Abstract
The role of estrogen receptor-α (ERα) signaling in immunometabolic function is established in females. However, its necessity in males, while appreciated, requires further study. Accordingly, we first determined whether lower metabolic function in male mice compared with females is related to reduced ERα expression. ERα protein expression in metabolically active tissues was lower in males than in females, and this lower expression was associated with worse glucose tolerance. Second, we determined whether ERα is required for optimal immunometabolic function in male mice consuming a chow diet. Despite lower expression of ERα in males, its genetic ablation (KO) caused an insulin-resistant phenotype characterized by enhanced adiposity, glucose intolerance, hepatic steatosis, and metaflammation in adipose tissue and liver. Last, we determined whether ERα is essential for exercise-induced metabolic adaptations. Twelve-week-old wild-type (WT) and ERα KO mice either remained sedentary (SED) or were given access to running wheels (WR) for 10 wk while fed an obesogenic diet. Body weight and fat mass were lower in WR mice regardless of genotype. Daily exercise obliterated immune cell infiltration and inflammatory gene transcripts in adipose tissue in both genotypes. In the liver, however, wheel running suppressed hepatic steatosis and inflammatory gene transcripts in WT but not in KO mice. In conclusion, the present findings indicate that ERα is required for optimal immunometabolic function in male mice despite their reduced ERα protein expression in metabolically active tissues. Furthermore, for the first time, we show that ERα signaling appears to be obligatory for exercise-induced prevention of hepatic steatosis.
Keywords: estrogen receptor-α, exercise, fatty liver, insulin resistance, metaflammation, obesity
INTRODUCTION
In the context of obesity, immunometabolic dysfunction is often triggered by ‘metaflammation’: grossly defined as an inflammatory process caused by an aberrant challenge to metabolic homeostasis (i.e., overnutrition) that is initiated and sustained by metabolic tissues (4, 23). Two metabolically active tissues that are highly susceptible to obesity-induced infiltration of immune cells and consequent release of proinflammatory cytokines are adipose tissue and liver. This shift to an inflammatory milieu is an early contributor to metabolic diseases including nonalcoholic fatty liver disease (NAFLD) and poses significant cardiovascular disease risk (16, 22, 27, 45, 59). Notably, one central instigator of metaflammation and associated disruption to immunometabolism is the loss of estrogen signaling. Estrogen action is facilitated by three estrogen receptors (ERs): ERα, ERβ, and plasma membrane-bound G protein-coupled ER (12, 54), although ERα is thought to be the primary mediator of estrogen effects on metabolic regulation and adipose/hepatic function (9, 14, 20, 31, 41, 48, 50; reviewed in Ref. (21). Indeed, in rodents, genetic ablation of ERα, but not ERβ, is associated with adipose tissue and liver dysfunction and metabolic impairments including insulin resistance and glucose intolerance (21, 41).
For a given body mass index, males are generally more insulin resistant than females, and this is generally explained by differences in sex hormones (15). However, whether this relative insulin resistance in males is also associated with lower expression of ERs is poorly described. In this regard, herein we demonstrate that ERα protein expression is lower in metabolically active tissues from males compared with females in both mice and swine. Importantly, despite lower ERα expression in males, its genetic deletion in mice provokes an insulin-resistant phenotype characterized by increased adiposity, glucose intolerance, and metaflammation in adipose tissue and liver, thus substantiating the key role of ERα signaling in maintaining metabolic function in sedentary males.
It is well established that increased levels of physical activity improve both adipose tissue (44, 60) and liver health (32–34, 46, 47); however, the mechanisms by which physical activity mediates improvements in these tissues have not been fully elucidated. Interestingly, estrogen is known to affect fuel partitioning via ERα (8), and some evidence suggests that daily activity may actually increase ERα expression in visceral adipose tissue (37). Thus, it is conceivable that ERα signaling mediates some of the beneficial effects of physical activity. As such, we reasoned that ERα signaling might be a critical nexus between increased physical activity and its beneficial effects within adipose tissue and liver. Specifically, we hypothesized that loss of ERα limits exercise-induced improvements in immunometabolic function, including the exercise-induced anti-inflammatory effects in adipose tissue and liver.
METHODS
All procedures were approved in advance by the University of Missouri Institutional Animal Care and Use Committee.
Animals
Heterozygote ERα−/+ male mice on a C57Bl/6 background were bred at our facility to produce homozygote ERα (KO) and littermate wild-type (WT) mice, as previously described (11, 35). Briefly, development of the ERα KO mouse was accomplished by homologous recombination and insertion of a neomycin sequence containing premature stop codons and polyadenylation sequences into a Not1 site in exon 2 of the mouse ER gene (11, 35, 51). Mice were kept in an environmentally controlled animal facility on a 12:12-h light-dark cycle from 700 to 1900 and housed from one to three per cage. All mice were euthanized following a 5-h fast, and tissues were harvested and either fixed in 10% formalin or snap-frozen in liquid nitrogen and stored at −80°C until analysis.
Experimental Protocols
Protocol 1.
Tissue lysates, including white adipose tissue (WAT), liver, and muscle from WT male and female mice on a C57Bl/6 background from a previously published study (64) were utilized to determine the expression levels of ERs between sexes. All mice were fed a normal chow diet (3.3 kcal/g food, 13% kcal fat, 57% kcal carbohydrate, and 30% kcal protein, no. 5001; LabDiet, St. Louis, MO) ad libitum for 20 wk. These mice underwent a glucose tolerance test at 25 wk of age to determine sex differences in metabolic function. Animals were euthanized at 26 wk of age and gonadal WAT, quadriceps muscle, and liver were collected, flash-frozen, and store at −80°C until further analysis. To enhance translatability, archived omental WAT and liver lysates from male (n = 5) and female (n = 7) Ossabaw swine (6 mo old) (42) were also incorporated in this protocol.
Protocol 2.
WT and ERα KO mice were bred at our facility (described above) and were fed normal chow diet ad libitum for 14 mo, providing a total of two groups (n = 6 or 7/group) to determine the role of ERα signaling against metaflammation and deterioration of glucose homeostasis. The following in vivo tests were performed: glucose tolerance test (at 14 mo of age) and body composition analysis via EchoMRI (at 14 mo of age). At 14 mo of age, mice were euthanized for tissue collection.
Protocol 3.
At 12 wk of age, WT and ERα KO mice were fed an obesogenic diet [4.65 kcal/g food; 46.0% kcal from fat, 36.0% kcal from carbohydrate with sucrose content (per weight) of 17.5% and high-fructose corn syrup content of 17.5% (Test Diet modified 58Y1; 5APC)] and were randomized to one of four groups (n = 7–10/group) for 10 wk: 1) WT sedentary (SED), 2) WT wheel running (WR), 3) KO SED, and 4) KO WR. All mice were fed an obesogenic diet to induce obesity and metabolic dysfunction in both WT and KO mice. In this way, we were able to examine the role of ERα signaling in modulating physical activity-induced metabolic benefits. Voluntary WR is a common approach to enhance daily physical activity without concomitant stress caused by forced exercise (i.e., swimming, treadmill running, etc.). Animals in the WR group were housed with running wheels connected to a Sunding bicycle computer (SD-548B; Dongguan Sunding Electron, TangXia, DongGuan, China) for determination of weekly running distance. Odometers were checked daily and reset every week. The following in vivo tests were performed: glucose tolerance test, insulin tolerance test, and body composition via EchoMRI. At 22 wk of age, mice were euthanized following a 5-h fast. Running wheels were also removed 5 h before euthanasia.
Experimental Procedures
Body composition.
Percent body fat (BF%) was measured with a nuclear magnetic resonance imaging whole body composition analyzer (EchoMRI 4in1/1100; Echo Medical Systems, Houston, TX) on conscious mice within 1 wk before euthanasia.
Glucose and insulin tolerance testing.
Glucose tolerance tests (protocols 1, 2, and 3) and insulin tolerance tests (protocols 2 and 3) were performed 1 wk and 3 wk before euthanasia, respectively. Briefly, after a 5-h fast, blood glucose was measured from the tail vein. The tail was nicked, and blood was sampled by a glucometer (Alpha Trak, Abbott Laboratories). A baseline measure of blood glucose was taken before administration of a sterile solution of 50% dextrose (2 g/kg body wt) via intraperitoneal (ip) injection, as previously performed (61, 65). Similarly, following baseline glucose measurement, 1 U insulin/kg body wt ip was injected. Glucose measurements were taken 15, 30, 45, 60, and 120 min after the glucose injection and 15, 30, 45, 60, 90, and 120 min after insulin injection. Glucose total area under curve (AUC) was calculated using the trapezoidal rule.
Fasting blood parameters.
