Abstract
Introduction
Many consequences of cerebrovascular disease are identifiable by magnetic resonance imaging (MRI), but variation in methods limits multicenter studies and pooling of data. The European Union Joint Program on Neurodegenerative Diseases (EU JPND) funded the HARmoNizing Brain Imaging MEthodS for VaScular Contributions to Neurodegeneration (HARNESS) initiative, with a focus on cerebral small vessel disease.
Methods
Surveys, teleconferences, and an in-person workshop were used to identify gaps in knowledge and to develop tools for harmonizing imaging and analysis.
Results
A framework for neuroimaging biomarker development was developed based on validating repeatability and reproducibility, biological principles, and feasibility of implementation. The status of current MRI biomarkers was reviewed. A website was created at www.harness-neuroimaging.org with acquisition protocols, a software database, rating scales and case report forms, and a deidentified MRI repository.
Conclusions
The HARNESS initiative provides resources to reduce variability in measurement in MRI studies of cerebral small vessel disease.
Keywords: Cerebrovascular disease, Stroke, Dementia, Magnetic resonance imaging, Radiology
1. Introduction
Vascular disease contributes to more than half of dementia cases, often in conjunction with Alzheimer's disease pathology [1]. Most of the vascular brain injury is caused by cerebral small vessel disease (cSVD) [2], which often goes clinically unrecognized until revealed by brain imaging. cSVD is strongly associated with cognitive impairment and future risk for cognitive decline and dementia [3], [4]. One of the challenging but intriguing aspects of research in this field is that cSVD has diverse manifestations, including brain infarcts, lacunes, white matter hyperintensity (WMH) of presumed vascular origin, perivascular spaces, and microbleeds [5]. In addition, several promising new imaging biomarkers are emerging for the diagnosis and monitoring of patients, as well as for studies into etiology and pathophysiology [6], [7].
Establishing the Standards for Reporting Vascular Changes on Neuroimaging (STRIVE) [5] was an important first step to harmonize neuroimaging assessment of cSVD. Terms and definitions for common cSVD lesion types, reporting standards, and suggestions for acquisition protocols were provided and are now commonly used in research practice. However, STRIVE did not address pathways for developing and validating new biomarkers, nor did it address sources of variability in measurement, which should be minimized to enhance the ability to detect biological differences in multicenter and longitudinal studies.
To fully realize the potential of neuroimaging biomarkers of cSVD for use in larger scale, multicenter studies including clinical trials with cSVD endpoints, we created the HARmoNizing Brain Imaging MEthodS for VaScular Contributions to Neurodegeneration (HARNESS) initiative. This initiative builds on the work of STRIVE by defining a framework for developing neuroimaging biomarkers of cSVD, reviewing the status of emerging neuroimaging biomarkers in this field, and developing and implementing standardized acquisition protocols and Web-based repositories to facilitate multicenter research.
2. Methods
2.1. HARNESS group composition
HARNESS was funded by the international Joint Program for Neurodegenerative Diseases initiative to analyze the role of neuroimaging biomarkers in neurodegeneration and dementia. The HARNESS members were invited to participate based on their contributions to cSVD research, including their participation in STRIVE, and to provide a balance of input from different geographic regions and research disciplines. HARNESS included 70 members from 29 institutions in 11 countries, representing disciplines including radiology, biomedical engineering, clinical trials, computer science, epidemiology, medical biophysics, neurology, stroke medicine, and psychiatry. Members were surveyed to identify important needs for harmonizing neuroimaging methods for cSVD and then subdivided into 11 working groups of 6–12 participants representing a range of disciplines, cSVD interests, and location to address these needs. The initiative commenced in July 2016 and culminated in an in-person conference in June 2017. Where appropriate, working groups identified relevant articles through literature searches, expert knowledge, and hand searching articles from reference lists, but formal systematic reviews and creation of evidence tables were considered out of scope.
3. Results
3.1. Neuroimaging biomarker framework for cSVD
We adopted the definition of a biomarker used by the Biomarkers Definitions Working Group [8]: “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention”. Inherent to this definition is that biomarkers may have different clinical purposes, including diagnosis, prognosis, monitoring, and measuring treatment response. Biomarkers have been used as surrogate endpoints for clinical trials, meaning that the biomarker substitutes for or represents a manifestation of the clinical endpoint, when the biomarker is expected to predict “clinical benefit or harm based on epidemiologic, therapeutic, pathophysiologic, or other scientific evidence” [9]. This might be considered the highest level of qualification for a biomarker. However, biomarkers have other important uses for investigation, diagnosis, and monitoring of disease even if they do not predict treatment response.
Validation is required to determine whether a biomarker can be considered fit for a specific purpose. Some regulatory authorities, such as the US Food and Drug Administration, define a formal process of biomarker qualification for use in evaluating therapeutics [10]. To our knowledge, no biomarker of cSVD, including WMH, lacunes, or microbleeds, has yet been submitted to and qualified by the US Food and Drug Administration for use in clinical trials, although they have been used as secondary endpoints in imaging substudies [11]. Qualification of an imaging marker that can be used as a trial endpoint would greatly accelerate the development of therapies for cSVD by improving selection criteria, reducing the size and cost of a trial, and increasing the specificity of the outcome.
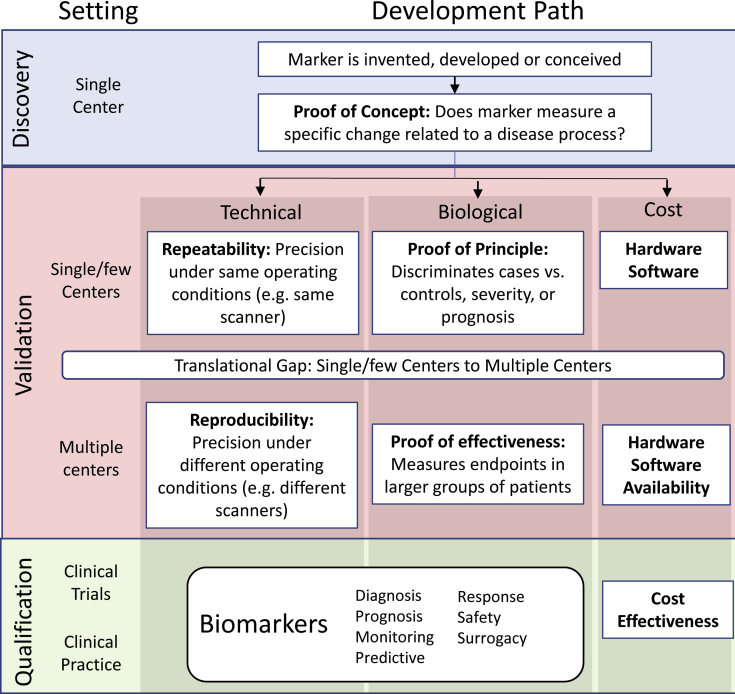
To facilitate validation of cSVD biomarkers, we present a framework for neuroimaging biomarker development in Fig. 1, adapted from consensus recommendations from the European Society of Radiology [12], and for development of imaging biomarkers for oncology [13]. Validation has technical aspects (e.g., can the same measurement be reproduced reliably on the same scanner or different scanners?), biological aspects (e.g., is the measurement different in patients with vs. without cSVD?), and feasibility of implementation (e.g., is the measurement practical and affordable?). In our version of this biomarker-development framework, we define proof of concept as validation of measurement of a specific change or process (e.g., arterial spin-labeling [ASL] magnetic resonance imaging [MRI] generates a signal that correlates with gold standard measurement of perfusion), whereas proof of principle refers to validation that the measurement distinguishes cases from controls or is associated with health outcomes (e.g., ASL-measured perfusion is different in cSVD patients compared with that in controls and is associated with worse prognosis) [12]. We define proof of effectiveness as the ability to measure the marker across larger groups of patients at multiple sites [12]. Repeatability refers to the precision of repeated measurements under the same conditions using the same scanner (with high repeatability conferring greater power to detect smaller within-individual changes over time, important for longitudinal studies), and reproducibility refers to the precision of replicate measurements on the same or similar objects (e.g., a phantom or human volunteers) using different scanners [12], [13]. For visual assessments by human raters, intrarater reliability refers to the precision of measurement by the same rater, whereas interrater reliability refers to the precision of measurements across different raters. The Quantitative Imaging Biomarker Alliance offers recommendations for study design and statistical approaches to technical validation [14]. Validation typically begins with relatively small, cross-sectional studies at single centers to demonstrate proof of concept, proof of principle, and initial technical validation before expanding to longitudinal studies and multicenter studies to demonstrate proof of effectiveness and reproducibility. Feasibility is then demonstrated by incorporation of the biomarker into clinical radiological practice or by qualification for use in clinical trials.
Fig. 1.
Imaging biomarker development framework for cerebral small vessel disease.
3.2. Survey of current cSVD biomarker development with specific considerations for selected emerging modalities
Commonly studied neuroimaging biomarkers of cSVD are lacunes, WMH of presumed vascular origin, and cerebral microbleeds. These lesions are typically reported in routine radiology practice and have been incorporated as secondary imaging endpoints in some clinical trials. For these markers, proof of concept, principle, and effectiveness have been established. Even so, longitudinal data on change over time and data on repeatability and reproducibility, so important for planning sample sizes in clinical trials, are relatively scant [15], [16].
