Abstract
Background: Middle East respiratory syndrome coronavirus (MERS-CoV) was first identified in humans in 2012. A systematic literature review was conducted to synthesize current knowledge and identify critical knowledge gaps.
Materials and Methods: We conducted a systematic review on MERS-CoV using PRISMA guidelines. We identified 407 relevant, peer-reviewed publications and selected 208 of these based on their contributions to four key areas: virology; clinical characteristics, outcomes, therapeutic and preventive options; epidemiology and transmission; and animal interface and the search for natural hosts of MERS-CoV.
Results: Dipeptidyl peptidase 4 (DPP4/CD26) was identified as the human receptor for MERS-CoV, and a variety of molecular and serological assays developed. Dromedary camels remain the only documented zoonotic source of human infection, but MERS-like CoVs have been detected in bat species globally, as well as in dromedary camels throughout the Middle East and Africa. However, despite evidence of camel-to-human MERS-CoV transmission and cases apparently related to camel contact, the source of many primary cases remains unknown. There have been sustained health care-associated human outbreaks in Saudi Arabia and South Korea, the latter originating from one traveler returning from the Middle East. Transmission mechanisms are poorly understood; for health care, this may include environmental contamination. Various potential therapeutics have been identified, but not yet evaluated in human clinical trials. At least one candidate vaccine has progressed to Phase I trials.
Conclusions: There has been substantial MERS-CoV research since 2012, but significant knowledge gaps persist, especially in epidemiology and natural history of the infection. There have been few rigorous studies of baseline prevalence, transmission, and spectrum of disease. Terms such as “camel exposure” and the epidemiological relationships of cases should be clearly defined and standardized. We strongly recommend a shared and accessible registry or database. Coronaviruses will likely continue to emerge, arguing for a unified “One Health” approach.
Keywords: MERS, MERS-CoV, coronaviruses, zoonotic disease, severe acute respiratory infection (SARI), MERS epidemiology
Introduction
September 2012 saw the identification of a novel human coronavirus, now known as Middle East respiratory syndrome coronavirus (MERS-CoV) (ProMED-mail 2012, Zaki et al. 2012). MERS-CoV is a beta-coronavirus distinct from but genetically related to the SARS (severe acute respiratory syndrome) coronavirus (Hilgenfeld and Peiris 2013, de Wit et al. 2016).
As MERS was identified a few years after the 2003 SARS outbreak, which spread across continents and raised pandemic concerns, there has been continuing concern about the pandemic potential of MERS-CoV (Hilgenfeld and Peiris 2013, de Wit et al. 2016). To assess and anticipate potential epidemic spread of MERS-CoV, it is essential to better understand the virology; human clinical characteristics, possible therapeutic and preventive options; epidemiology and transmission; and animal interfaces and possible origins and natural hosts.
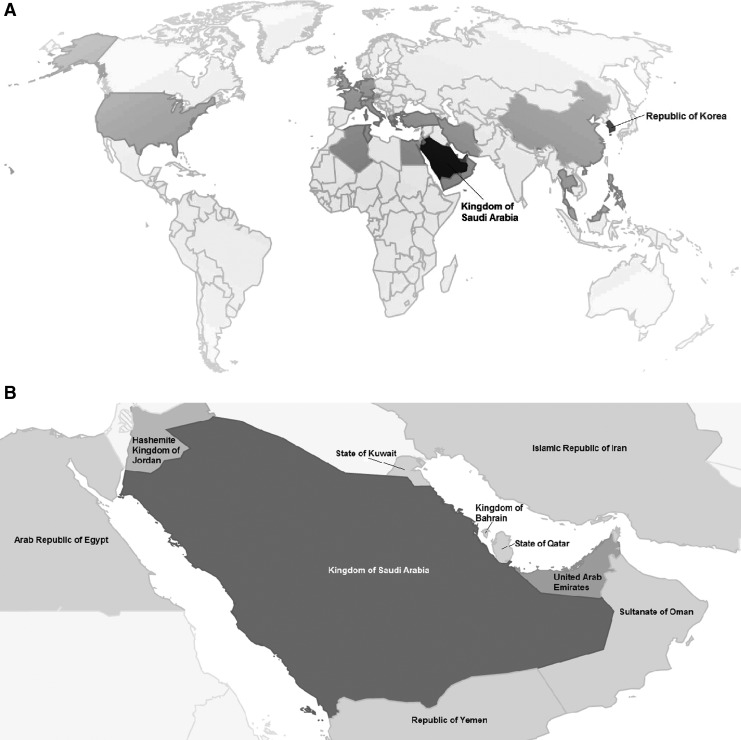
MERS was first identified in a patient with lower respiratory disease by a physician in Saudi Arabia, and subsequently, the virus was identified and characterized from this patient's sputum sample by researchers in Saudi Arabia and at Erasmus Medical Center in the Netherlands. Since then (to December 2018), 2266 laboratory-confirmed MERS-CoV cases and 804 deaths across 27 countries (35.5% crude case fatality rate [CFR]) have been reported to the WHO (Fig. 1), with the majority of cases (1888) and deaths (730) reported from Saudi Arabia (World Health Organization 2018a, World Health Organization/EMRO 2018). Cases continue to occur (World Health Organization/EMRO 2018).
FIG. 1.
(A) Map of countries having at least one laboratory-confirmed human MERS-CoV case, 2012–2016. Darker shades indicate greater numbers of laboratory-confirmed cases. (B) Inset of Fig. 1A showing Gulf countries having at least one laboratory-confirmed human MERS-CoV case. Darker shades indicate greater numbers of laboratory-confirmed cases. Data Source: World Health Organization (www.who.int/emergencies/mers-cov/en and www.who.int/emergencies/mers-cov/risk-assessment-july-2017.pdf). This map was generated using R software (R Foundation for Statistical Computing, Vienna, Austria) and the “googleVis” package (Gesmann et al. 2017). MERS-CoV, Middle East respiratory syndrome coronavirus.
Although the first known MERS-CoV cases were from Jordan (confirmed retrospectively, and the source is still unknown) (Hijawi et al. 2013), about 80% of MERS-CoV cases originate from or passed through Saudi Arabia. The infection is likely zoonotic; although origins and transmission are poorly understood, the dromedary camel is the one documented source of human zoonotic infection (Memish et al. 2014e).
A systematic review of MERS-CoV research was conducted to survey our current understanding of MERS-CoV and to identify research gaps in four major areas: (1) virology; (2) clinical characteristics, outcomes, and therapeutic and preventive options; (3) epidemiology and transmission; and (4) animal interface and the search for natural hosts of MERS-CoV.
Materials and Methods
We conducted a systematic literature review, using Embase, Google Scholar, MEDLINE/PubMed, supplemented by Thomson-Reuters “Web of Science”™ (all databases), to identify peer-reviewed published articles on MERS-CoV available by July 14, 2017. All searches were for articles published from 2012 onward, since MERS was first identified in 2012. The following search terms were used in all the databases for matches in title only (Google Scholar), title or abstract (Embase and MEDLINE/PubMed), or topic (“Web of Science”): “novel coronavirus,” “novel coronavirus EMC,” “novel coronavirus Erasmus,” “hCoV-EMC,” “MERS-CoV” (a PubMed “MeSH” term), “MERS coronavirus,” “Middle East Respiratory Syndrome,” and “Middle East Respiratory Syndrome Coronavirus” (a PubMed MeSH term).
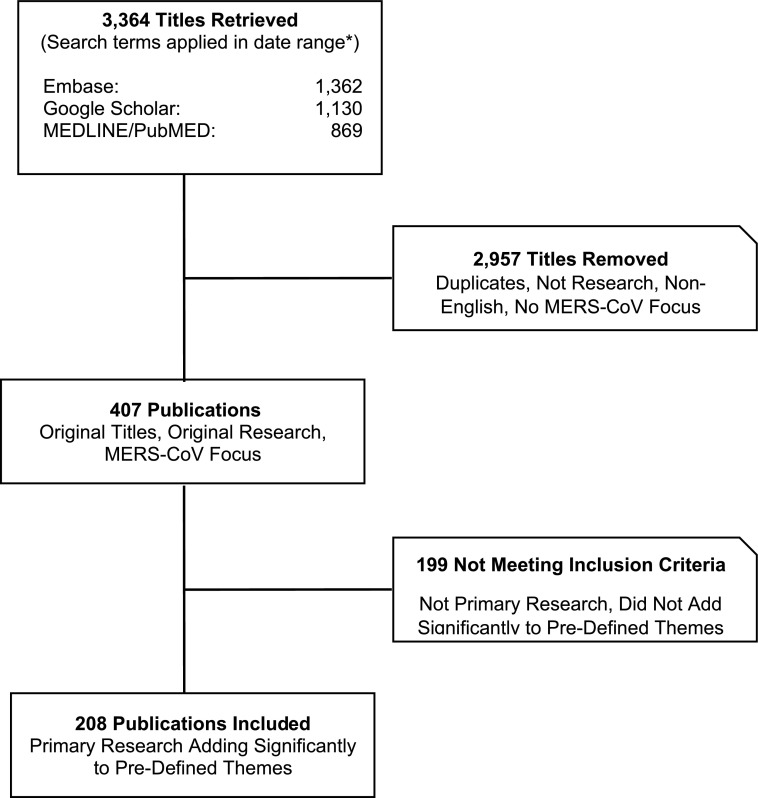
We reviewed the titles and abstracts of all resulting citations to identify relevant articles for this systematic review, following PRISMA guidelines (Fig. 2) (Moher et al. 2009). For all nonduplicate articles, excluding opinion and editorial pieces, on MERS-CoV or a related topic, we retrieved the full-text version and indexed the citation. Inclusion criteria were peer-reviewed primary research articles about MERS-CoV published between 2012 and mid-July 2017 (inclusive, with periodic updates to 2018) and adding unique information about MERS-CoV to the knowledge base within at least one of the four key themes: (1) virology; (2) clinical characteristics, outcomes, and therapeutic and preventive options; (3) epidemiology and transmission; and (4) animal interface and search for natural hosts of MERS-CoV.
FIG. 2.
Study selection diagram for systematic literature review on MERS-CoV (*For complete search terms and applicable date ranges, see the Materials and Methods section.).
Only English language publications (or with an English-language abstract available) were included. Of the 3364 titles retrieved, 407 were unique (nonduplicate), scientific research works relevant to MERS-CoV, and of these, 208 were originally included in the review based on the inclusion criteria (some interim updates have been made during article preparation).
Results
Virology
The pathogen causing MERS has been identified as a coronavirus, now named MERS-CoV, classified in the family Coronaviridae (subfamily Coronavirinae) (ICTV 2016). Coronaviruses are a family of enveloped, approximately spherical, positive-sense, single-stranded RNA viruses with genome length 26–32 kilobases (kb). Of the four described coronavirus genera, alpha- and beta-coronaviruses are believed to originate from bat species, whereas gamma- and delta-coronaviruses are believed to be of avian origin (Woo et al. 2012).
Within the beta-coronavirus genus, four lineages have been described: A, B (which includes SARS-CoV), C, and D. MERS-CoV is a beta-coronavirus of lineage C (Cotten et al. 2013), the first of this lineage known to infect humans. There are two main recognized clades (genetic sublineages) of MERS-CoV, clade A, which includes the earliest known MERS-CoV strains (EMC/2012 and Jordan-N3/2012), and clade B, which includes newer cases (Cotten et al. 2013), although additional genotypes are revealed by genomic sequencing (Chu et al. 2015), suggesting the existence of additional clades.
The genome of MERS-CoV is ∼30 kb and encodes 10 proteins: 2 replicase polyproteins (ORFs 1a and 1b), including the RNA-dependent RNA polymerase (RdRp); 4 structural proteins: envelope (E), M (membrane), N (nucleocapsid), and S (surface spike glycoprotein), and 4 nonstructural proteins (ORFs 3, 4a, 4b, and 5) (Kim et al. 2015; review: de Wit et al. 2016).
Particular attention has understandably focused on the S (spike) surface protein, which confers receptor specificity (Qian et al. 2013) and is considered the major determinant of coronavirus host range (Graham and Baric 2010, Hulswit et al. 2016). The main route of entry for MERS-CoV is dipeptidyl peptidase 4 (DPP4, also known as CD26) on the host cell surface (Raj et al. 2013), which is utilized by the receptor-binding domain of the MERS-CoV spike protein (Chen et al. 2013, Raj et al. 2013, 2014).
The virus readily replicates in A549 (human lung), Huh-7 (human liver), and the widely used Vero E6 (African green monkey kidney) cell lines (Raj et al. 2013, Eckerle et al. 2014), among others. Culture, in vitro and ex vivo, has also been used to help determine cell tropism and identify potential natural hosts of the virus. MERS-CoV can infect a variety of human cell types and tissues in culture, including macrophages, fibroblasts, and endothelial cells (Scobey et al. 2013, Tao et al. 2013, Hin et al. 2016). In an ex vivo study using human lung explants, MERS-CoV infected both alveolar epithelial cells and macrophages (Type II pneumocytes), with resulting alveolar damage (Hocke et al. 2013).
Interestingly, although kidney failure is frequently described in fatal cases and the virus grows well in a number of kidney and liver cell lines, in the one autopsy report that has been published, viral antigen was detected by immunohistochemical staining only in pneumocytes and lung interstitial epithelial cells, and not in the kidney or other extrapulmonary sites (Ng et al. 2016).
Replication was demonstrated in goat, camel (Eckerle et al. 2014), and equid origin cell lines (Meyer et al. 2015), but was not detected in cattle, sheep, or rodent cell lines tested (Eckerle et al. 2014).
Given the interest in bats as a natural host for many coronaviruses, especially since SARS, and the currently unknown origin of MERS-CoV, several bat cell lines have been tested to determine if they could support MERS-CoV replication (Müller et al. 2012, Cai et al. 2014, Eckerle et al. 2014).