Plasma glucose, cholesterol, triglycerides, and nonesterified fatty acid (NEFA) assays were performed by a commercial laboratory (Comparative Clinical Pathology Services, Columbia, MO) on an Olympus AU680 automated chemistry analyzer (Beckman-Coulter, Brea, CA) according to the manufacturer’s guidelines. Plasma insulin concentrations were determined using a commercially available, mouse-specific ELISA (Alpco Diagnostics, Salem, NH). Plasma estradiol was measured via radioimmunoassay at the VUMC Hormone Assay and Analytical Services Core (Vanderbilt University, Nashville, TN).
Histological assessments.
Optimal cutting temperature (OCT) compound or formalin-fixed samples were processed through paraffin embedding, sectioned at 5 µm, and stained with macrophage marker Mac-2 antibody (epididymal WAT), antibody CL8942AP, 1:1,000, Cedarlane), or hematoxylin and eosin (liver). Sections were evaluated via an Olympus BX34 photomicroscope (Olympus, Melville, NY), and images were taken with an Olympus SC30 Optical Microscope Accessory CMOS color camera. Objective quantification of macrophage infiltration in epididymal WAT was done by determining the positive Mac-2-stained area per ×10 fields of view using ImageJ software (NIH public domain; National Institutes of Health, Bethesda, MD). Crown-like structure density was defined as Mac-2-positive stained area (24). The average of three ×10 fields of view was determined for each animal. Adipocyte size was calculated based on 100 adipocytes per animal obtained from the same three ×10 fields. Briefly, cross-sectional areas of the adipocytes were obtained from perimeter tracings using ImageJ software as performed previously (61).
All histological assessments were performed by an investigator whom was blinded to the groups.
RNA extraction and quantitative real-time RT-PCR.
Epididymal WAT and liver lysates were homogenized in TRIzol solution using a tissue homogenizer (TissueLyser LT; Qiagen, Valencia, CA). Total RNA was isolated according to the Qiagen RNeasy lipid tissue protocol and assayed using a Nanodrop spectrophotometer (Thermo Scientific, Wilmington, DE) to assess purity and concentration. First-strand cDNA was synthesized from total RNA by using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Carlsbad, CA). Quantitative real-time PCR was performed as previously described, using the ABI StepOne Plus sequence detection system (Applied Biosystems) (61). All primers (Table 1) were purchased from IDT (Coralville, IA). A 20-μl reaction mixture containing 10 μl of iTaq UniverSYBR Green SMX (Bio-Rad, Hercules, CA) and the appropriate concentrations of gene-specific primers plus 4 μl of cDNA template were loaded in a single well of a 96-well plate. PCR reactions were performed in duplicate under thermal conditions as follows: 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 45 s. A dissociation melt curve analysis was performed to verify the specificity of the PCR products. 18S or RSP13 were used as housekeeping control genes. Cycle thresholds (CTs) were not different among the groups of animals. mRNA expression values are presented as 2ΔCT, whereby ΔCT = house-keeping CT − gene of interest CT. mRNA levels were expressed as fold change relative to the WT SED group, which was set at 1.
Table 1.
Gene primers
| Gene | Forward | Reverse |
|---|---|---|
| 18S | TCAAGAACGAAAGTCGGAGG | GGACATCTAAGGGCATCAC |
| Acc | CTGTATGAGAAAGGCTATGTG | AACCTGTCTGAAGAGGTTAG |
| Ccl2 | CAAGATGATCCCAATGAGTAG | TTGGTGACAAAAACTACAGC |
| Cd11c | ATGCCACTGTCTGCCTTCAT | GAGCCAGGTCAAAGGTGACA |
| Cidea | TGCTCTTCTGTATCGCCCAGT | GCCGTGTTAAGGAATCTGCTG |
| F4/80 | GTGCCATCATTGCGGGATTC | GACGGTTGAGCAGACAGTGA |
| Fas | GATTCAGGGAGTGGCTATTG | CATTCAGAATCGTGGCATAG |
| Srebf1 | CAGACCCTGGTGAGTGGAG | TCTGAGGGTGGAGGGGTAAG |
| Lgals3 | GAAAAGAGTACTAGAAGCGG | CATTTTCCTGATTAGTGCTCC |
| Itgam | AGCTTGGCTTTTTCAAGCGG | AAAGGCCGTTACTGAGGTGG |
| Tnfα | CTATGTCTCAGCCTCTTCTC | CATTTGGGAAACTTCTCATCC |
| Il6 | TCCAGTTGCCTTCTTGGGAC | AGTCTCCTCTCCGGACTTGT |
| Ccl5 | TCCCTGTCATTGCTTGCTCT | ATTTTCCCAGGACCGAGTGG |
| Cd68 | TGTTCAGCTCCAAGCCCAAA | GTACCGTCACAACCTCCCTG |
| Il1β | TGCCACCTTTTGACAGTGATG | TGATGTGCTGCTGCGAGATT |
| P22phox | ACTCTATCGCTGCAGGTGTG | AAGCTTCACCACAGAGGTCAG |
| P47phox | GGCACAAAGGACAATCCATCG | TTCCGTTTGGTGCTCTCTGT |
| Adipoq | GCACTGGCAAGTTCTACTGCAGCAA | GTAGGTGAAGAGAACGGCCTTGT |
| Leptin | CCTATTGATGGGTCTGCCCG | TGAGCGCTACCTGCATAGAC |
| Ifnγ | AGCAAGGCGAAAAAGGATGC | TCATTGAATGCTTGGCGCTG |
| Nlrp3 | GACACGAGTCCTGGTGACTTT | CAGACGTATGTCCTGAGCCAT |
Liver triglycerides.
Biochemical liver triglyceride content was determined as previously described (46). Briefly, ∼30 mg of frozen tissue was placed in 1 ml of lipid extraction solution [2:1 (vol/vol) chloroform-methanol], homogenized, and exposed to gentle agitation overnight at 4°C. One milliliter of 4 mM MgCl was added to each sample, and the samples were centrifuged for 1 h at 1,000 g at 4°C. The organic phase was removed, the remaining liquid was evaporated, and the sample was reconstituted in t-butanol-Triton X-114 [3:2 (vol/vol)]. After reconstitution of the sample, triglyceride content was measured using a commercially available assay (L-type triglyceride, Wako Life Sciences). Liver triglyceride concentrations were presented as milligrams per gram of liver wet weight.
Immunoblotting.
Immunoblotting was performed on epididymal WAT, inguinal WAT, liver, and quadriceps muscle lysates as previously described (17). ERα (Ab75635, 1:1,000) and ERβ (Ab3577, 1:1,000) primary antibodies were purchased from Abcam, and β-tubulin (no. 2146, 1:1,000), acetyl-CoA carboxylase (ACC; no. 3662, 1:1,000), phospho- (p)ACC at Ser79 (no. 3661 1:1,000), and farty acid synthase (FAS; no. 3180, 1:1,000) primary antibodies were purchased from Cell Signaling. Peroxisome proiferator-activated receptor-γ coactivator 1α (PGC1α; no. 516557, 1:1,000) and CD36 (sc-7309, 1:1,000) primary antibodies were purchased from Millipore, Sigma, and Santa Cruz Biotechnology, respectively. Intensities of individual protein bands were quantified using FluoroChem HD2 (AlphaView, v. 3.4.0.0) and expressed as ratio to control band β-tubulin or total protein stain (1% amido-black; Millipore, Sigma). Values are expressed as fold difference.
Ex vivo lipolysis.
Adipose tissue lysates were excised and cut into 10- to 20-mg pieces and placed in 0.297 ml of DMEM 1×, 1g/l glucose, 110 mg/l sodium pyruvate) with 2% free fatty acid-free BSA for 15 min at 37°C. Thereafter, isoproterenol (ISO, 10 µM) or PBS was added to medium and incubated at 37°C for 2 h under agitation. Following incubation, tissue lysates were removed, briefly washed with PBS, and flash-frozen in liquid nitrogen until further analysis. The incubation medium was collected and stored at −80°C for assessment of glycerol (Millipore Sigma). Glycerol is presented as millimoles per milligram of protein.
Statistical analysis.
Student’s t-tests were utilized for comparisons containing two groups (i.e., protocol 1: males vs. females, and protocol 2: WT vs. KO). A 2 × 2 (treatment × genotype) analysis of variance (ANOVA) was used to evaluate the effects of WR and ERα ablation on all dependent variables (i.e., protocol 3). Least significant difference post hoc tests were utilized for pairwise comparisons. All data are presented as means ± SE. For all statistical tests, significance was accepted at P < 0.05. All statistical analyses were performed with SPSS v. 20.0.