A recent systematic review highlighted the gaps in knowledge in repeatability and reproducibility of measurements of cSVD lesions, focusing mostly on quantitative biomarkers including volumes of WMH, lacunes, and brain [17]. The authors systematically searched the literature to identify information on scan-rescan repeatability (which they termed “within-center reproducibility”) and the effects of scanner vendor, field strength, sequence choices, and coil type. They found that the number of studies on repeatability and reproducibility varied widely by lesion type. The largest number of studies was found for measures of brain volume, probably because brain atrophy is an important biomarker for many neurological diseases in addition to cSVD, such as Alzheimer's disease, and because phantoms are available for measuring variations in geometric distortions across scanners. For WMH, lacunes, perivascular spaces, and microbleeds, there was only sparse information on repeatability with, relatively speaking, the greatest amount of information on WMH measurements cross-sectionally, but no repeatability data on longitudinal measurements.
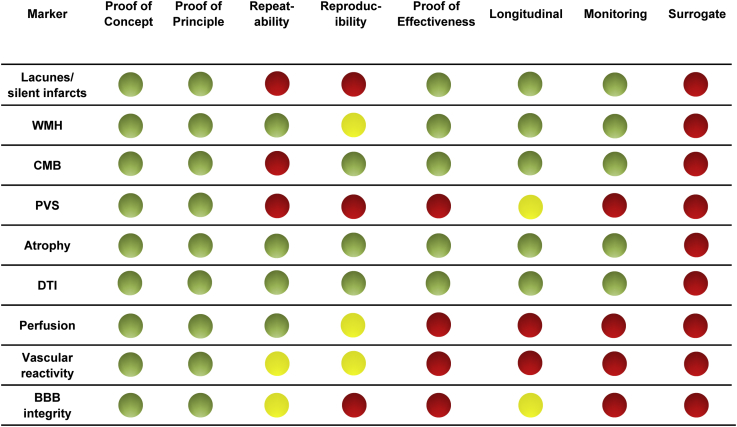
Fig. 2 provides an overview of the validation status of the best established cSVD markers and emerging modalities and techniques. Over time, the list of neuroimaging biomarkers of cSVD has grown substantially as our knowledge of cSVD pathophysiology [2], and ability to image it, has grown.
Fig. 2.
Schematic overview of the neuroimaging biomarker development status for cerebral small vessel disease. The green light indicates validation data from two or more studies from independent research groups; yellow light indicates support from a single study or conflicting evidence from multiple studies; and red light indicates there is currently insufficient evidence. Abbreviations: WMH, white matter hyperintensities of presumed vascular origin; CMB, cerebral microbleeds; PVS, perivascular spaces; DTI, diffusion tensor imaging; BBB, blood-brain barrier. Proof of concept: evidence that the marker measures a specific change or process related to cerebral small vessel disease. Proof of principle/mechanism: evidence that the marker differs between patients with and without cerebral small vessel disease. Proof of effectiveness: evidence from larger scale multiple center studies that the marker differs between patients with and without cerebral small vessel disease. Repeatability: precision of repeated measurements under the same conditions using the same scanner. Reproducibility: replicate measurements on the same or similar objects (e.g., a phantom or human volunteers) in different locations using different scanners. Longitudinal: the rate of change over time has been defined. Monitoring: evidence that longitudinal changes in the marker are associated with progression of cerebral small vessel disease. Surrogate: evidence that changes in the marker are strongly associated with clinical outcomes in cerebral small vessel disease, such that changes in the marker could be considered a substitute for a clinical endpoint.
Some markers have already received a large amount of attention, notably WMH (assessed visually or computationally), lacunes, and microbleeds (mainly visually with some emerging computational methods). Even so, some aspects of validation are lacking with few large comparisons of different volumetric tools and little longitudinal data, and none are yet adopted as confirmed surrogate outcomes in clinical trials. Nonetheless, they have already been the subject of many reviews [16], [17].
Hence, the list of biomarkers discussed in detail here represents the subset that the HARNESS group selected as the next most promising biomarkers for measuring unique aspects of cSVD pathophysiology, which have so far received less attention. The list is not exhaustive. Future research will likely add more modalities and lesion types. For example, microinfarcts have been visualized on MRI by several research groups and may be a frequent but underrecognized consequence of thrombosis or embolism of small arteries [18]. In addition, future research may clarify that biomarkers currently on the list are a poor fit for some purposes.
In the following sections, we review the state of imaging biomarker development for selected emerging modalities, along with considerations for further development and harmonization.
3.3. Structural imaging: perivascular spaces
Perivascular spaces are rapidly emerging as a novel marker of cSVD and are defined as “fluid-filled spaces that follow the typical course of a vessel as it goes through gray or white matter” [5]. Although long considered an innocuous phenomenon of aging, a converging body of proof of principle cross-sectional studies now suggests that a larger burden of perivascular spaces is associated with a higher likelihood of dementia, cognitive impairment, and stroke [19], [20], [21]. More importantly, these associations are independent from established markers of cSVD. Longitudinal studies of the appearance of perivascular spaces or their enlargement over time are lacking; therefore, the rate at which these spaces change over time is essentially unknown. One study showed that the 5-year incidence of new large perivascular spaces (defined as ≥3 mm diameter) in a general elderly population was 3.1% [21]; however, this size exceeds the generally accepted current width boundary between perivascular spaces and lacunes [5].
There are few data on the repeatability of measurements of perivascular spaces and reproducibility of measurement across scanners. For one automated method, repeatability was excellent with intraclass correlations of 0.92 for basal ganglia and 0.87 for centrum semiovale [22]. In contrast, intrarater reliability and interrater reliability for visual rating scales have been published by several groups and should be expected to be good to excellent (i.e., with kappa values of 0.5 or higher or intraclass correlation coefficients of 0.6 or higher). Rating on T2-weighted sequences is favored because perivascular spaces are well visible, but some studies have used high-resolution T1-weighted sequences instead. In one study, ratings on T1-weighted and T2-weighted sequences showed excellent correlation (intraclass correlation > 0.80) [23].
The HARNESS working group identified several difficulties in the quantification of perivascular spaces, which have so far hampered comprehensive understanding of their biological meaning. First, perivascular spaces, reflecting the virtual space between blood vessels and pia mater, by themselves are a physiologic finding. It is the enlargement of these spaces that can be seen on MRI, which is considered nonphysiologic. The question then remains what amount of enlargement should distinguish physiologic from nonphysiologic perivascular spaces? Originally, a convenience threshold was chosen such that any perivascular space visible on brain MRI was considered enlarged. However, increasing field strengths and other advances in imaging now allow much smaller perivascular spaces to become visible on MRI, indicating the need to use a more objective and reproducible threshold independent from imaging parameters.
Second, because perivascular spaces are defined by their intricate relation to brain vessels, they are ubiquitous in all brain regions. Yet, the extent of enlargement is different across brain regions and should be taken into account in their quantification. A working upper width limit of 3 mm is widely used to discriminate perivascular spaces from small lacunes [5], but, for example, it is well recognized that perivascular spaces of larger width are sometimes seen in the substantia innominata. Radiopathological correlation studies show that MRI can differentiate perivascular spaces from lacunes with good sensitivity and specificity using morphological and signal-intensity information [24], but more validation on correlations by region would be welcome. Similarly, the processes underlying their enlargement are thought to differ according to brain region; for example, in cerebral amyloid angiopathy, enlargement of perivascular spaces is seen in the centrum semiovale but not in the basal ganglia [25], [26].
Against this background, it is not surprising that the various efforts to quantify perivascular spaces have differed with respect to definition of enlargement, regions to be scored, and scoring system used [23], [27], [28], [29], [30]. Although work continues to identify the key features of these rating systems with respect to similarities, dissimilarities, strengths, weaknesses, and “translation” from one rating system to the other, we recommend that investigators use the rating system most relevant to their population, or that they are most comfortable with, while having a core understanding how that specific rating system relates to others available in the literature. Raters should be trained on a standardized data set with measurement of intrarater and interrater reliability and report these measures in publications; training tools are available on HARNESS website.
Parallel to this development of visual rating, there is now a strong focus on fully-automated quantification of perivascular spaces. These efforts have so far been hampered by similar methodological considerations as outlined previously in this article, but the recent introduction of machine learning algorithms in brain imaging holds great promise in overcoming these barriers [22], [31]. Just like how automated quantification of WMH resulted in dramatic improvement in our understanding of their role in neurodegenerative diseases particularly at the voxel level, automated detection, volumetrics, shape, density, and orientation of perivascular spaces could signify a paradigm shift in their position within the pantheon of cSVD markers.
3.4. Structural imaging: atrophy in the context of cSVD
Atrophy is now a well-established, measurable consequence of cSVD. Both cross-sectional and longitudinal studies show proof of principle that total brain volume is lower in cSVD and decreases more quickly in persons with enlarging WMH. The repeatability and reproducibility of brain volume measurements in the context of cSVD has been reviewed recently [17]. Here, we highlight specific aspects to be considered when implementing atrophy measurements in cSVD studies.
Given the complexity of brain anatomy, measures of brain volume should be obtained from 3D T1-weighted high-resolution isotropic sequences with quantitative computerized methods where possible. To capture chronic final effects, the image acquisitions should be performed remotely in time (probably 90 days or longer) from the occurrence of acute brain lesions.
