, Strand A, Furbank R, Stitt M. The role of inorganic phosphate in the development of freezing tolerance and the acclimatization of photosynthesis to low temperature is revealed by the pho mutants of Arabidopsis thaliana. Plant Journal. 2000;24:383–396. doi: 10.1046/j.1365-313x.2000.00888.x. [DOI] [PubMed] [Google Scholar]
- 7.Huner NPA, et al. Shedding some light on cold acclimation, cold adaptation, and phenotypic plasticity. Botany-Botanique. 2013;91:127–136. [Google Scholar]
- 8.Ensminger I, Busch F, Huner NPA. Photostasis and cold acclimation: sensing low temperature through photosynthesis. Physiologia Plantarum. 2006;126:28–44. [Google Scholar]
- 9.Steponkus PL. Role of the plasma membrane in freezing injury and cold acclimation. Annual Review of Plant Physiology. 1984;35:543–584. [Google Scholar]
- 10.Guo X, Xu S, Chong K. Cold signal shuttles from membrane to nucleus. Molecular Cell. 2017;66:7–8. doi: 10.1016/j.molcel.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Kaur N, Gupta AK. Signal transduction pathways under abiotic stresses in plants. Current Science. 2005;88:1771–1780. [Google Scholar]
- 12.BMEL. Ernte 2017 - Menge und Preise. Bundesministerium für Ernährung und Landwirtschaft (2017).
- 13.Degenkolbe T, et al. Differential remodeling of the lipidome during cold acclimation in natural accessions of Arabidopsis thaliana. Plant Journal. 2012;72:972–982. doi: 10.1111/tpj.12007. [DOI] [PubMed] [Google Scholar]
- 14.Klotke J, Kopka J, Gatzke N, Heyer AG. Impact of soluble sugar concentrations on the acquisition of freezing tolerance in accessions of Arabidopsis thaliana with contrasting cold adaptation - evidence for a role of raffinose in cold acclimation. Plant Cell and Environment. 2004;27:1395–1404. [Google Scholar]
- 15.Vega SE, del Rio AH, Bamberg JB, Palta JP. Evidence for the up-regulation of stearoyl-ACP (A9) desaturase gene expression during cold acclimation. American Journal of Potato Research. 2004;81:125–135. [Google Scholar]
- 16.Strand Å, et al. Acclimation of Arabidopsis leaves developing at low temperature. Increasing cytoplasmic volume accompanies increased activities of enzymes in the Calvin Cycle and in the sucrose-biosynthesis pathway. Plant Physiology. 1999;119:1387–1397. doi: 10.1104/pp.119.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arae T, et al. Co-ordinated regulations of mRNA synthesis and decay during cold acclimation in Arabidopsis cells. Plant and Cell Physiology. 2017;58:1090–1102. doi: 10.1093/pcp/pcx059. [DOI] [PubMed] [Google Scholar]
- 18.Caldana C, et al. High-density kinetic analysis of the metabolomic and transcriptomic response of Arabidopsis to eight environmental conditions. Plant Journal. 2011;67:869–884. doi: 10.1111/j.1365-313X.2011.04640.x. [DOI] [PubMed] [Google Scholar]
- 19.Thomashow MF, Gilmour SJ, Stockinger EJ, Jaglo-Ottosen KR, Zarka DG. Role of the Arabidopsis CBF transcriptional activators in cold acclimation. Physiologia Plantarum. 2001;112:171–175. [Google Scholar]
- 20.Jackson MW, Stinchcombe JR, Korves TM, Schmitt J. Costs and benefits of cold tolerance in transgenic Arabidopsis thaliana. Molecular Ecology. 2004;13:3609–3615. doi: 10.1111/j.1365-294X.2004.02343.x. [DOI] [PubMed] [Google Scholar]
- 21.Zuther E, Juszczak I, Lee YP, Baier M, Hincha DK. Time-dependent deacclimation after cold acclimation in Arabidopsis thaliana accessions. Scientific Reports. 2015;5:12199. doi: 10.1038/srep12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalberer SR, Wisniewski M, Arora R. Deacclimation and reacclimation of cold-hardy plants: Current understanding and emerging concepts. Plant Science. 2006;171:3–16. [Google Scholar]
- 23.Baier, M., Bittner, A., Prescher, A. & van Buer, J. Preparing plants for improved cold tolerance by priming. Plant Cell and Environment (2018, online first). [DOI] [PubMed]
- 24.van Buer, J., Cvetkovic, J. & Baier, M. Cold regulation of plastid ascorbate peroxidases serves as a priming hub controlling ROS signaling in Arabidopsis thaliana. Bmc Plant Biology16 (2016). [DOI] [PMC free article] [PubMed]
