Abstract
Bcl-2-associated athanogene 3 (BAG3) is a cochaperone protein and a central player of the cellular protein quality control system. BAG3 is prominently expressed in the heart and plays an essential role in cardiac protein homeostasis by interacting with chaperone heat shock proteins (HSPs) in large, functionally distinct multichaperone complexes. The BAG3 mutation of proline 209 to leucine (P209L), which resides in a critical region that mediates the direct interaction between BAG3 and small HSPs (sHSPs), is associated with cardiomyopathy in humans. However, the mechanism by which the BAG3 P209L missense mutation leads to cardiomyopathy remains unknown. To determine the molecular basis underlying the cardiomyopathy caused by the BAG3 P209L mutation, we generated a knockin (KI) mouse model in which the endogenous Bag3 gene was replaced with mutant Bag3 containing the P215L mutation, which is equivalent to the human P209L mutation. We performed physiological, histological, and biochemical analyses of Bag3 P209L KI mice to determine the functional, morphological, and molecular consequences of the P209L mutation. We found that Bag3 P209L KI mice exhibited normal cardiac function and morphology up to 16 mo of age. Western blot analysis further revealed that levels of sHSPs, stress-inducible HSPs, ubiquitinated proteins, and autophagy were unaffected in P209L mutant mouse hearts. In conclusion, the P209L mutation in Bag3 does not cause cardiomyopathy in mice up to 16 mo of age under baseline conditions.
NEW & NOTEWORTHY Bcl-2-associated athanogene 3 (BAG3) P209L mutation is associated with human cardiomyopathy. A recent study reported that transgenic mice overexpressing human BAG3 P209L in cardiomyocytes have cardiac dysfunction. In contrast, our P209L mice that express mutant BAG3 at the same level as that of wild-type mice displayed no overt phenotype. Our results suggest that human cardiomyopathy may result from species-specific requirements for the conserved motif that is disrupted by P209L mutation or from genetic background-dependent effects.
Keywords: Bcl-2-associated athanogene 3, cardiomyopathy, P209L mutation, small heat shock proteins
INTRODUCTION
Bcl-2-associated athanogene 3 (BAG3) is a member of a conserved family of cytoprotective molecular cochaperones, which are central players of the cellular protein quality control system (3, 23, 34, 40). BAG3 interacts with the ATPase domain of the molecular chaperone heat shock protein (HSP)70 via its BAG domain (35), thereby modulating chaperone protein activities (34). In addition to the conserved BAG domain, two conserved Ile-Pro-Val (IPV) motifs have recently been identified in the intermediate region of BAG3 that mediate the interaction of BAG3 with a number of small HSP [sHSPs; also known as HSP family B (HSPB)] family members (5, 13, 29). These multifaceted structural characteristics underlie the ability of BAG3 to assemble large chaperone-cochaperone complexes to mediate cellular adaptive responses to stressful stimuli (4, 8, 14, 29, 31, 33).
BAG3 is prominently expressed in cardiac and skeletal muscle tissues (31, 35). Human genetic studies have identified multiple mutations within different domains of BAG3 that are associated with cardiac myopathies (1, 6, 12, 26, 32, 36). A single amino acid substitution of glutamic acid 455 to lysine (E455K) in BAG3, which disrupts the interaction between BAG3 and HSP70 (9), is the unequivocal cause of human dilated cardiomyopathy (DCM) (36). We and others have reported that Bag3 global knockout mice exhibit impaired postnatal growth and premature lethality at 3–4 wk (9, 16, 39). We also reported that Bag3 cardiomyocyte-specific knockout (cKO) mice, as well as Bag3 E455K mutant mice, develop DCM (9). Further experiments revealed that Bag3 knockout or E455K mutation led to the destabilization of sHSPs (HSPB5, HSPB6, and HSPB8) in cardiomyocytes (9, 18), suggesting an essential role for BAG3 in stabilizing sHSPs and maintaining cardiomyocyte protein homeostasis.
Another single amino acid substitution of proline 209 to leucine (P209L) in BAG3 has been reported to be associated with severe childhood cardiomyopathy in humans by three independent groups (19, 27, 32). Interestingly, the P209 residue resides in the second IPV motif of BAG3 (Supplemental Fig. S1 in the Supplemental Material; Supplemental Material for the article is available online at the American Journal of Physiology-Heart and Circulatory Physiology website), which mediates the direct interaction between BAG3 and sHSPs (5, 13, 29). The foregoing evidence led us to hypothesize that the P209L mutation in BAG3 would result in cardiomyopathy because of the destabilization of sHSPs. Lending further credence to this notion, a recently reported cardiomyocyte-specific BAG3 P209L transgenic (TG) mouse model, in which human P209L mutant BAG3 protein was overexpressed in cardiomyocytes, displayed slow progression of cardiac complications with enlargement of the heart and decreased fractional shortening (FS) (28). To determine the molecular basis underlying the cardiomyopathy caused by the BAG3 P209L mutation, we generated a knockin (KI) mouse model in which the endogenous Bag3 gene was replaced with mutant Bag3 containing the P215L mutation (herein referred to as P209L), which is equivalent to the human P209L mutation. Surprisingly, Bag3 P209L KI mice display normal cardiac morphology and function up to 16 mo of age. Biochemical experiments revealed that the expression levels of sHSPs were unaffected in Bag3 P209K KI hearts and that the Bag3 P209L mutation did not impair stress-inducible HSP expression, ubiquitination, or autophagy. Taken together, our data suggest that the Bag3 P209L mutation alone does not lead to cardiomyopathy in mice up to 16 mo of age.
METHODS
Generation of Bag3 P209L KI mice.
