Abstract
Sphingolipids are both structural and bioactive compounds. In particular, ceramide and sphingosine 1‐phosphate regulate cell fate, inflammation and excitability. 1‐α,25‐dihydroxyvitamin D3 (1,25(OH)2D3) is known to play an important physiological role in growth and differentiation in a variety of cell types, including neural cells, through genomic actions mediated by its specific receptor, and non‐genomic effects that result in the activation of specific signalling pathways. 1,25(OH)2D3 and sphingolipids, in particular sphingosine 1‐phosphate, share many common effectors, including calcium regulation, growth factors and inflammatory cytokines, but it is still not known whether they can act synergistically. Alterations in the signalling and concentrations of sphingolipids and 1,25(OH)2D3 have been found in neurodegenerative diseases and fingolimod, a structural analogue of sphingosine, has been approved for the treatment of multiple sclerosis. This review, after a brief description of the role of sphingolipids and 1,25(OH)2D3, will focus on the potential crosstalk between sphingolipids and 1,25(OH)2D3 in neural cells.
Abbreviations
- 1,25(OH)2D3
1‐α,25‐dihydroxyvitamin D3
- AD
Alzheimer disease
- APP
amyloid precursor protein
- Aβ
amyloid β peptide
- BDNF
brain‐derived neurotrophic factor
- C1P
ceramide‐1‐phosphate
- CDase
ceramidase
- CERK
ceramide kinase
- CERS
ceramide synthase
- cGSN
cytoplasmatic gelsolin
- cPLA2
cytosolic phospholipase A2
- GBA1
lysosomal glucosylceramidase
- GC
glucosylceramide
- GCDase
glucosylceramidase
- GDNF
glial‐derived neutrophic factor
- HDAC
histone deacetylases
- KO
knockout
- LRM
lipid‐rich microdomains
- MS
multiple sclerosis
- NGF
nerve growth factor
- NPC
Niemann–Pick disease type C
- PD
Parkinson disease
- pGSN
plasma gelsolin
- S1P
sphingosine 1‐phosphate
- SL
sphingolipid
- SM
sphingomyelin
- SMase
sphingomyelinase
- aSMase
acid SMase
- nSMase
neutral SMase
- SPHK
sphingosine kinase
Tables of Links
| TARGETS | |
|---|---|
| GPCRs a | ERK2 |
| S1P1 receptor | Haem oxygenase |
| S1P2 receptor | HDAC |
| S1P3 receptor | iNOS |
| S1P4 receptor | JNK |
| S1P5 receptor | 3‐ketodihydrosphingosine reductase |
| Ligand‐gated ion channels b | Kinase suppressor of ras (KSR) |
| GluN3A | Lipid phosphate phosphatase |
| Voltage‐gated ion channels c | P38 MAPK |
| voltage‐gated calcium channels | PKA |
| Voltage‐gated potassium channels | PKC |
| Nuclear hormone receptors d | PLA2 |
| Vitamin D receptor (VDR) | Sphingolipid Δ4‐desaturase |
| Enzymes e | Sphingomyelin synthase |
| Akt (PKB) | Sphingomyelin phosphodiesterase |
| AMPK | Sphingosine 1‐phosphate lyase |
| Adenylate cyclase | Sphingosine 1‐phosphate phosphatase |
| BACE1 | SPHK1 |
| Caspase 9 | SPHK2 |
| Cathepsin D | SPT |
| CBS | UDP‐glucose ceramide glucosyltransferase |
| Acid ceramidase | Src |
| Alkaline ceramidase | Other protein targets f |
| Neutral ceramidase | Bcl‐xL |
| CERK | COUP‐TF1 |
| Ceramide synthase | Gα |
| CYP24A1 | RAGE |
| CYP27B1 | TNF‐α |
| ERK1 |
| LIGANDS | |
|---|---|
| Aβ | IL‐6 |
| BDNF | Miglustat |
| CNTF | MPP+ |
| Fingolimod | NADPH |
| FTY720‐phosphate | NGF |
| GDNF | NT‐3 |
| Glutathione | Sphingosine (SPH) |
| IGF‐1 | S1P |
| IL‐1α | Tau |
| IL‐1‐β | 1,25‐dihydroxyvitamin D3 |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,b,c,d,e,fAlexander et al., 2015a,b,c,d,e,f).
Roles of bioactive SLs in the nervous system
Sphingolipids (SLs) have long been regarded as inactive and stable structural components of the membrane, but some of them including ceramide, sphingosine, ceramide1‐phosphate (C1P) and sphingosine 1‐phosphate (S1P) are biologically active molecules. The cellular effect of SLs results from the combination of the effects of several interconvertible SLs (Figure 1), which are localized in distinct subcellular compartments and regulate distinct cellular processes and functions, including neural cell survival, apoptosis, autophagy, differentiation, migration, inflammation and neurotransmitter release (Table 1) (Colombaioni and Garcia‐Gil, 2004; Mencarelli and Martinez‐Martinez, 2013; Young et al., 2013; Shamseddine et al., 2015; Ghasemi et al., 2016).
Figure 1.
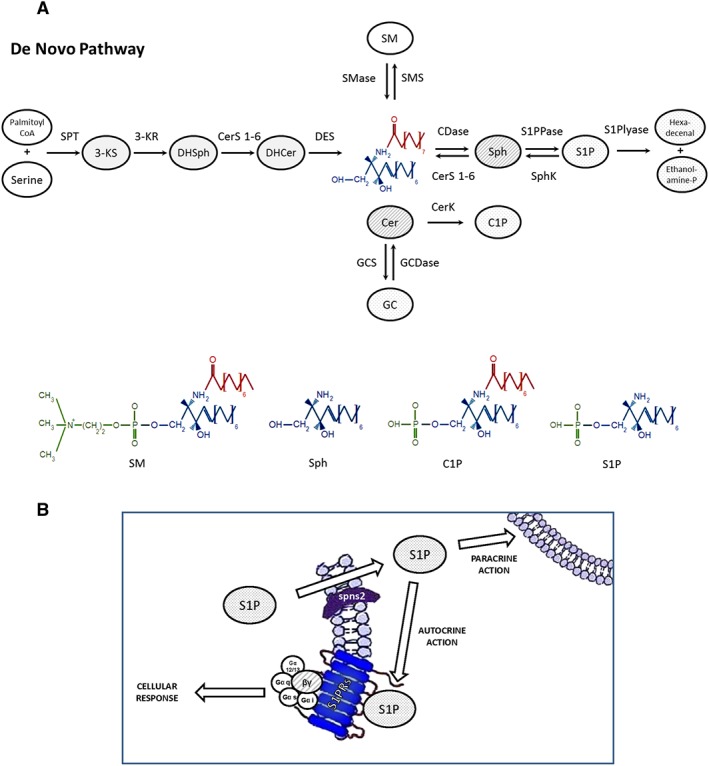
Metabolism of sphingolipids. (A). De novo synthesis of sphingolipids leads to the formation of ceramide (Cer) and sphingosine 1‐phosphate (S1P) through four reactions catalyzed by the serine palmitoyltransferase (SPT), which condenses palmitoyl‐CoA and serine into 3‐ketosphinganine (3‐KS), the 3‐ketosphinganine reductase (3‐KR), which generates sphinganine (DHSph), the (dihydro) ceramide synthase (CerS), which acylates sphinganine to dihydroceramide (DHCer), and the dihydroceramide desaturase (DES), which converts relatively inactive dihydroceramide to ceramide. The latter is converted to sphingosine (Sph) by ceramidase (CDase). Sphingosine can be converted to S1P by sphingosine kinase (SPHK) or to ceramide by CerS. The degradation of S1P is achieved by the reversible reaction catalyzed by the S1P phosphatase and the irreversible reaction catalyzed by S1P lyase, which produces hexadecenal and ethanolamine phosphate. The cell membrane constituent sphingomyelinase (SMase/SMPD) generates ceramide from sphingomyelin (SM). Phosphorylation of ceramide by ceramide kinase (CERK) generates ceramide 1‐phosphate (C1P). In the golgi, ceramide is converted to SM by SM synthase or to glucosylceramide (GC) by glucosylceramide synthase (GCS). GlcCer is then processed to more complex glycosphingolipids (not shown). Glucosylceramidase (GCDase; also called glucosylcerebrosidase) produces ceramide from glucosylCer. (B) S1P can be exported outside the cells by ABC transporters and the putative transporter Spinster 2 (spns2) and elicits autocrine or paracrine signalling by binding to and activating GPCRs (S1P1–5 receptors; S1PRs). G‐proteins are composed of three subunits: α, β and γ and are classified as G(q), G(i/o), G(12/13) and G(s) depending on the function of their α subunits.
Table 1.
Role of SLs in proliferation, survival, differentiation, neurodegeneration, ischaemia and inflammation, Cer, ceramide
| Cell/Tissue | Method | Effect | Mechanism | Reference |
|---|---|---|---|---|
| Proliferation | ||||
| Neural progenitor cultured cells | A, exogenous S1P | ↑ proliferation | – | Harada et al., 2004 |
| Oligodendrocyte precursors | A,siS1P1R | ↑ proliferation | S1P1R | Jung et al., 2007 |
| Neuronal progenitors retina | A | ↑ proliferation | S1P | Miranda et al., 2009 |
| Human neuroblastoma cell | A, siCERK | ↓ proliferation | ↓ CERK expression | Bini et al., 2012 |
| Neuronal progenitors retina | A | neuronal progenitors retina | C1P | Miranda et al., 2011 |
| Survival | ||||
| Photoreceptor | A | ↑ survival | C1P | Miranda et al., 2011 |
| SH‐5YSY, TNFα, | A,si CERK | ↑ survival | ↓ CERK expression | Barth et al., 2012 |
| Photoreceptor | A | ↑ survival | S1P | Miranda et al., 2009 |
| SH‐SY5Y, MPP+ | A | ↑ survival | S1P | Pyszko and Strosznajder, 2014 |
| Mature oligodendrocyte | A,si S1P5R | ↑ survival | S1P5R/AKT | Jaillard et al., 2005 |
| Drosophila mutants | B | ↑ photoreceptor survival | CDase expression | Acharya et al., 2003 |
| Retinitis pigmentosa mouse (eye) | B | ↑ photoreceptor survival | SPT inhibition | Strettoi et al., 2010 |
| Differentiation | ||||
| Neuronal progenitors retina | A | ↑ differentiation | S1P | Miranda et al., 2009 |
| PC12 | A | neurite retraction ↓differentiation | S1P/S1P2R | Toman et al., 2004 |
| PC12, dorsal root ganglion neurons | A | ↑ differentiation ↑neurite outgrowth | S1P/S1P1R | Toman et al., 2004 |
| Oligodendrocyte precursor, S1P1R KO mouse | B | ↓differentiation | ↓S1P1R | Dukala and Soliven, 2016 |
| Inflammation | ||||
| Microglial cultured cells, brain | A, B | ↓inflammation | C2‐Cer, ROS, MAPKs, PI3K/Akt, Jak/STAT | Jung et al., 2013 |
| Murine ischaemic brain, Cultured neurons | A,B,a | ↑inflammation, ↓inflammation | SPHK1inhibition, KO, SPHK2 inhibition, KO | Zheng et al., 2015 |
| Neurodegeneration | ||||
| Purkinje cell CERS1 mutants | A | ↓cell number, ↓ neurite branching | ↓C18Cer, ↑C16Cer, ↑Sph, ↑dhSph, ↑dhS1P, ↑S1P | Zhao et al., 2011 |
| Purkinje cell, aSMase KO | A | ↑ death | ↑SM | Horinouchi et al., 1995 |
| Neuroblastoma cell | A,siCERS2 | cell growth arrest, increased autophagy | ↓CERS2, ↑C16Cer, ↓ C24Cer, | Spassieva et al., 2009 |
| Ischaemia/Hypoxia | ||||
| Rat brain,glia, chronic hypoxia | B | – | ↑ Cer, ↑ aSMase, ↓GCS | Ohtani et al., 2004 |
| Hypoxia/reoxygenation, NT‐2 neuronal precursor cells | A | – | ↑ C14Cer, ↑C16 Cer, ↑ SMase, ↑CERS5 | Jin et al., 2008 |
| Ischaemia, rat hippocampus astrocyte | B | ↑TNFα, IL1, IL6 | ↑ nSMase | Gu et al., 2013 |
The intracellular levels of these bioactive SLs are fine‐tuned, and alterations in the SL profile in the nervous system contribute to the development of neurological and neuroinflammatory diseases such as photoreceptor degeneration, retinitis pigmentosa, Alzheimer disease (AD), Parkinson disease (PD), multiple sclerosis (MS) (Table 2) and major depression, Huntington disease and epilepsy (Acharya et al., 2003; Desplats et al., 2007; Haughey et al., 2010; Mielke and Lyketsos, 2010; Strettoi et al., 2010; Grimm et al., 2013; Halmer et al., 2014; Pyszko and Strosznajder, 2014; Vanni et al., 2014; Gulbins et al., 2015). Moreover, inherited defects of both the synthesis and catabolism of SLs cause varying degrees of dysfunction of the CNS, such as in inherited sensory and autonomic neuropathy, Niemann–Pick disease type A and B and lisosomal storage disorders (Sabourdy et al., 2015).
Table 2.
Involvement of SLs in neurodegenerative diseases
| Method | Effect | Mechanism | Reference | |
|---|---|---|---|---|
| AD | ||||
| Cortical neurons, Aβ 1–42 | A | ↑exosome release, ↑apoptosis | ↑nSMase, | Wang et al., 2012 |
| Human primary neurons, Aβ | A | ↑ apoptosis | ↑ nSMase | Jana and Pahan, 2004 |
| Cultured hippocampal neurons, Aβ | B | ↑apoptosis | ↑ C18Cer, C24Cer | Cutler et al., 2004 |
| Presenilin knock‐in mouse, primary cultured astrocytes | A | ↑cell death | ↑ C20Cer, C24Cer, ↑CERS1, ↑CERS4 | Wang et al., 2008 |
| Astrocytes, frontal cortex, CSF, patients | B | – | ↑Cer | Satoi et al., 2005 |
| White matter temporal cortex, white and grey matter, patients | B | – | ↑ C24:1Cer, ↓sulfatides | Han et al., 2002 |
| Medial frontal gyrus, patients | B | – | ↑ C24:0 Cer | Cutler et al., 2004 |
| Cultured neurons, Aβ, brain, patients | A, B | ↑Apoptosis | ↑a,nSMase, ↑acid CDase, ↑aSMase, ↑acid CDase, ↑Cer, ↓SM | He et al., 2010 |
| Entorhinal cortex,patients, Hippocampus, temporal grey matter | B | amyloid deposit | ↓SPHK1, ↓S1P1R, ↑SPL, ↓S1P, ↓SPHK1,2, ↑C16:0 Cer | Ceccom et al., 2014 |
| Brodman areas 46,10,20 patients, | B | – | ↑ PPAP2B, ↑ SPL, ↓acid CDase, ↑CERS1,2, ↓CERS6 | Katsel et al., 2007 |
| PD | ||||
| Anterior cingulate cortex, patients | B | – | ↓total Cer, ↓SM, ↑CERS1 expression | Abbott et al., 2014 |
| Anterior cingulate cortex, patients | B | ↑ autophagy ↑α‐synuclein | ↓glucosylCDase, ↓Cer, | Murphy et al., 2014 |
| MS | ||||
| White matter, patients | B | – | ↓S1P, ↑Sph, ↑C16Cer, ↑C18Cer | Qin et al., 2010 |
| Reactive astrocytes, patients | B | – | ↑ C18:0 Cer | Kim et al., 2012 |
| NPC | – | – | – | – |
| NPC−/− mouse brain | B | – | ↑glucosylCer, galactosylCer, glucosylSph, GM2, GM3 | Marques et al., 2015 |
| NPC−/− mouse brain with GBA2 deletion, | B | improved motor coordination | ↑glucosylCer, glucosylSph, =cholesterol, =gangliosides | Marques et al., 2015 |
| NPC1−/− mouse brain, miglustat Patients, plasma Patients + miglustat, plasma patients + miglustat, CSF | B | – | GCS inhibition, ↑ monohexylCer, ↑ monohexylCer, ↑C16:0Cer, ↓Sph, ↑S1P, ↓Cer, ↓GM1, ↓GM3, ↑monohexylCer | Fan et al., 2013 |
| NPC1−/− mouse brain, miglustat, | B | ↑synaptic plasticity | GCS inhibition | D'Arcangelo et al., 2016 |
| Purkinje neurons from NPC1−/− cat, miglustat | A/B | ↑survival | GCS inhibition | Stein et al., 2012 |
| NPC1−/− cat, miglustat | B | ↑lifespan, ↓ motor deficit | GCS inhibition | Stein et al., 2012 |
| Lymphocytes NPC patients, miglustat | A | correction of abnormal lipid trafficking | GCS inhibition | Lachmann et al., 2004 |
The table illustrates some examples of the involvement of SLs in neuroinflammation, ischaemia and in neurogenerative diseases such as PD, MS, AD and NPC, indicating the experimental method used (A: in vitro; B, in vivo; si: siRNA; Cer, ceramide; GBA2, non‐lysosomal glucosylCDase). The alterations in SL concentration or in expression/activity of enzymes involved in SL metabolism are listed under Mechanism.
