Abstract
The placenta sheds extracellular vesicles (EVs), including exosomes, into the maternal circulation. We recently demonstrated that this trafficking of EVs is bi-directional; with uptake of macrophage exosomes by the placenta inducing cytokine release. The specificity of this response is currently unknown. THP-1 cells were cultured as monocytes or differentiated to macrophages, and EVs isolated by ultra-centrifugation. The effect of EVs on human placental explants was measured by cytokine ELISA/luminex. Macrophage, but not monocyte, EVs induce the release of pro-inflammatory cytokines by the placenta. Thus, placental responses to immune cell EVs, including exosomes, reflects the phenotype of the source cell.
Keywords: Explants, trophoblast, exosomes, extracellular vesicles, immunology, reproductive immunology, macrophages, cytokines
Introduction
Extracellular vesicles (EVs), including microvesicles and exosomes, are intricately involved in cell-cell communication. Placental EVs have been demonstrated to interact with maternal immune cells, modulating their function; including inducing immune cell cytokine responses [1–5]. We recently published the first demonstration that EVs can also traffic from immune cells to the placenta, modifying placental function[6]. Specifically, we showed that macrophage exosomes are taken up by the placenta via clathrin-mediated endocytosis, and induce the release of pro-inflammatory cytokines [6].
Macrophages are the main professional antigen-presenting cell in the decidua, and are proposed to aid in tissue remodeling through phagocytosis and secretion of cytokines[7]. Co-culture and conditioned media experiments have previously shown that macrophages release molecules that exert a range of functional responses in the placenta, including trophoblast differentiation, migration and immunological responses [8,9]. This can be partly explained by cytokine secretion, but macrophage-placental communication could also involve EVs.
The specificity of the recently observed placental response to macrophage EVs is unknown; does the placenta mount a pro-inflammatory cytokine response to all immune-cell EVs? The goal of this current study was to determine how the activation status of the source cell affects EV function on the placenta; comparing the effect of EVs from activated macrophages and their non-activated monocytic pre-cursors.
Methods
Study Subjects
Term placentas from normal singleton pregnancies were obtained following elective Caesarean section without labor. The study was approved by Research Ethics Committee (13/LO/1712). Written informed consent was obtained.
Monocyte/macrophage culture
THP-1 cells were maintained in RPMI-Glutamax with 10% FCS and antibiotics. To model monocytes, THP-1s were cultured in suspension at 0.5×106/ml. For differentiation into macrophages, THP-1s were seeded at 1×106/ml with 50ng/ml Phorbol 12-myristate 13-acetate (PMA) [10] for 24h, washed and rested for 24h, before washing and changing to media with 10% exosome-depleted FBS (Thermo Fisher) for the 24h EV collection period.
EV isolation and quantification
EVs were purified from culture supernatants by sequential centrifugation; 1,000g for 10minx2, 10,000G for 15min, 100,000G for 90min (F-28/50 rotor), as previously described[6]. The EV pellet was resuspended in media used for placental explants; 1:1 serum-free Dulbecco’s Modified Eagles Medium (DMEM) and Ham’s F12 (F12) (DMEM/F12, Gibco, UK); and passed through a 0.2μM filter. EVs were quantified and sized using Nanoparticle Tracking Analysis (LM10; Nanosight). Samples were loaded by syringe pump and 3×90sec videos recorded and batch-analysed using instrument software (NTA 2.3). Single-use aliquots of EVs were stored at −80°C. All relevant experimental data is submitted to the EV-TRACK knowledgebase (EV-TRACK ID: EV180024)[11].
Western blotting
Western blotting was performed as previously described[6] on samples lysed in RIPA buffer, using the following antibodies: flotillin-1 (C-2, SantaCruz, 0.4μg/ml), MHC-I (HLA-A; EP1395Y, Abcam, 0.103μg/ml), TSG101 (C-2, SantaCruz, 1μg/ml), calnexin (#2433, CellSignallingTechnologies, 0.8μg/ml).
Explant culture
Three random placental tissue chunks were excised and pooled, then washed in PBS and dissected in DMEM/F12 with antibiotics. Placental villous explants (2–3mm) were placed one per well, in a 96-well plate. Treatments were performed in triplicate and randomized across the plate. EVs were added at 5×109–5×1010/ml, previously optimized to produce a dose response[6]. To check for endogenous cytokines in EV preps, EV-only control wells were incubated alongside explants and included in subsequent assays.