- 25.Hilker, M. et al. Priming and memory of stress responses in organisms lacking a nervous system. Biological Reviews of the Cambridge Philosophical Society91 (2016). [DOI] [PubMed]
- 26.Hahn A, et al. Plant core environmental stress response genes are systemically coordinated during abiotic stresses. International Journal Molecular Sciences. 2013;14:7617–7641. doi: 10.3390/ijms14047617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Op Den Camp RGL, et al. Rapid induction of distinct stress responses after the release of singlet oxygen in arabidopsis. Plant Cell. 2003;15:2320–2332. doi: 10.1105/tpc.014662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laloi C, et al. Cross-talk between singlet oxygen- and hydrogen peroxide-dependent signaling of stress responses in Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:672–677. doi: 10.1073/pnas.0609063103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Estavillo GM, et al. Evidence for a SAL1-PAP chloroplast retrograde pathway that functions in drought and high light signaling in Arabidopsis. Plant Cell. 2011;23:3992–4012. doi: 10.1105/tpc.111.091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee KP, Kim C, Landgraf F, Apel K. EXECUTER1- and EXECUTER2-dependent transfer of stress-related signals from the plastid to the nucleus of Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:10270–10275. doi: 10.1073/pnas.0702061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conrath U, Beckers GJM, Langenbach CJG, Jaskiewicz MR. Priming for enhanced defense. Annual Review of Phytopathology. 2015;53:97–119. doi: 10.1146/annurev-phyto-080614-120132. [DOI] [PubMed] [Google Scholar]
- 32.Hossain MA, et al. Heat or cold priming-induced cross-tolerance to abiotic stresses in plants: key regulators and possible mechanisms. Protoplasma. 2017;255:399–412. doi: 10.1007/s00709-017-1150-8. [DOI] [PubMed] [Google Scholar]
- 33.Thellier M, Luttge U. Plant memory: a tentative model. Plant Biology. 2013;15:1–12. doi: 10.1111/j.1438-8677.2012.00674.x. [DOI] [PubMed] [Google Scholar]
- 34.Lozano-Duran R, Zipfel C. Trade-off between growth and immunity: role of brassinosteroids. Trends in Plant Science. 2015;20:12–19. doi: 10.1016/j.tplants.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 35.van Hulten M, Pelser M, van Loon LC, Pieterse CMJ, Ton J. Costs and benefits of priming for defense in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:5602–5607. doi: 10.1073/pnas.0510213103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crisp PA, Ganguly D, Eichten SR, Borevitz JO, Pogson BJ. Reconsidering plant memory: Intersections between stress recovery, RNA turnover, and epigenetics. Science Advances. 2016;2:e1501340. doi: 10.1126/sciadv.1501340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boyes DC, et al. Growth stage-based phenotypic analysis of Arabidopsis: a model for high throughput functional genomics in plants. Plant Cell. 2001;13:1499–1510. doi: 10.1105/TPC.010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pena-Ahumada A, Kahmann U, Dietz KJ, Baier M. Regulation of peroxiredoxin expression versus expression of Halliwell-Asada-Cycle enzymes during early seedling development of Arabidopsis thaliana. Photosynthesis Research. 2006;89:99–112. doi: 10.1007/s11120-006-9087-3. [DOI] [PubMed] [Google Scholar]
- 39.Heiber I, Cai W, Baier M. Linking chloroplast antioxidant defense to carbohydrate availability: the transcript abundance of stromal ascorbate peroxidase is sugar-controlled via ascorbate biosynthesis. Molecular Plant. 2014;7:58–70. doi: 10.1093/mp/sst154. [DOI] [PubMed] [Google Scholar]
- 40.Eastmond PJ, et al. Postgerminative growth and lipid catabolism in oilseeds lacking the glyoxylate cycle. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:5669–5674. doi: 10.1073/pnas.97.10.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Hua J. A moderate decrease in temperature induces COR15a expression through the CBF signaling cascade and enhances freezing tolerance. Plant Journal. 2009;60:340–349. doi: 10.1111/j.1365-313X.2009.03959.x. [DOI] [PubMed] [Google Scholar]
