Abstract
Adolescents with type 2 diabetes (T2D) have severe insulin resistance (IR) secondary to obesity, genetics, and puberty, and IR predicts metabolic comorbidities. Adults with T2D have multitissue IR, which has guided therapeutic developments, but this is not established in youth. We sought to assess adipose, hepatic, and peripheral insulin sensitivity in adolescents with and without T2D. Twenty-seven youth with T2D [age: 15.6 ± 0.4 yr; female: 78%; body mass index (BMI) percentile: 96.1 (52.6, 95.9), late puberty; hemoglobin A1c (HbA1c) 7.3% (6.2, 10.1)] and 21 controls of similar BMI, pubertal stage, and habitual activity were enrolled. Insulin action was measured with a four-phase hyperinsulinemic-euglycemic clamp (basal, 10, 16, and 80 mU·m−2·min−1 for studying adipose, hepatic, and peripheral IR, respectively) with glucose and glycerol isotope tracers. Total fat mass, fat-free mass, liver fat fraction, and visceral fat were measured with dual-energy x-ray absorptiometry (DXA) and MRI, respectively. Free fatty acids (FFAs), lipid profile, and inflammatory markers were also measured. Adolescents with T2D had higher lipolysis (P = 0.012), endogenous glucose production (P < 0.0001), and lower glucose clearance (P = 0.002) during hyperinsulinemia than controls. In T2D, peripheral IR positively correlated to FFA (P < 0.001), inflammatory markers, visceral (P = 0.004) and hepatic fat (P = 0.007); hepatic IR correlated with central obesity (P = 0.004) and adipose IR (P = 0.003). Youth with T2D have profound multitissue IR compared with BMI-equivalent youth without T2D. The development of multitissue interactions appears crucial to the pathogenesis of T2D. Therapeutic targets on multitissue IR may be of benefit, deserving of further research.
Keywords: adolescence, hyperinsulinemic-euglycemic clamp, insulin resistance, isotopic tracers, type 2 diabetes
INTRODUCTION
Type 2 diabetes (T2D) that develops in youth, although still uncommon, is rapidly becoming one of the worst pediatric endocrine diseases of the modern era. Pediatric-onset T2D has increased in tandem with childhood obesity at a rate of ~5% per year since 2002 (13). Pediatric T2D, similar to adult disease, is characterized by primary insulin resistance (IR) and pancreatic β-cell insufficiency (16). IR increases along the spectrum of glucose tolerance in adolescents with obesity without T2D (4, 35) and IR in youth to be higher compared with adults (2). This more severe IR may hasten β-cell failure and contribute to the early development of T2D in youth (2, 18) and the more rapid failure rate of oral diabetes medications in youth with T2D (6, 49). However, after decades of research, the unique facets of T2D in youth are still not entirely understood (18). This underscores the urgency to understand T2D pathogenesis in youth better.
With the development of newer therapeutic options to target IR in specific organs, elucidating tissue-specific targets in T2D is a promising avenue for diabetes treatment. A high-dose hyperinsulinemic-euglycemic clamp (HE-clamp) is the most common procedure to assess peripheral IR, and this has been performed in both adults and youth (2, 11, 13, 18, 20, 21, 28, 35). The addition of isotope tracers and variations in insulin concentrations for a multiphase clamp allows for assessments of the effects of insulin on suppression of endogenous glucose production (EGP) as a measure of hepatic IR and suppression of lipolysis as a measure of adipose IR. Because of the intensity of the multiphase clamp, adipose and hepatic IR are often estimated using indices of fasting or percent suppression of lipolysis and EGP from a one-phase high-dose clamp (17, 35), which provides less physiologic insulin concentrations for suppression of lipolysis and EGP compared with the graded HE-clamp method (28, 36). Multiphase HE clamps with both measures have been performed in adults, and only EGP via tracers and three-phase clamps in youth with obesity with and without diabetes (2, 11, 20, 21, 28, 35). Therefore, our goal was to measure the effects of graded physiologic hyperinsulinemia on lipolysis, EGP, and peripheral glucose uptake in a group of adolescents who are overweight/obese with and without T2D prescribed glycemic control typical of modern youth.
PARTICIPANTS AND METHODS
Participants.
Adolescents with and without T2D were recruited from pediatric clinics at the Children’s Hospital Colorado and the Barbara Davis Center for a prospective, cross-sectional study of IR in youth as part of either the RESistance to InSulin in Type 1 ANd T2D (RESISTANT) (9) or the Androgens and Insulin Resistance Study (AIRS) (12, 14). The RESISTANT cohort included four groups of youth: T2D, type 1 diabetes, body mass index (BMI) >85 percentile no diabetes, and BMI <85 percentile no diabetes. The AIRS cohort included three groups: obese no diabetes with regular menses, obese with polycystic ovarian syndrome (PCOS), and BMI <85 percentile no diabetes with PCOS.
All participants with isotope tracer data in the T2D and overweight/obese no diabetes and no PCOS groups from RESISTANT and AIRS are included. Forty participants with and without T2D from the RESISTANT study and seven without diabetes or PCOS from AIRS are included in this analysis. Tracer data for women who are overweight, any male participants, and those with T2D from the RESISTANT study have never been published, whereas data from women with obesity from both cohorts have been published elsewhere (15).
Otherwise, inclusion criteria for the current analysis is the same as the parent study’s (which was identical other than diabetes or PCOS status) and notably included sedentary status to minimize impacts of physical activity on IR (<3 h exercise/week) and T2D defined as diabetes by American Diabetes Association criteria and absence of diabetes-associated autoantibodies. Exclusion criteria were alanine transferase (ALT) >80 IU/ml, blood pressure >140/90 mmHg, hemoglobin <9 mg/dl, serum creatinine >1.5 mg/dl, smoking, medications affecting IR (except metformin in T2D group), blood pressure, or lipids, and in T2D youth, HbA1c < 12%. In the T2D group, 21 participants were treated with metformin, and 13 participants received insulin treatment (1 bolus, 7 basal, and 5 basal-bolus injections). The study was approved by the University of Colorado Institutional Review Board. Informed consent was obtained from all participants ≥18 yr and parental consent and participant assent from all participants <18 yr.
Physical activity.
A 3-day pediatric activity recall assessing metabolic equivalents was completed (54), and participants wore an Actigraph GT3x accelerometer (Actigraph Corp, Pensacola, FL) for 7 days to assess habitual physical activity, and at least 3 days of data were collected on all participants and included for analysis. Data were corrected for wear time and categorized into age-appropriate activity levels: sedentary, light, lifestyle, moderate, vigorous, and very vigorous (25).
Insulin sensitivity.
To help minimize confounding effects on insulin sensitivity, the study day was preceded by 3 days of restricted physical activity and a fixed-macronutrient, weight-maintenance diet (55% carbohydrates, 30% fat, and 15% protein) that was adjusted for typical activity. The food was provided to the participants by the Metabolic Kitchen at the Clinical Translational Research Center, and participants were instructed to consume no additional calories. Studies were performed in the follicular phase for females when possible. For those with T2D and taking metformin, metformin was stopped for 72 h before the clamp. Participants with T2D were instructed to monitor blood glucose levels at least four times daily. Study day cancellation criteria included a fasting blood glucose greater than 200 mg/dl or random blood glucose greater than 300 mg/dl during the study diet or elevated urine ketones at admission, but this did not occur in any participants. There were no significant changes in blood glucose during the study diet. Participants were admitted to the inpatient Clinical Translational Research Center for an isocaloric meal, followed by 12 h of overnight monitored fasting. In participants with diabetes, the blood glucose concentrations were measured every 30 min starting at 8 PM, which was 2 h following the evening meal, using a bedside glucometer, and an overnight continuous insulin infusion was adjusted accordingly to normalize blood sugar levels gradually to a goal of 100 mg/dl before 6 AM. Our participants had no overnight hypoglycemic episodes, which can stimulate counterregulatory hormone secretion (1). The following morning, a four-phase HE-clamp was performed. Fasting blood samples were drawn before starting the isotopes infusion at 6 AM. A 2-h basal tracer equilibration phase then occurred, during which the overnight insulin infusion was continued to maintain normoglycemia only in those with T2D. Next, insulin doses for each additional 1.5-h phase were 10, 16, and 80 mU·m−2·min−1 to determine adipose, hepatic, and peripheral IR, respectively, based on our and others’ (15, 22) previous experience with the higher insulin requirements in pubertal youth. The goal insulin concentration for the highest stage was 200–300 mU/l to match the postprandial insulin concentrations found in this patient population (12, 43). Twenty percent dextrose (spiked with 6,6-2H2 glucose) was infused to maintain blood glucose at 95 mg/dl, with samples every 5 min for determination of glucose concentrations (Yellow Springs Instrument, Yellow Springs, OH).
