Abstract
Background
Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) is a functional long non-coding RNA involved in many biologic processes. The study was aimed to explore the functional roles of MALAT1 in osteosarcoma progression.
Material/Methods
A total of 76 osteosarcoma tissues and paired adjacent non-tumor tissues were collected from surgical resection. MALAT1, miRNAs, and genes mRNA expression levels were detected using quantitative real-time PCR (qRT-PCR). Protein expression level, cell proliferation, migration, and invasion were assessed using western blot, Cell Counting Kit-8 (CCK-8), wound-healing assay, and Matrigel invasion assay respectively. The target relationships among miRNAs, MALAT1, and mRNA were detected via dual-luciferase reporter assay.
Results
We found that MALAT1 was frequently upregulated in osteosarcoma samples and cell lines and a high level of MALAT1 predicted poor overall survival in osteosarcoma patients. Knockdown of MALAT1 inhibited proliferation, migration, and invasion of osteosarcoma cells. Further study showed a positive correlation between MALAT1 and c-Met or SOX4 expression. Mechanistic investigations demonstrated that MALAT1, as a competing endogenous RNA (ceRNA), regulated osteosarcoma proliferation and metastasis through competitively binding to miR-34a/c-5p and miR-449a/b.
Conclusions
Taken together, our study illustrates a new regulatory mechanism for MALAT1 and may provide a novel therapeutic strategy for the treatment of osteosarcoma.
MeSH Keywords: MicroRNAs, Neoplasm Metastasis, Osteosarcoma
Background
Osteosarcoma is a rare primary malignant bone tumor, particularly among children and adolescents [1–3]. Surgical removal combined with systemic multidrug chemotherapy is the most frequent approaches for osteosarcoma treatment. This strategy includes neoadjuvant chemotherapy followed by surgical removal of the primary tumor, along with the addition of adjuvant chemotherapy after surgery. With the introduction of neoadjuvant chemotherapy (including doxorubicin, cisplatin, methotrexate, ifosfamide and etoposide), long-term overall survival of osteosarcoma patients has dramatically improved to 65% to 70% [4]. However, patients with metastases have a poor prognosis, with overall survival of 10% to 30% [5]. Therefore, understanding of the molecular mechanisms underlying osteosarcoma metastasis may provide novel insights and help develop targeted therapeutic strategies.
Long non-coding RNAs (lncRNAs) are non-protein-coding transcripts that are longer than 200 nucleotides [6]. Emerging evidence demonstrated that lncRNAs not only play critical regulatory roles in tumorigenesis and tumor progression, but also serve as independent predictors for patient prognosis [7,8]. For example, non-small cell lung cancer patients with high lncRNA SNHG1 expression were significantly correlated with poor overall survival, and further analysis revealed lncRNA SNHG1 could promote cell proliferation via miR-101-3p/SOX9/Wnt/β-catenin axis [9].
Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) is a highly conserved and frequently upregulated lncRNA in mammals. Recently, MALAT1 has been reported to have important molecular functional during cancer progression in several human cancers [10,11]. High level of MALAT1 was associated with poor prognosis in oral squamous cell carcinoma and MALAT1 promoted cell migration and invasion through activation of β-catenin and NF-κB signaling pathways [12]. MALAT1 silencing suppressed human hematoma cell growth and metastasis via sponging miR-195 [13]. However, the functional role of MALAT1 in the carcinogenesis and tumor development of osteosarcoma remains unclear.
The c-Met proto-oncogene encodes the tyrosine kinase receptor of hepatocyte growth factor (HGF). Previous studies indicated that HGF/c-Met regulate cell migration and invasion in osteosarcoma [14–17]. SOX4 (SRY (sex determining region Y)-box 4) is a downstream target gene of TGF-β and WNT/β-catenin pathway, and has been reported to be a key regulator of epithelial-mesenchymal transition (EMT) and cancer metastasis [18–21]. However, a more detailed role of c-Met in the promotion cell metastasis in osteosarcoma has not been elucidated.
In the present study, we confirmed that MALAT1 were highly expressed in osteosarcoma tissues and cell lines. Knockdown of MALAT1 dramatically suppressed cell proliferation and metastasis. We also demonstrated that MALAT1 was a direct target of miR-34a/c-5p and miR-449a/b, and upregulated MALAT1 functions as a competing endogenous RNAs (ceRNA) of c-Met and SOX4. Taken together, this study provided a novel insight of MALAT1 in control of osteosarcoma metastasis.
Material and Methods
Cell culture and tissue samples
Human osteosarcoma cell lines (MNNG/HOS, Saos-2, U2OS, and MG-63) were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). Human osteoblast cell line hFOB 1.19 was purchased from the Cell Bank of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). All cells were maintained at 37°C in a humidified 5% CO2 atmosphere. A total of 76 osteosarcoma tissues and paired adjacent non-tumor tissues from surgical resection were collected at the First Affiliated Hospital, College of Medicine, Zhejiang University during January 2011 and December 2015. The clinical features of enrolled osteosarcoma patients are shown in Table 1. All samples were collected with informed consent and this study was approved by the Clinical Research Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University.
Table 1.
