Abstract
Type 2 diabetes mellitus (T2DM) is a systemic disease characterized by hyperglycemia, hyperlipidemia, and organismic insulin resistance. This pathological shift in both circulating fuel levels and energy substrate utilization by central and peripheral tissues contributes to mitochondrial dysfunction across organ systems. The mitochondrion lies at the intersection of critical cellular pathways such as energy substrate metabolism, reactive oxygen species (ROS) generation, and apoptosis. It is the disequilibrium of these processes in T2DM that results in downstream deficits in vital functions, including hepatocyte metabolism, cardiac output, skeletal muscle contraction, β-cell insulin production, and neuronal health. Although mitochondria are known to be susceptible to a variety of genetic and environmental insults, the accumulation of mitochondrial DNA (mtDNA) mutations and mtDNA copy number depletion is helping to explain the prevalence of mitochondrial-related diseases such as T2DM. Recent work has uncovered novel mitochondrial biology implicated in disease progressions such as mtDNA heteroplasmy, noncoding RNA (ncRNA), epigenetic modification of the mitochondrial genome, and epitranscriptomic regulation of the mtDNA-encoded mitochondrial transcriptome. The goal of this review is to highlight mitochondrial dysfunction observed throughout major organ systems in the context of T2DM and to present new ideas for future research directions based on novel experimental and technological innovations in mitochondrial biology. Finally, the field of mitochondria-targeted therapeutics is discussed, with an emphasis on novel therapeutic strategies to restore mitochondrial homeostasis in the setting of T2DM.
Keywords: diabetes mellitus, mitochondria dysfunction
INTRODUCTION
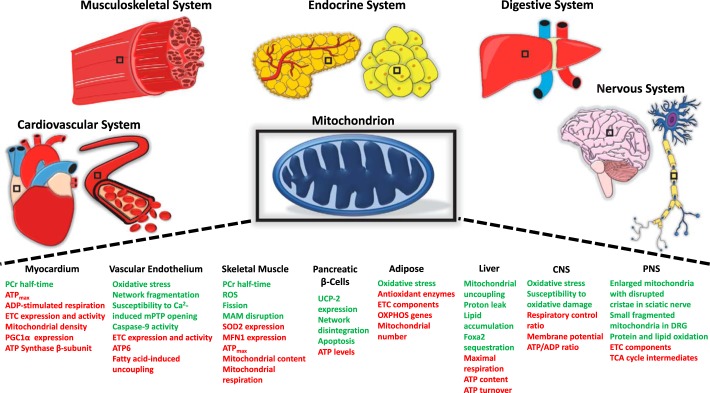
The International Diabetes Federation reports the number of adults 18–99 yr old with type 2 diabetes mellitus (T2DM) worldwide to be 451 million and predicts this figure will rise to 693 million by 2045 if trends continue (57). The increased risk of developing T2DM in both aging and obese populations, coupled with epidemiological data revealing rises in aged and obese demographics, necessitates deep understanding of the molecular changes underlying these links (99). Many have identified the mitochondrion as a locus of convergence for the host of dysregulated pathways in aging, obesity, and T2DM. Aging, for example, has been implicated in both mitochondria-intrinsic impairment such as mtDNA mutation and depletion and in mitochondria-related processes like apoptosis (16, 41, 92, 197). Obesity is characterized by an increased circulating free fatty acid concentration and accumulation of triacylglycerol in peripheral tissues that contribute to mitochondrial alterations, including increased lipotoxicity, elevated oxidative stress, and impaired energy substrate metabolism and oxidative phosphorylation (OXPHOS) (21, 33, 66, 94, 203). The role of mitochondrial dysfunction is of particular importance in T2DM due to its established association with insulin resistance, as previously reviewed (58, 105, 116, 152, 190). However, an up-to-date synthesis and organization of the many mitochondrial alterations in T2DM using an organ-based approach is urgently needed. The following sections will provide in intimate detail the structural, functional, and molecular changes accompanying mitochondrial dysfunction in T2DM across organ systems (Fig. 1). The organization of sections represents primary effector organs of T2DM pathogenesis with adaptations observed early on at the beginning to secondarily impacted organs that may not reach significant dysfunction until late in T2DM progression.
Fig. 1.
Mitochondria dysfunction across organ systems in type 2 diabetes mellitus. Structural, functional, and molecular changes to mitochondria in multiple diabetic models are displayed. Green font indicates an increase in the change, whereas red font indicates a decrease in the change. ATPmax, maximal ATP synthesis rate; CNS, central nervous system; DRG, dorsal root ganglia; ETC, electron transport chain; MAM, mitochondria-associated ER membrane; MFN1, mitofusin 1; mPTP, mitochondrial morphology and permeability transition pore; OXPHOS, oxidative phosphorylation; PCr, phosphocreatine; PGC-1α, peroxisomal proliferator activator receptor-γ coactivator-1α; PNS, peripheral nervous system; ROS, reactive oxygen species; SOD2, superoxide dismutase 2; UCP2, uncoupling protein 2.
MITOCHONDRIAL DYSFUNCTION IN THE T2DM DIGESTIVE SYSTEM
Mitochondrial Dysfunction in the T2DM Liver
Hepatic dysfunction in the form of nonalcoholic fatty liver disease (NAFLD) is commonly observed in patients with T2DM (107, 137, 150, 177, 183). Whether NAFLD is a cause or consequence of the diabetic pathology remains a topic of contention; however, the alterations in hepatic energy substrate metabolism and mitochondrial function in T2DM patients with NAFLD are well characterized. Decreased insulin sensitivity of the liver accompanied by increased hepatic fat storage are two such major metabolic changes found in the diabetic patient (87, 88, 137, 160). Mitochondria-intrinsic perturbations in obese, insulin-resistant patients with nonalcoholic steatohepatitis (NASH) include lower maximal respiration, increased mitochondrial uncoupling, and increased proton leak (85). These findings are further strengthened by the observation of decreased ATP content and turnover in the T2DM liver (160, 175).
Calcium homeostasis has also been shown to be an important contributor to mitochondrial function and insulin sensitivity in the liver (97, 146, 194). Notably, the endoplasmic reticulum (ER) has been shown to have functional association with mitochondria via mitofusin 2 tethering (49). These interactions at the mitochondria-associated ER membranes (MAMs) have been found to play a major role in insulin signaling, although whether the coupling of these organelles is increased or decreased in the insulin-resistant liver is debated (9, 185). Recent findings indicate that MAM disruption is observed in skeletal muscle of T2DM humans and mice (184). The influence of ER-mitochondria interactions on all other insulin-resistant organs in T2DM is currently unknown.
Mechanistic studies have uncovered a variety of potential molecular mediators to help explain changes in metabolism and mitochondrial dynamics in the diabetic liver. Members of the forkhead box transcription factor family have been shown to be among those mediators most prominently involved. It was found that Foxa2 is sequestered in the hepatocyte cytoplasm of insulin-resistant mice but that adenoviral introduction of a nuclear constitutively active form reverses many of the metabolic alterations in the T2DM liver described above (200). Conversely, Foxo1 knockout (KO) has been shown to reverse mitochondrial dysfunction and improve hepatic metabolism in insulin receptor substrate-1 and insulin receptor substrate-2 double-knockout (DKO) mice (35). Transcription factors of the PGC family have also been implicated in metabolic dysfunction of the diabetic liver (72, 86, 191). To highlight the important role of mitochondrial constituents in the diabetic liver, it was shown that long-chain acyl-CoA dehydrogenase-KO mice exhibit hepatic insulin resistance accompanied by hepatic steatosis (209). Furthermore, mitochondrial acyl-CoA:glycerol-sn-3-phosphate acyltransferase 1-KO mice were protected from hepatic triacylglycerol and diacylglycerol accumulation and insulin resistance following high-fat feeding, indicating its vital role in the development of diabetic liver metabolic dysfunction (122). Interestingly, liver-specific promoter methylation of important transcription factors, including PPARGC1A and mitochondrial transcription factor A, as well as mtDNA/nDNA ratio, have been reported to be associated with insulin levels and liver PPARGC1A mRNA levels in NAFLD (170). Further investigation of mitochondrial dynamics in the diabetic liver using techniques of epigenetics and epitranscriptomics may provide a clearer explanation of the altered transcriptomic and proteomic profiles associated with impaired mitochondrial function.
MITOCHONDRIAL DYSFUNCTION IN THE T2DM MUSCULOSKELETAL SYSTEM
Mitochondrial Dysfunction in T2DM Skeletal Muscle
Skeletal muscle insulin resistance is a central factor in the pathogenesis of T2DM. Because of their major role in systemic metabolism, skeletal muscle mitochondria in the T2DM setting have been thoroughly investigated. Many groups have come to an agreement on skeletal muscle mitochondrial dysfunction in the context of T2DM, although whether this is a cause or consequence of T2DM is still debated (10, 11, 22, 81, 113, 116, 138, 147, 148, 161, 206). This dysfunction has been characterized by a variety of methods both in vivo and ex vivo. In a study of T2DM patients and BMI-matched controls, the phosphocreatine (PCr) recovery half-time of vastus lateralis muscle from diabetic patients was 45% longer than that of controls (161). These results are further strengthened by a separate group’s finding of lower maximal ATP synthesis rate (ATPmax), as assessed by PCr recovery in vastus lateralis of T2DM patients (11). Phielix et al. (138) also found increased PCr half-time in diabetic vastus lateralis muscle and showed that ADP-stimulated basal respiration and FCCP-stimulated maximal respiration were decreased in mitochondria from the diabetic patient cohort. This decrease in mitochondrial respiratory capacity in diabetic patients is supported by the observation of decreased maximal ADP-stimulated respiration with pyruvate-malate combination in vastus lateralis mitochondria of diabetic patients (113). These functional impairments in mitochondria from diabetic skeletal muscle may be partly attributed to decreased subsarcolemmal mitochondria (SSM) electron transport chain (ETC) activity in vastus lateralis of T2DM patients compared with both obese and lean nondiabetic patients (148). Interestingly, many of these same mitochondrial abnormalities in hindlimb skeletal muscle of diabetic high-fat diet-fed mice, including decreased ADP-stimulated respiration, ETC complex I and III activities, and mitochondrial density, have also been reported (206). Whether mitochondrial dysfunction is the underlying cause or consequence of T2DM, the latter has been suggested based on findings of glucose intolerance preceding mitochondrial impairment in a diet-induced diabetic mouse model (22). In opposition to the above findings, some have been unable to find evidence of skeletal muscle mitochondrial dysfunction in the context of T2DM (24, 50). Although most studies focused on mitochondrial function in skeletal muscle have found some degree of dysfunction in T2DM, the ability of exercise interventions to prevent or reverse some aspects of this dysfunction is an area of active investigation and is addressed in a subsequent section.
It is important to consider the experimental techniques and normalization methods used to measure and interpret in vivo and ex vivo mitochondrial function. PCr half-time and ATPmax are in vivo assessments of mitochondrial function derived from phosphorus-31 magnetic resonance spectroscopy (31P-MRS) data obtained by fixing a surface coil in the middle of the vastus lateralis muscle and recording pre-exercise, during exercise, and postexercise (11, 50, 138, 161). Because of the inability to quantify the mitochondrial density of the precise muscle tissue providing the 31P-MRS values, these measures cannot distinguish whether increased PCr half-time or decreased ATPmax is due to decreased mitochondrial number or impaired mitochondrial function or exactly how much each may contribute. One method of determining ex vivo mitochondrial function is measuring oxygen consumption polarographically (22, 24, 113, 115, 138, 206). To ensure mitochondrial function rather than density is represented by using this technique, a variety of normalization methods have been employed to normalize for mitochondria content including citrate synthase activity, mtDNA copy number, isolated mitochondria protein concentration, or a combination of these parameters (24, 113, 138, 206). Another ex vivo analysis of mitochondria function can be found in the measurement of ETC complex activities following mitochondrial permeabilization. Optimal normalization of ETC complex activity data follows suit with those methods used for normalizing mitochondrial respiration data (113, 115, 148, 206). Whereas in vivo mitochondrial analysis provides a more physiologically relevant representation of mitochondrial function within its network and environment, ex vivo mitochondrial assessment allows one to control for mitochondrial density. A combination of in vivo and ex vivo mitochondrial analysis in the model being studied is ideal to provide the greatest insight into mitochondrial function. It is essential to consider both the technique to assess mitochondrial function and the method(s) of normalization when interpreting results of mitochondria function in all tissue types.
The shift in energy substrate availability for insulin-resistant skeletal muscle of T2DM patients and animal models necessitates an alteration in mitochondrial metabolism and is also associated with decreased expression of antioxidant genes in concert with increased oxidative stress (25). Multiple groups have converged on the important role peroxisomal proliferator activator receptor-γ coactivator-1α (PGC-1α) plays in mediating this metabolic change (117, 132). PGC-1α, a major promoter of oxidative metabolism and mitochondrial biogenesis, was found to be downregulated in vastus lateralis of T2DM patients when compared with controls with no family history of diabetes mellitus (132). Decreases in expression of PGC-1α-responsive genes involved in fatty acid oxidation (FAO), glycolysis, the tricarboxylic acid (TCA) cycle, and the ETC were also reported (132). The precise regulation of upstream and downstream pathways of the PGC-1 family of coactivators in health and metabolic disease has been reviewed previously (98). Gene Set Enrichment Analysis identified the OXPHOS gene set to have the most significantly changed expression in DNA microarray data from vastus lateralis samples of patients with T2DM relative to controls (117). In agreement with these findings, others have found downregulation of the ATP synthase gene transcript in skeletal muscle of T2DM subjects (172). Decreased FAO in vastus lateralis muscle of non-insulin-dependent diabetes mellitus patients has been observed by others; however, a concomitant increase in glucose oxidation was also reported (82). In addition to changes in the expression of oxidative metabolism components in the T2DM phenotype, it has been reported that the levels of phosphorylated ATP synthase β-subunit are significantly lower in T2DM (71). Others have reported increased expression of mtDNA-encoded cytochrome oxidase I, cytochrome oxidase III, and NADH dehydrogenase IV transcripts in T2DM skeletal muscle when normalized to mtDNA copy number, although this does not necessarily indicate the increased protein expression of these genes (8). Potential factors that may account for the increase in these gene transcripts important in OXPHOS that is contradictory to the above studies in skeletal muscle include the lack of controlling for age, sex, and other important patient characteristics between nondiabetic and T2DM groups as well as skeletal muscle samples being taken from either gastrocnemius or quadriceps instead of a single location in all subjects (8). Additional factors that may account for differences in mitochondrial function and gene expression between studies comparing skeletal muscle of nondiabetic and T2DM subjects include normalization methods and differences in fitness level as well as medication history of participants. The global decrease in expression of genes involved in oxidative metabolism of skeletal muscle mitochondria found in most studies investigating T2DM pathology may help explain the decreased basal and maximal respiration in this population described above.
It will be of great value to investigate additional mitochondrial dynamics, including calcium homeostasis, fission/fusion balance, network formation, and mitochondrial reticulum alterations in the setting of T2DM to determine how changes in these processes may influence metabolic dysregulation in skeletal muscle mitochondria. To this end, it was shown that right atrial tissue of T2DM patients displayed markedly reduced intrinsic contraction, increased oxidative stress, and mitochondria dysfunction associated with observations of mitochondrial network fragmentation and decreased expression of mitofusin-1 (115). Mitochondrial dynamics in T2DM has been reviewed previously (152, 199). Furthermore, the critical roles of calcium transport and homeostasis in both skeletal muscle and cardiac mitochondria as well as its tight linkage with OXPHOS has also been examined (42, 65, 129). Exploring how calcium handling is changed in T2DM may provide greater insight into the OXPHOS alterations observed. More recently, they have provided convincing evidence to support a mitochondrial reticulum in skeletal muscle in which proton-motive force is generated primarily in complex IV-rich paravascular mitochondria and conducted down complex V-rich I-band mitochondria, where it is used for ATP generation (64). The theoretical basis of this electrochemical transmission, identifying Na+ and/or K+ as essential ions in the conduction from the cell periphery to the I-band segments, has been reported (131).