RESULTS
ER Expression in Males and Females
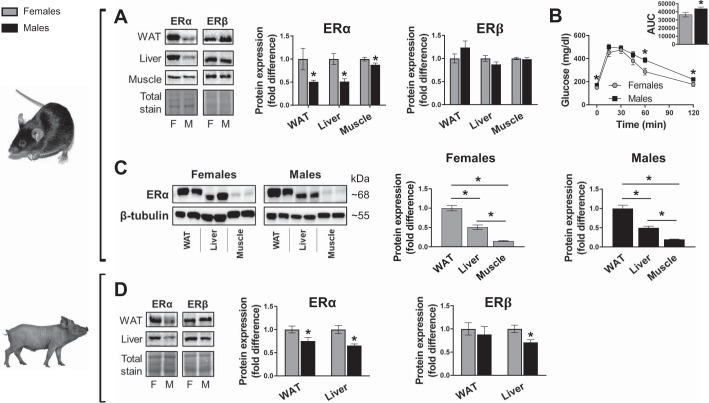
Given that ERα signaling has been implicated in the regulation of metabolic function and that premenopausal females are generally more insulin sensitive than males, we first determined whether sex differences in ERα protein expression manifest in insulin- sensitive tissues. ERα protein expression was significantly lower in male than in female littermate mice for WAT, liver, and muscle; whereas no differences in ERβ protein abundance were noted (Fig. 1A). Relative to age-matched females, male Ossabaw pigs also had significantly lower ERα protein expression in WAT and liver (Fig. 1D). Male pig livers also had lower ERβ expression (Fig. 1A). Compared with female mice, decreased ERα content in males was accompanied by worse glucose tolerance (Fig. 1B). Despite ERα abundance being higher in females in all tissues analyzed, in both sexes ERα protein followed the same tissue expression patterns, listed from highest to lowest expression: WAT > liver > muscle (Fig. 1C). Collectively, these data suggest that ERα expression is consistently lower in males than in females and that, of the tissues assessed, WAT displays the highest ERα expression levels regardless of sex.
Fig. 1.
Estrogen receptor (ER) expression in males and females. A: representative immunoblots and mean quantified protein expression for ERα and ERβ in adipose tissue, liver, and skeletal muscle from mice (top) and Ossabaw swine (bottom). B: glucose tolerance and area under the curve (AUC; inset) in 25-wk-old male and female wild-type C57Bl/6 mice consuming rodent chow diet. WAT, white adipose tissue; M, male; F, female. Mice: n = 10 F, n = 12 M. Pigs: n = 6–7 F, n = 5 M. Data are means ± SE; *P < 0.05 vs. females.
Loss of ERα Produces Metaflammation in Adipose Tissue and Liver
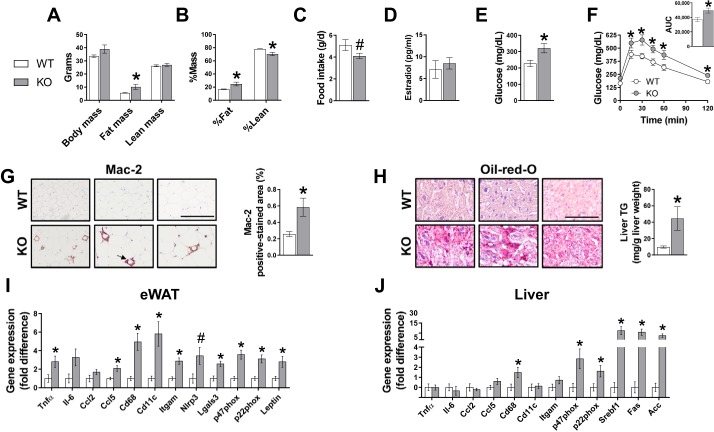
Despite low relative protein expression of ERα in males compared with females, ablation of ERα in male mice fed a low-fat rodent chow caused multiple features of metabolic dysfunction, including increased adiposity, glucose intolerance, hepatic steatosis, and increased inflammatory gene expression in epididymal WAT and liver compared with WT controls (Fig. 2). Circulating estradiol concentrations were not different between WT and KO animals (Fig. 2D), as previously reported in male mice (7).
Fig. 2.
Role of estrogen receptor-α (ERα) in adipose tissue and liver and metabolic function in chow-fed male mice. Body mass (A), body composition (B), food intake (C), fasting glucose (D), glucose tolerance (E) and area under the curve (AUC; inset), and representative white adipose tissue (WAT; F) histology sections immunostained for Mac-2 (arrow indicates positive-stained crown-like structures) and quantified Mac-2 positive-stained area. G: representative liver Oil-red-O histology sections and biochemically measured liver triglyceride (TG). H and I: WAT and liver gene expression. Histology sections are ×20 magnification; scale bar, 100 µm. WT, wild type; KO, ERα knockout. WT, n = 6; KO, n = 7. Data are means ± SE; *P < 0.05 vs. WT; #P = 0.06 vs. WT.
Role of ERα in Physical Activity-Induced Metabolic Adaptations
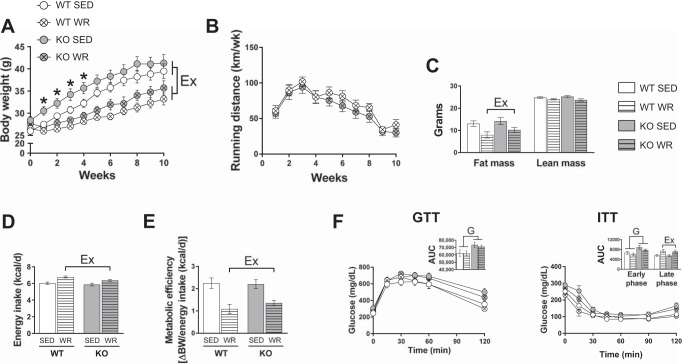
Exercise training has been shown to ameliorate obesity-induced metabolic dysfunction and metaflammation (44, 60). However, whether ERα signaling is required for this training-induced protection is not known. Accordingly, WR was utilized as a tool to attenuate the adverse effects of obesogenic diet in WT and KO mice. Early weight gain (week 1 to week 4) was greater in KO SED mice compared with WT SED, but final body weight was not different between WT or KO sedentary mice (Fig. 3A). WR mice exhibited less body weight gain than SED littermates (Table 2 and Fig. 3, A and B). Fat mass and lean mass did not differ between genotypes; however, there was a main effect of WR in reducing fat mass (Fig. 3C). WR mice consumed more energy than SED groups but had lower metabolic efficiency independent of genotype (Fig. 3, D and E). Despite no difference in total fat mass, KO animals had significantly greater epididymal adipose tissue and liver weights that were decreased with daily activity (Table 2). Increased heart weight was observed in WR mice vs. SED mice regardless of genotype, indicative of similar exercise training adaptations (Table 2).
Fig. 3.
Role of estrogen receptor-α (ERα) in systemic exercise adaptations in male mice. A and B: weekly body weight and weekly running distance. C: fat/lean mass. D and E: energy intake and metabolic efficiency. F and G: glucose tolerance and insulin tolerance with area under the curve (AUC; insets). WT, wild type; KO, ERα knockout; SED, sedentary; WR, wheel running; GTT, glucose tolerance test; ITT, insulin tolerance test. All groups were fed an obesogenic diet. WT, n = 7–13/group; KO, n = 8–11/group. Data are means ± SE. Ex, P < 0.05, main effect of exercise; G, P < 0.05, main effect of genotype.
Table 2.
Body and tissue weights
| WT (n = 7–9/group) |
KO (n = 8–10/group) |
ANOVA | |||
|---|---|---|---|---|---|
| Parameter | SED | WR | SED | WR | |
| Body weight | 39.8 ± 1.9 | 34.5 ± 1.5* | 42.4 ± 1.9 | 37.7 ± 2.0* | G: P = 0.17 A: P = 0.002 G×A: P = 0.76 |
| WAT | 2.13 ± 0.23 | 1.35 ± 0.19* | 2.53 ± 0.26 | 1.91 ± 0.22* |
G: P = 0.02 A: P = 0.001 G×A: P = 0.43 |
| Liver | 1.44 ± 0.14 | 1.21 ± 0.06* | 2.09 ± 0.21 | 1.71 ± 0.15* |
G: P = 0.0009 A: P = 0.03 G×A: P = 0.65 |
| BAT | 0.16 ± 0.01 | 0.11 ± 0.01* | 0.17 ± 0.02 | 0.14 ± 0.01* | G: P = 0.20 A: P = 0.008 G×A: P = 0.49 |
| Heart | 0.18 ± 0.01 | 0.20 ± 0.01* | 0.17 ± 0.01 | 0.19 ± 0.01* | G: P = 0.18 A: P = 0.02 G×A: P = 0.55 |
Values are means ± SE in grams. WAT, epididymal white adipose tissue; BAT, brown adipose tissue; WT, wild type; KO, estrogen receptor-α knockout; SED, sedentary; WR, wheel running. G, genotype; A, activity; G×A, genotype × activity interaction.
P < 0.05 vs. SED.
Bold P values indicate statistical significance.