Clinical features of 76 osteosarcoma patients.
| Features | Number of cases |
|---|---|
| Gender | |
| Male | 45 |
| Female | 31 |
| Age at diagnosis | |
| <18 | 36 |
| ≥18 | 40 |
| Histological subtype | |
| Osteoblastic | 13 |
| Chondroblastic | 21 |
| Fibroblastic | 17 |
| Mixed | 25 |
| Clinical stage | |
| I+IIA | 48 |
| IIB/III | 28 |
| Distant metastasis | |
| Absent | 30 |
| Present | 46 |
Plasmid construction, siRNA and miRNA transfection
Fragments of the 3′ untranslated regions (3′UTRs) of c-Met, SOX4, and MALAT1 containing the predicted miR-34a/c-5p and miR-449a/b binding sites were cloned and inserted into the pmirGLO dual-luciferase miRNA target expression vector at the SacI and XbaI restriction sites. miR-34a/c-5p and miR-449a/b mimics/inhibitors, miRNA negative control, c-Met, MALAT1 and SOX4 siRNA and siRNA control were synthesized by GenePharma Company (Shanghai, China). Transfection was performed using HiPerFect Transfection Reagent (Qiagen, Hilden, Germany) according to the manufacturer’s protocol.
RNA isolation and quantitative real-time PCR (qRT-PCR)
Total RNA from tissues and cell lines was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The gene expression was determined using SYBR Green PCR master mix (Applied Biosystems) and β-actin was used as an endogenous control. For miRNAs, qRT-PCR was carried out using Taqman miRNA Assays (Applied Biosystems, Foster City, CA, USA) and normalized to U6.
Dual-luciferase reporter assay
For luciferase assay, cells were plated in 24-well culture plates, and then co-transfected with pmirGLO-MALAT1-WT, pmirGLO-c-Met-WT, pmirGLO-SOX4-WT or -MUT reporter plasmids and miR-34a/c-5p or miR-449a/b mimic or negative control. Then, the cells were harvested and lysed for luciferase assay 24 hours after transfection. Luciferase activity was detected using the Dual Luciferase Reporter Assay System (Promega). Renilla luciferase was used for normalization.
Cell counting kit-8 (CCK-8) assay
After 24 hours of transfection, 4×103 cells/well were seeded in 96-well plates and then cultured for different time (24 hours, 48 hours, 72 hours, and 96 hours). Cell viability was assessed using the CCK-8 assay according to the manufacturer’s instructions. The optical density was measured at 450 nm using a spectrophotometer.
Cell migration and invasion assay
For migration assay, cells were incubated in serum-free medium for 48 hours after being scratched with a pipette tip. For the invasion assay, cells were seeded on the Matrigel-coated upper chamber (Corning Costar, Rochester, NY, USA) in serum-free medium. Complete medium containing 10% FBS was added to the lower chamber. After 24 hours incubation, cells were fixed in methanol and stained with 0.1% crystal violet for visualization.
Western blot analysis
Total protein lysates were prepared in RIPA lysis buffer and separated on 10% SDS-PAGE gel. Primary antibodies used in this study were as follows: rabbit mAb anti-c-Met (CST, #8198), mouse mAb anti-SOX4 (Santa Cruz, sc-130633) and rabbit mAb anti-β-actin (CST, #4970) were used for western blot analysis. Relative proteins expression levels were normalized to β-actin levels.
Statistical analysis
Each experiment was performed at least 3 times. Data was expressed as means ±SD. Statistical analysis were performed by Student’s t-test using SPSS18.0 software. P value <0.05 was considered statistically significant.
Results
MALAT1 was frequently upregulated and associated with poor overall survival in osteosarcoma patients
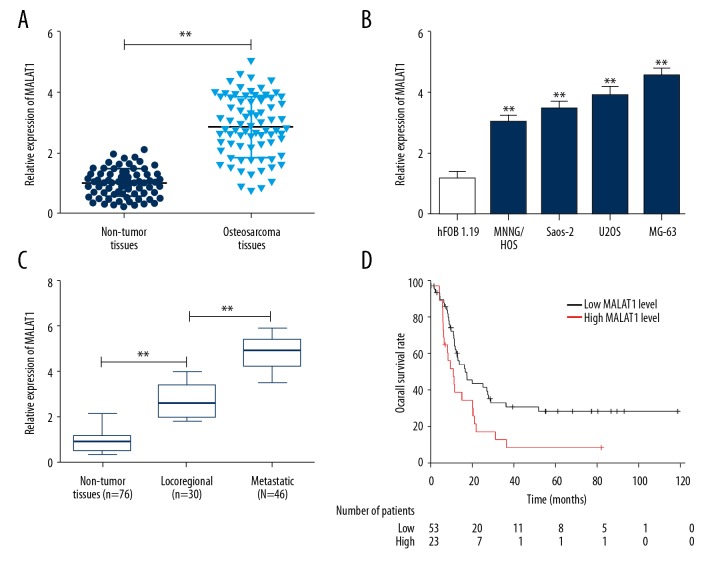
We first determined the expression of MALAT1 in 76 osteosarcoma tissues and paired adjacent non-tumor tissues using qRT-PCR. An obviously upregulated expression of MALAT1 was observed in osteosarcoma tissues compared with adjacent non-tumor tissues (Figure 1A; P<0.01). Similarly, expression of MALAT1 was increased in all 4 human osteosarcoma cell lines compared with normal osteoblastic cells (Figure 1B; P<0.01). We also determined the association between MALAT1 expression and osteosarcoma metastasis and found that MALAT1 level in metastatic osteosarcoma tissues was significantly increased compared with locoregional osteosarcoma tissues (Figure 1C; P<0.01). When correlated to disease outcome, high expression of MALAT1 in osteosarcoma patients was significantly associated with worse prognosis (Figure 1D; P=0.011). These results suggest that MALAT1 may function as a potential oncogene involved in osteosarcoma metastasis.
Figure 1.