Ability of Exercise to Reverse Mitochondrial Dysfunction in T2DM
Exercise training has been shown not only to improve insulin sensitivity in patients with T2DM but also to help restore mitochondrial function (69, 102, 110, 145, 182, 189). In fact, in vivo mitochondrial function, which was decreased in T2DM patients, was normalized by exercise training in T2DM subjects up to control levels (110). In a separate study, it was shown that a behavioral weight loss program in T2DM patients, including ≥4 days of exercise at 60–70% maximal heart rate, increased skeletal muscle mitochondrial density, size, cardiolipin content, mtDNA content, citrate synthase activity, and NADH oxidase activity (182). Van Tienen et al. (189) showed that after 1 yr of exercise training twice/wk, longstanding T2DM patients had an increased PCr recovery rate constant and increased mitochondrial density and complex I activity of vastus lateralis muscle. Low-volume, high-intensity exercise was also shown to increase maximal citrate synthase activity as well as upregulate subunits of ETC complexes II and III in vastus lateralis muscle of T2DM patients (102). Ten weeks of aerobic training on a stationary bike two to three times/wk was enough to increase respiration with complex I-supported substrates, ETCmax, and mitochondrial lipid oxidation in vastus lateralis from T2DM patients (69). Others have found low-intensity exercise in the form of walking >150 min/wk over the span of 4 mo to increase the expression of metabolic enzymes UCP3 and PPARδ (59). The positive effects of exercise extend beyond T2DM patients, as aerobic exercise programs have been shown to improve insulin sensitivity and skeletal muscle mitochondrial function in both healthy subjects and nondiabetic obese participants (69, 110). The precise contribution that physical inactivity of T2DM subjects has on skeletal muscle mitochondrial dysfunction has not been quantified; however, it is plausible that increasingly sedentary lifestyles of T2DM patients may play a significant role for changes in skeletal muscle mitochondrial density and function. Evidence in support of this hypothesis comes from studies in skeletal muscle showing that mitochondrial respiration is similar in T2DM and nondiabetic subjects when physical activity is controlled for and that mitochondrial function in T2DM is restored to control levels following an exercise training program (145, 189). The dynamic nature of the mitochondrion, and of mitochondrial metabolism more specifically, makes it susceptible to environmental insults but also to the positive impact of exercise training and lifestyle modification.
Exercise training has been found to have systemic positive effects in patients with T2DM, as evidenced by decreased liver fat, improved β-cell function, bolstered endothelial function, enhanced cardiac structure and function, and attenuation of diabetic peripheral neuropathy development (13, 20, 30, 40, 91, 213). The precise mechanisms linking exercise training to the improved function of organs negatively impacted by T2DM have not been fully elucidated, but we suspect that, as in skeletal muscle, improved mitochondrial function will be a significant contributor.
MITOCHONDRIAL DYSFUNCTION IN THE T2DM ENDOCRINE SYSTEM
Mitochondrial Dysfunction in T2DM Pancreatic β-Cells
Pancreatic β-cells serve as the body’s thermostat in sensing glucose level and responding to insulin needs of the organism. When either of these abilities to sense or to respond is impaired, there are consequences to systemic metabolism. In a state of metabolic homeostasis, temporary fluctuations in blood glucose levels are easily corrected by small alterations in insulin secretion. However, in the setting of T2DM, chronic exposure to hyperglycemia and hyperlipidemia impairs β-cell function. Multiple groups have highlighted mitochondrial structural or functional abnormalities as key factors in this impairment (6, 51). Pancreatic β-cells from diabetic patients were shown to have increased expression of ETC complexes I and V though decreased ATP levels and ATP/ADP ratio (6). They explained this paradox by showing increased uncoupling protein 2 (UCP-2) expression in diabetic islet cells of which glucose-stimulated insulin production was decreased (6). Interestingly, it has recently been reported that high-intensity CrossFit training 3 days/wk for 6 wk can reverse β-cell dysfunction in T2DM patients (123). In a Goto Kakizaki T2DM rat model it was shown that pancreatic β-cells displayed mitochondrial network disintegration (51). Others have found increased β-cell apoptosis in Zucker diabetic fatty rats and high-fat diet-induced models of T2DM (165, 169). Increased ETC-derived reactive oxygen species (ROS) in high-glucose-treated MIN6 pancreatic β-cells was shown to contribute to decreased glucose-induced insulin secretion (156). The important role of mitochondria in optimal β-cell function is further evidenced by the age-related loss of mtDNA corresponding with declining insulin secretion (43, 124). Previously, it had been shown that mtDNA depletion in a mouse pancreatic β-cell line prevented adequate glucose-stimulated insulin secretion (168). Altogether, pancreatic β-cell mitochondria play a dynamic role in the process of insulin secretion. This begs the question of whether mitochondria-targeted therapeutics may be a viable treatment strategy to improve β-cell function in T2DM patients.
Mitochondrial Dysfunction in T2DM Adipose Tissue
Often overlooked as a passive organ, adipose tissue is insulin-sensitive, highly dynamic, and acutely responsive to various environmental factors. The strong linkage between obesity and T2DM provides ample motivation into exploring how adipocyte populations are altered in the diabetic setting. One such alteration widely found in adipose tissue from T2DM patients and animal models is that of mitochondrial dysfunction or decrement (32, 37, 47, 93, 151). It was reported that obese T2DM patients displayed increased mitochondrial ROS when compared with nondiabetic normal weight controls and concomitantly that mitochondrial antioxidant enzymes were downregulated in this group (32). It has also been shown that many ETC components have decreased expression in visceral adipose mitochondria of women with T2DM (47). This is further supported by work showing decreased expression of OXPHOS genes in adipose tissue of T2DM patients (125). However, others have provided data to support the notion that mitochondrial dysfunction is only present in obese T2DM patients (31). In animal models of T2DM, the decrease in mitochondrial number and protein content have been shown to be corrected by either daily voluntary wheel running or treatment with the insulin-sensitizing agent rosiglitazone (37, 93, 151). Interestingly, one study looked at five groups of obese mice with increasing hyperglycemia and found that three classes of proteins with marked change in expression were those of metabolism, signal transduction, and transcription factors (120). In the 3T3-L1 adipocyte cell line, treatment with high glucose and high glucose plus high free fatty acids resulted in increased ROS levels, loss of mitochondrial membrane potential, and downregulation of NRF1 and PGC-1, two important OXPHOS transcription factors (61). These findings suggest that changes in adipose tissue composition and secretion of inflammatory molecules may contribute to mitochondrial dysfunction in other organs of the T2DM patient.
MITOCHONDRIAL DYSFUNCTION IN THE T2DM CARDIOVASCULAR SYSTEM
Mitochondrial Dysfunction in the T2DM Myocardium
Mitochondrial dysfunction in the type 2 diabetic human myocardium has been observed by multiple groups, although the negatively impacted mitochondria-intrinsic processes leading to this impairment are varied (2, 4, 45, 115). It has been identified that increased oxidative stress and mitochondrial network fragmentation in right atrial tissue of diabetic patients may be contributors to the observed dysfunction (115). Others have echoed this reported increase of ROS in the human diabetic heart (2, 4). Some argue that this increase in metabolic stress may render the myocardium more susceptible to Ca2+-induced mitochondrial permeability transition pore opening and further activation of the intrinsic apoptosis pathway (4). Indeed, this group found increased activity of caspase-9 in right atrial tissue from T2DM patients (4). Regarding the mitochondrial subpopulation most negatively impacted by the diabetic pathology, our laboratory has determined that cardiac SSM is most susceptible to T2DM insult (45). It was found that ETC complex I and IV activities and expression levels were decreased in SSM of T2DM patient right atrial tissue, whereas there was little to no difference in interfibrillar mitochondria (IFM) (45). The short- and long-term effects of insulin therapy or lifestyle modification on the levels of ROS and apoptosis activation in the diabetic human heart remain unexplored.
Murine models of T2DM have largely recapitulated the cardiac mitochondrial dysfunction described above (18, 23, 46, 84, 106, 109, 121, 181). These models may provide the researcher with more detailed insight into the mechanistic underpinnings of T2DM on cardiac mitochondria due to the ability to analyze the full heart and not atrial tissue alone. The in-depth understanding of mitochondrial-driven pathways such as energy substrate metabolism and ROS generation provided by T2DM animal models have proven invaluable. As an example, our laboratory observed downregulation of ATP6 in SSM of db/db hearts (164). Decreased expression of mtDNA-encoded genes in T2DM may also be a function of decreased binding of mtTFA to the D-loop of mtDNA to initiate transcription (80). Previously, we have shown that the SSM subpopulation from cardiac tissue in the db/db model had decreased size, internal complexity, state 3 respiration, and ETC complex activities (46). Others have characterized mitochondrial energy substrate metabolism in the insulin-resistant ob/ob heart, finding precipitously decreased glucose metabolism with concomitant increased rates of palmitate oxidation (109). As with the human disease, increased ROS generation has been established as a hallmark of the T2DM cardiac phenotype in animal models. It has been found that there is increased H2O2 production when db/db cardiac mitochondria were provided with glutamate and malate in combination (84). Other groups have also reported increased mitochondrial-derived ROS as well as higher lipid and protein peroxidation products (23, 106). Another group showed that mitochondrial ROS were dramatically increased in cardiac mitochondria of Zucker diabetic fatty rats (18). Interestingly, some have documented the increased presence of myocardial lipid droplets in the diabetic heart, situated even closer to the mitochondria (23, 121). Boudina et al. (23) make a striking argument for fatty acid-induced mitochondrial uncoupling in the db/db heart to explain how increased FAO and ETC complex activities lead to increased mitochondrial-generated ROS, lower ATP synthase F1 α-subunit expression, and impaired OXPHOS capacity. Similar to the skeletal muscle, which is discussed above, the myocardium has also been found to possess a mitochondrial reticulum, albeit with greater segmentation (63). Whether cardiac mitochondrial networks are altered in T2DM or have a reduced capacity to undergo dynamic disconnection to preserve function remains unknown. Although the above mentioned diabetic animal models clearly share many cardiac mitochondrial similarities with T2DM patients, there remains an open opportunity to more closely mimic the insulin-treated T2DM patient in an animal model and determine whether combining novel mitochondria targeted therapeutics with traditional insulin therapy would provide improved cardiac function in T2DM preclinical models.
Mitochondrial Dysfunction in T2DM Vascular Endothelium
Endothelial dysfunction has been found to accompany T2DM in both the human pathology and animal model (36, 83, 162). This impairment in endothelial function can be explained in part by altered mitochondrial processes. The most prevalent observation in diabetic endothelial cells is the increased accumulation of mitochondrial-derived ROS (36, 83, 162). It has been shown that mitochondrial fission contributes to increased mitochondrial ROS production by knocking down mitochondrial fission proteins Fis1 or Drp1 and finding no increase in ROS production when stressed with high glucose (162). In a T2DM animal model generated by streptozotocin (STZ) injection followed by a high-fat diet, coronary endothelial cells were found to have higher mitochondrial ROS levels and lower superoxide dismutase 2 expression than nondiabetic controls (36). Thus, the increased mitochondrial ROS observed in the T2DM vasculature could be a function of both structural abnormalities and decreased antioxidant defense.
MITOCHONDRIAL DYSFUNCTION IN THE T2DM NERVOUS SYSTEM
Mitochondrial Dysfunction in the T2DM Central Nervous System
The connection between T2DM and dementia has been thoroughly studied. In the Honolulu-Asia Aging Study, it was shown that T2DM was associated with increased risk of total dementia, Alzheimer’s disease, and vascular dementia (134). Risk of T2DM patients developing Alzheimer’s disease was compounded by the co-occurrence of the APOE-ε4 allele (134). Some have pointed to the alterations in the cerebral glucose metabolic rate in the frontal, temporal-parietal, and cingulate brain regions of prediabetic or T2DM patients as an important factor in declining cognitive function (12). This phenomenon has been more extensively investigated in diabetic animal models. It has been reported that brain mitochondria from sucrose-treated T2DM mice have a decreased respiratory control ratio, membrane potential, and ATP/ADP ratio when compared with nondiabetic mice (29). This observed brain mitochondrial dysfunction in T2DM may be attributed to higher ROS production in combination with greater susceptibility to oxidative damage (143, 158). The influences of aging and amyloid-β exposure on brain mitochondria from diabetic Goto Kakizaki rats were examined, and it was reported that function was most compromised when T2DM, aging, and amyloid-β exposure were all present (118). Others utilizing the same diabetic model found that amyloid-β1–40 exposure of brain mitochondria induced increased H2O2 production, followed by decreased respiratory control ratio and ATP content; however, treatment with the antioxidant CoQ10 attenuated these negative effects (119). It is important to note that some have documented no change in brain mitochondrial function or biogenesis of young (6-mo-old) diabetic Goto Kakizaki rats (159). There is a need to identify the specific pathways implicated in other pathologies that synergize with the T2DM phenotype to produce mitochondrial dysfunction in the brain.
Mitochondrial Dysfunction in the T2DM Peripheral Nervous System
Diabetic peripheral neuropathy is a major complication for many T2DM patients, and the impairment of peripheral neurons in diabetics has been shown to be linked to structural or functional abnormalities of their mitochondria. One group reported enlarged mitochondria containing disrupted cristae in sciatic nerves of galactose-fed diabetic rats and in sural nerves of T2DM patients (78). In contrast, others have observed more numerous smaller, fragmented mitochondria in dorsal root ganglion neurons of db/db mice when compared with controls (54). Others have observed increased mitochondria in dorsal root axons of db/db mice (192). Regarding other peripheral nerve mitochondrial changes in T2DM, it has been shown that there is decreased expression of ETC complex proteins in dorsal root ganglia of db/db mice (153). It has also been reported that TCA cycle intermediates are lower in sural and sciatic nerves as well as dorsal root ganglia of db/db mice (70). This downregulation of TCA cycle flux was in concordance with an increased presence of protein and lipid oxidation found in these tissues of diabetic mice (70). Determining the balance of mitochondrial fission and fusion in human T2DM peripheral nerves as well as how systemic antioxidant therapy may attenuate mitochondrial dysfunction in peripheral neurons remains unanswered for the field.
MITOCHONDRIAL DYSFUNCTION A UNIFYING THEME OF T2DM: THE ADAPTIVE THRESHOLD HYPOTHESIS
Mitochondrial dysfunction as a potentially unifying hypothesis accounting for both skeletal muscle insulin resistance and impaired pancreatic β-cell insulin secretion, two major events leading to T2DM, was first proposed by Lowell and Shulman (105) in a landmark viewpoint article in 2005. In the years since, organ-specific mitochondrial function in T2DM has been investigated much more thoroughly, as referenced in the above sections. Based on these findings, we suggest mitochondrial dysfunction to be a thread running deep in T2DM not only in organs that are primary drivers of the disease but also in those impacted secondarily. Current evidence supports a model where organs possess an adaptive threshold to genetic and environmental influences past the point where maladaptation occurs and the T2DM pathology progresses. Because certain organs play more prominent roles than others at different stages of T2DM pathogenesis, one would expect the adaptive thresholds of organs to be surpassed in a relatively predictive manner. However, because each organ functions in the context and environment of the organism, the adaptations and subsequent maladaptations of each organ in response to genetic and environmental insults leading to T2DM influence every other organ via the circulation. In this section, we present current evidence in support of this hypothesis.