As a broad generalization, the virus was reported to replicate well (roughly comparable with growth in Vero E6 cells) in kidney and lung cells from Rousettus aegyptiacus and other Old World pteropid (fruit) bats (Müller et al. 2012, Cai et al. 2014), to a lesser degree in cell lines from Old World insectivorous bats (Müller et al. 2012, Eckerle et al. 2014), and very little, if at all, in lung cells from several New World bats (Müller et al. 2012, Cai et al. 2014). However, as a caveat, no replication was found in the Rousettus aegyptiacus embryo cell lines tested (Cai et al. 2014).
Adaptation to cells from other bat species that are normally refractory to infection because of having a somewhat different DPP4 protein was recently demonstrated in vitro (Letko et al. 2018), resulting from selection of spike protein mutations that permitted binding to the new DPP4. A similar change, from bat to human-type receptor, was observed with SARS in 2003 (Letko et al. 2018).
Animal models for MERS-CoV include transgenic mice expressing human DPP4 (Cockrell et al. 2014, Zhao et al. 2014, Agrawal et al. 2015), which develop respiratory disease and are the most widely used model; marmosets (Falzarano et al. 2014); rhesus macaques (de Wit et al. 2013, Munster et al. 2013, Yao et al. 2014); camels (Barlan et al. 2014); horses (Barlan et al. 2014), and alpacas (Adney et al. 2016). However, there is no animal model that clearly mimics human clinical MERS (review: Baseler et al. 2016).
Diagnostic assays
The main laboratory assays for MERS-CoV are molecular tests to detect viral RNA, and serology (for prior infection) (CDC 2014, World Health Organization 2018b).
Reverse transcription PCR either real time or conventional, is now standard. For diagnostic screening, PCR primer sets to regions upstream of the E gene (“up-E”) are most frequently used, with ORF1a or ORF1b for confirmation (Corman et al. 2012a, 2012b). Sensitivity was reported as ∼3.4 viral gene copies/25 μL reaction for up-E and ∼64 copies for ORF1b, with specificity approaching 100% using a test panel of other known coronaviruses and clinical respiratory samples (Corman et al. 2012a). ORF1a PCR was reported to have comparable sensitivity and specificity to up-E (Corman et al. 2012b). Commercial kits are available, with comparable results reported (Corman et al. 2014c, Seong et al. 2016, Mohamed et al. 2017).
For sequencing, tests have been developed that may be better suited for strain differentiation, targeting regions of the RdRp (RNA polymerase) or N (nucleocapsid) genes (Corman et al. 2012b); there are a number of publications reporting on their use (Corman et al. 2012b; CDC 2014, Lu et al. 2014).
Another PCR test, targeting the S (spike) region, was developed to monitor circulation of MERS-CoV variants (Abd et al. 2013, Smits et al. 2015). Other types of nucleic acid tests have also been used (Shirato et al. 2014), as has immunohistochemistry (Ng et al. 2016).
Serologic samples are usually screened using enzyme-linked immunosorbent assay (ELISA) (Reusken et al. 2013a). If the sample is ELISA positive, CDC and other agencies formerly recommended confirmatory testing, first by immunofluorescence assay (IFA) and then by the gold standard plaque-reduction neutralization test or microneutralization (CDC 2014), but now recommend going directly to microneutralization assay (CDC 2014, revised). Other serological tests such as pseudoparticle neutralization (Perera et al. 2013) and array-based serology (Reusken et al. 2013a) have also been developed.
Unfortunately, there are pitfalls in serology, including cross-reactivity and false negatives. In 2014, CDC preliminarily reported a MERS-CoV case with a positive ELISA and IFA, but later retracted the case report due to a negative microneutralization result (Breakwell et al. 2015). Consequently, CDC no longer recommends IFA but instead now recommends using two different ELISA tests, with microneutralization for confirmation (CDC 2014, revised). Conversely, low or waning antibody response after infection, which would yield false negatives, has also been reported (Alshukairi et al. 2016, Choe et al. 2017). False negatives are further discussed below in the section on Host Response.
Clinical characteristics and outcomes
Clinical findings have been described and reviewed in detail (Assiri et al. 2013a, Saad et al. 2014, Rasmussen et al. 2015, World Health Organization 2015). The incubation period is ∼5–7 days (in some cases up to 12 days) (Assiri et al. 2013a, 2013b). Fever, cough, shortness of breath, and pneumonia (with abnormal chest radiography) are the most frequently noted signs; myalgia, diarrhea, vomiting, abdominal pain, chills or rigors, or malaise are also seen (Assiri et al. 2013a, Drosten et al. 2013, Eckerle et al. 2013, Guery et al. 2013, Ajlan et al. 2014, Arabi et al. 2014, Faure et al. 2014, Kapoor et al. 2014, Scheuplein et al. 2015, Hunter et al. 2016). Renal failure or pulmonary failure and shock were commonly described in severe and fatal cases. Overall CFR is ∼35.5% (World Health Organization 2018a).
Viral excretion from the respiratory tract has been documented through the first month of illness (Memish et al. 2014d, Poissy et al. 2014, Spanakis et al. 2014), with greater viral loads detected in lower respiratory tract specimens compared with upper respiratory tract (Drosten et al. 2013, Guery et al. 2013, Kapoor et al. 2014). Viral excretion for short periods was reported in urine and stool, although viral loads were low (Drosten et al. 2013). There was at least one report that MERS-CoV secondary cases (individuals infected by a primary case) clear infection earlier than primary cases (Memish et al. 2014d).
Disease outcomes differed by age and comorbidities (Assiri et al. 2013a, 2013b, Khalid et al. 2014, Saad et al. 2014, Alsahafi and Cheng 2016). Most cases in Saudi Arabia have occurred among men, ages 45 or older, and those living in areas of lower relative humidity and high temperature (Alghamdi et al. 2014). A review of 939 cases in Saudi Arabia found that CFR increases with age, from 12.5% among those 19 years or younger to 86.2% among those 80 years or older (Alsahafi and Cheng 2016). Mortality was more frequent and rapid in individuals with comorbidities such as diabetes, chronic renal disease, and, in one study, higher body mass index (Assiri et al. 2013a, 2013b, Al-Tawfiq et al. 2014, Khalid et al. 2014, Saad et al. 2014, Alsahafi and Cheng 2016). More severe disease has been associated with higher viral load (Feikin et al. 2015).
There are a handful of case reports on MERS outcomes in other vulnerable populations, including three cases in pregnant women and one in an HIV-positive individual.
In the three documented cases of pregnant women with MERS-CoV infection, one woman in her second trimester of pregnancy (∼5 months) experienced a stillbirth; the mother survived (Payne et al. 2014). The two other women were reported to be in their third trimester (32 weeks) when they presented with MERS. A healthy infant was delivered in each of these cases, although one mother died despite interferon (IFN) treatment (Malik et al. 2016), and one recovered despite the severity of her symptoms (Alserehi et al. 2016). Both were apparently healthy before infection and in their 30s. It is therefore possible that infection during the third trimester may be associated with better fetal outcomes, while other factors may be more critical for maternal outcome, but the numbers are too small to generalize.
There has been at least one published case of an individual with HIV/AIDS acquiring MERS-CoV (Shalhoub et al. 2015). This patient was probably infected with MERS-CoV during a hospital stay and subsequently shed virus for 38 days. The patient recovered after treatment (Shalhoub et al. 2015).
Host response
There have been a number of studies on immune function (Corman et al. 2016, Zhao et al. 2017). Neutralizing antibodies were detected in many patients, but several studies suggested that neutralizing antibodies induced during infection did not appear to be protective (Corman et al. 2016, Zhao et al. 2017), and it has been suggested that higher antibody titers may be more closely correlated with greater viral load and consequently worse prognosis (Zhao et al. 2017).
Kinetics of response may also be important. An antibody kinetics study with 17 patients in South Korea (from the same research group that later reported waning antibody titers in patients over a 1-year follow-up) found that most patients produced a robust neutralizing IgG antibody response beginning at about 2 weeks and peaking by about 3 weeks; delayed response was generally correlated with a less favorable prognosis (Park et al. 2015).
Persistence of antibody generally seems to follow a similar pattern. Detectable neutralizing antibody titers (≥20) have been reported in some patients up to 34 months (Payne et al. 2016). However, results varied considerably even within this group, and most reports indicate that duration of antibody response, as well as its magnitude, seemed most closely correlated with disease severity and that antibodies could be low or even absent in mild or asymptomatic infection (Alshukairi et al. 2016, Choe et al. 2017). These reports of low or waning antibody titers, especially in asymptomatic or mildly symptomatic individuals, may make serology problematic as a marker of population prevalence.
There is evidence that innate immunity, including IFNs and cytokines, and cellular immunity by CD8+ (presumably “killer”) T cells may play important roles in host defense and recovery (Faure et al. 2014, Zhao et al. 2017). Zhao et al. (2017) compared the role of antibodies and T cells in human MERS cases, and found that survivors mounted a vigorous CD8+ T cell response even in the absence of a detectable antibody response.
Macrophages also appear to play an important role, but there are limited and somewhat ambiguous data from the mouse model (Coleman et al. 2017), human cells (Zhou et al. 2014), and patients (Faure et al. 2014). With a number of viruses, it is not unusual for resting macrophages to be permissive for replication but for macrophages to show antiviral activity after activation (such as by IFN-γ) (Morse and Morahan 1981). Dendritic cells have been reported to produce IFN in response to infection (Scheuplein et al. 2015).
One challenge to overcome is the effect of the virus on host defenses. A caveat is that much of this research (although not all) has been done in vitro and may not necessarily be comparable with the immunological results described above in patients. MERS-CoV has been reported to inhibit host innate immunity (Lau et al. 2013). Impaired IFN and cytokine responses may mediate some of these effects, based on assays on cells from MERS patients (Faure et al. 2014, Scheuplein et al. 2015), although the numbers of patients were small. In vitro studies have shown that MERS-CoV could downregulate expression of IFN-stimulated gene subsets (Yang et al. 2013), chemokines (Mielech et al. 2014), and IFN-β. Another study found that MERS-CoV may cause delayed induction of proinflammatory cytokines together with suppression of the host's innate antiviral response (Lau et al. 2013).
Virus replication in human monocyte-derived macrophages has been described, with increased expression of tumor necrosis factor α (up to a 3-fold increase over normal levels), interleukin 6 (up to almost a 6-fold increase), interleukin 12, IFN-γ, and chemokines (some at a 10-fold or more increase and even more prominently than with SARS-CoV) being reported (Zhou et al. 2014).
Therapeutic and preventive options
Currently, the main treatment strategy is symptomatic supportive care; mechanical ventilation and ICU admission are usually required for severe cases.
Published data on applied therapeutic regimens for MERS-CoV patients are scarce, but there have been efforts to develop interventions.
Two case reports have documented the use of a combination of ribavirin and IFN α2b as a therapy for MERS-CoV patients, with divergent results. In one report, this therapy was given to five patients but all five died. However, all five patients had comorbidities and were diagnosed late in the course of their illness, suggesting the therapy may have been delivered too late to prove effective (Al-Tawfiq et al. 2014). In another report, this therapy was given to one patient who eventually recovered (Khalid et al. 2015).
In a retrospective cohort study of 44 patients with severe MERS (requiring ventilatory support), treatment with ribavirin and pegylated IFN α2a (note, not 2b) appeared to increase short-term survival (14 days), although by 28 days only 10 of the patients were still living (6/20 survivors in treated group compared with 4/24 surviving in the comparison group) (Omrani et al. 2014).
Among promising studies evaluating therapies in animal models, one in vivo study found that the combination of ribavirin and IFN α2b therapy significantly improved outcomes among MERS-CoV-infected rhesus monkeys (Falzarano et al. 2013). In another study, using the common marmoset model, lopinavir/ritonavir and IFN α1b (separately and in combination) also proved promising (Chan et al. 2015).
Although neutralizing antibodies induced during infection did not appear to be protective during natural infection (Corman et al. 2016, Zhao et al. 2017), human convalescent sera containing neutralizing antibodies conferred dose-dependent protection in a mouse model (Zhao et al. 2017). The readily available sera from MERS-immune camels apparently were also an effective treatment for MERS-infected mice and reduced the severity of pathological changes in murine lung tissue (Zhao et al. 2015). Several monoclonal antibodies (mAbs) (Mou et al. 2013, Ohnuma et al. 2013, Du et al. 2014, Jiang et al. 2014, Tang et al. 2014, Ying et al. 2014, Houser et al. 2016) and conventional antibodies (Zhao et al. 2013) have demonstrated effective neutralization of MERS-CoV, and could serve as potential therapeutic agents, at least in the interim.
High-throughput screening and other methods have identified additional promising compounds in several classes (Chan et al. 2013, de Wilde et al. 2013, 2014, Gao et al. 2013, Shirato et al. 2013, Adedeji et al. 2014, Dyall et al. 2014, Hart et al. 2014, Lu et al. 2014, Lundin et al. 2014, Tao et al. 2014, Wrensch et al. 2014, Corti et al. 2015, Liu et al. 2015). These include inhibitors against both viral (Kilianski et al. 2013, Lei et al. 2014, Kumar et al. 2017) and host (Gierer et al. 2015) proteases needed to process the virus. However, it was reported that inhibiting proprotein convertases inhibits host cell processing of the spike protein but did not affect viral infectivity or cell-to-cell spread (Gierer et al. 2015).
Other targets studied have included viral entry inhibitors (Zhao et al. 2013, Du et al. 2015) and protein 4a, which the researchers found suppressed innate antiviral response (Siu et al. 2014).
Vaccine development
Development of a human (and possibly camel) vaccine for MERS-CoV should be a key priority, given concerns about its spread and pandemic potential, but vaccine development can be a long process that is often driven by economics (Modjarrad 2016).
Much of the focus thus far has centered on the identification of the residues within the receptor-binding domain of the spike protein, conferring the best chance of protection (Du et al. 2013a, 2013b, Lan et al. 2014, Ma et al. 2014a, 2014b). A number of other antigens, some in viral vectors, have been tested in the laboratory (Song et al. 2013, Kim et al. 2014).
