Abstract
Definitive diagnosis of glomerular disease requires a kidney biopsy, an invasive procedure that may not be safe or feasible to perform in all patients. We developed a noninvasive, accurate, and economical diagnostic assay with easy commercial adaptability to detect recurrent focal segmental glomerulosclerosis (rFSGS) after kidney transplant. Since FSGS involves podocyte damage and death, our approach involved mRNA profiling of cultured podocytes treated with plasma from patients with rFSGS to identify upregulated genes involved in podocyte damage. For concept validation, three upregulated pro-apoptotic candidate genes (IL1β, BMF, and IGFBP3) were selected, and their promoter regions were cloned into a luciferase-based reporter vector and transfected into podocytes to generate stable podocyte cell lines. Strikingly, when exposed to rFSGS patient plasma, these cell lines showed increased reporter activity; in contrast, no reporter activity was noted with plasma from patients with non-recurrent FSGS or membranous nephropathy. Area under the receiver operating characteristics curves (AUCs) for models discriminating between rFSGS and other nephropathies (non-recurrent FSGS and membranous nephropathy) and between rFSGS and non-recurrent FSGS ranged from 0.81 to 0.86, respectively. Estimated sensitivities and specificities for the diagnosis of rFSGS were greater than 80% for the IL1β and BMF cell lines, and were slightly lower for the IGFBP3 cell line. Importantly, the novel approach outlined here for the diagnosis of rFSGS is widely applicable to the design of sensitive and specific diagnostic/prognostic assays for other glomerular diseases.
Keywords: rFSGS, kidney, diagnosis, luciferase, RNA Seq., podocytes, promoter
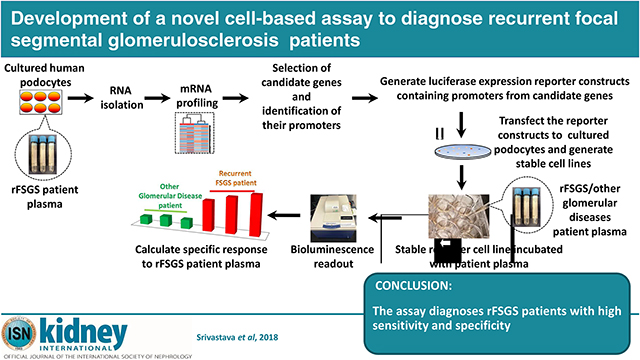
Graphical Abstract
Introduction:
Focal segmental glomerulosclerosis (FSGS) is not a single disease entity but a histologic pattern involving multiple etiologies resulting from podocyte injury and depletion. FSGS accounts for approximately 40% adult and around 20% pediatric cases of nephrotic syndrome. It is the leading glomerular cause of end-stage renal disease (ESRD) [1, 2] in the United States and it’s frequency in African Americans is roughly three and a half times higher than in Caucasians [3]. Despite its increasing prevalance [4], advances in the diagnosis and treatment of FSGS remain inadequate [5]. Since renal biopsy is the gold standard of diagnosis and many adults and pediatric patients are not routinely subjected to this procedure, the incidences and prevalence of FSGS may be substantially underdiagnosed.
In 2004 a working group proposed classification of FSGS into primary/idiopathic and secondary forms [6]. The secondary FSGS includes Familial/genetic forms, virus associated forms, drug-induced forms and forms mediated by adaptive structural-functional responses in the setting of congenital or acquired reduction in renal mass [5]. In contrast, the primary FSGS is considered to result from podocyte damage induced by plasma circulating factor(s), whose identity remains elusive [5]. The presence of circulating factor(s) lead to podocyte damage and proteinuria, immediately (within hours to weeks) following renal transplant, resulting in recurrent FSGS (rFSGS) [5]. Recurrence of FSGS in the allograft is associated with substantial co-morbidity and reduced graft survival. In fact, a study also showed that in comparison to non-recurrent FSGS, recurrent FSGS was associated with 5 fold hazard ratio of graft failure [7]. Currently there are no noninvasive diagnostic assays available to specifically detect the recurrence of FSGS in renal transplant patients. Therefore, a non-invasive assay that would predict FSGS recurrence would be immensely beneficial in not only reducing the overall incidence of recurrent FSGS but also in improved graft survival.
In this study, we report a novel concept that was used to develop a reporter-based assay, where chimeric cell lines were constructed that specifically responded to stimulation by rFSGS patient plasma and may thus serve as a noninvasive diagnostic tool to detect rFSGS. Since rFSGS patients can be segregated from all other (i.e., non-recurrent and secondary) FSGS patients [8], we used these patients to demonstrate the effectiveness of our approach. Importantly, the concept and approach discussed in this study will serve as the basis for developing similar assays for other nephrotic syndromes including primary FSGS, minimal change disease and membranous nephropathy in native kidneys, whose exact diagnosis currently requires invasive procedure such as renal biopsy.
Results :
The overall strategy and hypothesis to develop a cell-based assay for rFSGS:
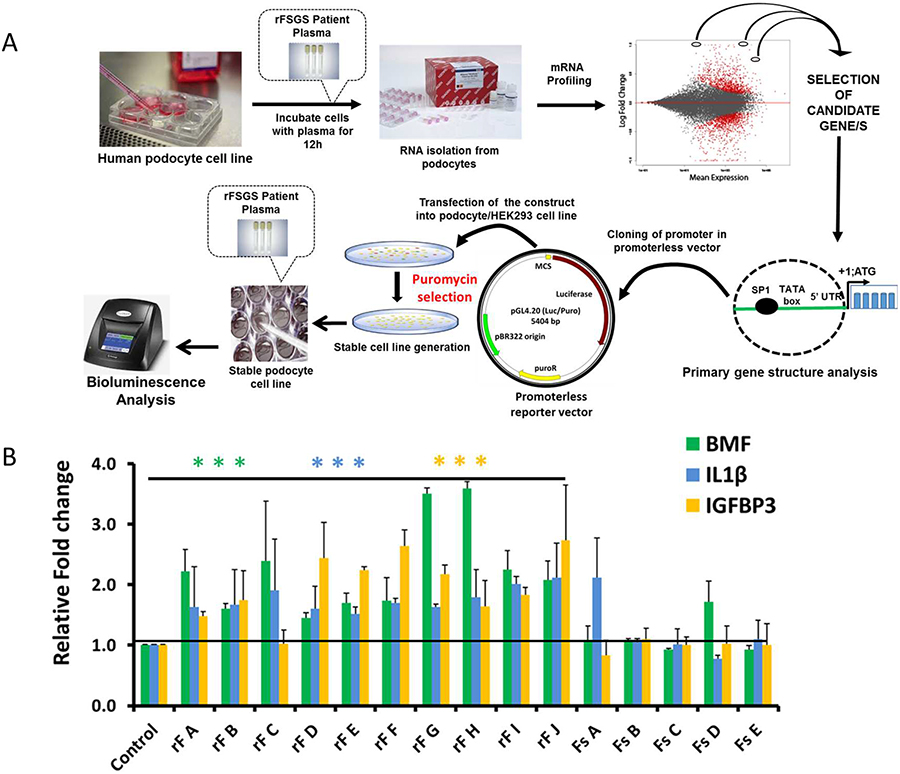
Since FSGS primarily targets podocytes, and the plasma from FSGS patients has been shown to induce podocyte damage [9, 10], we hypothesized that these patients have unique component/s in their plasma, which when added to cultured human podocytes can induce a specific set of genes and pathways involved in cellular damage including apoptosis. We further hypothesized that identification of these rFSGS responsive genes can be performed using the mRNA profiling of rFSGS plasma treated podocytes. Promoter sequences from these genes can then be used to construct reporter-based podocyte cell lines in which, the promoter activity can be selectively induced by treating with plasma from rFSGS patients (Fig. 1A). Furthermore, using these cell lines an assay can be developed that will serve as a diagnostic tool for detecting rFSGS. A schematic representation of this concept is presented in Fig. 1A.