Tracer infusion protocol.
Blood samples to measure background enrichment and concentration were obtained. Then, a bolus of 4.5 mg/kg [6,6-2H2] glucose (Isotec, Miamisville, IA), followed by a continuous infusion at 0.03 mg·kg−1·min−1 [6,6-2H2] glucose was paired with a primed (1.6 μmol/kg) then constant (0.11 μmol·kg−1·min−1 ) infusion of 2H5glycerol. During the last 30 min of each of the 4 phases, 4 samples each 10 min apart were drawn for glucose and glycerol concentrations and glucose and glycerol enrichment, and 3 samples for FFA and insulin concentrations (27).
Sample analysis.
Analyses were performed by standard methods at the University of Colorado Anschutz Research core laboratory or the Children’s Hospital Colorado clinical laboratory except where noted. Serum insulin, leptin, and adiponectin were analyzed with RIA (Millipore, Billerica, MA); plasma glycerol (R-Biopharm, Marshall, MI) and FFA (Wako Chemicals, Inc., Richmond, VA) were analyzed enzymatically. HbA1c was measured by Diabetes Control and Complications Trial (DCCT)-calibrated ion-exchange HPLC (Bio-Rad Laboratories, Hercules, CA). A complete blood count was measured by Automated Coulter and manual slide review; ALT and aspartate aminotransferase were measured by multipoint rate with P-5-P method (Vitros 5600, Ortho Clinical Diagnostics, Raritan, NJ); serum creatinine was measured by Jaffe kinetic alkaline picrate (Thermo Fisher Scientific, Waltham, MA). Total cholesterol, high-density lipoprotein (HDL) cholesterol and triglyceride assays were performed enzymatically on a Hitachi 917 autoanalyzer (Boehringer Mannheim Diagnostics, Indianapolis, IN). Low-density lipoprotein (LDL) cholesterol levels were calculated by the Friedewald equation, highly sensitive C-reactive protein (hs-CRP) via immunoturbidimetric assay (Beckman Coulter, Brea, CA), C-peptide, and IGF-1 via chemiluminescent immunoassay (DiaSorin, Stillwater, MN) and sex hormone binding globulin (SHBG) via chemiluminescent immunoassay (Beckman Coulter, Brea, CA).
Analysis of 2H5 glycerol and 6,6-2H2 glucose was done using gas chromatography mass spectrometry in the laboratory of B. Bergman, as described (8, 27).
Imaging techniques.
Body composition by DXA (Hologic, Waltham, MA) was performed. MRI was performed by a General Electric 3 Tesla magnet (GE, Milwaukee, WI) running Version 15M4 software. Visceral adiposity was measured using the gold standard of an MRI slice at lumbar vertebrae 4 and 5 (52). Hepatic fat fraction was determined using a modified Dixon method (16, 38).
Tracer calculations.
Glucose infusion rate (mg/kg·min) was measured based on steady-state measurements from the last 30 min of each phase of the clamp. All isotopic measurements were corrected for background enrichment. The glucose and glycerol rate of appearance (Ra), rate of disappearance, and metabolic clearance rate (MCR) over the last 30 min of each phase were calculated using the Steele nonsteady-state equation, accounting for “spiked” glucose in the 20% dextrose infusion (8). Because the participants with diabetes received insulin overnight, traditional calculations of percent suppression or IC50 cannot be performed as they require a true “basal,” which does not exist when a participant is given exogenous insulin, even if it is just for glucose control (41). Thus, to describe changes in Ra across the different insulin concentrations of each phase, the intercept and slope of the regression line for each individual’s data were also used to calculate the predicted Ra at the average insulin concentration for all participants during the 10 mU·m−2·min−1 phase for glycerol Ra (insulin 51 μU/ml) and the 16 mU·m−2·min−1 phase for EGP (insulin 71 μU/ml). As that relationship was not linear, data were log transformed. Negative EGP values in the obese group during the 80 mU·m−2·min−1 phase could not be log transformed, and thus the most negative EGP value was scaled to 0 and EGP values adjusted accordingly. Data were then reverse transformed for data presentation of the predicted Ra.
Statistical analysis.
The distributions of all variables were examined before analysis. Descriptive statistics presented as mean ± SD, median (Q1, Q3), or frequencies and percentages, as appropriate. For variables measured at only one time point, group comparisons were made using χ2 or Fisher’s exact test for proportions and the t-test or Kruskal-Wallis test for continuous variables. The associations between the tissue-specific IR (10 mU·m−2·min−1 phase glycerol Ra, 16 mU·m−2·min−1 EGP, and 80 mU·m−2·min−1 phase glucose infusion rate ) and body composition (visceral fat, liver fat, waist/hip ratio), inflammation (hs-CRP, IL-6, white blood cells) metabolic markers (adiponectin, leptin, HbA1c, and C-peptide), activity (3-day metabolic equivalents, time in lifestyle/light, time in moderate/vigorous) were examined using Spearman’s correlation coefficients.
Because of the influence of glucose concentrations in the measurement of basal EGP, we elected to control overnight blood glucose (56). This led to greater insulin concentrations during the basal phase in participants with diabetes, similar to our work in youth with type 1 diabetes (15, 38). Therefore, we also applied modeling techniques that are less reliant on measurements obtained during the basal phase, rather than utilizing percent suppression of basal or IC50 (41). By modeling the Ra from all clamp phases relative to the insulin concentration, we were able to best describe dynamic physiologic changes in glucose Ra in each individual and leverage the data from all four phases to understand fully tissue-specific IR across a physiologic spectrum of insulin concentrations. Repeated-measures mixed-effects models (MEM) were used to compare outcomes measured at multiple time points during the clamp. We modeled EGP and glycerol Ra, controlling for the phase of the clamp and insulin concentrations at each phase. The two-way interactions in the model can be interpreted as follows: 1) T2D × phase is the difference in the effect of each phase compared with the reference phase (phase 80) for a participant in T2D compared with the control group, 2) insulin × phase is the difference in the effect of insulin at each phase compared with the reference phase (phase 80) for both groups combined, and 3) insulin × T2D is the difference in the effect of insulin for the T2D compared with the control group. P values <0.05 were considered significant for main effects; P values <0.25 were considered significant for model interactions. All statistical analyses were performed with SAS Software, Version 9.4 (Cary, NC).
RESULTS
Twenty-seven adolescents with T2D and 21 youth who are overweight/obese without diabetes were enrolled. None of the youth without diabetes had impaired fasting glucose and only one of the youth with obesity had impaired glucose tolerance. Participants with T2D were slightly older than controls; however, sex, Tanner stage, BMI, and BMI percentile distributions were similar (Table 1). By design there were no differences in physical activity between groups. T2D had higher waist-to-hip ratio (P = 0.02), but other measures, including waist circumference, percent visceral, liver, and total fat were similar between groups. Data on typical dietary intake were not different between the groups (data not shown).
Table 1.
