Abstract
Respiratory syncytial virus (RSV) is a major cause of hospitalization for infants and young children worldwide. RSV is known to infect epithelial cells and increase the permeability of model airway epithelial monolayers in vitro. We hypothesized that RSV infection also induces airway barrier dysfunction in vivo. C57BL/6 mice were intranasally inoculated with RSV, and on day 4 post-inoculation were examined for viral replication, lung inflammation, and barrier integrity as well as the structure and molecular composition of epithelial junctions. In parallel, primary mouse tracheal epithelial cells (mTEC) were cultured for in vitro studies. RSV-infected mice lost weight and showed significant peribronchial inflammation compared with noninfected controls and UV-inactivated RSV-inoculated animals. RSV infection increased the permeability of the airway epithelial barrier and altered the molecular composition of epithelial tight junctions. The observed RSV-induced barrier disruption was accompanied by decreased expression of several tight-junction proteins and accumulation of cleaved extracellular fragments of E-cadherin in bronchoalveolar lavage and mTEC supernatants. Similarly, in vitro RSV infection of mTEC monolayers resulted in enhanced permeability and disruption of tight-junction structure. Furthermore, incubation of mTEC monolayers with a recombinant fragment of E-cadherin caused tight-junction disassembly. Taken together, these data indicate that RSV infection leads to airway barrier dysfunction in vivo, mediated by either decreased expression or cleavage of junctional proteins. Our observations provide further insights into the pathophysiology of RSV infection and provide a rationale for development of barrier-protecting agents to alleviate the pathogenic effects of RSV infection.
Keywords: adherens junctions, E-cadherin, epithelial barrier, inflammation, RSV, tight junctions
INTRODUCTION
It is estimated that each year as many as 126,000 infants are hospitalized in the United States due to lower respiratory tract infections or bronchiolitis. Respiratory syncytial virus (RSV) is the leading cause of lower respiratory tract infections worldwide, and strong associations between infection with RSV, persistent wheezing, and childhood asthma exist (36, 43, 51). The underlying pathogenic mechanisms, however, are not well understood. RSV is known to infect human, rat, and mouse airways, trigger mucosal inflammation (2, 23, 37, 38, 66), and induce production of proinflammatory cytokines, such as interleukin (IL)-6, IL-8, and granulocyte macrophage colony-stimulating factor, in human bronchial epithelial cells (34).
Dysfunction of the airway epithelial barrier is an emerging mechanism that may significantly contribute to the pathogenesis of respiratory viral infections (6, 13, 49, 52). Importantly, our previous in vitro studies demonstrated that RSV infection potently disrupts the integrity of the model human airway epithelial barrier (40, 42). Such barrier dysfunction could enhance sampling of luminal antigens by intraepithelial dendritic cells, leading to augmented immune responses and airway inflammation (12). In addition, barrier disruption may facilitate translocation of inhaled particles, allergens, and bacteria through the lung, potentially a risk factor for persistent wheeze later in life (49). Mouse models have been extensively used to study the effect of RSV in inflammation and immunity (8, 29, 58), but the effects of RSV on the structure and permeability of the airway epithelial barrier in vivo remain poorly characterized. There are no established animal models to investigate the pathophysiological mechanisms of the airway epithelial barrier disruption during RSV infection.
The integrity of the airway epithelial barrier is regulated by several mechanisms, among which assembly of the epithelial apical junctional complex (AJC) is of particular importance. AJC are composed of apically located tight junctions (TJs) and underlying adherens junctions (AJs) (60), both containing a large number of adhesion, scaffolding, signaling, and cytoskeletal proteins. TJs seal the paracellular space, limiting the passage of ions and uncharged solutes, whereas AJs are responsible for the initiation and maintenance of epithelial cell-cell contacts, enabling TJ assembly (12, 41). The adhesive properties of TJs are determined by three major types of transmembrane proteins: 1) members of the claudin family, 2) the TJ-associated MARVEL proteins (TAMP) family, which includes occludin, tricellulin, and Marvel D3, and 3) immunoglobulin-like proteins, which include junctional adhesion molecule A (JAM-A) and coxsackievirus and adenovirus receptor (CAR) (12, 41). Two major types of transmembrane adhesion proteins, E-cadherin, and nectins, are involved in the formation of epithelial AJs (18, 33). The cytoplasmic side of TJs is organized by multifunctional scaffolding proteins of the zonula occludens (ZO) family, whereas β-catenin, α-catenin, and p120 catenin form a complex with the cytoplasmic domain of E-cadherin. Cadherins mediate several physiological processes, including cell-cell adhesion and cell signaling, proliferation, and differentiation (48).
A number of previous in vitro studies suggest that bacterial and viral pathogens, allergens, and proinflammatory mediators disrupt the integrity of the airway epithelial barrier by decreasing expression of various AJC proteins (41, 47, 53). However, little is known about the changes in structure and composition of the epithelial AJC during pulmonary inflammation in vivo. The overall objective of this study was to establish a murine model to examine the effects of RSV infection on the structure and function of the airway epithelial barrier in vivo.
MATERIALS AND METHODS
Antibodies and chemicals.
The following primary monoclonal antibodies (mAbs) and polyclonal antibodies (pAbs) were used to detect junctional and signaling proteins by immunofluorescence labeling and immunoblotting: anti-occludin mAb, anti-ZO-1 pAb, anti-claudin-18 mAb (Thermo-Fisher Scientific, Waltham, MA); anti-claudin-1 pAb, anti-claudin-2 pAb, anti-GAPDH mAb (Abcam, Cambridge, MA); anti-β-catenin mAb, anti-E-cadherin mAb (BD Bioscience, San Jose, CA); and anti-soluble E-cadherin mAb (Santa Cruz Biotechnology, Dallas, TX). Anti-rabbit and anti-mouse secondary antibodies conjugated to Alexa 488 or Alexa 568 dyes were obtained from Thermo-Fisher Scientific. Mouse and rabbit secondary horseradish peroxidase-conjugated antibodies were purchased from GE Healthcare (Pittsburgh, PA). Recombinant human extracellular E-cadherin fragment was purchased from R&D Systems (Minneapolis, MN).
Virus.
Wild-type RSV strain A2 (RSV A2) stocks were grown as previously described (40). rgRSV244 (RSV derived from RSV A2 expressing the green fluorescent protein gene) was a kind gift from Mark Peeples (Nationwide Children’s Hospital Research Institute, Columbus, OH) and Peter Collins (National Institutes of Health, Bethesda, MD) (15). Ultraviolet (UV)-inactivated RSV (UV-RSV) was used as a negative control. UV inactivation was performed by exposure of RSV to UVB irradiation for 20 min. Inactivation of RSV replication was confirmed by a plaque-forming assay, as described below in Plaque-forming assay.
Animals.
C57BL/6 mice of both sexes (6–8 wk of age) were obtained from Jackson Laboratories (Bar Harbor, ME). The animals were inoculated intranasally with RSV A2 (3 × 105 to 9 × 107 pfu), UV-RSV, or an equal volume of supernatant from uninfected HEp-2 cells on day 0. Mice were weighed daily and euthanized on day 4 post-inoculation. This time point, which corresponded to the peak of RSV replication, was chosen according to previous studies (17, 55). Lungs were harvested for immunohistochemistry (IHC), hematoxylin and eosin (H&E) staining, plaque forming assay, and Western blot analysis. Five or six mice were used per group, and experiments were repeated three times. All procedures used in this study adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were reviewed and approved by the Institutional Animal Care and Use Committee of the Lerner Research Institute at the Cleveland Clinic.
Histopathology.
Sections of formalin-fixed, paraffin-embedded inflated lungs were stained with H&E (Leica Biosystems, Buffalo Grove, IL) according to the manufacturer’s protocol and examined using light microscopy. The slides were blinded, and the extent of pathology involving bronchioles, peribronchioles, and perivascular tissue was evaluated by analyzing five categories, similar to previously used semiquantitative, multiparametric scoring systems used for assessing pulmonary disease (1, 7, 14, 20, 30) (Table 1): 1) percentage of sites with peribronchiolar/peribronchial inflammation (0–3), 2) peribronchioles/peribronchial infiltrates (0–4), 3) percentage of sites with perivascular inflammation (0–3), 4) bronchiolar/bronchial luminal exudate (0–2), and 5) percentage of infiltrated alveoli areas (0–3). A total of 20 perivascular and peribronchial spaces per lung were counted for each animal.