At a given time point, volumetric measures reflect the sum of the individual's maximum brain volume growth (estimated by the intracranial cavity volume), the effect of age, and the effect of multiple potential diseases including cSVD, overt stroke, and neurodegenerative diseases such as Alzheimer's disease. Controlling for differences in head size, for example, by expressing volumes as a fraction of intracranial volume or including intracranial volume as a covariate, is mandatory in single time point analyses. Although controlling for intracranial volume is not strictly necessary for longitudinal analyses, investigators may still want to analyze it as a proxy for original maximum brain size, which reflects premorbid brain health and is associated with general intelligence [32]. In longitudinal analyses, the use of cross-timepoint registration pipelines rather than the repeated use of cross-sectional methods may reduce variability in measurement [33], [34], but the optimal approach remains to be confirmed.
Methods involving registration to a common template should be used cautiously given that brains with cSVD, often exhibiting large ventricles and white matter atrophy, can register poorly to atlases based on healthy individuals. This is a particularly challenging problem when cSVD is accompanied by larger destructive intracerebral hemorrhages or infarcts. The impact of brain tissue lesions on the different methods to assess brain volume is often unpredictable [35]. In particular, the presence of extensive WMH can lead to erratic behavior of most algorithms [36], [37], and if appropriate, they should be masked. In addition, algorithms may variably segment fluid-filled cavities within the brain (lacunes and enlarged perivascular spaces) as cerebrospinal fluid, gray matter, or white matter, requiring a systematic visual quality control of segmentation results [35], [38]. There is consensus that cavities resulting from infarction should be excluded from brain tissue estimates [5], depending on the question being asked; clearly, they do not represent spaces such as subarachnoid space or ventricles, nor do they represent normal brain tissue. They can be considered as part of the “total burden of brain injury” [39] in some analyses. Quantitative methods that can estimate perivascular space volume are emerging; when such measurements are made, we recommend that perivascular space volume be reported as a separate tissue class and not included in the total brain volume. Given the numerous sources of variation in gray to white contrast in cSVD, differential measures of gray and white matter volumes should be interpreted carefully [40]. The use of other computational volumetric markers, such as ventricle volumes, has not been validated in cSVD. All methods require visual checking and may need manual editing where automated segmentation has failed to identify the correct tissue.
3.5. Diffusion imaging metrics
Diffusion imaging provides data on the diffusion of water molecules within brain tissue. There are a large variety of techniques to analyze these data. Diffusion-weighted imaging is positive (i.e., shows increased signal) in the setting of recent infarction or microinfarction. Scalar measures describe diffusion properties on the voxel level, such as the extent or directionality. Diffusion tensor imaging (DTI) is the most useful model to derive these scalar metrics such as mean diffusivity (MD) or fractional anisotropy (FA). Tractography can be used to visualize fiber connections and analyze diffusion on the tract level. Global tractography in combination with graph theoretical network analysis allows assessment of the impact of cSVD on the level of brain networks.
Proof of principle that diffusion imaging metrics can serve as biomarkers of cSVD is well established by multiple studies associating diffusion imaging indices derived from the white matter or normal-appearing white matter (NAWM) with cSVD and cSVD risk factors. Most studies report cross-sectional associations between lower FA or higher MD and cognitive and gait impairments [41], [42]. MD is readily measured in the whole brain, tissue subregions, regions of interest, or tracts and shows the strongest associations with SVD lesion burden [43]. Recent, promising postprocessing methods to increase the reliability and ease of extraction of diffusion imaging metrics include histogram-derived diffusion imaging metrics, such as the peak width of the skeletonized MD distribution [44], and connectivity measures including those based on network theory [45], [46], [47]. Lower brain connectivity in strategic network locations, such as long-distance fibers connecting so-called network “hubs”, shows promise for prediction of speed and executive functioning [48], [49]. This is not an exhaustive list as there are several other promising diffusion imaging acquisition and analysis methods that show promise for development as biomarkers of cSVD [50], [51].
In contrast to the many cross-sectional studies, there are fewer studies evaluating diffusion imaging as a prognostic marker of disease progression [41]. The leukoaraiosis and disability study reported an association between NAWM MD at baseline and decline in the processing speed [52], whereas the Radboud University Nijmegen Diffusion Tensor and Magnetic Resonance Cohort study found no association between baseline NAWM MD and cognitive decline [53] or risk of dementia over 5 years [54]. Diffusion imaging–derived brain connectivity predicted conversion to dementia after 5 years [55]. Longitudinal studies of diffusion imaging changes over time are, at this time, relatively scarce [56], [57], [58], [59], [60] but promising, suggesting that changes over time can be detected on diffusion imaging with similar sensitivity as changes over time in WMH volume, requiring smaller sample sizes than required to detect atrophy or incident lacunes [61]. Progression over time in diffusion imaging metrics has been associated with increased risk of dementia [58] and gait decline [62].
The tissue correlate of altered diffusion metrics in cSVD is still debated. A recent study suggests that increased extracellular water content is a major contributor [50].
There are few studies on repeatability and reproducibility. Only one study with patients with cSVD showed high reproducibility of peak width of the skeletonized MD distribution in 7 patients with CADASIL scanned by using a 1.5T and 3T scanner (intraclass correlation 0.95) [44]. Other studies in healthy controls have shown good repeatability and reproducibility for FA and MD measurements (coefficient of variation ranging from 0.8% to 5.7%) [63], [64], [65]. Nonetheless, variation in scanner or scanner upgrades may bias measurements in longitudinal studies [63]; therefore, investigators ideally should avoid scanner upgrades or changing scanners between baseline and follow-up measurements in studies designed to detect small changes over time. Phantoms to estimate reproducibility are in development [66].
3.6. Perfusion and cerebrovascular reactivity
Perfusion and cerebrovascular reactivity (CVR) approaches are highly relevant in cSVD research because reduced tissue perfusion and impaired CVR are hallmark pathological features. These physiological forms of imaging introduce a unique set of challenges for study design, given the large variability in acquisition methods for perfusion especially CVR which are less well established than many structural imaging techniques. To image CVR, the investigator must choose among several experimental methods for stimulating changes in cerebral blood flow (CBF) and among several different acquisition types, such as blood oxygen level dependent (BOLD) or ASL. Because the vascular signal comes from only a proportion of voxel contents (the blood volume fraction in gray matter accounts for 5 to 10% of the tissue volume) and the changes in hemoglobin oxygenation are relatively small for BOLD-related techniques, attention must be paid to ensure sufficient signal-to-noise ratio to generate images of adequate quality.
Dynamic susceptibility contrast and ASL are examples of MRI acquisitions that yield perfusion-weighted images; the former relies on an exogenous gadolinium contrast agent, whereas the latter uses magnetically labeled arterial blood water that is proximal to the imaging volume to label blood and produces quantitative perfusion maps typically expressed in units of mL/100g tissue/minute.
ASL is a promising modality for repeated-measure studies because it does not require administration of an exogenous intravenous contrast agent. A fraction of cSVD articles on perfusion have thus far used ASL [67]; cross-sectional studies, for example, provide proof of principle by showing that a pattern of reduced frontal perfusion was associated with increased WMH volume [68]. Longitudinal studies are less common, however, one 4-year follow-up study reported that global CBF decreases were associated with higher baseline WMH but also that baseline CBF was not associated with greater WMH progression [69]. Another longitudinal study found that although lower baseline CBF predicted appearance of new WMH at 18 months, changes in CBF were not associated with new WMH [70]. Studies are needed on the association of baseline and longitudinal CBF and the prevalence and incidence of new brain infarcts and microinfarcts. Although white matter and subcortical tissue perfusion estimates are of particular interest in cSVD, these measurements are less robust than those in gray matter when using ASL [71] due to the lower CBF and longer arterial transit time.
A validation study of ASL found higher repeatability for pseudo-continuous ASL than for pulsed ASL or continuous ASL, with a coefficient of variation of 3.5% in gray matter and 8.0% in white matter [72]. There are few reproducibility studies across scanner types. One study found high reproducibility in eight volunteers scanned by using two General Electric (GE) 3T scanners [73]. Another study found that sequence parameter differences had a larger effect than hardware or software differences on General Electric, Philips, and Siemens scanners [74]. Phantoms for ASL have been developed but not yet widely adopted [75].
Unlike physiological imaging during a single “baseline” state, CVR involves physiological provocation to measure a vasoactive response, typically by breathing medical air enriched with carbon dioxide gas. Technical and paradigm details and considerations have been recently reviewed [76]. Multicontrast physiological imaging, combining perfusion and CVR maps in cSVD, is a promising technique [77]. At this time, relatively few CVR studies have focused explicitly on cSVD [78]. However, CVR imaging is being exploited as an imaging endpoint to assess the efficacy of vasodilatory drugs in a dose-escalation trial [79]. CVR appears to be a promising prognostic biomarker of cSVD brain changes, for example, as revealed by one longitudinal study that found impaired regional CVR was predictive of WMH lesion expansion at one-year follow-up [80]. A four-year longitudinal study showed that age-related decreases in CVR were associated with steeper declines in processing speed and episodic memory but not working memory or reasoning; however, the degree to which enlarging WMH or new infarction may have been associated with these changes was not assessed. The BOLD response to a visual stimulus has been shown to be a possible biomarker for cerebral amyloid angiopathy and could be a more easily implemented, well-tolerated alternative means to measure CVR, but is limited to the occipital lobe [81], [82], [83] and has not been compared directly to CVR measurement based on hypercapnia.