Participant characteristics and diet and physical activity data
| T2D | Obese Control | P Value | |
|---|---|---|---|
| Number | 27 | 21 | — |
| Age, years | 15.6 ± 0.2 | 14.3 ± 0.2 | 0.03 |
| Sex, female/male (% female) | 21/6 (78%) | 16/5 (76%) | 1.00 |
| BMI, kg/m2 | 31.2 (28.5, 38.8) | 31.8 (29.9, 34.6) | 0.88 |
| BMI %ile | 96.1 (52.6, 95.9) | 97.5 (51.2, 97.0) | 0.63 |
| Ethnicity | 0.92 | ||
| Single ethnicity, n | 26 | 19 | |
| Multiple ethnicity, n | 1 | 2 | |
| Non-hispanic white | 5 (17.9%) | 4 (17.4%) | |
| Hispanic white | 14 (50.0%) | 12 (52.2%) | |
| Black | 8 (28.6%) | 6 (26%) | |
| American Indian | 1 (3.5%) | 1 (4.4%) | |
| Tanner stage, 2/3/4/5 | 1/2/0/25 | 0/0/4/17 | 0.06 |
| Waist circumference, cm | 106.7 (97.3, 115.8) | 104.4 (88.8, 110.0) | 0.26 |
| Waist/hip ratio | 1.00 (0.90, 1.03) | 0.93 (0.89, 0.97) | 0.02 |
| Systolic blood pressure, mmHg | 119 ± 10 | 114 ± 10 | 0.15 |
| Diastolic blood pressure, mmHg | 68 ± 10 | 68 ± 5 | 0.79 |
| Body fat, % | 40.7 ± 5.1 | 42.9 ± 5.4 | 0.15 |
| Liver fat, % | 3.9 (1.8, 9.4) | 2.7 (1.8, 7.7) | 0.59 |
| Fatty liver disease, n (%) | 9 (33%) | 5 (24%) | 0.47 |
| Visceral fat, % | 15.0 (8, 21.4) | 27.7 (9.7, 41.7) | 0.21 |
| 3-day PAR (METS) | 61 (54, 70) | 58 (50, 64) | 0.25 |
| Accelerometer Data: | |||
| Sedentary, % | 68 ± 4 | 67 ± 3 | 0.80 |
| Lifestyle + light, % | 28 (19, 40) | 29 (23, 32) | 0.91 |
| Moderate + vigorous + very vigorous, % | 3 (1, 7) | 4 (3, 7) | 0.38 |
Demographics, physical characteristics, and lifestyle measurements in youth with obesity with and without T2D. Data are presented as median and interquartile range (25, 75) or as mean ± standard deviation. Bolded values indicate P value of <0.05. BMI, body mass index; METs, metabolic equivalents; PAR, pediatric activity recall; T2D, type 2 diabetes.
T2D had a higher HbA1c (P < 0.001), as expected (Table 2). There were no differences in leptin, total cholesterol, HDL, or triglycerides. However, adiponectin (P = 0.01) and LDL (P = 0.03) were lower in T2D. Additionally, aspartate aminotransferase (P = 0.04) and hs-CRP (P = 0.03) were lower in T2D youth compared with controls.
Table 2.
Fasting biochemical measurements
| T2D, n = 27 | Obese Control, n = 21 | P Value | |
|---|---|---|---|
| HbA1c, % | 7.3 (6.2, 10.1) | 5.4 (5.2, 5.5) | <0.001 |
| Leptin, ng/ml | 27.6 (20.2, 40.8) | 34.8 (27.1, 49.0) | 0.09 |
| Adiponectin, ng/ml | 4.5 (3.5, 8.3) | 7.9 (6.0, 10.4) | 0.01 |
| Cholesterol, ng/ml | 140 (131, 183) | 157 (127, 184) | 0.45 |
| HDL, mg/dl | 34 (31, 39) | 38 (32, 45) | 0.18 |
| LDL, mg/dl | 79 ± 26 | 95 ± 31 | 0.03 |
| Triglyceride, mg/dl | 108 (77, 231) | 100 (87, 165) | 0.95 |
| C-peptide, ng/ml | 2.5 (0.9, 3.9) | 2.8 (2.0, 3.5) | 0.47 |
| AST, U/l | 30 (19, 41) | 37 (30, 52) | 0.04 |
| ALT, U/l | 24 (16, 62) | 32 (24, 36) | 0.47 |
| WBC, 109cells/l | 7.9 (6.4, 9.8) | 6.8 (5.3, 7.9) | 0.05 |
| Platelets, 109 cells/l | 283 (253, 333) | 259 (243, 295) | 0.07 |
| hs-CRP, mg/l | 3.3 (1.0, 5.9) | 1.1 (0.4, 2.3) | 0.03 |
Laboratory measures drawn at 6 AM after a 12-h monitored inpatient fast. Data are presented as median and interquartile range (25%, 75%) or as mean ± standard deviation. Bolded values indicate P value of <0.05. ALT, alanine aminotransferase; AST, aspartate aminotransferase; hs-CRP, highly selective C-reactive protein; T2D, type 2 diabetes.
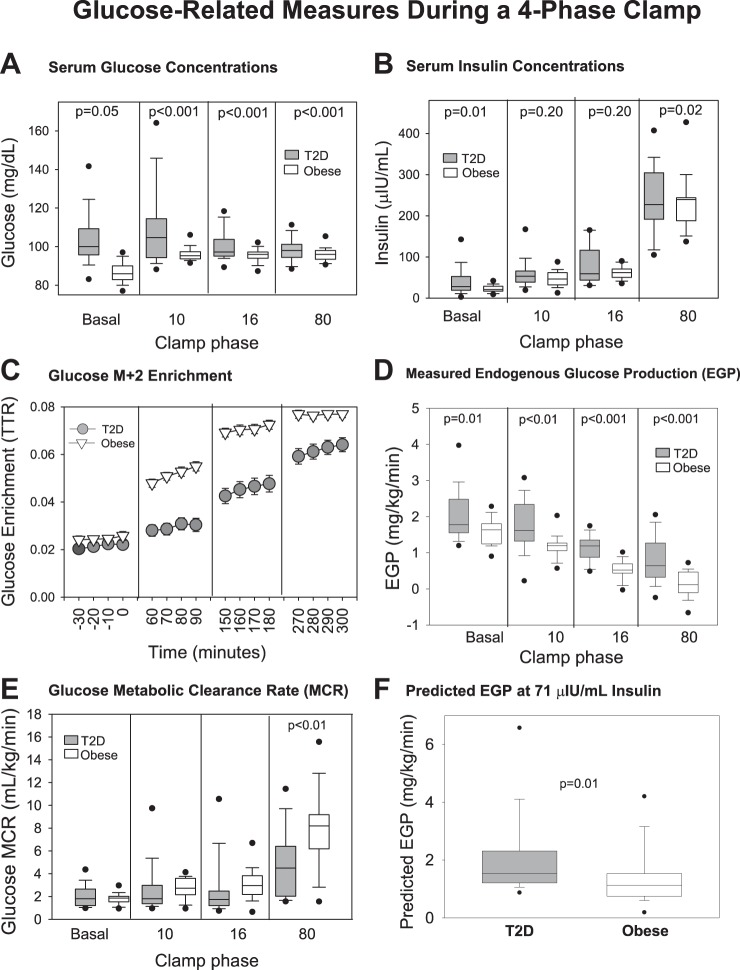
Glucose metabolism measurements are shown in Fig. 1. The glucose concentration was different between groups across all phases (Fig. 1A). The insulin concentrations (Fig. 1B) increased in both groups with the administration of increasing doses of insulin, and the change between phases was not different between groups (P = 0.31), although the groups were different at baseline and during the highest dose. Glucose enrichment expressed as the tracer/tracee ratio − background enrichment is shown in Fig. 1C. The EGP (Fig. 1D) generally decreased with increases in the insulin dose and despite the relatively small change in serum insulin concentrations between the 10 and 16 phase, there was still a nearly 50% decrease in EGP in those with T2D, indicating that the insulin dose was adequate to induce an effect. The observed EGP was higher in T2D during all phases compared with control group, with incomplete suppression even during the 80 mU·m−2·min−1 phase in T2D. Glucose MCR (Fig. 1E) generally increased with each increase in insulin dose but was lower at the 80 mU·m−2·min−1 phase (P = 0.002) in T2D and only compared at this time point as the relevance of MCR at lower doses of insulin is unclear. The predicted EGP (Fig. 1F; P = 0.02) was higher in T2D. The slopes of the lines to calculate predicted EGP between obese (−0.11 ± 0.02) and T2D (−0.07 ± 0.02) were different (P = 0.04) whereas the intercepts were not [2.24 ± 0.05 mg·kg−1·min−1 (obese) vs. 2.15 ± 0.07 (T2D), P = 0.34]. Furthermore, at the 16 mU·m−2·min−1 phase, the least squares mean of EGP was also higher (P < 0.001) (Table 3, bottom row) although, after adjusting for insulin concentrations, the overall curves from the MEM were not different between the groups (P = 0.71).