Table 1.
Lung inflammatory response was assessed by evaluating 5 parameters (0–15)
| I. Percentage of sites with peribronchiolar/peribronchial inflammation |
| 0) Absence of peristructial inflammatory cells |
| 1) <25% |
| 2) 25–50% |
| 3) >75% |
| II. Peribronchioles/peribronchial infiltrates |
| 0) Absence of peristructial inflammatory cells |
| 1) Few/occasional cuffing of peristructial cells, <1layer |
| 2) Most bronchi were surrounded by a ring of inflammatory cells 1 cell deep |
| 3) Most bronchi were surrounded by a ring of inflammatory cells 2–3 cells deep |
| 4) Most bronchi were surrounded by a ring of inflammatory cells >4 cells deep |
| III. Percentage of sites with perivascular inflammation |
| 0) None |
| 1) <10% |
| 2) 10–50% |
| 3) >50% |
| IV. Bronchiolar/bronchial luminal exudate |
| 0) None |
| 1) Minimal (<25% lumen occlusion) |
| 2) Heavy (>25% lumen occluded) |
| V. Percentage of infiltrated alveoli areas |
| 0) None |
| 1) <10% |
| 2) 10–50% |
| 3) >50% |
Extraction of RNA and quantitative real-time polymerase chain reaction analysis.
Total RNA was extracted from lung tissues using an RNeasy Mini kit, automated on the Qiacube (Qiagen). Amplification and copy number determination of RSV Nucleocapsid (N) copy number was determined using a commercially available Primerdesign Genesig Kit for Respiratory Syncytial Virus Type A (RSV-A) genomes from Primer Design (Oxford, UK). According to the manufacturer, the kit is designed to have the broadest detection profile possible while remaining specific to the RSV-A genome. The kit supplies a lyophilized standard that, when reconstituted, yields a quantified concentration of N copies per microliter. The reconstituted standard is then serially diluted to generate a standard curve, which is used for quantification of N copies in the test samples. This will also identify N in transcripts and genomic RNA.
Plaque-forming assay.
Plaque-forming assay [in plaque-forming units (pfu)] was performed as previously described (3, 40, 44). Briefly, HEp-2 cells were cultured until confluent and apically inoculated with diluted lung lysate. Cells were incubated at 37°C for 1 h, after which medium was removed and replaced with methylcellulose overlay medium. On day 2, the overlay was removed, and cells were washed, followed by incubation with crystal violet fixative/stain solution. Once cells were stained and solution was removed, plates were allowed to dry, and plaques were counted and used to calculate corresponding RSV titer in plaque forming unit per gram of tissue (3, 40, 44).
In vivo FITC-dextran permeability assay.
FITC-labeled dextran (4 kDa; Sigma, St. Louis, MO) was dissolved in sterile PBS at a concentration of 5 mg/ml and administered intranasally at a dose of 10 μg/g body wt. Mice were euthanized after 1 h, and blood was collected via cardiac puncture into BD Microtainer serum separators. Blood serum was collected after centrifugation. The FITC-dextran standard curve was prepared by serial dilution of the 10 mg/ml stock solution in PBS. Florescence intensity of the experimental samples and the standards were recorded at an excitation wavelength of 485 nm and an emission wavelength of 528 nm, using a FlexStation 3 (Molecular Devices, San Jose, CA). The fluorescence intensity of a blank serum obtained from mice that did not receive FITC-dextran was subtracted from all experimental samples.
Bronchoalveolar lavage fluid collection.
Mice were euthanized and immediately intratracheally intubated. Bronchoalveolar lavage (BAL) collection was performed with instillation of PBS. Cells were removed from BAL by centrifugation for 10 min at 700 rpm. Cell pellets were used for total cell count and for leukocyte counting after Kwik-Diff staining (Pierce Thermo Scientific, Waltham, MA). Cell-free BAL samples were subjected to gel electrophoresis and immunoblotting as described in Protein electrophoresis and immunoblotting. Total protein content in BAL samples was determined using a bicinchoninic acid (BCA) protein assay (Pierce Thermo Scientific), and samples with equal amount of total protein were loaded on the gel.
Isolation of mouse tracheal epithelial cells.
Tracheas were harvested from wild-type C57BL/6 mice. Mouse tracheal epithelial cells (mTECs) were purified and seeded on semipermeable membrane filters (63, 64). Cells were kept in submerged mTEC/Plus medium until confluent and then differentiated under air-liquid interface (ALI) conditions. Cells were infected with rgRSV244 at a multiplicity of infection (MOI) of 3.0 after cells became fully confluent and well differentiated, with an average transepithelial electrical resistance (TEER) of 1,500 Ω × cm2.
In vitro barrier permeability assays.
Permeability of cultured airway epithelial monolayers was determined by measuring TEER and transepithelial flux of a tracer molecule, sodium fluorescein, as described in our previous study (40).
Primary human bronchial epithelial cells.
Primary human bronchial epithelial cells were isolated from lungs of deidentified deceased pediatric donors, all of whom had died from nonpulmonary-related events. Cells were used between passages 3–5, cultured on semipermeable Transwell inserts (Corning, Tewksbury, MA), and differentiated at the air-liquid interface as previously described (16, 44).
Ethical statement.
Human tissue was provided by the International Institute for the Advancement of Medicine (IIAM) according to the procedures approved by the Cleveland Clinic Foundation.
Immunofluorescence labeling and confocal microscopy.
Whole lung tissues were fixed in paraformaldehyde (PFA) and embedded in paraffin. Four-micrometer-thick tissue sections were deparaffinized and boiled in antigen retrieval buffer (10 mM trisodium citrate, 0.05% Tween 20) for 30 min. Tissue sections were permeabilized in 0.1% Triton X-100 in distilled H2O for 10 min, followed by blocking with 10% normal donkey serum in PBS at room temperature for 60 min. Slides were incubated overnight at 4°C with the primary antibodies, followed by incubation with Alexa fluor-labeled secondary antibodies. Nuclei were stained with DAPI (Sigma-Aldrich), and slides were mounted using ProLong Gold Antifade reagent (Invitrogen, Camarillo, CA). Model airway epithelial cell monolayers cultured on Transwell inserts were fixed with methanol, as previously described (42). The fixed cells were incubated with specific primary antibodies, followed by incubation with Alexa fluor-labeled secondary antibodies, whereas nuclei were stained with DAPI. Immunofluorescence-labeled tissue sections and cell monolayers were examined using an upright fluorescent microscope or confocal microscope (Leica Microsystems, Wetslar, Germany). Cells were visualized using a ×40 or ×63/1.4 oil objective. The images were processed using Adobe Photoshop. Images shown are representative of at least three independent experiments, with multiple images taken per slide.
Protein electrophoresis and immunoblotting.
Mouse lung tissues were homogenized in a RIPA buffer. Equal concentrations of protein from each sample were resolved on SDS-PAGE and transferred to a PVDF membrane (Bio-Rad, Hercules, CA). Membranes were blocked with nonfat dry milk and incubated overnight at 4°C with primary antibodies, followed by 1-h incubation with secondary antibodies at room temperature. Membranes were exposed to enhanced chemoluminescence reagent (GE Healthcare), and protein bands were detected using X-ray film. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a lane protein-loading control. Signals of soluble E-cadherin fragments after electrophoresis and immunoblotting of BAL samples were detected using SuperSignalWest Pico chemiluminescence solutions (Thermo-Fisher) and quantified using a MyECL imager (Thermo Scientific).
Statistical analysis.
Data were analyzed using Prism software (GraphPad, San Diego, CA) and Microsoft Excel. Data are representative of three or more experiments and are presented as means ± SE. Data were evaluated statistically with ANOVA or Student’s t-test, with Bonferroni correction for multiple comparisons. Significance was considered at a P value <0.05.
