Abstract
Understanding the foreign body response (FBR) and desiging strategies to modulate such a response represent a grand challenge for implant devices and biomaterials. Here, the development of a microfluidic platform is reported, i.e., the FBR-on-a-chip (FBROC) for modeling the cascade of events during immune cell response to implants. The platform models the native implant microenvironment where the implants are interfaced directly with surrounding tissues, as well as vasculature with circulating immune cells. The study demonstrates that the release of cytokines such as monocyte chemoattractant protein 1 (MCP-1) from the extracellular matrix (ECM)-like hydrogels in the bottom tissue chamber induces trans-endothelial migration of circulating monocytes in the vascular channel toward the hydrogels, thus mimicking implant-induced inflammation. Data using patient-derived peripheral blood mononuclear cells further reveal interpatient differences in FBR, highlighting the potential of this platform for monitoring FBR in a personalized manner. The prototype FBROC platform provides an enabling strategy to interrogate FBR on various implants, including biomaterials and engineered tissue constructs, in a physiologically relevant and individual-specific manner.
Keywords: biomaterials, foreign body responses, immune responses, implants, organs-on-a-chip
Implantable devices and biomaterials have emerged as critical solutions for various healthcare problems and their use in either therapeutics or for preventive healthcare is well established.[1–6] However, adverse immune reactions against these non-self implants in the host body is often a major barrier to their success.[7–12] These adverse responses can produce dramatic negative outcomes such as excessive inflammation leading to severe tissue damage.[13,14] Chronic inflammation on the other hand, can be detrimental for the long-term functionality of the implants eventually leading to their failure.[7,9,10,13]
These responses are orchestrated by different components of the immune system and in particular macrophages, have been shown to play a pivotal role in the cascade of immunological responses towards implants.[15–19] Tissue-residing macrophages and recruited immune cells (particularly neutrophils and monocytes) from the circulation are amongst the first cells that react to tissue injury as well as to the introduced foreign body including different types of implants.[15,16,20] One of the persistent problems around the implants, especially those that are non-degradable or slowly degradable (e.g., polymeric/metallic implants), is the inability of the macrophages to resolve inflammation, provoking their tendency to maintain in the “frustrated phagocytosis” state.[21] During the beginning phase of inflammatory reactions to an injury, there is an increase of pro-inflammatory macrophages on the site, whereas a tolerogenic macrophage phenotype increases after this stage and it induces the forthcoming healing stage and allows the resolution of inflammation.[17,18,22] Under certain conditions such as existence of a “foreign body” or some pathologies, the host immune system fails to enhance regulatory/healing macrophage levels and switch to the pro-healing stage, which ultimately leads to persistent adverse immune reactions such as the aforementioned chronic inflammation, tissue damage, fibrotic capsule formation, and dysfunction of the implant.[17]
Development of strategies to properly design the implants and solutions to avoid or alleviate undesired foreign body response (FBR) represents one of the most critical challenges in implantable medical device field. Ideally, these characterizations should be completed at an early stage of product development and pre-operative material optimizations.[23] Nevertheless, there is currently no reliable approach to determine the adverse immune responses of the human body to foreign implants prior to their in vivo applications. Conventional cultures of immune cells directly with these implants[24–26] do not capture the dynamic process of the FBR. Various microfluidic platforms have been recently adapted to study immunological events such as inflammation and immunotherapy,[27–30] but they have rarely been used in screening the FBR to biomaterials and implants. Rodent models, including the one recently shown to recapitulate key aspects of human FBR,[19] are expensive and low-throughput.[31] None of the existing approaches allow for personalized screening of the FBR to implants. Indeed, literature[32,33] suggests a significant level of inter-individual variation that is driven by individual’s immunological profile. Therefore, there is a strong need for personalized assessment of FBR at low cost and in a higher-throughput/rapid manner to select the most suitable implant material with optimal parameters, for a given patient.
Here, we report the development of a physiologically relevant and microscopy-friendly in vitro microfluidic platform, the FBR-on-a-chip (FBROC), to reproduce the dynamic effects of circulating immune cells on the implant occurring in FBR. The device consisted of a bottom tissue chamber where the implant was introduced, an endothelium to model the vasculature barrier function, and a top vascular channel to populate with circulating monocytes. The monocyte-endothelium interaction, their trans-endothelial migration, and activation against titanium (Ti) microparticles were investigated under a set of different parameters including variations on flow, endothelium, chemoattractant, and Ti implants. Proof-of-concept experiments using human donor-derived monocytes were further performed to reveal inter-individual differences of FBR towards Ti microparticles in our FBROC platform.
Organ-on-a-chip platforms have been widely used to model the dynamic processes involved in the human system in vitro. The combination of biomimetic cell/extracellular matrix (ECM) arrangements with microfluidic devices can be used reproduce not only the tissue microarchitecture but also the physicochemical cues under physiologically relevant conditions.[34–38] As such, the organ-on-a-chip systems are demonstrated to be superior to conventional planar, static cell culture strategies, providing improved accuracy in predicting human responses to pharmaceutical compounds, chemicals/toxins, and biological species.[27,39–42]
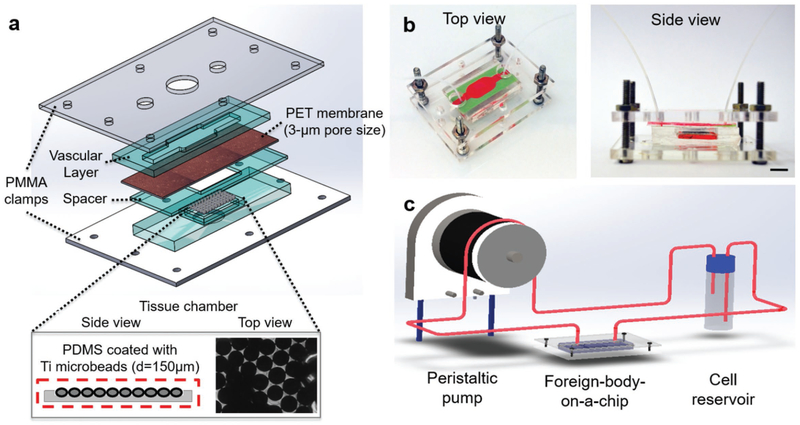
In this study, we have adapted this concept to study FBR in a physiologically relevant in vitro setting, through the development of a multilayered FBROC system to reproduce the innate immune cell interactions with implants (Figure 1a). The poly-dimethylsiloxane (PDMS)-based FBROC system consisted of a bottom tissue chamber where Ti microbeads were implanted as the foreign body. The bottom tissue chamber possessing Ti-beads was surrounded by a ring-channel containing GelMA hydrogel with or without monocyte chemoattractant protein-1 (MCP-1). The top chamber possessing an inlet and an outlet mimicked the vascular space, where immune cells (THP-1 monocytes) were allowed to circulate using a peristaltic pump. In between these two layers, a porous polyethylene terephthalate (PET) membrane was sandwiched, on top of which a monolayer of human umbilical vein endothelial cells (HUVECs) was populated to model the endothelial barrier between the vascular lumen and the surrounding tissue. In addition, a ring-shaped PDMS spacer having the same interior window size of the underlying tissue chamber was placed below the PET membrane to provide a desired space between the endothelial layer and the bottom tissue chamber. The layers were stacked together and clamped with a pair of transparent poly(methyl methacrylate) (PMMA) plates with screw/bolt sets to ensure hydraulic tightness, similar to the setups previously reported by us and others.[43,44] Figure 1a represents the schematics of different layers of the FBROC device, whereas Figure 1b demonstrates the top and side views of the actual device.