Bag3 P209L (equivalent to mouse P215L) KI mice were generated by standard techniques using a targeting construct, in which codon 215 CCC (encoding proline) in exon 3 of Bag3 was changed to CTC (encoding leucine) and which contained a neomycin selection cassette flanked by flippase recognition target (FRT) sites (9, 41). After electroporation of the targeting vector into R1 embryonic stem (ES) cells, G418-resistant ES cells were screened for homologous recombination by Southern blot analysis. One heterozygous recombinant ES clone was identified and microinjected into blastocysts from C57BL/6J mice to generate male chimeric mice. Male chimeric mice were bred with female C57BL/6J mice to generate germline-transmitted Bag3 P209L mice (Bag3m-neo/+). Bag3m-neo/+ mice were then crossed with FLP deleter mice (30) to remove the neomycin gene. Bag3m/+ heterozygous mutant mice were generated in a mixed 129/Sv × C57BL/6 background and were subsequently backcrossed into a C57BL/6 background for eight generations to control for potential background effects. Bagm/+ heterozygous mutant mice were subsequently intercrossed to generate homozygous Bag3m/m mice (Bag3 P209L KI mice). Genotyping of mice was confirmed by PCR analysis using mouse tail extracts and primers [forward (P1): 5′-AGGCACAGATGCAGCCAGTTAA-3′ and reverse (P2): 5′-GGTCTTCTGGGCTTGGTGGAA-3′]. Because a FRT site was left in the mutant allele once the neomycin cassette was removed, PCR products of the mutant allele with primers 1 and 2 are larger than those of the wild-type allele. All animal procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of California-San Diego.
Echocardiography.
Echocardiography was performed as previously described (9, 41). Briefly, mice were anesthetized with 1% isoflurane and underwent echocardiography using a FUJIFILM VisualSonics SonoSite Vevo 2100 ultrasound system with a 32- to 55-MHz linear transducer. Measurements of heart rate (HR), left ventricular (LV) internal dimensions at the end of diastole and systole (LVIDd and LVIDs, respectively), end-diastolic interventricular septum thickness (IVSd), and LV posterior wall thickness (LVPWd) were determined from the LV M-mode tracing. The percentage of FS was used as an indicator of systolic cardiac function. In the present study, serial echocardiographic measurements were performed for wild-type (+/+) control mice (n = 9, 5 male and 4 female mice) and heterozygous (m/+; n = 7 male mice) and homozygous (m/m; n = 13, 9 male and 4 female mice) Bag3 P209L KI mutant mice at 4, 11, and 16 mo of age.
Histology.
Hearts were isolated from age- and sex-matched littermates, washed in PBS, and fixed overnight in PBS containing 4% paraformaldehyde. Hearts were subsequently washed in PBS, dehydrated in 70% ethanol, embedded in paraffin, and coronally sectioned (10 μm thickness). Sections were stained with Masson’s trichrome or hematoxylin and eosin as previously described (9, 15, 38, 41), mounted, and imaged using a Hamamatsu NanoZoomer 2.0HT Slide Scanning System.
Quantitative real-time PCR.
Total RNA was isolated from the LV of mouse hearts using TRIzol reagent (ThermoFisher Scientific, Carlsbad, CA) according to the manufacturer’s instructions. cDNA was synthesized using Moloney murine leukemia virus reverse transcriptase (Bio-Rad, Hercules, CA). Primer sequences used for quantitative real-time PCR analysis are shown in Supplemental Table S2. RT-PCRs were performed using SsoFast EvaGreen Real-Time PCR Master Mix (Bio-Rad) in 96-well low-profile PCR plates in a Bio-Rad CFX96 Thermocycler. Samples were compared using the relative (comparative) threshold cycle (Ct) method after adjusting for 18S RNA (ΔCt) (10).
Protein isolation and Western blot analysis.
Protein isolation and Western blot analysis were performed as described in our previous studies (9, 20). Briefly, total protein extracts were prepared by suspending ground hearts in urea lysis buffer (8 M urea, 2 M thiourea, 3% SDS, 75 mM DTT, 0.03% bromophenol blue, and 0.05 M Tris·HCl, pH 6.8) followed by sonication to shear DNA (9, 41). Proteins were separated on 4–12% SDS-PAGE gels (ThermoFisher Scientific) and transferred overnight at 4°C onto PVDF membranes (Bio-Rad). After being blocked for 1 h in blocking buffer (Tris-buffered saline containing 0.1% Tween 20 and 5% dry milk), membranes were washed and incubated overnight at 4°C with the indicated primary antibody (detailed in Supplemental Table S3) in blocking buffer containing 2% dry milk. Blots were washed and incubated with horseradish peroxidase-conjugated secondary antibody generated in the rabbit (1:5,000) or mouse (1:2,000) (Dako, Santa Clara, CA) for 1 h at room temperature. Immunoreactive protein bands were visualized using enhanced chemiluminescence reagent (ThermoFisher Scientific).
Statistics.
Data are presented as means ± SE unless indicated otherwise. Statistical analysis was performed using GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA) with a two-tailed Student’s t-test or two-way ANOVA used for comparisons among groups. Survival data were calculated using Kaplan-Meier survival analysis with a log-rank statistical method. P values of <0.05 were considered statistically significant.
RESULTS
Bag3 P209L mutant mice are viable and have normal cardiac function and morphology.