Fig 1.
Glucose-related measures during a 4-phase hyperinsulinemic-euglycemic clamp. Data are shown by type 2 diabetes (T2D) (gray circles and boxes) and obese status (white triangles and boxes), and by phase of the clamp, named per insulin dose: serum glucose concentrations (A), serum insulin concentrations (B), glucose M+2 enrichment (C), measured endogenous glucose production (EGP) (D), glucose metabolic clearance rate (MCR) (E), predicted EGP at 71 μIU/ml insulin (F). For box plots, boxes are 25th–75th percentile, whiskers are 10th–90th percentile, and all other outliers are shown with black circles. P values are specific to the phase of the clamp in which they are located.
Table 3.
Results of repeated-measures models for clamp outcomes
| EGP | Glycerol Ra | [Glycerol] | [FFA] | |||||
|---|---|---|---|---|---|---|---|---|
| Effect | Estimate | P value | Estimate | P value | Estimate | P value | Estimate | P value |
| Intercept | 1.19 ± 0.35 | 2.58 ± 0.57 | 64.16 ± 13.90 | 164.84 ± 83.89 | ||||
| T2D | −0.49 ± 0.40 | 0.005 | −0.81 ± 0.67 | 0.04 | −37.63 ± 19.62 | 0.10 | −140.83 ± 119.64 | 0.09 |
| Insulin | −0.0013 ± 0.0013 | 0.74 | 0.00037 ± 0.0020 | 0.76 | −0.071 ± 0.039 | 0.68 | −0.24 ± 0.30 | 0.90 |
| Clamp phase | <.001 | <0.001 | <0.001 | <0.001 | ||||
| Basal phase | 0.94 ± 0.38 | 2.04 ± 0.79 | 22.85 ± 11.34 | 366.37 ± 103.04 | ||||
| 10 mU·m−2·min−1 phase | 0.82 ± 0.46 | 0.82 ± 0.68 | 6.62 ± 11.87 | 165.91 ± 112.51 | ||||
| 16 mU·m−2·min−1 phase | −0.27 ± 0.32 | 0.14 ± 0.68 | −3.52 ± 12.09 | 60.06 ± 113.93 | ||||
| T2D × phase | 0.71 | 0.14 | <0.001 | 0.03 | ||||
| T2D × basal phase | 0.034 ± 0.39 | 1.09 ± 0.88 | 44.44 ± 16.28 | 218.83 ± 123.58 | ||||
| T2D × 10 mU·m−2·min−1 phase | −0.32 ± 0.42 | −0.68 ± 0.66 | 15.33 ± 14.72 | −44.90 ± 111.93 | ||||
| T2D × 16 mU·m−2·min−1 phase | −0.07 ± 0.30 | −0.40 ± 0.63 | 13.54 ± 13.85 | 1.37 ± 103.47 | ||||
| Insulin × phase | 0.007 | 0.16 | 0.44 | 0.29 | ||||
| Insulin × basal phase | −0.00085 ± 0.0030 | −0.0093 ± 0.0078 | −0.084 ± 0.073 | −1.47 ± 0.96 | ||||
| Insulin × 10 mU·m−2·min−1 phase | 0.0017 ± 0.0036 | 0.0036 ± 0.0049 | 0.049 ± 0.092 | 0.96 ± 1.024 | ||||
| Insulin × 16 mU·m−2·min−1 phase | 0.0051 ± 0.0015 | 0.0080 ± 0.0042 | 0.043 ± 0.084 | 0.51 ± 0.95 | ||||
| Insulin × T2D | −0.0012 ± 0.0016 | 0.45 | −0.000070 ± 0.0024 | 0.97 | 0.098 ± 0.068 | 0.15 | 0.35 ± 0.46 | 0.29 |
| Least squares estimate of group difference at phase of primary interest (T2D-control) | 0.64 ± 0.11 | <0.001 | 1.50 ± 0.44 | 0.001 | 16.73 ± 10.10 | 0.10 | 165.68 ± 63.45 | 0.01 |
| Least squares estimate of group difference at phase of primary interest adjusted for metformin (T2D-control) | 0.52 ± 0.18 | 0.006 | 2.01 ± 0.55 | <0.001 | 47.72 ± 18.17 | 0.01 | 209.44 ± 79.04 | 0.009 |
Values are estimates ± SE. The 80 mU·m−2·min−1 phase of the clamp was treated as the reference category for phase, and controls were treated as the reference category for group. At 16 mU·m−2·min−1 phase, insulin 71 μU/ml for glucose Ra; at 10 mU·m−2·min−1 phase, insulin 51 μU/ml for glycerol Ra, glycerol, and FFA. The overall comparison of the entire curve from all four phases between groups is the T2D × phase, and the final output of the model at the phase of interest is the least squares estimate. The remainder of values include all of the parameter estimates from the model. EGP, endogenous glucose production; FFA, free fatty acid; Ra, rate of appearance; T2D, type 2 diabetes.