Figure 1.
Design of the FBROC device. a) Exploded schematic diagram showing the multilayer structure of the bioreactor, where an endothelialized porous membrane is sandwiched in between a vascular channel on top and a tissue chamber at the bottom, the latter of which implant of Ti microbeads was placed. b) Perspective- and side-view photographs showing the bioreactor in the multilayer configuration. c) Schematic diagram showing the operation of the FBROC device, where immune cells are circulated from the top vascular channel of the bioreactor to probe their interactions with the Ti microbeads in the bottom tissue chamber through the endothalial barrier.
In a typical experimental setting, the FBROC is connected to a reservoir hosting human monocytes, where the fluid flow is driven by a peristaltic pump allowing continuous circulation of the monocytes through the top vascular chamber (Figure 1c). The entire setup can be hosted in a regular cell culture incubator to ensure sufficient gas exchanges. The specific dimensions of the vascular chamber are indicated in Figure S1a in the Supporting Information. Optimized flow conditions and monocyte distribution inside the top vascular chamber of the bioreactor were obtained using computational fluid dynamics (CFD) simulation by COMSOL Multiphysics 3.5a (Comsol Inc., Burlington, MA, USA). Generally, fluid flow inside the microchannels is considered laminar due to the small channel dimensions and low fluid velocity.[34] Hence, the governing equations for obtaining flow conditions are continuity and momentum equations, i.e., Equations (1) and (2), respectively:
| (1) |
| (2) |
where is velocity vector and ρ, P, and ν are fluid density, pressure, and kinematic viscosity, respectively.[45] To obtain velocity distribution, Equations (1) and (2) are solved simultaneously via the finite-element method (FEM). Roswell Park Memorial Institute medium (RPMI) used as the cell culture medium is a homogenous, Newtonian, and non-compressible fluid.[46] Physical properties of the fluid were considered to be the same as those of water at 37 °C.[46]
Monocyte distribution was obtained by calculating trajectory of the THP-1 cells suspended throughout the culture medium by considering equation of motion for each set of particles acquired from Newton’s second law, hence:
| (3) |
where mP is monocyte’s mass and is total force experienced by them. The total force exerted on the monocytes was composed of three components: drag force, Brownian, and gravity. Therefore, the total force in the equation of motion can be written as:
| (4) |
where up denotes particle velocity, FD is drag force, g is the gravity acceleration, and ρp is the monocytes density. Drag force is obtained as:
| (5) |
where μ indicates fluid viscosity, urel = ufluid − up, and dp is monocyte diameter. For obtaining monocyte distribution, it was assumed that the impact of cell motion on the fluid flow was negligible.[47] Therefore, first-velocity field was estimated and hence, monocyte trajectories were computed.
The optimal inlet velocity was obtained by taking into consideration of factors including shear stress imposed on both monocytes and HUVECs, velocity profile inside the channel, distribution of the circulating monocytes, and duration for the monocytes to interact with and pass through the endothelial barrier. Average shear stress sensed by the vascular endothelial cells in the human body is between 5–10 dyne cm−2.[48] It is widely acknowledged that hemodynamic forces have a great impact on endothelial cells and are critical for normal vessel wall functionalities. For example, shear stress changes the shape and orientation of endothelial cells in culture; cells might not align in the direction of the flow under low-shear stress conditions, while elevated shear stress is detrimental to the cells.[49] On the other hand, shear stress also affects the monocyte-endothelium interactions. Monocyte rolling and arrest to the endothelium are influenced by both force and contact time applied to them,[46,50] where shear rate of 400 s−1 (translating to ≈3.6 dyne cm−2 in biological medium with a viscosity of 8.9 × 10−4 Pa·s, see Table 1) is considered as a threshold for monocyte adhesion.[49]
Table 1.
Parameters and constants used for modeling.
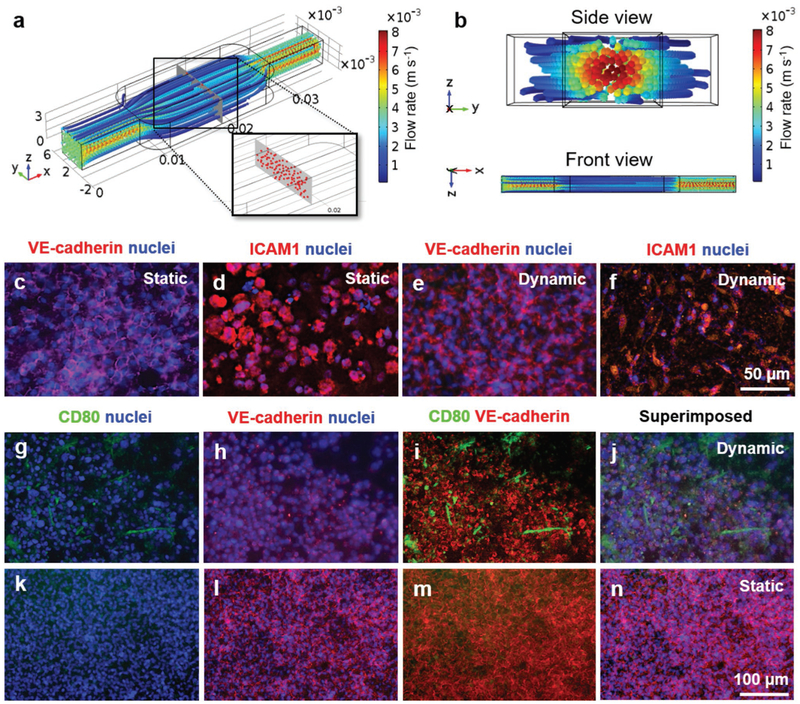
Streamline distribution of the medium flow inside the vascular channel is shown in Figure S1b in the Supporting Information, suggesting that the flow was largely laminar. Simulations of monocyte distribution inside the entire vascular chamber with a density of 1 × 106 cells mL−1 at the optimized flow rate of 400 μL min−1, in perspective and cross-sectional/front views, are depicted in Figure 2a,b, respectively, indicating that the cells could be uniformly distributed across the chamber emulating their homogeneous distributing profiles in circulation in blood vessels in the human system.
Figure 2.
Characterization of monocyte distribution, vascular barrier, and monocyte-endothelium interactions. a,b) Simulated distributions of the circulating immune cells in the top vascular channel of the bioreactor. c–f) Immunostaining of VE-cadherin and ICAM for confluent HUVECs cultured under (c,d) static and (e,f) dynamic conditions on the porous PET membrane. g–n) THP-1 monocyte interactions with the confluent endothelium under static and dynamic conditions.