Integration of T2DM Pathogenesis: Mitochondrial Dysfunction and Systemic Metabolism
Obesity-related T2DM is attributed partially to energy substrate imbalance both in the circulation and more importantly in the major effector organs of the diabetic condition: liver, skeletal muscle, and pancreatic β-islets. Energy substrate levels in the circulation are representative of external influences such as nutritional inputs and internal influences, including systemic energy substrate transport and metabolism in all bodily tissues. Excess nutrition and/or inactivity over an extended period in conjunction with genetic and epigenetic factors overwhelms the capacity of the organism to maintain strict control over circulating energy substrate levels.
In particular, increased hepatic lipid accumulation is a common observation in prediabetes and early-stage T2DM explained by higher circulating triacylglycerol levels (89). The culmination of lipid accumulation, aberrant lipid metabolism, and insulin resistance of the liver over time pushes the organ past its adaptive capability of increasing maximal mitochondrial respiration to respond to excess energy substrate accumulation, as in NAFLD to crossing its adaptive threshold characterized by decreased maximal respiration, mitochondrial uncoupling, and mitochondrial leakage, as in NASH (85). Interestingly, it was shown in the OLETF rat model of obesity that mitochondrial dysfunction may even precede NAFLD and hepatic insulin resistance (144). The impact of hepatic lipids on insulin resistance in T2DM has been definitively established and reviewed previously (136). Briefly, it has been demonstrated in vitro that palmitate treatment of hepatocyte cell lines and primary mouse hepatocytes reduces insulin receptor expression and activation of the insulin signaling cascade in a dose- and time-dependent fashion (154). Not only does the insulin-resistant fatty liver have a reduced capacity to efficiently convert the increased lipids being delivered to it by the circulation to energy via FAO and OXPHOS, but the lack of insulin signaling for the inhibition of gluconeogenesis also contributes to increased glucose production and secretion by the liver (111, 175). The downstream effects of increased circulating lipids, glucose, and inflammatory mediators following the development of insulin insensitivity in the liver help explain the tight inverse correlation found between intrahepatic triglyceride levels and whole body glucose disposal (74).
Increased circulating lipids following excess nutrition and chronically elevated circulating lipids and glucose following hepatic insulin resistance lead to the increased transport and altered metabolism of these substrates in skeletal muscle and pancreatic β-islets. Metabolic adaptations in skeletal muscle are first observed to accommodate the changing intracellular metabolite milieu; however, these adaptations, including increased shuttling of FFAs into cytotoxic lipid synthesis pathways and dysregulated energy substrate metabolism, lead to the adaptive threshold past the point at which there is an accumulation of inflammatory molecules, increased ROS, mitochondrial dysfunction, and insulin resistance (22, 90). The coexistence of hepatic and skeletal muscle insulin resistance contributes to hyperglycemia by both the increased production and decreased oxidation of glucose. As glucose production by the liver rises and its transport and catabolism by skeletal muscle falls, pancreatic β-cells must produce more and more insulin to achieve homeostatic levels. Long-term exposure to increasing concentrations of circulating glucose, FFAs, and inflammatory mediators contribute to β-cell,s reaching and surpassing their adaptive threshold from where lipotoxicity and glucotoxicity lead to increasing rates of ROS production and β-cell apoptosis (94, 165). The mechanisms of β-cell death in T2DM have been reviewed previously (52).
The progression along the adaptation/maladaptation continuum in each of the primary organs involved in T2DM pathogenesis, as described above, undoubtedly influences the function and thus progression along the adaptation/maladaptation continuum of organs secondarily impacted by prediabetic and diabetic conditions. Many of the negative effects observed in organs secondarily impacted by T2DM stem from the contribution of primary organs of T2DM pathogenesis to altered metabolite and inflammatory mediator profile of the systemic circulation. A case in point can be found in white adipose tissue (WAT). An adaptive response to declining glucose levels is the activation of WAT triglyceride lipase to mobilize FFAs to provide substrate for hepatic acetyl CoA (135). Insulin acts a negative regulator of this activation in WAT; however, in high-fat diet-fed mice and rats, WAT is not responsive to insulin’s inhibitor effect, and consequently WAT lipolysis is enhanced, leading to even greater levels of circulating FFAs (135). Others have added that adipose tissue mitochondria of T2DM patients have heightened levels of both ROS and lipid peroxidation products (32). Alterations in lipid metabolism and the overproduction of mitochondrial ROS have been shown to contribute to cardiac dysfunction in both db/db mouse and Zucker diabetic fatty rat models of T2DM as well (18, 121). The systemic metabolic effects of T2DM extend to peripheral nerves, where glycolytic intermediates are depleted and increased protein and lipid oxidation are observed (70). Because of the brain’s high dependence on glucose as a fuel source, it is not surprising that long-term exposure of the cerebrum to the T2DM-altered circulating milieu induces cerebral insulin resistance, reduced cerebral glucose metabolic rate, and increased risk of dementia (12). Because of the mitochondrion’s central role in glucose oxidation, FAO, OXPHOS, ROS generation, and apoptosis, the dysregulation of these pathways leading to increased circulating glucose and triglycerides, greater levels of intracellular and secreted cytotoxic lipid species and inflammatory mediators, and elevated mitochondria-derived ROS and apoptosis activation in key organs can all be linked to progression along the adaptation/maladaptation continuum of mitochondria across organs throughout T2DM progression.
mtDNA MUTATION AND VARIATION, NCRNA, EPIGENETICS, AND EPITRANSCRIPTOMICS: NEW PLAYERS IN MITOCHONDRIAL BIOLOGY
This section will cover novel experimental and technological innovations in mitochondrial biology. Athough we acknowledge that not much is known about the areas of ncRNA, epigenetics, and epitranscriptomics in the context of mitochondria, we present these topics as potential areas for future investigation in the setting of health and disease.
mtDNA Mutation and Variation in T2DM
The impact of mtDNA variants, whether homoplasmic or heteroplasmic, can be disastrous in the setting of metabolic disease. A litany of mtDNA mutations and single nucleotide polymorphisms have been found to be associated with T2DM (Table 1). Some groups have segregated their study populations into mitochondrial haplogroups based on mtDNA variants to determine whether a specific haplogroup is associated with increased or decreased risk of T2DM. One study showed that haplogroup J in a Finnish population was associated with maternally inherited T2DM (114). These results were further supported by a separate study that found haplogroup J1 to be associated with T2DM (56). Others have reported that subjects of the mitochondrial haplogroup B4 had an increased risk of T2DM, whereas those of haplogroup D4 carried a decreased risk (100). Interestingly, a lower prevalence (0.1–0.2%) of the A3243G mtDNA variant in T2DM UK white Caucasian patients has been previously reported in T2DM Asian patient populations (157). The role of mitochondrial genetics in conferring increased or decreased risk of T2DM has been clearly established. One very relevant question that remains to be answered is whether more metabolically active tissues that generate more mitochondrial ROS have increased rates of mtDNA heteroplasmy in T2DM.
Table 1.
mtDNA variations implicated in increased or decreased risk of T2DM
| mtDNA Alteration | Population | Variant Nature | Summary | Ref. No. |
|---|---|---|---|---|
| A3243G | Family | Heteroplasmic | Variant associated with familial DM and deafness | 187 |
| 4399–14821 Deletion | Family | Heteroplasmic | Deletion associated with maternal inheritance of DM and deafness | 14 |
| A3243G | Japanese | Heteroplasmic | Variant associated with familial DM | 77 |
| C1310T, G1438A, A12026G | Japanese | Homoplasmic | Variants associated with T2DM | 179 |
| A3243G | Chinese | Heteroplasmic | Variant associated with T2DM | 210 |
| T14577C | Family | Heteroplasmic | Variant associated with T2DM | 178 |
| A5178C | Japanese | N/A | Variant associated with maternally-inherited T2DM | 195 |
| T16189C | UK Caucasians | Mostly Homoplasmic | Variant associated with T2DM | 139 |
| G3316A, T3394C | Chinese | N/A | Variants associated with T2DM | 207 |
| C8684T | Japanese | N/A | Variant associated with T2DM | 67 |
| T3394C, A14693G, T16189C | Chinese | A14693G Homoplasmic | Variants associated with T2DM | 176 |
| T4216C, A4917G | Caucasian-Brazilian | Homoplasmic | Variants associated with T2DM | 44 |
| G5231A, A12358G, G12372A | Asian | N/A | Variants associated with resistance to T2DM | 60 |
| T16189C | Taiwanese | N/A | Variant associated with T2DM | 101 |
| T16189C | Asian | N/A | Variant associated with T2DM | 130 |
| T3394C, G4491A, T16189C, T16519C; C5178A, A10398G | Chinese Han | N/A | Variants T3394C, G4491A, T16189C, and T16519C are positively correlated with T2DM; variants C5178A, A10398G negatively correlated with T2DM | 96 |
| 16189–16193 Polycytosine Variant | Europid | N/A | Variant associated with T2DM | 205 |
DM, diabetes mellitus; N/A, not available; T2DM, type 2 diabetes mellitus. The mtDNA base pair changes are indicated, followed by the population being studied, the nature of the mtDNA base pair variation, and a summary of the findings.
ncRNA in Mitochondria: miRNA, lncRNA, and circRNA
Recent work has demonstrated that ncRNA species such as microRNA (miRNA), long noncoding RNA (lncRNA), and circular RNA (circRNA) are found in the mitochondria (19, 48, 76, 126, 141, 173). Our laboratory has shown that STZ-treated diabetic mice have increased expression of miRNA-378 in cardiac IFM (76). MiRNA-378 was found to bind and inhibit the translation of the mtDNA-encoded ATP6 mRNA transcript, which contributed to decreased ejection fraction and fractional shortening in diabetic hearts (76). Others have also shown that a nuclear genome-encoded miRNA species can translocate to the mitochondria to regulate mtDNA-encoded genes, as they report miR-181c to bind and inhibit the translation of mt-COX1 mRNA (48). Mitochondrial lncRNA have been found to be both of nuclear and mitochondrial origin (19, 126, 141). Because of their versatility of functions, including binding of mRNA, miRNA, and epigenetic modifiers, the potential differential expression of mitochondrial lncRNA across organ systems in the setting of T2DM may prove important. The function of lncRNA as competitive endogenous RNA that sequesters the activity of complementary miRNA has been reviewed previously (180). Although the stoichiometry of complementary lncRNA and miRNA species may not near the 1:1 ratio required for full selective miRNA inhibition in the cytoplasm due to the large pool of miRNA, the action of lncRNA sponging in the much smaller pool of mitochondrial miRNA may be more pronounced. There are a variety of challenges and potential confounding factors when performing miRNA, lncRNA, or circRNA profiling of cells or tissue samples that have been outlined previously (140, 167, 174). It is important to take into consideration sample processing and RNA extraction methods to ensure minimal degradation of ncRNA in the tissue being studied (140). Understanding the strengths and weaknesses of qRT-PCR, hybridization-based methods, and high-throughput sequencing (RNA-seq) is also imperative when designing miRNA profiling experiments (140). The low abundance of lncRNA in most tissue types increases difficulty of quantification; however, Clark et al. (38) recently developed the technique of capture sequencing that was shown to be superior to qRT-PCR and RNA-seq for detection and quantification of low expressed transcripts such as lncRNA. The low expression of circRNA coupled with the absence of a poly(A) tail prevented the detection of this ncRNA species previously, but new methods of linearizing these transcripts and optimizing RNA-seq library preparation are improving their detection and quantification (174).
Accumulating evidence over the past decade has supported the observation of systemic intercellular miRNA transport via exosomes. It was first demonstrated that mouse mast cells secrete exosomes harboring functional mRNA and miRNA that can be received by both mouse and human mast cells (186). Others have shown Epstein-Barr virus-infected B cells secrete exosomes carrying EBV-miRNAs that are delivered to monocyte-derived dendritic cells where they act on their target transcripts (133). Along the lines of immune cell communication, exosomal miRNA from T cells have been shown to be transported to antigen-presenting cells (112). This mode of intercellular communication has also been found within the cardiovascular system (15, 68). A recent report showed that the interorgan exosomal transport of miRNA-15a from pancreatic β-cells to retinal tissue may contribute to diabetic retinopathy in T2DM (79). Determining the role of interorgan exosome-mediated ncRNA transport in states of health and disease will further illuminate the deep interconnectedness of organ systems.
Mitochondrial Epigenetic and Epitranscriptomic Regulation
Two of the most cutting edge topics of mitochondrial biology are mitochondrial epigenetics and epitranscriptomics. Shock et al. (166) were the first to establish the presence of DNA methyltransferase 1 (DNMT1) in the mitochondria along with describing the mitochondrial targeting sequence in this isoform. They further showed the presence of 5-methylcytosine (5mC) and 5-hydroxymethylcytosine (5hmC) modifications of mtDNA (166). A later study reported 83 specific CpG sites found to be methylated in mtDNA (103). Another group has characterized the methylcytosine landscape of mtDNA from 39 separate human cell and tissue types (62). In support of mtDNA 5hmC modification, the presence of ten-eleven translocase, the enzyme responsible for 5mC hydroxylation to 5hmC, was observed in the mitochondria (53). The impact of T2DM on changes in mtDNA methylation and/or hydroxymethylation and how these changes effect mitochondrial function are wide-open questions for the field to investigate. There have been a variety of methods developed to detect mtDNA methylation, each with unique strengths and weaknesses (188). A couple potential challenges or confounding factors include incomplete mitochondrial purification or nuclear integration of mtDNA sequences (188).
It will also be of interest to look at cross-talk between the mitochondrion and nuclear epigenome in the setting of T2DM, and this dynamic in homeostasis and stress has been reviewed previously (108). Briefly, it has been shown that tissue-specific differentially methylated regions of the nuclear genome exist in a healthy state, accounting for the differences in function and thus gene expression in different tissues (104, 142). These tissue-specific methylation patterns represent the 70–80% of all CpGs that are methylated in most cell types and are the result of both the copying of methylation patterns following DNA replication primarily performed by DNMT1 and de novo methylation during development primarily attributed to DNMT3A and DNMT3B (127, 149, 212). DNA methylation has been shown to be dynamic, as CpG islands in the promoter regions of specific genetic loci may be more demethylated or heavily methylated to increase or decrease the expression of the specific gene in settings of disease such as that of T2DM. For instance, of the 41 genes reported to have differential expression between pancreatic β-cells of T2DM and nondiabetic subjects, 80% (34 genes) had an anticorrelation with their promoter methylation (193). Others found pancreatic duodenal homeobox 1 to be significantly downregulated in T2DM pancreatic β-cells, with 10 CpG sites in the distal promoter and enhancer regions of the gene shown to be much more heavily methylated in T2DM (202). Bisulfite sequencing of nuclear DNA isolated from cells or tissues of interest provides the average level of methylation for that population of cells at each desired site, which may then be correlated with expression of a specific gene. However, the average level of methylation at the exact distal and proximal gene regulatory elements and within the gene body itself required for a specific level of repression or de-repression of specific genes remains to be elucidated. Notably, recent advances in single-cell analysis have resulted in the ability to obtain methylome and transciptome data from the same cell (7, 39, 73). These illuminating single-cell multi-omics studies have further supported promoter methylation as a key influencer of gene repression (7, 73).