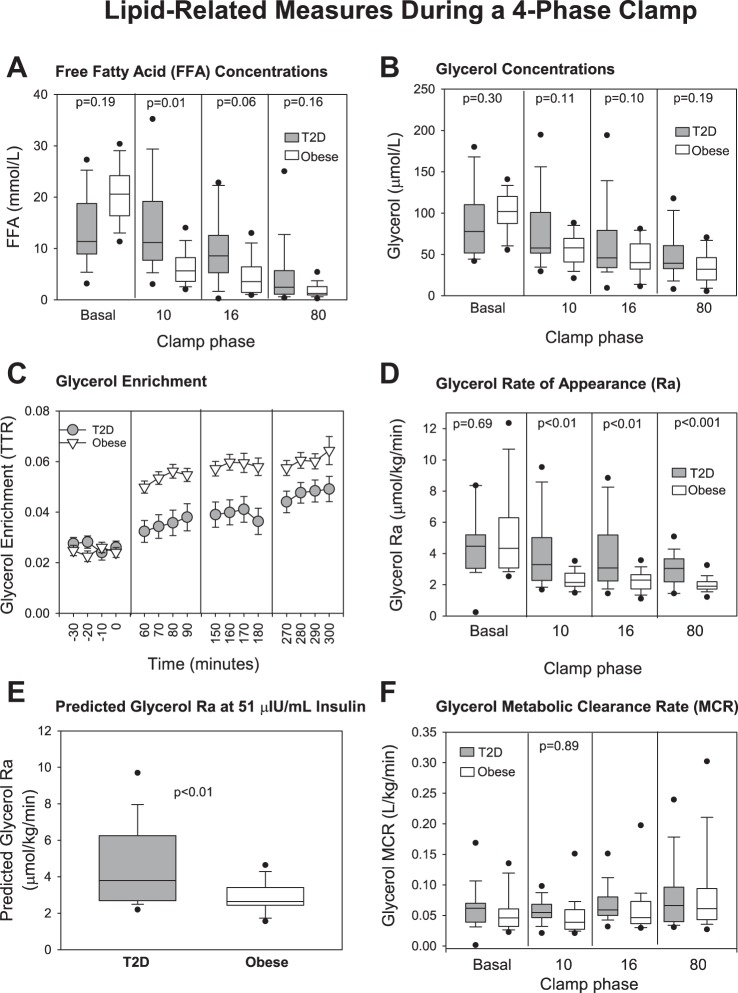
As expected, FFA concentrations (Fig. 2A) decreased with increasing insulin doses but decreased more slowly in T2D and were higher at the 10 mU·m−2·min−1 phase (P < 0.001) despite higher peripheral insulin concentrations. Glycerol concentrations (Fig. 2B) also decreased with advancing insulin infusion phases with smaller decrements in the T2D, although glycerol was not significantly higher in T2D at any phase. Glycerol Ra (Fig. 2D) was suppressed by the 10 mU·m−2·min−1 phase in the control group and was significantly lower in the control group during every phase of the clamp. Similarly, predicted glycerol Ra was higher in T2D (Fig. 2E; P = 0.006). Both the slopes and intercepts of the lines to calculate predicted glycerol Ra between obese and T2D were different [slope: −0.33 (−0.45, −0.25) obese vs. 2.18 (1.06, 3.02) P < 0.001; intercept 2.42 (1.84, 2.77 μmol·kg−1·min−1) obese vs. 1.57 (1.16, 1.92) T2D, P = 0.01]. Furthermore, the least squares mean of glycerol Ra (P = 0.001) and FFA (P < 0.001) were significantly higher in the group with T2D compared with controls at the 10 mU·m−2·min−1 phase (Table 3, bottom row). In the repeated-measures models the interactions of group and phase of the clamp were significant for glycerol and FFA concentrations (Table 3; P < 0.001 and P < 0.001, respectively) indicating significantly different trajectories between groups across the phases of the clamp but not glycerol Ra (P = 0.14). There was no effect of previous metformin therapy. The clearance of glycerol was not different between the groups (Fig. 2F).
Fig. 2.
Lipid-related measures during a 4-phase hyperinsulinemic-euglycemic clamp. Data are shown by type 2 diabetes (T2D) (gray circles and boxes) and obese status (white triangles and boxes), and by phase of the clamp, named per insulin dose as nonrepeated measures: free fatty acid (FFA) concentrations (A), glycerol concentrations (B), glycerol enrichment (C), glycerol rate of appearance (Ra) (D), predicted glycerol Ra at 51 μIU/ml insulin (E), glycerol metabolic clearance rate (MCR) (F). For box plots, boxes are 25th–75th percentile, whiskers are 10th–90th percentile, and all other outliers are shown with black circles. P values are specific to the phase of the clamp in which they are located.
Results from tissue-specific IR correlations per group are displayed in Table 4. In T2D youth, we found that MCR negatively correlated with FFA at the 80 mU·m−2·min−1 phase (P < 0.001), central obesity (waist-to-hip ratio, percent liver fat, and percent visceral fat), total cholesterol, triglycerides, and markers of inflammation, including ALT, hs-CRP, and white blood cells. On the other hand, MCR was positively correlated with physical activity in the past 3 days and adiponectin. EGP from phase 16 positively correlated with FFA during the same phase (P = 0.01) and waist-to-hip ratio but did not relate to MCR during the 80 phase. EGP had an inverse relationship with the hepatically produced IGF-1. MCR did not correlate with glycerol Ra at phase 10 (P = 0.15) nor EGP at phase 16 (P = 0.15, data not shown). Glycerol Ra positively correlated with predicted EGP (P = 0.003).
Table 4.
Correlation analysis between glycerol Ra, endogenous glucose production, and glucose clearance rate (MCR) and biochemical parameters in individuals with T2D and obesity
| T2D (r, P) | Obese Control (r, P) | |
|---|---|---|
| Glucose MCR, 80 mIU/m2/min phase | ||
| FFA, 80 mIU/m2/min phase | −0.748, <0.001 | −0.612, 0.008 |
| Glycerol Ra, 10 mIU/m2/min phase | −0.363, 0.15 | −0.485, 0.04 |
| Waist-to-hip ratio | −0.608, 0.003 | −0.060, 0.79 |
| Liver fat, % | −0.487, 0.03 | −0.171, 0.51 |
| Visceral fat, % | −0.576, 0.02 | −0.429, 0.26 |
| Total Cholesterol | −0.459, 0.02 | −0.132, 0.61 |
| Serum fasting triglycerides | −0.627, 0.001 | −0.251, 0.32 |
| Alanine transferase | −0.426, 0.03 | −0.423, 0.06 |
| Highly selective C-reactive protein | −0.475, 0.02 | −0.245, 0.34 |
| White blood cell count | −0.481, 0.02 | −0.135, 0.59 |
| 3-day activity (METs) | 0.488, 0.02 | 0.357, 0.22 |
| Adiponectin | 0.574, 0.004 | 0.318, 0.21 |
| EGP, 16 mIU/m2/min phase | ||
| FFA concentration at 16 mIU/m2/min | 0.650, 0.02 | 0.790, <0.001 |
| Waist-to-hip ratio | 0.817, 0.004 | 0.298, 0.21 |
| IGF-1 | −0.620, 0.02 | 0.100, 0.76 |
| SHBG | −0.172, 0.56 | −0.595, 0.007 |
| Glycerol Rate of Appearance, 10 mIU/m2/min phase | ||
| Predicted EGP | 0.680, 0.003 | −0.029, 0.900 |
Correlates of insulin sensitivity per tissue type are shown for youth with and without T2D. Data are shown as [correlation coefficient (r), P value]. Bolded values indicate significance. EGP, endogenous glucose production; FFA, free fatty acid; MCR, metabolic clearance rate; METs, metabolic equivalents; Ra, rate of appearance; T2D, type 2 diabetes; SHBG, sex hormone binding globulin.
In the controls, MCR negatively correlated with FFA at the 80 mU·m−2·min−1 phase (P = 0.008) and glycerol Ra from the phase 10 (P = 0.04). Furthermore, SHBG inversely correlated with EGP only in the control group.
DISCUSSION
The presence of overall IR is a key factor for developing T2D. Although the role of muscle/peripheral IR is well established, the interplay between and impact of muscle, adipose tissue, and liver IR on the natural history of T2D in youth has not been systematically elucidated. Separate studies in youth are needed because the natural history and underlying pathophysiology of T2D in youth differs dramatically from adults, as demonstrated in the recently published Restoring Insulin Secretion study (23, 43, 44, 48, 50). Almost all pediatric HE-clamp studies have utilized only high-dose insulin (80 mIU/m2/min), an approach that cannot simultaneously assess all insulin-responsive organs nor differentiate more subtle whole-body measures of IR reflecting hepatic and adipose tissues (3, 29, 30, 35). We used a state-of-the-art four-phase HE-clamp with tracers to assess quantitatively serum-measured adipose, hepatic, and peripheral IR in youth with diabetes, compared with control youth well-matched for BMI, pubertal stage, diet, and physical activity. We demonstrated that T2D youth had ~50% lower peripheral, adipose, and hepatic tracer-assessed insulin sensitivity compared with BMI-similar youth without diabetes, indicating early and significant IR. Furthermore, the peripheral and hepatic IR appear to associate with unchecked lipolysis.
Our findings of significantly decreased peripheral glucose uptake in T2D confirm previous work in another cohort of similarly aged youth and are congruent with other reports in youth (35, 39). It is well known that the majority of postprandial glucose uptake can be attributed to insulin action on skeletal muscle. Therefore, worsening peripheral IR in combination with worsening β-cell failure is a large contributor to the transition from normal glucose tolerance (NGT) to impaired glucose tolerance (IGT) and IGT to T2D (5, 31, 35). Our results are also in line with previous studies that peripheral IR in T2D correlated with adipose IR, abdominal obesity, liver, and visceral adiposity (33, 35, 52). In adults, β-cell decompensation occurs at a relatively lower degree of peripheral IR than seen in youth (2, 44). However, once T2D develops, the fall in β-cell function is more severe in youth with an estimated ~80% loss of β-cell function at the time of diabetes diagnosis (6, 18, 19, 49). This emphasizes the importance of treating IR before β-cell dysfunction in the pathogenesis of T2D development in youth. Therefore, an aggressive treatment targeting IR and β-cell function are crucial to prevent progression from prediabetes to diabetes in youth (43).