To create the functional endothelial barrier between the vascular chamber and the tissue chamber, HUVECs were seed on the surface of the PET membrane (3 μm in pore size) at a density of 7.5 × 104 cells cm−2. The formation of a continuous monolayer of HUVECs was observed after 72 h under static culture conditions. Similarly, when the HUVECs were cultured under dynamic condition at the flow rate of 400 μL min−1 for the same period, the continuous monolayer of HUVECs was formed, consistent with our previous observations[43] and literature.[51] The phenotype of the endothelial monolayer on the PET porous membrane was further characterized by immunostaining of HUVECs with endothelial biomarkers such as vascular endothelial-cadherin (VE-cadherin) and intercellular adhesion molecule 1 (ICAM1). VE-cadherin[52] and ICAM1[53] are endothelial-specific adhesion molecules that are consistently expressed on the membranes of and at junctions between endothelial cells. Fluorescence microscopy images of the immunostained endothelial monolayer formed on the PET porous membrane exhibited strong expression of VE-cadherin and ICAM1 under both static (Figure 2c,d, respectively) and dynamic (Figure 2e,f, respectively) conditions, indicating that the flow rate used did not have adverse effects on the function of the endothelial cells.
The endothelialized porous PET membrane was subsequently integrated into the FBROC platform to evaluate the monocyte-endothelium interactions. Human THP-1 monocytes were suspended in a 50:50 monocyte culture medium (RPMI 1640) and endothelial growth medium (EGM-1). We compared the expressions of CD80, a class of co-stimulatory receptors, on the monocytes under static and dynamic conditions. It has been shown that the freshly isolated monocytes do not express CD80 and CD80 expression is enhanced by stimulation in in vitro cultures[54] Immunostaining of THP-1 monocytes with CD80 antibody and fluorescence microscopy analyses performed after 4 d of dynamic culture revealed the expression of CD80 (Figure 2g–j), suggesting the activation and interaction of THP-1 monocytes with the HUVECs through attachment and spreading on the endothelial barrier under fluid flow, possibly induced by shear stress and the rolling effect.[55–57] On the contrary, under static culture condition (i.e., no fluid flow) minimum amount of expression of CD80 by THP-1 monocytes was observed on the endothelial barrier indicating that the TPH-1 cells became barely activated with limited interactions of these cells with the endothelium (Figure 2k–n). These results demonstrated the importance of creating a dynamic in vivo mimetic condition, since dynamic flow seems to be critical in reproducing the interactions between immune cells and endothelium, as widely reported.[55–57]
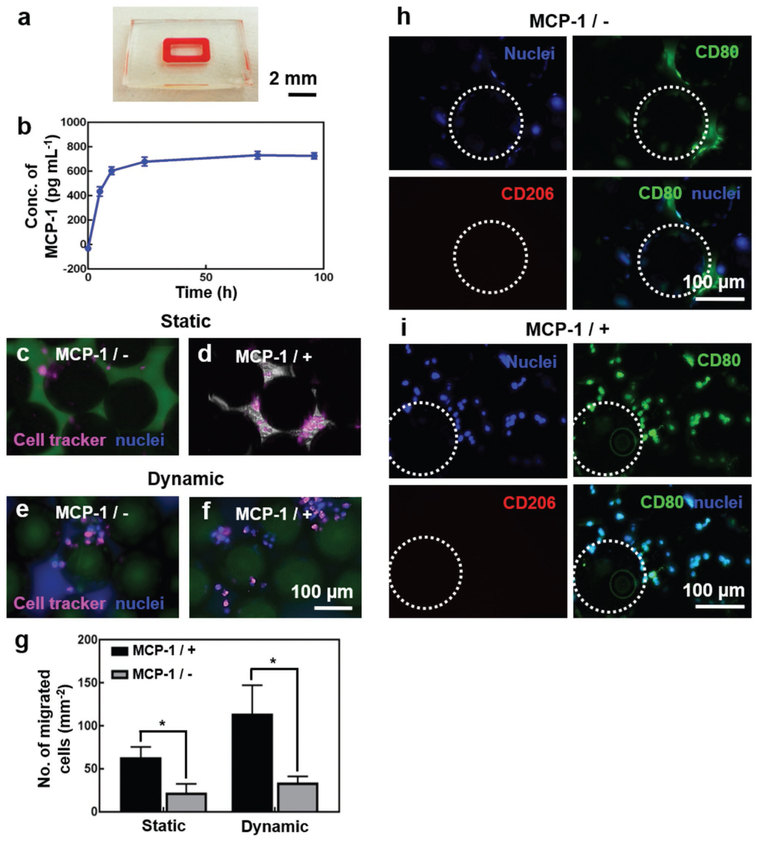
We next analyzed the migration of THP-1 monocytes through the endothelial barrier. Specifically, we compared the monocyte migration under static and dynamic conditions, as well as in the absence and presence of MCP-1. MCP-1 is a key chemokine regulating the migration/infiltration of immune cells such as monocytes and macrophages, and is released by the stromal tissues upon implantation of foreign materials during FBR.[58] To mimic such a process, we encapsulated MCP-1 in a ring of gelatin methacryloyl (GelMA) hydrogel at the bottom chamber of the device, surrounding the tissue chamber coated with Ti microbeads (Figure 3a). GelMA hydrogel is a porous and biocompatible material that has been used extensively in tissue engineering because it closely resembles some of the essential properties of the native ECM including inherent bioactivity and broadly tunable physicochemical behaviors.[59,60] The release of MCP-1 from the GelMA ring was quantified by collecting the medium outflow from the top vascular chamber under the same perfusion condition in the presence of the endothelial barrier, which showed a sustained release profile over the 4-d period with an initial burst release in the first day (Figure 3b).
Figure 3.
FBR of THP-1 monocytes to the Ti microbeads. a) Photograph showing the GelMA hydrogel ring in the bottom tissue chamber for MCP-1 release. b) MCP-1 release over a 96-h period. c–f) THP-1 monocyte trans-endothelial migration towards the bottom Ti microbeads under (c,d) static and (e,f) dynamic conditions, in the (c,e) absence and (d,f) presence of MCP-1. The cells were pre-labeled with cell tracker (pink) and post-labeled for nuclei (blue). g) Quantifications of the number of THP-1 monocyte migration. h,i) CD80 (green)/CD206 (red) expressions of activated THP-1 monocytes on the Ti microbeads, in the h) absence and i) presence of MCP-1, under dynamic conditions. The nuclei were counterstained in blue. The white dotted circles indicate the Ti microbeads. *p< 0.05.
When THP-1 monocytes pre-labeled with cell tracker red were cultured on the vascular channel on the top of the PET membrane with HUVECs monolayer under the static condition and in the absence of MCP-1, only very few cells could transmigrate through the endothelium to reach Ti microbeads in the bottom chamber at day 4 (Figure 3c). Whereas, increased number of THP-1 monocytes were found to have transmigrated through the endothelium when MCP-1 was slowly released from the GelMA ring for the same period of time (Figure 3d). However, under the dynamic flow condition, the transendothelial migration of THP-1 monocytes was significantly higher both in the presence and absence of MCP-1 when compared to those under static conditions (Figure 3e,f). The quantitative analyses of migration of THP-1 monocytes through the endothelial barrier under different conditions have been shown in Figure 3g. These results were consistent with our monocyte activation results (Figure 2g–n), where flow was found to be an important parameter.