The modulatory role of ceramide in growth and 1‐α,25‐dihydroxyvitamin D3 [1,25(OH)2D3]‐induced differentiation was first reported in leukaemic HL60 cells (Okazaki et al., 1989; Bielawska et al., 1992). Twenty years ago, it was proposed that the ratio of the intracellular content of S1P and ceramide was a major determinant of cell fate (Cuvillier et al., 1996): S1P enhances growth and survival, whereas its precursors (ceramide and sphingosine) promote growth arrest and cell death (Colombaioni and Garcia‐Gil, 2004; Mencarelli and Martinez‐Martinez, 2013; Shamseddine et al., 2015; Ghasemi et al., 2016). However, there is increasing evidence showing that the ceramides containing specific acyl chain lengths (ceramide species) have different functions (Ben‐David and Futerman, 2010; Hannun and Obeid, 2011): C18:0‐ceramide is synthesized by ceramide synthase 1 (CERS1), an isoenzyme abundant in the brain, and it has been suggested to act as a protective factor, because a lack of CERS1 caused neural death in mice cerebellum and impaired motor coordination (Zhao et al., 2011; Ginkel et al., 2012). However, CERS1 elimination decreases ganglioside levels, and this might be one of the causes of neural cell death in mice (Ginkel et al., 2012). Moreover, serum deprivation‐induced apoptosis in embryonic hippocampal cells induces an increase in C16:0‐ceramide and a decrease in C24:0‐ceramide (Garcia‐Gil et al., 2015). It is worth noting that compensatory mechanisms can occur following gene knockout (KO) or when the protein expression of a substance is reduced by administration of its siRNA. For example, the treatment of neuroblastoma cells with CERS2 siRNA results in an increase in the expression of CERS5 and CERS6 with a reduction in C24‐ceramide and sphingomyelin (SM) and increase in C14‐ and C16‐ceramide levels (Spassieva et al., 2009).
Other facts, in addition to the different acyl chain composition of the ceramides, increase the complexity of studying the role of SLs in cell fate: (i) S1P acts not only intracellularly but also as ligand of specific GPCRs (Maceyka et al., 2012) (Figure 1B); (ii) other SL metabolites, such as C1P, can also mediate apoptosis or proliferation depending on the cell type (Miranda et al., 2011; Bini et al., 2012; Presa et al., 2016); and (iii) the synthesis of SL and signalling can occur in different cellular compartments (Newton et al., 2015).
Ceramide and C1P
The apoptotic role of ceramide in the nervous system has been extensively reviewed (Colombaioni and Garcia‐Gil, 2004; Mencarelli and Martinez‐Martinez, 2013; Shamseddine et al., 2015). Ceramide is also involved in the control of autophagy (Daido et al., 2004; Spassieva et al., 2009), differentiation (Riboni et al., 1995), inflammation (Gu et al., 2013) and exosome release (Trajkovic et al., 2008; Wang et al., 2012). The generation of ceramide by activation of neutral SMase2 (nSMase2) is associated with an increase in dopamine uptake (Kim et al., 2010) and this modulates excitatory postsynaptic currents by controlling the insertion and clustering of NMDA receptors (Wheeler et al. 2009). Moreover, Caenorhabditis elegans mutants lacking ceramide synthase have been found to have defects in synaptic transmission and in synaptic vesicle cycling (Chan and Sieburth, 2012). Ceramide directly regulates the activity of several enzymes including cathepsin D, phospholipase A2, kinase suppressor of Ras, ceramide‐activated protein serine–threonine phosphatases 1 and 2A (PP1 and PP2A), PKC isoforms and ion channels, such as the potassium channel Kv1.3 (Bock et al., 2003). It is also able to form channels in mitochondria, which are involved in the release of pro‐apoptotic factors (Colombini, 2016). It directly inhibits mitochondrial complex III and increases the generation of ROS (García‐Ruiz et al., 1997). Ceramide inhibits the Akt signalling pathway, stimulates the stress‐activated kinase JNK and up‐regulates the apoptosis‐promoting variants Bcl‐xS and caspase‐9, while correspondingly down‐regulating the antiapoptotic variants Bcl‐xL and caspase‐9b (Chalfant et al., 2002).
There is accumulating evidence supporting the involvement of ceramide in the modulation of neural plasticity. For example, spatial memory and extinction learning are impaired when sphingomyelinase 2 (SMase2) is inhibited or ceramide levels are reduced (Tabatadze et al., 2010; Carrasco et al., 2012; Huston et al., 2016). Furthermore, genetic deletion of CERS1 is associated with deficits in motor learning and spatial working memory, as well as reduced anxiety (Ginkel et al., 2012).
Astrocytes are mediators of CNS responsiveness to inflammation and injury (Claycomb et al., 2013). They display increased ceramide following ischaemia/reperfusion with nSMase2‐dependent generation of the pro‐inflammatory cytokines TNF‐α, IL‐1 and IL‐6 (Gu et al., 2013). In neural stem and progenitor cells of the developing brain, ceramide influences cell polarity, motility and apoptosis (Bieberich, 2012) and induces ciliogenesis, a critical step in differentiation (He et al., 2014).
In contrast, the phosphorylated form of ceramide, C1P, induces proliferation and promotes the survival and differentiation of photoreceptors in rat retina neuronal cultures (Miranda et al., 2011), while inhibition or down‐regulation of ceramide kinase (CERK), which appears to be the only enzyme responsible for its synthesis, results in a decreased proliferation of human neuroblastoma cells (Bini et al., 2012). Moreover, C1P directly binds and activates α‐type cytosolic phospholipase A2 (cPLA2), so stimulating arachidonic acid release (Pettus et al., 2004). Activation of cPLA2 by C1P induces spinal neuronal death (Liu et al., 2014), while treatment of SH‐SY5Y cells with TNFα increases CERK activity. Depleting CERK activity blocks NADPH oxidase activation and eicosanoid biosynthesis and restores neuronal viability in the presence of TNFα (Barth et al., 2012). A CERK‐null mouse has been generated. Although CERK is highly expressed in Purkinje cells, this mouse did not display histological abnormalities or impairments in motor coordination, but their emotional behaviour was slightly affected (Mitsutake et al., 2007). Outside the nervous system, C1P stimulates the migration of macrophages via a specific plasma membrane receptor coupled to Gi proteins (Presa et al., 2016), and it is released from damaged myocardial cells possibly leading to the recruitment of stem/progenitor cells to damaged organs (Kim et al., 2013). Whether C1P is also released from the injured nervous system or whether it induces migration in neural stem cells is not known.
S1P
S1P modulates cell survival (Edsall et al., 1997), proliferation (Harada et al., 2004; Miranda et al., 2009), differentiation (Toman et al., 2004; Miranda et al., 2009) and migration (Novgorodov et al., 2007; Alfonso et al., 2015), calcium homeostasis (Sato et al., 2000; Giussani et al., 2007; Hagen et al., 2011), neurite retraction (Toman et al., 2004), angiogenic vascular maturation (Liu et al., 2000; Mizugishi et al., 2005) and cytoskeleton dynamics (Postma et al., 1996; Toman et al., 2004; Jaillard et al., 2005) (for recent reviews, see Bieberich 2012; Maceyka et al., 2012, Proia and Hla, 2015, Ghasemi et al., 2016). In addition, it is able to modulate excitability (Li et al., 2015a) by increasing glutamate release (Kajimoto et al., 2007; Kanno et al., 2010) and by regulating endocytosis and exocytosis (Chan and Sieburth, 2012; Shen et al., 2014; Riganti et al., 2016).
Regarding intracellular effects of S1P, S1P induces calcium release from the ER, inhibits histone deacetylases (HDAC), acts as a cofactor necessary for the E3 ligase activity of TNF receptor‐associated factor 2, activates recombinant human PKCδ and binds the mitochondrial protein prohibitin 2, a highly conserved protein that regulates mitochondrial assembly and function (Maceyka et al., 2012) (Figure 2). In agreement with the role of S1P in proliferation, sphingosine kinase 1(SPHK1) is overexpressed, while S1P lyase is often deleted in human cancers, including glioblastoma (Steck et al., 1995; van Brocklyn et al., 2005). SPHK1 overexpression is associated with resistance to chemotherapeutic drugs and to a poor prognosis (van Brocklyn et al., 2005).
Figure 2.
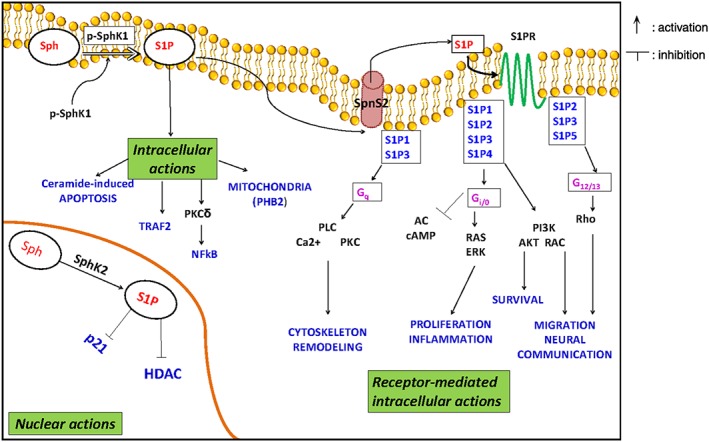
Biological function of S1P/S1P receptor signalling. Phosphorylation activates SphK1 and promotes its translocation to the membrane (dashed arrow), where S1P is generated. The bioactive lipid can be released and then bind to S1P receptors (S1PRs). Activation of each receptor subtype leads to distinct G‐protein‐mediated signalling pathways. S1P can be also formed by SPHK isoform 2 inside the nucleus, and in this compartment, it can inhibit p21 transcription and histone deacetylase activity (HDAC).
S1P functions not only inside cells but also as a ligand for five specific‐protein coupled receptors (as reviewed in Spiegel and Milstien, 2003). S1P can be exported outside the cells by transporters belonging to the ATP‐binding cassette family and the putative transporter Spinster 2 and, therefore, acts as an autocrine or paracrine factor (Maceyka et al., 2012) (Figure 1B). S1P receptors are expressed in CNS cells (neurons, oligodendrocytes, astrocytes and microglia). Signalling through S1P receptors involves the activation of Gi, Go, Gq or G12/13 (Figures 1B and 2) and, therefore, signal transduction pathways involving PLC, MAPKs, PI3K/Akt, Rac and Rho/Rho kinase (Spiegel and Milstien, 2003).
The GPCRs specific for S1P (S1P1–5 receptors) trigger different signalling pathways and are expressed and localized differently during tissue development or following stimulation. The S1P1 receptor regulates the migration of neural stem progenitor cells both during development (Alfonso et al., 2015) and in response to injury (Kimura et al., 2007). The S1P1 receptor is also involved in oligodendrocyte development, morphological maturation and early myelination (Jung et al., 2007; Dukala and Soliven, 2016), while activation of the S1P5 receptor on the oligodendrocyte progenitor cells leads to process retraction and inhibits migration (Jaillard et al., 2005; Novgorodov et al., 2007).
During nerve growth factor (NGF)‐induced neuronal differentiation, there is a relocalization of S1P receptors: while the S1P1 receptor, which induces neurite growth, is maintained in the plasma membrane, the S1P2 receptor is internalized (Toman et al., 2004) to prevent loss of neurites. Growth factors, such as NGF, increase SPHK activity and S1P formation and vice versa S1P can induce growth factor release (Yamagata et al., 2003; Sobue et al., 2005; Murakami et al., 2007). Deletion of genes encoding S1P1 receptors or both SPHK1 and SPHK2 in mice severely disrupts neurogenesis and angiogenesis leading to intrauterine death (Liu et al., 2000; Mizugishi et al., 2005) highlighting the role of S1P in the development of the nervous system.
1,25(OH)2D3 in nervous system physiology
The active form of vitamin D3 has hydroxyl groups in positions 1 and 25. The enzymes 1‐α hydroxylase (CYP27B1), required to synthesize 1,25(OH)2D3, and the 24‐hydroxylase (CYP24A1), needed to degrade 25‐(OH)D3 and 1,25(OH)2D3, are present in the brain (Zehnder et al., 2001; Naveilhan et al., 1993). The 1,25(OH)2D3 receptor (vitamin D receptor) is expressed in both neurons and glial cells (microglia, astrocytes, oligodendrocytes, Schwann cells) in different regions of the nervous system (DeLuca et al., 2013). Neural stem cells constitutively express vitamin D receptors, which can be up‐regulated by 1,25(OH)2D3 (Shirazi et al., 2015). Genomic 1,25(OH)2D3 –mediated effects require heterodimerization between the vitamin D receptor and the retinoid X receptor. This complex binds to response elements, thus regulating the transcription of genes (Christakos et al., 2016). It increases the transcription of the genes encoding growth factors, such as NGF, glial‐derived neutrophic factor (GDNF), neurotrophin 3, brain‐derived neurotrophic factor (BDNF) and ciliary neurotrophic factor, and for enzymes involved in the synthesis of neurotransmitters (tyrosine hydroxylase, tryptophan hydroxylase 2, glutamate decarboxylase), whereas it represses that of voltage‐dependent calcium channels (DeLuca et al., 2013; Patrick and Ames, 2014; Shirazi et al., 2015). The vitamin D receptor is also localized in the caveolae and induces rapid non‐genomic effects (Figure 3). Activation of PKA, Ca2+/calmodulin‐dependent PK, PI3K and MAPK p38 results in the phosphorylation of neurofilaments, and in the modulation of chloride, potassium and voltage‐dependent calcium channels in rat cortical neurons (Zanatta et al., 2012). In addition, other kinases including ERK1/ERK2, ERK5 and JNK1/JNK2 and PKC and other enzymes, such as PLA2, Src and p21ras (Bi et al., 2016; Hii and Ferrante, 2016), are also targets of 1,25(OH)2D3.
Figure 3.
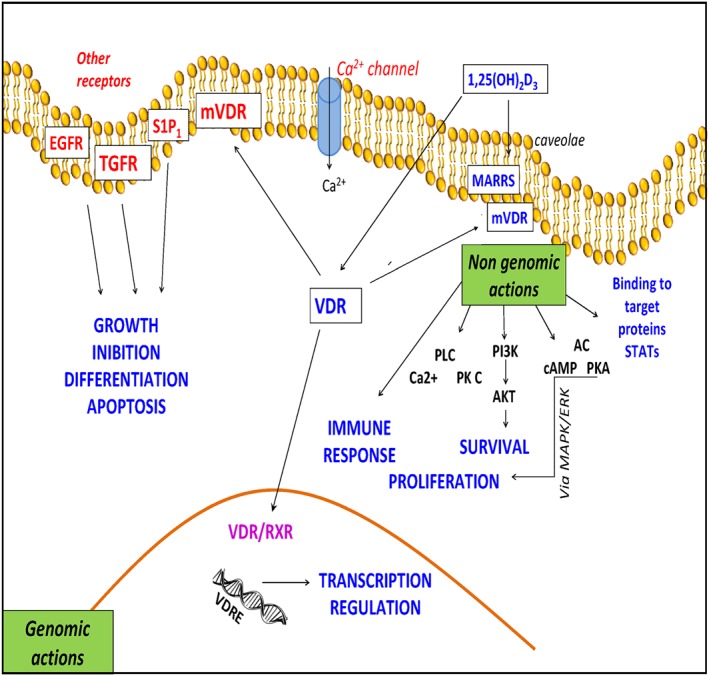
Biological function of 1,25(OH)2D3/vitamin D receptor (VDR) signalling. Non‐genomic rapid actions of 1,25(OH)2D3 are mediated by the membrane vitamin D3 receptor (mVDR), localized at the plasma membrane. An inactive form of VDR is present in the cytosol (VDR). mVDR activation through MARRS (membrane‐associated, rapid response steroid‐binding protein) promotes the MAPK cascade, Raf kinase with the consequent activation of PKC, PI3K and PKA. 1,25(OH)2D3 can interact with TGF and EGF receptors to modulate cell cycle processes. Activation of the GPCR S1P1 receptor (S1P1R) leads to a specific Raf‐MAPK–ERK cascade that may crosstalk with the classical VDR pathways. The genomic action of 1,25(OH)2D3 leads to the regulation of gene expression following the nuclear translocation of VDR, the formation of the complex of VDR and 9‐cis‐retinoic acid receptor (VDR/RXR), and its binding to the vitamin D3 response elements (VDREs).