Figure 1:
(A): Schematic of assay development: Total RNA was isolated from podocytes treated with rFSGS and control patient plasma and subjected to mRNA profiling. Candidate upregulated genes with proapoptotic function were selected from the list of DEGs (padj <0.05). Promoter regions from the candidate genes were cloned in a luciferase reporter vector and stable cell lines were constructed using puromycin as a selection marker. Stable cell lines expressing the promoter-driven reporter were treated with plasma from control or rFSGS patients and the reporter activity was measured using ONE-Glo(TM) EX Luciferase Assay System (Promega). Fold changes were calculated after normalizing with the control plasma. (B) Quantitative RT PCR of upregulated candidate genes: qPCR analysis using BMF, IL1β and IGFBP3 gene specific primers showed upregulation of candidate genes in rFSGS plasma (from patients rF A-J) treated podocytes, whereas minimal or no upregulation was noted in FSGS (Fs A-E) treated podocytes. The unpaired t test was performed (*P < 0.05, **P < 0.01 and ***P < 0.01).
Human Subjects:
The study was approved by the Institutional Review Board (IRB) of Medical University of South Carolina (MUSC) (IRB protocols #Pro00018380 and #Pro00019764) and all human subjects signed informed consent prior to the study. Collection of blood samples were performed according to the IRB guidelines provided by MUSC. Over the past two decades our group has been collecting and cataloging plasma primarily from FSGS patients. We used a very stringent criteria for selecting all the patients and controls used for this study. The patient designated of having rFSGS had to have confirmed diagnosis of FSGS in native kidneys, undergo kidney transplants and develop nephrotic range proteinuria and/or histologic findings of podocyte effacement or FSGS within few hours to days or weeks of the transplant (Table S1). The plasma from all the listed patients were collected immediately prior to plasmapheresis, which was initiated as soon as they were diagnosed with heavy proteinuria. We initially identified 30 patients with FSGS; 14 of these patients who received renal transplant showed recurrence of FSGS within hours to weeks. Control subjects included heart transplant recipients (to account for possible nonspecific effects of immunosuppressive drugs), non-recurrent FSGS patients and patient with histologic diagnosis of membranous nephropathy (to account for nonspecidic effects of nephrotic state). Details of the study subjects are provided in Table 1. Plasma samples obtained from the study subjects were aliquoted and stored at −80°C.
Table 1:
Baseline characteristics of all patients
| S.N. | Sex | Race/Ethnicity | Type of transplant | Pre-emptive | Age (Yr) | Pre Transplant Biopsy Proven diagnosis | Transplant | Post Transplant FSGS | *Proteinuria Post transplant (P/C) | Clinical Diagnosis FSGS/rFSGS/MGN/MG |
|---|---|---|---|---|---|---|---|---|---|---|
| Recurrent FSGS | ||||||||||
| rF A | M | African-American | Cadaver | NO | 12 | Y | Y | Y | 1.38 | rFSGS |
| rF B | M | Caucasian | Cadaver | NO | 60 | Y | Y | Y | 5.97 | rFSGS |
| rF C | M | Caucasian | Cadaver | NO | 53 | Y | Y | Y | 6.78 | rFSGS |
| rF D | M | African-American | Cadaver | NO | 28 | Y | Y | Y | 1.30 | rFSGS |
| rF E | M | Caucasian | Cadaver | NO | 55 | Y | Y | Y | 15.21 | rFSGS |
| rF F | M | Caucasian | Cadaver | YES | 62 | Y | Y | Y | 6.16 | rFSGS |
| rF G | M | Caucasian | Cadaver | NO | 46 | Y | Y | Y | 13 | rFSGS |
| rF H | M | African-American | Cadaver | NO | 63 | Y | Y | Y | 3 | rFSGS |
| rF I | M | African-American | Living donor | NO | 37 | Y | Y | Y | 1.3 | rFSGS |
| rF J | M | Caucasian | DNA* | NO | 56 | Y | Y | Y | NA | rFSGS |
| rF K | F | African-American | Cadaver | NO | 35 | Y | Y | Y | 7.14 | rFSGS |
| rF L | M | Caucasian | Cadaver | NO | 5 | Y | Y | Y | 32.69 | rFSGS |
| rF M | F | African-American | Cadaver | NO | 29 | Y | Y | Y | 15.3 | rFSGS |
| rF N | F | African-American | Cadaver | Yes | 46 | Y | Y | Y | 4 | rFSGS |
| FSGS controls | ||||||||||
| Fs A | F | African-American | 9 | Y | 28.5 | FSGS, | ||||
| Fs B | F | African-American | 84 | Y | 6.8 | FSGS | ||||
| Fs C | F | Caucasian | 67 | Y | 4.27 | FSGS | ||||
| Fs D | M | Philippine | 24 | Y | 10 | FSGS | ||||
| Fs E | M | Caucasian | 45 | Y | 11.7 | FSGS | ||||
| Fs F | M | African-American | 69 | Y | 4.87 | FSGS | ||||
| Fs G | M | Caucasian | 68 | Y | 0.6 | FSGS | ||||
| Fs H | M | African-American | 36 | Y | 14.73 | FSGS | ||||
| Fs I | M | Caucasian | 37 | Y | 15.69 | FSGS | ||||
| Fs J | F | African-American | 25 | Y | 0.88 | FSGS | ||||
| Fs K | M | Caucasian | 48 | Y | 20.9 | FSGS, collapsing | ||||
| Fs L | F | African-American | 39 | Y | 1.2 | FSGS, collapsing | ||||
| Fs M | M | African-American | 47 | Y | 3.5 | FSGS, collapsing | ||||
| Fs N | F | African-American | 34 | Y | 20.98 | FSGS, collapsing | ||||
| Fs O | M | African-American | 58 | Y | 22.7 | FSGS | ||||
| Fs P | M | Caucasian | 55 | Y | 0.6 | FSGS | ||||
| Other Nephropathy and non-nephropathy controls | ||||||||||
| MGN A | F | 61 | Y | 20 | MGN | |||||
| MGN B | M | 69 | Y | 13.7 | MGN | |||||
| MG1 | F | 47 | Y | DNA* | Myasthenia gravis | |||||
| Control | F | 20 | Y | DNA* | Heart Transplant | |||||
