To the Editor:
The most common genetic cause of emphysema and chronic obstructive pulmonary disease (COPD) is AAT (alpha-1 antitrypsin) deficiency (AATD) (1). The risk of lung disease in AATD is associated with decreased levels of circulating AAT, a proteinase inhibitor with high affinity for neutrophil-derived serine proteases such as elastase (2, 3). Identification of additional proteases targeted by AAT may provide further insight into the pathogenesis of emphysema. Our work suggests that AAT targets a protease involved in complement activation, MASP-2 (MBL [mannose-binding lectin]-associated serine protease 2). Some of the results of these studies have been previously reported in the form of an abstract (4).
In contrast to other MASP-1 or -3 isoforms, MASP-2 alone is sufficient to initiate the lectin pathway within the complement system, a network of zymogen enzymes (C1–C9) activated by auto- or enzymatic cleavage (Figure 1A) (5). Whereas its main function is to form the membrane attack complex (C5–C9) that is critical for pathogen killing, the complement has been recently implicated in sterile inflammation and autoimmunity (5). Although the role of the lectin complement pathway in COPD pathogenesis is understudied, emerging reports point to complement activation (C4 and C3) in sera of patients with COPD and AATD (6, 7), and in lungs of mice in models of cigarette smoke (CS)-induced emphysema (8).
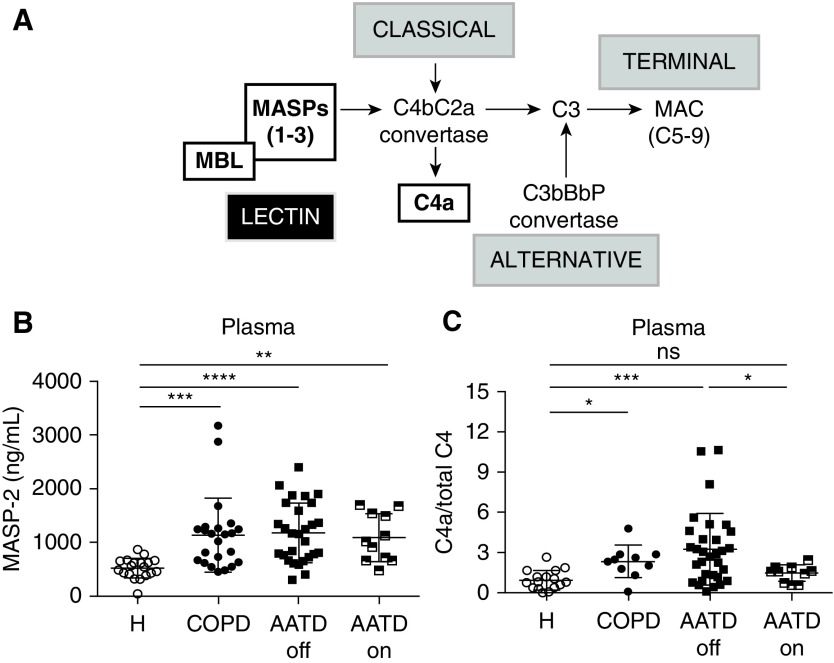
Figure 1.
Lectin pathway’s MASP-2 levels and C4a/total C4 ratios in plasma. (A) Simplified schematic depicting the classical, lectin, and alternative complement pathways. Activation of the lectin pathway requires MASPs binding to MBL to then cleave C4 and C2, to form C4bC2a convertase. The classical pathway also generates C4bC2a convertase via a different proteolytic cascade. The alternative pathway generates C3bBbP convertase, consisting of C3, factor B, and properdin. All three pathways cleave C3 and lead to the formation of the terminal complement C5–C9 complex known as membrane attack complex (MAC). (B and C) Circulating MASP-2 levels (B) and C4a/total C4 ratios (C) measured by ELISA (HycultBiotech and MyBiosource, respectively) in healthy subjects (H), subjects with chronic obstructive pulmonary disease (COPD), and subjects with alpha-1 antitrypsin (AAT) deficiency (AATD) off (AATD-off) or on (AATD-on) AAT augmentation therapy. Data are shown as mean ± 1 SD. Kruskal-Wallis, Dunn’s multiple comparisons, *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 versus H or AATD-off. MASP-2 = MBL-associated serine protease 2; MBL = mannose-binding lectin; ns = not significant.