The repeatability of CVR measurements has been investigated in healthy controls but not in patients with cSVD. In a study of 15 controls, the coefficient of variation ranged from 7.3% to 42.9% across 16 regions of interest, including cortical and subcortical gray matter and white matter [84]. The coefficient of variation was lower when using a paradigm that averaged two three-minute blocks of CO2 inhalation rather than three one-minute blocks [84].
A consensus group has provided recommendations for ASL imaging protocols [85]; however, long-label and long-delay ASL approaches may prove superior for CBF measurement in the white matter and subcortical gray matter. Multicenter studies using scanners from different vendors seem justifiable as long as key methods (including choice of pseudocontinuous ASL, readout strategy, labeling duration, and postlabeling delay time) are kept constant. For CVR imaging, there is a greater diversity of methods, and the different methods may suit specific patient populations. One published protocol [84] using three-minute CO2 blocks is being used in a multicenter trial.
3.7. Blood-brain barrier integrity
Although proof-of-concept evidence is very limited, proof-of-principle evidence from cross-sectional clinical studies suggests that blood-brain barrier (BBB) dysfunction determined by magnetic resonance (MR) is associated with imaging features of cSVD and that BBB leakage may contribute to tissue damage, development of cSVD features, and long-term adverse outcomes [86], [87]. Therefore, BBB permeability is an important target of measurement in studies of pathophysiology and treatment evaluation.
Dynamic contrast-enhanced MRI (DCE-MRI) using a standard dose of a gadolinium-based contrast agent (GBCA) is presently the most promising technique for quantitative imaging of subtle leakage [86] and has been applied in several studies of cSVD and related conditions [86], [88], [89], [90], [91]. However, while the technique is well-established in other conditions such as brain tumors, particular challenges emerge in cSVD due to the slow rate of leakage. For qualitative assessment, GBCA enhancement of cerebrospinal fluid on T2-weighted fluid-attenuated inversion recovery and T1-weighted imaging may provide a practical, but nonspecific, alternative [92], [93]. Other potential methods are difficult to quantify (e.g., dynamic susceptibility contrast MRI) [94], use ionising radiation [95], [96], or are at an early stage of development (compartmental ASL modeling [97], [98], [99]). Nevertheless, DCE-MRI is not routinely used in cSVD studies due to practical impediments (long scan time, exogenous contrast), lack of widespread expertise, and technical and physiological complexities and confounds [100], [101].
There are few studies of BBB permeability change over time in cSVD. A single study of 22 subjects with high WMH burden reported little overlap between regions of high white matter permeability between the first and second scans, but that high permeability was often seen along the border of WMH at either time [102].
Because there is no reliable convenient reference method for quantifying subtle BBB permeability, studies comparing DCE-MRI measurements with other measures of BBB integrity are few and inconclusive [103], [104]. The need for a second gadolinium administration is a barrier to conducting studies on repeatability, but one study showed good evidence of repeatability with coefficient of variation of 11.6% for white matter and 14.4% for gray matter at 3T [105]. Reproducibility across different MR hardware has not been investigated. Based on theoretical considerations and experimental observations, it is likely that measurements are influenced by MR field strength, scanner stability, spatial resolution, pulse sequence parameters, acquisition time, GBCA type, and pharmacokinetic model [100], [101], [106], [107]. The diversity of acquisition and analysis protocols described (sometimes incompletely) in the literature is, therefore, a key impediment to the interpretation and comparison of data from different studies and centers.
Our recommendation for future studies is to use a three-dimensional, MR acquisition with wide spatial coverage, precontrast T1 measurement, a minimum temporal resolution of around one minute, and a minimum DCE scan time of 15 minutes [108]. A vascular input function should be measured in the venous sinuses, and the permeability-surface area product for tissue regions or, where feasible, individual voxels should be estimated using an appropriate pharmacokinetic model, typically the Patlak model [109]; simulations may be performed to assess accuracy and precision. Results should be interpreted carefully, particularly when comparing data from different research groups or scanners. We identified three priorities for the development of this biomarker: (1) agreement by the wider cSVD and dementia imaging research community on an open-access, dynamic consensus protocol for DCE-MRI measurements of slow BBB leakage; (2) acquisition of data on repeatability and reproducibility; and (3) studies to assess accuracy, including theoretical work, comparison with independent measures of BBB integrity, and validation using MR test objects and histology. Further technical development to increase accuracy and precision, as well as continued development of alternative methods, is also encouraged.
3.8. Ultra-high-field MRI
Ultra-high-field MRI, in particular 7T MRI, is emerging as a new tool in cSVD research. The higher resolution, different tissue contrasts, and better signal-to-noise ratios of 7T MRI allow the investigator to probe aspects of cSVD that are difficult to assess at lower field strength. In addition to enhanced sensitivity for cSVD lesions such as microinfarcts and microbleeds and more precise assessment of atrophy [18], [110], with 7T MRI, it is possible to actually visualize the small vessels [111]. From both perforating arteries and veins, features such as vessel density, length, and tortuosity can be resolved [111], [112]. In addition, different aspects of vascular function, including blood flow, pulsatility of flow in small penetrating arteries (a possible indicator of vascular stiffness), vascular reactivity to vasoactive agents (e.g., carbon dioxide), or neuronal stimulation (i.e., functional MRI), can be assessed, making it possible to probe cSVD at the level of the small vessels themselves [111].
Despite the potential of 7T MRI in cSVD research, important steps have to be taken to validate these novel techniques. Of note, the European Ultrahigh-Field Imaging Network in Neurodegenerative Diseases (EUFIND), another Joint Program on Neurodegenerative Diseases initiative, has the goal of harmonizing 7T MRI protocols across more than 20 centers from Europe and the United States.
3.9. Tools to facilitate cSVD biomarker development and harmonization
The HARNESS initiative focused on three areas to provide tools for harmonization: MR acquisition, postprocessing, and common repositories for training and validation. These tools are made available to the research community at www.harness-neuroimaging.org.
The HARNESS website provides fully specified MR acquisition protocols suitable for research studies that have a focus on cSVD. Given the diversity of manifestations of cSVD and hypotheses that can be tested, there is no single MR acquisition protocol that can quantify all aspects of cSVD, and therefore, investigators must make choices regarding protocol composition, also accounting for issues of feasibility including acquisition time and cost. Therefore, instead of a single protocol, the HARNESS website provides several options that meet these criteria: (1) they adhere to STRIVE [5]; (2) they are suitable for identifying types of canonical cSVD lesions—lacunes and WMH of presumed vascular origin, recent small infarcts, microbleeds, atrophy, and DTI changes; (3) they have been tested on more than one scanner as part of an established multicenter study; and (4) the protocol developers are willing to share the protocol freely. There are also links to other websites and useful repositories of information.
Currently, protocols are available from the SVD@target study [84] (ISRCTN10514229) and the Canadian Dementia Imaging Protocol [113], with plans to add the protocol from the US National Institute of Neurological Disorders and Stroke MarkVCID Biomarker Consortium (https://markvcid.partners.org/) once it has been fully specified and tested. Sequence parameters with examination cards are provided for 3T for most of the major vendors, including General Electric, Phillips, and Siemens. The protocols are suitable for prospective research studies with quantitative imaging biomarkers but probably exceed most clinical stroke protocols in terms of acquisition time, spatial resolution, and inclusion of DTI. They have been implemented successfully in multicenter studies at research sites, but nonetheless may not be feasible for multicenter studies performed at predominantly clinical scan sites where the intent is to leverage clinical imaging without a focus on quantitative biomarkers.
Reducing imaging variability may be enhanced by following consensus recommendations [17] to perform automated quality checks for acquisition parameters and monitoring of images for artifacts, correction for gradient nonlinearities, a well-defined method for subject's positioning in the scanner, and a clear strategy for hardware replacement when needed.
The HARNESS software database serves as a searchable source for information on downloadable software tools for processing MR data for cSVD quantitative biomarkers, such as for segmenting WMH. There are many existing software libraries for neuroimaging analysis, but only HARNESS focuses exclusively on cSVD. Site users can search for software by image modality, measurement type, key words, availability (i.e., by download or by request to the developer), or operating system. Software developers control their own entries via password-protected accounts and must make their software available according to their own terms by providing a link or through contacting the developer. We are actively recruiting developers with tools to sell or share. Developers may access the site for information on how to create accounts.
To aid visual review for cSVD lesions according to STRIVE, the HARNESS site makes downloadable electronic documents available, including validated visual rating scale scores and instructions, case report forms, and training slides.
Training readers and software algorithms require access to independent MR data sets for measurements. The HARNESS site includes a Web-based repository with completely deidentified 3T MR data showing lacunes, WMH, microbleeds, and cortical superficial siderosis from patients with TIA, minor ischemic stroke, and cerebral amyloid angiopathy, with consensus “gold standard” measurements for comparison. This repository will be useful for independently confirming reliability of measurements within and across research groups and for derivation and validation of computerized algorithms for quantitative measurement (e.g., for segmenting WMH to determine location and overall volume), as well as for comparing WMH algorithms against an independent standard.