Fasting biochemistries are reported in Table 3. KO mice had greater total cholesterol and elevated LDL cholesterol vs. WT mice. Compared with SED mice, WR mice displayed lower LDL cholesterol despite no effect on total cholesterol levels independent of genotype (Table 3). WR mice had a ~1.6-fold reduction in homeostasis model assessment of insulin resistance (HOMA-IR) relative to SED mice, although this difference did not reach statistical significance (main effect of activity, P = 0.11; Table 3). Similarly, a main effect of WR in reducing plasma triglycerides and leptin concentrations were demonstrated irrespective of genotype (Table 3). Glucose AUC in response to a glucose tolerance test was higher in KO than in WT animals (Fig. 3F). Early-phase (0- to 60-min) blood glucose concentrations during an insulin tolerance test were higher in KO than in WT animals; whereas late-phase glucose AUC was higher in WR animals regardless of genotype (Fig. 3F). This late-phase increase in AUC in WR compared with SED animals is likely indicative of the counterregulatory response to insulin and not increased insulin resistance (1).
Table 3.
Plasma biochemistry
| WT (n = 7–13/group) |
KO (n = 8–9/group) |
ANOVA | |||
|---|---|---|---|---|---|
| Parameter | SED | WR | SED | WR | |
| Glucose, mg/dl | 289.1 ± 22.9 | 265.2 ± 15.4 | 294.9 ± 27.1 | 286.8 ± 11.8 | G: P = 0.52 A: P = 0.45 G×T: P = 0.71 |
| Insulin, mU/l | 2.2 ± 0.4 | 1.3 ± 0.3 | 2.3 ± 0.6 | 1.8 ± 0.4 | G: P = 0.49 A: P = 0.14 G×A: P = 0.65 |
| HOMA-IR, AU | 1.5 ± 0.2 | 0.9 ± 0.2 | 1.8 ± 0.5 | 1.3 ± 0.3 | G: P = 0.23 A: P = 0.11 G×A: P = 0.93 |
| Total cholesterol, mg/dl | 136.7 ± 15.9 | 123.7 ± 14.6 | 163.6 ± 16.1† | 160.1 ± 9.0† |
G: P = 0.03 A: P = 0.56 G×A: P = 0.73 |
| LDL cholesterol, mg/dl | 6.1 ± 0.6 | 4.8 ± 0.3* | 9.1 ± 0.9† | 7.4 ± 0.5*† |
G: P = 0.0001 A: P = 0.02 G×A: P = 0.71 |
| HDL cholesterol, mg/dl | 53.9 ± 4.9 | 56.1 ± 4.9 | 57.4 ± 4.4 | 65.3 ± 2.7 | G: P = 0.14 A: P = 0.24 G×A: P = 0.51 |
| Triglycerides, mg/dl | 101.3 ± 5.6 | 85.7 ± 1.8* | 94.8 ± 3.4 | 83.3 ± 3.8* | G: P = 0.28 A: P = 0.002 G×A: P = 0.61 |
| NEFA, mmol/l | 0.2 ± 0.04 | 0.2 ± 0.01 | 0.2 ± 0.03 | 0.2 ± 0.02 | G: P = 0.38 A: P = 0.78 G×A: P = 0.61 |
| Leptin, ng/ml | 1,040 ± 210 | 177 ± 74* | 1,099 ± 285 | 568 ± 167* | G: P = 0.31 A: P = 0.003 G×A: P = 0.46 |
Values are means ± SE. NEFA, nonesterified fatty acid; HOMA-IR, homeostasis model assessment of insulin resistance; WT, wild type; KO, estrogen receptor-α knockout; SED, sedentary; WR, wheel running. G, genotype; A, activity; G×A, genotype × activity interaction.
P < 0.05 vs. SED;
P < 0.05 vs. WT.
Bold P values indicate statistical significance.
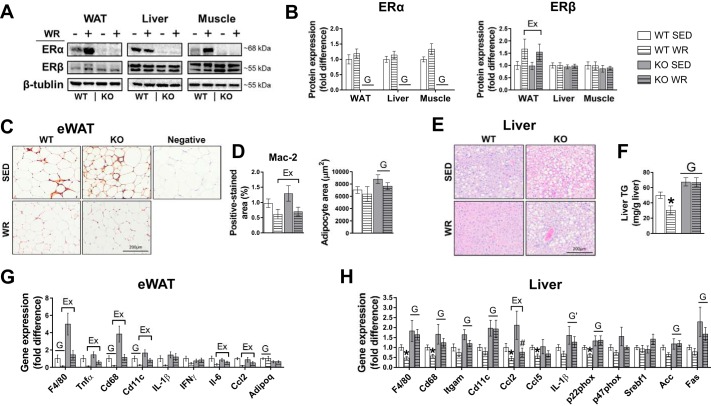
ERα protein expression in epididymal WAT, liver, and quadriceps muscle was absent in KO animals, thus validating the model (Fig. 4, A and B). Daily WR did not alter ERα expression in epididymal WAT or liver; however, an increase in ERα content in muscle from WT WR mice trended toward significance (P = 0.08; Fig. 4, A and B). ERβ protein expression was increased in WAT but not liver or muscle of WR animals independent of genotype (Fig. 4, A and B). Adipocyte size was greater in SED compared with WR mice, with no differences between genotypes (Fig. 4D). Histological examination of macrophage infiltration in WAT (i.e., via Mac-2 immunostaining) revealed increased density of crown-like structures in SED animals in both genotypes. However, compared with SED mice, positive crown-like structures were absent in WR groups (Fig. 4, C and D). Gene transcripts in WAT that are associated with immune cell infiltration and inflammation (F4/80, CD68, CD11c) were enhanced in KO vs. WT mice, and these markers were attenuated in WR animals (Fig. 4G). ERα KO mice had 30% greater hepatic triglyceride content than WT animals (Fig. 4, E and F). Daily WR reduced hepatic triglyceride content by ~50% in WT mice, whereas this effect was completely absent in KO WR animals. Histological examination of the liver further supported biochemically-measured liver triglyceride content, whereby KO animals displayed larger lipid vacuoles compared with WT mice, and daily WR reduced the size and number of lipid vacuoles in WT animals only (representative histology images, Fig. 4E). Liver mRNA expression of immune cell markers and inflammatory genes (F4/80, Cd68, Cd11c, Itgam) were increased in KO animals and were attenuated in WT WR mice but not in KO WR mice (Fig. 4H).
Fig. 4.
Role of estrogen receptor-α (ERα) in white adipose tissue (WAT)- and liver-associated exercise adaptations in male mice. A and B: representative immunoblots for ERα and ERβ and mean quantified expression. WT, wild type; KO, ERα knockout; SED, sedentary; WR, wheel running. C and D: representative WAT histology sections immunostained for Mac-2 (arrows indicate positive-stained crown-like structures), quantified Mac-2 positive-stained area, and adipocyte area. eWAT, epididymal WAT. E and F: representative liver hematoxylin and eosin-stained histology sections and biochemically measured liver triglycerides (TG). G and H: WAT and liver gene expression. WAT and liver histology sections are ×20 and ×10 magnification, respectively. All groups were fed an obesogenic diet. WT, n = 7–13/group; KO, n = 8–11/group. Data are means ± SE. Ex, P < 0.05, main effect of exercise; G, P < 0.05, main effect of genotype. G', P = 0.058, main effect of genotype.
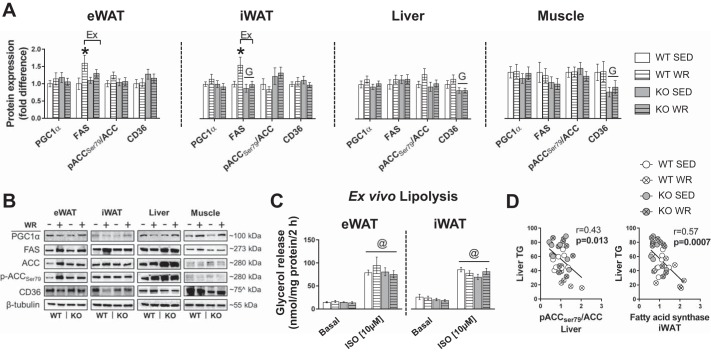
Next, we probed for molecular markers of lipid metabolism within WAT (epididymal and inguinal), liver, and quadriceps muscle. PGC1α protein expression did not differ by condition or genotype in epididymal and inguinal WAT, liver, or quadriceps muscle (Fig. 5, A and B). Compared with WT mice, KO mice had lower FAS expression (main effect genotype, P < 0.05) in inguinal WAT; whereas, relative to SED mice, WRs had increased FAS in both epididymal WAT and inguinal WAT (main effect of Ex, P < 0.05; Fig. 5, A and B). No differences in FAS were noted in the liver or muscle. Phosphorylation of ACC (i.e., the rate-limiting enzyme for fatty acid biosynthesis) at Ser79 was not different by genotype or WR in epididymal WAT, liver, or muscle; however, there was a trend for an increase in inguinal WAT in KO animals (genotype, P = 0.052; Fig. 5, A and B). CD36 protein expression was lower in liver and muscle from KO mice, with no effects of WR (Fig. 5, A and B). Ex vivo stimulated lipolysis did not reveal significant differences in glycerol release by genotype or WR in epididymal WAT or inguinal WAT (Fig. 5C).