- 42.Lee BH, Henderson DA, Zhu JK. The Arabidopsis cold-responsive transcriptome and its regulation by ICE1. Plant Cell. 2005;17:3155–3175. doi: 10.1105/tpc.105.035568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steponkus PL, Uemura M, Joseph RA, Gilmour SJ, Thomashow MF. Mode of action of the COR15a gene on the freezing tolerance of Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:14570–14575. doi: 10.1073/pnas.95.24.14570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laloi C, Przybyla D, Apel K. A genetic approach towards elucidating the biological activity of different reactive oxygen species in Arabidopsis thaliana. Journal of Experimental Botany. 2006;57:1719–1724. doi: 10.1093/jxb/erj183. [DOI] [PubMed] [Google Scholar]
- 45.Wan F, et al. Heterologous expression of Arabidopsis C-repeat binding factor 3 (AtCBF3) and cold-regulated 15A (AtCOR15A) enhanced chilling tolerance in transgenic eggplant (Solanum melongena L.) Plant Cell Reports. 2014;33:1951–1961. doi: 10.1007/s00299-014-1670-z. [DOI] [PubMed] [Google Scholar]
- 46.Baker SS, Wilhelm KS, Thomashow MF. The 5′-region of Arabidopsis thaliana Cor15a has cis-acting elements that confer cold-regulated, drought-regulated and ABA-regulated gene expression. Plant Molecular Biology. 1994;24:701–713. doi: 10.1007/BF00029852. [DOI] [PubMed] [Google Scholar]
- 47.Villand P, Olsen OA, Kleczkowski LA. Molecular characterization of multiple cDNA clones for ADP-glucose pyrophosphorylase from Arabidopsis thaliana. Plant Molecular Biology. 1993;23:1279–1284. doi: 10.1007/BF00042361. [DOI] [PubMed] [Google Scholar]
- 48.Sokolov LN, Dejardin A, Kleczkowski LA. Sugars and light/dark exposure trigger differential regulation of ADP-glucose pyrophosphorylase genes in Arabidopsis thaliana (thale cress) Biochemical Journal. 1998;336(Pt 3):681–687. doi: 10.1042/bj3360681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rook F, et al. Impaired sucrose-induction mutants reveal the modulation of sugar-induced starch biosynthetic gene expression by abscisic acid signalling. Plant Journal. 2001;26:421–433. doi: 10.1046/j.1365-313x.2001.2641043.x. [DOI] [PubMed] [Google Scholar]
- 50.Balazadeh S, et al. A gene regulatory network controlled by the NAC transcription factor ANAC092/AtNAC2/ORE1 during salt-promoted senescence. Plant Journal. 2010;62:250–264. doi: 10.1111/j.1365-313X.2010.04151.x. [DOI] [PubMed] [Google Scholar]
- 51.Kim JH, Chung KM, Woo HR. Three positive regulators of leaf senescence in Arabidopsis, ORE1, ORE3 and ORE9, play roles in crosstalk among multiple hormone-mediated senescence pathways. Genes & Genomics. 2011;33:373–381. [Google Scholar]
- 52.Hanaoka H, et al. Leaf senescence and starvation-induced chlorosis are accelerated by the disruption of an Arabidopsis autophagy gene. Plant Physiology. 2002;129:1181–1193. doi: 10.1104/pp.011024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woo HR, Kim JH, Nam HG, Lim PO. The delayed leaf senescence mutants of Arabidopsis, ore1, ore3, and ore9 are tolerant to oxidative stress. Plant and Cell Physiology. 2004;45:923–932. doi: 10.1093/pcp/pch110. [DOI] [PubMed] [Google Scholar]
- 54.Qiu, K. et al. EIN3 and ORE1 accelerate degreening during ethylene-mediated leaf senescence by directly activating chlorophyll catabolic genes in Arabidopsis. Plos Genetics11 (2015). [DOI] [PMC free article] [PubMed]
- 55.Kim JH, et al. Trifurcate feed-forward regulation of age-dependent cell death involving miR164 in Arabidopsis. Science. 2009;323:1053–1057. doi: 10.1126/science.1166386. [DOI] [PubMed] [Google Scholar]