DNA: Data not available
The plasma from rFSGS patients induces podocyte actin cytoskeleton damage:
Since plasma from rFSGS patients has been shown to induce podocyte actin cytoskeleton damage leading to podocyte loss [11], we first tested if the plasma from rFSGS patients to be used in this study induces podocyte cytoskeletal disorganization. Thus, cultured human podocytes were treated with plasma from three rFSGS patients (rF A, D and F), two non-recurrent FSGS patients (Fs A and B) and two membranous nephropathy (MGN) patients (MGN A and B). As expected, the immunofluorescence analyses revealed significant changes to the cytoplasmic distribution of actin stress fibers in rFSGS plasma when compared to the cells treated with non recurrent FSGS, MGN and control plasma, which showed predominantly cortical distribution (Fig. S1A). Quantification of changes in the actin cytoskeleton staining patterns showed >80% cells with altered actin cytoskeleton morphology depicting cellular damage in rFSGS plasma treated podocytes, whereas the podocytes treated with non-recurrent FSGS and MGN plasma showed < 30% damage and the control plasma showed <20% cellular damage (Fig. S1B). These results are consistent with previously published observations, where similar changes to podocyte actin cytoskeleton by rFSGS plasma demonstrating its potency have been reported [11, 12]. It should be noted that the control plasma was derived from a heart transplant patient, who had a similar drug profile as the rFSGS patients with no history of nephrotic disease.
Plasma from rFSGS patients induces differential expression of several genes in podocytes:
Since all forms of FSGS primarily target podocytes [9, 10], which ultimately leads to podocyte dysfunction and death, we next evaluated if treatment of human podocytes with rFSGS patients plasma induces genes involved with cellular damage/apoptosis. Indeed, 12 hour treatment of cultured podocytes with plasma from two different rFSGS patients (rF A and D) showed differential expression (DE) of several genes (supplementary info). Although several genes were differentially expressed, but to prove our concept and validate our approach, we selected BMF (BCl2 modifying factor), IL-1β (Interleukin 1 beta), IGFBP3 (Insulin growth factor binding protein 3) genes that were upregulated and have proapoptotic functions [13–15]. To further validate the upregulation of these three candidate genes, quantitative RT-PCR was performed using gene specific primers. Expectedly, podocytes treated with rFSGS patient plasma showed significantly increased expression of BMF, IL1β and IGFBP3 genes, when compared to the non-recurrent FSGS and control patient plamsa (Fig. 1B). Additionally, we selected LAMP3 (Lysosomal-Associated Membrane Protein 3) gene as a negative control, which was not upregulated in this analyses. Next, promoter regions from all the four genes were identified [based on, the published literature [16, 17] and selecting a region 2.5 to 4 kb upstream of transcription start site], PCR amplified (using primer sets listed in Table S3), and cloned into a promoterless vector {Promega (pGL4.20[luc2/puro])}. The final constructs were verified through sequencing and transfected into cultured human podocytes and stable cell lines for each promoter construct were created using puromycin selection.
The cell lines containing firefly reporter constructs selectively responded to rFSGS patient plasma:
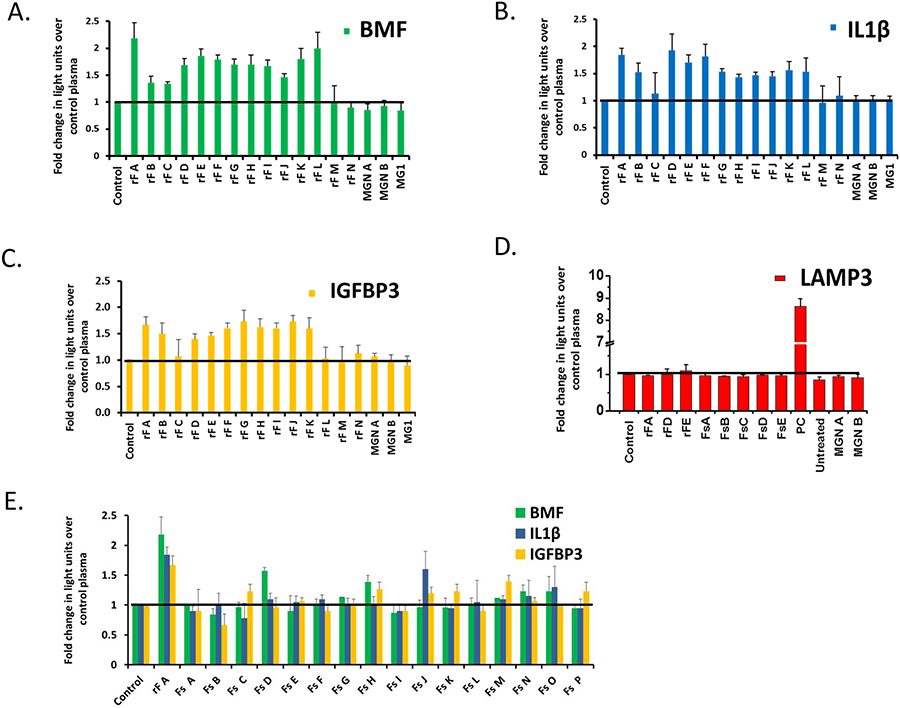
To determine whether the promoter driven reporter in these cell lines are activated by rFSGS plasma, we measured reporter activity of these cell lines following treatment with plasma from various rFSGS patients. In addition to the plasma from two rFSGS patients (rF A & D) that were used for mRNA profiling, plasma from 12 naïve rFSGS patients (rF B, C and E-N), were used. It should be noted that strict criteria were used to clinically confirm the diagnosis of FSGS recurrence in all these patients (Table 1 and Table S1). Remarkably, all the three cell lines (BMF, IL1β, and IGFBP3) showed significant increase in luciferase activity (over the control plasma), when plasma from the rFSGS patients was added to these cells (Fig. 2A–C). Additionally, although the response was variable for each patient (rF A-N), majority of rFSGS patients showed an increase in luciferase activity over the control in these cell lines (Fig. 2A–C). To further determine if the cellular response is independent of host cell line and specific to the reporter gene promoter, the reporter constructs were also transfected into HEK-293 and COS7 cells, and stable cell lines were created and tested in the same assay. Most notably, both the cell lines positively identified rFSGS patients and did not respond to plasma from either a control or non-glomerular disease patient (Fig. S2A and S2B). In contrast, the control LAMP3 podocyte reporter cell line, when treated with plasma from rFSGS, non-recurrent FSGS and other disease patients, did not show any induction of the reporter activity further confirming BMF, IL1β and IGFBP3 as genes responsive to rFSGS patient plasma (Fig. 2D). To further validate the LAMP3 reporter cell line, the induction of LAMP3 promoter with CD63 antibody was used as a positive control (Fig. 2D).