Fig. 5.
Role of estrogen receptor-α (ERα) and physical activity in regulating markers of lipid metabolism in adipose tissue, liver, and skeletal muscle. A and B: mean protein expression and representative immunoblots in epididymal (eWAT) and inguinal WAT (iWAT), liver, and quadriceps muscle. WT, wild type; KO, ERα knockout; SED, sedentary; WR, wheel running; PGC1α, peroxisome proliferator-activated receptor-γ coactivator 1α; FAS, fatty acid synthase; ACC, acetyl-CoA carboxylase. C: ex vivo lipolysis in eWAT and iWAT explants. D: association between liver triglycerides (TG) and lipogenic protein expression in adipose tissue and liver. ISO, isoproterenol. WT, n = 7–11/group; KO, n = 8–11/group. Data are means ± SE. Ex, P < 0.05 main effect of exercise; G, P < 0.05 main effect of genotype. ^kDa for CD36 in WAT was ~75, whereas in liver and quadriceps muscle, CD36 bands were ~90 kDa.
DISCUSSION
The significance of ERα signaling in females has been apparent for many years; however, increasing appreciation for its role in male metabolism is emerging (9, 18, 20, 41). In this regard, we report that, despite males having lower expression of ERα than females across species (i.e., mice and swine), loss of ERα increases adiposity, leads to deterioration of glucose tolerance, and provokes metaflammation in adipose tissue and liver. This suggests that ERα signaling is important for optimal immunometabolic function in males irrespective of its lower expression. Furthermore, we examined whether ERα signaling is required for physical activity adaptations in metabolically active tissues. In contrast to our hypothesis, ERα signaling was not required for the beneficial anti-inflammatory effects of physical activity in adipose tissue; however, in support of our hypothesis, ERα appeared to be obligatory for activity-induced prevention of NAFLD.
Here, we report that ERα expression is lower in males than in females in both murine and porcine species. On the other hand, in mice, ERβ was similar between males and females across multiple metabolically-active tissues, whereas male swine had lower ERβ expression in the liver but not in WAT (Fig. 1). We are not the first to report protein expression levels of ERs in swine (26, 29), although to our knowledge this is the first study showing sex differences in the abundance of these receptors in swine.
The importance of estrogen action in the male system has been demonstrated in humans with mutations in ERα and in genetically engineered murine models (reviewed in Refs. 5, 62). Indeed, ERα ablation in male mice, as shown herein and previously (9, 18, 20, 41), disrupts metabolic function and aggravates an inflammatory phenotype. In the present investigation, we did not find differences in circulating estradiol concentrations between WT and ERα KO mice (Fig. 2), suggesting that the immunometabolic manifestations observed may indeed be related to uncoupled estrogen signaling through ERα. In this context, prior literature shows that exogenous estradiol administration has no restorative effects in ERα KO mice corroborating that ERα is a critical signaling mediator in males (6, 39, 40). Furthermore, in male mice, removal of the aromatase gene, which encodes an enzyme responsible for estrogen synthesis, disrupts glucose homeostasis and causes insulin resistance, effects of which are largely attenuated by estradiol administration (58). Thus, experimental manipulation of either the ligand (i.e., estradiol) or ERα produces similar detrimental phenotypes.
This study highlights the importance of ERα signaling in hepatic lipid homeostasis. Mice null for ERα displayed hepatic steatosis and elevated circulating triglycerides, which are early manifestations of NAFLD and are considered the hepatic component of the metabolic syndrome. In addition, we found that ERα KO mice exhibited increased hepatic mRNA expression of multiple inflammatory transcripts that pose a significant risk for the development of nonalcoholic steatohepatitis (NASH) and cardiovascular diseases (38). In general, males are more susceptible to NASH than females (10), which could be related to their lower hepatic ERα expression (Fig. 1A). Our findings are in line with data from hepatocyte-specific ERα-deficient mice that display hepatic insulin resistance and NAFLD (69). Furthermore, estradiol-induced suppression of hepatic steatosis and attenuation of insulin resistance caused by ovariectomy require intact ERα (68). Taken together, our findings along with previously published literature substantiate the vital role of ERα signaling in liver disease prevention.
Presently, there are no long-term effective pharmacological treatments for NAFLD; however, aerobic exercise training is an established cornerstone for disease management that attenuates nutrient overload in the liver (2, 25, 56). Still, the signaling transducers that are responsible for physical activity-induced suppression of NAFLD are not fully elucidated. Herein, we present the novel finding that ERα signaling is necessary for the well-established reduction in liver steatosis caused by daily physical activity (Fig. 4, E and F). Importantly, physical activity levels were similar between WT and KO mice (Fig. 3B), suggesting that the lack of activity-induced amelioration of liver steatosis was not attributable to a lesser stimulus for adaptation in KO animals. In this regard, epidemiological studies suggest that individuals with ERα polymorphisms do not achieve the same degree of exercise benefits, including activity-associated cardiovascular and skeletal adaptations, as their ERα-intact counterparts (19, 30, 57). Despite these observations, the molecular mechanism(s) by which exercise interacts or signals through ERα is unknown. Here, we have uncovered an important target receptor that appears to be required for physical activity-induced benefits in the liver. It is possible that signaling via ERα is necessary for activity-induced increase in hepatic fatty acid oxidation and downregulation of de novo lipogenesis. Previous data have demonstrated that AMPK activity, a mediator of both fatty acid oxidative pathways and lipid synthetic machinery, is in part regulated by ERα (28, 43). Indeed, we found that phosphorylation of ACC at Ser79, a direct target of AMPK, was inversely associated with liver triglycerides (Fig. 5D), suggesting that in the context of increased physical activity the interaction between ERα and AMPK may be crucial in balancing cellular energy homeostasis. Nonetheless, future research should replicate our study in a hepatocyte-specific ERα KO model to determine whether primary deletion of ERα in hepatocytes prevents exercise-induced amelioration of NAFLD. Collectively, our findings establish the significance of ERα as a signal transducer mediating liver-specific exercise adaptations and sets forth the framework for additional mechanistic studies to further investigate this interaction (Fig. 6).
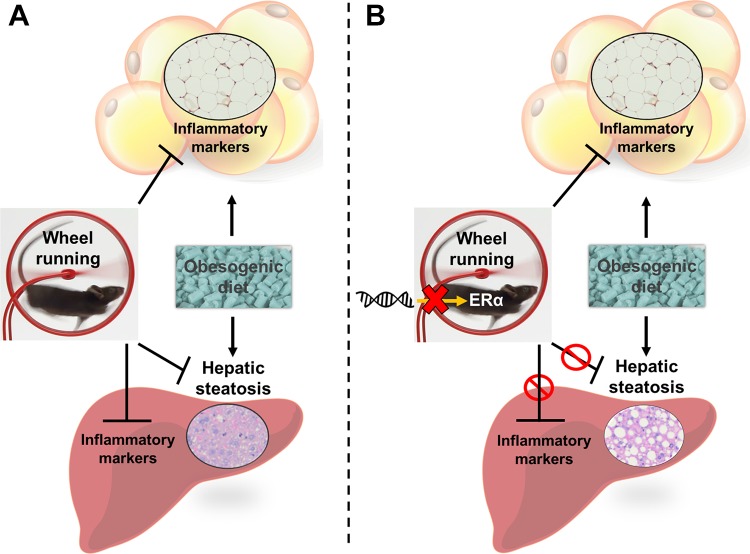
Fig. 6.
Summary of the interaction between daily exercise and estrogen receptor-α (ERα) signaling in liver and adipose tissue. A: voluntary wheel running attenuates metaflammation in adipose tissue and abrogates fatty liver in ERα-intact animals. B: genetic ablation of ERα prevents the reduction in fatty liver by daily exercise but not the anti-inflammatory effects in visceral adipose tissue.
Metaflammation has been implicated as a causal link between obesity and cardiometabolic complications (16, 27, 45, 59), with the attenuation of estrogen action being a significant contributor in animals and humans. Indeed, we found that whole body deletion of ERα triggered immunometabolic dysfunction characterized by increased adiposity and markers of immune cell infiltration and oxidative stress in WAT and liver. In obese adipose tissue, aggregates of immune cells surround and clear dead adipocytes, forming crown-like structures that trigger the production of proinflammatory cytokines and chemoattractants, thus perpetuating the inflammatory state (63, 66). In the present study, removal of ERα per se increased the abundance of crown-like structures and associated inflammation and thus phenocopied the effects often observed in obesity. Of note, this inflammatory phenotype caused by global ERα ablation has been recapitulated in adipocyte-specific ERα KO mice independently of sex (9), indicating that adipocyte ERα signaling is crucial for adipose tissue immunometabolic function.
