Abstract
Whether zinc (Zn2+) regulates barrier functions by modulating tight-junction (TJ) proteins when pathogens such as Shigella alter epithelial permeability is still unresolved. We investigated the potential benefits of Zn2+ in restoring impaired barrier function in vivo in Shigella-infected mouse tissue and in vitro in T84 cell monolayers. Basolateral Shigella infection triggered a time-dependent decrease in transepithelial resistance followed by an increase in paracellular permeability of FITC-labeled dextran and altered ion selectivity. This led to ion and water loss into the intestinal lumen. Immunofluorescence studies revealed redistribution of claudin-2 and -4 to an intracellular location and accumulation of these proteins in the cytoplasm following infection. Zn2+ ameliorated this perturbed barrier by redistribution of claudin-2 and -4 back to the plasma membrane and by modulating the phosphorylation state of TJ proteins t hough extracellular signal-regulated kinase (ERK)1/2 dependency. Zn2+ prevents elevation of IL-6 and IL-8. Mice challenged with Shigella showed that oral Zn2+supplementation diminished diverse pathophysiological symptoms of shigellosis. Claudin-2 and -4 were susceptible to Shigella infection, resulting in altered barrier function and increased levels of IL-6 and IL-8. Zn2+ supplementation ameliorated this barrier dysfunction, and the inflammatory response involving ERK-mediated change of phosphorylation status for claudin-2 and -4. Thus, Zn2+ may have potential therapeutic value in inflammatory diarrhea and shigellosis.
NEW & NOTEWORTHY Our study addresses whether Zn2+ could be an alternative strategy to reduce Shigella-induced inflammatory response and epithelial barrier dysfunction. We have defined a mechanism in terms of intracellular signaling pathways and tight-junction protein expression by Zn2+. Claudin-2 and -4 are susceptible to Shigella infection, whereas in the presence of Zn2+ they are resistant to infection-related barrier dysfunction involving ERK-mediated change of phosphorylation status of claudins.
Keywords: claudin, diarrhea, intestinal inflammation, Shigella flexneri, tight junction
INTRODUCTION
Globally, diarrheal disease is the ninth leading cause of death, with an estimated 1.3 million deaths per year, while it is the fourth leading cause in children under 5 yr, with roughly 500,000 deaths (12). Three etiologies, rotavirus, Cryptosporidium spp, and Shigella spp, together account for greater than 50% of diarrheal deaths in children less than 5 yr of age. Shigella is identified as one of the top four pathogens and major contributors to the global burden of diarrhea reported in the recent global enteric multicenter study (GEMS) (24, 25). Among 160 million cases of shigellosis reported, ~600,000 deaths have been estimated every year in developing countries; hence, Shigella infection constitutes a major health burden, particularly among young children (24). A current major concern surrounding Shigella is its capacity to rapidly acquire resistance against antibiotics (27). The rise in antibiotic resistance and the absence of an effective vaccine require the development of alternate strategies to control shigellosis (16, 27). The pathogenesis of Shigella is based on the bacteria’s ability to invade and replicate within the colonic epithelium, resulting in severe intestinal inflammatory responses contributing to intestinal barrier dysfunction (30, 31, 36). Thus, restoring altered barrier function could be a potential therapeutic goal in the treatment of shigellosis.
The World Health Organization and the United Nations Children’s Fund recommended in a joint statement in 2004 the use of Zn2+ supplementation for the treatment of acute diarrhea in developing countries. This recommendation was based on the strong biological and epidemiological evidence indicating that Zn2+ supplementation could significantly reduce the overall duration of diarrhea, stool volume, and frequency (6). Since the health authorities issued this endorsement, several reports on other effects of Zn2+ have been published. One emerging fact among the clinical studies is that the beneficial effects of Zn2+ apparently are not restricted to children who are Zn2+ deficient (5, 38). An emerging theme of basic studies on the action of Zn2+ is that the effects of Zn2+ may be pathogen specific, or, in the case of toxin-producing organisms, it is toxin specific. For example, Canani et. al found that Zn2+ blocked the secretory effects of cholera toxin and the Escherichia coli heat-labile enterotoxin (LT) that is mediated by cyclic adenosine monophosphate (cAMP) but not the effects of the E. coli heat-stable toxin (ST), mediated by cyclic guanosine monophosphate (cGMP) (7, 17). Another study suggests that Zn2+ may have pathogen-specific effects (8). It is unclear whether Zn2+ is equally effective against all types of infectious diarrhea or has specific effects on Shigella, or whether Zn2+ is active against specific pathogens. There are suggestions that Zn2+ can have effects on the pathogen as well as on the host, supporting the notion that Zn2+ may act in a pathogen-specific manner.
We initiated the present study to determine whether Zn2+ could target the host tight junction (TJ) and therefore mitigate intestinal inflammation in the context of shigellosis. Our interest in Zn2+ was prompted by our previous study that established that Zn2+ inhibits cAMP (cholera toxin)-induced Cl− secretion by inhibiting the basolateral K+ channels, which provides the driving force for Cl− secretion (i.e., water secretion) in rat ileum (16, 17). We hypothesized here that Zn2+could largely rescue the Shigella-induced perturbation of epithelial barrier functions and inflammatory responses and thus diminish disease severity. Using the T84 colonic carcinoma cell line as an in vitro model for trans-TJ study and a mouse model of shigellosis, we demonstrate that, as a result of Shigella infection, 1) transepithelial electrical resistance (TER), 2) epithelial permeability to uncharged macromolecules, and 3) selectivity for paracellular permeability of sodium to chloride and water flux and 4) proinflammatory cytokine levels were altered, all key determinants in the disease that disrupts barrier function. We were surprised to find that Zn2+ had mitigating effects on the intestinal cells to restore barrier function and proinflammatory cytokine responses induced by Shigella infection. Here, we report that Shigella infection caused a pronounced phosphorylation of extracellular signal-regulated kinase (ERK) to elicit barrier disruption dissociating claudin-2 by dephosphorylation and claudin-4 by phosphorylation from the cell periphery. This conspicuous activation of ERK signal transduction led to elevation of IL-6 and IL-8, whereas Zn2+ reversed this ERK activation and reinforced barrier integrity. The mechanism of Zn2+’s action clearly involved ERK-mediated phosphorylation of claudin-2 and -4. In vivo experiments in male C57BL/6 and BALB/c mice challenged intraperitoneally with S. flexneri 2a, along with in vitro studies in human colonic T84 cell monolayers infected with Shigella, demonstrate that Zn2+ can significantly reduce the dysregulated barrier function and inflammatory responses of the host correlating with disease severity.
MATERIALS AND METHODS
Reagents
Unless otherwise stated, all chemicals used in this study were obtained from Sigma-Aldrich (St. Louis, MO). Cell culture media and fetal bovine serum (FBS) were purchased from Cell Clone (Cat. No. cc3021) and HiMedia (Cat. No. RM9970), respectively. Penicillin-streptomycin, cDNA synthesis reagents (Cat. No. 11904-018), TRIzol (Cat. No. 15596-026), fluorescein isothiocynate (FITC 488; Cat. No. A11036), and Alexa fluor 568-conjugated secondary antibody were purchased from Invitrogen. Real-Time PCR Master Mix was purchased from Applied Biosystems (Cat. No. 4309155); 70-kDa rhodamine was purchased from Sigma, whereas FITC-4-kDa was obtained from Invitrogen. Claudin-2 and claudin-4 antibodies were purchased from Sydlabs (Natick, MA), and phosphorylated (p)ERK, ERK, Na+/K+-ATPase, and GAPDH were obtained from Cell Signaling Technology. Cystic fibrosis transmembrane conductance regulator (CFTR) 596 antibody was obtained from the University of North Carolina, Chapel Hill. Paraformaldehyde 16% solution (Cat. No. 50980487) was purchased from Fisher Scientific (Pittsburgh, PA). Subcellular proteome extraction kit, S-PEK (Cat. No. 539790) was purchased from Millipore (Billerica, MA). Polycarbonate membrane, 12-mm Snapwell-permeable support cell culture inserts (0.4-µm pore size; Coster, Cat. No. 3407) were from Sigma.
Shigella flexneri 2a Cultures and Infections
Shigella. flexneri 2a (NY 962/92) obtained from the Molecular Pathophysiology Division of the National Institute of Cholera and Enteric Diseases (NICED), Kolkata, India, was grown in Trypticase soy broth (TSB) at 37°C, as described by Pore et al. (33). Polarized T84 cell monolayers grown on transwell plates were infected by basolateral addition of bacterial culture of logarithmic phase (OD600 0.6) equivalent of to 200 multiplicity of infection (MOI) corresponding to 107 CFU, while shigellosis was generated in C57BL/6 and BALB/c male mice by intraperitoneal (ip) injection of 5 × 108 CFU Shigella. Briefly, S. flexneri 2a precultured in TSB with shaking at 37°C overnight. Aliquots of 100 μl of the precultures were inoculated into 10 ml of TSB for 2 h at 37°C early in the morning of the experimental day with shaking to the logarithmic phase (OD600, 0.6). Cultures were spun, and the pellets resuspended in antibiotic-free DMEM-F12 medium were administered to polarized T84 cells. For control experiments, the cells were incubated in antibiotic- and serum-free DMEM-F12 medium. No bacterial culture was used in these control experiments.
Zinc Formulation
Stock ZnCl2 (10 mM) solution was prepared in water. Serial dilutions were made to yield 200 μM in the culture medium and sterile PBS. Mice were administered three oral doses of ZnCl2 (2.5 µg/g body wt) in Ringer solution (100 µl using a 1-ml syringe) intragastrically via a fine polyethylene tube of external diameter 0.6 mm connected to the syringe every 24 h after onset of disease, as indicated in Fig. 7A.
Fig. 7.
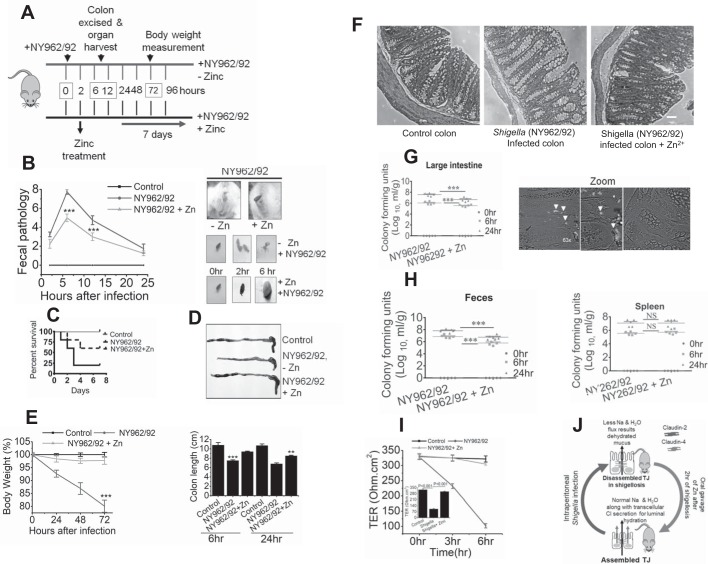
Zn2+ protects Shigella-mediated pathogenesis in adult mice. A: timeline of experimental procedure of shigellosis in adult mice. B: fecal pathology of uninfected (Control) and Shigella-infected in presence (NY962/29 +Zn) or absence (NY962/92) of Zn2+. Scores were determined at 2, 6, 12, and 24 h (left). Fecal pathology (left) was determined using the basis of stool color and stool consistency (right). C: Kaplan-Meier survival curve comparing survival of individuals by Shigella infection with or without Zn2+ administration. D, top: representative colon images at 24 h postinfection in uninfected (Control) or 24-h post-Shigella-infected in presence (NY962/92 + Zn) or absence (NY962/92 – Zn) of Zn2+. Bottom: bar graph summarizes colon lengths of uninfected (Control) and 6-h and 24-h post-Shigella infection. E: body weight changes were monitored at different postinfection time points. Data are means ± SE of 3 independent experiments; n = 6 mice per group. ***P < 0.001 vs. Control and in Shigella-infected colon + Zn2+ administration, **P < 0.01 vs. Shigella-infected colon. F: representative Hematoxylin-eosin staining of mouse colon. Scale bars in all images, 100 µm. G: effect of Zn2+ supplementation on bacterial colonization in mice subjected to intraperitoneal infection with S. flexneri 2a. Colony-forming units (CFU) in homogenates of large intestines. Phase images of postinfected tissue showing significant burden of green fluorescent protein (GFP)-positive NY962/92 along the villi at 3 h. time point, stained with anti-GFP (middle) and uninfected control (left). White arrows mark bacterial colonies. Zoomed image depicts magnified view of bacterial colonization; n = 3 mice per group. H: CFU in homogenates of feces and spleen (right) at each time point postinfection with NY962/92. Data represent mean of 3 independent experiments; n = 5 mice per each group. ***P < 0.001 vs. Shigella infection without Zn2+ administration at 6 and 24 h. P values were calculated using the Mann-Whitney U-test. I: transepithelial electrical resistance (TER) in mouse colon were measured in Ussing chambers. Data are means ± SE of 6 animals in each group. Inset: TER quantification derived from time course study. J: graphic demonstration of critical roles of claudin-2 and -4 in Shigella infection. Shigella infection alters intestinal permeability and mucosal dehydration by regulating tight junction (TJ) protein claudin-2 and -4 internalization into cytoplasm. Zn2+ supplementation helps reestablish altered intestinal permeability in shigellosis by reinstating claudin-2 and -4 on TJ membrane, resulting in decreased mouse mortality.