Cytokine measurement
24h supernatants were assayed for levels of IL-6, IL-8, IL-10, GM-CSF and IL-1β using commercial ELISA kits (Ready-SET-Go™, eBioscience). IL-10, TNF-α and IL-1α were measured by luminex assay (custom Bioplex, Bio-Rad).
Statistical analysis
D’Agostino-Pearson omnibus normality test was performed, followed by Friedman or Kruskal Wallis test, with Dunn’s multiple comparisons test to compare exosome-treated groups to the non-treated.
Results and Discussion
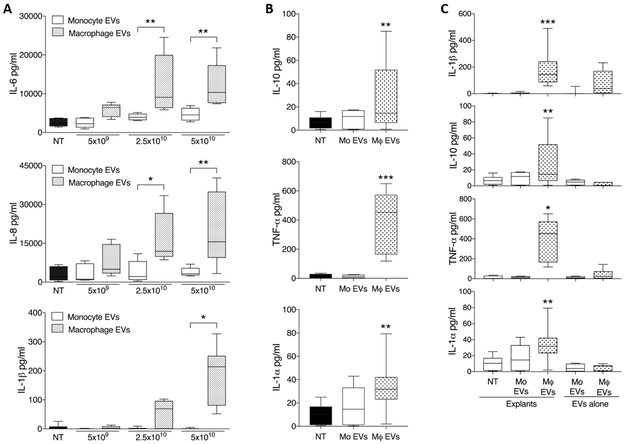
In this study, we demonstrate that the placenta mounts a pro-inflammatory response to EVs from activated macrophages, but not to those from monocytes. Placental explants released abundant amounts of IL-6, IL-8, IL-1ß and TNF-α, and lesser amounts of IL-10 and IL-1-α (Fig 2A-B) in response to culture with macrophage EVs, with no such response seen to monocyte EVs. No elevation in IFN-γ or GM-CSF was seen (data not shown). As shown previously for IL-6 and IL-8[6], elevated IL-1ß/TNF-α/IL-10/IL-1-α in supernatants were of placental origin (Fig 2C).
Figure 2. Placental release of cytokines in response to monocyte- and macrophage-derived EVs.
Term placental explants were treated with 5×109- 5×1010/ml EVs derived from monocytes or macrophages, and culture supernatants collected at 24h. Explants were in 150μl culture media, equating to 1.5×108, 3.75×109 and 7.5×109 EVs per explant for the three doses. For the highest concentration of EVs (5×1010/ml), the mean number of initial cells plated was 2.2×106 monocytes and 4.7×106 macrophages per explant. A) Cytokine levels in culture supernatants assayed by ELISA showed that macrophage EVs induced significant dose-dependent increases in the production of IL-6, IL-8 and IL-1ß, with no such response seen to monocyte EVs (n=5 placentas). B) Luminex performed for the highest exosome dose (5×1010/ml) additionally showed that IL-10 and TNF-α and IL-1-α were significantly elevated in response to macrophage EVs, but not monocyte EVs (n=8 placentas). Asterisks above individual columns indicates a significant difference from the non-treated (NT). C) To confirm that cytokines detected were of placental origin, as previously shown for IL-6/IL-8[6], control wells of exosomes alone were incubated alongside placental explants at the highest concentration, and assayed for IL-1ß/TNF-α/IL-10/IL-1-α by ELISA (n=8 placentas). At least two different EV preparations were used for each cytokine measurement. *p<0.05, **p<0.01, ***p<0.001.
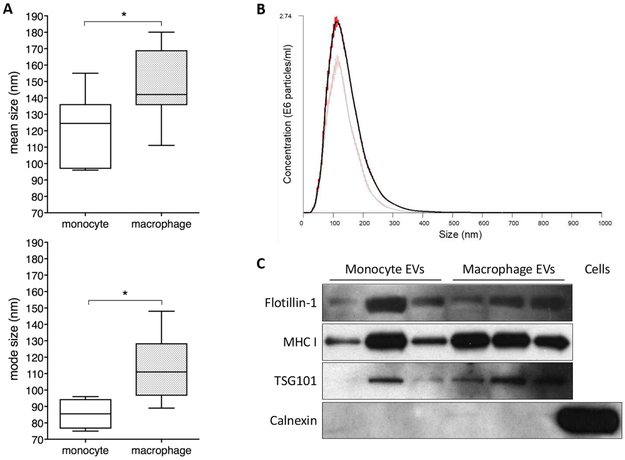
EVs encompassed the expected size range of exosome, with mode size of 96nm and 111nm for monocyte and macrophage EVs respectively (Fig 1A-B). The number and protein concentration of EVs recovered per million cells plated was 3.2×109/8.44μg for monocytes and 1.4×109/3.24μg for macrophages. EVs were positive for exosome markers flotillin-1, MHC-I and TSG101, and negative for calnexin, an indicator of contamination with cellular debris. Due to the ultracentifuge method of isolation used, other EV populations such as microvesicles may also be present. EVs from macrophages were slightly larger than those from monocytes (Fig 1B), perhaps reflecting their activated phenotype. Future investigations should interrogate the cargo of macrophage and monocyte EVs to determine the molecules responsible for the differential effect on the placenta.