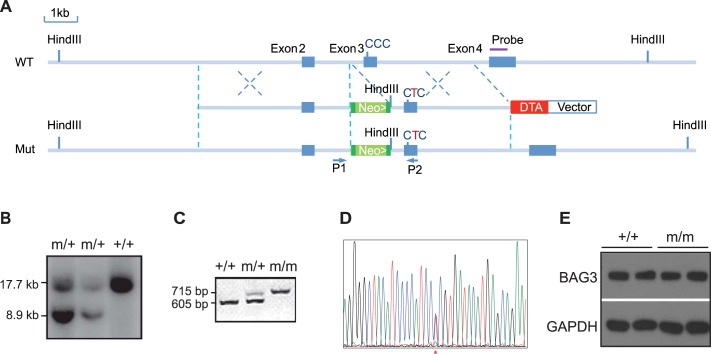
Multiple mutations in Bag3 have been associated with cardiomyopathy (1, 6, 12, 26, 32, 36). Three independent groups have identified a single amino acid substitution of proline 209 with leucine (P209L) in Bag3 as the cause of a severe childhood cardiomyopathy affecting cardiac structure and function (19, 27, 32). However, the pathogenic effects of this mutation are not known. To elucidate the biological and physiological consequences of the Bag3 P209L mutation, we generated Bag3 P209L KI mice, in which the endogenous Bag3 gene was replaced with mutant Bag3 containing the P215L mutation (equivalent to the human P209L mutation; Fig. 1, A–C). Sequencing the amplified PCR product of the genomic region containing the anticipated mutant site confirmed insertion of the correct mutation (Fig. 1D). Western blot analysis showed that BAG3 levels in the hearts of Bag3 homozygous (m/m) P209L KI mice were comparable with BAG3 protein levels in wild-type (+/+) control mice (Fig. 1E), suggesting that the P209L mutation does not impair the expression or stability of BAG3.
Fig. 1.
Generation of Bcl-2-associated athanogene 3 (Bag3) P209L knockin (KI) mice. A: targeting strategy. A restriction map of the relevant genomic region of Bag3 (top), targeting construct (middle), and mutated locus after recombination (bottom) is shown. Neo, neomycin resistance gene; DTA, diphtheria toxin A chain gene. Green boxes abutted to the Neo gene indicate flippase recognition target (FRT) sites. B: detection of wild-type (WT) (+) and mutant (m) alleles by Southern blot analysis with the probe diagrammatically represented in A after digestion with HindIII. C: genotyping of P209L KI mutant mice by PCR analysis of DNA isolated from tails using primers (P) diagrammatically represented in A. D: DNA sequencing confirmed insertion of the successful P215L mutation (equivalent to the human P209L mutation) from CCC to CTC (*). E: representative Western blot analysis of BAG3 in WT (+/+) and Bag3 P209L KI mutant (m/m) mouse hearts. GAPDH served as a loading control. n = 4 mice/group.
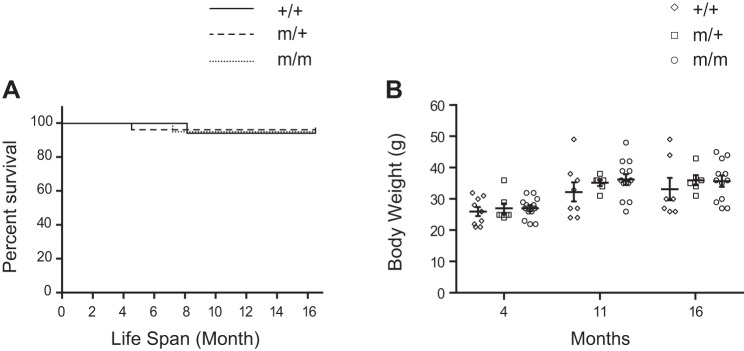
Bag3 P209L KI mice were born at the expected Mendelian ratios with 23% of an expected 25% pups (from 108 pups total) observed from Bag3m/+ heterozygous mutant crosses (Supplemental Table S1). Unlike Bag3 global knockout and E455K mutant mice that displayed lethality at 3–4 wk of age (9, 16, 39), we observed no sudden death or premature lethality of homozygous (m/m) or heterozygous (m/+) P209L KI mice compared with wild-type (+/+) littermates (Fig. 2A). We also observed no growth defects; body weight from 4 mo of age through 16 mo did not significantly differ between P209L KI mutants and wild-type littermates (Fig. 2B).
Fig. 2.
The Bcl-2-associated athanogene 3 (Bag3) P209L mutation does not impair survival or growth. A: Kaplan-Meier survival curves of wild-type (+/+) control mice (n = 17) and heterozygous (m/+) (n = 26) and homozygous (m/m) (n = 20) Bag3 P209L knockin (KI) mutant mice. B: body weight of +/+ control (n = 9) and m/+ (n = 7) and m/m (n = 13) Bag3 P209L KI mutant mice at 4, 11, and 16 mo of age. Data are presented as means ± SE.