Cell Culture
Wild-type human colonic T84 intestinal epithelial cells were obtained from the American Type Culture Collection (Rockville, MD). T84 cells were routinely maintained in 1:1 ratio of DMEM and Ham’s F-12 medium supplemented with 10% FBS, 100 µg/ml penicillin, and 100 µg/ml streptomycin, as described by us (42). Briefly, T84 cells between passages 7 and 22 were seeded onto polycarbonate membrane, 12-mm Snapwell permeable support cell culture inserts (0.4 µm pore size; Costar, Cat. No. 3407), and grown for 10 days until they reached a confluent, polarized, and differentiated state. Monolayer resistance was determined using an EVOM ohmmeter with STX2 electrodes (World Precision Instruments).
Paracellular Permeability Measurement
TER and mucosal-to-serosal unidirectional uncharged macromolecule fluxes were measured to assess the T84 cell monolayer integrity. Absolute TER values presented were calculated by subtracting the blank TER (i.e., filter/bathing solution) from that of total TER (i.e., with monolayer) and were normalized to the area of the monolayer. Cocultures of bacteria and T84 cells were incubated in the presence and absence of 200 µM Zn2+ for various time periods (2–12 h), and TER was measured. Paracellular permeability of T84 cell monolayers was measured using membrane-impermeable FITC-dextran (4 kDa) and rhodamine (70 kDa) (1 mg/ml Ringer solution). T84 monolayers grown on transwells were mounted under voltage clamp condition in an Ussing chamber. Mucosal and serosal sides were bathed with 5 ml of Ringer solution (in mM), consisting of 140 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 glucose, and 10 HEPES (pH 7.4). FITC-dextran and rhodamine (1 mg/ml each) were added to mucosal bathing solution. Three 1-h samples were collected from serosal bathing medium. Fluorescence intensity of the collected samples were analyzed in duplicates using a 96-well plate (150 µl/well) in a fluorometer (Appliskan, Thermo-Scientific). FITC-dextran was quantitated by measuring excitation and emission wavelengths of 490 and 520 nm, while rhodamine was quantitated by measuring excitation and emission wavelengths of 540 and 625 nm, respectively. Dextran fluxes were calculated from the amount of FITC-dextran and rhodamine-dextran in the basolateral compartment, and permeabilities were calculated as flux/concentration.
Electrophysiological Measurements
To determine NaCl dilution potential, T84 cell monolayers were mounted under voltage clamp conditions in Ussing chambers (Physiologic Instruments, San Diego, CA), as described previously (42). Inhibitors of Na-K-ATPase were added to uninfected and infected cells to determine whether they altered the dilution potential. Fluid resistance and electrode potentials were determined across blank filters in buffer A (120 mM NaCl), and the NaCl dilution potential was determined when buffer B (60 mM NaCl) replaced buffer A on the apical side of filters. The ion permeability ratio (β), permeability of Na+ over Cl− (PNa/PCl), and permeability of Cl− over Na+ (PCl/PNa) were determined using the Goldmann-Hodgkin-Kartz equation. Absolute permeability was calculated using the Kimizuka-Koketsu equation (20).
Measurement of Transepithelial Chloride Transport:
T84 cell monolayers grown on snapwell inserts and mouse ileal sections were mounted under voltage clamp condition in Ussing chambers as described previously (3, 19). Short-circuit current (Isc) was continuously measured, where the voltage was V2 − V1, where V1 represents basolateral and V2 represents apical (apical-basolateral) voltage. The switching of conventions results in switching polarity but nothing else and allows for a comparison of the properties of the channels, as we described before (19, 42). Mouse colon was partially stripped of serosal and muscle layers, and the mucosa was mounted in Ussing chambers and bathed at 37°C on both sides with -free Ringer solution, which was continually circulated with a gas lift oxygenated with 100% O2. Transepithelial potential was clamped to 0 mV using a VCC MC6 multichannel voltage-current clamp amplifier (Physiologic Instruments), as described in our earlier studies (19).
Water Flux
Serosal to mucosal osmotic gradient-driven water flux was measured in T84 cell monolayers grown in snapwells that were mounted under voltage-clamp conditions in Ussing chambers, as described previously (3, 42). Monolayers grown on transwell inserts were exposed to basolateral Shigella for 6 h and mounted in Ussing chambers (3). Serosal to mucosal osmotic gradient-driven water fluxes through TJs was induced by mucosal addition of 100 mM mannitol, while transcellular ion transport-driven water secretion was blocked by serosal ouabain (100 nM). Water evaporation of both chambers was minimized by adding 50 µl of warm sterile mineral oil (each side). Polyethylene glycol (PEG 4000, 1 mg/ml), a nonabsorbable marker, was added to both the mucosal and the serosal solutions, and its concentrations were measured turbidimetrically as described before (9). Turbidity with PEG was produced after addition of 30% trichloroacetic acid (TCA) and 6% BaCl2·2H2O to 5 ml of collected bathing solution after a 20-min incubation measured at 525 nm. Water transport from serosal to mucosal chamber was calculated from the change of PEG concentration (PEGI/PEGF) ratio on the mucosal side, as described (9, 34, 39, 43), where PEGI and PEGF are samples taken from the mucosal side at the beginning and end of the flux period, respectively. Water fluxes were measured for 3 h in control and T84 monolayer infected for 6 h with serosal S. flexneri in the presence and absence of mucosal Zn2+.
Bacterial Adhesion and Invasion Assays
The effect of Zn2+ on adherence or invasion of Shigella was assayed as described by Sakaguchi et al. (36). Bacterial cells from mid-log phase at an MOI of 200 were administered to T84 cells and incubated for 30 min at 37°C with 5% CO2. The monolayers were rinsed and subsequently lysed with 1% Triton X-100. Serial dilutions were made to determine the cell-associated bacteria. To determine the invasive bacterial population after 90 min of incubation, monolayers were extensively washed, and cells were treated with 500 μg/ml gentamicin for 90 min. After extensive washes, intracellular bacteria were released by 1% Triton X-100, and the cell lysates were diluted and plated for colony-forming units (CFU).
For qualitative analysis of bacterial adhesion and invasion, T84 cells were infected with green fluorescent protein (GFP)-expressing bacterial strains. Following incubation, cells were mounted and viewed under a Zeiss LSM 710 confocal microscope.
Electron Microscopy
T84 cells were grown to confluence for 10 days. Cells were cooled to 4°C for 6 h, and the monolayers were fixed with 3.75% gluteraldehyde in 0.1 M Na-cacodylate buffer (pH 7.4) for 20 h and washed in cacodylate buffer (3×). Cells were secondarily fixed with 1% osmium tetroxide for 1 h in the dark, rinsed in ice-cold distilled water (4×) and enbloc-stained with freshly prepared and filtered 0.5% uranyl acetate in distilled water for 1 h. Cells were dehydrated with sequential incubations in 70, 90, and 100% ethanol at room temperature. Epon-Araldite (Embed 812) was added to cells and allowed to polymerize at 60°C for 48 h. Ultrathin sections were cut and layered onto carbon-coated copper grid and stained with freshly prepared 2% lead acetate. Grids were examined with Tecnai T12 BioTwin, and images were captured digitally. Ultrastructure observations were made from multiple sites (>10 sites) of junctional complexes that were clearly identified.
Total-RNA Isolation and Real-Time Quantitative Polymerase Chain Reaction Analysis
Total RNA was isolated from T84 cell monolayers using TRIzol reagents (Sigma) and stored at −80°c. Total-RNA (10 μg) was used to synthesize cDNA, and real-time quantitative polymerase chain reaction (RT-qPCR) analyses were performed using the Step-One Plus system (Applied Biosystems AB7900 real-time PCR detection system), described earlier (42), under the following conditions: 95°C, 5 min; 40 cycles and 95°C, 3 s; 60°C 30 s. Amplification reactions were performed in a final volume of 25 µl containing cDNA from 30 ng of cDNA, 300 nM each forward and reverse primers (Table 1), and SYBR Green PCR Master Mix. Normalization of target genes were done by using GAPDH (forward 5′-AGCCACATCGCTCAGACAC-3′, 5′-GCCCAATACGACCAAATCC-3′). The resulting CT values were calculated using single data CT as 2−(Target-GAPDH) and expressed in arbitrary units (AU).
Table 1.
Sequences of forward and reverse primers of targeted human genes used for qPCR
| Primer Sequences |
|||
|---|---|---|---|
| Gene Name | 5′–3′ Forward | 3′–5′ Reverse | Length |
| Claudin-1 | AGGTGCTATCTGTTCAGTGATG | CTACACAAGGAAAGTCAGCCA | 22,21 |
| Claudin-2 | CCTCCATCCCACTCTTGTTATG | AGAAAGCAGGATGCAGGATG | 22,20 |
| Claudin-3 | CCAAGGCCAAGATCACCAT | GGTTGTAGAAGTCCCGGATAATG | 19,23 |
| Claudin-4 | GCCTTACTCCGCCAAGTATT | AGGGAAGAACAAAGCAGAGAG | 20,21 |
| Claudin-5 | TTGGCTGCTGCCTTACTT | ACCAGCTGTACACATCTTCC | 18,20 |
| Claudin-7 | GCTCTTACCCTAAGTCCAACTC | CTTTCAGGCATCTAGACACTCC | 22,22 |
| Claudin-8 | CTGGGTTCCGAGTTCATTAC | GGCATGGGTTGCCATTAT | 20,18 |
| Claudin-10a | AGACACAGGCTTCTTCCTAGA | GAGTGAGACAGGACATGAAAGG | 21,22 |
| Claudin-10b | AGACACAGGCTTCTTCCTAGA | GAGTGAGACAGGACATGAAAGG | 21,22 |
| Claudin-11 | CGAACTCCTGGACTCAAAGTATC | GGCTCCCATTGTCATCTGTATC | 23,23 |
| Claudin-14 | CACCAGCTGCCTACAAAGA | GACTCACACGTAGTCGTTCAG | 19,21 |
| Claudin-15 | GAAACCTTTGGCTTCTTCATGG | AGATGGTGTTGGTGGTGATG | 22,20 |
| Claudin-19 | GCAGAAAGATGAGGAGACAGAG | AGAGGGTAGGACCTCTGAATTA | 22,22 |
| Occludin | GGTTCACTTCTCCCAGTCTTTC | AGACACAATCAACAGGGTTAGG | 22,22 |
| Tricellulin | GTACTCGTGGTTGCTGGATTAG | GCCACCAATTAGAGTCCAGAAG | 22,22 |
Immunofluorescence
T84 cell monolayers grown in transwell inserts were fixed on ice in 3% paraformaldehyde solution in PBS (pH 7.4) at 4°C for 20 min. Monolayers were then washed in ice-cold PBS, quenched with 50 mM NH4Cl in PBS for 15 min on ice, and excised as circles from the inserts. The fixed monolayers were then permeabilized for 30 min in 0.1% saponin in PBS before being blocked for 30 min in PBS + 1% BSA supplemented with 10% FBS. Cell monolayers were incubated with primary antibody in PBS + 1% BSA for overnight at 4°C in a moist environment, as described previously (3, 19).
For staining of mouse intestinal tissue sections, the intestine was dissected, and the ileum was rinsed with ice-cold saline and fixed in 10% formalin before standard paraffin embedding as described earlier by us (3). Briefly, individual sections were heat fixed and deparaffinized followed by microwave treatment in 0.01 M sodium (pH 6.0) citrate solution for antigen recovery. Sections were then washed in PBS and blocked with 5% normal goat serum in PBS for 1 h at room temperature. Subsequently, sections were incubated with primary antibody diluted (1:50) in 5% normal goat serum in PBS overnight at 4°C followed by FITC- or Alexa-conjugated goat secondary antibody, and images were obtained using a Zeiss LSM 710 confocal microscope. CFTR was detected using the antibody at 1:200 dilution. Multiple images were quantified (semiquantitatively) to illustrate reproducible changes of TJ protein in the presence of Zn2+ in Shigella infection. We determined continuity of claudin-2 and -4 labeling in infected and uninfected cells (discontinuous vs. continuous claudin-2 and -4) in a plane perpendicular to the orientation of the TJ image. The percentage of cells with continuous or discontinuous label was expressed as a percentage of total number of cells in the field.