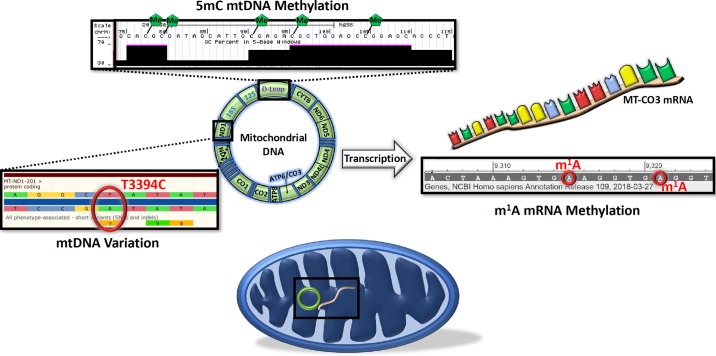
Methylation marks have also been observed on mtDNA-encoded mRNA transcripts (95, 155). The enzymes found in mitochondria to facilitate this mRNA N1-methyladenosine (m1A) modification are TRMT10C and TRMT61B (95, 155). Because of the powerful translation activation or inhibition of the m1A modification, depending on its position on the mRNA transcript, T2DM-induced changes in the mitochondrial epitranscriptome may help to further explain mitochondrial dysfunction in this condition (Fig. 2) (95, 155).
Fig. 2.
mtDNA variation and epigenetic and epitranscriptomic regulation of the mitochondrion. An example of mtDNA variation in the ND1 gene that is associated with increased risk of type 2 diabetes mellitus is shown (Ensembl; left) (96, 176, 207). An example of specific mtDNA CpG sites in the D-Loop found to be methylated is shown (UCSC Genome Browser; top) (103). An example of specific N1-methyladenosine (m1A) modifications of mtDNA-encoded MT-CO3 mRNA is shown (NCBI; right) (95). 5mC, 5-methylcytosine.
MITOCHONDRIA-TARGETED THERAPEUTICS FOR T2DM: RESTORING FUNCTION
Mitochondria-Targeted Antioxidant Therapy for T2DM
As described above, mitochondrial oxidative stress is a major contributor to mitochondrial dysfunction across organ systems in T2DM patients. This increase in ROS production in diabetic models is associated with alterations in both mitochondrial morphology and redox systems biology. In response to high-glucose treatment, it was found that the Clone 9 rat liver cell line and H9c2 rat myoblasts undergo dynamin-like GTPase DLP1/Drp1-mediated mitochondrial fragmentation, which is necessary for and precedes ROS overproduction (208). In a particularly illuminating study, Anderson et al. (3) found a strong link between high-fat diet with increased skeletal muscle mitochondria H2O2 emission, a more oxidized cellular redox state, and insulin resistance. They showed that mitochondrial H2O2 production was a major influencer of the intracellular redox environment by treating standard chow and high-fat diet-fed rats with the mitochondrial H2O2 scavenger SS31 and observing no change in oxidized glutathione or in reduced glutathione/oxidized glutathione ratio following acute glucose ingestion, whereas these measures were increased and decreased in both standard chow and high-fat diet-fed groups (3). The role of mitochondria in mediating insulin resistance in T2DM through changes in redox biology has been reviewed previously (58).
These observations have peaked the interest of many groups to determine the efficacy of mitochondria-targeted antioxidants, including mitoquinone (MitoQ), in diabetic models. MitoQ was found to decrease ROS, leukocyte-endothelium interactions, and TNFα level in leukocytes of T2DM patients (55). It was also shown to improve systemic insulin sensitivity and reduce pancreatic islet lipid peroxide levels in high-fat diet-fed obese mice (75). Further systemic benefit of MitoQ in T2DM is observed by improved renal function following administration to db/db mice (198, 201). Some attribute this positive effect to return of homeostasis in renal tubular cell mitophagy (201). Because of the presence of increased mitochondrial-derived ROS in T2DM musculoskeletal, cardiovascular, endocrine, and nervous systems, it is of high priority to determine whether MitoQ treatment helps to ameliorate the diabetes-induced mitochondrial dysfunction in these organ systems. Results indicating that catalase overexpression improves cardiomyocyte contractility in the agouti T2DM model hint at the potential benefit MitoQ may provide to the diabetic heart (204). Additional clinical and preclinical therapeutic approaches to repairing mitochondrial dysfunction in T2DM have been discussed previously (171).
Mitochondria-Targeted Metabolic Therapy for T2DM
Increased mitochondrial ROS in T2DM is intimately linked to changes in energy substrate metabolism and more inefficient OXPHOS. A common preventative or first-line T2DM therapeutic option is metformin, a member of the thiazolidinedione drug class. Metformin is a dynamic small molecule that has been reported to have multiple mechanisms of action in many different tissue types. These mechanisms of action share in common an alteration in energy substrate metabolism or OXPHOS. It was found that metformin activates AMP-activated protein kinase in hepatocytes and skeletal muscle with downstream effects that include decreased glucose production by the liver and increased glucose disposal into skeletal muscle (211). This observation of increased glucose uptake in skeletal muscle following metformin treatment is further supported by others reporting increased glucose transporter 4 expression in soleus muscle of STZ-induced diabetic rats following metformin administration (34). Another group has observed that metformin treatment in STZ-induced diabetic mice attenuated atherosclerosis by decreasing endothelial mitochondrial fission and mitochondrial-derived superoxide generation (196). Yet others have identified inhibition of ETC complex I and thus decreased OXPHOS as a mechanism of action for metformin (5, 26, 27, 128). It is important to maintain a systems mindset when making sense of how metformin acts to improve the T2DM condition by inhibiting its multiple targets across organ systems.
Mitochondria-Targeted Gene Therapy for Restoring Mitochondrial Omics in T2DM
T2DM is a highly complex polygenic disease with substantial environmental influences. Although there are indeed genetic drivers in T2DM pathogenesis, significant alterations have been found at the mitochondrial proteome and transcriptome levels in the diabetic condition (1, 17, 28, 46, 76, 163). Our laboratory has reported the upregulation of the mitochondrial RNA import constituent polynucleotide phosphorylase and downregulation of mitochondrial protein import constituent mitochondrial heat shock protein 70 in the db/db myocardium to help explain these observations (163, 164). Following transgenic manipulation of these genes to their physiological levels, not only were miRNA transcript and protein expression more normalized to nondiabetic values, but mitochondrial function was also improved (163, 164). As described above, others have shown human antigen R and G-rich RNA sequence-binding factor 1 to facilitate the import of lncRNA into the mitochondria (126). Determining whether these and other important mediators of mitochondrial transport are dysregulated in one or more tissue types of T2DM patients will uncover important insights into mitochondrial regulation. This will provide further validation for the premise of using gene therapy to correct mitochondrial “omics” and help restore mitochondrial function in T2DM.
CONCLUSION
Mitochondrial dysfunction is the common thread across organ systems in T2DM patients. Although the specific pathophysiology observed in each T2DM tissue type is unique due to tissue-specific gene expression patterns influenced by the diabetic systemic milieu, many cellular processes that are gone awry connect to the mitochondrion. This unifying principle of the T2DM phenotype creates a paradigm shift in which considering novel therapies for T2DM comorbidities, including obesity, cardiovascular disease, peripheral neuropathy, and Alzheimer’s disease, becomes increasingly focused. As new technologies such as epitranscriptomic analysis are being forged to uncover the amazing complexity of mitochondrial regulation, an expanding list of potential pharmacological targets is emerging. It will be the synergy between continuing to identify organism-wide driver pathways of T2DM-related mitochondrial dysfunction and advances in medicinal chemistry and mitochondrial drug delivery that may provide combination strategies to optimally treat this systemic condition.
GRANTS
This work was supported by R01-HL-128485 (J. M. Hollander), AHA-17PRE33660333 (Q. A. Hathaway), DGE-1144676 (Q. A. Hathaway), and the Community Foundation for the Ohio Valley Whipkey Trust.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.V.P., Q.A.H., and J.M.H. conceived and designed research; M.V.P. and Q.A.H. prepared figures; M.V.P., G.K.F., Q.A.H., A.J.D., A.K., and J.M.H. drafted manuscript; M.V.P., G.K.F., Q.A.H., A.J.D., A.K., and J.M.H. edited and revised manuscript; G.K.F., Q.A.H., A.J.D., A.K., and J.M.H. approved final version of manuscript.
REFERENCES
- 1.Akude E, Zherebitskaya E, Chowdhury SK, Smith DR, Dobrowsky RT, Fernyhough P. Diminished superoxide generation is associated with respiratory chain dysfunction and changes in the mitochondrial proteome of sensory neurons from diabetic rats. Diabetes 60: 288–297, 2011. doi: 10.2337/db10-0818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson EJ, Kypson AP, Rodriguez E, Anderson CA, Lehr EJ, Neufer PD. Substrate-specific derangements in mitochondrial metabolism and redox balance in the atrium of the type 2 diabetic human heart. J Am Coll Cardiol 54: 1891–1898, 2009. doi: 10.1016/j.jacc.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW III, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 119: 573–581, 2009. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson EJ, Rodriguez E, Anderson CA, Thayne K, Chitwood WR, Kypson AP. Increased propensity for cell death in diabetic human heart is mediated by mitochondrial-dependent pathways. Am J Physiol Heart Circ Physiol 300: H118–H124, 2011. doi: 10.1152/ajpheart.00932.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrzejewski S, Gravel SP, Pollak M, St-Pierre J. Metformin directly acts on mitochondria to alter cellular bioenergetics. Cancer Metab 2: 12, 2014. doi: 10.1186/2049-3002-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anello M, Lupi R, Spampinato D, Piro S, Masini M, Boggi U, Del Prato S, Rabuazzo AM, Purrello F, Marchetti P. Functional and morphological alterations of mitochondria in pancreatic beta cells from type 2 diabetic patients. Diabetologia 48: 282–289, 2005. doi: 10.1007/s00125-004-1627-9. [DOI] [PubMed] [Google Scholar]
- 7.Angermueller C, Clark SJ, Lee HJ, Macaulay IC, Teng MJ, Hu TX, Krueger F, Smallwood S, Ponting CP, Voet T, Kelsey G, Stegle O, Reik W. Parallel single-cell sequencing links transcriptional and epigenetic heterogeneity. Nat Methods 13: 229–232, 2016. doi: 10.1038/nmeth.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antonetti DA, Reynet C, Kahn CR. Increased expression of mitochondrial-encoded genes in skeletal muscle of humans with diabetes mellitus. J Clin Invest 95: 1383–1388, 1995. doi: 10.1172/JCI117790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arruda AP, Pers BM, Parlakgül G, Güney E, Inouye K, Hotamisligil GS. Chronic enrichment of hepatic endoplasmic reticulum-mitochondria contact leads to mitochondrial dysfunction in obesity. Nat Med 20: 1427–1435, 2014. doi: 10.1038/nm.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asmann YW, Stump CS, Short KR, Coenen-Schimke JM, Guo Z, Bigelow ML, Nair KS. Skeletal muscle mitochondrial functions, mitochondrial DNA copy numbers, and gene transcript profiles in type 2 diabetic and nondiabetic subjects at equal levels of low or high insulin and euglycemia. Diabetes 55: 3309–3319, 2006. doi: 10.2337/db05-1230. [DOI] [PubMed] [Google Scholar]
- 11.Bajpeyi S, Pasarica M, Moro C, Conley K, Jubrias S, Sereda O, Burk DH, Zhang Z, Gupta A, Kjems L, Smith SR. Skeletal muscle mitochondrial capacity and insulin resistance in type 2 diabetes. J Clin Endocrinol Metab 96: 1160–1168, 2011. doi: 10.1210/jc.2010-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baker LD, Cross DJ, Minoshima S, Belongia D, Watson GS, Craft S. Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch Neurol 68: 51–57, 2011. doi: 10.1001/archneurol.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balducci S, Iacobellis G, Parisi L, Di Biase N, Calandriello E, Leonetti F, Fallucca F. Exercise training can modify the natural history of diabetic peripheral neuropathy. J Diabetes Complications 20: 216–223, 2006. doi: 10.1016/j.jdiacomp.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Ballinger SW, Shoffner JM, Hedaya EV, Trounce I, Polak MA, Koontz DA, Wallace DC. Maternally transmitted diabetes and deafness associated with a 10.4 kb mitochondrial DNA deletion. Nat Genet 1: 11–15, 1992. doi: 10.1038/ng0492-11. [DOI] [PubMed] [Google Scholar]
- 15.Bang C, Batkai S, Dangwal S, Gupta SK, Foinquinos A, Holzmann A, Just A, Remke J, Zimmer K, Zeug A, Ponimaskin E, Schmiedl A, Yin X, Mayr M, Halder R, Fischer A, Engelhardt S, Wei Y, Schober A, Fiedler J, Thum T. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest 124: 2136–2146, 2014. doi: 10.1172/JCI70577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barazzoni R, Short KR, Nair KS. Effects of aging on mitochondrial DNA copy number and cytochrome c oxidase gene expression in rat skeletal muscle, liver, and heart. J Biol Chem 275: 3343–3347, 2000. doi: 10.1074/jbc.275.5.3343. [DOI] [PubMed] [Google Scholar]
- 17.Baseler WA, Dabkowski ER, Williamson CL, Croston TL, Thapa D, Powell MJ, Razunguzwa TT, Hollander JM. Proteomic alterations of distinct mitochondrial subpopulations in the type 1 diabetic heart: contribution of protein import dysfunction. Am J Physiol Regul Integr Comp Physiol 300: R186–R200, 2011. doi: 10.1152/ajpregu.00423.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beaudoin MS, Perry CG, Arkell AM, Chabowski A, Simpson JA, Wright DC, Holloway GP. Impairments in mitochondrial palmitoyl-CoA respiratory kinetics that precede development of diabetic cardiomyopathy are prevented by resveratrol in ZDF rats. J Physiol 592: 2519–2533, 2014. doi: 10.1113/jphysiol.2013.270538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bianchessi V, Badi I, Bertolotti M, Nigro P, D’Alessandra Y, Capogrossi MC, Zanobini M, Pompilio G, Raucci A, Lauri A. The mitochondrial lncRNA ASncmtRNA-2 is induced in aging and replicative senescence in Endothelial Cells. J Mol Cell Cardiol 81: 62–70, 2015. doi: 10.1016/j.yjmcc.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 20.Bloem CJ, Chang AM. Short-term exercise improves beta-cell function and insulin resistance in older people with impaired glucose tolerance. J Clin Endocrinol Metab 93: 387–392, 2008. doi: 10.1210/jc.2007-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonen A, Parolin ML, Steinberg GR, Calles-Escandon J, Tandon NN, Glatz JF, Luiken JJ, Heigenhauser GJ, Dyck DJ. Triacylglycerol accumulation in human obesity and type 2 diabetes is associated with increased rates of skeletal muscle fatty acid transport and increased sarcolemmal FAT/CD36. FASEB J 18: 1144–1146, 2004. doi: 10.1096/fj.03-1065fje. [DOI] [PubMed] [Google Scholar]