The combination of in vitro and in vivo experiments provides compelling evidence that 1,25(OH)2D3 has a crucial role in synaptic transmission and neuroplasticity (Smith et al., 2006; Grecksch et al., 2009; Groves et al., 2013; Eyles et al., 2013; Patrick and Ames, 2014; Latimer et al., 2014) as well as in proliferation, differentiation and neuroprotection, as summarized in Table 2. Increasing evidence derived from studies of 1,25(OH)2D3 deficiency and from vitamin D receptor polymorphisms suggests that 1,25(OH)2D3 influences susceptibility to a number of psychiatric and neurological diseases, which include AD, PD, schizophrenia, autism, depression, amyotrophic lateral sclerosis and epilepsy, and is especially strong for MS (Eyles et al., 2013; Peterson 2014; Spedding, 2014; Burton and Costello, 2015; Jiang et al., 2015; Shen and Ji, 2015a,b). The effect of 1,25(OH)2D3 deficiency has been studied in female rats or mice fed a 1,25(OH)2D3‐deficient diet during pregnancy. The overall brain size of the offspring of these animals was increased and they had larger lateral ventricles. These effects were not modified by the addition of 1,25(OH)2D3 to the diet after birth. In adult life, these rats demonstrated subtle alterations in learning and memory (Eyles et al., 2013; Fernandes de Abreu et al., 2010; Hawes et al., 2015). Interestingly, prenatal 1,25(OH)2D3‐depleted rats exhibited an impairment in latent inhibition, mimicking some features found in schizophrenia (Grecksch et al., 2009). Furthermore, in humans the offspring of mothers who have had insuffient 1,25(OH)2D3 during pregnancy have been found to have language impairments (Whitehouse et al., 2012). The administration of 1,25(OH)2D3 has been found to exert a neuroprotective effect on the cognitive decline in ageing rats (Latimer et al., 2014). The hormone prevents the development of and reversibly blocks the progression of the pathological manifestations of experimental allergic encephalomyelitis, which is the animal model of MS. This protective effect is absent in vitamin D receptor KO mice (DeLuca et al., 2013; Eyles et al., 2013). The effect of 1,25(OH)2D3 depends on its neuroimmunomodulatory properties (Eyles et al., 2013) and also on its action on neural cells. In fact, 1,25(OH)2D3 increases both neural stem cell proliferation and differentiation into neurons and oligodendrocytes, the myelinating cells of the CNS (de la Fuente et al., 2015; Shirazi et al., 2015).
In neurodegenerative diseases, including PD and AD, adult neurogenesis in the hippocampal dentate gyrus and in the subventricular zone is impaired (Winner and Winkler, 2015). Therefore, factors that can promote neurogenesis are considered potential treatments for these disorders. The anti‐proliferative and pro‐differentiating effects of 1,25(OH)2D3 in neural cells were first described more than 10 years ago (Brown et al., 2003; Ko et al., 2004) and were mediated through the regulation of cyclin expression and NGF production in cultured hippocampal cells (Brown et al., 2003; Ko et al., 2004). Recently, the CERK signalling pathway has been shown to be involved in the cell growth arrest promoted by 1,25(OH)2D3 in human neuroblastoma cells (Bini et al., 2012). In fact, the pharmacological inhibition and the silencing of CERK drastically reduced cell proliferation. 1,25(OH)2D3, and the vitamin D receptor agonist ZK191784 induced a significant decrease in CERK expression and C1P content. The involvement of the vitamin D receptor/COUP‐TFI/histone deacetylase complex in CERK regulation has also been reported by Bini et al. (2012). There are accumulating data suggesting that 1,25(OH)2D3 has complex effects on the neurogenesis of neural stem cells. Cui et al. (2007) have studied the effect of foetal 1,25(OH)2D3 deprivation, and observed the formation of an increased number of neurospheres in cultures from the neonatal subventricular zone. Exogenous 1,25(OH)2D3 added to the culture medium reduced the number of neurospheres number in control samples [in agreement with the presumed anti‐proliferative effect of 1,25(OH)2D3] but not in cultures from the hormone‐deprived pups (Cui et al., 2007). In contrast, in vivo experiments have shown that fetal 1,25(OH)2D3 deficiency leads to reduced neurogenesis in the dentate gyrus of the hippocampus (Keilhoff et al., 2010). In another model of 1,25(OH)2D3 deficiency, the 1α‐hydroxylase KO mice, 1,25(OH)2D3 increases the proliferation, but decreases the survival of neurons in the dentate gyrus in neonates (Zhu et al., 2012). The different effects probably depend on the time window of exposure and/or the different sensitivity to the hormone of distinct neurogenic niches.
Crosstalk between SLs and 1,25(OH)2D3 actions
One or more components of the signal transduction pathway promoted by 1,25(OH)2D3 affect the metabolism of SLs and vice versa (Figure 4). For example, 1,25(OH)2D3 regulates the expression of S1P‐ phosphatase 2 (Reardon et al., 2013) and of CERK (Bini et al., 2012). Cholecalciferol, the non hydroxylated precursor of 1,25(OH)2D3, induces activation of SMase, an increase in ceramide and cell death in human glioblastoma cells (Magrassi et al., 1998). However, 1,25(OH)2D3 also increases the transcription of neurotrophic factors, such as NGF and BDNF, which require SPHK activity to execute their neuroprotective or prodifferentiating activity (Edsall et al., 1997; Culmsee et al., 2002; Saini et al., 2005; Murakami et al., 2007) and to affect excitability (Zhang et al., 2008). Similarly, many protective or differentiating actions of 1,25(OH)2D3 in non‐neural cells are due to stimulation of SMase and the generation of SPHK and S1P (Okazaki et al., 1989; Kleuser et al., 1998; Manggau et al., 2001; Sauer et al., 2003).
Figure 4.
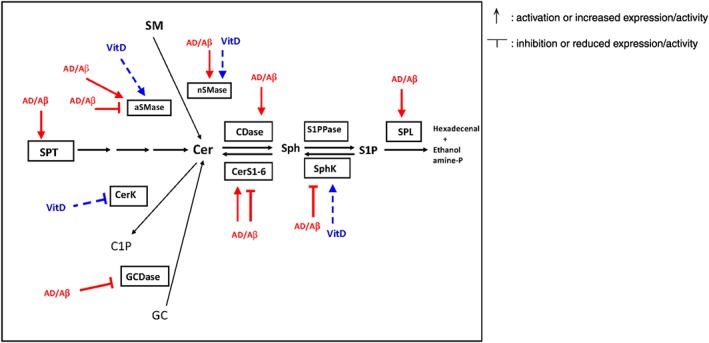
Effect of 1,25(OH)2D3 and Aβ on the key reactions involved in the sphingolipid metabolic pathway. The effect of 1,25(OH)2D3 is shown in blue and dashed lines, whereas the effect of Aβ treatment or alterations associated with AD on the key enzymes involved in the reactions are shown in red and continuous line.
1,25(OH)2D3 is able to modulate expression of S1P receptors: the hormone reduces the chemorepulsive S1P2 receptor levels on circulating osteoblast precursors (Kikuta et al., 2013) and decreases the expression of S1P3 receptors in human breast cancer cells (Dolezalova et al., 2003). Interestingly, vitamin D receptor expression is correlated with the calcitriol‐mediated reduction of migration in glioblastoma multiforme (Salomón et al., 2014), but it is not known whether this effect involves the differential expression of S1P receptors.
SLs and 1,25(OH)2D3 have some common targets, including cathepsins. Ceramide and C1P interact directly and activate cathepsin D (Heinrich et al., 2000; Zebrakovská et al., 2011), which is involved in cell death in many cell types. For example, gemcitabine activates acid SMase (aSMase), leading to the lysosomal accumulation of ceramide, cathepsin D activation and glioma cell death (Dumitru et al., 2009). Cathepsin D is able to migrate to the nucleus. Indeed, nuclear translocation of mitochondrial cytochrome C, lysosomal cathepsins B and D and other death‐promoting proteins has been observed within the first 60 min of generalized seizures (Zhao et al., 2010). Both cathepsin D and its inhibitor cystatin A have VRE in their promoters (Wang et al., 2005). This may explain, at least in part, the pro‐survival and pro‐death effects of 1,25(OH)2D3 on different cells.
Histone acetylation and methylation are often present at sites of vitamin D receptors, and 1,25(OH)2D3‐induced binding of the vitamin D receptor at these sites is associated with an increase in the level of histone modifications as well as in changes in chromatin packaging (Carlberg and Campbell, 2013). Similarly, S1P formed inside the nuclei by SPHK2 activation can inhibit HDACs and regulate gene transcription (Figure 2). Therefore, it could be possible that both 1,25(OH)2D3 and S1P epigenetically modulate the same genes (Hait et al., 2009; Huang et al., 2015a).
Furthermore, SLs are important components of lipid‐rich microdomains (LRM), also named lipid rafts, fluctuating nanoscale assemblies that can be stabilized to coalesce, forming platforms that function in membrane signalling and trafficking (Gulbins and Grassmé, 2002; Lingwood and Simons, 2010). LRM have been described in the plasma membrane, mitochondria and nuclei. In the inner nuclear membrane, LRM play a role in active chromatin anchoring, transcription factor binding and DNA duplication (Cascianelli et al., 2008; Albi and Villani, 2009; 2013; Cataldi et al., 2014). The vitamin D receptor seems to be partly localized in nuclear LRM (Marini et al., 2010; Bartoccini et al., 2011). Changes in SM levels and/or a shift in SM composition (from C24:0‐SM to C16:0‐SM) have been associated with a reduction in the expression of vitamin D receptors in nuclear LRM of tumour cells (Lazzarini et al., 2015) and embryonic hippocampal cell differentiation (Bartoccini et al., 2011). Whether these alterations in nuclear LRM are involved in neurodegeneration is not known. In the nervous system, LRM play a role in many processes, including neurotrophic factor signalling, cell adhesion and migration, axon guidance and myelin formation and stabilization (Aureli et al., 2015). Notably, recent evidence also suggests that LRM alterations are involved in neurodegenerative disorders including PD, AD, amyotrophic lateral sclerosis, Huntington's disease and prion diseases (Schengrund, 2010; Aureli et al., 2015; Marin et al., 2016).
Recent data have revealed that the crosstalk between S1P and 1,25(OH)2D3 also occurs in the extracellular fluids. It has been reported that patients with acute or chronic inflammation have low levels of plasma gelsolin (pGSN) (Osborn et al., 2008; Lee et al., 2009). Gelsolin has two isoforms with a similar structure and function: the cytoplasmic actin‐binding protein form, important for the regulation of cell shape and motility [cytoplasmatic gelsolin (cGSN)], and pGSN, a multifunctional protein that acts as an extracellular actin scavenger system crucial for the removal of actin released from injured cells (Chauhan et al., 2008; Carro, 2010). Although the functions of pGSN and the mechanisms of its protective action have not been clarified, it is clear that low levels of pGNS are an indicator of poor prognosis or critical care complications. Notably, pGNS is able to bind to S1P in humans. This pGSN‐S1P interaction in extracellular fluids may have several important consequences by impairing either the ability of gelsolin to bind actin or that of S1P to bind to S1P receptors. In fact, the pGSN‐S1P complex affects the S1P‐S1P1 receptor module that regulates lymphocyte distribution and the immunomodulatory balance at inflammatory sites (Bucki et al., 2010). It has been observed that patients suffering from lymphatic meningitis show low concentrations of pGSN and a high concentration of S1P in their CSF samples (Bucki et al., 2010). Notably, another recent study has demonstrated that 1,25(OH)2D3 treatment can affect either S1P and pGSN. In fact, the hormone alleviates inflammation in experimental allergic encephalomyelitis, a model of MS, and this therapeutic effect might be derived from the ability of the hormone to reduce S1P (which is elevated in the CSF and spinal cord of rats with experimental allergic encephalomyelitis). However, this effect might be limited by its simultaneous action in reducing pGSN and cGSN (Zhu et al., 2014).
Taken together, the accumulating evidence suggests that 1,25(OH)2D3 and SLs can converge and share some targets: (i) the activation of similar pathways (through activation of protein kinases); (ii) the modulation of enzyme expression/activity (i.e. cathepsin); (iii) the control of genes encoding for key enzymes of SLs metabolism and, probably, of S1P receptors by 1,25(OH)2D3; and (iv) the modulation by S1P‐dependent histone acetylation of vitamin D receptor‐dependent transcription. In addition, changes in the composition of SLs in LRM can also affect the localization and therefore the function of vitamin D receptors.
SLs/1,25(OH)2D3 crosstalk: potential role in neurogenerative diseases
AD
The actual most common form of dementia is AD, a neurodegenerative disorder of the CNS characterized by extracellular amyloid‐containing plaques, intracellular neurofibrillary tangles consisting of hyperphosphorylated tau protein and by the death of cholinergic neurons of the basal forebrain. Amyloid plaques are mainly formed by aggregated amyloid β peptide (Aβ) generated by the hydrolysis of the amyloid precursor protein (APP), first, by β‐secretase 1 and, then, by γ‐secretase. The fibrils of the senile plaques are mainly composed of the self‐assembled Aβ1–42 peptide that forms a heterogeneous mixture of oligomers and protofibrils. The small soluble Aβ1–42 oligomers are considered to be the major neurotoxic species in AD, and it has been hypothesized that cerebral accumulation of Aβ1–42 precedes and drives the deposition of the tau protein in neuronal perikarya and their processes (Selkoe and Hardy, 2016).
Alterations in the expression or in the activity of enzymes involved in the metabolism of SLs have been found in the brain of AD patients (Table 2, Figure 4). They include SMases (Katsel et al., 2007; He et al., 2010), ceramidase (CDase) (Huang et al., 2004), S1P lyase and SPHK (Ceccom et al., 2014), serine palmitoyl transferase, UDP‐glucose ceramide glucosyltransferase and CERS1,2,6 (Katsel et al., 2007; Couttas et al., 2016). Changes in SL content (ceramide, S1P, SM, gangliosides and sulfatides) have also been reported in animal models of AD (see ref. in Grimm et al., 2013) and in brain tissue and CSF of AD patients (Han et al., 2002; Cutler et al., 2004; Satoi et al., 2005; He et al., 2010; Mielke and Lyketsos, 2010; Couttas et al., 2014; Couttas et al., 2016; Fonteh et al., 2015).
The results of in vitro experiments indicate that Aβ1–42 directly binds and activates nSMase, decreasing SM content (Grimm et al., 2005). Aβ1–42 also activates aSMase through increased ROS accumulation via NADPH oxidase activation and reduced glutathion depletion (Jazvinšćak Jembrek et al., 2015). Ceramide generated by the degradation of SM due to the activation of SMase induces neuronal apoptosis (Jana and Pahan, 2004; Satoi et al., 2005; Malaplate‐Armand et al., 2006) or impairs autophagy (Yang et al., 2014). Also ceramide increases the stability, while S1P increases the activity of β‐secretase 1 (Puglielli et al., 2003; Takasugi et al., 2011). Moreover, SM decreases Aβ1–42 production by inhibiting the γ‐secretase (Grimm et al., 2005). Therefore, some SLs might be protective by lowering Aβ levels (either by decreasing its production or by increasing its clearance), while others might increase Aβ1–42 oligomerization and toxicity. Simultaneously, APP processing also leads to changes in lipid metabolism, resulting in complex regulatory feedback cycles, which appear to be dysregulated in AD (Grimm et al., 2013).