- 56.Pitsch NT, Witsch B, Baier M. Comparison of the chloroplast peroxidase system in the chlorophyte Chlamydomonas reinhardtii, the bryophyte Physcomitrella patens, the lycophyte Selaginella moellendorffii and the seed plant Arabidopsis thaliana. BMC Plant Biology. 2010;10:133. doi: 10.1186/1471-2229-10-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maruta T, Sawa Y, Shigeoka S, Ishikawa T. Diversity and evolution of ascorbate peroxidase functions in chloroplasts: More than just a classical antioxidant enzyme? Plant Cell Physiology. 2016;57:1377–1386. doi: 10.1093/pcp/pcv203. [DOI] [PubMed] [Google Scholar]
- 58.Danna CH, et al. Thylakoid-bound ascorbate peroxidase mutant exhibits impaired electron transport and photosynthetic activity. Plant Physiology. 2003;132:2116–2125. doi: 10.1104/pp.103.021717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kangasjärvi S, et al. Diverse roles for chloroplast stromal and thylakoid-bound ascorbate peroxidases in plant stress responses. Biochemical Journal. 2008;412:275–285. doi: 10.1042/BJ20080030. [DOI] [PubMed] [Google Scholar]
- 60.Maruta T, et al. H2O2-triggered retrograde signaling from chloroplasts to nucleus plays specific role in response to stress. Journal of Biological Chemistry. 2012;287:11717–11729. doi: 10.1074/jbc.M111.292847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maruta T, et al. Arabidopsis chloroplastic ascorbate peroxidase isoenzymes play a dual role in photoprotection and gene regulation under photooxidative stress. Plant and Cell Physiology. 2010;51:190–200. doi: 10.1093/pcp/pcp177. [DOI] [PubMed] [Google Scholar]
- 62.Zuo JR, Niu QW, Chua NH. An estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant Journal. 2000;24:265–273. doi: 10.1046/j.1365-313x.2000.00868.x. [DOI] [PubMed] [Google Scholar]
- 63.Hossain MA, Asada K. Inactivation of ascorbate peroxidase in spinach chloroplasts on dark addition of hydrogen peroxide: Its protection by ascorbate. Plant Cell and Environment. 1984;25:1285–1295. [Google Scholar]
- 64.Kitajima S. Hydrogen peroxide-mediated inactivation of two chloroplastic peroxidases, ascorbate peroxidase and 2-Cys peroxiredoxin. Photochemistry and Photobiology. 2008;84:1404–1409. doi: 10.1111/j.1751-1097.2008.00452.x. [DOI] [PubMed] [Google Scholar]
- 65.Baier, M., Pitsch, N. T., Mellenthin, M. & Guo, W. Regulation of genes encoding chloroplast antioxidant enzymes in comparison to regulation of the extra-plastidic antioxidant defense system. in Ascorbate-glutathione pathway and stress tolerance in plants (eds N. A. Anjum, M. -T. Chan, & S. Umar) pp 337–386 (2010).
- 66.Dekker JP, Boekema EJ. Supramolecular organization of thylakoid membrane proteins in green plants. Biochimica Et Biophysica Acta-Bioenergetics. 2005;1706:12–39. doi: 10.1016/j.bbabio.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 67.Mano J, Hideg E, Asada K. Ascorbate in thylakoid lumen functions as an alternative electron donor to photosystem II and photosystem I. Archives Biochemistry Biophysics. 2004;429:71–80. doi: 10.1016/j.abb.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 68.Polle A. Mehler reaction: Friend or foe in photosynthesis? Botanica Acta. 1996;109:84–89. [Google Scholar]
- 69.Mehler AH. Studies on reactions of illuminated chloroplasts .1. Mechanism of the reduction of oxygen and other Hill reagents. Archives of Biochemistry and Biophysics. 1951;33:65–77. doi: 10.1016/0003-9861(51)90082-3. [DOI] [PubMed] [Google Scholar]
- 70.Mano J, Ohno C, Domae Y, Asada K. Chloroplastic ascorbate peroxidase is the primary target of methylviologen-induced photooxidative stress in spinach leaves: its relevance to monodehydroascorbate radical detected with in vivo ESR. Biochimica et Biophysica Acta-Bioenergetics. 2001;1504:275–287. doi: 10.1016/s0005-2728(00)00256-5. [DOI] [PubMed] [Google Scholar]
- 71.Miyake C, Asada K. Inactivation mechanism of ascorbate peroxidase at low concentrations of ascorbate: Hydrogen peroxide decomposes compound I of ascorbate peroxidase. Plant Cell Physiology. 1996;37:423–430. [Google Scholar]
- 72.Kitajima S, Shimaoka T, Kurioka M, Yokota A. Irreversible cross-linking of heme to the distal tryptophan of stromal ascorbate peroxidase in response to rapid inactivation by H2O2. FEBS Journal. 2007;274:3013–3020. doi: 10.1111/j.1742-4658.2007.05829.x. [DOI] [PubMed] [Google Scholar]