Visceral adipose tissue inflammatory resolution via pharmacological and genetic approaches alleviates obesity-associated metabolic dysfunction (52). Along these lines, exercise training is an established tool that attenuates visceral adiposity and adipose tissue inflammation in both rodents and humans (3, 44, 60). These exercise-induced anti-inflammatory actions are also present in rodents that lack ovarian hormones (70); however, whether ERα signaling is necessary for the immunological benefits of daily exercise is not known. In this context, we report that voluntary WR attenuates adipose tissue, but not hepatic, inflammation regardless of ERα signaling. This, in turn, dissociates the anti-inflammatory effects of daily exercise and ERα signaling, suggesting that exercise training modifies alternative targets/pathways that improve WAT immunometabolism. Of note, ERβ was enhanced by daily WR in WAT but not in liver or muscle. Thus, it is possible that this upregulation of ERβ by daily exercise contributed to the attenuation of inflammation in adipose tissue. Interestingly, in vitro overexpression of ERβ inhibits adipogenesis via suppression of peroxisome proliferator-activated receptor-γ activity (13), an observation that has also been reported with acute and chronic exercise (53, 55).
Notably, we found that FAS expression was elevated in adipose tissue in WT WR but not in ERα KO WR mice (Fig. 5). This finding contrasts with previous work where exercise was reported to decrease adipose tissue lipogenesis (49) and conflicts with the general premise that increased expression of FAS is associated with insulin resistance (36). The increase in FAS was specific to adipose tissue, given that neither liver nor muscle had increased FAS expression in physically active animals. Importantly, elevated FAS expression in adipose tissue was not associated with increased inflammation but was inversely associated with liver triglyceride (Fig. 5D), suggesting a healthy lipid accrual phenotype in WT adipose tissue. Of note, adipose tissue lipid accretion would limit fatty acid flux to the liver and thus attenuate hepatic lipid accumulation. Taken together, our findings reveal that ERα is necessary for maintaining immunometabolic homeostasis in visceral WAT, but it is not required for the anti-inflammatory effects of daily exercise (Fig. 5).
Several aspects of this investigation warrant further consideration. Glucose tolerance was assessed via intraperitoneal glucose injection, which bypasses first liver response and the incretin effect. Thus, direct inferences regarding liver glucose homeostasis cannot be made in the current study. Additional studies using oral glucose administration or hyperinsulinemic-euglycemic clamp with glucose isotopes are needed to determine the role of ERα signaling in modulating physical activity-induced improvements in hepatic glucoregulation.
Although in the context of exercise we show that ERα signaling is indispensable for NAFLD reduction, we cannot rule out the possibility that other estrogen signaling mediators such as ERβ or G protein-coupled estrogen receptor (GPER) also exert biological effects in the liver. Indeed, it was recently suggested that ERβ agonism attenuates hepatic lipid accretion in a rodent model of NASH (67). However, given that, herein, exercising mice (i.e., voluntary WR) did not display differences in hepatic ERβ expression and that ERα ablation per se does not enhance its endogenous ligand (Fig. 2 and Ref. 7) it is unlikely that signaling through ERβ compensated for the loss of ERα action. A follow-up study could exercise ERα KO mice with or without ERβ agonism to determine whether forced signaling through ERβ restores exercise-induced amelioration of liver steatosis. GPER is also expressed in the liver (12, 54); however, there is paucity of experimental evidence revealing its biological role in this organ.
The unique finding that exercise-induced reduction in hepatic steatosis was absent in ERα-ablated mice suggests that this signaling node may be critical to the actions of exercise in the liver. Yet, as noted, the molecular mechanisms by which exercise interacts or signals through ERα are not known and cannot be deduced from this investigation. This is partly attributed to the fact that the present study was conducted using a whole body ERα KO mouse model, which impacts multiple organs and organ systems (7).
In conclusion, the present findings indicate that ERα is required for optimal immunometabolic function in male mice despite their reduced ERα protein expression in metabolically active tissues. Furthermore, for the first time, we show that ERα signaling appears to be obligatory for exercise-induced prevention of hepatic steatosis.
GRANTS
This study was supported, in part, by National Heart, Lung, and Blood Institute Grants K01 HL-125503 (to J. Padilla) and 1K08 HL-129074-01 (to C. Manrique-Acevedo), Veterans Affairs Merit Grant I01BX003271-01 (to R. S. Rector), and American Egg Board Grant no. 00050021 (to N. C. Winn). The Vanderbilt University Medical Center Hormone Assay and Analytical Services Core is supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-059637 and DK-020593.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.C.W., V.J.V.-P., and J.P. conceived and designed research; N.C.W., T.J.J., Z.I.G., R.P.C., and M.L.W. performed experiments; N.C.W., T.J.J., Z.I.G., R.P.C., M.L.W., and J.P. analyzed data; N.C.W., R.S.R., V.J.V.-P., and J.P. interpreted results of experiments; N.C.W. prepared figures; N.C.W. drafted manuscript; N.C.W., T.J.J., Z.I.G., R.P.C., J.A.K., D.B.L., C.M.-A., R.S.R., V.J.V.-P., and J.P. edited and revised manuscript; N.C.W., T.J.J., Z.I.G., R.P.C., M.L.W., J.A.K., D.B.L., C.M.-A., R.S.R., V.J.V.-P., and J.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We gratefully acknowledge the technical assistance of Grace Meers, Michelle Gastecki, Dusti Eaton, and Gabriela Lin. We also thank the University of Missouri Veterinary Medicine Diagnostic Laboratory for their technical assistance. This work was supported, in part, with resources and the use of facilities at the Harry S. Truman Memorial Veterans Hospital, Columbia, MO.
REFERENCES
- 1.Ayala JE, Samuel VT, Morton GJ, Obici S, Croniger CM, Shulman GI, Wasserman DH, McGuinness OP; NIH Mouse Metabolic Phenotyping Center Consortium . Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech 3: 525–534, 2010. doi: 10.1242/dmm.006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brouwers B, Hesselink MK, Schrauwen P, Schrauwen-Hinderling VB. Effects of exercise training on intrahepatic lipid content in humans. Diabetologia 59: 2068–2079, 2016. doi: 10.1007/s00125-016-4037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruun JM, Helge JW, Richelsen B, Stallknecht B. Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am J Physiol Endocrinol Metab 290: E961–E967, 2006. doi: 10.1152/ajpendo.00506.2005. [DOI] [PubMed] [Google Scholar]
- 4.Caputo T, Gilardi F, Desvergne B. From chronic overnutrition to metaflammation and insulin resistance: adipose tissue and liver contributions. FEBS Lett 591: 3061–3088, 2017. doi: 10.1002/1873-3468.12742. [DOI] [PubMed] [Google Scholar]
- 5.Cooke PS, Nanjappa MK, Ko C, Prins GS, Hess RA. Estrogens in male physiology. Physiol Rev 97: 995–1043, 2017. doi: 10.1152/physrev.00018.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Couse JE, Mahato D, Eddy EM, Korach KS. Molecular mechanism of estrogen action in the male: insights from the estrogen receptor null mice. Reprod Fertil Dev 13: 211–219, 2001. doi: 10.1071/RD00128. [DOI] [PubMed] [Google Scholar]
- 7.Curtis Hewitt S, Couse JF, Korach KS. Estrogen receptor transcription and transactivation: estrogen receptor knockout mice: what their phenotypes reveal about mechanisms of estrogen action. Breast Cancer Res 2: 345–352, 2000. doi: 10.1186/bcr79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Eon TM, Souza SC, Aronovitz M, Obin MS, Fried SK, Greenberg AS. Estrogen regulation of adiposity and fuel partitioning. Evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. J Biol Chem 280: 35983–35991, 2005. doi: 10.1074/jbc.M507339200. [DOI] [PubMed] [Google Scholar]