Western Blot Analysis
Subcellular fractions were prepared using a subcellular proteome extraction kit (S-PEK) to extract cytosol and membrane fractions of control T84 monolayers, and monolayers infected with S. flexneri in the presence and absence of Zn2+; 25-µg protein samples were resolved on 10% SDS-polyacrylamide gel and electroblotted. The blots were incubated with anti-claudin-2, anti-claudin-4, anti-pERK, and anti-ERK primary antibodies (1:500 dilution) overnight at 4°C. Proteins were incubated with goat anti-rabbit IgG conjugated to alkaline phosphatase (1:2,000) for 1 h at room temperature, and the blots were visualized using 5-bromo-4-chloro-3-indolyl phosphate (BCIP)/nitro blue tetrazolium (NBT), as we described before (3, 42).
Interleukin Measurement by ELISA
Interleukins were measured by double-sandwich ELISA technique following the manufacturer’s instructions (BD Biosciences). Media from the polarized T84 monolayers were collected from control, and monolayers infected with S. flexneri for 6 h in the presence and absence of mucosal Zn2+.
Animals and Ethics
C5BL/6 (B6) and BALB/c male mice purchased from Jackson Laboratory (Bar Harbor, ME) were housed in a pathogen-free barrier facility at NICED and used at the age from 6 to 12 wk. All animal experiments described were approved by the NICED Institutional Animal Care and Ethics Committee [NICED/CPCSEA/AW/(225)/2014-IAEC/MHK-1].
Fecal Pathology and Electrophysiological Studies in Mice
Pathological scores were defined according to stool consistency, appearance, and water content (47). Briefly, fresh feces were categorized by the following parameters: consistency (0 to 3; normal < loose < soft < hard), color (0 to 3; brown < yellow < light green < blue green), and cumulative numbers of diarrhea episodes (0 to 3). Mouse colon samples were mounted into Ussing chambers, and total transmural electrical resistance was recorded in open mode.
Statistical Analysis
Data were subjected to t-tests (for paired or unpaired samples as appropriate) and ANOVA for statistical analysis and presented as means ± SE. Statistical analyses were carried out using Origin 6.0 software. P ≤ 0.05 was considered significant. Survival analysis were performed through plotting of Kaplan-Meier curves. Mann-Whitney tests and Kaplan-Meier curves were performed with GraphPad Prism (v. 7.04, GraphPad Software).
RESULTS
Shigella Infection Provokes a Breach in the TJ Barrier that is Reversed by Zn2+
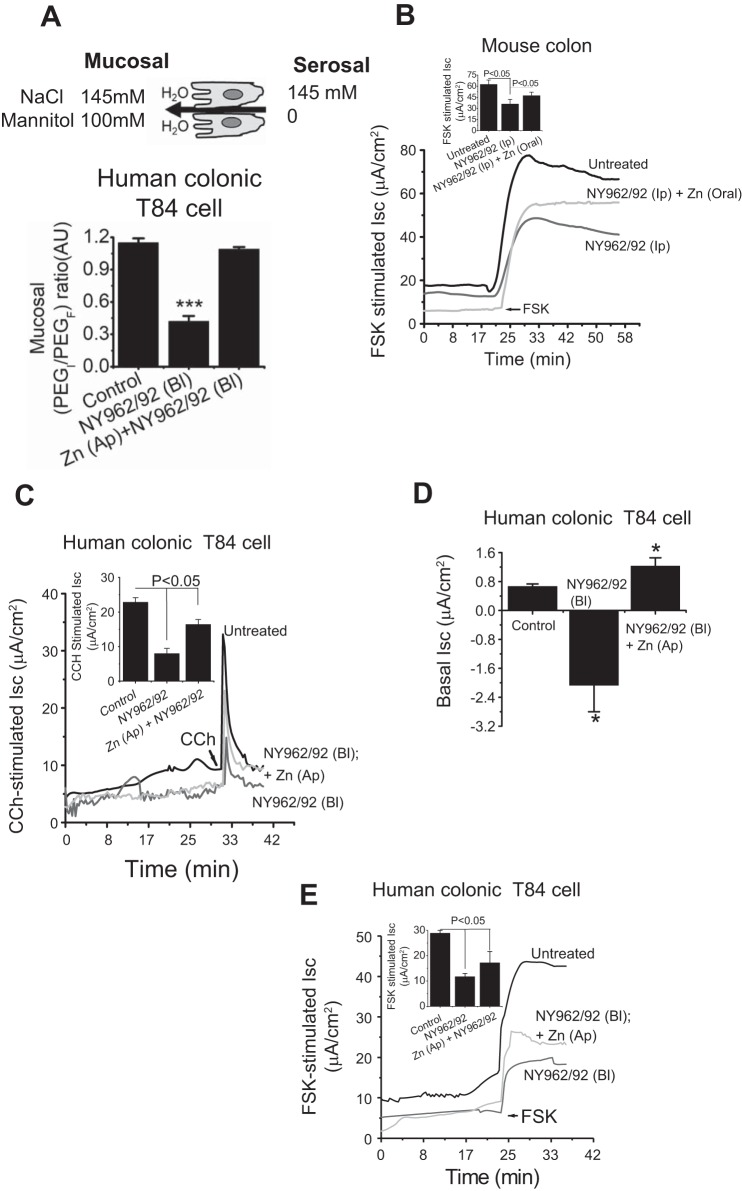
TJ alterations have been proposed to be involved in intestinal inflammation due to enteric infection as exemplified by decreases in TER. To determine whether site-specific interaction of Shigella would trigger TJ associated alterations, T84 cell monolayers were infected on either apical or basolateral compartments for different time intervals. We found no significant difference in TER between Shigella-infected and uninfected monolayers at the end of the 2-h infection period. However, by 6 h postinfection, reduction of TER was statistically significant compared with uninfected monolayers (Fig. 1A, inset). Dramatic deterioration of TER was observed in basolateral infection (72 ± 3.25%) compared with apical infection (36 ± 1.25%) over the 12-h observation period, suggesting that the effect induced by Shigella infection may be more persistent from the basolateral side compared with the apical side (Fig. 1A). Restriction of paracellular molecular flux is one of the most important characteristic of TJs. To trace TJ integrity as well as size-selective paracellular permeability, fluorescently labeled dextran of two different sizes (4 vs. 70 kDa) were used. When applied to T84 monolayers, Shigella infection caused a significant increase in paracellular permeability of 4-kDa dextran compared with either control or uninfected cell monolayers (1.52 ± 0.3 vs. 0.41 ± 0.053 µg/cm2 for 3 h, P < 0.001). In contrast with paracellular flux of 4-kDa FITC dextran, 70-kDa rhodamine had no flux at all even in monolayers infected with Shigella. FITC- and rhodamine-labeled dextran flux increased when cells were treated with EGTA. Calcium depletion by EGTA was used to establish the maximum flux via the paracellular pathway. The calcium-depleted cell monolayer had paracellular flux eight times higher than that of either control or Shigella-infected cells (Fig. 1B). The junctional paracellular gate exhibits complex features; hence, a single assay does not allow a meaningful conclusion. We determined the paracellular ionic conductance changes resulting from Shigella infection. The baseline value of ion selectivity (PNa/PCl) in T84 cells was 3.81 ± 0.11, consistent with cation selectivity reflected by diffusion potential of +7.32 ± 0.52 mV. The uninfected T84 monolayers showed higher permeability for Na+ (cation selective) than Cl− (Na+ permeability was threefold higher than Cl− permeability; Fig. 1C, inset, top left). As shown in Fig. 1C, the dilution potential obtained in Shigella-infected T84 monolayers was significantly less (less +Ve in mV) than uninfected cells, indicating that infection decreased relative permeability of Na+ cations compared with Cl− anions (Fig. 1C, middle).
Fig. 1.
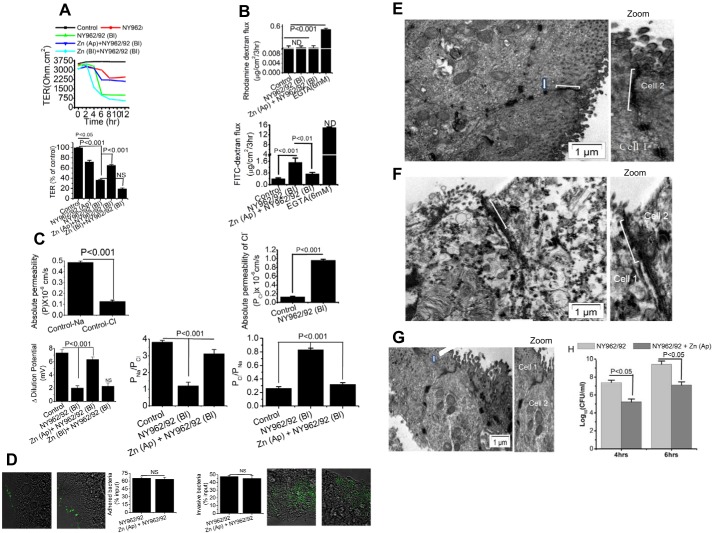
Apical Zn2+ application rescue severely affected paracellular transport function of Shigella infection in T84 cells. T84 cell monolayers used were grown 10 days postconfluence on transwell plates (Control). Infected monolayers were treated with 200 multiplicity of infection Shigella flexneri 2a (NY962/92) to either mucosal (Ap) or serosal (Bl) medium and in presence or absence of Zn2+ (200 μM). A: transepithelial electrical resistance (TER) quantification was derived from time course study when resistance reached a stable value of >500 Ohm·cm2. Top: representative time course of TER in response to Shigella infection and in presence of Zn2+. B: paracellular permeability of dextran across T84 monolayers. Shigella infection increased 4-kDa FITC-dextran flux (bottom) but not the 70-kDa rhodamine-dextran (top). Mucosal Zn2+ reversed Shigella-induced FITC-dextran flux. C, bottom: dilution potentials for Na+ and Cl− were measured on T84 cell monolayers infected with S. flexneri 2a in presence and absence of Zn2+. Permeability of Na+ over Cl− (PNa/PCl) and permeability of Cl− over Na+ (PCl/PNa) ratios were calculated as described in materials and methods. Top left: absolute permeability of Na+ and Cl− in uninfected (Control) T84 monolayers. Top right: absolute permebility of Cl− in uninfected (Control) and Shigella (NY962/92)-infected T84 monolayers. D: effect of Zn2+ on Shigella adhesion (left) and invasion (right) with visualization of bacteria labeled with green fluorescent protein (GFP) variant in infected T84 cells. Bar graph indicates that both adhesion and invasion of Shigella are similar in presence or absence of Zn2+ in T84 cell monolayers. Data are means ± SE; n = 5–6 independent experiments. NS, not significant. E and F: effects of Zn2+ on ultrastructure micrograph of tight junctions (TJ). T84 cells were treated with Zn2+ in presence of Shigella infection for 4 h and processed for transmission electron microscopy (TEM) as described in materials and methods. E: Control (uninfected) cells are oriented in such a way that the bottom right hand is apical and the top left is more basal. Bracket points toward a TJ that shows close apposition of plasma membrane leaflets of 2 cells. Arrow points to a desmosome with dense desmosomal plaques seen on either side of the intercellular space. F: TJ indicates location of discontinuous TJ zone with few membrane fusions, along with widening of gap between the 2 membranes due to Shigella infection. G: Zn2+ application brought about close membrane apposition with no apparent breakdown of intracellular membrane junction. Zoomed images depict detail in apical membrane TJ with altered regions indicated by brackets; n = 4 independent experiments. H: apical-to-basolateral bacterial translocation assay was performed with polarized T84 cells for 4 and 6 h. Basolateral medium was collected after 4 and 6 h and plated to obtain bacterial counts. Data plotted are means ± SE; n = 4–6 independent experiments.