Frequent upregulation of MALAT1 in osteosarcoma cell lines and tissues. (A) qRT-PCR was performed to detect the expression level of MALAT1 in 76 osteosarcoma tissues and paired adjacent non-tumor tissues. ** P<0.01 compared with non-tumor tissues. (B) qRT-PCR was performed to detect the expression level of MALAT1 in 4 human osteosarcoma cell lines and normal osteoblastic cell line hFOB 1.19. ** P<0.01 compared with hFOB 1.19. (C) Examination of MALAT1 expression in locoregional osteosarcoma patients and metastatic osteosarcoma patients. ** P<0.01 compared with non-tumor tissues or locoregional osteosarcoma tissues. (D) Kaplan-Meier curves for overall survival in osteosarcoma patients. All data are presented as mean ± standard deviation for 3 independent experiments.
Silence of MALAT1 suppressed proliferation, migration, and invasion of osteosarcoma cells
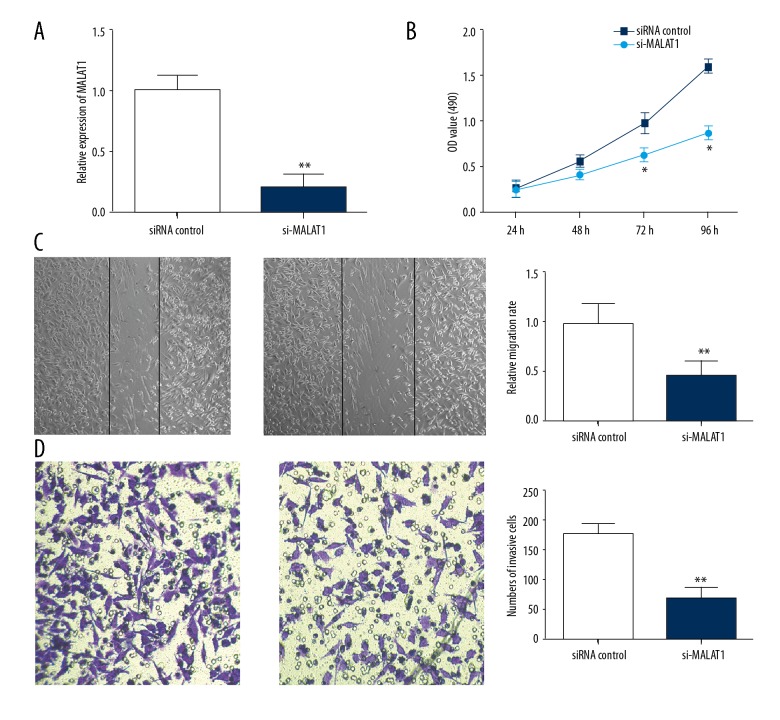
To determine the biological function of MALAT1 in osteosarcoma, siRNA against MALAT1 was transfected into MG63 cells and qRT-PCR was performed to confirm successful acquisition of MALAT1 silence (Figure 2A; P<0.01). CCK-8 assay demonstrated that the proliferation of MG-63 cells transfected with MALAT1 siRNA at 72 hours and 96 hours was significantly suppressed compared with siRNA control (Figure 2B). Transwell migration and invasion assays indicated that knockdown of MALAT1 significantly impaired the migration and invasion capacity of MG-63 cells compared with siRNA control (Figure 2C, 2D; both P<0.01).
Figure 2.
Silence of MALAT1 suppressed osteosarcoma cell proliferation, migration, and invasion. (A) MG-63 cells were transfected with MALAT1 siRNA, and siRNA control. Relative expression of MALAT1 was assessed using qRT-PCR. (B) CCK-8 assay was performed to evaluate the effect of MALAT1 on cell proliferation. (C) Wound-healing assay showed that silence of MALAT1 significantly suppressed osteosarcoma cell migration capacity. (D) Matrigel invasion assay showed that silence of MALAT1 significantly inhibited osteosarcoma cell invasion capacity. Data are presented as mean ± standard deviation for 3 independent experiments. * P<0.05, ** P<0.01 compared with siRNA control.
Silence of MALAT1 suppressed c-Met and SOX4 expression
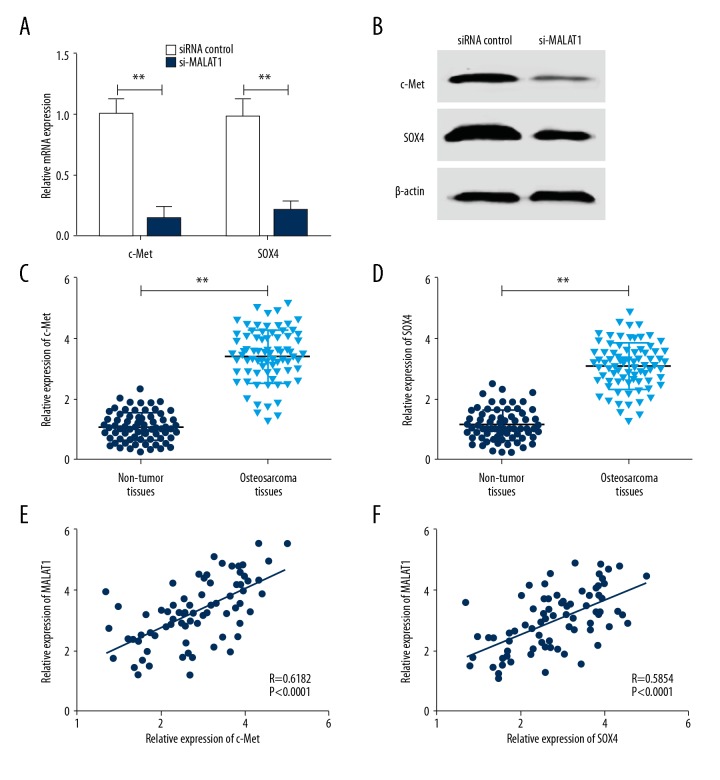
Abnormal expressions of c-Met and SOX4 have been correlated with tumorigenesis and tumor progression through the induction of an epithelial-to-mesenchymal transition and metastasis [22]. To investigate whether MALAT1 exert its regulatory effect through c-Met or SOX4, MALAT1 siRNA was transfected into MG-63 cells. As shown in Figure 3A and 3B, silence of MALAT1 reduced the expression of c-Met and SOX4 at both the mRNA and protein levels in MG-63 cells. To further explore the relationship between MALAT1 and these 2 genes, we determined the mRNA level in human osteosarcoma tissues using qRT-PCR. As shown in Figure 3C and 3D, c-Met and SOX4 mRNA levels in osteosarcoma tissues were significantly higher than that of adjacent non-tumor tissues. MALAT1 transcript level was obviously correlated with c-Met and SOX4 mRNA transcript levels. Person correlation analysis showed a significant positive correlation between MALAT1 level and c-Met or SOX4 mRNA expression in osteosarcoma tissues (Figure 3E, 3F; R=0.6182, P<0.0001; R=0.5854, P<0.0001, respectively).