A replication defective full-length cDNA clone of the MERS-CoV genome has been developed for identifying future vaccine candidates (Almazán et al. 2013). Vaccine candidates for dromedary camels have also been investigated, and have been reported to elicit protective responses (using the MERS-CoV spike protein in a poxvirus vector) (Haagmans et al. 2016).
There are special challenges to developing a MERS vaccine. The sporadic nature of human MERS-CoV infection and the difficulty of identifying suitable animal models for challenge studies make controlled trials difficult. Disease in camels usually appears mild (Adney et al. 2014), reducing the economic incentive for immunization. A camel vaccine could also be given in combination with a required vaccine, e.g., rabies, as suggested by Wirblich et al. (2017), or camelpox, as suggested by Haagmans et al. (2016).
Correlates of protection and duration of immunity are also uncertain. There are reports of strong antibody responses (Haagmans et al. 2016), but it has been suggested that the presence of neutralizing antibodies may not be protective in camels (Hemida et al. 2014) or humans (Corman et al. 2016, Zhao et al. 2017), and waning antibody titers have been reported in humans who have recovered from MERS-CoV infection (Choe et al. 2017). On the contrary, the reports of protection of mice by passive transfer of neutralizing antibodies suggest that this might be overcome by optimizing timing and dose (Zhao et al. 2017).
Research on MERS-CoV vaccine candidates is ongoing, and at least one candidate vaccine has now progressed to a Phase I human clinical trial (Modjarrad 2016, CEPI 2017). In a promising development, the recently launched Coalition for Epidemic Preparedness Innovations (CEPI) has listed MERS vaccine as one of its initial targets.
Epidemiology and transmission
The source of infection for most primary cases (the first known human case in a potential or putative chain of transmission) remains unknown. Baseline incidence and prevalence are unknown as only clinical cases or those found through contact tracing are generally identified. The few population-based studies that have been done appear to show low prevalence in the general population. A serological study of 10,009 individuals in Saudi Arabia found 15 seropositive for MERS-CoV, 7 of whom were occupationally camel-exposed (shepherds and slaughterhouse workers) (Müller et al. 2015), although the occupational samples were collected for a separate study and therefore may not be representative. In another study of 57,363 persons with suspected MERS-CoV infection tested by Saudi Arabia from April 2015 to February 2016, only 384 (0.7%) tested positive (Bin Saeed et al. 2017).
Most work has been done on symptomatic MERS patients because they are most likely to be detected and identified. Even so, one analysis suggested that at least 62% of even symptomatic cases were undetected (Cauchemez et al. 2014), and a study using data from travelers suggested that there may have been as many as 3250 severe cases from September 2012 to January 2016, about 2.3 times the number of cases confirmed (O'Hagan et al. 2016).
Determination of primary versus secondary cases is also challenging. One estimate is that 61% of MERS-CoV are primary cases, who tend to be mostly middle-aged males, compared with secondary cases who tend to be younger and distributed more evenly by sex (Gossner et al. 2016). The official overall CFR stands at ∼35.5% (World Health Organization 2018a). However, it has been suggested that the CFR among primary cases, estimated to be 74%, is likely inflated due to detection bias; CFR among secondary cases is estimated to be 20% (Cauchemez et al. 2014).
The main known exposures are health care-associated infections and contact with camels. Aside from the health care setting, the mechanisms for the introduction of the virus into human populations are poorly understood. Occupational exposure risks are also poorly characterized, both in health care and for those who work with camels.
As with SARS, many cases have been health care facility-associated (Drosten et al. 2015), but the mechanism of infection is unclear for many individuals (Fanoy et al. 2014). Active case finding, typically among perceived risk groups or those hospitalized with pneumonia, has been used with varying success to identify cases (Gierer et al. 2013, Aburizaiza et al. 2014, Memish et al. 2014a).
Nosocomial transmission due to inadequate infection control is well established as a significant driver of MERS-CoV infections in humans (Al-Abdallat et al. 2014, Maltezou and Tsiodras 2014, Oboho et al. 2015, Alraddadi et al. 2016, Assiri et al. 2016, Hastings et al. 2016), notably during the spring 2014 outbreak in Saudi Arabia and the late spring 2015 outbreak in South Korea. A review (Maltezou and Tsiodras 2014) of 536 known MERS-CoV cases at the time of the review using publicly available data found 11 possible or confirmed health care-associated transmission events leading to substantial morbidity and mortality, often involving patients having comorbidities.
Infection control lapses were apparently responsible for virtually all reported health care transmission events, highlighting the need for strengthened infection control capacity worldwide. However, specific breaches in infection control were usually not identified and probably varied (Maltezou and Tsiodras 2014). Some high-risk procedures were identified, usually aerosol generating procedures, including hemodialysis and respiratory intensive care (Assiri et al. 2013b).
Secondarily infected health care workers tended to have milder symptoms and better prognoses. In the 2014 outbreak in Saudi Arabia, one study analyzed 191 symptomatic cases of MERS-CoV and found that 20.9% were health care workers (additional infected health care workers were asymptomatic, so that 30.6% of the 255 total cases analyzed were health care workers), and most patients had contact with a health care facility (Oboho et al. 2015). In a cluster of 9 MERS-CoV cases in Jordan, 6 were health care workers at the outbreak hospital, and no MERS-CoV transmission was detected at 2 transfer hospitals with adequate infection control (Al-Abdallat et al. 2014).
A health care-associated outbreak occurred in South Korea in the late spring of 2015 through July 2015, arising from one infected individual returning from the Gulf states (Cowling et al. 2015, Hui et al. 2015, Park et al. 2015). An analysis of 185 South Korean MERS-CoV cases using publicly available data delineated three generations of secondary infections (more than half of the cases were from the second generation) (Lee and Wong 2015). Overall, 154 cases were hospital-associated (Lee and Wong 2015), primarily affecting non-health care workers, spread by conditions such as close contact with the patients in crowded hospital or emergency rooms (Lee 2015b) and patients switching facilities (Lee 2015a, 2015b).
Direct contact could be inferred in only about 10% of the cases, and some authors suggested that fomite transmission could be important (Lee and Wong 2015, Seo et al. 2016, also reviewed in Otter et al. 2016). Earlier reports demonstrated that MERS-CoV is relatively stable in the environment, reported recoverable on surfaces after 48 h at 20°C and 48% relative humidity and for at least 12 h at 30°C with 30% humidity (van Doremalen et al. 2013), or in liquid at 25°C after 2 h (Leclercq et al. 2014). In a study of four laboratory-confirmed MERS-CoV patient rooms in two South Korean hospitals, MERS-CoV viral RNA was found on environmental surfaces, including bedsheets and medical equipment, up to 5 days after a patient's last PCR-positive respiratory specimen; viable virus was also detected (Seo et al. 2016).
However, there were apparently few reports of infections in hospital laundry or maintenance workers, contrary to what would be expected for environmental transmission. It is possible that they were not identified or reported. We could find only two publications specifically mentioning infection in hospital cleaners (Hastings et al. 2016, Liu et al. 2016). Hastings et al. (2016) investigated a 2014 outbreak in King Fahd General Hospital and reported 1 MERS-CoV case among 1295 maintenance or housekeeping personnel. However, it appears that only symptomatic cases were tested, and specific details on this case were not provided.
Many reports from contact tracing identified family members, friends, coworkers, and other persons who had close contact with the patient. These include reports from Saudi Arabia (Memish et al. 2013b, Omrani et al. 2013, Drosten et al. 2014), as well as the United Kingdom (Pebody et al. 2012, Health Protection Agency (HPA) UK Novel Coronavirus Investigation Team 2013, Thomas et al. 2014), Iran (Yavarian et al. 2015), Tunisia (Abroug et al. 2014), China (Wu et al. 2015), Greece (Tsiodras et al. 2014), Germany (Buchholz et al. 2013, Reuss et al. 2014), Malaysia (Premila Devi et al. 2014), France (Mailles et al. 2013), and Italy (Puzelli et al. 2013).
Family clusters were identified in a number of these reports, but at most only a small percentage of contacts became cases, suggesting that close contact is not as important a risk factor as health care-associated transmission, and direct human-to-human transmission appears limited. Although the outbreak in South Korea also involved close contact among family members and other patients in hospital rooms, this may be more appropriately categorized as health care-associated transmission (Kang et al. 2017).
However, there is relatively little information on asymptomatic individuals, most of whom are detected by contact tracing. In one study, an asymptomatic health care worker excreted MERS-CoV for over 5 weeks (Al-Gethamy et al. 2015). In the spring 2014 outbreak in Saudi Arabia, an estimated 25.1% of laboratory-confirmed MERS-CoV was asymptomatic (Oboho et al. 2015). An analysis of 65 MERS-CoV patients in United Arab Emirates found that 35% were asymptomatic, and 1 individual yielded positive lower respiratory tract specimens for more than 2 weeks (Hosani et al. 2016). Al Hammadi et al. reported two asymptomatic men who had contact with infected camels; infection was confirmed by PCR in both men, and in all eight camels in the group (Al Hammadi et al. 2015). However, the actual frequency of asymptomatic individuals compared with symptomatic individuals is largely unknown.
Parameters for transmission dynamics are important for mathematical modeling of spread. Due to the heterogeneity in transmission, an important characteristic of infection dynamics, R0 (the basic reproductive rate in a susceptible population), has been difficult to pinpoint. Estimates have ranged from 0.50 to 0.69 (Breban et al. 2013, Poletto et al. 2014) to between 0.8 and 1.3 (Cauchemez et al. 2014). R0 > 1 is required for an outbreak to be self-sustaining (Anderson and May 1991). For specific locations, one model suggested an R0 of 3.5–6.7 in Jeddah and 2.0–2.8 in Riyadh (Majumder et al. 2014), but these likely did not correct for effect of superspreading in health care settings (Kucharski and Althaus 2015). One mathematical model suggested the risk of secondary transmission following an imported MERS-CoV case is 22.7% (95% CI: 19.2–25.1%), with the risks of generations 2, 3, and 4 estimated to be 10.5%, 6.1%, and 3.9%, respectively (Nishiura et al. 2015).
Travel-related cases
Despite the mass gathering of individuals at the annual Hajj pilgrimage (Khan et al. 2013), to date it has not resulted in any known MERS-CoV transmission events (Barasheed et al. 2014, Benkouiten et al. 2014, Gautret et al. 2014, Memish et al. 2014b, 2014c, Aberle et al. 2015, Annan et al. 2015), confirming modeling estimates for the 2014 Hajj (Lessler et al. 2014). Two travelers returning to the Netherlands from a separate pilgrimage (Umrah) in May 2014 were found to have MERS-CoV (Kraaij-Dirkzwager et al. 2014). Many of the travel-related cases have been tabulated (Pavli et al. 2014, Sridhar et al. 2015, Carias et al. 2016). In all these cases, casual transmission (outside of family and health care settings) has not been observed.
The animal interface and search for natural and reservoir hosts of MERS-CoV
Dromedary camels are the only documented zoonotic source of human infection, and there is considerable circumstantial evidence supporting camel-to-human transmission, including evidence of camels and humans becoming infected with an identical MERS-CoV (Azhar et al. 2014, Ferguson and Kerkhove 2014, Memish et al. 2014e). Identical genomic sequences of MERS-CoV were isolated from a camel owner who died from MERS-CoV and his camel who had rhinorrhea at that time; identical MERS-CoV RNA fragments were later detected in the air at the camel barn (Azhar et al. 2014). Serology suggested that the camel was infected before the owner (Azhar et al. 2014). Critics have suggested that the identical sequences may be due to cross-contamination (Drosten et al. 2014), but the authors maintain that the isolates were collected on different days and processed in different facilities. Other epidemiologic investigations have found similar evidence, but directionality was not implied (Ferguson and Kerkhove 2014, Memish et al. 2014e).
In outbreak sites, viral genomic sequences from camels have matched those published for humans, and one analysis of MERS-CoV among camels showed that many of the camel viruses can be classified into clades that correlate with human outbreaks (Alagaili et al. 2014). Interestingly, three dromedary camel strains of MERS-CoV had similar viral replication competence to human strains in Vero-E6 cells and human respiratory tract explants (Chan et al. 2014).
This evidence suggests that camels would appear involved in cross-species transmission of MERS-CoV, although the precise mechanism and frequency remain uncertain.
Occupational exposure to camels would seem a particularly strong risk factor for infection, but its relative importance and relative risk of different exposures are not well documented (Hemida et al. 2015). As camels are widely used in the region for a variety of purposes, many people have contact with camels, and exposure is often invoked in nonoccupational primary cases. Assessment of camel exposure is further complicated by the varying and sometimes vague definitions used to classify camel exposure, and the multiple types of possible exposures. Even so, it has been hypothesized that camel exposure is commonly underreported (Gossner et al. 2016). There is a critical need for clearer definitions and measurements of “camel exposure,” and to standardize methodology.
Evidence to date indicates that MERS-like coronaviruses have been circulating in camels since at least the 1980s, before the first detection of MERS-CoV in humans (Müller et al. 2014). Seropositivity has been found consistently in African and Middle Eastern camels since 1983 (Table 1). There is virologic evidence of MERS-CoV infection in camels in Qatar (Ferguson and Kerkhove 2014, Haagmans et al. 2014, Raj et al. 2014), Egypt (Chu et al. 2014, Ali et al. 2017a, 2017b), Oman (Nowotny and Kolodziejek 2014), Saudi Arabia (Hemida et al. 2014), Sudan, and elsewhere (Chu et al. 2015, Ali et al. 2017a).
Table 1.