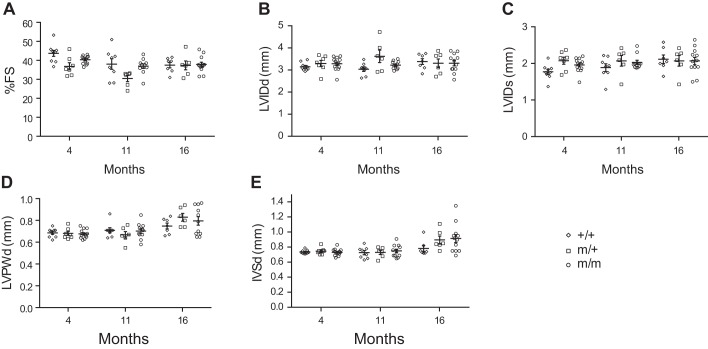
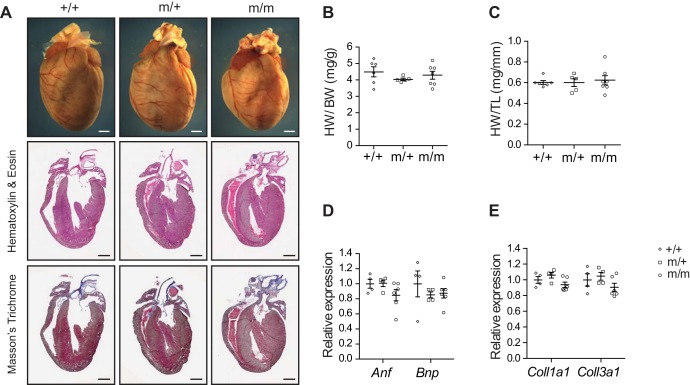
To determine whether the Bag3 P209L mutation impairs cardiac function, we performed echocardiographic analysis of P209L KI mutant and wild-type littermate control mice over the course of 16 mo (Fig. 3, A–E). Surprisingly, cardiac function of homozygous (m/m) and heterozygous (m/+) Bag3 P209L KI mutant mice was comparable with wild-type (+/+) littermates (FS: 37.9%, 37.4%, and 37.6%, respectively) as were LVIDd (3.32, 3.31, and 3.38 mm, respectively), LVIDs (2.07, 2.07, and 2.12 mm, respectively), LVPWd (0.84, 0.83, and 0.75 mm, respectively), and IVSd (0.91, 0.90, and 0.75 mm, respectively) at 16 mo (Fig. 3, A–E). We observed no significant change in any of these parameters irrespective of sex. In addition, morphological and histological analyses at 16 mo of age revealed no evidence of morphological defects or increased cardiac fibrosis in Bag3 P209L KI mice (Fig. 4A). There were also no changes in global indexes of cardiac hypertrophy as measured by the ratio of heart weight to body weight and heart weight to tibia length in Bag3m/m and Bag3m/+ P209L KI mice and wild-type control mice (Fig. 4, B and C). We also observed no changes in the expression levels of the cardiac fetal gene markers atrial natriuretic factor (Anf) and B-type natriuretic peptide (Bnp) as well as the profibrotic gene markers collagen α1 type I (Coll1a1) and type III (Coll3a1) (Fig. 4, D and E). Together, these results revealed no evidence of remodeling or fibrotic replacement of cardiomyocytes in Bag3 P209L KI mutants and demonstrated that the Bag3 P209L mutation in mice does not cause cardiomyopathy during aging.
Fig. 3.
Bcl-2-associated athanogene 3 (Bag3) P209L knockin (KI) mutant mice display normal cardiac function. A−E: echocardiographic analysis showing fractional shortening (FS; A), left ventricular internal diameter at diastole (LVIDd; B), left ventricular internal diameter at systole (LVIDs; C), left ventricular posterior wall thickness at diastole (LVPWd; D), and interventricular septum diameter at diastole (IVSd; E) in wild-type (+/+) control (n = 9) and heterozygous (m/+) (n = 7) and homozygous (m/m) (n = 13) Bag3 P209L-KI mutant mice at 4, 11, and 16 mo of age. Data are presented as means ± SE.
Fig. 4.
Bcl-2-associated athanogene 3 (Bag3) P209L knockin (KI) mutant mice display normal cardiac morphology and molecular markers. A: representative microscopic images of whole mouse hearts (top), cross-sectional views of hematoxylin and eosin-stained hearts (middle), and Masson’s trichrome-stained hearts (bottom) isolated from wild-type (+/+) control and heterozygous (m/+) and homozygous (m/m) Bag3 P209L KI mutant mice at 16 mo of age. Scale bars = 1 mm. n = 3 mice/group. B and C: heart weight-to-body weight ratio (HW/BW; B) and heart weight-to-tibia length ratio (HW/TL; C) of +/+ control (n = 6) and m/+ (n = 5) and m/m (n = 7) Bag3 P209L KI mutant mice at 16 mo of age. D and E: quantitative RT-PCR analysis of cardiac fetal gene markers (D) and profibrotic genes (E) in +/+ control and m/+ and m/m Bag3 P209L KI mutant mouse hearts at 16 mo of age. n = 4 hearts/group. Data are normalized to corresponding 18S levels, and mutant groups are expressed as fold changes versus control. Anf, atrial natriuretic factor; Bnp, B-type natriuretic peptide; Coll1a1, collagen α1 type I; Coll3a1, collagen α1 type III. Data are presented as means ± SE.
Protein levels of sHSPs are unaffected in BAG3 P209L KI hearts.
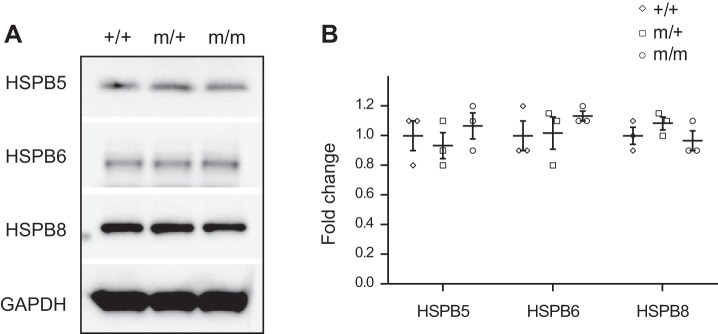
The P209 residue resides in the second IPV motif of BAG3, which is conserved between the human and mouse (Supplemental Fig. S1) and mediates the direct interaction between BAG3 and sHSPs (5, 13, 29). sHSPs, also known as HSPB, constitute an evolutionarily conserved yet diverse family of chaperones (7, 13, 24). Our previous findings demonstrated that HSPB5, HSPB6, and HSPB8, which interact with BAG3, became destabilized in Bag3 knockout and loss-of-function E455K mutant mice, suggesting that BAG3 is essential to maintain the protein stability of sHSPs (8). Thus, we performed Western blot analysis to determine whether protein levels of HSPB5, HSPB6, and HSPB8 are altered in Bag3 P209L mutant mice. The results revealed no difference in protein levels of HSPB5, HSPB6, and HSPB8 between Bag3m/m and Bag3m/+ P209L KI mice and wild-type control mice (Fig. 5), suggesting that the Bag3 P209L mutation does not impair the protein stability of HSPB5, HSPB6, and HSPB8.