Figure 3.
Silence of MALAT1 suppressed c-Met and SOX4 expression. (A) MALAT1 siRNA suppressed c-Met and SOX4 mRNA expression level in MG-63 cells compared with siRNA control. ** P<0.01 compared with siRNA control. (B) MALAT1 siRNA suppressed c-Met and SOX4 protein expression level in MG-63 cells compared with siRNA control. ** P<0.01 compared with siRNA control. (C) qRT-PCR was performed to detect the mRNA expression level of c-Met in 76 osteosarcoma tissues and paired adjacent non-tumor tissues. ** P<0.01 compared with non-tumor tissues. (D) qRT-PCR was performed to detect the mRNA expression level of SOX4 in 76 osteosarcoma tissues and paired adjacent non-tumor tissues. P<0.01 compared with non-tumor tissues. (E) Positive correlation between MALAT1 and c-Met mRNA level in 76 osteosarcoma tissues (R=0.6182, P<0.0001). (F) Positive correlation between MALAT1 and SOX4 mRNA level in 76 osteosarcoma tissues (R=0.5854, P<0.0001). All data are presented as mean ± standard deviation for 3 independent experiments.
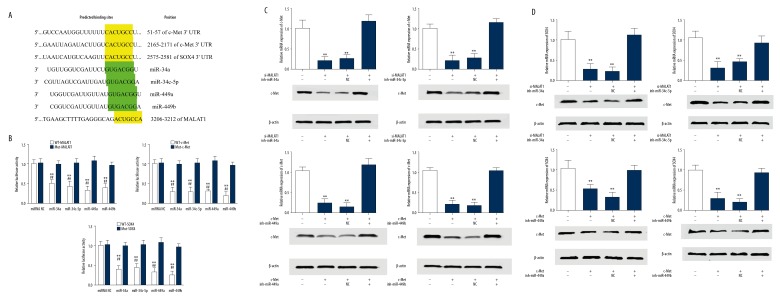
miR-34a/c-5p and miR-449a/b were predicted to be candidate miRNAs
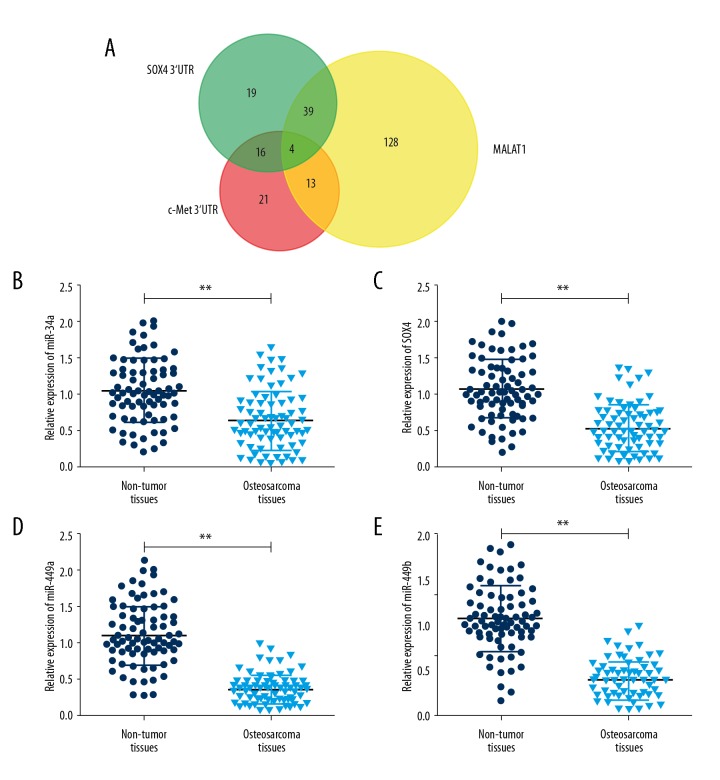
Accumulated evidence suggests lncRNAs could regulate their target genes by acting as either ceRNAs for microRNAs (miRNAs) or as miRNA sponges [23–25]. To explore whether MALAT1 functions as a ceRNA, we searched MALAT1 binding miRNAs using miRcode (http://www.mircode.org) and c-Met or SOX4 binding miRNAs using microRNA.org (http://www.microrna.org/) [26,27]. We identified 184 MALAT1 binding miRNAs, 54 c-Met binding miRNAs and 78 SOX4 binding miRNAs (Figure 4A). Among the miRNAs, miR-34a, miR-34c-5p, miR-449a, and miR-449b were identified as candidate miRNAs which were further confirmed to be downregulated in osteosarcoma cell lines compared with normal bones using Gene Expression Omnibus (GEO) dataset GSE28423 (Table 2) [28]. The qRT-PCR results showed the expression of miR-34a, miR-34c-5p, miR-449a, and miR-449b in osteosarcoma tissues were decreased 1.65-fold, 2.04-fold, 3.16-fold, and 2.75-fold compared with normal tissues, respectively (Figure 4B–4E). These findings suggest that MALAT1 may serve as a ceRNA for miR-34a/c-5p and miR-449a/b.