The differentiation of THP-1 monocytes into M1 or M2 phenotype was subsequently evaluated by immunostaining of the cells with CD80 or CD206 antibodies, respectively, after they have migrated through the endothelial barrier and attached onto the Ti microbeads, under dynamic flow condition at day 4. CD80 is M1-specific phenotype marker and thus overexpressed in pro-inflammatory (M1) subtype cell population, whereas CD206 is M2 phenotype marker whose expression is increased as the cells differentiate into anti-inflammatory alternatively activated (M2) subtype cell population.[61,62] Immunofluorescence studies revealed that the increased number of transendothelial-migrated THP-1 cells were differentiated into CD80-positive cells, primarily located in the tissue chamber containing Ti microbeads, both in the absence (Figure 3h) and presence (Figure 3i) of MCP-1, indicating that the differentiation of THP-1 was almost entirely towards the pro-inflammatory M1 phenotype and thus confirming the recognition of the Ti microbeads as foreign body by the THP-1 cells. As expected, the number of monocytes migrated across the endothelial barrier and attached to the Ti microbeads was higher when MCP-1 was released. Cytokine quantification at day 4 under dynamic culture in the presence of MCP-1 further revealed the overwhelming secretion of the pro-inflammatory cytokine interleukin (IL)-6 reaching a value of 403 ± 46.5 pg mL−1, where its baseline concentration in standard THP-1 monocyte culture has been well shown to be minimum.[63] This observation was consistent with the immunostaining results and the fact that IL-6 is a cytokine that is linked to pro-inflammatory responses by monocytes.[64]
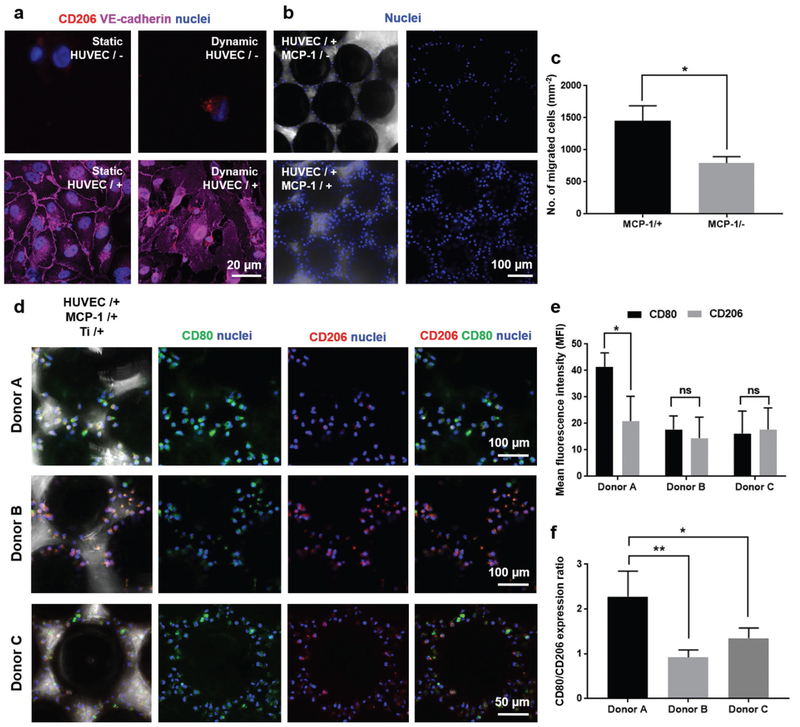
We finally extended our FBROC platform for studying human primary immune cell responses to implants. Instead of the human THP-1 monocyte cell line, we isolated primary human monocytes from human donor-derived peripheral blood mononuclear cells (PBMCs), and perfused these cells in the top vascular chamber of the FBROC device, containing the same Ti microbeads in the bottom tissue chamber. The presence of the endothelium and the dynamic flow seemed to be essential in activating the interactions of the human primary monocytes with the barrier as shown by CD206 staining on monocytes and VE-cadherin staining on HUVECs (Figure 4a). Similar to results obtained with THP-1 cell line, the release of MCP-1 from the bottom tissue chamber had a positive effect on inducing trans-endothelial migration of the human primary monocytes, as shown in both nuclei staining (Figure 4b) and quantification analyses (Figure 4c).
Figure 4.
Donor-specific FBR of patient PBMC-derived monocytes to the Ti microbeads. a) Monocyte/PET membrane interactions in the absence or presence of HUVECs under static or dynamic conditions. b) Trans-enothelial migration of monocytes onto Ti microbeads in the bottom tissue chamber in the absence or presence of MCP-1 under dynamic conditions. c) Quantification of trans-enothelial migration of monocytes onto Ti microbeads in the bottom tissue chamber in the absence or presence of MCP-1. d) CD206/CD80 expressions of activated monocytes from three different human donors on Ti microbeads in the presence of MCP-1 under dynamic conditions. e) Quantifications of CD206 and CD80 expressions of activated monocytes on the Ti microbeads in the presence of MCP-1 under dynamic conditions for monocytes derived from three different human donors. f) Quantifications of CD206/CD80 expression ratios of activated monocytes on the Ti microbeads in the presence of MCP-1 under dynamic conditions for monocytes derived from three different human donors. *p < 0.05 and **p < 0.01.
Similarly, the M1/M2 differentiation of the human primary monocytes was also analyzed and it was found that, for the specific donor shown in Figure S2 in the Supporting Information, in either absence or presence of Ti microbeads in the bottom tissue chamber of the bioreactor, the primary monocytes expressed both M1- and M2-associated surface markers approximately in equal ratio, with most cells exhibiting double stains. The differences of M1/M2 differentiation in response to the Ti microbeads between the human primary monocytes and the THP-1 monocytes could possibly be explained by the fact that, in the cell line, the expression of each marker is induced in response to different environmental stimuli, while in the primary cells, these markers are constitutively expressed and the intensities of expressions change when responding to different stimuli.[64–68] Indeed, when we examined the FBR of monocytes derived from three different donors, it was found that, interestingly, different individuals exhibited varying degrees of immune responses to the same Ti microbeads (Figure 4d). While two donors (Donors B and C) did not exhibit noticeable difference in M1/M2 differentiation of their PBMC-derived monocytes, one donor (Donar A) had strong pro-inflammatory reaction to the Ti microbeads (Figure 4e,f), clearly indicating that there is likely a “population spectrum” of responses to the same implant possibly due to differences in receptor expressions and cytokine profiles of the immune cells.[32,33] It should be noted that, this dataset achieved with human primary monocytes from healthy donors was different from that observed when human THP-1 monocyte cell line was used (which always showed pro-inflammatory phenotype). This fact further confirmed that the FBR is closely dependent on the specific populations of immune cells that confer variations in their reactions to foreign implants, which can be effectively tested on our FBROC platform.
In summary, we have reported the design and fabrication of multilayered FBROC system to mimic the immune cell-foreign body interactions in vitro using THP-1 monocytes circulating through the topmost vascular channel, separated from the bottom tissue chamber, containing Ti microbeads as the foreign body material, by a PET porous membrane coated with a monolayer of HUVECs. The addition of MCP-1 in the GelMA hydrogel, that functions as ECM surrounding the Ti microbeads, stimulated the monocyte-endothelial cell interactions and transendothelial migration of THP-1 cells as demonstrated by the immunostaining of cell-specific biomarkers. Similarly, we have observed that most of the transmigrated THP-1 cells differentiated into the pro-inflammatory M1 phenotype, thus confirming the recognition of the Ti microbeads as foreign body by the cells. In contrast to the THP-1 cell line, differentiation of primary human monocytes into M1 and M2 phenotypes vary from one donor to other, thus indicating the importance of personalized FBROC system to study the inter-patient difference in FBR. Once the FBR is screened on the FBROC platform, methods of immunomodulation[64,69–71] may then be accordingly personalized to mitigate the negative immune responses of the host towards the foreign body. Thus, we believe that the FBROC system has a potential to expand the studies of personalized FBR on vast categories of subjects including but not limited to, implants reported in the current work as well as biologically active materials and engineered tissues.