- 9.Davis KE, D Neinast M, Sun K, M Skiles W, D Bills J, A Zehr J, Zeve D, D Hahner L, W Cox D, M Gent L, Xu Y, V Wang Z, A Khan S, Clegg DJ. The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis. Mol Metab 2: 227–242, 2013. doi: 10.1016/j.molmet.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Lédinghen V, Ratziu V, Causse X, Le Bail B, Capron D, Renou C, Pilette C, Oules V, Gelsi E, Oberti F, Vallet-Pichard A, Le Provost N, Cadranel JF; Association Française pour l’Etude du Foie Groupe Epidémiologie et Evaluation; Association Nationale des Gastroentérologues des Hôpitaux généraux de France . Diagnostic and predictive factors of significant liver fibrosis and minimal lesions in patients with persistent unexplained elevated transaminases. A prospective multicenter study. J Hepatol 45: 592–599, 2006. doi: 10.1016/j.jhep.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Eddy EM, Washburn TF, Bunch DO, Goulding EH, Gladen BC, Lubahn DB, Korach KS. Targeted disruption of the estrogen receptor gene in male mice causes alteration of spermatogenesis and infertility. Endocrinology 137: 4796–4805, 1996. doi: 10.1210/endo.137.11.8895349. [DOI] [PubMed] [Google Scholar]
- 12.Enmark E, Gustafsson JA. Oestrogen receptors—an overview. J Intern Med 246: 133–138, 1999. doi: 10.1046/j.1365-2796.1999.00545.x. [DOI] [PubMed] [Google Scholar]
- 13.Foryst-Ludwig A, Clemenz M, Hohmann S, Hartge M, Sprang C, Frost N, Krikov M, Bhanot S, Barros R, Morani A, Gustafsson JA, Unger T, Kintscher U. Metabolic actions of estrogen receptor beta (ERbeta) are mediated by a negative cross-talk with PPARgamma. PLoS Genet 4: e1000108, 2008. doi: 10.1371/journal.pgen.1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geary N, Asarian L, Korach KS, Pfaff DW, Ogawa S. Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-alpha null mice. Endocrinology 142: 4751–4757, 2001. doi: 10.1210/endo.142.11.8504. [DOI] [PubMed] [Google Scholar]
- 15.Geer EB, Shen W. Gender differences in insulin resistance, body composition, and energy balance. Gend Med 6, Suppl 1: 60–75, 2009. doi: 10.1016/j.genm.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ginsberg HN, MacCallum PR. The obesity, metabolic syndrome, and type 2 diabetes mellitus pandemic. Part I. Increased cardiovascular disease risk and the importance of atherogenic dyslipidemia in persons with the metabolic syndrome and type 2 diabetes mellitus. J Cardiometab Syndr 4: 113–119, 2009. doi: 10.1111/j.1559-4572.2008.00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grunewald ZI, Winn NC, Gastecki ML, Woodford ML, Ball JR, Hansen SA, Sacks HS, Vieira-Potter VJ, Padilla J. Removal of interscapular brown adipose tissue increases aortic stiffness despite normal systemic glucose metabolism in mice. Am J Physiol Regul Integr Comp Physiol 314: R584–R597, 2018. doi: 10.1152/ajpregu.00332.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Handgraaf S, Riant E, Fabre A, Waget A, Burcelin R, Lière P, Krust A, Chambon P, Arnal JF, Gourdy P. Prevention of obesity and insulin resistance by estrogens requires ERα activation function-2 (ERαAF-2), whereas ERαAF-1 is dispensable. Diabetes 62: 4098–4108, 2013. doi: 10.2337/db13-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashi K, Maeda S, Iemitsu M, Otsuki T, Sugawara J, Tanabe T, Miyauchi T, Kuno S, Ajisaka R, Matsuda M. Estrogen receptor-alpha genotype affects exercise-related reduction of arterial stiffness. Med Sci Sports Exerc 40: 252–257, 2008. doi: 10.1249/mss.0b013e31815c04cf. [DOI] [PubMed] [Google Scholar]
- 20.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci USA 97: 12729–12734, 2000. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hevener AL, Clegg DJ, Mauvais-Jarvis F. Impaired estrogen receptor action in the pathogenesis of the metabolic syndrome. Mol Cell Endocrinol 418: 306–321, 2015. doi: 10.1016/j.mce.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hotamisligil GS. Inflammation and metabolic disorders. Nature 444: 860–867, 2006. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 23.Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature 542: 177–185, 2017. doi: 10.1038/nature21363. [DOI] [PubMed] [Google Scholar]
- 24.Huang ZH, Manickam B, Ryvkin V, Zhou XJ, Fantuzzi G, Mazzone T, Sam S. PCOS is associated with increased CD11c expression and crown-like structures in adipose tissue and increased central abdominal fat depots independent of obesity. J Clin Endocrinol Metab 98: E17–E24, 2013. doi: 10.1210/jc.2012-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson NA, Sachinwalla T, Walton DW, Smith K, Armstrong A, Thompson MW, George J. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology 50: 1105–1112, 2009. doi: 10.1002/hep.23129. [DOI] [PubMed] [Google Scholar]
- 26.Kalbe C, Mau M, Wollenhaupt K, Rehfeldt C. Evidence for estrogen receptor alpha and beta expression in skeletal muscle of pigs. Histochem Cell Biol 127: 95–107, 2007. doi: 10.1007/s00418-006-0224-z. [DOI] [PubMed] [Google Scholar]
- 27.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 116: 1494–1505, 2006. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JH, Meyers MS, Khuder SS, Abdallah SL, Muturi HT, Russo L, Tate CR, Hevener AL, Najjar SM, Leloup C, Mauvais-Jarvis F. Tissue-selective estrogen complexes with bazedoxifene prevent metabolic dysfunction in female mice. Mol Metab 3: 177–190, 2014. doi: 10.1016/j.molmet.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knapczyk-Stwora K, Durlej M, Duda M, Czernichowska-Ferreira K, Tabecka-Lonczynska A, Slomczynska M. Expression of oestrogen receptor α and oestrogen receptor β in the uterus of the pregnant swine. Reprod Domest Anim 46: 1–7, 2011. doi: 10.1111/j.1439-0531.2009.01505.x. [DOI] [PubMed] [Google Scholar]
- 30.Kondo H, Fujino H, Nagatomo F, Ishihara A. Influence of estrogen receptor α polymorphisms on bone density in response to habitual exercise in Japanese postmenopausal women. ScientificWorldJournal 2014: 593927, 2014. doi: 10.1155/2014/593927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang YQ, Akishita M, Kim S, Ako J, Hashimoto M, Iijima K, Ohike Y, Watanabe T, Sudoh N, Toba K, Yoshizumi M, Ouchi Y. Estrogen receptor beta is involved in the anorectic action of estrogen. Int J Obes Relat Metab Disord 26: 1103–1109, 2002. doi: 10.1038/sj.ijo.0802054. [DOI] [PubMed] [Google Scholar]
- 32.Linden MA, Fletcher JA, Morris EM, Meers GM, Kearney ML, Crissey JM, Laughlin MH, Booth FW, Sowers JR, Ibdah JA, Thyfault JP, Rector RS. Combining metformin and aerobic exercise training in the treatment of type 2 diabetes and NAFLD in OLETF rats. Am J Physiol Endocrinol Metab 306: E300–E310, 2014. doi: 10.1152/ajpendo.00427.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Linden MA, Fletcher JA, Morris EM, Meers GM, Laughlin MH, Booth FW, Sowers JR, Ibdah JA, Thyfault JP, Rector RS. Treating NAFLD in OLETF rats with vigorous-intensity interval exercise training. Med Sci Sports Exerc 47: 556–567, 2015. doi: 10.1249/MSS.0000000000000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linden MA, Sheldon RD, Meers GM, Ortinau LC, Morris EM, Booth FW, Kanaley JA, Vieira-Potter VJ, Sowers JR, Ibdah JA, Thyfault JP, Laughlin MH, Rector RS. Aerobic exercise training in the treatment of non-alcoholic fatty liver disease related fibrosis. J Physiol 594: 5271–5284, 2016. doi: 10.1113/JP272235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA 90: 11162–11166, 1993. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Menendez JA, Vazquez-Martin A, Ortega FJ, Fernandez-Real JM. Fatty acid synthase: association with insulin resistance, type 2 diabetes, and cancer. Clin Chem 55: 425–438, 2009. doi: 10.1373/clinchem.2008.115352. [DOI] [PubMed] [Google Scholar]
- 37.Metz L, Gerbaix M, Masgrau A, Guillet C, Walrand S, Boisseau N, Boirie Y, Courteix D. Nutritional and exercise interventions variably affect estrogen receptor expression in the adipose tissue of male rats. Nutr Res 36: 280–289, 2016. doi: 10.1016/j.nutres.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Neuschwander-Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology 52: 774–788, 2010. doi: 10.1002/hep.23719. [DOI] [PubMed] [Google Scholar]
- 39.Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of estrogen receptor-alpha gene expression in reproduction-related behaviors in female mice. Endocrinology 139: 5070–5081, 1998. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- 40.Ogawa S, Lubahn DB, Korach KS, Pfaff DW. Behavioral effects of estrogen receptor gene disruption in male mice. Proc Natl Acad Sci USA 94: 1476–1481, 1997. doi: 10.1073/pnas.94.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohlsson C, Hellberg N, Parini P, Vidal O, Bohlooly-Y M, Rudling M, Lindberg MK, Warner M, Angelin B, Gustafsson JA. Obesity and disturbed lipoprotein profile in estrogen receptor-alpha-deficient male mice. Biochem Biophys Res Commun 278: 640–645, 2000. doi: 10.1006/bbrc.2000.3827. [DOI] [PubMed] [Google Scholar]