We next examined whether Zn2+ can restore TJ function of T84 cell monolayers damaged by Shigella infection. As shown in Fig. 1A, addition of Zn2+ at a concentration of 200 µM to the apical bathing medium was able to increase TER by 17.29 ± 2.1% after 2 h of addition. Apical Zn2+ significantly restored TER by 65 ± 1.7% compared with basolateral shigella infection. However, basolateraily added Zn2+ had little or no protective effect on TER. The data suggested that basolateral shigella infection enabled apically added Zn2+ to access TJ to allow efficacy to restore the deteriorated barrier function. Apical addition of Zn2+, therefore, was used in subsequent studies. Zn2+ prevented the increase in paracellular permeability caused by Shigella infection, as shown by the passage of 4-kDa FITC-dextran (Fig. 1B). However, this recovery effect was not due to cytotoxic effects of Zn2+ on the viability of Shigella. Addition of 200 µM Zn2+ had no effect on the growth or bacterial viability of Shigella after incubation for 6 h (data not shown). To determine the protective role of Zn2+ on paracellular ionic conductivity altered due to Shigella infection, we applied an apical-to-basal chemical gradient of NaCl (120 mM NaCl at the apical side to 60 mM at the basal side) to the uninfected and Shigella-infected T84 monolayers in the presence or absence of Zn2+ and recorded the dilution potentials. As shown in Fig. 1C, Shigella infection caused significant reduction of dilution potential (+2.02 ± 0.35 vs. +7.32 ± 0.52 mV), with a decrease in PNa+/PCl−. Furthermore, anion selectivity (PCl−/PNa+) was threefold higher due to Shigella infection, which was attributable to a sixfold increase in absolute PCl (Fig. 1C, top right). As, calculated from the dilution potential, paracellular permeability of Na+ relative to Cl− was increased upon Zn2+ treatment. Bacterial adhesion and invasion play a key role in the pathogenesis of enteric infection. The barrier-protective role of Zn2+ could be due to reduction of adhesion and or invasion efficiency of Shigella strain. To examine this question, T84 cells were challenged in presence or absence of Zn2+ and the percentage of adhered and internalized bacteria were determined. As shown in Fig. 1D, we found that Zn2+ had no effect either in internalization or in adherence of Shigella to T84 cells, as revealed by qualitative analysis of GFP-labeled Shigella and quantitative analysis of bacterial count. Thus, restoration of TJ integrity by Zn2+was not due to the reduction of adhesion or invasion efficiency of Shigella strain.
The transmission electron microscopy (TEM) ultrastructure of T84 cells infected with Shigella infection is shown in Fig. 1, E–G. The presence of electron-dense material in the space between cells near the apical membrane reflects the TJ (Fig. 1E). In cells infected with Shigella, the TJ complex appeared reduced and widened and contained less electron-dense material (Fig. 1F). With 200 µM Zn2+ application, this effect was reduced (Fig. 1G). These results demonstrated that Zn2+ application resulted in restitution of distorted TJ morphology.
Zn2+Restores the Distribution Pattern of Claudin-4, limiting Shigella-Induced TJ Disruption
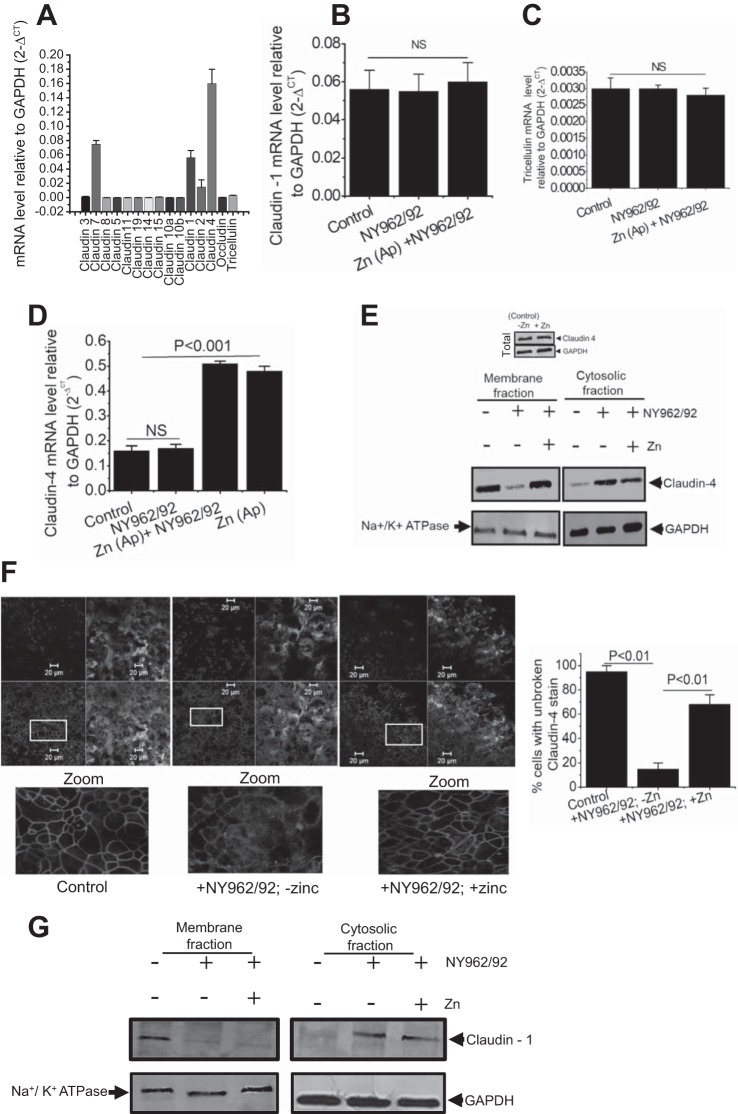
We investigated the effect of Zn2+ on the mRNA expression of TJ proteins in Shigella-infected T84 cells. RT-qPCR analyses revealed that claudin-1, -2, -4, and -7 are the major TJ mRNAs expressed in normal T84 cells. Among these four, claudin-4 is the predominantly expressed transcript in T84 cells (Fig. 2A). Thus, to elucidate the role of specific TJ protein genes, claudin-2 and -4 were chosen for further study. RT-qPCR analyses also revealed that Shigella infection did not affect either claudin-1 or tricellulin-specific mRNA abundance (Fig. 2, B and C). However, although Shigella infection did not alter claudin-4 and -2 mRNA abundance, their expression was significantly upregulated by Zn2+ (Figs. 2D and 4B).
Fig. 2.
Apical Zn2+ rescues claudin-4 relocalization to restore Shigella-mediated barrier defects. A: mRNA expression profile of tight junction (TJ) proteins in T84 cells. Expression of claudin-1 (B), tricellulin (C), and claudin-4 (D) with or without Shigella (NY962/92) infection and in presence or absence of Zn2+. Data are plotted means ± SE; n = 4–6 independent experiments. E: effect of Shigella infection in presence or absence of Zn2+ on claudin-4 protein level in cytosolic and membrane fractions of T84 cells, Na+-K+-ATPase and GAPDH were used as loading control. Representative blots are shown from 3 independent experiments. Top: representative Western blot for claudin-4 abundance in presence or absence of Zn2+ without Shigella infection. F: immunofluorescence images (XY) of T84 cells stained with anti-claudin-4 (red), apical membrane marker wheat germ agglutinin (green), and nuclear marker DAPI (blue). Claudin-4 staining at cell-cell contact (left) were disturbed by Shigella infection (middle), but in presence of Zn2+, claudin-4 is efficiently concentrated into cell-cell borders (right). Typical image represents 3 separate duplicate experiments. Bar graph represents quantification of multiple images based on continuity of claudin-4 stain (broken vs. unbroken) to illustrate reproducible changes of claudin-4 in uninfected (Control) and Shigella-infected T84 cell monolayers in presence and absence of Zn2+. Three fields from 2 independent experiments for each condition, ~75 cells were scored. Details of boxed areas are zoomed, showing arrangement of claudin-4 (bottom). G: effect of Shigella infection in presence or absence of Zn2+ on claudin-1 level in cytosolic and membrane fraction of T84 cells, representative Western blot from 2 independent experiments. Na+-K+-ATPase and GAPDH were used as loading control.
Fig. 4.
Altered distribution of claudin-2 is rescued by Zn2+ for restoration of paracellular permeability and barrier tightens. A: representative images of claudin-2 (red) and apical membrane marker wheat germ agglutinin (blue) in control (uninfected) and Shigella infected in the presence (NY962/92 + zinc) and absence (NY962/92 – zinc) of Zn2+ in T84 monolayer. Claudin-2 staining at cell-cell contacts is shown diminished by Shigella infection (XY). X-Z images showed that claudin-2-positive red strip in the apical region between neighboring cells were disturbed (NY962/92 – zinc) but relocalization of claudin-2 occurs in the presence of Zn2+ (NY962/92 + zinc). Bar graph represents quantification of multiple images based on continuity of claudin-2 stain (broken vs. unbroken). Three fields of view from 2 independent experiments for each condition, ~75 cells were scored. B: qRT-PCR showed that claudin-2 mRNA is markedly elevated by Zn2+ application in T84 cells. Data are means ± SE; n = 3 per group. C: effect of Shigella infection in presence or absence of Zn2+ on claudin-2 level in cytosolic and membrane fractions of T84 cells. Representative Western blot presented represent 3 independent experiments. Na+-K+-ATPase and GAPDH were used as loading control. Top: representative Western blot for claudin-2 abundance in presence and absence of Zn2+ without Shigella infection. D: T84 monolayers (top) and mouse distal colon (bottom); uninfected (left) or Shigella infected (right) were immunolabeled with anti-cystic fibrosis transmembrane conductance regulator (CFTR) antibody. Each image is representative of 3 separate experiments performed in duplicate.
Having shown that Zn2+ treatment influences claudin-4 mRNA expression and leads to accelerated TER and paracellular permeability along with charge selectivity across the Shigella-infected monolayers, we turned our attention first to claudin-4. Claudin-4 is considered to have distinct charge selectivity, either by tightening the paracellular pathway or by functioning as a paracellular pathway, regulating the passage of ions and small molecules (46). Since claudin-4 mRNA remained unaltered, we examined the ability of Shigella infection to regulate the subcellular distribution of claudin-4. Figure 2E demonstrated that claudin-4 was detected mostly in the membrane fraction and was visible as a strong band of ~22 kDa in uninfected cells. Upon infection, we detected redistribution of claudin-4 from the membrane to the cytosolic fraction at 6 h postinfection. Zn2+ treatment restored altered localization of claudin-4 at the membrane level (Fig. 2, E and F). We then asked whether the observed changes in claudin-4 mRNA expression in the presence of Zn2+ could be correlated in terms of protein levels. As shown in Fig. 2E, top, we detected no changes in claudin-4 protein amount due to Zn2+ treatment compared with control (without Zn2+). Thus, the increased claudin-4 mRNA cannot be interpreted in terms of protein level and must entail mislocalization (cytosolic vs. membrance fraction), as shown in Fig. 2E. Because claudin-1 mRNA expression was not altered in our study (Fig. 2B), we reasoned that Zn2+ may not have any effect on claudin-1 distribution. However, it is worth mentioning here that Sakaguchi et al. (36) reported alteration of claudin-1 distribution during Shigella infection. We found a reduction of membrane-associated claudin-1 in Shigella-infected monolayers, which was not recovered in the presence of Zn2+, suggesting that Zn2+ does not facilitate recovery of claudin-1 to the TJ complex (Fig. 2G).
Confocal microscopy of uninfected T84 monolayers revealed a typical chicken wire-like pattern of claudin-4 localization at the cell-cell boundary, indicating an intact TJ complex. However, xy plane images of Shigella-infected cells showed disrupted continuity of claudin-4 staining with evidence of cellular redistribution in the cytoplasm, concomitant with increased disorder across monolayers, as shown in Fig. 2F (middle image zoomed). To ensure reproducibility and accuracy of these findings, we quantified multiple images as described in materials and methods. The far right histogram in Fig. 2F demonstrates that Zn2+ supplementation to a substantial extent was able to recover 68% of the normal pattern (intactness) of claudin-4 staining, which was statistically significant compared with the absence of Zn2+ in Shigella infection (15 ± 5 vs. 68 ± 8% cells with unbroken claudin-4 stain). As a last measure, we investigated Shigella translocation across epithelial monolayers in the presence or absence of Zn2+ to determine whether Zn2+ supplementation could limit Shigella translocation. Figure 1H demonstrated that translocation across T84 monolayers started at ~4 h and reached a peak at 6 h in the absence of Zn2+. In contrast, translocation in the presence of Zn2+ significantly decreased at both time points, correlating with the barrier enhancing roles of Zn2+ with reduced translocation of pathogens from apical to basolateral pole due to improve TJ barrier.
Water Transport and Electrogenic Cl− Secretion During Shigella Infection With or Without Zn2+: Changed or Unchanged?