Fig. 5.
Protein levels of small heat shock proteins [sHSPs; also known as heat shock protein family B (HSPB)] are unchanged in Bcl-2-associated athanogene 3 (Bag3) P209L knockin (KI) mutant hearts. A and B: representative immunoblots (A) and corresponding quantitative densitometric analysis (B) of HSPB5, HSPB6, and HSPB8 in wild-type (+/+) control and heterozygous (m/+) and homozygous (m/m) Bag3 P209L KI mutant mouse hearts at 16 mo of age. GAPDH served as a loading control. The blots shown were derived from replicate samples run on parallel gels. Data are normalized to corresponding GAPDH, and mutant groups are expressed as fold changes versus control. n = 3 per group. Data are presented as means ± SE.
BAG3 P209L mutation does not impair stress-inducible HSP expression, ubiquitination, or autophagy.
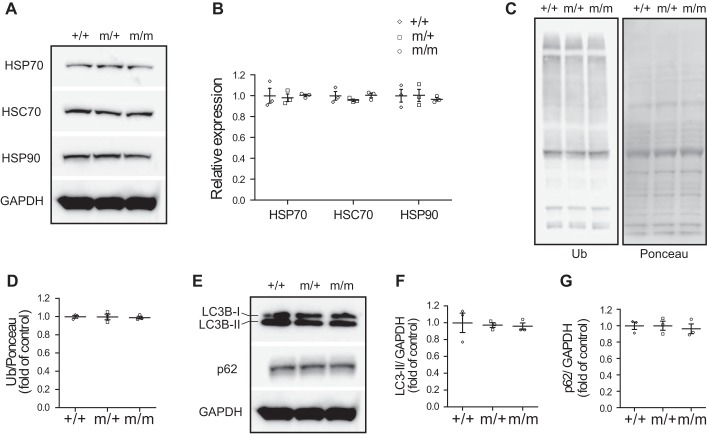
As a cochaperone protein, BAG3 interacts with ATP-dependent high-molecular-weight HSPs as well as ATP-independent sHSPs in functionally distinct multichaperone complexes (5, 31, 34) to facilitate protein homeostasis and autophagy (4, 8, 23). To determine the effects of the Bag3 P209L mutation on ATP-dependent high-molecular-weight HSP components in the chaperone complex, we evaluated protein samples from isolated hearts of 16-mo-old Bag3 P209L mutant and control mice. Western blot analysis revealed that the levels of stress-inducible HSP70 and HSP90 as well as constitutively expressed heat shock cognate 70 were unchanged in Bag3 P209L KI mice (Fig. 6, A and B). Furthermore, no change in protein ubiquitination, an indication for proteasomal degradation of misfolded proteins (2, 11, 25, 37), was observed in Bag3 P209L KI mutant hearts compared with wild-type control hearts (Fig. 6, C and D). We also determined whether the Bag3 P209L mutation affected autophagy by examining the levels of the autophagosome-associated protein light chain 3B (LC3B), which is present in cytosolic form (LC3B-I) until it becomes targeted to nascent autophagosomes (LC3B-II form) (21, 22). The results revealed that autophagic levels of LC3B-II as well as the selective autophagy receptor p62 were comparable in Bag3 P209L KI mutant and wild-type mouse hearts (Fig. 6, E–G). These results indicate that the Bag3 P209L mutation does not impair ATP-dependent high-molecular-weight HSP expression, protein ubiquitination, or autophagy.
Fig. 6.
The Bcl-2-associated athanogene 3 (Bag3) P209L knockin (KI) mutation does not impair stress-inducible heat shock protein (HSP) expression, ubiquitination, or autophagy in the heart. A and B: representative immunoblots (A) and corresponding quantitative densitometric analysis (B) of HSP70, heat shock cognate (HSC)70, and HSP90. The blots shown were derived from replicate samples run on parallel gels as described in Fig. 5A. C and D: representative immunoblots (C) and corresponding quantitative densitometric analysis (D) of ubiquitin (Ub). E–G: representative immunoblots (E) and corresponding quantitative densitometric analysis (F and G) of cardiac autophagy markers in wild-type (+/+) control and heterozygous (m/+) and homozygous (m/m) Bag3 P209L KI mutant mouse hearts at 16 mo of age after 18 h of starvation. GAPDH or Ponceau staining served as a loading control. The blots shown were derived from replicate samples run on parallel gels as described in Fig. 5A. Data were normalized to corresponding GAPDH levels or Ponceau staining, and mutant groups are expressed as fold changes versus control. n = 3 for each group. Data are presented as means ± SE.
DISCUSSION
BAG3 plays an integral role in cardiomyocyte protein homeostasis by interacting with both ATP-dependent high-molecular-weight HSPs and ATP-independent sHSPs in large, functionally distinct multichaperone complexes (4, 8, 14, 18, 29, 31, 33). The Bag3 P209L mutation, which resides in a critical region that mediates the direct interaction between BAG3 and sHSPS, is associated with cardiomyopathy in humans (19, 27, 32). To determine the molecular basis underlying the cardiomyopathy caused by the Bag3 P209L mutation, we generated and characterized a Bag3 P209L KI mouse model in which mutant Bag3 is expressed under the control of the endogenous locus. Surprisingly, Bag3 P209L KI mice were viable and displayed normal cardiac structure and function, even up to 16 mo of age. Biochemical analyses further revealed that levels of sHSPs, stress-inducible HSPs, ubiquitinated proteins, and autophagy were unaffected in P209L mutant mouse hearts, leading us to conclude that the Bag3 P209L mutation alone does not lead to cardiomyopathy in mice.