- 22.Bonnard C, Durand A, Peyrol S, Chanseaume E, Chauvin MA, Morio B, Vidal H, Rieusset J. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest 118: 789–800, 2008. doi: 10.1172/JCI32601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boudina S, Sena S, Theobald H, Sheng X, Wright JJ, Hu XX, Aziz S, Johnson JI, Bugger H, Zaha VG, Abel ED. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes 56: 2457–2466, 2007. doi: 10.2337/db07-0481. [DOI] [PubMed] [Google Scholar]
- 24.Boushel R, Gnaiger E, Schjerling P, Skovbro M, Kraunsøe R, Dela F. Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia 50: 790–796, 2007. doi: 10.1007/s00125-007-0594-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bravard A, Lefai E, Meugnier E, Pesenti S, Disse E, Vouillarmet J, Peretti N, Rabasa-Lhoret R, Laville M, Vidal H, Rieusset J. FTO is increased in muscle during type 2 diabetes, and its overexpression in myotubes alters insulin signaling, enhances lipogenesis and ROS production, and induces mitochondrial dysfunction. Diabetes 60: 258–268, 2011. doi: 10.2337/db10-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bridges HR, Jones AJ, Pollak MN, Hirst J. Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. Biochem J 462: 475–487, 2014. doi: 10.1042/BJ20140620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brunmair B, Staniek K, Gras F, Scharf N, Althaym A, Clara R, Roden M, Gnaiger E, Nohl H, Waldhäusl W, Fürnsinn C. Thiazolidinediones, like metformin, inhibit respiratory complex I: a common mechanism contributing to their antidiabetic actions? Diabetes 53: 1052–1059, 2004. doi: 10.2337/diabetes.53.4.1052. [DOI] [PubMed] [Google Scholar]
- 28.Bugger H, Chen D, Riehle C, Soto J, Theobald HA, Hu XX, Ganesan B, Weimer BC, Abel ED. Tissue-specific remodeling of the mitochondrial proteome in type 1 diabetic akita mice. Diabetes 58: 1986–1997, 2009. doi: 10.2337/db09-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carvalho C, Santos MS, Oliveira CR, Moreira PI. Alzheimer’s disease and type 2 diabetes-related alterations in brain mitochondria, autophagy and synaptic markers. Biochim Biophys Acta 1852: 1665–1675, 2015. doi: 10.1016/j.bbadis.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Cassidy S, Thoma C, Hallsworth K, Parikh J, Hollingsworth KG, Taylor R, Jakovljevic DG, Trenell MI. High intensity intermittent exercise improves cardiac structure and function and reduces liver fat in patients with type 2 diabetes: a randomised controlled trial. Diabetologia 59: 56–66, 2016. doi: 10.1007/s00125-015-3741-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chattopadhyay M, Guhathakurta I, Behera P, Ranjan KR, Khanna M, Mukhopadhyay S, Chakrabarti S. Mitochondrial bioenergetics is not impaired in nonobese subjects with type 2 diabetes mellitus. Metabolism 60: 1702–1710, 2011. doi: 10.1016/j.metabol.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 32.Chattopadhyay M, Khemka VK, Chatterjee G, Ganguly A, Mukhopadhyay S, Chakrabarti S. Enhanced ROS production and oxidative damage in subcutaneous white adipose tissue mitochondria in obese and type 2 diabetes subjects. Mol Cell Biochem 399: 95–103, 2015. doi: 10.1007/s11010-014-2236-7. [DOI] [PubMed] [Google Scholar]
- 33.Chavin KD, Yang S, Lin HZ, Chatham J, Chacko VP, Hoek JB, Walajtys-Rode E, Rashid A, Chen CH, Huang CC, Wu TC, Lane MD, Diehl AM. Obesity induces expression of uncoupling protein-2 in hepatocytes and promotes liver ATP depletion. J Biol Chem 274: 5692–5700, 1999. doi: 10.1074/jbc.274.9.5692. [DOI] [PubMed] [Google Scholar]
- 34.Cheng JT, Huang CC, Liu IM, Tzeng TF, Chang CJ. Novel mechanism for plasma glucose-lowering action of metformin in streptozotocin-induced diabetic rats. Diabetes 55: 819–825, 2006. doi: 10.2337/diabetes.55.03.06.db05-0934. [DOI] [PubMed] [Google Scholar]
- 35.Cheng Z, Guo S, Copps K, Dong X, Kollipara R, Rodgers JT, Depinho RA, Puigserver P, White MF. Foxo1 integrates insulin signaling with mitochondrial function in the liver. Nat Med 15: 1307–1311, 2009. doi: 10.1038/nm.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cho YE, Basu A, Dai A, Heldak M, Makino A. Coronary endothelial dysfunction and mitochondrial reactive oxygen species in type 2 diabetic mice. Am J Physiol Cell Physiol 305: C1033–C1040, 2013. doi: 10.1152/ajpcell.00234.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choo HJ, Kim JH, Kwon OB, Lee CS, Mun JY, Han SS, Yoon YS, Yoon G, Choi KM, Ko YG. Mitochondria are impaired in the adipocytes of type 2 diabetic mice. Diabetologia 49: 784–791, 2006. doi: 10.1007/s00125-006-0170-2. [DOI] [PubMed] [Google Scholar]
- 38.Clark MB, Mercer TR, Bussotti G, Leonardi T, Haynes KR, Crawford J, Brunck ME, Cao KA, Thomas GP, Chen WY, Taft RJ, Nielsen LK, Enright AJ, Mattick JS, Dinger ME. Quantitative gene profiling of long noncoding RNAs with targeted RNA sequencing. Nat Methods 12: 339–342, 2015. doi: 10.1038/nmeth.3321. [DOI] [PubMed] [Google Scholar]
- 39.Clark SJ, Argelaguet R, Kapourani CA, Stubbs TM, Lee HJ, Alda-Catalinas C, Krueger F, Sanguinetti G, Kelsey G, Marioni JC, Stegle O, Reik W. scNMT-seq enables joint profiling of chromatin accessibility DNA methylation and transcription in single cells. Nat Commun 9: 781, 2018. doi: 10.1038/s41467-018-03149-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen ND, Dunstan DW, Robinson C, Vulikh E, Zimmet PZ, Shaw JE. Improved endothelial function following a 14-month resistance exercise training program in adults with type 2 diabetes. Diabetes Res Clin Pract 79: 405–411, 2008. doi: 10.1016/j.diabres.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 41.Corral-Debrinski M, Horton T, Lott MT, Shoffner JM, Beal MF, Wallace DC. Mitochondrial DNA deletions in human brain: regional variability and increase with advanced age. Nat Genet 2: 324–329, 1992. doi: 10.1038/ng1292-324. [DOI] [PubMed] [Google Scholar]
- 42.Covian R, French S, Kusnetz H, Balaban RS. Stimulation of oxidative phosphorylation by calcium in cardiac mitochondria is not influenced by cAMP and PKA activity. Biochim Biophys Acta 1837: 1913–1921, 2014. doi: 10.1016/j.bbabio.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cree LM, Patel SK, Pyle A, Lynn S, Turnbull DM, Chinnery PF, Walker M. Age-related decline in mitochondrial DNA copy number in isolated human pancreatic islets. Diabetologia 51: 1440–1443, 2008. doi: 10.1007/s00125-008-1054-4. [DOI] [PubMed] [Google Scholar]
- 44.Crispim D, Canani LH, Gross JL, Tschiedel B, Souto KE, Roisenberg I. The European-specific mitochondrial cluster J/T could confer an increased risk of insulin-resistance and type 2 diabetes: an analysis of the m.4216T > C and m.4917A > G variants. Ann Hum Genet 70: 488–495, 2006. doi: 10.1111/j.1469-1809.2005.00249.x. [DOI] [PubMed] [Google Scholar]
- 45.Croston TL, Thapa D, Holden AA, Tveter KJ, Lewis SE, Shepherd DL, Nichols CE, Long DM, Olfert IM, Jagannathan R, Hollander JM. Functional deficiencies of subsarcolemmal mitochondria in the type 2 diabetic human heart. Am J Physiol Heart Circ Physiol 307: H54–H65, 2014. doi: 10.1152/ajpheart.00845.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dabkowski ER, Baseler WA, Williamson CL, Powell M, Razunguzwa TT, Frisbee JC, Hollander JM. Mitochondrial dysfunction in the type 2 diabetic heart is associated with alterations in spatially distinct mitochondrial proteomes. Am J Physiol Heart Circ Physiol 299: H529–H540, 2010. doi: 10.1152/ajpheart.00267.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dahlman I, Forsgren M, Sjögren A, Nordström EA, Kaaman M, Näslund E, Attersand A, Arner P. Downregulation of electron transport chain genes in visceral adipose tissue in type 2 diabetes independent of obesity and possibly involving tumor necrosis factor-alpha. Diabetes 55: 1792–1799, 2006. doi: 10.2337/db05-1421. [DOI] [PubMed] [Google Scholar]
- 48.Das S, Ferlito M, Kent OA, Fox-Talbot K, Wang R, Liu D, Raghavachari N, Yang Y, Wheelan SJ, Murphy E, Steenbergen C. Nuclear miRNA regulates the mitochondrial genome in the heart. Circ Res 110: 1596–1603, 2012. doi: 10.1161/CIRCRESAHA.112.267732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456: 605–610, 2008. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 50.De Feyter HM, van den Broek NM, Praet SF, Nicolay K, van Loon LJ, Prompers JJ. Early or advanced stage type 2 diabetes is not accompanied by in vivo skeletal muscle mitochondrial dysfunction. Eur J Endocrinol 158: 643–653, 2008. doi: 10.1530/EJE-07-0756. [DOI] [PubMed] [Google Scholar]
- 51.Dlasková A, Spacek T, Santorová J, Plecitá-Hlavatá L, Berková Z, Saudek F, Lessard M, Bewersdorf J, Jezek P. 4Pi microscopy reveals an impaired three-dimensional mitochondrial network of pancreatic islet beta-cells, an experimental model of type-2 diabetes. Biochim Biophys Acta 1797: 1327–1341, 2010. doi: 10.1016/j.bbabio.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 52.Donath MY, Ehses JA, Maedler K, Schumann DM, Ellingsgaard H, Eppler E, Reinecke M. Mechanisms of beta-cell death in type 2 diabetes. Diabetes 54, Suppl 2: S108–S113, 2005. doi: 10.2337/diabetes.54.suppl_2.S108. [DOI] [PubMed] [Google Scholar]
- 53.Dzitoyeva S, Chen H, Manev H. Effect of aging on 5-hydroxymethylcytosine in brain mitochondria. Neurobiol Aging 33: 2881–2891, 2012. doi: 10.1016/j.neurobiolaging.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Edwards JL, Quattrini A, Lentz SI, Figueroa-Romero C, Cerri F, Backus C, Hong Y, Feldman EL. Diabetes regulates mitochondrial biogenesis and fission in mouse neurons. Diabetologia 53: 160–169, 2010. doi: 10.1007/s00125-009-1553-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Escribano-Lopez I, Diaz-Morales N, Rovira-Llopis S, de Marañon AM, Orden S, Alvarez A, Bañuls C, Rocha M, Murphy MP, Hernandez-Mijares A, Victor VM. The mitochondria-targeted antioxidant MitoQ modulates oxidative stress, inflammation and leukocyte-endothelium interactions in leukocytes isolated from type 2 diabetic patients. Redox Biol 10: 200–205, 2016. doi: 10.1016/j.redox.2016.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Feder J, Ovadia O, Blech I, Cohen J, Wainstein J, Harman-Boehm I, Glaser B, Mishmar D. Parental diabetes status reveals association of mitochondrial DNA haplogroup J1 with type 2 diabetes. BMC Med Genet 10: 60, 2009. doi: 10.1186/1471-2350-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Federation ID. IDF Diabetes Atlas, 8th ed Brussels: International Diabetes Federation, 2017. [Google Scholar]
- 58.Fisher-Wellman KH, Neufer PD. Linking mitochondrial bioenergetics to insulin resistance via redox biology. Trends Endocrinol Metab 23: 142–153, 2012. doi: 10.1016/j.tem.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fritz T, Krämer DK, Karlsson HK, Galuska D, Engfeldt P, Zierath JR, Krook A. Low-intensity exercise increases skeletal muscle protein expression of PPARdelta and UCP3 in type 2 diabetic patients. Diabetes Metab Res Rev 22: 492–498, 2006. doi: 10.1002/dmrr.656. [DOI] [PubMed] [Google Scholar]
- 60.Fuku N, Park KS, Yamada Y, Nishigaki Y, Cho YM, Matsuo H, Segawa T, Watanabe S, Kato K, Yokoi K, Nozawa Y, Lee HK, Tanaka M. Mitochondrial haplogroup N9a confers resistance against type 2 diabetes in Asians. Am J Hum Genet 80: 407–415, 2007. doi: 10.1086/512202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao CL, Zhu C, Zhao YP, Chen XH, Ji CB, Zhang CM, Zhu JG, Xia ZK, Tong ML, Guo XR. Mitochondrial dysfunction is induced by high levels of glucose and free fatty acids in 3T3-L1 adipocytes. Mol Cell Endocrinol 320: 25–33, 2010. doi: 10.1016/j.mce.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 62.Ghosh S, Sengupta S, Scaria V. Comparative analysis of human mitochondrial methylomes shows distinct patterns of epigenetic regulation in mitochondria. Mitochondrion 18: 58–62, 2014. doi: 10.1016/j.mito.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 63.Glancy B, Hartnell LM, Combs CA, Femnou A, Sun J, Murphy E, Subramaniam S, Balaban RS. Power grid protection of the muscle mitochondrial reticulum. Cell Reports 19: 487–496, 2017. [Erratum in Cell Rep 23: 2832, 2018]. doi: 10.1016/j.celrep.2017.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Glancy B, Hartnell LM, Malide D, Yu ZX, Combs CA, Connelly PS, Subramaniam S, Balaban RS. Mitochondrial reticulum for cellular energy distribution in muscle. Nature 523: 617–620, 2015. doi: 10.1038/nature14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Glancy B, Willis WT, Chess DJ, Balaban RS. Effect of calcium on the oxidative phosphorylation cascade in skeletal muscle mitochondria. Biochemistry 52: 2793–2809, 2013. doi: 10.1021/bi3015983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gordon ES. Non-esterified fatty acids in the blood of obese and lean subjects. Am J Clin Nutr 8: 740–747, 1960. doi: 10.1093/ajcn/8.5.740. [DOI] [Google Scholar]