Location, Methods, and Results of Recent Middle East Respiratory Syndrome Coronavirus Camel Seroprevalence Studies
| Countries (reference) | Year(s) | Test(s) useda | No. of samples | No. of positives (%) |
|---|---|---|---|---|
| Egypt (Perera et al. 2013) | 2013 | ppNT | 110 | 108 (98.2) |
| Egypt, Somalia, Sudan (Müller et al. 2014) | 1983–1997 | rELISA | 189 | 159 (84.1) |
| MNT | 153 (81.0) | |||
| Egypt, Sudan, others (Ali et al. 2017a) | 2015–2016 | MNT | 1031 | 871 (84.5)b |
| Ethiopia (Reusken et al. 2014b) | 2010–2011 | S1 microarray | 188 | 181 (96.3)c |
| Jordan (Reusken et al. 2013b) | 2013 | Nt | 11 | 11 (100.0) |
| Kenya (Corman et al. 2014b) | 1992–2013 | rELISA | 774 | 228 (29.5) |
| IFA | 213 (27.5) | |||
| MNT | 133 (17.2) | |||
| Nigeria (Reusken et al. 2014b) | 2010–2011 | S1 microarray | 358 | 337 (94.1)c |
| Oman (Reusken et al. 2013c) | 2013 | PRNT | 50 | 50 (100.0) |
| Saudi Arabia (Alagaili et al. 2014) | 1992–2010 | ELISA | 264 | 230 (87.1) |
| 2013 | ELISA | 203 | 150 (73.9) | |
| Saudi Arabia (Hemida et al. 2014) | 1993 | ppNT | 131 | 118 (90.1) |
| Saudi Arabia (Hemida et al. 2013) | 2012–2013 | ppNT | 310 | 280 (90.3) |
| Tunisia (Reusken et al. 2014b) | 2009 | S1 microarray | 204 | 99 (48.5)c |
| United Arab Emirates (Meyer et al. 2014) | 2003, 2013 | rIFA | 651 | 632 (97.1) |
| Nt | 509 (80.5) | |||
| United Arab Emirates (Alexandersen et al. 2014) | 2005 | Nt | 11 | 9 (81.8) |
ppNT, pseudoparticle neutralization; rELISA, recombinant enzyme-linked immunosorbent assay; MNT, microneutralization; PRNT, plaque reduction neutralization test; rIFA, recombinant immunofluorescence assay; Nt, serum neutralization.
Seroprevalence reported higher in camels imported from Sudan (543/594; 88.7%) and East Africa (71/98; 72.4%) than resident (257/339; 5.8%); total 41/1078 nasal swabs reported PCR positive, 35/41 from Sudan.
The exact number was not reported, and has been imputed from the reported percentages.
Increasing genomic evidence suggests a diversity of coronaviruses in camels throughout the Middle East and Africa, including in major source countries for camel imports to the Middle East, cross-neutralizing with MERS-CoV but having divergent sequences and probably not human pathogens (Chu et al. 2015, 2018). This may explain the high apparent seropositivity in camels in Africa in the absence of known human cases of MERS.
Maintenance of the virus in the camel population remains an open question. Symptomatic MERS-CoV infection has been documented in both calves and adult camels (Adney et al. 2014), and viral shedding was detected in nasal secretions up to 7 days postinoculation with MERS-CoV (Adney et al. 2014), as well as in milk and possibly feces (Hemida et al. 2014, Reusken et al. 2014a). Camel milk has been suggested as a possible source of infection for both humans and camel foals (Hemida et al. 2014, Reusken et al. 2014a, van Doremalen et al. 2014). One study demonstrated that added MERS-CoV can survive in milk for at least 48 h at both 4°C and 22°C, although not detected after pasteurization (van Doremalen et al. 2014).
Other possible livestock hosts for MERS-CoV have been investigated, and have generally been negative (Hemida et al. 2013, Perera et al. 2013, Reusken et al. 2013b, 2013c, Alagaili et al. 2014, Alexandersen et al. 2014, van Doremalen et al. 2014, Meyer et al. 2015). One study found 15 seropositive alpacas, which are members of the camelid family, in Qatar (Reusken et al. 2016). Experimentally infected alpacas in one study demonstrated upper respiratory tract infection, viral shedding, and transmission to other alpacas (Adney et al. 2016).
The search for the origins and natural hosts of MERS-CoV continues. Bat species across the world are known to host a wide variety of coronaviruses, including beta-coronaviruses (the genus to which MERS-CoV belongs) (Anthony et al. 2013, Corman et al. 2013, Lelli et al. 2013, Memish et al. 2013a), and bats tend to show greater coronavirus diversity than other mammals (Drexler et al. 2014, Anthony et al. 2017). Consequently, much research has focused on the possible origin of MERS-CoV in bats. Possibly further supporting this hypothesis, in bat cell lines MERS-CoV has been reported to replicate only in those cell lines that express DPP4, the functional human receptor for the virus, and not in bat cell lines lacking DPP4 expression (Raj et al. 2013, Cai et al. 2014).
It is possible that MERS-CoV itself may be a relatively new introduction in humans. A phylogenetic analysis utilizing 65 complete or partially complete MERS-CoV genomic sequences (32 from Saudi Arabian cases in 2013) estimated that the most recent common ancestor was in March 2012 (95% CI: December 2011–June 2012) (Cotten et al. 2014).
One study, from analysis of an S1 subunit in its spike gene, suggested that MERS-CoV may have arisen from intraspike recombination between two ancestral coronaviruses (Corman et al. 2014).
The virus has not been isolated from a bat, although a portion of MERS-CoV RNA sequence apparently was identified in a bat (the insectivorous bat Taphozous perforatus, the Egyptian tomb bat) in Saudi Arabia in the same site as an early human case (100% nucleotide match, although this was only ∼180 nucleotides in the relatively well-conserved RdRp region) (Memish et al. 2013a). MERS-like coronaviruses, including the closely related HKU4, which also uses a DPP4 receptor, and HKU5, first identified by Woo et al. in 2006 (Woo et al. 2006, Lau et al. 2013, Matthews et al. 2014, Wang et al. 2014), and others have been identified in bat species in China (Matthews et al. 2014, Yang et al. 2014), Mexico (Anthony et al. 2013), South Africa (Ithete et al. 2013, Corman et al. 2014a), and Uganda (Anthony et al. 2017).
Discussion and Conclusions
Since the detection of MERS-CoV in 2012, a large body of research has been amassed, as evidenced by the hundreds of unique results from our database searches. The virus has rapidly been characterized, and its functional human receptor swiftly identified. Species and cellular tropism studies have complemented clinical investigations, and laboratory work has provided further insights on MERS-CoV pathogenesis.
Observational research from outbreaks in the Middle East and South Korea has generated a growing repository of clinical data, basic epidemiology, and genome sequences. Other studies have evaluated the roles of human/animal interfaces, health care settings, family clusters, and travel in MERS-CoV transmission. Furthermore, bats have been implicated as the possible origin of MERS-CoV, while dromedary camels have been shown to be likely sources of many human infections. There has been progress in developing therapeutics and vaccines. Although these have not yet progressed to full clinical trials, they could in the foreseeable future.
A major reason we undertook this review was to determine what gaps still exist. Of the publications we originally identified, the largest category (80 publications) was in laboratory or basic science studies, followed by surveillance (59), animal interfaces (55), clinical aspects and health care (50), therapeutics and vaccines (42), while human transmission and epidemiology was one of the sparsest (35, including investigations of hospital outbreaks and travel-related cases), noting that these categories are not necessarily exclusive and there is overlap.
In general, continued surveillance for MERS-CoV in affected countries should continue, prioritizing surveillance in humans, camels, and bats, given their apparent importance. As serology may not be reliable, due to cross-reactions and possibly waning antibodies, virologic surveillance and sequencing should also be attempted on selected specimens whenever feasible.
Although the role of bats is yet to be fully elucidated, and evidence is lacking that bats are a source of human infection, the diversity of coronaviruses, including MERS-like coronaviruses, in bat species across the globe indicates that continued research and ongoing surveillance of coronaviruses in bats are justified (Lau et al. 2013, Lelli et al. 2013, Yang et al. 2014).
A number of key gaps remain in epidemiology, including transmission, population-based incidence or prevalence rates, and geographic and demographic distributions of infections. Based on the limited human seroprevalence data so far, it seems likely that population prevalence is low, but the baseline should be established. It is also not clear whether seropositivity is a reliable indicator of prior infection. The finding of variable low antibody titers at 1 year postinfection (Choe et al. 2017) indicates that methods other than traditional serosurveys may be required (Reusken et al. 2013a, Malik et al. 2016). If serology is not reliable, this raises the question of how to screen populations. There are methods that are both more sensitive and more specific for antibody detection (Mishra et al. 2018), as well as assays of cellular immunity (Zhao et al. 2017), but are costlier and more difficult for population screening.
Contact tracing has identified asymptomatic infections, but studies of their frequency and transmissibility have been limited. Many of those infected are health care workers in whom these questions could be systematically studied. Relatively little is known about the natural history of human asymptomatic infections. Further studies of virus shedding, seroconversion, and transmission in asymptomatic infections are needed. Some evidence suggests that secondary cases have lower average CFRs and that subsequent links in the chain of MERS-CoV infection may decrease in severity.
Exposure in most primary cases is still largely inferential at best. While camels are apparently often the likely source, the definitions of camel exposure are varied, preventing clear epidemiological inferences. Definitions of the exposures and the methodology and metrics for determining exposure should be clarified and standardized. Even if camels are a principal source of human infections, this would still not explain a substantial portion of primary human cases that did not have apparent camel exposure before infection. This raises questions about how and where these other primary cases acquired MERS-CoV infection, whether through other animal species, unidentified zoonotic exposure, unrecognized infectious persons (such as asymptomatic infectious individuals), or environmental contamination.
Consequently, clarifying the routes of transmission remains crucial and should receive emphasis. To date, there is no compelling evidence of direct human-to-human transmission, such as through droplets, but secondary infection has been reported. It is often unclear how health care workers become infected. There is possible evidence of environmental contamination (fomites) in health care settings (nosocomial transmission) and close contact with an active MERS-CoV case.
Various infection control lapses have been documented, but rarely a specific event or route (Ben Embarek and Van Kerkhove 2015, Alraddadi et al. 2016, Van Kerkhove et al. 2016). Rigorous infection control therefore remains essential and could prevent many secondary cases. In the interim, better documentation or identification of the specific lapses that led to infection would be of great value in understanding mechanisms of transmission and is one of the greatest knowledge gaps. Much of the evidence for fomite transmission, for example, remains anecdotal (Van Kerkhove et al. 2016).
The mechanisms supporting chains of human MERS-CoV transmission need to be more fully evaluated. These gaps indicate a need for additional research, and also for consistent terminology and clear definitions. Although the epidemiological literature on MERS-CoV is large, much of it is fragmented and inconsistently reported. It is often difficult to deduce the sequence of infections, relationships between cases, or specific exposures. A comprehensive and consistent database or registry should be a priority.
Genome sequencing has proved useful in several respects. Genome sequencing was used to help determine viral circulation and ancestry (Cotten et al. 2014), and to differentiate apparently similar and serologically cross-reacting coronaviruses in camels, addressing the (still unresolved) question of why MERS seems regionally limited, while many camels are imported from other places (Chu et al. 2015, 2018). It has also been used to reconstruct the transmission events and help to establish chains of infection (Assiri et al. 2013b, Cotten et al. 2013), but it must be noted that there is a pressing need for more rigorous conventional epidemiology as well to validate the methodologies. These approaches might well serve a complementary function in advancing our understanding.
There remains a need for effective therapeutics and vaccines. Treatment remains symptomatic, with insufficient clinical data on other therapeutic options. These options will always be limited in number, but their development should be approached more systematically with shared protocols and critical comparison of results. As a number of drug and vaccine candidates have been identified, there may be more options available for future clinical trials. There appear to be efforts underway to develop some clinical trials based on standardized protocols. This is needed, despite the challenges presented by the sporadic nature of the cases, and the relative rarity of clinical MERS.
There were several limitations in this systematic review of MERS-CoV. Only publications in English or with English language abstracts were eligible for inclusion, although most of the cases have been in Arabic-speaking areas and Korea. However, hundreds of English language reports were available from countries around the world, including the Middle East and South Korea, minimizing this concern. Second, this review contains publications indexed by these databases by July 2017, so it is possible that some publications, especially recently published articles or manuscripts still in production, were unintentionally excluded from the review. We updated our literature review periodically during the preparation of this article to minimize the number of missed publications. Third, “gray literature” was not included, such as government reports and technical documents, and most meeting abstracts, although it is likely that important findings would have been submitted for journal publication.
As an emerging infection of zoonotic origin (Morse 1995), a unified One Health approach that engages the human health, livestock and companion animal, wildlife, and environment sectors will be crucial in dealing with MERS (Morse 2012, Morse et al. 2012). It could serve as an excellent case study for epidemiology and response to a new zoonotic, or emerging, infection.
Acknowledgments
We thank the WHO/EMRO for commissioning this work, and the anonymous reviewers of the article for thoughtful and incisive suggestions. SSM gratefully acknowledges support by the Arts & Letters Foundation.
Disclaimer
The funder had no role in developing the content of this article. The contents are the responsibility of the authors and do not necessarily reflect the views of the entities with which they are affiliated.
Author Disclosure Statement
No competing financial interests exist.