In addition to peripheral IR, we found that youth with T2D have significant hepatic IR (~50% higher EGP) during hyperinsulinemia, assessed by the gold-standard multiphase HE-clamp with a glucose tracer. Because of the challenges of measuring EGP in individuals with diabetes, the results were evaluated on 1) the raw data alone and 2) two distinct models, which incorporate insulin concentrations, and all three agree. Whereas we had patients perform a 3-day metformin washout, we also confirmed statistically that metformin treatment status did not have an effect on the results. A study in adolescents found a trend toward worse fasting hepatic IR in T2D using index of fasting glucose Ra versus NGT youth (5), but it was not statistically significant nor different than IGT youth, showing the value of adding assessments during hyperinsulinemia as in our study (5, 29). Several studies in youth (29) and adults (7) have reported elevated fasting EGP in T2D, arguing that EGP is higher in T2D, but are confounded by much higher fasting glucose concentrations. In a recent study, prediabetic youth with obesity had ~30% higher EGP compared with adults (2). The higher EGP we demonstrate in T2D youth contributes to their hyperglycemia, especially in the fasting state and therefore is an important target for future treatments.
We found that youth with T2D exhibited profound adipose IR during hyperinsulinemia and elevated glycerol Ra through all phases of the clamp after baseline compared with BMI-similar controls, despite similar BMI and body-fat distribution. In adults, a progressive decline in β-cell function that begins in individuals with NGT through T2D is associated with a progressive increase in FFAs and fasting adipose IR as assessed by oral glucose tolerance test (26). Another study also found that a fasting adipose IR index using tracers was tightly associated with fasting and 2-h glucose concentrations (37), arguing for the importance of the adipose IR we documented in youth on the pathophysiology of T2D. In support of this concept, in a large cohort of 962 youth with obesity, a fasting adipose IR index (fasting FFA × insulin) was increased across the spectrum of glucose tolerance status and correlated with a progressive reduction in FFA suppression during an oral glucose tolerance test (30). Similarly, in another cohort (35) that used a different adipose IR index [(1/glycerol Ra) found that IGT youth had more fasting adipose IR than NGT youth, and increased fasting adipose IR correlated with decreased β-cell function. Furthermore, NGT youth with obesity in that cohort (35) also had 32%–45% higher fasting glycerol Ra versus what was reported in adults with obesity (47), arguing for worse adipose IR in youth versus adults. Thus, our study confirms the literature with a more robust method than the previous approaches by using adipose-specific hyperinsulinemia and a glycerol tracer (24, 47) for assessing whole-body lipolytic rates and specifically compares youth with obesity with and without T2D of similar BMI. We also found that clamp phase-specific serum FFA, total cholesterol, and triglycerides had a tight relationship with peripheral and hepatic IR. The acute effect of elevated FFA on inducing peripheral and hepatic IR was demonstrated in a classic study by Boden et al. (10). Previous studies showed that elevated FFA is inversely associated with vascular dysfunction in healthy young adults (34, 39, 45, 46, 55), which may decrease muscle blood flow resulting in worsening peripheral IR and may include mitochondrial dysfunction and ectopic fat deposition in the visceral, liver, and muscle (52). Taken together, adipose IR might be an important future treatment target in youth.
Waist-to-hip ratio, hepatic fat, and visceral fat were associated with peripheral IR and waist-to-hip ratio with hepatic IR. More youth with T2D also met criteria for Nonalcoholic fatty liver disease (NAFLD) (hepatic fat ≥5.5%; 33% vs. 24%), and this is likely an underestimation because of metformin therapy in T2D. NAFLD is commonly reported in adolescents and adults with obesity; the prevalence increases across glucose tolerance status (12, 31), and hepatic and peripheral IR are associated with % hepatic fat in people with NAFLD (31, 32). Therefore, hepatic and visceral fat are also important treatment targets in the pathophysiology of T2D in youth.
We also found evidence of significant increased hs-CRP, a systemic inflammatory marker, in youth with T2D, which correlated with peripheral IR. Generalized inflammation is a common antecedent in obesity before development of T2D (40). Inflammation may influence peripheral IR by directly affecting the insulin signaling pathways or indirectly by inducing oxidative stress and inflammasome expression (51). However, hs-CRP did not relate to adipose or hepatic IR, possibly because blood tests poorly represent inflammation at the tissue level.
There are several limitations to our study. Because of the location of our institution, half of our participants were Caucasian who identified with a hispanic ethnicity; therefore, the results of this study may not represent the general population of youth with T2D whom include a larger proportions of blacks. We also were not able to perform a complete pancreatic clamp with somatostatin because of the pediatric age group and did not a priori collect samples for analysis of glucagon. Thus, we cannot fully attribute increased EGP to hepatic IR alone, as glucagon concentrations are unknown (42). All our measures of endogenous glycerol release and EGP showed significant differences between youth with and without T2D except for the MEM model, which compares the entire Ra/insulin curve. The MEM discrepancy may be related to altered endogenous insulin secretion in T2D, with direct portal/splanchnic delivery, which could affect visceral lipolysis rates and rates of hepatic glucose production. In our previous work in youth with type 1 diabetes (15) absent endogenous insulin secretion had significant findings on the MEM model. Whereas there are formulas to estimate portal insulin concentrations and insulin clearance, they have not been fully tested in youth with and without diabetes and this would be worthy of further research. Furthermore, some models require the repeated measurement of C-peptide, which we unfortunately did not collect. Half of our youth with T2D had prior metformin use with 72 h of medication withdrawal. We did find an effect of metformin in the glycerol concentrations, but a continued medication effect would have biased results toward the null and thus do not impact our findings. Finally, we acknowledge that the results presented are serum measures of EGP, lipolysis, and peripheral glucose uptake and not true intratissue measures as performed with microdialysis catheters, artero-venous catheters in the same limb, biopsies, or similar, more invasive techniques. However, none of these more invasive techniques are acceptable for use in children, and by utilizing identical techniques in a well-matched control group, the abnormalities in those with T2D are clear.
Conclusion.
We report for the first time, to our knowledge, that youth with T2D have profound multitissue IR, including adipose, hepatic, and muscle tissue, suggesting a similar pathogenesis to diabetes reported in adults. Unsuppressed FFA during hyperinsulinemia related to IR in other tissues suggesting FFA may induce IR in other organs. Further studies in larger and more diverse pediatric cohorts and future works on targeting the potential treatment targets we identified to interrupt the progression from IR to T2D and the associated rapid β-cell failure and early diabetes complications are needed.
GRANTS
This work is supported by National Center for Research Resources (NCRR) Grant No. K23-RR020038-01 and NIH/NCRR Colorado (to K. Nadeau); Colorado Clinical and Translational Sciences Institute (CTSI) CO-Pilot Grant TL1-RR025778, NIH/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) 1R56DK088971-01, JDRF5-2008-291, ADA 7-11-CD-08; NIDDK T32 DK063687, BIRCWH K12HD057022, NIDDK K23DK107871 (to M. Cree-Green). This research was also supported by NIH/ National Center for Advancing Translational Sciences (NCATS) Colorado Clinical and Translational Science Award (CTSA) Grant No. UL1-TR001082.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.J.N. conceived and designed research; M.C.-G., B.C.B., A.D.B., S.B., A.S., and K.J.N. performed experiments; M.C.-G., P.W., J.J.S., J.T., L.P., and K.J.N. analyzed data; M.C.-G., P.W., J.J.S., L.P., and K.J.N. interpreted results of experiments; M.C.-G., P.W., and K.J.N. prepared figures; M.C.-G., P.W., and K.J.N. drafted manuscript; M.C.-G., P.W., J.J.S., J.T., B.C.B., A.D.B., S.B., A.S., L.P., and K.J.N. edited and revised manuscript; M.C.-G., P.W., J.J.S., J.T., B.C.B., A.D.B., S.B., A.S., L.P., and K.J.N. approved final version of manuscript.