Figure 1. Characterisation of monocyte and macrophage EVs.
Phenotype of EVs released by THP-1 monocytes and macrophages was assessed by Nanoparticle Tracking Analysis (NTA) and western blotting for exosome markers. A) Mean and mode size of EVs released from monocytes (n=6) and macrophages (n=9). B) Representative NTA histogram demonstrating size distribution of EVs from macrophages (dark line) and monocytes (pale line). Red error bars indicate standard error of the mean. C) Western blotting for exosome markers flotillin-1, MHC-I and TSG101 indicate the presence of exosomes in EV preparations, and lack of calnexin indicates the absence of cellular debris. Yield of EVs per million cells plated at start of 24h collection period was 3.5×109 from monocytes and 1.9×109 from macrophages.
This current study suggests that the placental proinflammatory response is specific to EVs from activated macrophages, and not to EVs released by their resting circulating pre-cursors; blood monocytes. Preeclampsia is associated with increased numbers of decidual macrophages[13,14], and with increased monocyte/macrophage expression of pro-inflammatory markers[12,14], representing a potential involvement of pro-inflammatory macrophages in pregnancy complications. Pre-eclampsia is also assocated with increased placental pro-inflammatory cytokines, including several measured in this study (reviewed in[15]). Released EVs are thus a potential messaging system that can mediate maternal-placental signalling from monocytes that have differentiated into decidua-infiltrating macrophages.
Acknowledgments
BH and BK are supported by MRC The Gambia at LSHTM. The research was funded by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Imperial College Healthcare NHS Trust and Imperial College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. We thank Dr. Yanping Guo and the St. Mary’s NHLI FACS core facility for support and instrumentation for processing of luminex plates. We also thank Section of Paediatrics staff, especially the Kampmann team, and Karen Forbes at Leeds University for critical appraisal of the manuscript.
References
- [1].Southcombe J, Tannetta D, Redman C, Sargent I, The immunomodulatory role of syncytiotrophoblast microvesicles., PLoS One. 6 (2011) e20245. doi: 10.1371/journal.pone.0020245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Holder BS, Tower CL, Jones CJ, Aplin JD, Abrahams VM, Heightened pro-inflammatory effect of preeclamptic placental microvesicles on peripheral blood immune cells in humans, Biol Reprod. 86 (2012) 103. [DOI] [PubMed] [Google Scholar]
- [3].Holder BS, Tower CL, Forbes K, Mulla MJ, Aplin JD, Abrahams VM, Immune cell activation by trophoblast-derived microvesicles is mediated by syncytin 1, Immunology. 136 (2012) 184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Messerli M, May K, Hansson SR, Schneider H, Holzgreve W, Hahn S, Rusterholz C, Feto-maternal interactions in pregnancies: placental microparticles activate peripheral blood monocytes., Placenta. 31 (2010) 106–112. [DOI] [PubMed] [Google Scholar]
- [5].Joerger-Messerli MS, Hoesli IM, Rusterholz C, Lapaire O, Stimulation of monocytes by placental microparticles involves toll-like receptors and nuclear factor kappa-light-chain-enhancer of activated B cells, Front. Immunol 5 (2014). doi: 10.3389/fimmu.2014.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Holder B, Jones T, Sancho Shimizu V, Rice TF, Donaldson B, Bouqueau M, Forbes K, Kampmann B, Macrophage Exosomes Induce Placental Inflammatory Cytokines: A Novel Mode of Maternal-Placental Messaging, Traffic. 17 (2016) 168–178. doi: 10.1111/tra.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Abrahams VM, Kim YM, Straszewski SL, Romero R, Mor G, Macrophages and Apoptotic Cell Clearance During Pregnancy, Am. J. Reprod. Immunol 51 (2004) 275–282. doi: 10.1111/j.1600-0897.2004.00156.x. [DOI] [PubMed] [Google Scholar]