References
- Abd El Wahed A, Patel P, Heidenreich D, Hufert F, et al. . Reverse transcription recombinase polymerase amplification assay for the detection of Middle East respiratory syndrome coronavirus. PLoS Curr Outbreaks 2013; Available at: http://currents.plos.org/outbreaks/index.html%3Fp=22591.html accessed January, 2019 [DOI] [PMC free article] [PubMed]
- Aberle JH, Popow-Kraupp T, Kreidl P, Laferl H, et al. . Influenza A and B viruses but not MERS-CoV in Hajj Pilgrims, Austria, 2014. Emerg Infect Dis 2015; 21:726–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abroug F, Slim A, Ouanes-Besbes L, Kacem M-AH, et al. . Family cluster of Middle East respiratory syndrome coronavirus infections, Tunisia, 2013. Emerg Infect Dis 2014; 20:1527–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aburizaiza AS, Mattes FM, Azhar EI, Hassan AM, et al. . Investigation of anti–Middle East respiratory syndrome antibodies in blood donors and slaughterhouse workers in Jeddah and Makkah, Saudi Arabia, Fall 2012. J Infect Dis 2014; 209:243–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adedeji AO, Singh K, Kassim A, Coleman CM, et al. . Evaluation of SSYA10–001 as a replication inhibitor of Severe Acute Respiratory Syndrome, mouse hepatitis, and Middle East respiratory syndrome coronaviruses. Antimicrob Agents Chemother 2014; 58:4894–4898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adney DR, Bielefeldt-Ohmann H, Hartwig AE, Bowen RA. Infection, replication, and transmission of Middle East respiratory syndrome coronavirus in alpacas. Emerg Infect Dis 2016; 22:1031–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adney DR, van Doremalen N, Brown VR, Bushmaker T, et al. . Replication and shedding of MERS-CoV in upper respiratory tract of inoculated dromedary camels. Emerg Infect Dis 2014; 20:1999–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal AS, Garron T, Tao X, Peng B-H, et al. . Generation of a transgenic mouse model of Middle East respiratory syndrome coronavirus infection and disease. J Virol 2015; 89:3659–3670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajlan AM, Ahyad RA, Jamjoom LG, Alharthy A, et al. . Middle East respiratory syndrome coronavirus (MERS-CoV) infection: Chest CT findings. AJR Am J Roentgenol 2014; 203:782–787 [DOI] [PubMed] [Google Scholar]
- Al Hammadi ZM, Chu DKW, Eltahir YM, Hosani FA, et al. . Asymptomatic MERS-CoV infection in humans possibly linked to infected dromedaries imported from Oman to United Arab Emirates, May 2015. Emerg Infect Dis 2015; 21:2197–2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Abdallat MM, Payne DC, Alqasrawi S, Rha B, et al. . Hospital-associated outbreak of Middle East respiratory syndrome coronavirus: A serologic, epidemiologic, and clinical description. Clin Infect Dis 2014; 59:1225–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Gethamy M, Corman VM, Hussain R, Al-Tawfiq JA, et al. . A case of long-term excretion and subclinical infection with Middle East respiratory syndrome coronavirus in a healthcare worker. Clin Infect Dis 2015; 60:973–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Tawfiq JA, Hinedi K, Ghandour J, Khairalla H, et al. . Middle East respiratory syndrome coronavirus: A case-control study of hospitalized patients. Clin Infect Dis 2014; 59:160–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Tawfiq JA, Momattin H, Dib J, Memish ZA. Ribavirin and interferon therapy in patients infected with the Middle East respiratory syndrome coronavirus: An observational study. Int J Infect Dis 2014; 20:42–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagaili AN, Briese T, Mishra N, Kapoor V, et al. . Middle East respiratory syndrome coronavirus infection in dromedary camels in Saudi Arabia. MBio 2014; 5:e00884–00814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandersen S, Kobinger GP, Soule G, Wernery U. Middle East respiratory syndrome coronavirus antibody reactors among camels in Dubai, United Arab Emirates, in 2005. Transbound Emerg Dis 2014; 61:105–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alghamdi IG, Hussain II, Almalki SS, Alghamdi MS, et al. . The pattern of Middle East respiratory syndrome coronavirus in Saudi Arabia: A descriptive epidemiological analysis of data from the Saudi Ministry of Health. Int J Gen Med 2014; 7:417–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M, El-Shesheny R, Kandeil A, Shehata M, et al. . Cross-sectional surveillance of Middle East respiratory syndrome coronavirus (MERS-CoV) in dromedary camels and other mammals in Egypt, August 2015 to January 2016. Euro Surveill 2017a; 22:30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali MA, Shehata MM, Gomaa MR, Kandeil A, et al. . Systematic, active surveillance for Middle East respiratory syndrome coronavirus in camels in Egypt. Emerg Microbes Infect. 2017. January 4;6(1):e1. doi: 10.1038/emi.2016.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almazán F, DeDiego ML, Sola I, Zuñiga S, et al. . Engineering a replication-competent, propagation-defective Middle East respiratory syndrome coronavirus as a vaccine candidate. MBio 2013; 4:e00650–00613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alraddadi BM, Watson JT, Almarashi A, Abedi GR, et al. . Risk factors for primary Middle East respiratory syndrome coronavirus illness in humans, Saudi Arabia, 2014. Emerg Infect Dis 2016; 22:49–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsahafi AJ, Cheng AC. The epidemiology of Middle East respiratory syndrome coronavirus in the Kingdom of Saudi Arabia, 2012–2015. Int J Infect Dis 2016; 45:1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alserehi H, Wali G, Alshukairi A, Alraddadi B. Impact of Middle East respiratory syndrome coronavirus (MERS-CoV) on pregnancy and perinatal outcome. BMC Infect Dis 2016; 16:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshukairi AN, Khalid I, Ahmed WA, Dada AM, et al. . Antibody response and disease severity in healthcare worker MERS survivors. Emerg infect Dis 2016; 22:1113–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RM, May RM. Infectious Diseases of Humans: Dynamics and Control. Oxford, UK: Oxford University Press, 1991 [Google Scholar]
- Annan A, Owusu M, Marfo KS, Larbi R, et al. . High prevalence of common respiratory viruses and no evidence of Middle East respiratory syndrome coronavirus in Hajj pilgrims returning to Ghana, 2013. Trop Med Int Health 2015; 20:807–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony SJ, Gilardi K, Menachery VD, Goldstein T, et al. . Further evidence for bats as the evolutionary source of Middle East respiratory syndrome coronavirus. MBio 2017; 8:e00373–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony SJ, Johnson CK, Greig DJ, Kramer S, et al. . Global patterns in coronavirus diversity. Virus Evol 2017; 3:vex012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony SJ, Ojeda-Flores R, Rico-Chavez O, Navarrete-Macias I, et al. . Coronaviruses in bats from Mexico. J Gen Virol 2013; 94:1028–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabi YM, Arifi AA, Balkhy HH, Najm H, et al. . Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med 2014; 160:389–397 [DOI] [PubMed] [Google Scholar]
- Assiri A, Abedi GR, Saeed AAB, Abdalla MA, et al. . Multifacility outbreak of Middle East respiratory syndrome in Taif, Saudi Arabia. Emerg Infect Dis 2016; 22:32–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, Al-Rabiah FA, et al. . Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: A descriptive study. Lancet Infect Dis 2013a; 13:752–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assiri A, McGeer A, Perl TM, Price CS, et al. . Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med 2013b; 369:407–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azhar EI, El-Kafrawy SA, Farraj SA, Hassan AM, et al. . Evidence for camel-to-human transmission of MERS coronavirus. N Engl J Med 2014; 370:2499–2506 [DOI] [PubMed] [Google Scholar]
- Azhar EI, Hashem AM, El-Kafrawy SA, Sohrab SS, et al. . Detection of the Middle East respiratory syndrome coronavirus genome in an air sample originating from a camel barn owned by an infected patient. MBio 2014; 5:e01450–01414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barasheed O, Rashid H, Alfelali M, Tashani M, et al. . Viral respiratory infections among Hajj pilgrims in 2013. Virol Sin 2014; 29:364–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlan A, Zhao J, Sarkar MK, Li K, et al. . Receptor variation and susceptibility to Middle East respiratory syndrome coronavirus infection. J Virol 2014; 88:4953–4961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseler L, de Wit E, Feldmann H. A comparative review of animal models of Middle East respiratory syndrome coronavirus infection. Vet Pathol 2016; 53:521–531 [DOI] [PubMed] [Google Scholar]
- Ben Embarek P, Van Kerkhove MD. Middle East respiratory syndrome coronavirus (MERS-CoV): Current situation 3 years after the virus was first identified. Wkly Epidemiol Rec 2015; 90:245–250 [PubMed] [Google Scholar]
- Benkouiten S, Charrel R, Belhouchat K, Drali T, et al. . Respiratory viruses and bacteria among pilgrims during the 2013 Hajj. Emerg Infect Dis 2014; 20:1821–1827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bin Saeed AA, Abedi GR, Alzahrani AG, Salameh I, et al. . Surveillance and testing for Middle East respiratory syndrome coronavirus, Saudi Arabia, April 2015–February 2016. Emerg Infect Dis 2017; 23:682–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breakwell L, Pringle K, Chea N, Allen D, et al. . Lack of transmission among close contacts of patient with case of Middle East Respiratory Syndrome imported into the United States, 2014. Emerg Infect Dis 2015; 21:1128–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breban R, Riou J, Fontanet A. Interhuman transmissibility of Middle East respiratory syndrome coronavirus: Estimation of pandemic risk. Lancet 2013; 382:694–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briese T, Mishra N, Jain K, Zalmout IS, et al. . Middle East respiratory syndrome coronavirus quasispecies that include homologues of human isolates revealed through whole-genome analysis and virus cultured from dromedary camels in Saudi Arabia. MBio 2014; 5:e01146–01114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz U, Muller MA, Nitsche A, Sanewski A, et al. . Contact investigation of a case of human novel coronavirus infection treated in a German hospital, October–November 2012. Euro Surveill 2013; 18:20406. [PubMed] [Google Scholar]
- Cai Y, Yu S, Postnikova EN, Mazur S, et al. . CD26/DPP4 cell-surface expression in bat cells correlates with bat cell susceptibility to Middle East respiratory syndrome coronavirus (MERS-CoV) infection and evolution of persistent infection. PLoS One 2014; 9:e112060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carias C, O'Hagan JJ, Jewett A, Gambhir M, et al. . Exportations of symptomatic cases of MERS-CoV infection to countries outside the Middle East. Emerg Infect Dis 2016; 22:723–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauchemez S, Fraser C, Van Kerkhove MD, Donnelly CA, et al. . Middle East respiratory syndrome coronavirus: Quantification of the extent of the epidemic, surveillance biases, and transmissibility. Lancet Infect Dis 2014; 14:50–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. CDC Laboratory Testing for Middle East Respiratory Syndrome Coronavirus (MERS-CoV) 2014. (updated June 26, 2018). Available at: www.cdc.gov/coronavirus/mers/lab/lab-testing.html (Accessed August1, 2017. and July23, 2018)
- CEPI (Coalition for Epidemic Preparedness Innovations). Pipeline dataset for MERS-CoV. Available at: http://cepi.net/sites/default/files/Pipeline dataset for MERS-CoV.pdf (Accessed Aug. 1, 2017. and August8, 2018)
- Chan JF-W, Yao Y, Yeung M-L, Deng W, et al. . Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J Infect Dis 2015; 212:1904–1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JFW, Chan K-H, Kao RYT, To KKW, et al. . Broad-spectrum antivirals for the emerging Middle East respiratory syndrome. J Infect 2013; 67:606–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RWY, Hemida MG, Kayali G, Chu DKW, et al. . Tropism and replication of Middle East respiratory syndrome coronavirus from dromedary camels in the human respiratory tract: An in-vitro and ex-vivo study. Lancet Respir Med 2014; 2:813–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Rajashankar KR, Yang Y, Agnihothram SS, et al. . Crystal structure of the receptor-binding domain from newly emerged Middle East respiratory syndrome coronavirus. J Virol 2013; 87:10777–10783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe PG, Perera RAPM, Park WB, Song K-H, et al. . MERS-CoV antibody responses 1 year after symptom onset, South Korea, 2015. Emerg Infect Dis 2017; 23:1079–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu DK, Oladipo JO, Perera RA, Kuranga SA, et al. . Middle East respiratory syndrome coronavirus (MERS-CoV) in dromedary camels in Nigeria, 2015. Euro Surveill 2015; 20:30086. [DOI] [PubMed] [Google Scholar]
- Chu DKW, Hui KPY, Perera RAPM, Miguel E, et al. . MERS coronaviruses from camels in Africa exhibit region-dependent genetic diversity. Proc Natl Acad Sci U S A 2018; 115:3144–3149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu DKW, Poon LLM, Gomaa MM, Shehata MM, et al. . MERS coronaviruses in dromedary camels, Egypt. Emerg Infect Dis 2014; 20:1049–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockrell AS, Peck KM, Yount BL, Agnihothram SS, et al. . Mouse dipeptidyl peptidase 4 is not a functional receptor for Middle East respiratory syndrome coronavirus infection. J Virol 2014; 88:5195–5199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman CM, Sisk JM, Halasz G, Zhong J, et al. . CD8+ T cells and macrophages regulate pathogenesis in a mouse model of Middle East respiratory syndrome. J Virol 2017; 91:e01825–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman V, Eckerle I, Bleicker T, Zaki A, et al. . Detection of a novel human coronavirus by realtime reverse-transcription polymerase chain reaction. Euro Surveill 2012a; 17:20285. [DOI] [PubMed] [Google Scholar]
- Corman V, Müller M, Costabel U, Timm J, et al. . Assays for laboratory confirmation of novel human coronavirus (hCoV-EMC) infections. Euro Surveill 2012b; 17:20334. [DOI] [PubMed] [Google Scholar]