RESULTS
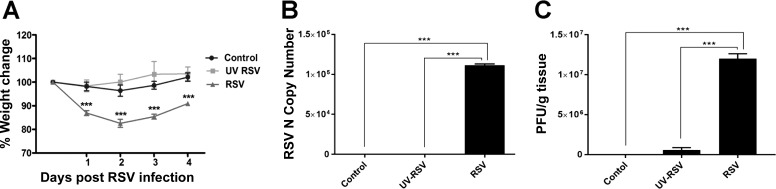
Our previous studies in cultured human airway epithelial cell monolayers demonstrated that RSV infection increases permeability of the epithelial barrier by triggering AJC disassembly (40, 42). The present study was designed to investigate whether RSV infection also results in airway barrier disruption in vivo. In initial experiments, we examined the effect of RSV infection on the body weight loss, RSV Nucleocapsid (N) copy number, and airway inflammation. Although previous studies had demonstrated that C57BL/6 mice are less susceptible to RSV compared with several other mouse strains (54, 59), the choice of this strain was dictated by our ultimate goal, to use various knockout mice to investigate the mechanisms of RSV infection in vivo. Knockout mice are predominantly available on the C57BL/6 background. Mice were intranasally inoculated with RSV A2 (3 × 105 to 9 × 107 pfu), UV-RSV, or an equal volume of supernatant from uninfected HEp-2 cells. Animal responses to RSV infection were evaluated by monitoring their body weight loss on days 1–4 post-inoculation and by histological analysis of lung slices obtained from euthanized animals on day 4 of RSV infection. We found that 4 days post-inoculation of 9 × 107 pfu RSV induced the most consistent and reproducible responses in mice. These responses were manifested as transient body weight loss and signs of pulmonary inflammation. Consistent with previous studies (5), mice infected with RSV lost 12 ± 2% body weight post-RSV inoculations, which was significantly higher than the 5 ± 2% body weight loss observed in control animals or the UV-RSV group (Fig. 1A). A quantitative RT-PCR analysis found increased mRNA expression of a specific viral N gene in lung homogenates of RSV-infected mice on day 4 post-inoculation, whereas no mRNA expression of the viral N gene was detected in control or UV-RSV inoculated mice (Fig. 1B). Consistent with these data, plaque-forming analysis demonstrated visible plaques in HEp-2 cells inoculated with diluted lung lysate from mice that were infected with live, replicating virus. By contrast, lung homogenates from control groups yielded no plaques, whereas UV-RSV group produced a negligible amount of small plaques, indicating lack of efficient viral replication (Fig. 1C).
Fig. 1.
Respiratory syncytial virus (RSV) causes body weight loss and replicates in the lung tissue of infected animals. C57BL/6 mice were infected intranasally with a 9 × 107 plaque-forming units (pfu) of RSV strain A2 or equal volume of supernatant from uninfected HEp-2 cells. A: mice were weighed daily, and percent of body weight change was calculated from body weights of mice recorded daily after infection relative to that at day 0 (before viral inoculation). B: RSV Nucleocapsid (N) copy number in lung homogenates of control and RSV-infected animals on day 4 after viral inoculation was determined by quantitative RT-PCR. C: lung homogenates of control, UV-RSV-, and live RSV-infected animals were subjected to plaque formation assay in HEp-2 cells. Data are presented as means ± SE; n ≥ 3, ***P < 0.001.
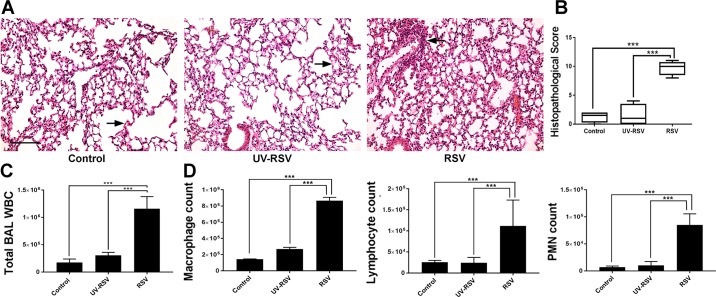
H&E staining demonstrated pulmonary inflammation in RSV-infected animals on day 4 post-inoculation, characterized by a marked peribronchial infiltration of immune cells (Fig. 2A). Histopathology scoring showed a significantly higher inflammatory response in RSV-infected animals compared with control mice or animals exposed to UV-RSV (Fig. 2B). Similar scoring systems have previously been used to semiquantitatively and multiparametrically assess pulmonary disease (1, 7, 14, 20, 30).
Fig. 2.
Respiratory syncytial virus (RSV) infection induces airway inflammation and leukocyte infiltration. A: photomicrographs of hematoxylin-eosin (H&E)-stained lung tissue section of control, UV-RSV, and live RSV-infected animals on day 4 post-inoculation. Arrow indicates histopathological changes including peribronchial inflammation and epithelial thickening. Scale bar, 40 μm. B: histopathological score of RSV-induced tissue damage/inflammation was calculated as described in Table 1. C: transmigrated leukocytes (WBC) were counted in the bronchoalveolar lavage (BAL) of control, UV-RSV, and live RSV-inoculated animals. D: different types of leukocytes were counted in BAL of control, UV-RSV, and live RSV-inoculated mice. Data are presented as means ± SE; n ≥ 3, ***P < 0.001.
Influx of leukocytes is a key indicator of airway inflammation. Previous studies have demonstrated that RSV-induced infiltration of neutrophils occurs in the early infectious stage and precedes the time point at which the highest viral load is measured in the BAL (5, 56). We found a marked increase in leukocyte count in the BAL in RSV-infected animals compared with control mice or mice inoculated with UV-RSV on day 4 post-RSV inoculation (Fig. 2, C and D). Consistent with previous reports (50), macrophages appeared to be a predominant leukocyte type in the BAL of infected animals; however, the influx of lymphocytes and neutrophils was also significantly increased in RSV-infected mice (Fig. 2D).
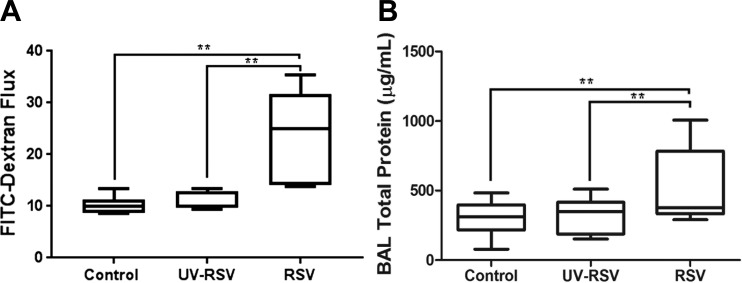
Next, we sought to determine whether RSV infection affects the integrity of the airway barrier by using two experimental approaches to evaluate barrier permeability. One approach examines the outside-in permeability by measuring transepithelial passage of an intranasally administered fluorescent tracer. The other approach determines the inside-out permeability by examining the passage of blood proteins into the bronchial lumen. To measure the outside-in permeability, mice were intranasally inoculated with either 10 μg/g FITC-dextran in PBS, or vehicle, on day 4 of viral infection. Blood was collected 1 h later, and FITC fluorescence intensity in the serum was measured. The serum level of FITC-dextran was significantly higher in RSV-infected animal as compared than in controls (Fig. 3A). BAL was collected and examined for total protein level. The total protein concentration was significantly increased in BAL of RSV-infected animals compared with control mice on day 4 post-RSV inoculation (Fig. 3B). Taken together, our data suggest that RSV infection increases airway permeability, enabling a bidirectional flux of large molecules and cells across the pulmonary barrier.
Fig. 3.
Respiratory syncytial virus (RSV) infection increases airway permeability and inflammation. C57BL/6 mice were infected intranasally with 9 × 107 plaque-forming units (pfu) of RSV strain A2, UV-RSV, or equal volume of supernatant from uninfected HEp-2 cells. A: mice were subjected to fluorescent dextran permeability assay 4 days post-viral inoculation, as described in materials and methods. Data are presented as means ± SE; n ≥ 5, *P < 0.05. B: total protein concentration was determined in collected bronchoalveolar lavage (BAL) samples of control and RSV-infected animals. Data are presented as means ± SE; n ≥ 3, **P < 0.01.