- [8].Rozner AE, Dambaeva SV, Drenzek JG, Durning M, Golos TG, Modulation of cytokine and chemokine secretions in rhesus monkey trophoblast co-culture with decidual but not peripheral blood monocyte-derived macrophages., Am. J. Reprod. Immunol 66 (2011) 115–27. doi: 10.1111/j.1600-0897.2010.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Buckley RJ, Whitley GS, Dumitriu IE, Cartwright JE, Macrophage polarisation affects their regulation of trophoblast behaviour, Placenta. 47 (2016) 73–80. doi: 10.1016/j.placenta.2016.09.004. [DOI] [PubMed] [Google Scholar]
- [10].Tsuchiya S, Kobayashi Y, Goto Y, Okumura H, Nakae S, Konno T, Tada K, Induction of maturation in cultured human monocytic leukemia cells by a phorbol diester., Cancer Res. 42 (1982) 1530–6. [PubMed] [Google Scholar]
- [11].Van Deun J, Mestdagh P, Agostinis P, Akay Ö, Anand S, Anckaert J, Martinez ZA, Baetens T, Beghein E, Bertier L, Berx G, Boere J, Boukouris S, Bremer M, Buschmann D, Byrd JB, Casert C, Cheng L, Cmoch A, Daveloose D, De Smedt E, Demirsoy S, Depoorter V, Dhondt B, Driedonks TAP, Dudek A, Elsharawy A, Floris I, Foers AD, Gärtner K, Garg AD, Geeurickx E, Gettemans J, Ghazavi F, Giebel B, Kormelink TG, Hancock G, Helsmoortel H, Hill AF, Hyenne V, Kalra H, Kim D, Kowal J, Kraemer S, Leidinger P, Leonelli C, Liang Y, Lippens L, Liu S, Lo Cicero A, Martin S, Mathivanan S, Mathiyalagan P, Matusek T, Milani G, Monguió-Tortajada M, Mus LM, Muth DC, Németh A, Nolte-’T Hoen ENM, O’Driscoll L, Palmulli R, Pfaffl MW, Primdal-Bengtson B, Romano E, Rousseau Q, Sahoo S, Sampaio N, Samuel M, Scicluna B, Soen B, Steels A, Swinnen JV, Takatalo M, Thaminy S, Théry C, Tulkens J, Van Audenhove I, Van Der Grein S, Van Goethem A, Van Herwijnen MJ, Van Niel G, Van Roy N, Van Vliet AR, Vandamme N, Vanhauwaert S, Vergauwen G, Verweij F, Wallaert A, Wauben M, Witwer KW, Zonneveld MI, De Wever O, Vandesompele J, Hendrix A, EV-TRACK: Transparent reporting and centralizing knowledge in extracellular vesicle research, Nat. Methods 14 (2017) 228–232. doi: 10.1038/nmeth.4185. [DOI] [PubMed] [Google Scholar]
- [12].Medeiros LTL, Peraçoli JC, Bannwart-Castro CF, Romão M, Weel IC, Golim MA, de Oliveira LG, Kurokawa CS, Medeiros Borges VT, Peraçoli MTS, Monocytes from Pregnant Women with Pre-Eclampsia are Polarized to a M1 Phenotype, Am. J. Reprod. Immunol 72 (2014) 5–13. doi: 10.1111/aji.12222. [DOI] [PubMed] [Google Scholar]
- [13].Reister F, Frank H-G, Heyl W, Kosanke G, Huppertz B, Schröder W, Kaufmann P, Rath W, The Distribution of Macrophages in Spiral Arteries of the Placental Bed in Pre-eclampsia Differs from that in Healthy Patients, Placenta. 20 (1999) 229–233. doi: 10.1053/plac.1998.0373. [DOI] [PubMed] [Google Scholar]
- [14].Schonkeren D, Van Der Hoorn ML, Khedoe P, Swings G, Van Beelen E, Claas F, Van Kooten C, De Heer E, Scherjon S, Differential distribution and phenotype of decidual macrophages in preeclamptic versus control pregnancies, Am. J. Pathol 178 (2011) 709–717. doi: 10.1016/j.ajpath.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].M.M. Keelan JA, Placental cytokines and preeclampsia., Front Biosci. 12 (2007) 2706–2727. doi: 10.2741/2266. [DOI] [PubMed] [Google Scholar]