- Corman VM, Albarrak AM, Omrani AS, Albarrak MM, et al. . Viral shedding and antibody response in 37 patients with Middle East respiratory syndrome coronavirus infection. Clin Infect Dis 2016; 62:477–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman VM, Ithete NL, Richards LR, Schoeman MC, et al. . Rooting the phylogenetic tree of Middle East respiratory syndrome coronavirus by characterization of a conspecific virus from an African bat. J Virol 2014a; 88:11297–11303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman VM, Meyer B, Jores J, Younan M, et al. . Antibodies against MERS coronavirus in dromedary camels, Kenya, 1992–2013. Emerg Infect Dis 2014b; 20:1319–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman VM, Ölschläger S, Wendtner C-M, Drexler JF, et al. . Performance and clinical validation of the RealStar® MERS-CoV Kit for detection of Middle East respiratory syndrome coronavirus RNA. J Clin Virol 2014c; 60:168–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman VM, Rasche A, Diallo TD, Cottontail VM, et al. . Highly diversified coronaviruses in neotropical bats. J Gen Virol 2013; 94:1984–1994 [DOI] [PubMed] [Google Scholar]
- Corti D, Zhao J, Pedotti M, Simonelli L, et al. . Prophylactic and postexposure efficacy of a potent human monoclonal antibody against MERS coronavirus. Proc Natl Acad Sci U S A 2015; 112:10473–10478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotten M, Watson SJ, Kellam P, Al-Rabeeah AA, et al. . Transmission and evolution of the Middle East respiratory syndrome coronavirus in Saudi Arabia: A descriptive genomic study. Lancet 2013; 382:1993–2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotten M, Watson SJ, Zumla AI, Makhdoom HQ, et al. . Spread, circulation, and evolution of the Middle East respiratory syndrome coronavirus. MBio 2014; 5:e01062–01013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowling BJ, Park M, Fang VJ, et al. . Preliminary epidemiological assessment of MERS-CoV outbreak in South Korea, May to June 2015. Euro Surveill 2015; 20:21163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wilde AH, Jochmans D, Posthuma CC, Zevenhoven-Dobbe JC, et al. . Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother 2014; 58:4875–4884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wilde AH, Raj VS, Oudshoorn D, Bestebroer TM, et al. . MERS-coronavirus replication induces severe in vitro cytopathology and is strongly inhibited by cyclosporin A or interferon-a treatment. J Gen Virol 2013; 94:1749–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit E, Rasmussen AL, Falzarano D, Bushmaker T, et al. . Middle East respiratory syndrome coronavirus (MERS-CoV) causes transient lower respiratory tract infection in rhesus macaques. Proc Natl Acad Sci U S A 2013; 110:16598–16603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: Recent insights into emerging coronaviruses. Nat Rev Microbiol 2016; 14:523–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexler JF, Corman VM, Drosten C. Ecology, evolution and classification of bat coronaviruses in the aftermath of SARS. Antiviral Res 2014; 101:45–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten C, Kellam P, Memish ZA. Evidence for camel-to-human transmission of MERS coronavirus (Letter). N Engl J Med 2014; 371:1359–1360 [DOI] [PubMed] [Google Scholar]
- Drosten C, Meyer B, Müller MA, Corman VM, et al. . Transmission of MERS-coronavirus in household contacts. N Engl J Med 2014; 371:828–835 [DOI] [PubMed] [Google Scholar]
- Drosten C, Muth D, Corman VM, Hussain R, et al. . An observational, laboratory-based study of outbreaks of Middle East respiratory syndrome coronavirus in Jeddah and Riyadh, Kingdom of Saudi Arabia, 2014. Clin Infect Dis 2015; 60:369–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten C, Seilmaier M, Corman VM, Hartmann W, et al. . Clinical features and virological analysis of a case of Middle East respiratory syndrome coronavirus infection. Lancet Infect Dis 2013; 13:745–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L, Kou Z, Ma C, Tao X, et al. . A truncated receptor-binding domain of MERS-CoV spike protein potently inhibits MERS-CoV infection and induces strong neutralizing antibody responses: Implication for developing therapeutics and vaccines. PLoS One 2013a; 8:e81587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L, G, Kou Z, Ma C, et al. . Identification of a receptor-binding domain in the S protein of the novel human coronavirus Middle East respiratory syndrome coronavirus as an essential target for vaccine development. J Virol 2013b; 87:9939–9942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L, Zhao G, Yang Y, Qiu H, et al. . A conformation-dependent neutralizing monoclonal antibody specifically targeting receptor-binding domain in Middle East respiratory syndrome coronavirus spike protein. J Virol 2014; 88:7045–7053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyall J, Coleman CM, Hart BJ, Venkataraman T, et al. . Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob Agents Chemother 2014; 58:4885–4893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckerle I, Corman VM, Müller MA, Lenk M, et al. . Replicative capacity of MERS coronavirus in livestock cell lines. Emerg Infect Dis 2014; 20:276–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckerle I, Müller MA, Kallies S, Gotthardt DN, et al. . In-vitro renal epithelial cell infection reveals a viral kidney tropism as a potential mechanism for acute renal failure during Middle East respiratory syndrome (MERS) coronavirus infection. Virol J 2013; 10:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falzarano D, de Wit E, Rasmussen AL, Feldmann F, et al. . Treatment with interferon-α2b and ribavirin improves outcome in MERS-CoV-infected rhesus macaques. Nat Med 2013; 19:1313–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falzarano D, Wit Ed, Feldmann F, Rasmussen AL, et al. . Infection with MERS-CoV causes lethal pneumonia in the common marmoset. PLoS Pathog 2014; 10:e1004250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanoy EB, van der Sande MA, Kraaij-Dirkzwager M, Dirksen K, et al. . Travel-related MERS-CoV cases: An assessment of exposures and risk factors in a group of Dutch travellers returning from the Kingdom of Saudi Arabia, May 2014. Emerg Themes Epidemiol 2014; 11:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure E, Poissy J, Goffard A, Fournier C, et al. . Distinct immune response in two MERS-CoV-infected patients: Can we go from bench to bedside? PLoS One 2014; 9:e88716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feikin DR, Alraddadi BM, Qutub M, Shabouni O, et al. . Association of higher MERS-CoV virus load with severe disease and death, Saudi Arabia, 2014. Emerg Infect Dis 2015; 21:2029–2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson NM, Kerkhove MDV. Identification of MERS-CoV in dromedary camels. Lancet Infect Dis 2014; 14:93–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Lu G, Qi J, Li Y, et al. . Structure of the fusion core and inhibition of fusion by a heptad repeat peptide derived from the S protein of Middle East respiratory syndrome coronavirus. J Virol 2013; 87:13134–13140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautret P, Charrel R, Benkouiten S, Belhouchat K, et al. . Lack of MERS coronavirus but prevalence of influenza virus in French pilgrims after 2013 Hajj. Emerg Infect Dis 2014; 20:728–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesmann M, de Castillo D. Introduction to googleVis 0.6.2. 2017. Available at https://cran.r-project.org/web/packages/googleVis/vignettes/googleVis.pdf [E-pub online]
- Gierer S, Hofmann-Winkler H, Albuali WH, Bertram S, et al. . Lack of MERS coronavirus neutralizing antibodies in humans, Eastern Province, Saudi Arabia. Emerg Infect Dis 2013; 19:2034–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierer S, Müller MA, Heurich A, Ritz D, et al. . Inhibition of proprotein convertases abrogates processing of the Middle Eastern respiratory syndrome coronavirus spike protein in infected cells but does not reduce viral infectivity. J Infect Dis 2015; 211:889–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossner C, Danielson N, Gervelmeyer A, Berthe F, et al. . Human-dromedary camel interactions and the risk of acquiring zoonotic Middle East respiratory syndrome coronavirus infection. Zoonoses Public Health 2016; 63:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham RL, Baric RS. Recombination, reservoirs, and the modular Spike: Mechanisms of coronavirus cross-species transmission. J Virol 2010; 84:3134–3146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guery B, Poissy J, el Mansouf L, Séjourné C, et al. . Clinical features and viral diagnosis of two cases of infection with Middle East respiratory syndrome coronavirus: A report of nosocomial transmission. Lancet 2013; 381:2265–2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haagmans BL, Al Dhahiry SHS, Reusken CBEM, Raj VS, et al. . Middle East respiratory syndrome coronavirus in dromedary camels: An outbreak investigation. Lancet Infect Dis 2014; 14:140–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haagmans BL, van den Brand JM, Raj VS, Volz A, et al. . An orthopoxvirus-based vaccine reduces virus excretion after MERS-CoV infection in dromedary camels. Science 2016; 351:77–81 [DOI] [PubMed] [Google Scholar]
- Hart BJ, Dyall J, Postnikova E, Zhou H, et al. . Interferon-β and mycophenolic acid are potent inhibitors of Middle East respiratory syndrome coronavirus in cell-based assays. J Gen Virol 2014; 95:571–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings DL, Tokars JI, Abdel Aziz IZAM, Alkhaldi KZ, et al. . Outbreak of Middle East respiratory syndrome at tertiary care hospital, Jeddah, Saudi Arabia, 2014. Emerg Infect Dis 2016; 22:794–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Protection Agency (HPA) UK Novel Coronavirus Investigation Team. Evidence of person-to-person transmission within a family cluster of novel coronavirus infections, United Kingdom, February 2013. Euro Surveill 2013; 8:20427. [DOI] [PubMed] [Google Scholar]
- Hemida MG, Al-Naeem A, Perera RAPM, Chin AWH, et al. . Lack of Middle East respiratory syndrome coronavirus transmission from infected camels. Emerg Infect Dis 2015; 21:699–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemida MG, Chu DKW, Poon LLM, Perera RAPM, et al. . MERS coronavirus in dromedary camel herd, Saudi Arabia. Emerg Infect Dis 2014; 20:1231–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemida MG, Perera RA, Wang P, Alhammadi MA, et al. . Middle East respiratory syndrome (MERS) coronavirus seroprevalence in domestic livestock in Saudi Arabia, 2010 to 2013. Euro Surveill 2013; 18:20659. [DOI] [PubMed] [Google Scholar]
- Hijawi B, Abdallat M, Sayaydeh A, Alqasrawi S, et al. . Novel coronavirus infections in Jordan, April 2012: Epidemiological findings from a retrospective investigation. East Mediterr Health J 2013; 19:S12–S18 [PubMed] [Google Scholar]
- Hilgenfeld R, Peiris M. From SARS to MERS: 10 years of research on highly pathogenic human coronaviruses. Antiviral Res 2013; 100:286–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hin Chu JZ, Wong BH-Y, Li C, Chan JF-W, et al. . Middle East respiratory syndrome coronavirus efficiently infects human primary T lymphocytes and activates the extrinsic and intrinsic apoptosis pathways. J Infect Dis 2016; 213:904–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocke AC, Becher A, Knepper J, Peter A, et al. . Emerging human Middle East respiratory syndrome coronavirus causes widespread infection and alveolar damage in human lungs. Am J Respir Crit Care Med 2013; 188:882–886 [DOI] [PubMed] [Google Scholar]
- Hosani FIA, Pringle K, Mulla MA, Kim L, et al. . Response to emergence of Middle East respiratory syndrome coronavirus, Abu Dhabi, United Arab Emirates, 2013–2014. Emerg Infect Dis 2016; 22:1162–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser KV, Gretebeck L, Ying T, Wang Y, et al. . Prophylaxis with a Middle East respiratory syndrome coronavirus (MERS-CoV)–specific human monoclonal antibody protects rabbits from MERS-CoV infection. J Infect Dis 2016; 213:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui DS, Perlman S, Zumla A. Spread of MERS to South Korea and China. Lancet Respir Med 2015; 3:509–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulswit RJ, de Haan CA, Bosch BJ. Coronavirus spike protein and tropism changes. Adv Virus Res 2016;96:29–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter JC, Nguyen D, Aden B, Al Bandar Z, et al. . Transmission of Middle East respiratory syndrome coronavirus infections in healthcare settings, Abu Dhabi. Emerg Infect Dis 2016; 22:647–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICTV. Virus Taxonomy: 2016 Release. Available at: https://talk.ictvonline.org/taxonomy (Accessed August1, 2017)
- Ithete NL, Stoffberg S, Corman VM, Cottontail VM, et al. . Close relative of Human Middle East respiratory syndrome coronavirus in bat, South Africa. Emerg Infect Dis 2013; 19:1698–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Wang N, Zuo T, Shi X, et al. . Potent neutralization of MERS-CoV by human neutralizing monoclonal antibodies to the viral spike glycoprotein. Sci Transl Med 2014; 6:234–259 [DOI] [PubMed] [Google Scholar]
- Kang CK, Song KH, Choe P, Park WB, et al. . Clinical and epidemiologic characteristics of spreaders of Middle East respiratory syndrome coronavirus during the 2015 outbreak in Korea. J Korean Med Sci 2017; 32:744–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor M, Pringle K, Kumar A, Dearth S, et al. . Clinical and laboratory findings of the first imported case of Middle East respiratory syndrome coronavirus to the United States. Clin Infect Dis 2014; 59:1511–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalid M, Al Rabiah F, Khan B, Al Mobeireek A, et al. . Ribavirin and interferon-a2b as primary and preventive treatment for Middle East respiratory syndrome coronavirus: A preliminary report of two cases. Antivir Ther 2015; 20:87–91 [DOI] [PubMed] [Google Scholar]
- Khalid M, Khan B, Rabiah FA, Alismaili R, et al. . Middle Eastern respiratory syndrome corona virus (MERS CoV): Case reports from a tertiary care hospital in Saudi Arabia. Ann Saudi Med 2014; 34:396–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan K, Sears J, Hu V, Brownstein J, et al. . Potential for the international spread of Middle East respiratory syndrome in association with mass gatherings in Saudi Arabia. PLoS Currents Outbreaks 2013; Available at: http://currents.plos.org/outbreaks/index.html%3Fp=21609.html accessed January, 2019 [DOI] [PMC free article] [PubMed]
- Kilianski A, Mielech AM, Deng X, Baker SC. Assessing activity and inhibition of Middle East respiratory syndrome coronavirus papain-like and 3C-like proteases using luciferase-based biosensors. J Virol 2013; 87:11955–11962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Okada K, Kenniston T, Raj VS, et al. . Immunogenicity of an adenoviral-based Middle East respiratory syndrome coronavirus vaccine in BALB/c mice. Vaccine 2014; 32:5975–5982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-J, Cho Y-J, Kim D-W, Yang J-S, et al. . Complete genome sequence of Middle East respiratory syndrome coronavirus KOR/KNIH/002_05_2015, isolated in South Korea. Genome Announc 2015; 3:e00787–00715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraaij-Dirkzwager M, Timen A, Dirksen K, Gelinck L, et al. . Middle East respiratory syndrome coronavirus (MERS-CoV) infections in two returning travellers in the Netherlands, May 2014. Euro Surveill 2014; 19:20817. [DOI] [PubMed] [Google Scholar]