Experimental Section
Materials:
Sylgard 184 Silicone Elastomer kit was purchased from Dow Corning Corporation (Midland, MI, USA) and PMMA sheets were obtained from McMaster-Carr (Elmhurst, IL, USA) and Goodfellow (Coraopolis, PA, USA). Gelatin from porcine skin (type-A, 300 bloom), methacrylic anhydride, 2-hydroxy-4′-(2-hydroxyethoxy)-2-methylpropiophenone (photoinitiator, PI, Irgacure 2959), Triton X-100, and bovine serum albumin (BSA) were purchased from Sigma–Aldrich (St. Louis, MO, USA). Dulbecco’s phosphate-buffered saline (DPBS), fetal bovine serum (FBS), trypsin-ethylenediaminetetraacetic acid (trypsin-EDTA), penicillin/streptomycin, 4′,6-diamidino-2-phenylindole (DAPI), Live/Dead Viability Kit, PrestoBlue Cell Viability Reagent, RPMI 1640 medium, and PET cell culture inserts were purchased from ThermoFisher Scientific (Waltham, MA, USA), whereas EGM-2 was obtained from Lonza (Walkersville, MD, USA). Medical-grade (Grade 2, Neyco) Ti microbeads were supplied by PROTiP Medical (Strasbourg, France). Primary antibodies against human VE-cadherin, ICAM-1, CD80, and CD206, as well as Alexa Fluor 594- and Alexa Fluor 555-conjugated secondary antibodies were purchased from Abcam (Cambridge, MA, USA). Mouse anti-human CD31 primary antibody was purchased from Dako (Santa Clara, CA, USA). Recombinant human CCL2/JE/MCP-1 and all enzyme-linked immunosorbent assay (ELISA) kits were obtained from R&D Systems (Minneapolis, MN, USA). All other chemicals used in this study were obtained from Sigma-Aldrich unless otherwise noted.
Synthesis of GelMA:
GelMA was synthesized according to the previously published protocol,[72–74] at a high degree of methacryloyl substitution (81.4 ± 0.4%). Briefly, 10 g of type A gelatin from porcine skin was dissolved in 100 mL of DPBS at 60 °C using magnetic stirrer and 8.0 mL of methacrylic anhydride was added drop wisely to the gelatin solution under continuous stirring condition. The reaction was carried out for 3 h at 50 °C and then quenched by a fivefold dilution of the reaction mixture with warm DPBS (40 °C). The product obtained was dialyzed against distilled water at 40 °C for 7 d using 12–14 kDa cut-off dialysis tubing to remove unreacted methacrylic anhydride. GelMA solution was finally lyophilized and stored at room temperature until further use.
Cell Culture:
HUVECs purchased from Angio-Proteomie (Boston, MA, USA) were cultured in EGM-2 while human monocytes (THP-1) obtained from ATCC (Manassas, VA, USA) were cultured in RPMI 1640 medium supplemented with 10% (v/v) FBS and 1% (v/v) penicillin-streptomycin. The cell cultures were maintained at 37 °C and 5% CO2 in a standard incubator. The medium was replaced every 2–3 d and the cells cultured in flasks were subcultured when they reached approximately 80% confluency. For on-chip experiments, a 1:1 volume mixture of the two media was used as the common medium, where no adverse effects were observed on either cell type.
Fabrication of the FBROC Device:
The FBROC device consisting of four layers was designed and fabricated as shown in Figure 1. Each layer of the chip was designed using CorelDraw (Corel Corp, Ottawa, ON, Canada) and imported to a laser cutting system (VLS 2.30 Desktop Laser, Universal Laser Systems Inc, Richmond, VA, USA) for cutting PMMA sheets (3 or 1.6 mm in thickness) into desired sizes and patterns to serve as the molds. PDMS precursor prepared by mixing the monomer and curing agent at the ratio of 10:1, was then poured onto the PMMA molds, cured at 85 °C for 2 h, and peeled off. The top layer consisted the vascular channel for circulating immune cells and the bottom layer consisted the tissue chamber coated with titanium beads (d = 150 μm). In between these two layers, a porous PET membrane of 3 μm in pore size was sandwiched, on top of which a monolayer of endothelial cells was populated to model the endothelial barrier between the vascular lumen and the surrounding tissue. In addition, a ring-shaped PDMS spacer having the same internal window size of the underlying tissue chamber was placed below the PET membrane. All these layers were held together tightly using a pair of PMMA (3 mm in thickness) clamped with sets of screws and bolts. The microfluidic chamber on top, composed of an inlet and an outlet, was connected to a peristaltic pump using turbo tubings thus allowing the circulation of THP-1 cells.
Fluid Dynamics Modeling and Simulation:
The flow velocity and shear stress profiles in the FBROC device were investigated with 3D CFD simulation using FEM implemented in COMSOL Multiphysics 3.5a. Equations of continuity (1) and momentum (2) were solved simultaneously to obtain fluid velocity distribution. Monocyte distribution was obtained by considering equation of motions and calculating three forces (i.e., drag, Brownian, and gravity) imposed on the cells via the Lagrangian method. Constants used for the simulation are provided in Table 1.
Morphological Observations:
To observe the morphology of HUVECs in the FBROC device, after 4 d of dynamic experiment, the device was disassembled and the HUVECs on the PET membrane were fixed with 4% (v/v) paraformaldehyde for 20 min. The cells were permeabilized with 0.1% (v/v) Triton X-100 in DPBS for 30 min and then blocked with 1%(w/v) BSA in DPBS, followed by F-actin staining by incubating the cells with Alexa Fluor 594-phalloidin (1:40 dilution in 0.1% (w/v) BSA) for 1 h at room temperature. After washing with DPBS, nuclei were counter-stained with DAPI for 5 min at room temperature. Finally, the cells were observed using AxioObserver D1 inverted fluorescence microscope (Zeiss, Thornwood, NY, USA).
Attraction of Circulating Monocytes:
MCP-1 was used as a chemoattractant to attract monocytes towards the bottom tissue chamber containing the Ti microbeads, simulating the cytokine released by the local tissue in response to the foreign body material. In both static and dynamic experiments, 50 ng mL−1 of MCP-1 was encapsulated in the 5 w/v% GelMA hydrogel containing 0.5% w/v PI. The GelMA hydrogel with MCP-1 was poured into ring-shaped channel surrounding the Ti microbeads in the bottom tissue chamber and then crosslinked by exposing under UV light (800 mW cm−2) for 30 s.
Transendothelial Migration of Monocytes through HUVEC Monolayers under Flow:
Migration of monocytes through the endothelium was monitored by labeling with cell tracker (CM Dil dye, Thermo Fisher). The cells suspended in the medium was first harvested by centrifuging at 200 g for 5 min. Harvested cells were mixed with CM Dil dye solution at the concentration of 1 μL mL−1 in DPBS and incubated at 37 °C for 5 min and then at 4 °C for 15 min. The monocytes were then suspended in the medium after washing with DPBS for further use. During dynamic experiments, these CM Dil-stained monocytes were allowed to circulate continuously through the upper vascular channel within the FBROC device. The THP-1 cells transmigrated across the endothelium and accumulated in the tissue chamber were observed using the fluorescence microscope.