Figure 4.
miR-34a/c-5p and miR-449a/b were predicted to be candidate miRNAs. (A) Venn diagram of overlapped binding miRNAs. mircode and microRNA.org were used to predict MALAT1 and c-Met and SOX4 binding miRNAs, respectively. (B) Relative expression of miR-34a was detected using qRT-PCR in 76 osteosarcoma tissues and paired adjacent non-tumor tissues. (C) Relative expression of miR-34c-5p was detected using qRT-PCR in 76 osteosarcoma tissues and paired adjacent non-tumor tissues. (D) Relative expression of miR-449a was detected using qRT-PCR in 76 osteosarcoma tissues and paired adjacent non-tumor tissues. (E) Relative expression of miR-449b was detected using qRT-PCR in 76 osteosarcoma tissues and paired adjacent non-tumor tissues. Data are presented as mean ± standard deviation for 3 independent experiments. ** P<0.01 compared with non-tumor tissues.
Table 2.
Expression levels of miR-34a/c-5p and miR-449a/b in GEO dataset GSE28423.
| miRNAs | P value | logFC |
|---|---|---|
| miR-34a | 1.71E-01 | −1.07912 |
| miR-449a | 1.71E-01 | −2.10129 |
| miR-34c-5p | 2.49E-01 | −1.45296 |
| miR-449b | 4.52E-01 | −0.38919 |
MALAT1 functioned as a ceRNA of c-Met and SOX4 by directly binding to miR-34a/c-5p and miR-449a/b
The predicted binding sites for miR-34a/c-5p and miR-449a/b in c-Met, SOX4, and MALAT1 are shown in Figure 5A. c-Met 3′UTR has 2 binding sites at position 51–57 and 2165–2171. SOX4 3′UTR and MALAT1 have 1 binding site at 2575–2581 and 3206–3212, respectively. To verify these predicted relationships, dual-luciferase reporter assay were employed and the results showed the luciferase activity of the wild type (WT) of MALAT1, c-Met 3′UTR,and SOX4 3′UTR reporter vectors, but not mutant (Mut) vectors, was significantly reduced after transfected with hsa-miR-34a, hsa-miR-34c-5p, hsa-miR-449a, or hsa-miR-449b mimics compared with miRNA negative control (Figure 5B). Knockdown of MALAT1 in MG-63 cells could significantly reduce c-Met expression in both mRNA and protein levels. However, co-transfection with MALAT1 siRNA and miRNA inhibitors (miR-34a/c-5p and miR-449a/b) obviously increased c-Met expression compared with that of MALAT1 siRNA alone or MALAT1 siRNA with miRNA negative control (Figure 5C). A similar result for SOX4 was observed after inhibition of both MALAT1 and miRNAs in MG-63 cells (Figure 5D). Taken together, these findings indicate that MALAT1 could effectively regulate c-Met and SOX4 expression by competitively binding to miR-34a/c-5p and miR-449a/b.
Figure 5.
MALAT1 functioned as a ceRNA of c-Met and SOX4 by directly binding to miR-34a/c-5p and miR-449a/b. (A) Putative binding sites of miR-34a, miR-34c-5p, miR-449a, and miR-449b in c-Met 3′UTR, SOX4 3′UTR, and MALAT1. (B) Dual-luciferase reporter assay in MG-63 cells, co-transfected of wild type (WT) or mutant (Mut) of MALAT1, c-Met 3′ UTR,or SOX4 3′UTR with miR-34a, miR-34c-5p, miR-449a, and miR-449b mimics or miRNA negative control. ** P<0.01 compared with miRNA negative control. ## P<0.01 compared with Mut vectors. (C) c-Met mRNA and protein expression level in MG-63 cells co-transfected with MALAT1 siRNA and miR-34a, miR-34c-5p, miR-449a, or miR-449b inhibitor. ** P<0.01 compared with siRNA control. (D) SOX4 mRNA and protein expression level in MG-63 cells co-transfected with MALAT1 siRNA and miR-34a, miR-34c-5p, miR-449a, or miR-449b inhibitor. ** P<0.01 compared with siRNA control. All data are presented as mean ± standard deviation for 3 independent experiments.