- 67.Guo LJ, Oshida Y, Fuku N, Takeyasu T, Fujita Y, Kurata M, Sato Y, Ito M, Tanaka M. Mitochondrial genome polymorphisms associated with type-2 diabetes or obesity. Mitochondrion 5: 15–33, 2005. doi: 10.1016/j.mito.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 68.Hergenreider E, Heydt S, Tréguer K, Boettger T, Horrevoets AJ, Zeiher AM, Scheffer MP, Frangakis AS, Yin X, Mayr M, Braun T, Urbich C, Boon RA, Dimmeler S. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol 14: 249–256, 2012. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- 69.Hey-Mogensen M, Højlund K, Vind BF, Wang L, Dela F, Beck-Nielsen H, Fernström M, Sahlin K. Effect of physical training on mitochondrial respiration and reactive oxygen species release in skeletal muscle in patients with obesity and type 2 diabetes. Diabetologia 53: 1976–1985, 2010. doi: 10.1007/s00125-010-1813-x. [DOI] [PubMed] [Google Scholar]
- 70.Hinder LM, Vivekanandan-Giri A, McLean LL, Pennathur S, Feldman EL. Decreased glycolytic and tricarboxylic acid cycle intermediates coincide with peripheral nervous system oxidative stress in a murine model of type 2 diabetes. J Endocrinol 216: 1–11, 2013. doi: 10.1530/JOE-12-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Højlund K, Wrzesinski K, Larsen PM, Fey SJ, Roepstorff P, Handberg A, Dela F, Vinten J, McCormack JG, Reynet C, Beck-Nielsen H. Proteome analysis reveals phosphorylation of ATP synthase beta -subunit in human skeletal muscle and proteins with potential roles in type 2 diabetes. J Biol Chem 278: 10436–10442, 2003. doi: 10.1074/jbc.M212881200. [DOI] [PubMed] [Google Scholar]
- 72.Holmström MH, Iglesias-Gutierrez E, Zierath JR, Garcia-Roves PM. Tissue-specific control of mitochondrial respiration in obesity-related insulin resistance and diabetes. Am J Physiol Endocrinol Metab 302: E731–E739, 2012. doi: 10.1152/ajpendo.00159.2011. [DOI] [PubMed] [Google Scholar]
- 73.Hu Y, Huang K, An Q, Du G, Hu G, Xue J, Zhu X, Wang CY, Xue Z, Fan G. Simultaneous profiling of transcriptome and DNA methylome from a single cell. Genome Biol 17: 88, 2016. doi: 10.1186/s13059-016-0950-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hwang JH, Stein DT, Barzilai N, Cui MH, Tonelli J, Kishore P, Hawkins M. Increased intrahepatic triglyceride is associated with peripheral insulin resistance: in vivo MR imaging and spectroscopy studies. Am J Physiol Endocrinol Metab 293: E1663–E1669, 2007. doi: 10.1152/ajpendo.00590.2006. [DOI] [PubMed] [Google Scholar]
- 75.Imai Y, Fink BD, Promes JA, Kulkarni CA, Kerns RJ, Sivitz WI. Effect of a mitochondrial-targeted coenzyme Q analog on pancreatic β-cell function and energetics in high fat fed obese mice. Pharmacol Res Perspect 6: e00393, 2018. doi: 10.1002/prp2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jagannathan R, Thapa D, Nichols CE, Shepherd DL, Stricker JC, Croston TL, Baseler WA, Lewis SE, Martinez I, Hollander JM. Translational Regulation of the Mitochondrial Genome Following Redistribution of Mitochondrial MicroRNA in the Diabetic Heart. Circ Cardiovasc Genet 8: 785–802, 2015. doi: 10.1161/CIRCGENETICS.115.001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kadowaki T, Kadowaki H, Mori Y, Tobe K, Sakuta R, Suzuki Y, Tanabe Y, Sakura H, Awata T, Goto Y, Hayakawa T, Matsuoka K, Kawamori R, Kamada T, Horai S, Nonaka I, Hagura R, Akanuma Y, Yazaki Y. A subtype of diabetes mellitus associated with a mutation of mitochondrial DNA. N Engl J Med 330: 962–968, 1994. doi: 10.1056/NEJM199404073301403. [DOI] [PubMed] [Google Scholar]
- 78.Kalichman MW, Powell HC, Mizisin AP. Reactive, degenerative, and proliferative Schwann cell responses in experimental galactose and human diabetic neuropathy. Acta Neuropathol 95: 47–56, 1998. doi: 10.1007/s004010050764. [DOI] [PubMed] [Google Scholar]
- 79.Kamalden TA, Macgregor-Das AM, Kannan SM, Dunkerly-Eyring B, Khaliddin N, Xu Z, Fusco AP, Yazib SA, Chow RC, Duh EJ, Halushka MK, Steenbergen C, Das S. Exosomal MicroRNA-15a Transfer from the Pancreas Augments Diabetic Complications by Inducing Oxidative Stress. Antioxid Redox Signal 27: 913–930, 2017. doi: 10.1089/ars.2016.6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kanazawa A, Nishio Y, Kashiwagi A, Inagaki H, Kikkawa R, Horiike K. Reduced activity of mtTFA decreases the transcription in mitochondria isolated from diabetic rat heart. Am J Physiol Endocrinol Metab 282: E778–E785, 2002. doi: 10.1152/ajpendo.00255.2001. [DOI] [PubMed] [Google Scholar]
- 81.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51: 2944–2950, 2002. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 82.Kelley DE, Simoneau JA. Impaired free fatty acid utilization by skeletal muscle in non-insulin-dependent diabetes mellitus. J Clin Invest 94: 2349–2356, 1994. doi: 10.1172/JCI117600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kizhakekuttu TJ, Wang J, Dharmashankar K, Ying R, Gutterman DD, Vita JA, Widlansky ME. Adverse alterations in mitochondrial function contribute to type 2 diabetes mellitus-related endothelial dysfunction in humans. Arterioscler Thromb Vasc Biol 32: 2531–2539, 2012. doi: 10.1161/ATVBAHA.112.256024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Koka S, Aluri HS, Xi L, Lesnefsky EJ, Kukreja RC. Chronic inhibition of phosphodiesterase 5 with tadalafil attenuates mitochondrial dysfunction in type 2 diabetic hearts: potential role of NO/SIRT1/PGC-1α signaling. Am J Physiol Heart Circ Physiol 306: H1558–H1568, 2014. doi: 10.1152/ajpheart.00865.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Koliaki C, Szendroedi J, Kaul K, Jelenik T, Nowotny P, Jankowiak F, Herder C, Carstensen M, Krausch M, Knoefel WT, Schlensak M, Roden M. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab 21: 739–746, 2015. doi: 10.1016/j.cmet.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 86.Koo SH, Satoh H, Herzig S, Lee CH, Hedrick S, Kulkarni R, Evans RM, Olefsky J, Montminy M. PGC-1 promotes insulin resistance in liver through PPAR-alpha-dependent induction of TRB-3. Nat Med 10: 530–534, 2004. doi: 10.1038/nm1044. [DOI] [PubMed] [Google Scholar]
- 87.Koska J, Stefan N, Permana PA, Weyer C, Sonoda M, Bogardus C, Smith SR, Joanisse DR, Funahashi T, Krakoff J, Bunt JC. Increased fat accumulation in liver may link insulin resistance with subcutaneous abdominal adipocyte enlargement, visceral adiposity, and hypoadiponectinemia in obese individuals. Am J Clin Nutr 87: 295–302, 2008. doi: 10.1093/ajcn/87.2.295. [DOI] [PubMed] [Google Scholar]
- 88.Kotronen A, Juurinen L, Tiikkainen M, Vehkavaara S, Yki-Järvinen H. Increased liver fat, impaired insulin clearance, and hepatic and adipose tissue insulin resistance in type 2 diabetes. Gastroenterology 135: 122–130, 2008. doi: 10.1053/j.gastro.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 89.Kotronen A, Seppälä-Lindroos A, Bergholm R, Yki-Järvinen H. Tissue specificity of insulin resistance in humans: fat in the liver rather than muscle is associated with features of the metabolic syndrome. Diabetologia 51: 130–138, 2008. doi: 10.1007/s00125-007-0867-x. [DOI] [PubMed] [Google Scholar]
- 90.Krebs M, Roden M. Molecular mechanisms of lipid-induced insulin resistance in muscle, liver and vasculature. Diabetes Obes Metab 7: 621–632, 2005. doi: 10.1111/j.1463-1326.2004.00439.x. [DOI] [PubMed] [Google Scholar]
- 91.Krotkiewski M, Lönnroth P, Mandroukas K, Wroblewski Z, Rebuffé-Scrive M, Holm G, Smith U, Björntorp P. The effects of physical training on insulin secretion and effectiveness and on glucose metabolism in obesity and type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 28: 881–890, 1985. doi: 10.1007/BF00703130. [DOI] [PubMed] [Google Scholar]
- 92.Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Van Remmen H, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla TA. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309: 481–484, 2005. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- 93.Laye MJ, Rector RS, Warner SO, Naples SP, Perretta AL, Uptergrove GM, Laughlin MH, Thyfault JP, Booth FW, Ibdah JA. Changes in visceral adipose tissue mitochondrial content with type 2 diabetes and daily voluntary wheel running in OLETF rats. J Physiol 587: 3729–3739, 2009. doi: 10.1113/jphysiol.2009.172601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee Y, Hirose H, Ohneda M, Johnson JH, McGarry JD, Unger RH. Beta-cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-beta-cell relationships. Proc Natl Acad Sci USA 91: 10878–10882, 1994. doi: 10.1073/pnas.91.23.10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li X, Xiong X, Zhang M, Wang K, Chen Y, Zhou J, Mao Y, Lv J, Yi D, Chen XW, Wang C, Qian SB, Yi C. Base-resolution mapping reveals distinct m1A methylome in nuclear- and mitochondrial-encoded transcripts. Mol Cell 68: 993–1005.e9, 2017. doi: 10.1016/j.molcel.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liao WQ, Pang Y, Yu CA, Wen JY, Zhang YG, Li XH. Novel mutations of mitochondrial DNA associated with type 2 diabetes in Chinese Han population. Tohoku J Exp Med 215: 377–384, 2008. doi: 10.1620/tjem.215.377. [DOI] [PubMed] [Google Scholar]
- 97.Lim JH, Lee HJ, Ho Jung M, Song J. Coupling mitochondrial dysfunction to endoplasmic reticulum stress response: a molecular mechanism leading to hepatic insulin resistance. Cell Signal 21: 169–177, 2009. doi: 10.1016/j.cellsig.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 98.Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab 1: 361–370, 2005. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 99.Lindström J, Tuomilehto J. The diabetes risk score: a practical tool to predict type 2 diabetes risk. Diabetes Care 26: 725–731, 2003. doi: 10.2337/diacare.26.3.725. [DOI] [PubMed] [Google Scholar]
- 100.Liou CW, Chen JB, Tiao MM, Weng SW, Huang TL, Chuang JH, Chen SD, Chuang YC, Lee WC, Lin TK, Wang PW. Mitochondrial DNA coding and control region variants as genetic risk factors for type 2 diabetes. Diabetes 61: 2642–2651, 2012. doi: 10.2337/db11-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liou CW, Lin TK, Huei Weng H, Lee CF, Chen TL, Wei YH, Chen SD, Chuang YC, Weng SW, Wang PW. A common mitochondrial DNA variant and increased body mass index as associated factors for development of type 2 diabetes: Additive effects of genetic and environmental factors. J Clin Endocrinol Metab 92: 235–239, 2007. doi: 10.1210/jc.2006-0653. [DOI] [PubMed] [Google Scholar]
- 102.Little JP, Gillen JB, Percival ME, Safdar A, Tarnopolsky MA, Punthakee Z, Jung ME, Gibala MJ. Low-volume high-intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. J Appl Physiol (1985) 111: 1554–1560, 2011. doi: 10.1152/japplphysiol.00921.2011. [DOI] [PubMed] [Google Scholar]
- 103.Liu B, Du Q, Chen L, Fu G, Li S, Fu L, Zhang X, Ma C, Bin C. CpG methylation patterns of human mitochondrial DNA. Sci Rep 6: 23421, 2016. doi: 10.1038/srep23421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lokk K, Modhukur V, Rajashekar B, Märtens K, Mägi R, Kolde R, Koltšina M, Nilsson TK, Vilo J, Salumets A, Tõnisson N. DNA methylome profiling of human tissues identifies global and tissue-specific methylation patterns. Genome Biol 15: 3248, 2014. [Erratum in Genome Biol 17: 224, 2016]. doi: 10.1186/gb-2014-15-4-r54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science 307: 384–387, 2005. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 106.Mariappan N, Elks CM, Sriramula S, Guggilam A, Liu Z, Borkhsenious O, Francis J. NF-kappaB-induced oxidative stress contributes to mitochondrial and cardiac dysfunction in type II diabetes. Cardiovasc Res 85: 473–483, 2010. doi: 10.1093/cvr/cvp305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Masuoka HC, Chalasani N. Nonalcoholic fatty liver disease: an emerging threat to obese and diabetic individuals. Ann N Y Acad Sci 1281: 106–122, 2013. doi: 10.1111/nyas.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Matilainen O, Quirós PM, Auwerx J. Mitochondria and epigenetics - crosstalk in homeostasis and stress. Trends Cell Biol 27: 453–463, 2017. doi: 10.1016/j.tcb.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 109.Mazumder PK, O’Neill BT, Roberts MW, Buchanan J, Yun UJ, Cooksey RC, Boudina S, Abel ED. Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes 53: 2366–2374, 2004. doi: 10.2337/diabetes.53.9.2366. [DOI] [PubMed] [Google Scholar]
- 110.Meex RC, Schrauwen-Hinderling VB, Moonen-Kornips E, Schaart G, Mensink M, Phielix E, van de Weijer T, Sels JP, Schrauwen P, Hesselink MK. Restoration of muscle mitochondrial function and metabolic flexibility in type 2 diabetes by exercise training is paralleled by increased myocellular fat storage and improved insulin sensitivity. Diabetes 59: 572–579, 2010. doi: 10.2337/db09-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell 6: 87–97, 2000. doi: 10.1016/S1097-2765(05)00015-8. [DOI] [PubMed] [Google Scholar]
- 112.Mittelbrunn M, Gutiérrez-Vázquez C, Villarroya-Beltri C, González S, Sánchez-Cabo F, González MA, Bernad A, Sánchez-Madrid F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun 2: 282, 2011. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mogensen M, Sahlin K, Fernström M, Glintborg D, Vind BF, Beck-Nielsen H, Højlund K. Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes. Diabetes 56: 1592–1599, 2007. doi: 10.2337/db06-0981. [DOI] [PubMed] [Google Scholar]
- 114.Mohlke KL, Jackson AU, Scott LJ, Peck EC, Suh YD, Chines PS, Watanabe RM, Buchanan TA, Conneely KN, Erdos MR, Narisu N, Enloe S, Valle TT, Tuomilehto J, Bergman RN, Boehnke M, Collins FS. Mitochondrial polymorphisms and susceptibility to type 2 diabetes-related traits in Finns. Hum Genet 118: 245–254, 2005. doi: 10.1007/s00439-005-0046-4. [DOI] [PubMed] [Google Scholar]
- 115.Montaigne D, Marechal X, Coisne A, Debry N, Modine T, Fayad G, Potelle C, El Arid JM, Mouton S, Sebti Y, Duez H, Preau S, Remy-Jouet I, Zerimech F, Koussa M, Richard V, Neviere R, Edme JL, Lefebvre P, Staels B. Myocardial contractile dysfunction is associated with impaired mitochondrial function and dynamics in type 2 diabetic but not in obese patients. Circulation 130: 554–564, 2014. doi: 10.1161/CIRCULATIONAHA.113.008476. [DOI] [PubMed] [Google Scholar]