Figure 2: Luciferase Reporter Assay:
The constructed reporter-based podocyte cell lines selectively responded to rFSGS patient plasma but not to control plasma or plasma derived from other nephropathy patients: Treatment of reporter cell lines BMF (A), IL1β (B) and IGFBP3 (C) with plasma from majority of rFSGS patients (A-N) showed elevated reporter activity (~1.5–2.5 fold increase over control), whereas the MGN plasma (MGN A, B) and MG (MG1) plasma showed no response. (D) The negative control, LAMP3 gene promoter reporter cell line was tested with plasma from control patient, rFSGS (rF A, D, E), FSGS (Fs A-E) and MGN (MGN A, B). CD63 Ab was used as a positive control (PC), to induce Lamp3 promoter reporter activity and untreated control cells were used as negative control. (E) The reporter cell lines were evaluated for their specificity by treating them with plasma from non-rFSGS (Fs A-P) patient and the maximal response was noted only from the rFSGS patient A (rF A), which was used as a positive control. The results are expressed as fold change in light units over control plasma, which is the ratio of absolute light units of the experimental samples to that of control plasma treated cell lines. Results represent 3 independent biological repeats and 2 technical repeats.
To further establish the specificity of our approach, we tested the response of these cell lines towards plasma from other glomerular disease patients including non-recurrent FSGS (Fs) and Membranous glomerulopathy (MGN). Unlike the rFSGS plasma, the plasma from these patients did not generate a consistent response with all the cell lines tested (Fig. 2E).
The cell-based assay detects rFSGS patients with high sensitivity and specificity:
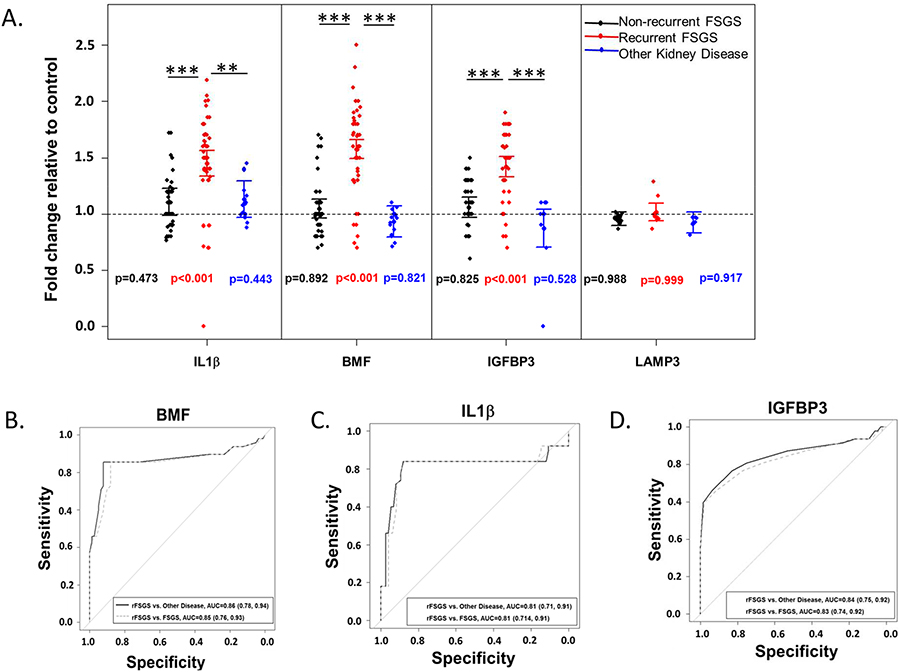
To evaluate the specificity of this assay, differences in mean fold-change response from BMF, IL1β and IGFBP3 cell lines between rFSGS patient samples and other nephropathies were evaluated. Table S4 shows the mean fold change relative to controls in IL1β, BMF, IGFBP3, and LAMP3 for rFSGS patient samples, non-recurrent FSGS samples, MGN and control samples [Myasthenia gravis (MG) and heart transplant]. The mean fold change in reporter activity with standard error for all promoters is presented in Fig. 3A. The initial comparison of relative fold-change in expression showed that relative to controls, there was a significant increase in the expression of BMF, IL1β and IGFBP3 (p < 0.001 for all) in rFSGS samples (Fig. 3A). In contrast, no significant change in the expression of LAMP3 reporter relative to controls in rFSGS samples was noted (p = 0.999). Additionally, there was no significant difference between samples from patients with non-recurrent FSGS or other nephropathies relative to controls (Fig. 3A). Further comparison of rFSGS with all the other samples showed a higher fold change for BMF, IL1β and IGFBP3 gene promoters in the rFSGS group (p < 0.001); whereas no differences were noted for LAMP3 gene promoter activity (p = 0.398). Individual comparisons of IL1β or BMF between groups showed that rFSGS samples exhibited higher relative reporter activity when compared to MGN/controls or non recurrent FSGS (p<0.001); whereas IGFBP3 promoter activity was different at the level of p<0.001 for non recurrent FSGS and p=0.015 for MGN/controls. To further evaluate the discriminative performance for each promoter, the area under the receiver operating characteristics curves (AUCs) for models discriminating between rFSGS versus nephropathies and rFSGS versus non-recurrent FSGS based on fold-change values for IL1β, BMF, and IGFBP3 promoter cell lines were estimated. The AUCs for these models ranged from 0.81 to 0.86 (Fig. 3B–D). Additionally, the estimated sensitivities across all models were greater than 80%. The specificities for IL1β and BMF were greater than 85% for rFSGS vs. all other nephropathies and for rFSGS vs. non-recurrent FSGS. However, the specificities for IGBFP3 were slightly lower at 70% for rFSGS vs. all other nephropathies and 64% for rFSGS vs. non-recurrent FSGS. Discriminative characteristics with 95% confidence intervals for all genes comparing rFSGS vs. all other nephropathies and rFSGS vs. non-recurrent FSGS are shown in Table 2.