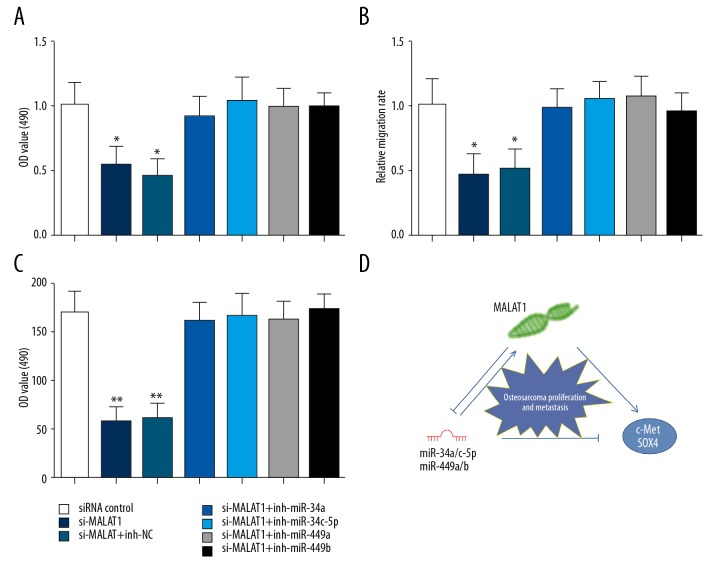
MALAT1 required miR-34a/c-5p or miR-449a/b to promote proliferation and metastasis of osteosarcoma cells
Next, we evaluated the influence of miR-34a/c-5p or miR-449a/b on the proliferation and metastasis of osteosarcoma cells induced by MALAT1. MALAT1 siRNA and miR-34a/c-5p or miR-449a/b inhibitors were co-transfected into MG-63 cells. CCK-8 assay showed that the inhibition of MALAT1 siRNA to cell proliferation could be obviously removed by co-transfected with miRNA inhibitors compared with miRNA negative control (Figure 6A). Transwell migration and invasion assays also demonstrated that miR-34a/c-5p or miR-449a/b could partly eliminate the effects of MALAT1 on migration and invasion of MG-63 cells (Figure 6B, 6C). These results suggest essential roles of miR-34a/c-5p and miR-449a/b in the regulation of MALAT1-mediated osteosarcoma cell proliferation and metastasis (Figure 6D).
Figure 6.
miR-34a/c-5p and miR-449a/b were essential for MALAT1-mediated osteosarcoma cell proliferation and metastasis. (A) The proliferation of MG-63 cells was measured using CCK-8 assays. (B) The migration of MG-63 cells was measured using wound-healing assay. (C) The invasion of MG-63 cells was measured using Matrigel invasion assay. (D) MALAT1 promoted the proliferation and metastasis of osteosarcoma cells by targeting c-Met and SOX4 via miR-34a/c-5p and miR-449a/b. Data are presented as mean ± standard deviation for 3 independent experiments. ** P<0.01 compared with siRNA control.
Discussion
Metastasis is the primary cause of mortality associated with cancer. Although metastasis only occurs to approximately 15% to 20% of osteosarcoma patients, 10% to 30% of long-term survival rates is extremely low compared with 65% and 70% in non-metastasis patients [29,30]. In this study, we demonstrated that MALAT1 is an oncogene associated with cell proliferation and metastasis in osteosarcoma, consistent with recent studies [31–34]. Except for proliferation and metastasis, MALAT1 was also reported to play an important role in cell differentiation, chemoresistance, and inflammatory response [35–37]. These findings inspired us to explore the underlying mechanism of MALAT1 regulating osteosarcoma cell metastasis.
It is believed that c-Met and SOX4 are important metastasis related genes which contribute to proliferation and metastasis in a variety of human malignancies. We further investigated whether MALAT1 promoting cell proliferation and metastasis is via regulating c-Met and SOX4. Knockdown of MALAT1 in MG-63 cells significantly reduced c-Met and SOX4 expression. Clinical data further showed a positive correlation between MALAT1 and c-Met or SOX4 expression. These results indicate MALAT1 might exert its functions through regulating c-Met and SOX4 in osteosarcoma.
The ceRNA hypothesis provides us a novel direction to understand lncRNA-miRNA-mRNA interaction in regulation of tumorigenesis and progression in human cancers [37,38]. In this study, we identified 4 candidate miRNAs: miR-34a, miR-34c-5p, miR-449a, and miR-449b using online tool and GEO dataset. Luciferase reporter assays showed a direct interaction between miR-34a, miR-34c-5p, miR-449a, or miR-449b and MALAT1, c-Met, or SOX4, respectively. When silence of those 4 miRNAs, MALAT1, c-Met, and SOX4 expression were increased compared with that of MALAT1 siRNA alone or MALAT1 siRNA with miRNA negative control. These findings indicate that MALAT1 could effectively regulate c-Met and SOX4 expression by competitively binding to miR-34a/c-5p and miR-449a/b.
MiR-34, a markedly conserved miRNA, has been regarded as a tumor suppressor in multiple cancers, including ovarian cancer, gastric cancer, and lung adenocarcinoma [39–41]. Bou Kheir et al. reported that downregulated miR-449 inhibits cell proliferation through regulation of a cohort of cancer-associated cell cycle regulators in gastric cancer [42]. Recently, several studies also suggest miR-34/449 family are essential for multi-ciliogenesis, spermatogenesis, motile ciliogenesis and normal brain development [43–45]. Our results showed miR-34a/c-5p and miR-449a/b were significantly downregulated and could partly eliminate the influences of MLAT1-induced proliferation and metastasis of osteosarcoma cells. Thus, miR-34 and miR-449 may also function as a tumor suppressor in osteosarcoma, and further studies are warranted to clarify the specific roles and underlying mechanisms of miR-34 and miR-449 in the development of osteosarcoma.
Conclusions
Our findings reveal a crucial role of MALAT1 and 2 miRNA clusters, miR-34a/c-5p and miR-449a/b in cell proliferation and metastasis of osteosarcoma. Our work also establishes a novel ceRNA interaction network and understanding such mechanisms may eventually lead to novel therapeutic strategies based on non-coding RNA for osteosarcoma patients.