- 116.Montgomery MK, Turner N. Mitochondrial dysfunction and insulin resistance: an update. Endocr Connect 4: R1–R15, 2015. doi: 10.1530/EC-14-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstråle M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34: 267–273, 2003. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 118.Moreira PI, Santos MS, Moreno AM, Seiça R, Oliveira CR. Increased vulnerability of brain mitochondria in diabetic (Goto-Kakizaki) rats with aging and amyloid-beta exposure. Diabetes 52: 1449–1456, 2003. doi: 10.2337/diabetes.52.6.1449. [DOI] [PubMed] [Google Scholar]
- 119.Moreira PI, Santos MS, Sena C, Nunes E, Seiça R, Oliveira CR. CoQ10 therapy attenuates amyloid beta-peptide toxicity in brain mitochondria isolated from aged diabetic rats. Exp Neurol 196: 112–119, 2005. doi: 10.1016/j.expneurol.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 120.Nadler ST, Stoehr JP, Schueler KL, Tanimoto G, Yandell BS, Attie AD. The expression of adipogenic genes is decreased in obesity and diabetes mellitus. Proc Natl Acad Sci USA 97: 11371–11376, 2000. doi: 10.1073/pnas.97.21.11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nakamura H, Matoba S, Iwai-Kanai E, Kimata M, Hoshino A, Nakaoka M, Katamura M, Okawa Y, Ariyoshi M, Mita Y, Ikeda K, Okigaki M, Adachi S, Tanaka H, Takamatsu T, Matsubara H. p53 promotes cardiac dysfunction in diabetic mellitus caused by excessive mitochondrial respiration-mediated reactive oxygen species generation and lipid accumulation. Circ Heart Fail 5: 106–115, 2012. [Erratum in Circ Heart Fail 7: 383, 2014]. doi: 10.1161/CIRCHEARTFAILURE.111.961565. [DOI] [PubMed] [Google Scholar]
- 122.Neschen S, Morino K, Hammond LE, Zhang D, Liu ZX, Romanelli AJ, Cline GW, Pongratz RL, Zhang XM, Choi CS, Coleman RA, Shulman GI. Prevention of hepatic steatosis and hepatic insulin resistance in mitochondrial acyl-CoA:glycerol-sn-3-phosphate acyltransferase 1 knockout mice. Cell Metab 2: 55–65, 2005. doi: 10.1016/j.cmet.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 123.Nieuwoudt S, Fealy CE, Foucher JA, Scelsi AR, Malin SK, Pagadala M, Rocco M, Burguera B, Kirwan JP. Functional high-intensity training improves pancreatic β-cell function in adults with type 2 diabetes. Am J Physiol Endocrinol Metab 313: E314–E320, 2017. doi: 10.1152/ajpendo.00407.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nile DL, Brown AE, Kumaheri MA, Blair HR, Heggie A, Miwa S, Cree LM, Payne B, Chinnery PF, Brown L, Gunn DA, Walker M. Age-related mitochondrial DNA depletion and the impact on pancreatic Beta cell function. PLoS One 9: e115433, 2014. doi: 10.1371/journal.pone.0115433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nilsson E, Jansson PA, Perfilyev A, Volkov P, Pedersen M, Svensson MK, Poulsen P, Ribel-Madsen R, Pedersen NL, Almgren P, Fadista J, Rönn T, Klarlund Pedersen B, Scheele C, Vaag A, Ling C. Altered DNA methylation and differential expression of genes influencing metabolism and inflammation in adipose tissue from subjects with type 2 diabetes. Diabetes 63: 2962–2976, 2014. doi: 10.2337/db13-1459. [DOI] [PubMed] [Google Scholar]
- 126.Noh JH, Kim KM, Abdelmohsen K, Yoon JH, Panda AC, Munk R, Kim J, Curtis J, Moad CA, Wohler CM, Indig FE, de Paula W, Dudekula DB, De S, Piao Y, Yang X, Martindale JL, de Cabo R, Gorospe M. HuR and GRSF1 modulate the nuclear export and mitochondrial localization of the lncRNA RMRP. Genes Dev 30: 1224–1239, 2016. doi: 10.1101/gad.276022.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 99: 247–257, 1999. doi: 10.1016/S0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- 128.Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J 348: 607–614, 2000. doi: 10.1042/bj3480607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pan X, Liu J, Nguyen T, Liu C, Sun J, Teng Y, Fergusson MM, Rovira II, Allen M, Springer DA, Aponte AM, Gucek M, Balaban RS, Murphy E, Finkel T. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat Cell Biol 15: 1464–1472, 2013. doi: 10.1038/ncb2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Park KS, Chan JC, Chuang LM, Suzuki S, Araki E, Nanjo K, Ji L, Ng M, Nishi M, Furuta H, Shirotani T, Ahn BY, Chung SS, Min HK, Lee SW, Kim JH, Cho YM, Lee HK; Study Group of Molecular Diabetology in Asia . A mitochondrial DNA variant at position 16189 is associated with type 2 diabetes mellitus in Asians. Diabetologia 51: 602–608, 2008. doi: 10.1007/s00125-008-0933-z. [DOI] [PubMed] [Google Scholar]
- 131.Patel KD, Glancy B, Balaban RS. The electrochemical transmission in I-Band segments of the mitochondrial reticulum. Biochim Biophys Acta 1857: 1284–1289, 2016. doi: 10.1016/j.bbabio.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci USA 100: 8466–8471, 2003. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL, de Gruijl TD, Würdinger T, Middeldorp JM. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci USA 107: 6328–6333, 2010. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Peila R, Rodriguez BL, Launer LJ; Honolulu-Asia Aging Study . Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes 51: 1256–1262, 2002. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 135.Perry RJ, Camporez JG, Kursawe R, Titchenell PM, Zhang D, Perry CJ, Jurczak MJ, Abudukadier A, Han MS, Zhang XM, Ruan HB, Yang X, Caprio S, Kaech SM, Sul HS, Birnbaum MJ, Davis RJ, Cline GW, Petersen KF, Shulman GI. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell 160: 745–758, 2015. doi: 10.1016/j.cell.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Perry RJ, Samuel VT, Petersen KF, Shulman GI. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 510: 84–91, 2014. doi: 10.1038/nature13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes 54: 603–608, 2005. doi: 10.2337/diabetes.54.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Phielix E, Schrauwen-Hinderling VB, Mensink M, Lenaers E, Meex R, Hoeks J, Kooi ME, Moonen-Kornips E, Sels JP, Hesselink MK, Schrauwen P. Lower intrinsic ADP-stimulated mitochondrial respiration underlies in vivo mitochondrial dysfunction in muscle of male type 2 diabetic patients. Diabetes 57: 2943–2949, 2008. doi: 10.2337/db08-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Poulton J, Luan J, Macaulay V, Hennings S, Mitchell J, Wareham NJ. Type 2 diabetes is associated with a common mitochondrial variant: evidence from a population-based case-control study. Hum Mol Genet 11: 1581–1583, 2002. doi: 10.1093/hmg/11.13.1581. [DOI] [PubMed] [Google Scholar]
- 140.Pritchard CC, Cheng HH, Tewari M. MicroRNA profiling: approaches and considerations. Nat Rev Genet 13: 358–369, 2012. doi: 10.1038/nrg3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rackham O, Shearwood AM, Mercer TR, Davies SM, Mattick JS, Filipovska A. Long noncoding RNAs are generated from the mitochondrial genome and regulated by nuclear-encoded proteins. RNA 17: 2085–2093, 2011. doi: 10.1261/rna.029405.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rakyan VK, Down TA, Thorne NP, Flicek P, Kulesha E, Gräf S, Tomazou EM, Bäckdahl L, Johnson N, Herberth M, Howe KL, Jackson DK, Miretti MM, Fiegler H, Marioni JC, Birney E, Hubbard TJ, Carter NP, Tavaré S, Beck S. An integrated resource for genome-wide identification and analysis of human tissue-specific differentially methylated regions (tDMRs). Genome Res 18: 1518–1529, 2008. doi: 10.1101/gr.077479.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Raza H, John A, Howarth FC. Increased oxidative stress and mitochondrial dysfunction in zucker diabetic rat liver and brain. Cell Physiol Biochem 35: 1241–1251, 2015. doi: 10.1159/000373947. [DOI] [PubMed] [Google Scholar]
- 144.Rector RS, Thyfault JP, Uptergrove GM, Morris EM, Naples SP, Borengasser SJ, Mikus CR, Laye MJ, Laughlin MH, Booth FW, Ibdah JA. Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. J Hepatol 52: 727–736, 2010. doi: 10.1016/j.jhep.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rector RS, Uptergrove GM, Borengasser SJ, Mikus CR, Morris EM, Naples SP, Laye MJ, Laughlin MH, Booth FW, Ibdah JA, Thyfault JP. Changes in skeletal muscle mitochondria in response to the development of type 2 diabetes or prevention by daily wheel running in hyperphagic OLETF rats. Am J Physiol Endocrinol Metab 298: E1179–E1187, 2010. doi: 10.1152/ajpendo.00703.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rieusset J, Fauconnier J, Paillard M, Belaidi E, Tubbs E, Chauvin MA, Durand A, Bravard A, Teixeira G, Bartosch B, Michelet M, Theurey P, Vial G, Demion M, Blond E, Zoulim F, Gomez L, Vidal H, Lacampagne A, Ovize M. Disruption of calcium transfer from ER to mitochondria links alterations of mitochondria-associated ER membrane integrity to hepatic insulin resistance. Diabetologia 59: 614–623, 2016. doi: 10.1007/s00125-015-3829-8. [DOI] [PubMed] [Google Scholar]
- 147.Ritov VB, Menshikova EV, Azuma K, Wood R, Toledo FG, Goodpaster BH, Ruderman NB, Kelley DE. Deficiency of electron transport chain in human skeletal muscle mitochondria in type 2 diabetes mellitus and obesity. Am J Physiol Endocrinol Metab 298: E49–E58, 2010. doi: 10.1152/ajpendo.00317.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes 54: 8–14, 2005. doi: 10.2337/diabetes.54.1.8. [DOI] [PubMed] [Google Scholar]
- 149.Robertson KD, Wolffe AP. DNA methylation in health and disease. Nat Rev Genet 1: 11–19, 2000. doi: 10.1038/35049533. [DOI] [PubMed] [Google Scholar]
- 150.Roden M. Mechanisms of Disease: hepatic steatosis in type 2 diabetes--pathogenesis and clinical relevance. Nat Clin Pract Endocrinol Metab 2: 335–348, 2006. doi: 10.1038/ncpendmet0190. [DOI] [PubMed] [Google Scholar]
- 151.Rong JX, Qiu Y, Hansen MK, Zhu L, Zhang V, Xie M, Okamoto Y, Mattie MD, Higashiyama H, Asano S, Strum JC, Ryan TE. Adipose mitochondrial biogenesis is suppressed in db/db and high-fat diet-fed mice and improved by rosiglitazone. Diabetes 56: 1751–1760, 2007. doi: 10.2337/db06-1135. [DOI] [PubMed] [Google Scholar]
- 152.Rovira-Llopis S, Bañuls C, Diaz-Morales N, Hernandez-Mijares A, Rocha M, Victor VM. Mitochondrial dynamics in type 2 diabetes: Pathophysiological implications. Redox Biol 11: 637–645, 2017. doi: 10.1016/j.redox.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Roy Chowdhury SK, Smith DR, Saleh A, Schapansky J, Marquez A, Gomes S, Akude E, Morrow D, Calcutt NA, Fernyhough P. Impaired adenosine monophosphate-activated protein kinase signalling in dorsal root ganglia neurons is linked to mitochondrial dysfunction and peripheral neuropathy in diabetes. Brain 135: 1751–1766, 2012. doi: 10.1093/brain/aws097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ruddock MW, Stein A, Landaker E, Park J, Cooksey RC, McClain D, Patti ME. Saturated fatty acids inhibit hepatic insulin action by modulating insulin receptor expression and post-receptor signalling. J Biochem 144: 599–607, 2008. doi: 10.1093/jb/mvn105. [DOI] [PubMed] [Google Scholar]
- 155.Safra M, Sas-Chen A, Nir R, Winkler R, Nachshon A, Bar-Yaacov D, Erlacher M, Rossmanith W, Stern-Ginossar N, Schwartz S. The m1A landscape on cytosolic and mitochondrial mRNA at single-base resolution. Nature 551: 251–255, 2017. doi: 10.1038/nature24456. [DOI] [PubMed] [Google Scholar]
- 156.Sakai K, Matsumoto K, Nishikawa T, Suefuji M, Nakamaru K, Hirashima Y, Kawashima J, Shirotani T, Ichinose K, Brownlee M, Araki E. Mitochondrial reactive oxygen species reduce insulin secretion by pancreatic beta-cells. Biochem Biophys Res Commun 300: 216–222, 2003. doi: 10.1016/S0006-291X(02)02832-2. [DOI] [PubMed] [Google Scholar]
- 157.Saker PJ, Hattersley AT, Barrow B, Hammersley MS, Horton V, Gillmer MD, Turner RC. UKPDS 21: low prevalence of the mitochondrial transfer RNA gene (tRNA(Leu(UUR))) mutation at position 3243bp in UK Caucasian type 2 diabetic patients. Diabet Med 14: 42–45, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 158.Santos MS, Santos DL, Palmeira CM, Seiça R, Moreno AJ, Oliveira CR. Brain and liver mitochondria isolated from diabetic Goto-Kakizaki rats show different susceptibility to induced oxidative stress. Diabetes Metab Res Rev 17: 223–230, 2001. doi: 10.1002/dmrr.200. [DOI] [PubMed] [Google Scholar]
- 159.Santos RX, Correia SC, Alves MG, Oliveira PF, Cardoso S, Carvalho C, Seiça R, Santos MS, Moreira PI. Mitochondrial quality control systems sustain brain mitochondrial bioenergetics in early stages of type 2 diabetes. Mol Cell Biochem 394: 13–22, 2014. doi: 10.1007/s11010-014-2076-5. [DOI] [PubMed] [Google Scholar]
- 160.Schmid AI, Szendroedi J, Chmelik M, Krssák M, Moser E, Roden M. Liver ATP synthesis is lower and relates to insulin sensitivity in patients with type 2 diabetes. Diabetes Care 34: 448–453, 2011. doi: 10.2337/dc10-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Schrauwen-Hinderling VB, Kooi ME, Hesselink MK, Jeneson JA, Backes WH, van Echteld CJ, van Engelshoven JM, Mensink M, Schrauwen P. Impaired in vivo mitochondrial function but similar intramyocellular lipid content in patients with type 2 diabetes mellitus and BMI-matched control subjects. Diabetologia 50: 113–120, 2007. doi: 10.1007/s00125-006-0475-1. [DOI] [PubMed] [Google Scholar]