Intestinal mucus is composed of water and ions that facilitate the clearance of pathogenic organisms. It is also likely that a defect in ion and water transport results in luminal surface dehydration that affects the efficiency of mucus clearance. This could be an effective strategy of pathogenic bacteria during infection that contributes to the pathogenesis. To examine the effect of Shigella infection on luminal surface dehydration, T84 monolayers were infected with Shigella strain. The analysis of water flux was carried out in Ussing chambers as described elsewhere (9, 42). For induction of water flux by an osmotic gradient, 100 mM mannitol was added to the apical solution. Under these conditions, decreased water flux was observed in Shigella-infected cells compared with uninfected control, whereas Zn2+ treatment influenced osmotically driven water transport (Fig. 3A). Calculated PEGI/PEGF was 1.08 ± 0.04 in uninfected control that was reduced to 0.42 ± 0.05 upon Shigella infection. Zn2+ restored this aberrant change of PEGI/PEGF in Shigella-infected cells. We next examined the effect of Shigella infection on Cl− secretion by cAMP and calcium stimulation, because the hydration status of the luminal surface is principally regulated by Cl− export through CFTR and Ca2+-activated chloride channels. Figure 3B demonstrated that Shigella-infected mouse colon had reduced ion transport responses to the cAMP-elevating agent forskolin (FSK) compared with uninfected control. Similarly, T84 monolayers had reduced FSK-stimulated Isc due to Shigella infection (Fig. 3E). Shigella infection also suppressed transport responses to the calcium-elevating agent carbachol (CCH) in T84 cells compared with uninfected control, suggesting failure of adequate luminal hydration (Fig. 3C). These reduced ion transport responses were restored by Zn2+ application (Fig. 3, B, C, and E). Interestingly, baseline Isc showed a change of secretion to absorption compared with uninfected T84 monolayers; however, Zn2+ application elevates this baseline Isc toward secretion (Fig. 3D). These results suggest that Shigella infection caused a reduction of Cl− secretion while inducing absorption, resulting in a reduced driving force for water to enter the lumen, resulting in a dehydrated luminal surface. This scenario may be a disease mechanism that provides an ideal environment for Shigella infection.
Fig. 3.
Zn2+ prevents reduction of secretagogue-stimulated Cl− and paracellular water transport in intestinal epithelial cells of Shigella (NY962/92) infection. A: summarized effects of Zn2+ on paracellular water transport (serosal-to-mucosal) in human colonic T84 cell monolayers infected with Shigella. Top: illustration of the cell indicates the direction of osmotic gradient and water transport. Water transport is reflected by the change in polyethylene glycol (PEG) concentration ratio indicating volume in the mucosal side relative to volume in the serosal side. During water secretion to the mucosal side, PEGF falls and the ratio becomes greater than 1. B: representative current recording of Cl− transport of the effect of calcium and cAMP in mouse intestine and T84 monolayers (C and E). FSK, forskolin; CCH, carbachol. Inset figures represent data pooled from 3 monolayers and expressed means ± SE; n = 6. D: bar graph showing quantification of basal short-circuit current (Isc) changes elicited by Shigella infection alone and Shigella infection in presence of Zn2+. Data are pooled from 3 monolayers in each condition and results are expressed as means ± SE. *P < 0.05 vs. Control.
Luminal Surface Dehydration Due to Shigella Infection Is Dependent on Claudin-2, CFTR, and Anoctamin Dysfunction
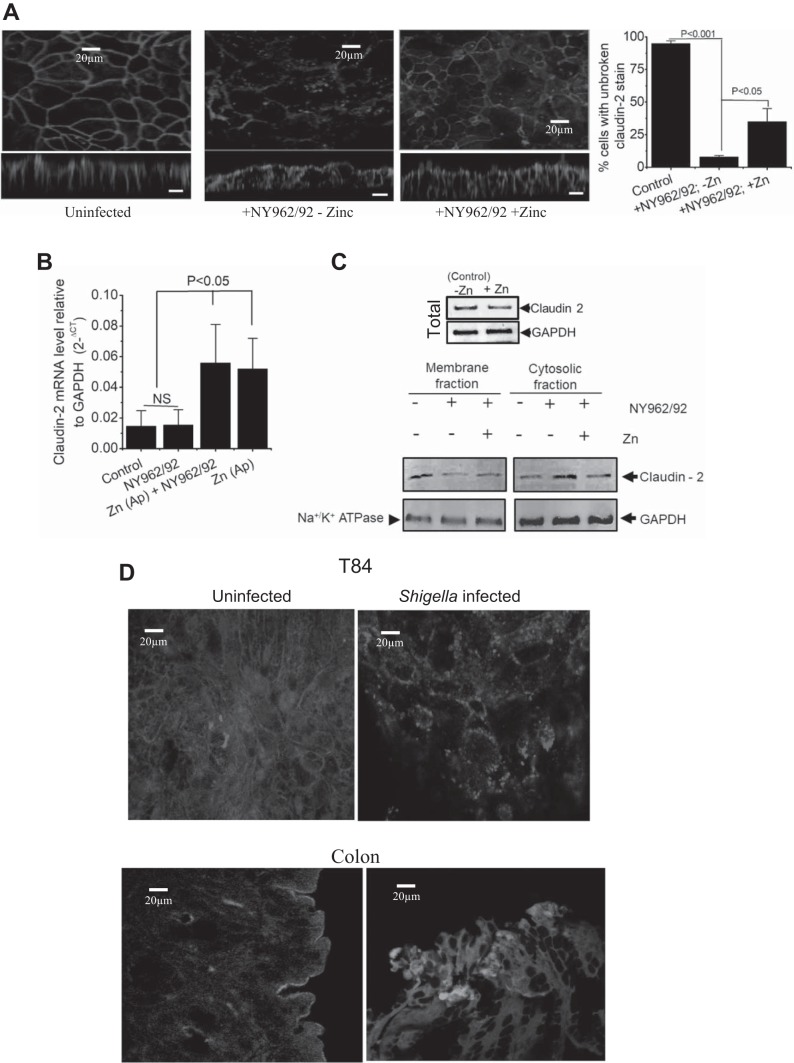
Claudin-2 localization is modified in Shigella infection.
Figure 4A demonstrated that Shigella infection drastically disrupted the overall pattern of claudin-2 staining, causing redistribution from the cell border to a broader and cytoplasmic staining but was localized in punctuated aggregates. Furthermore, xz plane images showed distortion of claudin-2-positive staining as long vertical strips that were in the apical regions between two neighboring cells. When cells were infected in the presence of Zn2+, there was slightly more specific junctional staining of claudin-2 as sharp continuous bands. Zn2+-treated cells showed partial preservation of the pattern of claudin-2, albeit with evident damage by infection, indicating that Zn2+ can regulate claudin-2 dynamics. The data were analyzed semiquantitatively by measuring continuity of claudin-2 stain as a measure of junction intactness described in materials and methods and presented in a histogram in Fig. 4A, far right. We observed that Zn2+ supplementation decreased the discontinuous claudin-2 stain by 35% compared with the absence of Zn2+ (8 ± 2 vs. 38 ± 10% cells, with continuous intact claudin-2 stain). Since claudin-2 mRNA was altered by Zn2+ application (Fig. 4B), we examined whether Zn2+ application increased levels of claudin-2 on the plasma membrane by Western blot analysis of membrane- and cytosol-associated protein fractions. As demonstrated in Fig. 4C, claudin-2 expression was reduced in membrane fractions after 6-h exposure to Shigella. Surprisingly, upon exposure of apical Zn2+ claudin-2, although present in the membrane-associated fraction, was also found to be present in the cytoplasm. We next examined whether increase in claudin-2 mRNA level in presence of Zn2+ correlated with change in protein levels. Figure 4C, top, shows the effect of Zn2+ on the claudin-2 protein abundance. Somewhat to our surprise, claudin-2 protein did not change in abundance.
Effects of Zn2+ on CFTR localization in Shigella infection.
The luminal Cl− channel exerts a major influence on the capacity of the lumen to maintain normal hydration, with both CFTR and anoctamin(s) functioning as Cl− channels. In Ussing chamber experiments (Fig. 3, B, C, and E), the intracellular cAMP-elevating agent FSK and Ca2+-elevating agent CCH, stimulated electrogenic Cl− secretion in colonic cells was diminished by Shigella infection. This scenario could result in dehydration of the luminal surface, concentration of mucins within the mucus layer and adhesion of mucus to the epithelial surface to provide a nidus for infection. We next asked whether Shigella infection could disrupt trafficking of the major Cl− transporting protein CFTR. The cellular localization of CFTR in control and Shigella-infected cells was performed and analyzed by confocal xy images. These confocal images demonstrated that CFTR was predominantly expressed on the surface of uninfected cells; however, infection with Shigella resulted in loss of apical CFTR staining in T84 cells and mouse intestine (Fig. 4D).
Pro- and anti-inflammatory cytokine production during shigella infection and control by Zn2+.
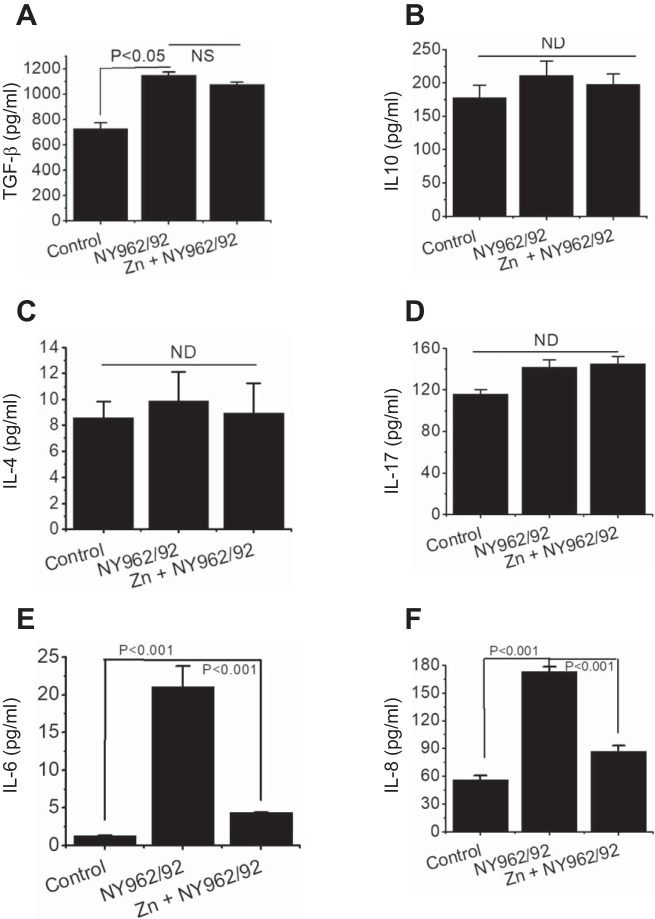
Shigella infection is accompanied by activation of intestinal epithelial cells, resulting in production of local cytokines that may function as a signal to other immune cells to incite inflammation in the gastrointestinal mucosa. To elucidate the cytokine patterns in the presence or absence of Zn2+, the production of gut-specific pro- and anti-inflammatory cytokines was measured. We used ELISA specific for human transforming growth factor-β (TGFβ), IL-4, IL-6, IL-8, IL-10, and IL-17 in human colonic T84 cells 6 h after Shigella infection. As shown in Fig. 5, E and F, T84 cell monolayers infected with Shigella showed roughly 16- and 3-fold increases in IL-6 (1.3 ± 0.03 vs. 21.06 ± 2.75 pg/ml), and IL-8 (56.5 ± 4.2 vs. 173 ± 5.23 pg/ml) production, respectively, compared with noninfected cells. Interestingly, the application of Zn2+ inhibited these elevated inflammatory responses (Fig. 5, E and F). In contrast to the effects on IL-6 and IL-8, IL-4, IL-10, and IL-17 production were unaltered in the presence of Zn2+ following Shigella infection. The TGFβ level was increased following infection, but Zn2+ was not able to diminish TGFβ levels. These data strongly indicate that IL-6, IL-8, and TGFβ are important cytokines in Shigella pathogenesis and suggest that Zn2+ may provide a therapeutic opportunity in protecting intestinal epithelial tissue damage by reducing local cytokine levels against Shigella infection.
Fig. 5.
Effects of Shigella (NY962/92) infection on interleukin (IL) production by human intestinal T84 cells Interleukin assessment of transforming growth factor-β (TGFβ; A), IL-10 (B), IL-4 (C), IL-17 (D), IL-6 (E), and IL-8 (F) secreted by uninfected (control) and Shigella-infected T84 monolayers for 6 h in the presence (NY962/92 + Zn) and absence (NY962/92 – Zn) of Zn2+. Zn 2+ reversed IL-6 and IL-8 secretion to basal level. Data presented represent means ± SE of 3 independent experiments per group. ND, not determined.
Role of signaling intermediate inhibition of MAPK prevents shigella-induced alteration of barrier function.