4. Summary and conclusions
The HARNESS initiative was a multidisciplinary consensus process with input from a large number of neuroimaging researchers investigating cSVD. Our group developed a framework for neuroimaging biomarker development closely aligned with those proposed in other areas of imaging research. The HARNESS website (www.harness-neuroimaging.org) was created to facilitate harmonized neuroimaging methods for cSVD research. The site includes cSVD-appropriate MR acquisition protocols aligned with STRIVE [5], a searchable database of software programs for analyzing brains with cSVD, visual rating scales and case report forms, and a repository of 100 deidentified scans demonstrating different cSVD lesion types. These tools and resources are made available to the research community via the site and can be easily updated by contributors.
In this rapidly evolving field, we found that the degree of biomarker validation—technical, biological, and clinical and feasibility—varied by cSVD lesion and measurement type. In general, visually diagnosed cSVD lesions such as lacunes, WMH, and microbleeds have the greatest amount of clinical validation, as prognostic markers, and data are available on incidence and change over time and are already being used in multicenter studies and reported in routine clinical practice. Even so, none of these markers has yet been qualified for use in clinical trials by regulatory agencies, and more work is needed to standardize and compare current volumetric tools. Other markers are at a less advanced stage of biomarker development. Atrophy has been extensively studied but almost always in the context of Alzheimer's disease and not cSVD. Among the emerging cSVD markers, there are relatively more data on diffusion imaging and perivascular space imaging, but more longitudinal data and multicenter data on reproducibility are needed. Measurements of brain perfusion, vascular reactivity, and BBB integrity are promising but are at an even earlier stage of development. For these cSVD manifestations, innovation to overcome technical and feasibility barriers, rather than harmonizing to a best protocol, is the most important next step in development.
We found that technical validation often lagged clinical validation. However, estimates of repeatability and reproducibility are critically important to estimate minimum detectable differences over time and variability in measurement in multicenter studies, which are essential for sample size calculations for multicenter longitudinal trials. This lag in technical validation likely reflects the difficulty in obtaining funding for technical studies compared with clinical studies, the burden on research subjects to undergo multiple scans, and the general lack of nonhuman phantoms for studies of reproducibility. In contrast to volumetric imaging and functional MRI, phantoms for other measurements are less well developed. One research group has developed a phantom for iron deposits that mimic mineral deposits and microbleeds, not currently available for purchase [114]; otherwise, we are not aware of any other phantoms that recreate specific aspects of cSVD. Technical validation for neuroimaging biomarkers of cSVD would be enhanced by creating funding opportunities specifically for this purpose.
Research in Context.
-
1.
Systematic review: Working groups identified relevant papers through literature searches, expert knowledge, and hand searching articles from reference lists, but formal systematic reviews and creation of evidence tables were not within scope.
-
2.
Interpretation: To help harmonize methods for neuroimaging research on cerebral small vessel disease we developed a framework for neuroimaging biomarker development, reviewed the status of development of established and emerging neuroimaging biomarkers of cerebral small vessel disease within this framework, and created a website (www.harness-neuroimaging.org) with MR acquisition protocols, a searchable database of software for quantitative brain imaging analysis of cerebral small vessel disease, visual rating scales and case report forms, and a repository of deidentified scans demonstrating different lesion types.
-
3.
Future directions: The HARNESS initiative provides resources to reduce variability in measurement in MRI studies of cerebral small vessel disease that should facilitate multicenter studies and clinical trials.
Acknowledgment
Funding sources: This work was funded by the European Union Joint Program on Neurodegenerative Diseases (EU JPND). The funder had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Footnotes
Declarations of interest: The authors report no conflicts of interest.
References
- 1.Arvanitakis Z., Capuano A.W., Leurgans S.E., Bennett D.A., Schneider J.A. Relation of cerebral vessel disease to Alzheimer's disease dementia and cognitive function in elderly people: A cross-sectional study. Lancet Neurol. 2016;15:934–943. doi: 10.1016/S1474-4422(16)30029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wardlaw J.M., Smith C., Dichgans M. Mechanisms of sporadic cerebral small vessel disease: Insights from neuroimaging. Lancet Neurol. 2013;12:483–497. doi: 10.1016/S1474-4422(13)70060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vermeer S.E., Prins N.D., den Heijer T., Hofman A., Koudstaal P.J., Breteler M.M. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 4.Debette S., Beiser A., DeCarli C., Au R., Himali J.J., Kelly-Hayes M. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: the Framingham Offspring Study. Stroke. 2010;41:600–606. doi: 10.1161/STROKEAHA.109.570044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wardlaw J.M., Smith E.E., Biessels G.J., Cordonnier C., Fazekas F., Frayne R. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. The Lancet Neurol. 2013;12:822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blair G.W., Hernandez M.V., Thrippleton M.J., Doubal F.N., Wardlaw J.M. Advanced neuroimaging of cerebral small vessel disease. Curr Treat Options Cardiovasc Med. 2017;19:56. doi: 10.1007/s11936-017-0555-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith E.E., Beaudin A.E. New insights into cerebral small vessel disease and vascular cognitive impairment from MRI. Curr Opin Neurol. 2018;31:36–43. doi: 10.1097/WCO.0000000000000513. [DOI] [PubMed] [Google Scholar]
- 8.Biomarkers Definitions Working G Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 9.Robb M.A., McInnes P.M., Califf R.M. Biomarkers and surrogate endpoints: Developing common terminology and definitions. JAMA. 2016;315:1107–1108. doi: 10.1001/jama.2016.2240. [DOI] [PubMed] [Google Scholar]
- 10.Buckler A.J., Bresolin L., Dunnick N.R., Sullivan D.C., Aerts H.J., Bendriem B. Quantitative imaging test approval and biomarker qualification: interrelated but distinct activities. Radiology. 2011;259:875–884. doi: 10.1148/radiol.10100800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dufouil C., Chalmers J., Coskun O., Besancon V., Bousser M.G., Guillon P. Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: the PROGRESS (Perindopril Protection Against Recurrent Stroke Study) Magnetic Resonance Imaging Substudy. Circulation. 2005;112:1644–1650. doi: 10.1161/CIRCULATIONAHA.104.501163. [DOI] [PubMed] [Google Scholar]
- 12.European Society of Radiology ESR statement on the stepwise development of imaging biomarkers. Insights Imaging. 2013;4:147–152. doi: 10.1007/s13244-013-0220-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Connor J.P., Aboagye E.O., Adams J.E., Aerts H.J., Barrington S.F., Beer A.J. Imaging biomarker roadmap for cancer studies. Nat Rev Clin Oncol. 2017;14:169–186. doi: 10.1038/nrclinonc.2016.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sullivan D.C., Obuchowski N.A., Kessler L.G., Raunig D.L., Gatsonis C., Huang E.P. Metrology standards for quantitative imaging biomarkers. Radiology. 2015;277:813–825. doi: 10.1148/radiol.2015142202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt R., Berghold A., Jokinen H., Gouw A.A., van der Flier W.M., Barkhof F. White matter lesion progression in LADIS: Frequency, clinical effects, and sample size calculations. Stroke. 2012;43:2643–2647. doi: 10.1161/STROKEAHA.112.662593. [DOI] [PubMed] [Google Scholar]