Here, we report that Bag3 P209L KI mice express the mutant BAG3 at the same level as that of wild-type mice. However, in contrast to a recent study that reported significant systolic dysfunction by 8 mo of age in a cardiomyocyte-specific BAG3 P209L transgenic mouse model, in which human P209L mutant BAG3 protein was overexpressed in cardiomyocytes (28), our Bag3 P209L KI mice displayed no overt phenotype. It is possible that the observed phenotype in BAG3 P209L transgenic mice is due to overexpression of human BAG3 protein. Indeed, it has been reported that overexpression of wild-type human BAG3 in cardiomyocytes also leads to cardiac dysfunction (17).
Human genetic studies have revealed that the P209L heterozygous mutation in BAG3 was associated with early onset severe cardiomyopathy (19, 27, 32). However, both our Bag3 P209L heterozygous and homozygous mutant mice displayed no cardiac phenotype. There are several possible explanations for our observation, one of which is that it may take a considerable amount of time to form aberrant protein aggregates and eventually lead to cardiomyopathy. We only evaluated the cardiac structure and function of Bag3 P209L KI mice, generated in a pure C57/B6 genetic background, at baseline conditions up to 16 mo of age. It is possible that Bag3 P209L KI mice may eventually develop cardiomyopathy over a longer time period or under stress conditions. It is also possible that unknown genetic modifier(s) could be required to manifest a cardiomyopathy phenotype, which could be validated by studying Bag3 P209L mutant mice in different inbred mouse strains. Another potential explanation is that different genomic features exist between the human and mouse. The P209L mutation may lead to changes in mutant Bag3 transcription, translation, or protein stability in humans. Indeed, it has been shown that the protein level of BAG3 is reduced in patients carrying the P209L mutation (19). We did not observe a difference in BAG3 protein levels between Bag3 P209L KI mice and wild-type control mice. In conclusion, our results suggest that human cardiomyopathy may result from species-specific requirements for the conserved motif in Bag3 that is disrupted by the P209L mutation in cardiac function or from genetic background-dependent effects.
GRANTS
This work was supported by grants from the National Heart, Lung, and Blood Institute (NHLBI) and Foundation Leducq (TNE-13CVD04, to J. Chen), an American Heart Association Endowed Chair in Cardiovascular Research (to J. Chen), NHLBI Grant K99-HL-143210 (to X. Fang), European Commission’s Marie Sklodowska-Curie Individual Fellowship (Titin Signals) 656636 (to J. Bogomolovas), and by Shenzhen Basic Research Foundation Grants KCYJ20160428154108239 and KQJSCX20170330155020267 (to K. Ouyang).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
X.F., K.O., and J.C. conception and design of research; X.F., J.B., P.S.Z., Y.M., X.M., Z.C., L.Z., and M.Z. performed experiments; X.F. analyzed data; X.F. and J.C. interpreted results of experiments; X.F. prepared figures; X.F. drafted manuscript; X.F., J.V., K.O., and J.C. edited and revised manuscript; X.F., J.B., P.S.Z., Y.M., X.M., Z.C., L.Z., M.Z., J.V., K.O., and J.C. approved final version of manuscript.
Supplemental Data
Figure S1 and Tables S1-S3 - .pdf (144 KB)
REFERENCES
- 1.Arimura T, Ishikawa T, Nunoda S, Kawai S, Kimura A. Dilated cardiomyopathy-associated BAG3 mutations impair Z-disc assembly and enhance sensitivity to apoptosis in cardiomyocytes. Hum Mutat 32: 1481–1491, 2011. doi: 10.1002/humu.21603. [DOI] [PubMed] [Google Scholar]
- 2.Breusing N, Grune T. Regulation of proteasome-mediated protein degradation during oxidative stress and aging. Biol Chem 389: 203–209, 2008. doi: 10.1515/BC.2008.029. [DOI] [PubMed] [Google Scholar]
- 3.Broadley SA, Hartl FU. The role of molecular chaperones in human misfolding diseases. FEBS Lett 583: 2647–2653, 2009. doi: 10.1016/j.febslet.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 4.Carra S. The stress-inducible HspB8-Bag3 complex induces the eIF2alpha kinase pathway: implications for protein quality control and viral factory degradation? Autophagy 5: 428–429, 2009. doi: 10.4161/auto.5.3.7894. [DOI] [PubMed] [Google Scholar]
- 5.Carra S, Seguin SJ, Lambert H, Landry J. HspB8 chaperone activity toward poly(Q)-containing proteins depends on its association with Bag3, a stimulator of macroautophagy. J Biol Chem 283: 1437–1444, 2008. doi: 10.1074/jbc.M706304200. [DOI] [PubMed] [Google Scholar]
- 6.Chami N, Tadros R, Lemarbre F, Lo KS, Beaudoin M, Robb L, Labuda D, Tardif JC, Racine N, Talajic M, Lettre G. Nonsense mutations in BAG3 are associated with early-onset dilated cardiomyopathy in French Canadians. Can J Cardiol 30: 1655–1661, 2014. doi: 10.1016/j.cjca.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 7.Chis R, Sharma P, Bousette N, Miyake T, Wilson A, Backx PH, Gramolini AO. α-Crystallin B prevents apoptosis after H2O2 exposure in mouse neonatal cardiomyocytes. Am J Physiol Heart Circ Physiol 303: H967–H978, 2012. doi: 10.1152/ajpheart.00040.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doong H, Vrailas A, Kohn EC. What’s in the ‘BAG’?−a functional domain analysis of the BAG-family proteins. Cancer Lett 188: 25–32, 2002. doi: 10.1016/S0304-3835(02)00456-1. [DOI] [PubMed] [Google Scholar]