To understand the underlying mechanisms of attenuated TJ barrier by Zn2+ in Shigella infection, we examined the involvement of mitogen-activated protein kinase (MAPK) signaling pathways. T84 cells were stimulated with Shigella following pretreatment with PD98059 (20 µmol/l), a MAPK inhibitor and SP600125 (10 µmol/l), a c-Jun NH2-terminal kinase (JNK) inhibitor. While measuring TER, we verified that treatment with PD98059 alone had no detectable effect on basal epithelial permeability and TER (Fig. 6A), consistent with previous findings (10, 11). Interestingly, pretreatment of polarized T84 monolayers with PD98059 abolished the Shigella-induced decrease in TER and increased flux of 4-kDa FITC-dextran (Fig. 6, A and B). This observation was not due to loss of Shigella-host cell interaction, as PD98059 at a concentration of 20µmol/l did not inhibit the adhesion or invasion of Shigella (Fig. 6D) as reported previously (23). However, pretreatment with SP600125 was unable to restore the Shigella-mediated decrease in TER and increased flux of FITC-dextran, an indication of involvement of ERK pathways in Shigella-induced barrier dysfunction (Fig. 6, A and B, top).
Fig. 6.
Tight junction barrier defect in T84 cells due to Shigella infection is regulated by extracellular signal-regulated kinase (ERK) signaling pathway for claudin-2 and -4 phosphorylation status. Effects of apical (Ap) Zn2+and basolateral (Bl) PD98059 (MAPK inhibitor) on transepithelial electrical resistance (TER; A), 4-kDa FITC-dextran flux (B) in uninfected (Control) and Shigella-infected (NY962/92) T84 monolayers. Anthrapyrazolone (SP600125, 10 μM) failed to inhibit Shigella-mediated decrease in TER and increase permeability of 4-kDa FITC dextran in T84 monolayers (top A and B, respectively). C: claudin-2 (top) and -4 (bottom) localization in uninfected and Shigella-infected T84 monolayers treated with or without PD98059 or SP600125. Bar graph represents quantification of multiple images based on continuity of claudin-2 and -4 stains (broken vs. unbroken) in uninfected (Control) and Shigella-infected T84 cell monolayers in presence of PD98059 and SP600125. D: effect of PD98059 on Shigella adhesion (top) and invasion (bottom) in infected T84 cell monolayers. Both adhesion and invasion of Shigella are not inhibited by PD98059 in T84 cell monolayers. Data are means ± SE of 3 independent experiments. NS, not significant. E: effect of Shigella infection on ERK phosphorylation in presence or absence of Zn2+ in T84 cell monolayers as assessed by Western blot of phospho (p)-ERK. Densitometric quantification (ratio of ratios) of p-ERK levels relative to total GAPDH [(p-ERK/GAPDH)/(total ERK/GAPDH)]in triplicate experiments. F: immunoprecipated (IP) proteins with anti-claudin-2 (top) and anti-claudin-4 (bottom) antibody from T84 cell lysate of uninfected and Shigella-infected in presence or absence of Zn2+ were examined for Ser/Thr phosphorylation by Western blotting using phosphor-antibody. Whole cell lysate was used as loading control. Representative blots represent 3 independent experiments. G: effects of PD98059 on IL-6 and IL-8 production in uninfected (Control) and Shigella-infected T84 monlayers in presence (NY962/92 + PD98059) or absence (NY962/92) of PD98059. Results are expressed as means ± SE. NS, P > 0.05, which is considered not statistically significant.
To determine whether the observed recovery of TER by PD98059 treatment also required claudin-2 and -4, we performed immunofluoresence staining of T84 monolayers preincubated with PD98059 followed by Shigella infection for 6 h. In control, noninfected cells, the labeling for claudin-2 and -4 was distributed along the cell periphery in a continuous pattern. Shigella infection not only resulted in the loss of normal distribution and disrupted cell border staining but also increased intracellular staining of claudin-2 and -4. However, in the presence of PD98059, the typical chicken wire pattern of claudin-2 and -4 at the cell-cell boundary was evident, indicating restoration of barrier function (Fig. 6C). Surprisingly, pretreatment with SP600125 was unable to recover the significant loss of cell-cell contacts and this typical pattern of TJ upon Shigella infection. Consistent with this observation, our quantitative confocal image analysis further confirmed that PD98059 significantly (P < 0.05) restored claudin-2 and -4 staining at the junction, whereas SP600125 was unable to recover the significant loss of cell-cell contacts for claudin-2 and -4 (Fig. 6C, far right). The involvement of ERK activity in the production of IL-8 and IL-6 has been reported (23, 40, 41). PD98059 pretreatment inhibited IL-6 and IL-8 production resulting from Shigella infection (Fig. 6G). This corroborates the effect of Zn2+ presented in Fig. 5, E and F, along with its effect on TER and paracellular flux studies. Collectively, these findings demonstrate the involvement of MAPK but not the JNK pathway activation in Shigella-mediated barrier dysfunction.
Does Zn2+ Prevent Shigella-Induced Phosphorylation of Claudins and Its Disengagement from the TJ Complex Through the ERK Pathway?
To explain how Zn2+ might reverse the barrier defect in shigellosis, we turned our attention to ERK1/2, which has been reported to modulate TJ function and expression (26, 35). Figure 6E shows that phosphorylation of ERK1/2 (p-ERK1/2) increased 6 h postinfection with Shigella compared with uninfected control. By contrast, Zn2+ treatment induced a marked decrease in the phosphorylation level of ERK (Fig. 6E). The total amount of ERK1/2 did not change, suggesting increased activation of ERK. To explore the molecular mechanism connecting Zn2+ to phosphorylated TJ proteins, the phosphorylation status of claudin-2 and -4 was assessed. Figure 6F demonstrates the levels of bead-bound serine/threonine phosphorylation in claudin-2 and -4. Comparison of phosphorylations reveals that claudin-2 phosphorylation was completely retained in the presence of Zn2+, whereas claudin-4 phosphorylation was increased when cells were infected with Shigella. In contrast, the total amount was unchanged in both claudin-2 and claudin -4. Together, data from both immunolocalization presented in Figs. 2F and 4A and phosphorylation experiments suggested that decrement and increment of claudin-2 and -4 phosphorylation and dephosphorylation, respectively, in the presence of Zn2+ is associated with preferential plasma membrane localization.
Oral Gavage of Zn2+ Counteracts Bacterial Colonization and Diverse Pathophysiological Symptoms Provoked upon Intraperitoneal Shigella Infection
Because in vitro studies revealed that Zn2+ effectively recovers compromised barrier function due to Shigella infection, a functional role for Zn2+ was next examined in vivo. Both C57BL/6 and BALB/c mice were infected intraperitoneally with 106, 107, 108, and 109 CFU of Shigella and a dose of 108 was used for subsequent studies according to methods published earlier (47) at 0 h as shown in Fig. 7A. Mice were oral gavaged with 200 µM of Zn2+ after they had shown severe diarrhea symptoms at ~2 h, which peaked at 6 h and continued until 12 h, as scored by fecal pathology (color and consistency). Animals receiving Zn2+ showed a significant reduction in diarrheal scores (Fig. 7B) whereas ~80% of mice infected with Shigella died within 4 days. In contrast, the oral Zn2+ administration group had an increase in survival; 65% of mice survived (Fig. 7C), suggesting that Zn2+ administration resulted in improved survival compared with mice not receiving oral Zn2+. Furthermore, examining stool consistency in the colon, we found uninfected mouse colon contained formed pellets of stool. However, the colons of animals infected with Shigella contained semisolid stool, and formed stool pellets were seen in Zn2+-treated mouse colon (Fig. 7B, top). Pathological studies on colon and body weight revealed the protective effect of Zn2+ on colon length and body weight upon Shigella infection. Histological assessment of mouse colon showed goblet cell hyperplasia; however, no epithelial shedding or detachment was seen following Shigella infection for 6 h (Fig. 7, D, E, and F). To confirm whether Shigella had invaded and colonized the host through the intraperitoneal route and to show the protective effect of Zn2+ supplementation, we determined the number of bacteria colonies in mucosal large intestine and systemic compartments at different postinfection time points. As expected, we found significant numbers of Shigella organisms in large intestine and feces from 0 to 24 h after mice were challenged with Shigella. Similar patterns of colonization were seen in systemic (i.e, spleen) tissues. Interestingly, we found that Zn2+ supplementation significantly diminished colonic colonization of Shigella and eventually reduced bacterial shedding (Fig. 7, G and H). We expected diarrhea to lead to weight loss, and infected mice lost nearly one-third of their body weight by 3 days of infection (Fig. 7E). Our studies revealed that Zn2+ supplementation prevented weight loss and improved survival rates in mice challenged intraperitoneally with Shigella. To visualize S. flexneri 2a within intestinal tissue from infected mice, we injected GFP-tagged bacteria intraperitoneally, and infection was allowed to proceed for 2 h. Confocal microscopy images revealed distribution of GFP-tagged S. flexneri 2a along intestinal villi. GFP was detected mostly within villi as well as at the bases in small intestine, consistent with mucosal colonization (Fig. 7G, middle).
Zn2+ Nullified Increased Intestinal Permeability and Paracellular Transport Dysfunction in Shigella-Infected Mice
The role of Zn2+ supplementation on epithelial barrier disruption was studied by electrophysiological observations on colon of Shigella-challenged mice. Barrier function of the mucosal epithelium was assessed in Ussing chamber studies. Shigella-infected colon showed significant loss of electrical resistance (103 ± 7.16 vs. 326 ± 9.98 Ω·cm2). However, Zn2+-supplemented colon after Shigella infection showed no significant decrease in TER (Fig. 7I), consistent with restoration of epithelial barrier properties.
DISCUSSION
We (17, 18) established previously that Zn2+ inhibits secretory diarrhea and provides substantial benefit by inhibiting Cl− secretion and stimulating sodium absorption in intestinal epithelial cells. We have shown here that Zn2+ protects against disruption of the intestinal TJ barrier that could conceivably modulate immune and inflammatory responses to shigellosis in a way that is beneficial to the host. There are three major findings presented by our study relevant for Zn2+-mediated modulation of the TJ barrier to attenuate intestinal inflammation. First, our results indicate that infection of enterocytes with Shigella changes the phosphorylation status of claudin-2 and -4, leading to its disengagement from the TJ complex. These observations, together with the associated disruption, contribute to altering paracellular charge selectivity and permeability to ions and macromolecules. We further demonstrate that Zn2+ restores this impaired paracellular transport activity by influencing the phosphorylation status of claudins-2 and -4. Second, an ERK1/2-dependent phosphorylation at serine/threonine residues is essential for the pathogen to modulate the physiological behavior of claudin-2 and -4. Zn2+ treatment inhibits phosphorylation of ERK1/2 that leads to prevent the disengagement of claudin-2 and -4 by differences in phosphorylation states. Third, Zn2+ counteracted IL-6 and IL-8 levels, the major cytokines that are produced in substantially higher amounts in Shigella-infected T84 cell monolayers. The cytokine levels are correlated with the intestinal barrier defect, in concert with decreased Na+ cation permeability over Cl− anion and increase in transepithelial flux of small-sized molecule of 4-kDa. Our results indicated that ERK1/2 inactivation by Zn2+ attenuates IL-6 and, IL-8 overproduction to limit inflammation.