In this study, we investigated whether MASP-2 and the lectin pathway are activated in COPD and AATD plasma and lung tissue.
Samples were obtained from subjects with COPD (n = 38, age = 64 ± 10 yr, 21 men, FEV1% predicted = 45 ± 27), AATD (n = 47) off AAT augmentation therapy (AATD-off, n = 30, age = 67 ± 9 yr, 18 men, FEV1% predicted = 73 ± 32) or on augmentation therapy (AATD-on, n = 17, age = 58 ± 14 yr, 8 men, FEV1% predicted = 33 ± 11), and healthy never-smokers (n = 23, age = 40 ± 8.5 yr, 5 men). Subjects with active infections in the past 3 months were excluded. The subjects’ smoking status was recorded as active smoker, ex-smoker, never smoker, or unknown (the latter group was excluded from multivariate analyses). Statistical analyses were performed in R and Prism (GraphPad Software). Deidentified plasma samples were obtained from Leiden University (n = 8), Hannover University (n = 15), the Medical University of South Carolina (n = 24), and National Jewish Health (n = 62), and lung samples were obtained from the Lung Tissue Research Consortium and Hannover University. The institutional review board at National Jewish Health approved the study as exempt human subjects research.
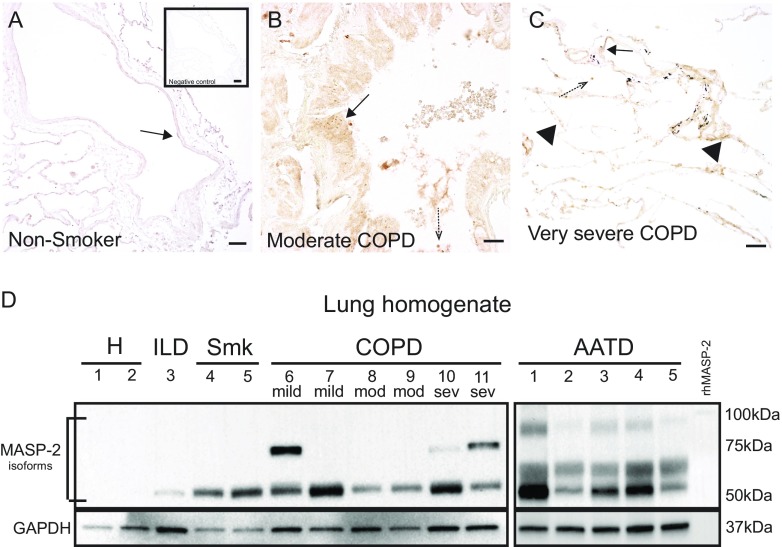
Plasma MASP-2 levels in subjects with COPD (1,135 ± 670 ng/ml), AATD-off (1,176 ± 541 ng/ml), or AATD-on (1,085 ± 428 ng/ml) were significantly increased compared with those in healthy volunteers (521.5 ± 179 ng/ml) (Figure 1B). MASP-2 levels were not significantly different between males and females (1,326 ± 1,153 ng/ml vs. 887 ± 568 ng/ml; P = 0.051, t test) or among never-smokers, ex-smokers, and active smokers with COPD (983 ± 590 ng/ml vs. 1,154 ± 653 ng/ml vs. 1,205 ± 33 ng/ml, respectively; P = 0.53, Kruskal-Wallis test). The downstream product of MASP-2 proteolytic activity, the cleaved C4a fragment (expressed relative to total C4) was also elevated in COPD (2.33 ± 1.1) and AATD-off (3.25 ± 2.6), but not AATD-on (1.44 ± 0.6), plasma compared with that from healthy individuals (0.96 ± 0.7) (Figure 1C). Plasma MASP-2 tended to be positively associated with C4a levels (r = 0.24, P = 0.06). MASP-2 immunostaining in the airways and lung parenchyma was markedly increased in subjects with COPD of any severity, as defined by Global Initiative for Chronic Obstructive Lung Disease criteria (Figures 2A–2C). Interestingly, lower-molecular-weight MASP-2 isoforms (∼65 and ∼50 kD), indicative of MASP-2 activation, were primarily present in COPD and AATD lungs, rather than in control lungs (Figure 2D).
Figure 2.