Figure 3: The BMF, IL1β and IGFBP3 reporter cell lines detect rFSGS patients with high specificity:
(A) To evaluate the specificity of this assay, differences in mean fold-change response from BMF, IL1β and IGFBP3 cell lines between rFSGS patient samples and other nephropathies were statistically analyzed after pooling the data and were categorized into three groups (Black, FSGS; Red, rFSGS and Blue, MGN/MG/Control plasma). The fold change for BMF, IL1β and IGFBP3 promoter cell lines treated with rFSGS plasma were significantly higher when compared to non-rFSGS/MGN patient plasma. Points are values averaged within subject for each group and the lines represent the 95% confidence interval for mean FC. The horizontal dashed line represents 1-fold change (control). P-values beside the points for each group represent the p-value for comparison to control. Significant differences for pairwise group comparisons are signified by the vertical lines where * is p<0.05, ** is p < 0.01, and *** is p < 0.001. All p-values are corrected for multiple comparisons using Tukey’s honestly significant difference. In contrast, no significant change in the expression of LAMP3 relative in rFSGS samples vs other kidney diseases were observed (p=0.999). Additionally, no differences were observed between samples from patients with FSGS or other nephropathies in this cell line. (B-D) To further evaluate the discriminative performance for each promoter, sensitivity and specificty values for IL1β, BMF, and IGFBP3 promoter cell lines were estimated using receiver operator characteristic curves. The AUCs for model fits discriminating between rFSGS and all other nephropathies and between rFSGS and non-recurrent FSGS ranged from 0.81 to 0.86 respectively, for IL1β and BMF reporter cell lines, whereas these were slightly lower for the IGFBP3 cell line (70% and 64% respectively). AUC, area under the curve.
Table 2:
Area under the receiver operating characteristics curve (AUC), sensitivity, and specificity discriminating between rFSGS and other kidney disease (includes FSGS and MGN), and controls and between rFSGS and FSGS based on FC values of a specific gene. Sensitivity and specificity are estimated from a logistic regression model using predicted probability of having rFSGS as 0.5. We present 95% Wald confidence intervals for AUC and exact 95% confidence intervals for sensitivity and specificity:
| Comparison | Gene | AUC | Sensitivity | Specificity |
|---|---|---|---|---|
| rFSGS vs. Other disease | IL1β | 0.81 (0.71, 0.91) | 0.78 (0.64, 0.88) | 0.87 (0.77, 0.95) |
| BMF | 0.86 (0.78, 0.94) | 0.83 (0.70, 0.93) | 0.91 (0.82, 0.97) | |
| IGFBP3 | 0.84 (0.75, 0.92) | 0.80 (0.75, 0.96) | 0.70 (0.57, 0.81) | |
| rFSGS vs. FSGS | IL1β | 0.81 (0.71, 0.91) | 0.84 (0.71, 0.93) | 0.85 (0.70, 0.93) |
| BMF | 0.85 (0.76, 0.93) | 0.85 (0.72, 0.94) | 0.88 (0.76, 0.95) | |
| IGFBP3 | 0.83 (0.74, 0.92) | 0.80 (0.81, 0.95) | 0.64 (0.49, 0.77) |
Discussion :
Although tissue biopsies are expensive and involve invasive procedures, they are a gold standard of diagnosis for many renal diseases. For glomerular diseases such as FSGS, no other diagnostic procedure closely matches the specificity of a renal biopsy. In this study, we demonstrate a novel concept that was used to develop a noninvasive, accurate and economical diagnostic assay to detect patients in which FSGS can recur but remain underdiagnosed.
Predicting recurrence of FSGS has been a challenging quest for many investigators due to unknown features of humoral factor(s) that are likely responsible for FSGS recurrence and loss of renal allograft [18]. Several approaches have been developed but none of them could be successfully adopted as a routine diagnostic tool in detecting rFSGS. Although the discovery of suPAR as a circulating factor and a biomarker for rFSGS gained a lot of attention, subsequent studies [19–21] concluded that suPAR could be the marker of progression of nephrotic syndrome, but not specifically the FSGS or rFSGS [21, 22], and hence its use in clinical diagnosis of rFSGS remains questionable. Apart from suPAR, glomerular enlargement assay [23], an antibody [24] and cell-culture (5) based approaches were proposed to diagnose rFSGS. However, these assays are time consuming, technically challenging, and involve a panel of antibodies that make them expensive to perform and therefore, unlikely to be adopted commercially. Moreover, due to these limitations these assays will not likely be tested against large sample databases. To overcome these issues, we adopted an innovative approach, which may eventually lead to the development of assays for many different glomerular diseases including primary FSGS in native kidneys. Since rFSGS can be segregated from non-recurrent FSGS and other glomerular diseases (Table S1), we choose rFSGS to demonstrate our proof of concept. Another issue that has compounded the diagnosis of rFSGS is the employment of nonstringent criteria for its diagnosis. In this study, we were extremely cautious in categorizing the rFSGS and strict criteria were enforced, for pre and post transplant diagnosis of FSGS (Table S1). This is also the reason for the limited number of rFSGS patients used in this study; despite this, our study is still one of the largest collection of rFSGS samples (based on the patient database of two largest FSGS cohorts from CureGN and NEPTUNE).
In this study, we describe a novel podocyte cell-based approach (Fig. 1A), which can be easily adapted commercially and has superior detection efficiency in diagnosing rFSGS patients. This is the first study describing a reporter-based system to detect a glomerular disease. While previous approaches have primarily relied on directly analyzing human plasma, we performed profiling of human podocytes that were treated with rFSGS or control patient plasma. Through comparisons with controls, we selected rFSGS responsive genes, whose promoter regions were used to construct three reporter cell lines (BMF, IL1β and IGFBP3) that specifically responded to plasma from rFSGS patients. Importantly, a control cell line (LAMP3) derived from the promoter of a non-responsive rFSGS gene did not respond in this assay. Most notably, the assay responded to patient plasma that was frozen for years, further indicating the feasibility of this approach in a commercial setup that may require transportation of plasma to various centers where diagnosis can be made.
Since we expected a variable response due to patient diversity and possibly different circulating factors, we intended to select multiple promoters for constructing cell lines that are the backbone of this assay. Our statistical analyses further confirms the specificity of these multiple reporter cell lines, which individually provide more than 80% specificity for diagnosis. While we selected only three gene promoters to prove the effectiveness of our concept, it is possible that additional gene promoters can be identified and included in this assay to further strengthen the specificity of this assay. Although specific, it is to be noted that the magnitude of response in this luciferase-based assay was nominal (1.5–2 fold only); however, this response was maintained across the entire cohort tested. Additionally, since this assay was shown to be independent of the host cell line, to further increase the assay response, we can screen multiple cell lines to select a cell line that will generate maximal response from these promoters. This also suggests that further optimization may be required prior to determining its commercial value.