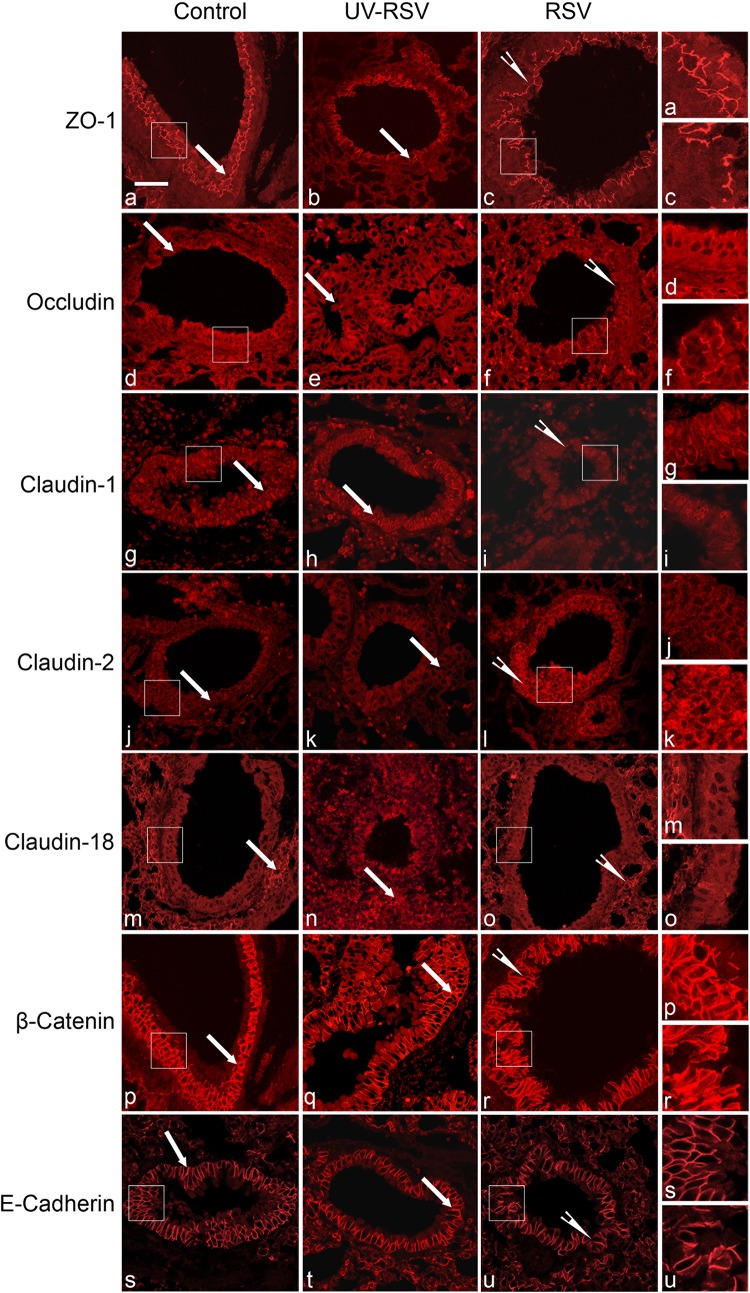
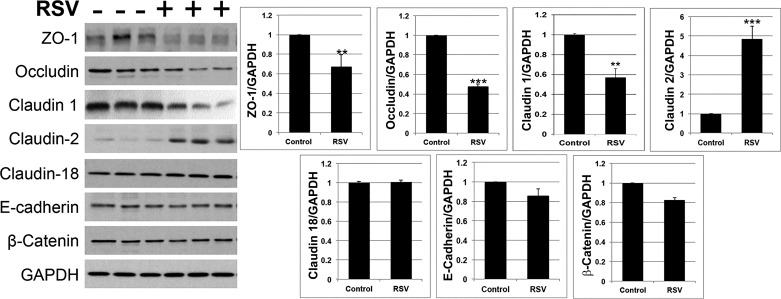
We then sought to determine the mechanisms that might underlie RSV-induced disruption of the pulmonary epithelial barrier in vivo. Since AJC disassembly was shown to be essential for a viral-dependent increase in permeability of the model pulmonary epithelial barrier (40, 42), we predicted that similar events would take place in the airways of RSV-infected mice. Immunofluorescence labeling and confocal microscopy were used to evaluate AJC integrity in bronchiolar epithelium of control and RSV-infected animals on day 4 post-viral inoculations by examining localization of different TJ (ZO-1, occludin, claudin-2, and claudin-18) and AJ (E-cadherin and β-catenin) proteins. Immunolabeling of control mouse bronchioles demonstrated a well-developed AJC in the stratified epithelium that lines the bronchiolar lumen (Fig. 4, arrows). RSV infection caused several major changes in this AJC organization. First, the intensity and complexity of ZO-1 strands in the most apical epithelial layers was decreased during RSV infection (Fig. 4, arrowheads). Second, occludin became mislocalized in RSV-infected airway epithelial cells, disappearing from the most apical layer and but remaining along the lateral contacts in the more basal layers on the stratified airway epithelium. Finally, the increased labeling intensity of another TJ protein, claudin-2, was observed in bronchioles of RSV-infected animals (Fig. 4). Such an increase in claudin-2 was noticeable for both the membrane-localized and the cytoplasmic fractions of this protein. By contrast, localization of claudin-1, claudin-18, and AJ proteins E-cadherin and β-catenin was not significantly changed during RSV infection (Fig. 4). We also used a quantitative immunoblotting approach to examine the effects of RSV on the expression of different junctional proteins. Consistent with the immunolabeling data, protein expression of ZO-1 and occludin was significantly decreased, whereas expression of claudin-2 was markedly increased in lung homogenates of RSV-infected mice (Fig. 5). Additionally, claudin-1 expression was significantly decreased during RSV infection, whereas levels of claudin-18, β-catenin, and E-cadherin proteins remained unchanged (Fig. 5).
Fig. 4.
Respiratory syncytial virus (RSV) infection disrupts the integrity of airway epithelial junctions. Lung tissue sections of control, UV-RSV, and live RSV-infected animals were subjected to immunofluorescence labeling for tight-junction (TJ) proteins [zonula occludens-1 (ZO-1), occludin, claudin-1, claudin-2, claudin-18) and adherens junctions (AJ) (β-catenin, E-cadherin) proteins. Labeled sections were examined by confocal microscopy. Arrows indicate intact junctional complexes in airways of control animals. Arrowheads point to disrupted TJ structure and increased claudin-2 labeling in tissue sections of RSV-infected mice. Scale bar, 40 μm. Images are representative of ≥3 experiments, with >5 mice per group.
Fig. 5.
Respiratory syncytial virus (RSV) infection alters expression of selective tight-junction (TJ) proteins in murine lungs. Total lysates of lung tissue harvested from mock and RSV-infected animals on day 4 post-viral inoculation were subjected to SDS-gel electrophoresis and immunoblotting analysis for different apical junctional complex (AJC) proteins. Representative immunoblot images (A) and densitometric quantification of protein band intensities (B) are shown. Data are presented as means ± SE; n ≥ 3, **P < 0.01, ***P < 0.001 vs. control group.
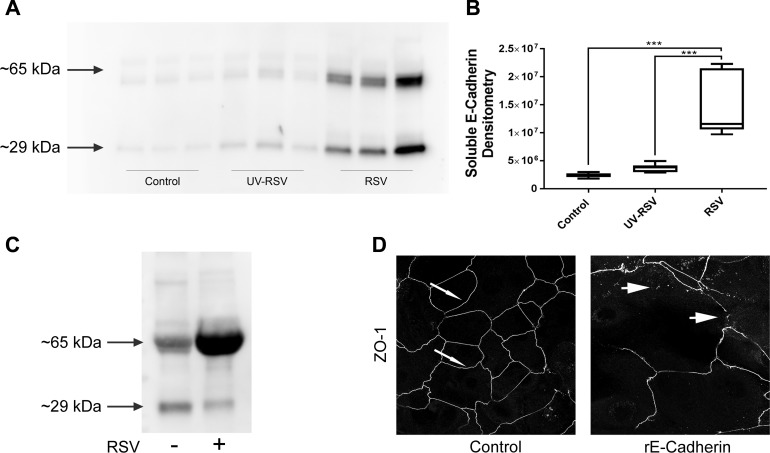
We also made the serendipitous observation that murine BAL contains soluble fragments of E-cadherin (sE-cad), recognized by a monoclonal antibody against the extracellular fragment of this AJ protein. RSV infection significantly increased the concentration of sE-cad in the BAL (Fig. 6, A and B), which could be indicative of accelerated cleavage of E-cadherin in the infected lungs. Likewise, marked release of similar E-cadherin fragments into cell culture medium of RSV-exposed mTEC monolayers was also detected (Fig. 6C). Next, we sought to elucidate whether the observed release of sE-cad fragments could contribute to the disruption of the airway epithelial barrier. Recombinant extracellular E-cadherin fragment (10 µg/ml) was added to human primary bronchial epithelial monolayers before they formed a tight barrier, and the effects of such sE-cad addition on the TJ assembly was determined 4 days later by immunolabeling of ZO-1. Control epithelial cells demonstrated well-defined continuous junctional labeling of ZO-1, which are characteristics of intact TJs (Fig. 6D, arrows). By contrast, sE-cad-exposed epithelial cell monolayers demonstrated discontinuous ZO-1 labeling at the cell-cell contact areas, thereby indicating defects in TJ assembly (Fig. 6D, arrowheads).