Expression of mannose-binding lectin (MBL)–associated serine protease 2 (MASP-2) in lung tissue. (A–C) Immunohistochemistry of MASP-2 (anti-human MASP-2 antibody 1:200; MyBioSource) in the airways (solid arrows), alveolar septa (arrowheads), and infiltrating leukocytes (dashed arrows) of nonsmokers (A), subjects with moderate chronic obstructive pulmonary disease (COPD) (B), and subjects with very severe COPD (C). Lungs from nonsmokers were used as a negative control (A, inset) and subjected to the same staining protocol, with the exception of the primary antibody (Vectasin ABC kit; Vector Laboratories). Scale bars, 50 μm. (D) Representative immunoblot of MASP-2 (anti-human MASP-2 antibody, 1:1,000; MyBioSource) expression in lung tissue homogenates from healthy subjects (H), active smokers (Smk), subjects with interstitial lung disease (ILD), subjects with COPD (of the indicated severity based on FEV1% predicted), and subjects with alpha-1 antitrypsin (AAT) deficiency (AATD) on AAT augmentation therapy. Note the two activated MASP-2 isoforms (∼65 and ∼50 kD) with lower molecular weight than the zymogen protein (recombinant human MASP-2, rhMASP-2, 80 kD).
Our data suggest that MASP-2 levels and activity are increased in subjects with COPD and AATD. The marked effect of AAT augmentation therapy on the abundance of C4a fragments generated, rather than on the MASP-2 levels, suggests that AAT inhibits MASP-2’s activity rather than its expression.
Our study has several limitations, including the small size of our groups and differences in demographics (with a predominance of young age and females in the control group), smoking status (fewer ex-smokers in the AATD-off group), and disease severity (milder COPD in the AATD-off group). Our statistical analyses did not include clinical covariates (e.g., treatment strategy and exacerbation frequency) and were not powered to investigate a relationship between MASP-2 and clinical markers of disease severity. For example, in multivariate linear regression models controlled for age, sex, AAT augmentation, and smoking status, MASP-2 was inversely associated with FEV1% predicted (β = −0.0056) and DlCO% predicted (β = −0.0049), but it made a statistically insignificant contribution to the models (P = 0.06 and 0.16, respectively). Also, we did not evaluate the contribution of the classical pathway to C4 cleavage in this study. We plan to study the contribution of individual pathways to C3a and terminal complement activation during CS exposure using mice deficient in classical (C1q−/−), lectin (MASP-2−/−), or alternative (fD−/−) pathways.
Despite its limitations, to our knowledge, our study provides the first evidence that MASP-2 and the lectin complement pathway are activated in COPD and may be a target for AAT antiprotease activity. Although AAT has the highest inhibitory activity against neutrophil elastase, it also inhibits other proteases, such as the metalloproteinases and caspase-3 and -6 (9, 10). AAT antiprotease activity is weakened by alterations of protein conformation due to genetic (e.g., the single point mutation PiZZ) or posttranslational (e.g., CS-induced oxidation) modifications (2, 11). Because individuals with diminished AAT antiprotease activity are at risk for systemic and pulmonary inflammation and injury (12), our results suggest that excessive MASP-2–driven C4 activation may play an important role in terminal complement signaling via the membrane attack complex or intracellular injury pathways activated by C4a docking on structural cells. The higher MASP-2 levels and activation in COPD lungs not only corroborate our results in plasma but also suggest that, in addition to its activation in sera, MASP-2 may be activated in situ, a concept that was recently investigated in models of ischemic myocardial and kidney injury and in lung epithelial cells (13–15). Our findings indicate that COPD, similarly to other conditions associated with excessive complement activation (e.g., immune complex–mediated vasculitis), may benefit from studies of MASP-2– and complement-targeted therapies.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank E. Varano for her assistance with clinical data and samples (Alpha-1 Foundation Research Registry, Medical University of South Carolina).
Footnotes
Supported by a 2016 Laurell’s Award (European Respiratory Society and Grifols, to K.A.S.) and European Research Council consolidator grant XHaLe (D.J.). This study used specimens and data provided by the Lung Tissue Research Consortium (NHLBI).