- 73.Exposito-Rodriguez M, Laissue PP, Yvon-Durocher G, Smirnoff N, Mullineaux PM. Photosynthesis-dependent H2O2 transfer from chloroplasts to nuclei provides a high-light signalling mechanism. Nature Communications. 2017;8:49. doi: 10.1038/s41467-017-00074-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rossel JB, et al. Systemic and intracellular responses to photooxidative stress in Arabidopsis. Plant Cell. 2007;19:4091–4110. doi: 10.1105/tpc.106.045898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhu Y, Yang H, Mang HG, Hua J. Induction of BAP1 by a moderate decrease in temperature is mediated by ICE1 in Arabidopsis. Plant Physiology. 2011;155:580–588. doi: 10.1104/pp.110.169466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mittler R, et al. Gain- and loss-of-function mutations in Zat10 enhance the tolerance of plants to ablotic stress. FEBS Letters. 2006;580:6537–6542. doi: 10.1016/j.febslet.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hua J, Grisafi P, Cheng SH, Fink GR. Plant growth homeostasis is controlled by the Arabidopsis BON1 and BAP1 genes. Genes & Development. 2001;15:2263–2272. doi: 10.1101/gad.918101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baxter A, Mittler R, Suzuki N. ROS as key players in plant stress signalling. Journal of Experimental Botany. 2014;65:1229–1240. doi: 10.1093/jxb/ert375. [DOI] [PubMed] [Google Scholar]
- 79.Foyer CH, Noctor G. Stress-triggered redox signalling: what’s in pROSpect? Plant Cell and Environment. 2016;39:951–964. doi: 10.1111/pce.12621. [DOI] [PubMed] [Google Scholar]
- 80.Springthorpe V, Penfield S. Flowering time and seed dormancy control use external coincidence to generate life history strategy. eLife. 2015;4:e05557. doi: 10.7554/eLife.05557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Penfield S, Springthorpe V. Understanding chilling responses in Arabidopsis seeds and their contribution to life history. Philosophical Transactions of the Royal Society B-Biological Sciences. 2012;367:291–297. doi: 10.1098/rstb.2011.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Olivas NHD, et al. Natural variation in life history strategy of Arabidopsis thaliana determines stress responses to drought and insects of different feeding guilds. Molecular Ecology. 2017;26:2959–2977. doi: 10.1111/mec.14100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sung SB, Amasino RM. Vernalization and epigenetics: how plants remember winter. Current Opinion in Plant Biology. 2004;7:4–10. doi: 10.1016/j.pbi.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 84.Bastow R, et al. Vernalization requires epigenetic silencing of FLC by histone methylation. Nature. 2004;427:164–167. doi: 10.1038/nature02269. [DOI] [PubMed] [Google Scholar]
- 85.Onouchi H, Coupland G. The regulation of flowering time of Arabidopsis in response to daylength. Journal of Plant Research. 1998;111:271–275. [Google Scholar]
- 86.Lievre M, Granier C, Guedon Y. Identifying developmental phases in the Arabidopsis thaliana rosette using integrative segmentation models. New Phytologist. 2016;210:1466–1478. doi: 10.1111/nph.13861. [DOI] [PubMed] [Google Scholar]
- 87.Alcazar R, Reymond M, Schmitz G, de Meaux J. Genetic and evolutionary perspectives on the interplay between plant immunity and development. Current Opinion in Plant Biology. 2011;14:378–384. doi: 10.1016/j.pbi.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 88.Baier M, Dietz K-J. Primary structure and expression of plant homologues of animal and fungal thioredoxin-dependent peroxide reductases and bacterial alkyl hydroperoxide reductases. Plant Molecular Biology. 1996;31:553–564. doi: 10.1007/BF00042228. [DOI] [PubMed] [Google Scholar]
- 89.Bartelheimer, M., Schmid, C., Storf, J., Hell, K. & Bauer, S. Interspecific competition in Arabidopsis thaliana: A knowledge gap is starting to close. Progress in Botany, 303–319 (2015).