- 16.Chappell F.M., Del Carmen Valdes Hernandez M., Makin S.D., Shuler K., Sakka E., Dennis M.S. Sample size considerations for trials using cerebral white matter hyperintensity progression as an intermediate outcome at 1 year after mild stroke: Results of a prospective cohort study. Trials. 2017;18:78. doi: 10.1186/s13063-017-1825-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Guio F., Jouvent E., Biessels G.J., Black S.E., Brayne C., Chen C. Reproducibility and variability of quantitative magnetic resonance imaging markers in cerebral small vessel disease. J Cereb Blood flow Metab. 2016;36:1319–1337. doi: 10.1177/0271678X16647396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Veluw S.J., Shih A.Y., Smith E.E., Chen C., Schneider J.A., Wardlaw J.M. Detection, risk factors, and functional consequences of cerebral microinfarcts. Lancet Neurol. 2017;16:730–740. doi: 10.1016/S1474-4422(17)30196-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hilal S., Tan C.S., Adams H.H.H., Habes M., Mok V., Venketasubramanian N. Enlarged perivascular spaces and cognition: A meta-analysis of 5 population-based studies. Neurology. 2018;91:e832–e842. doi: 10.1212/WNL.0000000000006079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown R., Benveniste H., Black S.E., Charpak S., Dichgans M., Joutel A. Understanding the role of the perivascular space in cerebral small vessel disease. Cardiovasc Res. 2018;114:1462–1473. doi: 10.1093/cvr/cvy113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding J., Sigurethsson S., Jonsson P.V., Eiriksdottir G., Charidimou A., Lopez O.L. Large perivascular spaces visible on magnetic resonance imaging, cerebral small vessel disease progression, and risk of dementia: The Age, Gene/Environment Susceptibility-Reykjavik Study. JAMA Neurol. 2017;74:1105–1112. doi: 10.1001/jamaneurol.2017.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dubost F., Yilmaz P., Adams H., Bortsova G., Ikram M.A., Niessen W. Enlarged perivascular spaces in brain MRI: Automated quantification in four regions. NeuroImage. 2019;185:534–544. doi: 10.1016/j.neuroimage.2018.10.026. [DOI] [PubMed] [Google Scholar]
- 23.Adams H.H., Hilal S., Schwingenschuh P., Wittfeld K., van der Lee S.J., DeCarli C. A priori collaboration in population imaging: The Uniform Neuro-Imaging of Virchow-Robin Spaces Enlargement consortium. Alzheimers Dement. 2015;1:513–520. doi: 10.1016/j.dadm.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bokura H., Kobayashi S., Yamaguchi S. Distinguishing silent lacunar infarction from enlarged Virchow-Robin spaces: A magnetic resonance imaging and pathological study. J Neurol. 1998;245:116–122. doi: 10.1007/s004150050189. [DOI] [PubMed] [Google Scholar]
- 25.Banerjee G., Kim H.J., Fox Z., Jager H.R., Wilson D., Charidimou A. MRI-visible perivascular space location is associated with Alzheimer's disease independently of amyloid burden. Brain. 2017;140:1107–1116. doi: 10.1093/brain/awx003. [DOI] [PubMed] [Google Scholar]
- 26.Charidimou A., Boulouis G., Pasi M., Auriel E., van Etten E.S., Haley K. MRI-visible perivascular spaces in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology. 2017;88:1157–1164. doi: 10.1212/WNL.0000000000003746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu Y.C., Dufouil C., Soumare A., Mazoyer B., Chabriat H., Tzourio C. High degree of dilated Virchow-Robin spaces on MRI is associated with increased risk of dementia. J Alzheimer's Dis. 2010;22:663–672. doi: 10.3233/JAD-2010-100378. [DOI] [PubMed] [Google Scholar]
- 28.Patankar T.F., Mitra D., Varma A., Snowden J., Neary D., Jackson A. Dilatation of the Virchow-Robin space is a sensitive indicator of cerebral microvascular disease: study in elderly patients with dementia. AJNR Am J Neuroradiol. 2005;26:1512–1520. [PMC free article] [PubMed] [Google Scholar]
- 29.Chen W., Song X., Zhang Y. Alzheimer's Disease Neuroimaging Initiative. Assessment of the Virchow-Robin Spaces in Alzheimer disease, mild cognitive impairment, and normal aging, using high-field MR imaging. AJNR Am J Neuroradiol. 2011;32:1490–1495. doi: 10.3174/ajnr.A2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Potter G.M., Chappell F.M., Morris Z., Wardlaw J.M. Cerebral perivascular spaces visible on magnetic resonance imaging: development of a qualitative rating scale and its observer reliability. Cerebrovasc Dis. 2015;39:224–231. doi: 10.1159/000375153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ballerini L., Lovreglio R., Valdes Hernandez M.D.C., Ramirez J., MacIntosh B.J., Black S.E. Perivascular spaces segmentation in brain MRI using optimal 3D filtering. Scientific Rep. 2018;8:2132. doi: 10.1038/s41598-018-19781-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Royle N.A., Booth T., Valdes Hernandez M.C., Penke L., Murray C., Gow A.J. Estimated maximal and current brain volume predict cognitive ability in old age. Neurobiol Aging. 2013;34:2726–2733. doi: 10.1016/j.neurobiolaging.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reuter M., Schmansky N.J., Rosas H.D., Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61:1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Durand-Dubief F., Belaroussi B., Armspach J.P., Dufour M., Roggerone S., Vukusic S. Reliability of longitudinal brain volume loss measurements between 2 sites in patients with multiple sclerosis: Comparison of 7 quantification techniques. AJNR Am J Neuroradiol. 2012;33:1918–1924. doi: 10.3174/ajnr.A3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valdes Hernandez Mdel C., Armitage P.A., Thrippleton M.J., Chappell F., Sandeman E., Munoz Maniega S. Rationale, design and methodology of the image analysis protocol for studies of patients with cerebral small vessel disease and mild stroke. Brain Behav. 2015;5:e00415. doi: 10.1002/brb3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wardlaw J.M., Valdes Hernandez M.C., Munoz-Maniega S. What are white matter hyperintensities made of? Relevance to vascular cognitive impairment. J Am Heart Assoc. 2015;4:001140. doi: 10.1161/JAHA.114.001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valdes Hernandez Mdel C., Gonzalez-Castro V., Ghandour D.T., Wang X., Doubal F., Munoz Maniega S. On the computational assessment of white matter hyperintensity progression: Difficulties in method selection and bias field correction performance on images with significant white matter pathology. Neuroradiology. 2016;58:475–485. doi: 10.1007/s00234-016-1648-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valdes Hernandez Mdel C., Maconick L.C., Munoz Maniega S., Wang X., Wiseman S., Armitage P.A. A comparison of location of acute symptomatic vs. 'silent' small vessel lesions. Int J Stroke. 2015;10:1044–1050. doi: 10.1111/ijs.12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dickie D.A., Valdes Hernandez M.D.C., Makin S.D., Staals J., Wiseman S.J., Bastin M.E. The brain health index: Towards a combined measure of neurovascular and neurodegenerative structural brain injury. Int J Stroke. 2018;13:849–856. doi: 10.1177/1747493018770222. [DOI] [PubMed] [Google Scholar]
- 40.Levy-Cooperman N., Ramirez J., Lobaugh N.J., Black S.E. Misclassified tissue volumes in Alzheimer disease patients with white matter hyperintensities: Importance of lesion segmentation procedures for volumetric analysis. Stroke. 2008;39:1134–1141. doi: 10.1161/STROKEAHA.107.498196. [DOI] [PubMed] [Google Scholar]
- 41.Pasi M., van Uden I.W., Tuladhar A.M., de Leeuw F.E., Pantoni L. White matter microstructural damage on diffusion tensor imaging in cerebral small vessel disease: Clinical consequences. Stroke. 2016;47:1679–1684. doi: 10.1161/STROKEAHA.115.012065. [DOI] [PubMed] [Google Scholar]
- 42.Cremers L.G., de Groot M., Hofman A., Krestin G.P., van der Lugt A., Niessen W.J. Altered tract-specific white matter microstructure is related to poorer cognitive performance: The Rotterdam Study. Neurobiol Aging. 2016;39:108–117. doi: 10.1016/j.neurobiolaging.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 43.Maniega S.M., Valdes Hernandez M.C., Clayden J.D., Royle N.A., Murray C., Morris Z. White matter hyperintensities and normal-appearing white matter integrity in the aging brain. Neurobiol Aging. 2015;36:909–918. doi: 10.1016/j.neurobiolaging.2014.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baykara E., Gesierich B., Adam R., Tuladhar A.M., Biesbroek J.M., Koek H.L. A novel imaging marker for small vessel disease based on skeletonization of white matter tracts and diffusion histograms. Ann Neurol. 2016;80:581–592. doi: 10.1002/ana.24758. [DOI] [PubMed] [Google Scholar]
- 45.Lawrence A.J., Chung A.W., Morris R.G., Markus H.S., Barrick T.R. Structural network efficiency is associated with cognitive impairment in small-vessel disease. Neurology. 2014;83:304–311. doi: 10.1212/WNL.0000000000000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reijmer Y.D., Fotiadis P., Martinez-Ramirez S., Salat D.H., Schultz A., Shoamanesh A. Structural network alterations and neurological dysfunction in cerebral amyloid angiopathy. Brain. 2015;138:179–188. doi: 10.1093/brain/awu316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tuladhar A.M., van Dijk E., Zwiers M.P., van Norden A.G., de Laat K.F., Shumskaya E. Structural network connectivity and cognition in cerebral small vessel disease. Hum Brain Mapp. 2016;37:300–310. doi: 10.1002/hbm.23032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reijmer Y.D., Fotiadis P., Piantoni G., Boulouis G., Kelly K.E., Gurol M.E. Small vessel disease and cognitive impairment: The relevance of central network connections. Hum Brain Mapp. 2016;37:2446–2454. doi: 10.1002/hbm.23186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tuladhar A.M., Lawrence A., Norris D.G., Barrick T.R., Markus H.S., de Leeuw F.E. Disruption of rich club organisation in cerebral small vessel disease. Hum Brain Mapp. 2017;38:1751–1766. doi: 10.1002/hbm.23479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duering M., Finsterwalder S., Baykara E., Tuladhar A.M., Gesierich B., Konieczny M.J. Free water determines diffusion alterations and clinical status in cerebral small vessel disease. Alzheimers Dement. 2018;14:764–774. doi: 10.1016/j.jalz.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang H., Schneider T., Wheeler-Kingshott C.A., Alexander D.C. NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 2012;61:1000–1016. doi: 10.1016/j.neuroimage.2012.03.072. [DOI] [PubMed] [Google Scholar]