Recently, it has been demonstrated that exosome release in neural cells requires SMase activity (Wang et al., 2012). The role of exosomes in AD is controversial. One study has shown that in vitro neuronal exosomes are able to capture Aβ, and their infusion into brains of AD mice decreases Aβ and amyloid depositions (Yuyama et al., 2015). More recently, it has been suggested that ceramide‐enriched exosomes promote the aggregation of Aβ (Dinkins et al., 2016), since an AD mouse model lacking nSMase2 exhibits a decreased exosome release associated with a reduced plaque burden and improved cognition (Dinkins et al., 2016).
Increasing evidence derived from epidemiological studies indicates that 1,25(OH)2D3 deficiency and vitamin D receptor polymorphisms influence susceptibility to AD (Gezen‐Ak et al., 2012; Annweiler et al., 2014), whereas Aβ1–42 may disrupt the hormone‐vitamin D receptor pathway and cause defective utilization of 1,25(OH)2D3 by suppressing the level of vitamin D receptors, and by elevating the level of 24‐hydroxylase, and, thereby, increasing the catabolism of the hormone (Dursun et al., 2011; 2013a). In addition to its neuroprotective effects involving calcium homeostasis, a decrease in ROS and inflammation, 1,25(OH)2D3 is able to exert other specific effects important for AD. For example, 1,25(OH)2D3 may regulate the expression of many genes associated with AD and attenuate the build up of Aβ deposits either by enhancing their clearance (transport to the blood or to the CSF) or by stimulating the phagocytosis of Aβ. It is likely that the hormone alters APP processing and prevents the defects in ACh by increasing the activity of choline acetyltransferase (thus ACh synthesis) in the brain (Briones and Darwish, 2012; Annweiler et al., 2014; Durk et al., 2014; Landel et al., 2016).
Epigenetic modifications are involved in the regulation of many genes, and aberrant epigenetic changes are associated with AD. For example, hyperacetylation of histone H4 at lysine 12 in peripheral monocytes appears to be an early event in AD‐pathology (Plagg et al., 2015). Recently, HDAC inhibitors have emerged as promising compounds for restoring the cognitive deficits in a mouse model of AD (Kilgore et al., 2010). Therefore, upon treatment with 1,25(OH)2D3 or/and FTY7120, a possible effect on acetylation and DNA methylation of AD‐related genes (i.e. β‐secretase 1) could result in beneficial effects against Aβ‐induced toxicity.
Niemann–Pick disease type C (NPC)
Niemann–Pick disease type C (NPC) is an autosomal recessive storage disorder due to mutations of two proteins NPC1 and NPC2, which mediate intracellular cholesterol trafficking in mammals. NPC is characterized by abnormal sequestration of unesterified cholesterol within the late endolysosomes and the accumulation of sphingosine and gangliosides, the formation of meganeurites and neurofibrillary tangles, neuroinflammation and axonal dystrophy. As the disease progresses, neuronal death of Purkinje cells of the cerebellum becomes prominent. AD and NPC share some molecular pathways, including abnormal cholesterol metabolism, and the involvement of Aβ and tau (Malnar et al., 2014; Vanier, 2015). Miglustat is an inhibitor of the enzyme glucosylceramide synthase that converts ceramide into glucosyl ceramide (Figure 1), the first step in the synthesis of gangliosides. Miglustat has neuroprotective effects on NPC models (Table 2). It has been approved for use in several cases of gangliosidosis and, recently, for NPC (Patterson et al., 2015). Therefore, SLs appear to play a role in the pathogenesis of NPC. Indeed, it has been demonstrated that SM inhibits, while ceramide increases NPC2‐mediated cholesterol transport (Abdul‐Hammed et al., 2010). Moreover, Lloyd‐Evans et al. (2008) have proposed that sphingosine, by altering calcium homeostasis, could play a role in the onset of NPC.
It is possible that 1,25(OH)2D3 and S1P could have some protective effect on NPC. It is already known that stem cells induce survival of cerebellar NPC1−/− cells (Lee et al., 2010) by increasing S1P and, as discussed above, that 1,25(OH)2D3 is able to reduce Aβ load in AD models. In addition, autophagy is dysregulated in NPC, and 1,25(OH)2D3 exerts some neuroprotective effects through the modulation of autophagy (Li et al., 2015a).
PD
PD is a neurodegenerative disease characterized by loss of dopamine cells in the basal ganglia, and the accumulation and/or aggregation of α‐synuclein. Mutations in genes causing lysosomal storage disorders, such as those encoding GCDase A, aSMase and NPC1, may increase the risk for developing PD (for a recent review, see Migdalska‐Richards and Schapira, 2016). Moreover, reduced GCDase activity (GBA1) has been found in patients with sporadic PD (Murphy et al., 2014; Table 2). The concentration of glucosyl ceramide and that of α‐synuclein are inversely correlated. Total ceramide and SM levels are reduced in the anterior cingulate cortex of PD patients compared with controls (Abbott et al., 2014). A shift towards ceramide containing short acyl chains and an up‐regulation of the expression of the Cer1S gene (which could be a compensatory effect to the reduction in ceramide) have also been reported (Abbott et al., 2014). S1P and 1,25(OH)2D3 have a neuroprotective effect on cellular models of PD (Shinpo et al., 2000; Smith et al., 2006; Pyszko and Strosznajder, 2014; see Table 3). Also 1,25(OH)2D3 has shown neuroprotection in different animal models of PD that have been correlated with increases in GDNF, increases in tyroxine hydroxylase expressing cells and a decrease in inflammation (Wang et al., 2001; Kim et al., 2006; Smith et al., 2006). Furthermore, 1,25(OH)2D3 supplementation was associated with a significantly reduced risk of PD (Shen and Ji, 2015a). The mechanism by which a deficiency in GCDase increases the risk of developing PD is still unclear, but it is known that glucosyl ceramide can stabilize α‐synuclein oligomers (Mazzulli et al., 2011) and that activation of GCDase reduces the accumulation of α‐synuclein and restores lysosomal function in vitro (Mazzulli et al., 2016). Indeed, small increases in glucosyl ceramide and glucosyl sphingosine have been reported in primary cultured cortical neurons with GCDase knockdown (Mazzulli et al., 2011), in dopaminergic neurons harbouring heterozygote GCDase/GBA1 mutations (Schöndorf et al., 2014) and in the hippocampus of PD patients without a GBA1 mutation (Rocha et al., 2015) (Table 2). Another possibility is that the changes in SL metabolism derived from GCDase deficiency impair autophagy, which has been suggested to contribute to α‐synuclein accumulation in cellular and animal models of GCase deficiency (Mazzulli et al., 2011; Schöndorf et al., 2014).
Table 3.
Effects of 1,25(OH)2D3 on nervous system differentiation, protection and proliferation
| Differentiation | |||
|---|---|---|---|
| Cell | Effect | Mechanism | References |
| Primary embryonic hippocampal | – | – | – |
| cells | ↑ neurite outgrowth | ↑NGF | Brown et al., 2003 |
| OPC | ↑ differentiation | ↑MBP | de la Fuente et al., 2015 |
| Neuronal stem cell | ↑ differentiation to | – | – |
| – | oligodendrocytes | ↑ CNTF | Shirazi et al., 2015 |
| Schwann cells | ↑ differentiation | ↑ IGF‐1 | Hao et al., 2015 |
| HN9.10e | ↑ neurite outgrowth | ↑ NGF, Bcl‐2 | Marini et al., 2010 |
| Protection | |||
|---|---|---|---|
| Animal/Cell | Stimulus | Mechanism | References |
| Murine experimental | – | – | – |
| allergic encephalomyelitis | MBP | nd | Lemire and Archer, 1991 |
| Dopaminergic cell | MPTP+, sulfoximine | ↓ROS, ↑glutathion | Shinpo et al., 2000 |
| Mesencephalic cell | 6OH‐DA, | ↑TH, ↑arborization | Wang et al., 2001 |
| Hippocampal neuron | NMDA, glutamate | ↓LVCC | Brewer et al., 2001 |
| Substantia nigra | Zn | ↓lipid peroxidation, | Lin et al., 2003 |
| – | – | ↑ DA | – |
| Cortex | ischaemia | ↑HO‐1, ↓ GFAP | Oermann et al., 2004 |
| Cortical neuron | glutamate | ↑MAP2, ↑GAP‐43, | Taniura et al., 2006 |
| – | – | ↑synapsin 1, ↑VDR | – |
| Rat, substantia nigra | 6OH‐DA | ↑DA | Smith et al., 2006 |
| rat, mice | MPTP | ↓ microglia activation, | Kim et al., 2006 |
| – | – | ↓TNFα mRNA, ↓INFγ mRNA | – |
| cortical cells | cyanide | ↓ uncoupling, ↑Ikkb | Li et al., 2008 |
| hippocampus | glutamate, ischaemia | ↓ caspase‐3 | Kajta et al., 2009 |
| mesencephalic neuron | – | ↑GDNF | Orme et al., 2013 |
| rat hippocampus | ischaemia/reperfusion | GluN3A, ERK, pCREB | Fu et al., 2013 |
| SH‐SY5Y | rotenone | ↑ autophagy | Jang et al., 2014 |
| – | – | ↑LC3, beclin, AMPK | – |
| Neuron–glia | endotoxin | ↓ MAPK, ↓iNOS, | Huang et al., 2015b |
| – | – | ↓IL‐6, ↓MIP‐2 mRNA | – |
| mouse | MPTP | ↑ autophagy | Li et al., 2015b |
| Cortex slices | hyperhomocysteinaemia | ↓ ROS, ↓ iNOS | Longoni et al., 2016 |
| Schwann | high glucose | ↑ CBS, ↑H2S, ↓ ROS | Zhang et al., 2016 |
| – | methylglyoxal | – | – |
| Tg2576 and TgCRND8 mice | – | ↓plaque formation, | Durk et al., 2014 |
| – | – | ↓ lower soluble Aβ levels, | – |
| – | – | ↑ P‐glycoprotein | – |
| AD mouse | (AbPP) | ↓decrease memory deficit | Yu et al., 2011 |
| – | – | ↓plaque formation, ↑NGF | – |
| – | – | ↓ inflammation | – |
| Ageing rats | – | ↓decrease memory deficit | Latimer et al., 2014 |
| – | – | Modulation pro‐inflammatory cytokines | Briones and Darwish, 2012 |
| – | – | ↓decrease amyloid | – |
| – | – | ↓decrease amyloid | Yu et al., 2011 |
| Hippocampal neurons and cortical neurons | Aβ | ↓cytotoxicity ↓iNOS | Dursun et al., 2011, 2013a,b |
| – | – | ↓ LVCC A1C, ↑VDR | |
| Mouse retina | – | ↑ phagocytosis, ↓ Aβ | Lee et al., 2012 |
| AD macrophages | – | Modulation IL‐1, IL‐1R | Mizwicki et al., 2012, 2013 |
| – | – | ↑ phagocytosis, ↓ Aβ | – |
| bEnd.3 cells | – | ↑Aβ1–40 brain‐to‐blood efflux | – |
| – | – | of amyloid‐β (Aβ) peptide | Guo et al., 2016 |
| – | – | LRP1 and RAGE regulation | – |
| Proliferation | |||
|---|---|---|---|
| Cell/animal | Action | Mechanism | References |
| Neuroblastoma cells | ↓ | CERK | Bini et al., 2012 |
| – | – | nd | Gumireddy et al., 2003a,b |
| – | – | – | Celli et al., 1999a,b |
| – | – | – | Stio et al., 2001 |
| Stem cells | ↑ | ↑NT‐3, BDNF, GDNF and CNTF | Shirazi et al., 2015 |
| Primary embryonic | ↓ | – | Brown et al., 2003 |
| hippocampal cells | – | – | – |
| 1,25(OH)2D3 ‐deprived | – | – | – |
| embryos E19, brain | ↑ | ↑cyclin D | Ko et al., 2004 |
| – | – | ↓ cyclin B, ↓p21 | – |
| Glioblastoma cells | ↑, no effect | – | Diesel et al., 2005 |
| 1,25(OH)2D3‐deprived | ↓ | – | Cui et al., 2007 |
| neuroprogenitors, SVZ | – | – | – |
| 1α‐hydroxylase knockout | ↑ | – | Zhu et al., 2012 |
| Mouse, dentate gyrus | – | – | – |
The table illustrates the effect of 1,25(OH)2D3 in cell types and the mechanism involved. The noxius agent is listed under Stimulus. ↑, increase; ↓, decrease; 6‐OHDA, 6‐hydroxydopamine; AMPK, AMP‐dependent PK; CNTF, ciliary derived neurotrophic factor; bEnd.3, mouse brain microvascular endothelial cell line; DRG, dorsal root ganglion; HO, hemoxygenase; iNOS, inducible nitric oxide synthase; LRP1, low‐density lipoprotein receptor‐related protein 1; MBP, myelin basic protein; MPTP, 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine; nd, not determined; OPC, oligodendrocyte precursor cell; RAGE, receptor for advanced glycation end products, SVZ, subventricular zone.
SLs, in particular S1P, and 1,25(OH)2D3 display their neuroprotective actions through common effectors such as calcium regulation, synaptic modulation and growth factor expression, but whether 1,25(OH)2D3 and SLs could act synergistically on neuroprotection and/or neurogenesis in neurodegenerative diseases, such as AD, PD and NPC, is still unknown and deserves further investigation. Preliminary results in our laboratory indicate that the crosstalk between SLs and 1,25(OH)2D3 leads to a specific balance between neurodegeneration/neuroprotection in neuronal cells. In particular, in human SH‐SY5Y differentiated cells, we found that 1,25(OH)2D3 treatment counteracts the down‐regulation of S1P1‐mediated signalling promoted by Aβ1–42 (Pierucci et al., 2017).
Potential implications for 1,25(OH)2D3 and FTY720 supplementation in AD
Several observations in clinical trials have demonstrated that 1,25(OH)2D3 supplementation may have protective effects in AD; however, in other studies, no beneficial outcome has been reported (DeLuca et al., 2013; Landel et al., 2016), and evidence for a correlation between hypovitaminosis D and reduced neuroprotection against AD or AD progression is missing. Similarly, the ability of 1,25(OH)2D3 supplementation to prevent other neurodegenerative diseases, such as MS, needs further investigation. Moreover, some data suggest that the combination of 1,25(OH)2D3 supplementation with the anti‐neurodegenerative drug nemantidine could counteract the cognitive decline better than that of the single compound (Annweiler et al., 2014).
On the contrary, the neuroprotective effect of S1P analogues on neurodegenerative diseases is well established. Fingolimod, the commercial name of FTY720, is an analogue of sphingosine, which acts as an immunosuppressant and has recently been approved for the treatment of MS. The phosphorylation of FTY720 by SPHK generates FTY720‐phosphate, a molecule structurally similar to S1P that can bind to all the S1P receptors, except the S1P2 receptor. In lymph nodes, it acts as a highly potent functional antagonist of the S1P1 receptor, leading to S1P1 receptor internalization in T cells that become unable to egress from the nodes. Also FTY720 is active on different cells of the nervous system, including neurons, astrocytes, oligodendrocytes and microglia (Brunkhorst et al., 2014), and its protective function affects the process of myelination, the activation of microglia, proliferation and migration of precursor cells, neuronal differentiation and survival (Kawabori et al., 2013). In vivo, it has been shown that experimental allergic encephalomyelitis was attenuated by FTY720 supplementation, and no effect was observed on astrocytes that did not express S1P1 receptors. However, neurons lacking S1P1 receptors were positively affected by the compound (Choi et al., 2011). In vitro, FTY720 decreases Aβ production in cultured neuronal cells (Takasugi et al., 2013).
Regarding its therapeutic potential in AD, it has been reported that when FTY720 supplementation was given to rats injected with Aβ1–42, there was a reduction in cell death in the hippocampus and cortex as well as an increase in memory compared with control rats (Asle‐Rousta et al., 2013; Hemmati et al., 2013). The in vivo beneficial effect on the nervous system is due to many factors including an increase in BDNF production, which leads to an increase in striatum size (Deogracias et al., 2012) and facilitates neuronal repair in diseases associated with decreased levels of BDNF, such as Huntington's disease (Di Pardo et al., 2014; Miguez et al., 2015). FTY720 also reduces α‐synuclein aggregation, in part by increasing BDNF levels (Vidal‐Martínez et al., 2016).
Recently, it has been shown that FTY720 also has inhibitory effects on epigenetic modifications by reducing HDAC and regulating gene expression programmes associated with memory and learning (Hait et al., 2014). All together, these observations lead us to speculate that 1,25(OH)2D3 supplementation and FTY720 could act synergistically in the prevention of neurodegenerative diseases. Preliminary studies in vivo performed in our laboratory suggest the possibility of an interaction between the hormone and FTY720. In fact, damage was reduced when 1,25(OH)2D3 supplementation in mice injected with a submaximal dose of Aβ1–42 was combined with FTY720 treatment. Further investigations are in progress (Meacci et al., personal communication).