- 90.Barenfaller K, et al. A long photoperiod relaxes energy management in Arabidopsis leaf six. Current Plant Biology. 2016;2:34–45. [Google Scholar]
- 91.Rudnik R, Bulcha JT, Reifschneider E, Ellersiek U, Baier M. Specificity versus redundancy in the RAP2.4 transcription factor family of Arabidopsis thaliana: transcriptional regulation of genes for chloroplast peroxidases. Bmc Plant Biology. 2017;17:144. doi: 10.1186/s12870-017-1092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hiltscher H, et al. The radical induced cell death protein 1 (RCD1) supports transcriptional activation of genes for chloroplast antioxidant enzymes. Frontiers in Plant Science. 2014;5:475. doi: 10.3389/fpls.2014.00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Heiber I, et al. The redox imbalanced mutants of Arabidopsis differentiate signaling pathways for redox regulation of chloroplast antioxidant enzymes. Plant Physiology. 2007;143:1774–1788. doi: 10.1104/pp.106.093328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Asada K. The water-water cycle as alternative photon and electron sinks. Philosophical Transactions of the Royal Society London B-Series Biological Sciences. 2000;355:1419–1431. doi: 10.1098/rstb.2000.0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Noctor G, Reichheld JP, Foyer CH. ROS-related redox regulation and signaling in plants. Seminars in Cell & Developmental Biology. 2018;80:3–12. doi: 10.1016/j.semcdb.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 96.Asada K. The water-water cycle in chloroplasts: Scavenging of active oxygen and dissipation of excess photons. Annual Review Plant Physiology Plant Molecular Biology. 1999;50:601–639. doi: 10.1146/annurev.arplant.50.1.601. [DOI] [PubMed] [Google Scholar]
- 97.Duan M, et al. Antisense-mediated suppression of tomato thylakoidal ascorbate peroxidase influences anti-oxidant network during chilling stress. Plant Physiology Biochemistry. 2012;58:37–45. doi: 10.1016/j.plaphy.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 98.Sun WH, et al. Overexpression of tomato tAPX gene in tobacco improves tolerance to high or low temperature stress. Biologia Plantarum. 2010;54:614–620. [Google Scholar]
- 99.Murgia I, et al. Arabidopsis thaliana plants overexpressing thylakoidal ascorbate peroxidase show increased resistance to Paraquat-induced photooxidative stress and to nitric oxide-induced cell death. Plant Journal. 2004;38:940–953. doi: 10.1111/j.1365-313X.2004.02092.x. [DOI] [PubMed] [Google Scholar]
- 100.Juszczak I, Rudnik R, Pietzenuk B, Baier M. Natural genetic variation in the expression regulation of the chloroplast antioxidant system among Arabidopsis thaliana accessions. Physiologia Plantarum. 2012;146:53–70. doi: 10.1111/j.1399-3054.2012.01602.x. [DOI] [PubMed] [Google Scholar]
- 101.Karimi M, Inze D, Depicker A. GATEWAYTM vectors for Agrobacterium-mediated plant transformation. Trends in Plant Science. 2002;7:193–195. doi: 10.1016/s1360-1385(02)02251-3. [DOI] [PubMed] [Google Scholar]
- 102.Mellenthin M, Ellersiek U, Börger A, Baier M. Expression of the Arabidopsis sigma factor SIG5 is photoreceptor and photosynthesis controlled. Plants. 2014;3:359–391. doi: 10.3390/plants3030359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Abel, S. & Theologis, A. In Arabidopsis Protocols: Methods in Molecular Biology Vol. 82 (eds Martinez-Zapater J. M. & Salinas J.) (Humana Press, 1998).
- 104.Jefferson RA, Kavanagh TA, Bevan MW. GUS fusion: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Curtis MD, Grossniklaus U. A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiology. 2003;133:462–469. doi: 10.1104/pp.103.027979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bernhardt K, Vigelius SK, Wiese J, Linka N, Weber APM. Agrobacterium-mediated Arabidopsis thaliana transformation: an overview of T-DNA binary vectors, floral dip and screening for homzygeous lines. Journal of Endocytobiosis and Cell Research. 2012;22:19–28. [Google Scholar]
- 107.Harrison SJ, et al. A rapid and robust method of identifying transformed Arabidopsis thaliana seedlings following floral dip transformation. Plant Methods. 2006;2:19. doi: 10.1186/1746-4811-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Arvidsson S, Kwasniewski M, Riano-Pachon DM, Mueller-Roeber B. QuantPrime - a flexible tool for reliable high-throughput primer design for quantitative PCR. BMC Bioinformatics. 2008;9:465. doi: 10.1186/1471-2105-9-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.