- 52.Jokinen H., Schmidt R., Ropele S., Fazekas F., Gouw A.A., Barkhof F. Diffusion changes predict cognitive and functional outcome: The LADIS study. Ann Neurol. 2013;73:576–583. doi: 10.1002/ana.23802. [DOI] [PubMed] [Google Scholar]
- 53.van Uden I.W., van der Holst H.M., Schaapsmeerders P., Tuladhar A.M., van Norden A.G., de Laat K.F. Baseline white matter microstructural integrity is not related to cognitive decline after 5 years: The RUN DMC study. BBA Clin. 2015;4:108–114. doi: 10.1016/j.bbacli.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Uden I.W., van der Holst H.M., Tuladhar A.M., van Norden A.G., de Laat K.F., Rutten-Jacobs L.C. White matter and hippocampal volume predict the risk of dementia in patients with cerebral small vessel disease: The RUN DMC Study. J Alzheimers Dis. 2016;49:863–873. doi: 10.3233/JAD-150573. [DOI] [PubMed] [Google Scholar]
- 55.Tuladhar A.M., van Uden I.W., Rutten-Jacobs L.C., Lawrence A., van der Holst H., van Norden A. Structural network efficiency predicts conversion to dementia. Neurology. 2016;86:1112–1119. doi: 10.1212/WNL.0000000000002502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ritchie S.J., Bastin M.E., Tucker-Drob E.M., Maniega S.M., Engelhardt L.E., Cox S.R. Coupled changes in brain white matter microstructure and fluid intelligence in later life. J Neurosci. 2015;35:8672–8682. doi: 10.1523/JNEUROSCI.0862-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ritchie S.J., Tucker-Drob E.M., Cox S.R., Dickie D.A., Del C.V.H.M., Corley J. Risk and protective factors for structural brain ageing in the eighth decade of life. Brain Struct Funct. 2017;222:3477–3490. doi: 10.1007/s00429-017-1414-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zeestraten E.A., Lawrence A.J., Lambert C., Benjamin P., Brookes R.L., Mackinnon A.D. Change in multimodal MRI markers predicts dementia risk in cerebral small vessel disease. Neurology. 2017;89:1869–1876. doi: 10.1212/WNL.0000000000004594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zeestraten E.A., Benjamin P., Lambert C., Lawrence A.J., Williams O.A., Morris R.G. Application of diffusion tensor imaging parameters to detect change in longitudinal studies in cerebral small vessel disease. PLoS One. 2016;11:e0147836. doi: 10.1371/journal.pone.0147836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Groot M., Cremers L.G., Ikram M.A., Hofman A., Krestin G.P., van der Lugt A. White matter degeneration with aging: Longitudinal diffusion MR imaging analysis. Radiology. 2016;279:532–541. doi: 10.1148/radiol.2015150103. [DOI] [PubMed] [Google Scholar]
- 61.Benjamin P., Zeestraten E., Lambert C., Ster I.C., Williams O.A., Lawrence A.J. Progression of MRI markers in cerebral small vessel disease: Sample size considerations for clinical trials. J Cereb Blood flow Metab. 2016;36:228–240. doi: 10.1038/jcbfm.2015.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van der Holst H.M., Tuladhar A.M., Zerbi V., van Uden I.W.M., de Laat K.F., van Leijsen E.M.C. White matter changes and gait decline in cerebral small vessel disease. NeuroImage Clin. 2018;17:731–738. doi: 10.1016/j.nicl.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takao H., Hayashi N., Kabasawa H., Ohtomo K. Effect of scanner in longitudinal diffusion tensor imaging studies. Hum Brain Mapp. 2012;33:466–477. doi: 10.1002/hbm.21225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vollmar C., O'Muircheartaigh J., Barker G.J., Symms M.R., Thompson P., Kumari V. Identical, but not the same: Intra-site and inter-site reproducibility of fractional anisotropy measures on two 3.0T scanners. Neuroimage. 2010;51:1384–1394. doi: 10.1016/j.neuroimage.2010.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou X., Sakaie K.E., Debbins J.P., Narayanan S., Fox R.J., Lowe M.J. Scan-rescan repeatability and cross-scanner comparability of DTI metrics in healthy subjects in the SPRINT-MS multicenter trial. Magn Reson Imaging. 2018;53:105–111. doi: 10.1016/j.mri.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Provenzale J.M., Taylor B.A., Wilde E.A., Boss M., Schneider W. Analysis of variability of fractional anisotropy values at 3T using a novel diffusion tensor imaging phantom. Neuroradiol J. 2018;31:581–586. doi: 10.1177/1971400918789383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shi Y., Thrippleton M.J., Makin S.D., Marshall I., Geerlings M.I., de Craen A.J. Cerebral blood flow in small vessel disease: A systematic review and meta-analysis. J Cereb Blood flow Metab. 2016;36:1653–1667. doi: 10.1177/0271678X16662891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crane D.E., Black S.E., Ganda A., Mikulis D.J., Nestor S.M., Donahue M.J. Gray matter blood flow and volume are reduced in association with white matter hyperintensity lesion burden: A cross-sectional MRI study. Front Aging Neurosci. 2015;7:131. doi: 10.3389/fnagi.2015.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van der Veen P.H., Muller M., Vincken K.L., Hendrikse J., Mali W.P., van der Graaf Y. Longitudinal relationship between cerebral small-vessel disease and cerebral blood flow: The second manifestations of arterial disease-magnetic resonance study. Stroke. 2015;46:1233–1238. doi: 10.1161/STROKEAHA.114.008030. [DOI] [PubMed] [Google Scholar]
- 70.Promjunyakul N., Lahna D., Kaye J.A., Dodge H.H., Erten-Lyons D., Rooney W.D. Characterizing the white matter hyperintensity penumbra with cerebral blood flow measures. NeuroImage Clin. 2015;8:224–229. doi: 10.1016/j.nicl.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Osch M.J., Teeuwisse W.M., van Walderveen M.A., Hendrikse J., Kies D.A., van Buchem M.A. Can arterial spin labeling detect white matter perfusion signal? Magn Reson Med. 2009;62:165–173. doi: 10.1002/mrm.22002. [DOI] [PubMed] [Google Scholar]
- 72.Chen Y., Wang D.J., Detre J.A. Test-retest reliability of arterial spin labeling with common labeling strategies. J Magn Reson Imaging. 2011;33:940–949. doi: 10.1002/jmri.22345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu B., Lou X., Wu X., Ma L. Intra- and interscanner reliability and reproducibility of 3D whole-brain pseudo-continuous arterial spin-labeling MR perfusion at 3T. J Magn Reson Imaging. 2014;39:402–409. doi: 10.1002/jmri.24175. [DOI] [PubMed] [Google Scholar]
- 74.Mutsaerts H.J., van Osch M.J., Zelaya F.O., Wang D.J., Nordhoy W., Wang Y. Multi-vendor reliability of arterial spin labeling perfusion MRI using a near-identical sequence: Implications for multi-center studies. Neuroimage. 2015;113:143–152. doi: 10.1016/j.neuroimage.2015.03.043. [DOI] [PubMed] [Google Scholar]
- 75.Noguchi T., Yoshiura T., Hiwatashi A., Togao O., Yamashita K., Kobayashi K. Quantitative perfusion imaging with pulsed arterial spin labeling: a phantom study. Magn Reson Med Sci. 2007;6:91–97. doi: 10.2463/mrms.6.91. [DOI] [PubMed] [Google Scholar]
- 76.Liu P., De Vis J.B., Lu H. Cerebrovascular reactivity (CVR) MRI with CO2 challenge: A technical review. Neuroimage. 2018 doi: 10.1016/j.neuroimage.2018.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rane S., Koh N., Boord P., Madhyastha T., Askren M.K., Jayadev S. Quantitative cerebrovascular pathology in a community-based cohort of older adults. Neurobiol Aging. 2018;65:77–85. doi: 10.1016/j.neurobiolaging.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Blair G.W., Doubal F.N., Thrippleton M.J., Marshall I., Wardlaw J.M. Magnetic resonance imaging for assessment of cerebrovascular reactivity in cerebral small vessel disease: A systematic review. J Cereb Blood flow Metab. 2016;36:833–841. doi: 10.1177/0271678X16631756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Blair G.W., Appleton J.P., Law Z.K., Doubal F., Flaherty K., Dooley R. Preventing cognitive decline and dementia from cerebral small vessel disease: The LACI-1 Trial. Protocol and statistical analysis plan of a phase IIa dose escalation trial testing tolerability, safety and effect on intermediary endpoints of isosorbide mononitrate and cilostazol, separately and in combination. Int J Stroke. 2018;13:530–538. doi: 10.1177/1747493017731947. [DOI] [PubMed] [Google Scholar]
- 80.Sam K., Crawley A.P., Conklin J., Poublanc J., Sobczyk O., Mandell D.M. Development of white matter hyperintensity is preceded by reduced cerebrovascular reactivity. Ann Neurol. 2016;80:277–285. doi: 10.1002/ana.24712. [DOI] [PubMed] [Google Scholar]