Human Primary Monocytes:
Buffy coats form healthy donors were obtained from the National Blood Service (National Blood Service, Sheffield, UK) following ethics committee approval (2009/D055). PBMCs were isolated by Histopaque-1077 (Sigma–Aldrich) density gradient centrifugation. Monocytes were isolated from PBMCs by positive selection of CD14+ cells using the MACS magnetic cell separation system (Miltenyi Biotec) as described before.[77,78] This method routinely yielded > 95% pure monocytes as determined by flow cytometric analysis of CD14 expression. All methods were performed in accordance with the relevant guidelines and regulations. It was likely that the phenotypes of the primary monocytes were different from those freshly isolated due to cell manipulation during the multistep isolation procedure, which however, would be consistent among the cells from each donor and should not affect the results.
ELISA:
All reagents were brought to the room temperature before starting the assay. A 200-μL volume of standard or sample was added to each monoclonal MCP-1-specific antibody-precoated 96 microplate well and incubated for 2 h at room temperature. Then each well was aspirated and washed three times with washing buffer. Next, a 200-μL volume of MCP-1 conjugate (polyclonal antibody specific for human MCP-1 conjugate) was added to each well and incubated 1 h at room temperature, which was then followed by three washes. Stabilized hydrogen peroxide and stabilized chromogen (tetramethylbenzidine) were mixed within 15 min of use in equal volumes to reconstitute the substrate solution. A 200-μL volume of this solution was added to each well and incubated for 30 min at room temperature protected from light. Finally, 50 μL of 2N sulfuric acid as the stop solution was added to each well. The optical density of each well was determined by a microplate reader (BioTek, VT, US) at 450 nm with 540 nm as wavelength correction. Samples were run in triplicates unless otherwise stated. ELISA against IL-6 was performed in the same manner.
Immunocytochemical Analyses:
To demonstrate the functions of HUVECs, the cells on the PET membrane were immunostained for VE-cadherin, CD31, and ICAM-1. At designated time points, HUVECs on the PET membrane were washed with DPBS and fixed with 4% (w/v) paraformaldehyde for 20 min, followed by incubation with permealization buffer (0.1% (v/v) Triton X-100 in DPBS) for 30 min at room temperature. The cells were blocked with 1% (w/v) BSA in DPBS for 1 h at room temperature and incubated overnight with the primary antibody (1:200 dilution) at 4 °C. After washing with DPBS, the samples were incubated with the secondary antibody at 1:200 dilution (Alexa Fluor 555-conjugated goat anti-rabbit for VE-cadherin and Alexa Fluor 594-conjugated goat anti-mouse for CD31 or ICAM-1) for 1 h at room temperature. After washing with DPBS, nuclei were counter-stained with DAPI and then examined under fluorescence microscope. Similarly, the differentiation of THP-1 monocytes or human primary monocytes into M1 or M2 phenotypes was evaluated by immunostaining of the cells with CD80 (1:80 dilution) and CD206 (1:100 dilution) antibodies. For quantifying the expression ratios of CD80/CD206 on primary monocytes, mean fluorescence intensities of the respective channels were calculated using ImageJ.
Statistical Analysis:
Sample sizes were three in all cases. Data were presented as mean ± standard deviations. Statistical analysis was performed using unpaired t-tests. The statistical significance was determined with p < 0.05 and < 0.01.
Supplementary Material
Acknowledgements
F.S., M.R., H.L., and S.S.H. contributed equally to this work. The authors acknowledge funding from the National Institutes of Health (AR057837, DE021468, AR068258, AR066193, and EB022403). Y.S.Z. acknowledges funds from the National Institutes of Health (CA201603, EB026175, and EB025270) and the New England Anti-Vivisection Society (NEAVS). This project further received funding from the European Union’s FP7 and Horizon 2020 research and innovation programme under grant agreement no. 602694 (IMMODGEL) and no. 760921 (PANBioRA). S.S.R. acknowledges funding from the National Institutes of Health (EB026824 and AR074234). S.M. acknowledges NEAVS and the American Fund for Alternatives to Animal Research (AFAAR) for Postdoctoral Fellowship. The affiliation of Ege University was added for O.Y.-C. after initial online publication on January 29, 2019.
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Conflict of Interest
The authors declare no conflict of interest.
Contributor Information
Fatemeh Sharifi, Division of Engineering in Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Cambridge, MA 02139, USA; School of Mechanical Engineering, Sharif University of Technology, Tehran 11155-8639, Iran.
Dr. Su Su Htwe, Immunology and Immuno-bioengineering Group, School of Life Science, Faculty of Medicine and Health Sciences, University of Nottingham, Nottingham NG7 2RD, UK
Dr. Martina Righi, Division of Engineering in Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Cambridge, MA 02139, USA; The BioRobotics Institute, Sant’Anna School of Advanced Studies, Piaggio 56025, Italy
Prof. Hua Liu, Division of Engineering in Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Cambridge, MA 02139, USA; Dr. Li Dak Sum & Yip Yio Chin Center for Stem Cells, and Regenerative Medicine, Zhejiang University School of Medicine, Hangzhou 310012, P. R. China; Key Laboratory of Tissue Engineering and Regenerative, Medicine of Zhejiang Province, Zhejiang University School of Medicine, Hangzhou 310012, P. R. China
Anna Pietralunga, Division of Engineering in Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Cambridge, MA 02139, USA.