Although our sample size was small due to the limited number of available rFSGS patients for this study, nevertheless our statistical results found high sensitivities and specificities for the selected genes. This provides strong support for the effectiveness of our approach and concept. Strikingly, the assay was able to differentiate between rFSGS from non-recurrent FSGS, a finding of major importance for the optimal management of these patients. We are soliciting multiple centers nationwide to obtain sufficient samples to further power this study and we believe that further improvements will result in its commercial reality. In conclusion, we present a novel concept that was used to develop a cost-effective and technically simple assay to specifically diagnose rFSGS patients. Once developed, this assay can be performed on all patients with suspected FSGS and nephrotic range proteinuria. Ultimately, this assay will not only be able to predict recurrence of FSGS in allografts but also constitute a non invasive tool for diagnosis of primary FSGS in native kidneys caused by permeability factor(s).
Methods :
Cell culture and immunofluorescence microscopy and antibodies:
Human podocytes were cultured using RPMI medium supplemented with 10% FBS, 2 g/liter of NaHCO3, insulin-transferrin-selenium (ITS) supplement and 200 units/ml penicillin and streptomycin as described previously at 33°C and 5% CO 2 [25]. Podocytes were differentiated by thermoswitching to 37°C and removing the ITS supplement from the RPMI media as described previously [25]. Podocytes were grown to 80–90% confluency on glass coverslips coated with collagen. Podocytes were serum starved over-night and treated with 4% patient plasma in serum free RPMI medium for 12–16 hrs. It is evident to mention that the plasma for all the listed patients were collected during plasmapheresis. The patients underwent plasmapheresis as soon as they were diagnosed with heavy proteinuria, and the plasma samples were collected immediately before the plasmapheresis was initiated. Podocytes were fixed with 4% paraformaldehyde and immunostained with Alexa-Fluor-488 phalloidin and DAPI. Representative images from three independent experiments are shown (Fig. S1A). Fluorescence microscopy was performed using Leica Microscope, DMI 4000B and ImageJ software was used to finalize these images. The human embryonic kidney (HEK293) and COS7 cells were cultured in DMEM supplemented with 10% FBS and 1% penicillin and streptomycin. CD63 antibody was procured commercially from ThermoFisher Scientific, Cat No: 10628D.
mRNA profiling of plasma treated podocytes:
RNA isolated from the human podocytes treated with control (one control) and two rFSGS patients (rF A and rF D) plasma were subjected to RNA Seq (mRNA profiling) from the Novogen Genome Sequencing Company or at MUSC facility. RNA samples were isolated from independent experiments performed in triplicate. Quantitative and qualitative analyses of RNA was performed using Nanodrop, agarose gel electrophoresis and Agilent 2100 at Novogen and processed for RNA Seq. Briefly, library construction was performed using poly-T oligo-attached magnetic beads. After qualitative and quantitative (library effective concentration >2nM) assessment of appropriate libraries were fed into HiSeq/MiSeq Illumina platform for the RNA sequencing. Downstream bioinformatics analyses was performed by using a combination of programs including Bowtie2, Tophat2, HTseq, Cufflink and the wrapped scripts at Novagen. Reference genome and gene model annotation files were acquired from NCBI/UCSC/Ensemble genome browser. Indexes of the reference genome were built using Bowtie v2.0.6 and paired-end clean reads were aligned to the reference genome using TopHat v2.0.9. Gene expression level quantification was done using HTSeq v0.6.1 and for each gene read numbers counts were mapped, whereas, Reads Per Kilobase of exon model (RPKM) of each gene was calculated based on the length of the gene and expression levels were analyzed [26]. DESeq2 R package (2_1.6.3) database was used for the differential expression (DE) analyses. The resulting P-values were adjusted using the Benjamini and Hochberg’s approach and adjusted P-value <0.05 found by DESeq2 were assigned as DE. Analyses of DE of two conditions was performed using the DEGSeq R package (1.12.0). The P-values were also adjusted using Benjamini & Hochberg method. Corrected P-value of 0.005 and log2 (Fold change) of 1 were set as threshold for significantly differential expression.
Identification of candidate gene promoters, cloning and generation of stable podocyte cell lines:
Three upregulated candidate genes BMF (BCl2 modifying factor), IL1β (Interleukin1-beta), and IGFBP3 (Insulin like growth factor binding protein 3), which are also involved in cellular apoptosis, were selected from the RNA-Seq data (Geo submission # GSE117669; the top 10 upregulated and downregulated genes are shown in Table S2). LAMP3 gene promoter was chosen as a negative experimental control since it was not upregulated in the profiling data. The promoter regions (2.5 to 4 kb upstream of transcription start site) for these genes were PCR amplified using primers listed in Table S3 and cloned upstream of a luciferase reporter ORF in a promoter less vector pGL4.20Luc/Puro (Promega) [16, 17]. Promoter sequence were obtained from ensemble database and the promoter analyses software Proscan programme v1.7 (https://wwwbimas.cit.nih.gov/cgi-bin/molbio/proscan) was used to confirm the sequence characteristic such as regulatory elements of a promoter like TATA box, transcription factor binding sites etc. Amplified promoter sequences were obtained after extensive PCR optimization and further confirmed by restriction digestion and sequencing. IL1β gene promoter was cloned at NheI/BglII sites (primers 2F/R), whereas BMF and IGFBP3 promoters were cloned at NheI/HindIII sites (primers 1F/R and primers 3F/R respectively) and LAMP3 at NheI/EcoRV sites (primers 4F/R). Stable podocyte cell lines expressing luciferase constructs were generated by transfecting these constructs in cultured podocytes using Lipofectamine-2000 (Thermo Scientific) followed by selection with puromycin. Control stable podocyte cell line was also prepared using blank luciferase vector. In addition to the podocyte cell lines, we also made chimeric human embryonic kidney (HEK293) and COS7 cell lines with the promoters of BMF and IL1β.
Luciferase Assay:
Stable cell lines expressing luciferase constructs were tested for bioluminescence response upon treatment with the plasma of study subjects. Briefly, the cells were cultured in 24 well plates to achieve 90% confluency. The cells were washed with serum free medium, starved overnight in serum free RPMI medium and treated with patient plasma (4% patient or control plasma) diluted in 500 Δl of serum free RPMI without phenol red indicator (to avoid interference in luminescence readings). Cells were incubated further for 12–16 hours. Then luciferase assay was performed using the “ONEGlo(TM) EX Luciferase Assay System” (Promega # E8130) as per the manufacturer’s instructions. Briefly, cells were washed and 150Δl of 1X passive lysis buffer (Promega # E194A) was added to each well and incubated further for 15 min at 4°C with mild shaking. The entire lysate was collected in microtubes and spun at 5000 rpm for 10 minutes at 4°C. 80Δl of supernatant was dispensed in the 96 well white opaque tissue culture plate. 80Δl/well of One Glow reagent (Promega) was added and incubated for 2–3 mins at RT before recording luminescence in a luminometer (Berthold Centro XS3 LB960-DLR Ready-Berthold Technologies, Germany) at an integration time of 1 second.