In conclusion, the potential for expanding the use of 1,25(OH)2D3 to treat neurodegenerative diseases is worth investigating. Additionally, the therapeutic potential of the structural analogues of 1,25(OH)2D3 (see ref. in Leyssens et al., 2014) remains unexplored. In the long term, 1,25(OH)2D3 and its analogues might provide valuable tools either for basic research into the elucidation of the mechanisms of neuroprotection and for subsequent designer drug development. The combined treatments with 1,25(OH)2D3 and agonists/antagonists of S1P1–5 receptors and the improvement in the characterization and quantification of ceramide species may offer significant advances in terms of understanding, and the ability to predict, the protein aggregation‐induced toxicity in vivo.
Author contributions
M.G.‐G. and E.M. conceived the outline of the review and wrote the manuscript. F.P. and A.V. contributed to some parts of the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
We thank Dr Alessia Frati for her contribution to the design of the figures. This work was supported by local grants from the University of Pisa to MGG and from the University of Firenze and MIUR to E.M.
Garcia‐Gil, M. , Pierucci, F. , Vestri, A. , and Meacci, E. (2017) Crosstalk between sphingolipids and vitamin D3: potential role in the nervous system. British Journal of Pharmacology, 174: 605–627. doi: 10.1111/bph.13726.
References
- Acharya U, Patel S, Koundakjian E, Nagashima K, Han X, Acharya JK (2003). Modulating sphingolipid biosynthetic pathway rescues photoreceptor degeneration. Science 299: 1740–1743. [DOI] [PubMed] [Google Scholar]
- Abbott SK, Li H, Muñoz SS, Knoch B, Batterham M, Murphy KE et al. (2014). Altered ceramide acyl chain length and ceramide synthase gene expression in Parkinson's disease. Mov Disord 29: 518–526. [DOI] [PubMed] [Google Scholar]
- Abdul‐Hammed M, Breiden B, Adebayo MA, Babalola JO, Schwarzmann G, Sandhoff K (2010). The roles of endosomal membrane lipids and NPC2 in cholesterol transfer and membrane fusion. J Lipid Res 51: 1747–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albi E, Villani M (2009). Nuclear lipid microdomains regulate cell function. Commun Integr Biol 2: 23–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albi E, Lazzarini A, Lazzarini R, Floridi A, Damaskopoulou E, Curcio F et al. (2013). Nuclear lipid microdomain as place of interaction between sphingomyelin and DNA during liver regeneration. Int J Mol Sci 14: 6529–6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfonso J, Penkert H, Duman C, Zuccotti A, Monyer H (2015). Downregulation of sphingosine 1‐phosphate receptor 1 promotes the switch from tangential to radial migration in the OB. J Neurosci 35: 13659–13672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Peters JA, Kelly E, Marrion N, Benson HE, Faccenda E et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Ligand‐gated ion channels. Br J Pharmacol 172: 5870–5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Catterall WA, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015c). The Concise Guide to PHARMACOLOGY 2015/16: Voltage‐gated ion channels. Br J Pharmacol 172: 5904–5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Cidlowski JA, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015d). The Concise Guide to PHARMACOLOGY 2015/16: Nuclear hormone receptors. Br J Pharmacol 172: 5956–5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015e). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015f). The Concise Guide to PHARMACOLOGY 2015/16: Overview. Br J Pharmacol 172: 5729–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annweiler C, Karras SN, Anagnostis P, Beauchet O (2014). Vitamin D supplements: a novel therapeutic approach for Alzheimer patients. Front Pharmacol 5: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asle‐Rousta M, Kolahdooz Z, Oryan S, Ahmadiani A, Dargahi L (2013). FTY720 (fingolimod) attenuates beta‐amyloid peptide (Aβ42)‐induced impairment of spatial learning and memory in rats. J Mol Neurosci 50: 524–532. [DOI] [PubMed] [Google Scholar]
- Aureli M, Grassi S, Prioni S, Sonnino S, Prinetti A (2015). Lipid membrane domains in the brain. Biochim Biophys Acta 1851: 1006–1016. [DOI] [PubMed] [Google Scholar]
- Bartoccini E, Marini F, Damaskopoulou E, Lazzarini R, Cataldi S, Cascianelli G et al. (2011). Nuclear lipid microdomains regulate nuclear vitamin D3 uptake and influence embryonic hippocampal cell differentiation. Mol Biol Cell 22: 3022–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth BM, Gustafson SJ, Hankins JL, Kaiser JM, Haakenson JK, Kester M et al. (2012). Ceramide kinase regulates TNFα‐stimulated NADPH oxidase activity and eicosanoid biosynthesis in neuroblastoma cells. Cell Signal 24: 1126–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben‐David O, Futerman AH (2010). The role of the ceramide acyl chain length in neurodegeneration: involvement of ceramide synthases. Neuromolecular Med 124: 341–350. [DOI] [PubMed] [Google Scholar]
- Bi X, Shi Q, Zhang H, Bao Y, Hu D, Pohl N et al. (2016). c‐Jun NH2‐teminal kinase 1 interacts with vitamin D receptor and affects vitamin D‐mediated inhibition of cancer cell proliferation. J Steroid Biochem Mol Biol 163: 164–172. [DOI] [PubMed] [Google Scholar]
- Bieberich E (2012). It's a lipid's world: bioactive lipid metabolism and signaling in neural stem cell differentiation. Neurochem Res 37: 1208–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielawska A, Linardic CM, Hannun YA (1992). Modulation of cell growth and differentiation by ceramide. FEBS Lett 307: 211–214. [DOI] [PubMed] [Google Scholar]
- Bini F, Frati A, Garcia‐Gil M, Battistini C, Granado M, Martinesi M et al. (2012). New signalling pathway involved in the anti‐proliferative action of vitamin D₃ and its analogues in human neuroblastoma cells. A role for ceramide kinase. Neuropharmacology 63: 524–537. [DOI] [PubMed] [Google Scholar]
- Bock J, Szabó I, Gamper N, Adams C, Gulbins E (2003). Ceramide inhibits the potassium channel Kv1.3 by the formation of membrane platforms. Biochem Biophys Res Commun 305: 890–897. [DOI] [PubMed] [Google Scholar]
- Brewer LD, Thibault V, Chen KC, Langub MC, Landfield PW, Porter NM (2001). Vitamin D hormone confers neuroprotection in parallel with downregulation of L‐type calcium channel expression in hippocampal neurons. J Neurosci 21: 98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briones TL, Darwish H (2012). Vitamin D mitigates age‐related cognitive decline through the modulation of pro‐inflammatory state and decrease in amyloid burden. J Neuroinflammation 9: 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J, Bianco JI, McGrath JJ, Eyles DW (2003). 1,25‐dihydroxyvitamin D3 induces nerve growth factor, promotes neurite outgrowth and inhibits mitosis in embryonic rat hippocampal neurons. Neurosci Lett 343: 139–143. [DOI] [PubMed] [Google Scholar]
- Brunkhorst R, Vutukuri R, Pfeilschifter W (2014). Fingolimod for the treatment of neurological diseases‐state of play and future perspectives. Front Cell Neurosci 8: 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucki R, Kulakowska A, Byfield FJ, Zendzian‐Piotrowska M, Baranowski M, Marzec M et al. (2010). Plasma gelsolin modulates cellular response to sphingosine 1‐phosphate. Am J Physiol Cell Physiol 299: C1516–C1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton JM, Costello FE (2015). Vitamin D in multiple sclerosis and central nervous system demyelinating disease – a review. J Neuroophthalmol 35: 194–200. [DOI] [PubMed] [Google Scholar]
- Carlberg C, Campbell MJ (2013). Vitamin D receptor signaling mechanisms: integrated actions of a well‐defined transcription factor. Steroids 78: 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco P, Sahún I, McDonald J, Ramírez S, Jacas J, Gratacós E et al. (2012). Ceramide levels regulated by carnitine palmitoyltransferase 1C control dendritic spine maturation and cognition. J Biol Chem 287: 21224–21232. doi: 10.1074/jbc.M111.337493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carro E (2010). Gelsolin as therapeutic target in Alzheimer's disease. Expert Opin Ther Targets 14: 585–592. [DOI] [PubMed] [Google Scholar]
- Cascianelli G, Villani M, Tosti M, Marini F, Bartoccini E, Magni MV et al. (2008). Lipid microdomains in cell nucleus. Mol Biol Cell 19: 5289–5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldi S, Codini M, Cascianelli G, Tringali S, Tringali AR, Lazzarini A et al. (2014). Nuclear lipid microdomain as resting place of dexamethasone to impair cell proliferation. Int J Mol Sci 15: 19832–19846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccom J, Loukh N, Lauwers‐Cances V, Touriol C, Nicaise Y, Gentil C et al. (2014). Reduced sphingosine kinase‐1 and enhanced sphingosine 1‐phosphate lyase expression demonstrate deregulated sphingosine 1‐phosphate signaling in Alzheimer's disease. Acta Neuropathol Commun 2: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celli A, Treves C, Nassi P, Stio M (1999b). Role of 1,25‐dihydroxyvitamin D3 and extracellular calcium in the regulation of proliferation in cultured SH‐SY5Y human neuroblastoma cells. Neurochem Res 24: 691–698. [DOI] [PubMed] [Google Scholar]
- Celli A, Treves C, Stio M (1999a). Vitamin D receptor in SH‐SY5Y human neuroblastoma cells and effect of 1,25‐dihydroxyvitamin D3 on cellular proliferation. Neurochem Int 34: 117–124. [DOI] [PubMed] [Google Scholar]
- Chalfant CE, Rathman K, Pinkerman RL, Wood RE, Obeid LM, Ogretmen B et al. (2002). De novo ceramide regulates the alternative splicing of caspase 9 and Bcl‐x in A549 lung adenocarcinoma cells. Dependence on protein phosphatase‐1. J Biol Chem 277: 12587–12595. [DOI] [PubMed] [Google Scholar]
- Chan JP, Sieburth D (2012). Localized sphingolipid signaling at presynaptic terminals is regulated by calcium influx and promotes recruitment of priming factors. J Neurosci 32: 17909–17920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan V, Ji L, Chauhan A (2008). Anti‐amyloidogenic, anti‐oxidant and anti‐apoptotic role of gelsolin in Alzheimer's disease. Biogerontology 9: 381–389. [DOI] [PubMed] [Google Scholar]
- Choi JW, Gardell SE, Herr DR, Rivera R, Lee CW, Noguchi K et al. (2011). FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1‐phosphate receptor 1 (S1P1) modulation. Proc Natl Acad Sci U S A 108: 751–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G (2016). Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev 96: 365–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claycomb KI, Johnson KM, Winokur PN, Sacino AV, Crocker SJ (2013). Astrocyte regulation of CNS inflammation and remyelination. Brain Sci 3: 1109–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombaioni L, Garcia‐Gil M (2004). Sphingolipid metabolites in neural signalling and function. Brain Res Brain Res Rev 46: 328–355. [DOI] [PubMed] [Google Scholar]
- Colombini M (2016). Ceramide channels and mitochondrial outer membrane permeability. J Bioenerg Biomembr . doi: 10.1007/s10863-016-9646-z. [DOI] [PubMed] [Google Scholar]
- Couttas TA, Kain N, Daniels B, Lim XY, Shepherd C, Kril J et al. (2014). Loss of the neuroprotective factor sphingosine 1‐phosphate early in Alzheimer's disease pathogenesis. Acta Neuropathol Commun 2: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couttas TA, Kain N, Suchowerska AK, Quek LE, Turner N, Fath T et al. (2016). Loss of ceramide synthase 2 activity, necessary for myelin biosynthesis, precedes tau pathology in the cortical pathogenesis of Alzheimer's disease. Neurobiol Aging 43: 89–100. [DOI] [PubMed] [Google Scholar]
- Culmsee C, Gerling N, Lehmann M, Nikolova‐Karakashian M, Prehn JH, Mattson MP et al. (2002). Nerve growth factor survival signaling in cultured hippocampal neurons is mediated through TrkA and requires the common neurotrophin receptor P75. Neuroscience 115: 1089–1108. [DOI] [PubMed] [Google Scholar]
- Cui X, McGrath JJ, Burne TH, Mackay‐Sim A, Eyles DW (2007). Maternal vitamin D depletion alters neurogenesis in the developing rat brain. Int J Dev Neurosci 25: 227–232. [DOI] [PubMed] [Google Scholar]
- Cutler RG, Kelly J, Storie K, Pedersen WA, Tammara A, Hatanpaa K et al. (2004). Involvement of oxidative stress‐induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer's disease. Proc Natl Acad Sci U S A 101: 2070–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S et al. (1996). Suppression of ceramide‐mediated programmed cell death by sphingosine‐1‐phosphate. Nature 381: 800–803. [DOI] [PubMed] [Google Scholar]
- D'Arcangelo G, Grossi D, Racaniello M, Cardinale A, Zaratti A, Rufini S et al. (2016). Miglustat reverts the impairment of synaptic plasticity in a mouse model of NPC disease. Neural Plast 2016 .3830424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daido S, Kanzawa T, Yamamoto A, Takeuchi H, Kondo Y, Kondo S (2004). Pivotal role of the cell death factor BNIP3 in ceramide‐induced autophagic cell death in malignant glioma cells. Cancer Res 64: 4286–4293. [DOI] [PubMed] [Google Scholar]
- DeLuca GC, Kimball SM, Kolasinski J, Ramagopalan SV, Ebers GC (2013). Review: the role of vitamin D in nervous system health and disease. Neuropathol Appl Neurobiol 39: 458–484. [DOI] [PubMed] [Google Scholar]
- de la Fuente AG, Errea O, van Wijngaarden P, Gonzalez GA, Kerninon C, Jarjour AA et al. (2015). Vitamin D receptor‐retinoid X receptor heterodimer signaling regulates oligodendrocyte progenitor cell differentiation. J Cell Biol 211: 975–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deogracias R, Yazdani M, Dekkers MP, Guy J, Ionescu MC, Vogt KE et al. (2012). Fingolimod, a sphingosine‐1 phosphate receptor modulator, increases BDNF levels and improves symptoms of a mouse model of Rett syndrome. Proc Natl Acad Sci U S A 109: 14230–14235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplats PA, Denny CA, Kass KE, Gilmartin T, Head SR, Sutcliffe JG et al. (2007). Glycolipid and ganglioside metabolism imbalances in Huntington's disease. Neurobiol Dis 27: 265–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pardo A, Amico E, Favellato M, Castrataro R, Fucile S, Squitieri F (2014). FTY720 (fingolimod) is a neuroprotective and disease‐modifying agent in cellular and mouse models of Huntington disease. Hum Mol Genet 23: 2251–2265. [DOI] [PubMed] [Google Scholar]
- Diesel B, Radermacher J, Bureik M, Bernhardt R, Seifert M, Reichrath J et al. (2005). Vitamin D(3) metabolism in human glioblastoma multiforme: functionality of CYP27B1 splice variants, metabolism of calcidiol, and effect of calcitriol. Clin Cancer Res 11: 5370–5380. [DOI] [PubMed] [Google Scholar]
- Dinkins MB, Enasko J, Hernandez C, Wang G, Kong J, Helwa I et al. (2016). Neutral sphingomyelinase‐2 deficiency ameliorates Alzheimer's disease pathology and improves cognition in the 5XFAD mouse. J Neurosci 36: 8653–8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolezalova H, Shankar G, Huang MC, Bikle DD, Goetzl EJ (2003). Biochemical regulation of breast cancer cell expression of S1P2 (Edg‐5) and S1P3 (Edg‐3) G protein‐coupled receptors for sphingosine 1‐phosphate. J Cell Biochem 88: 732–743. [DOI] [PubMed] [Google Scholar]