Acknowledgments
We thank Dr. Shoucheng Wu of the First Affiliated Hospital, College of Medicine, Zhejiang University for his helpful suggestions and advice during this project.
Footnotes
Source of support: Departmental sources
Conflicts of interest
None.
References
- 1.Federman N, Bernthal N, Eilber FC, et al. The multidisciplinary management of osteosarcoma. Curr Treat Options Oncol. 2009;10:82–93. doi: 10.1007/s11864-009-0087-3. [DOI] [PubMed] [Google Scholar]
- 2.Bleyer A, O’Leary M, Barr R, et al. Cancer epidemiology in older adolescents and young adults 15 to 29 years of age, including SEER incidence and survival: 1975–2000. National Cancer Institute, NIH; Bethesda, MD, USA: 2006. [Google Scholar]
- 3.Gatta G, van der Zwan JM, Casali PG, et al. Rare cancers are not so rare: The rare cancer burden in Europe. Eur J Cancer. 2011;47:2493–511. doi: 10.1016/j.ejca.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Isakoff MS, Bielack SS, Meltzer P, et al. Osteosarcoma: Current treatment and a collaborative pathway to success. J Clin Oncol. 2015;33:3029–35. doi: 10.1200/JCO.2014.59.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duchman KR, Gao Y, Miller BJ. Prognostic factors for survival in patients with high-grade osteosarcoma using the Surveillance, Epidemiology, and End Results (SEER) Program database. Cancer Epidemiol. 2015;39:593–99. doi: 10.1016/j.canep.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Kapranov P, Cheng J, Dike S, et al. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science. 2007;316:1484–88. doi: 10.1126/science.1138341. [DOI] [PubMed] [Google Scholar]
- 7.Liz J, Esteller M. lncRNAs and microRNAs with a role in cancer development. Biochim Biophys Acta. 2016;1859:169–76. doi: 10.1016/j.bbagrm.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. Cancer Discov. 2011;1:391–407. doi: 10.1158/2159-8290.CD-11-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui Y, Zhang F, Zhu C, et al. Upregulated lncRNA SNHG1 contributes to progression of non-small cell lung cancer through inhibition of miR-101-3p and activation of Wnt/β-catenin signaling pathway. Oncotarget. 2017;8:17785–94. doi: 10.18632/oncotarget.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guffanti A, Iacono M, Pelucchi P, et al. A transcriptional sketch of a primary human breast cancer by 454 deep sequencing. BMC Genomics. 2009;10:163. doi: 10.1186/1471-2164-10-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ji P, Diederichs S, Wang W, et al. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22:8031–41. doi: 10.1038/sj.onc.1206928. [DOI] [PubMed] [Google Scholar]
- 12.Zhou X, Liu S, Cai G, et al. Long-non coding RNA MALAT1 promotes tumor growth and metastasis by inducing epithelial mesenchymal transition in oral squamous cell carcinoma. Sci Rep. 2015;5:15972. doi: 10.1038/srep15972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu D, Zhu Y, Pang J, et al. Knockdown of long non-coding RNA MALAT1 inhibits growth and motility of human hepatoma cells via modulation of miR-195. J Cell Biochem. 2018;119:1368–80. doi: 10.1002/jcb.26297. [DOI] [PubMed] [Google Scholar]
- 14.Coltella N, Manara MC, Cerisano V, et al. Role of the MET/HGF receptor in proliferation and invasive behavior of osteosarcoma. FASEB J. 2003;17:1162–64. doi: 10.1096/fj.02-0576fje. [DOI] [PubMed] [Google Scholar]
- 15.Niu G, Li B, Sun J, et al. miR-454 is down-regulated in osteosarcomas and suppresses cell proliferation and invasion by directly targeting c-Met. Cell Prolif. 2015;48:348–55. doi: 10.1111/cpr.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Sun X, Wu J, et al. MicroRNA-613 suppresses proliferation, migration and invasion of osteosarcoma by targeting c-MET. Am J Cancer Res. 2016;6(12):2869–79. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Husmann K, Ducommun P, Sabile AA, et al. Signal transduction and downregulation of C-MET in HGF stimulated low and highly metastatic human osteosarcoma cells. Biochem Biophys Res Commun. 2015;464:1222–27. doi: 10.1016/j.bbrc.2015.07.108. [DOI] [PubMed] [Google Scholar]
- 18.Kuwahara M, Yamashita M, Shinoda K, et al. The transcription factor Sox4 is a downstream target of signaling by the cytokine TGF-beta and suppresses T(H)2 differentiation. Nat Immunol. 2012;13:778–86. doi: 10.1038/ni.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van der Flier LG, Sabates-Bellver J, Oving I, et al. The intestinal Wnt/TCF signature. Gastroenterology. 2007;132:628–32. doi: 10.1053/j.gastro.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 20.Bu P, Wang L, Chen KY, et al. miR-1269 promotes metastasis and forms a positive feedback loop with TGF-β. Nat Commun. 2015;6:6879. doi: 10.1038/ncomms7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tiwari N, Tiwari VK, Waldmeier L, et al. Sox4 is a master regulator of epithelial-mesenchymal transition by controlling Ezh2 expression and epigenetic reprogramming. Cancer Cell. 2013;23:768–83. doi: 10.1016/j.ccr.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 22.Vervoort SJ, van Boxtel R, Coffer PJ. The role of SRY-related HMG box transcription factor 4 (SOX4) in tumorigenesis and metastasis: Friend or foe? Oncogene. 2013;32:3397–409. doi: 10.1038/onc.2012.506. [DOI] [PubMed] [Google Scholar]