- 162.Shenouda SM, Widlansky ME, Chen K, Xu G, Holbrook M, Tabit CE, Hamburg NM, Frame AA, Caiano TL, Kluge MA, Duess MA, Levit A, Kim B, Hartman ML, Joseph L, Shirihai OS, Vita JA. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation 124: 444–453, 2011. doi: 10.1161/CIRCULATIONAHA.110.014506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shepherd DL, Hathaway QA, Nichols CE, Durr AJ, Pinti MV, Hughes KM, Kunovac A, Stine SM, Hollander JM. Mitochondrial proteome disruption in the diabetic heart through targeted epigenetic regulation at the mitochondrial heat shock protein 70 (mtHsp70) nuclear locus. J Mol Cell Cardiol 119: 104–115, 2018. doi: 10.1016/j.yjmcc.2018.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Shepherd DL, Hathaway QA, Pinti MV, Nichols CE, Durr AJ, Sreekumar S, Hughes KM, Stine SM, Martinez I, Hollander JM. Exploring the mitochondrial microRNA import pathway through Polynucleotide Phosphorylase (PNPase). J Mol Cell Cardiol 110: 15–25, 2017. doi: 10.1016/j.yjmcc.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shimabukuro M, Zhou YT, Levi M, Unger RH. Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci USA 95: 2498–2502, 1998. doi: 10.1073/pnas.95.5.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Shock LS, Thakkar PV, Peterson EJ, Moran RG, Taylor SM. DNA methyltransferase 1, cytosine methylation, and cytosine hydroxymethylation in mammalian mitochondria. Proc Natl Acad Sci USA 108: 3630–3635, 2011. doi: 10.1073/pnas.1012311108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sims D, Sudbery I, Ilott NE, Heger A, Ponting CP. Sequencing depth and coverage: key considerations in genomic analyses. Nat Rev Genet 15: 121–132, 2014. doi: 10.1038/nrg3642. [DOI] [PubMed] [Google Scholar]
- 168.Soejima A, Inoue K, Takai D, Kaneko M, Ishihara H, Oka Y, Hayashi JI. Mitochondrial DNA is required for regulation of glucose-stimulated insulin secretion in a mouse pancreatic beta cell line, MIN6. J Biol Chem 271: 26194–26199, 1996. doi: 10.1074/jbc.271.42.26194. [DOI] [PubMed] [Google Scholar]
- 169.Sone H, Kagawa Y. Pancreatic beta cell senescence contributes to the pathogenesis of type 2 diabetes in high-fat diet-induced diabetic mice. Diabetologia 48: 58–67, 2005. doi: 10.1007/s00125-004-1605-2. [DOI] [PubMed] [Google Scholar]
- 170.Sookoian S, Rosselli MS, Gemma C, Burgueño AL, Fernández Gianotti T, Castaño GO, Pirola CJ. Epigenetic regulation of insulin resistance in nonalcoholic fatty liver disease: impact of liver methylation of the peroxisome proliferator-activated receptor γ coactivator 1α promoter. Hepatology 52: 1992–2000, 2010. doi: 10.1002/hep.23927. [DOI] [PubMed] [Google Scholar]
- 171.Sorrentino V, Menzies KJ, Auwerx J. Repairing Mitochondrial Dysfunction in Disease. Annu Rev Pharmacol Toxicol 58: 353–389, 2018. doi: 10.1146/annurev-pharmtox-010716-104908. [DOI] [PubMed] [Google Scholar]
- 172.Sreekumar R, Halvatsiotis P, Schimke JC, Nair KS. Gene expression profile in skeletal muscle of type 2 diabetes and the effect of insulin treatment. Diabetes 51: 1913–1920, 2002. doi: 10.2337/diabetes.51.6.1913. [DOI] [PubMed] [Google Scholar]
- 173.Sun X, Wang L, Ding J, Wang Y, Wang J, Zhang X, Che Y, Liu Z, Zhang X, Ye J, Wang J, Sablok G, Deng Z, Zhao H. Integrative analysis of Arabidopsis thaliana transcriptomics reveals intuitive splicing mechanism for circular RNA. FEBS Lett 590: 3510–3516, 2016. doi: 10.1002/1873-3468.12440. [DOI] [PubMed] [Google Scholar]
- 174.Szabo L, Salzman J. Detecting circular RNAs: bioinformatic and experimental challenges. Nat Rev Genet 17: 679–692, 2016. doi: 10.1038/nrg.2016.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Szendroedi J, Chmelik M, Schmid AI, Nowotny P, Brehm A, Krssak M, Moser E, Roden M. Abnormal hepatic energy homeostasis in type 2 diabetes. Hepatology 50: 1079–1086, 2009. doi: 10.1002/hep.23093. [DOI] [PubMed] [Google Scholar]
- 176.Tang DL, Zhou X, Li X, Zhao L, Liu F. Variation of mitochondrial gene and the association with type 2 diabetes mellitus in a Chinese population. Diabetes Res Clin Pract 73: 77–82, 2006. doi: 10.1016/j.diabres.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 177.Targher G, Byrne CD. Clinical Review: Nonalcoholic fatty liver disease: a novel cardiometabolic risk factor for type 2 diabetes and its complications. J Clin Endocrinol Metab 98: 483–495, 2013. doi: 10.1210/jc.2012-3093. [DOI] [PubMed] [Google Scholar]
- 178.Tawata M, Hayashi JI, Isobe K, Ohkubo E, Ohtaka M, Chen J, Aida K, Onaya T. A new mitochondrial DNA mutation at 14577 T/C is probably a major pathogenic mutation for maternally inherited type 2 diabetes. Diabetes 49: 1269–1272, 2000. doi: 10.2337/diabetes.49.7.1269. [DOI] [PubMed] [Google Scholar]
- 179.Tawata M, Ohtaka M, Iwase E, Ikegishi Y, Aida K, Onaya T. New mitochondrial DNA homoplasmic mutations associated with Japanese patients with type 2 diabetes. Diabetes 47: 276–277, 1998. doi: 10.2337/diab.47.2.276. [DOI] [PubMed] [Google Scholar]
- 180.Thomson DW, Dinger ME. Endogenous microRNA sponges: evidence and controversy. Nat Rev Genet 17: 272–283, 2016. doi: 10.1038/nrg.2016.20. [DOI] [PubMed] [Google Scholar]
- 181.Tocchetti CG, Caceres V, Stanley BA, Xie C, Shi S, Watson WH, O’Rourke B, Spadari-Bratfisch RC, Cortassa S, Akar FG, Paolocci N, Aon MA. GSH or palmitate preserves mitochondrial energetic/redox balance, preventing mechanical dysfunction in metabolically challenged myocytes/hearts from type 2 diabetic mice. Diabetes 61: 3094–3105, 2012. doi: 10.2337/db12-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Toledo FG, Menshikova EV, Ritov VB, Azuma K, Radikova Z, DeLany J, Kelley DE. Effects of physical activity and weight loss on skeletal muscle mitochondria and relationship with glucose control in type 2 diabetes. Diabetes 56: 2142–2147, 2007. doi: 10.2337/db07-0141. [DOI] [PubMed] [Google Scholar]
- 183.Tolman KG, Fonseca V, Dalpiaz A, Tan MH. Spectrum of liver disease in type 2 diabetes and management of patients with diabetes and liver disease. Diabetes Care 30: 734–743, 2007. doi: 10.2337/dc06-1539. [DOI] [PubMed] [Google Scholar]
- 184.Tubbs E, Chanon S, Robert M, Bendridi N, Bidaux G, Chauvin MA, Ji-Cao J, Durand C, Gauvrit-Ramette D, Vidal H, Lefai E, Rieusset J. Disruption of Mitochondria-Associated Endoplasmic Reticulum Membrane (MAM) Integrity Contributes to Muscle Insulin Resistance in Mice and Humans. Diabetes 67: 636–650, 2018. doi: 10.2337/db17-0316. [DOI] [PubMed] [Google Scholar]
- 185.Tubbs E, Theurey P, Vial G, Bendridi N, Bravard A, Chauvin MA, Ji-Cao J, Zoulim F, Bartosch B, Ovize M, Vidal H, Rieusset J. Mitochondria-associated endoplasmic reticulum membrane (MAM) integrity is required for insulin signaling and is implicated in hepatic insulin resistance. Diabetes 63: 3279–3294, 2014. doi: 10.2337/db13-1751. [DOI] [PubMed] [Google Scholar]
- 186.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9: 654–659, 2007. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 187.van den Ouweland JM, Lemkes HH, Ruitenbeek W, Sandkuijl LA, de Vijlder MF, Struyvenberg PA, van de Kamp JJ, Maassen JA. Mutation in mitochondrial tRNA(Leu)(UUR) gene in a large pedigree with maternally transmitted type II diabetes mellitus and deafness. Nat Genet 1: 368–371, 1992. doi: 10.1038/ng0892-368. [DOI] [PubMed] [Google Scholar]
- 188.van der Wijst MG, Rots MG. Mitochondrial epigenetics: an overlooked layer of regulation? Trends Genet 31: 353–356, 2015. doi: 10.1016/j.tig.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 189.van Tienen FH, Praet SF, de Feyter HM, van den Broek NM, Lindsey PJ, Schoonderwoerd KG, de Coo IF, Nicolay K, Prompers JJ, Smeets HJ, van Loon LJ. Physical activity is the key determinant of skeletal muscle mitochondrial function in type 2 diabetes. J Clin Endocrinol Metab 97: 3261–3269, 2012. doi: 10.1210/jc.2011-3454. [DOI] [PubMed] [Google Scholar]
- 190.Vial G, Dubouchaud H, Leverve XM. Liver mitochondria and insulin resistance. Acta Biochim Pol 57: 389–392, 2010. [PubMed] [Google Scholar]
- 191.Vianna CR, Huntgeburth M, Coppari R, Choi CS, Lin J, Krauss S, Barbatelli G, Tzameli I, Kim YB, Cinti S, Shulman GI, Spiegelman BM, Lowell BB. Hypomorphic mutation of PGC-1beta causes mitochondrial dysfunction and liver insulin resistance. Cell Metab 4: 453–464, 2006. doi: 10.1016/j.cmet.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Vincent AM, Edwards JL, McLean LL, Hong Y, Cerri F, Lopez I, Quattrini A, Feldman EL. Mitochondrial biogenesis and fission in axons in cell culture and animal models of diabetic neuropathy. Acta Neuropathol 120: 477–489, 2010. doi: 10.1007/s00401-010-0697-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Volkmar M, Dedeurwaerder S, Cunha DA, Ndlovu MN, Defrance M, Deplus R, Calonne E, Volkmar U, Igoillo-Esteve M, Naamane N, Del Guerra S, Masini M, Bugliani M, Marchetti P, Cnop M, Eizirik DL, Fuks F. DNA methylation profiling identifies epigenetic dysregulation in pancreatic islets from type 2 diabetic patients. EMBO J 31: 1405–1426, 2012. doi: 10.1038/emboj.2011.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wang CH, Wei YH. Role of mitochondrial dysfunction and dysregulation of Ca2+ homeostasis in the pathophysiology of insulin resistance and type 2 diabetes. J Biomed Sci 24: 70, 2017. doi: 10.1186/s12929-017-0375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wang D, Taniyama M, Suzuki Y, Katagiri T, Ban Y. Association of the mitochondrial DNA 5178A/C polymorphism with maternal inheritance and onset of type 2 diabetes in Japanese patients. Exp Clin Endocrinol Diabetes 109: 361–364, 2001. doi: 10.1055/s-2001-17407. [DOI] [PubMed] [Google Scholar]
- 196.Wang Q, Zhang M, Torres G, Wu S, Ouyang C, Xie Z, Zou MH. Metformin Suppresses Diabetes-Accelerated Atherosclerosis via the Inhibition of Drp1-Mediated Mitochondrial Fission. Diabetes 66: 193–205, 2017. doi: 10.2337/db16-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wang Y, Michikawa Y, Mallidis C, Bai Y, Woodhouse L, Yarasheski KE, Miller CA, Askanas V, Engel WK, Bhasin S, Attardi G. Muscle-specific mutations accumulate with aging in critical human mtDNA control sites for replication. Proc Natl Acad Sci USA 98: 4022–4027, 2001. doi: 10.1073/pnas.061013598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ward MS, Flemming NB, Gallo LA, Fotheringham AK, McCarthy DA, Zhuang A, Tang PH, Borg DJ, Shaw H, Harvie B, Briskey DR, Roberts LA, Plan MR, Murphy MP, Hodson MP, Forbes JM. Targeted mitochondrial therapy using MitoQ shows equivalent renoprotection to angiotensin converting enzyme inhibition but no combined synergy in diabetes. Sci Rep 7: 15190, 2017. doi: 10.1038/s41598-017-15589-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Williams M, Caino MC. Mitochondrial Dynamics in Type 2 Diabetes and Cancer. Front Endocrinol (Lausanne) 9: 211, 2018. doi: 10.3389/fendo.2018.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wolfrum C, Asilmaz E, Luca E, Friedman JM, Stoffel M. Foxa2 regulates lipid metabolism and ketogenesis in the liver during fasting and in diabetes. Nature 432: 1027–1032, 2004. doi: 10.1038/nature03047. [DOI] [PubMed] [Google Scholar]
- 201.Xiao L, Xu X, Zhang F, Wang M, Xu Y, Tang D, Wang J, Qin Y, Liu Y, Tang C, He L, Greka A, Zhou Z, Liu F, Dong Z, Sun L. The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox Biol 11: 297–311, 2017. doi: 10.1016/j.redox.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Yang BT, Dayeh TA, Volkov PA, Kirkpatrick CL, Malmgren S, Jing X, Renström E, Wollheim CB, Nitert MD, Ling C. Increased DNA methylation and decreased expression of PDX-1 in pancreatic islets from patients with type 2 diabetes. Mol Endocrinol 26: 1203–1212, 2012. doi: 10.1210/me.2012-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yang S, Zhu H, Li Y, Lin H, Gabrielson K, Trush MA, Diehl AM. Mitochondrial adaptations to obesity-related oxidant stress. Arch Biochem Biophys 378: 259–268, 2000. doi: 10.1006/abbi.2000.1829. [DOI] [PubMed] [Google Scholar]
- 204.Ye G, Metreveli NS, Donthi RV, Xia S, Xu M, Carlson EC, Epstein PN. Catalase protects cardiomyocyte function in models of type 1 and type 2 diabetes. Diabetes 53: 1336–1343, 2004. doi: 10.2337/diabetes.53.5.1336. [DOI] [PubMed] [Google Scholar]
- 205.Ye Z, Gillson C, Sims M, Khaw KT, Plotka M, Poulton J, Langenberg C, Wareham NJ. The association of the mitochondrial DNA OriB variant (16184-16193 polycytosine tract) with type 2 diabetes in Europid populations. Diabetologia 56: 1907–1913, 2013. doi: 10.1007/s00125-013-2945-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yokota T, Kinugawa S, Hirabayashi K, Matsushima S, Inoue N, Ohta Y, Hamaguchi S, Sobirin MA, Ono T, Suga T, Kuroda S, Tanaka S, Terasaki F, Okita K, Tsutsui H. Oxidative stress in skeletal muscle impairs mitochondrial respiration and limits exercise capacity in type 2 diabetic mice. Am J Physiol Heart Circ Physiol 297: H1069–H1077, 2009. doi: 10.1152/ajpheart.00267.2009. [DOI] [PubMed] [Google Scholar]
- 207.Yu P, Yu DM, Liu DM, Wang K, Tang XZ. Relationship between mutations of mitochondrial DNA ND1 gene and type 2 diabetes. Chin Med J (Engl) 117: 985–989, 2004. [PubMed] [Google Scholar]
- 208.Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci USA 103: 2653–2658, 2006. doi: 10.1073/pnas.0511154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Zhang D, Liu ZX, Choi CS, Tian L, Kibbey R, Dong J, Cline GW, Wood PA, Shulman GI. Mitochondrial dysfunction due to long-chain Acyl-CoA dehydrogenase deficiency causes hepatic steatosis and hepatic insulin resistance. Proc Natl Acad Sci USA 104: 17075–17080, 2007. doi: 10.1073/pnas.0707060104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zhong S, Ng MC, Lo YM, Chan JC, Johnson PJ. Presence of mitochondrial tRNA(Leu(UUR)) A to G 3243 mutation in DNA extracted from serum and plasma of patients with type 2 diabetes mellitus. J Clin Pathol 53: 466–469, 2000. doi: 10.1136/jcp.53.6.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108: 1167–1174, 2001. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ziller MJ, Gu H, Müller F, Donaghey J, Tsai LT, Kohlbacher O, De Jager PL, Rosen ED, Bennett DA, Bernstein BE, Gnirke A, Meissner A. Charting a dynamic DNA methylation landscape of the human genome. Nature 500: 477–481, 2013. doi: 10.1038/nature12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zoppini G, Targher G, Zamboni C, Venturi C, Cacciatori V, Moghetti P, Muggeo M. Effects of moderate-intensity exercise training on plasma biomarkers of inflammation and endothelial dysfunction in older patients with type 2 diabetes. Nutr Metab Cardiovasc Dis 16: 543–549, 2006. doi: 10.1016/j.numecd.2005.09.004. [DOI] [PubMed] [Google Scholar]