Fig. 6.
Respiratory syncytial virus (RSV) infection induces accumulation of soluble E-cadherin in the bronchoalveolar lavage (BAL). BAL fluid collected from control, UV-RSV, and live RSV-infected mice (A and B) as well as cell culture medium collected from control, UV-RSV, and live RSV-infected mouse tracheal epithelial cell monolayers (mTEC; C) were analyzed for the presence of soluble E-cadherin fragments. Representative immunoblot images (A and C) and densitometric quantification of protein band intensity (B) are shown. D: recombinant extracellular E-cadherin fragment (10 µg/ml) was added to nonconfluent human primary airway epithelial cells. Cells were immunofluorescently stained for tight-junction (TJ) protein zonula occludens-1 (ZO-1) after 4 days. Scale bar, 40 μm. Data are presented as means ± SE, n ≥ 3. ***P < 0.001.
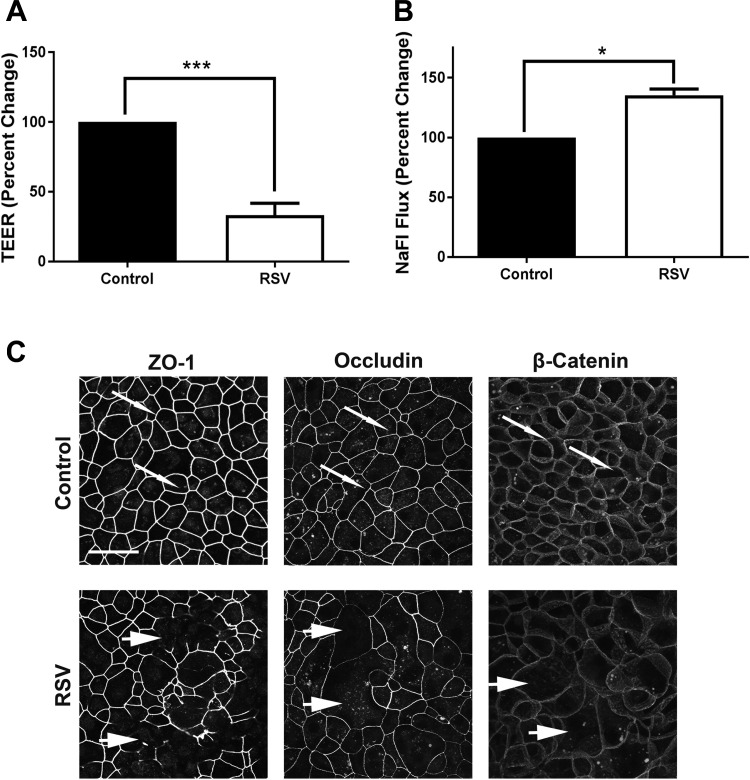
Finally, we asked whether the observed RSV-induced disruption of the airway epithelial barrier and AJC disassembly in vivo reflect the direct effects of the virus on epithelial junctions or represent indirect consequences of tissue inflammation. To address this, we infected mTEC monolayers with RSV in vitro and examined the effects of the viral infection on structure and functions of epithelial AJC. This in vitro model of the airway barrier recapitulated several effects of RSV observed in vivo without the inflammatory response initiated in the milieu of the lung. These include release of cleaved extracellular fragment of E-cadherin (Fig. 6C), increase in epithelial permeability as manifested by the decreased TEER, and increased sodium fluorescein flux (Fig. 7, A and B) as well as selectively disrupted TJ, but not AJ, integrity (Fig. 7C, arrowheads). Together, these data suggest that RSV is directly capable of targeting epithelial junctions both in vitro and in vivo, leading to the dysfunction of the airway epithelial barrier.
Fig. 7.
Respiratory syncytial virus (RSV) infection induces paracellular permeability and disrupts tight-junction (TJ) structure in cultured mouse tracheal epithelial cell (mTEC) monolayers. Confluent mTEC monolayers were infected with RSV or left untreated. On day 4 post-inoculation, barrier properties of the cells were investigated by either transepithelial electrical resistance (TEER) measurement (A) or fluorescent dextran permeability assay (B). Data are presented as percent change vs. control ± SE; n ≥ 3, *P < 0.05, and ***P < 0.001 vs. control group. C: cells were fixed on day 4 post-infection and immunolabeled for different apical junctional complex (AJC) proteins. Arrows indicate normal TJs and adherens junctions (AJs) in control cell monolayers. Arrowheads point to areas of TJ disassembly in RSV-infected cells. Scale bar, 40 μm. Images are representative of 3 independent experiments.
DISCUSSION
The airway epithelial barrier serves as the first line of defense against inhaled allergens, particles, and viruses. Disruption of this barrier is an important contributor to the pathogenesis of pulmonary diseases. The present study describes, for the first time, the effects of RSV infection on the airway epithelial barrier in vivo, which involves increased epithelial permeability and altered structure and molecular composition of the epithelial junctions. Our study demonstrates that RSV infection increases bidirectional flux of substances across murine airways, evidenced by increased uptake of the inhaled tracer from the environment, as well as the passage of proteins into the bronchial lumen (Fig. 3). These observations suggest that leakiness of the airway barrier in response to RSV infection occurs at both epithelial and endothelial barriers. Furthermore, our data (Fig. 1), together with previous studies (21, 38), indicate that RSV triggers the inflammatory response in the infected lungs. As a result, the dysfunction of the two major pulmonary barriers could reflect both a direct effect of viral infection and a consequence of released inflammatory cytokines and chemokines in response to viral infection. Importantly, our previous examination of cell necrosis (LDH) and apoptosis (caspase activation) showed no evidence of RSV-induced cell death in vitro (40), which is consistent with other reports that RSV can cause long-lasting infection and inflammation of the airway epithelium without inducing apoptosis (65).
The observed disruption of the airway barrier in RSV-infected animals was accompanied by the altered structure and composition of epithelial TJs (Figs. 4 and 5). Specifically, our data indicate the abnormal distribution of the most apical TJs encircling the bronchial lumen without marked alterations in lateral intercellular contacts formed by AJ proteins. Furthermore, we found altered molecular composition of TJs in RSV-infected airways manifested by marked upregulation of claudin-2 expression and downregulation of ZO-1, occludin, and claudin-1 proteins. The observed alterations in TJ structure are consistent with previous studies that examined the effects of RSV on AJs in model epithelial cell monolayers (40, 42). However, the described changes in molecular composition of TJs represent a specific response to RSV infection in vivo (21) since these data did not completely recapitulate viral effects on TJs in cultured cell monolayers. For example, our previous study of RSV infection of cultured bronchial epithelial cell monolayers found neither downregulation of ZO-1 and occludin (40) nor upregulation of claudin-2 (data not shown). Another study that examined RSV interactions with model nasal epithelial monolayers reported upregulation of claudin-4 without altered expression of other AJC proteins (28). A possible reason for such a discrepancy between in vitro and in vivo effects of RSV on epithelial junctions is that in an in vitro system epithelial cells are exposed to RSV alone, whereas in vivo they are effected by both virus and the inflammatory milieu created by viral infection. The inflammatory environment is likely to contain different cytokines such as IL-4, IL-13, and IFNγ, known to disrupt TJ integrity and decrease expression of different junctional proteins (4, 47, 61).
The described changes in the molecular organization of epithelial TJs triggered by RSV infection are likely to play causal roles in leakiness of the airway epithelial barrier. Indeed, this evidence suggests that ZO-1 and occludin act as positive regulators of epithelial barrier integrity. Studies in different types of epithelial monolayers in vitro demonstrated that loss of ZO-1 increases paracellular permeability to both ions and large uncharged molecules (19, 22, 35). Likewise, occludin depletion was shown to attenuate the establishment of tight paracellular barrier in vitro (39), and downregulation of claudin-1 was associated with increased permeability of airway epithelial monolayers (11).