- 9.Fang X, Bogomolovas J, Wu T, Zhang W, Liu C, Veevers J, Stroud MJ, Zhang Z, Ma X, Mu Y, Lao DH, Dalton ND, Gu Y, Wang C, Wang M, Liang Y, Lange S, Ouyang K, Peterson KL, Evans SM, Chen J. Loss-of-function mutations in co-chaperone BAG3 destabilize small HSPs and cause cardiomyopathy. J Clin Invest 127: 3189–3200, 2017. doi: 10.1172/JCI94310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang X, Stroud MJ, Ouyang K, Fang L, Zhang J, Dalton ND, Gu Y, Wu T, Peterson KL, Huang HD, Chen J, Wang N. Adipocyte-specific loss of PPARγ attenuates cardiac hypertrophy. JCI Insight 1: e89908, 2016. doi: 10.1172/jci.insight.89908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang X, Trexler C, Chen J. Ushering in the cardiac role of Ubiquilin1. J Clin Invest 128: 5195–5197, 2018. doi: 10.1172/JCI124567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldman AM, Begay RL, Knezevic T, Myers VD, Slavov DB, Zhu W, Gowan K, Graw SL, Jones KL, Tilley DG, Coleman RC, Walinsky P, Cheung JY, Mestroni L, Khalili K, Taylor MR. Decreased levels of BAG3 in a family with a rare variant and in idiopathic dilated cardiomyopathy. J Cell Physiol 229: 1697–1702, 2014. doi: 10.1002/jcp.24615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuchs M, Poirier DJ, Seguin SJ, Lambert H, Carra S, Charette SJ, Landry J. Identification of the key structural motifs involved in HspB8/HspB6-Bag3 interaction. Biochem J 425: 245–257, 2009. doi: 10.1042/BJ20090907. [DOI] [PubMed] [Google Scholar]
- 14.Gamerdinger M, Hajieva P, Kaya AM, Wolfrum U, Hartl FU, Behl C. Protein quality control during aging involves recruitment of the macroautophagy pathway by BAG3. EMBO J 28: 889–901, 2009. doi: 10.1038/emboj.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirai M, Arita Y, McGlade CJ, Lee KF, Chen J, Evans SM. Adaptor proteins NUMB and NUMBL promote cell cycle withdrawal by targeting ERBB2 for degradation. J Clin Invest 127: 569–582, 2017. doi: 10.1172/JCI91081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Homma S, Iwasaki M, Shelton GD, Engvall E, Reed JC, Takayama S. BAG3 deficiency results in fulminant myopathy and early lethality. Am J Pathol 169: 761–773, 2006. doi: 10.2353/ajpath.2006.060250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inomata Y, Nagasaka S, Miyate K, Goto Y, Hino C, Toukairin C, Higashio R, Ishida K, Saino T, Hirose M, Tsumura H, Sanbe A. Bcl-2-associated athanogene 3 (BAG3) is an enhancer of small heat shock protein turnover via activation of autophagy in the heart. Biochem Biophys Res Commun 496: 1141–1147, 2018. doi: 10.1016/j.bbrc.2018.01.158. [DOI] [PubMed] [Google Scholar]
- 18.Judge LM, Perez-Bermejo JA, Truong A, Ribeiro AJ, Yoo JC, Jensen CL, Mandegar MA, Huebsch N, Kaake RM, So PL, Srivastava D, Pruitt BL, Krogan NJ, Conklin BR. A BAG3 chaperone complex maintains cardiomyocyte function during proteotoxic stress. JCI Insight 2: e94623, 2017. doi: 10.1172/jci.insight.94623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kostera-Pruszczyk A, Suszek M, Płoski R, Franaszczyk M, Potulska-Chromik A, Pruszczyk P, Sadurska E, Karolczak J, Kamińska AM, Rędowicz MJ. BAG3-related myopathy, polyneuropathy and cardiomyopathy with long QT syndrome. J Muscle Res Cell Motil 36: 423–432, 2015. doi: 10.1007/s10974-015-9431-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin Q, Zhao G, Fang X, Peng X, Tang H, Wang H, Jing R, Liu J, Lederer WJ, Chen J, Ouyang K. IP3 receptors regulate vascular smooth muscle contractility and hypertension. JCI Insight 1: e89402, 2016. doi: 10.1172/jci.insight.89402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maejima Y, Chen Y, Isobe M, Gustafsson AB, Kitsis RN, Sadoshima J. Recent progress in research on molecular mechanisms of autophagy in the heart. Am J Physiol Heart Circ Physiol 308: H259–H268, 2015. doi: 10.1152/ajpheart.00711.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell 140: 313–326, 2010. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizushima W, Sadoshima J. BAG3 plays a central role in proteostasis in the heart. J Clin Invest 127: 2900–2903, 2017. doi: 10.1172/JCI95839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morrison LE, Whittaker RJ, Klepper RE, Wawrousek EF, Glembotski CC. Roles for alphaB-crystallin and HSPB2 in protecting the myocardium from ischemia-reperfusion-induced damage in a KO mouse model. Am J Physiol Heart Circ Physiol 286: H847–H855, 2004. doi: 10.1152/ajpheart.00715.2003. [DOI] [PubMed] [Google Scholar]
- 25.Nandi D, Tahiliani P, Kumar A, Chandu D. The ubiquitin-proteasome system. J Biosci 31: 137–155, 2006. doi: 10.1007/BF02705243. [DOI] [PubMed] [Google Scholar]