Ion selectivity plays an integral role in determining the net transport through the paracellular pathway, and thus, altered ion selectivity implicates change in TJ functions (44). Numerous studies indicate the predominant role of claudins to determine the permeability and charge selectivity of the paracellular pathway to small ions (2, 37, 46). We considered whether Shigella infection simply disrupted TJ integrity and then paracellular permeability to ions would be disturbed. Indeed, this was the case that the permeability ratio (expressed as PNa/PCl) was decreased; conversely, PCl−/PNa+ was increased in Shigella-infected cells compared with the uninfected cells (Fig. 1C, middle and right). In the intestine, claudin-1, -3, -4, -5, and -8 tighten TJ (decrease paracellular permeability), whereas claudin-2 forms charge-selective paracellular pores. We speculated that internalization of claudin-2 and -4 was important and was closely associated with Shigella-induced barrier loss. In fact, we observed derangement of both claudin-2 and -4 distributions throughout T84 cell monolayers infected with Shigella. This correlated with an increased flux of 4-kDa dextran, decreased TER, and dilution potential along with Na+ permeability. Our results indicate that Shigella infection induces disruption of TJ complex, resulting in internalization of claudin-2 and -4 that may be central to Shigella-mediated barrier loss. The electron microscopy data support this observation by demonstrating a poorly visualized TJ in the Shigella-infected T84 monolayers compared with the Zn2+-supplemented group. Claudin-2 was shown to mediate water permeability in cultured cell monolayers (34) and is one of the main claudins responsible for paracellular transport of Na+. We found a reduction of both water permeability and Na+ selectivity across T84 monolayers infected with Shigella, which could arguably have been due to the primary defect in claudin-2 localization. This is because Shigella infection caused a derangement of claudin-2 distribution throughout the monolayers and was detected mostly in the cytosolic fraction at 6 h postinfection, as is evident from our immunolocalization and Western blotting analysis (Fig. 4, A and C). Relevant to our observation is a study by Muto et al. (29), demonstrating a significant decrease in net Na+ and water reabsorption in the kidney proximal tubule of claudin-2 knockout mice. Claudin-4 is a known determinant of paracellular sealing (45) and might be necessary and responsible for epithelial paracellular transport by altering TER and solute permeability. Our observation that Shigella infection caused a reduction of TER that coincided with the increase of 4-kDa dextran permeability, a measure of barrier dysfunction. qRT-PCR revealed the highest expression of claudin-4 than other claudins examined in T84 cell monolayers in our study, which correlated with the highest TER in T84 monolayers under basal condition, indicating that claudin-4 may have a tendency for TER of TJ. It is not surprising to conclude that claudin-4 is a powerful effector of paracellular sealing, as uncharged solute permeability supported by immunofluorescence microscopy with anti-claudin-4 revealed that Shigella infection began to be distributed in the cytoplasm with concomitant disappearance from junctional complex. Furthermore, disintegration of claudin-4 upon Shigella infection elevated the permeation of Cl− (Fig. 1C, right) and enhanced the predisposition of the TJ for Cl−. Taking all together, we conclude that most pronounced effects of Shigella infection on TJ proteins involve a disintegration of claudin-2 and -4 that is accompanied by compromised functional barrier properties, including paracellular sealing, size, and charge selectivity of the paracellular permeability of intestinal epithelium. What is being emphasized here is that claudin-2 and -4 are more susceptible to Shigella infection than uninfected T84 cell monolayers.
The permeability of the paracellular pathway can be modulated by transcellular transport processes. Transcellular Cl− secretion is the principal determinant of luminal hydration. With Cl− secretion, paracellular movement of Na+ follows. The resulting accumulation of luminal NaCl provides an osmotic gradient for the diffusion of water. Cl− secretion involves the concerted effort of several transporters. The apically located cAMP-dependent CFTR is responsible for the majority of apical Cl− secretion. Therefore, it is important to address the issue whether Shigella infection caused dehydration of intestinal lumen by altering transcellular and paracellular transport function to facilitate enteric infection. These were directly tested by electrophysiological measurement of transcellular Cl− transport, osmotically driven water transport, and dilution potential together with paracellular permeability in intestinal T84 cells, as presented in Figs. 1 and 3. We have shown that the decrease of Cl− transport, accompanied by a decrease in water transport and dilution potential together with paracellular permeability to Na+, might lead to dehydration of the mucus layer and provide a nidus for bacterial colonization to induce inflammation, thus aggravating severe mucosal damage. Consistent with the decrease in FSK- and CCH-stimulated transcellular Cl− transport (Figs. 3, B, C, and E, and 4D), Shigella infection caused an elevation of basal absorption that turned from secretion (Fig. 3D), leading to additional dehydration. Although we have not pinpointed whether the altered basal absorption was due to altered function of epithelial sodium channels, previous findings have demonstrated inhibited electroneutral NaCl absorption due to Shiga toxin purified from S. dysenteriae 1 (21). We have given a simple explanation as to why this could be the case to correlate dysfunctional ion transport and promotion of infection. Our studies do not establish how Shigella infection diminished transcellular transport of Cl−, but one possibility could be due to loss of apical localization of major Cl−-transporting protein CFTR, as evident from our immunolocalization study presented in Fig. 4D (22). Our observation suggests that Shigella infection might disrupt CFTR localization, thus decreasing the amount of CFTR in the apical membrane, decreasing Cl− secretion. This could explain why Cl− secretion was significantly reduced in T84 cells infected with Shigella by FSK stimulation. We conclude that elevation of basal absorption turning from secretion, as either a direct or indirect consequence of the lack of CFTR function, may be due to a constitutive increase of absorptive machinery that remains to be identified. Our results demonstrated that Zn2+ was not only able to rescue the reduced dilution potential but also reversed the baseline cation-selective paracellular pathway and transcellular transport of Cl−, dysregulated by Shigella infection. While these findings reflect the results of the passive transport of Na+, they also indicate that the TJ protein responsible for transport of cation and preservation of barrier tightening had recovered.
To study the mechanistic insight of the protective role of Zn2+ on altered TJ protein in more detail, we investigated the link between claudin-2 and -4 and their modulation in Shigella-infected barrier integrity. Here, the data demonstrated a molecular link between intracellular TJ disassembly and changes in the phosphorylation status of claudin-2 and -4, leading to its disengagement from the TJ complex to contribute to the impairment of the epithelial fence and inflammation. The mRNA expression and protein levels, as well as cellular distribution of claudin-2 and -4, were measured in T84 cells following Shigella exposure. Transcriptional analysis of infected cells revealed no modulation of gene expression by virulent Shigella alone; however, in the presence of Zn2+, expression levels of claudin-2 and -4 in Shigella-infected cells were upregulated, although the levels of claudin-1 and tricellulin remained unaltered, indicating that Zn2+ can regulate transcription factors for claudin-2 and -4. At present, we have no way to distinguish what signals are involved in the increase in claudin-2 and -4 mRNA. To our knowledge, this is the first report of changes in these transcript levels in response to zinc. However, changes in mRNA and protein level do not correlate, perhaps due to the regulation control at different levels. Further studies are necessary to address which transcription regulator(s) mediates the effects of Zn2+ on claudin-2 and -4 expressions. In Fig. 4B, Zn2+ markedly increases claudin 2 mRNA over control, but it was not reflected in protein expression. One possible explanation could be the fact that all mRNAs that are transcribed are not translated; thus, the number of mRNA copies does not necessarily reflect the number of functional protein molecules (14, 15). Here, we also provide the first evidence that barrier functions, as well as paracellular water transport, were affected due to Shigella infection. Zn2+ treatment on infected enterocytes modulated this Shigella-mediated perturbed water transport by regulating the paracellular water channel claudin-2.
A dysfunctional epithelial cell barrier may be one of the predisposing factors leading to inflammation. Our results indicate that infection of T84 cells with Shigella elevated the secretion of IL-6 and IL-8 in culture medium at concentrations ranges from 20 to 160 pg/ml, respectively. Interestingly, in the presence of Zn2+ these effects were attenuated. Our data do not establish a role for IL-6 or IL-8 in mediating barrier dysfunction of Shigella infection. However, the elevation of IL-6 and -8 levels and claudin-2 and -4 disintegrations from TJ complex in infection and increased ERK phosphorylation may suggest that the two could be linked. MAPK pathways were linked with interleukin secretion and barrier breaching with variable effects ranging from downregulation to upregulation of TJ proteins (1, 4, 13, 28, 32). We suspect that a possible mechanism could be the MAPK-mediated regulation of TJ function interrupted by Zn2+ to mislead bacterial signaling to disrupt barrier function. Here, we found that infection with Shigella leads to pronounced phosphorylation of ERK, whereas treatment with Zn2+ reversed the phosphorylation level to the basal state (Fig. 6E). The Shigella-infected cells that possess upregulated MAPK activity exhibited altered claudin-2 and -4 distributions and decreased barrier properties, suggesting that activation of MAPK, which was assessed by phosphorylated ERK levels, seems to be required for disruption of TJ barrier properties. Indeed, TER and paracellular permeability of the monolayers returned to basal levels after treatment with Zn2+ or PD98059. In addition, we found that inhibition of the MAPK/ERK1/2 pathway with PD98059 triggered redistribution of claudin-2 and -4 to the TJ levels. This is comparable to the effect of Zn2+ and correlated with the apparent increase and decrease of serine/threonine phosphorylation of claudin-2 and -4, respectively, along with their basal staining patterns at the cell border. These observations indicate that the MAPK/ERK signaling pathway is a determining factor for Zn2+-mediated protection of intestinal TJ and barrier properties against Shigella infection. On the basis of these results, we propose a model in Fig. 7J, schematically representing the potential mechanism by which Zn2+ protects against the disruption of intestinal TJ barrier and development of intestinal inflammation during Shigella infection.
Finally, we sought to use an in vivo pathophysiologically relevant model of shigellosis to determine potential efficacy, target engagement of Zn2+, and key pathophysiological events that link intestinal barrier dysfunction and to determine whether Zn2+ significantly abrogated Shigella-induced intestinal pathogenesis. C57BL/6 and BALB/c mice, following intraperitoneal challenge with virulent S. flexneri 2a, displayed acute and severe diarrhea with symptoms that included body weight loss, shortened colon length, and a greatly reduced survival rate of 80% at day 3 after infection, as observed (47). In contrast, when these mice were orally gavaged with Zn2+ 2 h postinfection after the onset of diarrhea, there was increase in electrical resistance and restricted paracellular permeability to ions and noncharged macromolecules (data not shown), along with normalization of transcellular transport of basal as well as cAMP-stimulated Cl− secretion and significantly reduced diarrheal scores, suggesting the role of Zn2+ in intestinal fluid and electrolyte balance and intestinal barrier integrity. More interestingly, Zn2+-treated mice resulted in increased survival compared with the untreated group. All together, these findings suggest that Zn2+ treatment can improve intestinal barrier integrity in shigellosis, resulting in increased survival.
In conclusion, our study has put forth an insight to the protective mechanism of Zn2+ to attune the pathogenesis of Shigella both in an in vitro T84 cell model and in an in vivo murine model. We identified that ERK phosphorylation by Shigella infection is the determining factor to remove claudin-2 and -4 from TJ complex to alter barrier function. However, for claudin-2 and -4, redistribution back to the plasma membrane occurs only when Zn2+ is present, thus accounting for its therapeutic efficacy in acute inflammatory diarrhea.
GRANTS
We gratefully acknowledge financial support, in part, by the Government of India, Ministry of Science and Technology, Department of Biotechnology Grant BT/PR6462/FNS/20/669/2012 and Ramalingaswami Grant BT/HD/35/02/07/2009 (to K. M. Hoque). P. Sarkar was supported by a Dept. of Science & Technology, Govt. of India (DST)-INSPIRE fellowship, and I. A. Sheikh, J. Aoun, and T. Saha were supported by Indian Council of Medical Research, Govt. of India, and University Grant Commission Fellowships, respectively. We gratefully acknowledge the financial support, in part, by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-104791 9 (to V. M. Rajendran).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.M.H. conceived and designed research; P.S., T.S., I.A.S., S.C., and J.A. performed experiments; P.S., I.A.S., S.C., and K.M.H. analyzed data; P.S., V.M.R., and K.M.H. interpreted results of experiments; P.S., T.S., J.A., and K.M.H. prepared figures; P.S. and K.M.H. drafted manuscript; M.K.C., V.M.R., N.A.A., S.D., and K.M.H. edited and revised manuscript; P.S., T.S., I.A.S., S.C., J.A., M.K.C., V.M.R., N.A.A., and K.M.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Arpita Sarbajna and Bivash Ranjan Mallick, National Institute of Cholera, and Enteric Diseases electron microscope technicians for help in carrying out electron microscopy experiments. This work is in partial fulfillment of the PhD degree for P. Sarkar.
Present address of I. A. Sheikh: Dept. of Pediatrics, Univ. of Arizona, Tucson, AZ.