- 23.Wang H, Huo X, Yang XR, et al. STAT3-mediated upregulation of lncRNA HOXD-AS1 as a ceRNA facilitates liver cancer metastasis by regulating SOX4. Mol Cancer. 2017;16:136. doi: 10.1186/s12943-017-0680-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu XS, Wang F, Li HF, et al. LncRNA-PAGBC acts as a microRNA sponge and promotes gallbladder tumorigenesis. EMBO Rep. 2017;18:1837–53. doi: 10.15252/embr.201744147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui B, Li B, Liu Q, et al. lncRNA CCAT1 promotes glioma tumorigenesis by sponging miR-181b. J Cell Biochem. 2017;118:4548–57. doi: 10.1002/jcb.26116. [DOI] [PubMed] [Google Scholar]
- 26.Jeggari A, Marks DS, Larsson E. miRcode: A map of putative microRNA target sites in the long non-coding transcriptome. Bioinformatics. 2012;28:2062–63. doi: 10.1093/bioinformatics/bts344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Betel D, Wilson M, Gabow A, et al. The microRNA.org resource: Targets and expression. Nucleic Acids Res. 2008;36:D149–53. doi: 10.1093/nar/gkm995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Namløs HM, Meza-Zepeda LA, Barøy T, et al. Modulation of the osteosarcoma expression phenotype by microRNAs. PLoS One. 2012;7:e48086. doi: 10.1371/journal.pone.0048086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: An analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–90. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 30.Kager L, Zoubek A, Pötschger U, et al. Primary metastatic osteosarcoma: Presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol. 2003;21:2011–18. doi: 10.1200/JCO.2003.08.132. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Zhang Y, Yang T, et al. Long non-coding RNA MALAT1 for promoting metastasis and proliferation by acting as a ceRNA of miR-144-3p in osteosarcoma cells. Oncotarget. 2017;8:59417–34. doi: 10.18632/oncotarget.19727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu K, Huang J, Ni J, et al. MALAT1 promotes osteosarcoma development by regulation of HMGB1 via miR-142-3p and miR-129-5p. Cell Cycle. 2017;16:578–87. doi: 10.1080/15384101.2017.1288324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huo Y, Li Q, Wang X, et al. MALAT1 predicts poor survival in osteosarcoma patients and promotes cell metastasis through associating with EZH2. Oncotarget. 2017;8:46993–7006. doi: 10.18632/oncotarget.16551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai X, Liu Y, Yang W, et al. Long noncoding RNA MALAT1 as a potential therapeutic target in osteosarcoma. J Orthop Res. 2016;34:932–41. doi: 10.1002/jor.23105. [DOI] [PubMed] [Google Scholar]
- 35.Han X, Yang F, Cao H, et al. Malat1 regulates serum response factor through miR-133 as a competing endogenous RNA in myogenesis. FASEB J. 2015;29:3054–64. doi: 10.1096/fj.14-259952. [DOI] [PubMed] [Google Scholar]
- 36.Li P, Zhang X, Wang H, et al. MALAT1 Is associated with poor response to oxaliplatin-based chemotherapy in colorectal cancer patients and promotes chemoresistance through EZH2. Mol Cancer Ther. 2017;16:739–51. doi: 10.1158/1535-7163.MCT-16-0591. [DOI] [PubMed] [Google Scholar]
- 37.Puthanveetil P, Chen S, Feng B, et al. Long non-coding RNA MALAT1 regulates hyperglycaemia induced inflammatory process in the endothelial cells. J Cell Mol Med. 2015;19:1418–25. doi: 10.1111/jcmm.12576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salmena L, Poliseno L, Tay Y, et al. ceRNA hypothesis: The Rosetta Stone of a hidden RNA language? Cell. 2011;146:353–58. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505:344–52. doi: 10.1038/nature12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corney DC, Hwang CI, Matoso A, et al. Frequent downregulation of miR-34 family in human ovarian cancers. Clin Cancer Res. 2010;16:1119–28. doi: 10.1158/1078-0432.CCR-09-2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ji Q, Hao X, Meng Y, et al. Restoration of tumor suppressor miR-34 inhibits human p53-mutant gastric cancer tumorspheres. BMC Cancer. 2008;8:266. doi: 10.1186/1471-2407-8-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daugaard I, Knudsen A, Kjeldsen TE, et al. The association between miR-34 dysregulation and distant metastases formation in lung adenocarcinoma. Exp Mol Pathol. 2017;102:484–91. doi: 10.1016/j.yexmp.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 42.Bou Kheir T, Futoma-Kazmierczak E, Jacobsen A, et al. miR-449 inhibits cell proliferation and is down-regulated in gastric cancer. Mol Cancer. 2011;10:29. doi: 10.1186/1476-4598-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chevalier B, Adamiok A, Mercey O, et al. miR-34/449 control apical actin network formation during multiciliogenesis through small GTPase pathways. Nat Commun. 2015;6:8386. doi: 10.1038/ncomms9386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song R, Walentek P, Sponer N, et al. miR-34/449 miRNAs are required for motile ciliogenesis by repressing cp110. Nature. 2014;510:115–20. doi: 10.1038/nature13413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu J, Bao J, Kim M, et al. Two miRNA clusters, miR-34b/c and miR-449, are essential for normal brain development, motile ciliogenesis, and spermatogenesis. Proc Natl Acad Sci USA. 2014;111:E2851–57. doi: 10.1073/pnas.1407777111. [DOI] [PMC free article] [PubMed] [Google Scholar]