Prof. Ozlem Yesil-Celiktas, Division of Engineering in Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Cambridge, MA 02139, USA; Department of Bioengineering, Faculty of Engineering, Ege University, Bornova 35100, Izmir, Turkey
Dr. Sushila Maharjan, Division of Engineering in Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Cambridge, MA 02139, USA; Research Institute for Bioscience and Biotechnology, Lalitpur 44600, Nepal
Dr. Byung-Hyun Cha, Division of Engineering in Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Cambridge, MA 02139, USA
Dr. Su Ryon Shin, Division of Engineering in Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Cambridge, MA 02139, USA
Prof. Mehmet Remzi Dokmeci, Division of Engineering in Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Cambridge, MA 02139, USA; Center for Minimally Invasive Therapeutics (C-MIT), University of California-Los Angeles, Los Angeles, CA 90095, USA; Department of Radiology, David Geffen School of Medicine, University of California-Los Angeles, Los Angeles, CA 90095, USA; Department of Bioengineering, Department of Chemical and Biomolecular Engineering, Henry Samueli School of Engineering and Applied Sciences, University of California, Los Angeles, Los Angeles, CA 90095, USA; California NanoSystems Institute (CNSI), University of California, Los Angeles, Los Angeles, CA 90095, USA; Department of Bioindustrial Technologies, Konkuk University, Seoul 05029, Republic of Korea
Dr. Nihal Engin Vrana, Biomatériaux et Bioingénierie, Institut National de la Santé et de la Recherche Médicale (INSERM), 67085 Strasbourg, France; Protip Medical, 67000 Strasbourg, France; Fédération de Médecine Translationnelle de Strasbourg, Fédération des Matériaux et Nanoscience d’Alsace (FMNA), Faculté de Chirurgie Dentaire, Université de Strasbourg, 67000 Strasbourg, France
Prof. Amir M. Ghaemmaghami, Immunology and Immuno-bioengineering Group, School of Life Science, Faculty of Medicine and Health Sciences, University of Nottingham, Nottingham NG7 2RD, UK
Prof. Ali Khademhosseini, Division of Engineering in Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Cambridge, MA 02139, USA; Center for Minimally Invasive Therapeutics (C-MIT), University of California-Los Angeles, Los Angeles, CA 90095, USA; Department of Radiology, David Geffen School of Medicine, University of California-Los Angeles, Los Angeles, CA 90095, USA; Department of Bioengineering, Department of Chemical and Biomolecular Engineering, Henry Samueli School of Engineering and Applied Sciences, University of California, Los Angeles, Los Angeles, CA 90095, USA; California NanoSystems Institute (CNSI), University of California, Los Angeles, Los Angeles, CA 90095, USA; Department of Bioindustrial Technologies, Konkuk University, Seoul 05029, Republic of Korea
Prof. Yu Shrike Zhang, Division of Engineering in Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Cambridge, MA 02139, USA
References
- [1].Fukumitsu K, Yagi H, Soto-Gutierrez A, Transplant Proc. 2011, 43, 2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Langer R, Vacanti JP, Science 1993, 260, 920. [DOI] [PubMed] [Google Scholar]
- [3].Rafii S, Lyden D, Nat. Med 2003, 9, 702. [DOI] [PubMed] [Google Scholar]
- [4].Breitbach M, Bostani T, Roell W, Xia Y, Dewald O, Nygren JM, Fries JW, Tiemann K, Bohlen H, Hescheler J, Welz A, Bloch W, Jacobsen SE, Fleischmann BK, Blood 2007, 110, 1362. [DOI] [PubMed] [Google Scholar]
- [5].Nakamura A, Akahane M, Shigematsu H, Tadokoro M, Morita Y, Ohgushi H, Dohi Y, Imamura T, Tanaka Y, Bone 2010, 46, 418. [DOI] [PubMed] [Google Scholar]
- [6].Fernández-Ruiz M, Kumar D, Humar A, Clin. Transl. Immunol 2014, 3, e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Badylak SF, Gilbert TW, Semin Immunol. 2008, 20, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Martins PN, Pratschke J, Pascher A, Fritsche L, Frei U, Neuhaus P, Tullius SG, Transplantation 2005, 79, 127. [DOI] [PubMed] [Google Scholar]
- [9].Anderson JM, Problems in General Surgery 1994, 11, 147. [Google Scholar]
- [10].Anderson JM, Rodriguez A, Chang DT, Semin Immunol. 2008, 20, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ward WK, J. Diab. Sci. Tech 2008, 2, 768. [Google Scholar]
- [12].Williams DF, Biomaterials 2008, 29, 2941. [DOI] [PubMed] [Google Scholar]
- [13].Anderson J, Cramer S, Host Response to Biomaterials 2015, 13. [Google Scholar]
- [14].Remes A, Williams DF, Biomaterials 1992, 13, 731. [DOI] [PubMed] [Google Scholar]
- [15].Anderson JM, Defife K, Mcnally A, J. Mater. Sci.: Mater. Med 1999, 10, 579. [DOI] [PubMed] [Google Scholar]
- [16].Xia Z, Triffitt JT, Biomed. Mater 2006, 1, R1. [DOI] [PubMed] [Google Scholar]
- [17].Badylak SF, Valentin JE, Ravindra AK, Mccabe GP, Stewart-Akers AM, Tissue Eng., Part A 2008, 14, 1835. [DOI] [PubMed] [Google Scholar]
- [18].Scatena M, Eaton KV, Jackson MF, Lund SA, Giachelli CM, in The Immune Response to Implanted Materials and Devices, Springer, Switzerland, 2017, p. 37. [Google Scholar]
- [19].Doloff JC, Veiseh O, Vegas AJ, Tam HH, Farah S, Ma M, Li J, Bader A, Chiu A, Sadraei A, Nat. Mater 2017, 16, 671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Anderson JM, Jiang S, in The Immune Response to Implanted Materials and Devices, Vol. 15, Springer, Switzerland: 2017. [Google Scholar]
- [21].Klopfleisch R, Jung F, J. Biomed. Mater. Res. A 2017, 105A, 927. [DOI] [PubMed] [Google Scholar]
- [22].Kzhyshkowska J, Gudima A, Riabov V, Dollinger C, Lavalle P, Vrana NE, J. Leukocyte Biol 2015, 98, 953. [DOI] [PubMed] [Google Scholar]
- [23].Williams D, J. Biomed. Eng 1989, 77, 185. [DOI] [PubMed] [Google Scholar]
- [24].Smith MJ, White KL, Smith DC, Bowlin GL, Biomaterials 2009, 30, 149. [DOI] [PubMed] [Google Scholar]
- [25].Panilaitis B, Altman GH, Chen J, Jin H-J, Karageorgiou V, Kaplan DL, Biomaterials 2003, 24, 3079. [DOI] [PubMed] [Google Scholar]
- [26].Feito M, Vila M, Matesanz M, Linares J, Gongalves G, Marques P, Vallet-Regí M, Rojo J, Portolés M, J. Colloid Interface Sci 2014, 432, 221. [DOI] [PubMed] [Google Scholar]
- [27].Biselli E, Agliari E, Barra A, Bertani FR, Gerardino A, De Ninno A, Mencattini A, Di Giuseppe D, Mattei F, Schiavoni G, Sci. Rep 2017, 7, 12737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Irimia D, Wang X, Trends Biotechnol. 2018, 36, 923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Moore N, Doty D, Zielstorff M, Kariv I, Moy L, Gimbel A, Chevillet J, Lowry N, Santos J, Mott V, Lab Chip; 2018, 78, 1844. [DOI] [PubMed] [Google Scholar]
- [30].Heintze JM, Nat. Rev Nephrol 2018, 14, 354. [DOI] [PubMed] [Google Scholar]
- [31].Mestas J, Hughes CCW, J. Immunol 2004, 172, 2731. [DOI] [PubMed] [Google Scholar]