- 42.Olver TD, Grunewald ZI, Jurrissen TJ, MacPherson REK, LeBlanc PJ, Schnurbusch TR, Czajkowski AM, Laughlin MH, Rector RS, Bender SB, Walters EM, Emter CA, Padilla J. Microvascular insulin resistance in skeletal muscle and brain occurs early in the development of juvenile obesity in pigs. Am J Physiol Regul Integr Comp Physiol 314: R252–R264, 2018. doi: 10.1152/ajpregu.00213.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pedram A, Razandi M, O’Mahony F, Harvey H, Harvey BJ, Levin ER. Estrogen reduces lipid content in the liver exclusively from membrane receptor signaling. Sci Signal 6: ra36, 2013. doi: 10.1126/scisignal.2004013. [DOI] [PubMed] [Google Scholar]
- 44.Porter JW, Rowles JL III, Fletcher JA, Zidon TM, Winn NC, McCabe LT, Park YM, Perfield JW II, Thyfault JP, Rector RS, Padilla J, Vieira-Potter VJ. Anti-inflammatory effects of exercise training in adipose tissue do not require FGF21. J Endocrinol 235: 97–109, 2017. doi: 10.1530/JOE-17-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reaven GM. Insulin resistance: the link between obesity and cardiovascular disease. Med Clin North Am 95: 875–892, 2011. doi: 10.1016/j.mcna.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 46.Rector RS, Thyfault JP, Morris RT, Laye MJ, Borengasser SJ, Booth FW, Ibdah JA. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima Fatty rats. Am J Physiol Gastrointest Liver Physiol 294: G619–G626, 2008. doi: 10.1152/ajpgi.00428.2007. [DOI] [PubMed] [Google Scholar]
- 47.Rector RS, Uptergrove GM, Morris EM, Borengasser SJ, Laughlin MH, Booth FW, Thyfault JP, Ibdah JA. Daily exercise vs. caloric restriction for prevention of nonalcoholic fatty liver disease in the OLETF rat model. Am J Physiol Gastrointest Liver Physiol 300: G874–G883, 2011. doi: 10.1152/ajpgi.00510.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ribas V, Nguyen MT, Henstridge DC, Nguyen AK, Beaven SW, Watt MJ, Hevener AL. Impaired oxidative metabolism and inflammation are associated with insulin resistance in ERα-deficient mice. Am J Physiol Endocrinol Metab 298: E304–E319, 2010. doi: 10.1152/ajpendo.00504.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richard D, Trayhurn P. Effect of exercise training on the rates of fatty acid synthesis in mice. Can J Physiol Pharmacol 62: 695–699, 1984. doi: 10.1139/y84-114. [DOI] [PubMed] [Google Scholar]
- 50.Roesch DM. Effects of selective estrogen receptor agonists on food intake and body weight gain in rats. Physiol Behav 87: 39–44, 2006. doi: 10.1016/j.physbeh.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 51.Rubanyi GM, Freay AD, Kauser K, Sukovich D, Burton G, Lubahn DB, Couse JF, Curtis SW, Korach KS. Vascular estrogen receptors and endothelium-derived nitric oxide production in the mouse aorta. Gender difference and effect of estrogen receptor gene disruption. J Clin Invest 99: 2429–2437, 1997. doi: 10.1172/JCI119426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rull A, Camps J, Alonso-Villaverde C, Joven J. Insulin resistance, inflammation, and obesity: role of monocyte chemoattractant protein-1 (or CCL2) in the regulation of metabolism. Mediators Inflamm 2010: 326580, 2010. doi: 10.1155/2010/326580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sakurai T, Endo S, Hatano D, Ogasawara J, Kizaki T, Oh-ishi S, Izawa T, Ishida H, Ohno H. Effects of exercise training on adipogenesis of stromal-vascular fraction cells in rat epididymal white adipose tissue. Acta Physiol (Oxf) 200: 325–338, 2010. doi: 10.1111/j.1748-1716.2010.02159.x. [DOI] [PubMed] [Google Scholar]
- 54.Sharma G, Mauvais-Jarvis F, Prossnitz ER. Roles of G protein-coupled estrogen receptor GPER in metabolic regulation. J Steroid Biochem Mol Biol 176: 31–37, 2018. doi: 10.1016/j.jsbmb.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shen Y, Zhou H, Jin W, Lee HJ. Acute exercise regulates adipogenic gene expression in white adipose tissue. Biol Sport 33: 381–391, 2016. doi: 10.5604/20831862.1224395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sullivan S, Kirk EP, Mittendorfer B, Patterson BW, Klein S. Randomized trial of exercise effect on intrahepatic triglyceride content and lipid kinetics in nonalcoholic fatty liver disease. Hepatology 55: 1738–1745, 2012. doi: 10.1002/hep.25548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suuriniemi M, Mahonen A, Kovanen V, Alén M, Lyytikäinen A, Wang Q, Kröger H, Cheng S. Association between exercise and pubertal BMD is modulated by estrogen receptor alpha genotype. J Bone Miner Res 19: 1758–1765, 2004. doi: 10.1359/JBMR.040918. [DOI] [PubMed] [Google Scholar]
- 58.Toda K, Toda A, Ono M, Saibara T. Lack of 17β-estradiol reduces sensitivity to insulin in the liver and muscle of male mice. Heliyon 4: e00772, 2018. doi: 10.1016/j.heliyon.2018.e00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vieira-Potter VJ. Inflammation and macrophage modulation in adipose tissues. Cell Microbiol 16: 1484–1492, 2014. doi: 10.1111/cmi.12336. [DOI] [PubMed] [Google Scholar]
- 60.Vieira VJ, Valentine RJ, Wilund KR, Antao N, Baynard T, Woods JA. Effects of exercise and low-fat diet on adipose tissue inflammation and metabolic complications in obese mice. Am J Physiol Endocrinol Metab 296: E1164–E1171, 2009. doi: 10.1152/ajpendo.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wainright KS, Fleming NJ, Rowles JL, Welly RJ, Zidon TM, Park YM, Gaines TL, Scroggins RJ, Anderson-Baucum EK, Hasty AH, Vieira-Potter VJ, Padilla J. Retention of sedentary obese visceral white adipose tissue phenotype with intermittent physical activity despite reduced adiposity. Am J Physiol Regul Integr Comp Physiol 309: R594–R602, 2015. doi: 10.1152/ajpregu.00042.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walker VR, Korach KS. Estrogen receptor knockout mice as a model for endocrine research. ILAR J 45: 455–461, 2004. doi: 10.1093/ilar.45.4.455. [DOI] [PubMed] [Google Scholar]
- 63.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796–1808, 2003. doi: 10.1172/JCI200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Winn NC, Grunewald ZI, Gastecki ML, Woodford ML, Welly RJ, Clookey SL, Ball JR, Gaines TL, Karasseva NG, Kanaley JA, Sacks HS, Vieira-Potter VJ, Padilla J. Deletion of UCP1 enhances ex vivo aortic vasomotor function in female but not male mice despite similar susceptibility to metabolic dysfunction. Am J Physiol Endocrinol Metab 313: E402–E412, 2017. doi: 10.1152/ajpendo.00096.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Winn NC, Vieira-Potter VJ, Gastecki ML, Welly RJ, Scroggins RJ, Zidon TM, Gaines TL, Woodford ML, Karasseva NG, Kanaley JA, Sacks HS, Padilla J. Loss of UCP1 exacerbates Western diet-induced glycemic dysregulation independent of changes in body weight in female mice. Am J Physiol Regul Integr Comp Physiol 312: R74–R84, 2017. doi: 10.1152/ajpregu.00425.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112: 1821–1830, 2003. doi: 10.1172/JCI200319451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang B, Zhang CG, Ji LH, Zhao G, Wu ZY. Estrogen receptor β selective agonist ameliorates liver cirrhosis in rats by inhibiting the activation and proliferation of hepatic stellate cells. J Gastroenterol Hepatol 33: 747–755, 2018. doi: 10.1111/jgh.13976. [DOI] [PubMed] [Google Scholar]
- 68.Zhu L, Brown WC, Cai Q, Krust A, Chambon P, McGuinness OP, Stafford JM. Estrogen treatment after ovariectomy protects against fatty liver and may improve pathway-selective insulin resistance. Diabetes 62: 424–434, 2013. doi: 10.2337/db11-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu L, Martinez MN, Emfinger CH, Palmisano BT, Stafford JM. Estrogen signaling prevents diet-induced hepatic insulin resistance in male mice with obesity. Am J Physiol Endocrinol Metab 306: E1188–E1197, 2014. doi: 10.1152/ajpendo.00579.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zidon TM, Park YM, Welly RJ, Woodford ML, Scroggins RJ, Britton SL, Koch LG, Booth FW, Padilla J, Kanaley JA, Vieira-Potter VJ. Voluntary wheel running improves adipose tissue immunometabolism in ovariectomized low-fit rats. Adipocyte 7: 20–34, 2018. doi: 10.1080/21623945.2017.1402991. [DOI] [PMC free article] [PubMed] [Google Scholar]