- 26.Norton N, Li D, Rieder MJ, Siegfried JD, Rampersaud E, Züchner S, Mangos S, Gonzalez-Quintana J, Wang L, McGee S, Reiser J, Martin E, Nickerson DA, Hershberger RE. Genome-wide studies of copy number variation and exome sequencing identify rare variants in BAG3 as a cause of dilated cardiomyopathy. Am J Hum Genet 88: 273–282, 2011. doi: 10.1016/j.ajhg.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Odgerel Z, Sarkozy A, Lee HS, McKenna C, Rankin J, Straub V, Lochmüller H, Paola F, D’Amico A, Bertini E, Bushby K, Goldfarb LG. Inheritance patterns and phenotypic features of myofibrillar myopathy associated with a BAG3 mutation. Neuromuscul Disord 20: 438–442, 2010. doi: 10.1016/j.nmd.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quintana MT, Parry TL, He J, Yates CC, Sidorova TN, Murray KT, Bain JR, Newgard CB, Muehlbauer MJ, Eaton SC, Hishiya A, Takayama S, Willis MS. Cardiomyocyte-specific human Bcl2-associated anthanogene 3 P209L expression induces mitochondrial fragmentation, Bcl2-associated anthanogene 3 haploinsufficiency, and activates p38 signaling. Am J Pathol 186: 1989–2007, 2016. doi: 10.1016/j.ajpath.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rauch JN, Tse E, Freilich R, Mok SA, Makley LN, Southworth DR, Gestwicki JE. BAG3 is a modular, scaffolding protein that physically links heat shock protein 70 (Hsp70) to the small heat shock proteins. J Mol Biol 429: 128–141, 2017. doi: 10.1016/j.jmb.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodríguez CI, Buchholz F, Galloway J, Sequerra R, Kasper J, Ayala R, Stewart AF, Dymecki SM. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat Genet 25: 139–140, 2000. doi: 10.1038/75973. [DOI] [PubMed] [Google Scholar]
- 31.Rosati A, Graziano V, De Laurenzi V, Pascale M, Turco MC. BAG3: a multifaceted protein that regulates major cell pathways. Cell Death Dis 2: e141, 2011. doi: 10.1038/cddis.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Selcen D, Muntoni F, Burton BK, Pegoraro E, Sewry C, Bite AV, Engel AG. Mutation in BAG3 causes severe dominant childhood muscular dystrophy. Ann Neurol 65: 83–89, 2009. doi: 10.1002/ana.21553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su F, Myers VD, Knezevic T, Wang J, Gao E, Madesh M, Tahrir FG, Gupta MK, Gordon J, Rabinowitz J, Ramsey FV, Tilley DG, Khalili K, Cheung JY, Feldman AM. Bcl-2-associated athanogene 3 protects the heart from ischemia/reperfusion injury. JCI Insight 1: e90931, 2016. doi: 10.1172/jci.insight.90931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takayama S, Reed JC. Molecular chaperone targeting and regulation by BAG family proteins. Nat Cell Biol 3: E237–E241, 2001. doi: 10.1038/ncb1001-e237. [DOI] [PubMed] [Google Scholar]
- 35.Takayama S, Xie Z, Reed JC. An evolutionarily conserved family of Hsp70/Hsc70 molecular chaperone regulators. J Biol Chem 274: 781–786, 1999. doi: 10.1074/jbc.274.2.781. [DOI] [PubMed] [Google Scholar]
- 36.Villard E, Perret C, Gary F, Proust C, Dilanian G, Hengstenberg C, Ruppert V, Arbustini E, Wichter T, Germain M, Dubourg O, Tavazzi L, Aumont MC, DeGroote P, Fauchier L, Trochu JN, Gibelin P, Aupetit JF, Stark K, Erdmann J, Hetzer R, Roberts AM, Barton PJ, Regitz-Zagrosek V, Cardiogenics Consortium; Aslam U, Duboscq-Bidot L, Meyborg M, Maisch B, Madeira H, Waldenström A, Galve E, Cleland JG, Dorent R, Roizes G, Zeller T, Blankenberg S, Goodall AH, Cook S, Tregouet DA, Tiret L, Isnard R, Komajda M, Charron P, Cambien F. A genome-wide association study identifies two loci associated with heart failure due to dilated cardiomyopathy. Eur Heart J 32: 1065–1076, 2011. doi: 10.1093/eurheartj/ehr105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, Li J, Zheng H, Su H, Powell SR. Proteasome functional insufficiency in cardiac pathogenesis. Am J Physiol Heart Circ Physiol 301: H2207–H2219, 2011. doi: 10.1152/ajpheart.00714.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu T, Mu Y, Bogomolovas J, Fang X, Veevers J, Nowak RB, Pappas CT, Gregorio CC, Evans SM, Fowler VM, Chen J. HSPB7 is indispensable for heart development by modulating actin filament assembly. Proc Natl Acad Sci USA 114: 11956–11961, 2017. doi: 10.1073/pnas.1713763114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Youn DY, Lee DH, Lim MH, Yoon JS, Lim JH, Jung SE, Yeum CE, Park CW, Youn HJ, Lee JS, Lee SB, Ikawa M, Okabe M, Tsujimoto Y, Lee JH. Bis deficiency results in early lethality with metabolic deterioration and involution of spleen and thymus. Am J Physiol Endocrinol Metab 295: E1349–E1357, 2008. doi: 10.1152/ajpendo.90704.2008. [DOI] [PubMed] [Google Scholar]
- 40.Young JC, Agashe VR, Siegers K, Hartl FU. Pathways of chaperone-mediated protein folding in the cytosol. Nat Rev Mol Cell Biol 5: 781–791, 2004. doi: 10.1038/nrm1492. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Z, Stroud MJ, Zhang J, Fang X, Ouyang K, Kimura K, Mu Y, Dalton ND, Gu Y, Bradford WH, Peterson KL, Cheng H, Zhou X, Chen J. Normalization of Naxos plakoglobin levels restores cardiac function in mice. J Clin Invest 125: 1708–1712, 2015. doi: 10.1172/JCI80335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 and Tables S1-S3 - .pdf (144 KB)