- [32].Buscher K, Ehinger E, Gupta P, Pramod AB, Wolf D, Tweet G, Pan C, Mills CD, Lusis AJ, Ley K, Nat. Commun 2017, 8, 16041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Martín-Fuentes P, Civeira F, Recalde D, García-Otín AL, Jarauta E, Marzo I, Cenarro A, J. Immunol 2007, 779, 3242. [DOI] [PubMed] [Google Scholar]
- [34].Huh D, Hamilton GA, Ingber DE, Trends Cell Biol. 2011, 21, 745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Moraes C, Mehta G, Lesher-Perez SC, Takayama S, Ann. Biomed. Eng 2012, 40, 1211. [DOI] [PubMed] [Google Scholar]
- [36].Bhatia SN, Ingber DE, Nat. Biotechnol 2014, 32, 760. [DOI] [PubMed] [Google Scholar]
- [37].Yum K, Hong SG, Healy KE, Lee LP, Biotechnol. J 2014, 9, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zheng F, Fu F, Cheng Y, Wang C, Zhao Y, Gu Z, Small 2016, 12, 2253. [DOI] [PubMed] [Google Scholar]
- [39].Esch EW, Bahinski A, Huh D, Nat. Rev. Drug Discovery 2015, 14, 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Skardal A, Shupe T, Atala A, Drug Discovery Today 2016, 21, 1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Li Y, Zhu K, Liu X, Zhang YS, Curr. Drug Metab 2018, 19, 100. [DOI] [PubMed] [Google Scholar]
- [42].Zhang YS, Zhang Y-N, Zhang W, Drug Discovery Today 2017, 22, 1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zhang YS, Aleman J, Shin SR, Kilic T, Kim D, Shaegh S. a. M., Massa S, Riahi R, Chae S-K, Hu N, Avci H, Zhang W, Silvestri A, Manbohi A, Polini A, Calzone G, Shaikh N, Sanati A, Alerasool P, Bhise NS, Budina E, Pourmand A, Skardal A, Shupe T, Bishop C, Dokmeci MR, Atala A, Khademhosseini A, Proc. Natl. Acad. Sci. USA 2017, 114, E2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Skardal A, Murphy SV, Devarasetty M, Mead I, Kang H-W, Seol Y-J, Zhang YS, Shin S-R, Zhao L, Aleman J, Hall A, Shupe T, Kleensangb A, Dokmeci MR, Lee SJ, Jackson J, Yoo J, Hartung T, Khademhosseini A, Soker S, Bishop C, Atala A, Sci. Rep 2017, 7, 8837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].White FM, Corfield I, Viscous Fluid Flow, Vol. 3, McGraw-Hill, New York: 2006. [Google Scholar]
- [46].Rinker KD, Prabhakar V, Truskey GA, Biophys. J 2001, 80, 1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Russel WB, Saville DA, Schowalter WR, Colloidal Dispersions, Cambridge University Press, Cambridge: 1989. [Google Scholar]
- [48].Dewey CF, Bussolari SR, Gimbrone MA, Davies PF, J. Biomech. Eng 1981, 103, 177. [DOI] [PubMed] [Google Scholar]
- [49].Grigioni M, Daniele C, D’avenio G, Barbaro V, J. Biomech 1999, 32, 1107. [DOI] [PubMed] [Google Scholar]
- [50].Theilmeier G, Lenaerts T, Remacle C, Collen D, Vermylen J, Hoylaerts MF, Blood 1999, 94, 2725. [PubMed] [Google Scholar]
- [51].Galbraith CG, Skalak R, Chien S, Cell Motil. Cytoskeleton 1998, 40, 317. [DOI] [PubMed] [Google Scholar]
- [52].Vestweber D, Arterioscler. Thromb. Vasc. Biol 2008, 28, 223. [DOI] [PubMed] [Google Scholar]
- [53].Auerbach SD, Yang L, Luscinskas FW, in Adhesion Molecules: Function and Inhibition, Springer, New York, 2007, p. 99. [Google Scholar]
- [54].Fleischer J, Soeth E, Reiling N, Grage-Griebenow E, Flad HD, Ernst M, Immunology 1996, 89, 592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hsiai TK, Cho SK, Wong PK, Ing M, Salazar A, Sevanian A, Navab M, Demer LL, Ho C-M, FASEB J. 2003, 17, 1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Dong C, Lei XX, J. Biomech 2000, 33, 35. [DOI] [PubMed] [Google Scholar]
- [57].Campbell JJ, Hedrick J, Zlotnik A, Siani MA, Thompson DA, Butcher EC, Science 1998, 279, 381. [DOI] [PubMed] [Google Scholar]
- [58].Deshmane SL, Kremlev S, Amini S, Sawaya BE, J. Interferon Cytokine Res 2009, 29, 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Loessner D, Meinert C, Kaemmerer E, Martine LC, Yue K, Levett PA, Klein TJ, Melchels FPW, Khademhosseini A, Hutmacher DW, Nat. Protoc 2016, 11, 727. [DOI] [PubMed] [Google Scholar]
- [60].Yue K, Li X, Schrobback K, Sheikhi A, Annabi N, Leijten J, Zhang W, Zhang YS, Hutmacher DW, Klein TJ, Biomaterials 2017, 139, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Zhou Y, Yoshida S, Kubo Y, Yoshimura T, Kobayashi Y, Nakama T, Yamaguchi M, Ishikawa K, Oshima Y, Ishibashi T, Mol. Med. Rep 2017, 15, 3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Jaguin M, Houlbert N, Fardel O, Lecureur V, Cell. Immunol 2013, 281, 51. [DOI] [PubMed] [Google Scholar]
- [63].Baqui A. a. M. A., Meiller TF, Chon JJ, Turng BF, Falkler WA Jr., Oral Microbiol. Immunol 1998, 13, 173. [DOI] [PubMed] [Google Scholar]
- [64].Cha B-H, Shin SR, Leijten J, Li Y-C, Singh S, Liu JC, Annabi N, Abdi R, Dokmeci MR, Vrana NE, Ghaemmaghami AM, Khademhosseini A, Adv. Healthcare Mater 2017, 6, 1700289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Rostam HM, Singh S, Salazar F, Magennis P, Hook A, Singh T, Vrana NE, Alexander MR, Ghaemmaghami AM, Immunobiology 2016, 221, 1237. [DOI] [PubMed] [Google Scholar]
- [66].Mendoza-Coronel E, Ortega E, Front. Immunol 2017, 8, 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Genin M, Clement F, Fattaccioli A, Raes M, Michiels C, BMC Cancer 2015, 15, 577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].An S, Kim S, Huh Y, Lee TR, Kim H-K, Park K-L, Eun HC, Contact Dermatitis 2009, 60, 185. [DOI] [PubMed] [Google Scholar]
- [69].Dollinger C, Ndreu-Halili A, Uka A, Singh S, Sadam H, Neuman T, Rabineau M, Lavalle P, Dokmeci MR, Khademhosseini A, Adv. Biosyst 2017, 1, 1700041. [Google Scholar]
- [70].Rieger E, Dupret-Bories A, Salou L, Metz-Boutigue M-H, Layrolle P, Debry C, Lavalle P, Vrana NE, Nanoscale 2015, 7, 9908. [DOI] [PubMed] [Google Scholar]
- [71].Singh S, Awuah D, Rostam HM, Emes RD, Kandola NK, Onion D, Htwe SS, Rajchagool B, Cha B-H, Kim D, ACS Biomater. Sci. Eng 2017, 3, 969. [DOI] [PubMed] [Google Scholar]
- [72].Nichol JW, Koshy ST, Bae H, Hwang CM, Yamanlar S, Khademhosseini A, Biomaterials 2010, 31, 5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Loessner D, Meinert C, Kaemmerer E, Martine LC, Yue K, Levett PA, Klein TJ, Melchels FP, Khademhosseini A, Hutmacher DW, Nat. Protoc 2016, 11, 727. [DOI] [PubMed] [Google Scholar]
- [74].Liu W, Heinrich MA, Zhou Y, Akpek A, Hu N, Liu X, Guan X, Zhong Z, Jin X, Khademhosseini A, Zhang YS, Adv. Healthcare Mater 2017, 6, 1601451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Norouzi N, Bhakta HC, Grover WH, PLoS One 2017, 12, e0180520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Geissmann F, Jung S, Littman DR, Immunity 2003, 19, 71. [DOI] [PubMed] [Google Scholar]
- [77].García-Nieto S, Johal RK, Shakesheff KM, Emara M, Royer P-J, Chau DY, Shakib F, Ghaemmaghami AM, PLoS One 2010, 5, e10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Zoladek AB, Johal RK, Garcia-Nieto S, Pascut F, Shakesheff KM, Ghaemmaghami AM, Notingher I, Analyst 2010, 135, 3205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.