- Dukala DE, Soliven B (2016). S1P1 deletion in oligodendroglial lineage cells: Effect on differentiation and myelination. Glia 64: 570–582. [DOI] [PubMed] [Google Scholar]
- Dumitru CA, Sandalcioglu IE, Wagner M, Weller M, Gulbins E (2009). Lysosomal ceramide mediates gemcitabine‐induced death of glioma cells. J Mol Med 87: 1123–1132. [DOI] [PubMed] [Google Scholar]
- Durk MR, Han K, Chow EC, Ahrens R, Henderson JT, Fraser PE et al. (2014). 1α,25‐Dihydroxyvitamin D3 reduces cerebral amyloid‐β accumulation and improves cognition in mouse models of Alzheimer's disease. J Neurosci 34: 7091–7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dursun E, Gezen‐Ak D, Yilmazer S (2011). A novel perspective for Alzheimer's disease: vitamin D receptor suppression by amyloid‐β and preventing the amyloid‐β induced alterations by vitamin D in cortical neurons. J Alzheimers Dis 23: 207–219. [DOI] [PubMed] [Google Scholar]
- Dursun E, Gezen‐Ak D, Yilmazer S (2013a). Beta amyloid suppresses the expression of the vitamin d receptor gene and induces the expression of the vitamin D catabolic enzyme gene in hippocampal neurons. Dement Geriatr Cogn Disord 36: 76–86. [DOI] [PubMed] [Google Scholar]
- Dursun E, Gezen‐Ak D, Yilmazer S (2013b). A new mechanism for amyloid‐β induction of iNOS: vitamin D‐vitamin D receptor pathway disruption. J Alzheimers Dis 36: 459–474. [DOI] [PubMed] [Google Scholar]
- Edsall LC, Pirianov GG, Spiegel S (1997). Involvement of sphingosine 1‐phosphate in nerve growth factor‐mediated neuronal survival and differentiation. J Neurosci 17: 6952–6960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyles DW, Burne TH, McGrath JJ (2013). Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol 34: 47–64. [DOI] [PubMed] [Google Scholar]
- Fan M, Sidhu R, Fujiwara H, Tortelli B, Zhang J, Davidson C et al. (2013). Identification of Niemann–Pick C1 disease biomarkers through sphingolipid profiling. J Lipid Res 54: 2800–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes de Abreu DA, Nivet E, Baril N, Khrestchatisky M, Roman F, Féron F (2010). Developmental vitamin D deficiency alters learning in C57Bl/6J mice. Behav Brain Res 208: 603–608. [DOI] [PubMed] [Google Scholar]
- Fonteh AN, Ormseth C, Chiang J, Cipolla M, Arakaki X, Harrington MG (2015). Sphingolipid metabolism correlates with cerebrospinal fluid Beta amyloid levels in Alzheimer's disease. PLoS One 10: e0125597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Xue R, Gu J, Xiao Y, Zhong H, Pan X et al. (2013). Neuroprotective effect of calcitriol on ischemic/reperfusion injury through the NR3A/CREB pathways in the rat hippocampus. Mol Med Rep 8: 1708–1714. [DOI] [PubMed] [Google Scholar]
- Garcia‐Gil M, Lazzarini A, Lazzarini R, Floridi E, Cataldi S, Floridi A et al. (2015). Serum deprivation alters lipid profile in HN9.10e embryonic hippocampal cells. Neurosci Lett 589: 83–87. [DOI] [PubMed] [Google Scholar]
- García‐Ruiz C, Colell A, Marí M, Morales A, Fernández‐Checa JC (1997). Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. Role of mitochondrial glutathione. J Biol Chem 272: 11369–11377. [DOI] [PubMed] [Google Scholar]
- Gezen‐Ak D, Dursun E, Bilgiç B, Hanağasi H, Ertan T, Gürvit H et al. (2012). Vitamin D receptor gene haplotype is associated with late‐onset Alzheimer's disease. Tohoku J Exp Med 228: 189–196. [DOI] [PubMed] [Google Scholar]
- Ghasemi R, Dargahi L, Ahmadiani A (2016). Integrated sphingosine‐1 phosphate signaling in the central nervous system: from physiological equilibrium to pathological damage. Pharmacol Res 104: 156–164. [DOI] [PubMed] [Google Scholar]
- Ginkel C, Hartmann D, vom Dorp K, Zlomuzica A, Farwanah H, Eckhardt M et al. (2012). Ablation of neuronal ceramide synthase 1 in mice decreases ganglioside levels and expression of myelin‐associated glycoprotein in oligodendrocytes. J Biol Chem 287: 41888–41902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani P, Ferraretto A, Gravaghi C, Bassi R, Tettamanti G, Riboni L et al. (2007). Sphingosine‐1‐phosphate and calcium signaling in cerebellar astrocytes and differentiated granule cells. Neurochem Res 32: 27–37. [DOI] [PubMed] [Google Scholar]
- Grecksch G, Rüthrich H, Höllt V, Becker A (2009). Transient prenatal vitamin D deficiency is associated with changes of synaptic plasticity in the dentate gyrus in adult rats. Psychoneuroendocrinology 34 (Suppl 1): S258–S264. [DOI] [PubMed] [Google Scholar]
- Grimm MO, Grimm HS, Pätzold AJ, Zinser EG, Halonen R, Duering M et al. (2005). Regulation of cholesterol and sphingomyelin metabolism by amyloid‐beta and presenilin. Nat Cell Biol 7: 1118–1123. [DOI] [PubMed] [Google Scholar]
- Grimm MO, Zimmer VC, Lehmann J, Grimm HS, Hartmann T (2013). The impact of cholesterol, DHA, and sphingolipids on Alzheimer's disease. Biomed Res Int 2013: 814390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves NJ, Kesby JP, Eyles DW, McGrath JJ, Mackay‐Sim A, Burne TH (2013). Adult vitamin D deficiency leads to behavioural and brain neurochemical alterations in C57BL/6 J and BALB/c mice. Behav Brain Res 241: 120–131. [DOI] [PubMed] [Google Scholar]
- Gu L, Huang B, Shen W, Gao L, Ding Z, Wu H et al. (2013). Early activation of nSMase2/ceramide pathway in astrocytes is involved in ischemia‐associated neuronal damage via inflammation in rat hippocampi. J Neuroinflammation 10: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulbins E, Grassmé H (2002). Ceramide and cell death receptor clustering. Biochim Biophys Acta 1585: 139–145. [DOI] [PubMed] [Google Scholar]
- Gulbins E, Walter S, Becker KA, Halmer R, Liu Y, Reichel M et al. (2015). A central role for the acid sphingomyelinase/ceramide system in neurogenesis and major depression. J Neurochem 134: 183–192. [DOI] [PubMed] [Google Scholar]
- Gumireddy K, Ikegaki N, Phillips PC, Sutton LN, Reddy CD (2003a). Effect of 20‐epi‐1alpha,25‐dihydroxyvitamin D3 on the proliferation of human neuroblastoma: role of cell cycle regulators and the Myc‐Id2 pathway. Biochem Pharmacol 65: 1943–1955. [DOI] [PubMed] [Google Scholar]
- Gumireddy K, Reddy GS, Ikegaki N, Binderup L, Sutton LN, Phillips PC et al. (2003b). Anti‐proliferative effects of 20‐epi‐vitamin‐D3 analogue, KH1060 in human neuroblastoma: induction of RAR‐beta and p21(Cip1). Cancer Lett 190: 51–60. [DOI] [PubMed] [Google Scholar]
- Guo YX, He LY, Zhang M, Wang F, Liu F, Peng WX (2016). 1,25‐Dihydroxyvitamin D3 regulates expression of LRP1 and RAGE in vitro and in vivo, enhancing Aβ1‐40 brain‐to‐blood efflux and peripheral uptake transport. Neuroscience 322: 28–38. [DOI] [PubMed] [Google Scholar]
- Halmer R, Walter S, Faßbender K (2014). Sphingolipids: important players in multiple sclerosis. Cell Physiol Biochem 34: 111–118. [DOI] [PubMed] [Google Scholar]
- Hagen N, Hans M, Hartmann D, Swandulla D, van Echten‐Deckert G (2011). Sphingosine‐1‐phosphate links glycosphingolipid metabolism to neurodegeneration via a calpain‐mediated mechanism. Cell Death Differ 18: 1356–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK et al. (2009). Regulation of histone acetylation in the nucleus by sphingosine‐1‐phosphate. Science 325: 1254–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hait NC, Wise LE, Allegood JC, O'Brien M, Avni D, Reeves TM et al. (2014). Active, phosphorylated fingolimod inhibits histone deacetylases and facilitates fear extinction memory. Nat Neurosci 17: 971–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, M Holtzman D, McKeel DW Jr, Kelley J, Morris JC (2002). Substantial sulfatide deficiency and ceramide elevation in very early Alzheimer's disease: potential role in disease pathogenesis. J Neurochem 82: 809–818. [DOI] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM (2011). Many ceramides. J Biol Chem 28632: 27855–27862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao W, Tashiro S, Hasegawa T, Sato Y, Kobayashi T, Tando T et al. (2015). Hyperglycemia promotes Schwann cell de‐differentiation and de‐myelination via sorbitol accumulation and Igf1 protein down‐regulation. J Biol Chem 290: 17106–17115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada J, Foley M, Moskowitz MA, Waeber C (2004). Sphingosine‐1‐phosphate induces proliferation and morphological changes of neural progenitor cells. J Neurochem 88: 1026–1039. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Bandaru VV, Bae M, Mattson MP (2010). Roles for dysfunctional sphingolipid metabolism in Alzheimer's disease neuropathogenesis. Biochim Biophys Acta 1801: 878–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes JE, Tesic D, Whitehouse AJ, Zosky GR, Smith JT, Wyrwoll CS (2015). Maternal vitamin D deficiency alters fetal brain development in the BALB/c mouse. Behav Brain Res 286: 192–200. [DOI] [PubMed] [Google Scholar]
- He X, Huang Y, Li B, Gong CX, Schuchman EH (2010). Deregulation of sphingolipid metabolism in Alzheimer's disease. Neurobiol Aging 31: 398–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Wang G, Wakade S, Dasgupta S, Dinkins M, Kong JN et al. (2014). Primary cilia in stem cells and neural progenitors are regulated by neutral sphingomyelinase 2 and ceramide. Mol Biol Cell 25: 1715–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich M, Wickel M, Winoto‐Morbach S, Schneider‐Brachert W, Weber T, Brunner J et al. (2000). Ceramide as an activator lipid of cathepsin D. Adv Exp Med Biol 477: 305–315. [DOI] [PubMed] [Google Scholar]
- Hemmati F, Dargahi L, Nasoohi S, Omidbakhsh R, Mohamed Z, Chik Z et al. (2013). Neurorestorative effect of FTY720 in a rat model of Alzheimer's disease: comparison with memantine. Behav Brain Res 252: 415–421. [DOI] [PubMed] [Google Scholar]
- Hii CS, Ferrante A (2016). The non‐genomic actions of vitamin D. Nutrients 8: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Tanimukai H, Liu F, Iqbal K, Grundke‐Iqbal I, Gong CX (2004). Elevation of the level and activity of acid ceramidase in Alzheimer's disease brain. Eur J Neurosci 20: 3489–3497. [DOI] [PubMed] [Google Scholar]
- Huang YN, Ho YJ, Lai CC, Chiu CT, Wang JY (2015b). 1,25‐Dihydroxyvitamin D3 attenuates endotoxin‐induced production of inflammatory mediators by inhibiting MAPK activation in primary cortical neuron–glia cultures. J Neuroinflammation 12: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi K, Erlich S, Perl DP, Ferlinz K, Bisgaier CL, Sandhoff K et al. (1995). Acid sphingomyelinase deficient mice: a model of types A and B Niemann–Pick disease. Nat Genet 10: 288–293. [DOI] [PubMed] [Google Scholar]
- Huang J, Yang G, Huang Y, Kong W, Zhang S (2015a). 1,25(OH)2D3 inhibits the progression of hepatocellular carcinoma via downregulating HDAC2 and upregulating P21(WAFI/CIP1). Mol Med Rep 13: 1373–1380. [DOI] [PubMed] [Google Scholar]
- Huston JP, Kornhuber J, Mühle C, Japtok L, Komorowski M, Mattern C et al. (2016). A sphingolipid mechanism for behavioral extinction. J Neurochem 137: 589–603. [DOI] [PubMed] [Google Scholar]
- Jaillard C, Harrison S, Stankoff B, Aigrot MS, Calver AR, Duddy G et al. (2005). Edg8/S1P5: an oligodendroglial receptor with dual function on process retraction and cell survival. J Neurosci 25: 1459–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana A, Pahan K (2004). Fibrillar amyloid‐beta peptides kill human primary neurons via NADPH oxidase‐mediated activation of neutral sphingomyelinase. Implications for Alzheimer's disease. J Biol Chem 279: 51451–51459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang W, Kim HJ, Li H, Jo KD, Lee MK, Song SH et al. (2014). 1,25‐Dyhydroxyvitamin D3 attenuates rotenone‐induced neurotoxicity in SH‐SY5Y cells through induction of autophagy. Biochem Biophys Res Commun 451: 142–147. [DOI] [PubMed] [Google Scholar]
- Jazvinšćak Jembrek M, Hof PR, Šimić G (2015). Ceramides in Alzheimer's disease: key mediators of neuronal apoptosis induced by oxidative stress and Aβ accumulation. Oxid Med Cell Longev 2015: 346783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang P, Zhu WY, He X, Tang MM, Dang RL, Li HD et al. (2015). Association between vitamin D receptor gene polymorphisms with childhood temporal lobe epilepsy. Int J Environ Res Public Health 12: 13913–13922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Hou Q, Mullen TD, Zeidan YH, Bielawski J, Kraveka JM et al. (2008). Ceramide generated by sphingomyelin hydrolysis and the salvage pathway is involved in hypoxia/reoxygenation‐induced Bax redistribution to mitochondria in NT‐2 cells. J Biol Chem 283: 26509–26517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung CG, Kim HJ, Miron VE, Cook S, Kennedy TE, Foster CA et al. (2007). Functional consequences of S1P receptor modulation in rat oligodendroglial lineage cells. Glia 55: 1656–1667. [DOI] [PubMed] [Google Scholar]
- Jung JS, Shin KO, Lee YM, Shin JA, Park EM, Jeong J et al. (2013). Anti‐inflammatory mechanism of exogenous C2 ceramide in lipopolysaccharide‐stimulated microglia. Biochim Biophys Acta 1831: 1016–1026. [DOI] [PubMed] [Google Scholar]
- Kajimoto T, Okada T, Yu H, Goparaju SK, Jahangeer S, Nakamura S (2007). Involvement of sphingosine‐1‐phosphate in glutamate secretion in hippocampal neurons. Mol Cell Biol 27: 3429–3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajta M, Makarewicz D, Ziemińska E, Jantas D, Domin H, Lasoń W et al. (2009). Neuroprotection by co‐treatment and post‐treating with calcitriol following the ischemic and excitotoxic insult in vivo and in vitro. Neurochem Int 55: 265–274. [DOI] [PubMed] [Google Scholar]
- Kanno T, Nishizaki T, Proia RL, Kajimoto T, Jahangeer S, Okada T et al. (2010). Regulation of synaptic strength by sphingosine 1‐phosphate in the hippocampus. Neuroscience 171: 973–980. [DOI] [PubMed] [Google Scholar]
- Katsel P, Li C, Haroutunian V (2007). Gene expression alterations in the sphingolipid metabolism pathways during progression of dementia and Alzheimer's disease: a shift toward ceramide accumulation at the earliest recognizable stages of Alzheimer's disease? Neurochem Res 32: 845–856. [DOI] [PubMed] [Google Scholar]
- Kawabori M, Kacimi R, Karliner JS, Yenari MA (2013). Sphingolipids in cardiovascular and cerebrovascular systems: pathological implications and potential therapeutic targets. World J Cardiol 5: 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilhoff G, Grecksch G, Becker A (2010). Haloperidol normalized prenatal vitamin D depletion‐induced reduction of hippocampal cell proliferation in adult rats. Neurosci Lett 476: 94–98. [DOI] [PubMed] [Google Scholar]