- 81.Dumas A., Dierksen G.A., Gurol M.E., Halpin A., Martinez-Ramirez S., Schwab K. Functional magnetic resonance imaging detection of vascular reactivity in cerebral amyloid angiopathy. Ann Neurol. 2012;72:76–81. doi: 10.1002/ana.23566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peca S., McCreary C.R., Donaldson E., Kumarpillai G., Shobha N., Sanchez K. Neurovascular decoupling is associated with severity of cerebral amyloid angiopathy. Neurology. 2013;81:1659–1665. doi: 10.1212/01.wnl.0000435291.49598.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van Opstal A.M., van Rooden S., van Harten T., Ghariq E., Labadie G., Fotiadis P. Cerebrovascular function in presymptomatic and symptomatic individuals with hereditary cerebral amyloid angiopathy: A case-control study. Lancet Neurol. 2017;16:115–122. doi: 10.1016/S1474-4422(16)30346-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thrippleton M.J., Shi Y., Blair G., Hamilton I., Waiter G., Schwarzbauer C. Cerebrovascular reactivity measurement in cerebral small vessel disease: Rationale and reproducibility of a protocol for MRI acquisition and image processing. Int J Stroke. 2018;13:195–206. doi: 10.1177/1747493017730740. [DOI] [PubMed] [Google Scholar]
- 85.Alsop D.C., Detre J.A., Golay X., Gunther M., Hendrikse J., Hernandez-Garcia L. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med. 2015;73 doi: 10.1002/mrm.25197. spcone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Farrall A.J., Wardlaw J.M. Blood-brain barrier: Ageing and microvascular disease--systematic review and meta-analysis. Neurobiol Aging. 2009;30:337–352. doi: 10.1016/j.neurobiolaging.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 87.Raja R., Rosenberg G.A., Caprihan A. MRI measurements of blood-brain barrier function in dementia: A review of recent studies. Neuropharmacology. 2018;134:259–271. doi: 10.1016/j.neuropharm.2017.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Munoz Maniega S., Chappell F.M., Valdes Hernandez M.C., Armitage P.A., Makin S.D., Heye A.K. Integrity of normal-appearing white matter: Influence of age, visible lesion burden and hypertension in patients with small-vessel disease. J Cereb Blood Flow Metab. 2017;37:644–656. doi: 10.1177/0271678X16635657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang C.E., Wong S.M., van de Haar H.J., Staals J., Jansen J.F., Jeukens C.R. Blood-brain barrier leakage is more widespread in patients with cerebral small vessel disease. Neurology. 2017;88:426–432. doi: 10.1212/WNL.0000000000003556. [DOI] [PubMed] [Google Scholar]
- 90.Topakian R., Barrick T.R., Howe F.A., Markus H.S. Blood-brain barrier permeability is increased in normal-appearing white matter in patients with lacunar stroke and leucoaraiosis. J Neurol Neurosurg Psychiatry. 2010;81:192–197. doi: 10.1136/jnnp.2009.172072. [DOI] [PubMed] [Google Scholar]
- 91.Israeli D., Tanne D., Daniels D., Last D., Shneor R., Guez D. The application of MRI for depiction of subtle blood brain barrier disruption in stroke. Int J Biol Sci. 2010;7:1–8. doi: 10.7150/ijbs.7.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wardlaw J.M., Doubal F., Armitage P., Chappell F., Carpenter T., Munoz Maniega S. Lacunar stroke is associated with diffuse blood-brain barrier dysfunction. Ann Neurol. 2009;65:194–202. doi: 10.1002/ana.21549. [DOI] [PubMed] [Google Scholar]
- 93.Freeze W.M., Schnerr R.S., Palm W.M., Jansen J.F., Jacobs H.I., Hoff E.I. Pericortical enhancement on delayed postgadolinium fluid-attenuated inversion recovery images in normal aging, mild cognitive impairment, and Alzheimer disease. AJNR Am J Neuroradiol. 2017;38:1742–1747. doi: 10.3174/ajnr.A5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thornhill R.E., Chen S., Rammo W., Mikulis D.J., Kassner A. Contrast-enhanced MR imaging in acute ischemic stroke: T2* measures of blood-brain barrier permeability and their relationship to T1 estimates and hemorrhagic transformation. AJNR Am J Neuroradiol. 2010;31:1015–1022. doi: 10.3174/ajnr.A2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Iannotti F., Fieschi C., Alfano B., Picozzi P., Mansi L., Pozzilli C. Simplified, noninvasive PET measurement of blood-brain barrier permeability. J Comput Assist Tomogr. 1987;11:390–397. doi: 10.1097/00004728-198705000-00004. [DOI] [PubMed] [Google Scholar]
- 96.Caserta M.T., Caccioppo D., Lapin G.D., Ragin A., Groothuis D.R. Blood-brain barrier integrity in Alzheimer's disease patients and elderly control subjects. J Neuropsychiatry Clin Neurosci. 1998;10:78–84. doi: 10.1176/jnp.10.1.78. [DOI] [PubMed] [Google Scholar]
- 97.Gregori J., Schuff N., Kern R., Gunther M. T2-based arterial spin labeling measurements of blood to tissue water transfer in human brain. J Magn Reson Imaging. 2013;37:332–342. doi: 10.1002/jmri.23822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu P., Uh J., Lu H. Determination of spin compartment in arterial spin labeling MRI. Magn Reson Med. 2011;65:120–127. doi: 10.1002/mrm.22601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schmid S., Teeuwisse W.M., Lu H., van Osch M.J. Time-efficient determination of spin compartments by time-encoded pCASL T2-relaxation-under-spin-tagging and its application in hemodynamic characterization of the cerebral border zones. Neuroimage. 2015;123:72–79. doi: 10.1016/j.neuroimage.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 100.Armitage P.A., Farrall A.J., Carpenter T.K., Doubal F.N., Wardlaw J.M. Use of dynamic contrast-enhanced MRI to measure subtle blood-brain barrier abnormalities. Magn Reson Imaging. 2011;29:305–314. doi: 10.1016/j.mri.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Heye A.K., Thrippleton M.J., Armitage P.A., Valdes Hernandez M.D.C., Makin S.D., Glatz A. Tracer kinetic modelling for DCE-MRI quantification of subtle blood-brain barrier permeability. Neuroimage. 2016;125:446–455. doi: 10.1016/j.neuroimage.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huisa B.N., Caprihan A., Thompson J., Prestopnik J., Qualls C.R., Rosenberg G.A. Long-term blood-brain barrier permeability changes in Binswanger disease. Stroke. 2015;46:2413–2418. doi: 10.1161/STROKEAHA.115.009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Montagne A., Barnes S.R., Sweeney M.D., Halliday M.R., Sagare A.P., Zhao Z. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85:296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Taheri S., Gasparovic C., Shah N.J., Rosenberg G.A. Quantitative measurement of blood-brain barrier permeability in human using dynamic contrast-enhanced MRI with fast T1 mapping. Magn Reson Med. 2011;65:1036–1042. doi: 10.1002/mrm.22686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wong S.M., Jansen J.F.A., Zhang C.E., Staals J., Hofman P.A.M., van Oostenbrugge R.J. Measuring subtle leakage of the blood-brain barrier in cerebrovascular disease with DCE-MRI: Test-retest reproducibility and its influencing factors. J Magn Reson Imaging. 2017;46:159–166. doi: 10.1002/jmri.25540. [DOI] [PubMed] [Google Scholar]
- 106.Larsson H.B., Courivaud F., Rostrup E., Hansen A.E. Measurement of brain perfusion, blood volume, and blood-brain barrier permeability, using dynamic contrast-enhanced T(1)-weighted MRI at 3 tesla. Magn Reson Med. 2009;62:1270–1281. doi: 10.1002/mrm.22136. [DOI] [PubMed] [Google Scholar]
- 107.Barnes S.R., Ng T.S., Montagne A., Law M., Zlokovic B.V., Jacobs R.E. Optimal acquisition and modeling parameters for accurate assessment of low Ktrans blood-brain barrier permeability using dynamic contrast-enhanced MRI. Magn Reson Med. 2016;75:1967–1977. doi: 10.1002/mrm.25793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.van de Haar H.J., Jansen J.F.A., Jeukens C., Burgmans S., van Buchem M.A., Muller M. Subtle blood-brain barrier leakage rate and spatial extent: Considerations for dynamic contrast-enhanced MRI. Med Phys. 2017;44:4112–4125. doi: 10.1002/mp.12328. [DOI] [PubMed] [Google Scholar]
- 109.Sourbron S.P., Buckley D.L. Classic models for dynamic contrast-enhanced MRI. NMR Biomed. 2013;26:1004–1027. doi: 10.1002/nbm.2940. [DOI] [PubMed] [Google Scholar]
- 110.De Guio F., Reyes S., Vignaud A., Duering M., Ropele S., Duchesnay E. In vivo high-resolution 7 Tesla MRI shows early and diffuse cortical alterations in CADASIL. PLoS One. 2014;9:e106311. doi: 10.1371/journal.pone.0106311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zwanenburg J.J.M., van Osch M.J.P. Targeting cerebral small vessel disease with MRI. Stroke. 2017;48:3175–3182. doi: 10.1161/STROKEAHA.117.016996. [DOI] [PubMed] [Google Scholar]
- 112.Kuijf H.J., Bouvy W.H., Zwanenburg J.J., Razoux Schultz T.B., Viergever M.A., Vincken K.L. Quantification of deep medullary veins at 7 T brain MRI. Eur Radiol. 2016;26:3412–3418. doi: 10.1007/s00330-016-4220-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Duchesne S., Chouinard I., Potvin O., Bartha R., Bellec P., Collins L. The Canadian Dementia Imaging Protocol: Harmonizing national cohorts. J Magn Reson Imaging. 2019;49:456–465. doi: 10.1002/jmri.26197. [DOI] [PubMed] [Google Scholar]
- 114.Valdes Hernandez Mdel C., Glatz A., Kiker A.J., Dickie D.A., Aribisala B.S., Royle N.A. Differentiation of calcified regions and iron deposits in the ageing brain on conventional structural MR images. J Magn Reson Imaging. 2014;40:324–333. doi: 10.1002/jmri.24348. [DOI] [PubMed] [Google Scholar]