Quantitative real-time PCR:
To evaluate the expression of BMF, IL1β and IGFBP3 genes, the cultured human podocyte cells were incubated with plasma from rFSGS and FSGS patients. Quantitative real-time PCR (qPCR) was performed using CFX96 real time thermal cycler (Biorad) and the gene-specific primers designed using Generunner (Table S5). Three independent RNA samples were used for analysis. The gene encoding ACTIN protein was used for calibration. Data analyses were performed using threshold cycle (ΔΔCT) method described by Livak and Schmittgen [27].
Statistical analyses:
The data were collected for experiments examining fold change in the expression of BMF, IL1β and IGFBP3 relative to healthy controls in patients with rFSGS, FSGS, MGN, and controls. The luminescence readings were normalized with control readings and relative light units were calculated and plotted as shown in the graphs. Descriptive statistics were calculated for patient samples including the proportion of rFSGS, FSGS, MGN and control patients and the mean fold-change relative to control in IL1β, BMF, IGFBP3 and LAMP3 for each group. The primary goal of this analyses was to determine if there are significant differences in fold-change in luminescence (relative to controls) of IL1β, BMF, IGFBP3 or LAMP3 between rFSGS patients and patients with either FSGS or other nephropathies like MGN. A linear mixed model approach was used to estimate mean fold change relative to controls in samples from rFSGS and patients without recurrent FSGS. The model included a fixed effect for disease type and random subject and batch effects to account for measurements collected on the same subject and samples evaluated in the same experiment. Pairwise comparisons between rFSGS, FSGS, nephropathies patients (like MGN) and controls were conducted to estimate differences in gene expression between the different groups using the Tukey honestly significant difference (HSD) adjustment to control for multiple comparisons. Generalized linear mixed regression models (GLMMs) were also fit to compare the ability of each gene to discriminate between rFSGS versus other nephropathies or control and between rFSGS and FSGS. Area under the receiver operating characteristics curve (AUC), sensitivity, and specificity with 95% confidence interval for each gene were estimated from the GLMM models. All analyses were conducted in SAS v. 9.4 (SAS Institute, Cary NC).
Supplementary Material
Figure S1. Actin cytoskeleton staining: (A) Plasma from rFSGS patients induced actin cytoskeletal reorganization. Representative images of the actin cytoskeleton staining with phalloidin (Green) and DAPI (Blue) of the podocytes treated with rFSGS patient or control plasma are shown in the left panel, FSGS and MGN patients are shown in the right panel. (B) Quantification of altered actin cytoskeleton morphology depicting cellular damage upon treatment of podocytes with rFSGS patients or control plasma. The cellular damage was assessed at >80% in podocytes treated with rFSGS patient plasma, whereas for FSGS and MGN plasma treated podocytes, <30% overall cellular damage was observed (p≤0.05, 2-tailed t-test, >10 cells per experimental condition were evaluated from three experimental repeats). Bar = 25 Δm. *P < 0.05, **P < 0.01 and ***P < 0.01 according to the unpaired t test.
Figure S2. Plasma induced reporter activity is independent of host cell line. The promoter reporter constructs were transfected into (A) HEK293 and (B) Cos7 cell lines. Similar to the chimeric podocyte cell lines, these cell lines also responded selectively to the plasma from rFSGS patients (A to E) but not to the control, FSGS (A to E) or MGN/MG patient/s plasma. (C, D) Differences in mean fold-change response from BMFand IL1β promoter HEK and Cos7 cell lines were statistically analyzed. The data were categorized into three groups (Black, FSGS; Red, rFSGS and Blue, MGN/MG plasma) and the statistics was done using one way ANNOVA with multiple comparison (*P < 0.05, **P < 0.01 and ***P < 0.0). The p values within the group has been calculated according to the unpaired t test.
Table S1: The table shows period of recurrence of FSGS in patients (rF A-N) following renal transplant. The period denotes when the clinical diagnosis was made. In some cases, (*) although patients redeveloped FSGS, but the official reporting was delayed.
Table S2: The table shows top 10 upregulated and downregulated genes from the RNA Seq analyses of patient rF A.
Table S3: List of oligonucleotides used to amplify promoter regions of BMF (1F/R), IL1β (2F/R), IGFBP3 (3F/R) and LAMP3 (4F/R) genes.
Table S4: Mean fold-change in the expression relative to controls by disease status. Values are reported as mean (SE) and are estimated from the linear mixed model.
Table S5: List of oligonucleotides used for RT PCR.
Acknowledgements:
This work was supported in whole or in part by the NIH and NIDDK Grants, 2R01DK087956–06A1 to D. N. and by the South Carolina Clinical & Translational Research Institute, Medical University of South Carolina’s CTSA, NIH/NCRR Grant Number UL1RR029882. The authors also thank the American Society of Nephrology for the Ben J. Lipps Research Fellowship to A.K.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The authors declare that there are no competing interests. Inventors have filed a patent application related to chimeric cell lines containing the candidate gene promoter-reporter constructs.