Originally Published in Press as DOI: 10.1164/rccm.201807-1380LE on December 17, 2018
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Stoller JK, Aboussouan LS. A review of α1-antitrypsin deficiency. Am J Respir Crit Care Med. 2012;185:246–259. doi: 10.1164/rccm.201108-1428CI. [DOI] [PubMed] [Google Scholar]
- 2.Ogushi F, Fells GA, Hubbard RC, Straus SD, Crystal RG. Z-type alpha 1-antitrypsin is less competent than M1-type alpha 1-antitrypsin as an inhibitor of neutrophil elastase. J Clin Invest. 1987;80:1366–1374. doi: 10.1172/JCI113214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molloy K, Hersh CP, Morris VB, Carroll TP, O’Connor CA, Lasky-Su JA, et al. Clarification of the risk of chronic obstructive pulmonary disease in α1-antitrypsin deficiency PiMZ heterozygotes. Am J Respir Crit Care Med. 2014;189:419–427. doi: 10.1164/rccm.201311-1984OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mikosz A, Strange CB, Janciauskiene S, Stolk J, Braubach P, Jonigk DD, et al. Lectin complement pathway activation in COPD and alpha-1 antitrypsin (A1AT) deficiency [abstract] Am J Respir Crit Care Med. 2018;197:A7131. [Google Scholar]
- 5.Merle NS, Noe R, Halbwachs-Mecarelli L, Fremeaux-Bacchi V, Roumenina LT. Complement system part II: role in immunity. Front Immunol. 2015;6:257. doi: 10.3389/fimmu.2015.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kew RR, Ghebrehiwet B, Janoff A. Cigarette smoke can activate the alternative pathway of complement in vitro by modifying the third component of complement. J Clin Invest. 1985;75:1000–1007. doi: 10.1172/JCI111760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Littleton ET, Bevis L, Hansen LJ, Peakman M, Mowat AP, Mieli-Vergani G, et al. Alpha 1-antitrypsin deficiency, complement activation, and chronic liver disease. J Clin Pathol. 1991;44:855–858. doi: 10.1136/jcp.44.10.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan X, Shan M, You R, Frazier MV, Hong MJ, Wetsel RA, et al. Activation of C3a receptor is required in cigarette smoke-mediated emphysema. Mucosal Immunol. 2015;8:874–885. doi: 10.1038/mi.2014.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serban KA, Petrache I. Alpha-1 antitrypsin and lung cell apoptosis. Ann Am Thorac Soc. 2016;13:S146–S149. doi: 10.1513/AnnalsATS.201505-312KV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrache I, Fijalkowska I, Medler TR, Skirball J, Cruz P, Zhen L, et al. Alpha-1 antitrypsin inhibits caspase-3 activity, preventing lung endothelial cell apoptosis. Am J Pathol. 2006;169:1155–1166. doi: 10.2353/ajpath.2006.060058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alam S, Li Z, Janciauskiene S, Mahadeva R. Oxidation of Z α1-antitrypsin by cigarette smoke induces polymerization: a novel mechanism of early-onset emphysema. Am J Respir Cell Mol Biol. 2011;45:261–269. doi: 10.1165/rcmb.2010-0328OC. [DOI] [PubMed] [Google Scholar]
- 12.Fregonese L, Stolk J. Hereditary alpha-1-antitrypsin deficiency and its clinical consequences. Orphanet J Rare Dis. 2008;3:16. doi: 10.1186/1750-1172-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kulkarni HS, Elvington ML, Liszewski MK, Brody SL, Atkinson JP. Human airway epithelial cells have multiple sources and stores of the intracellular complement protein C3 [abstract] Am J Respir Crit Care Med. 2017;195:A5206. [Google Scholar]
- 14.Schwaeble WJ, Lynch NJ, Clark JE, Marber M, Samani NJ, Ali YM, et al. Targeting of mannan-binding lectin-associated serine protease-2 confers protection from myocardial and gastrointestinal ischemia/reperfusion injury. Proc Natl Acad Sci USA. 2011;108:7523–7528. doi: 10.1073/pnas.1101748108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roos A, Rastaldi MP, Calvaresi N, Oortwijn BD, Schlagwein N, van Gijlswijk-Janssen DJ, et al. Glomerular activation of the lectin pathway of complement in IgA nephropathy is associated with more severe renal disease. J Am Soc Nephrol. 2006;17:1724–1734. doi: 10.1681/ASN.2005090923. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.