M.C.G. and K.J.N. are the guarantors of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
ACKNOWLEDGMENTS
The authors thank the participants and their families and the Clinical Translational Research Center nurses and staff.
REFERENCES
- 1.Arslanian S, Heil BV, Kalhan SC. Hepatic insulin action in adolescents with insulin-dependent diabetes mellitus: relationship with long-term glycemic control. Metabolism 42: 283–290, 1993. doi: 10.1016/0026-0495(93)90075-Y. [DOI] [PubMed] [Google Scholar]
- 2.Arslanian S, Kim JY, Nasr A, Bacha F, Tfayli H, Lee S, Toledo FGS. Insulin sensitivity across the lifespan from obese adolescents to obese adults with impaired glucose tolerance: Who is worse off? Pediatr Diabetes 19: 205–211, 2018. doi: 10.1111/pedi.12562. [DOI] [PubMed] [Google Scholar]
- 3.Bacha F, Gungor N, Lee S, Arslanian SA. Progressive deterioration of β-cell function in obese youth with type 2 diabetes. Pediatr Diabetes 14: 106–111, 2013. doi: 10.1111/j.1399-5448.2012.00915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bacha F, Gungor N, Lee S, Arslanian SA. In vivo insulin sensitivity and secretion in obese youth: what are the differences between normal glucose tolerance, impaired glucose tolerance, and type 2 diabetes? Diabetes Care 32: 100–105, 2009. doi: 10.2337/dc08-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bacha F, Lee S, Gungor N, Arslanian SA. From pre-diabetes to type 2 diabetes in obese youth: pathophysiological characteristics along the spectrum of glucose dysregulation. Diabetes Care 33: 2225–2231, 2010. doi: 10.2337/dc10-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bacha F, Pyle L, Nadeau K, Cuttler L, Goland R, Haymond M, Levitsky L, Lynch J, Weinstock RS, White NH, Caprio S, Arslanian S; TODAY Study Group . Determinants of glycemic control in youth with type 2 diabetes at randomization in the TODAY study. Pediatr Diabetes 13: 376–383, 2012. doi: 10.1111/j.1399-5448.2011.00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basu R, Basu A, Johnson CM, Schwenk WF, Rizza RA. Insulin dose-response curves for stimulation of splanchnic glucose uptake and suppression of endogenous glucose production differ in nondiabetic humans and are abnormal in people with type 2 diabetes. Diabetes 53: 2042–2050, 2004. doi: 10.2337/diabetes.53.8.2042. [DOI] [PubMed] [Google Scholar]
- 8.Bergman BC, Howard D, Schauer IE, Maahs DM, Snell-Bergeon JK, Eckel RH, Perreault L, Rewers M. Features of hepatic and skeletal muscle insulin resistance unique to type 1 diabetes. J Clin Endocrinol Metab 97: 1663–1672, 2012. doi: 10.1210/jc.2011-3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bjornstad P, Truong U, Pyle L, Dorosz JL, Cree-Green M, Baumgartner A, Coe G, Regensteiner JG, Reusch JE, Nadeau KJ. Youth with type 1 diabetes have worse strain and less pronounced sex differences in early echocardiographic markers of diabetic cardiomyopathy compared to their normoglycemic peers: a RESistance to InSulin in Type 1 ANd Type 2 diabetes (RESISTANT) Study. J Diabetes Complications 30: 1103–1110, 2016. doi: 10.1016/j.jdiacomp.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boden G. Obesity and free fatty acids. Endocrinol Metab Clin North Am 37: 635–646, 2008. doi: 10.1016/j.ecl.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conte C, Fabbrini E, Kars M, Mittendorfer B, Patterson BW, Klein S. Multiorgan insulin sensitivity in lean and obese subjects. Diabetes Care 35: 1316–1321, 2012. doi: 10.2337/dc11-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cree-Green M, Bergman BC, Coe GV, Newnes L, Baumgartner AD, Bacon S, Sherzinger A, Pyle L, Nadeau KJ. Hepatic steatosis is common in adolescents with obesity and PCOS and relates to de novo lipogenesis but not insulin resistance. Obesity (Silver Spring) 24: 2399–2406, 2016. doi: 10.1002/oby.21651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cree-Green M, Gupta A, Coe GV, Baumgartner AD, Pyle L, Reusch JE, Brown MS, Newcomer BR, Nadeau KJ. Insulin resistance in type 2 diabetes youth relates to serum free fatty acids and muscle mitochondrial dysfunction. J Diabetes Complications 31: 141–148, 2017. doi: 10.1016/j.jdiacomp.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cree-Green M, Newcomer BR, Coe G, Newnes L, Baumgartner A, Brown MS, Pyle L, Reusch JE, Nadeau KJ. Peripheral insulin resistance in obese girls with hyperandrogenism is related to oxidative phosphorylation and elevated serum free fatty acids. Am J Physiol Endocrinol Metab 308: E726–E733, 2015. doi: 10.1152/ajpendo.00619.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cree-Green M, Stuppy JJ, Thurston J, Bergman BC, Coe GV, Baumgartner AD, Bacon S, Scherzinger A, Pyle L, Nadeau KJ. Youth with type 1 diabetes have adipose, hepatic, and peripheral insulin resistance. J Clin Endocrinol Metab 103: 3647–3657, 2018. doi: 10.1210/jc.2018-00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cree-Green M, Triolo TM, Nadeau KJ. Etiology of insulin resistance in youth with type 2 diabetes. Curr Diab Rep 13: 81–88, 2013. doi: 10.1007/s11892-012-0341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D’Adamo E, Cali AM, Weiss R, Santoro N, Pierpont B, Northrup V, Caprio S. Central role of fatty liver in the pathogenesis of insulin resistance in obese adolescents. Diabetes Care 33: 1817–1822, 2010. doi: 10.2337/dc10-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Adamo E, Caprio S. Type 2 diabetes in youth: epidemiology and pathophysiology. Diabetes Care 34, Suppl 2: S161–S165, 2011. doi: 10.2337/dc11-s212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeFronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58: 773–795, 2009. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia 53: 1270–1287, 2010. doi: 10.1007/s00125-010-1684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeFronzo RA, Simonson D, Ferrannini E. Hepatic and peripheral insulin resistance: a common feature of type 2 (non-insulin-dependent) and type 1 (insulin-dependent) diabetes mellitus. Diabetologia 23: 313–319, 1982. doi: 10.1007/BF00253736. [DOI] [PubMed] [Google Scholar]
- 22.Druet C, Tubiana-Rufi N, Chevenne D, Rigal O, Polak M, Levy-Marchal C. Characterization of insulin secretion and resistance in type 2 diabetes of adolescents. J Clin Endocrinol Metab 91: 401–404, 2006. doi: 10.1210/jc.2005-1672. [DOI] [PubMed] [Google Scholar]
- 23.Elder DA, Herbers PM, Weis T, Standiford D, Woo JG, D’Alessio DA. β-cell dysfunction in adolescents and adults with newly diagnosed type 2 diabetes mellitus. J Pediatr 160: 904–910, 2012. doi: 10.1016/j.jpeds.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fabbrini E, Magkos F, Conte C, Mittendorfer B, Patterson BW, Okunade AL, Klein S. Validation of a novel index to assess insulin resistance of adipose tissue lipolytic activity in obese subjects. J Lipid Res 53: 321–324, 2012. doi: 10.1194/jlr.D020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc 30: 777–781, 1998. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 26.Gastaldelli A, Gaggini M, DeFronzo RA. Role of adipose tissue insulin resistance in the natural history of type 2 diabetes: results from the San Antonio Metabolism study. Diabetes 66: 815–822, 2017. doi: 10.2337/db16-1167. [DOI] [PubMed] [Google Scholar]