In contrast to occludin, ZO-1, and claudin-1, claudin-2 belongs to a subset of TJ proteins that antagonize tight epithelial barrier formation. Claudin-2 is considered a pore-forming, or leaky, claudin, since its overexpression increases paracellular permeability (10, 24, 25). Claudin-2-deficient mice demonstrated decreased permeability and altered ion selectivity of TJs in renal proximal tubules (31). A body of evidence indicates that claudin-2 expression is upregulated by different proinflammatory mediators and environmental stressors and that such upregulation mediates the increased permeability of different epithelial barriers in inflammatory and infectious diseases (26). It is reasonable to suggest that increased claudin-2 expression contributes to a leaky airway epithelial barrier in RSV-infected lungs.
Altered molecular composition of TJs is not the only mechanism contributing to the disruption of the airway epithelial barrier during RSV infection; the other likely involves cleavage of E-cadherin and release soluble extracellular fragments of this AJ protein. Such E-cadherin cleavage was characteristic of RSV infection both in vivo and in vitro (Fig. 6), and these findings complement significant literature describing accumulation of sE-cad under different inflammatory and infectious conditions. For example, cleavage of E-cadherin has been identified as a mechanism of disruption of the intestinal epithelial barrier in response to Candida albicans (9). In that study, Frank et al. detect a 35-kDa fragment derived from the intracellular portion of E-cadherin and an 89-kDa extracellular fragment of E-cadherin. Cellular proteases such as matrix metalloproteinases, disintegrin and metalloproteinases (ADAMs), and γ-secretase have been shown to cleave the E-cadherin extracellular domain (9, 27, 57). It has been suggested that the cleaved sE-cad may act as paracrine/autocrine signaling molecule by disrupting intercellular contacts influencing epithelial homeostasis (32). For instance, Wheelock et al. reported that a fragment of E-cadherin disrupts adhesion of cells in a human mammary carcinoma line. Similarly, a recombinant fragment resembling cleaved E-cadherin has been shown to promote TJ disruption and promote dissemination of ovarian carcinoma cells (45, 57). In good agreement with these studies, addition of a recombinant sE-cad fragment attenuated TJ assembly in primary human bronchial epithelial cell monolayers (Fig. 6D). It should be noted that in our study released sE-cad fragments were not accompanied by gross abnormalities of AJ structure or E-cadherin expression in RSV-infected airways or cultured cell monolayers (Figs. 4 and 5). This may indicate that only a small fraction of E-cadherin is cleaved in RSV-infected airway epithelium under our experimental conditions. However even this limited cleavage may have functional consequences by inducing focal disruption of the epithelial barrier either via decreasing concentration of adhesive E-cadherin at the plasma membrane or via disrupting intercellular adhesion by competing sE-cad fragments.
The present study, while describing an essential mechanism relevant to human RSV infection, has limitations. For example, young adult mice (6–8 wk old) were used instead of neonates, whereas infants are the most susceptible age group in humans. Paradoxically, neonatal mice appear to be resistant to RSV infection (46), which may reflect some unknown peculiarities in their developmental process. It will be important to correlate age-dependent responses of mice to RSV infections and the structure and functions of the AJC in their airway epithelium. RSV infection during infancy has been shown to increase the risk of persistent wheezing, asthma, and allergy sensitization later in life (51, 62). Therefore, it would be interesting to examine whether RSV infection at a younger age can induce long-lasting-damage of the airway barrier that persists after clearance of the infection. Furthermore, it is crucial to study whether outside-in airway leakiness persists after RSV clearance and to investigate inhaled allergen sensitization as a potential consequence of AJC disruption.
In conclusion, our data provide new insights into the impact of RSV infection on the airway epithelial barrier. We have demonstrated that RSV infection specifically alters the structure and composition of airway epithelial junctions. This is associated with increased permeability of the epithelial barrier, resulting in a leaky airway, which could potentially promote penetration of inhaled allergens and particles into subepithelial space. These data lay the groundwork for future investigations elucidating the molecular mechanisms by which RSV induces junctional disassembly in epithelial cells and how this may promote allergen sensitization. Understanding the mechanisms of RSV-induced disruption of the airway epithelial barrier may help develop future therapeutic approaches to treat acute and chronic sequelae of RSV infection.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants K12-HD-068373 (to F. Rezaee), K08-AI-112781 (to F. Rezaee), and RO1-HL-061007 (to G. Piedimonte). This work used the Leica SP8 confocal microscope that was purchased with funding from NIH SIG Grant 1S10-OD-019972-01.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
F.R. conceived and designed research; C.C.S., D.T.L., T.J.H., V.B., and F.R. performed experiments; C.C.S., D.T.L., T.J.H., A.I.I., G.P., and F.R. analyzed data; C.C.S., T.J.H., A.I.I., G.P., and F.R. interpreted results of experiments; C.C.S., D.T.L., and F.R. prepared figures; C.C.S. and F.R. drafted manuscript; C.C.S., D.T.L., T.J.H., A.I.I., G.P., and F.R. edited and revised manuscript; and C.C.S., D.T.L., T.J.H., V.B., A.I.I., G.P., and F.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Cornelia Bergmann, Dept. of Neurosciences, and the Lerner Research Institute for help with RSV plaque-forming assays.
REFERENCES
- 1.Barends M, Van Oosten M, De Rond CG, Dormans JA, Osterhaus AD, Neijens HJ, Kimman TG. Timing of infection and prior immunization with respiratory syncytial virus (RSV) in RSV-enhanced allergic inflammation. J Infect Dis 189: 1866–1872, 2004. doi: 10.1086/386341. [DOI] [PubMed] [Google Scholar]
- 2.Becker S, Reed W, Henderson FW, Noah TL. RSV infection of human airway epithelial cells causes production of the beta-chemokine RANTES. Am J Physiol Lung Cell Mol Physiol 272: L512–L520, 1997. doi: 10.1152/ajplung.1997.272.3.L512. [DOI] [PubMed] [Google Scholar]
- 3.Boukhvalova MS, Yim KC, Prince GA, Blanco JC. Methods for monitoring dynamics of pulmonary RSV replication by viral culture and by real-time reverse transcription-PCR in vivo: Detection of abortive viral replication. Curr Protoc Cell Biol 46: 26.6.1-26.6.19, 2010. doi: 10.1002/0471143030.cb2606s46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruewer M, Utech M, Ivanov AI, Hopkins AM, Parkos CA, Nusrat A. Interferon-γ induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. FASEB J 19: 923–933, 2005. doi: 10.1096/fj.04-3260com. [DOI] [PubMed] [Google Scholar]