REFERENCES:
- 1.Kitiyakara C, Eggers P, and Kopp JB, Twenty-one-year trend in ESRD due to focal segmental glomerulosclerosis in the United States. Am J Kidney Dis, 2004. 44(5): p. 815–25. [PubMed] [Google Scholar]
- 2.Kitiyakara C, Kopp JB, and Eggers P, Trends in the epidemiology of focal segmental glomerulosclerosis. Semin Nephrol, 2003. 23(2): p. 172–82. [DOI] [PubMed] [Google Scholar]
- 3.Haas M, et al. , Changing etiologies of unexplained adult nephrotic syndrome: a comparison of renal biopsy findings from 1976–1979 and 1995–1997. Am J Kidney Dis, 1997. 30(5): p. 621–31. [DOI] [PubMed] [Google Scholar]
- 4.Reiser J, Nast CC, and Alachkar N, Permeability factors in focal and segmental glomerulosclerosis. Adv Chronic Kidney Dis, 2014. 21(5): p. 417–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg AZ and Kopp JB, Focal Segmental Glomerulosclerosis. Clin J Am Soc Nephrol, 2017. 12(3): p. 502–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Agati VD, et al. , Pathologic classification of focal segmental glomerulosclerosis: a working proposal. Am J Kidney Dis, 2004. 43(2): p. 368–82. [DOI] [PubMed] [Google Scholar]
- 7.Cosio FG and Cattran DC, Recent advances in our understanding of recurrent primary glomerulonephritis after kidney transplantation. Kidney Int, 2017. 91(2): p. 304–314. [DOI] [PubMed] [Google Scholar]
- 8.Sprangers B, Meijers B, and Appel G, FSGS: Diagnosis and Diagnostic Work-Up. Biomed Res Int, 2016. 2016: p. 4632768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris JJ, et al. , Active proteases in nephrotic plasma lead to a podocin-dependent phosphorylation of VASP in podocytes via protease activated receptor-1. J Pathol, 2013. 229(5): p. 660–71. [DOI] [PubMed] [Google Scholar]
- 10.Kachurina N, et al. , Novel unbiased assay for circulating podocyte-toxic factors associated with recurrent focal segmental glomerulosclerosis. Am J Physiol Renal Physiol, 2016. 310(10): p. F1148–56. [DOI] [PubMed] [Google Scholar]
- 11.Coward RJ, et al. , Nephrotic plasma alters slit diaphragm-dependent signaling and translocates nephrin, Podocin, and CD2 associated protein in cultured human podocytes. J Am Soc Nephrol, 2005. 16(3): p. 629–37. [DOI] [PubMed] [Google Scholar]
- 12.Fornoni A, et al. , Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci Transl Med, 2011. 3(85): p. 85ra46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee HS, et al. , Regulation of apoptosis and inflammatory responses by insulin-like growth factor binding protein 3 in fibroblast-like synoviocytes and experimental animal models of rheumatoid arthritis. Arthritis Rheumatol, 2014. 66(4): p. 863–73. [DOI] [PubMed] [Google Scholar]
- 14.Martin DS, et al. , Apoptotic changes in the aged brain are triggered by interleukin-1beta-induced activation of p38 and reversed by treatment with eicosapentaenoic acid. J Biol Chem, 2002. 277(37): p. 34239–46. [DOI] [PubMed] [Google Scholar]
- 15.Puthalakath H, et al. , Bmf: a proapoptotic BH3-only protein regulated by interaction with the myosin V actin motor complex, activated by anoikis. Science, 2001. 293(5536): p. 1829–32. [DOI] [PubMed] [Google Scholar]
- 16.Shirakawa F, et al. , The human prointerleukin 1 beta gene requires DNA sequences both proximal and distal to the transcription start site for tissue-specific induction. Mol Cell Biol, 1993. 13(3): p. 1332–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, et al. , Bmf is a possible mediator in histone deacetylase inhibitors FK228 and CBHA-induced apoptosis. Cell Death Differ, 2006. 13(1): p. 129–40. [DOI] [PubMed] [Google Scholar]
- 18.Savin VJ, McCarthy ET, and Sharma M, Permeability factors in nephrotic syndrome and focal segmental glomerulosclerosis. Kidney Research and Clinical Practice, 2012. 31(4): p. 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei C, et al. , Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med, 2011. 17(8): p. 952–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallon L and Quaggin SE, SuPAR and FSGS: is the jury still out? Nat Rev Nephrol, 2017. 13(9): p. 593. [DOI] [PubMed] [Google Scholar]
- 21.Kronbichler A, et al. , Soluble Urokinase Receptors in Focal Segmental Glomerulosclerosis: A Review on the Scientific Point of View. J Immunol Res, 2016. 2016: p. 2068691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spinale JM, et al. , A reassessment of soluble urokinase-type plasminogen activator receptor in glomerular disease. Kidney Int, 2015. 87(3): p. 564–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee HS and Lim SD, The significance of glomerular hypertrophy in focal segmental glomerulosclerosis. Clin Nephrol, 1995. 44(6): p. 349–55. [PubMed] [Google Scholar]
- 24.Delville M, et al. , A circulating antibody panel for pretransplant prediction of FSGS recurrence after kidney transplantation. Sci Transl Med, 2014. 6(256): p. 256ra136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagner MC, et al. , Ischemic injury to kidney induces glomerular podocyte effacement and dissociation of slit diaphragm proteins Neph1 and ZO-1. J Biol Chem, 2008. 283(51): p. 35579–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mortazavi A, et al. , Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods, 2008. 5(7): p. 621–8. [DOI] [PubMed] [Google Scholar]
- 27.Livak KJ and Schmittgen TD, Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods, 2001. 25(4): p. 402–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Actin cytoskeleton staining: (A) Plasma from rFSGS patients induced actin cytoskeletal reorganization. Representative images of the actin cytoskeleton staining with phalloidin (Green) and DAPI (Blue) of the podocytes treated with rFSGS patient or control plasma are shown in the left panel, FSGS and MGN patients are shown in the right panel. (B) Quantification of altered actin cytoskeleton morphology depicting cellular damage upon treatment of podocytes with rFSGS patients or control plasma. The cellular damage was assessed at >80% in podocytes treated with rFSGS patient plasma, whereas for FSGS and MGN plasma treated podocytes, <30% overall cellular damage was observed (p≤0.05, 2-tailed t-test, >10 cells per experimental condition were evaluated from three experimental repeats). Bar = 25 Δm. *P < 0.05, **P < 0.01 and ***P < 0.01 according to the unpaired t test.
Figure S2. Plasma induced reporter activity is independent of host cell line. The promoter reporter constructs were transfected into (A) HEK293 and (B) Cos7 cell lines. Similar to the chimeric podocyte cell lines, these cell lines also responded selectively to the plasma from rFSGS patients (A to E) but not to the control, FSGS (A to E) or MGN/MG patient/s plasma. (C, D) Differences in mean fold-change response from BMFand IL1β promoter HEK and Cos7 cell lines were statistically analyzed. The data were categorized into three groups (Black, FSGS; Red, rFSGS and Blue, MGN/MG plasma) and the statistics was done using one way ANNOVA with multiple comparison (*P < 0.05, **P < 0.01 and ***P < 0.0). The p values within the group has been calculated according to the unpaired t test.
Table S1: The table shows period of recurrence of FSGS in patients (rF A-N) following renal transplant. The period denotes when the clinical diagnosis was made. In some cases, (*) although patients redeveloped FSGS, but the official reporting was delayed.
Table S2: The table shows top 10 upregulated and downregulated genes from the RNA Seq analyses of patient rF A.
Table S3: List of oligonucleotides used to amplify promoter regions of BMF (1F/R), IL1β (2F/R), IGFBP3 (3F/R) and LAMP3 (4F/R) genes.
Table S4: Mean fold-change in the expression relative to controls by disease status. Values are reported as mean (SE) and are estimated from the linear mixed model.
Table S5: List of oligonucleotides used for RT PCR.