- Kucharski A, Althaus C. The role of superspreading in Middle East respiratory syndrome coronavirus (MERS-CoV) transmission. Euro Surveill 2015; 20:21167. [DOI] [PubMed] [Google Scholar]
- Kumar V, Shin JS, Shie JJ, Ku KB, et al. . Identification and evaluation of potent Middle East respiratory syndrome coronavirus (MERS-CoV) 3CL(Pro) inhibitors. Antiviral Res 2017; 141:101–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan J, Deng Y, Chen H, Lu G, et al. . Tailoring subunit vaccine immunity with adjuvant combinations and delivery routes using the Middle East respiratory coronavirus (MERS-CoV) receptor-binding domain as an antigen. PLoS One 2014; 9:e112602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau SKP, Lau CCY, Chan K-H, Li CPY, et al. . Delayed induction of proinflammatory cytokines and suppression of innate antiviral response by the novel Middle East respiratory syndrome coronavirus: Implications for pathogenesis and treatment. J Gen Virol 2013; 94:2679–2690 [DOI] [PubMed] [Google Scholar]
- Lau SKP, Li KSM, Tsang AKL, Lam CSF, et al. . Genetic characterization of Betacoronavirus Lineage C viruses in bats reveals marked sequence divergence in the spike protein of Pipistrellus bat coronavirus HKU5 in Japanese pipistrelle: Implications for the origin of the novel Middle East Respiratory Syndrome coronavirus. J Virol 2013; 87:8638–8650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq I, Batejat C, Burguiere AM, Manuguerra J-C. Heat inactivation of the Middle East respiratory syndrome coronavirus. Influenza Other Respir Viruses 2014; 8:585–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. Better understanding on MERS corona virus outbreak in Korea. J Korean Med Sci 2015a; 30:835–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH. Emergency medical services in response to the Middle East respiratory syndrome outbreak in Korea. J Korean Med Assoc 2015b; 58:611–616 [Google Scholar]
- Lee SS, Wong NS. Probable transmission chains of Middle East respiratory syndrome coronavirus and the multiple generations of secondary infection in South Korea. Int J Infect Dis 2015; 38:65–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei J, Mesters JR, Drosten C, Anemüller S, et al. . Crystal structure of the papain-like protease of MERS coronavirus reveals unusual, potentially druggable active-site features. Antiviral Res 2014; 109:72–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelli D, Papetti A, Sabelli C, Rosti E, et al. . Detection of coronaviruses in bats of various species in Italy. Viruses 2013; 5:2679–2689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessler J, Ridriguez-Barraquer I, Cummings D, Garske T, et al. . Estimating potential incidence of MERS-CoV associated with Hajj pilgrims to Saudi Arabia, 2014. PLoS Currents Outbreaks 2014. Available at: http://currents.plos.org/outbreaks/index.html%3Fp=44225.html accessed January, 2019 [DOI] [PMC free article] [PubMed]
- Letko M, Miazgowicz K, McMinn R, Seifert SN, et al. . Adaptive evolution of MERS-CoV to species variation in DPP4. Cell Rep 2018; 24:1730–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Xia S, Sun Z, Wang Q, et al. . Testing of Middle East respiratory syndrome coronavirus replication inhibitors for the ability to block viral entry. Antimicrob Agents Chemother 2015; 59:742–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Chan T-C, Chu Y-T, Wu J T-S, et al. . Comparative epidemiology of human infections with Middle East respiratory syndrome and Severe Acute respiratory syndrome coronaviruses among healthcare personnel. PLoS One 2016; 11:e0149988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Liu Q, Zhu Y, Chan K-H, et al. . Structure-based discovery of Middle East respiratory syndrome coronavirus fusion inhibitor. Nat Commun 2014; 5:3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Whitaker B, Sakthivel SKK, Kamili S, et al. . Real-time reverse transcription-PCR assay panel for Middle East respiratory syndrome coronavirus. J Clin Microbiol 2014; 52:67–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin A, Dijkman R, Bergstrom T, Kann N, et al. . Targeting membrane-bound viral RNA synthesis reveals potent inhibition of diverse coronaviruses including the Middle East respiratory syndrome virus. PLoS Pathog 2014; 10:e1004166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Li Y, Wang L, Zhao G, et al. . Intranasal vaccination with recombinant receptor-binding domain of MERS-CoV spike protein induces much stronger local mucosal immune responses than subcutaneous immunization: Implication for designing novel mucosal MERS vaccines. Vaccine 2014a; 32:2100–2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Wang L, Tao X, Zhang N, et al. . Searching for an ideal vaccine candidate among different MERS coronavirus receptor-binding fragments—The importance of immunofocusing in subunit vaccine design. Vaccine 2014b; 32:6170–6176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailles A, Blanckaert K, Chaud P, van der Werf S, et al. . First cases of Middle East respiratory syndrome coronavirus (MERS-CoV) infections in France, investigations and implications for the prevention of human-to-human transmission, France, May 2013. Euro Surveill 2013; 18:20502. [PubMed] [Google Scholar]
- Majumder M, Rivers C, Lofgren E, Fisman D. Estimation of MERS-coronavirus reproductive number and case fatality rate for the spring 2014 Saudi Arabia outbreak: Insights from publicly available data. PLoS Currents Outbreaks 2014; Available at: http://currents.plos.org/outbreaks/index.html%3Fp=40801.html accessed January, 2019 [DOI] [PMC free article] [PubMed]
- Malik A, El Masry KM, Ravi M, Sayed F. Middle East respiratory syndrome coronavirus during pregnancy, Abu Dhabi, United Arab Emirates, 2013. Emerg Infect Dis 2016; 22:515–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik M, Elkholy AA, Khan W, Hassounah S, et al. . Middle East respiratory syndrome coronavirus: Current knowledge and future considerations. East Mediterr Health J 2016; 22:537–546 [PubMed] [Google Scholar]
- Maltezou HC, Tsiodras S. Middle East respiratory syndrome coronavirus: Implications for health care facilities. Am J Infect Control 2014; 42:1261–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KL, Coleman CM, van der Meer Y, Snijder EJ, et al. . The ORF4b-encoded accessory proteins of Middle East respiratory syndrome coronavirus and two related bat coronaviruses localize to the nucleus and inhibit innate immune signalling. J Gen Virol 2014; 94:874–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memish ZA, Al-Tawfiq JA, Makhdoom HQ, Al-Rabeeah AA, et al. . Screening for Middle East respiratory syndrome coronavirus infection in hospital patients and their healthcare worker and family contacts: A prospective descriptive study. Clin Microbiol Infect 2014a; 20:469–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memish ZA, Almasri M, Turkestani A, Al-Shangiti AM, et al. . Etiology of severe community-acquired pneumonia during the 2013 Hajj—Part of the MERS-CoV surveillance program. Int J Infect Dis 2014b; 25:186–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memish ZA, Assiri A, Almasri M, Alhakeem RF, et al. . Prevalence of MERS-CoV nasal carriage and compliance with the Saudi health recommendations among pilgrims attending the 2013 Hajj. J Infect Dis 2014c; 210:1067–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memish ZA, Assiri AM, Al-Tawfi JA. Middle East respiratory syndrome coronavirus (MERS-CoV) viral shedding in the respiratory tract: An observational analysis with infection control implications. Int J Infect Dis 2014d; 29:307–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memish ZA, Cotten M, Meyer B, Watson SJ, et al. . Human infection with MERS coronavirus after exposure to infected camels, Saudi Arabia, 2013. Emerg Infect Dis 2014e; 20:1012–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memish ZA, Mishra N, Olival KJ, Fagbo SF, et al. . Middle East respiratory syndrome coronavirus in bats, Saudi Arabia. Emerg Infect Dis 2013a; 19:1819–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memish ZA, Zumla AI, Al-Hakeem RF, Al-Rabeeah AA, et al. . Family cluster of Middle East respiratory syndrome coronavirus infections. N Engl J Med 2013b; 368:2487–2494 [DOI] [PubMed] [Google Scholar]
- Meyer B, García-Bocanegra I, Wernery U, Wernery R, et al. . Serologic assessment of possibility for MERS-CoV infection in equids. Emerg Infect Dis 2015; 21:181–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B, Müller MA, Corman VM, Reusken CBEM, et al. . Antibodies against MERS coronavirus in dromedaries, United Arab Emirates, 2003 and 2013. Emerg Infect Dis 2014; 20:552–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielech AM, Kilianski A, Baez-Santos YM, Mesecar AD, et al. . MERS-CoV papain-like protease has deISGylating and deubiquitinating activities. Virology 2014; 450–451:64–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra N, Caciula A, Price A, Thakkar R, et al. . Diagnosis of Zika virus infection by peptide array and enzyme-linked immunosorbent assay. MBio 2018; 9:e00095–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modjarrad K. MERS-CoV vaccine candidates in development: The current landscape. Vaccine 2016; 34:2982–2987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed DH, AlHetheel AF, Mohamud HS, Aldosari K, et al. . Clinical validation of 3 commercial real-time reverse transcriptase polymerase chain reaction assays for the detection of Middle East respiratory syndrome coronavirus from upper respiratory tract specimens. Diagn Microbiol Infect Dis 2017; 87:320–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, et al. . Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009; 339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse SS. Factors in the emergence of infectious diseases. Emerg Infect Dis 1995; 1:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse SS. Public health disease surveillance networks. Microbiol Spectr 2012; 1:OH-0002-2012 [DOI] [PubMed] [Google Scholar]
- Morse SS, Mazet JAK, Woolhouse M, Parrish CR, et al. . Prediction and prevention of the next pandemic zoonosis. Lancet 2012; 380:1956–1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse SS, Morahan PS. Activated macrophages mediate interferon-independent inhibition of Herpes simplex virus. Cell Immunol 1981; 58:72–84 [DOI] [PubMed] [Google Scholar]
- Mou H, Raj VS, van Kuppeveld FJM, Rottier PJM, et al. . The receptor binding domain of the new Middle East respiratory syndrome coronavirus maps to a 231-residue region in the spike protein that efficiently elicits neutralizing antibodies. J Virol 2013; 87:9379–9383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller MA, Corman VM, Jores J, Meyer B, et al. . MERS coronavirus neutralizing antibodies in camels, Eastern Africa, 1983–1997. Emerg Infect Dis 2014; 20:2093–2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller MA, Meyer B, Corman VM, Al-Masri M, et al. . Presence of Middle East respiratory syndrome coronavirus antibodies in Saudi Arabia: A nationwide, cross-sectional, serological study. Lancet Infect Dis 2015; 15:559–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller MA, Raj VS, Muth D, Meyer B, et al. . Human coronavirus EMC does not require the SARS-Coronavirus receptor and maintains broad replicative capability in mammalian cell lines. MBio 2012; 3:e00515–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster VJ, de Wit E, Feldmann H. Pneumonia from human coronavirus in a macaque model. N Engl J Med 2013; 368:1560–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng DL, Al Hosani F, Keating MK, Gerber SI, et al. . Clinicopathologic, immunohistochemical, and ultrastructural findings of a fatal case of Middle East respiratory syndrome coronavirus infection in the United Arab Emirates, April 2014. Am J Pathol 2016; 186:652–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiura H, Mizumoto K, Ejima K, Zhong Y, et al. . Assessing the risk of observing multiple generations of Middle East respiratory syndrome (MERS) cases given an imported case. Euro Surveill 2015; 20:21181. [DOI] [PubMed] [Google Scholar]
- Nowotny N, Kolodziejek J. Middle East respiratory syndrome coronavirus (MERS-CoV) in dromedary camels, Oman, 2013. Euro Surveill 2014; 19:20781. [DOI] [PubMed] [Google Scholar]
- O'Hagan JJ, Carias C, Rudd JM, Pham HT, et al. . Estimation of severe Middle East respiratory syndrome cases in the Middle East, 2012–2016. Emerg Infect Dis 2016; 22:1797–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oboho IK, Tomczyk SM, Al-Asmari AM, Banjar AA, et al. . 2014 MERS-CoV outbreak in Jeddah—A link to health care facilities. N Engl J Med 2015; 372:846–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnuma K, Haagmans BL, Hatano R, Raj VS, et al. . Inhibition of Middle East respiratory syndrome coronavirus infection by Anti-CD26 monoclonal antibody. J Virol 2013; 87:13892–13899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omrani AS, Matin MA, Haddad Q, Al-Nakhli D, et al. . A family cluster of Middle East respiratory syndrome coronavirus infections related to a likely unrecognized asymptomatic or mild case. Int J Infect Dis 2013; 17:e668–e672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omrani AS, Saad MM, Baig K, Bahloul A, et al. . Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: A retrospective cohort study. Lancet Infect Dis 2014; 14:1090–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otter J, Donskey C, Yezli S, Douthwaite S, et al. . Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: The possible role of dry surface contamination. J Hosp Infect 2016; 92:235–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HY, Lee EJ, Ryu YW, Kim Y, et al. . Epidemiological investigation of MERS-CoV spread in a single hospital in South Korea, May to June 2015. Euro Surveill 2015; 20:21169. [DOI] [PubMed] [Google Scholar]
- Park WB, Perera RAPM, Choe PG, Lau EH. Kinetics of serologic responses to MERS coronavirus infection in humans, South Korea. Emerg Infect Dis 2015; 21:2186–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavli A, Tsiodras S, Maltezou HC. Middle East respiratory syndrome coronavirus (MERS-CoV): Prevention in travelers. Travel Med Infect Dis 2014; 12:602–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne DC, Iblan I, Alqasrawi S, Al Nsour M, et al. . Stillbirth during infection with Middle East respiratory syndrome coronavirus. J Infect Dis 2014; 209:1870–1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne DC, Iblan I, Alqasrawi S, et al. . Persistence of antibodies against Middle East Respiratory Syndrome coronavirus. Emerg Infect Dis 2016; 22:1824–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pebody R, Chand M, Thomas H, Green H, et al. . The United Kingdom public health response to an imported laboratory confirmed case of a novel coronavirus in September 2012. Euro Surveill 2012; 17:20292. [PubMed] [Google Scholar]