- 5.Castro SM, Guerrero-Plata A, Suarez-Real G, Adegboyega PA, Colasurdo GN, Khan AM, Garofalo RP, Casola A. Antioxidant treatment ameliorates respiratory syncytial virus-induced disease and lung inflammation. Am J Respir Crit Care Med 174: 1361–1369, 2006. doi: 10.1164/rccm.200603-319OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Comstock AT, Ganesan S, Chattoraj A, Faris AN, Margolis BL, Hershenson MB, Sajjan US. Rhinovirus-induced barrier dysfunction in polarized airway epithelial cells is mediated by NADPH oxidase 1. J Virol 85: 6795–6808, 2011. doi: 10.1128/JVI.02074-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Estripeaut D, Torres JP, Somers CS, Tagliabue C, Khokhar S, Bhoj VG, Grube SM, Wozniakowski A, Gomez AM, Ramilo O, Jafri HS, Mejias A. Respiratory syncytial virus persistence in the lungs correlates with airway hyperreactivity in the mouse model. J Infect Dis 198: 1435–1443, 2008. doi: 10.1086/592714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer JE, Johnson JE, Kuli-Zade RK, Johnson TR, Aung S, Parker RA, Graham BS. Overexpression of interleukin-4 delays virus clearance in mice infected with respiratory syncytial virus. J Virol 71: 8672–8677, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frank CF, Hostetter MK. Cleavage of E-cadherin: a mechanism for disruption of the intestinal epithelial barrier by Candida albicans. Transl Res 149: 211–222, 2007. doi: 10.1016/j.trsl.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Furuse M, Furuse K, Sasaki H, Tsukita S. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J Cell Biol 153: 263–272, 2001. doi: 10.1083/jcb.153.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gan H, Wang G, Hao Q, Wang QJ, Tang H. Protein kinase D promotes airway epithelial barrier dysfunction and permeability through down-regulation of claudin-1. J Biol Chem 288: 37343–37354, 2014. doi: 10.1074/jbc.A113.511527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Georas SN, Rezaee F. Epithelial barrier function: at the front line of asthma immunology and allergic airway inflammation. J Allergy Clin Immunol 134: 509–520, 2014. doi: 10.1016/j.jaci.2014.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo-Parke H, Canning P, Douglas I, Villenave R, Heaney LG, Coyle PV, Lyons JD, Shields MD, Power UF. Relative respiratory syncytial virus cytopathogenesis in upper and lower respiratory tract epithelium. Am J Respir Crit Care Med 188: 842–851, 2013. doi: 10.1164/rccm.201304-0750OC. [DOI] [PubMed] [Google Scholar]
- 14.Haeberle HA, Kuziel WA, Dieterich HJ, Casola A, Gatalica Z, Garofalo RP. Inducible expression of inflammatory chemokines in respiratory syncytial virus-infected mice: role of MIP-1α in lung pathology. J Virol 75: 878–890, 2001. doi: 10.1128/JVI.75.2.878-890.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hallak LK, Spillmann D, Collins PL, Peeples ME. Glycosaminoglycan sulfation requirements for respiratory syncytial virus infection. J Virol 74: 10508–10513, 2000. doi: 10.1128/JVI.74.22.10508-10513.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harford TJ, Rezaee F, Scheraga RG, Olman MA, Piedimonte G. Asthma predisposition and respiratory syncytial virus infection modulate transient receptor potential vanilloid 1 function in children’s airways. J Allergy Clin Immunol 141: 414–416.e4, 2018. doi: 10.1016/j.jaci.2017.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hashimoto K, Mori S, Hashimoto Y, Kaneko H, Ishibashi K, Ishioka K, Kawasaki Y, Peebles RS Jr, Munakata M, Hosoya M, Suzutani T. DSCG reduces RSV-induced illness in RSV-infected mice. J Med Virol 81: 354–361, 2009. doi: 10.1002/jmv.21378. [DOI] [PubMed] [Google Scholar]
- 18.Indra I, Hong S, Troyanovsky R, Kormos B, Troyanovsky S. The adherens junction: a mosaic of cadherin and nectin clusters bundled by actin filaments. J Invest Dermatol 133: 2546–2554, 2013. doi: 10.1038/jid.2013.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivanov AI, Young C, Den Beste K, Capaldo CT, Humbert PO, Brennwald P, Parkos CA, Nusrat A. Tumor suppressor scribble regulates assembly of tight junctions in the intestinal epithelium. Am J Pathol 176: 134–145, 2010. doi: 10.2353/ajpath.2010.090220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jafri HS, Chavez-Bueno S, Mejias A, Gomez AM, Rios AM, Nassi SS, Yusuf M, Kapur P, Hardy RD, Hatfield J, Rogers BB, Krisher K, Ramilo O. Respiratory syncytial virus induces pneumonia, cytokine response, airway obstruction, and chronic inflammatory infiltrates associated with long-term airway hyperresponsiveness in mice. J Infect Dis 189: 1856–1865, 2004. doi: 10.1086/386372. [DOI] [PubMed] [Google Scholar]
- 21.Kast JI, McFarlane AJ, Głobińska A, Sokolowska M, Wawrzyniak P, Sanak M, Schwarze J, Akdis CA, Wanke K. Respiratory syncytial virus infection influences tight junction integrity. Clin Exp Immunol 190: 351–359, 2017. doi: 10.1111/cei.13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S, Kim GH. Roles of claudin-2, ZO-1 and occludin in leaky HK-2 cells. PLoS One 12: e0189221, 2017. doi: 10.1371/journal.pone.0189221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King KA, Hu C, Rodriguez MM, Romaguera R, Jiang X, Piedimonte G. Exaggerated neurogenic inflammation and substance P receptor upregulation in RSV-infected weanling rats. Am J Respir Cell Mol Biol 24: 101–107, 2001. doi: 10.1165/ajrcmb.24.2.4264. [DOI] [PubMed] [Google Scholar]
- 24.Koval M. Claudin heterogeneity and control of lung tight junctions. Annu Rev Physiol 75: 551–567, 2013. doi: 10.1146/annurev-physiol-030212-183809. [DOI] [PubMed] [Google Scholar]
- 25.Krause G, Winkler L, Mueller SL, Haseloff RF, Piontek J, Blasig IE. Structure and function of claudins. Biochim Biophys Acta 1778: 631–645, 2008. doi: 10.1016/j.bbamem.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 26.Luettig J, Rosenthal R, Barmeyer C, Schulzke JD. Claudin-2 as a mediator of leaky gut barrier during intestinal inflammation. Tissue Barriers 3: e977176, 2015. doi: 10.4161/21688370.2014.977176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maretzky T, Reiss K, Ludwig A, Buchholz J, Scholz F, Proksch E, de Strooper B, Hartmann D, Saftig P. ADAM10 mediates E-cadherin shedding and regulates epithelial cell-cell adhesion, migration, and β-catenin translocation. Proc Natl Acad Sci USA 102: 9182–9187, 2005. doi: 10.1073/pnas.0500918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masaki T, Kojima T, Okabayashi T, Ogasawara N, Ohkuni T, Obata K, Takasawa A, Murata M, Tanaka S, Hirakawa S, Fuchimoto J, Ninomiya T, Fujii N, Tsutsumi H, Himi T, Sawada N. A nuclear factor-κB signaling pathway via protein kinase C δ regulates replication of respiratory syncytial virus in polarized normal human nasal epithelial cells. Mol Biol Cell 22: 2144–2156, 2011. doi: 10.1091/mbc.e10-11-0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muñoz JL, McCarthy CA, Clark ME, Hall CB. Respiratory syncytial virus infection in C57BL/6 mice: clearance of virus from the lungs with virus-specific cytotoxic T cells. J Virol 65: 4494–4497, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy BR, Sotnikov A, Paradiso PR, Hildreth SW, Jenson AB, Baggs RB, Lawrence L, Zubak JJ, Chanock RM, Beeler JA, Prince GA. Immunization of cotton rats with the fusion (F) and large (G) glycoproteins of respiratory syncytial virus (RSV) protects against RSV challenge without potentiating RSV disease. Vaccine 7: 533–540, 1989. doi: 10.1016/0264-410X(89)90278-8. [DOI] [PubMed] [Google Scholar]
- 31.Muto S, Hata M, Taniguchi J, Tsuruoka S, Moriwaki K, Saitou M, Furuse K, Sasaki H, Fujimura A, Imai M, Kusano E, Tsukita S, Furuse M. Claudin-2-deficient mice are defective in the leaky and cation-selective paracellular permeability properties of renal proximal tubules. Proc Natl Acad Sci USA 107: 8011–8016, 2010. doi: 10.1073/pnas.0912901107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nava P, Kamekura R, Nusrat A. Cleavage of transmembrane junction proteins and their role in regulating epithelial homeostasis. Tissue Barriers 1: e24783, 2013. doi: 10.4161/tisb.24783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niessen CM. Tight junctions/adherens junctions: basic structure and function. J Invest Dermatol 127: 2525–2532, 2007. doi: 10.1038/sj.jid.5700865. [DOI] [PubMed] [Google Scholar]
- 34.Noah TL, Becker S. Respiratory syncytial virus-induced cytokine production by a human bronchial epithelial cell line. Am J Physiol Lung Cell Mol Physiol 265: L472–L478, 1993. doi: 10.1152/ajplung.1993.265.5.L472. [DOI] [PubMed] [Google Scholar]
- 35.Odenwald MA, Choi W, Buckley A, Shashikanth N, Joseph NE, Wang Y, Warren MH, Buschmann MM, Pavlyuk R, Hildebrand J, Margolis B, Fanning AS, Turner JR. ZO-1 interactions with F-actin and occludin direct epithelial polarization and single lumen specification in 3D culture. J Cell Sci 130: 243–259, 2017. doi: 10.1242/jcs.188185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peebles RS., Jr Viral infections, atopy, and asthma: is there a causal relationship? J Allergy Clin Immunol 113, Suppl 1: S15–S18, 2004. doi: 10.1016/j.jaci.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 37.Piedimonte G. Contribution of neuroimmune mechanisms to airway inflammation and remodeling during and after respiratory syncytial virus infection. Pediatr Infect Dis J 22, Suppl 2: S66–S75, 2003. doi: 10.1097/01.inf.0000053888.67311.1d. [DOI] [PubMed] [Google Scholar]