- Kikuta J, Kawamura S, Okiji F, Shirazaki M, Sakai S, Saito H et al. (2013). Sphingosine‐1‐phosphate‐mediated osteoclast precursor monocyte migration is a critical point of control in antibone‐resorptive action of active vitamin D. Proc Natl Acad Sci U S A 110: 7009–7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgore M, Miller CA, Fass DM, Hennig KM, Haggarty SJ, Sweatt JD et al. (2010). Inhibitors of class 1 histone deacetylases reverse contextual memory deficits in a mouse model of Alzheimer's disease. Neuropsychopharmacology 35: 870–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Schneider G, Abdel‐Latif A, Mierzejewska K, Sunkara M, Borkowska S et al. (2013). Ceramide‐1‐phosphate regulates migration of multipotent stromal cells and endothelial progenitor cells‐‐implications for tissue regeneration. Stem Cells 313: 500–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Ryu SY, Yun I, Kim WJ, Lee KS, Park JW et al. (2006). 1alpha,25‐dihydroxyvitamin D(3) protects dopaminergic neurons in rodent models of Parkinson's disease through inhibition of microglial activation. J Clin Neurol 2: 252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK, Ahn KH, Ji JE, Choi JM, Jeon HJ, Jung SY et al. (2010). Neutral sphingomyelinase 2 induces dopamine uptake through regulation of intracellular calcium. Cell Signal 22: 865–870. [DOI] [PubMed] [Google Scholar]
- Kim S, Steelman AJ, Zhang Y, Kinney HC, Li J (2012). Aberrant upregulation of astroglial ceramide potentiates oligodendrocyte injury. Brain Pathol 22: 41–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A, Ohmori T, Ohkawa R, Madoiwa S, Mimuro J, Murakami T et al. (2007). Essential roles of sphingosine 1‐phosphate/S1P1 receptor axis in the migration of neural stem cells toward a site of spinal cord injury. Stem Cells 25: 115–124. [DOI] [PubMed] [Google Scholar]
- Kleuser B, Cuvillier O, Spiegel S (1998). 1Alpha,25‐dihydroxyvitamin D3 inhibits programmed cell death in HL‐60 cells by activation of sphingosine kinase. Cancer Res 58: 1817–1824. [PubMed] [Google Scholar]
- Ko P, Burkert R, McGrath J, Eyles D (2004). Maternal vitamin D3 deprivation and the regulation of apoptosis and cell cycle during rat brain development. Brain Res Dev Brain Res 153: 61–68. [DOI] [PubMed] [Google Scholar]
- Lachmann RH, te Vruchte D, Lloyd‐Evans E, Reinkensmeier G, Sillence DJ, Fernandez‐Guillen L et al. (2004). Treatment with miglustat reverses the lipid‐trafficking defect in Niemann–Pick disease type C. Neurobiol Dis 16: 654–658. [DOI] [PubMed] [Google Scholar]
- Landel V, Annweiler C, Millet P, Morello M, Féron F (2016). Vitamin D, cognition, and Alzheimer's disease: the therapeutic benefit is in the D‐tails. J Alzheimers Dis 53: 419–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latimer CS, Brewer LD, Searcy JL, Chen KC, Popović J, Kraner SD et al. (2014). Vitamin D prevents cognitive decline and enhances hippocampal synaptic function in aging rats. Proc Natl Acad Sci U S A 111: E4359–E4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzarini A, Macchiarulo A, Floridi A, Coletti A, Cataldi S, Codini M et al. (2015). Very‐long‐chain fatty acid sphingomyelin in nuclear lipid microdomains of hepatocytes and hepatoma cells: can the exchange from C24:0 to C16:0 affect signal proteins and Vit D receptor? Mol Biol Cell 26: 2418–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Lee JK, Min WK, Bae JH, He X, Schuchman EH et al. (2010). Bone marrow‐derived mesenchymal stem cells prevent the loss of Niemann–Pick type C mouse Purkinje neurons by correcting sphingolipid metabolism and increasing sphingosine‐1‐phosphate. Stem Cells 28: 821–831. [DOI] [PubMed] [Google Scholar]
- Lee PS, Sampath K, Karumanchi SA, Tamez H, Bhan I, Isakova T et al. (2009). Plasma gelsolin and circulating actin correlate with hemodialysis mortality. J Am Soc Nephrol 20: 1140–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V, Rekhi E, Hoh Kam J, Jeffery G (2012). Vitamin D rejuvenates aging eyes by reducing inflammation, clearing amyloid beta and improving visual function. Neurobiol Aging 33: 2382–2389. [DOI] [PubMed] [Google Scholar]
- Lemire JM, Archer DC (1991). 1,25‐dihydroxyvitamin D3 prevents the in vivo induction of murine experimental autoimmune encephalomyelitis. J Clin Invest 87: 1103–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyssens C, Verlinden L, Verstuyf A (2014). The future of vitamin D analogs. Front Physiol 5: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Prabhakaran K, Zhang X, Zhang L, Liu H, Borowitz JL et al. (2008). 1Alpha,25‐dihydroxyvitamin D3 attenuates cyanide‐induced neurotoxicity by inhibiting uncoupling protein‐2 up‐regulation. J Neurosci Res 86: 1397–1408. [DOI] [PubMed] [Google Scholar]
- Li H, Jang W, Kim HJ, Jo KD, Lee MK, Song SH et al. (2015b). Biochemical protective effect of 1,25‐dihydroxyvitamin D3 through autophagy induction in the MPTP mouse model of Parkinson's disease. Neuroreport 26: 669–674. [DOI] [PubMed] [Google Scholar]
- Li C, Li JN, Kays J, Guerrero M, Nicol GD (2015a). Sphingosine 1‐phosphate enhances the excitability of rat sensory neurons through activation of sphingosine 1‐phosphate receptors 1 and/or 3. J Neuroinflammation 12: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AM, Fan SF, Yang DM, Hsu LL, Yang CH (2003). Zinc‐induced apoptosis in substantia nigra of rat brain: neuroprotection by vitamin D3. Free Radic Biol Med 34: 1416–1425. [DOI] [PubMed] [Google Scholar]
- Lingwood D, Simons K (2010). Lipid rafts as a membrane‐organizing principle. Science 327: 46–50. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP et al. (2000). Edg‐1, the G protein‐coupled receptor for sphingosine‐1‐phosphate, is essential for vascular maturation. J Clin Invest 106: 951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu NK, Deng LX, Zhang YP, Lu QB, Wang XF, Hu JG et al. (2014). Cytosolic phospholipase A2 protein as a novel therapeutic target for spinal cord injury. Ann Neurol 75: 644–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd‐Evans E, Morgan AJ, He X, Smith DA, Elliot‐Smith E, Sillence DJ et al. (2008). Niemann–Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nat Med 14: 1247–1255. [DOI] [PubMed] [Google Scholar]
- Longoni A, Kolling J, dos Santos TM, dos Santos JP, da Silva JS, Pettenuzzo L et al. (2016). 1,25‐Dihydroxyvitamin D3 exerts neuroprotective effects in an ex vivo model of mild hyperhomocysteinemia. Int J Dev Neurosci 48: 71–79. [DOI] [PubMed] [Google Scholar]
- Maceyka M, Harikumar KB, Milstien S, Spiegel S (2012). Sphingosine‐1‐phosphate signaling and its role in disease. Trends Cell Biol 22: 50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrassi L, Adorni L, Montorfano G, Rapelli S, Butti G, Berra B et al. (1998). Vitamin D metabolites activate the sphingomyelin pathway and induce death of glioblastoma cells. Acta Neurochir 140: 707–714. [DOI] [PubMed] [Google Scholar]
- Malaplate‐Armand C, Florent‐Béchard S, Youssef I, Koziel V, Sponne I, Kriem B et al. (2006). Soluble oligomers of amyloid‐beta peptide induce neuronal apoptosis by activating a cPLA2‐dependent sphingomyelinase‐ceramide pathway. Neurobiol Dis 23: 178–189. [DOI] [PubMed] [Google Scholar]
- Malnar M, Hecimovic S, Mattsson N, Zetterberg H (2014). Bidirectional links between Alzheimer's disease and Niemann–Pick type C disease. Neurobiol Dis 72 (Pt A): 37–47. [DOI] [PubMed] [Google Scholar]
- Manggau M, Kim DS, Ruwisch L, Vogler R, Korting HC, Schäfer‐Korting M et al. (2001). 1Alpha,25‐dihydroxyvitamin D3 protects human keratinocytes from apoptosis by the formation of sphingosine‐1‐phosphate. J Invest Dermatol 117: 1241–1249. [DOI] [PubMed] [Google Scholar]
- Marin R, Fabelo N, Fernández‐Echevarría C, Canerina‐Amaro A, Rodríguez‐Barreto D, Quinto‐Alemany D et al. (2016). Lipid raft alterations in aged‐associated neuropathologies. Curr Alzheimer Res 13: 973–984. [DOI] [PubMed] [Google Scholar]
- Marini F, Bartoccini E, Cascianelli G, Voccoli V, Baviglia MG, Magni MV et al. (2010). Effect of 1alpha,25‐dihydroxyvitamin D3 in embryonic hippocampal cells. Hippocampus 20: 696–705. [DOI] [PubMed] [Google Scholar]
- Marques AR, Aten J, Ottenhoff R, van Roomen CP, Herrera Moro D, Claessen N et al. (2015). Reducing GBA2 activity ameliorates neuropathology in Niemann–Pick type C mice. PLoS One 10: e0135889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzulli JR, Xu YH, Sun Y, Knight AL, McLean PJ, Caldwell GA et al. (2011). Gaucher disease glucocerebrosidase and α‐synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell 146: 37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzulli JR, Zunke F, Tsunemi T, Toker NJ, Jeon S, Burbulla LF et al. (2016). Activation of β‐glucocerebrosidase reduces pathological α‐synuclein and restores lysosomal function in Parkinson's patient midbrain neurons. J Neurosci 36: 7693–7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mencarelli C, Martinez‐Martinez P (2013). Ceramide function in the brain: when a slight tilt is enough. Cell Mol Life Sci 70: 181–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM, Lyketsos CG (2010). Alterations of the sphingolipid pathway in Alzheimer's disease: new biomarkers and treatment targets? Neuromolecular Med 12: 331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migdalska‐Richards A, Schapira AH (2016). The relationship between glucocerebrosidase mutations and Parkinson disease. J Neurochem 139 (Suppl 1): 77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda GE, Abrahan CE, Politi LE, Rotstein NP (2009). Sphingosine‐1‐phosphate is a key regulator of proliferation and differentiation in retina photoreceptors. Invest Ophthalmol Vis Sci 50: 4416–4428. [DOI] [PubMed] [Google Scholar]
- Miranda GE, Abrahan CE, Agnolazza DL, Politi LE, Rotstein NP (2011). Ceramide‐1‐phosphate, a new mediator of development and survival in retina photoreceptors. Invest Ophthalmol Vis Sci 52: 6580–6588. [DOI] [PubMed] [Google Scholar]
- Miguez A, García‐Díaz Barriga G, Brito V, Straccia M, Giralt A, Ginés S et al. (2015). Fingolimod (FTY720) enhances hippocampal synaptic plasticity and memory in Huntington's disease by preventing p75NTR up‐regulation and astrocyte‐mediated inflammation. Hum Mol Genet 24: 4958–4970. [DOI] [PubMed] [Google Scholar]
- Mitsutake S, Yokose U, Kato M, Matsuoka I, Yoo JM, Kim TJ et al. (2007). The generation and behavioral analysis of ceramide kinase‐null mice, indicating a function in cerebellar Purkinje cells. Biochem Biophys Res Commun 363: 519–524. [DOI] [PubMed] [Google Scholar]
- Mizwicki MT, Liu G, Fiala M, Magpantay L, Sayre J, Siani A et al. (2013). 1α,25‐dihydroxyvitamin D3 and resolvin D1 retune the balance between amyloid‐β phagocytosis and inflammation in Alzheimer's disease patients. J Alzheimers Dis 34: 155–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizwicki MT, Menegaz D, Zhang J, Barrientos‐Durán A, Tse S, Cashman JR et al. (2012). Genomic and nongenomic signaling induced by 1α,25(OH)2‐vitamin D3 promotes the recovery of amyloid‐β phagocytosis by Alzheimer's disease macrophages. J Alzheimers Dis 29: 51–62. [DOI] [PubMed] [Google Scholar]
- Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, Proia RL (2005). Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol 25: 11113–11121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Ichihara M, Sobue S, Kikuchi R, Ito H, Kimura A et al. (2007). RET signaling‐induced SPHK1 gene expression plays a role in both GDNF‐induced differentiation and MEN2‐type oncogenesis. J Neurochem 102: 1585–1594. [DOI] [PubMed] [Google Scholar]
- Murphy KE, Gysbers AM, Abbott SK, Tayebi N, Kim WS, Sidransky E et al. (2014). Reduced glucocerebrosidase is associated with increased α‐synuclein in sporadic Parkinson's disease. Brain 137 (Pt 3): 834–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveilhan P, Neveu I, Baudet C, Ohyama KY, Brachet P, Wion D (1993). Expression of 25(OH) vitamin D3 24‐ hydroxylase gene in glial cells. Neuroreport 5: 255–257. [DOI] [PubMed] [Google Scholar]
- Newton J, Lima S, Maceyka M, Spiegel S (2015). Revisiting the sphingolipid rheostat: evolving concepts in cancer therapy. Exp Cell Res 333: 195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novgorodov AS, El‐Alwani M, Bielawski J, Obeid LM, Gudz TI (2007). Activation of sphingosine‐1‐phosphate receptor S1P5 inhibits oligodendrocyte progenitor migration. FASEB J 21: 1503–1514. [DOI] [PubMed] [Google Scholar]
- Oermann E, Bidmon HJ, Witte OW, Zilles K (2004). Effects of 1alpha,25 dihydroxyvitamin D3 on the expression of HO‐1 and GFAP in glial cells of the photothrombotically lesioned cerebral cortex. J Chem Neuroanat 28: 225–238. [DOI] [PubMed] [Google Scholar]
- Okazaki T, Bell RM, Hannun YA (1989). Sphingomyelin turnover induced by vitamin D3 in HL‐60 cells. Role in cell differentiation. J Biol Chem 264: 19076–19080. [PubMed] [Google Scholar]
- Orme RP, Bhangal MS, Fricker RA (2013). Calcitriol imparts neuroprotection in vitro to midbrain dopaminergic neurons by upregulating GDNF expression. PLoS One 8: e62040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn TM, Verdrengh M, Stossel TP, Tarkowski A, Bokarewa M (2008). Decreased levels of the gelsolin plasma isoform in patients with rheumatoid arthritis. Arthritis Res Ther 10: R117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani R, Tomimoto H, Kondo T, Wakita H, Akiguchi I, Shibasaki H et al. (2004). Upregulation of ceramide and its regulating mechanism in a rat model of chronic cerebral ischemia. Brain Res 1023: 31–40. [DOI] [PubMed] [Google Scholar]
- Patrick RP, Ames BN (2014). Vitamin D hormone regulates serotonin synthesis. Part 1: relevance for autism. FASEB J 28: 2398–2413. doi: 10.1096/fj.13-246546. [DOI] [PubMed] [Google Scholar]
- Patterson MC, Mengel E, Vanier MT, Schwierin B, Muller A, Cornelisse P et al. (2015). Stable or improved neurological manifestations during miglustat therapy in patients from the international disease registry for Niemann‐Pick disease type C: an observational cohort study. Orphanet J Rare Dis 10: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson AL (2014). A review of vitamin D and Parkinson's disease. Maturitas 78: 40–44. [DOI] [PubMed] [Google Scholar]
- Pettus BJ, Bielawska A, Subramanian P, Wijesinghe DS, Maceyka M, Leslie CC et al. (2004). Ceramide 1‐phosphate is a direct activator of cytosolic phospholipase A2. J Biol Chem 279: 11320–11326. [DOI] [PubMed] [Google Scholar]
- Pierucci F, Garcia‐Gil M, Frati A, Bini F, Martinesi M, Vannini E et al. (2017). Vitamin D(3) protects against Aβ peptide cytotoxicity in differentiated human neuroblastoma SH‐ SY5Y cells: A role for S1P1/p38MAPK/ATF4 axis. Neuropharmacology 116: 328–342. [DOI] [PubMed] [Google Scholar]
- Plagg B, Ehrlich D, Kniewallner KM, Marksteiner J, Humpel C (2015). Increased acetylation of histone H4 at lysine 12 (H4K12) in monocytes of transgenic Alzheimer's mice and in human patients. Curr Alzheimer Res 12: 752–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma FR, Jalink K, Hengeveld T, Moolenaar WH (1996). Sphingosine‐1‐phosphate rapidly induces Rho‐dependent neurite retraction: action through a specific cell surface receptor. EMBO J 15: 2388–2392. [PMC free article] [PubMed] [Google Scholar]
- Presa N, Gomez‐Larrauri A, Rivera IG, Ordoñez M, Trueba M, Gomez‐Muñoz A (2016). Regulation of cell migration and inflammation by ceramide 1‐phosphate. Biochim Biophys Acta 1861: 402–409. [DOI] [PubMed] [Google Scholar]