- Perera RA, Wang P, Gomaa MR, El-Shesheny R, et al. . Seroepidemiology for MERS coronavirus using microneutralisation and pseudoparticle virus neutralisation assays reveal a high prevalence of antibody in dromedary camels in Egypt, June 2013. Euro Surveill 2013; 18:20574. [DOI] [PubMed] [Google Scholar]
- Poissy J, Goffard A, Parmentier-Decrucq E, Favory R, et al. . Kinetics and pattern of viral excretion in biological specimens of two MERS-CoV cases. J Clin Virol 2014; 61:275–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poletto C, Pelat C, Lévy-Bruhl D, Yazdanpanah Y, et al. . Assessment of the Middle East respiratory syndrome coronavirus (MERS-CoV) epidemic in the Middle East and risk of international spread using a novel maximum likelihood analysis approach. Euro Surveill 2014; 19:20699. [DOI] [PubMed] [Google Scholar]
- Premila Devi J, Noraini W, Norhayati R, Chee Kheong C, et al. . Laboratory-confirmed case of Middle East respiratory syndrome coronavirus (MERS-CoV) infection in Malaysia: Preparedness and response, April 2014. Euro Surveill 2014; 19:20797. [DOI] [PubMed] [Google Scholar]
- ProMED-mail. NOVEL CORONAVIRUS—SAUDI ARABIA: HUMAN ISOLATE; Available at www.promedmail.org/direct.php?id=20120920.1302733 Archive Number: 20120920.21302733. (Accessed August1, 2017) [Google Scholar]
- Puzelli S, Azzi A, Santini M, Di Martino A, et al. . Investigation of an imported case of Middle East respiratory syndrome coronavirus (MERS-CoV) infection in Florence, Italy, May to June 2013. Euro Surveill 2013; 18:20564. [DOI] [PubMed] [Google Scholar]
- Qian Z, Dominguez SR, Holmes KV. Role of the spike glycoprotein of human Middle East respiratory syndrome coronavirus (MERS-CoV) in virus entry and syncytia formation. PLoS One 2013; 8:e76469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj VS, Farag EABA, Reusken CBEM, Lamers MM, et al. . Isolation of MERS coronavirus from dromedary camel, Qatar, 2014. Emerg Infect Dis 2014; 20:1339–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj VS, Mou H, Smits SL, Dekkers DHW, et al. . Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 2013; 495:251–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj VS, Smits SL, Provacia LB, van den Brand JMA, et al. . Adenosine deaminase acts as a natural antagonist for dipeptidyl peptidase 4-mediated entry of the Middle East respiratory syndrome coronavirus. J Virol 2014; 88:1834–1838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SA, Gerber SI, Swerdlow DL. Middle East respiratory syndrome coronavirus: Update for clinicians. Clin Infect Dis 2015; 60:1686–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusken C, Mou H, Godeke G, van der Hoek L, et al. . Specific serology for emerging human coronaviruses by protein microarray. Euro Surveill 2013a; 18:20441. [DOI] [PubMed] [Google Scholar]
- Reusken CB, Ababneh M, Raj VS, Meyer B, et al. . Middle East respiratory syndrome coronavirus (MERS-CoV) serology in major livestock species in an affected region in Jordan, June to September 2013. Euro Surveill 2013b; 18:20662. [DOI] [PubMed] [Google Scholar]
- Reusken CB, Farag EA, Jonges M, Godeke GJ, et al. . Middle East respiratory syndrome coronavirus (MERS-CoV) RNA and neutralising antibodies in milk collected according to local customs from dromedary camels, Qatar, April 2014. Euro Surveill 2014a; 19:20829. [DOI] [PubMed] [Google Scholar]
- Reusken CBEM, Feyisa A, Messadi L, Ularamu H, et al. . Geographic distribution of MERS coronavirus among dromedary camels, Africa. Emerg Infect Dis 2014b; 20:1370–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusken CBEM, Haagmans BL, Müller MA, Gutierrez C, et al. . Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: A comparative serological study. Lancet Infect Dis 2013c; 13:859–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusken CBEM, Schilp C, Raj VS, Bruin ED, et al. . MERS-CoV infection of alpaca in a region where MERS-CoV is endemic. Emerg Infect Dis 2016; 22:1129–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuss A, Litterst A, Drosten C, Seilmaier M, et al. . Contact investigation for imported case of Middle East respiratory syndrome, Germany. Emerg Infect Dis 2014; 20:620–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad M, Omrania AS, Baig K, Bahloul A, et al. . Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: A single-center experience in Saudi Arabia. Int J Infect Dis 2014; 29:301–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuplein VA, Seifried J, Malczyk AH, Miller L, et al. . High secretion of interferons by human plasmacytoid dendritic cells upon recognition of Middle East respiratory syndrome coronavirus. J Virol 2015; 89:3859–3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scobey T, Yount BL, Sims AC, Donaldson EF, et al. . Reverse genetics with a full-length infectious cDNA of the Middle East respiratory syndrome coronavirus. Proc Natl Acad Sci U S A 2013; 110:16157–16162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo YB, Heo JY, Song M-S, Lee J, et al. . Environmental contamination and viral shedding in MERS patients during MERS-CoV outbreak in South Korea. Clin Infect Dis 2016; 62:755–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong M-W, Lee SJ, Cho SI, Ko K, et al. . External quality assessment of MERS-CoV molecular diagnostics during the 2015 Korean outbreak. Ann Lab Med 2016; 36:230–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalhoub S, AlZahrani A, Simhairi R, Mushtaq A. Successful recovery of MERS CoV pneumonia in a patient with acquired immunodeficiency syndrome: A case report. J Clin Virol 2015; 62:69–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirato K, Kawase M, Matsuyama S. Middle East respiratory syndrome coronavirus infection mediated by the transmembrane serine protease TMPRSS2. J Virol 2013; 87:12552–12561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirato K, Yano T, Senba S, Akachi S, et al. . Detection of Middle East respiratory syndrome coronavirus using reverse transcription loop-mediated isothermal amplification (RT-LAMP). Virol J 2014; 11:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu K-L, Yeung ML, Kok K-H, Yuen K-S, et al. . Middle East respiratory syndrome coronavirus 4a protein is a double-stranded RNA-binding protein that suppresses PACT-induced activation of RIG-I and MDA5 in the innate antiviral response. J Virol 2014; 88:4866–4876 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Smits SL, Raj VS, Pas SD, Reusken CBEM, et al. . Reliable typing of MERS-CoV variants with a small genome fragment. J Clin Virol 2015; 64:83–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song F, Fux R, Provacia LB, Volz A, et al. . Middle East respiratory syndrome coronavirus spike protein delivered by modified vaccinia virus Ankara efficiently induces virus-neutralizing antibodies. J Virol 2013; 87:11950–11954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanakis N, Tsiodras S, Haagmans BL, Raj VS, et al. . Virological and serological analysis of a recent Middle East respiratory syndrome coronavirus infection case on a triple combination antiviral regimen. Int J Antimicrob Agents 2014; 44:528–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar S, Brouqui P, Parola P, Gautret P. Imported cases of Middle East respiratory syndrome: An update. Travel Med Infect Dis 2015; 13:106–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X-C, Agnihothram SS, Jiao Y, Stanhope J, et al. . Identification of human neutralizing antibodies against MERS-CoV and their role in virus adaptive evolution. Proc Natl Acad Sci U S A 2014; 111:e2018–e2026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X, Hill TE, Morimoto C, Peters CJ, et al. . Bilateral entry and release of Middle East respiratory syndrome coronavirus induces profound apoptosis of human bronchial epithelial cells. J Virol 2013; 87:9953–9958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X, Mei F, Agrawal A, Peters CJ, et al. . Blocking of exchange proteins directly activated by cAMP leads to reduced replication of Middle East respiratory syndrome coronavirus. J Virol 2014; 88:3902–3910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas HL, Zhao H, Green HK, Boddington NL, et al. . Enhanced MERS coronavirus surveillance of travelers from the Middle East to England. Emerg Infect Dis 2014; 20:1562–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiodras S, Baka A, Mentis A, Iliopoulos D, et al. . A case of imported Middle East respiratory syndrome coronavirus infection and public health response, Greece, April 2014. Euro Surveill 2014; 19:20782. [DOI] [PubMed] [Google Scholar]
- van Doremalen N, Bushmaker T, Karesh WB, Munster VJ. Stability of Middle East respiratory syndrome coronavirus in milk. Emerg Infect Dis 2014; 20:1263–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N, Bushmaker T, Munster VJ. Stability of Middle East respiratory syndrome coronavirus (MERS-CoV) under different environmental conditions. Euro Surveill 2013; 18:20590. [DOI] [PubMed] [Google Scholar]
- van Doremalen N, Miazgowicz KL, Milne-Price S, Bushmaker T, et al. . Host species restriction of Middle East respiratory syndrome coronavirus through its receptor, dipeptidyl peptidase 4. J Virol 2014; 88:9220–9232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kerkhove MD, Peiris MJS, Malik MR, Ben Embarek P. Interpreting results from environmental contamination studies of Middle East respiratory syndrome coronavirus. Clin Infect Dis 2016; 63:1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Qi J, Yuan Y, Xuan Y, et al. . Bat origins of MERS-CoV supported by bat coronavirus HKU4 usage of human receptor CD26. Cell Host Microbe 2014; 16:328–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirblich C, Coleman CM, Drishya K, Abraham TS, et al. . One-health: A safe, efficient, dual-use vaccine for humans and animals against Middle East respiratory syndrome coronavirus and rabies virus. J Virol 2017; 91:e02040–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo P, Lau S, Lam C, Lau C, et al. . Discovery of seven novel mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J Virol 2012; 86:3995–4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo PCY, Lau SKP, Li KSM, Poon RWS, et al. . Molecular diversity of coronaviruses in bats. Virology 2006; 351:180–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization 2015. Clinical management of severe acute respiratory infection when Middle East respiratory syndrome coronavirus (MERS-CoV) infection is suspected. 2015. Available at [E-pub online: www.who.int/csr/disease/coronavirus_infections/case-management-ipc/en]
- World Health Organization 2018a. WHO MERS-CoV global summary and assessment of risk—August 2018. Available at [E-pub online: www.who.int/csr/disease/coronavirus_infections/risk-assessment-august-2018.pdf; MERS; webpage online: www.who.int/emergencies/mers-cov/en] [Google Scholar]
- World Health Organization 2018b. Laboratory testing for Middle East respiratory syndrome coronavirus—Interim guidance (revised), January 2018. Available at [E-pub online: www.who.int/csr/disease/coronavirus_infections/mers-laboratory-testing/en]
- World Health Organization/EMRO (Eastern Mediterranean Regional Office) 2018. Available at MERS situation update [E-pub online: www.emro.who.int/health-topics/mers-cov/situation-update.html]
- Wrensch F, Winkler M, Pöhlmann S. IFITM proteins inhibit entry driven by the MERS-coronavirus spike protein: Evidence for cholesterol-independent mechanisms. Viruses 2014; 6:3683–3698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Yi L, Zou L, Zhong H, et al. . Imported case of MERS-CoV infection identified in China, May 2015: Detection and lesson learned. Euro Surveill 2015; 20:21158. [DOI] [PubMed] [Google Scholar]
- Yang L, Ren X, Wu Z, Yang F, et al. . MERS-related betacoronavirus in Vespertilio superans bats, China. Emerg Infect Dis 2014; 20:1260–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Du L, Liu C, Wang L, et al. . Receptor usage and cell entry of bat coronavirus HKU4 provide insight into bat-to-human transmission of MERS coronavirus. Proc Natl Acad Sci U S A 2014; 111:12516–12521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Zhang L, Geng H, Deng Y, et al. . The structural and accessory proteins M, ORF 4a, ORF 4b, and ORF 5 of Middle East respiratory syndrome coronavirus (MERS-CoV) are potent interferon antagonists. Protein Cell 2013; 4:951–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Bao L, Deng W, Xu L, et al. . An animal model of MERS produced by infection of rhesus macaques with MERS coronavirus. J Infect Dis 2014; 209:236–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavarian J, Rezaei F, Shadab A, Soroush M, et al. . Cluster of middle east respiratory syndrome coronavirus infections in Iran, 2014. Emerg Infect Dis 2015; 21:362–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying T, Du L, Ju TW, Prabakaran P, et al. . Exceptionally potent neutralization of Middle East respiratory syndrome coronavirus by human monoclonal antibodies. J Virol 2014; 88:7796–7805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus ADME, et al. . Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 2012; 367:1814–1820 [DOI] [PubMed] [Google Scholar]
- Zhao G, Du L, Ma C, Li Y, et al. . A safe and convenient pseudovirus-based inhibition assay to detect neutralizing antibodies and screen for viral entry inhibitors against the novel human coronavirus MERS-CoV. Virol J 2013; 10:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Alshukairi AN, Baharoon SA, Ahmed WA, et al. . Recovery from the Middle East respiratory syndrome is associated with antibody and T cell responses. Sci Immunol 2017; 2:eaan5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Li K, Wohlford-Lenane C, Agnihothram SS, et al. . Rapid generation of a mouse model for Middle East respiratory syndrome. Proc Natl Acad Sci U S A 2014; 111:4970–4975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Perera RAPM, Kayali G, Meyerholz D, et al. . Passive immunotherapy with dromedary immune serum in an experimental animal model for Middle East respiratory syndrome coronavirus infection. J Virol 2015; 89:6117–6120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Chu H, Li C, Wong BH, et al. . Active replication of Middle East respiratory syndrome coronavirus and aberrant induction of inflammatory cytokines and chemokines in human macrophages: Implications for pathogenesis. J Infect Dis 2014; 209:1331–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]