- 38.Piedimonte G, Hegele RG, Auais A. Persistent airway inflammation after resolution of respiratory syncytial virus infection in rats. Pediatr Res 55: 657–665, 2004. doi: 10.1203/01.PDR.0000112244.72924.26. [DOI] [PubMed] [Google Scholar]
- 39.Raleigh DR, Marchiando AM, Zhang Y, Shen L, Sasaki H, Wang Y, Long M, Turner JR. Tight junction-associated MARVEL proteins marveld3, tricellulin, and occludin have distinct but overlapping functions. Mol Biol Cell 21: 1200–1213, 2010. doi: 10.1091/mbc.e09-08-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rezaee F, DeSando SA, Ivanov AI, Chapman TJ, Knowlden SA, Beck LA, Georas SN. Sustained protein kinase D activation mediates respiratory syncytial virus-induced airway barrier disruption. J Virol 87: 11088–11095, 2013. doi: 10.1128/JVI.01573-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rezaee F, Georas SN. Breaking barriers. New insights into airway epithelial barrier function in health and disease. Am J Respir Cell Mol Biol 50: 857–869, 2014. doi: 10.1165/rcmb.2013-0541RT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rezaee F, Harford TJ, Linfield DT, Altawallbeh G, Midura RJ, Ivanov AI, Piedimonte G. cAMP-dependent activation of protein kinase A attenuates respiratory syncytial virus-induced human airway epithelial barrier disruption. PLoS One 12: e0181876, 2017. doi: 10.1371/journal.pone.0181876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rezaee F, Linfield DT, Harford TJ, Piedimonte G. Ongoing developments in RSV prophylaxis: a clinician’s analysis. Curr Opin Virol 24: 70–78, 2017. doi: 10.1016/j.coviro.2017.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rezaee F, Meednu N, Emo JA, Saatian B, Chapman TJ, Naydenov NG, De Benedetto A, Beck LA, Ivanov AI, Georas SN. Polyinosinic:polycytidylic acid induces protein kinase D-dependent disassembly of apical junctions and barrier dysfunction in airway epithelial cells. J Allergy Clin Immunol 128: 1216–1224.e11, 2011. doi: 10.1016/j.jaci.2011.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosso M, Majem B, Devis L, Lapyckyj L, Besso MJ, Llauradó M, Abascal MF, Matos ML, Lanau L, Castellví J, Sánchez JL, Pérez Benavente A, Gil-Moreno A, Reventós J, Santamaria Margalef A, Rigau M, Vazquez-Levin MH. E-cadherin: a determinant molecule associated with ovarian cancer progression, dissemination and aggressiveness. PLoS One 12: e0184439, 2017. doi: 10.1371/journal.pone.0184439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruckwardt TJ, Malloy AM, Gostick E, Price DA, Dash P, McClaren JL, Thomas PG, Graham BS. Neonatal CD8 T-cell hierarchy is distinct from adults and is influenced by intrinsic T cell properties in respiratory syncytial virus infected mice. PLoS Pathog 7: e1002377, 2011. doi: 10.1371/journal.ppat.1002377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saatian B, Rezaee F, Desando S, Emo J, Chapman T, Knowlden S, Georas SN. Interleukin-4 and interleukin-13 cause barrier dysfunction in human airway epithelial cells. Tissue Barriers 1: e24333, 2013. doi: 10.4161/tisb.24333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saito M, Tucker DK, Kohlhorst D, Niessen CM, Kowalczyk AP. Classical and desmosomal cadherins at a glance. J Cell Sci 125: 2547–2552, 2012. doi: 10.1242/jcs.066654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sajjan U, Wang Q, Zhao Y, Gruenert DC, Hershenson MB. Rhinovirus disrupts the barrier function of polarized airway epithelial cells. Am J Respir Crit Care Med 178: 1271–1281, 2008. doi: 10.1164/rccm.200801-136OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharma A, Wu W, Sung B, Huang J, Tsao T, Li X, Gomi R, Tsuji M, Worgall S. Respiratory syncytial virus (RSV) pulmonary infection in humanized mice induces human anti-rsv immune responses and pathology. J Virol 90: 5068–5074, 2016. doi: 10.1128/JVI.00259-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med 161: 1501–1507, 2000. doi: 10.1164/ajrccm.161.5.9906076. [DOI] [PubMed] [Google Scholar]
- 52.Singh D, McCann KL, Imani F. MAPK and heat shock protein 27 activation are associated with respiratory syncytial virus induction of human bronchial epithelial monolayer disruption. Am J Physiol Lung Cell Mol Physiol 293: L436–L445, 2007. doi: 10.1152/ajplung.00097.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soong G, Parker D, Magargee M, Prince AS. The type III toxins of Pseudomonas aeruginosa disrupt epithelial barrier function. J Bacteriol 190: 2814–2821, 2008. doi: 10.1128/JB.01567-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stark JM, McDowell SA, Koenigsknecht V, Prows DR, Leikauf JE, Le Vine AM, Leikauf GD. Genetic susceptibility to respiratory syncytial virus infection in inbred mice. J Med Virol 67: 92–100, 2002. doi: 10.1002/jmv.2196. [DOI] [PubMed] [Google Scholar]
- 55.Stokes KL, Chi MH, Sakamoto K, Newcomb DC, Currier MG, Huckabee MM, Lee S, Goleniewska K, Pretto C, Williams JV, Hotard A, Sherrill TP, Peebles RS Jr, Moore ML. Differential pathogenesis of respiratory syncytial virus clinical isolates in BALB/c mice. J Virol 85: 5782–5793, 2011. doi: 10.1128/JVI.01693-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stoppelenburg AJ, Salimi V, Hennus M, Plantinga M, Huis in ‘t Veld R, Walk J, Meerding J, Coenjaerts F, Bont L, Boes M. Local IL-17A potentiates early neutrophil recruitment to the respiratory tract during severe rsv infection. PloS One 8: e78461, 2013. doi: 10.1371/journal.pone.0078461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Symowicz J, Adley BP, Gleason KJ, Johnson JJ, Ghosh S, Fishman DA, Hudson LG, Stack MS. Engagement of collagen-binding integrins promotes matrix metalloproteinase-9-dependent E-cadherin ectodomain shedding in ovarian carcinoma cells. Cancer Res 67: 2030–2039, 2007. doi: 10.1158/0008-5472.CAN-06-2808. [DOI] [PubMed] [Google Scholar]
- 58.Tang YW, Neuzil KM, Fischer JE, Robinson FW, Parker RA, Graham BS. Determinants and kinetics of cytokine expression patterns in lungs of vaccinated mice challenged with respiratory syncytial virus. Vaccine 15: 597–602, 1997. doi: 10.1016/S0264-410X(96)00214-9. [DOI] [PubMed] [Google Scholar]
- 59.Taylor G. Animal models of respiratory syncytial virus infection. Vaccine 35: 469–480, 2017. doi: 10.1016/j.vaccine.2016.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 9: 799–809, 2009. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 61.Utech M, Ivanov AI, Samarin SN, Bruewer M, Turner JR, Mrsny RJ, Parkos CA, Nusrat A. Mechanism of IFN-γ-induced endocytosis of tight junction proteins: myosin II-dependent vacuolarization of the apical plasma membrane. Mol Biol Cell 16: 5040–5052, 2005. doi: 10.1091/mbc.e05-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu P, Hartert TV. Evidence for a causal relationship between respiratory syncytial virus infection and asthma. Expert Rev Anti Infect Ther 9: 731–745, 2011. doi: 10.1586/eri.11.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.You Y, Brody SL. Culture and differentiation of mouse tracheal epithelial cells. Methods Mol Biol 945: 123–143, 2012. doi: 10.1007/978-1-62703-125-7_9. [DOI] [PubMed] [Google Scholar]
- 64.You Y, Richer EJ, Huang T, Brody SL. Growth and differentiation of mouse tracheal epithelial cells: selection of a proliferative population. Am J Physiol Lung Cell Mol Physiol 283: L1315–L1321, 2002. doi: 10.1152/ajplung.00169.2002. [DOI] [PubMed] [Google Scholar]
- 65.Zhang L, Peeples ME, Boucher RC, Collins PL, Pickles RJ. Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J Virol 76: 5654–5666, 2002. doi: 10.1128/JVI.76.11.5654-5666.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Y, Luxon BA, Casola A, Garofalo RP, Jamaluddin M, Brasier AR. Expression of respiratory syncytial virus-induced chemokine gene networks in lower airway epithelial cells revealed by cDNA microarrays. J Virol 75: 9044–9058, 2001. doi: 10.1128/JVI.75.19.9044-9058.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]