REFERENCES
- 1.Aggarwal S, Suzuki T, Taylor WL, Bhargava A, Rao RK. Contrasting effects of ERK on tight junction integrity in differentiated and under-differentiated Caco-2 cell monolayers. Biochem J 433: 51–63, 2011. doi: 10.1042/BJ20100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angelow S, Yu ASL. Claudins and paracellular transport: an update. Curr Opin Nephrol Hypertens 16: 459–464, 2007. doi: 10.1097/MNH.0b013e32820ac97d. [DOI] [PubMed] [Google Scholar]
- 3.Aoun J, Hayashi M, Sheikh IA, Sarkar P, Saha T, Ghosh P, Bhowmick R, Ghosh D, Chatterjee T, Chakrabarti P, Chakrabarti MK, Hoque KM. Anoctamin 6 contributes to Cl− secretion in accessory cholera enterotoxin (Ace)-stimulated diarrhea: an essential role for phosphatidylinositol 4,5-bisphosphate (PIP2) signaling in cholera. J Biol Chem 291: 26816–26836, 2016. doi: 10.1074/jbc.M116.719823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basuroy S, Seth A, Elias B, Naren AP, Rao R. MAPK interacts with occludin and mediates EGF-induced prevention of tight junction disruption by hydrogen peroxide. Biochem J 393: 69–77, 2006. doi: 10.1042/BJ20050959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatnagar S, Bahl R, Sharma PK, Kumar GT, Saxena SK, Bhan MK. Zinc with oral rehydration therapy reduces stool output and duration of diarrhea in hospitalized children: a randomized controlled trial. J Pediatr Gastroenterol Nutr 38: 34–40, 2004. doi: 10.1097/00005176-200401000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Bhutta ZA, Bird SM, Black RE, Brown KH, Gardner JM, Hidayat A, Khatun F, Martorell R, Ninh NX, Penny ME, Rosado JL, Roy SK, Ruel M, Sazawal S, Shankar A. Therapeutic effects of oral zinc in acute and persistent diarrhea in children in developing countries: pooled analysis of randomized controlled trials. Am J Clin Nutr 72: 1516–1522, 2000. doi: 10.1093/ajcn/72.6.1516. [DOI] [PubMed] [Google Scholar]
- 7.Canani RB, Cirillo P, Buccigrossi V, Ruotolo S, Passariello A, De Luca P, Porcaro F, De Marco G, Guarino A. Zinc inhibits cholera toxin-induced, but not Escherichia coli heat-stable enterotoxin-induced, ion secretion in human enterocytes. J Infect Dis 191: 1072–1077, 2005. doi: 10.1086/428504. [DOI] [PubMed] [Google Scholar]
- 8.Canani RB, Ruotolo S, Buccigrossi V, Passariello A, Porcaro F, Siani MC, Guarino A. Zinc fights diarrhoea in HIV-1-infected children: in-vitro evidence to link clinical data and pathophysiological mechanism. AIDS 21: 108–110, 2007. doi: 10.1097/QAD.0b013e328011849a. [DOI] [PubMed] [Google Scholar]
- 9.Chakrabarti MK, Haque KM, Chakrabarty M, Mahalanabis D. Effect of reducing sodium or glucose concentration in a hypo-osmolar ORS (oral rehydration salts) on absorption efficiency: marker perfusion study in rat jejunum. Dig Dis Sci 50: 241–245, 2005. doi: 10.1007/s10620-005-1589-x. [DOI] [PubMed] [Google Scholar]
- 10.Cong X, Zhang Y, Li J, Mei M, Ding C, Xiang R-L, Zhang L-W, Wang Y, Wu L-L, Yu G-Y. Claudin-4 is required for modulation of paracellular permeability by muscarinic acetylcholine receptor in epithelial cells. J Cell Sci 128: 2271–2286, 2015. doi: 10.1242/jcs.165878. [DOI] [PubMed] [Google Scholar]
- 11.Czerucka D, Dahan S, Mograbi B, Rossi B, Rampal P. Implication of mitogen-activated protein kinases in T84 cell responses to enteropathogenic Escherichia coli infection. Infect Immun 69: 1298–1305, 2001. doi: 10.1128/IAI.69.3.1298-1305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gill CJ, Thea DM, Hibberd P. Diarrhoeal disease trends in the GBD 2015 study: optimism tempered by scepticism. Lancet Infect Dis 17: 884–885, 2017. doi: 10.1016/S1473-3099(17)30336-5. [DOI] [PubMed] [Google Scholar]
- 13.González-Mariscal L, Tapia R, Chamorro D. Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta 1778: 729–756, 2008. doi: 10.1016/j.bbamem.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 14.Greenbaum D, Colangelo C, Williams K, Gerstein M. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol 4: 117, 2003. doi: 10.1186/gb-2003-4-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gygi SP, Rochon Y, Franza BR, Aebersold R. Correlation between protein and mRNA abundance in yeast. Mol Cell Biol 19: 1720–1730, 1999. doi: 10.1128/MCB.19.3.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoque KM, Binder HJ. Zinc in the treatment of acute diarrhea: current status and assessment. Gastroenterology 130: 2201–2205, 2006. doi: 10.1053/j.gastro.2006.02.062. [DOI] [PubMed] [Google Scholar]
- 17.Hoque KM, Rajendran VM, Binder HJ. Zinc inhibits cAMP-stimulated Cl secretion via basolateral K-channel blockade in rat ileum. Am J Physiol Gastrointest Liver Physiol 288: G956–G963, 2005. doi: 10.1152/ajpgi.00441.2004. [DOI] [PubMed] [Google Scholar]
- 18.Hoque KM, Sarker R, Guggino SE, Tse CM. A new insight into pathophysiological mechanisms of zinc in diarrhea. Ann N Y Acad Sci 1165: 279–284, 2009. doi: 10.1111/j.1749-6632.2009.04442.x. [DOI] [PubMed] [Google Scholar]
- 19.Hoque KM, Woodward OM, van Rossum DB, Zachos NC, Chen L, Leung GPH, Guggino WB, Guggino SE, Tse C-M. Epac1 mediates protein kinase A-independent mechanism of forskolin-activated intestinal chloride secretion. J Gen Physiol 135: 43–58, 2010. doi: 10.1085/jgp.200910339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kahle KT, Macgregor GG, Wilson FH, Van Hoek AN, Brown D, Ardito T, Kashgarian M, Giebisch G, Hebert SC, Boulpaep EL, Lifton RP. Paracellular Cl− permeability is regulated by WNK4 kinase: insight into normal physiology and hypertension. Proc Natl Acad Sci USA 101: 14877–14882, 2004. doi: 10.1073/pnas.0406172101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kandel G, Donohue-Rolfe A, Donowitz M, Keusch GT. Pathogenesis of Shigella diarrhea. XVI. Selective targetting of Shiga toxin to villus cells of rabbit jejunum explains the effect of the toxin on intestinal electrolyte transport. J Clin Invest 84: 1509–1517, 1989. doi: 10.1172/JCI114327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klumperman J, Boekestijn JC, Mulder AM, Fransen JA, Ginsel LA. Intracellular localization and endocytosis of brush border enzymes in the enterocyte-like cell line Caco-2. Eur J Cell Biol 54: 76–84, 1991. [PubMed] [Google Scholar]
- 23.Köhler H, Rodrigues SP, McCormick BA. Shigella flexneri interactions with the basolateral membrane domain of polarized model intestinal epithelium: role of lipopolysaccharide in cell invasion and in activation of the mitogen-activated protein kinase ERK. Infect Immun 70: 1150–1158, 2002. doi: 10.1128/IAI.70.3.1150-1158.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acácio S, Biswas K, O’Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382: 209–222, 2013. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 25.Kotloff KL, Winickoff JP, Ivanoff B, Clemens JD, Swerdlow DL, Sansonetti PJ, Adak GK, Levine MM. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull World Health Organ 77: 651–666, 1999. [PMC free article] [PubMed] [Google Scholar]
- 26.Li D, Mrsny RJ. Oncogenic Raf-1 disrupts epithelial tight junctions via downregulation of occludin. J Cell Biol 148: 791–800, 2000. doi: 10.1083/jcb.148.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18: 268–281, 2012. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 28.Mitic LL, Anderson JM. Molecular architecture of tight junctions. Annu Rev Physiol 60: 121–142, 1998. doi: 10.1146/annurev.physiol.60.1.121. [DOI] [PubMed] [Google Scholar]
- 29.Muto S, Hata M, Taniguchi J, Tsuruoka S, Moriwaki K, Saitou M, Furuse K, Sasaki H, Fujimura A, Imai M, Kusano E, Tsukita S, Furuse M. Claudin-2-deficient mice are defective in the leaky and cation-selective paracellular permeability properties of renal proximal tubules. Proc Natl Acad Sci USA 107: 8011–8016, 2010. doi: 10.1073/pnas.0912901107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phalipon A, Sansonetti PJ. Shigella’s ways of manipulating the host intestinal innate and adaptive immune system: a tool box for survival? Immunol Cell Biol 85: 119–129, 2007. doi: 10.1038/sj.icb7100025. [DOI] [PubMed] [Google Scholar]
- 31.Philpott DJ, Edgeworth JD, Sansonetti PJ. The pathogenesis of Shigella flexneri infection: lessons from in vitro and in vivo studies. Philos Trans R Soc Lond B Biol Sci 355: 575–586, 2000. doi: 10.1098/rstb.2000.0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinton P, Braicu C, Nougayrede JP, Laffitte J, Taranu I, Oswald IP. Deoxynivalenol impairs porcine intestinal barrier function and decreases the protein expression of claudin-4 t hough a mitogen-activated protein kinase-dependent mechanism. J Nutr 140: 1956–1962, 2010. doi: 10.3945/jn.110.123919. [DOI] [PubMed] [Google Scholar]
- 33.Pore D, Chowdhury P, Mahata N, Pal A, Yamasaki S, Mahalanabis D, Chakrabarti MK. Purification and characterization of an immunogenic outer membrane protein of Shigella flexneri 2a. Vaccine 27: 5855–5864, 2009. doi: 10.1016/j.vaccine.2009.07.054. [DOI] [PubMed] [Google Scholar]
- 34.Rosenthal R, Milatz S, Krug SM, Oelrich B, Schulzke J-D, Amasheh S, Günzel D, Fromm M. Claudin-2, a component of the tight junction, forms a paracellular water channel. J Cell Sci 123: 1913–1921, 2010. doi: 10.1242/jcs.060665. [DOI] [PubMed] [Google Scholar]
- 35.Samak G, Aggarwal S, Rao RK. ERK is involved in EGF-mediated protection of tight junctions, but not adherens junctions, in acetaldehyde-treated Caco-2 cell monolayers. Am J Physiol Gastrointest Liver Physiol 301: G50–G59, 2011. doi: 10.1152/ajpgi.00494.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakaguchi T, Köhler H, Gu X, McCormick BA, Reinecker HC. Shigella flexneri regulates tight junction-associated proteins in human intestinal epithelial cells. Cell Microbiol 4: 367–381, 2002. doi: 10.1046/j.1462-5822.2002.00197.x. [DOI] [PubMed] [Google Scholar]
- 37.Amasheh S, Meiri N, Gitter AH, Schöneberg T, Mankertz J, Schulzke JD, Fromm M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci 115: 4969–4976, 2002. doi: 10.1242/jcs.00165. [DOI] [PubMed] [Google Scholar]
- 38.Sazawal S, Black RE, Bhan MK, Bhandari N, Sinha A, Jalla S. Zinc supplementation in young children with acute diarrhea in India. N Engl J Med 333: 839–844, 1995. doi: 10.1056/NEJM199509283331304. [DOI] [PubMed] [Google Scholar]
- 39.Schedl HP. Use of polyethylene glycol and phenol red as unabsorbed indicators for intestinal absorption studies in man. Gut 7: 159–163, 1966. doi: 10.1136/gut.7.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sengupta TK, Schmitt EM, Ivashkiv LB. Inhibition of cytokines and JAK-STAT activation by distinct signaling pathways. Proc Natl Acad Sci USA 93: 9499–9504, 1996. doi: 10.1073/pnas.93.18.9499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sengupta TK, Talbot ES, Scherle PA, Ivashkiv LB. Rapid inhibition of interleukin-6 signaling and Stat3 activation mediated by mitogen-activated protein kinases. Proc Natl Acad Sci USA 95: 11107–11112, 1998. doi: 10.1073/pnas.95.19.11107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sheikh IA, Koley H, Chakrabarti MK, Hoque KM. The Epac1 signaling pathway regulates Cl− secretion via modulation of apical KCNN4c channels in diarrhea. J Biol Chem 288: 20404–20415, 2013. doi: 10.1074/jbc.M113.467860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ulyatt MJ. The use of polyethylene glycol as a marker for measuring rumen water volume and the rate of flow of water from the rumen of grazing sheep. N Z J Agric Res 7: 713–722, 1964. doi: 10.1080/00288233.1964.10416400. [DOI] [Google Scholar]
- 44.Van Itallie CM, Anderson JM. Measuring size-dependent permeability of the tight junction using PEG profiling. Methods Mol Biol 762: 1–11, 2011. doi: 10.1007/978-1-61779-185-7_1. [DOI] [PubMed] [Google Scholar]
- 45.Van Itallie CM, Fanning AS, Anderson JM. Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins. Am J Physiol Renal Physiol 285: F1078–F1084, 2003. doi: 10.1152/ajprenal.00116.2003. [DOI] [PubMed] [Google Scholar]
- 46.Van Itallie C, Rahner C, Anderson JM. Regulated expression of claudin-4 decreases paracellular conductance t hough a selective decrease in sodium permeability. J Clin Invest 107: 1319–1327, 2001. doi: 10.1172/JCI12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang JY, Lee SN, Chang SY, Ko HJ, Ryu S, Kweon MN. A mouse model of shigellosis by intraperitoneal infection. J Infect Dis 209: 203–215, 2014. doi: 10.1093/infdis/jit399. [DOI] [PubMed] [Google Scholar]