- 27.Gilker CD, Pesola GR, Matthews DE. A mass spectrometric method for measuring glycerol levels and enrichments in plasma using 13C and 2H stable isotopic tracers. Anal Biochem 205: 172–178, 1992. doi: 10.1016/0003-2697(92)90595-X. [DOI] [PubMed] [Google Scholar]
- 28.Groop LC, Bonadonna RC, DelPrato S, Ratheiser K, Zyck K, Ferrannini E, DeFronzo RA. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest 84: 205–213, 1989. doi: 10.1172/JCI114142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gungor N, Bacha F, Saad R, Janosky J, Arslanian S. Youth type 2 diabetes: insulin resistance, beta-cell failure, or both? Diabetes Care 28: 638–644, 2005. doi: 10.2337/diacare.28.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hershkop K, Besor O, Santoro N, Pierpont B, Caprio S, Weiss R. Adipose insulin resistance in obese adolescents across the spectrum of glucose tolerance. J Clin Endocrinol Metab 101: 2423–2431, 2016. doi: 10.1210/jc.2016-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hong BS, Li Y, Lai S, Liu J, Guan H, Ke W, He X, Li Y. Ectopic fat deposition on insulin sensitivity: correlation of hepatocellular lipid content and M Value. J Diabetes Res 2016: 3684831, 2016. doi: 10.1155/2016/3684831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kato K, Takamura T, Takeshita Y, Ryu Y, Misu H, Ota T, Tokuyama K, Nagasaka S, Matsuhisa M, Matsui O, Kaneko S. Ectopic fat accumulation and distant organ-specific insulin resistance in Japanese people with nonalcoholic fatty liver disease. PLoS One 9: e92170, 2014. doi: 10.1371/journal.pone.0092170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kato K, Takeshita Y, Misu H, Zen Y, Kaneko S, Takamura T. Liver steatosis is associated with insulin resistance in skeletal muscle rather than in the liver in Japanese patients with non-alcoholic fatty liver disease. J Diabetes Investig 6: 158–163, 2015. doi: 10.1111/jdi.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation 113: 1888–1904, 2006. doi: 10.1161/CIRCULATIONAHA.105.563213. [DOI] [PubMed] [Google Scholar]
- 35.Kim JY, Nasr A, Tfayli H, Bacha F, Michaliszyn SF, Arslanian S. Increased lipolysis, diminished adipose tissue insulin sensitivity, and impaired β-cell function relative to adipose tissue insulin sensitivity in obese youth with impaired glucose tolerance. Diabetes 66: 3085–3090, 2017. doi: 10.2337/db17-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korenblat KM, Fabbrini E, Mohammed BS, Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology 134: 1369–1375, 2008. doi: 10.1053/j.gastro.2008.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malin SK, Kashyap SR, Hammel J, Miyazaki Y, DeFronzo RA, Kirwan JP. Adjusting glucose-stimulated insulin secretion for adipose insulin resistance: an index of β-cell function in obese adults. Diabetes Care 37: 2940–2946, 2014. doi: 10.2337/dc13-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nadeau KJ, Regensteiner JG, Bauer TA, Brown MS, Dorosz JL, Hull A, Zeitler P, Draznin B, Reusch JE. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. J Clin Endocrinol Metab 95: 513–521, 2010. doi: 10.1210/jc.2009-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nadeau KJ, Zeitler PS, Bauer TA, Brown MS, Dorosz JL, Draznin B, Reusch JE, Regensteiner JG. Insulin resistance in adolescents with type 2 diabetes is associated with impaired exercise capacity. J Clin Endocrinol Metab 94: 3687–3695, 2009. doi: 10.1210/jc.2008-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 286: 327–334, 2001. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 41.Pyle L, Bergman BC, Nadeau KJ, Cree-Green M. Modeling changes in glucose and glycerol rates of appearance when true basal rates of appearance cannot be readily determined. Am J Physiol Endocrinol Metab 310: E323–E331, 2016. doi: 10.1152/ajpendo.00368.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramnanan CJ, Edgerton DS, Kraft G, Cherrington AD. Physiologic action of glucagon on liver glucose metabolism. Diabetes Obes Metab 13, Suppl 1: 118–125, 2011. doi: 10.1111/j.1463-1326.2011.01454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.RISE Consortium Impact of insulin and metformin versus metformin alone on β-cell function in youth with impaired glucose tolerance or recently diagnosed type 2 diabetes. Diabetes Care 41: 1717–1725, 2018. doi: 10.2337/dc18-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.RISE Consortium Metabolic contrasts between youth and adults with impaired glucose tolerance or recently diagnosed type 2 diabetes: i. observations using the hyperglycemic clamp. Diabetes Care 41: 1696–1706, 2018. doi: 10.2337/dc18-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steer P, Basu S, Lithell H, Vessby B, Berne C, Lind L. Acute elevations of medium- and long-chain fatty acid have different impacts on endothelium-dependent vasodilation in humans. Lipids 38: 15–19, 2003. doi: 10.1007/s11745-003-1025-9. [DOI] [PubMed] [Google Scholar]
- 46.Steinberg HO, Tarshoby M, Monestel R, Hook G, Cronin J, Johnson A, Bayazeed B, Baron AD. Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. J Clin Invest 100: 1230–1239, 1997. doi: 10.1172/JCI119636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.ter Horst KW, van Galen KA, Gilijamse PW, Hartstra AV, de Groot PF, van der Valk FM, Ackermans MT, Nieuwdorp M, Romijn JA, Serlie MJ. Methods for quantifying adipose tissue insulin resistance in overweight/obese humans. Int J Obes 41: 1288–1294, 2017. doi: 10.1038/ijo.2017.110. [DOI] [PubMed] [Google Scholar]
- 48.Tfayli H, Arslanian S. Pathophysiology of type 2 diabetes mellitus in youth: the evolving chameleon. Arq Bras Endocrinol Metabol 53: 165–174, 2009. doi: 10.1590/S0004-27302009000200008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.TODAY Study Group Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and β-cell function in TODAY. Diabetes Care 36: 1749–1757, 2013. doi: 10.2337/dc12-2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.TODAY Study Group; Zeitler P, Hirst K, Pyle L, Linder B, Copeland K, Arslanian S, Cuttler L, Nathan DM, Tollefsen S, Wilfley D, Kaufman F. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med 366: 2247–2256, 2012. doi: 10.1056/NEJMoa1109333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med 17: 179–188, 2011. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weiss R, Dufour S, Taksali SE, Tamborlane WV, Petersen KF, Bonadonna RC, Boselli L, Barbetta G, Allen K, Rife F, Savoye M, Dziura J, Sherwin R, Shulman GI, Caprio S. Prediabetes in obese youth: a syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet 362: 951–957, 2003. doi: 10.1016/S0140-6736(03)14364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weston AT, Petosa R, Pate RR. Validation of an instrument for measurement of physical activity in youth. Med Sci Sports Exerc 29: 138–143, 1997. doi: 10.1097/00005768-199701000-00020. [DOI] [PubMed] [Google Scholar]
- 55.Widlansky ME, Gutterman DD. Regulation of endothelial function by mitochondrial reactive oxygen species. Antioxid Redox Signal 15: 1517–1530, 2011. doi: 10.1089/ars.2010.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolfe RR, Shaw JH, Jahoor F, Herndon DN, Wolfe MH. Response to glucose infusion in humans: role of changes in insulin concentration. Am J Physiol 250: E306–E311, 1986. doi: 10.1152/ajpendo.1986.250.3.E306. [DOI] [PubMed] [Google Scholar]