- Proia RL, Hla T (2015). Emerging biology of sphingosine‐1‐phosphate: its role in pathogenesis and therapy. J Clin Invest 125: 1379–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglielli L, Ellis BC, Saunders AJ, Kovacs DM (2003). Ceramide stabilizes beta‐site amyloid precursor protein‐cleaving enzyme 1 and promotes amyloid beta‐peptide biogenesis. J Biol Chem 278: 19777–19783. [DOI] [PubMed] [Google Scholar]
- Pyszko JA, Strosznajder JB (2014). The key role of sphingosine kinases in the molecular mechanism of neuronal cell survival and death in an experimental model of Parkinson's disease. Folia Neuropathol 52: 260–269. [DOI] [PubMed] [Google Scholar]
- Qin J, Berdyshev E, Goya J, Natarajan V, Dawson G (2010). Neurons and oligodendrocytes recycle sphingosine 1‐phosphate to ceramide: significance for apoptosis and multiple sclerosis. J Biol Chem 285: 14134–14143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha EM, Smith GA, Park E, Cao H, Brown E, Hallett P et al. (2015). Progressive decline of glucocerebrosidase in aging and Parkinson's disease. Ann Clin Transl Neurol 2: 433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon BJ, Hansen JG, Crystal RG, Houston DK, Kritchevsky SB, Harris T et al. (2013). Vitamin D‐responsive SGPP2 variants associated with lung cell expression and lung function. BMC Med Genet 14: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riboni L, Prinetti A, Bassi R, Caminiti A, Tettamanti G (1995). A mediator role of ceramide in the regulation of neuroblastoma Neuro2a cell differentiation. J Biol Chem 270: 26868–26875. [DOI] [PubMed] [Google Scholar]
- Riganti L, Antonucci F, Gabrielli M, Prada I, Giussani P, Viani P et al. (2016). Sphingosine‐1‐phosphate (S1P) impacts presynaptic functions by regulating synapsin I localization in the presynaptic compartment. J Neurosci 36: 4624–4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabourdy F, Astudillo L, Colacios C, Dubot P, Mrad M, Ségui B et al. (2015). Monogenic neurological disorders of sphingolipid metabolism. Biochim Biophys Acta 1851: 1040–1051. [DOI] [PubMed] [Google Scholar]
- Saini HS, Coelho RP, Goparaju SK, Jolly PS, Maceyka M, Spiegel S et al. (2005). Novel role of sphingosine kinase 1 as a mediator of neurotrophin‐3 action in oligodendrocyte progenitors. J Neurochem 95: 1298–1310. [DOI] [PubMed] [Google Scholar]
- Salomón DG, Fermento ME, Gandini NA, Ferronato MJ, Arévalo J, Blasco J et al. (2014). Vitamin D receptor expression is associated with improved overall survival in human glioblastoma multiforme. J Neurooncol 118: 49–60. [DOI] [PubMed] [Google Scholar]
- Sato K, Ui M, Okajima F (2000). Differential roles of Edg‐1 and Edg‐5, sphingosine 1‐phosphate receptors, in the signaling pathways in C6 glioma cells. Brain Res Mol Brain Res 85: 151–160. [DOI] [PubMed] [Google Scholar]
- Satoi H, Tomimoto H, Ohtani R, Kitano T, Kondo T, Watanabe M et al. (2005). Astroglial expression of ceramide in Alzheimer's disease brains: a role during neuronal apoptosis. Neuroscience 130: 657–666. [DOI] [PubMed] [Google Scholar]
- Sauer B, Ruwisch L, Kleuser B (2003). Antiapoptotic action of 1alpha,25‐dihydroxyvitamin D3 in primary human melanocytes. Melanoma Res 13: 339–347. [DOI] [PubMed] [Google Scholar]
- Schöndorf DC, Aureli M, McAllister FE, Hindley CJ, Mayer F, Schmid B et al. (2014). iPSC‐derived neurons from GBA1‐associated Parkinson's disease patients show autophagic defects and impaired calcium homeostasis. Nat Commun 5: 4028–4045. [DOI] [PubMed] [Google Scholar]
- Schengrund CL (2010). Lipid rafts: keys to neurodegeneration. Brain Res Bull 82: 7–17. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, Hardy J (2016). The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol Med 8: 595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamseddine AA, Airola MV, Hannun YA (2015). Roles and regulation of neutral sphingomyelinase‐2 in cellular and pathological processes. Adv Biol Regul 57: 24–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Giordano F, Wu Y, Chan J, Zhu C, Milosevic I et al. (2014). Coupling between endocytosis and sphingosine kinase 1 recruitment. Nat Cell Biol 16: 652–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Ji HF (2015a). Associations between vitamin D status, supplementation, outdoor work and risk of Parkinson's disease: a meta‐analysis assessment. Nutrients 7: 4817–4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Ji HF (2015b). Vitamin D deficiency is associated with increased risk of Alzheimer's disease and dementia: evidence from meta‐analysis. Nutr J 14: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinpo K, Kikuchi S, Sasaki H, Moriwaka F, Tashiro K (2000). Effect of 1,25‐dihydroxyvitamin D(3) on cultured mesencephalic dopaminergic neurons to the combined toxicity caused by L‐buthionine sulfoximine and 1‐methyl‐4‐phenylpyridine. J Neurosci Res 62: 374–382. [DOI] [PubMed] [Google Scholar]
- Shirazi HA, Rasouli J, Ciric B, Rostami A, Zhang GX (2015). 1,25‐Dihydroxyvitamin D3 enhances neural stem cell proliferation and oligodendrocyte differentiation. Exp Mol Pathol 98: 240–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MP, Fletcher‐Turner A, Yurek DM, Cass WA (2006). Calcitriol protection against dopamine loss induced by intracerebroventricular administration of 6‐hydroxydopamine. Neurochem Res 31: 533–539. [DOI] [PubMed] [Google Scholar]
- Sobue S, Hagiwara K, Banno Y, Tamiya‐Koizumi K, Suzuki M, Takagi A et al. (2005). Transcription factor specificity protein 1 (Sp1) is the main regulator of nerve growth factor‐induced sphingosine kinase 1 gene expression of the rat pheochromocytoma cell line, PC12. J Neurochem 95: 940–949. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassieva SD, Mullen TD, Townsend DM, Obeid LM (2009). Disruption of ceramide synthesis by CERS2 down‐regulation leads to autophagy and the unfolded protein response. Biochem J 424: 273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spedding S (2014). Vitamin D and depression: a systematic review and meta‐analysis comparing studies with and without biological flaws. Nutrients 6: 1501–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel S, Milstien S (2003). Sphingosine‐1‐phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol 4: 397–407. [DOI] [PubMed] [Google Scholar]
- Steck PA, Ligon AH, Cheong P, Yung WK, Pershouse MA (1995). Two tumor suppressive loci on chromosome 10 involved in human glioblastomas. Genes Chromosomes Cancer 12: 255–261. [DOI] [PubMed] [Google Scholar]
- Stein VM, Crooks A, Ding W, Prociuk M, O'Donnell P, Bryan C et al. (2012). Miglustat improves purkinje cell survival and alters microglial phenotype in feline Niemann–Pick disease type C. J Neuropathol Exp Neurol 71: 434–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stio M, Celli A, Treves C (2001). Synergistic anti‐proliferative effects of vitamin D derivatives and 9‐cis retinoic acid in SH‐SY5Y human neuroblastoma cells. J Steroid Biochem Mol Biol 77: 213–222. [DOI] [PubMed] [Google Scholar]
- Strettoi E, Gargini C, Novelli E, Sala G, Piano I, Gasco P et al. (2010). Inhibition of ceramide biosynthesis preserves photoreceptor structure and function in a mouse model of retinitis pigmentosa. Proc Natl Acad Sci U S A 107: 18706–18711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabatadze N, Savonenko A, Song H, Bandaru VV, Chu M, Haughey NJ (2010). Inhibition of neutral sphingomyelinase‐2 perturbs brain sphingolipid balance and spatial memory in mice. J Neurosci Res 88: 2940–2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasugi N, Sasaki T, Suzuki K, Osawa S, Isshiki H, Hori Y et al. (2011). BACE1 activity is modulated by cell‐associated sphingosine‐1‐phosphate. J Neurosci 31: 6850–6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasugi N, Sasaki T, Ebinuma I, Osawa S, Isshiki H, Takeo K et al. (2013). FTY720/fingolimod, a sphingosine analogue, reduces amyloid‐β production in neurons. PLoS One 8: e64050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniura H, Ito M, Sanada N, Kuramoto N, Ohno Y, Nakamichi N et al. (2006). Chronic vitamin D3 treatment protects against neurotoxicity by glutamate in association with upregulation of vitamin D receptor mRNA expression in cultured rat cortical neurons. J Neurosci Res 83: 1179–1189. [DOI] [PubMed] [Google Scholar]
- Toman RE, Payne SG, Watterson KR, Maceyka M, Lee NH, Milstien S et al. (2004). Differential transactivation of sphingosine‐1‐phosphate receptors modulates NGF‐induced neurite extension. J Cell Biol 166: 381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F et al. (2008). Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319: 1244–1247. [DOI] [PubMed] [Google Scholar]
- Van Brocklyn JR, Jackson CA, Pearl DK, Kotur MS, Snyder PJ, Prior TW (2005). Sphingosine kinase‐1 expression correlates with poor survival of patients with glioblastoma multiforme: roles of sphingosine kinase isoforms in growth of glioblastoma cell lines. J Neuropathol Exp Neurol 64: 695–705. [DOI] [PubMed] [Google Scholar]
- Vanier MT (2015). Complex lipid trafficking in Niemann–Pick disease type C. J Inherit Metab Dis 38: 187–199. [DOI] [PubMed] [Google Scholar]
- Vanni N, Fruscione F, Ferlazzo E, Striano P, Robbiano A, Traverso M et al. (2014). Impairment of ceramide synthesis causes a novel progressive myoclonus epilepsy. Ann Neurol 76: 206–212. [DOI] [PubMed] [Google Scholar]
- Vidal‐Martínez G, Vargas‐Medrano J, Gil‐Tommee C, Medina D, Garza NT, Yang B et al. (2016). FTY720/fingolimod reduces synucleinopathy and improves gut motility in A53T mice: contributions of pro‐brain‐derived neurotrophic factor (pro‐bdnf) and mature BDNF. J Biol Chem 291: 20811–20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Silva J, Dasgupta S, Bieberich E (2008). Long‐chain ceramide is elevated in presenilin 1 (PS1M146V) mouse brain and induces apoptosis in PS1 astrocytes. Glia 56: 449–456. [DOI] [PubMed] [Google Scholar]
- Wang G, Dinkins M, He Q, Zhu G, Poirier C, Campbell A et al. (2012). Astrocytes secrete exosomes enriched with proapoptotic ceramide and prostate apoptosis response 4 (PAR‐4): potential mechanism of apoptosis induction in Alzheimer disease (AD). J Biol Chem 287: 21384–21395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TT, Tavera‐Mendoza LE, Laperriere D, Libby E, MacLeod NB, Nagai Y et al. (2005). Large‐scale in silico and microarray‐based identification of direct1,25‐dihydroxyvitamin D3 target genes. Mol Endocrinol 19: 2685–2695. [DOI] [PubMed] [Google Scholar]
- Wang JY, Wu JN, Cherng TL, Hoffer BJ, Chen HH, Borlongan CV et al. (2001). Vitamin D(3) attenuates 6‐hydroxydopamine‐induced neurotoxicity in rats. Brain Res 904: 67–75. [DOI] [PubMed] [Google Scholar]
- Wheeler D, Knapp E, Bandaru VV, Wang Y, Knorr D, Poirier C et al (2009). Tumor necrosis factor‐alpha‐induced neutral sphingomyelinase‐2 modulates synaptic plasticity by controlling the membrane insertion of NMDA receptors. J Neurochem 109: 1237–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse AJ, Holt BJ, Serralha M, Holt PG, Kusel MM, Hart PH (2012). Maternal serum vitamin D levels during pregnancy and offspring neurocognitive development. Pediatrics 129: 485–493. [DOI] [PubMed] [Google Scholar]
- Winner B, Winkler J (2015). Adult neurogenesis in neurodegenerative diseases. Cold Spring Harb Perspect Biol 7: a021287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata K, Tagami M, Torii Y, Takenaga F, Tsumagari S, Itoh S et al. (2003). Sphingosine 1‐phosphate induces the production of glial cell line‐derived neurotrophic factor and cellular proliferation in astrocytes. Glia 41: 199–206. [DOI] [PubMed] [Google Scholar]
- Yang DS, Stavrides P, Saito M, Kumar A, Rodriguez‐Navarro JA, Pawlik M et al. (2014). Defective macroautophagic turnover of brain lipids in the TgCRND8 Alzheimer mouse model: prevention by correcting lysosomal proteolytic deficits. Brain 137 (Pt 12): 3300–3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MM, Kester M, Wang HG (2013). Sphingolipids: regulators of crosstalk between apoptosis and autophagy. J Lipid Res 54: 5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Gattoni‐Celli M, Zhu H, Bhat NR, Sambamurti K, Gattoni‐Celli S et al. (2011). Vitamin D3‐enriched diet correlates with a decrease of amyloid plaques in the brain of AβPP transgenic mice. J Alzheimers Dis 25: 295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuyama K, Sun H, Usuki S, Sakai S, Hanamatsu H, Mioka T et al. (2015). A potential function for neuronal exosomes: sequestering intracerebral amyloid‐β peptide. FEBS Lett 589: 84–88. [DOI] [PubMed] [Google Scholar]
- Zanatta L, Goulart PB, Gonçalves R, Pierozan P, Winkelmann‐Duarte EC, Woehl VM et al. (2012). 1α,25‐dihydroxyvitamin D(3) mechanism of action: modulation of L‐type calcium channels leading to calcium uptake and intermediate filament phosphorylation in cerebral cortex of young rats. Biochim Biophys Acta 1823: 1708–1719. [DOI] [PubMed] [Google Scholar]
- Zebrakovská I, Máša M, Srp J, Horn M, Vávrová K, Mareš M (2011). Complex modulation of peptidolytic activity of cathepsin D by sphingolipids. Biochim Biophys Acta 1811: 1097–1104. [DOI] [PubMed] [Google Scholar]
- Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM et al. (2001). Extrarenal expression of 25‐hydroxyvitamin d(3)‐1 alpha‐hydroxylase. J Clin Endocrinol Metab 86: 888–894. [DOI] [PubMed] [Google Scholar]
- Zhao S, Aviles ER Jr, Fujikawa DG (2010). Nuclear translocation of mitochondrial cytochrome c, lysosomal cathepsins B and D, and three other death‐promoting proteins within the first 60 minutes of generalized seizures. J Neurosci Res 88: 1727–1737. [DOI] [PubMed] [Google Scholar]
- Zhao L, Spassieva SD, Jucius TJ, Shultz LD, Shick HE, Macklin WB et al. (2011). A deficiency of ceramide biosynthesis causes cerebellar purkinje cell neurodegeneration and lipofuscin accumulation. PLoS Genet 7: e1002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Chi XX, Nicol GD (2008). Brain‐derived neurotrophic factor enhances the excitability of rat sensory neurons through activation of the p75 neurotrophin receptor and the sphingomyelin pathway. J Physiol 586: 3113–3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zhuang XD, Meng FH, Chen L, Dong XB, Liu GH et al. (2016). Calcitriol prevents peripheral RSC96 Schwann neural cells from high glucose & methylglyoxal‐induced injury through restoration of CBS/H2S expression. Neurochem Int 92: 49–57. [DOI] [PubMed] [Google Scholar]
- Zheng S, Wei S, Wang X, Xu Y, Xiao Y, Liu H et al. (2015). Sphingosine kinase 1 mediates neuroinflammation following cerebral ischemia. Exp Neurol 272: 160–169. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Qin Z, Gao J, Yang M, Qin Y, Shen T et al. (2014). Vitamin D therapy in experimental allergic encephalomyelitis could be limited by opposing effects of sphingosine 1‐phosphate and gelsolin dysregulation. Mol Neurobiol 50: 733–743. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Zhou R, Yang R, Zhang Z, Bai Y, Chang F et al. (2012). Abnormal neurogenesis in the dentate gyrus of adult mice lacking 1,25‐dihydroxy vitamin D3 (1,25‐(OH)2 D3). Hippocampus 22: 421–433. [DOI] [PubMed] [Google Scholar]


